- 1Action Lab, Faculty of Information Technology, Monash University, Melbourne, VIC, Australia
- 2Deakin University School of Public Health and Social Development, Deakin University, Melbourne, VIC, Australia
- 3Monash Addiction Research Centre, Eastern Health Clinical School, Monash University, Melbourne, VIC, Australia
Introduction: Australia has one of the highest rates of opioid prescribing and prescription opioid-related harm in the world. Although effective for pain relief, the use of prescription opioids is a leading cause of preventable morbidity and mortality. Barriers exist for consumers identifying their own risk factors, accessing naloxone (opioid overdose antidote) and overdose prevention education. This study aimed to co-design a digital Opioid Safety Toolkit for national dissemination through pharmacies to encourage three consumer opioid safety behaviours: (1) uptake of naloxone, (2) creating a safety plan, and (3) discussing their use of opioids, including any concerns with their healthcare professional.
Methods: The digital Toolkit was co-designed and developed using a novel approach to digital health intervention design combining the Theoretical Domains Framework (TDF) and Double-Diamond design process. Co-design involved a series of seven iterative workshops with consumers (4) and professionals (3). Workshops focused on identifying factors influencing opioid safety behaviours, exploring design preferences, sense-checking, and ideation of the user flow. User testing was conducted with the penultimate version of the Toolkit.
Results: 13 consumers with lived experience of prescription opioid use and 14 professionals including prescribers, pharmacists, pain specialists, researchers and consumer advocates participated in up to three separate workshops. 15 consumers participated in user testing interviews. Analysis of workshops identified factors promoting safety behaviours including increased public awareness of naloxone, understanding personal risk (TDF domain of Knowledge); healthcare professional's role in education and consumers' experience of stigma (Social/professional role and identity); use of conversational aids to scaffold conversations, material resources and data ownership (Environment, context and resources). User testing elicited feedback pertaining to the information and resources on the website and the overall user interface and experience.
Discussion: The Toolkit was co-designed with consumers and professionals to facilitate opioid safety behaviours. The Toolkit includes evidence-based information, tools for risk assessment and screening, opioid use monitoring, conversation aids, and a safety plan. The Toolkit is being disseminated nationally through Australian pharmacies following a randomized controlled trial that demonstrated the Toolkit promotes safety behaviours, is easy to use and acceptable to those with lived experience of prescription opioid use and professionals.
1 Introduction
Opioid use and related harms are a global public health challenge, resulting in considerable health, economic and societal impacts. One of the most substantial harms is fatal and non-fatal overdoses. While the types of opioids involved in opioid-related deaths vary across countries, in Australia, harms associated with opioids are predominantly related to prescription opioids (1). Opioid overdose deaths in Australia have increased two-fold between 2002 and 2019 (2), with the majority involving prescription opioids (1, 3). Unlike the US and Canada, Australia did not see an escalation of opioid deaths during the COVID pandemic, opioid overdose deaths declined slightly during 2020–2021 (4, 5). However, preliminary estimates for 2022 indicate an upward trend (4). Furthermore, each day, there are around 150 hospitalizations and 14 emergency department presentations involving opioids (6), highlighting the need to identify and empower those at risk of prescription opioid related harm.
Opioids are commonly prescribed to treat both acute and chronic pain, with the latter being one of the most common reasons people seek medical care (7). Opioid prescribing has increased substantially over the past three decades and Australia has one the highest rates of opioid prescribing per capita, exceeding countries such as the US (8), with approximately 3 million Australians prescribed opioids, and 1.9 million adults initiating opioids each year (9). Long-term prescription opioid use is associated with harms including dependence, morbidity, and mortality (10).
Over the past decade there has been considerable research to understand and measure opioid-related risk among people who are prescribed opioids. For example, an Australian cohort study found 40% were prescribed high opioid doses (above 90 mg oral morphine equivalent) (11), with higher opioid dose associated with higher odds of multiple physical and mental health issues, nonmedical opioid use and opioid dependence (11). Similarly, a large proportion of those prescribed opioids had increased risk of overdose due to meeting criteria for previous alcohol use disorder (one third) or taking concurrent benzodiazepines (one third) (12–14). Other overdose risk factors include concurrent respiratory conditions and taking an opioid dose above 50 mg of oral morphine equivalents (15).
Despite an estimated four out of five people who are prescribed opioids for chronic pain having at least one opioid overdose risk factor, recognition of opioid-related risk and knowledge about signs and symptoms of opioid overdose in this population is low (15, 16). There are a range of barriers to help-seeking for this population. For example, even though one in three people in a sample of people who were prescribed opioids met criteria for opioid use disorder (OUD), less than 5% had received evidence-based treatment for it, with seeking help from a health professional reported by only a small fraction of people who were worried about their own opioid use (17).
As opioid-related risk is dynamic, routine monitoring and assessment is recommended. Provision of naloxone, an opioid overdose antidote, has been shown to reduce the likelihood of later emergency presentations, and has been shown to be acceptable for people who are prescribed opioids for pain (15, 18). An Australian Government pilot program made naloxone free from participating community pharmacies, without a prescription, however it was estimated that only 2% of at-risk people who were prescribed opioids in this study received naloxone (19). The evaluation recommended that dedicated efforts were needed to upscale overdose prevention for this group. Further, self-administered screening tools to identify OUD and monitor outcomes with opioids have been developed and shown to be acceptable to people who are prescribed opioids for pain (20–23). Taken together, the foundation, or basic tools to help identify and respond to opioid-related risk among people who are prescribed opioids have been developed, and the digitization and dissemination of these tools could promote the uptake of naloxone.
Digital health interventions aim to increase reach, equality, and use of evidence-based information compared to their non-digital counterparts (24). Digital health interventions for opioid risk management include exploration of technologies to prevent, predict, detect, and respond to opioid misuse and overdose (25, 26). For example, technologies for prevention of opioid overdose have included computational methods to predict relapse and recovery factors, digitally mediated peer sponsorship, and design of drug checking test result displays to depict uncertainty (27–29). While for detection of and response to opioid overdose there is research around hypothetical and deployed web-based apps which alert volunteers to nearby potential overdoses (30, 31). However, two recent systematic reviews found a lack of studies reporting satisfaction, acceptability, willingness of consumers to engage with the digital technologies (25, 26).
Researchers have called for future work to use co-design approaches with consumers who are prescribed opioids for pain relief, especially those with chronic pain (32, 33). However, most of the research to date concerning the design of digital health interfaces has focused on those who use illicit opioids or misuse prescription opioids, rather than those prescribed opioids for pain relief. Furthermore, these studies tend to focus on the design of digital interventions at a local level (e.g., crowdsourcing opioid antidote provision in confined areas (34) rather than design for implementation at an organisational or systems level (e.g., nationwide) (35, 36).
A critical reason for failure to implement and scale digital health interventions is the lack of consideration for contextual and implementation factors across individual (behavioural), organization, and system levels during the design process. Mohr (37) highlights that technology-enabled services are often developed by those outside implementation settings, neglecting key considerations like requirements, processes, and service needs (38). Implementation science is the scientific study of what works to embed evidence-based interventions, programs and policies in real-world settings (39). Several theories, models and frameworks allow researchers and practitioners to understand contextual factors and develop strategies to influence adoption, ongoing maintenance, and scale of interventions (40). For example, determinant frameworks focus on predicting or explaining factors affecting implementation by identifying contextual barriers and facilitators thereby helping plan for solutions to problems before they arise [e.g., the Theoretical Domains Framework (TDF)] (40, 41).
Researchers and practitioners see the potential benefits in combining human-computer interaction (HCI) and implementation science (42, 43). However, most of the research to date focuses on how implementation science can benefit from HCI, rather than how HCI may benefit from implementation science. For example, promoting iterative design and evaluation in implementation planning (37) and embedding human-centered and user-centered approaches to the development of implementation strategies (42–44). Waddell et al. (46) addressed this gap by proving an approach to combining an implementation science framework (the non-adoption, abandonment, scale-up, spread, and sustainability framework) within a design process, the Double Diamond (45–47). Although providing a systematic scaffolding through which to design for contextual factors across individual, organisational and system levels, the authors call for future work to use different implementation science frameworks depending on the design problem.
This research addresses a specific gap in the literature, the lack of digital public health interventions to empower people who have been prescribed opioids for pain relief to engage in safety behaviours. As such, the aim of this study was to co-design an opioid safety Toolkit with consumers and experts for national dissemination in pharmacies. The design of the Toolkit focused on promoting three safety behaviours (having naloxone at home, creating a safety plan, and speaking to a healthcare professional about their use of opioids, including any concerns with their opioids). Specifically, the objectives of the study were to (1) digitize pre-existing validated tools to reduce opioid safety risk factors, (2) explore the barriers and facilitators to opioid safety behaviours using a novel approach to combing implementation science and design methods, (3) design an opioid safety Toolkit that the addresses the elicited barriers and facilitators, meets the needs of end-users and is implementable in the real-world.
2 Method
2.1 Study design
This study adapted a novel co-design approach that combined the Double Diamond design process alongside a behavioural and implementation science framework, the Theoretical Domains Framework (TDF) (40) (Figure 1). The Double Diamond (45) is a process framework that includes four phases from a design problem through exploration (discovery), synthesis (definition), ideation (development) and solution testing and refinement (delivery) to arrive at a design solution. Although visually represented as sequential steps, the Double Diamond can include iteration across and within the phases. As explored by Waddell et al. (46), the Double Diamond can serve as an overall process in which implementation science frameworks can be embedded (46). Given the initial focus of the Toolkit was to encourage behaviour change among prescription opioid consumers and by extension healthcare professionals, this study embedded the TDF.
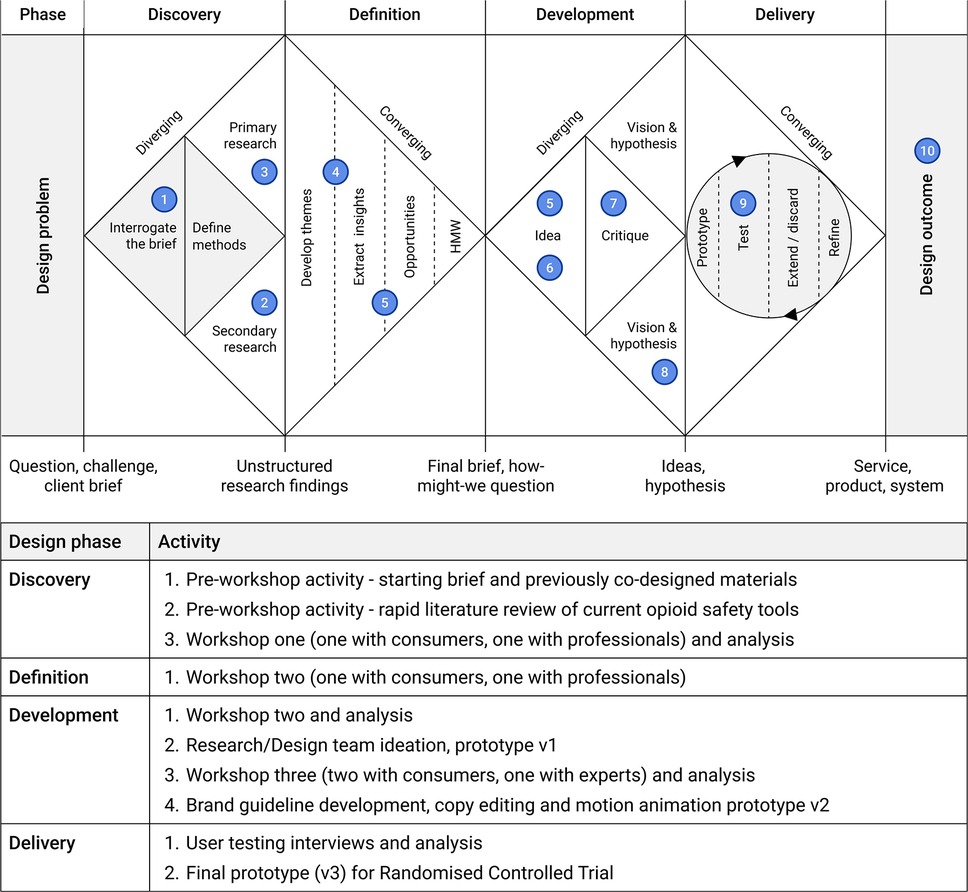
Figure 1. The opioid safety toolkit design process as guided by the double diamond process and relevant activities. Adapted with permission from “How to apply a design thinking, HCD, UX or any creative process from scratch” by Dan Nessler.
The TDF is the result of the expert synthesis and simplification of behaviour change theories. The framework is used to explore and inform intervention and implementation design by eliciting data with respect to people's values, beliefs, experiences and motivations that underpin behaviour (40). The TDF consists of 14 domains and 84 component constructs that can act as barriers and facilitators to behaviours, such as knowledge, skills, environment, reinforcement, and social influences. These factors are commonly used to develop theory-informed behavioural interventions, such as public health campaigns and the uptake of new policies or procedures as it is readily mapped to the Behaviour Change Techniques Taxonomy via the Theory and Techniques Tool (48–51).
2.2 Participants
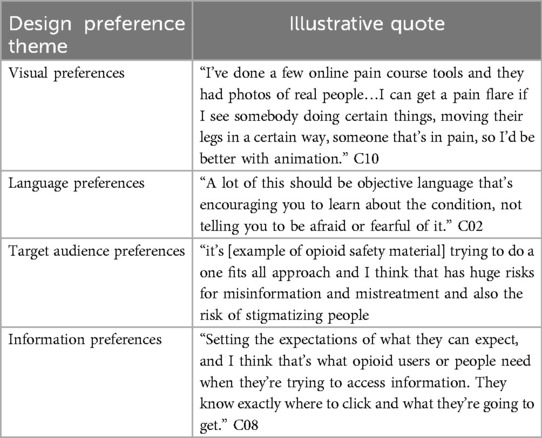
To maximize the real-world implementation of the Toolkit, the project team partnered with two expert organizations across the design process, representing both consumers and pharmacists. Painaustralia is a leading advocacy group working to improve the quality of life of people living with chronic pain. The Pharmaceutical Society of Australia is the peak body for Australian pharmacists. Participants were recruited using purposive and snowball sampling (52, 53). For consumers, the recruitment and sampling strategy was purposefully broad and inclusive to encourage a range of views of people who had been prescribed opioids for pain relief. Specifically, the inclusion criteria for both the co-design workshops and user testing were for people living in Australia, with lived experience of being prescribed opioids for noncancer pain relief. Exclusion criteria were people who did not have proficient English language skills to engage in the recruitment, workshops or interviews. For professionals the inclusion criteria included healthcare professionals who were responsible for prescribing or dispensing opioids for pain relief to consumers or who were members of professional or consumer advocacy groups. Exclusion criteria were professionals not practicing in Australia. Consumer participants were recruited through online advertisements via Painaustralia's LinkedIn and Facebook pages, short term consumers were invited through authors' and staff at Painaustralia's consumer networks. Health professionals and stakeholders were invited through authors' and staff at the Pharmaceutical Society of Australia's professional networks and snowball recruitment via email. Consumer participants included: (i) consumers who had been using opioids for over 3 months (long-term), (ii) consumers who had been using prescription opioids for 2-weeks to 3 months, (iii) consumers who had been using opioids for less than 2 weeks (short-term) or who were carers of people who had been prescribed opioids. Professional participants included prescribers, pharmacists, researchers, or consumer or healthcare professional advocates. Healthcare professionals from rural areas, and those with expertise working with first nations people were purposively recruited.
2.2.1 Ethical considerations
Ethics approval was granted by Monash University (ID: 40628). All participants provided written informed content to prior to participation. Consumers were contacted by Painaustralia staff who have a high level of content knowledge about opioid prescriptions and chronic pain and ensured through discussion over the phone that the participant was eligible and interested in participating. “Short-term” consumers were contacted by the research team via email and were available to answer any questions over the phone. Professional participants were contacted via email and a researcher was available to answer any questions via email or phone. Privacy and confidentiality of participants was maintained by removing identifying information during the transcription process. Participant codes were assigned to participants for all analysis and reporting. All participants who participated in the co-design received $100AUD (∼$65USD) gift card for their time. Participants in the user-testing received $50AUD (∼$32.50USD) gift card for their time.
2.3 Overview of the co-design process
The prescription Opioid Safety Toolkit (the Toolkit) was designed and developed over 9 months using co-design processes. A total of seven 2-h workshops, three with professionals and three with consumers (workshop 3 was repeated with a different cohort of consumers for a total of four workshops) were conducted. User testing interviews were later conducted to test the usability of the Toolkit. All workshops and interviews were conducted online using the video conference platform Zoom (version 6) and digital whiteboard Miro. The Miro whiteboard was controlled by the researchers who screen-shared the whiteboard during the co-design workshops so that all participants could see participants' responses to activities in real-time.
2.3.1 Pre-workshop activities (1, 2)—discovery
A range of previously co-designed Australian resources were identified as starting materials to include in the Toolkit, alongside examples of international resources that had been developed to increase opioid safety. The local resources were developed with Australian consumers and healthcare professionals to promote opioid safety behaviours (20–23). They included the validated Routine Opioid Outcome Monitoring (ROOM) tool which -was specifically developed to screen for prescription opioid-related risks and clinical outcomes, comprising content and language salient to people prescribed opioids for chronic non-cancer pain (20, 23). In addition, a consumer-facing leaflet and educational videos were developed in consultation with pharmacists and consumers to educate consumers on the storage and use of naloxone (54). A rapid literature review of existing opioid safety resources was conducted, and these materials were used to inform activities in Workshop 1.
2.3.2 Workshop One (3)—discovery
Barriers and facilitators for the safety behaviours of interest (having naloxone at home, creating a safety plan, and speaking to a healthcare professional) were identified using three design methods. Firstly, “think aloud” (55) was used to explore consumers' and professionals' reflections on available opioid safety materials. Secondly, “journey mapping” (56) visually represented consumers' experience being prescribed opioids for pain relief (Supplementary Figure S1). Thirdly, “brainstorming” explored where and how safety behaviours might be embedded in the consumer journey for maximum uptake. Workshop transcripts were analyzed using the TDF to elicit the specific barriers and facilitators to engaging in safety behaviours. Design preferences were inductively analyzed and broadly covered the preferred language and visual design style (color, typography and visual imagery).
2.3.3 Workshop Two (4,5)—definition and development
This workshop sense-checked barriers and facilitators to safety behaviours, and the language and design preferences identified in Workshop One. Participants were presented with a summary of Workshop One findings, then asked if there was nuance missing, or if anything stood out as particularly important. Workshop Two also focused on developing solutions to the issues identified in Workshop One. Participants reviewed key challenges, such as the need to tailor information based on how long someone has been on a medication. They then brainstormed solutions such as constructing screening questions to enable personalized information delivery. Finally, they helped map the Toolkit's user flow, determining the content and its optimal presentation order.
2.3.4 Research/design team ideation (6)—development of v1 prototype
Following Workshop Two, the research/design team took the potential screening questions and user flows developed by the participants and used them to inform the design of an early low fidelity prototype (v1) of the Toolkit on Figma, an online collaborative design and prototyping tool (Supplementary Figure S2). Barriers and facilitators aligned with the TDF were mapped to relevant Behaviour Change Techniques (BCTs) using the Theory and Techniques Tool (51).
2.3.5 Workshop three (7)—development of v2 prototype
This phase aimed to critique the low-fidelity prototype and refine the language and design preferences of the Toolkit. Three workshops were run, each tailored to the participant group: (i) long-term consumers of opioids, (ii) professional groups, and (iii) short-term consumers of opioids and carers. The long-term consumers and professional groups, provided feedback on each page of the v1 prototype—what their initial reactions were, what they thought the page was asking them to do, and specific feedback such as whether anything was missing, whether the flow was logical, and their preferences about the user interface design. Participants were then shown examples of language that could be used in the Toolkit (e.g., about naloxone uptake) and to provide feedback and reflections on how the language made them feel, who it was coming from, and what they thought they should do next (i.e., did it elicit behaviour change).
A new group of short-term consumers of opioids and carers were included for this workshop phase. The addition of short-term consumers was in response to participants' belief that different information should be provided to short-term consumers compared to those that had medium- or long-term prescription opioid use. These participants were first oriented to the aims of the project and the previous co-design findings. They were then shown one of the existing opioid resources to check if they identified similar barriers and facilitators to safety behaviours as previous workshop participants. Finally, they were also asked to provide page by page feedback on the v1 prototype of the Toolkit.
2.3.6 Development of brand guideline, copy editing, and motion animation and development of prototype v2 (8)—development
A brand guideline was developed using synthesized feedback on preferred language and visual design style (color, typography and visual imagery). The brand guidelines served as a resource to communicate the design preferences of the workshop participants, including the general look and feel, as well as the specific colors, logos, typography, and iconography. This brand guideline was used to communicate with a copy editor who was employed to refine the language of the Toolkit in line with the learnings from the workshops. The guideline was also used to communicate with a motion animation professional who was employed to update the pre-existing opioid education videos (see pre-workshop activities section) in line with the Toolkit's branding. The v1 low-fidelity prototype (i.e., a simplified representation of the Toolkit including the user-flow and basic layout) was updated to create a more refined prototype (v2) in Figma (Supplementary Figure S2, Supplementary Figure S3). The v2 prototype incorporated participant feedback and included the brand guidelines.
2.3.7 User testing interviews (9)—delivery
The v2 prototype subsequently underwent user testing with participants who had not yet interacted with the Toolkit and represented a diverse range of consumer types. During the interview, participants were provided with a link to the prototype and asked to interact with the Toolkit using “think aloud” (55) to share their thoughts as they progressed through the pages. Participants were prompted to share all thoughts, no matter how small. Interviews were recorded and transcribed and researchers took notes during the session. Feedback gathered from the interview participants was then analyzed and further refinements were made to v2 prototype's information architecture (user flow) and user interface.
2.3.8 V3 prototype development (10)
The engineering team developed a mobile- and desktop-responsive web application based on the prototypes, analysis and feedback from previous phases.
2.4 Analysis
All workshops and interviews were video and audio recorded (via Zoom) and transcribed verbatim (using Rev.com). For the workshops, qualitative analysis was based on Atkins et al. (40) recommendations for conducting TDF-informed analysis and included both inductive and deductive approaches using NVivo software. After reading workshop transcripts to ensure familiarity, three members of the research team (AW, JW, DB) individually coded a subset of workshops (approximately 10%). Deductive coding was based on direct content analysis (57) wherein data was directly coded to the TDF domains, while any remaining transcript content was inductively coded (58). Team members met to compare and discuss coding choices and reach a consensus. One researcher (AW) coded the remaining transcripts using the same approach and developed inductive themes within each TDF domain. These themes were discussed with the overall research team who interrogated and confirmed the themes. User testing interviews were inductively analyzed (58). Three researchers (AW, DB, and CP) iteratively developed codes following each interview based on positive and negative feedback, one researcher (AW) subsequently analyzed the transcripts based on the elicited codes using NVivo.
3 Results
A total of 27 people participated in the workshops including 13 consumers and 14 professionals (denoted as C and P, respectively) while 15 consumers participated in the user testing interviews (Table 1). Short-term consumers included those with prescriptions for either acute or chronic pain, medium and long-term consumers included those with prescriptions for chronic pain. One professional provided feedback via email only. Demographics of participants and the workshops or interviews they attended are included in the supplementary materials (Supplementary Table S1, Supplementary Table S2).
3.1 Workshop results
Analysis of the workshops identified five TDF domains and subsequent sub-themes—Knowledge (increasing public awareness, understanding personal risk); Social/Professional Role and Identity (healthcare professionals role and responsibilities, experiences of stigma); Environment, Context and Resources (a conversational aid, material resources and data ownership); Social Support (others need to know how to use naloxone); and Beliefs about Capabilities (consumers are experts in their own experience).
3.1.1 Knowledge
3.1.1.1 Public awareness
Consumers felt it was crucial that the general community know about the importance of having naloxone on hand for opioid safety and its availability at pharmacies. For them, increasing public awareness should result in the people who are prescribed opioids for pain keeping naloxone in their first aid kit and knowing how to use it in case of an emergency. Importantly, their explanation of naloxone's importance included an additional message—that opioid overdose could happen to anyone, rather than only to people who were misusing or using illicit opioid substances.
“I think educating the wider community so that naloxone is not looked at as it's the druggies, that it is part of your First Aid thing and so that everyone realizes that it could just save someone in your family or one of your friends or something.” C06
Professionals said it was crucial that for safety behaviours to change, especially increasing naloxone uptake, there would need to be a public health campaign. Interestingly, all experts agreed there would need to be a campaign that reached both consumers and professionals using the same visuals and branding to ensure consistency. This finding aligned with consumer's request that experts be provided with education and training to be prepared for any potential conversations with consumers, especially around naloxone uptake. Likewise, consumers felt that consistent visuals and branding of a resource being promoted to both professionals and consumers would increase the credibility and therefore make it more likely for professionals to support them if they were to discuss the Toolkit with their own healthcare professional.
“So the primary issue with naloxone is normalizing it to such degree that people are comfortable with it being part of their life. So for both people who are using opioids and also the prescribers and dispensers and other health professionals who work in that space” P12
3.1.1.2 Understanding personal risk
Consumers felt a lack of knowledge of the risks associated with opioid use was a barrier to engaging in safety behaviours. Many of the consumers thought that those with new prescriptions should have the risks explained to them, by healthcare professional or through provision of resources. Although most long-term opioid consumers in the workshops felt they knew “enough” information about the risks associated with prescription opioids, some lamented having to find out information about risks themselves often after trial and error with medications or in some cases accidental overdose (e.g., sedation and slowed breathing). Interestingly, even though most long-term opioid prescription consumers felt although they knew “enough” information about risks, they expressed that it was still helpful for them to see the information again.
“Speaking for myself, probably be helpful for us just to see it. I mean, we might already have all this information sorted, but we might've forgotten something or there might be a little prompt that we need.” C07
Likewise, healthcare professionals felt it was crucial for consumers to understand their own individual risk factors associated with opioid prescriptions. In contrast to consumers, professionals felt any resource must go beyond the usual risk factors such as dose and type of medication, to include increased knowledge around how risk could change over time, and around personalized risk factors such as changes in context.
“People could have been on a stable dose for a long time, but other things have changed, and that's where they're not understanding the risks.” P02
Professionals reported experiences of consumers not wanting to engage in conversations about risk, while consumers highlighted how risk-based language could be off-putting. So, both consumers and professionals highlighted the importance of using supportive language to speak about risk factors to not alarm consumers who have been prescribed opioids for acute pain, or to discourage longer-term users with judgmental language.
“I thought let's not start off with the deaths right up front because you're going to scare the bejesus out of everybody. Maybe we'll go with the short-term harms, like constipation and everything” P04
“I think we have to be careful that we are balancing that we're telling people that it is okay to take something if they need it as part of a greater pain management strategy and everything else, but just making sure that we're not sending them the message that we're trying to talk them out of taking an opioid because sometimes it is appropriate” P10
3.1.2 Social/professional role and identity
3.1.2.1 Healthcare professionals role and responsibilities
Consumers felt it was the responsibility of healthcare professionals to educate consumers about opioids and the need for naloxone in the home. Some consumers felt the discussion should start with the prescriber (often their general practitioner), while others felt it could start with their pharmacist. Some consumers were concerned that pharmacists were too busy to have the conversation. A potential mitigation strategy was explored and most agreed that discussions should happen multiple times with different professionals and could also include allied health professionals (e.g., physiotherapists) to ensure the consumer was guaranteed to have the conversation at least once, and ideally with different healthcare professionals.
“Your doctor should have told you about naloxone. [To say]‘Go and talk to your pharmacist’” It just leaves any responsibility for overdose prevention out of the responsibility of the hands of prescribers…I think it should say, “Hopefully your doctor would've mentioned naloxone or suggest you get it from your pharmacy, if not, get it from your pharmacy.” C09
“It's really important that we've got to get that message about safety, but that means all of us have to be involved, particularly the healthcare professional.” P03
Consumers and healthcare professionals agreed it would be crucial for healthcare professionals, especially pharmacists, to receive training in how to have conversations around overdose and the importance of naloxone uptake. Related to the below theme of past negative experiences, many expressed a preference for pharmacists to lead the conversation, but to do so in non-stigmatizing ways. Professionals agreed that healthcare professionals should be the cornerstone of opioid safety conversations that cover the risks associated, alternative treatment options, and the need for naloxone in the home with family members who know how to use it. Some consumers and professionals felt opioid risk and naloxone conversations should happen with every opioid prescription. Most consumers and professionals agreed that in an ideal world there would be trained professionals similar to diabetes educators who would provide specific education for consumers. Healthcare professionals also endorsed the need to use non-stigmatizing language that empowered consumers to ask questions about opioid safety, believing this could open the door for consumers to consider reducing their opioid prescription in the future.
“In an ideal world when you're prescribed an opiate for chronic pain, then it would be great if you could see a pain educator who could be a pharmacist trained to just answer questions. You could have family members attend it with you” P10
“Targeting, again, healthcare professionals to treat patients without stigma and without judgment and empowering and enabling the public in somehow getting to say it's okay to ask questions.” P02
3.1.2.2 Experiences of stigma
Almost all consumers recounted negative experiences with healthcare professionals and prescription opioids. Some felt like they had been forced to wait prolonged periods to pick up their prescription as some form of test by the pharmacist. Others had experiences of professionals minimizing their experience of pain (e.g., being told to try mindfulness instead of their prescription) or that they need psychological help. These past negative experiences with professionals resulted in consumers being wary of approaching opioid safety discussions with their professionals for fear of repeated negative experiences.
“They don't understand the importance of it I think, how absolutely critical it is to get those medications… I have felt tested by a pharmacist to see how much I'm going to put up with, how long I'm going to wait, when am I going to come back?” C01
“I had another doctor say, ‘They're doing all this stuff about brain plasticity. So just stop being in pain.'”
“One of the things that I hate doing when I go to the chemist is knowing what to say because there is that level of judgment sometimes” C03
Professionals too had heard examples of consumers facing stigma from other healthcare professionals. All agreed it was the responsibility of healthcare professionals to provide education around opioid safety and naloxone using non-stigmatizing language. Some community pharmacists had experiences of consumers assuming they were being chastised by the pharmacist for bringing up these conversations while other professionals spoke about the need to educate consumers about their right to seek out other professionals if they were facing stigma.
“Some patients when you talk about the locks [a safety behaviour to keep opioids in a locked cabinet], and they immediately say, ‘you're assuming that I'm drug dependent person, a drug user’. And then you've got them really offside” P12
“Or even giving the person the right or it's okay to change your health professional if you're not happy with the way that you are being potentially stigmatized or… with pharmacies, that's certainly one of the things that we hear back” P02
3.1.3 Environment, context and resources
3.1.3.1 A conversational aid
Consumers discussed how an online resource could facilitate their conversations with healthcare professionals. For most, it was crucial to be able to approach conversations around prescription opioid use, naloxone and safe use with their own information to ensure more equal conversations. Some consumers specifically requested scripted examples of how to speak to their healthcare professional about the risks associated with opioids, while others wanted examples of how to ask for naloxone in pharmacy settings.
“When you go to speak to your clinician that you feel actually armed with enough information that you can have a discussion with them rather than feeling like you don't know anything and they know everything” C10
The ROOM Tool was seen as useful for supporting conversations, especially as mandated 12-month review and second opinion are now required to enable ongoing opioid prescribing by their general practitioner. Some consumers felt the ROOM Tool would give credibility to their signs and symptoms associated with their opioid use. A minority were concerned that the use of numbers (i.e., using numbers to depict their pain on a scale) to measure pain didn't adequately capture the nuance of their experience, while others felt it would provide a starting point to explore their pain experience.
“Rather than just you saying, ‘I'm in a lot of pain,’ if you can say, ‘Look, I filled this in and this is something…that clinicians have developed,’ it might have more credibility.” C04
“C07: Well, it's just people who've been using opioids for a while, and maybe people who are thinking of tapering because if they're already thinking of tapering, and then they take that questionnaire, that maybe helps them with some ways to verbalize that to their GP maybe C01: Or a way for them to assess how the opioids are affecting them.”
3.1.3.2 Material resources and data ownership
Some professionals were particularly concerned with data ownership indicating the importance of consumers having ultimate control of any information they enter into the Toolkit. They also felt that a living document that tracked their medication could promote opioid safety behaviours. Consumers were less concerned by data ownership, and hopeful that if they were to present their data via a Toolkit to a healthcare professional it would be a credible source and “believed”.
“It's a living document that the consumer has ownership of. So if the consumer's aware of what they can do, and they keep on getting their chronic pain meds because they're knowing they're using them safely. So that's an incentive for them.” P08
3.1.4 Social support
3.1.4.1 Others need to know how to use naloxone
Consumers and professionals agreed that any resource promoting the uptake of naloxone must include instructions for household members on how to access and use naloxone, and engage in related behaviours such as calling the ambulance and putting the person in the recovery position. Consumers and professionals alike felt these behaviours were likely unknown and needed to be explicitly provided.
“I guess the other thing that goes with that is that whatever material we produce hopefully would be useful for families well, to read it, and to understand, because it's pretty hard to do naloxone education if you don't involve family” P12
3.1.5 Beliefs about capabilities
3.1.5.1 Consumers are experts in their own experience
A major barrier to engaging in opioid safety behaviours for long-term consumers was their belief that they were immune to the risks associated with taking prescription opioids. Consumers with new prescriptions, older people, and people who use illicit opioids were viewed as more likely to lack opioid safety behaviour knowledge and therefore at the greatest risk. Conversely, almost all long-term consumers were confident in their own knowledge of their condition and medications, often describing how their own understanding exceeded that of their treating physician or other healthcare professionals, and not perceiving themselves to be at risk.
“Some of us are on pharmaceutical doses that are quite low because we need this to manage our daily life and we're not escalating. We may be slightly dependent, but we're not at risk of overdosing. It's the opioid naive people in my humble opinion, who are at risk and those who engage in risky behaviours such as your illicit users or the elderly.” C02
3.1.6 Inductive analysis—design preferences
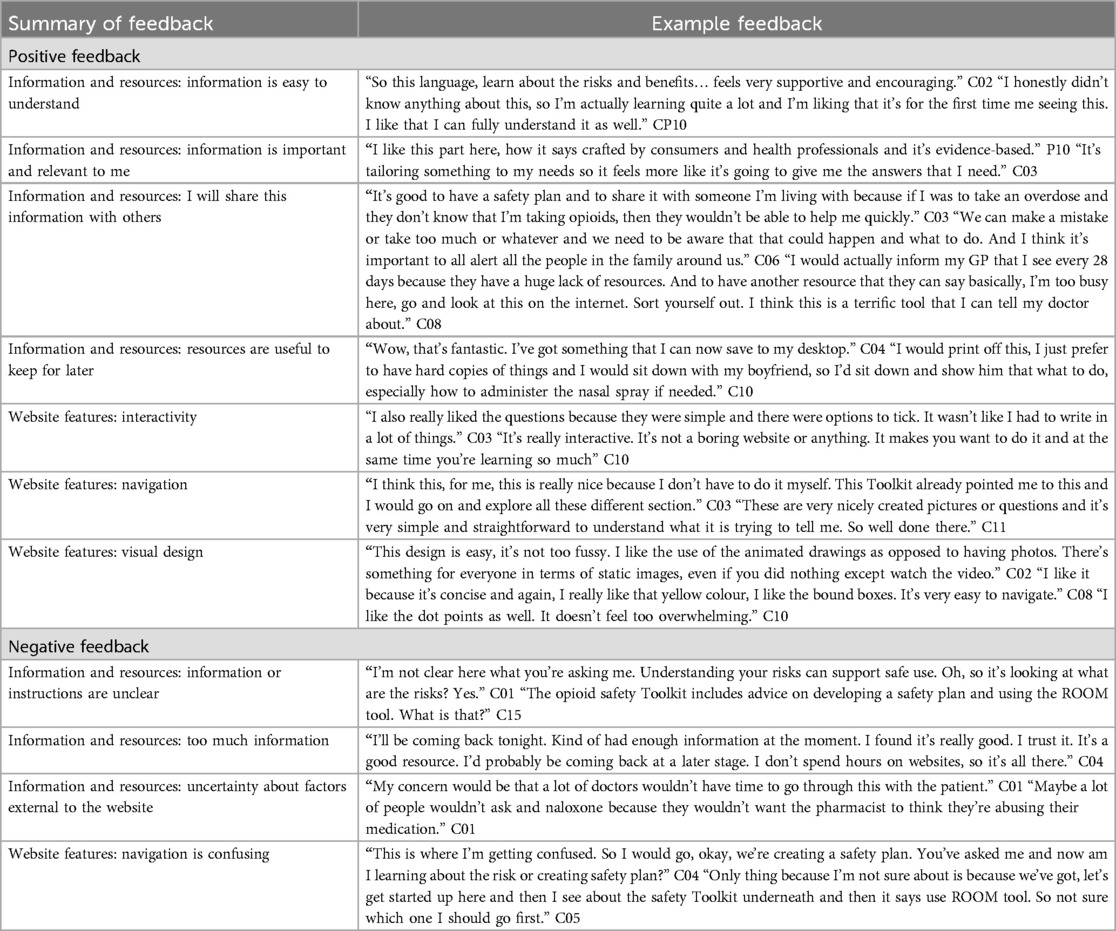
Design preferences were explored throughout each workshop and analysis grouped into four key areas—visuals, language, target audience, and information (Table 2). First, the visual preferences included: (i) the use of vector-based illustrations and animations (as opposed to photography), (ii) the use of digital, animated, and print-based media, (iii) a preference for a positive, optimistic and motivating online digital space where users felt safe, and (iv) an easy to navigate and interact website with limited cognitive load. As one consumer explained, design preferences were intrinsically linked to their experience of pain with the potential for design decisions to influence their ability to process the information.
Second, the preferences related to language included: (i) using non-targeting/non-stigmatizing language, (ii) avoiding too much text, (iii) avoiding overly clinical language, (iv) using simple and easy-to-understand language, and (v) correctly using and defining common terms and acronyms. Briefly, language and messaging was designed to be non-stigmatizing by validating participants perspectives that for some people opioids are important part of pain management (e.g., “For some people, opioids are the best option for pain relief”), and ensuring existing stigmatizing language used in resources were not included (i.e., listing harms before benefits of opioids, NPS MedicineWise) (59).
Third, preferences related to the target audience included: (i) explicitly defining the target user, (ii) screening individuals to identify their level of risk, and (iii) giving guidance based on their level of risk. Finally, information preferences included: (i) catering to a wide range of audiences from different cultural backgrounds, (ii) giving tailored information, (iii) providing evidence-based, factual information, (iv) providing a clear call to action or next step, and (v) being relevant to the Australian context.
3.2 User testing
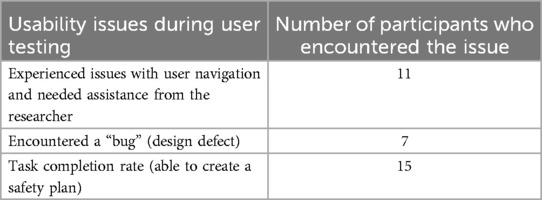
User testing was conducted with 15 consumers with lived experience of being prescribed opioids for pain relief including 12 long- (more than three months), 1 medium- (2 weeks to three months), and 2 short-term (less than 2 weeks) (Table 1; Supplementary Table S3). User testing feedback provided an additional opportunity to refine the Toolkit (Supplementary Figure S3). Feedback related to either information and resources presented (e.g., instructions provided), or specific features of the website (e.g., interactivity). This section details the positive and negative feedback elicited through user testing (Table 3), quantitative user testing (Table 4) and modifications made a result (Table 5).
3.2.1 Information and resources
Participants in the user testing interviews were generally positive about the Toolkit's information and resources, with most finding the information easy to understand, tailored, relevant and important non-stigmatizing and encouraging in tone. A major positive aspect of the Toolkit identified by participants was the ability to download and keep the resources created (their completed opioid safety plan and their personal answers to the ROOM tool). Most participants spoke about their intention to download the safety plan and share details of emergency naloxone use with their family and/or friends. Others shared that they planned to download and take the ROOM tool to their next doctor's appointment as a conversational guide. In terms of the information presented, some consumers found the website to have too much information to take in in a single session. While other consumers found aspects of the information and instructions unclear thus being usure of what they were being asked to do. In response, modifications were made to the copy on the website. Labels on navigation buttons were updated for clarity. Signposting copy was also added to prime consumers on what they can expect to see next. While additional copy was added to better explain the ROOM tool and its purpose.
3.2.2 Website features
Think-aloud activity in the user testing uncovered a number of positive and negative aspects of the website's user experience and interactivity. Overall, participants appreciated the use of bright colors and icons throughout the design with the layout enhancing information provision. Positively, many participants found the interactivity aspects interesting and simple to use. Similarly, some found the navigation overall simple to follow throughout most of the website with users being funneled to specific tailored information based on their inputs. Others found this funneling confusing or annoying, indicating they expected to be directed to different information based on their inputs. In response, changes were made to the user flow to better tailor information to the needs of consumers, including adding additional navigation buttons, a menu visible on all pages of the Toolkit, and a progress bar to indicate users' progression throughout the creation of their safety plan and the ROOM tool.
Finally, a dissemination plan was created to ensure pharmacists across Australia are prepared for consumers to request naloxone, while consumer specific social media content was developed to increase awareness of the Toolkit among consumers.
3.3 The opioid safety toolkit
The final web application (60) (Figure 2) includes different user flows to direct consumers to different experiences based on their needs, updated versions of the previously co-designed resources (ROOM tool, consumer-facing leaflet and videos), newly co-designed resources to increase safety behaviours, and a naloxone pharmacy finder tool. Specific Behaviour Change Techniques are included in the tool including information about health consequences, instruction on how to perform the behaviour, demonstration of the behaviour, credible source, social support (practical), and restructuring the physical environment (see Table 6 for operationalisation).
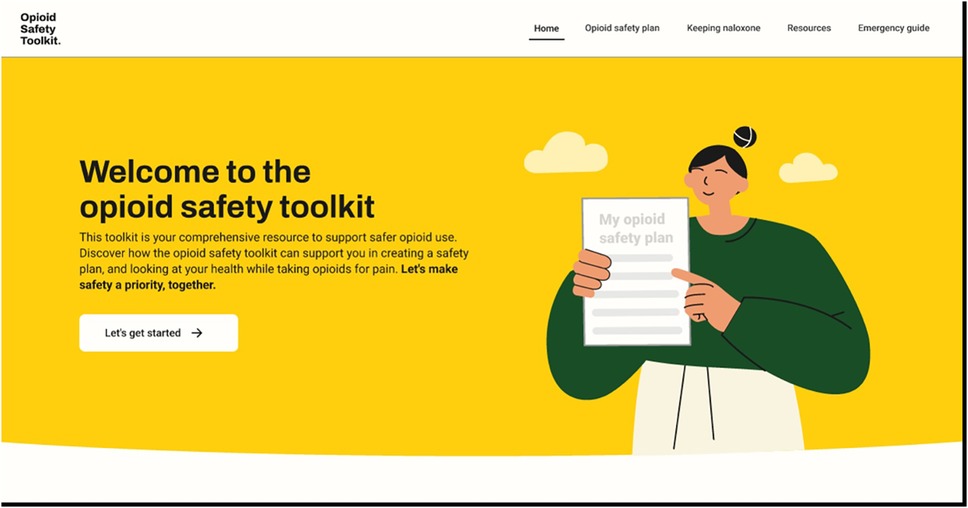
Figure 2. Opioid safety toolkit webpage “Screenshot from:Opiod Safety Toolkit, https://saferopioiduse.com.au/”.
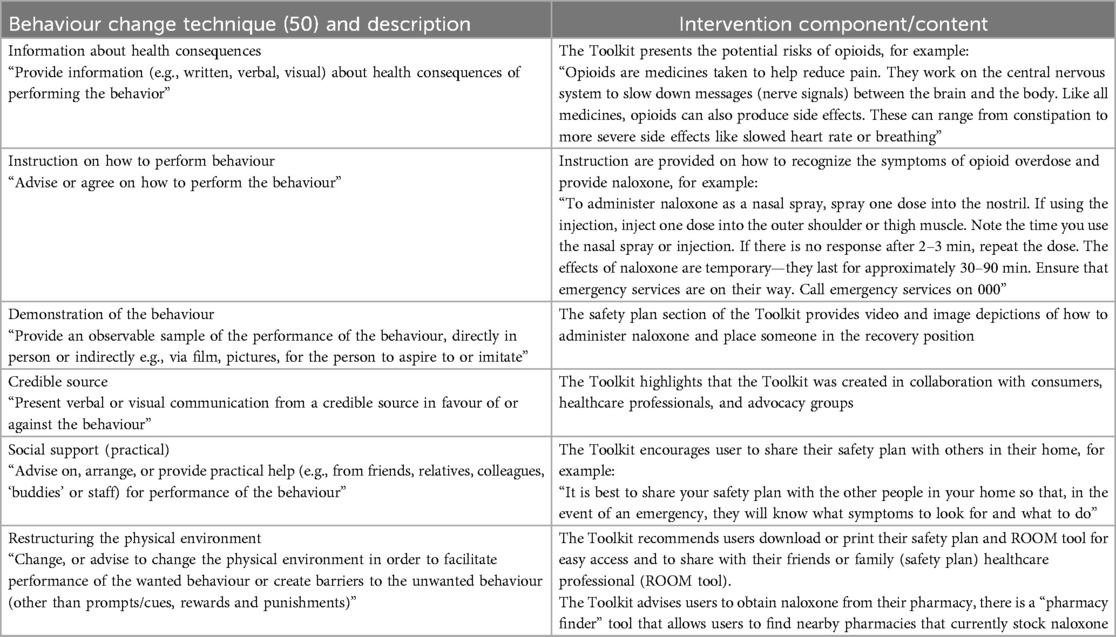
Table 6. Behaviour change techniques employed in the intervention component or content of the opioid safety toolkit.
4 Discussion
This study aimed to design and develop a digital Opioid Safety Toolkit for people who are prescribed opioids for pain. A secondary aim was to extend the integration of implementation science and HCI by embedding a well-known behavioural and implementation science framework, the TDF, with the Double Diamond design process (40, 45, 46). Through participatory co-design approaches underpinned by the TDF, nuanced barriers and facilitators to engaging in opioid safety behaviours (i) making a safety plan, (ii) speaking with their healthcare professional about opioid safety, and (iii) having naloxone in the home and ensuring others know how to use it were identified and embedded within the final Toolkit. This research addresses gaps in literature by designing digitally mediated naloxone interventions for people who have been prescribed opioids for pain, rather than for people who use illicit opioids or misuse opioids, including their risk reduction perspectives (25). Furthermore, this extends previous community pharmacy naloxone intervention research that has predominantly been based in the United States (61). Themes relating to the barriers, facilitators and design of the digital opioid safety Toolkit emerged from workshops with consumers and experts. Using the TDF as a lens to interpret the deductively coded data, dominant themes included Knowledge; Social/Professional Role and Identity; Environment, Context and Resources; Social Support; and Beliefs about Capabilities. Inductive themes within each domain related to the need for increased public awareness and understanding personalised risk (Knowledge), healthcare professionals' role and experiences of stigma (Social/Professional Role and Identity), the need for a conversational aid and material resources and data ownership (Environment, Context and Resources), the need for others to know how to use naloxone (Social Support), and consumers' expertise in their own lives (Beliefs about Capabilities).
Past experiences of stigma were discussed by all consumers and most experts as one of the main barriers to consumers engaging in opioid safety behaviours. Research on stigma and OUD suggests that stigma is experienced in complex ways, conceptualised through an interplay of individual, social, and societal levels (62, 63). For instance, stigma has been shown to influence the way doctors interact with patients who are using prescription opioids (64, 65) and can decrease the likelihood of requesting naloxone from a pharmacist (66). Design considerations had a great effect on consumers’ perception of stigma across workshops. This finding was related to the consumers' belief about their own capabilities, with long-term consumers believing that they were immune to opioid risks and therefore not in need of any additional information. Consumers' propensity to underestimate their personal opioid overdose risk are supported by previous research wherein patients with non-cancer pain did not consider themselves at risk of overdose (67–69). This perception of risk also aligns with the general populations propensity to see their own risk for negative health consequences as low (70, 71).
To address these barriers, the Toolkit needed to include language that would validate the experiences of people prescribed long-term prescription opioids. For example, positive framing of opioids (that acknowledges pain relief properties), rather than risk-based framing, was seen to be more acceptable and less stigmatising. This is in direct opposition to currently available Australian opioid education resources which tend to rely heavily on risk-based language (59). This finding was integrated into the design through different user flows for consumers based on the length of their prescription. As such, the design results in tailored and personalised information ensuring long-term consumers only access necessary information that leads them to create an opioid safety plan and engage with the ROOM tool. In contrast, people taking opioids in the short term (commencing opioids in the last 2 weeks) receive more information regarding the risks, benefits, alternative treatment options for opioids, and set expectations for a short duration of use. However, as discovered in the user testing the user flow was too prescriptive and as a result confusing for some users, a solution to this was the additional of a navigation bar to allow users to also move freely throughout the Toolkit. Furthermore, the majority of people who are prescribed opioids in Australia are prescribed for non-cancer chronic pain (9). As such, it was important to consider the pain experiences of consumers in the design of the Toolkit. For example, a couple of consumers commented that seeing imagery of real people could exacerbate their pain symptoms. As such the design only included vector-based illustrations as to not inadvertently cause pain to consumers interacting with the Toolkit.
Healthcare professionals' role was seen to both help and hinder opioid safety behaviours. On one hand, consumers were clear in their preference for healthcare professionals to initiate conversations about opioid safety. This is consistent with past research that showed a high level of acceptability by consumers for healthcare professionals providing naloxone (15). The work by Nielsen et al. (15) was conducted to explore healthcare professionals concerns that conversations with consumers would result in offence being caused to consumers. A perception that has persisted, and echoed in both the workshops and user testing interviews. The fear of causing offence may be one of the barriers limiting the distribution of naloxone through the Australia Government's take home naloxone program. Previous research has advocated for the use of multifaceted interventions that address both the demand and supply of naloxone uptake (36). Findings from this work supports the need for multifaceted approaches to address low provision for people prescribed opioids, and are reflected in the dissemination plan for the Toolkit.
In this study, a facilitator for opioid safety behaviours was a conversational aid to support consumers to know how to speak to their healthcare professional (supporting the demand side). This finding was triangulated in the user testing interviews with the majority of consumers expressing their intention to use the ROOM tool as a conversational aid with their clinician. Future scalability of the Toolkit relies on buy-in from multiple stakeholder groups, including healthcare professionals, administrators and government policy makers. To ensure scalability, the provision of naloxone and information regarding its importance must become standard practice. To achieve this we also focus on the supply side with a healthcare professional facing campaign (the details of which will be reported elsewhere).
This campaign aims to increase healthcare professionals' awareness of the Toolkit, encourages consumers to obtain naloxone from their pharmacists, and builds on previous work which included developing a language guide for pharmacists on how to initiate conversations with consumers about opioid safety (54). The dissemination plan follows the developed brand guidelines to increase credibility across assets and includes text messages for pharmacies to send on dispensing opioids for a consumer which integrates into their current text message services, print posters, presentations at national pharmacy conferences, advertisements in industry magazines, and a dedicated website for pharmacists with additional resources. The dissemination plan will be evaluated by triangulating qualitative and quantitative metrics across key assets and activities. For example, website analytics for the dedicated pharmacist resource including traffic and resource downloads; the number of pharmacies that send text messages; Electronic Direct Mail open rates; and naloxone distribution supply data. Additional dissemination factors will be evaluated for consumers. For example, patient reach will be evaluated through website traffic to the toolkit as well as SMS open rates.
4.1 Strengths and limitations
A key strength of this study is the inclusion of consumers with lived experience of being prescribed opioids for pain relief and experts who are responsible for prescribing or dispensing prescription opioids as well as academics and consumer and professional body advocates. Co-design with this diverse group of stakeholders resulted in nuanced insights across individual, organisational and system level factors influencing safety behaviours. The TDF combined with the Double Diamond provided useful guidance throughout the study. The Double Diamond itself was useful in developing the aims and activities of the workshops (e.g., exploration, synthesis, ideation, testing), while the TDF helped to explore factors affecting opioid safety behaviours from multiple perspectives. This triangulation in data has been useful in ensuring the overall design met the needs of multiple stakeholders. For example, themes around stigma, health professional role and beliefs about capabilities were better understood by exploring the ways in which barriers and facilitators to behaviour impact both consumer and healthcare professionals' behaviour. Furthermore, the use of the TDF as a lens for analysis aided the selection of evidence-based behaviour change techniques (Table 4), that mean the intervention itself grounded in behaviour change theory.
Finally, limitations to this study should be noted. The consumer participants (13 in the workshops and 15 in interviews) included in this study are unlikely to reflect the broad and diverse experiences of Australians who are prescribed opioids for pain relief. Although attempts were made to include participants from diverse jurisdictions, majority were from the east coast of Australia and are likely to have an interest in the topic area. Similarly, the expert participants in this study are likely to introduce some selection bias in that those who participated are more likely to be interested in prescription opioid safety. Additional research has included a broader sample so findings relating to effectiveness are generalizable. This includes those who are less interested in or do not have expertise in the subject area through a randomized controlled trial and participant qualitative interviews of their experience with the Toolkit (the results of which will be published elsewhere). Furthermore, as this study included co-design and user testing, it is not possible to discuss the Toolkit's effect on the safety behaviours themselves. These results will be published elsewhere.
5 Conclusion
Through a co-design process with consumers and professionals, a digital Opioid Safety Toolkit was designed that can help consumers identify their own opioid risk factors and monitor their outcomes with opioids over time. The digital Toolkit is currently being disseminated in pharmacies nationally in Australia, following a randomised controlled trial confirming its efficacy and acceptability. The co-design process adapted a novel approach to designing with implementation in mind by combining the Double Diamond with the TDF (40, 45, 46). Results from the co-design activities indicate multiple intersecting themes influencing behaviour change techniques and design elements embedded throughout the Toolkit. Translation of previously co-designed evidence-based tools into a web-based Toolkit has the potential to increase the reach, uptake and impact of safety behaviours to empower and support consumers with lived experience of being prescribed opioids.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Monash University Human Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AW: Investigation, Conceptualization, Writing – original draft, Project administration, Methodology, Writing – review & editing, Formal analysis, Supervision. JW: Investigation, Writing – original draft, Writing – review & editing, Formal analysis, Project administration, Methodology, Conceptualization, Supervision. DB: Conceptualization, Visualization, Writing – review & editing, Writing – original draft, Software, Formal analysis. CP: Writing – review & editing, Writing – original draft, Software, Validation, Formal analysis. LP: Writing – original draft, Project administration, Funding acquisition, Methodology, Writing – review & editing, Conceptualization. TL: Writing – review & editing, Writing – original draft, Project administration. PO: Writing – original draft, Conceptualization, Resources, Writing – review & editing, Funding acquisition, Supervision, Methodology. JS: Formal analysis, Visualization, Software, Writing – original draft, Writing – review & editing. LK: Writing – original draft, Writing – review & editing. SN: Funding acquisition, Resources, Writing – review & editing, Conceptualization, Methodology, Writing – original draft, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding was provided by the Australian Government Department of Health and Aged Care. SN and LP are supported by the NHMRC, LP is an NMHRC Emerging Leader (#2016909) and SN is an NMHRC Leadership Fellow (#2024894). Beyond funding support, the funders had no direct role in the study design, data collection, analysis, interpretation, or writing the manuscript.
Acknowledgments
We gratefully acknowledge staff of Painaustralia and the Pharmaceutical Society of Australia, especially Monika Boogs, Jarrod McMaugh, and Elli Williams. We gratefully acknowledge the participants of the study. We thank Mariano Araiza (Motion Designer) for producing the intervention video animation. We thank Karli Renee Gilchrist (Copywriter) for copywriting services for the intervention. We thank Krista Crawford for administrative support. We thank Roisin McNaney for her feedback on the draft manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdgth.2025.1600836/full#supplementary-material
References
1. Roxburgh A, Hall WD, Dobbins T, Gisev N, Burns L, Pearson S, et al. Trends in heroin and pharmaceutical opioid overdose deaths in Australia. Drug Alcohol Depend. (2017) 179:291–8. doi: 10.1016/j.drugalcdep.2017.07.018
2. Chrzanowska A, Man N, Akhurst J, Sutherland R, Degenhardt L, Peacock A. Trends in Overdose and Other Drug-Induced Deaths in Australia 2002–2021. Sydney: National Drug and Alcohol Research Centre; UNSW Sydney (2023).
4. Chrzanowska A, Man N, Sutherland R, Degenhardt L, Peacock A. Trends in Overdose and Other Drug-induced Deaths in Australia, 2003-2022. Sydney: National Drug and Alcohol Research Centre, UNSW Sydney (2024). p. 1–4. doi: 10.26190/unsworks/30158
5. Nguyen T, Buxton JA. Pathways between COVID-19 public health responses and increasing overdose risks: a rapid review and conceptual framework. Int J Drug Policy. (2021) 93:103236. doi: 10.1016/j.drugpo.2021.103236
6. Australian Institute of Health Welfare. Opioid Harm in Australia: and Comparisons Between Australia and Canada. Canberra: AIHW (2018).
7. Wager TD. Managing pain. Cerebrum. (2022) 2022:cer-03-22. Available at: http://europepmc.org/abstract/MED/35813306 (Accessed January 05, 2025).35813306
8. Ju C, Wei L, Man KKC, Wang Z, Ma TT, Chan AYL, et al. Global, regional, and national trends in opioid analgesic consumption from 2015 to 2019: a longitudinal study. Lancet Public Health. (2022) 7(4):e335–46. doi: 10.1016/S2468-2667(22)00013-5
9. Lalic S, Ilomäki J, Bell JS, Korhonen MJ, Gisev N. Prevalence and incidence of prescription opioid analgesic use in Australia. Br J Clin Pharmacol. (2019) 85(1):202–15. doi: 10.1111/bcp.13792
10. Hsu DJ, McCarthy EP, Stevens JP, Mukamal KJ. Hospitalizations, costs and outcomes associated with heroin and prescription opioid overdoses in the United States 2001–12. Addiction. (2017) 112(9):1558–64. doi: 10.1111/add.13795
11. Campbell G, Nielsen S, Larance B, Bruno R, Mattick R, Hall W, et al. Pharmaceutical opioid use and dependence among people living with chronic pain: associations observed within the pain and opioids in treatment (POINT) cohort. Pain Med. (2015) 16(9):1745–58. doi: 10.1111/pme.12773
12. Larance B, Campbell G, Peacock A, Nielsen S, Bruno R, Hall W, et al. Pain, alcohol use disorders and risky patterns of drinking among people with chronic non-cancer pain receiving long-term opioid therapy. Drug Alcohol Depend. (2016) 162:79–87. doi: 10.1016/j.drugalcdep.2016.02.048
13. Nielsen S, Lintzeris N, Bruno R, Campbell G, Larance B, Hall W, et al. Benzodiazepine use among chronic pain patients prescribed opioids: associations with pain, physical and mental health, and health service utilization. Pain Med. (2015) 16(2):356–66. doi: 10.1111/pme.12594
14. Campbell G, Bruno R, Lintzeris N, Cohen M, Nielsen S, Hall W, et al. Defining problematic pharmaceutical opioid use among people prescribed opioids for chronic noncancer pain: do different measures identify the same patients? Pain. (2016) 157(7):1489–98. doi: 10.1097/j.pain.0000000000000548
15. Nielsen S, Peacock A, Lintzeris N, Bruno R, Larance B, Degenhardt L. Knowledge of opioid overdose and attitudes to supply of take-home naloxone among people with chronic noncancer pain prescribed opioids. Pain Med. (2018) 19(3):533–40. doi: 10.1093/pm/pnx021
16. Lintzeris N, Santo T Jr, Nielsen S, Degenhardt L, Campbell G. Estimating centre for disease control and prevention-defined overdose risk in people prescribed opioids for chronic non-cancer pain: implications for take-home naloxone provision. Intern Med J. (2019) 49(8):1054–5. doi: 10.1111/imj.14386
17. Larance B, Campbell G, Moore T, Nielsen S, Bruno R, Lintzeris N, et al. Concerns and help-seeking among patients using opioids for management of chronic noncancer pain. Pain Med. (2019) 20(4):758–69. doi: 10.1093/pm/pny078
18. Coffin PO, Behar E, Rowe C, Santos G-M, Coffa D, Bald M, et al. Nonrandomized intervention study of naloxone coprescription for primary care patients receiving long-term opioid therapy for pain. Ann Intern Med. (2016) 165(4):245–52. doi: 10.7326/M15-2771
19. Salom CL, Maravilla JC, Thomas N, Juckel J, Daly C, Peacock A, et al. Evaluation of the pharmaceutical benefits scheme subsidised take home naloxone pilot. Brisbane, Australia: The University of Queensland (2021).
20. Nielsen S, Picco L, Campbell G, Lintzeris N, Larance B, Farrell M, et al. Development of a brief patient-administered screening tool for prescription opioid dependence for primary care settings. Pain Med. (2020) 21(2):e79–88. doi: 10.1093/pm/pnz213
21. Nielsen S, Picco L, Kowalski M, Sanfilippo P, Wood P, Larney S, et al. Routine opioid outcome monitoring in community pharmacy: outcomes from an open-label single-arm implementation-effectiveness pilot study. Res Social Adm Pharm. (2020) 16(12):1694–701. doi: 10.1016/j.sapharm.2020.02.009
22. Picco L, Middleton M, Bruno R, Kowalski M, Nielsen S. Validation of the OWLS, a screening tool for measuring prescription opioid use disorder in primary care. Pain Med. (2020) 21(11):2757–64. doi: 10.1093/pm/pnaa275
23. Picco L, Middleton M, Bruno R, Kowalski M, Nielsen S. Validity and reliability of the computer-administered routine opioid outcome monitoring (ROOM). Tool Pain Med. (2020) 21(12):3645–54. doi: 10.1093/pm/pnaa297
24. Lee M, Lee H, Kim Y, Kim J, Cho M, Jang J, et al. Mobile app-based health promotion programs: a systematic review of the literature. Int J Environ Res Public Health. (2018) 15(12):2838. doi: 10.3390/ijerph15122838
25. Tas B, Lawn W, Traykova EV, Evans RAS, Murvai B, Walker H, et al. A scoping review of mHealth technologies for opioid overdose prevention, detection and response. Drug Alcohol Rev. (2023) 42(4):748–64. doi: 10.1111/dar.13645
26. Kiburi SK, Ngarachu E, Tomita A, Paruk S, Chiliza B. Digital interventions for opioid use disorder treatment: a systematic review of randomized controlled trials. J Subst Abuse Treat. (2023) 144:108926. doi: 10.1016/j.jsat.2022.108926
27. Chancellor S, Nitzburg G, Hu A, Zampieri F, Choudhury MD. Discovering alternative treatments for opioid use recovery using social Media. Proceedings of the 2019 CHI Conference on Human Factors in Computing Systems; Glasgow, Scotland UK: Association for Computing Machinery; (2019). p. Paper 124.
28. MacLean D, Gupta S, Lembke A, Manning C, Heer J. Forum77: an analysis of an online health forum dedicated to addiction recovery. Proceedings of the 18th ACM Conference on Computer Supported Cooperative Work & Social Computing; Vancouver, BC, Canada: Association for Computing Machinery (2015). p. 1511–26
29. Weatherston J, Perin C, Hore DK, Wallace B, Storey M-AD. An unquantified uncertainty visualization design space during the opioid crisis. Extended Abstracts of the 2020 CHI Conference on Human Factors in Computing Systems (2020).
30. Aizen R, Marcu G, Misra A, Sieber G, Schwartz DG, Roth AM, et al. Designing an emergency response community for opioid overdoses in Philadelphia. Extended Abstracts of the 2018 CHI Conference on Human Factors in Computing Systems (2018).
31. Nandakumar R, Gollakota S, Sunshine JE. Opioid overdose detection using smartphones. Sci Transl Med. (2019) 11(474):eaau8914. doi: 10.1126/scitranslmed.aau8914
32. Magee MR, Gholamrezaei A, McNeilage AG, Sim A, Dwyer L, Ferreira ML, et al. A digital video and text messaging intervention to support people with chronic pain during opioid tapering: content development using co-design. JMIR Form Res. (2022) 6(11):e40507. doi: 10.2196/40507
33. Marcelo AC, Ho EK, Hunter DJ, Hilmer SN, Jokanovic N, Prior J, et al. TANGO: development of consumer information leaflets to support TAperiNG of opioids in older adults with low back pain and hip and knee osteoarthritis. Drugs Aging. (2023) 40(4):343–54. doi: 10.1007/s40266-023-01011-x
34. Schwartz DG, Ataiants J, Roth A, Marcu G, Yahav I, Cocchiaro B, et al. Layperson reversal of opioid overdose supported by smartphone alert: a prospective observational cohort study. eClinicalMedicine. (2020) 25:100474. doi: 10.1016/j.eclinm.2020.100474
35. Haegerich TM, Paulozzi LJ, Manns BJ, Jones CM. What we know, and don’t know, about the impact of state policy and systems-level interventions on prescription drug overdose. Drug Alcohol Depend. (2014) 145:34–47. doi: 10.1016/j.drugalcdep.2014.10.001
36. National Academies of Sciences E, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Committee on Pain Management and Regulatory Strategies to Address Prescription Opioid Abuse. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington, D.C.: National Academies Press (US) (2017).
37. Mohr DC, Lyon AR, Lattie EG, Reddy M, Schueller SM. Accelerating digital mental health research from early design and creation to successful implementation and sustainment. J Med Internet Res. (2017) 19(5):e153. doi: 10.2196/jmir.7725
38. Eccles MP, Mittman BS. Welcome to implementation science. Implement Sci. (2006) 1(1):1. doi: 10.1186/1748-5908-1-1
39. Nilsen P. Making sense of implementation theories, models and frameworks. Implement Sci. (2015) 10(1):1–13. doi: 10.1186/s13012-015-0242-0
40. Atkins L, Francis J, Islam R, O’Connor D, Patey A, Ivers N, et al. A guide to using the theoretical domains framework of behaviour change to investigate implementation problems. Implement Sci. (2017) 12(1):77. doi: 10.1186/s13012-017-0605-9
41. Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci. (2012) 7(1):37. doi: 10.1186/1748-5908-7-37
42. Dopp AR, Parisi KE, Munson SA, Lyon AR. Aligning implementation and user-centered design strategies to enhance the impact of health services: results from a concept mapping study. Implement Sci Commun. (2020) 1(1):17. doi: 10.1186/s43058-020-00020-w
43. Lyon AR, Brewer SK, Arean PA. Leveraging human-centered design to implement modern psychological science: return on an early investment. Am Psychol. (2020) 75(8):1067–79. doi: 10.1037/amp0000652
44. Chen E, Neta G, Roberts MC. Complementary approaches to problem solving in healthcare and public health: implementation science and human-centered design. Transl Behav Med. (2021) 11(5):1115–21. doi: 10.1093/tbm/ibaa079
45. Bristish Design Council. What is the Framework for Innovation? Design Council’s Evolved Double Diamond Internet. London: British Design Council (2015). Available at: https://www.designcouncil.org.uk/news-opinion/what-framework-innovation-design-councils-evolved-double-diamond (Accessed August 08, 2023).
46. Waddell A, Seguin JP, Wu L, Stragalinos P, Wherton J, Watterson JL, et al. Leveraging implementation science in human-centred design for digital health. Proceedings of the CHI Conference on Human Factors in Computing Systems (2024). p. 1–17
47. Greenhalgh T, Wherton J, Papoutsi C, Lynch J, Hughes G, A’Court C, et al. Beyond adoption: a new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies. J Med Internet Res. (2017) 19(11):e367. doi: 10.2196/jmir.8775
48. Waddell A, Spassova G, Sampson L, Jungbluth L, Dam J, Bragge P. Co-designing a theory-informed intervention to increase shared decision-making in maternity care. Health Res Policy Syst. (2023) 21(1):1–13. doi: 10.1186/s12961-023-00959-x
49. Bravington A, Chen H, Dyson J, Jones L, Dalgliesh C, Bryan A, et al. Translating qualitative data into intervention content using the theoretical domains framework and stakeholder co-design: a worked example from a study of cervical screening attendance in older women. BMC Health Serv Res. (2022) 22(1):610. doi: 10.1186/s12913-022-07926-2
50. Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically-clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. (2013) 46(1):81–95. doi: 10.1007/s12160-013-9486-6
51. Johnston M, Carey RN, Connell Bohlen LE, Johnston DW, Rothman AJ, de Bruin M, et al. Development of an online tool for linking behavior change techniques and mechanisms of action based on triangulation of findings from literature synthesis and expert consensus. Transl Behav Med. (2021) 11(5):1049–65. doi: 10.1093/tbm/ibaa050
52. Creswell JW, Creswell JD. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. 5th ed. London: Morgan Kaufmann Publishers (2018).
53. Berkwits M, Inui TS. Making use of qualitative techniques. J Gen Intern Med. (1998) 13(3):195–9. doi: 10.1046/j.1525-1497.1998.00054.x
54. Volpe I, Nielsen S, Manning V, Savic M, McMaugh J. Overdose Prevention for People Prescribed Opioids for Chronic Pain: Enhancing Community Pharmacists’ Capacity to Respond. Melbourne, Australia: Turning Point (2020).
56. Stickdorn M, Schneider J. This is Service Design Thinking: Basics, Tools and Cases. Hoboken, USA: Wiley (2011).
57. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. (2005) 15(9):1277–88. doi: 10.1177/1049732305276687
58. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. (2006) 3(2):77–101. doi: 10.1191/1478088706qp063oa
59. NPS MedicineWise. Opioid Medicines and Chronic Non-Cancer Pain. Sydney, Australia: Australian Commission on Safety and Quality in Health Care (2019). Available at: https://www.nps.org.au/consumers/opioid-medicines (Accessed August 01, 2023).
60. Action Lab and Monash Addiction Research Centre. Opioid Safety Toolkit. Melbourne, VIC: Monash University (2024). Available at: https://opioid.com.au (Accessed August 01, 2023).
61. Cid A, Daskalakis G, Grindrod K, Beazely MA. What is known about community pharmacy-based take-home naloxone programs and program interventions? A scoping review. Pharmacy (Basel). (2021) 9(1):1–32. doi: 10.3390/pharmacy9010030
62. Judd H, Yaugher AC, Shay O, Meier S, L C. Understanding stigma through the lived experiences of people with opioid use disorder. Drug Alcohol Depend. (2023) 249:110873. doi: 10.1016/j.drugalcdep.2023.110873
63. Cheetham A, Picco L, Barnett A, Lubman DI, Nielsen S. The impact of stigma on people with opioid use disorder, opioid treatment, and policy. Subst Abuse Rehabil. (2022) 13:1–12. doi: 10.2147/SAR.S304566
64. Fomiatti R, Farrugia A, Fraser S, Dwyer R, Neale J, Strang J. Addiction stigma and the production of impediments to take-home naloxone uptake. Health. (2020) 26(2):139–61. doi: 10.1177/1363459320925863
65. Strang J, McDonald R, Campbell G, Degenhardt L, Nielsen S, Ritter A, et al. Take-home naloxone for the emergency interim management of opioid overdose: the public health application of an emergency medicine. Drugs. (2019) 79(13):1395–418. doi: 10.1007/s40265-019-01154-5
66. Natale I, Harvey C, Wood P, Anderson K. “It can save your life, that’s all I know,” barriers and facilitators for engagement in take-home naloxone for people receiving opioid substitution treatment in regional Australia: an explorative study. Qual Res Med Healthc. (2023) 7(2):10868. doi: 10.4081/qrmh.2023.10868
67. Mueller SR, Koester S, Glanz JM, Gardner EM, Binswanger IA. Attitudes toward naloxone prescribing in clinical settings: a qualitative study of patients prescribed high dose opioids for chronic non-cancer pain. J Gen Intern Med. (2017) 32(3):277–83. doi: 10.1007/s11606-016-3895-8
68. Nichols MA, Kepley KL, Rosko KS, Hudmon KS, Curran GM, Ott CA, et al. Community pharmacist-provided opioid intervention frequencies and barriers. J Am Pharm Assoc (2003). (2023) 63(1):336–42. doi: 10.1016/j.japh.2022.10.004
69. Schofield J, Parkes T, Mercer F, Foster R, Hnízdilová K, Matheson C, et al. Feasibility and acceptability of an overdose prevention intervention delivered by community pharmacists for patients prescribed opioids for chronic non-cancer pain. Pharmacy. (2023) 11(3):1–17. doi: 10.3390/pharmacy11030088
70. Ferrer R, Klein WM. Risk perceptions and health behavior. Curr Opin Psychol. (2015) 5:85–9. doi: 10.1016/j.copsyc.2015.03.012
71. Weinstein ND. Unrealistic optimism about future life events. J Pers Soc Psychol. (1980) 39(5):806–20. doi: 10.1037/0022-3514.39.5.806
72. Nessler D. How to apply a design thinking, HCD, UX or any creative process from scratch. Medium. (2016) Available at: https://medium.com/digital-experience-design/how-to-apply-a-design-thinking-hcd-ux-or-any-creative-process-from-scratch-b8786efbf812 (Accessed September 08, 2024).
Keywords: opioids, co-design, naloxone, health literacy, implementation science, human-computer interaction, behavioural science
Citation: Waddell A, Watterson JL, Basur D, Prawira CO, Picco L, Lam T, Olivier P, Seguin JP, Kay L and Nielsen S (2025) An evidence-based digital prescription opioid safety toolkit for national dissemination: co-design and user testing. Front. Digit. Health 7:1600836. doi: 10.3389/fdgth.2025.1600836
Received: 27 March 2025; Accepted: 9 June 2025;
Published: 11 July 2025.
Edited by:
Aikaterini Kassavou, University of Bedfordshire, United KingdomReviewed by:
Lindsey Hohmann, Auburn University, United StatesDeepika Rao, Dartmouth College, United States
Copyright: © 2025 Waddell, Watterson, Basur, Prawira, Picco, Lam, Olivier, Seguin, Kay and Nielsen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alex Waddell, YWxleC53YWRkZWxsQG1vbmFzaC5lZHU=
 Alex Waddell
Alex Waddell Jessica L. Watterson1,2
Jessica L. Watterson1,2