- 1Department of Psychology, University of South Carolina, Columbia, SC, United States
- 2Institute for Mind and Brain, University of South Carolina, Columbia, SC, United States
- 3Carolina Autism and Neurodevelopment Research Center, University of South Carolina, Columbia, SC, United States
- 4Linguistics Program, University of South Carolina, Columbia, SC, United States
Developmental cognitive neuroscience studies the evolution of the bidirectional links between biology and cognition during development. An area of recent focus is the impact of social factors on the biology-cognition link. Indeed, recent calls-to-action encourage a more dynamic approach to investigating mechanisms related to the development of the social brain. To address this need, we utilized a burgeoning innovation in cognitive neuroscience known as “hyperscanning”, which allows for real-time synchronized measurements of biological signals (e.g., brain signals via electroencephalography, EEG; cardiac activity via electrocardiogram, ECG) across two people engaged in social interaction. The potential of hyperscanning has yet to be tapped for research with diverse and developmental populations underrepresented in neuroscience (and science broadly), including pediatric clinical and racial minority populations. The present manuscript provides proof-of-concept for the use of naturalistic and inclusive hyperscanning paradigms. For this research, we adapted a collaborative conversation task that allowed us to examine differences in synchronized measures of sociocognitive mechanisms (specifically, motivation and language) across different social contexts (familiar child dyads, stranger child dyads, familiar adult-child dyads, and stranger adult dyads). Preliminary results from a pilot study with 45 racially diverse autistic and non-autistic participants indicate that, at the group level, youth are less accurate and need more hints than adults, peer dyads (i.e., child-child, adult-adult) are more approach-motivated, and dyad features (e.g., familiarity) influence how linguistically aligned individuals are during the task. Additionally, we provide initial evidence for within-person biology-behavior links and asymmetrical between-person alignment of approach motivational brain states that indicate that one's current motivation state was predicted to be opposite of their partner and vary subtly across social contexts. Overall, this hyperscanning task is sensitive to developmental and contextual factors and will propel our understanding of social and cognitive processes. We encourage cognitive developmentalists to consider recommendations laid out in the current proof-of-concept to take actionable steps in moving the field toward more inclusive and pervasive research.
1 Introduction
Early developmental theories emphasize the role of social connections (e.g., relationships) on cognitive development (Gauvain, 2001). For instance, classic Vygotskyian theory postulates that learning emerges from social interactions, where cognitive abilities are established through conversation and collaboration with others (Vygotsky, 1978). Bronfenbrenner's ecological systems theory further emphasizes that development is dependent and contingent on the nature of certain kinds of direct and indirect relationships, and that these relationships continue to evolve across the lifespan (Bronfenbrenner and Morris, 2006). Considering the multifaceted nature of cognitive development, modern developmental psychology has expanded beyond behavioral responses to integrate and understand how biological foundations inform and influence cognition (Johnson, 2011; Miller, 2022).
Indeed, since the late 1970s, brain measurement techniques such as electroencephalography (EEG) and functional neuroimaging (e.g., functional near-infrared spectroscopy, fNIRS; functional magnetic resonance imaging, fMRI) began providing evidence of the maturation and functional organization of the developing brain that supports cognitive development (Blakemore and Frith, 2005; Immordino-Yang et al., 2025; Immordino-Yang and Damasio, 2007) – subsequently leading to the establishment of the field of developmental cognitive neuroscience. While this field has generated important research that studies underlying mechanisms of different aspects of cognition, including mechanisms supporting sociocognition (Johnson, 2011), many paradigms fail to consider how these mechanisms might differ or develop in real-world settings that are inherently varied for individuals from different backgrounds. More specifically, sociocognition does not operate in isolation and requires flexible engagement during real-world settings that are inherently dynamic and complex (Salley and Colombo, 2016). While there is merit and importance in discovering individual and group-level biological and behavioral responses to manipulated and well-controlled experimental stimuli (e.g., static images of faces, videos of biological motion, speech sounds), understanding how cognitive mechanisms required for interactive and rapidly changing social dynamics emerge and change over different developmental contexts is a crucial next step for the field (Redcay and Schilbach, 2019).
One innovative approach that is rapidly growing in cognitive neuroscience involves “hyperscanning” techniques where brain responses from two people are recorded at the same time (Babiloni and Astolfi, 2014; Dumas et al., 2011). This method is particularly valuable for gaining a deeper understanding of the development of sociocognition as it permits analysis at the individual level (i.e., one person's response), sequentially (i.e., unidirectional relationships from one person to another), or simultaneously (i.e., bidirectional relationships between people). Hyperscanning is often conducted within interactive settings, such as between romantic partners during physical connection (Nelson et al., 2024), high school students and teachers during lectures (Bevilacqua et al., 2018), and individuals engaged in cooperative decision-making (Hu et al., 2018). Although dual fMRI hyperscanning is possible (Montague et al., 2002; Speer et al., 2024), most developmentally focused work has utilized either fNIRS or EEG (see Killeen and Teti, 2012; Liao et al., 2015; McDonald and Perdue, 2018), where infants, children, and adolescents can interact in more ecological and familiar environments. Indeed, EEG hyperscanning is positioned to address a variety of sociocognitive and cognitive processes, including attention, communication, cooperation/shared actions, and decision making (Liu et al., 2018).
As examples pertinent to cognitive development, EEG hyperscanning can capture correlates across multiple sociocognitive mechanisms (e.g., motivation, language) between parents and children during collaborative puzzle building (Atzaba-Poria et al., 2017) and directed gaze tasks (Leong et al., 2017). Additionally, a smaller body of work has begun to investigate the role of collaboration in clinical populations defined by social impairments or differences. For instance, by definition, “social communication impairments” are diagnostic of autism spectrum disorder (American Psychiatric Association, 2013) and include difficulties in understanding, responding to, and utilizing social cues with other people (Bradshaw et al., 2021; Bishop et al., 2016). While social communication often relies on nonverbal behaviors (e.g., eye contact, gestures, facial expressions), non-visual verbal communication is also unique in autism. During conversation, autistic people are known to perseverate on topics and initiate topic shifts less frequently yet offer similar number of conversation turns to partners (see systematic review in Sng et al., 2018). Burgeoning work targeting shared brain responses within goal-directed settings indicates unique autistic neural signatures within cooperative interactions between parents and autistic children (Wang et al., 2020) and autistic and non-autistic peers (Chen et al., 2025). Although these studies are a crucial first-step in better understanding the biological correlates of sociocognitive mechanisms in real-time social interactions, there still exists gaps to better understand how these mechanisms are related to other sociocognitive mechanisms (e.g., language) and shift developmentally, across different social contexts (e.g., parent-child, peer-peer), varied neurodevelopment trajectories (e.g., atypical-typical, atypical-atypical, typical-typical), and diverse sociocultural backgrounds (e.g., race, ethnicity, socioeconomic status).
1.1 Current objective
We argue that hyperscanning is a methodological breakthrough that will transform developmental cognitive psychology, and, relevant to our own scientific goals, our understanding of sociocognitive mechanisms during social interactions. However, to fully endorse hyperscanning as an innovation, the technique should improve upon our existing knowledge of cognition, be appropriate (i.e., feasible, practical, relevant) for most people, and be adaptable for different social contexts. For instance, best practices would ensure that methods can accommodate individuals with sensory sensitivities (e.g., autistic participants), technological hurdles (e.g., afro-textured hair, locs, cornrows, twists), cultural sensitivity (e.g., ensuring that stimuli or experimental design are appropriate across cultures), and be developmentally appropriate and engaging for individuals of all ages.
To illustrate the opportunities of hyperscanning relevant to cognitive development, we provide a detailed empirical example that describes the adaptation of a collaborative conversation task to be used with EEG hyperscanning across a variety of populations. As proof-of-concept, the current study set out to establish the feasibility of our adapted task across four different social contexts (child-child familiar, child-child stranger, adult-child familiar, and adult-adult stranger dyads). We also recruited diverse participants that varied in autism diagnosis and race/ethnicity (Black, White, Hispanic/Latine) to provide evidence for how cognitive development research can design hyperscanning studies that are clinically and culturally sensitive. Finally, as described more in-depth below, we sought to understand the effectiveness of hyperscanning during real-time collaborative conversations by analyzing biological and behavioral measurements of two sociocogntive mechanisms: motivation and language.
1.1.1 Developing a new collaborative conversation task
In the current study, we adapted our task (Figure 1) from the dyadic collaborative DiapixUK task (Baker and Hazan, 2011; Van Engen et al., 2010). We chose this task because it was originally designed to capture multiple spontaneous speech dialogues that may occur during conversations geared toward jointly solving problems. More specifically, the task involves giving two participants (i.e., dyad) slightly different cartoon images and asking them to work together to find 12 differences without being able to see each other's image (Baker and Hazan, 2011). Despite how natural it is for most people to engage in conversation with others, there are complex sociocognitive mechanisms that underly this seemingly natural ability (Gandolfi et al., 2023). For instance, successful communication requires conversational partners to work together to establish mutual understanding (Clark, 1996; Clark and Marshall, 1981; Pickering and Garrod, 2004). Additionally, building mutual understanding during conversation, especially one that is aimed at solving a problem, requires a desire or motivation to understand each other (Tomasello, 2014). In this way, using spontaneous but goal-directed conversations could be helpful for understanding how individuals work toward mutual understanding through aligning at several linguistic levels (e.g., lexical, syntactic; Pickering and Garrod, 2004) or increasing motivation to move toward a shared goal (i.e., approach motivation; Tomasello, 2014).
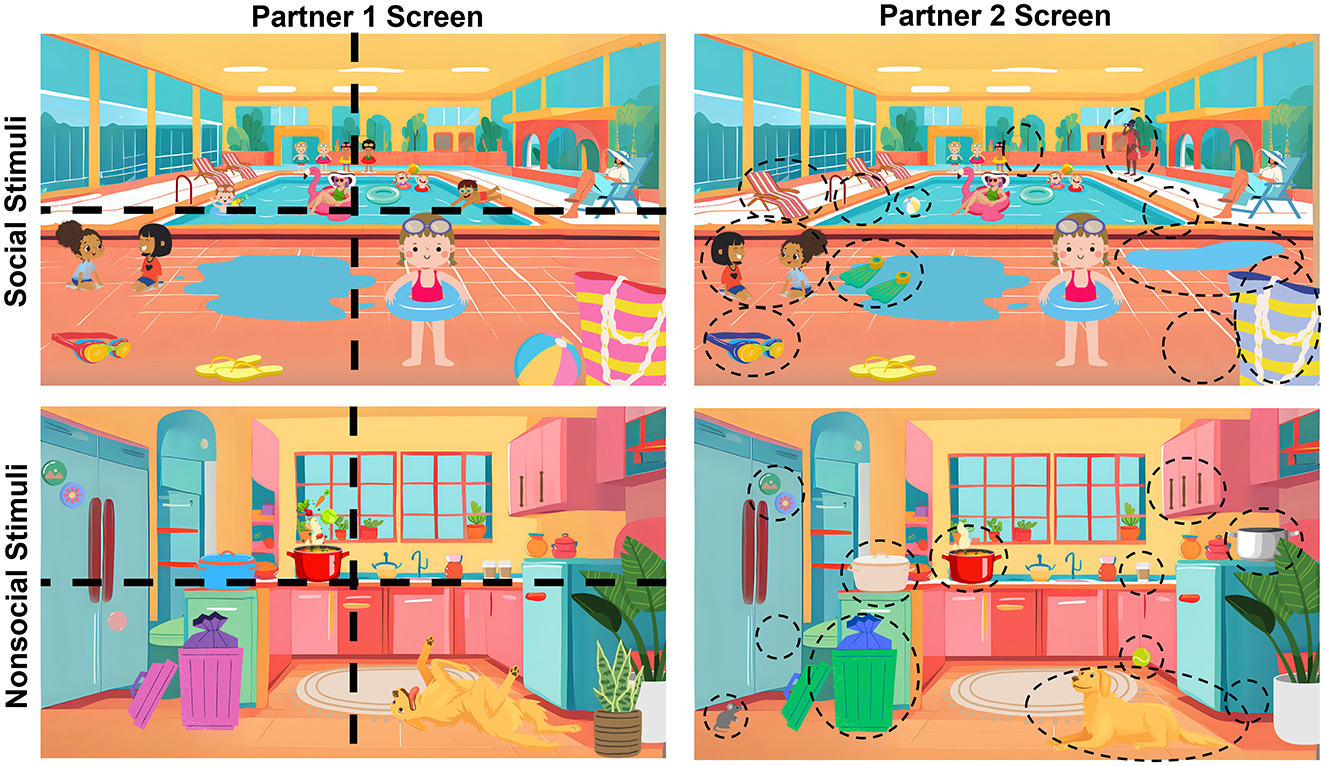
Figure 1. Examples of social and nonsocial stimuli for the collaborative conversation task. Dashed lines represent how images were divided into quadrants to ensure equal number of differences (three per quadrant). Dashed circles represent differences across the pairs of images.
Our adapted task differed from the DiapixUK task in four ways. First, the stimuli set in the DiapixUK task only contained social images (i.e., images with people). Certain populations (e.g., autistic individuals) may orient or process social images differently than other populations (Dawson et al., 1998; Kaiser et al., 2010). As such, we created social and nonsocial sets of stimuli to better control for such differences. Second, many images in the DiapixUK task also contained written words (e.g., street/store signs, speech bubbles). We wanted to make stimuli that were more inclusive to individuals regardless of age, reading ability, and reading speed. As such, our images did not contain any words or explicit representations of speech. Third, in the DiapixUK task participants must work until they finish all 12 differences or until 15 minutes have passed (Baker and Hazan, 2011). We opted to not require completion of all differences to reduce any potential frustration and maintain steady conversation. Instead, participants were given a set amount of time to find as many differences as possible and were told that although they may not find all of the differences their goal was to work together to find as many as possible. Finally, in the DiapixUK task, dyads are given a specific strategy to follow to increase linguistic comparability across dyads: participants are instructed to start in the top-left corner of the picture and continue counterclockwise (Baker and Hazan, 2011). We chose not to give specific strategies to better delineate how different social contexts influenced approaches for the task and to identify unique brain mechanisms that might be related to strategies spontaneously created and mutually agreed upon by the dyads. Instead, research assistants offered brief hints asking participants to think about discussing certain properties (e.g., “think about different colors”, “think about the number of things”) or attempting to engage a quiet participant (e.g., “describe what is on your screen”, “what do you see?”). These metrics were recorded to understand potential accommodations needed for different dyad compositions.
1.1.2 Using EEG hyperscanning to measure sociocognitive mechanisms in an ecologically valid way
EEG is a noninvasive and relatively inexpensive way to capture millisecond level postsynaptic changes in the brain that occur in different contexts (e.g., at rest, time-locked stimuli). In this way, EEG hyperscanning generates temporally rich data that can be linked across multiple sources (e.g., behaviors, linguistic output, facial expressions) and other physiological signals (e.g., heart rate, respiratory rate; Bell and Cuevas, 2012) to address critical questions about the development of cognition. Another important advantage of using EEG over other cognitive neuroscience techniques is that recent recommendations have been developed to mitigate systemic exclusion of minoritized participants. For instance, use of high-density EEG nets improve signal-to-noise ratio caused by motion or poor adherence of electrodes to the scalp (Choy et al., 2022), pulling hair through EEG nets to help electrodes make contact with the scalp (Hudac et al., 2022; Magstim EGI, 2023), and offering Black hair care products to help with participant comfortability (Brown, 2023).
Importantly, using a collaborative task, such as the one reported here (based on the Diapix task), in a hyperscanning EEG setting allows for a rich variety of outcomes that can be analyzed individually or together. Behaviors may include quantitative outcomes such as accuracy, number of hint prompts needed, or qualitative coding of strategies (e.g., visually scanning counter-clockwise or top to bottom). More detailed analysis of the language used by each person provides an opportunity to evaluate communication by each person and linguistic alignment within the dyad (e.g., agreeing on labels unique to the picture, using similar sentence structures). To better understand biological mechanisms driving sociocognitive processes, measures like EEG or heart rate can be extracted at the individual-level but also examined at the dyad-level (e.g., neural and/or heart rate synchrony). For parsimony, we opt to focus on two specific outcomes that are related to sociocognitive mechanisms, are important for building mutual understanding, and may drive collaborative conversations (i.e., language, motivation). More specifically, the current proof-of-concept reports on linguistic alignment (at the word and sentence levels) and EEG-correlates of approach motivation as proxies of sociocognitive mechanisms. Additionally, we elaborate on other potential analytic opportunities in the discussion.
1.1.2.1 Frontal alpha asymmetry as a biological measure of approach motivation
EEG data collected during collaborative conversations across diverse populations could provide useful insight into how neural mechanisms that support social interactions and sociocognitive abilities like the motivation to understand others emerge and change dynamically from person to person. While humans are motivated to form and maintain social relationships throughout their life (Baumeister and Leary, 1995), individuals may vary in how motivated they are to do so which, in turn, affects social behavior and cognition, distinctly compared to other individual differences (e.g., personality traits; Neel et al., 2016). Social approach and withdraw motivational states have been used to describe individuals' desires to engage or disengage with other people (Nikitin and Schoch, 2021). Frontal alpha asymmetry (FAA) is an EEG correlate that has been connected to approach/withdraw processes (Harmon-Jones and Gable, 2018; Smith et al., 2017) and predicts social-decision making differently in adolescents and adults (Revilla et al., 2024). Additionally, more positive FAA (indicating approach motivation), has been linked to social communication abilities in autism (Bitsika et al., 2024). As such, FAA may be an important marker of motivational brain states that support sociocognition and influence conversations geared toward building mutual understanding and enhancing collaboration.
1.1.2.2 Linguistic alignment as a behavioral measure of linguistic communication
Language is both social and cognitive in nature, particularly during interactive and collaborative conversation. It is arguably the main instrument humans rely on for social interaction and is therefore a critical aspect of sociocognition. During a conversation, interlocutors (i.e., conversational partners) must cooperate to achieve a communicative goal (Clark, 1996). This cooperation includes navigating turn-taking (Goodwin, 1981; Levinson, 2016; Wilson and Wilson, 2005) and convergence upon shared aspects of the conversation (Garrod and Anderson, 1987; Pickering and Garrod, 2004, 2021). Linguistic alignment refers to the reuse or convergence upon linguistic choices between speakers (Garrod and Pickering, 2007; Pickering and Garrod, 2004, 2021). According to the interactive alignment model (Pickering and Garrod, 2004), alignment supports efficient language processing through automatic priming mechanisms. While alignment occurs at multiple levels of linguistic representation, the present paper focuses on lexical (word) and syntactic (grammatical structure) alignment across various social contexts (e.g., dyad types).
Lexical alignment occurs when speakers reuse or repeat the word choices of their conversational partner within a conversation (Brennan and Clark, 1996). For example, two speakers may converge on the use of “bunny” rather than “rabbit” or “hare” when referring to the same animal. Lexical alignment can benefit communication by increasing the efficiency of aligning representations, which is the ultimate goal of linguistic communication (Garrod and Pickering, 2004; Pickering and Garrod, 2004). However, strict lexical repetition has limited communicative utility in conversation and can interfere with communicative efficiency as it doesn't introduce new information (Almor, 1999; Almor and Nair, 2007). Therefore, both too little and too much repetition can hinder communication efficiency.
Syntactic alignment occurs when speakers reuse a grammatical structure that has been recently produced or comprehended (Branigan et al., 2000; Levelt and Kelter, 1982). For example, after hearing a prepositional object dative structure such as “He gave the toy to the teacher”, another speaker will be more likely to reuse the same structure, as in, “She sent the letter to her mother”, rather than use the alternative double-object dative structure as in, “She sent her mother the letter” (Branigan et al., 2000). Unlike lexical alignment, which has been shown to have a clear communicative function, it is unclear whether syntactic alignment has a direct function or merely reflects automatic priming mechanisms (Ostrand and Chodroff, 2021; Ostrand and Ferreira, 2019).
There are currently mixed findings demonstrating that individuals with differing sociocognitive abilities (e.g., autistic individuals) also differ in linguistic alignment. For instance, similar amounts of lexical alignment between age-matched autistic individuals and non-autistic individuals in constrained sentence production tasks have been reported (Branigan et al., 2016). However, other research reports that autistic individuals show reduced lexical alignment compared to non-autistic individuals in less constrained dialogue contexts (Stabile and Eigsti, 2022). This further emphasizes the need to design tasks that are both ecologically valid and inclusive so that we may better understand how sociocognitive mechanisms develop to support real-time social interactions.
2 Materials and methods
2.1 Participants
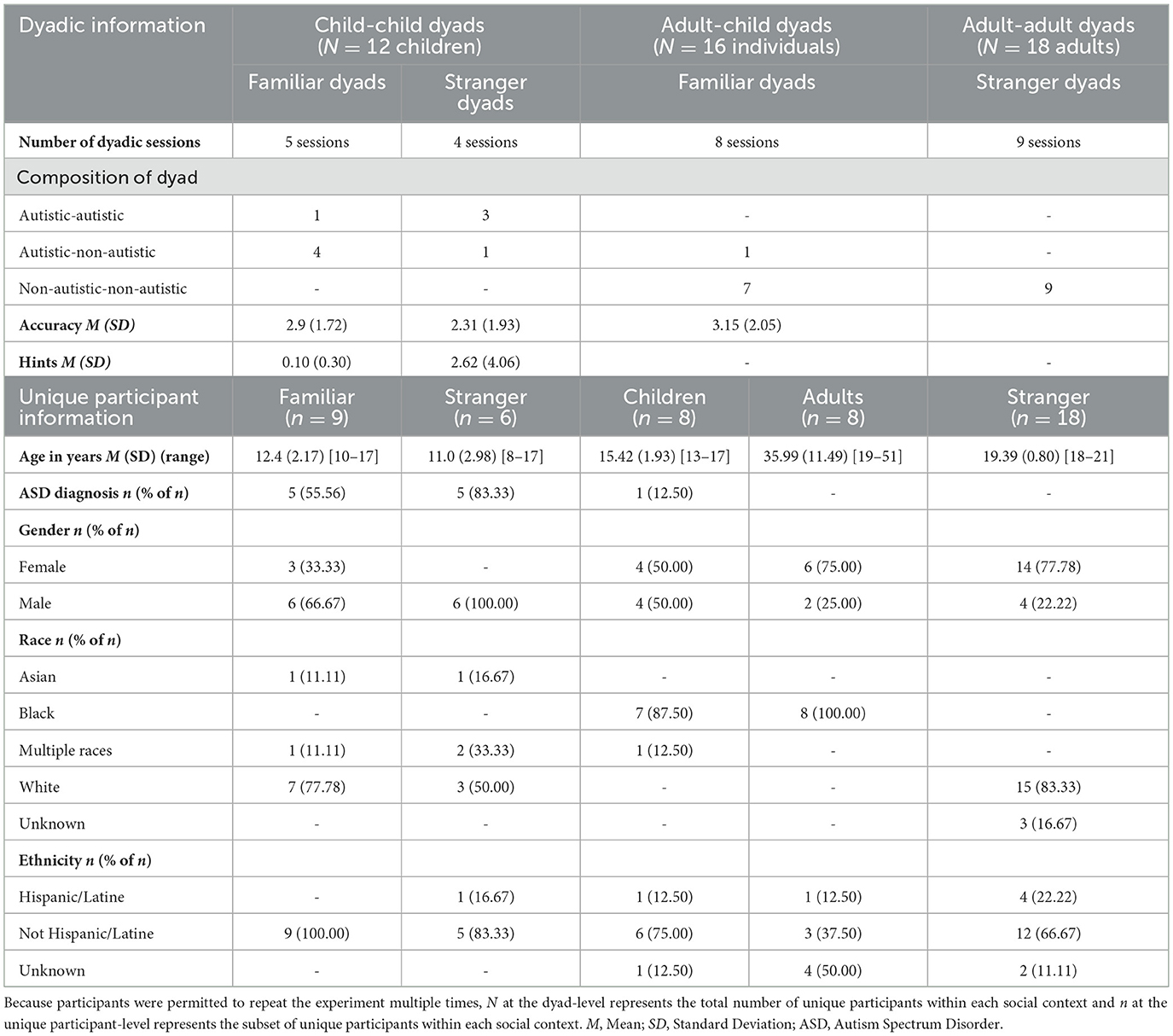
Full demographic information can be found in Table 1. Forty-five participants completed our adapted collaborative conversation task across 26 dyadic sessions for three different ongoing studies at a university in the southeastern United States. Given that the goal of the current study was to provide proof-of-concept for adapting a task for hyperscanning EEG settings and diverse populations, we only used data that was available for processing from the ongoing studies and no a priori power analyses were conducted to establish appropriate sample sizes. Social context was determined by the nature of the ongoing studies' design. As such, we evaluate data from four different social contexts: (1) five child-child familiar dyads (comprised of 3 sibling dyads and 2 friend dyads), (2) four child-child stranger dyads, (3) eight adult-child familiar dyads where adults were an adult family member (5 parent, 2 older sibling, 1 grandparent), and (4) nine adult-adult stranger dyads. All child-child dyads had at least one autistic participant. One child-child familiar and three child-child stranger dyads had two autistic participants. One adult-child familiar dyad had an autistic child. All autism diagnoses were confirmed via parent-report. Participants were permitted to complete multiple studies. Four individuals completed this task twice as a child-child stranger and familiar dyads, and one autistic child completed this task four times (three times in child-child stranger dyads and once in an adult-child familiar dyad).
Participants were recruited from flyers distributed among the community, a theater summer camp for autistic students (organized and led by the authors' research group), and a local research registry. Adult-adult dyads were recruited via the university's participant research pool for undergraduate students or by word-of-mouth. For adult-child and child-child dyads, the ongoing studies recruited existing caregiver-child and friend dyads, respectively, that were established before the start of data collection. Child-child stranger and adult-adult dyads were assigned as partners based on availability. Child-child and adult-child dyads were compensated for their time via gift cards. Adult-adult dyad participants (n = 15) were compensated via course credit except for two participants who were compensated via gift cards and one participant who requested to volunteer.
2.2 Stimulus design and procedures
2.2.1 Collaborative conversation task
Stimuli for the collaborative conversation task were designed as cartoon illustrations in Canva using a paid subscription. For some illustrations, we used Canva's built-in AI image generator “MagicMedia” to help create custom backgrounds (e.g., pool, kitchen) and cartoon images (e.g., person wearing sunhat, dog rolled over). Similar to the DiapixUK task (Baker and Hazan, 2011), each stimuli set contained 12 differences, corresponding to three differences within each quadrant of the image (see Figure 1). Differences were created by manipulating the presence, color, orientation, and number of items within the pairs of images. To increase accessibility of this task, we opted to exclude any written text in the stimuli. Stimuli also varied in content such that participants saw an equal number of social (i.e., containing people) and nonsocial stimuli (i.e., not containing people). A total of 18 stimuli sets were made to permit repeat testing up to three times with a set of 6 stimuli.
In the collaborative conversation task, participants within a dyad were seated facing a separate computer screen in the same room with a partition between them, such that they could not see each other's screens. Instructions were explained by a research assistant via a practice example. Dyads were told they would only see one image and their partner had a different version of the image. Dyads were instructed that they would have a few minutes to work together to find all 12 differences for each image. Each block of the task (i.e., one pair of stimuli) lasted 90 seconds and participants were told that the goal of the task was to work together to find as many of the 12 differences as possible. No specific strategy was given to participants, although they were allowed to request help from research assistants sitting in the room with them. Given that dyads completing the original Diapix task found 10 differences in no less than 5.34 min (Van Engen et al., 2010), we did not anticipate dyads being able to find 12 differences in 90 s. Research assistants remained in the room during the task to confirm for participants each time they spotted a correct difference and offer hints, as needed. Dyad accuracy was calculated by summing the number of times research assistants marked the dyad successfully found a difference. Number of hints needed per dyad was also calculated by summing the number of times research assistants offered strategic help. Due to differences in study setup and timing, child-child and adult-adult dyads completed six blocks (three social image pairs, three nonsocial image pairs) and adult-child dyads completed four blocks (two social, two nonsocial); however, for a more accurate comparison across social contexts, we report on data from only the first four blocks. Overall, participant feedback revealed that the majority (>65%) of participants enjoyed or had fun with the task (see Supplementary Table 1).
2.2.2 EEG acquisition and processing
Continuous EEG was recorded from high-density 128-channel geodesic sensor nets using Net Station 5.3 software integrated with two identical EEG high-impedance 400-series amplifiers (Magstim-EGI, Eugene OR USA). Across all 26 dyadic sessions, 55.56% of participants self-reported having thick, textured, curly, or protective hair (e.g., locs, braids, twists). During acquisition, EEG signals were referenced to the vertex electrode, analog filtered (0.1 Hz high-pass, 100 Hz elliptical low-pass), amplified, and digitized with a sampling rate of 250 Hz. Data was only analyzed during the 90 s per block (360 s for 4 blocks), even if participants were still talking after the block was over. Standard post-processing procedures included bandpass filtering between 0.1 Hz−40 Hz and automated artifact correction using the “clean_rawdata” plugin in EEGLAB (Delorme and Makeig, 2004). In line with Delorme's (2023) examination of preprocessing standards, channels were rejected using a rejection threshold of 0.9. Large artifacts were then removed via spectrum thresholding using the “pop_rejcont” function of EEGLAB (frequency range: 20–40 Hz, threshold: 10 dB). All removed channels were interpolated, and data was re-referenced to average. There was a difference in the amount of data lost by social context, F(2, 49) = 6.25, p = 0.0038, such that adult-child data loss (M = 13%, SD = 9%) was significantly less compared to child-child (M = 23%, SD = 8%), p = 0.0037) and adult-adult dyads (M = 20%, SD = 7%), p < 0.0321 with Tukey HSD correction.
As noted above, we selected FAA as our primary neural correlate of approach motivation. To confirm FAA was an appropriate outcome of interest, Supplementary Figure 1 demonstrates individual power spectrum data and topographic plots for the entire sample (N = 45, across 26 dyad sessions) across delta (1–4 Hz), theta (4–7 Hz), alpha (8–12 Hz), and beta (12–21 Hz) frequency bands. Specifically, this figure demonstrates typical attenuation of power over each frequency range (Panel B) and evidence of increased relative alpha power compared to lower frequency bands (e.g., delta, theta) across all social contexts (Panel C). With additional evidence from clear frontal activation of alpha (Panel A), we determined it was appropriate to continue with FAA as our EEG outcome of interest.
EEG for each block within the collaborative conversation task was epoched with 500 ms windows and decomposed by frequency using the default EEGLAB settings for time-frequency analyses. Relative and absolute alpha (8–12 Hz) power were then averaged across channels into two frontal clusters (Magstim-EGI 128-channel net: left channels = 23, 24, 26, 27, 33; right channels = 2, 3, 122, 123, 124) for each trial. FAA was calculated by subtracting the natural log-transformed alpha of the left cluster from the right [ln(right) – ln(left)]. Preliminary models indicated that both relative and absolute alpha exhibited significant social contexts differences between left and right hemispheres, p < 0.0001. To be consistent with the literature (Vincent et al., 2021), FAA extracted from relative alpha power was utilized throughout the remainder of the study.
2.2.3 Linguistic acquisition and processing
Participant conversations during the collaborative task were recorded in video form using web-cameras connected to computers recording EEG data. As part of the Net Station 5.3 software, videos are automatically recorded at the start of EEG recording. After each session, audio was extracted from each video file and conversations were initially transcribed using Microsoft Word's automatic transcription feature, including separation of each speaker with timestamps. Transcripts were manually reviewed (authors C.M.N. and J.M.) and content was edited (i.e., adding in any words or phrases that were missed by the transcription software). Lastly, to prepare transcripts for the automated pipeline used to extract linguistic alignment, the text was edited for punctuation, ensuring that each utterance ended with a period and that all text was lowercase excluding proper nouns (see Supplementary material 1 for an example).
All data described in the current manuscript was taken from ongoing studies; thus, only a subset of dyads were included in the linguistic analysis based upon those that had finished transcriptions at the time of the current analyses. This included the entire sample of nine child-child dyads, five adult-child dyads, and eight adult-adult dyads. Lexical and syntactic alignment scores were obtained from the transcripts using Analyzing Linguistic Interactions with Generalizable techNiques (ALIGN; Duran et al., 2019), an open-source Python library which extracts alignment scores based on the cosine similarity between interlocutor's turns. Importantly, ALIGN produces cosine similarity scores of lexical, syntactic, and semantic (conceptual) alignment at two different levels: (1) for the entire conversation between dyads, used in the current analyses to understand group-level differences; and (2) for each conversational turn participants make within the dyad, used in the current analyses to understand dyad level and moment by moment changes in alignment. Cosine similarity is a measure of how similar two vectors are (representing two data points, such as words) in a multi-dimensional space, in terms of directionality, calculated as the cosine of the angle between the vectors (Jurafsky and Martin, 2008). Lexical alignment is quantified as the cosine similarity of the lemmatized (uninflected base, e.g., “dogs” becomes “dog”) lexical items between speakers (Duran et al., 2019). Syntactic alignment is quantified as the cosine similarity of part-of-speech (POS)-tagged word forms between speakers (Duran et al., 2019). ALIGN segments the lemmatized and POS-tagged word forms from each speaker's utterance into series of short chunks of different lengths, known as n-grams (Duran et al., 2019). For the current paper, we selected bi-grams (i.e., two-word sequences) to evaluate lexical and syntactic alignment at the conversation and turn-by-turn level. In both lexical and syntactic alignment, scores may range from 0 to 1, where scores closer to 1 indicate stronger alignment between partners (Duran et al., 2019).
2.3 Analytic plan
Our objective was to provide initial evidence of how this collaborative conversation task generates useful measures of sociocognition in dyadic interactions across a diverse range of participants. Thus, our analyses explored social context differences based upon the dyad partners: child-child familiar, child-child stranger, adult-child familiar, and adult-adult stranger. All analyses were performed using R (version 4.3.1) and estimated marginal means and standard errors are reported with false-discovery rate correction (Benjamini and Hochberg, 1995) for all post-hoc testing.
First, dyad-level differences in behavior (accuracy, hints), neural approach motivation (FAA), and linguistic (lexical alignment, syntactic alignment) outcomes were examined. Base models were linear mixed-effects models that were computed using restricted maximum likelihood with Nelder-Mead optimization via the ‘lme4' package (Bates et al., 2015). For each outcome, we examined interacting effects of social context (i.e., different dyad type) and block (via blocks of experiment) while controlling for main effects of autism diagnosis and age. To account for the nested structure of the data, random intercepts for participants nested within dyads were included. Because dyad-level linguistic outcomes were measured across the entire conversation, linear mixed-effects models did not include effects of block and contained a random intercept per dyad.
Second, within-person cross-measure analyses were evaluated by examining how an individual's neural motivation state (FAA) predicted their own linguistic alignment at the turn-by-turn level. We evaluated cross-measure relationships at concurrent time (i.e., association within same 10 second bin; Figure 2A). To ensure 10 second bins aligned across linguistic and neural data, we edited the linguistic transcripts to allow for the participant information to include block and bin number (see Supplementary material 2). To illustrate and understand associations between brain state and behavior, we used packages ‘ggplot2' (Wickham, 2016) and ‘ggpubr' (Kassambara, 2025) to report Pearson's correlation coefficients with false-discovery rate to correct p-values for multiple comparisons. Then, linear mixed-effects models estimated the extent by which FAA predicted linguistic outcomes, separately for lexical and syntactic alignment. Models included fully interacting effects of social context and lag while controlling for age and autism diagnosis.
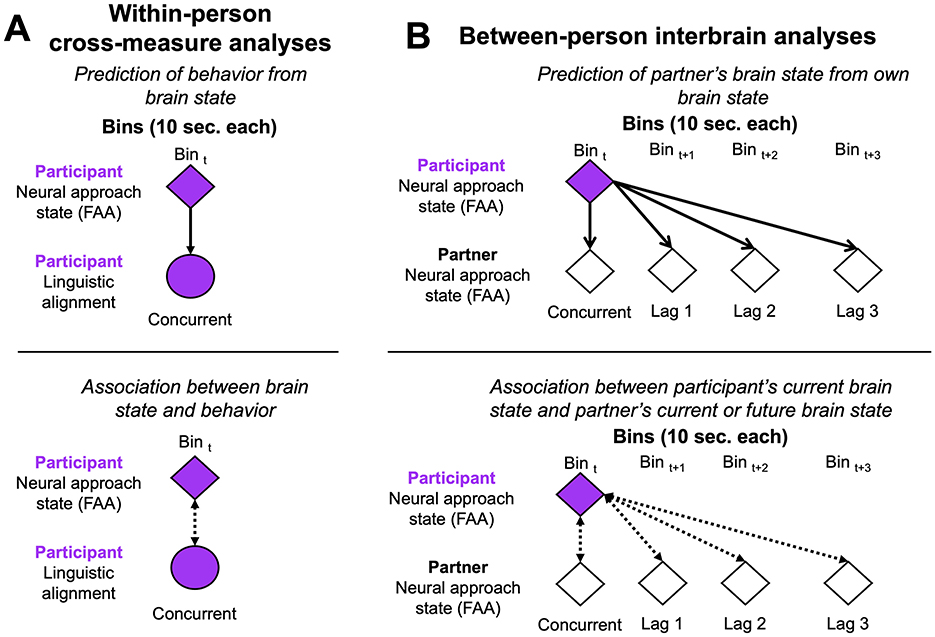
Figure 2. Overview of time-based analytic plan. (A) Illustrates within-person cross measure analyses where behavior was predicted from (top) and associated with (bottom) brain state at concurrent time. (B) Illustrates between-person interbrain analyses where participant's partner's brain state was predicted from (top) and associated with (bottom) their own current or future brain states.
Lastly, between-person interbrain analyses were evaluated to better understand neural agreement between partners and how this may change over subsequent time. Adopting an approach used previously in EEG hyperscanning (Nelson et al., 2024), we examined concurrent and lagged similarities in FAA between each participant and their partner (Figure 2B). First, we examined two different measurements of associations: (1) concordance correlation coefficients (scale limits = −1 to 1; Lin, 1989, 2000) to measure agreement between participant-partner in a way to capture accuracy and magnitude (i.e., are FAA values equal in value?), and (2) Pearson correlations to measure linear association to capture the direction of correlations. In other words, while concordance correlation coefficients can describe whether dyad partners are in a similar motivation state, Pearson correlations can describe whether the state is “approach” or “withdraw”. For each association analysis, we examined concurrent associations and opted to use lagged time at one-, two-, and three-step lags to determine how a participant's current motivation was influenced by their partner in the moment and from their partner's past motivation. Any bin where one partner had missing data was removed from analyses. Concordance correlation coefficients were extracted using package ‘epiR' (Stevenson and Sergeant, 2025) for each person and inputted into the base linear mixed-effects model with lag as an interacting factor to test concordance differences by social context. Pearson's correlation coefficients were extracted with the same method as described above with false-discovery rate corrected p-values to visualize relationships. Lastly, we used linear mixed-effects models to test whether a participant's neural motivation state was predicted by their partner's neural motivation state at both concurrent and lagged time (Figure 2B). Models included fully interacting effects of social context, partner's current brain state, and lag while controlling for age and autism diagnosis.
3 Results
3.1 Dyad-level characterization by social context
Full model results for linear mixed-effects models are reported in Supplementary Table 2. Autism diagnosis and age were not significant predictors of any outcome measure within dyad-level analyses (p > 0.235) and, thus, are not further discussed.
3.1.1 Accuracy and number of hints needed
While no dyads found all 12 differences, all dyads managed to correctly find at least one difference by the second block, though three child-child dyads (1 familiar, 2 stranger) did not correctly find any difference in the first 90-s block. Overall, dyads became more accurate across blocks (p < 0.0002), suggesting improvement. However, accuracy varied by social context and block (p = 0.012), highlighting three patterns: (a) linear improvement in adult-child familiar dyads, (b) best performance in middle blocks for child-child stranger dyads, and (c) minimal improvement in child-child familiar and adult-adult stranger dyads (Figure 3).
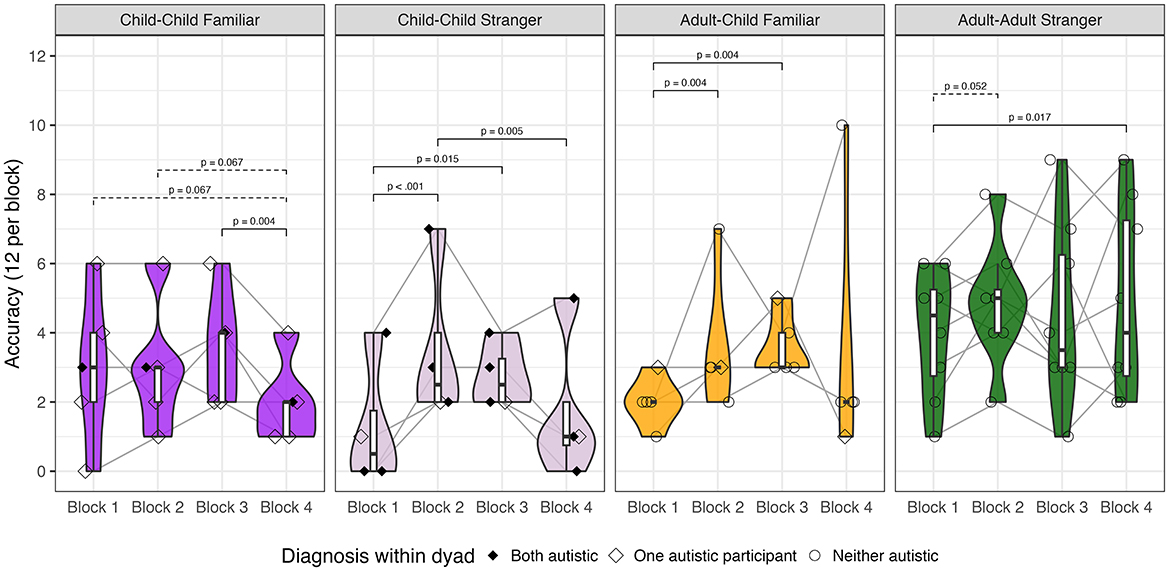
Figure 3. Group-level accuracy by block. Within each social context (i.e., type of dyad), accuracy is presented as boxplots to demonstrate quartile range and violin plots to demonstrate density. Shapes indicate whether both participants were autistic (filled diamond), one participant was autistic (hollow diamond), or neither participant was autistic (hollow circle). FDR-corrected pairwise differences are noted between blocks within social context.
A trend indicated that child-child stranger dyads required more hints than other social contexts (p = 0.0591), driven in part by two dyads that needed 8 hints and 34 hints, overall. Two child-child familiar dyads requested a hint in the second block. None of the adult-child familiar or adult-adult stranger dyads required hints.
3.1.2 Neural state of approach motivation (FAA)
Figure 4 demonstrates topographic plots and violin plots of FAA for all social contexts across blocks. Overall, FAA decreased across block (p < 0.0001, see Supplementary Table 4 for descriptive statistics), indicating reduction in approach motivation across the task. FAA significantly differed by social context (p = 0.0423), such that peer dyads (child-child familiar, child-child stranger, and adult-adult stranger) exhibited more approach-like motivation (i.e., positive FAA; p < 0.0160), whereas adult-child familiar dyads had more neutral brain states (i.e., zero-like FAA values; p = 0.450).
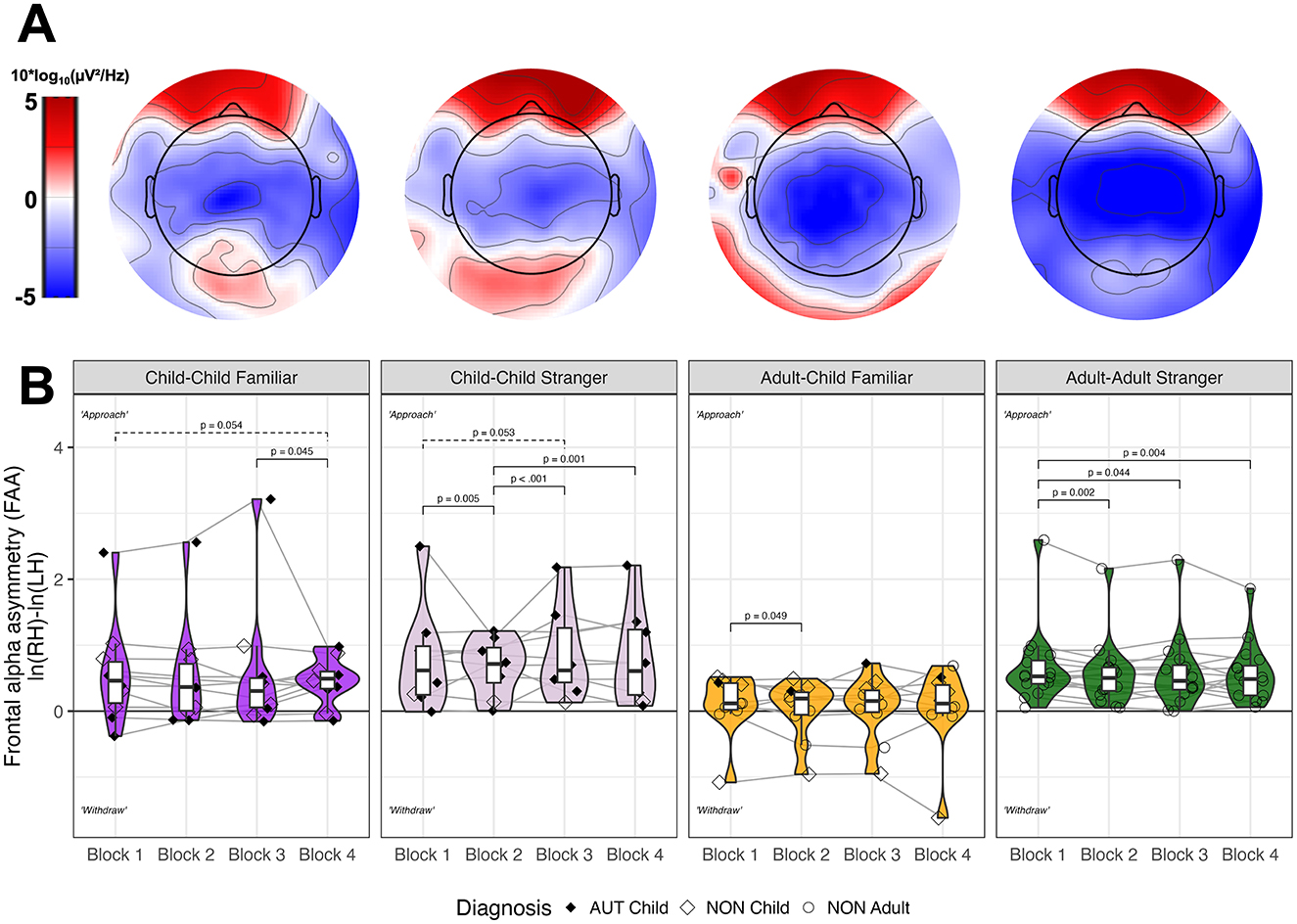
Figure 4. Neural patterns across social context. (A) Topographic plots illustrate distribution of 10*log10 of alpha band (8–12 Hz) power spectral density (μV2/Hz) across frontal electrodes. (B) Frontal alpha asymmetry (FAA) is plotted as the natural log of relative alpha power for right compared to left [hemisphere i.e., ln(RH)-ln(LH)] for each individual by social context, averaged within each block. Boxplots demonstrate quartile range and violin plots demonstrate density. Shapes indicate whether an individual is an autistic child (filled diamond), non-autistic child (hollow diamond), or non-autistic adult (hollow square). FDR-corrected pairwise differences are noted between blocks within social context.
3.1.3 Lexical and syntactic alignment
Descriptively, lexical alignment at the conversation level was moderate (ranging from 0.1 to 0.84) across social context (p = 0.85, see Figure 5). In contrast, dyads exhibited moderate to strong syntactic alignment at the conversation level, ranging from 0.53 to 0.97. Syntactic alignment varied by social context (p = 0.0474) and post-hoc comparisons suggested this effect was driven by lower syntactic alignment in child-child stranger relative to familiar dyads (p = 0.0438, uncorrected).
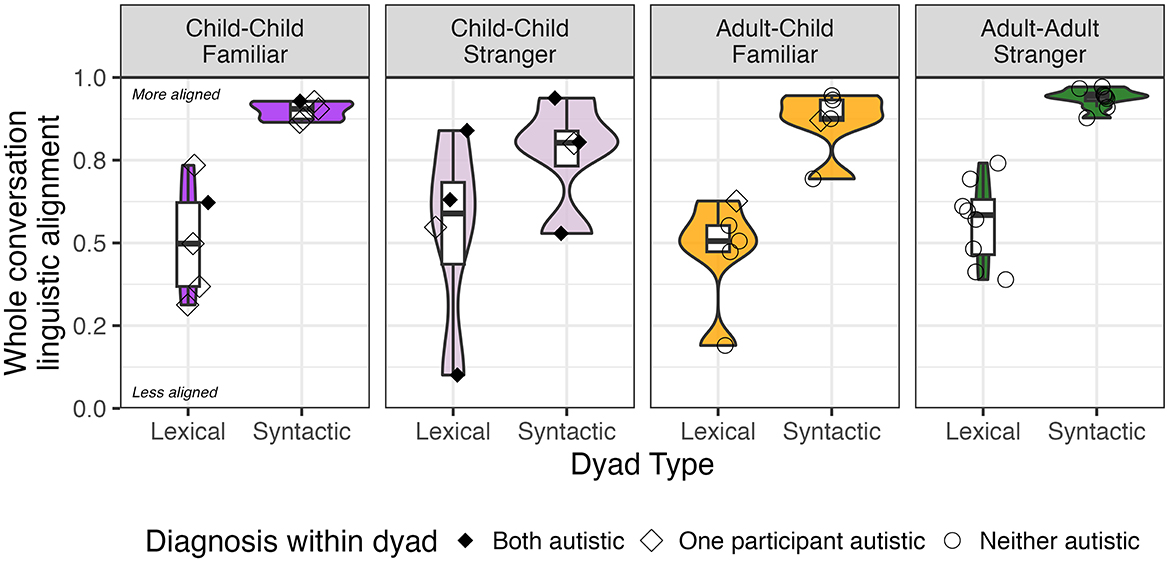
Figure 5. Group-level syntactic and lexical alignment at the conversation level by dyad type and dyad familiarity. Values range from 0 (no alignment) to 1 (most alignment). Boxplots demonstrate quartile range and violin plots demonstrate density. Shapes indicate whether both participants were autistic (filled diamond), one participant was autistic (hollow diamond), or if neither participant was autistic (hollow circle).
3.2 Within-person cross-measure analyses between neural and linguistic outcomes
Because these analyses required neural and linguistic data within each bin, we first confirmed sufficient data was retained per social context. The least amount of data was retained for the child-child stranger dyads (76.4%) relative to child-child familiar (90.1%), adult-child familiar (92.2%), and adult-adult stranger (93.4%) dyads (p < 0.0001). Cross-measure Pearson correlations were extracted and illustrated in Figure 6 for both lexical and syntactic alignment at a turn-by-turn level. Notably, negative relationships were noted such that increased approach motivation (i.e., positive FAA) was related to reduced linguistic alignment in adult-adult stranger dyads (both lexical and syntactic alignment) and adult-child familiar (syntactic alignment only). Lastly, across all participants, the linear mixed-effects model confirmed the directionality of these associations. Specifically, models indicated that increased approach motivation predicted reduced lexical alignment, F(1, 1371) = 5.46, p = 0.020, and reduced syntactic alignment, F(1, 1371) = 5.06, p = 0.025. These effects did not vary by social context within the model (p > 0.32).
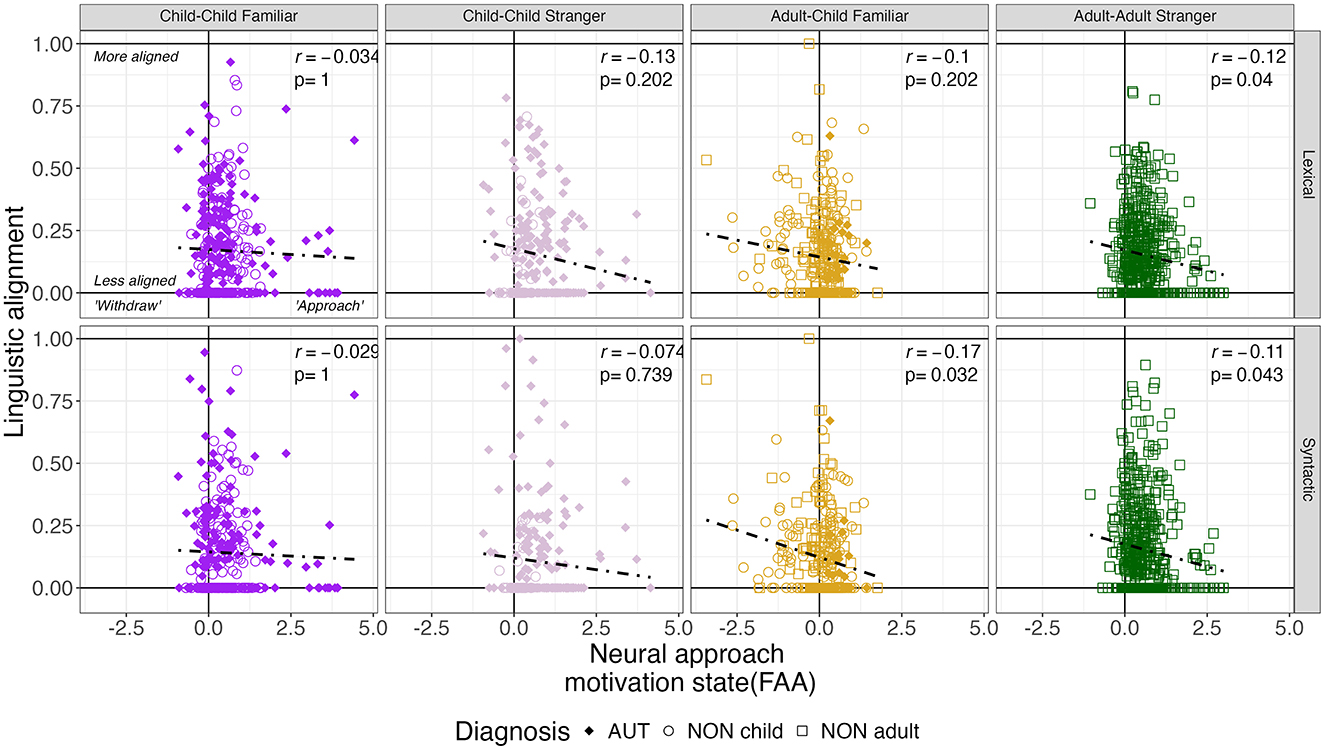
Figure 6. Cross-measure associations between neural motivation states and linguistic alignment. Pearson correlation coefficients and FDR-corrected p-values demonstrate significant relationships between the frontal alpha asymmetry (FAA) and turn-by-turn lexical and syntactic alignment. Shapes indicate whether an individual is an autistic child (filled diamond), non-autistic child (hollow diamond), or non-autistic adult (hollow square).
3.3 Between-person interbrain synchrony of approach motivation (FAA)
3.3.1 Interbrain agreement (concordance correlation coefficients)
The linear mixed-effects model of concordance correlation coefficients indicated an interaction between social context and lag, F(9, 618) = 2.37, p = 0.012, largely driven by effects at the concurrent time (Figure 7A). Specifically, at the same timepoint, child-child stranger (trend, p = 0.055), adult-child familiar, and adult-adult stranger dyads were more concordant (i.e., agreement in approach/withdraw motivation in each partner) than child-child familiar dyads (p < 0.004). These effects were reduced at the first lag (p < 0.024) and ameliorated at subsequent lags (p > 0.31). An overall effect of lag indicated that concordance decreased over lag, F(3, 621) = 10.16, p < 0.0001, suggesting that a participant's current approach motivation state was less concordant with their partner's approach motivation states further in the past.
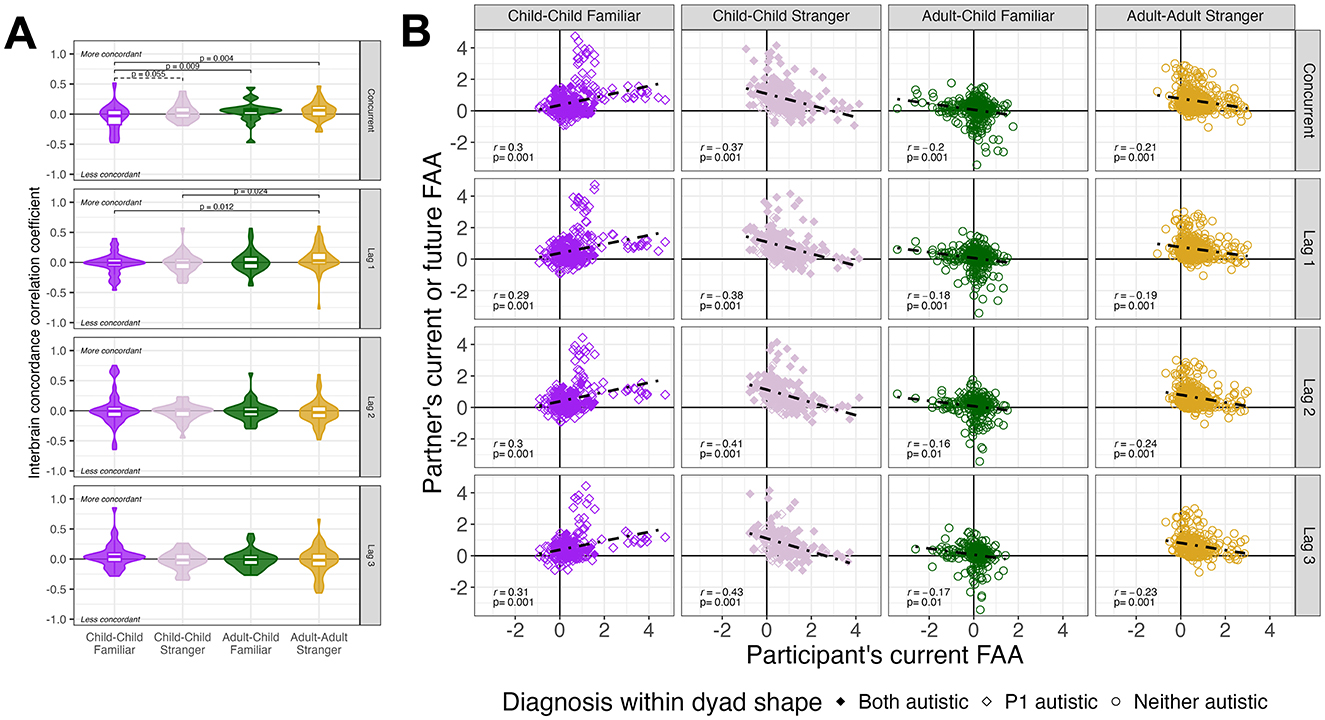
Figure 7. Interbrain synchrony in approach motivation (frontal alpha asymmetry, FAA) via concordance correlation coefficients (A) and Pearson correlations (B). Facet rows represent how participant's current FAA predicts partner's concurrent FAA and subsequent 10-s lags. (A) Boxplots demonstrate quartile range and violin plots demonstrate density of concordance correlation coefficients (i.e., agreement between participant-partner in accuracy and magnitude). Values closer to 1 indicate partners who are more concordant. Values closer to −1 indicate partners who are less concordant. FDR-corrected pairwise differences are noted between blocks within social context. (B) Pearson correlation coefficients and FDR-corrected p-values demonstrate linear associations between participant-partner. Larger correlation values indicate stronger association between participant (x-axis) and partner (y-axis) at concurrent time (top row) and subsequent lags. Shapes indicate whether an individual is an autistic child (filled diamond), non-autistic child (hollow diamond), or non-autistic adult (hollow square).
3.3.2 Linear associations (Pearson correlations)
As shown in Figure 7B via Pearson correlation tests, it is evident that direction of interbrain linear associations varied by social context. Child-child familiar dyads had positive correlations, where a participant's current increased state of approach motivation (i.e., positive FAA) was associated with increased states of approach motivation from partners at concurrent and lagged time. In contrast, at most lag points, child-child stranger, adult-child familiar, and adult-adult stranger dyads had negative correlations, where a participant's current state of approach motivation is associated with partner's neutral or withdraw motivation states.
3.3.3 Prediction of participant's current motivational state from partner's concurrent and prior motivational state
The linear mixed-effects model did not reveal any significant effects of lag (p > 0.76); thus results are discussed at the concurrent bin. The model confirmed an effect of partner, F(9, 9984) = 42.08, p < 0.0001, indicating that, across the full sample, participant's current state of approach motivation was reliably predicted by their partner's current brain state. This effect was moderated by social context, as indicated by an interaction between social context and partner's current state, F(9, 9984) = 54.92, p < 0.0001. To interrogate this interaction, negative slopes are predicted in all social contexts (Figure 8) with a graded pattern of strength for between-dyad differences (p < 0.0001): adult-adult stranger (slope = −0.58), child-child stranger (slope = −0.52), adult-child familiar (slope = −0.43), and child-child familiar (slope = −0.28). Pairwise comparisons indicated the following patterns: (1) when partners elicited strong or weak withdraw-like motivation (e.g., model-estimated negative FAA at −1 or −2), all dyads were predicted to elicit approach-like motivation, though approach motivation was stronger for child-child stranger and adult-adult stranger dyads than other dyads (positive FAA), p > 0.0129; (2) when partners elicited a neutral motivational state (e.g., model predicted FAA at 0), we note that all social contexts were predicted to elicit approach-like motivation except for adult-child familiar which was predicted to elicit a neutral state (p = 0.25); (3) when partners elicited weak approach-like motivation (model-estimated FAA at 1), only adult-adult strangers were predicted to elicit approach motivation (p = 0.0244), while other dyads were predicted to elicit a neutral state (p > 0.068); and lastly, (4) when partners elicited strong approach-like motivation (model-predicted FAA at 2), most participants were in a neutral motivational state (p > 0.635) except adult-child familiar dyads, which were predicted to elicit withdraw-like motivation (p = 0.042).
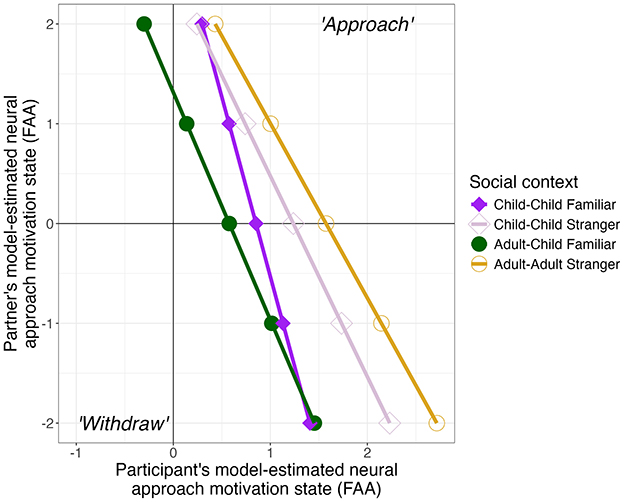
Figure 8. Model-estimated predictions of how partner's neural motivational state predicts participant's current motivation state (frontal alpha asymmetry, FAA). Shape depicts social context differences: child-child familiar (filled diamond), child-child stranger (hollow diamond), adult-child familiar (filled circle), and adult-adult stranger (hollow circle).
4 Discussion
Innovations in hyperscanning (i.e., dyadic neuroscience) will propel the field of cognitive development to address critical questions about the mechanisms and cognitive processes that support social interactions. Social interactions are inherently dynamic and are shaped by numerous aspects, such as context, mood, and social partner reciprocity. Importantly, these questions require an active and ecological approach that will require thoughtful modifications to accommodate the developmental needs of children, adolescents, and young adults within a lens of clinical and cultural sensitivity. Although we will continue to need well-controlled experiments, including classic “single-brain” cognitive neuroscience studies to understand the nature of cognitive development, these findings must align and apply to non-controlled, real-time interactions that are meaningful and adaptable for diverse groups of people.
In this paper, we described a new hyperscanning collaborative conversation task that successfully captures data relevant to evaluating biological and behavioral mechanisms related to sociocognition. As part of this proof-of-concept, we used different analytic techniques to explore differences in dyad-level behavior and sociocognitive processes including motivation and language across individuals who varied in age (ages 8 to 50), autism diagnosis, and racial/ethnic background. Additionally, we provide initial evidence that this task is sufficiently challenging but feasible for autistic and non-autistic youth and that dyads are approach-motivated in collaborative peer-based social contexts. Different social contexts such as familiarity also influenced how linguistically aligned individuals were during the task. We also demonstrate preliminary evidence that adult-adult stranger dyads' withdraw motivation was related to greater lexical alignment, in the moment. Finally, we found that dyads' brain states were aligned in different ways across social contexts. Within the discussion of our findings, we emphasize relevant solutions to address barriers involved in hyperscanning.
4.1 Study design and implementation with clinical and cultural sensitivity
Despite psychologists and developmental cognitive neuroscientists advocating for research that is mindful of the influence clinical and cultural backgrounds can have on cognition (Garcini et al., 2022; Masuda et al., 2020), successful execution of such research has been sparse, and diverse perspectives have historically been underrepresented (Dotson and Duarte, 2020). This manuscript presents a collaborative conversation task as an example of how researchers might be able to adapt EEG hyperscanning tasks with clinical and cultural sensitivity in mind. Indeed, regardless of age, autism diagnosis, or racial/ethnic background all participants in the current study completed all blocks of the task and contributed useful data which may be attributed to our clinically and culturally sensitive approach to study design and implementation. Of note, only adult-child familiar dyads showcased linear improvement, whereas child-child stranger dyads performed the best during the middle blocks and needed the most hints. This highlights that social context needs to be considered when designing task stimuli, block length, and overall task length.
To ensure that our task was feasible for all ages and individuals who may vary in reading ability, we opted to create stimuli that did not contain written text (e.g., street signs, speech bubbles). Indeed, all pairs were successful by the second block, though three child-child dyads (1 familiar, 2 stranger) did not successfully identify one of the 12 differences in the first block. We were also purposeful in the amount of time we allowed dyads to work to find differences in a set of images so that we minimized frustration that might occur in participants with varying processing speeds (e.g., autistic adolescents). Unlike the Diapix task (Baker and Hazan, 2011; Van Engen et al., 2010), we gave dyads 90 seconds for each set of images and emphasized that the goal of the task was not to find all the differences but instead to work together to do the best they could. This was particularly helpful for the child-child dyads, as they needed the most scaffolding (i.e., greater number of hints), got the most incorrect, but still evaluated the task as a mostly positive experience (see Supplementary Table 2). While we only describe results from the first four blocks to simplify findings, we anticipated child-child dyads may struggle and asked them to complete six total blocks so that they had ample time to get used to the task requirements. Given that child-child stranger dyads who were mostly autistic participants, struggled the most to correctly identify a difference, future studies employing similar tasks in similar populations may consider extending the amount of time given to solve one image as well as using different time limits for different populations based on their abilities. Finally, we allowed participants to complete this task multiple times with different partners to aid in recruitment and data collection of populations that are more difficult to recruit (i.e., Black and autistic populations). We did not anticipate learning effects to exist in our sample as research suggests there were no learning effects in the DiapixUK task when participants completed it multiple times (see Baker and Hazan, 2011) and our task on average was only slightly longer (9 min) than the quickest time adults solved a Diapix task (5.34 min). However, it is still possible that learning effects occurred. While outside the scope of this paper, future research may want to only allow participants to complete the task once or validate that the task they are using does not demonstrate learning effects across multiple sessions.
In addition to task completion across all participants, we also note that EEG procedures were successfully adopted across the entire sample, including our adult-child dyads that were all Black participants. EEG research has often failed to include Black participants because of systemic exclusion of minoritized participants and known technological hurdles with afro textured hair. We adapted techniques recommended by previous literature (see Brown, 2023; Hudac et al., 2022; Magstim EGI, 2023) to ensure we were culturally sensitive and acceptable. For example, screening calls and pre-visit information included sharing videos of net application and describing the wet EEG net procedures. At the visit, participants were allowed to touch and explore the net before initial placement, which was also an aid for autistic children. As needed, we explained how we place the net with textured or protective hair by slowly shifting hair through the gaps between electrodes to improve contact with the scalp. Longer hair like locs and braids were often pulled partially through the net creating small loops or even pulled entirely. Additionally, at every step of the session, we ensured participants' comfortability by explaining the procedures and reminding them that they may stop at any point. Finally, at the end of the adult-child familiar sessions we explicitly asked participants how the EEG cap felt over their hair (see Supplementary Table 4). No participant expressed any concern with EEG procedures, and many indicated that they felt comfortable with the research team and positive about the session.
4.2 Opportunities for innovations in hyperscanning (dyadic EEG) and linguistic data processing and analysis
Using a collaborative conversation task allowed for a varied and rich set of data that is relevant to real-time cognitive processing. In fact, we provide initial evidence that our adapted task highlights significant and interesting developmental differences. Different analytic techniques demonstrated initial dyad-level behavioral, neural, and linguistic differences, within-person cross-measure relationships between neural and linguistic outcomes, and between-person interbrain neural alignment. However, given that the results described here are solely for demonstrating proof-of-concept and are based on small and unequal sample sizes across groups, we caution over-interpretation of the current findings. Future studies that aim to use this task or adapt similar tasks should aim to recruit an equal number of dyads when investigating dyad group differences. Additionally, in line with recent developmental neuroscience work, future work should aim to recruit larger sample sizes (e.g., > 50 participants) as they increase the likelihood for detecting small effect sizes and lead to more reliable effect sizes in general (Morales et al., 2025). Further, dyad composition within groups was not completely controlled for in the current study as we used data from separate already established studies. For instance, child-child and adult-child dyads varied in how far apart they were in age which could lead to differences in interactions and confound results. Future studies may aim to better control for such differences statistically or in their recruitment strategies.
4.2.1 EEG data collection and processing
Despite EEG being sensitive to motion artifacts that may be particularly salient in a collaborative task where participants are talking, we successfully retained at least 77% of data across all social contexts which was sufficient for our analyses. Importantly, we were successful in using data from all of our autistic participants, including those with sensory sensitivities, and data from all participants with hairstyles that have historically been avoided in neuroscience. Our high data retention may also be linked to innovations in our field over the past decade to carefully improve data processing procedures, including the automated artifact correction procedures we used within EEGLAB (Delorme and Makeig, 2004). Other automated pipelines are regularly being adapted and improved to ensure efficiencies and minimal data loss across a variety of populations (e.g., HAPPE; Gabard-Durnam et al., 2018), which is a necessary consideration for more ecological EEG paradigms.
FAA was our neural outcome of choice because of its known links to approach and withdraw motivational processes that may be relevant to understanding how socicognitive processes emerge and develop over time (Harmon-Jones and Gable, 2018; Smith et al., 2017). We confirmed this was an appropriate outcome by visualizing power from varied frequency ranges (see Supplementary Figure 1). While beta (12–21 Hz) was another frequency band that demonstrated increases in power relative to others, it was outside the scope of the current study to examine changes in beta across social contexts. However, future research may benefit from further examining beta using our collaborative conversation task given that recent hyperscanning research has revealed strong beta interbrain synchrony in parietal and occipital electrode clusters across collaboration, competition, and single participation/passive observation tasks (Léné et al., 2021).
4.2.2 Linguistic data collection and processing
Linguistic alignment is important for supporting efficient language processing (Pickering and Garrod, 2004). In line with the Diapix task (Baker and Hazan, 2011; Van Engen et al., 2010), we found that our adapted collaborative conversation task elicited interactive speech between interlocutors, suitable for the analysis of similar linguistic features. While the ALIGN pipeline (Duran et al., 2019) allowed for a quick and relatively easy way to extract linguistic alignment features, transcribing the conversations was more time intensive. In the current study, we used free auto-transcription features as a first step in transcribing each session. This method may be more accessible and inexpensive to labs that are new to transcribing; however, fixing errors that were made by the auto-transcription process was the most time-consuming and larger studies may require more personnel to complete this process efficiently. Thus, researchers interested in more efficient automated pipelines may consider paid and established transcript software (e.g., Sonix, Otter.ai, Trint). While we also had two researchers review transcripts to ensure accuracy, calculating interrater reliability in future studies may also be important for demonstrating accurate transcribing procedures across sessions.
4.2.3 Analytic approaches for investigating biological-behavioral mechanisms of sociocognition
4.2.3.1 Using dyad-level motivation and language outcomes to investigate sociocognitive differences across social contexts
We provided preliminary evidence that neural mechanisms related to approach motivation (i.e., FAA) varied across social context with a caution against overinterpretation due to significantly different data loss across social contexts, small sample sizes, and unbalanced dyad compositions. Neural markers from peer dyads (i.e., child-child, adult-adult) demonstrated more approach motivation compared to the adult-child familiar dyads who had overall more neutral motivation. While all of our adult-adult dyads were strangers, our child-child familiar dyads were either peer friends or relatives. As such, a deeper understanding of how differing social relationships (e.g., friends, families) influence the development of cognitive mechanisms is needed. Indeed, this may be particularly relevant for adolescent development as recent research has found that daily friend support influences adolescent well-being regardless of same-day parent support, but consistent parent support may be protective to a global lack of support from friends (Schacter and Margolin, 2019). In contrast, our adult-child familiar dyads were more diverse in their composition (i.e., parent-child, sibling-child, grandparent-child) which may explain the overall neutral motivational states. Other hyperscanning research suggests that mother and child negativity during collaborative puzzle-solving interactions predicts more negative FAA (i.e., withdraw motivation; Atzaba-Poria et al., 2017), highlighting a unique mechanism for mother-child relationships. Given that there is limited research examining FAA in the context of other family dynamics, further investigating FAA using our collaborative conversation task in a wide range and larger sample of dyads may better highlight how neural mechanisms related to sociocognition develop and differ across more nuanced social contexts. Finally, while we discuss FAA as a direct marker of approach motivation in the current manuscript, FAA may instead reflect more indirect emotional and motivational processes. More recent research suggests that FAA is an indicator of broad changes in self-regulatory processes that may be context dependent (Perone and Vaughan, 2024). While we captured moment-by-moment shifts in FAA during our task, future research may aim to evaluate the change in FAA from before the onset of the task to better understand how changes in FAA are task-specific. Additionally, although most participants in the current study enjoyed the task, evaluating stress levels and engagement throughout the task may aid in interpreting effects of FAA across the task.
In exploring how the adapted collaborative conversation task influenced linguistic alignment, we evaluated lexical and syntactic alignment across social contexts. While our goal was to provide proof of concept for adapting a collaborative conversation task to evaluate sociocognitive mechanisms across diverse dyad types, our analyses were largely exploratory and should be interpreted with caution. Future interpretations of linguistic alignment using this task or other similar tasks would benefit from better established effect size ranges. Additionally, careful experimental design (e.g., balanced comparison groups) will enable clearer interpretations in the future. Despite this, the current results highlighted two interesting patterns. First, we found moderate lexical alignment at the conversation level that remained consistent across social contexts. While it may be surprising that lexical alignment did not vary by social context given previous research suggesting that dynamics of a conversation, including familiarity with each other, influence linguistic alignment (Riordan et al., 2014), we believe that this may have been due to the nature of the collaborative conversation task. More recent research has found that children's lexical alignment in the Diapix task is stronger compared to a play-based conversation task which is hypothesized to be related to the differing task requirements (Chieng et al., 2024). For instance, in a task where partners cannot see each other's screens and their goal is to figure out how their screens differ, accurate verbal information is necessary to be successful, regardless of social context. Additionally, stimuli were kept relatively simple to ensure all participants would be able to complete the task and, thus, converging on the same word for an object in the stimuli may not have been particularly difficult in this study.
Second, we found strong syntactic alignment at the conversation level in all social contexts except for child-child stranger dyads. Unlike lexical alignment, syntactic alignment refers to the similarity of grammatical structures between partners. While aligning syntactically may be easy as a result of automatic priming, greater syntactic alignment may reflect an effort to increase communication efficiency and may, therefore, require more effort (Ostrand and Chodroff, 2021; Ostrand and Ferreira, 2019). These effects may also be driven by the fact that our child-child stranger dyads were mostly autistic-autistic dyads (3/4 dyads). Due to sample size, we caution over-interpretation, however, our preliminary results are in contrast with other work indicating similar syntactic alignment in autistic and non-autistic individuals (Fusaroli et al., 2023; Slocombe et al., 2013). Prior research suggests that syntactic alignment potentially involves mentalizing and imitation skills (Hopkins et al., 2016), which implicate long-standing theories that mentalizing may be more difficult for autistic children (Baron-Cohen et al., 1985; Happé, 1993; Tager-Flusberg, 1993). Yet, few studies provide evidence of a direct link between conversational discourse features and theory of mind; one study found this relationship may be mediated by linguistic ability (Capps et al., 1998) though another study found this relationship was independent of individual differences, including language, cognitive skills, and age (Hale and Tager-Flusberg, 2005). Quality of social relationships may also be related to mentalizing abilities in autism (Hunsche and Kerns, 2025) which could also explain why there was decreased syntactic alignment in child-child stranger compared to child-child familiar dyads. Interaction quality may be less specific to autism and more impacted by individual differences (Stroth et al., 2022; Bishop et al., 2016) – making it increasingly important to develop and utilize tools that can measure and incorporate real-time strategies and adaptions. Lastly, it will be critical to consider the “double-empathy problem” (Milton, 2012), which suggests that difficulties within social interactions may be due to communication mismatch between autistic and non-autistic people. Continuing to examine communication using this naturalistic collaboration task within different dyadic social contexts will uncover how and why different social strategies may improve mutual understanding and a shared context (e.g., norms surrounding communication style, lower social pressure, increased shared empathy).
4.2.3.2 Using within-person cross-measure correlations to better understand associations between motivation and language
Ecological approaches to capture real-world execution of cognitive and sociocognitive skills require a clear link between biological mechanisms and behavior. Here, we focused on how neurobiological motivation states lead to the use of language, specifically linguistic alignment between a participant and their dyad partner as a first step. Importantly, because these analyses rely on sufficient joined data between measurements, we opted to average neural and language data across 10 second bins and retained 75–92% of data across all social contexts. We found negative linear associations for adult-adult stranger and adult-child familiar dyads, such that neurobiological states of withdraw motivation (i.e., decreased FAA) were related to increased linguistic alignment. While this result was surprising, this may suggest that as partners are feeling withdrawn from the task or each other, they are attempting to align linguistically to continue moving toward shared understanding. Given that only adult-adult stranger and adult-child familiar dyads demonstrated this relationship, this finding might point out a specific mechanism that is not fully present until adulthood or is perhaps led by the adult participant (Misiek et al., 2020). There may be additional development considerations related to the motivational state for alignment. Although alignment is evident in adult-child conversations (Chieng et al., 2024) and autistic peer child conversations (Branigan et al., 2016), simulation studies have also suggested that it is more difficult to align to child conversation (French et al., 2024).
“Grounding theory” provides an alternative perspective, which is that as dyads work to find mutual knowledge (e.g., find the differences, in our task), linguistic alignment can be an implicit mechanism to showcase mutual understanding (Clark and Brennan, 1991; Riordan et al., 2014). It may be the case that a person who is not leading the conversation may be in a withdraw-like state while their language indicates alignment behaviors. In other words, they may align linguistically with their partner so that they can accomplish the task at hand but may not be taking the lead in the conversation which may reflect more withdraw-like motivational brain states. To better understand this perspective, a more in-depth analysis of individual conversations is required in which EEG data could be modeled based upon the participant's current mode (Almor, 2008; Boiteau et al., 2014). This was beyond the scope of this proof-of-concept paper; however, to illustrate individual fluctuations, Supplementary Figure 2 showcases one participant who completed the collaborative conversation task in multiple contexts: child-child stranger dyad and adult-child familiar dyad. Finally, as mentioned previously, FAA may reflect broader emotional, motivational, or self-regulatory states. Alpha more generally is also influenced by cognitive load (Klimesch et al., 1993). As such, the negative relationship between FAA and linguistic alignment may be explained by participant's internal emotional state throughout the task or by task difficulty. Given that interpretations of FAA remain relatively mixed in the existing research, future research should aim to continue to examine the effects of FAA during collaborative tasks, relationships between FAA and other cognitive mechanisms like language, and changes of FAA across periods of rest and task-based effort.
There are other possible within-person cross-measures that could be collected and analyzed in this task. For instance, we also collected heart rate and other qualitative data (e.g., strategies used). These outcomes may be particularly useful to consider when examining biology-behavior links underlying sociocognition occurring in real-time social interactions. For instance, research suggests that individual differences in heart rate variability are linked to performance on executive function tasks and prefrontal cortical activity highlighting a specific brain to heart to behavior pathway relevant to cognitive development (Thayer et al., 2009). Thus, examining pathways like these in real-time social interactions across development may provide insight into how they emerge and promote successful social interaction.
4.2.3.3 Using hyperscanning to better understand interbrain neural synchrony between dyads
To demonstrate the options and opportunities for hyperscanning and fully probe the data, we used several statistical methods to evaluate interbrain synchrony of approach motivation at concurrent and lagged time. First, we utilized concordance correlation coefficients to interpret the relationships in agreement based upon accuracy and magnitude of neurobiological states. We found concordance within the same time bin for adult-child familiar and adult-adult stranger dyads, with a trending effect for child-child stranger dyads, and no concordance for child-child familiar dyads. Although age was not significant in these models, there may be developmental changes related to concordance, such that adults may be more adept at implicitly modeling and adapting to their partner's brain state particularly in higher-frequency bands like alpha. Indeed, some research has found that adolescents' neural states during cooperation tasks are more in-sync than adults but only for delta and theta frequency bands (Yang et al., 2023). To better understand how individuals are biologically wired to build mutual understanding, future research should continue to examine developmental trajectories of interbrain synchrony in alpha or FAA. Concordance effects also decreased over lag, such that there was less agreement between the participant's current brain state and the partner's prior brain states. This may indicate that we selected an appropriate time-bin to showcase concurrent similarities, but it may be important to examine smaller time-bins or perhaps use a moving-window average in the future.
Pearson correlations also indicated social context differences, such that most dyads had increased approach motivation when their partner had decreased approach motivation. This may be attributed to the participant perceiving a decline in their partner's motivation and consequently increasing their own efforts and motivation to compensate for the imbalance. However, this could also be related to turn-taking that was inherent in our collaborative conversation task. Speaking or response preparation is cognitively demanding and interferes with visual task performance (Almor, 2008) and reduction in alpha power has been correlated to speech and motor response preparation (Babiloni et al., 1999; Bögels et al., 2015). Therefore, in the current study, FAA may be reduced in participants who were listening and preparing to respond to their partner's questions. While conversational roles (e.g., speaker, listener) can be extracted from transcription data, our goal here was to establish feasibility of certain analytic techniques. Therefore, we opted to not evaluate conversational roles in this way but they should be examined in future studies to further examine this interpretation.
Only the child-child familiar dyads showcased a different positive association, such that participant and partner's neurobiological states of approach motivation were aligned in direction (i.e., both in approach, neutral, or withdraw). While it may be expected for brains to be positively aligned in a task that is asking individuals to build mutual understanding, our findings may suggest that who your partnered with is important for alignment of brain states. For example, previous research suggests that adolescent friendship influences and supports the development of reward and motivational processes in the brain (Güroglu, 2022). Given that our child-child familiar dyads were the only familiar social context in which dyads were close friends or peer-aged family, positive associations between motivational brain states may be unique to friendship social interactions. There remains a clear need to better understand how motivational mechanisms support sociocognitive development in real-time social interaction and across different familiar social contexts (e.g., friends vs. family).
Lastly, we confirmed the directionality of these associations by analyzing model-predicted estimates to better understand the direct influence of partners on participants' motivational approach state. Broadly speaking, one's current motivation state was predicted to be opposite of their partner (or neutral), but slopes were stronger for stranger dyads than familiar dyads. These asymmetrical findings align with our current and other correlational findings (e.g., Balconi and Vanutelli, 2016; Dumas et al., 2010) and give additional credence to evaluating interbrain synchrony as a method to capture sociocognitive developmental mechanisms involved during conversation and other social interactions. There are many real-world implications for interbrain synchronization—improved social bonds and communication (Balconi and Fronda, 2020; Grootjans et al., 2024; Nelson et al., 2024), enhanced classroom efficacy and learning outcomes (Dikker et al., 2017; Mendoza-Armenta et al., 2024), and optimized group or team performance (Liu et al., 2023; Moerel et al., 2025; Reinero et al., 2021). Although these implications are relevant for youth, dyadic neuroscience remains an underutilized method in cognitive psychology, particularly across different social contexts, developmental ages, and clinical populations. For instance, our Pearson correlations in the current study indicate that child-child dyads (including autistic-autistic and autistic-non-autistic pairs) do showcase interbrain alignment, extending prior evidence that synchrony was absent in autistic-non-autistic dyads (Chen et al., 2025).
4.3 Recommendations for scientists adapting cognitive science tasks for developmental populations
Our collaborative conversation task was engaging for most of our participants across varied ages, which aided in ensuring participants were focused and attentive to the task and improved data quality. While hyperscanning research may be naturally more engaging because of the inherent interactivity of the method, traditional cognitive science tasks could also benefit from designing more engaging and developmentally appropriate tasks (e.g., dynamic stimuli, gameifying tasks). For instance, one procedure we have implemented to ensure our materials are engaging and developmentally appropriate involves systematically asking participants for feedback immediately. There are limited examples of community-based approaches for neuroscience or involving child or adolescent advisory boards in studies of cognitive development (Choudhury et al., 2012). Yet, we encourage researchers to consider creative ways to amplify child and family voices to ensure research is designed with developmentally appropriate stimuli and expectations.
Additionally, developmental theories emphasize that cognitive development is influenced by interactions across multiple types of environments that naturally vary across communities and cultures (Bronfenbrenner and Morris, 2006). As such, recruiting diverse developmental samples will be important for increasing generalizability of developmental cognitive psychology research. While most of the dyad data presented here was collected from ongoing studies whose recruitment goals are geared toward recruiting autistic and Black participants, our adult-adult dyads were mostly White, neurotypical, college students which limits generalizability. In addition to collecting more detailed demographic information from participants (e.g., socioeconomic status) and stratifying recruitment to ensure your sample represents population-level demographics, some potentially helpful suggestions for increasing generalizability include: (1) establishing cross-site collaborations, including international collaborations, and expanding the physical boundaries of research procedures (Bhavnani et al., 2022), (2) joining and developing outreach within diverse community settings to build reciprocal relationships, (3) offering non-traditional compensation, such as tickets to local attractions, respite night coupons, and reimburse for transportation, lodging, and other expenses incurred by participating (e.g., vouchers for hair styling products), and 4) designing research projects with clear objectives to meet community needs of underrepresented samples.
5 Conclusions
The next frontier of cognitive development will require using real-time and real-world approaches to understand cognitive mechanisms, yet the adoption of dyadic neuroscience for developmental populations is underutilized. While we provide an initial proof-of-concept for using this collaborative conversation task, we caution that these findings should be confirmed in larger samples with consistent characteristics. Notwithstanding this limitation, our study is one of the first to detail methodological considerations and provide proof-of-concept for an adapted collaborative conversation task that aims to investigate sociocognitive mechanisms in an ecologically valid way. We provide preliminary evidence that suggests social contexts drive differences across biological and behavioral measures of real-time sociocognition. Importantly, we also demonstrate that such studies are feasible for diverse populations and can generate more generalizable cognitive development research. Finally, we suggest researchers consider all elements when designing studies to ensure that tasks are clinically and culturally sensitive, engaging, and developmentally appropriate. As such, the current study demonstrates how important it is for the field to disseminate useful and actionable steps to make research more inclusive and innovative when examining biology-behavior links that underly inherently dynamic and complex real-world situations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the University of South Carolina's Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
CN: Data curation, Methodology, Visualization, Supervision, Writing – original draft, Formal analysis, Writing – review & editing, Investigation. SW: Writing – review & editing, Validation, Methodology, Resources, Writing – original draft. JM: Data curation, Writing – review & editing, Investigation, Writing – original draft, Project administration, Methodology. AA: Writing – original draft, Methodology, Writing – review & editing, Validation. CH: Methodology, Writing – original draft, Supervision, Software, Investigation, Conceptualization, Funding acquisition, Visualization, Validation, Formal analysis, Writing – review & editing, Resources, Data curation, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This funding was supported by a McCausland Fellowship and a pilot grant from the University of South Carolina's Office of the Vice President for Research (awards to CH). Authors AA and SW were partially supported by the U.S. National Science Foundation Linguistics Program Doctoral Dissertation Research Improvement Grant No. BCS-2444223. Additionally, author SW was partially supported by a graduate assistantship from the Aging Brain Cohort Study at the University of South Carolina, a SPARC Graduate Research Grant from the Office of the Vice President for Research at the University of South Carolina, and the Russell J. and Dorothy S. Bilinski Dissertation Fellowship.
Acknowledgments
We would like to thank our participants for their time and valuable contribution to this study. We also would like to thank Janae Hersey and Ezra Wingard for their assistance with data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Gen AI was used in the creation of this manuscript. The authors used Gen AI via Canva's built-in AI image generators to assist in creating illustrations for the stimuli used in the experimental task. More specifically, the authors used the “MagicMedia” tool that is powered by GPT-4.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdpys.2025.1644956/full#supplementary-material
References
Almor, A. (1999). Noun-phrase anaphora and focus: the informational load hypothesis. Psychol. Rev. 106, 748–765. doi: 10.1037//0033-295X.106.4.748
Almor, A. (2008). Why does language interfere with vision-based tasks? Exp. Psychol. 55, 260–268. doi: 10.1027/1618-3169.55.4.260
Almor, A., and Nair, V. A. (2007). The form of referential expressions in discourse. Lang. Linguist. Compass 1, 84–99. doi: 10.1111/j.1749-818X.2007.00009.x
American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association.
Atzaba-Poria, N., Deater-Deckard, K., and Bell, M. A. (2017). Mother–child interaction: links between mother and child frontal electroencephalograph asymmetry and negative behavior. Child Dev. 88, 544–554. doi: 10.1111/cdev.12583
Babiloni, C., Carducci, F., Cincotti, F., Rossini, P. M., Neuper, C., Pfurtscheller, G., et al. (1999). Human movement-related potentials vs desynchronization of EEG alpha rhythm: a high-resolution EEG study. Neuroimage 10, 658–665. doi: 10.1006/nimg.1999.0504
Babiloni, F., and Astolfi, L. (2014). Social neuroscience and hyperscanning techniques: past, present and future. Neurosci. Biobehav. Rev. 44, 76–93. doi: 10.1016/j.neubiorev.2012.07.006
Baker, R., and Hazan, V. (2011). DiapixUK: task materials for the elicitation of multiple spontaneous speech dialogs. Behav. Res. Methods 43, 761–770. doi: 10.3758/s13428-011-0075-y
Balconi, M., and Fronda, G. (2020). The use of hyperscanning to investigate the role of social, affective, and informative gestures in non-verbal communication: electrophysiological (EEG) and inter-brain connectivity evidence. Brain Sci. 10:29. doi: 10.3390/brainsci10010029
Balconi, M., and Vanutelli, M. E. (2016). Competition in the brain: the contribution of EEG and fNIRS modulation and personality effects in social ranking. Front. Psychol. 7:1587. doi: 10.3389/fpsyg.2016.01587
Baron-Cohen, S., Leslie, A. M., and Frith, U. (1985). Does the autistic child have a “theory of mind”? Cognition 21, 37–46. doi: 10.1016/0010-0277(85)90022-8
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Baumeister, R. F., and Leary, M. R. (1995). The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychol. Bull. 117, 497–529. doi: 10.1037//0033-2909.117.3.497
Bell, M. A., and Cuevas, K. (2012). Using EEG to study cognitive development: issues and practices. J. Cogn. Dev. 13, 281–294. doi: 10.1080/15248372.2012.691143
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B 57, 289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
Bevilacqua, D., Davidesco, I., Wan, L., Chaloner, K., Rowland, J., Ding, M., et al. (2018). Brain-to-brain synchrony and learning outcomes vary by student–teacher dynamics: evidence from a real-world classroom electroencephalography study. J. Cogn. Neurosci. 31, 401–411. doi: 10.1162/jocn_a_01274
Bhavnani, S., Parameshwaran, D., Sharma, K. K., Mukherjee, D., Divan, G., Patel, V., et al. (2022). The acceptability, feasibility, and utility of portable electroencephalography to study resting-state neurophysiology in rural communities. Front. Hum. Neurosci. 16:802764. doi: 10.3389/fnhum.2022.802764
Bishop, S. L., Havdahl, K. A., Huerta, M., and Lord, C. (2016). Subdimensions of social-communication impairment in autism spectrum disorder. J. Child Psychol. Psychiatry 57, 909–916. doi: 10.1111/jcpp.12510
Bitsika, V., Sharpley, C. F., Evans, I. D., and Vessey, K. A. (2024). Neurological validation of ASD diagnostic criteria using frontal alpha and theta asymmetry. J. Clin. Med. 13:4876. doi: 10.3390/jcm13164876
Blakemore, S.-J., and Frith, U. (2005). The Learning Brain: Lessons for Education. Oxford: Blackwell Publishing.
Bögels, S., Magyari, L., and Levinson, S. C. (2015). Neural signatures of response planning occur midway through an incoming question in conversation. Sci. Rep. 5:12881. doi: 10.1038/srep12881
Boiteau, T. W., Malone, P. S., Peters, S. A., and Almor, A. (2014). Interference between conversation and a concurrent visuomotor task. J. Exp. Psychol. Gen. 143, 295–311. doi: 10.1037/a0031858
Bradshaw, J., McCracken, C., Pileggi, M., Brane, N., Delehanty, A., Day, T., et al. (2021). Early social communication development in infants with autism spectrum disorder. Child Dev. 92, 2224–2234. doi: 10.1111/cdev.13683
Branigan, H. P., Pickering, M. J., and Cleland, A. A. (2000). Syntactic co-ordination in dialogue. Cognition 75, B13–B25. doi: 10.1016/S0010-0277(99)00081-5
Branigan, H. P., Tosi, A., and Gillespie-Smith, K. (2016). Spontaneous lexical alignment in children with an autistic spectrum disorder and their typically developing peers. J. Exp. Psychol. Learn. Mem. Cogn. 42, 1821–1831. doi: 10.1037/xlm0000272
Brennan, S. E., and Clark, H. H. (1996). Conceptual pacts and lexical choice in conversation. J. Exp. Psychol. Learn. Mem. Cogn. 22, 1482–1493. doi: 10.1037//0278-7393.22.6.1482
Bronfenbrenner, U., and Morris, P. A. (2006). “The bioecological model of human development,” in Handbook of Child Psychology, 6th Edn., Vol. 1, Eds. W. Damon and R. M. Lerner (Hoboken, NJ: Wiley), 793–828.
Brown, L. (2023). Assessing Field Standard Practices for Incorporating Black Individuals in EEG Research (Master's thesis). Purdue University, West Lafayette, IN. Available online at: https://hammer.purdue.edu/articles/thesis/ASSESSING_FIELD_STANDARD_PRACTICES_FOR_INCORPORATING_BLACK_INDIVIDUALS_IN_EEG_RESEARCH/22696240?file=40376909 (Accessed October 20, 2025).
Capps, L., Kehres, J., and Sigman, M. (1998). Conversational abilities among children with autism and children with developmental delays. Autism 2, 325–344. doi: 10.1177/1362361398024002
Chen, I.-C., Hsu, H.-C., Chen, C.-L., Chang, M.-H., Wei, C.-S., and Chuang, C.-H. (2025). Interbrain synchrony attenuation during a peer cooperative task in young children with autistic traits – An EEG hyperscanning study. Neuroimage 312:121217. doi: 10.1016/j.neuroimage.2025.121217
Chieng, A. C. J., Wynn, C. J., Wong, T. P., Barrett, T. S., and Borrie, S. A. (2024). Lexical alignment is pervasive across contexts in non-WEIRD adult–child interactions. Cogn. Sci. 48:e13417. doi: 10.1111/cogs.13417
Choudhury, S., McKinney, K. A., and Merten, M. (2012). Rebelling against the brain: public engagement with the ‘neurological adolescent.' Soc. Sci. Med. 74, 565–573. doi: 10.1016/j.socscimed.2011.10.029
Choy, T., Baker, E., and Stavropoulos, K. (2022). Systemic racism in EEG research: considerations and potential solutions issues of systemic bias in neuroscience: Recruitment and retention. Affect. Sci. 3, 239–247. doi: 10.1007/s42761-021-00050-0
Clark, H. H., and Brennan, S. E. (1991). “Grounding in communication,” in Perspectives on Socially Shared Cognition, Eds. L. B. Resnick, J. M. Levine, and S. D. Teasley (Washington, DC: American Psychological Association), 127–149.
Clark, H. H., and Marshall, C. R. (1981). “Definite reference and mutual knowledge,” in Elements of Discourse Understanding, Eds. A. K. Joshi, B. L. Webber, and I. A. Sag (Cambridge: Cambridge University Press), 10–63.
Dawson, G., Meltzoff, A. N., Osterling, J., Rinaldi, J., and Brown, E. (1998). Children with autism fail to orient to naturally occurring social stimuli. J. Autism Dev. Disord. 28, 479–485. doi: 10.1023/A:1026043926488
Delorme, A., and Makeig, S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21. doi: 10.1016/j.jneumeth.2003.10.009
Dikker, S., Wan, L., Davidesco, I., Kaggen, L., Oostrik, M., McClintock, J., et al. (2017). Brain-to-brain synchrony tracks real-world dynamic group interactions in the classroom. Curr. Biol. 27, 1375–1380. doi: 10.1016/j.cub.2017.04.002
Dotson, V. M., and Duarte, A. (2020). The importance of diversity in cognitive neuroscience. Ann. N. Y. Acad. Sci. 1464, 181–191. doi: 10.1111/nyas.14268
Dumas, G., Lachat, F., Martinerie, J., Nadel, J., and George, N. (2011). From social behaviour to brain synchronization: review and perspectives in hyperscanning. IRBM 32, 48–53. doi: 10.1016/j.irbm.2011.01.002
Dumas, G., Nadel, J., Soussignan, R., Martinerie, J., and Garnero, L. (2010). Inter-brain synchronization during social interaction. PLoS ONE 5:e12166. doi: 10.1371/journal.pone.0012166
Duran, N. D., Paxton, A., and Fusaroli, R. (2019). ALIGN: analyzing linguistic interactions with generalizable techNiques—A Python library. Psychol. Methods 24, 419–438. doi: 10.1037/met0000206
French, D., D'Mello, S., and von der Wense, K. (2024). “Aligning to adults is easy, aligning to children is hard: A study of linguistic alignment in dialogue systems,” in Proceedings of the 1st Human-Centered Large Language Modeling Workshop (ACL), 81–87.
Fusaroli, R., Weed, E., Rocca, R., Fein, D., and Naigles, L. (2023). Repeat after me? Both children with and without autism commonly align their language with that of their caregivers. Cogn. Sci. 47:e13369. doi: 10.1111/cogs.13369
Gabard-Durnam, L. J., Mendez Leal, A. S., Wilkinson, C. L., and Levin, A. R. (2018). The Harvard automated processing pipeline for electroencephalography (HAPPE): standardized processing software for developmental and high-artifact data. Front. Neurosci. 12:97. doi: 10.3389/fnins.2018.00097
Gandolfi, G., Pickering, M. J., and Garrod, S. (2023). Mechanisms of alignment: shared control, social cognition and metacognition. Philos. Trans. R. Soc. B Biol. Sci. 378:20210362. doi: 10.1098/rstb.2021.0362
Garcini, L. M., Arredondo, M. M., Berry, O., Church, J. A., Fryberg, S., Thomason, M. E., et al. (2022). Increasing diversity in developmental cognitive neuroscience: a roadmap for increasing representation in pediatric neuroimaging research. Dev. Cogn. Neurosci. 58:101167. doi: 10.1016/j.dcn.2022.101167
Garrod, S., and Anderson, A. (1987). Saying what you mean in dialogue: a study in conceptual and semantic co-ordination. Cognition 27, 181–218. doi: 10.1016/0010-0277(87)90018-7
Garrod, S., and Pickering, M. J. (2004). Why is conversation so easy? Trends Cogn. Sci. 8, 8–11. doi: 10.1016/j.tics.2003.10.016
Garrod, S., and Pickering, M. J. (2007). “Alignment in dialogue,” in The Oxford Handbook of Psycholinguistics, Eds. M. G. Gaskell and G. Altmann (Oxford: Oxford University Press), 443–451.
Goodwin, C. (1981). Conversational Organization: Interaction Between Speakers and Hearers. New York, NY: Academic Press.
Grootjans, Y., Harrewijn, A., Fornari, L., Janssen, T., de Bruijn, E. R. A., van Atteveldt, N., et al. (2024). Getting closer to social interactions using electroencephalography in developmental cognitive neuroscience. Dev. Cogn. Neurosci. 67:101391. doi: 10.1016/j.dcn.2024.101391
Güroglu, B. (2022). The power of friendship: the developmental significance of friendships from a neuroscience perspective. Child Dev. Perspect. 16, 110–117. doi: 10.1111/cdep.12450
Hale, C. M., and Tager-Flusberg, H. (2005). Social communication in children with autism: the relationship between theory of mind and discourse development. Autism 9, 157–178. doi: 10.1177/1362361305051395
Happé, F. G. (1993). Communicative competence and theory of mind in autism: a test of relevance theory. Cognition 48, 101–119. doi: 10.1016/0010-0277(93)90026-R
Harmon-Jones, E., and Gable, P. A. (2018). On the role of asymmetric frontal cortical activity in approach and withdrawal motivation: an updated review of the evidence. Psychophysiology 55:e12879. doi: 10.1111/psyp.12879
Hopkins, Z., Yuill, N., and Keller, B. (2016). Children with autism align syntax in natural conversation. Appl. Psycholinguist. 37, 347–370. doi: 10.1017/S0142716414000599
Hu, Y., Pan, Y., Shi, X., Cai, Q., Li, X., and Cheng, X. (2018). Inter-brain synchrony and cooperation context in interactive decision making. Biol. Psychol. 133, 54–62. doi: 10.1016/j.biopsycho.2017.12.005
Hudac, C. M., Wallace, J. S., Ward, V. R., Friedman, N. R., Delfin, D., and Newman, S. D. (2022). Dynamic cognitive inhibition in the context of frustration: increasing racial representation of adolescent athletes using mobile community-engaged EEG methods. Front. Neurol. 13:918075. doi: 10.3389/fneur.2022.918075
Hunsche, M. C., and Kerns, C. M. (2025). How social cognition relates to different dimensions of social connection among autistic individuals: a systematic review. Clin. Psychol. Sci. Pract. doi: 10.1037/cps0000265
Immordino-Yang, M. H., and Damasio, A. (2007). We feel, therefore we learn: the relevance of affective and social neuroscience to education. Mind Brain Educ. 1, 3–10. doi: 10.1111/j.1751-228X.2007.00004.x
Immordino-Yang, M. H., Darling-Hammond, L., and Krone, C. R. (2025). “Nurturing nature: How brain development is inherently social and emotional, and what this means for education,” in Social and Emotional Learning, 1st Edn., Ed. K. Wentzel (New York, NY: Routledge), 63–83.
Jurafsky, D., and Martin, J. H. (2008). Speech and Language Processing: An Introduction to Natural Language Processing, Computational Linguistics, and Speech Recognition. 2nd Edn. Upper Saddle River, NJ: Prentice Hall.
Kaiser, M. D., Hudac, C. M., Shultz, S., Lee, S. M., Cheung, C., Berken, A. M., et al. (2010). Neural signatures of autism. Proc. Natl. Acad. Sci. U. S. A. 107, 21223–21228. doi: 10.1073/pnas.1010412107
Kassambara, A. (2025). ggpubr: 'ggplot2' Based Publication Ready Plots. R package version 0.6.1. Available online at: https://rpkgs.datanovia.com/ggpubr/
Killeen, L. A., and Teti, D. M. (2012). Mothers' frontal EEG asymmetry in response to infant emotion states and mother–infant emotional availability, emotional experience, and internalizing symptoms. Dev. Psychopathol. 24, 9–21. doi: 10.1017/S0954579411000629
Klimesch, W., Schimke, H., and Pfurtscheller, G. (1993). Alpha frequency, cognitive load and memory performance. Brain Topogr. 5, 241–251. doi: 10.1007/BF01128991
Léné, P., Karran, A. J., Labonté-Lemoyne, E., Sénécal, S., Fredette, M., Johnson, K. J., et al. (2021). Is there collaboration specific neurophysiological activation during collaborative task activity? An analysis of brain responses using electroencephalography and hyperscanning. Brain Behav. 11:e2270. doi: 10.1002/brb3.2270
Leong, V., Byrne, E., Clackson, K., Georgieva, S., Lam, S., and Wass, S. (2017). Speaker gaze increases information coupling between infant and adult brains. Proc. Natl. Acad. Sci. U. S. A. 114, 13290–13295. doi: 10.1073/pnas.1702493114
Levelt, W. J. M., and Kelter, S. (1982). Surface form and memory in question answering. Cogn. Psychol. 14, 78–106. doi: 10.1016/0010-0285(82)90005-6
Levinson, S. C. (2016). Turn-taking in Human Communication – Origins and Implications for Language Processing. Trends Cogn. Sci. 20, 6–14. doi: 10.1016/j.tics.2015.10.010
Liao, Y., Acar, Z. A., Makeig, S., and Deak, G. (2015). EEG imaging of toddlers during dyadic turn-taking: mu-rhythm modulation while producing or observing social actions. Neuroimage 112, 52–60. doi: 10.1016/j.neuroimage.2015.02.055
Lin, L. I.-K. (1989). A concordance correlation coefficient to evaluate reproducibility. Biometrics 45, 255–268. doi: 10.2307/2532051
Lin, L. I.-K. (2000). A note on the concordance correlation coefficient. Biometrics 56, 324–325. doi: 10.1111/j.0006-341X.2000.00324.x
Liu, D., Liu, S., Liu, X., Zhang, C., Li, A., Jin, C., et al. (2018). Interactive brain activity: review and progress on EEG-based hyperscanning in social interactions. Front. Psychol. 9:1862. doi: 10.3389/fpsyg.2018.01862
Liu, Q., Cui, H., Huang, B., Huang, Y., Sun, H., Ru, X., et al. (2023). Inter-brain neural mechanism and influencing factors underlying different cooperative behaviors: a hyperscanning study. Brain Struct. Funct. 229, 75–95. doi: 10.1007/s00429-023-02700-4
Magstim, EGI, and Hudac, C. M. (2023). Magstim EGI Webinar: Inclusive HD-EEG Research for all People. Available online at: https://www.youtube.com/watch?v=4SOk3c2N0To (Accessed October 20, 2025).
Masuda, T., Batdorj, B., and Senzaki, S. (2020). Culture and attention: future directions to expand research beyond the geographical regions of WEIRD cultures. Front. Psychol. 11:1394. doi: 10.3389/fpsyg.2020.01394
McDonald, N. M., and Perdue, K. L. (2018). The infant brain in the social world: moving toward interactive social neuroscience with functional near-infrared spectroscopy. Neurosci. Biobehav. Rev. 87, 38–49. doi: 10.1016/j.neubiorev.2018.01.007
Mendoza-Armenta, A. A., Blanco-Téllez, P., García-Alcántar, A. G., Ceballos-González, I., Hernández-Mustieles, M. A., Ramírez-Mendoza, R. A., et al. (2024). Implementation of a real-time brain-to-brain synchrony estimation algorithm for neuroeducation applications. Sensors 24:1776. doi: 10.3390/s24061776
Miller, P. H. (2022). Developmental theories: past, present, and future. Dev. Rev. 66:101049. doi: 10.1016/j.dr.2022.101049
Milton, D. E. M. (2012). On the ontological status of autism: the ‘double empathy problem'. Disabil. Soc. 27, 883–887. doi: 10.1080/09687599.2012.710008
Misiek, T., Favre, B., and Fourtassi, A. (2020). “Development of multi-level linguistic alignment in child-adult conversations,” in Proceedings of the Workshop on Cognitive Modeling and Computational Linguistics, 54–58.
Moerel, D., Grootwagers, T., Quek, G. L., Smit, S., and Varlet, M. (2025). Unlocking information alignment between interacting brains with EEG hyperscanning. bioRxiv. doi: 10.1101/2025.01.07.631802
Montague, P., Berns, G. S., Cohen, J. D., McClure, S. M., Pagnoni, G., Dhamala, M., et al. (2002). Hyperscanning: simultaneous fMRI during linked social interactions. Neuroimage 16, 1159–1164. doi: 10.1006/nimg.2002.1150
Morales, S., Oh, L., Cox, K., Rodriguez-Sanchez, R., Nadaya, G., Buzzell, G. A., et al. (2025). Generalizability of developmental EEG: demographic reporting, representation, and sample size. Dev. Cogn. Neurosci. 74:101567. doi: 10.1016/j.dcn.2025.101567
Neel, R., Kenrick, D. T., White, A. E., and Neuberg, S. L. (2016). Individual differences in fundamental social motives. J. Pers. Soc. Psychol. 110, 887–907. doi: 10.1037/pspp0000068
Nelson, C. M., O'Reilly, C., Xia, M., and Hudac, C. M. (2024). Coupling up: a dynamic investigation of romantic partners' neurobiological states during nonverbal connection. Behav. Sci. 14:1133. doi: 10.3390/bs14121133
Nikitin, J., and Schoch, S. (2021). “Social approach and avoidance motivations,” in Handbook of Solitude: Psychological Perspectives on Social Isolation, Social Withdrawal, and Being Alone, 2nd Edn., Eds. R. J. Coplan and J. C. Bowker (Hoboken, NJ: Wiley Blackwell), 191–208.
Ostrand, R., and Chodroff, E. (2021). It's alignment all the way down, but not all the way up: speakers align on some features but not others within a dialogue. J. Phon. 88:101074. doi: 10.1016/j.wocn.2021.101074
Ostrand, R., and Ferreira, V. S. (2019). Repeat after us: syntactic alignment is not partner-specific. J. Mem. Lang. 108:104037. doi: 10.1016/j.jml.2019.104037
Perone, S., and Vaughan, A. M. (2024). Frontal alpha asymmetry dynamics: a window into active self-regulatory processes. Biol. Psychol. 193:108872. doi: 10.1016/j.biopsycho.2024.108872
Pickering, M. J., and Garrod, S. (2004). Toward a mechanistic psychology of dialogue. Behav. Brain Sci. 27, 169–189. doi: 10.1017/S0140525X04000056
Pickering, M. J., and Garrod, S. (2021). Understanding Dialogue. Cambridge: Cambridge University Press.
Redcay, E., and Schilbach, L. (2019). Using second-person neuroscience to elucidate the mechanisms of social interaction. Nat. Rev. Neurosci. 20, 495–505. doi: 10.1038/s41583-019-0179-4
Reinero, D. A., Dikker, S., and Van Bavel, J. J. (2021). Inter-brain synchrony in teams predicts collective performance. Soc. Cogn. Affect. Neurosci. 16, 43–57. doi: 10.1093/scan/nsaa135
Revilla, R., Nelson, C. M., Friedman, N. R., Braun, S. S., and Hudac, C. M. (2024). Frontal alpha asymmetry predicts subsequent social decision-making: a dynamic multilevel, neural, and developmental perspective. Dev. Cogn. Neurosci. 69:101434. doi: 10.1016/j.dcn.2024.101434
Riordan, M. A., Kreuz, R. J., and Olney, A. M. (2014). Alignment is a function of conversational dynamics. J. Lang. Soc. Psychol. 33, 465–481. doi: 10.1177/0261927X13512306
Salley, B., and Colombo, J. (2016). Conceptualizing social attention in developmental research. Soc. Dev. 25, 687–703. doi: 10.1111/sode.12174
Schacter, H. L., and Margolin, G. (2019). The interplay of friends and parents in adolescents' daily lives: towards a dynamic view of social support. Soc. Dev. 28, 708–724. doi: 10.1111/sode.12363
Slocombe, K. E., Alvarez, I., Branigan, H. P., Jellema, T., Burnett, H. G., Fischer, A., et al. (2013). Linguistic alignment in adults with and without Asperger's syndrome. J. Autism Dev. Disord. 43, 1423–1436. doi: 10.1007/s10803-012-1698-2
Smith, E. E., Reznik, S. J., Stewart, J. L., and Allen, J. J. B. (2017). Assessing and conceptualizing frontal EEG asymmetry: an updated primer on recording, processing, analyzing, and interpreting frontal alpha asymmetry. Int. J. Psychophysiol. 111, 98–114. doi: 10.1016/j.ijpsycho.2016.11.005
Sng, C. Y., Carter, M., and Stephenson, J. (2018). A systematic review of the comparative pragmatic differences in conversational skills of individuals with autism. Autism Dev. Lang. Impair. 3, 1–24. doi: 10.1177/2396941518803806
Speer, S. P. H., Mwilambwe-Tshilobo, L., Tsoi, L., Burns, S. M., Falk, E. B., and Tamir, D. I. (2024). Hyperscanning shows friends explore and strangers converge in conversation. Nat. Commun. 15:7781. doi: 10.1038/s41467-024-51990-7
Stabile, M., and Eigsti, I.-M. (2022). Lexical alignment and communicative success in autism spectrum disorder. J. Speech Lang. Hear. Res. 65, 4300–4305. doi: 10.1044/2022_JSLHR-22-00314
Stevenson, M., and Sergeant, E. (2025). epiR: Tools for the Analysis of Epidemiological Data. R package version 2.0.88. Available online at: https://CRAN.R-project.org/package=epiR
Stroth, S., Niehaus, H., Wolff, N., Poustka, L., Roessner, V., Kamp-Becker, I., et al. (2022). Subdimensions of social-communication behavior in autism—A replication study. JCPP Adv. 2:e12077. doi: 10.1002/jcv2.12077
Tager-Flusberg, H. (1993). “What language reveals about the understanding of minds in children with autism,” in Understanding Other Minds: Perspectives from Autism, Eds. S. Baron-Cohen, H. Tager-Flusberg, and D. Cohen (Oxford: Oxford University Press), 138–157.
Thayer, J. F., Hansen, A. L., Saus-Rose, E., and Johnsen, B. H. (2009). Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann. Behav. Med. 37, 141–153. doi: 10.1007/s12160-009-9101-z
Van Engen, K. J., Baese-Berk, M., Baker, R. E., Choi, A., Kim, M., Bradlow, A. R., et al. (2010). The wildcat corpus of native-and foreign-accented English: communicative efficiency across conversational dyads with varying language alignment profiles. Lang. Speech 53, 510–540. doi: 10.1177/0023830910372495
Vincent, K. M., Xie, W., and Nelson, C. A. (2021). Using different methods for calculating frontal alpha asymmetry to study its development from infancy to 3 years of age in a large longitudinal sample. Dev. Psychobiol. 63:e22163. doi: 10.1002/dev.22163
Vygotsky, L. S. (1978). Mind in Society: The Development of Higher Psychological Processes. Cambridge, MA: Harvard University Press.
Wang, Q., Han, Z., Hu, X., Feng, S., Wang, H., Liu, T., et al. (2020). Autism symptoms modulate interpersonal neural synchronization in children with autism spectrum disorder in cooperative interactions. Brain Topogr. 33, 112–122. doi: 10.1007/s10548-019-00731-x
Wilson, M., and Wilson, T. P. (2005). An oscillator model of the timing of turn-taking. Psychon. Bull. Rev. 12, 957–968. doi: 10.3758/BF03206432
Keywords: sociocognitive development, EEG hyperscanning, linguistic alignment, clinically and culturally sensitive methods, collaborative conversation
Citation: Nelson CM, Wilson SC, McFadden J, Almor A and Hudac CM (2025) Dyadic neuroscience is the next scientific frontier of sociocognitive development: a proof-of-concept for a collaborative conversation task in clinical and underrepresented populations. Front. Dev. Psychol. 3:1644956. doi: 10.3389/fdpys.2025.1644956
Received: 11 June 2025; Accepted: 10 October 2025;
Published: 05 November 2025.
Edited by:
Catherine Sandhofer, University of California, Los Angeles, United StatesReviewed by:
Stephanie M. Carlson, University of Minnesota Twin Cities, United StatesEfstratia Papoutselou, University of Nottingham, United Kingdom
Copyright © 2025 Nelson, Wilson, McFadden, Almor and Hudac. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cailee M. Nelson, Y2FpbGVlbkBtYWlsYm94LnNjLmVkdQ==; Caitlin M. Hudac, Y2h1ZGFjQG1haWxib3guc2MuZWR1
 Cailee M. Nelson
Cailee M. Nelson Sarah C. Wilson
Sarah C. Wilson Jackson McFadden
Jackson McFadden Amit Almor
Amit Almor Caitlin M. Hudac
Caitlin M. Hudac