- 1Technology in Semiconductors and Devices Laboratory, Department of ECE, School of Engineering, Tezpur University, Tezpur, Assam, India
- 2Department of Engineering “Enzo Ferrari”, University of Modena and Reggio Emilia, Modena, Italy
- 3Quest-Global, Bengaluru, Karnataka, India
- 4Department of ECE, SRM Institute of Science and Technology-Vadapalani Campus, Chennai, Tamil Nadu, India
- 5Department of Electronics, North Lakhimpur University, Lakhimpur, Assam, India
Dielectric modulation in field-effect transistors (FETs) for label-free biosensing have been extensively explored to date, mostly due to the availability of semiconductor device technology computer-aided design (TCAD) tools. Of these works, many reports have revolved around TCAD simulations and focused on ideal or slightly deviated-from-ideal conditions, rather than on the inclusion of non-idealities to create actual biosensing test scenarios. This perspective presents a status of label-free dielectric-modulated biosensing in FETs. It highlights the five most important but rarely used or missing non-idealities in semiconductor TCAD tools, viz., multispecies representation, biomolecular kinematics, cavity profile, hybridization, and transient response. To better align TCAD frameworks with experimental studies, this article recommends adopting method-specific TCAD-integrated modeling (MSTIM) approaches.
1 Introduction
Dielectric modulation in field-effect transistors (FETs) for biosensing purposes has garnered widespread interest since the demonstration of the phenomenon (Im et al., 2007). While the fabrication of the biosensor using a conventional CMOS fabrication process flow was significant in the timeline, it was the simulation framework that inspired research work on dielectric-modulated (DM) biosensing using FETs.
Technology computer-aided design (TCAD) tools for semiconductor devices have revolutionized the electronics design and technology sector on many fronts while reducing financial and design costs (SDU Manual, 2022; Sarkar, 2018). Major functional advantages of TCAD tools include (a) design and simulation of two-dimensional and three-dimensional device architectures; (b) flexibility and options of using several physics-based models to describe device principles; (c) parametric visualization of electrical parameters; (d) definition of new material properties for simulation; (e) device-to-circuit and mixed mode analyses.
The ability of the TCAD tools to conveniently design and simulate FETs has shaped the way research has progressed in the area of DM label-free biosensing using FETs. The design of cavities in the gate dielectric of FETs for dielectric-modulated biosensing has led to the emergence of different configurations of DM biosensors across devices of multiple architectures and principles of operation, which include metal oxide semiconductor (MOS) FETs (Das et al., 2025; Yojo et al., 2021), tunnel FETs (Vadizadeh et al., 2025; Elshafie et al., 2025; Kondavitee et al., 2025; Hussian et al., 2024), fin-shaped FETs (Gandhi and Kondekar, 2025), nanotube FETs (Hadded et al., 2024; Ho et al., 2024), and junctionless FETs (Singh et al., 2024; Son et al., 2025). While the number of studies of FET architectures for biosensing is exceptional, there has been slow progress in the design of simulation environments with near-practical test scenarios.
The major challenge in most TCAD tools is the absence of full-scale biomolecular kinematics and electrochemical models. This roadblock in the evolution of the TCAD environment includes the non-idealities encountered in real-world DM biosensing applications. Another concern is that while few articles have attempted to explore this area and presented interesting solutions, the same does not apply to benchmarking the simulation environments for such works. This has not only affected the way in which TCAD simulations are carried out for DM biosensors but also has led to methods that have become repeatable and mostly predictable.
The primary objective of this article is to assess the current state of the art in FET-based DM biosensors and present the gaps in TCAD frameworks that can be explored to propel the research in this area with better test scenarios and methods. To avoid ambiguity, it is to be noted that any one of the terminologies—label-free biosensors, dielectric-modulated (DM) biosensors, FET-based DM biosensors, or FET-based biosensors—refers to cavity-embedded FET-based label-free dielectric-modulated biosensors.
2 Current status, gaps, and challenges
2.1 Architecture
In the context of label-free biosensing, dielectric modulation refers to the principle by which altering the material properties (dielectric constant,
As mentioned in Section 1, the currently proposed TCAD analyses of DM biosensors originate from the work of Im et al. (2007). The fabricated DM FET-based biosensor reported by the authors had a partially etched chromium cavity (leading to an air gap) beneath the gold electrodes. To demonstrate dielectric modulation in a biotin–streptavidin system, the reported FET was fabricated with a cavity length of 200 nm in experiments (400 nm using a TCAD tool) and a cavity height of 15 nm (Im et al., 2007). Using a dielectric constant of 2.1 in the cavity, TCAD simulations showed a shift in the threshold voltage of the device; a similar trend was observed for the experimental measurement. The report further proved that the biotin–streptavidin binding can be reversibly broken, showing that after it is broken,
Based on the work mentioned in the preceding paragraph, it is a fundamental design principle in current TCAD frameworks that while creating any FET-based DM biosensor in a TCAD tool, the gate dielectric region beneath the gate material is divided into at least two dielectric materials. One material that represents the actual gate dielectric partially fills the region, provides support to the gate material in principle, and has a fixed dielectric constant. The other dielectric material is used to represent the etched cavity—its dielectric constant (
While several works have focused on specific biomolecules such as breast cancer markers (Prasanthi et al., 2025), uricase, APTES, bacteriophage, keratin (Mohanty et al., 2021), and SARS-CoV-2 (Yadav et al., 2021), others have demonstrated the performance of DM FET biosensors using values of dielectric constants and charge (Raut et al., 2025; Goswami and Bhowmick, 2019). Sensitivity in such biosensors is measured according to the relative change in an electrical parameter like drain current and threshold voltage with reference to an air-filled cavity, mathematically described by the following form:
2.2 Considerations and deviations in TCAD simulations
This section highlights the methods through which key conditions are currently considered in TCAD simulations and their deviations from practical scenarios, if any. These methods and conditions are discussed in a tabular format (Table 1) to fill the gaps and missing cases in TCAD frameworks that may be reliably used to create test scenarios that are close to actual scenarios.
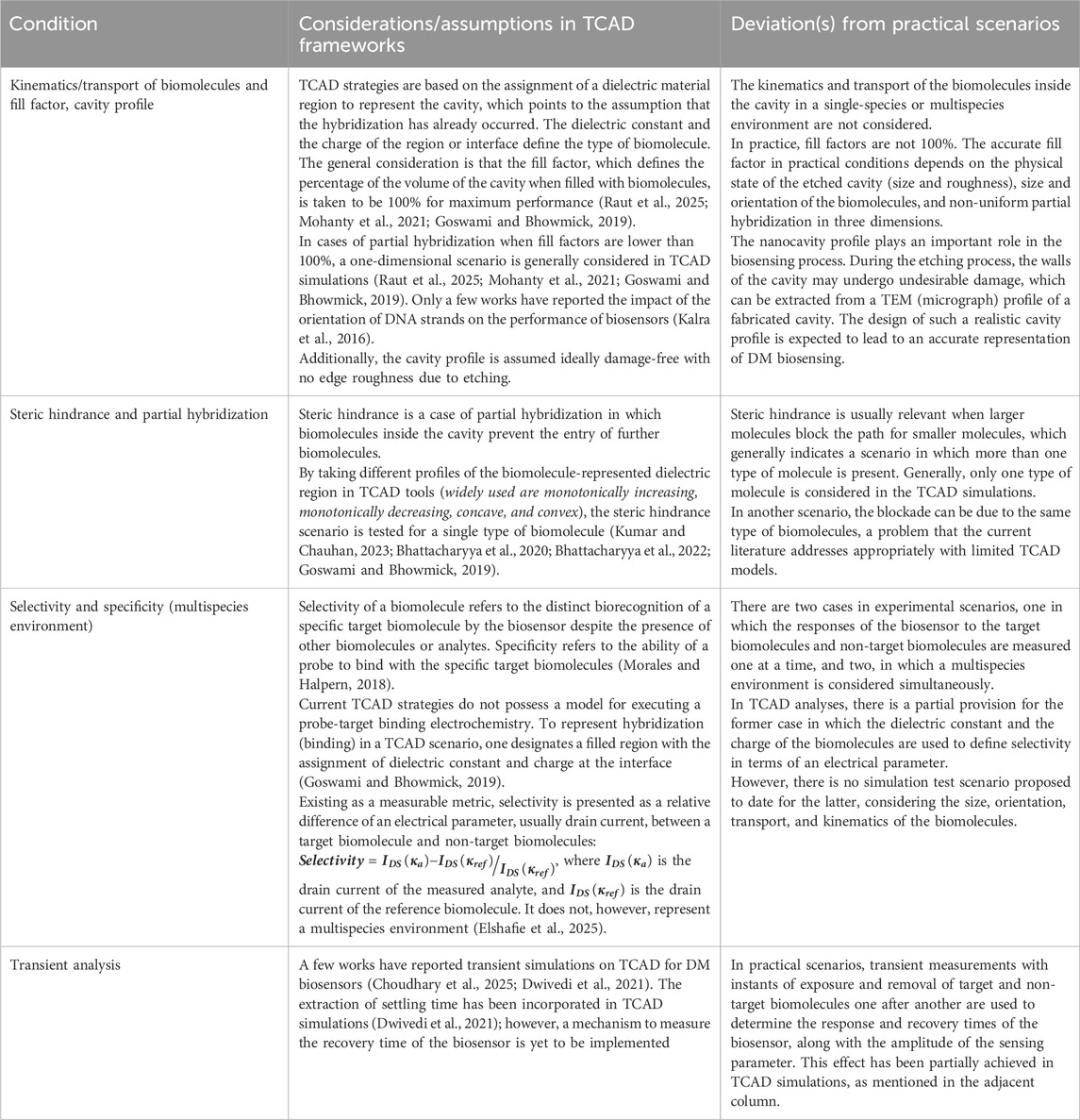
Table 1. TCAD strategies for DM FET-based biosensors, their current status, and deviations from practical scenarios.
3 Method-specific TCAD-integrated modeling (MSTIM): perspectives for better models
It is evident from Section 2 that advances in the domain of DM biosensors using TCAD tools have occurred from the standpoint of architectures and types of devices and placement of the cavity. The primary purpose of TCAD tools is to create predictive frameworks for real-world scenarios so that time, effort, and cost are minimized and optimization is done before fabricating a device. Progress in the areas of design of experiment-mimicking models and measurement methods using TCAD tools is expected to complement the work in experimental biosensing.
Current considerations in TCAD simulations (Refer to Table 1) are yet to include several practical conditions. Some innovation prospects in TCAD frameworks are method-inclusive with sufficient considerations for non-idealities, such as the impact of the method of loading on steric hindrance (including size of biomolecules, and cavity profiles), partial hybridization (including real experimental profiles), and response and recovery times (reference to transient analyses). Therefore, a method-specific TCAD-integrated modeling (MSTIM) approach is proposed with emphasis on a holistic TCAD framework. Here, the terminology method is inclusive of all variables (related to functionalization, kinematics, transport, cavity profile, shape, properties of biomolecules, device design, and measurement) that may decide the process of biosensing. Figure 1 shows the basis for an MSTIM approach, highlighting the importance of the design of the cavity with possibilities of including cavity designs based on actual post-etching profiles. Similarly, the representation of receptor layers may vary from a single layer to multi-wall layers, depending on the method of receptor functionalization. The inclusion of the size and shape of biomolecules in TCAD structural designs is another possibility. For cavities that are only 10–15 nm in height, the length or diameter and orientation of the molecules (bioanalytes and receptors), along with that of any self-assembled layers, is crucial for accurate determination of the fill factors. In addition to the dimensions of molecules, the selectivity, specificity, and time required for attaining equilibrium conditions may lead to scenarios where the entire receptor layer is not bound by bioanalytes. The scenario is particularly important in a multispecies biomolecule environment, which remains underrepresented in current TCAD frameworks.
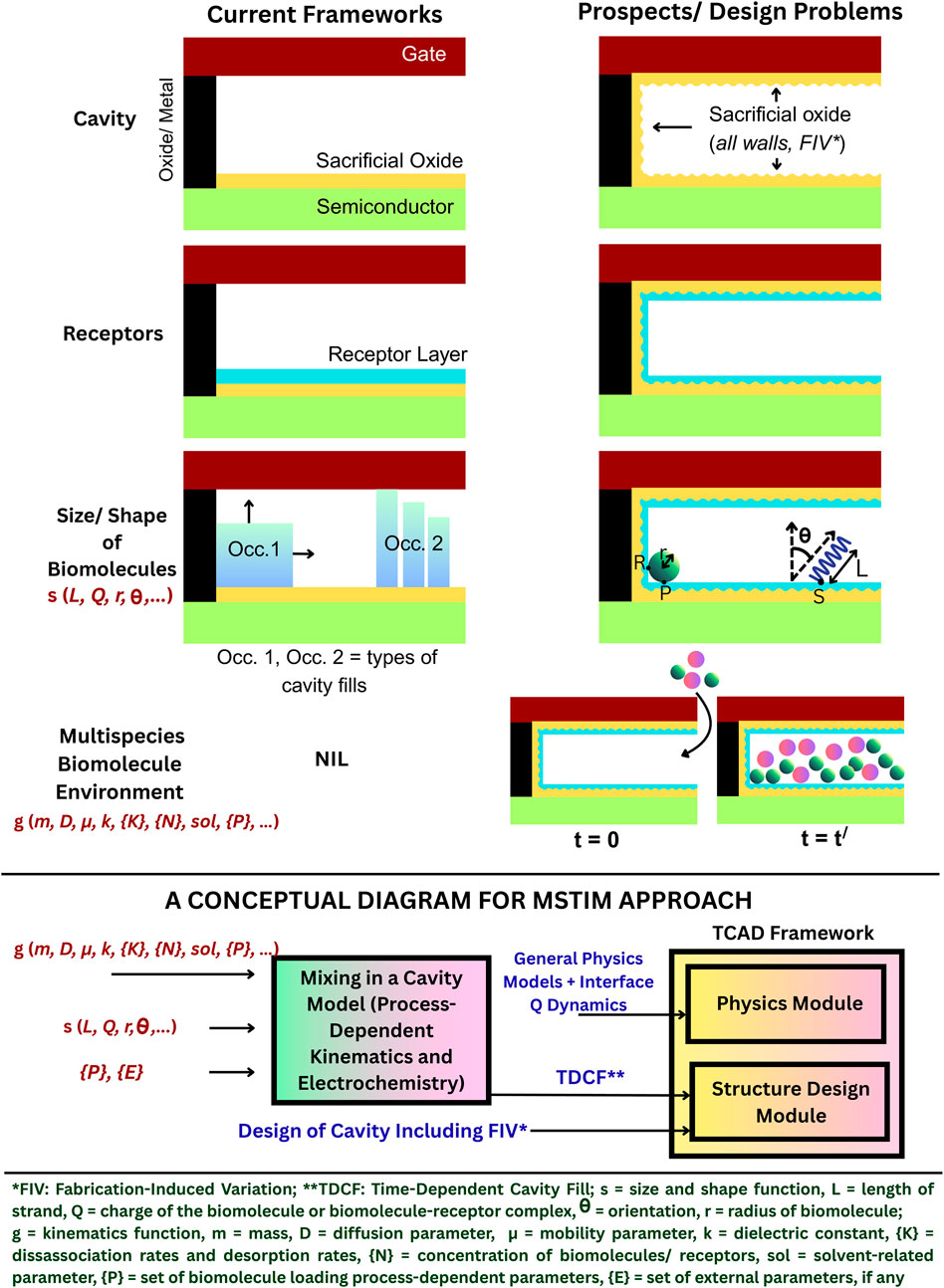
Figure 1. Summary of the MSTIM approach toward building robust TCAD frameworks for DM FET biosensors, showing the gaps and the need for external models.
One of the important scenarios in a multispecies environment is the mixing-in-a-box problem (e.g., rapid mixing process or diffusion-limited process) (Chanda et al., 2023). The process by which the biomolecules move and interact inside a cavity when inserted is important in small cavities. Such analyses using the Langmuir kinematics and diffusion-limited process have been reported for ion-sensing field-effect transistors (ISFETs) (Chanda et al., 2023; Wadhwa et al., 2024); however, similar kinematics and transport mechanisms have not been used in the case of DM FET biosensors.
Existing works on FET-based DM biosensors using TCAD tools generally refer to the dry or near-dry sensing of biomolecules (referring to a state in which a dry cavity is occupied with either vacuum or biomolecules or both, during measurement) instead of wet sensing as in ISFETs (Hu et al., 2023). However, the so-called dry sensing in FET-based DM biosensors depends on several wet steps (Im et al., 2007), which, if considered, can make the TCAD frameworks robust and more realistic. Interestingly, during the functionalization of the cavity in such biosensors, the receptors and bioanalytes are transported to the cavity using appropriate buffer solutions. The cavity is dried with ultra-pure nitrogen after every step of functionalization, or at the end (Im et al., 2007). This indicates that models for Langmuir kinematics and diffusion-limited processes in ISFETs can be applied to DM FET-based biosensors to determine the filling of the cavity (time-dependent cavity fill: TDCF). On the other hand, apart from kinematics and transport, measurement models can account for buffer residues due to incomplete or faulty nitrogen drying, an aspect that has not yet been discussed in reported works.
Dielectric-modulated FET (DM FET) biosensors currently rely almost exclusively on aqueous-phase insertion of biomolecules into cavities. While effective, such wet-loading often suffers from uneven filling, nonspecific adsorption, and structural instability of biomolecules during processing. To move beyond these limitations, it is worth considering dry and near-dry loading methods that, although not yet implemented in DM-FETs, have shown promise in other biomolecule immobilization and pharmaceutical biomolecule or drug delivery technologies.
Aerosol or electrospray deposition may provide localized, reproducible loading by rapidly evaporating nebulized droplets, but shear forces and solvent compatibility with semiconductor surfaces are concerns (Mensink et al., 2017; Kavadiya and Biswas, 2018). Lyophilization–infiltration cycles offer a hybrid route to combine penetration with stability, although multiple drying steps may complicate device fabrication (Nadkarni et al., 2024; Kawasaki et al., 2019; Gaidhani et al., 2015). Vacuum-assisted dry loading could allow insertion of lyophilized biomolecules into cavities without exposing the dielectric to swelling or liquid-induced defects, although its efficiency in nanoscale pores remains unknown (Nadkarni et al., 2024; Mensink et al., 2017). Supercritical CO2-assisted insertion is particularly attractive for its ability to deeply penetrate cavities and leave no solvent residues, but adapting high-pressure processes to semiconductor devices poses engineering challenges (do Nascimento Junior et al., 2021). Sublimation-based or electric-field–assisted sublimation deposition (Guo et al., 2015) has not yet been demonstrated in cavity-based FET biosensors, but conceptually it could inspire solvent-free loading strategies, especially for smaller biomolecular species or for protective coatings that help stabilize larger biomolecules. The feasibility depends heavily on adapting the chemistry to preserve bioactivity during the sublimation process.
Although academic in principle, these methods highlight unexplored opportunities for DM FET biosensors. If adapted successfully, they could offer more uniform, residue-free, and stable biomolecule loading than conventional aqueous approaches. Systematic studies are needed to assess their feasibility. They represent a forward-looking direction for enhancing the robustness and reproducibility of label-free DM FET biosensing.
Although TCAD tools are devoid of method-specific models, externally designed time-dependent models can be used to determine the orientation and position of receptors and biomolecules inside the cavity so that their profile can be included in TCAD simulations.
4 Future directions
The domain of FET-based DM label-free biosensors is promising and has been explored to the best of the potential of the TCAD tools so far. With innovation in experiments growing with time, there is a need for progress in simulation frameworks for such biosensors. The current status of the DM biosensors reveals a one-directional progress, and therefore, the domain demands for innovation in TCAD strategies for DM biosensing. Method-specific models incorporating variables that describe the kinematics, nature of molecules, cavity design parameters, and other external parameters are expected to assist experimental studies. Holistic TCAD frameworks supported by such MSTIM approaches are expected to revolutionize the FET-based DM biosensing research. TCAD models could explore other bioanalyte loading methods that are widely used in other biomolecule immobilization strategies or in the pharmaceutical industries. While assessment is required to test alternative strategies, the process involved therein may open new avenues in modeling DM FET biosensors.
The advent of artificial intelligence (AI) and machine learning (ML) in the exploration of semiconductor devices is a boon to the industry. At a time when such state-of-the-art technologies are being applied to experiments in biosensing, TCAD frameworks must be built with better models and approaches so that the prediction of the performance of these biosensors is more efficient.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
RG: Conceptualization, Writing – review and editing, Investigation, Supervision, Writing – original draft. VMU: Writing – original draft, Resources, Investigation, Writing – review and editing. SM: Writing – original draft, Supervision, Writing – review and editing. DD: Writing – review and editing, Investigation, Writing – original draft. PD: Investigation, Writing – review and editing, Writing – original draft. HC: Writing – original draft, Investigation, Writing – review and editing. RG: Writing – original draft, Writing – review and editing, Investigation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bhattacharyya, A., Chanda, M., and De, D. (2020). Analysis of partial hybridization and probe positioning on sensitivity of a dielectric modulated junctionless label free biosensor. IEEE Trans. Nanotechnol. 19, 719–727. doi:10.1109/tnano.2020.3025544
Bhattacharyya, A., De, D., and Chanda, M. (2022). Sensitivity measurement for bio-TFET considering repulsive steric effects with better accuracy. IEEE Trans. Nanotechnol. 21, 100–109. doi:10.1109/tnano.2022.3148922
Chanda, M., Patel, S. D., Bhattacharyya, A., and Sahay, S. (2023). Impact of transport mechanism on binding kinematics and sensitivity of FET biosensors. IEEE Trans. Electron Devices 71 (1), 359–367. doi:10.1109/ted.2023.3281539
Choudhary, V., Kumar, M., Chugh, N., and Madan, J. (2025). B-VDL-TFET: an ultra-scaled, high-sensitivity sensor for breast cancer detection. IEEE Sensors J. 25, 29820–29829. doi:10.1109/jsen.2025.3581081
Das, S. K., Biswal, S. M., Giri, L., Pahi, I., and Nanda, U. (2025). Development and investigation of DMDG-MOSFET biosensor for charged biomolecule detection. Phys. Scr. 100 (2), 025001. doi:10.1088/1402-4896/ad9784
do Nascimento Junior, D. R., Tabernero, A., Cabral Albuquerque, E. C. D. M., and Vieira de Melo, S. A. B. (2021). Biopesticide encapsulation using supercritical CO2: a comprehensive review and potential applications. Molecules 26 (13), 4003. doi:10.3390/molecules26134003
Dwivedi, P., Singh, R., Sengar, B. S., Kumar, A., and Garg, V. (2021). A new simulation approach of transient response to enhance the selectivity and sensitivity in tunneling field effect transistor-based biosensor. IEEE Sensors J. 21 (3), 3201–3209. doi:10.1109/jsen.2020.3028153
Elshafie, H., Basha, A. A., Alqahtani, A. S., Mubarakali, A., Parthasarathy, P., and Venkatesh, M. (2025). Performance optimization of High-κ engineered TM-DG-InP/GaAs-TFET for ultra-sensitive biosensing applications: mechanistic insights and TCAD simulation analysis. ECS J. Solid State Sci. Technol. 14 (5), 057002. doi:10.1149/2162-8777/add49b
Elshafie, H., Alqahtani, A. S., Mubarakali, A., Rag, A., Venkatesh, M., and Parthasarathy, P. (2025). Label-free biomolecule detection with InP/AlGaAs charge plasma dielectric-modulated vertical TFET using TaN as metal gate. IEEE Access 13, 89667–89684. doi:10.1109/access.2025.3570233
Gaidhani, K. A., Harwalkar, M., Bhambere, D., and Nirgude, P. S. (2015). Lyophilization/Freeze Drying–A review. World J. Pharm. Res. 4 (8), 516–543.
Gandhi, N., and Kondekar, P. N. (2025). Artificial neural network-assisted optimization of DM JLNC-FinFET biosensor with raised source/drain architecture. IEEE Sensors J. 25, 32851–32860. doi:10.1109/jsen.2025.3592016
Goswami, R., and Bhowmick, B. (2019). Comparative analyses of circular gate TFET and heterojunction TFET for dielectric-modulated label-free biosensing. IEEE Sensors J. 19 (21), 9600–9609. doi:10.1109/jsen.2019.2928182
Guo, S., Wang, Y., Zhou, D., and Li, Z. (2015). Electric field-assisted matrix coating method enhances the detection of small molecule metabolites for mass spectrometry imaging. Anal. Chem. 87 (12), 5860–5865. doi:10.1021/ac504761t
Hadded, A., Ayed, M. B., and Alshaya, S. A. (2024). High sensitivity and specificity in healthcare: design and validation of a novel SiNW-FET biosensor for viral detection. IEEE Access 12, 112308–112319. doi:10.1109/access.2024.3442428
Ho, R., Fuller, S., Lee, H. S., and Shulaker, M. M. (2024). Biosensor chip for point-of-care diagnostics: carbon nanotube sensing platform for bacterial detection and identification. IEEE Trans. Nanotechnol. 23, 281–285. doi:10.1109/tnano.2024.3380997
Hu, B., Sun, H., Tian, J., Mo, J., Xie, W., Song, Q. M., et al. (2023). Advances in flexible graphene field-effect transistors for biomolecule sensing. Front. Bioeng. Biotechnol. 11, 1218024. doi:10.3389/fbioe.2023.1218024
Hussian, A., Alkhammash, H. I., Wani, M. S., and Loan, S. A. (2024). Metal strip implanted tunneling field-effect transistor biosensor as a label-free biosensor. ACS Appl. Bio Mater. 7 (7), 4633–4641. doi:10.1021/acsabm.4c00483
Im, H., Huang, X. J., Gu, B., and Choi, Y. K. (2007). A dielectric-modulated field-effect transistor for biosensing. Nat. Nanotechnol. 2 (7), 430–434. doi:10.1038/nnano.2007.180
Kalra, S., Kumar, M. J., and Dhawan, A. (2016). Dielectric-modulated field effect transistors for DNA detection: impact of DNA orientation. IEEE Electron Device Lett. 37 (11), 1485–1488. doi:10.1109/led.2016.2613110
Kavadiya, S., and Biswas, P. (2018). Electrospray deposition of biomolecules: applications, challenges, and recommendations. J. Aerosol Sci. 125, 182–207. doi:10.1016/j.jaerosci.2018.04.009
Kawasaki, H., Shimanouchi, T., and Kimura, Y., (2019). Recent development of optimization of lyophilization process. J. Chem., 9502856, 14 pages. doi:10.1155/2019/9502856
Kondavitee, G. S., Kumar, R. A., Shaikh, M. R. U., Alkhammash, H. I., Wadhwa, G., Ramesh, M., et al. (2025). Novel label-free detection of cancer biomarkers using T-Shape channel and L-Shape nano-cavity in si: HfO 2 ferroelectric DM-JLTFET with gate staggering biosensor. IEEE Sensors J., 1. doi:10.1109/jsen.2025.3562321
Kumar, S., and Chauhan, R. K. (2023). Biosensor sensitivity and steric hindrance: a comparison of InGaAs pocket and conventional TFET designs. Micro Nanostructures 182, 207644. doi:10.1016/j.micrna.2023.207644
Mensink, M. A., Frijlink, H. W., van der Voort Maarschalk, K., and Hinrichs, W. L. (2017). How sugars protect proteins in the solid state and during drying (review): mechanisms of stabilization in relation to stress conditions. Eur. J. Pharm. Biopharm. 114, 288–295. doi:10.1016/j.ejpb.2017.01.024
Mohanty, S. S., Mishra, S., Mohapatra, M., and Mishra, G. P. (2021). Impact of bio-target location and their fill-in factor on the sensitivity of hetero channel double gate MOSFET label-free biosensor. Adv. Nat. Sci. Nanosci. Nanotechnol. 12 (2), 025012. doi:10.1088/2043-6262/ac0799
Morales, M. A., and Halpern, J. M. (2018). Guide to selecting a biorecognition element for biosensors. Bioconjugate Chem. 29 (10), 3231–3239. doi:10.1021/acs.bioconjchem.8b00592
Nadkarni, A., Rana, D., Desai, N., Benival, D., Joshi, V., Salave, S., et al. (2024). Advanced characterization and sample preparation strategies for nanoformulations. J. Nanotheranostics 5 (3), 104–127. doi:10.3390/jnt5030008
Prasanthi, L., Prakash, M. D., Tammireddy, S. S., Satyanarayana, V., Panigrahy, A. K., Patta, S., et al. (2025). Advanced hybrid dielectric OTFT biosensor for early breast cancer diagnosis with enhanced sensitivity. Results Eng. 27, 107037. doi:10.1016/j.rineng.2025.107037
Raut, P., Panda, D. K., Nanda, U., and Hsu, C. C. (2025). Simulation and modeling of high-sensitive JL-TFET based biosensor for label free detection of biomolecules. Microsyst. Technol. 31 (5), 1103–1111. doi:10.1007/s00542-024-05638-7
Sarkar, A. (2018). Device simulation using silvaco ATLAS tool. In: Technology computer aided design. Boca Raton, FL: CRC Press. p. 203–252.
Singh, D., Patil, G. C., and Choudhury, B. D. (2024). Split gate bulk-planar junctionless FET based biosensor for label-free detection of biomolecules. IEEE Sensors J. 24, 28611–28618. doi:10.1109/jsen.2024.3439575
Son, J., Heo, C., Kim, H., Meyyappan, M., and Kim, K. (2025). A novel bulk planar junctionless field-effect transistor for high-performance biosensing. Biosensors 15 (3), 135. doi:10.3390/bios15030135
Vadizadeh, M. (2025). “Design and Performance of a Dielectrically Modulated Electron-Hole Bilayer Tunneling FET Biosensor: Effects of Nanogap Cavity on Sensitivity and Voltage Requirements,” in IEEE Sensors Journal 25, 29576–29583. doi:10.1109/JSEN.2025.3582331
Wadhwa, G., Thakur, A., Taibi, A., and Proto, A. (2024). Performance analysis based on biomolecule position and pH-sensing mechanism for vertical TFET. Phys. Scr. 100 (1), 015029. doi:10.1088/1402-4896/ad9a13
Yadav, S., Gedam, A., and Tirkey, S. (2021). A dielectric modulated biosensor for SARS-COV-2. IEEE Sensors J. 21 (13), 14483–14490. doi:10.1109/jsen.2020.3019036
Keywords: TCAD, dielectric-modulated biosensor, steric hindrance, kinematics, selectivity, sensitivity, cavity profile, hybridization
Citation: Goswami R, Menon U V, Mitra SK, Deb D, Das PS, Choudhury H and Gautam RV (2025) Beyond ideal models: non-idealities in TCAD simulations of dielectric-modulated FETs for label-free biosensing. Front. Electron. 6:1686130. doi: 10.3389/felec.2025.1686130
Received: 14 August 2025; Accepted: 10 September 2025;
Published: 09 October 2025.
Edited by:
Dimitrios Koutsouras, University of Bath, United KingdomReviewed by:
Angsuman Sarkar, Kalyani Government Engineering College (KGEC), IndiaCopyright © 2025 Goswami, Menon U, Mitra, Deb, Das, Choudhury and Gautam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rupam Goswami, cnVwYW0yMUB0ZXp1LmVybmV0Lmlu
†ORCID: Unnikrishnan Vivek Menon, orcid.org/0000-0001-5946-115X
 Rupam Goswami
Rupam Goswami Vivek Menon U
Vivek Menon U Suman Kumar Mitra3
Suman Kumar Mitra3