- 1Azrieli Faculty of Medicine, Bar-Ilan University, Zefat, Israel
- 2Department of Radiology, Mayo Clinic, Rochester, MN, United States
- 3The Charles Bronfman Institute of Personalized Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 4The Windreich Department of Artificial Intelligence and Human Health, Mount Sinai Medical Center, New York, NY, United States
- 5The Hasso Plattner Institute for Digital Health at Mount Sinai, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Background: Large language models (LLMs) offer promise for enhancing clinical care by automating documentation, supporting decision-making, and improving communication. However, their integration into real-world healthcare workflows remains limited and under characterized. This systematic review aims to evaluate the literature on real-world implementation of LLMs in clinical workflows, including their use cases, clinical settings, observed outcomes, and challenges.
Methods: We searched MEDLINE, Scopus, Web of Science, and Google Scholar for studies published between January 2015 and April 2025 that assessed LLMs in real-world clinical applications. Inclusion criteria were peer-reviewed, full-text studies in English reporting empirical implementation of LLMs in clinical settings. Study quality and risk of bias were assessed using the PROBAST tool.
Results: Four studies published between 2024 and 2025 met inclusion criteria. All used generative pre-trained transformers (GPTs). Reported applications included outpatient communication, mental health support, inbox message drafting, and clinical data extraction. LLM deployment was associated with improvements in operational efficiency, user satisfaction, and reduced workload. However, challenges included performance variability across data types, limitations in generalizability, regulatory delays, and lack of post-deployment monitoring.
Conclusions: Early evidence suggests that LLMs can enhance clinical workflows, but real-world adoption remains constrained by systemic, technical, and regulatory barriers. To support safe and scalable use, future efforts should prioritize standardized evaluation metrics, multi-site validation, human oversight, and implementation frameworks tailored to clinical settings.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/recorddashboard, PROSPERO CRD420251030069.
Introduction
The integration of large language models (LLMs) into clinical practice has sparked interest across the healthcare community (1). These technologies have the potential to enhance diagnostic accuracy, reduce administrative burden, and support clinical decision-making (2). However, while LLMs have demonstrated impressive performance in controlled retrospective settings (3, 4), their translation into clinical workflows remains inconsistent and underexplored (5).
Despite exponential growth, there remains a significant gap between developed models and real-world translation (6). The majority of explored use cases are still at the proof-of-concept stage, due to regulatory uncertainties, technical deployment barriers, privacy concerns and variable institutional readiness (7, 8).
Moreover, evaluation metrics vary widely across studies, with many reporting model performances in silico without assessing usability, safety, or effectiveness in real-world clinical workflow (9, 10). There is also a lack of robust post-deployment monitoring systems to better understand the impact and shifting performance of these models.
This systematic review aims to evaluate the existing literature on LLM integration into real-world clinical settings. Specifically, we assess the extent of their deployment, the clinical settings in which they are applied, the tasks they are used for, and the outcomes associated with their use. By doing so, we aim to guide future research and adoption strategies.
Methods
Literature search
We systematically searched the literature to identify studies describing the application of LLMs in a real-world setting. We searched MEDLINE, Google Scholar, Scopus, and the Web of Science for papers published from January 2015 to April 2025. The full search process, including Boolean operators presented here and also detailed in the Supplementary Materials.
(“large language model” OR “large language models” OR ChatGPT OR “GPT-4” OR “GPT-3” OR BERT OR “transformer model” OR “foundation model”) AND (“real-world evidence” OR “real world application” OR “clinical implementation” OR “routine practice” OR “clinical use” OR deployment OR “workflow integration”) AND (“clinical practice” OR “healthcare setting” OR hospital OR “medical setting”) AND (“original research” OR “observational study” OR “clinical study” OR “implementation study”)
In addition, we checked the reference lists of selected publications and the “Similar Articles” feature in PubMed, to identify additional publications. Ethical approval was not required, as this is a systematic review of previously published research and does not include individual participant information. Our study followed the Preferred Reporting Items for Systematic Reviews and meta-analyses (PRISMA) guidelines (11). The study is registered with PROSPERO (CRD420251030069).
Study selection
We included studies conducted in real-world clinical care settings, such as hospitals, clinics, ambulatory, inpatient, outpatient, emergency, and primary care involving clinicians and/or patients. The intervention was an LLM-enabled tool integrated into live workflows. Eligible comparators included usual care pre–post designs. Outcomes encompassed workflow, efficiency, usability, adoption, clinical impact, and safety vs. risk. We excluded simulation-only studies (including vignette-based evaluations not used to guide real patient care), bench evaluations without deployment, and non-LLM NLP. All search results were imported into a single CSV table and deduplicated. Two authors (YA and VS) independently screened titles and abstracts for relevance. Potentially eligible articles were retrieved in full text and assessed by YA and VS. Discrepancies were resolved by a third author (EK).
Inclusion and exclusion criteria
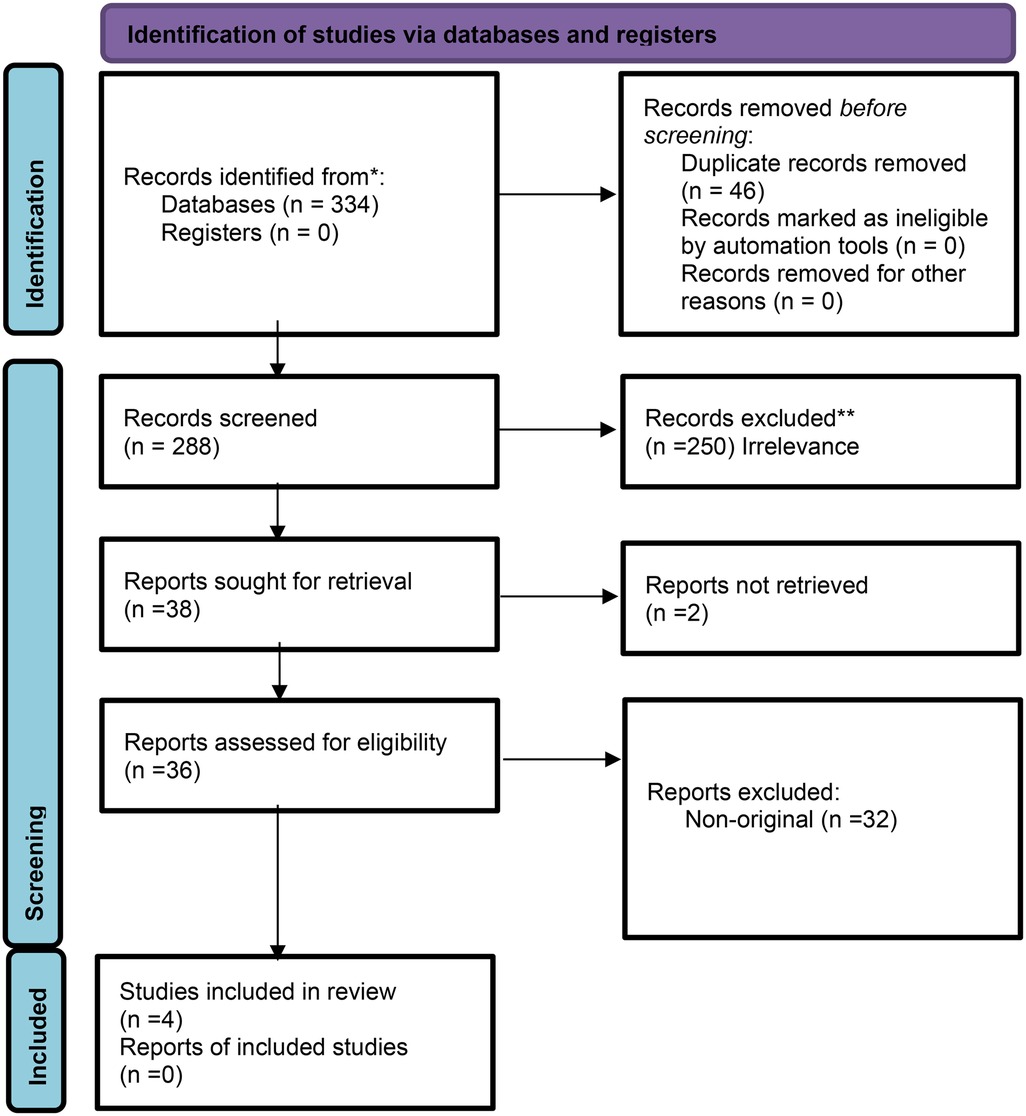
Full-text peer-reviewed publications in English focusing on LLMs integration and deployment in real-world clinical workflow were included. We excluded non-English articles, non-original research, non-peer-reviewed publications, studies that did not assess LLMs, and studies that did not explicitly assess LLMs in real-world settings. Figure 1 presents the flow diagram of the screening and inclusion process.
Quality assessment
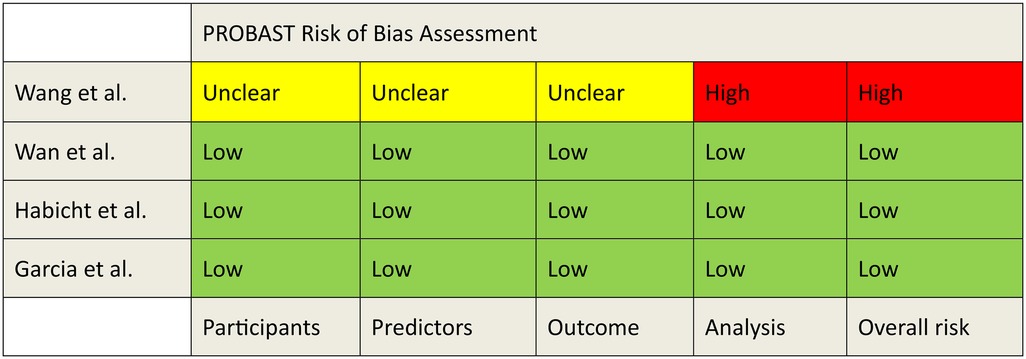
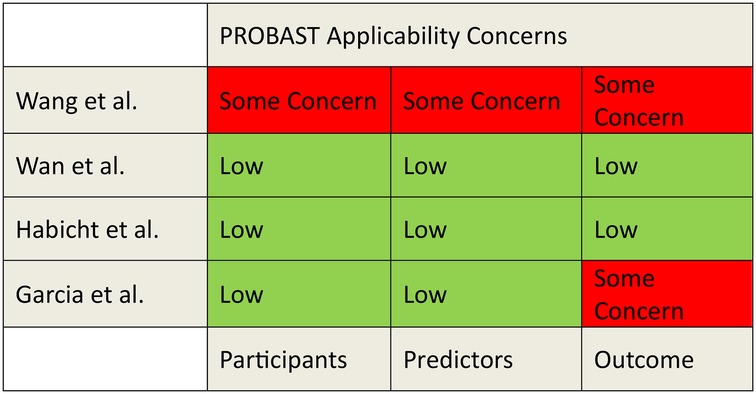
The risk of bias and applicability was evaluated using the PROBAST tool (Figures 2, 3). A detailed assessment of the studies using the PROBAST tool is detailed in the Supplementary Material.
Fundamental concepts
An overview of fundamental concepts in AI is included in the Supplementary Material, along with visual hierarchy shown in Supplementary Figure S1.
Results
Study selection and characteristics
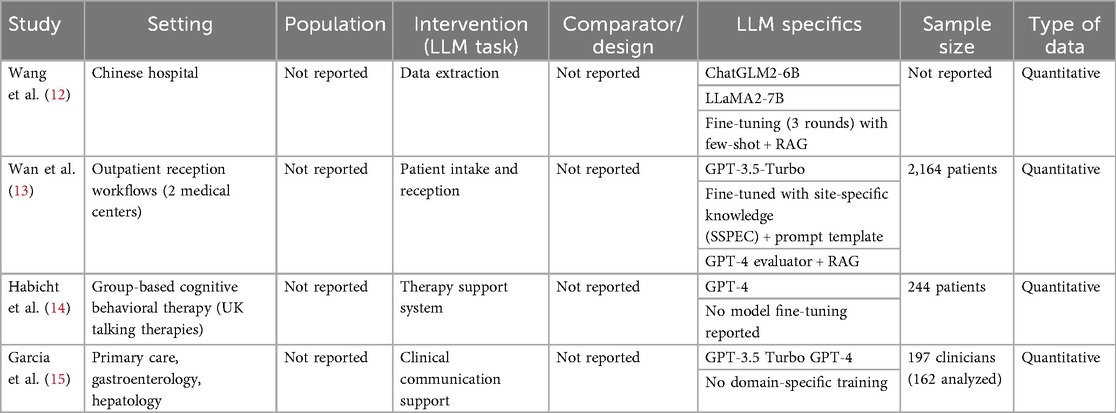
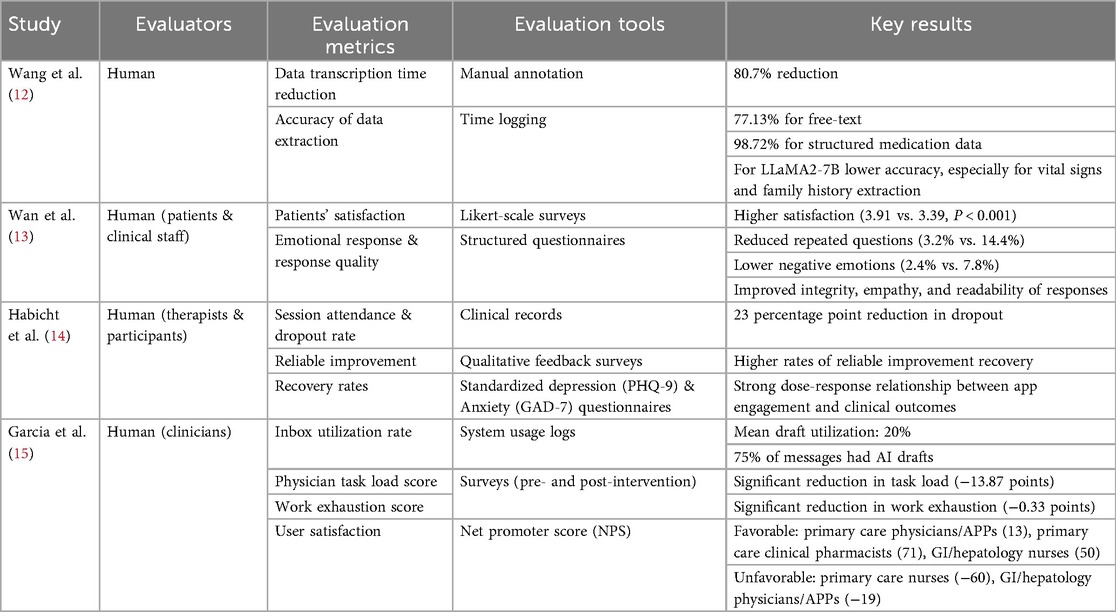
Four studies were included in this review, published between March 2024 and March 2025. All studies utilized generative pre-trained transformers (GPT) (100%). Two studies focused on patient services, including LLM-supported communication during outpatient intake and response to patient messages (50%). Two studies' focus areas were on data extraction (50%), one applied LLM as a support tool (25%) (Table 1).
The results of the studies are summarized in Table 2. Figure 4 provides an overview of the characteristics of the included studies.
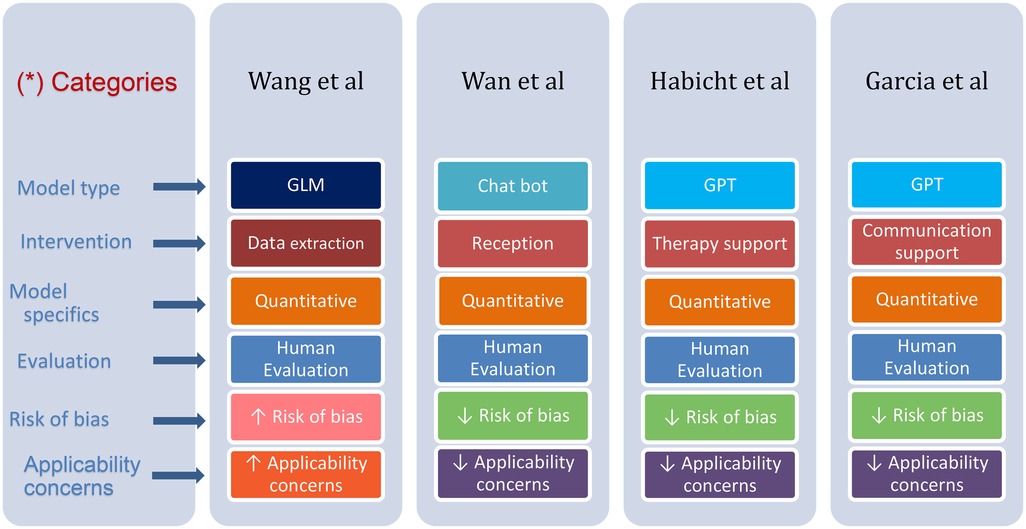
Figure 4. Differences and similarities in various parameters presented in the reviewed studies. (*) Each row has its distinctive color, where color tone change emphasizes a difference.
Descriptive summary of results
Wang et al. (12) evaluated ChatGLM2-6B for real-world data extraction, and did not report a patient count. The model achieved an 80.7% reduction in transcription time. The accuracy varied by data type, 77.13% for free text and 98.72% for structured medication data. In comparison, the LLaMA2-7B model showed lower accuracy, especially for vital signs and family history (Table 2).
Wan et al. (13) randomized 2,164 outpatients across two medical centers to nurse-only vs. nurse–SSPEC workflows. The nurse–SSPEC model improved patient satisfaction, reduced repeated questions (3.2% vs. 14.4%), and lowered negative patient emotions (2.4% vs. 7.8%). It also enhanced response quality in integrity, empathy, and readability (Table 2).
Habicht et al. (14) assessed a GPT-4–powered AI tool in group-based CBT (n = 244 patients) and found it improved clinical outcomes. The AI group had more session attendance, fewer missed appointments, and a 23-percentage point lower dropout rate than those using standard worksheets—higher engagement correlated with better adherence and outcomes. Qualitative feedback also noted improved self-awareness, mindfulness, and practical use of CBT techniques (Table 2).
Garcia et al. (15) evaluated GPT-3.5 Turbo and GPT-4 for generating draft replies to patient messages in gastroenterology, hepatology, and primary care. The AI drafts improved efficiency and reduced clinician workload without compromising communication quality. Enrolled 197 clinicians, of whom 162 were included in the final analysis; draft utilization averaged 20%, with 75% of messages receiving AI-generated replies. While time spent on inbox tasks did not significantly change, clinicians reported reduced task load and work exhaustion. User feedback raised concerns about message tone, length, and relevance (Table 2).
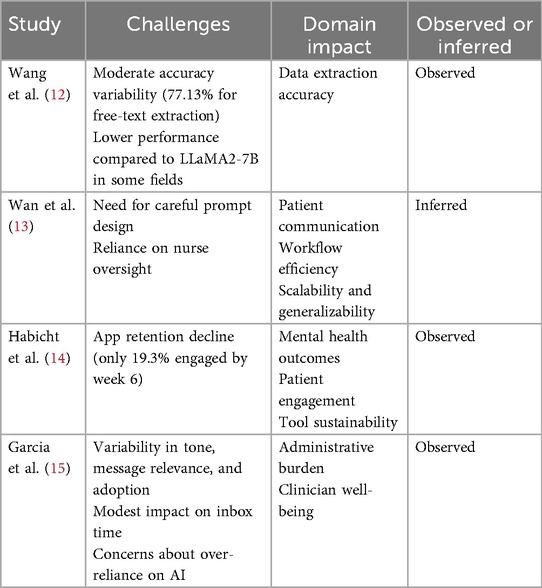
Limitations and challenges discussed or inferred in the reviewed studies are presented in Table 3.
Discussion
LLMs show promise in real-world clinical workflows (16), with the potential to enhance many fields in clinical care. We illustrate this in Figure 5, showcasing various clinical domains with key results from the reviewed studies (Figure 5). However, their implementation remains early-stage and context-dependent. Our synthesis across four deployed implementations indicates consistent benefits in task burden and clinician experience, alongside improvements in selected patient-facing outcomes. At the same time, effects remain context-dependent, varying by role, setting, task type, and integration depth. In this context, our synthesis indicates that LLMs function less as universal accelerators and more as context-sensitive amplifiers of specific tasks, with the clearest benefits emerging when models are embedded in existing tools and supervised. Several studies demonstrated clear empirical benefits. For example, Habicht et al. (14) showed that GPT-4 as a therapy support tool reduced therapy dropout rates and improved clinical outcomes in group interventions. Wan et al. (13) reported that the site-specific LLM chatbot (SSPEC) reduced repeated interactions and negative patient emotions. Garcia et al. (15) found that GPT-generated draft replies decreased clinician task load and work exhaustion across several clinical settings. These findings suggest that the value of LLMs is realized not simply by model capability but by deliberate product-workflow fit, a bounded task, appropriate timing in the clinical journey, and clear human supervision and handoff.
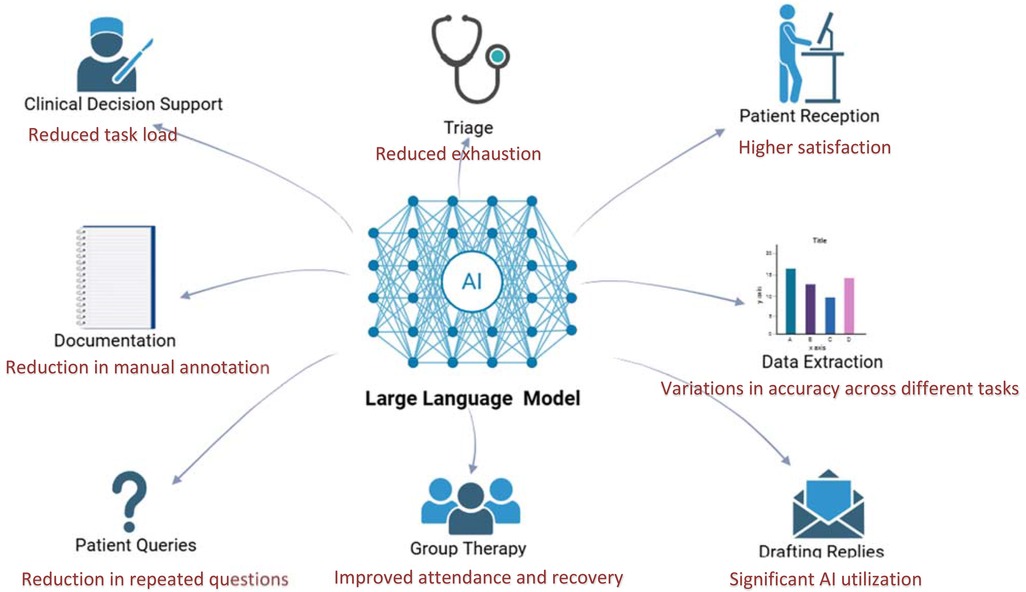
Figure 5. LLM application in various fields in real-world setting, integrated with summarized results from reviewed studies. Created using Biorender, licensed under Academic License.
Across included studies, three implementation patterns consistently enabled success. Retrieval-augmented generation (RAG) with prompt templates to ground outputs in local knowledge, guardrails, and escalation tiers (including evaluator models and human handoffs) to manage safety and appropriateness, and EHR integration to address privacy and reduce workflow friction. Conversely, role-specific dissatisfaction, and retention decay in patient-facing flows emerged as failure modes when these patterns were absent or inconsistently applied.
Despite encouraging signals, translation and scale remain constrained. In Wang et al. (12), while ChatGLM2-6B achieved a reduction in transcription time, accuracy varied significantly across data types. LLaMA2-7B performed notably worse in comparison. These discrepancies accentuate the importance of task specificity and local calibration in AI deployment.
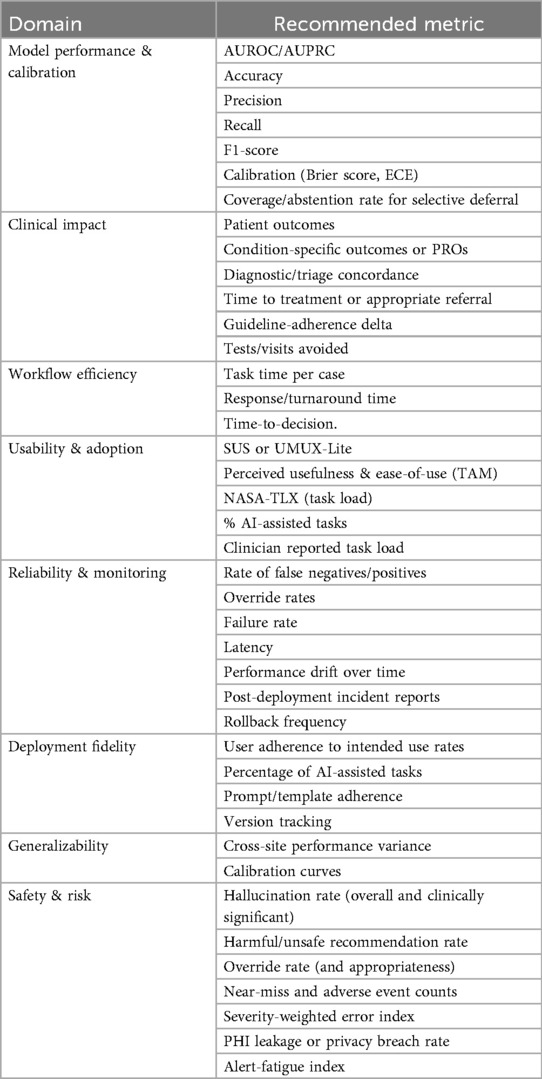
While LLMs can improve operational efficiency and clinical outcomes, widespread adoption is limited by systemic barriers and arbitrary evaluation metrics (17). These include heterogeneous EHR systems and unclear standards for performance evaluation. Future research should focus on efficient prospective validation and developing clear standardized evaluation metrics. We propose a list of possible standardized evaluation domains and metrics detailed in Table 4.
Translating LLMs from experimental settings into clinical practice remains a challenge. Clinical implementation is frequently delayed by regulatory barriers, including classification as software-as-a-medical-device (SaMD), which necessitates lengthy regulatory approval processes and extensive local validation (18). These processes contribute to version lag, whereby newer models become available before prior versions are deployed or evaluated. Furthermore, performance may degrade over time due to evolving clinical documentation, user behavior, or patient populations (8). These challenges underscore the need for post-deployment monitoring frameworks and standardized scientific reporting to ensure model safety, performance, and generalizability in clinical environments (17, 19).
Strategies for the future
Several strategic approaches should be considered to facilitate the broader adoption of LLM technologies in clinical workflows. Local adaptation of models is essential, as performance can vary significantly depending on institutional characteristics such as data quality, documentation styles, and patient demographics. Ensuring that models are trained and validated on local data can improve generalizability and clinical relevance. Also, incorporating a human expert oversight framework can improve safety, accountability, and user trust.
This review highlights the need for comparable outcome definitions. Studies frequently mix denominators, time windows, and units, hindering synthesis. Outcomes are also unevenly distributed. Workflow or experience measures are common, while downstream clinical outcomes and severity-weighted safety indicators are rare. To improve comparability, a standardized evaluation set with consistent units, denominators, and time windows are required, with selective deferral and severity-aware incident metrics. Adopting shared reporting conventions would make future evidence more cumulative, enable meta-analytic techniques where appropriate, and clarify trade-offs between efficiency, quality, and risk across sites.
AI tools should be designed with task specificity; models tailored to distinct clinical functions, such as triage, documentation, or medication extraction, are more likely to achieve meaningful utility. This can be achieved using fine-tuning (20) or Retriever-Augmented Generation (RAG) (21).
A standardized set of metrics should be developed to support consistency and facilitate future evaluations. We propose our set of metrics in Table 4. Also, involving clinicians and end users in the development and implementation process ensures that tools are aligned with real-world workflow needs, increasing user acceptance. Collecting user feedback during deployment can guide iterative model refinement and usability improvements.
Safety mechanisms and override options are crucial to prevent unintended consequences such as clinical errors, automation over-reliance, data bias, and alert fatigue (22). These risks can disrupt workflows and raise ethical concerns about accountability. Human oversight and ongoing monitoring are essential to ensure AI supports, rather than compromises, clinical care (19).
Ensuring that AI tools are clinically effective requires more than technical performance. This includes interoperability with EHRs, standardized evaluation metrics, and human-centered design features like transparency and clinician oversight. By applying these strategies, AI can move beyond experimental use to become a trusted part of routine clinical care.
This review has several limitations. First, the number of eligible studies evaluating LLMs in real-world clinical workflows remains limited, reflecting the early stage of implementation research in this domain. Our search emphasized real-world clinical settings, which may have excluded studies that did not explicitly use setting descriptors. Nonetheless, multi-database coverage and related-article screening reduce the likelihood of missing eligible deployments. Second, the heterogeneity in study design, evaluation methods, clinical settings, and outcome reporting precluded formal meta-analysis. Third, most included studies were conducted in high-resource settings, potentially limiting the generalizability of findings to low- and middle-income countries. Additionally, some of the studies relied on self-reported outcomes or lacked long-term follow-up, which may introduce reporting bias or fail to capture sustained clinical impact. Finally, despite efforts to capture a comprehensive set of studies, some relevant work may have been missed due to language restrictions, database coverage, or publication lag.
Conclusion
LLMs demonstrate early but uneven success in real-world integration, with empirical improvements in efficiency and user satisfaction. They demonstrate encouraging but context-dependent benefits in real-world clinical workflows, and their effects varied by role, task, and site, underscoring that outcomes depend as much on implementation choices as on the underlying model family. However, challenges related to generalizability, interoperability, and evaluation must be addressed to ensure scalable and safe adoption. LLMs' outcomes are situation-specific and site-specific, underscoring the need for multi-site validation, transparent version reporting, and post-deployment monitoring for safety and equity. For health systems, the immediate implication is to prioritize implementation architecture, such as RAG pipelines, EHR-proximal integration, and measure outcomes with standardized units and denominators. Future research should prioritize prospective multi-center validation, using standardized metrics, and end-user collaboration with evaluation of role-specific impacts and comparative studies of design patterns to support the responsible and effective use of AI in clinical care.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
YA: Data curation, Formal analysis, Writing – original draft. VS: Writing – review & editing. BG: Writing – review & editing. PK: Writing – review & editing. GN: Writing – review & editing. EK: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdgth.2025.1659134/full#supplementary-material
Abbreviations
AI, artificial intelligence; CBT, cognitive behavioral therapy; EHR, electronic health record; GPT, generative pre-trained transformer; LLM, large language model; NHS, National Health Service; NLP, natural language processing; PHQ-9, patient health questionnaire-9; PROBAST, prediction model risk of bias assessment tool; SSPEC, site-specific prompt engineering chatbot; AUROC, area under the receiver operating characteristic curve; AUPRC, area under the precision–recall curve; ECE, expected calibration error; SUS, system usability scale; UMUX-lite, usability metric for user experience, short form; TAM, technology acceptance model; NASA-TLX, NASA task load index; PRO/PROs, patient-reported outcome/patient-reported outcomes; PHI, protected health information; SES, socioeconomic status; NCC-MERP, national coordinating council for medication error reporting and prevention.
References
1. Bekbolatova M, Mayer J, Ong CW, Toma M. Transformative potential of AI in healthcare: definitions, applications, and navigating the ethical landscape and public perspectives. Healthcare. (2024) 12(2):125. doi: 10.3390/healthcare12020125
2. Faiyazuddin M, Rahman SJQ, Anand G, Siddiqui RK, Mehta R, Khatib MN, et al. The impact of artificial intelligence on healthcare: a comprehensive review of advancements in diagnostics, treatment, and operational efficiency. Health Sci Rep. (2025) 8(1):e70312. doi: 10.1002/hsr2.70312
3. Poon H. Multimodal generative AI for precision health. NEJM AI. (2023) 1(5):AI-S2300233. doi: 10.1056/AI-S2300233
4. Beam AL, Kohane IS. Making the most of AI in health care: strategic principles for implementation. NEJM AI. (2023) 1(2):AIp2300031. doi: 10.1056/AIp2300031
5. Wang S, Aggarwal A, Singh S, Morency L, Kitani K, Wu D, et al. Red teaming ChatGPT in medicine to yield real-world insights on model behavior. NPJ Digit Med. (2025) 8(1):56. doi: 10.1038/s41746-025-01542-0
6. Meng X, Yan X, Zhang K, Liu D, Cui X, Yang Y, et al. The application of large language models in medicine: a scoping review. iScience. (2024) 27(5):109713. doi: 10.1016/j.isci.2024.109713
7. Hoffman J, Wenke R, Angus RL, Shinners L, Richards B, Hattingh L. Overcoming barriers and enabling artificial intelligence adoption in allied health clinical practice: a qualitative study. Digit Health. (2025) 11:20552076241311144. doi: 10.1177/20552076241311144
8. Omar M, Soffer S, Agbareia R, Bragazzi NL, Apakama DU, Horowitz CR, et al. Sociodemographic biases in medical decision making by large language models. Nat Med. (2025) 31(6):1873–81. doi: 10.1038/s41591-025-03626-6; Epub ahead of print.40195448
9. Lee J, Park S, Shin J, Cho B. Analyzing evaluation methods for large language models in the medical field: a scoping review. BMC Med Inform Decis Mak. (2024) 24(1):366. doi: 10.1186/s12911-024-02709-7
10. Artsi Y, Klang E, Collins JD, Glicksberg BS, Korfiatis P, Nadkarni GN, et al. Large language models in radiology reporting—a systematic review of performance, limitations, and clinical implications. Intell Based Med. (2025) 12:100287. doi: 10.1016/j.ibmed.2025.100287
11. PRISMA. Transparent reporting of systematic reviews and meta-analyses [Internet]. (2021). Available online at: https://www.prisma-statement.org/ (Accessed April 10, 2025).
12. Wang B, Lai J, Cao H, Jin F, Li Q, Tang M, et al. Enhancing the interoperability and transparency of real-world data extraction in clinical research: evaluating the feasibility and impact of a ChatGLM implementation in Chinese hospital settings. Eur Heart J Digit Health. (2024) 5(6):712–24. doi: 10.1093/ehjdh/ztae066
13. Wan P, Huang Z, Tang W, Zhang X, Cao Y, Xu Y, et al. Outpatient reception via collaboration between nurses and a large language model: a randomized controlled trial. Nat Med. (2024) 30(12):2878–85. doi: 10.1038/s41591-024-03148-7
14. Habicht J, Dina LM, McFadyen J, Stylianou M, Harper R, Hauser TU, et al. Generative AI-enabled therapy support tool for improved clinical outcomes and patient engagement in group therapy: real-world observational study. J Med Internet Res. (2025) 27:e60435. doi: 10.2196/60435
15. Garcia P, Ma SP, Shah S, Smith M, Jeong Y, Devon-Sand A, et al. Artificial intelligence-generated draft replies to patient inbox messages. JAMA Netw Open. (2024) 7(3):e243201. doi: 10.1001/jamanetworkopen.2024.3201
16. Sarker IH. Machine learning: algorithms, real-world applications and research directions. SN Comput Sci. (2021) 2(3):160. doi: 10.1007/s42979-021-00592-x
17. Ho CN, Tian T, Ayers AT, Aaron RE, Phillips V, Wolf RM, et al. Qualitative metrics from the biomedical literature for evaluating large language models in clinical decision-making: a narrative review. BMC Med Inform Decis Mak. (2024) 24(1):357. doi: 10.1186/s12911-024-02757-z
18. Longhurst CA, Singh K, Chopra A, Atreja A, Brownstein JS. A call for artificial intelligence implementation science centers to evaluate clinical effectiveness. NEJM AI. (2024) 1(8):e2400223. doi: 10.1056/AIp2400223
19. Ratwani RM, Classen D, Longhurst C. The compelling need for shared responsibility of AI oversight: lessons from health IT certification. J Am Med Assoc. (2024) 332(10):787–8. doi: 10.1001/jama.2024.12630
20. Anisuzzaman DM, Malins JG, Friedman PA, Attia ZI. Fine-tuning large language models for specialized use cases. Mayo Clin Proc Digit Health. (2024) 3(1):100184. doi: 10.1016/j.mcpdig.2024.11.005
21. Thomo A. Pubmed retrieval with RAG techniques. Stud Health Technol Inform. (2024) 316:652–3. doi: 10.3233/SHTI240498
Keywords: large language models, real-world application, clinical implementation, artificial intelligence, healthcare workflows
Citation: Artsi Y, Sorin V, Glicksberg BS, Korfiatis P, Nadkarni GN and Klang E (2025) Large language models in real-world clinical workflows: a systematic review of applications and implementation. Front. Digit. Health 7:1659134. doi: 10.3389/fdgth.2025.1659134
Received: 3 July 2025; Accepted: 11 September 2025;
Published: 30 September 2025.
Edited by:
Seedahmed S. Mahmoud, Shantou University, ChinaReviewed by:
Katherine Blondon, Hôpitaux universitaires de Genève (HUG), SwitzerlandUrs Fisch, University Hospital of Basel, Switzerland
Copyright: © 2025 Artsi, Sorin, Glicksberg, Korfiatis, Nadkarni and Klang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaara Artsi, eWFhcmEuYXJ0c2k3N0BnbWFpbC5jb20=
 Yaara Artsi
Yaara Artsi Vera Sorin2
Vera Sorin2 Benjamin S. Glicksberg
Benjamin S. Glicksberg Girish N. Nadkarni
Girish N. Nadkarni