- 1Institute for Medical Information Processing, Biometry, and Epidemiology, Ludwig-Maximilians-Universität München, Munich, Germany
- 2Institute of Ethics, History and Theory of Medicine, Ludwig-Maximilians-Universität München, Munich, Germany
Background: Patient preferences are a critical component of shared decision-making (SDM), particularly when choosing between treatment options with differing risks and outcomes. Many methods exist to elicit these preferences, but their complexity, usability, and acceptance vary.
Objective: We aim to gain insight into the acceptance, effort and preferences of participants regarding five different methods of preference assessment. Additionally, we investigate the influence of health status, experiences within the health system and of demographic factors on the results.
Methods: We conducted a cross-sectional online survey including five preference elicitation Methods: best-worst scaling, direct weighting, PAPRIKA (Potentially All Pairwise Rankings of all Possible Alternatives), time trade-off, and standard gamble. The questionnaire was distributed via academic and patient advocacy mailing lists, reaching both healthy individuals and those with acute or chronic illnesses. Participants rated each method using six standardized statements on a 5-point Likert scale. Additional items assessed general acceptance of algorithm-assisted preference assessments and the clarity of the questionnaire.
Results: Of 258 initiated questionnaires, 123 (48%) were completed and included in the analysis. Participants were diverse in age, gender, and health status, but predominantly highly educated and digitally literate. Across all measures, the PAPRIKA method received the highest ratings for clarity, usability, and perceived ability to express preferences. Simpler methods (best-worst scaling, direct weighting) were rated as less useful for capturing nuanced preferences, while abstract utility-based methods (standard gamble, time trade-off) were seen as cognitively demanding. Subgroup analyses showed minimal variation across demographic groups. Most participants (82%) could imagine using at least one of the presented methods in real clinical settings, but also emphasized the importance of physician involvement in interpreting results.
Conclusion: The interactive PAPRIKA method best balanced cognitive demand and expressiveness and was preferred by most participants. Structured methods for preference elicitation may enhance SDM when integrated into clinical workflows and supported by healthcare professionals. Further research is needed to evaluate their use in real-world decisions and among more diverse patient populations.
Introduction
The number of prognostic and predictive models aiming to inform on treatment decisions has been steeply rising within the last two decades (1). Many of them successfully use traditional and advanced statistical methods, which also include machine learning and artificial intelligence, to allow health care professionals (HCP) and patients to better estimate the patient's prognosis and potential outcome of different therapies (2).
While on the one hand the use of algorithms could lead to a mechanization within the relationship between HPC and the individual patient, on the other hand these algorithms could also be an opportunity to strengthen shared decision-making (SDM) (3, 4). A plethora of studies have shown that SDM is a key component of high-quality healthcare (5–9).
SDM is considered the final step completing the implementation of evidence-based medicine (10). It relies on understanding patient values and preferences and including them into the decision process (11). Patient values and preferences are the unique understandings, preferences, concerns, expectations and life circumstances of each patient (12). Values are defined as a patient's attitudes and perceptions about certain healthcare options, and preferences are their preferred choices after accounting for their values (13).
These preferences have to be assessed. While there is research on what patients value in healthcare (14) and qualitative evidence on how patient preferences are integrated into healthcare (11) questions to elicit patient preferences can also be included either directly into algorithms that inform treatment decisions or within the process of applying the algorithm within the clinical workup. This way these preferences receive the same attention as other clinical factors and it would become part of the routine to assess them in a structured manner.
To achieve this benefit, patient preferences need to be assessed in a valid and approachable way to truly reflect a patient's values. Many methods for the assessment of preferences are available from decision theory, but they need to be adapted to the medical context.
After finalizing a scoping review on how patient preferences have been incorporated into medical decision algorithms and models (15, 16) we chose five assessment methods for patient preferences to further investigate: Best-worst scaling (17), direct weighting (18), Potentially All Pairwise Rankings of all possible Alternatives (PAPRIKA) (19), time trade-off (20) and standard gamble (21). The selection of the methods reflects the diversity of approaches and cover a wide spectrum of complexity and cognitive demand.
Direct Weighting (DW) and Best-Worst Scaling (BWS) are methods for attribute-weighting the importance of treatment attributes. In DW patients directly attribute weights to given criteria, e.g., by distributing points adding up to 100. DW is an intuitive approach in clinical research and quality-of-life studies (18). It has also been used for prostate cancer screening decision analysis tool (22). BWS was developed in mathematical psychology (17) and has widespread in marketing (23), social care (24), health preferences research (25) and more. In BWS criteria are split into small sets. Patients then chose the most and least important options from these sets. This generates relative importance scores to rank items and estimate preference weights. BWS can, for example, be included in decision aid for psychiatry treatment choices (26).
Standard Gamble (SG) and Time Trade-off (TTO) qualify preferences by using the assessment of individual health utility. SG measures preferences under uncertainty. Patients are asked to choose between living in a certain health state for sure, or taking a risky treatment with a probability p of full health and (1-p) immediate death. By varying p until the patient is indifferent between the certain health state and the probability of immediate death (1-p) the utility value of the health state is derived on a scale from 0 (equivalent to death) and 1 (equivalent to perfect health) (21). This method was for example used in a decision aid for prostate cancer treatment (27). The TTO method measures how much lifetime in full health is considered equivalent to a longer time in a less desirable state by the patient (20). Simes et al. applied TTO to statistical decision theory with utility elicitation (28). Both methods originated in health economics (29), and are widely used for quality-of-life estimation (30) and health technology assessment (31). TTO is frequently used for value set construction (32, 33).
PAPRIKA (Potentially All Pairwise RanKings of all possible Alternatives), which infers criterion weights from a sequence of pairwise comparisons where scenarios differ on exactly two attributes (19). PAPRIKA is a relatively new method, developed in 2008 and patented in 2010 by the company 1,000 minds and has gained popularity in many domains, such as clinical (34) and guideline development (35) contexts because it aligns with multi-attribute treatment decisions and can provide transparent weighting structure.
Each method differs in cognitive burden, output (utilities vs. weights/rankings), and fit with clinical workflows (33, 36–38). While each of these methods can be used to assess patient preferences on an individual level, prior work suggests method acceptability can vary by patient population and context (38).
Within the EPAMeD project (Ethics and practice of algorithm-supported decision making in medicine) we are aiming to develop an algorithmic tool for decision support for patients with multiple sclerosis. Here, we describe the adaption of several methods for preference assessment to a hypothetical treatment decision scenario and the evaluation and acceptance of these methods within a group of patients and healthy persons.
Aims and objectives
In this study, we compare a wide range of methods that vary in cognitive complexity and level of detail for eliciting preferences. These methods are presented to patients with different chronic or acute health conditions, as well as to healthy individuals.
We aim to gain insight into the acceptance, effort and preferences of participants regarding the different methods. Additionally, we investigate the influence of health status, experiences within the health system and of demographic factors on the results.
Materials and methods
Study design
This is a cross-sectional questionnaire study in volunteers conducted via an online survey platform over a one-month period.
Questionnaire
The online questionnaire was in German language and consisted of 18 pages (Supplementary File S1). The phrasing reported here is an English translation. The first page described the topic and project. The second page assessed demographic data of the participant.
The third page introduced the hypothetical scenario. It asked participants to imagine they had a severe progressing disease which leads to severe limitations in mobility, need for care and early death when untreated. The time to progression however is very individual. There are three therapeutic options for the disease with different effectiveness, application routes and side effects. Participants are then asked to imagine they have to choose one treatment together with their treating doctor, who is using structured methods to support the decision.
The following pages presented the instances of the five investigated methods adapted to the hypothetical scenario and three treatment options with varying levels of the attributes: Effectiveness, side effects and application route.
In DW, participants indicated relative importance by allocating a fixed budget of points across the three attributes. Operationally, they were asked to distribute 100 points across the attributes (effectiveness, side effects and application route) so that the total summed to 100, placing more points on attributes that mattered more to them.
In BWS, respondents evaluated small sets of attributes and identified, within each set, the most important and the least important item. For example, given a set containing reduction of symptoms, avoiding side effects, and mode of administration, they selected one as most important, one as least important, and one as nether important nor not important.
In PAPRIKA, we presented pairwise comparisons between hypothetical treatments that differed on two attributes. For instance, contrasting an option with a higher effectiveness requiring monthly infusion against an option with a moderate effectiveness with daily oral pills. Participants could then indicate which option they preferred, or whether the methods were equally desirable.
For the utility-based methods, TTO elicited the point of indifference between living a longer in a less desirable health state and a shorter time in full health. Participants were asked, if they would rather live 10 years with a given combination of attributes representing a specific treatment regimen vs. 10-X years in full health. X was decreased by one year per iteration until indifference. This was performed for all three hypothetical treatment options.
SG elicited utilities by offering a choice between a certain health state and a risky treatment resulting in full health with probability p and immediate death with probability (1−p). Patients were asked if they want to provide a risk p. If the answer was yes, the participants were able to adjust the point of indifference.
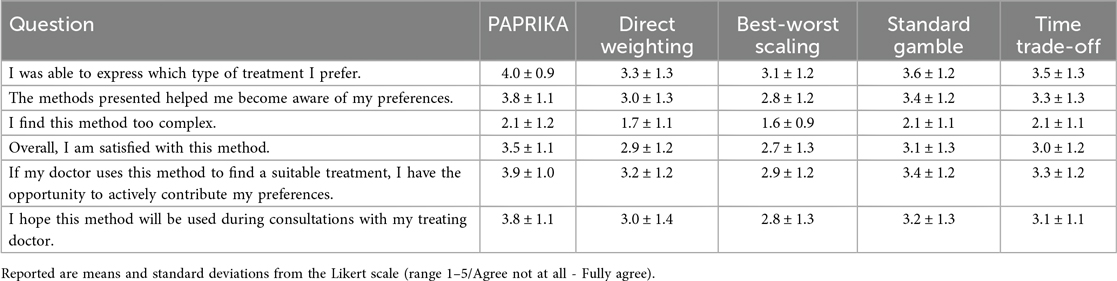
By including methods that differ in their cognitive demands and ease of use, our study aims to identify approaches that balance methodological rigor with acceptability in different patient groups. Furthermore, the study predicts the practicality of these methods in real-world SDM contexts, including their ability to help patients to weigh treatment options and effectively express their preferences. After the presentation of each method, where participants could enter their choices, they were shown a page with six statements to rate the method on a 5-point Likert scale (Agree not at all - Fully agree). The statements regarding each method respectively were:
1. I was able to express which type of treatment I prefer.
2. I have thoroughly weighed the different aspects of a possible treatment using this method.
3. I find this method too complex.
4. Overall, I am satisfied with this method.
5. If my doctor uses this method to find a suitable treatment, I have the opportunity to actively contribute my preferences.
6. I hope this method will be used during consultations with my treating doctor.
Additionally, participants were asked to rate three statements regarding the acceptance and satisfaction with shared decision-making and the overall concept of algorithm-assisted assessment of patient preferences, as well as three statements regarding the clarity of the questionnaire. We again used a 5-point Likert scale (Agree not at all - Fully agree). The statements were:
1. It is important to me to be able to express my preferences in detail when choosing a therapy
2. The methods presented helped me become aware of my preferences
3. I would prefer to complete the preference assessment calmly at home rather than in a 15-minute conversation with my doctor
4. The questions in the survey were clear to me
5. There were words or expressions that I did not understand
6. There was enough context to be able to answer the questions
One more question was asked with yes and no as possible answers:
(1) Can you imagine that your preferences for selecting a therapy could be assessed using one of the methods shown earlier?
Finally, there was an option to leave comments in free text. The full questionnaire (original version and English translation) is provided in Supplementary File S1.
To ensure feasibility and consistency we performed a pre-test of the questionnaire with ten participants. The pretest resulted into modifications mainly of wording and presentation of scales. Results of the pretest are not included in the data reported in this manuscript.
Participants
To include a broad range of individuals—both healthy and those with chronic or acute conditions—we distributed our questionnaire via multiple channels: the informational newsletter of Ludwig Maximilian University (Munich, Germany), the mailing list of our Ph.D. Program in Epidemiology & Public Health, and the mailing list of Selbsthilfezentrum München, a patient advocacy organization. All recipients were motivated to further share the link in order to reach more and more diverse participants. While participants could choose not to answer single questions their responses were not included into the analysis if they did not follow through the questionnaire to the end.
The sample size calculation was based on the Friedman test. With a sample size of 100 participants, the estimated power to detect a difference of at least 0.5 points on the Likert scale between methods is approximately 93%.
Analysis
Data collected on nominal or ordinal scale is reported using percentages, responses collected on the Likert scale are reported by calculating the means and standard deviations (sd). Depending on group sizes, results are also reported in subgroups according to demographic data. To identify relevant differences the Friedman tests and Wilcoxon tests were used. However, due to the explorative nature of this study we are not formally setting a level of significance. Spearman correlation coefficients were calculated to identify correlations between Likert scales and demographic data.
Comments were analyzed using thematic analysis following the approach described by Braun and Clarke (39). Two researchers (JF and VSH) independently reviewed all comments, conducted open coding, and iteratively developed a set of themes that captured recurring patterns in the data. Discrepancies were discussed until consensus was reached.
Software
The questionnaire was developed using REDCap Version 14.6.7 and provided on the servers of the Institute for Medical Information Processing, Biometry and Epidemiology, LMU, Munich.
All statistical analyses were performed using R version 4.3.1.
Results
The questionnaire was online for one month between February 5th, 2025 and March 4th, 2025. The questionnaire was started 258 times, 123 participants completed the questionnaire (48%).
Demographics and personal information
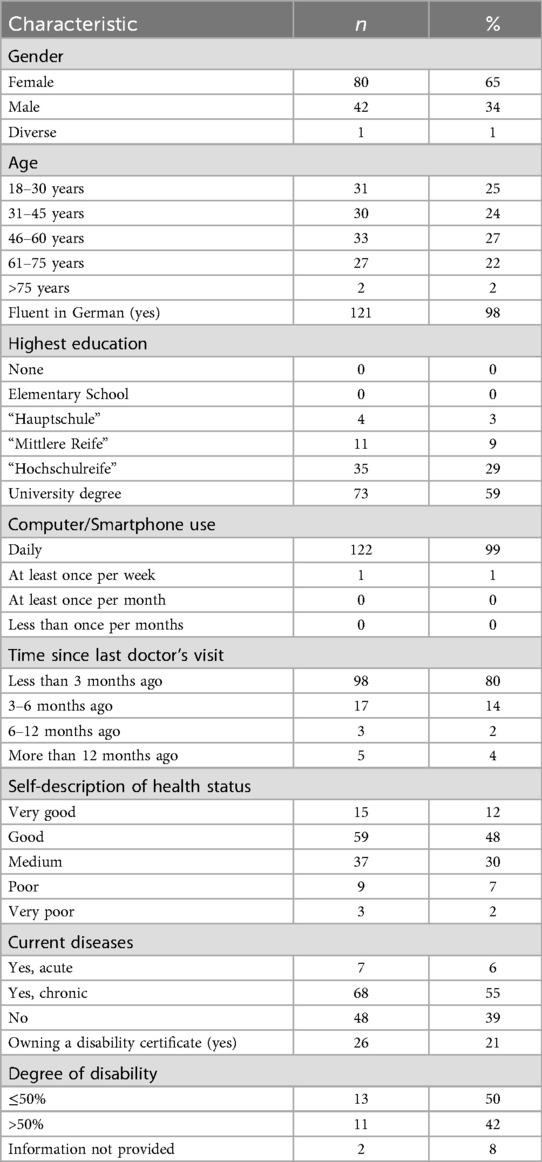
The majority of participants was female (65%), regarding age the participants were distributed almost evenly between four age groups between 18 and 75 years, only two participants were older than 75 years. The majority of participants was fluent in German (98%) and held a high school (29%) or university diploma (60%). Almost all participants except for one stated to be using a computer or smartphone daily. 80% of participants had consulted a doctor within the last three months, 55% reported to be chronically ill, with 21% (n = 26) owning a disability certificate (German “Schwerbehindertenausweis”) with a median grade of disability of 50% (range 40%–100%). Detailed results are reported in Table 1.
Evaluation of the five presented methods
The participants were shown five methods which were adapted to the hypothetical scenario. After the presentation of each method they were asked to evaluate the method by agreeing or disagreeing with six statements on a 5-point Likert scale.
The results show a clear preference of the participants for the PAPRIKA method. PAPRIKA was rated better regarding five statements with similar or smaller standard deviation than the other methods.
The standard gamble and time trade-off methods received very similar ratings regarding all statements, which were worse than the PAPRIKA ratings but better than the ratings for direct weighting and best-worst scaling. Ratings for these very simple methods were worse for all statements except for the statement regarding complexity. Table 2 shows the mean Likert scores for each statement by method.
The Friedman test showed that the differences between methods were very large (p < 0.0001 for each statement). Post-hoc comparisons indicated that PAPRIKA differed substantially from all other methods for almost all statements.
Subgroup analyses
There were no relevant differences between genders regarding the evaluation of methods. Only male and female participants were compared due to the small number of diverse participants (n = 1).
In general, the age group 46–60 years evaluated the methods more critical than the other three age groups. For PAPRIKA differences between the overall mean and the age group means were rarely larger than ±0.5 points on the Likert scale. Older participants rated the more parsimonious methods (Direct Weighting and Best-worst Scaling) generally better than younger participants. The Spearman correlation coefficient between age groups and Likert scale results of the single statements showed low to moderate correlations between −0.4 and +0.4. Only the correlation between age and the wish to use Direct Weighting for future treatment decisions was higher (0.44).
Overall, the mean ratings on the Likert scale for the different methods did not vary substantially across the self-reported health status. Spearman correlations did not exceed ± 0.2. The only exception was the question of whether participants found the method too complex, which was generally rated higher by those who reported poor or very poor health.
Only very small differences could be detected between the mean Likert scale ratings of participants with or without chronic diseases. Participants who reported to own a disability certificate on the other hand rated all suggested methods better than participants without a disability certificate.
Very few participants reported having visited a doctor more than six months ago (n = 8). No relevant differences in the mean rating of methods were observed between participants who visited a doctor up to three months or three to six months ago.
Only 15 participants did not receive higher education. If a participant had a university degree or not was strongly correlated to the age group, indicating that many students took part in the study. Overall, this made a subgroup analysis of the level of education uninformative.
Due to the small numbers of participants who did not speak German fluently (n = 2) and who did not use a smartphone or computer daily (n = 1) we did not perform subgroup analyses on those.
Overall evaluation of algorithm-assisted assessment of patient preferences
Following the presentation and rating of the five methods we asked the participants some general questions regarding their acceptance and satisfaction with the overall concept of algorithm-assisted assessment of patient preferences. Full results are shown in Table 3.
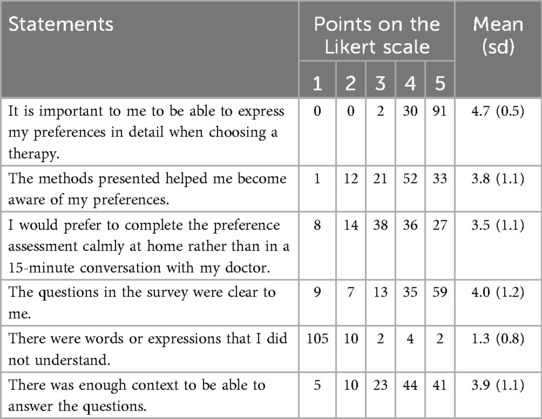
Table 3. Results of the overall evaluation of algorithm-assisted assessment of patient preferences and of the questions regarding the clarity of the questionnaire.
Most participants agreed strongly with the statement “It is important to me to be able to express my preferences in detail when choosing a therapy.” (mean 4.7, sd 0.5).
Most participants agreed with the statement “The methods presented helped me become aware of my preferences” (mean 3.8, sd 1.1).
Also, the statement “I would prefer to complete the preference assessment calmly at home rather than in a 15-minute conversation with my doctor.” was rather agreed to by the majority of patients (mean 3.5, sd 1.1).
The question “Can you imagine that your preferences for selecting a therapy could be assessed using one of the methods shown earlier?” was answered with “yes” by 82% (n = 101) of the participants.
Clarity of the questionnaire
As one way of validation we asked three questions to confirm the questionnaire was understandable to the participants. Most participants agreed to the statements about clarity of the questions (mean 4.0, sd 1.2) and sufficiency of context (mean 3.9, sd 1.1). Only few participants reported not to have understood words or expressions in the questionnaire (mean 1.3, sd 0.8). Full results are reported in Table 3.
Comments
In the last section of the questionnaire participants had the opportunity to leave a free text comment. Many of them were insightful regarding either the participants experience with the questionnaire or attitudes toward the methods. Overall, 28 comments were made. Thematic analysis identified four overarching themes:
1. Involvement of a doctor: Seven participants found the methods presented helpful to support decision making but pointed out that for discussion and decision-making a doctor needs to be involved.
2. Struggling with abstraction in the scenario: Seven participants described that they struggled with the hypothetical scenario.
3. Perceived burden: Three participants stated that they found the time trade-off and standard gamble methods too theoretical or psychologically burdensome.
4. Balancing usefulness and complexity: Two participants described PAPRIKA as useful but challenging as comparisons have to be read closely to identify the respective differences.
Nine participants left other comments containing singular topics like own experiences, confusion about specific methods (e.g., TTO), the level of detail in the information provided, or the wish for prevention instead of treatment.
Discussion
Our study aimed to compare a variety of methods to assess patient preferences for therapy selection. The methods were presented to patients with different chronic or acute health issues as well as healthy individuals. With our study setup we reached healthy as well as chronically ill individuals and also a small number of participants with an acute disease. The participants were diverse regarding age, gender and health status but rather homogeneous regarding education and digital literacy.
Across all aspects we assessed, the participants showed a strong preference for the PAPRIKA method. The simpler methods (direct weighting and best-worst scaling) were perceived as too restrictive regarding the expression of individual preferences and balancing different aspects of the treatment options.
The more abstract methods (standard gamble and time trade-off) were rated better than the simple methods but worse than PAPRIKA. The comments hint that the abstract methods were perceived as too theoretical and too mentally burdensome as these methods focus on a trade-off in life years.
Obviously, these kinds of tools to aid shared decision making are not made for all patients and all healthcare situations. From a clinical perspective there needs to be sufficient time to make a shared decision. The presented methods are not meant to be applied in emergency situations. Also, the decision needs to be preference-sensitive, meaning there need to be two or more treatment options that are studied well enough to provide adequate information on likely treatment outcome and adverse event rates. This is often the case in chronic diseases with variable prognoses as multiple sclerosis and Parkinson's disease (40).
For patients who are comfortable using technology and prefer a more structured kind of decision making our study shows that a tool like PAPRIKA is the most agreed to. It needs to be determined if structured methods to assess patient preferences are also accepted by patients with a lower level of formal education who are as well proficient using digital tools. This group was underrepresented in our sample likely due to our ways of distributing our questionnaire.
Up until now in the medical field the PAPRIKA method has been mainly used for patient prioritization (34, 41, 42), medical research (43–45), health technology prioritization (46–48) and to inform public health decisions (49, 50). Applications directly including patients into their therapy decision do currently not exist on an individual level or stay theoretical (51).
Developing a patient preference assessment tool for a specific treatment decision according to our results must consider two major challenges:
1. It must enhance SDM. This could be validated using the SDM-Q-9 questionnaire. This questionnaire is made to measure level of SDM perceived by patients (52). It is regularly used (53) and translated into many languages (54–56). Future studies should assess whether using a method like PAPRIKA leads to higher SDM-Q-9 scores, indicating an improved shared decision process.
2. If therapeutic characteristics are presented, they must be based on the highest quality of evidence available. A tool like PAPRIKA which is modeled to assess patient preferences for a specific health care situation, needs to include high quality information e.g., on relapse rates or probability of adverse events to be helpful.
Both aspects make it inevitable to closely tie tools for assessing patient preferences into the clinical workflow. They cannot be stand-alone. They need to be introduced to the patients by healthcare professionals and the results need to be discussed with healthcare professionals. This is also a result of our study: While a majority of participants would agree to use structured methods for the assessment of their preferences and found them helpful to become aware of their own preferences there was a strong urge to discuss the results with the treating doctor.
As an additional tool for assessing preferences especially for informing changes of therapeutic measures the integration of wearable biomonitors may enhance the quality of therapy-related decision-making. Continuous, objective data on parameters such as activity, sleep, or physiological stress can provide patients with a more concrete understanding of how their condition and potential treatments affect daily life.
The presented methods can also be included into existing prognostic and predictive models ensuring that recommended treatments and treatment outcomes align with the patient's preferences, providing increased patient satisfaction, treatment adherence, and better health outcomes.
Limitations
Our study has two main limitations: The sample composition and the hypothetical nature of the scenario.
While our sample of participants was diverse in terms of sex, age, and health status, the majority was highly educated with strong digital literacy. Thus, we were not able to capture the opinions of less educated persons and those who might be less comfortable using digital tools and devices. This bias could mean that the methods, especially the more complex ones, may appear more acceptable in our sample than they would in a less educated population. Therefore, our findings might represent a best-case scenario of understanding and engaging with these tools.
A participant in our study is in many ways different from a specific patient who needs to weigh treatment options for a specific disease. Patients experience the symptoms and know about their severity and perseverance. They know to what extend the disease influences their day to day life and are thus better able to trade-off between symptoms and potential adverse events of a therapy. These layers of knowledge had to be substituted for the participants or imagined by the participants making the setup more complex and – although inevitable for the studied subject – limit generalizability.
Additionally, we did not attempt to determine which method produces the most accurate preferences or whether all methods yield similar preference rankings for an individual. Our focus was on user experience and acceptance.
Conclusion
In conclusion, our study suggests that an interactive trade-off method (e.g., PAPRIKA) best achieved the balance of detail and usability needed for capturing patient preferences in medical decisions. Participants were willing and able to engage with such a tool, especially when they could do so on their own time, but still value physician guidance in interpreting the results. Future work should focus on integrating this approach into clinical workflows and testing it in more diverse patient populations and real decision-making scenarios.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The requirement of ethical approval was waived by Ethikkommission bei der LMU München 25-0479-KB for the studies involving humans because anonymous, prospective questionnaire study in volunteers. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin due to the anonymous online design of the study a signature was not possible. Participants were informed about the study's rationale and storage and analysis of the provided data.
Author contributions
JF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. AW: Conceptualization, Funding acquisition, Methodology, Project administration, Writing – original draft, Writing – review & editing. VH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding was provided by Bundesministerium für Bildung und Forschung (BMBF [01GP2207]) to support the conduct of the EPAMed research project. The funder had no influence on study methods or results.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Generative AI was used to optimize language.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdgth.2025.1641765/full#supplementary-material
References
1. de Jong Y, Ramspek CL, Zoccali C, Jager KJ, Dekker FW, van Diepen M. Appraising prediction research: a guide and meta-review on bias and applicability assessment using the Prediction model Risk Of Bias ASsessment Tool (PROBAST). Nephrology (Carlton). (2021) 26(12):939–47. doi: 10.1111/nep.13913
2. Alowais SA, Alghamdi SS, Alsuhebany N, Alqahtani T, Alshaya AI, Almohareb SN, et al. Revolutionizing healthcare: the role of artificial intelligence in clinical practice. BMC Med Educ. (2023) 23(1):689. doi: 10.1186/s12909-023-04698-z
3. Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. (2012) 27(10):1361–7. doi: 10.1007/s11606-012-2077-6
4. Stiggelbout AM, Van der Weijden T, De Wit MP, Frosch D, Légaré F, Montori VM, et al. Shared decision making: really putting patients at the centre of healthcare. Br Med J. 2012;344:e256. doi: 10.1136/bmj.e256
5. Barry MJ, Edgman-Levitan S. Shared decision making–pinnacle of patient-centered care. N Engl J Med. (2012) 366(9):780–1. doi: 10.1056/NEJMp1109283
6. Gionfriddo MR, Leppin AL, Brito JP, Leblanc A, Shah ND, Montori VM. Shared decision-making and comparative effectiveness research for patients with chronic conditions: an urgent synergy for better health. J Comp Eff Res. (2013) 2(6):595–603. doi: 10.2217/cer.13.69
7. Loh A, Simon D, Wills CE, Kriston L, Niebling W, Härter M. The effects of a shared decision-making intervention in primary care of depression: a cluster-randomized controlled trial. Patient Educ Couns. (2007) 67(3):324–32. doi: 10.1016/j.pec.2007.03.023
8. Wehking F, Debrouwere M, Danner M, Geiger F, Buenzen C, Lewejohann J-C, et al. Impact of shared decision making on healthcare in recent literature: a scoping review using a novel taxonomy. J Public Health. (2024) 32(12):2255–66. doi: 10.1007/s10389-023-01962-w
9. Bruch JD, Khazen M, Mahmic-Kaknjo M, Légaré F, Ellen ME. The effects of shared decision making on health outcomes, health care quality, cost, and consultation time: an umbrella review. Patient Educ Couns. (2024) 129:108408. doi: 10.1016/j.pec.2024.108408
10. Moleman M, Regeer BJ, Schuitmaker-Warnaar TJ. Shared decision-making and the nuances of clinical work: concepts, barriers and opportunities for a dynamic model. J Eval Clin Pract. (2021) 27(4):926–34. doi: 10.1111/jep.13507
11. Tringale M, Stephen G, Boylan A-M, Heneghan C. Integrating patient values and preferences in healthcare: a systematic review of qualitative evidence. BMJ Open. (2022) 12(11):e067268. doi: 10.1136/bmjopen-2022-067268
12. Straus SE, Glasziou P, Richardson WS, Haynes RB. Evidence-based Medicine E-book: How to Practice and Teach EBM. Edinburgh: Elsevier Health Sciences (2018).
13. Llewellyn-Thomas HA, Crump RT. Decision support for patients: values clarification and preference elicitation. Med Care Res Rev. (2013) 70(1 Suppl):50s–79. doi: 10.1177/1077558712461182
14. Bastemeijer CM, Voogt L, van Ewijk JP, Hazelzet JA. What do patient values and preferences mean? A taxonomy based on a systematic review of qualitative papers. Patient Educ Couns. (2017) 100(5):871–81. doi: 10.1016/j.pec.2016.12.019
15. Fusiak J, Mansmann U, Hoffmann VS. Methods to incorporate patient preferences into medical decision algorithms and models, and their quantification, balancing, and evaluation: a scoping review protocol. JBI Evid Synth. (2024) 22(12):2593–600. doi: 10.11124/JBIES-23-00498
16. Fusiak J, Sarpari K, Ma I, Mansmann U, Hoffmann VS. Practical applications of methods to incorporate patient preferences into medical decision models: a scoping review. BMC Med Inform Decis Mak. (2025) 25(1):109. doi: 10.1186/s12911-025-02945-5
17. Flynn TN, Louviere JJ, Peters TJ, Coast J. Best–worst scaling: what it can do for health care research and how to do it. J Health Econ. (2007) 26(1):171–89. doi: 10.1016/j.jhealeco.2006.04.002
18. Browne JP, O'Boyle CA, McGee HM, McDonald NJ, Joyce CRB. Development of a direct weighting procedure for quality of life domains. Qual Life Res. (1997) 6(4):301–9. doi: 10.1023/A:1018423124390
19. Hansen P, Ombler F. A new method for scoring additive multi-attribute value models using pairwise rankings of alternatives. J Multi-Criteria Dec Anal. (2008) 15(3-4):87–107. doi: 10.1002/mcda.428
20. Lugnér AK, Krabbe PFM. An overview of the time trade-off method: concept, foundation, and the evaluation of distorting factors in putting a value on health. Expert Rev Pharmacoecon Outcomes Res. (2020) 20(4):331–42. doi: 10.1080/14737167.2020.1779062
21. Gafni A. The standard gamble method: what is being measured and how it is interpreted. Health Serv Res. (1994) 29(2):207–24.8005790
22. Cunich M, Salkeld G, Dowie J, Henderson J, Bayram C, Britt H, et al. Integrating evidence and individual preferences using a web-based multi-criteria decision analytic tool. Patient Patient Cent Outcomes Res. (2011) 4(3):153–62. doi: 10.2165/11587070-000000000-00000
23. Cohen E. Applying best-worst scaling to wine marketing. Int J Wine Bus Res. (2009) 21:8–23. doi: 10.1108/17511060910948008
24. Potoglou D, Burge P, Flynn T, Netten A, Malley J, Forder J, et al. Best-worst scaling vs. discrete choice experiments: an empirical comparison using social care data. Soc Sci Med. (2011) 72(10):1717–27. doi: 10.1016/j.socscimed.2011.03.027
25. Marley AAJ, Flynn TN, Louviere JJ. Probabilistic models of set-dependent and attribute-level best–worst choice. J Math Psychol. (2008) 52(5):281–96. doi: 10.1016/j.jmp.2008.02.002
26. Henshall C, Marzano L, Smith K, Attenburrow M-J, Puntis S, Zlodre J, et al. A web-based clinical decision tool to support treatment decision-making in psychiatry: a pilot focus group study with clinicians, patients and carers. BMC Psychiatry. (2017) 17(1):265. doi: 10.1186/s12888-017-1406-z
27. Knight SJ, Nathan DP, Siston AK, Kattan MW, Elstein AS, Collela KM, et al. Pilot study of a utilities-based treatment decision intervention for prostate cancer patients. Clin Prostate Cancer. (2002) 1(2):105–14. doi: 10.3816/CGC.2002.n.012
28. Simes RJ. Treatment selection for cancer patients: application of statistical decision theory to the treatment of advanced ovarian cancer. J Chronic Dis. (1985) 38(2):171–86. doi: 10.1016/0021-9681(85)90090-6
29. Lipman SA, Brouwer WBF, Attema AE. What is it going to be, TTO or SG? A direct test of the validity of health state valuation. Health Econ. (2020) 29(11):1475–81. doi: 10.1002/hec.4131
30. Martin AJ, Glasziou PP, Simes RJ, Lumley T. A comparison of standard gamble, time trade-off, and adjusted time trade-off scores. Int J Technol Assess Health Care. (2000) 16(1):137–47. doi: 10.1017/S0266462300161124
31. Meregaglia M, Nicod E, Drummond M. The estimation of health state utility values in rare diseases: do the approaches in submissions for NICE technology appraisals reflect the existing literature? A scoping review. Eur J Health Econ. (2023) 24(7):1151–216. doi: 10.1007/s10198-022-01541-y
32. Sun S, Chuang LH, Sahlén KG, Lindholm L, Norström F. Estimating a social value set for EQ-5D-5l in Sweden. Health Qual Life Outcomes. (2022) 20(1):167. doi: 10.1186/s12955-022-02083-w
33. Luo N, Vasan Thakumar A, Cheng LJ, Yang Z, Rand K, Cheung YB, et al. Developing an EQ-5D-5l value set for Singapore. Pharmacoeconomics. (2025). doi: 10.1007/s40273-025-01519-7
34. Powers J, McGree JM, Grieve D, Aseervatham R, Ryan S, Corry P. Managing surgical waiting lists through dynamic priority scoring. Health Care Manag Sci. (2023) 26(3):533–57. doi: 10.1007/s10729-023-09648-1
35. Cai RA, Chaplin H, Livermore P, Lee M, Sen D, Wedderburn LR, et al. Development of a benchmarking toolkit for adolescent and young adult rheumatology services (BeTAR). Pediatr Rheumatol. (2019) 17(1):23. doi: 10.1186/s12969-019-0323-8
36. Martelli N, Hansen P, van den Brink H, Boudard A, Cordonnier AL, Devaux C, et al. Combining multi-criteria decision analysis and mini-health technology assessment: a funding decision-support tool for medical devices in a university hospital setting. J Biomed Inform. (2016) 59:201–8. doi: 10.1016/j.jbi.2015.12.002
37. Kim SH, Lee SI, Jo MW. Feasibility, comparability, and reliability of the standard gamble compared with the rating scale and time trade-off techniques in Korean population. Qual Life Res. (2017) 26(12):3387–97. doi: 10.1007/s11136-017-1676-4
38. Németh B, Molnár A, Bozóki S, Wijaya K, Inotai A, Campbell JD, et al. Comparison of weighting methods used in multicriteria decision analysis frameworks in healthcare with focus on low- and middle-income countries. J Comp Eff Res. (2019) 8(4):195–204. doi: 10.2217/cer-2018-0102
39. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. (2006) 3(2):77–101. doi: 10.1191/1478088706qp063oa
40. Heesen C, Solari A. Editorial: shared decision-making in neurology. Front Neurol. (2023) 14:1222433. doi: 10.3389/fneur.2023.1222433
41. Liberman AL, Pinto D, Rostanski SK, Labovitz DL, Naidech AM, Prabhakaran S. Clinical decision-making for thrombolysis of acute Minor stroke using adaptive conjoint analysis. Neurohospitalist. (2019) 9(1):9–14. doi: 10.1177/1941874418799563
42. Fitzgerald A, de Coster C, McMillan S, Naden R, Armstrong F, Barber A, et al. Relative urgency for referral from primary care to rheumatologists: the priority referral score. Arthritis Care Res (Hoboken). (2011) 63(2):231–9. doi: 10.1002/acr.20366
43. Taylor WJ, Singh JA, Saag KG, Dalbeth N, MacDonald PA, Edwards NL, et al. Bringing it all together: a novel approach to the development of response criteria for chronic gout clinical trials. J Rheumatol. (2011) 38(7):1467–70. doi: 10.3899/jrheum.110274
44. Babashahi S, Hansen P, Sullivan T. Creating a priority list of non-communicable diseases to support health research funding decision-making. Health Policy. (2021) 125(2):221–8. doi: 10.1016/j.healthpol.2020.12.003
45. Baggott C, Hansen P, Hancox RJ, Hardy JK, Sparks J, Holliday M, et al. What matters most to patients when choosing treatment for mild-moderate asthma? Results from a discrete choice experiment. Thorax. (2020) 75(10):842–8. doi: 10.1136/thoraxjnl-2019-214343
46. Golan O, Hansen P. Which health technologies should be funded? A prioritization framework based explicitly on value for money. Isr J Health Policy Res. (2012) 1(1):44. doi: 10.1186/2045-4015-1-44
47. Khanal S, Nghiem S, Miller M, Scuffham P, Byrnes J. Development of a prioritization framework to aid healthcare funding decision making in health technology assessment in Australia: application of multicriteria decision analysis. Value Health. (2024) 27(11):1585–93. doi: 10.1016/j.jval.2024.07.003
48. Moreno-Calderón A, Tong TS, Thokala P. Multi-criteria decision analysis software in healthcare priority setting: a systematic review. Pharmacoeconomics. (2020) 38(3):269–83. doi: 10.1007/s40273-019-00863-9
49. Chan AHY, Tao M, Marsh S, Petousis-Harris H. Vaccine decision making in New Zealand: a discrete choice experiment. BMC Public Health. (2024) 24(1):447. doi: 10.1186/s12889-024-17865-8
50. Shmueli A, Golan O, Paolucci F, Mentzakis E. Efficiency and equity considerations in the preferences of health policy-makers in Israel. Isr J Health Policy Res. (2017) 6:18. doi: 10.1186/s13584-017-0142-7
51. Anaka M, Chan D, Pattison S, Thawer A, Franco B, Moody L, et al. Patient priorities concerning treatment decisions for advanced neuroendocrine tumors identified by discrete choice experiments. Oncologist. (2024) 29(3):227–34. doi: 10.1093/oncolo/oyad312
52. Kriston L, Scholl I, Hölzel L, Simon D, Loh A, Härter M. The 9-item shared decision making questionnaire (SDM-Q-9). development and psychometric properties in a primary care sample. Patient Educ Couns. (2010) 80(1):94–9. doi: 10.1016/j.pec.2009.09.034
53. Doherr H, Christalle E, Kriston L, Härter M, Scholl I. Use of the 9-item shared decision making questionnaire (SDM-Q-9 and SDM-Q-doc) in intervention studies-a systematic review. PLoS One. (2017) 12(3):e0173904. doi: 10.1371/journal.pone.0173904
54. de Filippis R, Aloi M, Pilieci AM, Boniello F, Quirino D, Steardo L, et al. Psychometric properties of the 9-item shared decision-making questionnaire (SDM-Q-9): validation of the Italian version in a large psychiatric clinical sample. Clin Neuropsychiatry. (2022) 19(4):264–71. doi: 10.36131/cnfioritieditore20220408
55. Baicus C, Balanescu P, Gurghean A, Badea CG, Padureanu V, Rezus C, et al. Romanian version of SDM-Q-9 validation in internal medicine and cardiology setting: a multicentric cross-sectional study. Rom J Intern Med. (2019) 57(2):195–200. doi: 10.2478/rjim-2019-0002
Keywords: patient preference, shared decision making, medical decision aids, potentially all pairwise rankings of all possible alternatives (PAPRIKA) method, questionnaire method
Citation: Fusiak J, Wolkenstein A and Hoffmann VS (2025) Assessing patient preferences for medical decision making - a comparison of different methods. Front. Digit. Health 7:1641765. doi: 10.3389/fdgth.2025.1641765
Received: 5 June 2025; Accepted: 27 October 2025;
Published: 13 November 2025.
Edited by:
Kelly Smith, University of Toronto, CanadaReviewed by:
Robet L. Drury, ReThink Health, United StatesSarah Pila, Northwestern University, United StatesCopyright: © 2025 Fusiak, Wolkenstein and Hoffmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Verena S. Hoffmann, dmhvZmZtYW5uQGliZS5tZWQudW5pLW11ZW5jaGVuLmRl
 Jakub Fusiak
Jakub Fusiak Andreas Wolkenstein
Andreas Wolkenstein Verena S. Hoffmann
Verena S. Hoffmann