- 1APDM Wearable Technologies—a Clario Company, Portland, OR, United States
- 2Department of Neurology, Oregon Health & Science University, Portland, OR, United States
- 3Department of Neurology, The University of Chicago, Chicago, IL, United States
- 4Department of Electrical and Computer Engineering, Portland State University, Portland, OR, United States
Background: Recent findings suggest that a single gait assessment in a clinic may not reflect everyday mobility.
Objective: We compared gait measures that best differentiated individuals with spinocerebellar ataxia (SCA) from age-matched healthy controls (HC) during a supervised gait test in the clinic vs. a week of unsupervised gait during daily life.
Methods: Twenty-six individuals with SCA types 1, 2, 3, and 6, and 13 (HC) wore three Opal inertial sensors (on both feet and lower back) during a 2-minute walk in the clinic and for seven days in daily life. Seventeen gait measures were analyzed to investigate the group differences using Mann–Whitney U-tests and area under the curve (AUC).
Results: Ten gait measures were significantly worse in SCA than HC for the clinic test (p < 0.003), but only 3 were worse in daily life (p < 0.003). Only a few gait measures consistently discriminated groups in both environments. Specifically, variability in Swing Time and Double Support Time had AUCs of 0.99 (p < 0.0001) and 0.96 (p < 0.0001) in the clinic, and 0.84 (p < 0.0003) and 0.80 (p < 0.002) in daily life, respectively. Clinical gait measures showed stronger correlations with clinical outcomes (ie, SARA and FARS-ADL; r = 0.50–0.77) than between daily life gait measures (r = 0.31–0.49). Gait activity in daily life was not statistically significant between the SCA and HC groups (p > 0.06).
Conclusions: Digital gait measures discriminate SCA in both environments. In-clinic measures are more sensitive, while daily life measures provide ecological validity, highlighting a trade-off and offering complementary insights.
Introduction
Gait impairment is an early sign of spinocerebellar ataxia (SCA) that increases in severity with disease progression (1–3). Moreover, gait and balance impairments are among the most debilitating impairments exhibited with SCA, with deleterious impacts on daily function, fall risk, and quality of life (4). In clinical trials of ataxia, disease severity and progression are most commonly assessed using the Scale for Assessment and Rating of Ataxia (SARA) (5), which categorizes the severity of gait, balance, and other motor impairments on an ordinal scale. However, the SARA has several limitations, including high variance and subjectivity in scoring, low sensitivity, the need for large sample sizes in clinical trials, and the need for clinical specialists to administer testing (6–8). Given these limitations, there is a need for a quantitative assessment of SCA gait to evaluate therapeutic efficacy in both the clinic and daily living environments.
Advancements in wearable technologies have overcome financial and logistical limitations that have hindered the use of quantitative movement assessment in clinical trials, allowing for objective ataxic movements and gait measurement that is feasible in both clinical and daily life settings (9–26). Ilg et al. (11) found that gait variability measures captured in the laboratory and at home were able to discriminate between patients with cerebellar ataxia from healthy controls. Similar gait variability measures have been reported in laboratory gait assessment of prodromal and manifest SCA using wearable sensors (16, 21). Additionally, recent longitudinal studies indicate that gait variability may show sensitivity to progression in cerebellar ataxia patients, and that clinical trial sample size may be significantly reduced with the implementation of wearable sensors to capture accurate and objective measures reflective of motor symptom progression in SCA (27, 28). Daily life monitoring outside the clinic may be particularly useful as limited access to clinical specialists makes in-clinic assessment challenging. Furthermore, daily monitoring of gait using wearables in the home environment offers a comprehensive and real-life view of disease severity (11, 29).
Despite increasing adaptation of wearable technologies in SCA, relatively few studies have investigated whether the most discriminative gait features identified in clinical settings remain the same in real-world daily life settings. For example, Iig et al. (11) found that gait variability measures such as lateral step deviation and composite score (lateral step deviation and stride length variability) were statistically significant between cerebellar ataxia and healthy subjects in both in-clinic and real-life daily walking conditions, with higher effect sizes observed in the clinic settings. Similarly, Seemann et al. (29) found that while in-clinic measures showed a higher effect size compared to daily life gait measures to discriminate cerebellar ataxia from healthy subjects, daily life gait measures were more sensitive to detect longitudinal change over 1 year. These findings show how gait characteristics change across different environmental contexts. While prior studies have largely focused on degenerative cerebellar ataxia broadly, there is limited evidence directly comparing discriminative gait measures in both in-clinic and daily life settings within specific spinocerebellar ataxia subtypes (SCA1, SCA2, SCA3, and SCA6).
The purpose of this study was to identify the gait measures that best discriminate between individuals diagnosed with SCA and age-and sex-matched healthy controls (HC) from a 2-minute walking test at a natural pace in the clinic using wearable inertial sensors. We then compared these prescribed task measures to gait measures collected over a week of free-living activity from daily life. We explored whether the gait measures that are most discriminative between SCA from HC during in-clinic settings are consistent when assessed in daily life. We hypothesized that: (1) distinct gait measures would best discriminate SCA from HC in clinical and daily life settings, and (2) gait characteristics would differ in the same subjects tested in the clinic and daily life.
Methods
Participants
Participants were recruited as part of a larger study (IDEA, Instrumented Data Exchange for Ataxia) aimed to examine the gait and balance progression of spinocerebellar ataxia genotypes 1,2,3, and 6 (SCA1-6). Participants enrolled at the OHSU and University of Chicago sites were given the opportunity to participate in 7–14 days of daily life monitoring of their gait quality, immediately following their clinic visits. As part of the larger study's inclusion criteria, participants were limited to those able to walk independently in the clinic, back and forth a 10-meter path for 2 minutes. Exclusion criteria were having a head injury, vestibular dysfunction, stroke, or other neurological condition or musculoskeletal disorder impairing mobility.
Clinical assessment
All subjects were assessed by a neurologist-specialist using a standardized, validated, eight-domain ratingscale (score range 0–40)—the scale for the assessment and rating of ataxia (SARA), Inventory of Non-Ataxia Signs (INAS), Activities-Specific Balance Confidence Scale (ABC), Patient-Reported Outcome Measure (PROM), and Friedreich's Ataxia Rating Scale—Activities of Daily Living (FARs ADL).
Clinic gait data collection
In the clinic, data from 3, synchronized, inertial measurement units (IMUs) (Opals by APDM Wearable Technologies- a Clario Company, Portland, OR, USA): one on top of each foot and one over the lower lumbar with an elastic belt were used in this substudy. Each Opal IMU includes a tri-axial accelerometer, gyroscope, and magnetometer with a sampling rate of 128 Hz. Participants completed the 2-minute walk test over a 10-meter pathway as part of a larger battery of tests. The same synchronized sensors and data algorithms were used to derive the same gait measures during the prescribed and daily-life walking.
Daily-life gait data collection
Immediately after testing in the clinic, subjects were asked to wear instrumented socks (30) on each foot and one Opal sensor over the lower lumbar area with an elastic belt. For daily wear, the Opal IMUs were reconfigured to fit comfortably on each foot within a neoprene wrap, with a battery in a pocket above the lateral malleolus, ensuring the system is easy to use and unobtrusive More details in Shah et al. (30). Subjects were instructed to wear the sensors for at least 8 hours a day for at least 7 days. Data were stored in Opal's internal memory. After 7–14 days of data collection, the socks were returned, and the data were uploaded to a secure database for further processing.
Measures of gait
In total, 17 gait measures were extracted from the gait in the clinic and daily life. The algorithms for extracting spatial and temporal measures of gait were the same across both clinic and daily life settings, as described and verified in prior studies (31, 32). For daily life gait analysis, the algorithm detects walking bouts using inertial sensor data from the feet and identifies turns based on pelvic yaw rotation (31). Steps are grouped into walking bouts if the interval between steps is less than 2.5 s, and bouts with at least 3 steps lasting at least 3 s are processed using Mobility Lab's commercial algorithms (33–37). The analysis algorithm employs the Unscented Kalman Filter to integrate accelerometer, gyroscope, and magnetometer data, precisely estimating each foot's orientation and trajectory (36, 37). The complete list of measures and definitions is provided in Supplementary Table S1.
Participant gait data were included in the clinic 2-minute walk test if they had at least 25 gait cycles and a total test duration of 110 s or more. For daily life data, inclusion required at least 20 hours of recorded activity over a minimum of 4 days, with at least 20 walking bouts.
Statistical analysis
Non-parametric statistics were used to evaluate both between-group and within-group differences. Further, the Area Under Curve (AUC) of the Receiver Operating Characteristics (ROC) was used to calculate the between-group discriminatory ability of gait measures. To assess whether gait measures differed by environment, paired Wilcoxon tests were used within each group. To examine the association between gait measures and clinical scores, the Pearson correlation coefficient was used. All statistical analyses were conducted using R software (Version 4.2.0), with statistical significance set at p < 0.003 based on Bonferroni's correction (0.05/17 = 0.003, due to 17 measures) to control for multiple comparisons.
Results
Demographics and gait activity
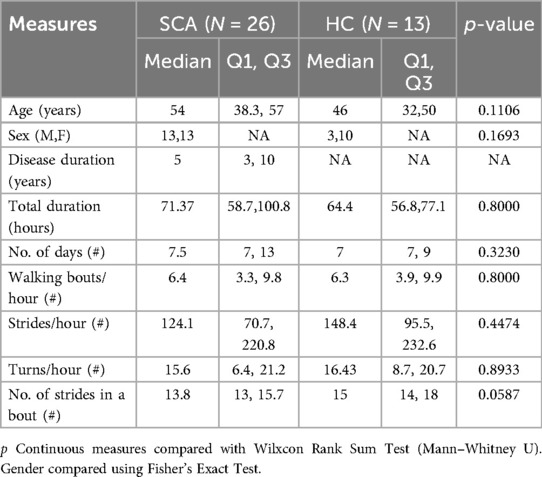
This study included 39 people, 26 of whom were diagnosed SCA patients (10 SCA1, 9 SCA2, 4 SCA3, and 3 SCA4) and an additional 13 age and gender similar HC. Age and gender were similar between groups (see Table 1). A total of 3,074 hours of data were collected in daily life, containing 476,477 strides. Activity measures were not different between groups, including stride/hour and turns/hour (see Table 1). The frequency distribution of the median number of strides per bout for the SCA and HC groups is shown in Supplementary Figure S1.
SCA and HC discriminative ability of clinic vs. daily life gait measures
Measures collected in the clinic consistently outperformed those collected in daily life (Table 2). Overall, there were 10 measures in-clinic, and 3 measures in daily life showed an AUC ≥ 0.8 (Figure 1). Despite the differences in discriminative ability, two measures of gait variability, specifically, the Double Support and Swing Time Standard Deviations (SD), performed strongly in both environments. Double Support Time SD (%) demonstrated an AUC of 0.99 and 0.84 in the clinic and daily life, respectively, while Swing Time SD (%) achieved AUCs of 0.96 and 0.8.
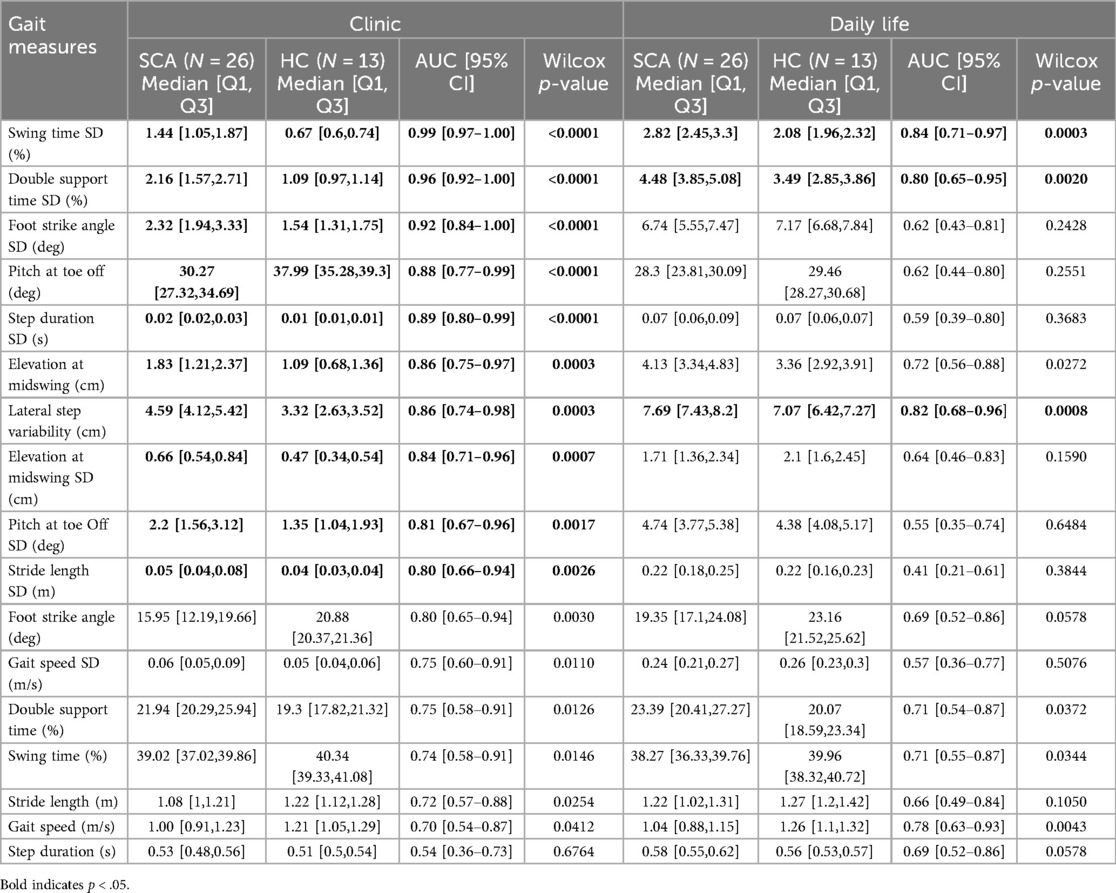
Table 2. More gait measures distinguished SCA from healthy control gait in the clinic prescribed 2-minute walk than in daily life walking. Medians and first and third quartiles of gait measures compared between the SCA and HC groups with AUC and Wilcox p-values.

Figure 1. AUC plots distinguishing gait measures of those with SCA vs. healthy controls when measured in the clinic (top) vs. in daily life (bottom).
Gait characteristics differed for the clinic vs. the daily life environments
Most (13/17) gait measures significantly differed in the same people with SCA, and 11/17 differed in HC when collected in the clinic vs. daily life (see Supplementary Table S2 and Figure 2A for examples). Although Double Support Time and Swing Time variability were significantly different values when collected in the clinic vs. daily life, both environments showed a statistically significant difference in these gait measures between the SCA and HC cohorts (Figure 2B). Nevertheless, a few measures were significantly different between SCA and HC when collected in the clinic, but not in daily life (Figure 2C).
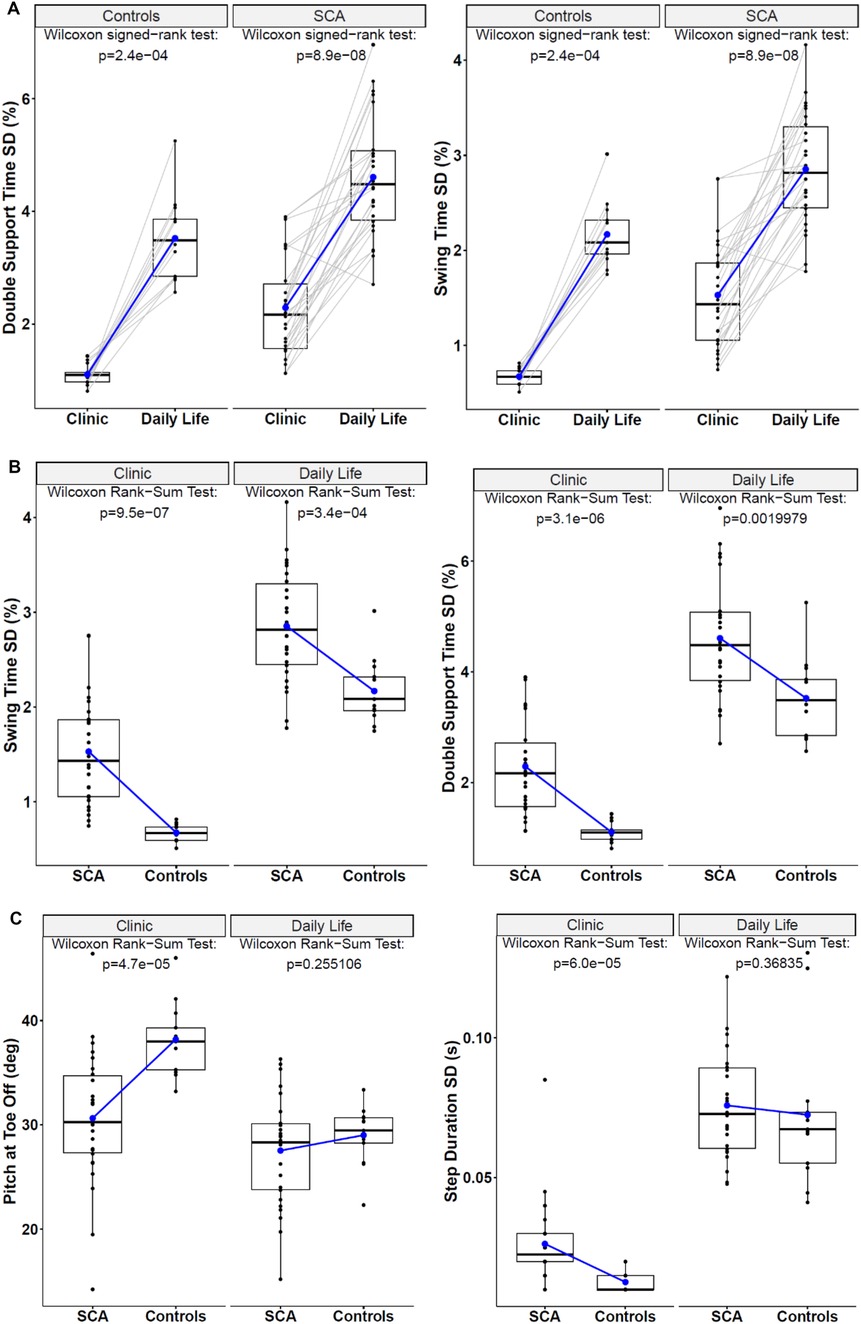
Figure 2. (A) Paired box plots demonstrate how the two most discriminative gait measures differed for individual subjects across the clinic vs. daily life environments. (B) Box plots of the most discriminative gait measures to compare SCA and HC in both the clinic and daily life environments. (C) Examples of Box-Plots for Gait measures that were discriminative to SCA vs. HC in the clinic but not in daily life.
Gait measures were significantly correlated with clinical and patient-reported outcomes
The most discriminative variability measures [Double Support Time SD(%) and Swing Time SD (%)] were also significantly correlated with SARA scores and Patient Reported Outcomes such as PROM, ABC, and also Disease Duration in the SCA population (Figure 3). Double support time variability collected in the clinic generally had stronger correlations with clinical measures compared to Double support time variability collected in daily life (i.e., r = 0.76 and 0.77 in the clinic vs. r = 0.31 and 0.45 in daily life with SARA total). The one exception is correlation with the PROM Physical component 2, which was significantly correlated with both discriminative measures in both the clinic and daily life (r = 0.57–0.68).
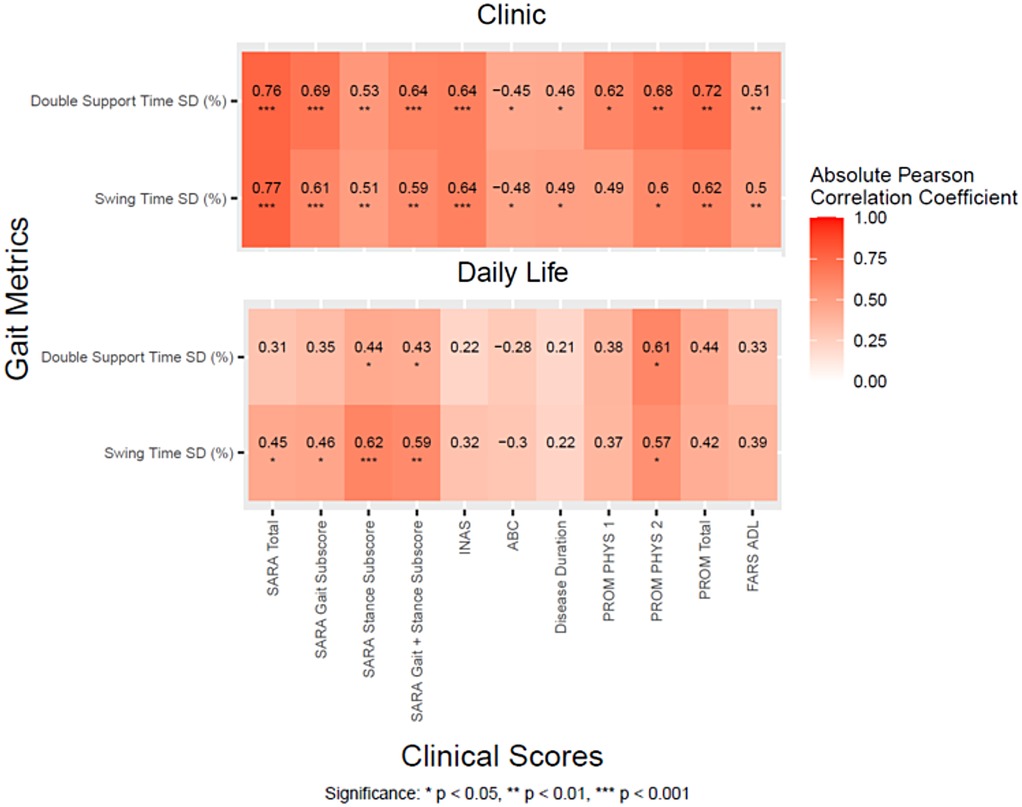
Figure 3. Correlation heatmaps between the two most discriminative gait measures and clinical scores for the SCA cohort during daily life and in the clinic.
Discussion
This study aimed to identify the most discriminatory gait measures for use in clinical trials for SCA 1,2,3, and 6 from body-worn, inertial sensors during a 2-minute, in-clinic walking at natural pace assessment and during a week of walking during daily life. We found that the prescribed walking task in the clinic yielded more discriminative measures than the unprescribed walking in daily life. Yet, two gait timing variability measures were consistently the top discriminative measures for SCA in both settings.
The top three discriminative gait measures between SCA and HC in the clinic and in the home environment were in the variability domain. Swing time SD and Double Support SD were among the top discriminators in both environments, along with foot strike angle SD (clinic) and lateral step variability (daily life). These gait variability measures are consistent with results from previous studies in SCA, showing increased spatiotemporal variability. Shah et al. (16) found that toe-out angle variability and double-support time variability were the most sensitive and specific 2-minute-walk-test gait features of SCA using wearable inertial sensors, with similar findings demonstrated in pre-manifest SCA2 subjects (21). Ilg et al. (11) found that lateral step deviation had an AUC of 0.86 when distinguishing patients with cerebellar ataxia compared to healthy controls in a daily living environment. These findings highlight the clinical significance of gait variability measures collected both during prescribed walking tasks in the clinic and during spontaneous walking in daily living for assessing natural history and intervention studies in ataxia.
Several key differences between in-clinic and home data were observed. First, the clinic 2-minute walk test data showed greater sensitivity to SCA compared to daily life data, as demonstrated by greater AUC values. In fact, 11 out of 17 measures in the clinic and 3 out of 17 measures in daily life had an AUC ≥ 0.8 for discriminating SCA from HC. Second, digital gait measures showed a greater correlation with clinical scales and patient-reported outcomes overall compared to the home environment. This suggests that gait variability measured at home reflects aspects of motor function less aligned with patient perception and clinician-reported performance-related motor assessment.
Gait data captured in a prescribed task in the clinic reflects the patient's capacity to perform gait, whereas data captured in the daily living environment, without task constraints, reflects a patient's actual functional performance in their own environment. The benefits of gait data captured in a clinical task, like the 2-minute walk, include high discriminative ability, correlation with clinical scales, and reliable measurement with relatively quick assessment time. However, patients walk differently in the clinical environment when observed by clinicians and asked to concentrate on their gait in a novel environment (30).
Although variability inherent in passive gait assessment means that more subjects are needed to differentiate ataxic gait from normal, comprehensive daily living gait quality assessment reflects an individual's actual functional mobility in their home and local environment, which provides valuable insights that complement in-clinic assessment. Thus, there are benefits and drawbacks for both in-clinic (e.g., Hawthorne effect, patient burden) (38) and daily living assessment (high variability due to distractions and dual-tasking, large datasets) (39). Results from this study suggest that both in-clinic and daily-living gait variability measures offer utility for clinical trials; however, daily-living assessment may be considered supplemental to a clinic-prescribed gait test, given the limited studies of real-life gait assessment in SCA to date.
There are several limitations to the current study. First, the sample size is limited, with only a few subjects for each of the 4 subtypes of SCA. Future research should aim to gather a larger sample for each subtype of SCA and include additional types, as the discriminative power of gait may vary between SCA subtypes. Second, this study did not take into account other signs of ataxia (ie, upper limb coordination) that could be quantified using body-worn sensors, which may enhance the discriminative power, validity, and reliability for SCA, and allow testing of nonambulatory patients. Future research should explore combining gait and balance measures to develop a composite standing and walking balance score, potentially more sensitive and specific to SCA than a single measure (40). Third, we did not compare similar gait bout lengths between clinical and daily life settings, as we only had data for 2-minute gait bouts in the clinic and observed only a few bouts as long as 2-minute gait bouts in daily life. We have previously shown that people tend to walk faster during a prescribed, self-paced gait test than during daily life when they are distracted and tend to have shorter gait bouts. Forth, due to the small number of participants within each SCA subtype, we were unable to conduct meaningful subtype-specific analyses. Therefore, future studies should include larger cohorts for each subtype to allow more detailed, SCA subtype-specific investigations. Lastly, future studies should include longitudinal progression data to identify which discriminative measures most effectively quantify disease progression.
This study has identified a set of objective and discriminative, digital gait measures from body-worn inertial sensors collected during free living in daily life and during a self-paced, prescribed 2-minute walk in the clinic. The variability of gait timing measures was discriminative for SCA in both daily life and the clinic, but in-clinic measures showed greater discriminative power and higher correlations with clinical scales and patient-reported outcomes. Future research involving tracking disease progression, validity, and reliability of a larger cohort of people with SCA is needed to identify the most useful digital gait biomarkers for clinical trials.
Data availability statement
The datasets presented in this article are not readily available due to patient privacy. De-identified gait and SARA data will be made available with the sponsor and PIs consent. Requests to access the datasets should be directed toY2dvbWV6QGJzZC51Y2hpY2Fnby5lZHU=.
Ethics statement
This study involved human participants and was approved by Oregon Health & Science University and The University of Chicago. The study was conducted in accordance with their institutions ethical committees. Written informed consent to participate was provided by the participants.
Author contributions
VS: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. DM: Data curation, Formal analysis, Software, Visualization, Writing – original draft, Writing – review & editing. AJ: Writing – original draft, Writing – review & editing. HC: Methodology, Project administration, Writing – review & editing. JM: Data curation, Investigation, Writing – review & editing. ME-G: Data curation, Investigation, Writing – review & editing. KS: Resources, Writing – review & editing. DS: Methodology, Project administration, Writing – review & editing. PC-K: Methodology, Project administration, Writing – review & editing. FH: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing – review & editing. CG: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This IDEA (Instrumented Data Exchange for Ataxia) study was sponsored by Biogen and Pfizer. The funders had no role in study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
The authors thank all participants for generously donating their time to participate.
Conflict of interest
Authors VS, DM, AJ, JM, ME-G, KS and FH are employees at APDM Wearable Technologies, a Clario Company.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdgth.2025.1590150/full#supplementary-material
Supplemental Table S1 | Gait measures and their definitions.
Supplemental Table S2 | S2A. Paired Wilcoxon results of comparing Clinic versus Daily Life measures for the SCA participants. Bold indicates p<0.003. S2B. Paired Wilcoxon results of comparing Clinic versus Daily Life measures for the HC participants. Bold indicates p<0.003.
Supplemental Figure S1 | The frequency distribution of the median number of strides per bout for the SCA and HC groups.
Abbreviations
SARA, scale for the assessment and rating of ataxia; INAS, inventory of non-ataxia signs; ABC, activities-specific balance confidence scale; PROM, patient-reported outcome measure; FARS ADL, Friedreich's ataxia rating scale—activities of daily living.
References
1. Buijsen RAM, Toonen LJA, Gardiner SL, van Roon-Mom WMC. Genetics, mechanisms, and therapeutic progress in polyglutamine spinocerebellar ataxias. Neurotherapeutics. (2019) 16:263–86. doi: 10.1007/s13311-018-00696-y
2. Luo L, Wang J, Lo RY, Figueroa KP, Pulst SM, Kuo P-H, et al. The initial symptom and motor progression in spinocerebellar ataxias. Cerebellum. (2017) 16:615–22. doi: 10.1007/s12311-016-0836-3
3. Globas C, Tezenas du Montcel S, Baliko L, Boesch S, Depondt C, DiDonato S, et al. Early symptoms in spinocerebellar ataxia type 1, 2, 3, and 6. Mov Disord. (2008) 23:2232–8. doi: 10.1002/mds.22288
4. Sánchez-López CR, Perestelo-Pérez L, Escobar A, López-Bastida J, Serrano-Aguilar P. Health-related quality of life in patients with spinocerebellar ataxia. Neurología. (2017) 32:143–51. doi: 10.1016/j.nrleng.2015.09.002
5. Schmitz-Hubsch T, Tezenas Du Montcel S, Baliko L, Berciano J, Boesch S, Depondt C. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. (2006) 66:1717–20. doi: 10.1212/01.wnl.0000219042.60538.92
6. Jacobi H, Tezenas du Montcel S, Bauer P, Giunti P, Cook A, Labrum R, et al. Long-term disease progression in spinocerebellar ataxia types 1, 2, 3, and 6: a longitudinal cohort study. Lancet Neurol. (2015) 14:1101–8. doi: 10.1016/S1474-4422(15)00202-1
7. Ashizawa T, Oz G, Paulson HL. Spinocerebellar ataxias: prospects and challenges for therapy development. Nat Rev Neurol. (2018) 14:590–605. doi: 10.1038/s41582-018-0051-6
8. Perez-Lloret S, Van de Warrenburg B, Rossi M, Rodríguez-Blázquez C, Zesiewicz T, Saute JAM, et al. Assessment of ataxia rating scales and cerebellar functional tests: critique and recommendations. Mov Disord. (2021) 36:283–97. doi: 10.1002/mds.28313
9. Serrao M, Pierelli F, Ranavolo A, Draicchio F, Conte C, Don R, et al. Gait pattern in inherited cerebellar ataxias. Cerebellum. (2012) 11:194–211. doi: 10.1007/s12311-011-0296-8
10. Zesiewicz TA, Stephenson JB, Hun Kim S, Sullivan KL, Jahan I, Huang Y, et al. Longitudinal gait and balance decline in friedreich’s ataxia: a pilot study. Gait Posture. (2017) 55:25–30. doi: 10.1016/j.gaitpost.2017.03.019
11. Ilg W, Seemann J, Giese M, Traschütz A, Schöls L, Timmann D, et al. Real-life gait assessment in degenerative cerebellar ataxia: toward ecologically valid biomarkers. Neurology. (2020) 95:e1199–210. doi: 10.1212/WNL.0000000000010176
12. Milne SC, Murphy A, Georgiou-Karistianis N, Yiu EM, Delatycki MB, Corben LA. Psychometric properties of outcome measures evaluating decline in gait in cerebellar ataxia: a systematic review. Gait and Posture. (2018) 61:149–62. doi: 10.1016/j.gaitpost.2017.12.031
13. Shirai S, Yabe I, Matsushima M, Ito YM, Yoneyama M, Sasaki H. Quantitative evaluation of gait ataxia by accelerometers. J Neurol Sci. (2015) 358:253–8. doi: 10.1016/j.jns.2015.09.004
14. Terayama K, Sakakibara R. Wearable gait sensors to measure ataxia due to spinocerebellar degeneration. Neurol Clin Neurosci. (2018) 6:9–12. doi: 10.1111/ncn3.12174
15. Rochester L, Galna B, Lord S, Mhiripiri D, Eglon G, Chinnery PF. Gait impairment precedes clinical symptoms in spinocerebellar ataxia type 6. Mov Disord. (2014) 29:252–5. doi: 10.1002/mds.25706
16. Shah VV, Rodriguez-Labrada R, Horak FB, McNames J, Casey H, Floyd KH, et al. Gait variability in spinocerebellar ataxia assessed using wearable inertial sensors. Mov Disord. (2021) 36:2922–31. doi: 10.1002/mds.28740
17. Buckley E, Mazzà C, Mcneill A. A systematic review of the gait characteristics associated with cerebellar ataxia. Gait Posture. (2018) 60:154–63. doi: 10.1016/j.gaitpost.2017.11.024
18. Ilg W, Milne S, Schmitz-Hübsch T, Alcock L, Beichert L, Bertini E, et al. Quantitative gait and balance outcomes for ataxia trials: consensus recommendations by the ataxia global initiative working group on digital-motor biomarkers. Cerebellum. (2024) 23:1566–92. doi: 10.1007/s12311-023-01625-2
19. Caliandro P, Iacovelli C, Conte C, Simbolotti C, Rossini PM, Padua L, et al. Trunk-lower limb coordination pattern during gait in patients with ataxia. Gait Posture. (2017) 57:252–7. doi: 10.1016/j.gaitpost.2017.06.267
20. Milne SC, Hocking DR, Georgiou-Karistianis N, Murphy A, Delatycki MB, Corben LA. Sensitivity of spatiotemporal gait parameters in measuring disease severity in friedreich ataxia. Cerebellum. (2014) 13:677–88. doi: 10.1007/s12311-014-0583-2
21. Velázquez-Pérez L, Rodriguez-Labrada R, González-Garcés Y, Arrufat-Pie E, Torres-Vega R, Medrano-Montero J, et al. Prodromal spinocerebellar ataxia type 2 subjects have quantifiable gait and postural sway deficits. Mov Disord. (2020) 36(2):471–80. doi: 10.1002/mds.28343
22. Schniepp R, Wuehr M, Schlick C, Huth S, Pradhan C, Dieterich M, et al. Increased gait variability is associated with the history of falls in patients with cerebellar ataxia. J Neurol. (2014) 261:213–23. doi: 10.1007/s00415-013-7189-3
23. Serrao M, Chini G, Casali C, Conte C, Rinaldi M, Ranavolo A, et al. Progression of gait ataxia in patients with degenerative cerebellar disorders: a 4-year follow-up study. Cerebellum. (2017) 16:629–37. doi: 10.1007/s12311-016-0837-2
24. Matsushima A, Yoshida K, Genno H, Murata A, Matsuzawa S, Nakamura K, et al. Clinical assessment of standing and gait in ataxic patients using a triaxial accelerometer. Cerebellum Ataxias. (2015) 2:9. doi: 10.1186/s40673-015-0028-9
25. Németh AH, Antoniades CA, Dukart J, Minnerop M, Rentz C, Schuman B-J, et al. Using smartphone sensors for ataxia trials: consensus guidance by the ataxia global initiative working group on digital-motor biomarkers. Cerebellum. (2024) 23:912–23. doi: 10.1007/s12311-023-01608-3
26. Manohar R, Yang FX, Stephen CD, Schmahmann JD, Eklund NM, Gupta AS. At-home wearables and machine learning capture motor impairment and progression in adult ataxias. Brain. (2024) awaf154. doi: 10.1093/brain/awaf154
27. Seemann J, Daghsen L, Cazier M, Lamy J-C, Welter M-L, Giese MA, et al. Digital gait measures capture 1-year progression in early-stage spinocerebellar ataxia type 2. Mov Disord. (2024) 39:788–97. doi: 10.1002/mds.29757
28. Fox SH, Katzenschlager R, Lim S-Y, Barton B, De Bie RMA, Seppi K, et al. International Parkinson and movement disorder society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson's disease. Mov Disord. (2018) 33:1248–66. doi: 10.1002/mds.27372
29. Seemann J, Beyme T, John N, Harmuth F, Giese M, Schöls L, et al. Capture of longitudinal change in real-life walking in cerebellar ataxia increases patient relevance and effect size. Mov Disord. (2025) 40(7):1343–55. doi: 10.1002/mds.30230
30. Shah VV, McNames J, Mancini M, Carlson-Kuhta P, Spain RI, Nutt JG, et al. Laboratory versus daily life gait characteristics in patients with multiple sclerosis, Parkinson’s disease, and matched controls. J Neuroeng Rehabil. (2021) 17:1–12. doi: 10.1186/s12984-020-00781-4
31. Shah VV, McNames J, Mancini M, Carlson-Kuhta P, Spain RI, Nutt JG, et al. Quantity and quality of gait and turning in people with multiple sclerosis, Parkinson’s disease and matched controls during daily living. J Neurol. (2020) 267:1188–96. doi: 10.1007/s00415-020-09696-5
32. Shah VV, McNames J, Mancini M, Carlson-Kuhta P, Nutt JG, El-Gohary M, et al. Digital biomarkers of mobility in Parkinson’s disease during daily living. J Parkinsons Dis. 10(3):1099–1111. doi: 10.3233/JPD-201914
33. Mancini M, King L, Salarian A, Holmstrom L, McNames J, Horak FB. Mobility lab to assess balance and gait with synchronized body-worn sensors. Journal of Bioengineering and Biomedical Sciences. (2013):1–5. doi: 10.4172/2155-9538.s1-007
34. Washabaugh EP, Kalyanaraman T, Adamczyk PG, Claflin ES, Krishnan C. Validity and repeatability of inertial measurement units for measuring gait parameters. Gait Posture. (2017) 55:87–93. doi: 10.1016/j.gaitpost.2017.04.013
35. Morris R, Stuart S, McBarron G, Fino PC, Mancini M, Curtze C. Validity of mobility lab (version 2) for gait assessment in young adults, older adults and Parkinson’s disease. Physiol Meas. (2019) 40:0–8. doi: 10.1088/1361-6579/ab4023
36. Wan EA, Van Der Merwe R. The unscented kalman filter for nonlinear estimation. Proceedings of the IEEE 2000 Adaptive Systems for Signal Processing, Communications, and Control Symposium (Cat. No. 00EX373) (2000). p. 153–8
37. Van Der Merwe R, Wan E. Sigma-Point Kalman Filters for Probabilistic Inference in Dynamic State-Space Models (PhD dissertation). Oregon Health and Science University (2004).
38. Geh CLM, Beauchamp MR, Crocker PRE, Carpenter MG. Assessed and distressed: white-coat effects on clinical balance performance. J Psychosom Res. (2011) 70:45–51. doi: 10.1016/j.jpsychores.2010.09.008
39. Adams JL, Lizarraga KJ, Waddell EM, Myers TL, Jensen-Roberts S, Modica JS, et al. Digital technology in movement disorders: updates, applications, and challenges. Curr Neurol Neurosci Rep. (2021) 21:1–11. doi: 10.1007/s11910-021-01101-6
Keywords: gait, IMUs, wearable technology, daily life monitoring, spinocerebellar ataxia
Citation: Shah VV, Muzyka D, Jagodinsky A, Casey H, McNames J, El-Gohary M, Sowalsky K, Safarpour D, Carlson-Kuhta P, Horak FB and Gomez CM (2025) Clinic vs. daily life gait characteristics in patients with spinocerebellar ataxia. Front. Digit. Health 7:1590150. doi: 10.3389/fdgth.2025.1590150
Received: 8 March 2025; Accepted: 28 July 2025;
Published: 3 September 2025.
Edited by:
Ankit Vijayvargiya, Dublin City University, IrelandReviewed by:
Praveen Shukla, Manipal University Jaipur, IndiaFrancisco Anaya Reyes, Insight Centre for Data Analytics, Ireland
Copyright: © 2025 Shah, Muzyka, Jagodinsky, Casey, McNames, El-Gohary, Sowalsky, Safarpour, Carlson-Kuhta, Horak and Gomez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vrutangkumar V. Shah, c2hhaHZyQG9oc3UuZWR1
 Vrutangkumar V. Shah
Vrutangkumar V. Shah Daniel Muzyka1
Daniel Muzyka1 Hannah Casey
Hannah Casey Delaram Safarpour
Delaram Safarpour Patricia Carlson-Kuhta
Patricia Carlson-Kuhta Fay B. Horak
Fay B. Horak