- Department of Urology, The Seventh Affiliated Hospital of Sun Yat-sen University, Shenzhen, China
Background: Mobile health (mHealth) is an accessible strategy to deliver health information and is becoming increasingly popular as a form of follow-up among medical staff. However, the effects of mobile health on the physical and mental health outcomes of patients with prostate cancer after discharge from the hospital remain unclear. This meta-analysis evaluated the current evidence regarding the effects of mHealth interventions on the outcomes of patients with prostate cancer.
Methods: Four databases (PubMed, Cochrane Central electronic database, EMBASE, and Web of Science) were searched from inception to 8 November 2024 for randomized controlled trials (RCTs) comparing the effects of mobile health vs. usual care on the outcomes of patients with prostate cancer. Pooled outcome measures were determined using random effects models.
Results: In total, 11 RCTs, including 1,368 patients, met the criteria for inclusion in this meta-analysis. The meta-analysis revealed a significant effect of mHealth interventions on long-term bowel function outcomes (standard mean difference = 0.19, 95% confidence interval = 0.01–0.37, P = 0.04, I2 = 0.00%) compared with the usual standard care or no mHealth. However, no significant differences were observed in the following outcomes: short-term and long-term effects on anxiety, depression, self-efficacy, psychological distress, and urinary and hormonal function, and short-term effects on bowel function.
Conclusions: mHealth interventions can significantly improve long-term bowel function outcomes. However, more research is needed to confirm other physical and mental health outcomes.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, PROSPERO (CRD420250651320).
1 Introduction
Prostate cancer (PC) is the second-most prevalent cancer in men worldwide (1). GLOBOCAN 2020 estimates reported 1,414,259 new cases of prostate cancer globally in 2020, with a higher prevalence in developed countries (2). During active treatment (prostatectomy, chemotherapy, radiotherapy, and/or hormone therapy) and the years after (survivorship), many patients experience symptom distress, including physical (urinary symptoms, bowel symptoms, sexual dysfunction, hormonal imbalance symptoms, etc.), psychological (anxiety, depression, etc.), and social aspects (stigma, social alienation, etc.), which can negatively affect quality of life (QOL) (3–5). Studies have shown that the incidence of urinary incontinence among localized prostate cancer was 4%–31%, the incidence of fecal incontinence was 4%–10%, and the incidence of sexual dysfunction was 11%–87%. These symptoms can even last as long as 10 years (6–9). Compared to men with localized disease, men with advanced prostate cancer experience greater pain and fatigue, higher levels of psychosocial distress, increased risk of suicide, and poorer health-related quality of life (10–12). Many of these symptoms persist for years, even after treatment is completed. As a result, many patients suffer from socioeconomic loss, resulting in significant life changes. Therefore, a strategy to reduce symptom distress during treatment or after discharge is of great clinical value.
Mobile health (mHealth) is an accessible strategy for delivering health information and has been widely used during the COVID-19 pandemic (13, 14). A recent meta-analysis revealed that mHealth interventions may provide an acceptable and feasible strategy to deliver continuity of health support to patients between medical appointments (15). Moreover, these interventions offer a convenient way to support self-management of cancer-related symptoms (16). Whether an individual receives treatment for PC upon diagnosis or is actively monitored over time, mHealth applications offer great opportunities for men to receive personalized, timely, high-quality, and evidence-based care (17). However, patients with prostate cancer have multiple and complex symptoms during and after treatment, with a long recovery period. After discharge, patients still need continuous and comprehensive interventions and nursing for rehabilitation training, prevention of complications, psychology, and other aspects (18). Therefore, mHealth interventions offer patients with PC an alternative form of medical care through a variety of communication technologies, including telephone, mail, remote video, or mobile applications, to treat patients at a distance.
However, the short-term and long-term effects of remote interventions on symptom management in patients with PC are unclear, and less attention has been paid to the long-term effects of the interventions. A newly published scoping review has shown that the current application of mHealth interventions in PC survivorship care pathways remains poor, with 10 mHealth apps identified and only one still in use, and the long-term effects of these apps are currently unknown (18). This research highlights the benefits of mHealth among patients with PC and shows long-term (12-month) improvements in urinary irritation, bowel function, hormonal function, and depression scores (19). However, a randomized controlled trial from Australia revealed that 12 months of telephone intervention was not accompanied by overall improvements in QOL or psychological distress (20).
A literature review found that most studies on the impact of mHealth on symptom management for patients with prostate cancer have evaluated the long-term effects of the intervention by following up patients for 3 months after the intervention (20–22). Therefore, this study focused on the short- and long-term effects of the intervention at a node of 3 months. Given these findings, it is necessary to update meta-analyses to comprehensively analyze the impact of mHealth interventions on symptom management of patients with PC.
2 Methods
2.1 Protocol registration
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic review and Meta-Analyses (PRISMA) guidelines (23). The protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) (Registration ID: CRD420250651320; available at: https://www.crd.york.ac.uk/prospero/).
2.2 Study selection
The PubMed, Cochrane Central electronic database, EMBASE, and Web of Science were searched from inception to 8 November 2024 using a series of keywords related to mobile health and prostate cancer. The details of the search strategy are presented in the Supplementary Appendix. To avoid missing any relevant studies, manual searches of the reference lists of all included articles were also performed, as were searches of the reference lists of previous related meta-analyses or systematic reviews.
The inclusion criteria for studies were as follows: (1) population: adults (age > 18 years) with a prostate cancer diagnosis who were undergoing or completed active prostate cancer treatment (surgery, chemotherapy, and/or radiotherapy); (2) intervention: mHealth interventions including but not limited to smartphone apps, SMS, or wearable devices targeting symptom monitoring or self-management; (3) control group comprised of those receiving the usual care (e.g., standard follow-up) or non-digital interventions (e.g., paper diaries); (4) primary outcomes: mental (depression, anxiety, and distress) or physical (urinary function, sexual function, bowel function, and hormonal function) health outcomes based on standardized scales with a secondary outcome of quality of life; (5) study design: randomized controlled trials (RCTs). This meta-analysis focused on the short-term and long-term effects of the intervention at the node of 3 months. The short-term effect was measured after the end of the intervention, and the measured outcome indicators were followed up for ≤3 months. The long-term effect was measured after the end of the intervention, and the measured outcome indicators were followed up for >3 months (we selected the longest time point as the data outcome).
Studies were excluded from this meta-analysis according to the following exclusion criteria: (1) publication in any language other than English or (2) presentation of incomplete data or duplicated data.
EndNote X9 (Clarivate Analytics, https://www.endnote.com) was used to manage references. Two authors (HSC and HH) independently examined titles and abstracts to select eligible trials. In cases of discrepancies between the two authors' selections, a third author (YML) was consulted to resolve the differences. Full-text articles were then retrieved and reviewed using the same method to determine which trials met the inclusion criteria for this study.
2.3 Data extraction
Two authors (HH and HHL) independently extracted data from the included trials using a data extraction table that included general information (title, first author, publication date, and study location), trial characteristics (sample size, method of randomization, allocation, blinding method, incomplete outcomes data, selective reporting, and other), subject characteristics (country, neoplasm staging, treatment condition, and age), intervention (type of intervention, intervention and follow-up durations, frequency of mHealth use, intervener, and theoretical framework), and outcomes (outcome measures). Discrepancies between authors were resolved through consultation with a third author (HSC).
2.4 Risk of bias assessment
In this study, two reviewers (HSC and HH) assessed the quality of the included studies using the Risk of Bias 2 (RoB 2) tool, as recommended by the Cochrane Handbook (24). This tool evaluates the risk of bias in five domains: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in the measurement of the outcome, and bias in the selection of the reported result. An overall risk of bias assessment was also conducted for each study. Disagreements with respect to the methodological quality were resolved by discussion and mutual consultation (YML).
2.5 Statistical analysis
The effects of mHealth on the outcomes of patients with PC were evaluated on the basis of the included studies. Continuous variables were evaluated and combined using mean difference (MD) when units of measurement were consistent across studies or could be converted to a common scale. When the units could not be directly compared or converted, the standardized mean difference (SMD) was used (24). The statistical parameter I2 was used to examine the heterogeneity of the effect sizes, with values higher than 50% indicating substantial heterogeneity (24). In this study, considering the existence of clinical heterogeneity (e.g., mode of intervention, participant characteristics), we employed the DerSimonian and Laird random effects model for our meta-analysis (24). A sensitivity analysis was conducted by removing the RCTs that included interventions that relied entirely on mHealth, those that included interventions that were delivered by peer support volunteers, or those that included a theory or framework referenced across the studies to detect the stability of the results. If the number of included studies for the outcome indicators was more than 10, a funnel plot and Egger’s test were used to detect publication bias. The level of statistical significance was set at P < 0.05 and all statistical tests were two-sided. Statistical analyses were performed using STATA version 17.0 (Stata Corp LP, College Station, TX, USA).
3 Results
3.1 Study identification and selection
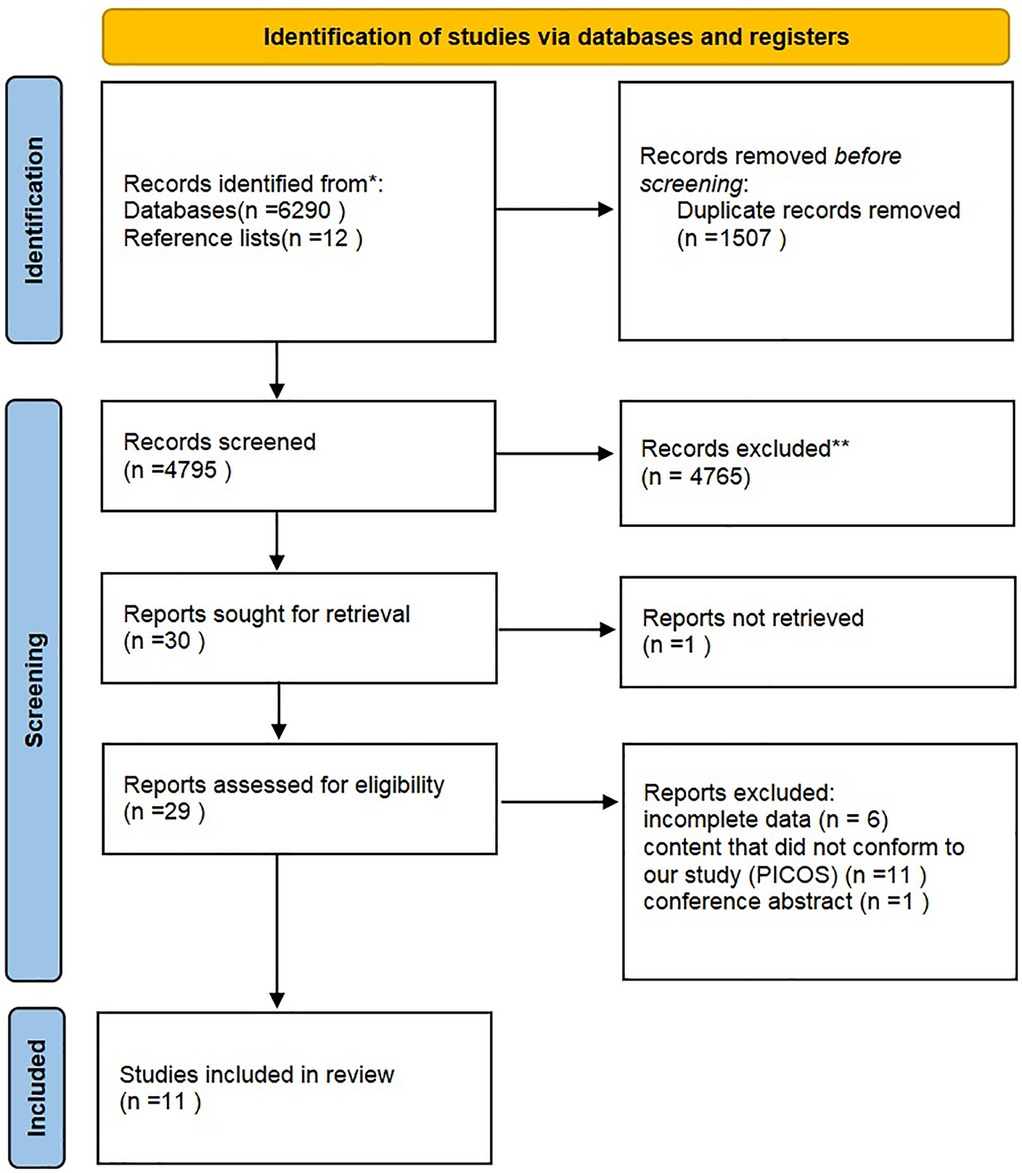
The initial search identified 6,302 articles. After screening of titles and abstracts, 6,273 articles were excluded, including 1,507 duplicates, and 4,766 articles that did not meet the inclusion criteria. The full texts of 29 articles were reviewed, and 18 of these articles were excluded due to incomplete data (N = 6) (25–29), content that did not conform to our study (PICOS) (N = 11) (30–40), or being a conference abstract (N = 1) (37). Finally, 11 RCTs were included in the present meta-analysis (Figure 1).
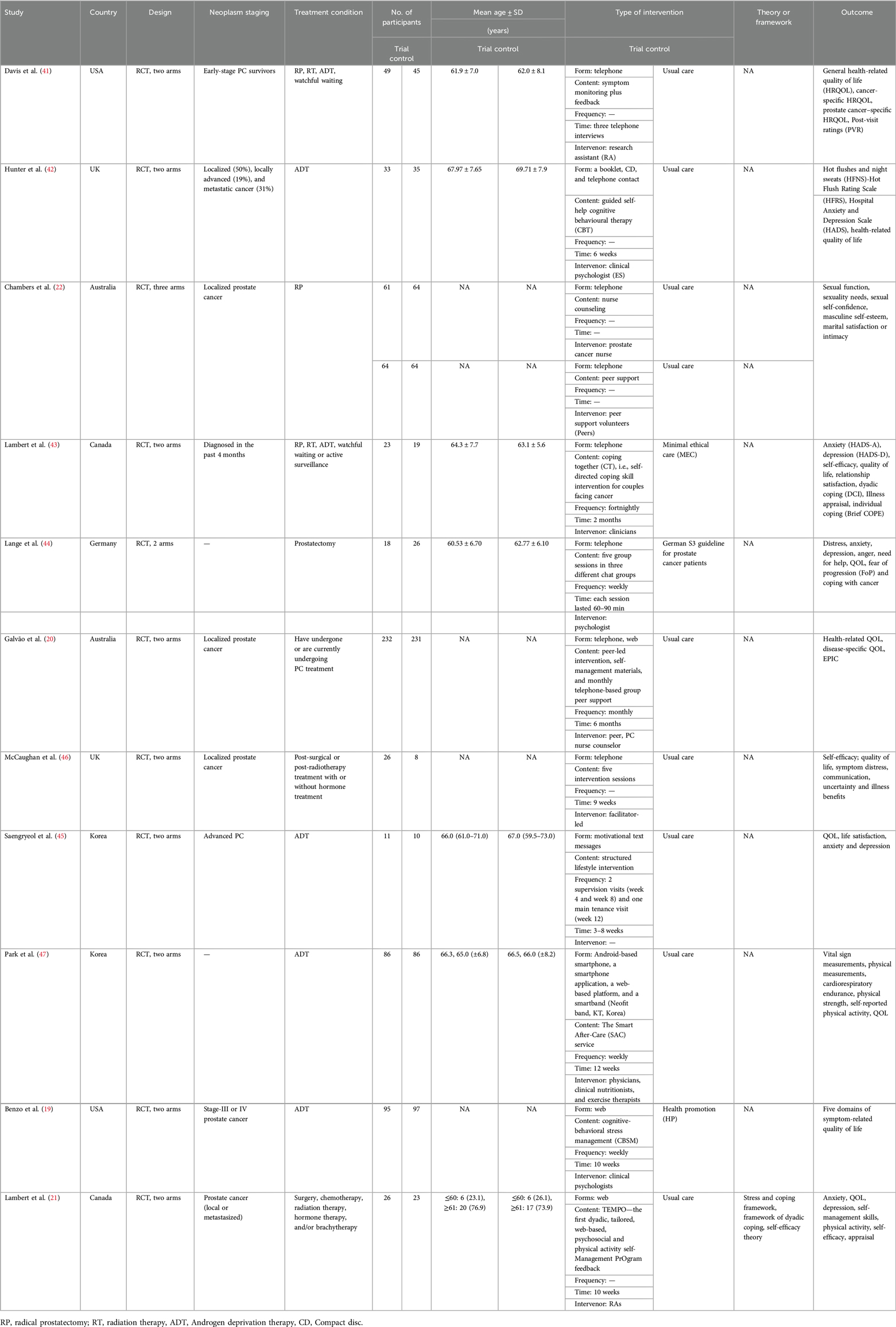
The characteristics of the included RCTs are summarized in Table 1. The 11 RCTs included two studies (18%) conducted in Australia, Canada, the UK, the USA, and Korea, and one (9%) study conducted in Germany. All RCTs were published in English and conducted between 2013 and 2022. A total of 1,368 patients diagnosed with prostate cancer were included. Among the 11 included RCTs, the study of Galvão et al. (20) had the largest sample size with 463 individuals. Except for two RCTs, which used an Internet intervention, all other mHealth interventions were performed over the telephone. Interventions ranged from 6 weeks to 6 months in duration, and the studies were delivered by professional technical personnel (including clinical psychologists, clinicians, physicians, clinical nutritionists, nurses, and research assistants) (n = 10) and peer support volunteers (n = 1). Most interventions were delivered by health and social care professionals, and only one relied entirely on mHealth. One theory or framework was referenced across studies (21). The control group received the usual care, ethical care, guidelines for patients with prostate cancer, or health promotion.
3.2 Risk of bias assessment
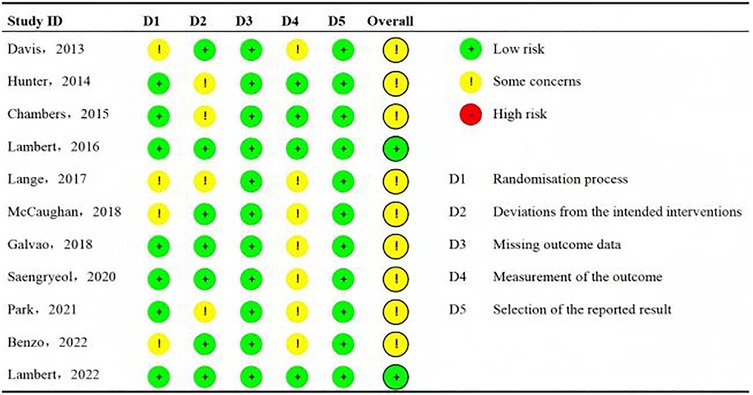
The Cochrane Collaboration RoB tool was used to assess the RoB of the included studies. The domains for assessment included selection bias, including sequence generation and allocation sequence concealment; performance or detection bias via blinding of participants, personnel, and outcome assessors; attrition bias via incomplete outcome data; and reporting bias via selective outcome reporting. The criteria for low, unclear, and high RoB within and across the studies followed the Cochrane Handbook for Systematic Reviews of Interventions. RoB was independently assessed by authors HSC (five articles), HH (three articles), and HHL (three articles). YML reviewed all RoB assessments to confirm accuracy. In addition, data integrity and selective reporting were both assessed as low risk. The results are presented in Figure 2.
3.3 Meta-analysis of primary outcomes
Six RCTs (20, 21, 42–45) (n = 687 participants) reported the short-term effect of the intervention on anxiety, and the meta-analysis demonstrated no significant difference between the mHealth intervention group and the control group [SMD = −0.07, 95% confidence interval (CI) = −0.43 to 0.29, P = 0.70, I2 = 63.30%] (Table 2). The long-term effects of the interventions on anxiety were measured in two studies (n = 419 participants) (20, 42), and the meta-analysis demonstrated no significant difference between the mHealth intervention group and the control group (SMD = −0.11, 95% CI = −0.30 to 0.08, P = 0.26, I2 = 0.00%) (Table 3).
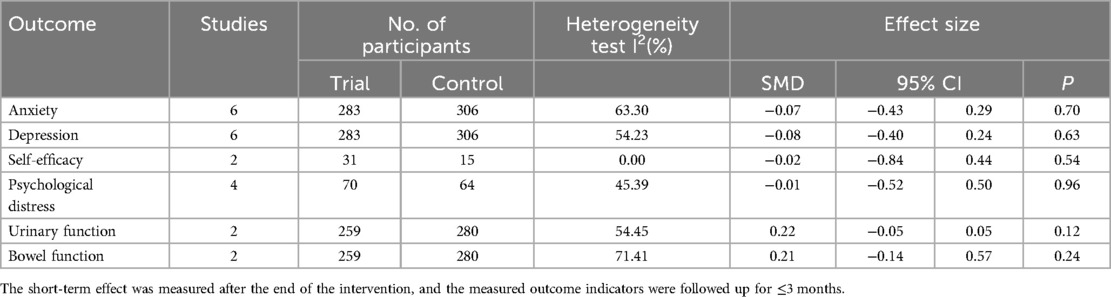
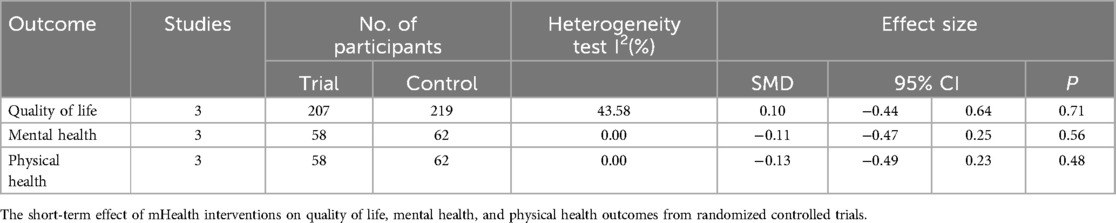
Table 2. The short-term effect of mHealth interventions on anxiety, depression, self-efficacy, psychological distress, urinary function, and bowel function outcomes from randomized controlled trials.
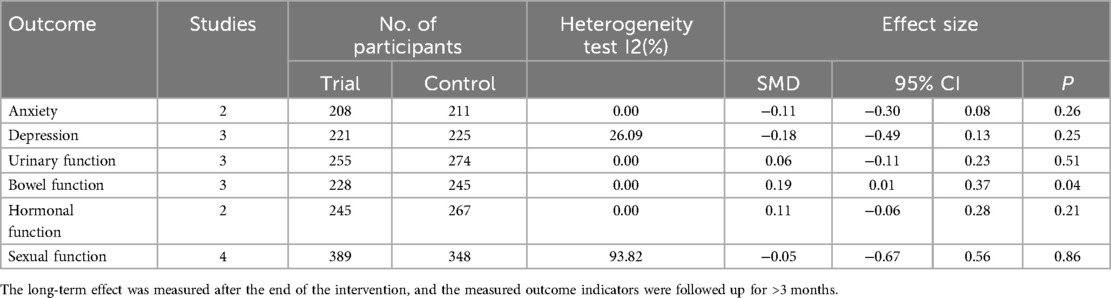
Table 3. The long-term effect of mHealth interventions on anxiety, depression, urinary function, bowel function, hormonal function and sexual function outcomes from randomized controlled trials.
Six RCTs (20, 21, 42–45) (n = 589 participants) reported the short-term effect of the intervention on depression, and the meta-analysis demonstrated no significant difference between the mHealth intervention group and the control group (SMD = −0.08, 95% CI = −0.40 to 0.24, P = 0.63, I2 = 54.23%) (Table 2). The long-term effects of the interventions on depression were measured in three studies (19, 20, 42) (n = 446 participants), and the meta-analysis demonstrated no significant difference between the mHealth intervention group and the control group (SMD = −0.18, 95% CI = −0.49 to 0.13, P = 0.25, I2 = 26.09%) (Table 3).
Two RCTs (43, 46) (n = 46 participants) reported the short-term effect of the interventions on self-efficacy, and the meta-analysis demonstrated no significant difference between the mHealth intervention group and the control group (SMD = −0.02, 95% CI = −0.84 to 0.44, P = 0.54, I2 = 0.00%) (Table 2).
Four RCTs (21, 43, 44, 46) (n = 134 participants) reported the short-term effect of the interventions on psychological distress, and the meta-analysis demonstrated no significant difference between the mHealth intervention group and the control group (SMD = −0.01, 95% CI = −0.52 to 0.50, P = 0.96, I2 = 45.39%) (Table 2).
Two RCTs (20, 47) (n = 539 participants) reported the short-term effect of the interventions on urinary function, and the meta-analysis demonstrated no significant difference between the mHealth intervention group and the control group (SMD = 0.22, 95% CI = −0.05 to 0.50, P = 0.12, I2 = 54.45%) (Table 2). The long-term effects of the interventions on urinary function were measured in three studies (19, 20, 41) (n = 529 participants), and the meta-analysis demonstrated no significant difference between the mHealth intervention group and the control group (SMD = 0.06, 95% CI = −0.11 to 0.23, P = 0.51, I2 = 0.00%) (Table 3).
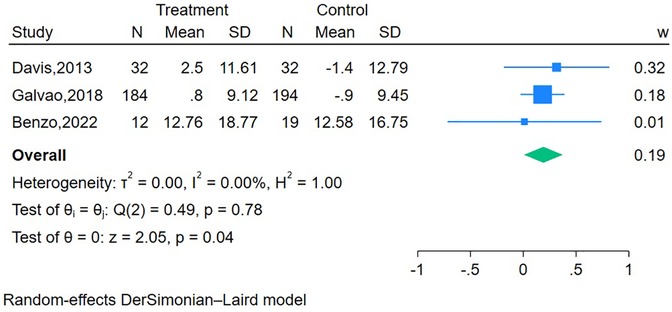
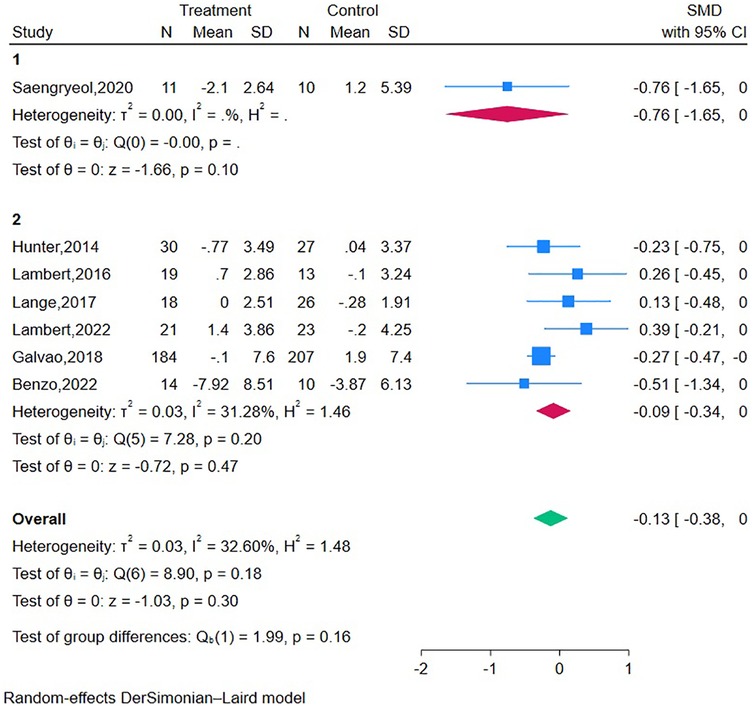
Two RCTs (20, 47) (n = 539 participants) reported the short-term effect of the interventions on bowel function, and the meta-analysis demonstrated no significant difference between the mHealth intervention group and the control group (SMD = 0.21, 95% CI = −0.14 to 0.57, P = 0.24, I2 = 71.41%) (Table 2). The long-term effects of the interventions on bowel function were measured in three studies (19, 20, 41) (n = 473 participants), and the meta-analysis demonstrated a significant difference between the mHealth intervention group and the control group (SMD = 0.19, 95% CI = 0.01–0.37, P = 0.04, I2 = 0.00%) (Figure 3).
The long-term effects of the interventions on hormonal function were measured in two studies (19, 20) (n = 512 participants), and the meta-analysis demonstrated no significant difference between the mHealth intervention group and the control group (SMD = 0.11, 95% CI = −0.06 to 0.28, P = 0.21, I2 = 0.00%) (Table 3).
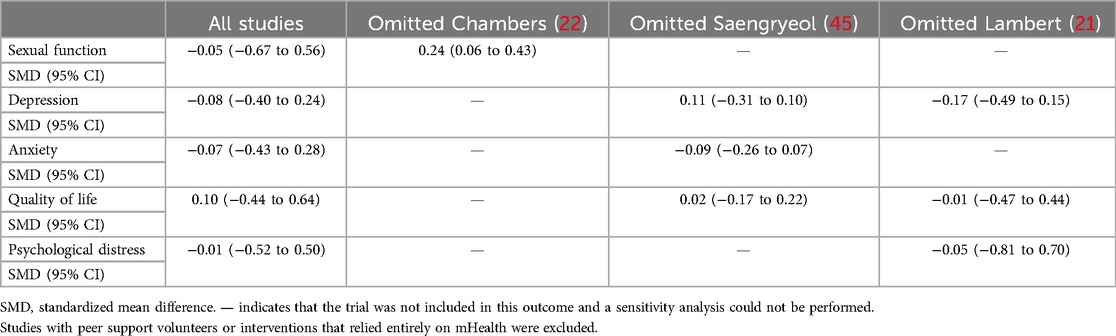
The long-term effects of the interventions on sexual function were measured in four studies (19, 20, 22, 41) (n = 737 participants), and the meta-analysis demonstrated no significant difference between the mHealth intervention group and the control group (SMD = −0.05, 95% CI = −0.67to 0.56, P = 0.86, I2 = 93.82%) (Table 3).
3.4 Meta-analysis of secondary outcomes
As for the secondary outcomes (Table 4), the meta-analysis demonstrated no significant differences in the short-term effects of the interventions on quality of life, mental health, and physical health. Three health-related QOL (HRQOL) measures, validated in patients with prostate cancer, were used: the physical and mental scales of the Assessment of Quality of Life-8 Dimensions (AQoL-8D) (43), the 8-item Short Form Health Survey (SF-8) (44), and the 12-item Short Form Health Survey (SF-12) (21).
Three RCTs (19, 20, 41) reported that the mHealth interventions were delivered by professional technical personnel, and one RCT (22) reported that the mHealth intervention was delivered by peer support volunteers. In the sensitivity analyses (Table 5), after removing the RCT of Chambers et al. (22), the sexual function differences became statistically significant. However, since only three studies were included, further research is needed to explore these findings. Subgroup analysis was conducted on data of tumor stage and intervention type (Figure 4). Relevant hypothesis tests indicated no significant differences.
3.5 Publication bias
Fewer than 10 articles were included for each outcome, and thus, an assessment of publication bias could not be conducted.
4 Discussion
This meta-analysis included 11 RCTs involving 1,368 participants and provided evidence that mHealth interventions significantly improve long-term bowel function outcomes. Moreover, in the sensitivity analyses, the sexual function differences became statistically significant. However, mHealth interventions did not significantly reduce symptom distress (anxiety, depression, self-efficacy, psychological distress, urinary function, bowel function, and hormonal function outcomes) or improve quality of life.
Our findings are consistent with previous systematic literature reviews (48), which concluded that technology-based interventions (TBIs) cannot improve health outcomes (anxiety, depression, and HRQOL) among patients with prostate cancer. However, the present meta-analysis included more patient outcomes, such as self-efficacy, psychological distress, urinary function, bowel function, and hormonal function, to further explore the short-term and long-term effects of mHealth interventions.
The mHealth interventions did not improve anxiety, depression, or psychological distress symptoms. This result may be due to a floor effect, whereby participants' baseline anxiety, depression, and psychological distress symptom scores were within the healthy range. The incidence of anxiety, depression, and psychological distress among patients with prostate cancer ranges from 15% to 22% (49, 50), 14.7% to 65.9% (51, 52), and 21% to 28% (53, 54), respectively. The studies in this review did not recruit patients with anxiety or depression. In primary care, some evidence suggests that mHealth interventions can decrease anxiety, depression, and psychological distress symptoms, and there is growing evidence of their benefits in cancer care (16, 55). However, more research is needed to evaluate their effectiveness in patients with prostate cancer experiencing anxiety, depression, and psychological distress.
The present study did not find that mHealth interventions can significantly improve patients' urinary, bowel, and hormonal function outcomes, probably due to the small number of included studies (only two or three RCTs) or the fact that the included population did not comprise patients with advanced prostate cancer. Moreover, the content and frequency of mHealth interventions may not have been standardized across studies, as latest RCTs indicate that targeting a web-based intervention to recipients most likely to benefit patients with elevated levels of symptom burden and can improve urinary irritation, bowel function, hormonal function, and depression symptoms in men with PC (19). For the symptoms mentioned above, data from the studies incorporated were inconsistent, making conclusions regarding their management through mHealth interventions difficult to draw. However, the long-term effects of mHealth interventions on bowel function had a significant positive impact. Several hypotheses could explain why we observed improvements over time in these conditions. First, we hypothesize that the “attention” provided to participants (60–90 min/session in the intervention group and 0–60 min/session in the control group) by the study staff could have played a role in the improvement of symptoms over time in these conditions. Second, participation bias (e.g., participants' desire to reduce their stress) may also partially explain why individuals in these conditions improved their outcomes. Finally, we also hypothesize that improvement in bowel function across conditions could be attributed to the overlapping content presented (e.g., education component, attention, and social support). This is consistent with previous work that showed mHealth interventions as a possible method for reducing the severity of participants' irritable bowel syndrome symptoms (56), and a review that concluded that mHealth interventions were associated with significant reductions in bowel symptoms and improvement in quality of life that tended to continue up to 12 months of follow-up (57). More research is needed to elucidate and disentangle the effects of mHealth interventions on symptom-related quality of life among prostate cancer survivors.
Quality of life is related to physical health, psychological conditions, social relationships, and environment (58). mHealth interventions likely cannot improve quality of life and self-efficacy because mHealth interventions fail to improve the symptom-related distress and psychological distress of patients with prostate cancer. This result may be due to the quality of life and the self-efficacy of these men already being at normative levels at baseline, and they may not have needed an intervention. However, a recent review found that mHealth interventions are promising for improving the QOL of patients with cancer, and the strongest evidence exists for physical activity interventions, followed by mindfulness and cognitive behavioral therapy (59). This meta-analysis only included two articles on physical activity interventions and one article on cognitive behavioral therapy interventions, thus, robust conclusions cannot be drawn from the available evidence.
The latest studies show that cancer survivors benefit variably from mHealth tools (15, 16, 60). To maximize the effects of such tools, future research should consider the following aspects. First, positive associations have primarily been demonstrated between mHealth/digital literacy levels and the utilization of the Internet as part of information-seeking related to healthcare and prostate cancer; however, PC survivors' mHealth literacy levels were likely to be at a novice level. Therefore, it is necessary for us to reliably measure mHealth literacy, improve our ability to identify low-literacy target groups, and develop and test interventions to effect change (61). Second, another research study found that mHealth intervention personalization improves engagement and efficacy (62). Future studies should consider using the technology acceptance model to codesign mHealth interventions with end-users and analyze end-user personalization regarding engagement and health outcomes (15). Third, a retrospective study demonstrated that utilizing mobile Internet management for the ongoing care of patients who have undergone radical prostatectomy yields significant benefits because the invasive nature of radical prostatectomy often induces psychological stress (63). Future research could start with the treatment methods adopted by patients and analyze whether different treatment approaches have an impact on the physical and mental outcomes of patients. Fourth, future mobile health interventions should be based on patients' unique needs and preferences, considering critical factors for implementing mHealth interventions, including perceived utility, ease of use, and resolving technical barriers such as privacy, cost, and security, as well as the remote monitoring of patients' progress. Importantly, intervention measures designed jointly with end-users can enhance participation.
The present meta-analysis had several limitations. First, due to the limited number of included articles, we could not fully evaluate publication bias, which resulted in low statistical efficiency. Second, most interventions were delivered over a relatively short period, typically ranging from 6 to 10 weeks; most frequently, the interventions were provided weekly. However, the lack of data prevented the comparison of mHealth interventions by length or frequency of the intervention. In the same way, evidence was insufficient to enable comparison by neoplasm staging and perform subgroup analyses, which would have been useful because an RCT suggests that different forms of interventions may be more or less effective according to disease stage (64). Finally, different instruments were used to assess patient outcomes, which made a comparison of the results difficult.
5 Conclusions
The results of this systematic review and meta-analysis indicate that patients with prostate cancer benefit less from mHealth interventions both mentally (anxiety, depression, self-efficacy, psychological distress, and quality of life) and physically (urinary function and hormonal function). However, mHealth interventions significantly improved long-term bowel function outcomes. Further, we need studies with sufficient statistical power and length of follow-up to generate much-needed definitive evidence concerning the efficacy of mHealth interventions for the management of symptoms in patients with prostate cancer.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
HSC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. HH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft. HHL: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft. YZ: Data curation, Investigation, Methodology, Software, Writing – original draft. NL: Investigation, Software, Writing – original draft. YML: Conceptualization, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Department of Urology, Seventh Affiliated Hospital of Sun Yat-sen University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdgth.2025.1584764/full#supplementary-material
Abbreviations
PRISMA, Preferred Reporting Items for Systematic review and Meta-Analyses guidelines; RCTs, randomized controlled trials; PC, prostate cancer; QOL, quality of life.
References
1. Brausi M, Hoskin P, Andritsch E, Banks I, Beishon M, Boyle H, et al. ECCO essential requirements for quality cancer care: prostate cancer. Crit Rev Oncol Hematol. (2020) 148:102861. doi: 10.1016/j.critrevonc.2019.102861
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Mundle R, Afenya E, Agarwal N. The effectiveness of psychological intervention for depression, anxiety, and distress in prostate cancer: a systematic review of literature. Prostate Cancer Prostatic Dis. (2021) 24(3):674–87. doi: 10.1038/s41391-021-00342-3
4. Spratt DE, Shore N, Sartor O, Rathkopf D, Olivier K. Treating the patient and not just the cancer: therapeutic burden in prostate cancer. Prostate Cancer Prostatic Dis. (2021) 24(3):647–61. doi: 10.1038/s41391-021-00328-1
5. Kazlauskas E, Patasius A, Kvedaraite M, Nomeikaite A, Rudyte M, Smailyte G. Icd-11 adjustment disorder following diagnostic procedures of prostate cancer: a 12-month follow-up study. J Psychosom Res. (2023) 168:111214. doi: 10.1016/j.jpsychores.2023.111214
6. Al Awamlh BA, Wallis CJD, Penson DF, Huang LC, Zhao Z, Conwill R, et al. Functional outcomes after localized prostate cancer treatment. Jama. (2024) 331(4):302–17. doi: 10.1001/jama.2023.26491
7. Wallis CJD, Glaser A, Hu JC, Huland H, Lawrentschuk N, Moon D, et al. Survival and complications following surgery and radiation for localized prostate cancer: an international collaborative review. Eur Urol. (2018) 73(1):11–20. doi: 10.1016/j.eururo.2017.05.055
8. Nam RK, Cheung P, Herschorn S, Saskin R, Su J, Klotz LH, et al. Incidence of complications other than urinary incontinence or erectile dysfunction after radical prostatectomy or radiotherapy for prostate cancer: a population-based cohort study. Lancet Oncol. (2014) 15(2):223–31. doi: 10.1016/s1470-2045(13)70606-5
9. Ficarra V, Novara G, Rosen RC, Artibani W, Carroll PR, Costello A, et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol. (2012) 62(3):405–17. doi: 10.1016/j.eururo.2012.05.045
10. Holm M, Doveson S, Lindqvist O, Wennman-Larsen A, Fransson P. Quality of life in men with metastatic prostate cancer in their final years before death—a retrospective analysis of prospective data. BMC Palliat Care. (2018) 17(1):126. doi: 10.1186/s12904-018-0381-6
11. Noriega Esquives B, Lee TK, Moreno PI, Fox RS, Yanez B, Miller GE, et al. Symptom burden profiles in men with advanced prostate cancer undergoing androgen deprivation therapy. J Behav Med. (2022) 45(3):366–77. doi: 10.1007/s10865-022-00288-4
12. Crump C, Stattin P, Brooks JD, Sundquist J, Bill-Axelson A, Edwards AC, et al. Long-term risks of depression and suicide among men with prostate cancer: a national cohort study. Eur Urol. (2023) 84(3):263–72. doi: 10.1016/j.eururo.2023.04.026
13. Tosoni S, Voruganti I, Lajkosz K, Mustafa S, Phillips A, Kim SJ, et al. Patient consent preferences on sharing personal health information during the COVID-19 pandemic: “the more informed we are, the more likely we are to help”. BMC Med Ethics. (2022) 23(1):53. doi: 10.1186/s12910-022-00790-z
14. Rittberg R, Mann A, Desautels D, Earle CC, Navaratnam S, Pitz M. Canadian cancer centre response to COVID-19 pandemic: a national and provincial response. Curr Oncol. (2020) 28(1):233–51. doi: 10.3390/curroncol28010026
15. Singleton AC, Raeside R, Hyun KK, Partridge SR, Di Tanna GL, Hafiz N, et al. Electronic health interventions for patients with breast cancer: systematic review and meta-analyses. J Clin Oncol. (2022) 40(20):2257–70. doi: 10.1200/jco.21.01171
16. Ream E, Hughes AE, Cox A, Skarparis K, Richardson A, Pedersen VH, et al. Telephone interventions for symptom management in adults with cancer. Cochrane Database Syst Rev. (2020) 6(6):Cd007568. doi: 10.1002/14651858.CD007568.pub2
17. Jamnadass E, Rai BP, Veneziano D, Tokas T, Rivas JG, Cacciamani G, et al. Do prostate cancer-related mobile phone apps have a role in contemporary prostate cancer management? A systematic review by Eau young academic urologists (Yau) urotechnology group. World J Urol. (2020) 38(10):2411–31. doi: 10.1007/s00345-020-03197-w
18. Ogunsanya ME, Sifat M, Bamidele OO, Ezenwankwo EF, Clifton S, Ton C, et al. Mobile health (Mhealth) interventions in prostate cancer survivorship: a scoping review. J Cancer Surviv. (2023) 17(3):557–68. doi: 10.1007/s11764-022-01328-3
19. Benzo RM, Moreno PI, Noriega-Esquives B, Otto AK, Penedo FJ. Who benefits from an Ehealth-based stress management intervention in advanced prostate cancer? Results from a randomized controlled trial. Psychooncology. (2022) 31(12):2063–73. doi: 10.1002/pon.6000
20. Galvão DA, Newton RU, Girgis A, Lepore SJ, Stiller A, Mihalopoulos C, et al. Randomized controlled trial of a peer led multimodal intervention for men with prostate cancer to increase exercise participation. Psychooncology. (2018) 27(1):199–207. doi: 10.1002/pon.4495
21. Lambert SD, Duncan LR, Culos-Reed SN, Hallward L, Higano CS, Loban E, et al. Feasibility, acceptability, and clinical significance of a dyadic, web-based, psychosocial and physical activity self-management program (tempo) tailored to the needs of men with prostate cancer and their caregivers: a multi-center randomized pilot trial. Curr Oncol. (2022) 29(2):785–804. doi: 10.3390/curroncol29020067
22. Chambers SK, Occhipinti S, Schover L, Nielsen L, Zajdlewicz L, Clutton S, et al. A randomised controlled trial of a couples-based sexuality intervention for men with localised prostate cancer and their female partners. Psychooncology. (2015) 24(7):748–56. doi: 10.1002/pon.3726
23. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
24. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (updated August 2023). Cochrane (2023). Available at: https://training.cochrane.org/handbook (Accessed November 08, 2024).
25. Giesler RB, Given B, Given CW, Rawl S, Monahan P, Burns D, et al. Improving the quality of life of patients with prostate carcinoma: a randomized trial testing the efficacy of a nurse-driven intervention. Cancer. (2005) 104(4):752–62. doi: 10.1002/cncr.21231
26. Gong R, Heller A, Moreno PI, Yanez B, Penedo FJ. Low social well-being in advanced and metastatic prostate cancer: effects of a randomized controlled trial of cognitive behavioral stress management. Int J Behav Med. (2024) 35(14):1–14. doi: 10.1007/s12529-024-10270-w
27. Langlais CS, Chen YH, Van Blarigan EL, Kenfield SA, Kessler ER, Daniel K, et al. Quality of life of prostate cancer survivors participating in a remotely delivered web-based behavioral intervention pilot randomized trial. Integr Cancer Ther. (2022) 21:15347354211063500. doi: 10.1177/15347354211063500
28. Tagai EK, Miller SM, Hudson SV, Diefenbach MA, Handorf E, Bator A, et al. Improved cancer coping from a web-based intervention for prostate cancer survivors: a randomized controlled trial. Psychooncology. (2021) 30(9):1466–75. doi: 10.1002/pon.5701
29. Wennerberg C, Hellström A, Schildmeijer K, Ekstedt M. Effects of web-based and Mobile self-care support in addition to standard care in patients after radical prostatectomy: randomized controlled trial. Jmir Cancer. (2023) 9:e44320. doi: 10.2196/44320
30. Heydenreich M, Puta C, Gabriel HHW, Dietze A, Wright P, Zermann D. Does trunk muscle training with an oscillating rod improve urinary incontinence after radical prostatectomy? A prospective randomized controlled trial. Clin Rehabil. (2020) 34(3):320–33. doi: 10.1177/0269215519893096
31. Aliperti L, Patil D, Mehta A, Filson C, Crociani C, Sanda M, et al. Long-term health related quality of life in prostate cancer patients requiring radiotherapy after radical prostatectomy. J Urol. (2017) 197(4):e360. doi: 10.1016/j.juro.2017.02.863
32. Dronca R, Rao R, Maniaci M, Odedina F, Kaninjing E, Ashing K, et al. Feasibility of patient-centered home care (PCHC) to reduce disparities in black men (BM) with advanced prostate cancer (CAP): an Iccare Consortium for Prostate Cancer in Black Men Project. Cancer Epidemiol Biomarkers Prev. (2023) 32(1):B046. doi: 10.1158/1538-7755.DISP22-B046
33. Xu S, Guo P, Fuller GP, Dobias CA, Idiagbonya E, Song L. Neighborhood deprivation and living with prostate cancer: patients’ and Partners’ psychosocial behavioral Status, symptoms, and quality of life. Cancer Epidemiol Biomarkers Prev. (2022) 31(1 SUPPL):PO-176. doi: 10.1158/1538-7755.DISP21-PO-176
34. Evans HEL, Galvão DA, Forbes CC, Girard D, Vandelanotte C, Newton RU, et al. Acceptability and preliminary efficacy of a web- and telephone-based personalised exercise intervention for individuals with metastatic prostate cancer: the exerciseguide pilot randomised controlled trial. Cancers (Basel). (2021) 13(23):5925. doi: 10.3390/cancers13235925
35. Badger TA, Segrin C, Figueredo AJ, Harrington J, Sheppard K, Passalacqua S, et al. Psychosocial interventions to improve quality of life in prostate cancer survivors and their intimate or family partners. Qual Life Res. (2011) 20(6):833–44. doi: 10.1007/s11136-010-9822-2
36. Chambers SK, Occhipinti S, Foley E, Clutton S, Legg M, Berry M, et al. Mindfulness-based cognitive therapy in advanced prostate cancer: a randomized controlled trial. J Clin Oncol. (2017) 35(3):291–7. doi: 10.1200/JCO.2016.68.8788
37. Goode PS, Johnson TM 2nd, Newman DK, Vaughan CP, Echt KV, Markland AD, et al. Perioperative mobile telehealth program for post-prostatectomy incontinence: a randomized clinical trial. J Urol. (2022) 208(2):379–87. doi: 10.1097/ju.0000000000002697
38. Song L, Guo P, Tan X, Chen RC, Nielsen ME, Birken SA, et al. Enhancing survivorship care planning for patients with localized prostate cancer using a couple-focused web-based, Mhealth program: the results of a pilot feasibility study. J Cancer Surviv. (2021) 15(1):99–108. doi: 10.1007/s11764-020-00914-7
39. Wittmann D, Mehta A, Bober SL, Zhu Z, Daignault-Newton S, Dunn RL, et al. Truenth sexual recovery intervention for couples coping with prostate cancer: randomized controlled trial results. Cancer. (2022) 128(7):1513–22. doi: 10.1002/cncr.34076
40. Wootten AC, Meyer D, Abbott JM, Chisholm K, Austin DW, Klein B, et al. An online psychological intervention can improve the sexual satisfaction of men following treatment for localized prostate cancer: outcomes of a randomised controlled trial evaluating my road ahead. Psychooncology. (2017) 26(7):975–81. doi: 10.1002/pon.4244
41. Davis KM, Dawson D, Kelly S, Red S, Penek S, Lynch J, et al. Monitoring of health-related quality of life and symptoms in prostate cancer survivors: a randomized trial. J Support Oncol. (2013) 11(4):174–82. doi: 10.12788/j.suponc.0013
42. Hunter M, Stefanopoulou E, Yousaf O, Grunfeld E. A randomised controlled trial of a cognitive behavioural intervention for men who have hot flushes following prostate cancer treatment (Mancan). Psychooncology. (2014) 23:126–7. doi: 10.1111/j.1099-1611.2014.3694
43. Lambert SD, McElduff P, Girgis A, Levesque JV, Regan TW, Turner J, et al. A pilot, multisite, randomized controlled trial of a self-directed coping skills training intervention for couples facing prostate cancer: accrual, retention, and data collection issues. Support Care Cancer. (2016) 24(2):711–22. doi: 10.1007/s00520-015-2833-3
44. Lange L, Fink J, Bleich C, Graefen M, Schulz H. Effectiveness, acceptance and satisfaction of guided chat groups in psychosocial aftercare for outpatients with prostate cancer after prostatectomy. Internet Interv Appl Inf Technol Mental Behav Health. (2017) 9:57–64. doi: 10.1016/j.invent.2017.06.001
45. Saengryeol P, Kwangnam K, Hyun Kyu A, Jong Won K, Gyurang M, Byung Ha C, et al. Impact of lifestyle intervention for patients with prostate cancer. Am J Health Behav. (2020) 44(1):90–9. doi: 10.5993/AJHB.44.1.10
46. McCaughan E, Curran C, Northouse L, Parahoo K. Evaluating a psychosocial intervention for men with prostate cancer and their partners: outcomes and lessons learned from a randomized controlled trial. Appl Nurs Res. (2018) 40:143–51. doi: 10.1016/j.apnr.2018.01.008
47. Park YH, Lee JI, Lee JY, Cheong IY, Hwang JH, Seo SI, et al. Internet of things-based lifestyle intervention for prostate cancer patients on androgen deprivation therapy: a prospective, multicenter, randomized trial. Am J Cancer Res. (2021) 11(11):5496–507.34873475
48. Qan'ir Y, Song L. Systematic review of technology-based interventions to improve anxiety, depression, and health-related quality of life among patients with prostate cancer. Psychooncology. (2019) 28(8):1601–13. doi: 10.1002/pon.5158
49. James C, Brunckhorst O, Eymech O, Stewart R, Dasgupta P, Ahmed K. Fear of cancer recurrence and PSA anxiety in patients with prostate cancer: a systematic review. Support Care Cancer. (2022) 30(7):5577–89. doi: 10.1007/s00520-022-06876-z
50. Meissner VH, Peter C, Ankerst DP, Schiele S, Gschwend JE, Herkommer K, et al. Prostate cancer-related anxiety among long-term survivors after radical prostatectomy: a longitudinal study. Cancer Med. (2023) 12(4):4842–51. doi: 10.1002/cam4.5304
51. Watts S, Leydon G, Birch B, Prescott P, Lai L, Eardley S, et al. Depression and anxiety in prostate cancer: a systematic review and meta-analysis of prevalence rates. BMJ Open. (2014) 4(3):e003901. doi: 10.1136/bmjopen-2013-003901
52. Zhao X, Sun M, Yang Y. Effects of social support, hope and resilience on depressive symptoms within 18 months after diagnosis of prostate cancer. Health Qual Life Outcomes. (2021) 19(1):15. doi: 10.1186/s12955-020-01660-1
53. Ilie G, Rendon R, Mason R, MacDonald C, Kucharczyk MJ, Patil N, et al. A comprehensive 6-Mo prostate cancer patient empowerment program decreases psychological distress among men undergoing curative prostate cancer treatment: a randomized clinical trial. Eur Urol. (2023) 83(6):561–70. doi: 10.1016/j.eururo.2023.02.009
54. Occhipinti S, Zajdlewicz L, Coughlin GD, Yaxley JW, Dunglison N, Gardiner RA, et al. A prospective study of psychological distress after prostate cancer surgery. Psychooncology. (2019) 28(12):2389–95. doi: 10.1002/pon.5263
55. Penedo FJ, Oswald LB, Kronenfeld JP, Garcia SF, Cella D, Yanez B. The increasing value of Ehealth in the delivery of patient-centred cancer care. Lancet Oncol. (2020) 21(5):e240–e51. doi: 10.1016/s1470-2045(20)30021-8
56. Tayama J, Hamaguchi T, Koizumi K, Yamamura R, Okubo R, Kawahara JI, et al. Efficacy of an Ehealth self-management program in reducing irritable bowel syndrome symptom severity: a randomized controlled trial. Sci Rep. (2024) 14(1):4. doi: 10.1038/s41598-023-50293-z
57. Knowles SR, Mikocka-Walus A. Utilization and efficacy of internet-based Ehealth technology in gastroenterology: a systematic review. Scand J Gastroenterol. (2014) 49(4):387–408. doi: 10.3109/00365521.2013.865259
58. WHOQOL Group. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med. (1998) 28(3):551–8.9626712
59. Buneviciene I, Mekary RA, Smith TR, Onnela JP, Bunevicius A. Can Mhealth interventions improve quality of life of cancer patients? A systematic review and meta-analysis. Crit Rev Oncol Hematol. (2021) 157:103123. doi: 10.1016/j.critrevonc.2020.103123
60. Leach CR, Hudson SV, Diefenbach MA, Wiseman KP, Sanders A, Coa K, et al. Cancer health self-efficacy improvement in a randomized controlled trial. Cancer. (2022) 128(3):597–605. doi: 10.1002/cncr.33947
61. Jackson SR, Yu P, Armany D, Occhipinti S, Chambers S, Leslie S, et al. Ehealth literacy in prostate cancer: a systematic review. Patient Educ Couns. (2024) 123:108193. doi: 10.1016/j.pec.2024.108193
62. Carter DD, Robinson K, Forbes J, Hayes S. Experiences of mobile health in promoting physical activity: a qualitative systematic review and meta-ethnography. PLoS One. (2018) 13(12):e0208759. doi: 10.1371/journal.pone.0208759
63. Peng S, Wei Y, Ye L, Jin X, Huang L. Application of mobile internet management in the continuing care of patients after radical prostatectomy. Sci Rep. (2024) 14(1):31520. doi: 10.1038/s41598-024-83303-9
Keywords: prostate cancer, mobile health (mHealth), symptom management, long-term effects, short-term effects, meta-analysis
Citation: Chen HS, He H, Lin HH, Zhang Y, Li N and Li YM (2025) Effectiveness of mobile health in symptom management of prostate cancer patients: a systematic review and meta-analysis. Front. Digit. Health 7:1584764. doi: 10.3389/fdgth.2025.1584764
Received: 2 March 2025; Accepted: 11 April 2025;
Published: 7 May 2025.
Edited by:
Avishek Choudhury, West Virginia University, United StatesReviewed by:
Tanmoy Sarkar Pias, Virginia Tech, United StatesHao Chen, Xiamen University of Technology, China
Copyright: © 2025 Chen, He, Lin, Zhang, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai Shan Chen, Y2hlbmhhaXNoYW5Ac3lzdXNoLmNvbQ==; Ya Mei Li, bGl5YW1laUBzeXN1c2guY29t
 Hai Shan Chen
Hai Shan Chen Hua He
Hua He