- 1Department of Applied Health Sciences, University of Birmingham, Birmingham, United Kingdom
- 2Birmingham Health Partners, Birmingham, United Kingdom
- 3Birmingham Voluntary Services Council, Birmingham, United Kingdom
- 4West Midlands Health Technology Innovation Accelerator, Birmingham, United Kingdom
- 5University Hospitals Birmingham NHS Foundation Trust, Birmingham, United Kingdom
- 6Medical Devices Testing and Evaluation Centre, UHB NFT, Birmingham, United Kingdom
Co-production is increasingly being used to develop sustainable improvements in health service delivery that are shaped by the experiences and needs of a diverse range of stakeholders including patients and healthcare providers. The process also offers a compelling means of fundamentally addressing the key issues of acceptability and applicability of digital health tools that contribute to ongoing inequity in the use of digital health technologies. However, creating and moderating hybrid digital health co-production teams is hindered by heightened obstacles to inclusivity and equitability of the cost and complexity of digital healthcare, and the diverse digital experience amongst the relevant stakeholders. With previous examples of co-production that involve direct interaction between developers and diverse groups of patients and staff rare, this integrative review has collated the latest evidence on engaging these diverse stakeholders in healthcare innovation, with best practice in co-production, and presents it within a framework representing the five core steps of co-production: Set-up, Discovery, Definition, Development, and Delivery. This guidance includes structured and tailored training in co-production and the concepts of digital health, surfacing and challenging existing assumptions around data security and confidentiality, defining funding models, introducing and refining protypes of increasing sophistication, and structured implementation and evaluation of both the co-production process and its outputs.
1 Introduction
The capability of digital health technologies to automate and streamline effective and equitable care is recognised globally and they are beginning to transform the way medical professionals deliver care and patients manage their health in a range of settings and locations (1). However, the shift towards digitally enabled health care is a complex process involving technologies of varying functionality and purpose, and incorporating significant changes to pathways, workflows, patient engagement, and broader systems of delivery (2, 3). Implicit within this digital transformation is that relevant technologies are available and applicable to all levels of society, yet discrepancies exist in the extent to which patients access and utilise digital health technologies (4), where it is impacted by their affordability (5) and patients varying levels of confidence and sophistication (6). These differences are compounded by the growing sophistication in the functionality of devices and the infrastructure they require meaning that underserved populations (which we define here as those who are economically deprived and/or from ethnic minorities that are engaged less effectively by formal healthcare interventions (7), frequently miss out on the comparative advantages of digital health afforded those that are better educated or of higher socio-economic status (8). This divide in the access and utilisation of digital health technologies have multiple and widely understood social determinants relating to resource, education, ethnicity, digital literacy, and connectivity (9). There are a number of ways these issues might be addressed including the provision of free data, hardware, and tailored training (10), amongst which and arguably the most fundamental, is to ensure that the design of any digital health tool or solution is directly compatible with the diverse range of patients its intended to serve (11). In other areas of healthcare, the needs of a diverse range of stakeholders including patients and healthcare providers have been successfully accommodated through the use of co-production (12, 13). The process of co-production has multiple definitions, with some 60 being noted in a recent review which recommended that future work should instead of being caught up in agreeing on the precise definition instead focus on the shared core principles of co-production (14). Therefore for the purposes of this review we define it as the process or methodology that encourages participants to identify a problem before empowering them to solve it, an iterative process involving open and equitable interaction between service users and those involved in producing or providing a service (15).
There is growing evidence of the benefits of co-production in developing digital health solutions (16–18). The success of co-production is predicated on transparent communication (19), the mutual exchange of knowledge (20), and equitability of decision-making authority (21). However, co-production is vulnerable to a number of challenges associated with the ability and opportunity to participate and the accommodation of stakeholders from necessarily diverse backgrounds (22). This means that although the potential of co-production is widely understood, its practical application often falls short, leading to power imbalances amongst stakeholders, the tokenistic involvement of patients, and co-produced solutions that lack sustainability (23). This is particularly true of co-production in digital health where obstacles to inclusivity and equitability are heightened by the cost and complexity of digital healthcare, and greater diversity of experience in digital technologies amongst the relevant stakeholders including diverse and underserved populations, health care staff of various role and responsibility, and technology developers (12, 19, 24, 25).
There is growing recognition that more robust strategies are needed to pursue inclusive digital co-production, though there is little specific evidence to draw on (25–29). There are though lessons that might be learnt from combining successful strategies for engaging diverse patient populations in health and care improvement initiatives with the latest evidence of effective co-production (22, 30). This review collates these two strands of evidence, presenting them within the five core steps of co-production. In this way we provide practicable insight into how the challenges to inclusive digital co-production can be addressed.
2 Methods
The work consists of an integrative review of research relating to inclusive co-production activities in (digital) health that includes best practice and latest evidence of optimum engagement activities with a range of stakeholders including underserved populations (31). The intention was not to identify every piece of work that has been conducted in co-design and -production, but to follow best practice in conducting integrative evidence reviews, summarizing the empirical and theoretical literature illustrated by recent and relevant examples to map this evidence against the five core steps of the co-production process as outlined in Section 2.2 (32).
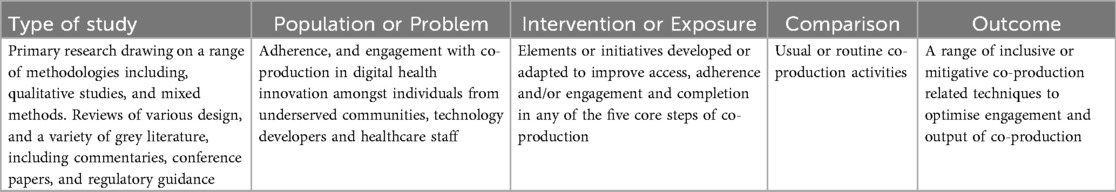
Ultimately, we describe the challenges to inclusive co-production, and where possible the measures that might be taken to mitigate them. Study eligibility criteria were established using the Population, Intervention, Comparison, Outcome, and Study design (PICO) framework (33) (see Table 1) and we have described our search in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (34).
2.1 Search strategy
The literature was searched in June 2025 from 2000 onwards for recent examples and evidence of best practice in co-production or otherwise engaging a diverse range of stakeholders in health-related innovation. This timespan allows us to describe recent research relevant to current models of (digital) co-production and the latest understanding of the challenges to inclusive co-production. We created a search for one database and adapted it for use in the others used the following electronic medical databases: MEDLINE, and PubMed, and supplemented by citation searches and hand searches of including of Google Scholar. The inclusion criteria for our review comprised primary research that were peer-reviewed and relevant grey literature including regulatory guidance, only work published in English was considered. The search terms can be found in Supplementary File 1.
2.2 Data extraction and synthesis
The data was extracted and placed against the five core steps of co-production by the first author in discussion with the third author. A primarily narrative approach consistent with the recommended analytical method for narrative synthesis was used to summarise the nature and effect of the evidence for inclusive co-production within the five steps (31). The criteria for selecting the included work were based on their relevance to the design and delivery of future inclusive digital co-production activities. We extracted data that included (i) programme overview (ii) author and publication date (iii) nature of evidence (iv) country of origin (v) summary of recommendations.
3 Results
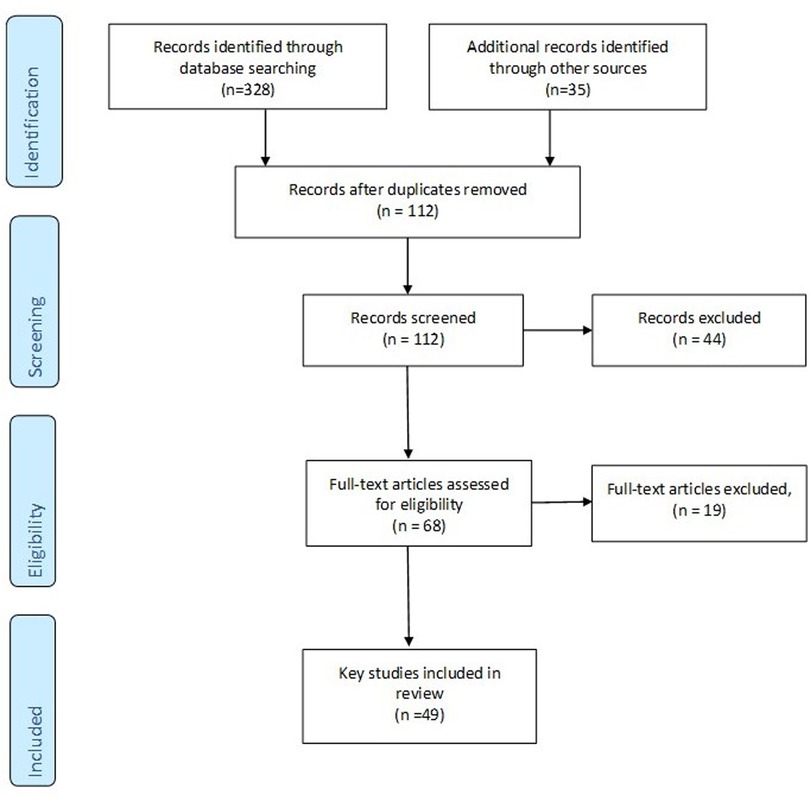
A total of 49 papers were selected for inclusion were included in the review. We initially retrieved 128 articles and after duplicates, protocols, or exclusion because they were not specific or relevant to one of the co-production steps or otherwise inclusive considerations were left with 49 papers explored in the review. The PRISMA Flow Diagram is shown in Figure 1.
The papers were authored across a total of 17 countries: the majority (22) were authored in Norh America [17 in the United States of America (USA), five in Canada], and 25 in Europe (including seven in the UK, and four in the Netherlands), with other countries including Australia and Malaysia. The work included consisted of reviews of various design, regulatory guidelines, white papers, commentaries, and primary research. A summary table of study characteristics can be found in Supplementary File 2.
3.1 Considerations to support inclusive digital co-production
Below we first reiterate the key principles of co-production as they pertain to the five core steps of the process. We then collate the current evidence by each of the five recognised steps of co-production.
3.1.1 The principles of co-production
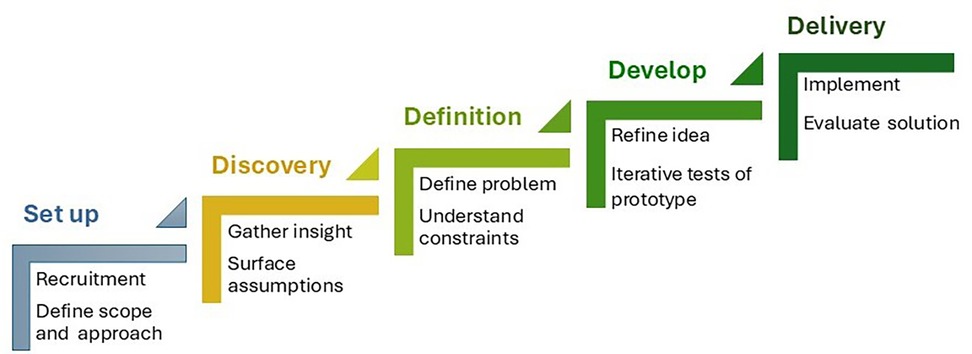
A number of frameworks and methodologies have emerged to underpin co-production (35) and a recent systematic review of co-production in healthcare identified the same shared principles of various co-production approaches required in the democratic mobilisation of knowledge to improve health care and delivery including: bringing people together as active and equal partners, valuing all knowledge, using a creative approach, and iterative prototyping techniques (36). In operationalising these elements, all share versions of the same five core steps, namely: Set-up, this is where a range of participants are recruited reflective of all stakeholders, including user groups and broader community. It involves clear and collaborative agreement of the intended aims and outcomes of the process, including levels of involvement, the decision-making process, and the use of training to support the process and upskilling of participants; Discovery, where you gain an understanding of the context and issues at hand including exploring various perspectives, concerns and assumptions, and preferences and priorities; Definition, where the insights gained are prioritised, themes, patterns, and key problems are identified, and the feasibility of various solutions understood; Development, this is the opportunity to generate ideas and creative solutions. Early protypes might be developed to support learning through doing and help identify risks and previously unforeseen consequences. As ideas coalesce buy-in from external groups might be sought; and Delivery, the final step involves producing and launching the final solution, and map next steps and future sustainability. It is also an opportune moment to consider the performance and continuance of the co-production process (12, 37) Figure 2.
3.1.2 The latest evidence in support of inclusive digital co-production
Below recent evidence of best practice in stakeholder engagement and digital health co-production is presented within each of the five key steps and constructs. This evidence is summarised in Table 1 and further explored below Table 2.
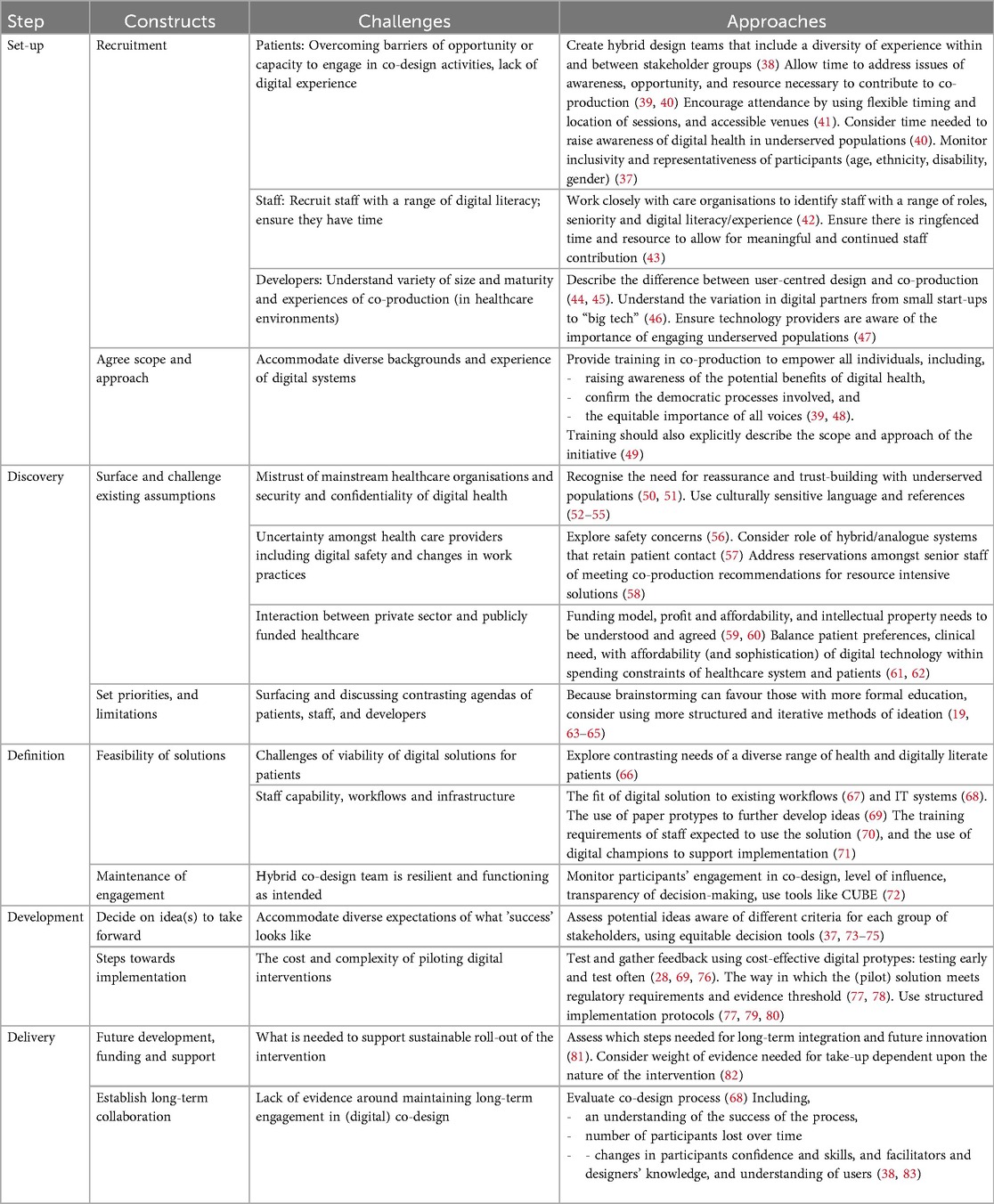
Table 2. Summary of considerations for inclusive digital co-production [after Man 2019 (37)].
3.1.3 Set-up
This first step consists of the recruitment of diverse stakeholders and the delineation of the scope and approach particular to the co-production initiative. In the context of digital health it has been recommended that hybrid co-production teams are created consisting of at least three diverse, stakeholder groups: patients and/or citizens, a range of senior decision-makers and providers from health and social care organisations, and digital health technology developers and suppliers from companies of various expertise, size, and maturity (38). These three groups are hereon referred to as “patients”, “staff” and “developers”.
There is consensus that the recruitment of patients or citizens to any healthcare related activity can be improved by addressing issues of awareness, opportunity, and resource (39, 40). To ensure engagement of all patient participants including underserved populations it is likely that some basic training in the core elements of digital health technologies is warranted (39). This might also include a lengthier period of formative design and development as the process continues to allow individuals to become adjusted to the concepts involved (40). Participation can be supported by flexible approaches in the timing, mode, and location of co-production activities, such as utilising community-situated venues and timing co-production activities to accommodate responsibilities of work and family (41). The payment of travel and expenses and the provision of vouchers is commonplace in patient engagement in health improvement activities, often guided by national bodies (84) but in the context of digital co-production this might usefully involve the provision of hardware or data packages to support familiarisation with digital technology. Finally, to ensure the intended diversity it is important to monitor the demographics of those enrolled (37).
The importance of enlisting a broad range of staff, representative of a range of digital experience, roles, and responsibilities, is understood: not only for the value of their individual experience, but also their subsequent influence on the acceptance of the digital solution amongst colleagues (42). There is evidence that it tends to be the digitally literate staff that enlist or support digital co-production with those less digitally inclined often overlooked (42). Similar to patients, to encourage the involvement of staff with limited digital experience some education may be needed as to the key concepts and capabilities of digital health. There are also more broadly recognised barriers to recruiting and retaining staff for healthcare initiatives relating to time and resource, with the understanding that meaningful participation can be encouraged by providing them with dedicated time and cover, acknowledging their participation as continuous professional development (43).
Arguably the least understood or explored group of stakeholders in digital co-production are those developing digital health technologies. Although many companies in the sector will be aware and have experience of user-centred design, the closely related process used in computer sciences (45); it is distinct from co-production in that understanding the needs of users occurs apart from the design process (whereas users are integrally involved in the design process in co-production) (44). The range of companies involved can range from non-profit social enterprise companies to international “Big Tech” such as Google and Palantir, with global interests in the use of data banks, aggregation platforms, and artificial intelligence (46). This can lead to significant variation in the understanding of patients and the healthcare environment, with similar differences in the resource and opportunity to engage in iterative co-production (47).
As with newly combined co-production teams in other domains of healthcare, this step should include the provision of training in what the process entails, the principles of equality and equitability, and the ask of their time and resource (48). This is also a timely opportunity for transparent and consensual agreement of the scope of the work to align the ambitions of those participating (49, 73).
3.1.4 Discovery
The discovery step contains the discussion of challenges, assumptions and the setting of priorities for the co-production team. In a growing number of patients, particularly those from underserved populations this might involve addressing or overcoming issues of mistrust in centralised care organisations (41), as well as concerns around the security and confidentiality of digital health tools and data (50, 51, 85, 86). There is evidence that moderators might help to build trust by accommodating participants cultural backgrounds, primary languages, and cultural and faith practices (52–55).
Hesitancy towards digital health has also been reported amongst care providers, with concerns around the safety and reliability of digital health tools (56), with the recognition that moderators might start with an explicit discussion of the potential issues of using digital health tools (87). These include perceptions that digital solutions will reduce the in-person patient contact that many value and the role of hybrid/analogue or digitally-enhanced systems (38). There are also previously reported suspicions amongst senior-decision that co-production risks their commitment to potentially expensive or resource intensive solutions in order to meet emerging needs and preferences (58).
It is important that the potential funding model for the solution is discussed particularly for private sector technology developers, and commissioners where the additional cost can render digital solutions unsustainable (59). Although there are recommendations for the need for adaptable and value-based financing mechanisms, evidence of successful funding models is lacking (61, 88). Associated with this is the need to explore the expected profit margins (89), and a strategy agreed for where intellectual property lies (60), particularly in publicly funded and hybrid public/privately funded healthcare systems (61). These discussions require balancing what is desirable vs. what is affordable, not only to the health service but also patients, particularly underserved populations (62).
To support these initial conversations and potential solutions co-production initiatives typically use methods that involve abstraction and verbal communication such as open brainstorming (19). However, such approaches are more familiar to those exposed to formal education, and it has been suggested that carefully constraining the issue under discussion and using iterative sessions better suits a broader range of participants and provides more workable solutions (63, 64). This includes individual sessions structured around who is the target user, why they would use the tool/solution, the context in which they will use it, and early thoughts as to how the project team will gauge success of the solution (65).
3.1.5 Definition
This step involves discussing the feasibility of the proposed solutions in terms of their fit, predominantly with patients and existing health services, though with implications for those developing and producing the technology (90). For underserved populations there are a number of well-rehearsed barriers to the take-up of digital health offers including access to hardware such as smart phones or PCs, the cost of data packages, and reliable internet connectivity and broader issues of digital and health literacy (66).
Health service representatives have a different set of constraints to consider. These relate to the characteristics on individual providers, their work practices, as well as the compatibility with existing care pathways and workflows (67). It is at this step where the training requirements of existing staff might be considered with acknowledged difficulties of self or experiential learning in delivering novel digital offers (70), and whether digital champions might be used to support patients and staff (71). This is also the point at which the impact on existing work processes must be understood as well as broader considerations of infrastructure including the necessary data assets, compatibility with existing IT systems, and the return on investment (68). These decision can be supported by paper prototyping, including sketches, diagrams, and storyboards are fast and inexpensive to create, and recommended for these earliest stages where the design direction is vague (69).
There are potentially difficult conversations to be had during this step where conflicting agendas are being aligned and difficult decisions being made, and there are recommendations that this is an appropriate time to understand the level of continued engagement across stakeholders. There are tools developed for this purpose that enable an understanding of levels of ownership, responsibility, and interactivity in the co-production team (37, 72).
3.1.6 Development
This step involves deciding which idea or ideas should be taken forward and refined, alongside developing the plan for implementation. In making these choices it is important to recognise that each group of stakeholders will have varying priorities, and proposed solutions might be usefully scored on the different attributes valued by each (37), and categorised within the three domains of good design in healthcare; efficiency, safety, and usability (74). There are tools available designed to support equitable decision making amongst diverse groups which may be appropriate in this instance (91). These were borne of multi-criteria decision making that is more commonly understood within the field of operational research where alternatives are analysed with respect to a set of multiple (and often conflicting) criteria (75).
Refinement of the solution or tool requires digital rapid prototyping techniques are used to test more realistic and solid ideas; they should be realistic enough to accurately test most interface elements (69). They can be built using purposely developed prototyping tools and software (e.g., Marvel or Proto) or simple versions can also be made using presentation software like PowerPoint or Keynote (69). They are a cost-effective means of creating a prototype which would then be explored and further improved through a multi-phase (and pre-clinical) testing process (28, 76, 92, 93). Finally a high-fidelity prototype might be produced, and while valuable are also expensive and time-consuming and best used to refine near final versions or where complex interventions require accurate simulation (69).
A pre-determined time period of implementation should be agreed, with clearly defined roles and responsibilities of the stakeholders involved (78). A number of structured implementation frameworks for digital health have been developed (77, 79, 80). The implementation of novel digital health technologies is complicated by the intended functionality and identity of the intended user e.g., patients vs. providers) and it can evolve as they interact with surrounding system and processes (94). Though there is a lack of standardisation of which frameworks apply in which context, its recognised that they should involve a mixed method analysis of the impact, uptake, user experience and working mechanisms of the digital solution (77, 80) including a prior data-collection plan, agreed criteria for success or failure (78),.
3.1.7 Delivery
The final step considers the long-term sustainability of the implementation, including assessing aspects of interoperability and integration with existing workflows, how well they support patient engagement and empowerment, and the degree to which the data collected can be used to evidence success and inform further innovation (68). In the UK the National Institute for Clinical and Health Excellence has created a useful three-tiered “Evidence Standards Framework” for digital health, where the level of evidence required to demonstrate effectiveness varies based on the technology's function and potential risk with one Tier 1 being the lowest (82). Specifically, these tiers are (1) Information and support tools (e.g., health tracking apps, symptom diaries); (2) technology used for health behaviour management, or preventative care; and the highest is Tier (3) Technologies that provide treatment or diagnosis (e.g., medical devices, digital therapeutics) (82).
It is also important to grasp the opportunity to learn more of which elements of co-production have proven most effective and how successfully a diverse range of stakeholders have been engaged (83). For example metrics might include the number of participants that remained throughout the process, or self-reported changes in their self-efficacy (38). This includes what moderators have learnt from the process, including their understanding of individual stakeholder groups and how they might be combined (83). Part of this process includes how the co-production dialogue can become continual, where previous co-production initiatives have been criticised for being short-term in their approach instead of continuing to provide feedback and providing tangible demonstration of the value placed on their time and input (95).
4 Discussion
Although the promise of digital co-production is apparent there are risks that health inequalities could be reinforced if the structural barriers that challenge the meaningful participation of diverse stakeholders remain unaddressed (96, 97). Previous examples of co-production that involve direct interaction between developers and underserved groups are rare (24). As we have described, the role of training and scope setting is key, both to manage expectations and to ensure consensual and transparent objectives. However, though there are several examples of regional training offers in the UK, there is no nationally or internationally recognised criteria for training in co-production (98).
The cost of digital solutions and the potential profits involved for private developers means it is particularly important that viable, cost-effective solutions that satisfy all parties are reached (61, 89). The inclusion of diverse populations in co-production might mean that the solutions proposed and developed have broader buy-in across diverse patient populations, it does not mean that they are independently capable of overcoming some of the social and environmental determinants of digital exclusion (99). These are without the power of health and social care organisations but should be coordinated, or otherwise supported by centrally mandated policies of national governments, such as subsidised coverage, limited data costs, and the use of open source software (39, 100). Such moves might be tempered by the lack of proven cost-effectiveness of digital interventions (101).
In thinking of the future for digital co-production, specific evidence relating to successful strategies is scarce with a lack of consensus over the metrics needed to support its long-term sustainability (102). This has led to calls for the reporting of (digital) co-production to be standardised to include two key perspectives: the impact on those participating e.g., whether they would remain involved or otherwise encourage others to participate, and the efficacy and sustainability of the outcomes (102). It is increasingly recognised that central bodies and health organisations should develop more flexible, and service specific approaches to promote a continuing co-production dialogue (81). This is particularly true when considering underserved and diverse patient populations where their involvement is likely their first interaction with co-production and may shape their ongoing relationship with health services (9, 103). This would ideally benefit from universal recommendations for digital inclusivity and the approaches that encourage it to be enshrined in policies that enables inclusive digital transformation and fosters innovation (104, 105). However, perhaps hindered by the paucity of evidence on best practice, policies specific to digital health co-production are yet to emerge.
Finally, it is worth noting that despite the majority of the work we have drawn upon being conducted in high-income countries digital health interventions in LMICs face many of the same barriers to digital health equity as those in HICs. These include inadequate infrastructure, limited digital literacy, regulatory challenges, lack of engagement, and high cost (106–109). Though there are as yet few examples of digital health co-production in LMICs, the potential of co-production to develop equitable care is increasingly being recognised (110). Although there may be heightened challenges to co-production in LMICs relating to more rigid hierarchical structures, socio-cultural beliefs, and political interference, there remains the potential for those interested to learn from the successful inclusive strategies highlighted here (111, 112).
4.1 Strengths and limitations
By placing our findings and recommendations in the context of the five core steps of co-production we have fulfilled our aim of producing a concise and coherent review of the challenges to inclusive co-production and how they might be overcome. The range of countries represented further demonstrates the international recognition of the value of co-production in a range of services including digital health. Though we would argue that the identified principles, tools and strategies that support inclusive co-production can be applied across multiple HICs and LMICs, we also acknowledge the value of tailoring initiatives to local populations and their particular sensitivities and needs. It is acknowledged that collective terms such as “underserved populations” as used here in reality describes a heterogenous group defined by socio-economic status, demographic characteristics and broader cultural factors (7). The implementation of any digital health co-production activity should necessarily accommodate the specific context and socio-cultural sensitivities of the target group (113).
4.2 Conclusions
With previous examples of co-production that involve direct interaction between developers and diverse groups of patients and staff rare, this integrative review has collated the latest evidence on engaging these diverse stakeholders in healthcare innovation, with best practice in co-production, and presents it within a framework representing the five core steps of co-production: Set-up, Discovery, Definition, Development, and Delivery. This guidance includes structured and tailored training in co-production and the concepts of digital health, surfacing and challenging existing assumptions around data security and confidentiality, defining funding models, introducing and refining protypes of increasing sophistication, and structured implementation and evaluation of both the co-production process and its outputs.
Author contributions
IL: Conceptualization, Writing – review & editing, Writing – original draft. GD: Writing – review & editing. HJ: Conceptualization, Writing – review & editing. SB: Project administration, Writing – review & editing. SD: Funding acquisition, Writing – review & editing. LH: Writing – review & editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by Innovate UK.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdgth.2025.1636469/full#supplementary-material
References
2. The Good Things Foundation. Digital Inclusion in Health and Care: Lessons Learned from the NHS Widening Digital Participation Programme (2017–2020). London: The Good Health Foundation London (2020).
3. Asthana S, Jones R, Sheaff R. Why does the NHS struggle to adopt eHealth innovations? A review of macro, meso and micro factors. BMC Health Serv Res. (2019) 19(1):1–7. doi: 10.1186/s12913-019-4790-x
4. van Deursen AJAM, van Dijk JAGM. The first-level digital divide shifts from inequalities in physical access to inequalities in material access. New Media Soc. (2018) 21(2):354–75. doi: 10.1177/1461444818797082
5. Hollimon LA, Taylor KV, Fiegenbaum R, Carrasco M, Garchitorena Gomez L, Chung D, et al. Redefining and solving the digital divide and exclusion to improve healthcare: going beyond access to include availability, adequacy, acceptability, and affordability. Front Digit Health. (2025) 7:1508686. doi: 10.3389/fdgth.2025.1508686
6. Hargittai E, Piper AM, Morris MR. From internet access to internet skills: digital inequality among older adults. Univers Access Inf Soc. (2019) 18(4):881–90. doi: 10.1007/s10209-018-0617-5
7. Bonevski B, Randell M, Paul C, Chapman K, Twyman L, Bryant J, et al. Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol. (2014) 14(1):1–29. doi: 10.1186/1471-2288-14-42
8. Perrin A. Digital gap Between Rural and Nonrural America Persists. Washington DC: The Pew Research Centre (2019).
9. Wang K, Chen XS, Gu D, Smith BD, Dong Y, Peet JZ. Examining first and second-level digital divide at the intersection of race/ethnicity, gender, and socioeconomic Status: an analysis of the national health and aging trends study. Gerontologist. (2024) 64(9):gnae079. doi: 10.1093/geront/gnae079
10. Cullen R. The digital divide: a global and national call to action. Electronic Library. (2003) 21(3):247–57. doi: 10.1108/02640470310480506
11. Wilson S, Tolley C, Mc Ardle R, Lawson L, Beswick E, Hassan N, et al. Recommendations to advance digital health equity: a systematic review of qualitative studies. NPJ Digit Med. (2024) 7(1):173. doi: 10.1038/s41746-024-01177-7
12. Kilfoy A, Hsu T-CC, Stockton-Powdrell C, Whelan P, Chu CH, Jibb L. An umbrella review on how digital health intervention co-design is conducted and described. NPJ Digit Med. (2024) 7(1):374. doi: 10.1038/s41746-024-01385-1
13. Palmer VJ, Weavell W, Callander R, Piper D, Richard L, Maher L, et al. The participatory zeitgeist: an explanatory theoretical model of change in an era of coproduction and codesign in healthcare improvement. Med Humanit. (2019) 45(3):247–57. doi: 10.1136/medhum-2017-011398
14. Masterson D, Areskoug Josefsson K, Robert G, Nylander E, Kjellström S. Mapping definitions of co-production and co-design in health and social care: a systematic scoping review providing lessons for the future. Health Expect. (2022) 25(3):902–13. doi: 10.1111/hex.13470
15. Robert G, Locock L, Williams O, Cornwell J, Donetto S, Goodrich J. Co-Producing and Co-Designing. Cambridge: Cambridge University Press (2022).Available online at: https://www.cambridge.org/core/product/157832BBAE1448211365D396CD110900
16. Lember V. The Increasing Role of Digital Technologies in co-production and co-creation. Co-production and co-creation. London: Routledge (2018). p. 115–27.
17. Sanz MF, Acha BV, García MF. Co-design for people-centred care digital solutions: a literature review. Int J Integr Care. (2021) 21(2). doi: 10.5334/ijic.5573
18. Noorbergen TJ, Adam MT, Teubner T, Collins CE. Using co-design in mobile health system development: a qualitative study with experts in co-design and mobile health system development. JMIR Mhealth Uhealth. (2021) 9(11):e27896. doi: 10.2196/27896
19. Smith H, Budworth L, Grindey C, Hague I, Hamer N, Kislov R, et al. Co-production practice and future research priorities in United Kingdom-funded applied health research: a scoping review. Health Res Policy Syst. (2022) 20(1):36. doi: 10.1186/s12961-022-00838-x
20. Albert A, Islam S, Haklay M, McEachan RR. Nothing about US without US: a co-production strategy for communities, researchers and stakeholders to identify ways of improving health and reducing inequalities. Health Expect. (2023) 26(2):836–46. doi: 10.1111/hex.13709
21. Oliver K, Kothari A, Mays N. The dark side of coproduction: do the costs outweigh the benefits for health research? Health Res Policy Syst. (2019) 17:1–10. doi: 10.1186/s12961-019-0432-3
22. Makey LM, Walsh CL, Salih I. Co-production: what it is and how it can ensure inclusive practice for service users and staff. Nurs Manage. (2024) 31(5):18–23. doi: 10.7748/nm.2022.e2046
23. Gremyr A, Andersson Gäre B, Thor J, Elwyn G, Batalden P, Andersson A-C. The role of co-production in learning health systems. Int J Qual Health Care. (2021) 33(Supplement_2):ii26–32. doi: 10.1093/intqhc/mzab072
24. Bucher A, Chaudhry BM, Davis JW, Lawrence K, Panza E, Baqer M, et al. How to design equitable digital health tools: a narrative review of design tactics, case studies, and opportunities. PLOS Digit Health. (2024) 3(8):e0000591. doi: 10.1371/journal.pdig.0000591
25. Perikangas S, Tuurnas S. Design for inclusive digital co-production. Public Manag Rev. (2024) 26(6):1731–51. doi: 10.1080/14719037.2023.2224819
26. Wu Y, Li Y, Baskys A, Chok J, Hoffman J, Roosan D. Health disparity in digital health technology design. Health Technol (Berl). (2024) 14(2):239–49. doi: 10.1007/s12553-024-00814-1
27. Moore G, Wilding H, Gray K, Castle D. Participatory methods to engage health service users in the development of electronic health resources: systematic review. J Particip Med. (2019) 11(1):e11474. doi: 10.2196/11474
28. The Kings Fund. Designing Inclusive and Trusted Digital Health Services with People and Communities. The Kings Fund London 2025 Available online at: https://www.kingsfund.org.uk/insight-and-analysis/long-reads/inclusive-digital-services-people-communities
29. Nickel GC, Wang S, Kwong JCC, Kvedar JC. The case for inclusive co-creation in digital health innovation. NPJ Digit Med. (2024) 7(1):251. doi: 10.1038/s41746-024-01256-9
30. Bibbins-Domingo K, Helman A, Dzau VJ. The imperative for diversity and inclusion in clinical trials and health research participation. JAMA. (2022) 327(23):2283–4. doi: 10.1001/jama.2022.9083
31. Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Inf Libr J. (2009) 26(2):91–108. doi: 10.1111/j.1471-1842.2009.00848.x
32. Whittemore R, Knafl K. The integrative review: updated methodology. J Adv Nurs. (2005) 52(5):546–53. doi: 10.1111/j.1365-2648.2005.03621.x
33. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10(10):Ed000142. doi: 10.1002/14651858.ED000142
34. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J. (2009) 339. doi: 10.1136/bmj.b2535
35. Pearce T, Maple M, Shakeshaft A, Wayland S, McKay K. What is the co-creation of new knowledge? A content analysis and proposed definition for health interventions. Int J Environ Res Public Health. (2020) 17(7):2229. doi: 10.3390/ijerph17072229
36. Grindell C, Coates E, Croot L, O’Cathain A. The use of co-production, co-design and co-creation to mobilise knowledge in the management of health conditions: a systematic review. BMC Health Serv Res. (2022) 22(1):877. doi: 10.1186/s12913-022-08079-y
37. Man M, Abrams T, McLeod R. Implementing and evaluating co-design. London: New Philanthropy Capital. (2019).
38. Duffy A, Christie GJ, Moreno S. The challenges toward real-world implementation of digital health design approaches: narrative review. JMIR Hum Factors. (2022) 9(3):e35693. doi: 10.2196/35693
39. Badr J, Motulsky A, Denis J-L. Digital health technologies and inequalities: a scoping review of potential impacts and policy recommendations. Health Policy. (2024) 146:105122. doi: 10.1016/j.healthpol.2024.105122
40. Avila-Garcia P, Hernandez-Ramos R, Nouri SS, Cemballi A, Sarkar U, Lyles CR, et al. Engaging users in the design of an mHealth, text message-based intervention to increase physical activity at a safety-net health care system. JAMIA Open. (2019) 2(4):489–97. doi: 10.1093/jamiaopen/ooz049
41. Richmond J, Anderson A, Cunningham-Erves J, Ozawa S, Wilkins CH. Conceptualizing and measuring trust, mistrust, and distrust: implications for advancing health equity and building trustworthiness. Annu Rev Public Health. (2023) 45:465–84. doi: 10.1146/annurev-publhealth-061022-044737
42. Galvagno M, Dalli D. Theory of value co-creation: a systematic literature review. Manag Serv Qual. (2014) 24(6):643–83. doi: 10.1108/MSQ-09-2013-0187
43. Browne S, Dooley S, Geraghty A, Dominguez Castro P, Reynolds C, Perrotta C, et al. Reflections on recruiting healthcare professionals as research participants: learning from the ONSPres study. HRB Open Res. (2022) 5:47. doi: 10.12688/hrbopenres.13499.1
44. Bekker M, Long J. User involvement in the design of human—computer interactions: some similarities and differences between design approaches. In: McDonald S, Waern Y, Cockton G, editors. People and Computers XIV—usability or Else! Proceedings of HCI 2000. London: Springer (2000). doi: 10.1007/978-1-4471-0515-2_10
45. Darejeh A, Singh D. A review on user interface design principles to increase software usability for users with less computer literacy. J Comput Sci. (2013) 9(11):1443. doi: 10.3844/jcssp.2013.1443.1450
46. Schuhmacher A, Haefner N, Honsberg K, Goldhahn J, Gassmann O. The dominant logic of big tech in healthcare and pharma. Drug Discov Today. (2023) 28(2):103457. doi: 10.1016/j.drudis.2022.103457
47. Chang BL, Bakken S, Brown SS, Houston TK, Kreps GL, Kukafka R, et al. Bridging the digital divide: reaching vulnerable populations. J Am Med Inform Assoc. (2004) 11(6):448–57. doi: 10.1197/jamia.M1535
48. Zogas A, Sitter KE, Barker AM, Fix GM, Khanna A, Herbst AN, et al. Strategies for engaging patients in co-design of an intervention. Patient Educ Couns. (2024) 123:108191. doi: 10.1016/j.pec.2024.108191
49. Jagannathan K, Arnott JC, Wyborn C, Klenk N, Mach KJ, Moss RH, et al. Great expectations? Reconciling the aspiration, outcome, and possibility of co-production. Curr Opin Environ Sustain. (2020) 42:22–9. doi: 10.1016/j.cosust.2019.11.010
50. Lyles CR, Nguyen OK, Khoong EC, Aguilera A, Sarkar U. Multilevel determinants of digital health equity: a literature synthesis to advance the field. Annu Rev Public Health. (2023) 44(1):383–405. doi: 10.1146/annurev-publhealth-071521-023913
51. Sultan A. Improving Cybersecurity Awareness in Underserved Populations. UC Berkely: Center for Long Term Cybersecurity (2019). https://cltc berkeley edu/wpcontent/uploads/2019/04/CLTC_Underserved_Populations pdf
52. Berry LL, Awdish RLA, Letchuman S, Steffensen KD. Trust-based partnerships are essential - and achievable - in health care service. Mayo Clin Proc. (2021) 96(7):1896–906. doi: 10.1016/j.mayocp.2021.03.035
53. Nandyal S, Strawhun D, Stephen H, Banks A, Skinner D. Building trust in American hospital-community development projects: a scoping review. J Community Hosp Intern Med Perspect. (2021) 11(4):439–45. doi: 10.1080/20009666.2021.1929048
54. Webb Hooper M, Mitchell C, Marshall VJ, Cheatham C, Austin K, Sanders K, et al. Responding to healthcare distrust among underserved communities: phase II. Psychooncology. (2022) 31(1):3–8. doi: 10.1002/pon.5841
55. Jones J, Barry MM. Factors influencing trust and mistrust in health promotion partnerships. Glob Health Promot. (2018) 25(2):16–24. doi: 10.1177/1757975916656364
56. Borges do Nascimento IJ, Abdulazeem H, Vasanthan LT, Martinez EZ, Zucoloto ML, Østengaard L, et al. Barriers and facilitators to utilizing digital health technologies by healthcare professionals. NPJ Digit Med. (2023) 6(1):161. doi: 10.1038/s41746-023-00899-4
57. Herlitz L, Crellin N, Vindrola-Padros C, Ellins J, Georghiou T, Litchfield I, et al. Patient and staff experiences of using technology-enabled and analogue models of remote home monitoring for COVID-19 in England: a mixed-method evaluation. Int J Med Inf. (2023) 179:105230. doi: 10.1016/j.ijmedinf.2023.105230
58. Steen T, Brandsen T, Verschuere B. The dark side of co-creation and co-production: seven evils. Co-production and co-creation: Lonodon: Routledge. (2018):284–93. doi: 10.4324/9781315204956-45
59. Kotenko NV, Bohnhardt V. Digital health projects financing: challenges and opportunities. Health Econ Manag Rev. (2021) 1:100–7. doi: 10.21272/hem.2021.1-10
60. Tekic A, Willoughby KW. Configuring intellectual property management strategies in co-creation: a contextual perspective. Innovation. (2020) 22(2):128–59. doi: 10.1080/14479338.2019.1585189
61. Charle-Maachi C, Moreau-Gaudry A, Sainati D, Camus D, Adenot I, Barthelemy C-E, et al. What value do digital health solutions bring, what are the funding mechanisms and evaluations? Therapies. (2022) 77(1):133–47. doi: 10.1016/j.therap.2021.12.010
62. Latonero M, Aneja U. Co-designing Digital Interventions and Technology Projects with Civil Society [Internet]. Geneva: World Economic Forum (2021).
63. Harrington CN, Erete S, Piper A. Deconstructing community-based collaborative design: towards more equitable participatory design engagements. Proc ACM Hum Comput Interact. (2019) 3(216):1–25. doi: 10.1145/3359318
64. Sparrey C. Learning Activity: Structured Brainstorming for the Co-production of Real-World Products. Knowledge, Innovation, and Impact: A Guide for the Engaged Health Researcher: A Guide for the Engaged Health Researcher. New York, NY: Springer (2020). p. 187–90.
65. Chokshi S, Mann D. Four phases for user-centered digital development: integrating academic and industry approaches to health information technology. JMIR Hum Factors. (2018) 5(4):e11048. doi: 10.2196/11048
66. Litchfield I, Shukla D, Greenfield S. Impact of COVID-19 on the digital divide: a rapid review. BMJ Open. (2021) 11(10):e053440. doi: 10.1136/bmjopen-2021-053440
67. Abernethy A, Adams L, Barrett M, Bechtel C, Brennan P, Butte A, et al. The promise of digital health: then, now, and the future. NAM Perspect. (2022) 2022:10.31478/202206e.
68. Marwaha JS, Landman AB, Brat GA, Dunn T, Gordon WJ. Deploying digital health tools within large, complex health systems: key considerations for adoption and implementation. NPJ Digit Med. (2022) 5(1):13. doi: 10.1038/s41746-022-00557-1
69. Koru UX. The Role of Rapid Prototyping Techniques in Transforming Digital. Pune: Healthcare Koru Ltd (2025). Available online at: https://www.koruux.com/blog/rapid-prototyping-techniques-in-healthcare/
70. Longhini J, Rossettini G, Palese A. Digital health competencies among health care professionals: systematic review. J Med Internet Res. (2022) 24(8):e36414. doi: 10.2196/36414
71. Pettersen S, Eide H, Berg A. The role of champions in the implementation of technology in healthcare services: a systematic mixed studies review. BMC Health Serv Res. (2024) 24(1):456. doi: 10.1186/s12913-024-10867-7
72. Moriau L, Tondeur J, Bertone J, Huysmans M, Temmerman M, Meurs P. The engagement CUBE: a dialogical tool for designing, facilitating and monitoring engaged research and teaching strategies. Int J Sustain High Educ. (2022) 23(4):783–98.
73. Pierce JH, Weir C, Taft T, Richards Ii W, McFarland MM, Kawamoto K, et al. Shared decision-making tools implemented in the electronic health record: scoping review. J Med Internet Res. (2025) 27:e59956.39983125
74. Bate P, Robert G. Bringing User Experience to Healthcare Improvement: The Concepts, Methods and Practices of Experience-based design. Boca Raton, FL: CRC Press (2023).
75. Wallenius J, Dyer JS, Fishburn PC, Steuer RE, Zionts S, Deb K. Multiple criteria decision making, multiattribute utility theory: recent accomplishments and what lies ahead. Manage Sci. (2008) 54(7):1336–49.
76. Kushniruk A, Borycki E, Kuwata S, Kannry J. Predicting changes in workflow resulting from healthcare information systems: ensuring the safety of healthcare. Healthc Q. (2006) 9 Spec No:114–8. doi: 10.12927/hcq.18469
77. Kip H, Beerlage-de Jong N, van Gemert-Pijnen L, Kelders SM. The CeHRes roadmap 2.0: update of a holistic framework for development, implementation, and evaluation of eHealth technologies. J Med Internet Res. (2025) 27:e59601. doi: 10.2196/59601
78. NHS AI and Digital Regulations Service. Piloting Digital Technologies in a Health or Care Service. London: National Health Service London (2024).
79. Mosch LK, Poncette A-S, Spies C, Weber-Carstens S, Schieler M, Krampe H, et al. Creation of an evidence-based implementation framework for digital health technology in the intensive care unit: qualitative study. JMIR formative Research. (2022) 6(4):e22866. doi: 10.2196/22866
80. Soobiah C, Cooper M, Kishimoto V, Bhatia RS, Scott T, Maloney S, et al. Identifying optimal frameworks to implement or evaluate digital health interventions: a scoping review protocol. BMJ Open. (2020) 10(8):e037643. doi: 10.1136/bmjopen-2020-037643
81. Pestoff V. Collective action and the sustainability of co-production. Public Manag Rev. (2014) 16(3):383–401.
82. National Institute for Health and Care Excellence in Health Care. Evidence Standards Framework (ESF) for Digital Health Technologies. London: NICE (2022). [Available online at: https://www.nice.org.uk/about/what-we-do/our-programmes/evidence-standards-framework-for-digital-health-technologies
83. von Huben A, Howell M, Norris S, Wong KC, Tang J, Kazi S, et al. Stakeholder preferences for attributes of digital health technologies to consider in health service funding. Int J Technol Assess Health Care. (2023) 39(1):e12.36786028
84. National Institute for Health and Care Excellence in Health Care Payment Guidance for Researchers and Professionals Involving People in Research. London: NICE (2024). [Available online at: https://www.nihr.ac.uk/payment-guidance-researchers-and-professionals
85. Koehle H, Kronk C, Lee YJ. Digital health equity: addressing power, usability, and trust to strengthen health systems. Yearb Med Inform. (2022) 31(01):020–32.
86. Satariano A. Palantir Wins Major UK Health Contract Despite Criticism. New York: The New York Times (2023).
87. Park JI, Lee HY, Kim H, Lee J, Shinn J, Kim H-S. Lack of acceptance of digital healthcare in the medical market: addressing old problems raised by Various clinical professionals and developing possible solutions. jkms. (2021) 36(37):e253–0. doi: 10.3346/jkms.2021.36.e253
88. Chahal BPS, Sharma U, Bansal B. Innovative Financing Models and Future Directions in Healthcare: Evaluating the Impact of Financial Strategies on Digital Health Outcomes and Innovation. Driving Global Health and Sustainable Development Goals With Smart Technology. Palmdale: IGI Global Scientific Publishing (2025). p. 267–302.
89. Lydon C. Digital healthcare market predicted to hit $836bn by 2031. Digital Health [Internet]. (2024). [No vol number] Available online at: https://www.digitalhealth.net/2024/07/digital-healthcare-market-predicted-to-hit-836bn-by-2031/ (Accessed June 01, 2025).
90. Institute of M. In: Grossmann C, Powers B, McGinnis JM, editors. Digital Infrastructure for the Learning Health System: The Foundation for Continuous Improvement in Health and Health Care: Workshop Series Summary. Washington, DC: National Academies Press IGI Global Scientific Publishing (2011).
91. Ishizaka A, Siraj S. Are multi-criteria decision-making tools useful? An experimental comparative study of three methods. Eur J Oper Res. (2018) 264(2):462–71.
92. Koivumaa J. Sprint: how to solve big problems and test new ideas in just five days. New York: Simon and Shuster. (2017).
93. Health Foundation for Western and Central New York. CCWB Learning Hub: Prototyping – What Works with Low/No Cost Methods. New York: Health Foundation (2025). [Available online at: https://hfwcny.org/program/co-creating-well-being/ccwb-learning-hub-prototyping-what-works-with-low-no-cost-methods/
94. Lawler EK, Hedge A, Pavlovic-Veselinovic S. Cognitive ergonomics, socio-technical systems, and the impact of healthcare information technologies. Int J Ind Ergon. (2011) 41(4):336–44.
95. Turnhout E, Metze T, Wyborn C, Klenk N, Louder E. The politics of co-production: participation, power, and transformation. Curr Opin Environ Sustain. (2020) 42:15–21.
96. Williams O, Sarre S, Papoulias SC, Knowles S, Robert G, Beresford P, et al. Lost in the shadows: reflections on the dark side of co-production. Health Res Policy Syst. (2020) 18:1–10.31900230
97. Mulvale G, Robert G. Special issue- engaging vulnerable populations in the co-production of public services. International Journal of Public Administration. (2021) 44(9):711–4.
98. Birmingham SEND. Birmingham SEND Co-production Training 2025 Birmingham City Council Birmingham 2025 [Available online at: https://www.localofferbirmingham.co.uk/2025/03/11/birmingham-send-co-production-training-2025/ (Accessed June 01, 2025).
99. Warschauer M, Tate T. Digital Divides and Social Inclusion. Handbook of Writing, Literacies, and Education in Digital Cultures. London: Routledge (2017). p. 63–75.
100. Paton C, Braa J, Muhire A, Marco-Ruiz L, Kobayashi S, Fraser H, et al. Open source digital health software for resilient, accessible and equitable healthcare systems. Yearb Med Inform. (2022) 31(01):067–73.
101. Gentili A, Failla G, Melnyk A, Puleo V, Tanna GLD, Ricciardi W, et al. The cost-effectiveness of digital health interventions: a systematic review of the literature. Front Public Health. (2022) 10:787135.36033812
102. Nordin A, Kjellstrom S, Robert G, Masterson D, Areskoug Josefsson K. Measurement and outcomes of co-production in health and social care: a systematic review of empirical studies. BMJ Open. (2023) 13(9):e073808.37739472
103. Lee EWJ, McCloud RF, Viswanath K. Designing effective eHealth interventions for underserved groups: five lessons from a decade of eHealth intervention design and deployment. J Med Internet Res. (2022) 24(1):e25419.34994700
104. Marques IC, Ferreira JJ. Digital transformation in the area of health: systematic review of 45 years of evolution. Health Technol (Berl). (2020) 10(3):575–86.
105. Wagg S, Simeonova B. A policy-level perspective to tackle rural digital inclusion. Inf Technol People. (2022) 35(7):1884–911.
106. Jones-Esan L, Somasiri N, Lorne K. Enhancing Healthcare Delivery Through Digital Health Interventions: A Systematic Review on Telemedicine and Mobile Health Applications in Low and Middle-Income Countries (LMICs). York: York St John University (2024).
107. Yew SQ, Trivedi D, Adanan NIH, Chew BH. Facilitators and barriers to the implementation of digital health technologies in hospital settings in lower-and middle-income countries since the onset of the COVID-19 pandemic: scoping review. J Med Internet Res. (2025) 27:e63482.40053793
108. Kozlakidis Z, Wootton T, Sargsyan K. Digital health: needs, trends, applications. In: Kozlakidis Z, Muradyan A, Sargsyan K, editors. Digitalization of Medicine in Low- and Middle-Income Countries: Paradigm Changes in Healthcare and Biomedical Research. Cham: Springer International Publishing (2024). p. 5–12.
109. Moetlhoa B, Nxele SR, Maluleke K, Mathebula E, Marange M, Chilufya M, et al. Barriers and enablers for implementation of digital-linked diagnostics models at point-of-care in South Africa: stakeholder engagement. BMC Health Serv Res. (2024) 24(1):216.38365781
110. Singh DR, Sah RK, Simkhada B, Darwin Z. Potentials and challenges of using co-design in health services research in low- and middle-income countries. Glob Health Res Policy. (2023) 8(1):5.36915174
111. Egid BR, Roura M, Aktar B, Quach JA, Chumo I, Dias S, et al. “You want to deal with power while riding on power”: global perspectives on power in participatory health research and co-production approaches. BMJ Glob Health. (2021) 6(11):e006978.34764147
112. Mukherjee AS, Sahay S, Kumar R, Banta R, Joshi N. “A living lab within a lab”: approaches and challenges for scaling digital public health in resource-constrained settings. Front Public Health. (2023) 11:1187069.37608976
Keywords: digital health, digital inclusion, co-production, health inequalities, patient engagement
Citation: Litchfield I, Delanerolle G, Juffs H, Bloxham S, Dunning S and Harper L (2025) Increasing the inclusivity of digital health co-production: an integrative review. Front. Digit. Health 7:1636469. doi: 10.3389/fdgth.2025.1636469
Received: 27 May 2025; Accepted: 22 September 2025;
Published: 17 October 2025.
Edited by:
Yalini Senathirajah, University of Pittsburgh, United StatesReviewed by:
Sylvie Occelli, Institute of Social Economic Research of Piedmont, ItalyTathagata Bhattacharjee, University of London, United Kingdom
Copyright: © 2025 Litchfield, Delanerolle, Juffs, Bloxham, Dunning and Harper. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ian Litchfield, aS5saXRjaGZpZWxkQGJoYW0uYWMudWs=
 Ian Litchfield
Ian Litchfield Gayathri Delanerolle1,2
Gayathri Delanerolle1,2