- 1Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea
- 2Department of Clinical Research Design and Evaluation, SAIHST, Sungkyunkwan University, Seoul, Republic of Korea
- 3Department of Digital Health, SAIHST, Sungkyunkwan University, Seoul, Republic of Korea
- 4Center for Clinical Epidemiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea
- 5Centre for Heart Lung Innovation, St. Paul’s Hospital, University of British Columbia, Vancouver, BC, Canada
- 6Department of Respiratory Medicine, Kyoto University, Graduate School of Medicine, Kyoto, Japan
- 7Respiratory Unit, Fondazione IRCCS San Gerardo dei Tintori, Monza, Italy
- 8School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy
- 9Division of Respiratory Medicine, Department of Medicine, University of British Columbia, Vancouver, BC, Canada
Advances made in digital health in recent years have the potential to improve the care of patients living with chronic obstructive pulmonary disease (COPD) for whom substantial disability still exists. In particular, telehealth and telerehabilitation programs, wearable devices, and apps have been studied as novel methods of providing care to COPD patients who may have limited access to clinical centers or who may benefit from an increased level of monitoring. Many of these interventions gained traction during the COVID-19 pandemic when mandated social isolation required the rapid implementation of remote care models. While these digital health interventions have since demonstrated promise in delivering care to otherwise isolated communities, the ongoing need for more evidence proving their positive impact on important clinical outcomes remains a barrier to their full implementation. How to best integrate digital health solutions into existing care models requires greater consideration of the technological, financial, and labor demands such solutions may entail.
Introduction
Chronic obstructive pulmonary disease (COPD) is defined as a heterogeneous lung condition due to abnormalities of the airways (bronchitis) and/or alveoli (emphysema). Persistent and often progressive airflow obstruction that marks the disease results in chronic respiratory symptoms (dyspnoea, cough, sputum production, and/or exacerbations) (1). COPD is the third leading cause of death worldwide (2) and it is estimated that there are 480 million people suffering from COPD all over the world (3). By 2050, this estimate is expected to reach 600 million people (3). Compared to the unaffected population, patients with COPD are at higher risk for the development of coexisting extrapulmonary co-morbidities that are associated with poor outcomes, including cardiovascular disease, lung cancer, diabetes, osteoporosis and death (4). Therefore, COPD is associated with a range of potential impairments, including respiratory, physical, social, and emotional, reducing quality of life and limiting daily activities. However, COPD management is complex and an individualized approach to care depends on the condition's severity, including the degree of symptoms, exacerbation frequency and severity, and hospital admissions (1). Targeted COPD medications such as inhalers, while the backbone of therapy, are plagued by slow development (5), thus novel strategies to improve care are desperately needed.
The digital health revolution that has evolved over the past few decades may provide opportunities to improve COPD care. Digital health includes various solutions that utilize digital technologies to meet health needs, including health information technology, telehealth and telemedicine, mobile health, wearable devices, artificial intelligence, machine learning, the Internet of Things, and digital therapeutics. These technologies have the potential to change how diseases are screened, diagnosed, and followed, allowing for more precise profiling of disease progression and thus improving management. This review provides an overview of the best studied digital health opportunities, including its various forms and applications in COPD management. To provide context for emerging digital health technologies in this field, we first offer readers an overview of COPD, from its pathological hallmarks, clinical presentation, and current management strategies both for long-term maintenance care and acute exacerbations. We then narrate the evolution of and evidence for telehealth, telerehabilitation, wearable devices, and apps that have been the most rigorously studied to date in COPD. Finally, we address the substantial barriers that exist that must be addressed prior to the full implementation of digital health technologies in COPD, barriers present at patient, health care provider, and systemic levels. Our narrative review is thus designed to provide both a broad overview of COPD digital health technologies and guidance for future research to improve their implementation in patient care.
Chronic COPD management
The physiological hallmark of COPD is airway obstruction, which is predominantly the consequence of inflammation and fibrosis of the small airways (chronic bronchitis) and the loss of alveolar attachments (emphysema) resulting in a reduction in forced expiratory volume in 1 s (FEV1) and the FEV1/forced vital capacity (FVC) ratio. For almost 50 years, COPD has been widely accepted as a self-inflicted condition caused by tobacco smoking (6). COPD was considered a disease occurring in susceptible individuals in whom smoking induced an abnormal inflammatory response (7) that damaged the airways and alveoli, accelerating the physiologic decline of lung function with age. The resulting airflow limitation and chronic respiratory symptoms are difficult to reverse and may periodically be manifested as exacerbations (8). However, recent data suggest that about a third of COPD patients worldwide are non-smokers (9). In addition to genetic disorders such as alpha-1 antitrypsin deficiency, other environmental pollutants, such as smoke from biomass fuel used for cooking and heating (9) and air pollution (10), are also considered major environmental risk factors for COPD in many places around the globe. Moreover, extrapulmonary comorbid conditions are highly prevalent among patients with COPD but are largely unrelated to lung function (4). Therefore, COPD can be seen as the pulmonary component of a systemic and multimorbid syndrome (11).
The Global Initiative for Chronic Obstructive Lung Disease suggests an initial approach to disease management based on the intensity of symptoms and the history of exacerbations, with a subsequent algorithm that includes using blood eosinophil counts for adjustment of therapy (1). A long-acting muscarinic antagonist (LAMA) is the initial drug of choice for patients with mild disease and no exacerbations (1). If the patient has more severe dyspnea, severe airflow obstruction, and lung hyperinflation, combining a LAMA with a selective long-acting beta2-agonist (LABA) is more effective; the two agents can be provided in a single inhaler to simplify treatment (1). It is reasonable to start therapy with a combination of a LABA and an inhaled glucocorticoid in patients with a history of asthma, wheezing, rhinitis, polyps, or allergies; a history of exacerbations; an elevated blood eosinophil count (>150 per cubic millimeter); or a combination of these findings. If exacerbations continue (two or more or one requiring hospitalization), the combination of a LAMA, a LABA, and an inhaled glucocorticoid in a single canister decreases the risk of exacerbations, improves lung function, and may decrease the risk of death (1). Adjunct therapies that have been found to be beneficial in patients with COPD include pulmonary rehabilitation, a multidisciplinary exercise training program that can help to relieve symptoms and improve exercise capacity (12).
Acute COPD exacerbations
An exacerbation of COPD (AE-COPD) is defined as an event characterized by increased dyspnea and/or cough and sputum that worsens in <14 days which may be accompanied by tachypnea and/or tachycardia and is often associated with local and systemic inflammation caused by infection, pollution, or other insult of the airways (13). AE-COPD are clinically relevant events in the natural history of COPD because they negatively impact health status, lung function, rates of hospitalizations, and disease progression (14). Nevertheless, patients with COPD are also at increased risk of other acute events, particularly decompensated heart failure (15, 16), pneumonia (17), and pulmonary embolism (18, 19) that may also mimic or aggravate an AE-COPD.
Exacerbations are considered to be mild when only worsening symptoms are reported; moderate when the patient receives antibiotics, systemic glucocorticoids, or both; and severe when the patient visits an emergency department or is hospitalized (20). They may also increase the risk of myocardial infarction, stroke, pulmonary embolism, and death (18, 21, 22). A subset of patients with COPD are prone to developing frequent exacerbations (23). Frequent exacerbations are associated with worsening health status, more rapid decline in lung function and are a driver of health care costs, accounting for more than 20% of all readmissions occurring within 30 days after a hospitalization for the same diagnosis (24, 25). Therapies for AE-COPD include short-acting inhaled beta2-agonists and short-acting anticholinergic agents, while systemic glucocorticoids help to improve airflow, gas exchange, and symptoms (26, 27). Antibiotics, particularly for patients with increased sputum purulence and volume, may also reduce treatment failure and improve short-term mortality rates (28). Long-term therapies that can help prevent AE-COPD include chronic macrolides (29) and phosphodiesterase-4 inhibitors such as roflumilast (30).
Despite these drug interventions, many patients with COPD continue to face poor quality of life (31) and reduced lifespans (32). Ultimately, clinicians and allied health providers must navigate the complex journey of both acute and chronic management in COPD within the limitations of these treatments. In the next section, we present the evidence surrounding the most widely studied digital health interventions that have the potential to improve COPD care.
Telehealth and telerehabilitation
The telecommunications revolution of the 20th and 21st centuries has propelled the telehealth movement forward with extraordinary rapidity. Where once a simple telephone may have been considered the pinnacle technology connecting patient with doctor (33), we now have at our fingertips a plethora of video communication interfaces, electronic medical record systems, and real-time monitoring devices that can instantly transmit important health information to and from a patient. In their ideal forms, these technologies bridge significant divides that may hamper patient care, including physical distance from providers and healthcare facilities and lengthy wait times.
For patients with COPD, who are often elderly, may have considerable respiratory symptoms that limit their ability to attend in-person appointments, and who require complex multidisciplinary management, telehealth may serve a particularly useful role in their care. The introduction of telehealth into COPD management largely occurred around the turn of the century following the expansion of Internet access across the globe and the proliferation of mobile devices. One of the first studies ever to evaluate the potential of telehealth in COPD was conducted in the United Kingdom in the late 1990s where video phones installed in patient homes allowed a nursing team to monitor vital signs, peak expiratory flow rates, and respiratory symptom scores during acute exacerbations (34). While this low-bandwidth technology often did not have sufficient image resolution for the nursing staff to accurately assess respiratory rate, it nonetheless demonstrated the feasibility of a telehealth approach to home monitoring. Subsequently, numerous trials emerged that studied remote monitoring of vital signs in patients with COPD (35) and integrating such monitoring systems with either telephone or video linkages to provider teams (36–39). Although initial assessments of the efficacy of these small programs were promising, with reductions of hospitalizations, emergency room visits, readmissions, and overall health care utilization and costs reported (40–44), the integration of modern technology into COPD care was certainly not universally welcomed. Practitioners in this early phase voiced concerns about the impact technology had on the efficiency of care and on the patient interaction experience, particularly when technical problems disrupted visits (45–48). Studies demonstrating no improvement in the quality of life (49, 50) of patients with COPD also called into question the benefit of these programs.
Proposed models of telehealth in COPD care have included not just these remote monitoring systems connected to a health care team, but also electronic tools to predict the onset of acute exacerbations (51–59), virtual education programs to assist patients with important elements of disease management such as inhaler technique (60–64), and telerehabilitation (65–70). Patient perceptions of these programs has generally rated high, noting convenience (71, 72), self-empowerment (72–74), and sense of security (73–77) as being positive attributes of telehealth. Nonetheless, the growing body of literature on telehealth in COPD has still not yet shown clear benefits when it comes to key outcomes such as exacerbations, hospitalizations, and mortality. Supplementary Table S1 provides an overview summary of relevant trials. A 2013 randomized controlled trial from Scotland (n = 128 in the telemonitoring group and n = 128 in the usual care group) demonstrated no significant difference in the mean number of COPD admissions over one year nor in the number of days to hospital admission (78). In fact, a subsequent randomized controlled trial published in 2016 evaluating telemonitoring with a link to a hospital-based care team suggested potential harm associated with telehealth: hospital admissions at six months was significantly higher in the telemonitoring group compared to the control group (p = 0.026) (79). The PROMETE II trial, a randomized controlled trial comparing home telemonitoring in 237 patients with severe-very severe COPD to routine clinical practice failed to show any benefit in exacerbation rates, hospitalization days, intensive care unit admissions, and quality of life scores (80). Results were similar in the 2018 CHROMED trial which did not find any benefit to telemonitoring in terms of time to hospitalization and rate of exacerbations in 312 patients with COPD (although a significant difference was demonstrated in hospital readmission rate, p = 0.017) (81). Multiple studies evaluating cost-effectiveness of such programs also appear to have mixed results, with some studies demonstrating no difference (82–85), improved cost-effectiveness (86–88), and worse cost-effectiveness (89) compared to usual care. Altogether, the evidence compiled would not suggest that telehealth programs provide tangible long-term advantages yet, with the caveat that our understanding of which subgroups of COPD patients may in fact benefit is still quite limited (90).
Of all the methods in which telehealth can be implemented in COPD care, telerehabilitation is perhaps the best studied. Pulmonary rehabilitation in COPD has well-established benefits when it comes to improving health-related quality of life and exercise capacity (91) and reducing mortality following exacerbations (92). In reality, though, access to pulmonary rehabilitation for many patients remains elusive, particularly for those living in rural and sparsely populated regions (93, 94), and utilization of pulmonary rehabilitation remains disturbingly low (95). Delivery of equivalent services through a virtual interface could potentially bring this valuable resource to a greater number of eligible COPD patients. Early evidence of the benefit of such programs was demonstrated in a 2017 multicentre randomized controlled trial evaluating a smartphone-based telecoaching program in 343 patients with COPD (96). Patients in the intervention arm had a greater increase in steps per day and 6-minute walk distance compared to the usual care group. Other studies have since demonstrated that enrollment in a telerehabilitation program can reduce hospital readmissions (67, 70). Subsequent trials comparing home-based to hospital-based rehabilitation programs have demonstrated non-inferiority in terms of reducing the risk of COPD exacerbations and hospitalizations (69) and improvements in 6-minute walk distance (66, 68, 97) and COPD Assessment Test scores (97). To date, no safety issues have been identified with these home-based telerehabilitation programs (98). However, the lack of cost-effectiveness analyses and the wide variability in programs studied are ongoing limitations that should be addressed (98, 99).
Regardless of these inherent gaps in knowledge, the rapid implementation of telehealth in COPD care was necessitated by the COVID-19 pandemic during which in-person assessments, diagnostic testing, and pulmonary rehabilitation programs were drastically reduced or halted entirely overnight. Health care systems were forced to immediately adapt to pandemic conditions in the interests of protecting patients from contracting the virus, with virtual video and telephone consultations substantially increasing in the first year of the pandemic (100, 101). While a full evaluation of these measures is still ongoing, preliminary reports attest to the feasibility of their emergency implementation and the positive reception of such measures in both patients and providers. The majority of clinicians have reported that they were still able to assess symptom severity and provide smoking cessation counseling despite remote care (102), while patients felt positively that telemedicine allowed them to continue accessing health care without being exposed to COVID-19 (103) and provided them with a sense of comfort (104). Still, access to telehealth was patchy in the first days of the pandemic, with one study reporting that only 12.6% of patients with COPD had regular access to medical visits during the lockdown period despite 59.1% of physicians transitioning to telehealth platforms (104). As the experience with virtual platforms grows in health care systems around the globe, telehealth will nonetheless form the foundation of care delivery that will allow for flexibility with fewer disruptions to patient care in times of emergency.
Wearable devices
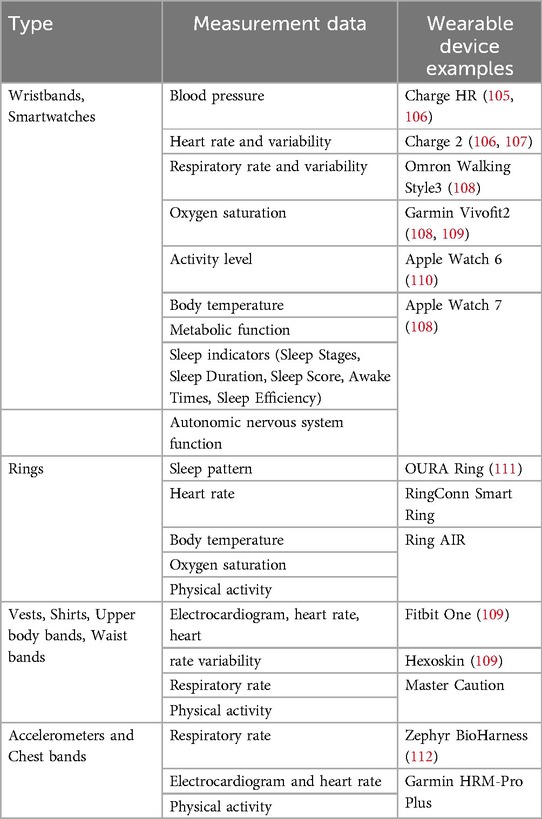
The symptoms and conditions of COPD patients including vital signs, oxygen saturation, sleep pattern, and physical activity can be monitored by wearable devices. Unsurprisingly, there has been a sustained demand for the development of tools for symptom monitoring and health management in COPD.
Among wearable devices (Table 1), wristbands and smartwatches are the most commonly used, which can measure blood pressure, heart rate and variability, respiratory rate and variability, oxygen saturation, physical activity, body temperature, metabolic function, sleep indicators, and autonomic nervous system function. Wearables worn on the upper body and chest can also accurately measure heart rate, respiratory rate, and activity level. In particular, chest-worn wearables have the advantage of being able to collect more accurate data during exercise or daily activities. Wearables worn on the finger can measure sleep patterns, heart rate, and body temperature, and are useful for monitoring the quality of sleep and overall health status of the user. Each type of wearable device plays an important role in monitoring and managing COPD patients. Wearables for monitoring vital signs track basic indicators of health status in real time, and physical activity wearables help enhance the user's activity level and facilitate various exercise programs.
Research prior to 2019 primarily focused on the validity and accuracy of sensor-based remote monitoring devices compared with gold standard medical equipment (113, 114). Subsequent studies aimed at using these devices to develop a prediction model of COPD exacerbations by collecting short-term (30 days) (115) and long-term (90 days) patient monitoring data (116). The feasibility of continuously and reliably capturing audio, heart rate, and physical activity through smartwatches (117), moreover using these devices to provide education and self-monitoring interventions (118), were also demonstrated in COPD patients. Rubio et al., for example, showed the potential of using home monitoring of resting breathing rate data to supervise recovery from COPD exacerbations (116). Monitors were able to provide accurate assessments of breathing rate, although some patient feedback criticized the monitors for being intrusive during the acute illness phase.
Although promising, the widespread adoption of wearable devices by patients with COPD has not been universal, hampered by a lack of evidence that these devices can meaningfully improve important clinical outcomes, inaccessibility, and inaccuracy concerns. Walker et al., reported that remote monitoring of lung function by forced oscillation technique and cardiac parameters did not change time to first hospitalization and quality of life over 9 months in a randomized controlled trial (81). A survey for devices listed up to June 2019 reported that the devices with the most technological promise and compatibility with daily living had high or unlisted prices, placing them out of reach for many patients (119). While studies by Buekers et al. (114) and Chan et al. (120) monitored oxygen saturation using consumer-based wearable finger and smartphone oximeters, technical limitations were reported, including incomplete data recording during moderate to vigorous physical activity and exercise. As noted in a systematic review, which compiled data from seven papers published prior to 2021, oxygen saturation and respiratory rate devices were valid compared to other respiratory monitoring devices, but were not accurate in predicting exacerbation events (121). Meanwhile, although Pipek et al. demonstrated that a wearable device (Apple Watch 6) can be a reliable way to obtain heart rate and oxygen saturation in patients with lung diseases in a controlled environment (110), subsequent publications called into question these findings. A study conducted by Stove et al. raised concerns that wearable devices such as the Apple Watch Series 7 and Garmin Vivosmart 4 should not be relied upon for monitoring oxygen saturation during pulmonary rehabilitation. Specifically, their research revealed that these devices tended to overestimate oxygen saturation levels in individuals with COPD when levels were below 95%, while underestimating oxygen saturation when levels were above 95% at rest and immediately after the 30 s sit-to-stand test and the 6 min walk test (108).
Other research followed utilizing consumer-based activity trackers as tools for measuring physical activity (113) and as coaching devices for promoting physical activity (122) in patients with COPD. The initial studies focused on evaluating these devices' accuracy and feasibility. In a single-arm feasibility study, 19 patients with COPD wore a commercial activity monitor every day (median 42 days) during pulmonary rehabilitation. These patients were able to increase their exercise steps throughout the pulmonary rehabilitation process without sacrificing non-exercise physical activity. These patients demonstrated improved dyspnea and quality of life following use of the device, confirming that wearable technology can support effective remote walking exercise prescriptions and engagement during pulmonary rehabilitation (123). However, in another study involving 122 patients divided into standard care, self-monitoring, and telemonitoring groups, COPD patients in the remote or self-monitoring groups did not experience improved outcomes or reduced healthcare utilization compared to standard care, despite regular use of technology. This was attributed to low primary healthcare utilization, the absence of structured training components, and inadequate integration of technology with action plans (124).
More recently, attempts to integrate physiological features captured by wearable devices with environmental monitoring to read toxic and noxious exposures have aimed to improve our abilities to predict COPD exacerbations. For instance, Wu et al., used a wearable device, home air quality sensing device, a smartphone app, and a supervised prediction algorithm to achieve excellent performance (accuracy of 92.1%) in predicting whether a patient would experience an acute exacerbation of COPD within the next seven days (125). These more sophisticated algorithms integrating multiple technologies may prove beneficial in future, yet at present clinicians remain somewhat mixed on their use in clinical practice. In one mixed-method survey, most clinicians attested to not employing these technologies routinely in their patient panels and reported low confidence in their overall effectiveness (109). Healthcare practitioners report a lack of guidance as to how to implement these technologies in their clinical practices and seek more information on their use (105). As one scoping review has outlined, strategies in addition to technology are needed to effectively support health management and physical activity monitoring in COPD patients (126). Specifically the authors note these key areas that must be addressed and achieved by wearable technology innovators: (1) Monitoring and tracking activity and health, (2) Enhancing motivation for physical activity, (3) Ensuring acceptability of the device, (4) Addressing technical issues with the device, (5) Establishing appropriate and achievable health goals, (6) Integrating the device into daily life and routine, and (7) Recognizing the physical and psychological benefits of device usage (126). Physiological data measurement during exercise remains inaccurate with evidence coming from small trials, and current health behavior theories are not yet well integrated into self-management, resulting in low effectiveness. In future, large-scale studies focusing on multiple outcomes will be required, and the release of consumer-based wearable devices equipped with predictive models remain goals for the community.
Apps
Today, mobile health (mHealth) apps, either on computer, tablet, or smartphone interfaces, are used for a wide variety of health services and for patient education. In their ideal form, these apps can help patients monitor their COPD, manage their symptoms, identify warning signs that should trigger a higher level of care, and receive education and treatment plans. From 2014 to 2017, research primarily focused on the design and the feasibility of these apps. Programs delivered through apps were provided to patients through a combination of online and offline methods, through both Web and phone interfaces, and designed to promote their physical activity (127–129). These studies demonstrated encouraging results that these interventions could improve physical activity levels in patients with COPD, and that both patients and physiotherapists had positive attitudes towards the apps. Nonetheless, demonstrating concrete improvements in outcomes such as symptom reduction or hospitalizations was elusive. For instance, Farmer et al. performed a randomized controlled trial in 2017 using a tablet-based system that included a symptom diary, vital sign monitor, and education modules, but did not find any benefit in terms of patients' St. George's Respiratory Questionnaire scores or hospital admission rates (130). A subsequent systematic review of apps during this era demonstrated only a weak association with a lower risk for hospital admission and no significant impact on length of hospitalization (131). Other systematic reviews noted that health-related quality of life and physical activity could be improved by smart technology apps, but that these effects were not sustained over longer periods of time (132).
With sensor modules that can detect changes in vital signs now being integrated into smartphone technology, though, the promise of apps to predict adverse outcomes in COPD such as exacerbations may be on the horizon. Apps that allow patients to log their symptoms daily in conjunction with heart rate and oxygen saturation monitoring that could alert a patient to worrying trends may conceivably allow early detection of exacerbations and therefore prompt immediate treatment and prevention of hospitalizations. Early trials suggested that these apps were feasible with excellent compliance rates amongst patients who would be required to report their symptoms on a regular basis (133–135). Interestingly, these apps can capture a far greater number of clinical worsening events than patients ultimately recalled themselves and reported to their doctors (136). Criner et al. demonstrated that one such app could shorten the time to which a patient could receive clinical direction from their primary care provider for an exacerbation event (135). The EmmaCOPD app which monitors both symptom worsening and step count decline to prompt a clinical intervention resulted in a reduction in the number of hospitalized days and exacerbation frequency (137). Further machine learning models utilized in another trial by Chmiel et al. allowed a digital health app to use symptom data to predict an exacerbation event within three days with a sensitivity of 0.551 and a specificity of 0.759 (138). No doubt, these algorithms will need to be refined in future as technologies develop to improve risk prediction models.
More evidence that apps can be successfully integrated into COPD management with beneficial results have come in the domain of pulmonary rehabilitation. The Kaia COPD app containing an exercise training program was recently demonstrated in a randomized controlled trial to significantly improve symptom scores and allowed patients to maintain physical activity after completing pulmonary rehabilitation courses (139). These promising results in improving exercise in patients with COPD compared to control groups have been reflected in other trials as well (140, 141). Mobile apps also appear to be effective in empowering patients through education. Both quantitative assessments and qualitative interviews with patients following an mHealth app intervention, for example, found significant improvements in disease awareness and self-efficacy (142). Coping abilities and knowledge of COPD management have also been reported to increase following mHealth app use (143, 144).
In general, patient attitudes towards the use of apps in their COPD care are positive (145), but in reality they are faced with a number of choices available on public markets which may lack oversight by health care professionals. Thirteen COPD-related apps were reviewed by Quach et al. in 2023, who found that none of the apps reported data that supported their efficacy (146). Nine of the apps were furthermore found to have outdated or incorrect information about COPD. Only three of the apps provided clear descriptions of their data security features. The medical community must therefore be aware of the proliferation of apps that might in fact be harmful for patients and only apps that have been carefully screened, demonstrate evidence of benefit, and guarantee privacy and security should be promoted to patients. Not only should these apps be constructed with input from health professionals who can ascertain the quality of the information provided, but they should also be the product of a close patient-provider-technology partnership to ensure a patient-oriented design. Iterative multidisciplinary processes in app design where patients are consulted along each step have been described, which may provide the best chance at successful acceptance of apps in patients with COPD (147, 148). These partnerships may also be able to address issues such as poor readability and a lack of cultural sensitivity that have been reported with certain apps (149).
The quality of mHealth apps can be assessed using the Mobile App Rating Scale (MARS) which consists of 23 items covering four objective quality dimensions (engagement, functionality, aesthetics, and information quality) and one subjective quality scale (150). With this comprehensive scale, two assessors on our team independently evaluated the quality of six accessible and downloadable COPD apps (AioCare Patient, COPD Manage, NIH: COPD, NHS Wales: COPDhub, COPD Pocket Consultant Guide, and Chronic Lung Disease Treatment). Inter-rater reliability measured by the Intraclass Correlation Coefficient (ICC) was 0.76 (95% CI 0.67–0.83), indicating good reliability. We found that COPD apps tended to focus most on functionalities such as performance, navigation, or usability (Figure 1). However, these apps showed relatively low quality in the engagement dimension such as fun, interest, or interactivity. The focus on clinical information accuracy and self-management functionality in COPD mHealth applications potentially overlooks a critical aspect of user interest. Thus, integration of enticing engagement and gamification elements into app architecture may be necessary for sustaining long-term user adherence and optimization of therapeutic outcomes.
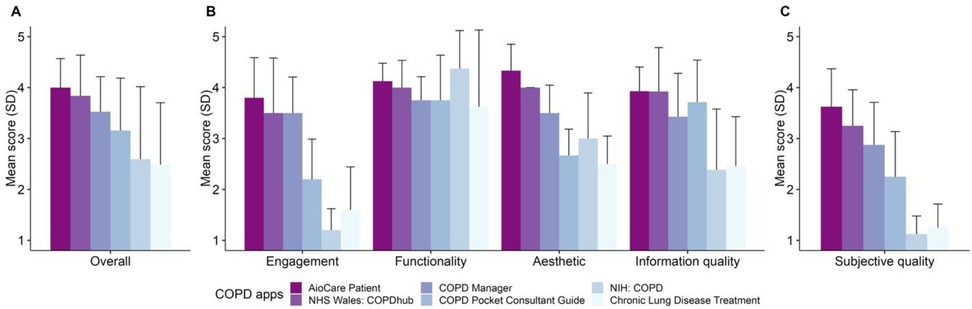
Figure 1. COPD apps by MARS scores. (A) Indicates overall app quality by the MARS score. (B) Indicates scores by individual objective domains, including engagement (covering fun, interest, and interactivity), functionality (covering performance, usability, and navigation), aesthetics (covering layout, graphics, and visual appeal), and information quality (covering accuracy, credibility, and evidence bias). (C) Provides the MARS score related to subjective quality. Error bars indicate standard deviation. All questions were evaluated using a 5-point Likert scale.
Barriers and controversies in digital health
Despite the rapid development and evolution of digital technologies in the health care sphere, the barriers that prevent their full implementation in COPD remain relatively fixed over time (48, 151–157). Until these barriers are effectively addressed by our community, it is likely that digital health will remain at the fringes of COPD care. Here, we consider barriers to digital health arising from three levels of care: those related to systemic and technical problems and those at the level of healthcare providers (HCPs) and patients (Figure 2).
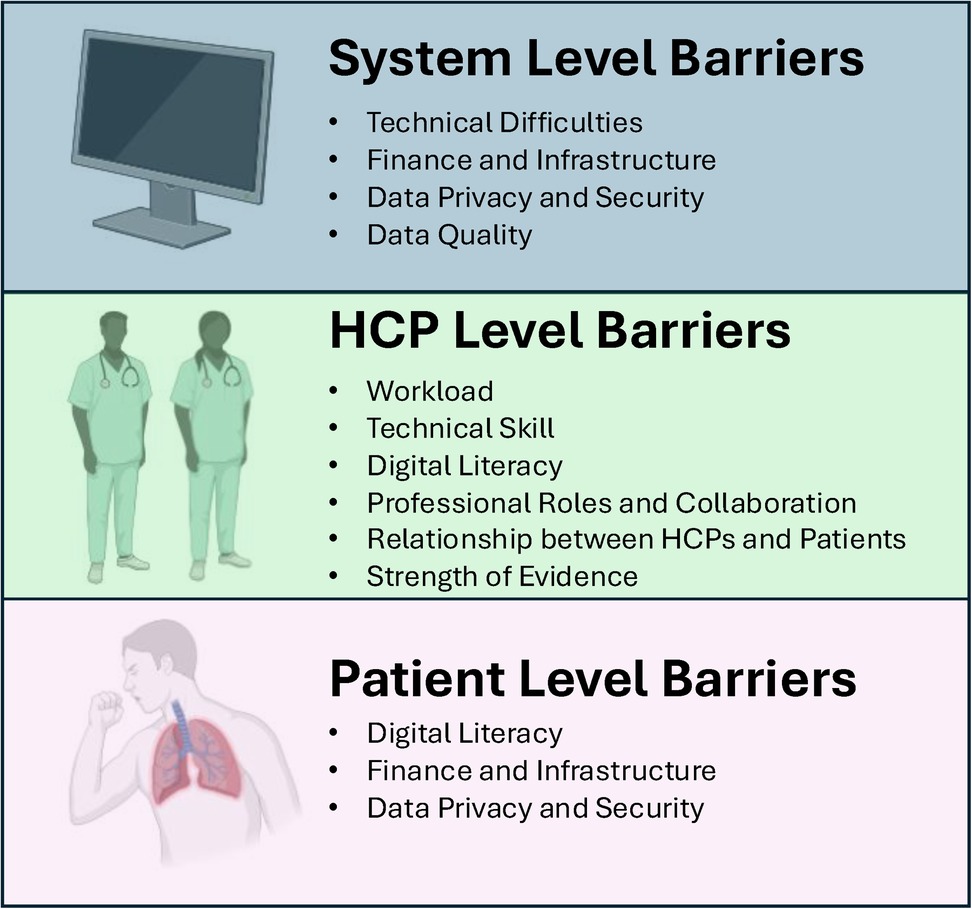
Figure 2. Levels of barriers to digital health. Created using Biorender.
HCP level barriers
For HCPs, it is essential that digital technologies be seamlessly integrated into what is already a demanding and strenuous workload (151–155). HCPs see themselves as battling a “sea of data,” (155) therefore management of patients' data taken via digital health devices as a part of their daily routine may be perceived as yet another burden to their workload. Devices which constantly monitor patients' oxygen saturation, heart rate, temperature, and symptoms related to COPD exacerbations produce unprecedented amounts of data. A high degree of triggered alarms turn out to be false, resulting in unnecessary clinical work (158). HCPs have voiced concerns about the sustainability of digital health given these added burdens (159). Whether new artificial intelligence technologies can assist HCPs in making sense of these burgeoning datasets is still unclear. Ultimately, appropriate staffing support and compensation would certainly have to be addressed for sustainability to be achieved. Who and what entities will shoulder the financial and technical burden of infrastructure, data management, privacy and security, and quality control has yet to be worked out by the majority of health care systems (151, 153, 154).
Technical skill (151, 153, 154) and digital literacy (151) are also well recognized barriers to full implementation. For many HCPs, the digital frontier represents a shift in practice for which insufficient training has been provided. Facility in using these devices, comfort in addressing technical problems when they arise, not to mention keeping up to date with their latest advances, are all required for HCPs to fully adopt digital health into their practices. At the same time, feedback from tele-rehabilitation programs also suggests that HCPs feel discomfort with the type of care provided through advanced technologies, that particular communication skills are required to master the provider-patient relationship in this novel form (151, 153, 154). When care is provided through screens, HCPs have noted the loss of personal interactions, social contexts, and direct contact through touch and sight, all of which they place value in guiding their understanding of each patient (151, 154). Without this critical accompanying information, concerns regarding safety are additionally raised. HCPs voice worry about providing rehabilitation in remote settings without the guidance of direct observation which may make them blind to each patient's particular safety needs and physically unable to help them if the patient worsens (153). In the face of these limitations, HCPs consider the evidence for digital health technologies in improving COPD outcomes insufficient to counter their apprehensions (155). As more is learned about digital health and better training is provided to HCPs on digital health technologies, fear of and doubt over the unfamiliar may recede. According to Sharma et al. (154), the introduction of telehealth can be experienced as threatening to HCPs, but at the same time HCPs who had experience in providing telehealth had fewer and milder concerns. This suggests that this unfamiliarity to digital health may be overcome through suitable training and support.
Patient level barriers
In a certain sense, patient concerns with respect to digital health mirror those of their HCPs. Inadequate facility with and access to digital technologies and concerns about data confidentiality and security impact digital health uptake as much for patients as it does for HCPs. First and foremost, uptake requires a certain degree of overall health literacy, defined as “the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions” (160). Health literacy, though, remains low in patients with COPD (161–163). This is particularly true for vulnerable groups, such as those of older age, with low education and income levels, and from minority and underrepresented communities (162–165). Those with low health literacy have been shown to be less engaged in self-management plans (163). Even if a minimum level of health literacy is attained, though, further literacy in digital technologies is still needed. Electronic health (eHealth) literacy is defined as “a patient's capacity to seek, locate, understand and evaluate health information from the Internet and apply such knowledge when addressing or solving a disease-related health and concern” (166). Many of the same risk factors (e.g., older age, low income and education levels) for poor health literacy also contribute to poor eHealth literacy (167, 168). Patients with COPD have been shown to have low to moderate eHealth literacy (169, 170), with technophobia, a negative attitude towards aging, lower perceptions of self-efficacy, greater severity of COPD, low technology use, low income, and low education levels significantly associated with worse eHealth literacy scores (169–172). Efforts to increase digital health uptake must therefore address these particular risk factors.
Ultimately, any digital health technology has to be usable for a patient (173). Previous qualitative investigations have noted that patients with COPD often think that telehealth screen fonts are too small for them to read (174, 175) and that touchscreens can be difficult to use particularly if they had manual dexterity problems (176). Excessive keys and buttons can overwhelm users and cause confusion (175). Even the act of downloading an app or creating a login and account can be problematic for users with little experience with smart technologies (173, 177). Technology design that is performed with ongoing, iterative contributions from end-users is thus essential, as is continuous usability testing to ensure that even those with limited eHealth and digital literacy can still navigate them easily. Additionally, to address usability problems, designers must identify where dissonance appears between a system's intended use and the users' actual interpretation and experience in both physical and cognitive aspects. Naranjo-Rojas et al. propose “think-aloud” methods that allow participants to vocalize their concerns and challenges as they use technologies in real time to facilitate the improvement of digital health technologies (177). Displays and interfaces that are accessible, intuitive, organized, and visually appealing are thus essential elements of digital health design.
Access to necessary technologies also remains patchy amongst patients with COPD, reflecting the inequities that exist in the digital health realm. Melzer et al. reported that only 54% of veterans with COPD had a computer at home, only 54% had a smartphone, and only 36% had reliable in-home Internet, resulting in 25% having overall low use of technology (169). Other groups have reported even lower rates of technology ownership. For instance, Alwashmi et al. reported that only 23% of their cohort of patients with COPD owned a smartphone (173). Compounding these inequities are often financial pressures as medical technologies designed for remote patient monitoring carry either high or undisclosed costs that may be out of range for many patients (178). It is not surprising then that patients have expressed they cannot afford the costs that come with digital health technologies (173). Unless greater availability of emerging technology is granted to a wider swath of the COPD patient population, much of the digital health revolution will bypass them.
System level barriers
Healthcare systems, from the limitations of their technological capacities to the complexities of their organizational and financial structures, also pose significant barriers to the implementation of digital health. While there are no structured articles that have investigated systemic barriers specifically pertaining to digital health in COPD management, key themes emerge in the current literature that highlight the structural limitations facing the digital health revolution. First, we must ask whether our current technologies are sophisticated enough to provide smooth and seamless services to patients living with COPD. Second, are the vast number of health care organizations, all of whom may have differing attitudes towards digital health, budgetary constraints, and their own unique electronic medical systems, ready to implement new digital health programs and technologies? Finally, can healthcare systems ensure the proper data security and privacy provisions that patients will rightfully demand as these technologies are implemented? These concerns are not unique to COPD care, but reflect larger barriers that must be overcome for the entire field of digital health.
Internet accessibility and stability are certainly barriers in promoting digital health. Smaradottir et al. pointed out in an evaluation of a tablet-based telemedicine program for COPD that lapsed data transmission and poor quality image and sound during videoconferencing, due to unstable and insufficient mobile network coverage, were significant system level problems impacting the use of the program (179). Other qualitative studies have echoed these technical concerns, with patients voicing that malfunctioning technology increased their stress and was a barrier to adherence (180). These are concerns not just of patients, but also of HCPs (181). The stability and accessibility of internet service are especially critical when HCPs monitor vital signs in remote settings. Even if the internet infrastructure itself functions properly, digital health technologies cannot work efficiently if programs are incompatible with older operating systems that some patients may have (152).
As we have previously mentioned, digital health solutions must be integrated into existing and already high workloads for physicians and allied health care professionals. However, equally important is integration into existing practice cultures, where the adoption of digital health programs may not be welcome by all and where previously defined clinical roles may now be thrown into question by new technologies. Interviews with focus groups have revealed that the introduction of telehealth systems can result in perceptions that previously valued skills and expertise were now being undermined and replaced (154). Others have noted conflicts between those championing digital health technologies and hospital management and other HCPs who may be more resistant (153). Compounding these issues is the need to adapt existing technologies to a wide plethora of discrepant electronic health record systems, with each healthcare system or hospital having their own unique platforms (182).
Resolution of these conflicts will be necessary before full implementation of digital health in COPD is achieved. Moreover, prioritizing newer technologies obviously demands significant financial resources, which many healthcare systems may be too strapped at present to promise investment. Who would bear these financial responsibilities and how digital health services will be integrated into current medical billing systems to ensure fair compensation remains in question. Conflicting evidence in the literature that providing telehealthcare to patients with COPD may or may not in fact be very cost-effective is yet one more argument against the implementation of digital health solutions (183, 184). A recent 2024 systematic review of randomized controlled trials (184) found that in studies that conducted full economic analyses, the incremental cost-effectiveness ratio of interventions such as electronic patient diaries, real-time monitoring, and teleconsultations was between 3530.93€ to 286,369.28€/quality-adjusted life year. Real-time monitoring and teleconsultations appeared to be the most cost-effective interventions, while electronic patient diaries had a less consistent signal for cost-effectiveness. Nonetheless, study comparisons were hampered by the diversity in methods for cost evaluation and time period of assessment, as well as by many studies having only performed partial economic analyses that solely took into account the cost of the intervention rather than the consequences of the intervention. The authors also noted that most studies have been performed in high income countries, limiting generalizability to low and middle-income countries. These limitations demonstrate the need for further work on the cost-effectiveness question.
Maintaining these digital health systems and preventing them from cybersecurity threats (185) is not just an important factor in promoting digital health to HCPs, but also an issue that has been identified by patients as critical to their support of these new solutions (186). Both patients and providers must feel secure that data remain private and confidential in an age where cyberattacks against health care systems have become increasingly common, disruptive, and costly for all. This requires collaborative work between device and app developers, HCPs, and information and technology cybersecurity experts (185), as well as with regulatory bodies. Both the European Union and the U.S. Food and Drug Administration, for instance, have issued guidance on cybersecurity requirements for medical devices (187, 188). Despite these guidelines, though, developers must continually adapt to a rapidly changing security landscape and ensure that their technologies remain ahead of threats. Contingency plans for when data are compromised would certainly help to improve management of these unfortunate breaches. A separate but related issue would be the question of legal liability associated with these digital health solutions. Licensure and jurisdiction for telemedicine, informed patient consent, maintenance of quality control standards and records, data ownership rights, conflicts of interest, and malpractice have all been raised as legal issues that require concrete guidance in order for digital health solutions to be fully implemented (189–193).
Future directions for digital health in COPD
The barriers facing the digital health revolution are complex and unsolved at this moment and are certainly not unique to COPD. Large-scale interventions to healthcare systems require time, effort, cost, and significant cultural shifts. Marwaha et al. suggest nine key factors that all healthcare systems and providers should comprehensively consider prior to adopting digital health solutions: production selection, financial value, clinical value, data asset requirements, internal champions, executive sponsors, institutional priorities, implementation resources, and long-term operational strategies (194). Using this kind of scheme will help us to select and implement the most effective digital tools into our daily practice. Patients, health care providers, and health care systems will ultimately require far more rigorous research into the efficacy of these technologies. Do they substantially improve outcomes in metrics that are meaningful to patients living with COPD, such as quality of life, symptom burdens, hospitalizations, and mortality? Will they significantly reduce the enormous cost burden of COPD care that many health jurisdictions now face or will be facing in the coming decades? Answering these questions will certainly require more randomized controlled trials powered to clinically important outcomes with subsequent cost-effectiveness analyses. However, they will also require close partnerships between researchers and end-users, both at the patient and HCP levels. Novel technologies will not be successfully implemented without ongoing iterative input from these stakeholders to ensure they address their needs and technological capabilities.
In due time, we may face yet another pandemic where we will be required to treat patients remotely again. Also, as society ages, there will no doubt be an increasing number of patients who cannot commute to healthcare facilities, necessitating a wider adoption of telehealth solutions. Overcoming these current challenges will lead us a better healthcare system in which providers, patients, and digital health technologies can work together in harmony.
Author contributions
HYP: Data curation, Investigation, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing, Conceptualization, Formal analysis, Project administration. SK: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. ML: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. HR: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. YH: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. FL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. JML: Conceptualization, Data curation, Investigation, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. JML is supported by the Canada Research Chairs Program and the GlaxoSmithKline Professorship in COPD.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdgth.2025.1640585/full#supplementary-material
Abbreviations
AE-COPD, Acute exacerbation of chronic obstructive pulmonary disease; COPD, chronic obstructive pulmonary disease; eHealth, electronic health; FEV1, forced expiratory volume in 1s; FVC, forced vital capacity; HCP, health care providers; LABA, long-acting beta2-agonist; LAMA, long-acting muscarinic antagonist; MARS, Mobile App Rating Scale; mHealth, mobile health.
References
1. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease 2025 Report. Available online at: https://goldcopd.org/2025-gold-report/ (Accessed June 03, 2025).
2. Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. (2017) 389(10082):1931–40. doi: 10.1016/S0140-6736(17)31222-9
3. Boers E, Barrett M, Su JG, Benjafield AV, Sinha S, Kaye L, et al. Global burden of chronic obstructive pulmonary disease through 2050. JAMA Netw Open. (2023) 6(12):e2346598-e. doi: 10.1001/jamanetworkopen.2023.46598
4. Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. (2008) 32(4):962–9. doi: 10.1183/09031936.00012408
5. Cazzola M, Ora J, Calzetta L, Rogliani P, Matera MG. The future of inhalation therapy in chronic obstructive pulmonary disease. Curr Res Pharmacol Drug Discov. (2022) 3:100092. doi: 10.1016/j.crphar.2022.100092
6. Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. (1977) 1(6077):1645–8. doi: 10.1136/bmj.1.6077.1645
7. Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. (2004) 350(26):2645–53. doi: 10.1056/NEJMoa032158
8. Kim V, Aaron SD. What is a COPD exacerbation? Current definitions, pitfalls, challenges and opportunities for improvement. Eur Respir J. (2018) 52(5):1801261. doi: 10.1183/13993003.01261-2018
9. Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. (2009) 374(9691):733–43. doi: 10.1016/S0140-6736(09)61303-9
10. Sin DD, Doiron D, Agusti A, Anzueto A, Barnes PJ, Celli BR, et al. Air pollution and COPD: GOLD 2023 committee report. Eur Respir J. (2023) 61(5):2202469. doi: 10.1183/13993003.02469-2022
11. Fabbri LM, Rabe KF. From COPD to chronic systemic inflammatory syndrome? Lancet. (2007) 370(9589):797–9. doi: 10.1016/S0140-6736(07)61383-X
12. McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. (2015) 2015(2):Cd003793. doi: 10.1002/14651858.CD003793.pub3
13. Celli BR, Fabbri LM, Aaron SD, Agusti A, Brook R, Criner GJ, et al. An updated definition and severity classification of chronic obstructive pulmonary disease exacerbations: the Rome proposal. Am J Respir Crit Care Med. (2021) 204(11):1251–8. doi: 10.1164/rccm.202108-1819PP
14. Anzueto A. Impact of exacerbations on COPD. Eur Respir Rev. (2010) 19(116):113–8. doi: 10.1183/09059180.00002610
15. Hesse K, Bourke S, Steer J. Heart failure in patients with COPD exacerbations: looking below the tip of the iceberg. Respir Med. (2022) 196:106800. doi: 10.1016/j.rmed.2022.106800
16. Stolz D, Breidthardt T, Christ-Crain M, Bingisser R, Miedinger D, Leuppi J, et al. Use of B-type natriuretic peptide in the risk stratification of acute exacerbations of COPD. Chest. (2008) 133(5):1088–94. doi: 10.1378/chest.07-1959
17. Torres A, Blasi F, Dartois N, Akova M. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax. (2015) 70(10):984–9. doi: 10.1136/thoraxjnl-2015-206780
18. Couturaud F, Bertoletti L, Pastre J, Roy PM, Le Mao R, Gagnadoux F, et al. Prevalence of pulmonary embolism among patients with COPD hospitalized with acutely worsening respiratory symptoms. JAMA. (2021) 325(1):59–68. doi: 10.1001/jama.2020.23567
19. Jiménez D, Agustí A, Tabernero E, Jara-Palomares L, Hernando A, Ruiz-Artacho P, et al. Effect of a pulmonary embolism diagnostic strategy on clinical outcomes in patients hospitalized for COPD exacerbation: a randomized clinical trial. JAMA. (2021) 326(13):1277–85. doi: 10.1001/jama.2021.14846
20. Wedzicha JA, Calverley PMA, Albert RK, Anzueto A, Criner GJ, Hurst JR, et al. Prevention of COPD exacerbations: a European respiratory society/American thoracic society guideline. Eur Respir J. (2017) 50(3):1602265. doi: 10.1183/13993003.02265-2016
21. Rothnie KJ, Connell O, Müllerová H, Smeeth L, Pearce N, Douglas I, et al. Myocardial infarction and ischemic stroke after exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc. (2018) 15(8):935–46. doi: 10.1513/AnnalsATS.201710-815OC
22. Hoogendoorn M, Hoogenveen RT, Rutten-van Mölken MP, Vestbo J, Feenstra TL. Case fatality of COPD exacerbations: a meta-analysis and statistical modelling approach. Eur Respir J. (2011) 37(3):508–15. doi: 10.1183/09031936.00043710
23. Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. (2010) 363(12):1128–38. doi: 10.1056/NEJMoa0909883
24. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the medicare fee-for-service program. N Engl J Med. (2009) 360(14):1418–28. doi: 10.1056/NEJMsa0803563
25. Dransfield MT, Kunisaki KM, Strand MJ, Anzueto A, Bhatt SP, Bowler RP, et al. Acute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2017) 195(3):324–30. doi: 10.1164/rccm.201605-1014OC
26. Walters JA, Tan DJ, White CJ, Gibson PG, Wood-Baker R, Walters EH. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. (2014) 2014(9):Cd001288. doi: 10.1002/14651858.CD001288.pub4
27. Niewoehner DE, Erbland ML, Deupree RH, Collins D, Gross NJ, Light RW, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of veterans affairs cooperative study group. N Engl J Med. (1999) 340(25):1941–7. doi: 10.1056/NEJM199906243402502
28. Ram FS, Rodriguez-Roisin R, Granados-Navarrete A, Garcia-Aymerich J, Barnes NC. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. (2006) 2(2):Cd004403. doi: 10.1002/14651858.CD004403.pub2
29. Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA Jr, Criner GJ, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. (2011) 365(8):689–98. doi: 10.1056/NEJMoa1104623
30. Martinez FJ, Calverley PM, Goehring UM, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet. (2015) 385(9971):857–66. doi: 10.1016/S0140-6736(14)62410-7
31. Roberts MH, Mannino DM, Mapel DW, Lunacsek O, Amin S, Farrelly E, et al. Disease burden and health-related quality of life (HRQoL) of chronic obstructive pulmonary disease (COPD) in the US—evidence from the medical expenditure panel survey (MEPS) from 2016 to 2019. Int J Chron Obstruct Pulmon Dis. (2024) 19:1033–46. doi: 10.2147/COPD.S446696
32. Vogelmeier CF, Friedrich FW, Timpel P, Kossack N, Diesing J, Pignot M, et al. Impact of COPD on mortality: an 8-year observational retrospective healthcare claims database cohort study. Respir Med. (2024) 222:107506. doi: 10.1016/j.rmed.2023.107506
33. Aronson SH. The lancet on the telephone 1876–1975. Med Hist. (1977) 21(1):69–87. doi: 10.1017/S0025727300037182
34. Mair FS, Wilkinson M, Bonnar SA, Wootton R, Angus RM. The role of telecare in the management of exacerbations of chronic obstructive pulmonary disease in the home. J Telemed Telecare. (1999) 5(Suppl 1):S66–7. doi: 10.1258/1357633991932603
35. de Lusignan S, Althans A, Wells S, Johnson P, Vandenburg M, Robinson J. A pilot study of radiotelemetry for continuous cardiopulmonary monitoring of patients at home. J Telemed Telecare. (2000) 6(Suppl 1):S119–22. doi: 10.1258/1357633001934384
36. Johnston B, Wheeler L, Deuser J, Sousa KH. Outcomes of the kaiser permanente tele-home health research project. Arch Fam Med. (2000) 9(1):40–5. doi: 10.1001/archfami.9.1.40
37. Dale J, Connor S, Tolley K. An evaluation of the west surrey telemedicine monitoring project. J Telemed Telecare. (2003) 9(Suppl 1):S39–41. doi: 10.1258/135763303322196295
38. Koizumi T, Takizawa M, Nakai K, Yamamoto Y, Murase S, Fujii T, et al. Trial of remote telemedicine support for patients with chronic respiratory failure at home through a multistation communication system. Telemed J E Health. (2005) 11(4):481–6. doi: 10.1089/tmj.2005.11.481
39. Polisena J, Tran K, Cimon K, Hutton B, McGill S, Palmer K, et al. Home telehealth for chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Telemed Telecare. (2010) 16(3):120–7. doi: 10.1258/jtt.2009.090812
40. Vontetsianos T, Giovas P, Katsaras T, Rigopoulou A, Mpirmpa G, Giaboudakis P, et al. Telemedicine-assisted home support for patients with advanced chronic obstructive pulmonary disease: preliminary results after nine-month follow-up. J Telemed Telecare. (2005) 11(Suppl 1):86–8. doi: 10.1258/1357633054461697
41. Paré G, Sicotte C, St-Jules D, Gauthier R. Cost-minimization analysis of a telehomecare program for patients with chronic obstructive pulmonary disease. Telemed J E Health. (2006) 12(2):114–21. doi: 10.1089/tmj.2006.12.114
42. de Toledo P, Jiménez S, del Pozo F, Roca J, Alonso A, Hernandez C. Telemedicine experience for chronic care in COPD. IEEE Trans Inf Technol Biomed. (2006) 10(3):567–73. doi: 10.1109/TITB.2005.863877
43. Sorknaes AD, Madsen H, Hallas J, Jest P, Hansen-Nord M. Nurse tele-consultations with discharged COPD patients reduce early readmissions–an interventional study. Clin Respir J. (2011) 5(1):26–34. doi: 10.1111/j.1752-699X.2010.00187.x
44. McLean S, Nurmatov U, Liu JL, Pagliari C, Car J, Sheikh A. Telehealthcare for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. (2011) 2011(7):Cd007718. doi: 10.1002/14651858.CD007718.pub2
45. Mair FS, Goldstein P, May C, Angus R, Shiels C, Hibbert D, et al. Patient and provider perspectives on home telecare: preliminary results from a randomized controlled trial. J Telemed Telecare. (2005) 11(Suppl 1):95–7. doi: 10.1258/1357633054461976
46. Hibbert D, Mair FS, May CR, Boland A, O'Connor J, Capewell S, et al. Health professionals’ responses to the introduction of a home telehealth service. J Telemed Telecare. (2004) 10(4):226–30. doi: 10.1258/1357633041424386
47. Mair FS, Hiscock J, Beaton SC. Understanding factors that inhibit or promote the utilization of telecare in chronic lung disease. Chronic Illn. (2008) 4(2):110–7. doi: 10.1177/1742395308092482
48. Brewster L, Mountain G, Wessels B, Kelly C, Hawley M. Factors affecting front line staff acceptance of telehealth technologies: a mixed-method systematic review. J Adv Nurs. (2014) 70(1):21–33. doi: 10.1111/jan.12196
49. Lewis KE, Annandale JA, Warm DL, Hurlin C, Lewis MJ, Lewis L. Home telemonitoring and quality of life in stable, optimised chronic obstructive pulmonary disease. J Telemed Telecare. (2010) 16(5):253–9. doi: 10.1258/jtt.2009.090907
50. Whitten P, Mickus M. Home telecare for COPD/CHF patients: outcomes and perceptions. J Telemed Telecare. (2007) 13(2):69–73. doi: 10.1258/135763307780096249
51. Pépin JL, Degano B, Tamisier R, Viglino D. Remote monitoring for prediction and management of acute exacerbations in chronic obstructive pulmonary disease (AECOPD). Life (Basel). (2022) 12(4):499. doi: 10.3390/life12040499
52. Cooper CB, Sirichana W, Arnold MT, Neufeld EV, Taylor M, Wang X, et al. Remote patient monitoring for the detection of COPD exacerbations. Int J Chron Obstruct Pulmon Dis. (2020) 15:2005–13. doi: 10.2147/COPD.S256907
53. Orchard P, Agakova A, Pinnock H, Burton CD, Sarran C, Agakov F, et al. Improving prediction of risk of hospital admission in chronic obstructive pulmonary disease: application of machine learning to telemonitoring data. J Med Internet Res. (2018) 20(9):e263. doi: 10.2196/jmir.9227
54. Miłkowska-Dymanowska J, Białas AJ, Obrębski W, Górski P, Piotrowski WJ. A pilot study of daily telemonitoring to predict acute exacerbation in chronic obstructive pulmonary disease. Int J Med Inform. (2018) 116:46–51. doi: 10.1016/j.ijmedinf.2018.04.013
55. Shah SA, Velardo C, Farmer A, Tarassenko L. Exacerbations in chronic obstructive pulmonary disease: identification and prediction using a digital health system. J Med Internet Res. (2017) 19(3):e69. doi: 10.2196/jmir.7207
56. Al Rajeh AM, Hurst JR. Monitoring of physiological parameters to predict exacerbations of chronic obstructive pulmonary disease (COPD): a systematic review. J Clin Med. (2016) 5(12):108. doi: 10.3390/jcm5120108
57. Riis HC, Jensen MH, Cichosz SL, Hejlesen OK. Prediction of exacerbation onset in chronic obstructive pulmonary disease patients. J Med Eng Technol. (2016) 40(1):1–7. doi: 10.3109/03091902.2015.1105317
58. Mohktar MS, Redmond SJ, Antoniades NC, Rochford PD, Pretto JJ, Basilakis J, et al. Predicting the risk of exacerbation in patients with chronic obstructive pulmonary disease using home telehealth measurement data. Artif Intell Med. (2015) 63(1):51–9. doi: 10.1016/j.artmed.2014.12.003
59. Jensen MH, Cichosz SL, Dinesen B, Hejlesen OK. Moving prediction of exacerbation in chronic obstructive pulmonary disease for patients in telecare. J Telemed Telecare. (2012) 18(2):99–103. doi: 10.1258/jtt.2011.110607
60. Nohra RG, Chaaban T, Sacre H, Salameh P, Aoun Bacha Z, Le Bon Chami B, et al. Evaluating the feasibility and pretesting the impact of an educational and telemonitoring program for COPD patients in Lebanon. Int J Chron Obstruct Pulmon Dis. (2022) 17:949–65. doi: 10.2147/COPD.S339592
61. Trosini-Désert V, Lafoeste H, Regard L, Malrin R, Galarza-Jimenez MA, Amarilla CE, et al. A telemedicine intervention to ensure the correct usage of inhaler devices. Telemed J E Health. (2020) 26(11):1336–44. doi: 10.1089/tmj.2019.0246
62. Press VG, Arora VM, Kelly CA, Carey KA, White SR, Wan W. Effectiveness of virtual vs in-person inhaler education for hospitalized patients with obstructive lung disease: a randomized clinical trial. JAMA Netw Open. (2020) 3(1):e1918205. doi: 10.1001/jamanetworkopen.2019.18205
63. Locke ER, Thomas RM, Woo DM, Nguyen EHK, Tamanaha BK, Press VG, et al. Using video telehealth to facilitate inhaler training in rural patients with obstructive lung disease. Telemed J E Health. (2019) 25(3):230–6. doi: 10.1089/tmj.2017.0330
64. Thomas RM, Locke ER, Woo DM, Nguyen EHK, Press VG, Layouni TA, et al. Inhaler training delivered by internet-based home videoconferencing improves technique and quality of life. Respir Care. (2017) 62(11):1412–22. doi: 10.4187/respcare.05445
65. Hartman M, Mináriková J, Batalik L, Pepera G, Su JJ, Formiga MF, et al. Effects of home-based training with internet telehealth guidance in COPD patients entering pulmonary rehabilitation: a systematic review. Int J Chron Obstruct Pulmon Dis. (2023) 18:2305–19. doi: 10.2147/COPD.S425218
66. Hansen H, Torre A, Kallemose T, Ulrik CS, Godtfredsen NS. Pulmonary telerehabilitation vs. Conventional pulmonary rehabilitation—a secondary responder analysis. Thorax. (2023) 78(10):1039–42. doi: 10.1136/thorax-2023-220065
67. Zanaboni P, Dinesen B, Hoaas H, Wootton R, Burge AT, Philp R, et al. Long-term telerehabilitation or unsupervised training at home for patients with chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med. (2023) 207(7):865–75. doi: 10.1164/rccm.202204-0643OC
68. Hansen H, Bieler T, Beyer N, Kallemose T, Wilcke JT, Østergaard LM, et al. Supervised pulmonary tele-rehabilitation versus pulmonary rehabilitation in severe COPD: a randomised multicentre trial. Thorax. (2020) 75(5):413–21. doi: 10.1136/thoraxjnl-2019-214246
69. Vasilopoulou M, Papaioannou AI, Kaltsakas G, Louvaris Z, Chynkiamis N, Spetsioti S, et al. Home-based maintenance tele-rehabilitation reduces the risk for acute exacerbations of COPD, hospitalisations and emergency department visits. Eur Respir J. (2017) 49(5):1602129. doi: 10.1183/13993003.02129-2016
70. Bhatt SP, Patel SB, Anderson EM, Baugh D, Givens T, Schumann C, et al. Video telehealth pulmonary rehabilitation intervention in chronic obstructive pulmonary disease reduces 30-day readmissions. Am J Respir Crit Care Med. (2019) 200(4):511–3. doi: 10.1164/rccm.201902-0314LE
71. Schmidt HC, Christensen HM. Patient-assessed quality of virtual consultations as follow-up on long-term oxygen therapy for patients with COPD. Respir Care. (2023) 68(8):1097–105. doi: 10.4187/respcare.10575
72. Odeh B, Kayyali R, Nabhani-Gebara S, Philip N, Robinson P, Wallace CR. Evaluation of a telehealth service for COPD and HF patients: clinical outcome and patients’ perceptions. J Telemed Telecare. (2015) 21(5):292–7. doi: 10.1177/1357633X15574807
73. Gorst SL, Coates E, Armitage CJ. It’s sort of a lifeline": chronic obstructive pulmonary disease patients’ experiences of home telehealth. Health Psychol. (2016) 35(1):60–8. doi: 10.1037/hea0000246
74. Krag T, Jørgensen EH, Phanareth K, Kayser L. Experiences with in-person and virtual health care services for people with chronic obstructive pulmonary disease: qualitative study. JMIR Rehabil Assist Technol. (2023) 10:e43237. doi: 10.2196/43237
75. Nissen L, Lindhardt T. A qualitative study of COPD-patients’ experience of a telemedicine intervention. Int J Med Inform. (2017) 107:11–7. doi: 10.1016/j.ijmedinf.2017.08.004
76. Fitzsimmons DA, Thompson J, Bentley CL, Mountain GA. Comparison of patient perceptions of telehealth-supported and specialist nursing interventions for early stage COPD: a qualitative study. BMC Health Serv Res. (2016) 16(1):420. doi: 10.1186/s12913-016-1623-z
77. Lundell S, Modig M, Holmner Å, Wadell K. Perceptions of home telemonitoring use among patients with chronic obstructive pulmonary disease: qualitative study. JMIR Mhealth Uhealth. (2020) 8(6):e16343. doi: 10.2196/16343
78. Pinnock H, Hanley J, McCloughan L, Todd A, Krishan A, Lewis S, et al. Effectiveness of telemonitoring integrated into existing clinical services on hospital admission for exacerbation of chronic obstructive pulmonary disease: researcher blind, multicentre, randomised controlled trial. Br Med J. (2013) 347:f6070. doi: 10.1136/bmj.f6070
79. Chatwin M, Hawkins G, Panicchia L, Woods A, Hanak A, Lucas R, et al. Randomised crossover trial of telemonitoring in chronic respiratory patients (TeleCRAFT trial). Thorax. (2016) 71(4):305–11. doi: 10.1136/thoraxjnl-2015-207045
80. Ancochea J, García-Río F, Vázquez-Espinosa E, Hernando-Sanz A, López-Yepes L, Galera-Martínez R, et al. Efficacy and costs of telehealth for the management of COPD: the PROMETE II trial. Eur Respir J. (2018) 51(5):1800354. doi: 10.1183/13993003.00354-2018
81. Walker PP, Pompilio PP, Zanaboni P, Bergmo TS, Prikk K, Malinovschi A, et al. Telemonitoring in chronic obstructive pulmonary disease (CHROMED). A randomized clinical trial. Am J Respir Crit Care Med. (2018) 198(5):620–8. doi: 10.1164/rccm.201712-2404OC
82. Jódar-Sánchez F, Ortega F, Parra C, Gómez-Suárez C, Bonachela P, Leal S, et al. Cost-utility analysis of a telehealth programme for patients with severe chronic obstructive pulmonary disease treated with long-term oxygen therapy. J Telemed Telecare. (2014) 20(6):307–16. doi: 10.1177/1357633X14544421
83. Stoddart A, van der Pol M, Pinnock H, Hanley J, McCloughan L, Todd A, et al. Telemonitoring for chronic obstructive pulmonary disease: a cost and cost-utility analysis of a randomised controlled trial. J Telemed Telecare. (2015) 21(2):108–18. doi: 10.1177/1357633X14566574
84. Clarke M, Fursse J, Brown-Connolly NE, Sharma U, Jones R. Evaluation of the national health service (NHS) direct pilot telehealth program: cost-effectiveness analysis. Telemed J E Health. (2018) 24(1):67–76. doi: 10.1089/tmj.2016.0280
85. Esteban C, Antón A, Moraza J, Iriberri M, Larrauri M, Mar J, et al. Cost-effectiveness of a telemonitoring program (telEPOC program) in frequently admitted chronic obstructive pulmonary disease patients. J Telemed Telecare. (2021):1357633 × 211037207. doi: 10.1177/1357633X211037207
86. Hofer F, Achelrod D, Stargardt T. Cost-utility analysis of telemonitoring interventions for patients with chronic obstructive pulmonary disease (COPD) in Germany. Appl Health Econ Health Policy. (2016) 14(6):691–701. doi: 10.1007/s40258-016-0267-9
87. Witt Udsen F, Lilholt PH, Hejlesen OK, Ehlers LH. Subgroup analysis of telehealthcare for patients with chronic obstructive pulmonary disease: the cluster-randomized Danish Telecare North Trial. Clinicoecon Outcomes Res. (2017) 9:391–401. doi: 10.2147/CEOR.S139064
88. Ney JP, Robinson SA, Richardson CR, Moy ML. Can technology-based physical activity programs for chronic obstructive pulmonary disease be cost-effective? Telemed J E Health. (2021) 27(11):1288–92. doi: 10.1089/tmj.2020.0398
89. Hofer F, Schreyögg J, Stargardt T. Effectiveness of a home telemonitoring program for patients with chronic obstructive pulmonary disease in Germany: evidence from the first three years. PLoS One. (2022) 17(5):e0267952. doi: 10.1371/journal.pone.0267952
90. Janjua S, Carter D, Threapleton CJ, Prigmore S, Disler RT. Telehealth interventions: remote monitoring and consultations for people with chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. (2021) 7(7):Cd013196. doi: 10.1002/14651858.CD013196.pub2
91. Puhan MA, Gimeno-Santos E, Cates CJ, Troosters T. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. (2016) 12(12):Cd005305. doi: 10.1002/14651858.CD005305.pub4
92. Lindenauer PK, Stefan MS, Pekow PS, Mazor KM, Priya A, Spitzer KA, et al. Association between initiation of pulmonary rehabilitation after hospitalization for COPD and 1-year survival among medicare beneficiaries. JAMA. (2020) 323(18):1813–23. doi: 10.1001/jama.2020.4437
93. Kahn PA, Mathis WS. Accessibility of pulmonary rehabilitation in the US. JAMA Netw Open. (2024) 7(2):e2354867. doi: 10.1001/jamanetworkopen.2023.54867
94. Malla G, Bodduluri S, Sthanam V, Sharma G, Bhatt SP. Access to pulmonary rehabilitation among medicare beneficiaries with chronic obstructive pulmonary disease. Ann Am Thorac Soc. (2023) 20(4):516–22. doi: 10.1513/AnnalsATS.202204-318OC
95. Bhatt SP, Westra J, Kuo YF, Sharma G. Pulmonary rehabilitation utilization in older adults with chronic obstructive pulmonary disease, 2013 to 2019. Ann Am Thorac Soc. (2024) 21(5):740–7. doi: 10.1513/AnnalsATS.202307-601OC
96. Demeyer H, Louvaris Z, Frei A, Rabinovich RA, de Jong C, Gimeno-Santos E, et al. Physical activity is increased by a 12-week semiautomated telecoaching programme in patients with COPD: a multicentre randomised controlled trial. Thorax. (2017) 72(5):415–23. doi: 10.1136/thoraxjnl-2016-209026
97. Alwakeel AJ, Sicondolfo A, Robitaille C, Bourbeau J, Saad N. The accessibility, feasibility, and safety of a standardized community-based tele-pulmonary rehab program for chronic obstructive pulmonary disease: a 3-year real-world prospective study. Ann Am Thorac Soc. (2022) 19(1):39–47. doi: 10.1513/AnnalsATS.202006-638OC
98. Cox NS, Dal Corso S, Hansen H, McDonald CF, Hill CJ, Zanaboni P, et al. Telerehabilitation for chronic respiratory disease. Cochrane Database Syst Rev. (2021) 1(1):Cd013040. doi: 10.1002/14651858.CD013040.pub2
99. Michaelchuk W, Oliveira A, Marzolini S, Nonoyama M, Maybank A, Goldstein R, et al. Design and delivery of home-based telehealth pulmonary rehabilitation programs in COPD: a systematic review and meta-analysis. Int J Med Inform. (2022) 162:104754. doi: 10.1016/j.ijmedinf.2022.104754
100. Pinnock H, Murphie P, Vogiatzis I, Poberezhets V. Telemedicine and virtual respiratory care in the era of COVID-19. ERJ Open Res. (2022) 8(3):00111-2022. doi: 10.1183/23120541.00111-2022
101. Boyce DM, Thomashow BM, Sullivan J, Tal-Singer R. New adopters of telemedicine during the coronavirus-19 pandemic in respondents to an online community survey: the case for access to remote management tools for individuals with chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis. (2021) 8(2):213–8. doi: 10.15326/jcopdf.2020.0181
102. Wu F, Burt J, Chowdhury T, Fitzpatrick R, Martin G, van der Scheer JW, et al. Specialty COPD care during COVID-19: patient and clinician perspectives on remote delivery. BMJ Open Respir Res. (2021) 8(1):e000817. doi: 10.1136/bmjresp-2020-000817
103. Madawala S, Quach A, Lim JY, Varatharaj S, Perera B, Osadnik C, et al. Healthcare experience of adults with COPD during the COVID-19 pandemic: a rapid review of international literature. BMJ Open Respir Res. (2023) 10(1):e001514. doi: 10.1136/bmjresp-2022-001514
104. Volpato E, Centanni S, Banfi P, D'Antonio S, Peterle E, Bugliaro F, et al. Narrative analysis of the impact of COVID-19 on patients with chronic obstructive pulmonary disease, their caregivers, and healthcare professionals in Italy. Int J Chron Obstruct Pulmon Dis. (2021) 16:2181–201. doi: 10.2147/COPD.S312372
105. Wilde LJ, Percy C, Clark C, Ward G, Wark PA, Sewell L. Views and experiences of healthcare practitioners supporting people with COPD who have used activity monitors: “More than just steps”. Respir Med. (2023) 218:107395. doi: 10.1016/j.rmed.2023.107395
106. Buekers J, Arbillaga-Etxarri A, Gimeno-Santos E, Donaire-Gonzalez D, Chevance G, Aerts J-M, et al. Heart rate and oxygen uptake kinetics obtained from continuous measurements with wearable devices during outdoor walks of patients with COPD. Digit Health. (2023) 9:20552076231162989. doi: 10.1177/20552076231162989
107. Michaelchuk W, Colella TJ, Goldstein RS, Brooks D. Wearable device for sedentary behavior change in chronic obstructive pulmonary disease is feasible and acceptable. Can J Respir Crit Care Sleep Med. (2023) 7(2):79–85. doi: 10.1080/24745332.2023.2177213
108. Støve MP, Graversen AH, Sørensen J. Assessment of noninvasive oxygen saturation in patients with COPD during pulmonary rehabilitation: smartwatch versus pulse oximeter. Respir Care. (2023) 68(8):1041–8. doi: 10.4187/respcare.10760
109. Althobiani MA, Khan B, Shah AJ, Ranjan Y, Mendes RG, Folarin A, et al. Clinicians’ perspectives of wearable technology to detect and monitor exacerbations of chronic obstructive pulmonary disease: mixed-method survey. Int J Chron Obstruct Pulmon Dis. (2023) 18:1401–12. doi: 10.2147/COPD.S405386
110. Pipek LZ, Nascimento RFV, Acencio MMP, Teixeira LR. Comparison of SpO2 and heart rate values on apple watch and conventional commercial oximeters devices in patients with lung disease. Sci Rep. (2021) 11(1):18901. doi: 10.1038/s41598-021-98453-3
111. Lippi L, Turco A, Folli A, D’Abrosca F, Curci C, Mezian K, et al. Technological advances and digital solutions to improve quality of life in older adults with chronic obstructive pulmonary disease: a systematic review. Aging Clin Exp Res. (2023) 35(5):953–68. doi: 10.1007/s40520-023-02381-3
112. Tiwari A, Liaqat S, Liaqat D, Gabel M, de Lara E, Falk TH, editors. Remote copd severity and exacerbation detection using heart rate and activity data measured from a wearable device. 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC). IEEE (2021).
113. Blondeel A, Demeyer H, Janssens W, Troosters T. Accuracy of consumer-based activity trackers as measuring tool and coaching device in patients with COPD and healthy controls. PLoS One. (2020) 15(8):e0236676. doi: 10.1371/journal.pone.0236676
114. Buekers J, Theunis J, De Boever P, Vaes AW, Koopman M, Janssen EV, et al. Wearable finger pulse oximetry for continuous oxygen saturation measurements during daily home routines of patients with chronic obstructive pulmonary disease (COPD) over one week: observational study. JMIR Mhealth Uhealth. (2019) 7(6):e12866. doi: 10.2196/12866
115. Lin W-Y, Verma VK, Lee M-Y, Lin H-C, Lai C-S. Prediction of 30-day readmission for COPD patients using accelerometer-based activity monitoring. Sensors. (2019) 20(1):217. doi: 10.3390/s20010217
116. Rubio N, Parker RA, Drost EM, Pinnock H, Weir CJ, Hanley J, et al. Home monitoring of breathing rate in people with chronic obstructive pulmonary disease: observational study of feasibility, acceptability, and change after exacerbation. Int J Chron Obstruct Pulmon Dis. (2017) 12:1221–31. doi: 10.2147/COPD.S120706
117. Wu R, Liaqat D, de Lara E, Son T, Rudzicz F, Alshaer H, et al. Feasibility of using a smartwatch to intensively monitor patients with chronic obstructive pulmonary disease: prospective cohort study. JMIR Mhealth Uhealth. (2018) 6(6):e10046. doi: 10.2196/10046
118. Orme MW, Weedon AE, Saukko PM, Esliger DW, Morgan MD, Steiner MC, et al. Findings of the chronic obstructive pulmonary disease-sitting and exacerbations trial (COPD-SEAT) in reducing sedentary time using wearable and mobile technologies with educational support: randomized controlled feasibility trial. JMIR Mhealth Uhealth. (2018) 6(4):e9398. doi: 10.2196/mhealth.9398
119. Fan KG, Mandel J, Agnihotri P, Tai-Seale M. Remote patient monitoring technologies for predicting chronic obstructive pulmonary disease exacerbations: review and comparison. JMIR Mhealth Uhealth. (2020) 8(5):e16147. doi: 10.2196/16147
120. Chan C, Inskip J, Kirkham A, Ansermino J, Dumont G, Li L, et al. A smartphone oximeter with a fingertip probe for use during exercise training: usability, validity and reliability in individuals with chronic lung disease and healthy controls. Physiotherapy. (2019) 105(3):297–306. doi: 10.1016/j.physio.2018.07.015
121. Mehdipour A, Wiley E, Richardson J, Beauchamp M, Kuspinar A. The performance of digital monitoring devices for oxygen saturation and respiratory rate in COPD: a systematic review. COPD. (2021) 18(4):469–75. doi: 10.1080/15412555.2021.1945021
122. Bentley CL, Powell L, Potter S, Parker J, Mountain GA, Bartlett YK, et al. The use of a smartphone app and an activity tracker to promote physical activity in the management of chronic obstructive pulmonary disease: randomized controlled feasibility study. JMIR Mhealth Uhealth. (2020) 8(6):e16203. doi: 10.2196/16203
123. Ward S, Orme M, Zatloukal J, Singh S. Adherence to walking exercise prescription during pulmonary rehabilitation in COPD with a commercial activity monitor: a feasibility trial. BMC Pulm Med. (2021) 21:1–9. doi: 10.1186/s12890-021-01406-9
124. Stamenova V, Liang K, Yang R, Engel K, van Lieshout F, Lalingo E, et al. Technology-enabled self-management of chronic obstructive pulmonary disease with or without asynchronous remote monitoring: randomized controlled trial. J Med Internet Res. (2020) 22(7):e18598. doi: 10.2196/18598
125. Wu C-T, Li G-H, Huang C-T, Cheng Y-C, Chen C-H, Chien J-Y, et al. Acute exacerbation of a chronic obstructive pulmonary disease prediction system using wearable device data, machine learning, and deep learning: development and cohort study. JMIR Mhealth Uhealth. (2021) 9(5):e22591. doi: 10.2196/22591
126. Wilde LJ, Sewell L, Percy C, Ward G, Clark C. What are the experiences of people with COPD using activity monitors?: a qualitative scoping review. COPD. (2022) 19(1):88–98. doi: 10.1080/15412555.2022.2033192
127. Wang CH, Chou PC, Joa WC, Chen LF, Sheng TF, Ho SC, et al. Mobile-phone-based home exercise training program decreases systemic inflammation in COPD: a pilot study. BMC Pulm Med. (2014) 14:142. doi: 10.1186/1471-2466-14-142
128. van der Weegen S, Verwey R, Spreeuwenberg M, Tange H, van der Weijden T, de Witte L. It’s LiFe! Mobile and web-based monitoring and feedback tool embedded in primary care increases physical activity: a cluster randomized controlled trial. J Med Internet Res. (2015) 17(7):e184. doi: 10.2196/jmir.4579
129. Vorrink S, Huisman C, Kort H, Troosters T, Lammers JW. Perceptions of patients with chronic obstructive pulmonary disease and their physiotherapists regarding the use of an eHealth intervention. JMIR Hum Factors. (2017) 4(3):e20. doi: 10.2196/humanfactors.7196
130. Farmer A, Williams V, Velardo C, Shah SA, Yu LM, Rutter H, et al. Self-management support using a digital health system compared with usual care for chronic obstructive pulmonary disease: randomized controlled trial. J Med Internet Res. (2017) 19(5):e144. doi: 10.2196/jmir.7116
131. Yang F, Wang Y, Yang C, Hu H, Xiong Z. Mobile health applications in self-management of patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis of their efficacy. BMC Pulm Med. (2018) 18(1):147. doi: 10.1186/s12890-018-0671-z
132. McCabe C, McCann M, Brady AM. Computer and mobile technology interventions for self-management in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. (2017) 5(5):Cd011425. doi: 10.1002/14651858.CD011425.pub2
133. Hardinge M, Rutter H, Velardo C, Shah SA, Williams V, Tarassenko L, et al. Using a mobile health application to support self-management in chronic obstructive pulmonary disease: a six-month cohort study. BMC Med Inform Decis Mak. (2015) 15:46. doi: 10.1186/s12911-015-0171-5
134. Rodriguez Hermosa JL, Fuster Gomila A, Puente Maestu L, Amado Diago CA, Callejas González FJ, Malo De Molina Ruiz R, et al. Compliance and utility of a smartphone app for the detection of exacerbations in patients with chronic obstructive pulmonary disease: cohort study. JMIR Mhealth Uhealth. (2020) 8(3):e15699. doi: 10.2196/15699
135. Criner GJ, Cole T, Hahn KA, Kastango K, Eudicone JM, Gilbert I. Use of a digital chronic obstructive pulmonary disease respiratory tracker in a primary care setting: a feasibility study. Pulm Ther. (2021) 7(2):533–47. doi: 10.1007/s41030-021-00168-3
136. Rodríguez Hermosa JL, Fuster Gomila A, Puente Maestu L, Amado Diago CA, Callejas González FJ, Malo De Molina Ruiz R, et al. Assessing the usefulness of the prevexair smartphone application in the follow-up high-risk patients with COPD. Int J Chron Obstruct Pulmon Dis. (2021) 16:53–65. doi: 10.2147/COPD.S279394
137. van Buul AR, Derksen C, Hoedemaker O, van Dijk O, Chavannes NH, Kasteleyn MJ. Ehealth program to reduce hospitalizations due to acute exacerbation of chronic obstructive pulmonary disease: retrospective study. JMIR Form Res. (2021) 5(3):e24726. doi: 10.2196/24726
138. Chmiel FP, Burns DK, Pickering JB, Blythin A, Wilkinson TM, Boniface MJ. Prediction of chronic obstructive pulmonary disease exacerbation events by using patient self-reported data in a digital health app: statistical evaluation and machine learning approach. JMIR Med Inform. (2022) 10(3):e26499. doi: 10.2196/26499
139. Spielmanns M, Gloeckl R, Jarosch I, Leitl D, Schneeberger T, Boeselt T, et al. Using a smartphone application maintains physical activity following pulmonary rehabilitation in patients with COPD: a randomised controlled trial. Thorax. (2023) 78(5):442–50. doi: 10.1136/thoraxjnl-2021-218338
140. Wang L, Guo Y, Wang M, Zhao Y. A mobile health application to support self-management in patients with chronic obstructive pulmonary disease: a randomised controlled trial. Clin Rehabil. (2021) 35(1):90–101. doi: 10.1177/0269215520946931
141. Park SK, Bang CH, Lee SH. Evaluating the effect of a smartphone app-based self-management program for people with COPD: a randomized controlled trial. Appl Nurs Res. (2020) 52:151231. doi: 10.1016/j.apnr.2020.151231
142. Alharbey R, Chatterjee S. An mHealth assistive system “MyLung” to empower patients with chronic obstructive pulmonary disease: design science research. JMIR Form Res. (2019) 3(1):e12489. doi: 10.2196/12489
143. Kooij L, Vos PJE, Dijkstra A, van Harten WH. Effectiveness of a mobile health and self-management app for high-risk patients with chronic obstructive pulmonary disease in daily clinical practice: mixed methods evaluation study. JMIR Mhealth Uhealth. (2021) 9(2):e21977. doi: 10.2196/21977
144. Glynn L, Mc Cann M, Mc Cabe C. Smartphone applications supporting self-management programme for adults with chronic obstructive pulmonary disease: a scoping review. PLOS Digit Health. (2024) 3(6):e0000532. doi: 10.1371/journal.pdig.0000532
145. Wu RC, Ginsburg S, Son T, Gershon AS. Using wearables and self-management apps in patients with COPD: a qualitative study. ERJ Open Res. (2019) 5(3):00036-2019. doi: 10.1183/23120541.00036-2019
146. Quach S, Benoit A, Oliveira A, Packham TL, Goldstein R, Brooks D. Features and characteristics of publicly available mHealth apps for self-management in chronic obstructive pulmonary disease. Digit Health. (2023) 9:20552076231167007. doi: 10.1177/20552076231167007
147. Korpershoek YJG, Hermsen S, Schoonhoven L, Schuurmans MJ, Trappenburg JCA. User-centered design of a mobile health intervention to enhance exacerbation-related self-management in patients with chronic obstructive pulmonary disease (copilot): mixed methods study. J Med Internet Res. (2020) 22(6):e15449. doi: 10.2196/15449
148. Davies A, Mueller J, Hennings J, Caress AL, Jay C. Recommendations for developing support tools with people suffering from chronic obstructive pulmonary disease: co-design and pilot testing of a Mobile health prototype. JMIR Hum Factors. (2020) 7(2):e16289. doi: 10.2196/16289
149. Owens OL, Beer JM, Reyes LI, Gallerani DG, Myhren-Bennett AR, McDonnell KK. Mindfulness-based symptom and stress management apps for adults with chronic lung disease: systematic search in app stores. JMIR Mhealth Uhealth. (2018) 6(5):e124. doi: 10.2196/mhealth.9831
150. Hee Ko KK, Kim SK, Lee Y, Lee JY, Stoyanov SR. Validation of a Korean version of mobile app rating scale (MARS) for apps targeting disease management. Health Informatics J. (2022) 28(2):14604582221091975. doi: 10.1177/14604582221091975
151. Alwashmi MF, Fitzpatrick B, Davis E, Gamble JM, Farrell J, Hawboldt J. Perceptions of health care providers regarding a mobile health intervention to manage chronic obstructive pulmonary disease: qualitative study. JMIR Mhealth Uhealth. (2019) 7(6):e13950. doi: 10.2196/13950
152. Robinson SA, Shimada SL, Sliwinski SK, Wiener RS, Moy ML. Stakeholder perceptions of a web-based physical activity intervention for COPD: a mixed-methods study. J Clin Med. (2023) 12(19):6296. doi: 10.3390/jcm12196296
153. Damhus CS, Emme C, Hansen H. Barriers and enablers of COPD telerehabilitation—a frontline staff perspective. Int J Chron Obstruct Pulmon Dis. (2018) 13:2473–82. doi: 10.2147/COPD.S167501
154. Sharma U, Clarke M. Nurses’ and community support workers’ experience of telehealth: a longitudinal case study. BMC Health Serv Res. (2014) 14:164. doi: 10.1186/1472-6963-14-164
155. Slevin P, Kessie T, Cullen J, Butler MW, Donnelly SC, Caulfield B. Exploring the barriers and facilitators for the use of digital health technologies for the management of COPD: a qualitative study of clinician perceptions. QJM. (2020) 113(3):163–72. doi: 10.1093/qjmed/hcz241
156. Blakey JD, Bender BG, Dima AL, Weinman J, Safioti G, Costello RW. Digital technologies and adherence in respiratory diseases: the road ahead. Eur Respir J. (2018) 52(5):1801147. doi: 10.1183/13993003.01147-2018
157. Ramachandran HJ, Oh JL, Cheong YK, Jiang Y, Teo JYC, Seah CWA, et al. Barriers and facilitators to the adoption of digital health interventions for COPD management: a scoping review. Heart Lung. (2023) 59:117–27. doi: 10.1016/j.hrtlng.2023.02.004
158. Al Rajeh A, Steiner MC, Aldabayan Y, Aldhahir A, Pickett E, Quaderi S, et al. Use, utility and methods of telehealth for patients with COPD in England and Wales: a healthcare provider survey. BMJ Open Respir Res. (2019) 6(1):e000345. doi: 10.1136/bmjresp-2018-000345
159. van Lieshout F, Yang R, Stamenova V, Agarwal P, Cornejo Palma D, Sidhu A, et al. Evaluating the implementation of a remote-monitoring program for chronic obstructive pulmonary disease: qualitative methods from a service design perspective. J Med Internet Res. (2020) 22(10):e18148. doi: 10.2196/18148
160. Ratzen SC, Parker RM. Introduction. In: Selden CR, Zorn M, Ratzan SC, Parker RM, editors. National Library of Medicine Current Bibliographies in Medicine: Health Literacy. Bethesda, MD: National Institutes of Health, U.S. Departmennt of Health and Human Services (2000). p. 5–7.
161. Roberts NJ, Ghiassi R, Partridge MR. Health literacy in COPD. Int J Chron Obstruct Pulmon Dis. (2008) 3(4):499–507. doi: 10.2147/copd.s1088
162. Yadav UN, Lloyd J, Hosseinzadeh H, Baral KP, Bhatta N, Harris MF. Levels and determinants of health literacy and patient activation among multi-morbid COPD people in rural Nepal: findings from a cross-sectional study. PLoS One. (2020) 15(5):e0233488. doi: 10.1371/journal.pone.0233488
163. Azkan Ture D, Bhattacharya S, Demirci H, Yildiz T. Health literacy and health outcomes in chronic obstructive pulmonary disease patients: an explorative study. Front Public Health. (2022) 10:846768. doi: 10.3389/fpubh.2022.846768
164. Paasche-Orlow MK, Parker RM, Gazmararian JA, Nielsen-Bohlman LT, Rudd RR. The prevalence of limited health literacy. J Gen Intern Med. (2005) 20(2):175–84. doi: 10.1111/j.1525-1497.2005.40245.x
165. Nutbeam D. The evolving concept of health literacy. Soc Sci Med. (2008) 67(12):2072–8. doi: 10.1016/j.socscimed.2008.09.050
166. Norman CD, Skinner HA. eHEALS: the eHealth literacy scale. J Med Internet Res. (2006) 8(4):e27. doi: 10.2196/jmir.8.4.e27
167. Shiferaw KB, Tilahun BC, Endehabtu BF, Gullslett MK, Mengiste SA. E-health literacy and associated factors among chronic patients in a low-income country: a cross-sectional survey. BMC Med Inform Decis Mak. (2020) 20(1):181. doi: 10.1186/s12911-020-01202-1
168. Milanti A, Chan DNS, Parut AA, So WKW. Determinants and outcomes of eHealth literacy in healthy adults: a systematic review. PLoS One. (2023) 18(10):e0291229. doi: 10.1371/journal.pone.0291229
169. Melzer AC, Hagedorn H, Nelson D, Kaplan A, Campbell M, Fu SS. Use of information and communication technology among patients with chronic obstructive pulmonary disease who smoke: a mixed methods study. Ann Am Thorac Soc. (2023) 20(3):381–9. doi: 10.1513/AnnalsATS.202208-740OC
170. Stellefson ML, Shuster JJ, Chaney BH, Paige SR, Alber JM, Chaney JD, et al. Web-based health information seeking and eHealth literacy among patients living with chronic obstructive pulmonary disease (COPD). Health Commun. (2018) 33(12):1410–24. doi: 10.1080/10410236.2017.1353868
171. Jiang Y, Gao J, Sun P, Nan J, Zou X, Sun M, et al. Factors associated with the e-health literacy among older adults with chronic obstructive pulmonary disease: a cross-sectional study. Telemed J E Health. (2024) 30(4):e1138–e47. doi: 10.1089/tmj.2023.0394
172. Witry M, Comellas A, Simmering J, Polgreen P. The association between technology use and health Status in a chronic obstructive pulmonary disease cohort: multi-method study. J Med Internet Res. (2018) 20(4):e125. doi: 10.2196/jmir.9382
173. Alwashmi MF, Fitzpatrick B, Farrell J, Gamble JM, Davis E, Nguyen HV, et al. Perceptions of patients regarding mobile health interventions for the management of chronic obstructive pulmonary disease: mixed methods study. JMIR Mhealth Uhealth. (2020) 8(7):e17409. doi: 10.2196/17409
174. Jiang Y, Sun P, Chen Z, Guo J, Wang S, Liu F, et al. Patients’ and healthcare providers’ perceptions and experiences of telehealth use and online health information use in chronic disease management for older patients with chronic obstructive pulmonary disease: a qualitative study. BMC Geriatr. (2022) 22(1):9. doi: 10.1186/s12877-021-02702-z
175. Chien SY. Mobile app for patients with chronic obstructive pulmonary diseases during home-based exercise care: usability study. JMIR Hum Factors. (2024) 11:e60049. doi: 10.2196/60049
176. Vatnøy TK, Thygesen E, Dale B. Telemedicine to support coping resources in home-living patients diagnosed with chronic obstructive pulmonary disease: patients’ experiences. J Telemed Telecare. (2017) 23(1):126–32. doi: 10.1177/1357633X15626854
177. Naranjo-Rojas A, Perula-de Torres L, Cruz-Mosquera FE, Molina-Recio G. Usability of a mobile application for the clinical follow-up of patients with chronic obstructive pulmonary disease and home oxygen therapy. Int J Med Inform. (2023) 175:105089. doi: 10.1016/j.ijmedinf.2023.105089
178. United States Public Health Service Office of the Surgeon General. Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Service. Washington: U.S. Department of Health, Education, and Welfare (1964). Contract No.: PHS Publication No. 1103.
179. Smaradottir B, Gerdes M, Fensli R, Martinez S. Usability evaluation of a COPD remote monitoring application. Stud Health Technol Inform. (2015) 210:845–9.25991274
180. Hoaas H, Andreassen HK, Lien LA, Hjalmarsen A, Zanaboni P. Adherence and factors affecting satisfaction in long-term telerehabilitation for patients with chronic obstructive pulmonary disease: a mixed methods study. BMC Med Inform Decis Mak. (2016) 16:26. doi: 10.1186/s12911-016-0264-9
181. Korpershoek YJ, Holtrop T, Vervoort SC, Schoonhoven L, Schuurmans MJ, Trappenburg JC. Early-stage feasibility of a mobile health intervention (Copilot) to enhance exacerbation-related self-management in patients with chronic obstructive pulmonary disease: multimethods approach. JMIR Form Res. (2020) 4(11):e21577. doi: 10.2196/21577
182. Adler-Milstein J, Holmgren AJ, Kralovec P, Worzala C, Searcy T, Patel V. Electronic health record adoption in US hospitals: the emergence of a digital “advanced use” divide. J Am Med Inform Assoc. (2017) 24(6):1142–8. doi: 10.1093/jamia/ocx080
183. Witt Udsen F, Lilholt PH, Hejlesen O, Ehlers L. Cost-effectiveness of telehealthcare to patients with chronic obstructive pulmonary disease: results from the Danish ‘TeleCare north’ cluster-randomised trial. BMJ Open. (2017) 7(5):e014616. doi: 10.1136/bmjopen-2016-014616
184. Ferreira MAM, Dos Santos AF, Sousa-Pinto B, Taborda-Barata L. Cost-effectiveness of digital health interventions for asthma or COPD: systematic review. Clin Exp Allergy. (2024) 54(9):651–68. doi: 10.1111/cea.14547
185. Williams PA, Woodward AJ. Cybersecurity vulnerabilities in medical devices: a complex environment and multifaceted problem. Med Devices (Auckl). (2015) 8:305–16. doi: 10.2147/MDER.S50048
186. Houser SH, Flite CA, Foster SL. Privacy and security risk factors related to telehealth services—a systematic review. Perspect Health Inf Manag. (2023) 20(1):1f.37215337
187. U.S. Food & Drug Administration. Cybersecurity in Medical Devices: Quality System Considerations and Content of Premarket Submissions. Rockvile, MD: Department of Health and Human Services (2023). p. 1–64.
188. Biasin E, Yaşar B, Kamenjašević E. New cybersecurity requirements for medical devices in the EU: the forthcoming European health data space, data act, and artificial intelligence act. Law Technol Humans. (2023) 5(2):43–58. doi: 10.5204/lthj.3068
189. Mariño RJ, Zaror C. Legal issues in digital oral health: a scoping review. BMC Health Serv Res. (2024) 24(1):6. doi: 10.1186/s12913-023-10476-w
190. Cordeiro JV. Digital technologies and data science as health enablers: an outline of appealing promises and compelling ethical, legal, and social challenges. Front Med (Lausanne). (2021) 8:647897. doi: 10.3389/fmed.2021.647897
191. Goldstein SP, Nebeker C, Ellis RB, Oser M. Ethical, legal, and social implications of digital health: a needs assessment from the society of behavioral medicine to inform capacity building for behavioral scientists. Transl Behav Med. (2024) 14(3):189–96. doi: 10.1093/tbm/ibad076
192. Fields BG. Regulatory, legal, and ethical considerations of telemedicine. Sleep Med Clin. (2020) 15(3):409–16. doi: 10.1016/j.jsmc.2020.06.004
193. Becker CD, Dandy K, Gaujean M, Fusaro M, Scurlock C. Legal perspectives on telemedicine part 1: legal and regulatory issues. Perm J. (2019) 23:18–293. doi: 10.7812/TPP/18-293
Keywords: COPD, telehealth, digital health, apps, wearable devices
Citation: Park HY, Kong S, Lee M, Ryu H, Hamakawa Y, Luppi F and Leung JM (2025) Digital health technologies for improving the management of people with chronic obstructive pulmonary disease. Front. Digit. Health 7:1640585. doi: 10.3389/fdgth.2025.1640585
Received: 3 June 2025; Accepted: 21 July 2025;
Published: 6 August 2025.
Edited by:
Guido Iaccarino, Federico II University Hospital, ItalyReviewed by:
Vineet Mishra, HCL Corporation Pvt Ltd, IndiaUmut Elmas, University of Naples Federico II, Italy
Copyright: © 2025 Park, Kong, Lee, Ryu, Hamakawa, Luppi and Leung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Janice M. Leung, SmFuaWNlLkxldW5nQGhsaS51YmMuY2E=
 Hye Yun Park
Hye Yun Park Sunga Kong
Sunga Kong Mangyeong Lee
Mangyeong Lee Hyein Ryu
Hyein Ryu Yoko Hamakawa5,6
Yoko Hamakawa5,6 Fabrizio Luppi
Fabrizio Luppi Janice M. Leung
Janice M. Leung