- 1Special Medicine Department, Medical College, Qingdao University, Qingdao, China
- 2Clinical Medicine Department, Medical College, Qingdao University, Qingdao, China
- 3Physiology Department, Medical College, Qingdao University, Qingdao, China
Background: Insulin resistance is a metabolic disorder that occurs in type 2 diabetes mellitus and obesity. Genetic factors such as β3-adrenoceptor polymorphism (Trp64Arg) may be involved in IR and insulin secretion. However, their association is controversial. Therefore, the current meta-analysis was conducted to clarify the relationship between the Trp64Arg and IR.
Methods: The literature search was performed in PubMed, Embase, and Web of Science using the keywords “Receptors, Adrenergic, beta-3, Receptors, Adrenergic, Insulin Resistance, Protein-Coupled Receptor Kinase 3” from 2005 to February 7, 2021. We used a random-effects model to calculate the pooled effect size. We conducted subgroup analysis and regression analysis to identify sources of heterogeneity; and Egger’s test and funnel plot were used to test publication bias. Finally, we conducted a sensitivity analysis.
Results: We included eight papers with 1,586 subjects. There was a positive correlation between Trp64Arg mutation and insulin level (standardized mean difference = 0.20, 95% confidence intervals: 0.00 to 0.39, I2 = 57.6%, p = 0.016). However, there was no association between Trp64Arg and the homeostasis model (HOMA-IR) assessment. Egger’s tests showed no publication bias; the sensitivity analysis showed that our results were stable. Regression analysis revealed no source of heterogeneity.
Conclusion: Trp64Arg may be associated with IR. European ancestry, obesity, plasma insulin level, and test status may be potential factors affecting the relationship between Trp64Arg and IR.
Introduction
Insulin resistance (IR) is a metabolic disorder that can lead to type 2 diabetes and obesity (1). It can occur in the liver, white adipose tissues, and skeletal muscle (2–4). In clinical practice and epidemiological studies, fasting insulin levels and homeostasis model assessment (HOMA-IR) are commonly used substitute indices of IR (5). There is evidence to suggest that genetic factors are involved in IR and insulin secretion (6).
β-Adrenoceptors are the targets of endogenous catecholamines noradrenaline and adrenaline (7). β3-Adrenergic receptor gene (ADRB3) is a member of the β-adrenoceptor system (7) and plays an essential role in metabolic disorders (8). ADRB3 is expressed in human visceral adipose and is associated with increased lipolysis. In turn, increased lipolysis can lead to skeletal muscle IR (9). ADRB3 dysfunction may lead to IR and obesity and may be a candidate gene for obesity and IR (5). Winden et al. (10) reported that the ADRB3 polymorphism is the replacement of tryptophan (Trp64Trp) by arginine at position 64 (Trp64Arg) of the β3-adrenergic receptor. Studies showed that the Trp64Arg mutation was associated with type 2 diabetes mellitus, IR, and body weight increases (11, 12). Subsequently, many studies investigated the relationship between this common variant and various metabolic syndrome phenotypes, including elevated body mass index
(BMI); Trp64Arg mutation had a specific effect on BMI (13–15). Similarly, the relationship between IR or its related indices and Trp64Arg was also studied. In 2005, a meta-analysis indicated that the Trp64Arg variant was associated with IR (5). However, studies after 2005 remained controversial, and there has been no meta-analysis to clarify the relationship between the Trp64Arg and IR. For example, Højlund et al. (16) demonstrated that Trp64Arg might not increase IR, and another study suggested that Trp64Arg had no relationship with IR (17). However, another study showed that a mutation group of patients with Trp64Arg had higher insulin and HOMA-IR levels (18).
Therefore, to help resolve the discrepancies among these studies, we analyzed the association between Trp64Arg and IR or its related indices in this meta-analysis.
Research Methods
Search Strategy
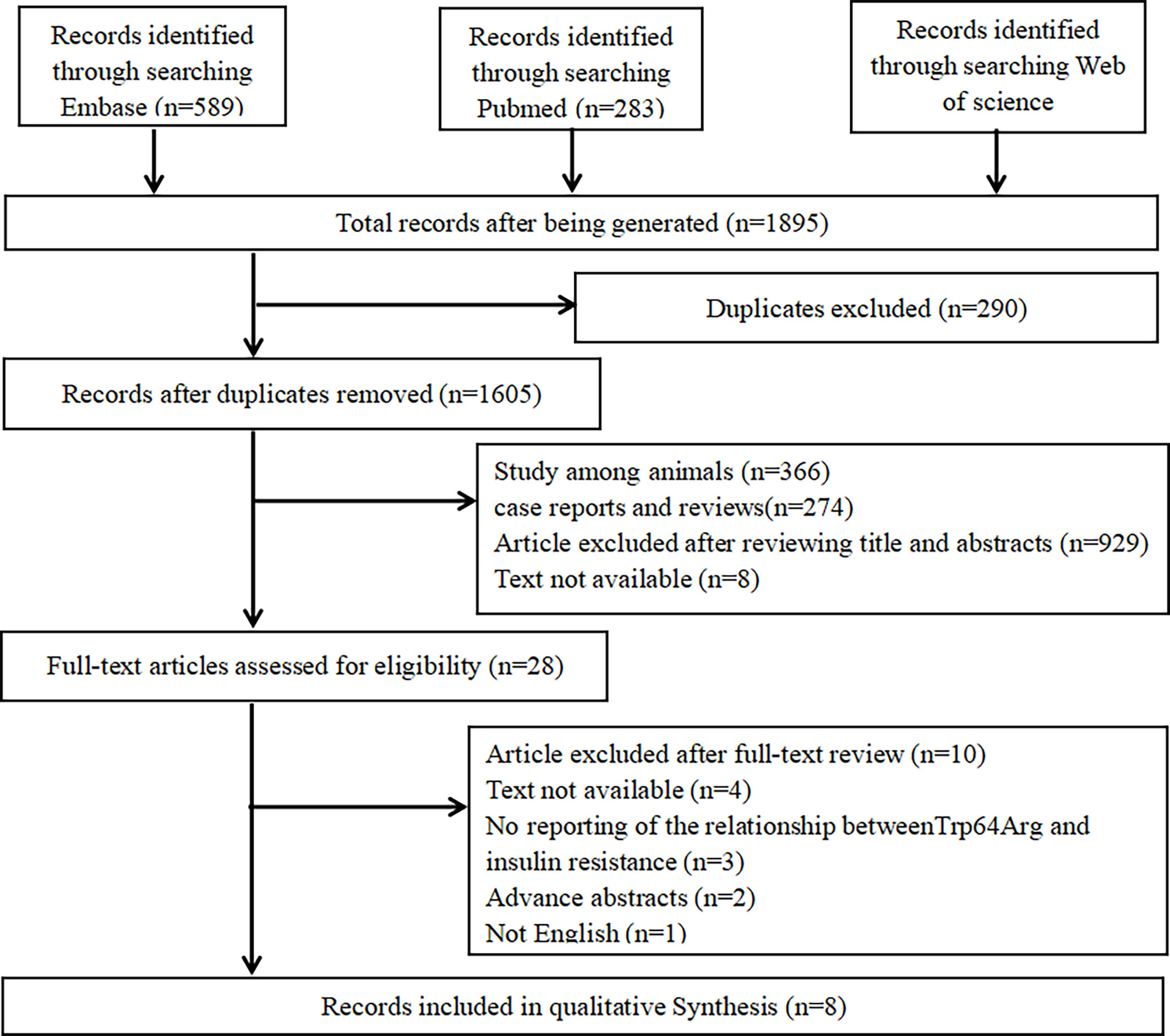
We performed the meta-analysis based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (19). A literature research was performed in PubMed, Web of Science, and Embase. Two groups of keywords and their major subheading terms were used to search relevant studies including receptors, adrenergic, beta-3 (e.g., “beta-3 Adrenergic Receptors,” “Receptors, Adrenergic,” “G-Protein-Coupled Receptor Kinase 3,” “TRP64Arg,” “TRP64Arg polymorphisms,” “ADRB3,” “adrenergic receptor gene,” “adrenergic receptor gene,” and “β3-AR”) and IR (e.g., “Resistance, insulin,” “Insulin Sensitivity,” and “Sensitivity, Insulin”). The date range was from January 1, 2005 to February 7, 2021. More details are shown in Figure 1.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: 1) studies of humans; 2) inclusion of means, the standard deviation (SD) of related indices of IR, including insulin level and HOMA-IR by genotypes and by subgroups; 3) publication in English; and 4) observational studies with subjects ≥10. The exclusion criteria were as follows: 1) case reports, reviews, comments, protocols, meeting abstracts, and meta-analyses; 2) no study of the correlation between Trp64Arg and IR; 3) other interventions; and 4) inability to assess the full text.
Data Extraction and Quality Assessment
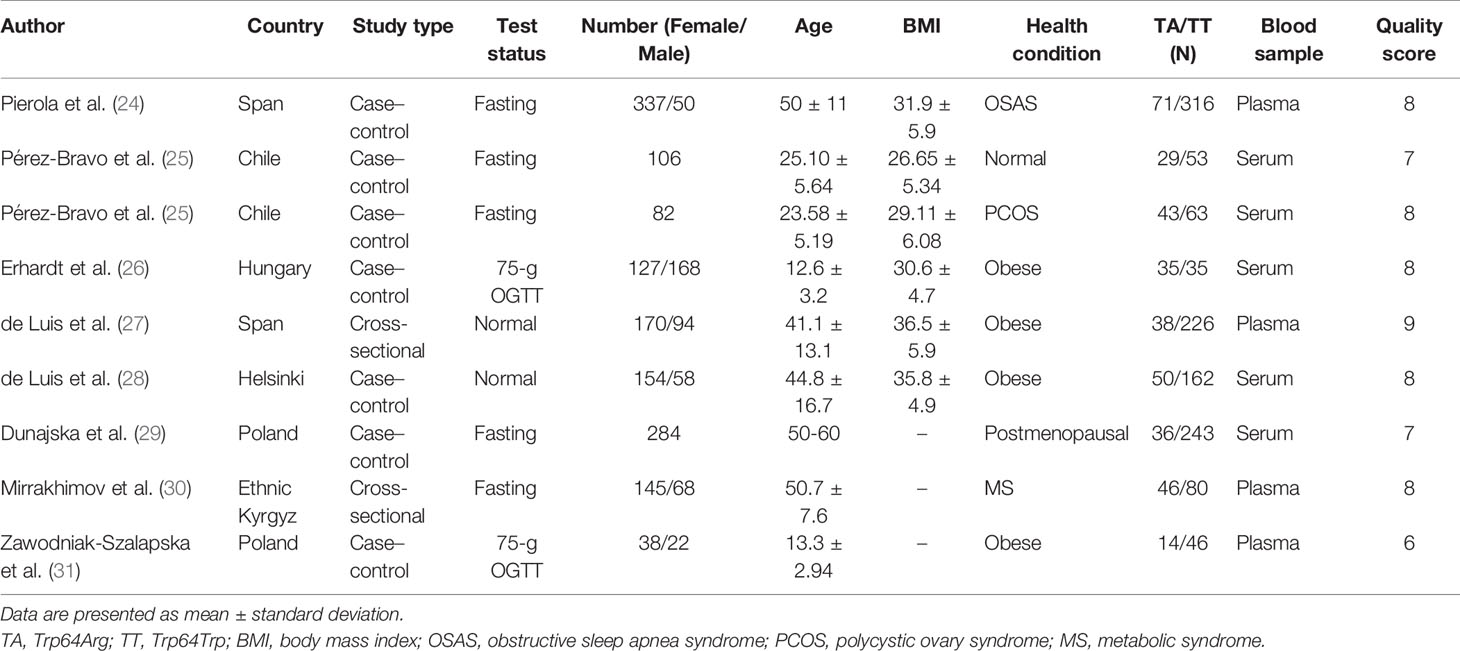
For data extraction, two independent reviewers reviewed all studies and extracted the data in a standardized format. The data were collected after all the disagreements were resolved. The collected information included the first author of the studies, the year of publication, country, sample size, the number of men and women, and the effect value. Subjects’ characteristics such as age, BMI, health condition, and test status of insulin were also extracted as potential variables for further subgroup analysis. To unify the units of insulin, the conversion factor 6.945 (1 mU/L = 6.945 pM) was applied to transform insulin concentration from picomolar into milliunits per liter (20). The qualification of the cross-sectional studies was based on Agency for Healthcare Research and Quality (21), and Newcastle-Ottawa Scale was used to assess the qualifications of case–control studies. All assessments were performed by two authors independently. Specific criteria and scores are presented in Supplementary Table 1.
Statistical Analysis
The associations between Trp64Arg and IR were analyzed using the random-effects model, which used the mean values and their SD. Forest plots were also drawn. I2 was used to show the heterogeneity; I2 ≥50% and p-value <0.05 indicated significant heterogeneity (22). If the results showed heterogeneity, meta-regression analysis was used to explore its sources (23). We conducted influence analysis to determine the impact of a single study on the overall results. If the results showed that one study impacted on the overall results, we conducted a sensitivity analysis to determine the stability of the results. Cumulative analysis was performed to evaluate the trend of pooled effect estimates over time. Publication bias was analyzed using funnel plots and Egger’s tests. We also performed subgroup analysis based on age, country, BMI, blood samples, and test status. All the analyses were performed using Stata software 12.0.
Results
Literature Search
Search details are shown in Table 1. We searched PubMed, Web of Science, and Embase. In total, 1,895 potential studies were filtered from online databases, and 290 studies were removed because of duplication, 1,577 ineligible studies were removed after scanning the title and abstracts, and 20 were removed after scanning the full texts. Finally, eight studies met the selection criteria (24–31).
Study Characteristics
All of the studies were published from 2005 to 2021 and included 362 subjects with the Trp64Arg variant and 1,224 subjects with normal genotype. The characteristics of the studies are presented in Table 1. Six case–control studies (24–26, 28, 29, 31) included 1,196 individuals and two cross-sectional studies (27, 30) that included 390 individuals. Four studies were conducted among obese populations (26–28, 31), one study was conducted among the normal (25) population, and the other four studies were conducted among people with obstructive sleep apnea syndrome (24), polycystic ovary syndrome (25), postmenopausal status (29), and metabolic syndrome (30). The age ranged from 13 to 60 years. More details are presented in Table 1.
Overall Analysis
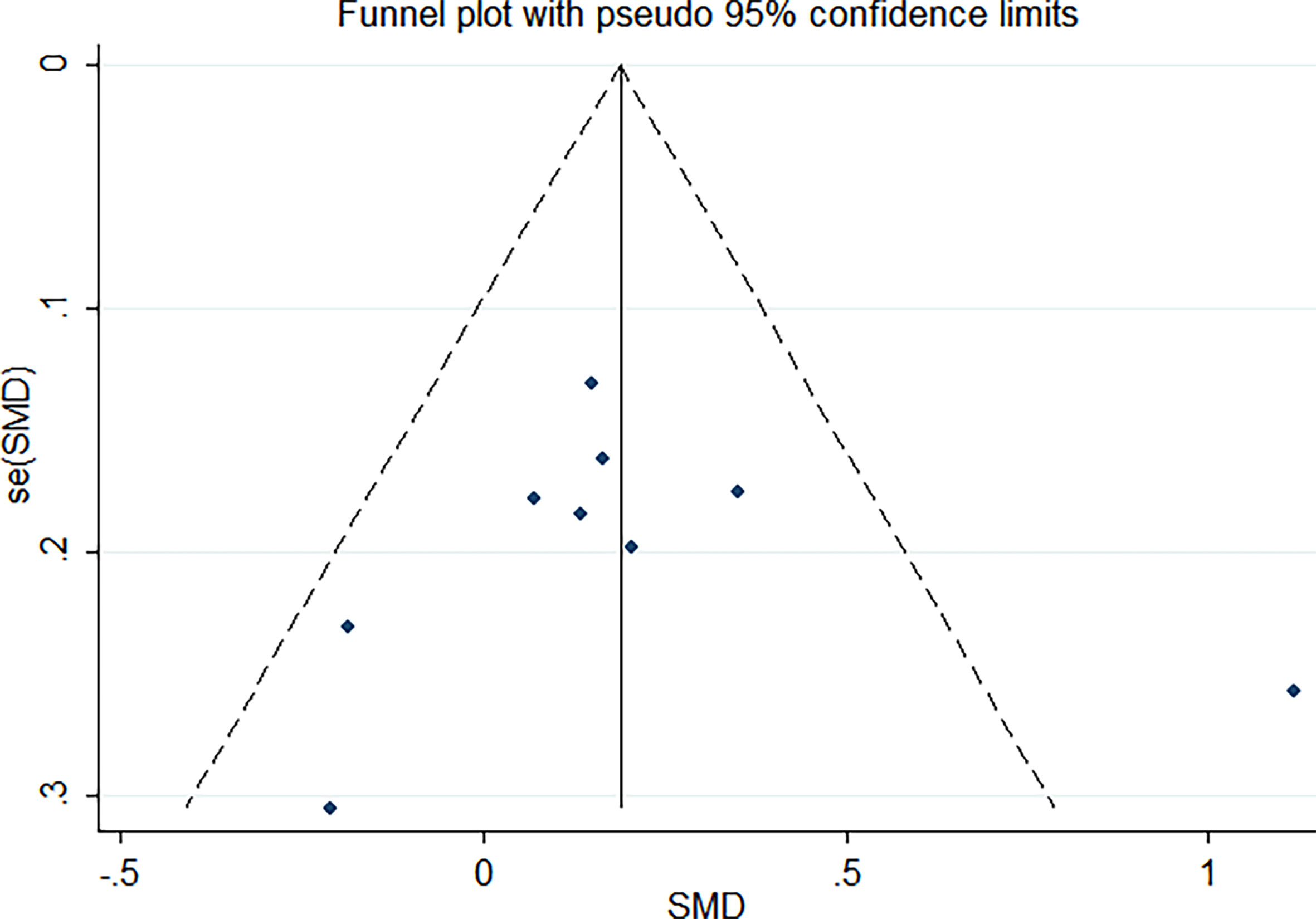
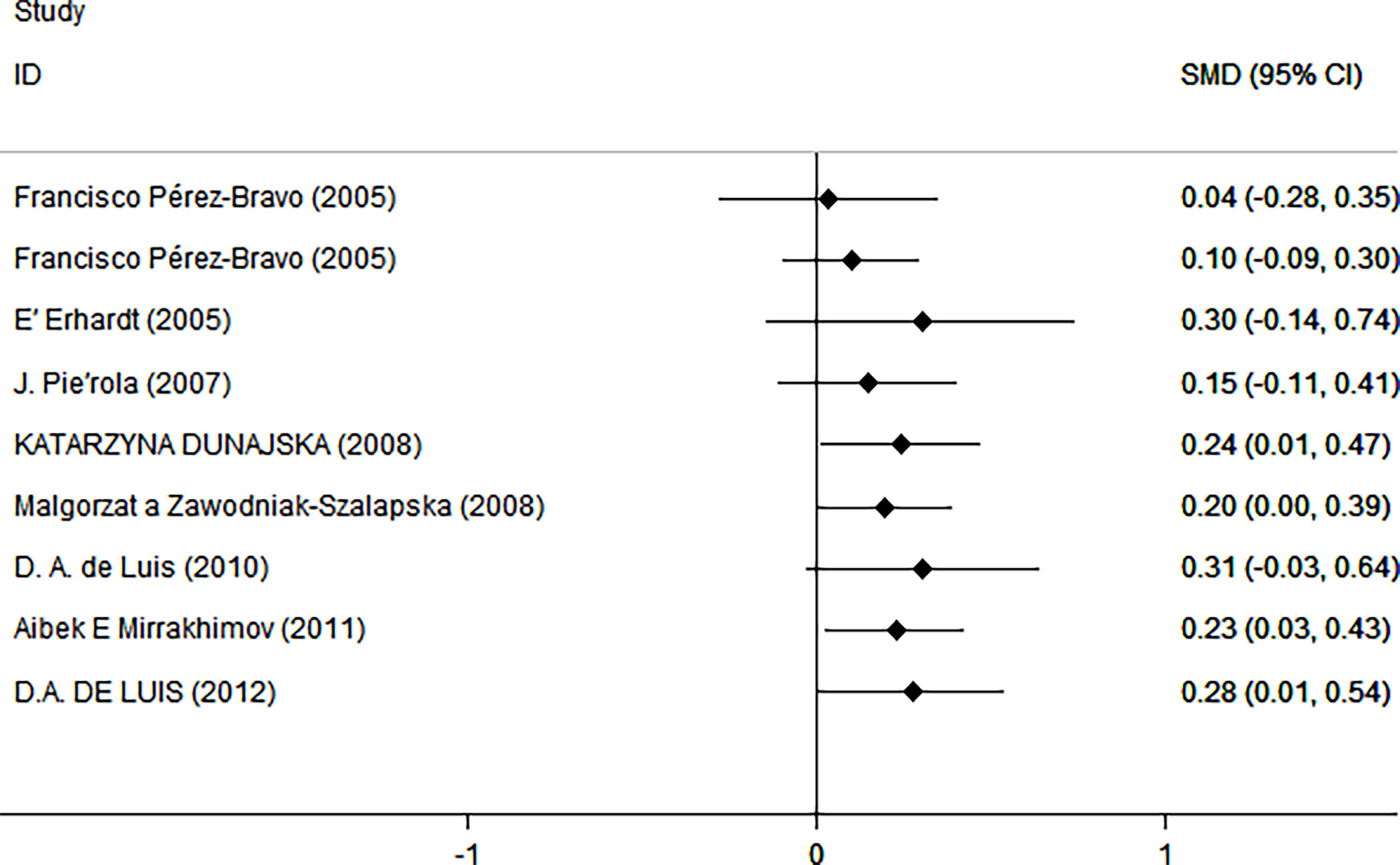
As shown in Figure 2, there was a positive correlation between insulin and Trp64Arg variant genotype (standardized mean difference (SMD) = 0.20, 95% confidence interval (CI): 0.00 to 0.39). There was heterogeneity among studies (I2 = 57.6%, p = 0.016). In the influence analysis, we found that Erhardt’s study (7) had a greater impact on the stability of the combined result (Supplementary Figure 1). The sensitivity analysis showed that after excluding that study, the re-fitted result remained statistically significant, with an SMD and 95% CI of 0.13 (0.01, 0.26) (Supplementary Figure 2). Egger’s test and the funnel plot showed no publication bias (p = 0.796) (Figure 3 and Supplementary Figure 3).
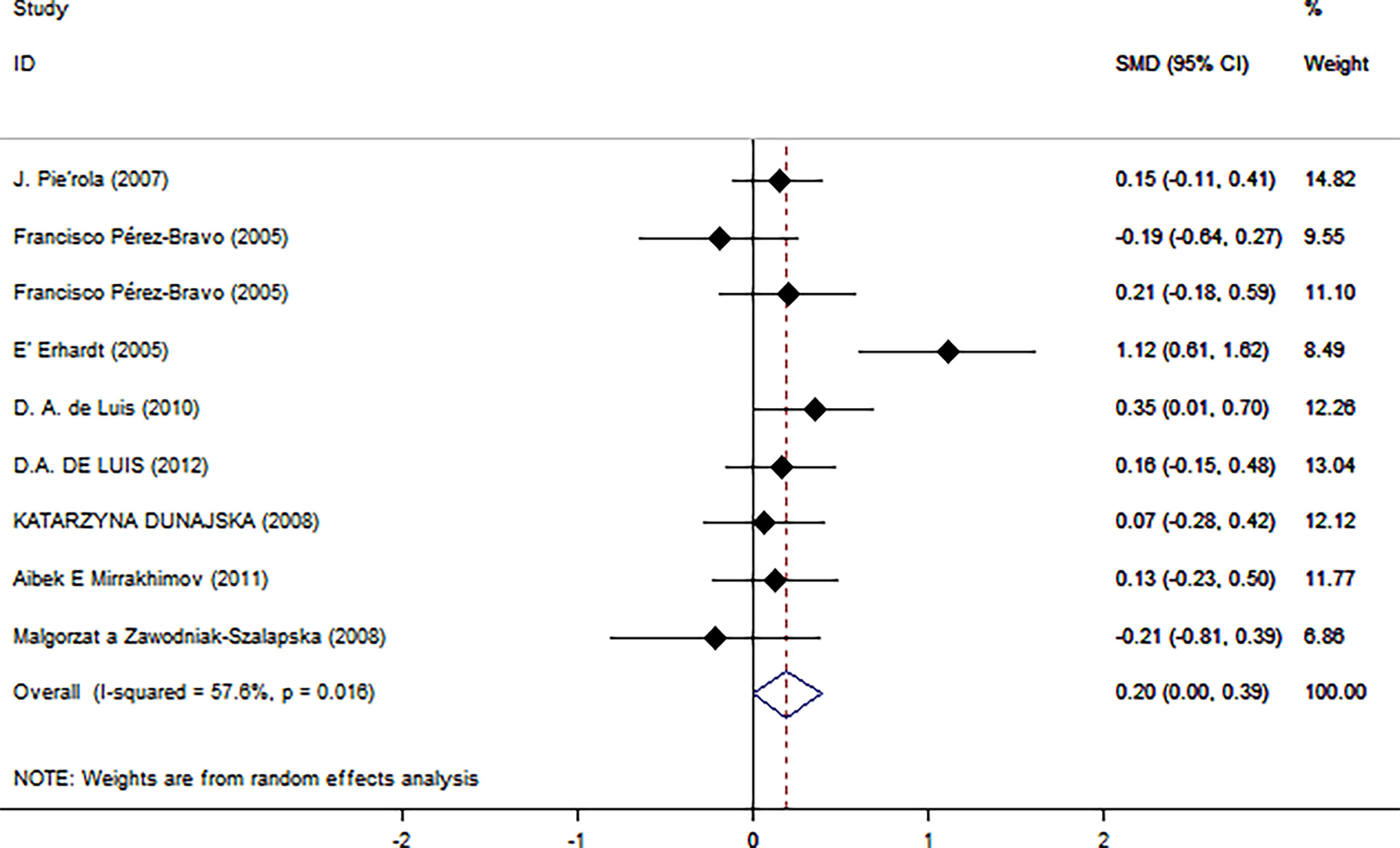
Figure 2 Insulin level as an index of insulin resistance. Forest plot showing the effect size of the correlation between Trp64Arg mutation and insulin resistance. 95% confidence intervals (CIs) are expressed in bars (each group) and diamond (all studies). Summary estimates are analyzed using a random-effects model. SMD, standardized mean difference.
Subgroup Analysis
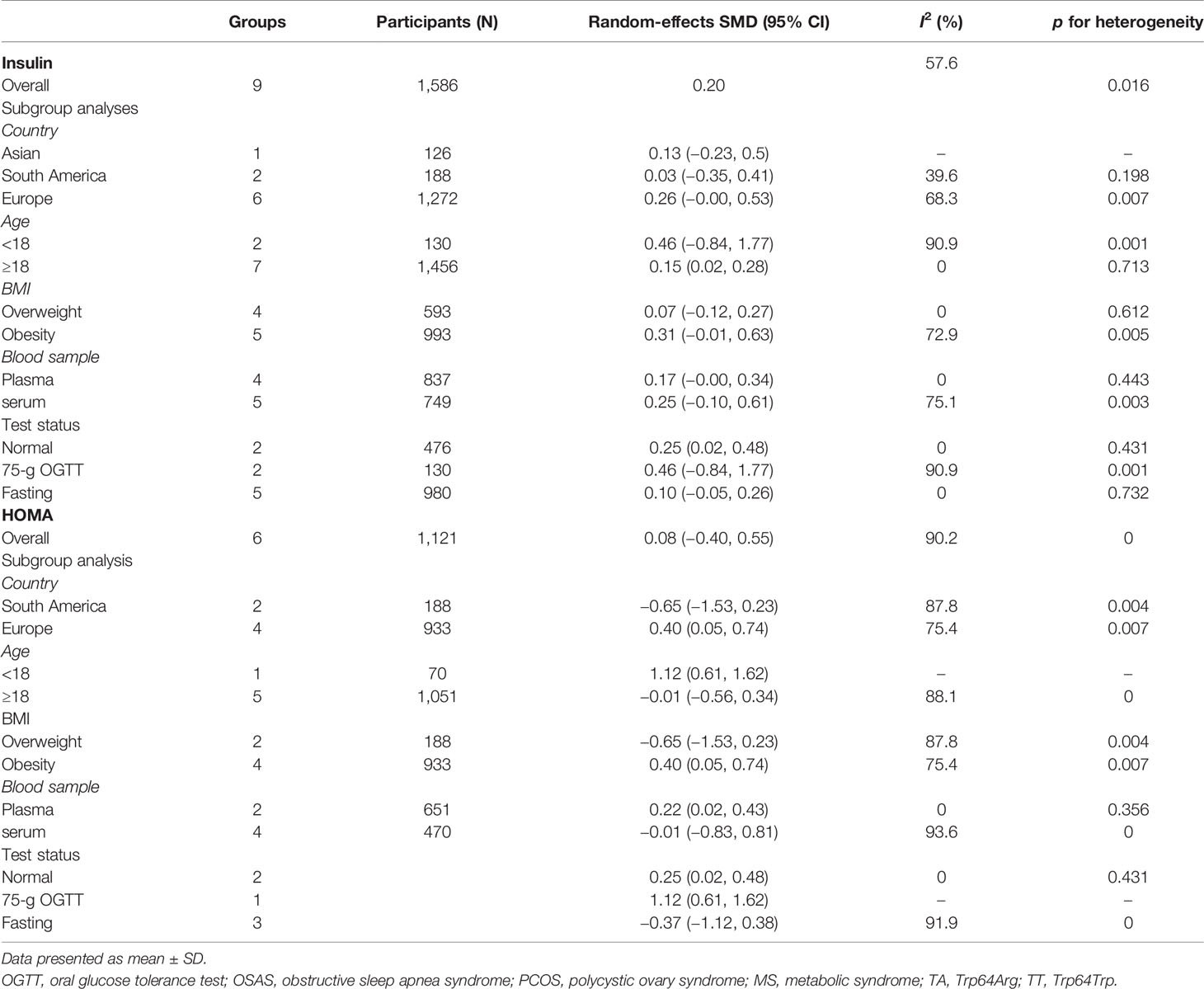
Table 2 summarizes the results of the subgroup analysis of the relationship between Trp64Arg and insulin levels and HOMA-IR based on the participants’ characteristics. First, we found that the associations between Trp64Arg genotype and insulin levels differed depending on test status. Specifically, all subjects were divided into three groups: the fasting group, the 75-g oral glucose tolerance test (OGTT) (1.75 g/kg ideal body weight, max. 75 g) group, and the normal group. Studies conducted under the normal test status revealed a positive correlation between Trp64Arg mutation and insulin levels (SMD = 0.25, 95% CI: 0.02 to 0.48, I2 = 0%, p = 0.431) (Figure 4). However, no association was found between Trp64Arg and insulin levels in the subgroup of fasting condition and 75-g OGTT condition. We also conducted subgroup analysis based on blood sample (plasma or serum), ethnicity (Asian, South American, and European), and BMI (overweight or obesity) (32). However, we did not find differences among these factors.
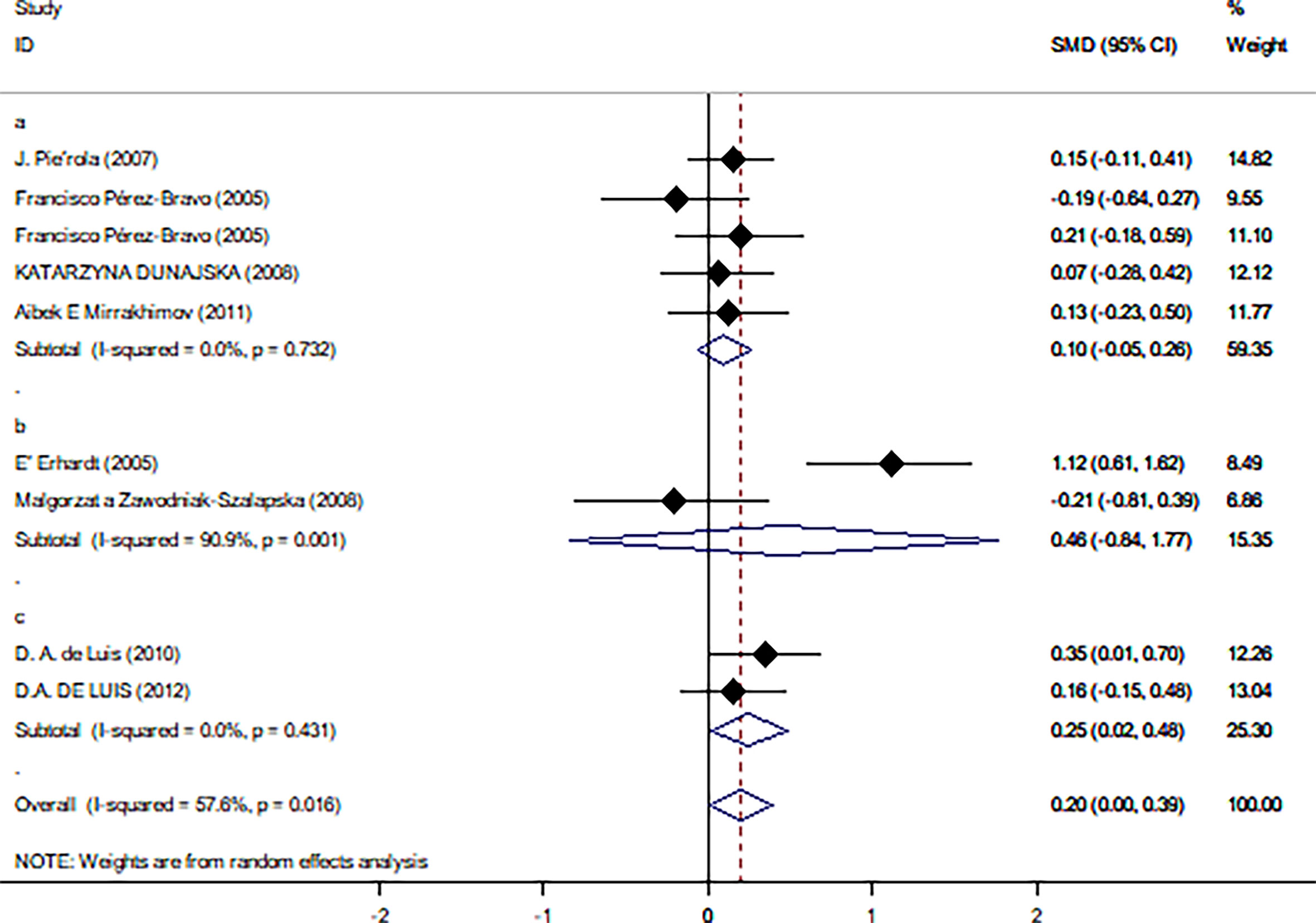
Figure 4 Forest plot of correlations between Trp64Arg mutation and insulin resistance-based onset status. 95% confidence intervals (CI) are expressed in bars (each group) and diamond (all studies). Summary estimates were analyzed using a random-effects model. Pierola et al., Pérez-Bravo et al., Dunajska et al., and Mirrakhimov et al. presented data from people with fasting test status; Erhardt et al. and Zawodniak-Szalapska et al. presented data from people with 75-g OGTT test status. de Luis et al. presented data from people with normal test status. SMD, standardized mean difference; OGTT, oral glucose tolerance test.
Next, we used HOMA-IR as an index of IR (Figure 5). A positive relationship was found only for normal test status (SMD = 0.25, 95% CI: 0.02 to 0.48, I2 = 0%, p = 0.431), and not for other test status. We also found a positive relationship only in plasma blood samples (SMD = 0.22, 95% CI: 0.02 to 0.43, I2 = 0%, p = 0.356), obese populations (SMD = 0.40, 95% CI: 0.05 to 0.74, I2 = 75.4%, p = 0.007), and European ethnicity (SMD = 0.40, 95% CI: 0.05 to 0.74, I2 = 75.4%, p = 0.007) (Table 2).
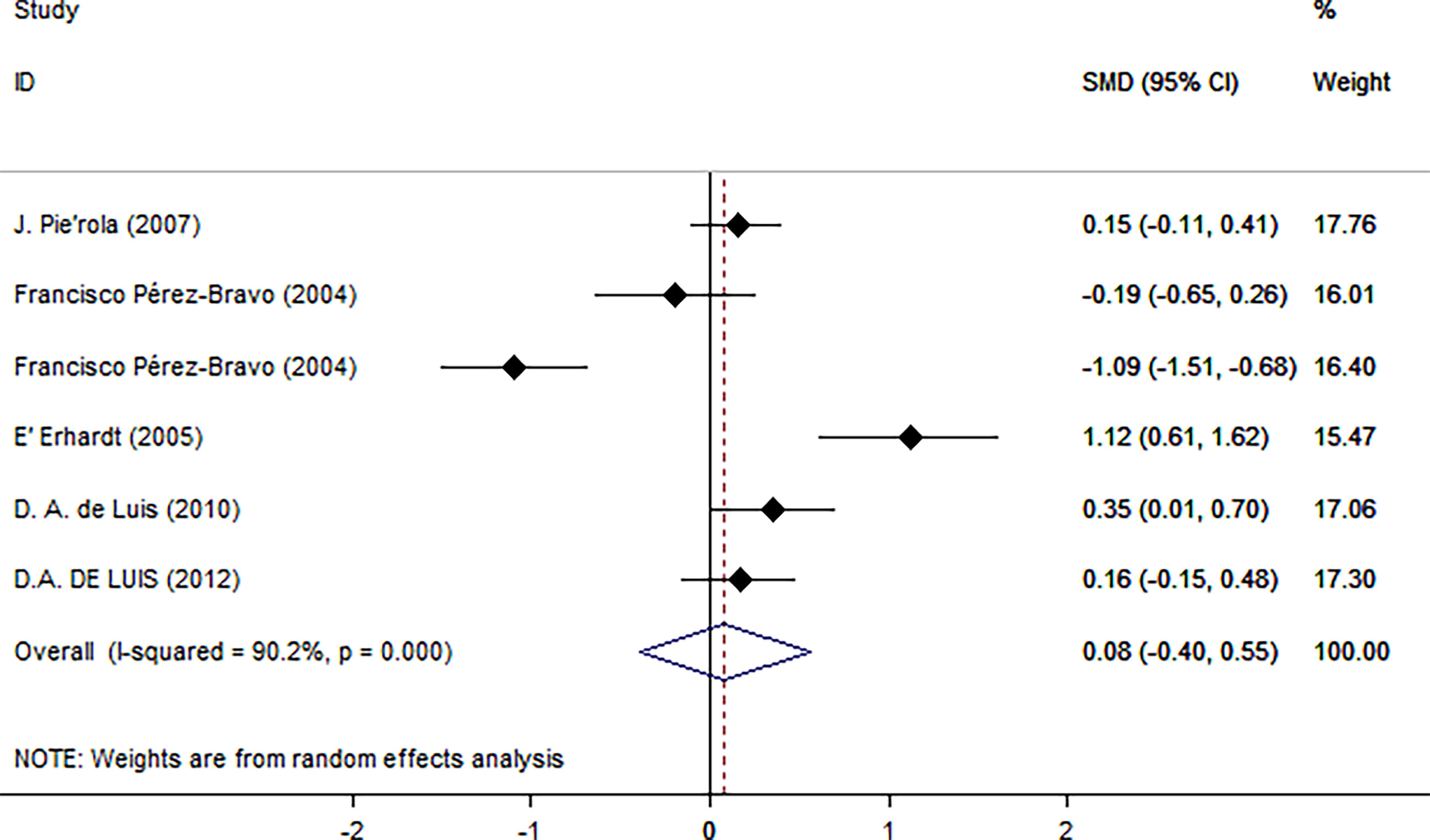
Figure 5 Using HOMA as an index of insulin resistance. Forest plot showing the effect size of the correlation between Trp64Arg mutation and insulin resistance. 95% confidence intervals (CIs) are expressed in bars (each group) and diamond (all studies). Summary estimates are analyzed using a random-effects model. SMD, standardized mean difference.
Meta-Regression Analysis
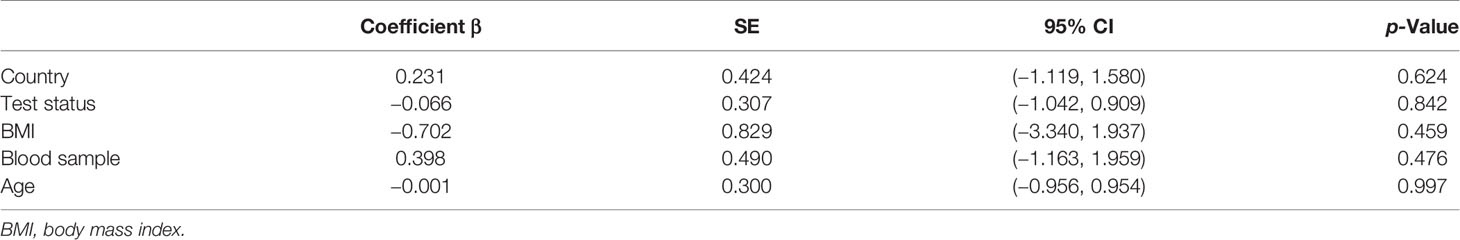
To identify sources of heterogeneity, we conducted a meta-regression analysis. Covariates including age, country, blood sample, test status, and BMI were explored. None of these factors was the source of the heterogeneity (Table 3).
Cumulative Analysis
To evaluate the trend of pooled effect estimates over time, we did a cumulative analysis. The results showed that the pooled result was stable over time (Figure 6).
Discussion
ADRB3 is an important regulator of many physiological functions, such as thermogenesis of brown adipose tissue and lipolysis of white adipose tissue. There are many variations in ADRB3 polymorphism; among these mutations, the most common ADRB3 mutation is Trp64Arg (33). Strosberg et al. mentioned that the allele Arg64 genotype is expressed in nearly all populations of the world (34); the allele can be activated by its agonists (33). Researches showed that ADRB3 polymorphic variants are associated with many diseases, such as cardiovascular diseases, obesity, diabetes, and other disorders (35). This provides insight into the potential pathophysiological effects of ADRB3 polymorphism in the development of these diseases.
We found that the Trp64Arg variant had a positive correlation with IR. The results of the cumulative analysis show that the pooled result was stable over time. Subgroup analysis suggested that test status was a factor that may influence the relationship between Trp64Arg and IR. When using HOMA-IR as an index of IR, subgroup analysis showed that the association between Trp64Arg and IR might be affected by the type of blood sample, obesity, and ethnicity. Heterogeneity in studies is expected in meta-analyses (36); exploring the potential sources of heterogeneity is an essential part of the analysis. What should be noted is that there was heterogeneity between studies in our meta-analysis. Therefore, we used meta-regression analysis to identify the covariates that may have a substantial impact on the heterogeneity, including age, ethnicity, test status, BMI, and source of a blood sample. However, none of these covariates affected the heterogeneity in the meta-regression analysis. Subsequently, considering the heterogeneity of the meta-analysis included in the study, we conducted subgroup analyses based on test status, blood sample source, ethnicity, and BMI.
Subgroup analyses suggested that test status might affect the relationship between the Trp64Arg variant and IR. Adeva-Andany et al. (37) mentioned in their review that dietary ingredients can affect the degree of insulin sensitivity; therefore, dietary ingredients under normal test status may influence the association between the Trp64Arg variant and IR. Some ingredients in the diet can affect IR; for example, Rivellese et al. mentioned that saturated fatty can increase IR; thus, subjects included in the researches may eat saturated fatty acids when they are under normal test status (38). In addition, the concentration of free fatty acids in type 2 diabetes or obese patients is increased; free fatty acids are a major factor contributing to IR (39). On the other hand, people with normal test status are characterized by obesity, which may be another cause of the differences (27, 28). Moreover, this finding can also support the conclusion that the effect mutation on IR may be affected by obesity (33). Papandreou et al. found that several plasma amino acids may be associated with IR, including branched-chain amino acids, aromatic amino acids, alanine, proline, and glutamine (40, 41). These findings may explain the potential influence of blood samples on the relationship. The allele frequency of Trp64Arg varies among different ethnicities (42). These findings suggest that ethnicity may influence the relationship between the Trp64Arg variant and IR.
In the influence analysis, we found that Erhardt’s study had a more significant impact on the pooled results, which may affect the stability of the results (Supplementary Figure 1). After this study was excluded, the results remained statistically significant, suggesting that the results were stable (Supplementary Figure 2). Egger’s test and funnel plots showed that there was no publication bias.
A previous meta-analysis showed that Trp64Arg had a small association with BMI (43). When using HOMA-IR as an index of IR, BMI affected the relationship between Trp64Arg and IR. Considering that IR is associated with obesity (44), further subgroup analysis indicated that the association between Trp64Arg and IR was significant in obesity. Therefore, we speculate that obesity may be the cause of the Trp64Arg mutation that leads to IR. Garcia-Rubi et al. (45) found that obese women with Trp64Arg mutation had greater IR than had women who carried Trp64Arg genotype based on age, body composition, and physical activity. However, Urhammer et al. (46) found that Trp64Arg may be associated with IR and was not affected by BMI. If the relationship between IR and Trp64Arg mutation can be attributed to obesity, we should pay attention to the underlying mechanism. The Trp64Arg variant has been shown to reduce lipolysis (47). Another study found that the Trp64Arg mutation can influence body weight loss (48). These findings suggest that the underlying mechanism may be related to impaired lipolysis, which leads to the increased adipocytes and then obesity, which may lead to IR and hyperinsulinemia.
In addition to IR, the Trp64Arg mutation is associated with insulin secretion. In a study of male twins who carried the Trp64Arg mutation, IR and insulin secretion (determined by homeostasis model assessment) were significantly lower (49). Furthermore, Christiansen et al. demonstrated that Trp64Arg polymorphism had no effects on plasma glucose responses but associated with decreased insulin secretion (50). The result showed that Trp64Arg may affect IR by affecting insulin secretion rather than blood glucose level.
In summary, our study further clarified the relationship between Trp64Arg and IR and explained it at the gene level. It provides a direction for genetic research on the identification of therapeutic targets for clinical treatment of IR. However, the included studies are all case–control studies and cross-sectional studies; therefore, it is difficult to draw causal inferences. There may be selection bias in population selection, in either case–control studies or cross-sectional studies. And, measurement error may also exist. Finally, the number of articles included in our study is small, so more studies need to be included for further analysis.
Conclusion
Our meta-analysis suggested that the Trp64Arg mutation in the beta-adrenergic receptor had a positive correlation with IR. Ethnicity, obesity status, blood sample source, and test status may be the potential factors affecting the relationship between Trp64Arg and IR.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
HW and CZ had the same contribution to the literature. HW and CZ designed the work, collected the data, conducted data analysis, and wrote the manuscript. ML, QL, QZ, DL, and Z-YM reviewed and edited the manuscript. JD had full access over all data in the study and is ultimately responsible for the decision to submit and publish the final version. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by two grants (to JD) from the National Natural Science Foundation of China (No. 31872791) and Natural Science Foundation of Shandong Province of China (No. ZR2019MC046).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.708139/full#supplementary-material
Supplementary Figure 1 | Impact analysis of individual studies. The study by E´ Erhardt had a significant effect on the pooled result.
Supplementary Figure 2 | Forest plot after excluding a single study with high impact. 95% confidence intervals (CI) are expressed in bars (each group) and diamond (all studies). Summary estimates are analyzed using a random-effects model. SMD, standardized mean difference.
Supplementary Figure 3 | Egger’s publication bias plot. p=0.796.
References
1. Brown AE, Walker M. Genetics of Insulin Resistance and the Metabolic Syndrome. Curr Cardiol Rep (2016) 18:75. doi: 10.1007/s11886-016-0755-4
2. Mauriège P, Bouchard C. Trp64Arg Mutation in Beta 3-Adrenoceptor Gene of Doubtful Significance for Obesity and Insulin Resistance. Lancet (1996) 348:698–9. doi: 10.1016/S0140-6736(05)65601-2
3. Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, et al. Glucose and Free Fatty Acid Metabolism in Non-Insulin-Dependent Diabetes Mellitus. Evidence for Multiple Sites of Insulin Resistance. J Clin Invest (1989) 84:205–13. doi: 10.1172/JCI114142
4. Kahn CR, Neville DM Jr, Roth J. Insulin-Receptor Interaction in the Obese-Hyperglycemic Mouse. A Model of Insulin Resistance. J Biol Chem (1973) 248:244–50. doi: 10.1016/S0021-9258(19)44468-2
5. Zhan S, Ho SC. Meta-Analysis of the Association of the Trp64Arg Polymorphism in the Beta3 Adrenergic Receptor With Insulin Resistance. Obes Res (2005) 13:1709–19. doi: 10.1038/oby.2005.209
6. Lehtovirta M, Kaprio J, Forsblom J, Eriksson J, Tuomilehto L, Groop L. Insulin Sensitivity and Insulin Secretion in Monozygotic and Dizygotic Twins. Diabetologia (2000) 43:285–93. doi: 10.1007/s001250050046
7. Leineweber K, Büscher R, Bruck H, Brodde OE. Beta-Adrenoceptor Polymorphisms. Naunyn Schmiedebergs Arch Pharmacol (2004) 369:1–22. doi: 10.1007/s00210-003-0824-2
8. Artunc F, Schleicher E, Weigert C, Fritsche A, Stefan N, Häring HU. The Impact of Insulin Resistance on the Kidney and Vasculature. Nat Rev Nephrol (2016) 12:721–37. doi: 10.1038/nrneph.2016.145
9. Emorine L, Blin N, Strosberg AD. The Human Beta 3-Adrenoceptor: The Search for a Physiological Function. Trends Pharmacol Sci (1994) 15:3–7. doi: 10.1016/0165-6147(94)90118-X
10. Widén E, Lehto M, Kanninen T, Walston J, Shuldiner AR, Groop LC. Association of a Polymorphism in the Beta 3-Adrenergic-Receptor Gene With Features of the Insulin Resistance Syndrome in Finns. N Engl J Med (1995) 333:348–51. doi: 10.1056/NEJM199508103330604
11. Walston J, Silver K, Bogardus C, Knowler WC, Celi FS, Austin S, et al. Time of Onset of Non-Insulin-Dependent Diabetes Mellitus and Genetic Variation in the Beta 3-Adrenergic-Receptor Gene. N Engl J Med (1995) 333:343–7. doi: 10.1056/NEJM199508103330603
12. Clément K, Vaisse C, Manning BS, Basdevant A, Guy-Grand B, Ruiz J, et al. Genetic Variation in the Beta 3-Adrenergic Receptor and an Increased Capacity to Gain Weight in Patients With Morbid Obesity. N Engl J Med (1995) 333:352–4. doi: 10.1056/NEJM199508103330605
13. Fujisawa T, Ikegami H, Kawaguchi Y, Ogihara T. Meta-Analysis of the Association of Trp64Arg Polymorphism of Beta 3-Adrenergic Receptor Gene With Body Mass Index. J Clin Endocrinol Metab (1998) 83:2441–4. doi: 10.1210/jc.83.7.2441
14. Allison D, Heo M, Faith M, Pietrobelli AJIJOO, et al. Meta-Analysis of the Association of the Trp64Arg Polymorphism in the β 3 Adrenergic Receptor With Body Mass Index. J Clin Endocrinol Metab (1998) 22:559–66. doi: 10.1038/sj.ijo.0800625
15. Kurokawa N, Nakai K, Kameo S, Liu ZM, Satoh G. Association of BMI With the Beta3-Adrenergic Receptor Gene Polymorphism in Japanese: Meta-Analysis. Obes Res (2001) 9:741–5. doi: 10.1038/oby.2001.102
16. Højlund K, Christiansen C, Bjørnsbo KS, Poulsen P, Bathum L, Henriksen JE, et al. Energy Expenditure, Body Composition and Insulin Response to Glucose in Male Twins Discordant for the Trp64Arg Polymorphism of the Beta3-Adrenergic Receptor Gene. Diabetes Obes Metab (2006) 8:322–30. doi: 10.1111/j.1463-1326.2005.00509.x
17. de Luis DA, Aller R, Izaola O, Gonzalez Sagrado M, Conde R. Relation of Trp64Arg Polymorphism of Beta 3-Adrenergic Receptor Gene to Adipocytokines and Fat Distribution in Obese Patients. Ann Nutr Metab (2008) 52:267–71. doi: 10.1159/000144047
18. De Luis Román DA, Primo D, Izaola O, Aller R. Relation of Trp64Arg Polymorphism of Beta 3 Adrenoreceptor Gene With Metabolic Syndrome and Insulin Resistance in Obese Women. Nutr Hosp (2017) 34:383–8. doi: 10.20960/nh.384
19. Liberati A, Altman DG, Altman J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. J Clin Epidemiol (2009) 62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006
20. Table. SydPath. (2017). Available at: https://www.who.int/news-room/fact-sheets/detail/e-coli. [Accessed March 5, 2021].
21. Bindman AB. The Agency for Healthcare Research and Quality and the Development of a Learning Health Care System. JAMA Intern Med (2017) 177:909–10. doi: 10.1001/jamainternmed.2017.2589
22. Higgins JP, Thompson SG. Quantifying Heterogeneity in a Meta-Analysis. Stat Med (2002) 21:1539–58. doi: 10.1002/sim.1186
23. Patsopoulos NA, Evangelou E, Ioannidis JP, et al. Sensitivity of Between-Study Heterogeneity in Meta-Analysis: Proposed Metrics and Empirical Evaluation. Int J Epidemiol (2008) 37:1148–57. doi: 10.1093/ije/dyn065
24. Piérola J, Barceló A, de la Peña M, Barbé F, Soriano JB, Sánchez Armengo AI, et al. Beta3-Adrenergic Receptor Trp64Arg Polymorphism and Increased Body Mass Index in Sleep Apnoea. Eur Respir J (2007) 30:743–7. doi: 10.1183/09031936.00152006
25. Pérez-Bravo F, Echiburú B, Maliqueo M, Santos JL, Sir-Petermann T. Tryptophan 64 –> Arginine Polymorphism of Beta-3-Adrenergic Receptor in Chilean Women With Polycystic Ovary Syndrome. Clin Endocrinol (Oxf) (2005) 62:126–31. doi: 10.1111/j.1365-2265.2004.02183.x
26. Erhardt E, Czakó M, Csernus K, Molnár D, Kosztolányi G. The Frequency of Trp64Arg Polymorphism of the Beta3-Adrenergic Receptor Gene in Healthy and Obese Hungarian Children and its Association With Cardiovascular Risk Factors. Eur J Clin Nutr (2005) 59:955–9. doi: 10.1038/sj.ejcn.1602164
27. de Luis DA, Ballesteros M, Ruiz E, Muñoz C, Penacho A, Iglesias P, et al. Polymorphism Trp64Arg of Beta 3 Adrenoreceptor Gene: Allelic Frequencies and Influence on Insulin Resistance in a Multicenter Study of Castilla-León. Nutr Hosp (2010) 25:299–303.
28. de Luis DA, Aller R, Izaola O, Gonzalez Sagrado M, Conde R, Castro MJ, et al. Interaction of -55CT Polymorphism of UCP3 Gene With Trp64Arg Polymorphism of Beta3adrenoreceptor Gene on Insulin Resistance in Obese Patients. Eur Rev Med Pharmacol Sci (2012) 16:610–6.
29. Dunajska K, Lwow F, Milewicz A, Jedrzejuk D, Laczmanski L, Belowska-Bien K, et al. Beta(3)-Adrenergic Receptor Polymorphism and Metabolic Syndrome in Postmenopausal Women. Gynecol Endocrinol (2008) 24:133–8. doi: 10.1080/09513590801921686
30. Mirrakhimov AE, Kerimkulova AS, Lunegova OS, Moldokeeva CB, Zalesskaya YV, Abilova SS, et al. An Association Between TRP64ARG Polymorphism of the B3 Adrenoreceptor Gene and Some Metabolic Disturbances. Cardiovasc Diabetol (2011) 10:89. doi: 10.1186/1475-2840-10-89
31. Zawodniak-Szałapska M, Stawerska R, Brzeziańska E, Pastuszak-Lewandoska D, Lukamowicz J, Cypryk K, et al. Association of Trp64Arg Polymorphism of Beta3-Adrenergic Receptor With Insulin Resistance in Polish Children With Obesity. J Pediatr Endocrinol Metab (2008) 21:147–54. doi: 10.1515/JPEM.2008.21.2.147
32. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Johnson CLJAD. CDC Growth Charts: United States. (2000) 8:1–27.
33. Yang LK, Tao YX. Physiology and Pathophysiology of the Beta(3)-Adrenergic Receptor, in G Protein Signaling Pathways in Health and Disease. Tao YX, editor (2019), pp. 91–112.
34. Strosberg AD. Association of Beta 3-Adrenoceptor Polymorphism With Obesity and Diabetes: Current Status. Trends Pharmacol Sci (1997) 18:449–54. doi: 10.1016/S0165-6147(97)90681-7
35. Yang L-K, Tao Y-X. Physiology and Pathophysiology of the β3-Adrenergic Receptor. Prog Mol Biol Transl Sci (2019) 161:91–112. doi: 10.1016/bs.pmbts.2018.09.003
36. Lee YH. Meta-Analysis of Genetic Association Studies. Ann Lab Med (2015) 35:283–7. doi: 10.3343/alm.2015.35.3.283
37. Adeva-Andany MM, González-Lucán M, Fernandez-Fernandez C, Carneiro-Freire N, Seco-Filgueira M, Pedre-Pineiro AM. Diet Composition Determines Insulin Sensitivity and Cardiovascular Risk in Humans. Clin Nutr ESPEN (2019) 33:29–38. doi: 10.1016/j.clnesp.2019.05.014
38. Rivellese A, De Natale C, Lilli S. Type of Dietary Fat and Insulin Resistance. Ann N Y Acad Sci (2002) 967:329–35. doi: 10.1111/j.1749-6632.2002.tb04288.x
39. Rachek LI. Free Fatty Acids and Skeletal Muscle Insulin Resistance. Prog Mol Biol Transl Sci (2014) 121:267–92. doi: 10.1016/B978-0-12-800101-1.00008-9
40. Tai ES, Tan ML, Stevens RD, Low YL, Muehlbauer MJ, Goh DL, et al. Insulin Resistance Is Associated With a Metabolic Profile of Altered Protein Metabolism in Chinese and Asian-Indian Men. Diabetologia (2010) 53:757–67. doi: 10.1007/s00125-009-1637-8
41. Würtz P, Soininen P, Kangas AJ, Rönnemaa T, Lehtimäki T, Kähönen M, et al. Branched-Chain and Aromatic Amino Acids are Predictors of Insulin Resistance in Young Adults. Diabetes Care (2013) 36:648–55. doi: 10.2337/dc12-0895
42. Silver K, Walston J, Wang Y, Dowse G, Zimmet P, Shuldiner AR. Molecular Scanning for Mutations in the Beta 3-Adrenergic Receptor Gene in Nauruans With Obesity and Noninsulin-Dependent Diabetes Mellitus. J Clin Endocrinol Metab (1996) 81:4155–8. doi: 10.1210/jc.81.11.4155
43. Shuldiner AR, Sabra M. Trp64Arg Beta3-Adrenoceptor: When Does a Candidate Gene Become a Disease-Susceptibility Gene? Obes Res (2001) 9:806–9. doi: 10.1038/oby.2001.109
44. Barazzoni R, Gortan Cappellari G, Ragni M, Nisoli E. Insulin Resistance in Obesity: An Overview of Fundamental Alterations. Eat Weight Disord (2018) 23:149–57. doi: 10.1007/s40519-018-0481-6
45. García-Rubi E, Starling RD, Tchernof A, Matthews DE, Walston JD, Shuldiner AR, et al. Trp64Arg Variant of the Beta3-Adrenoceptor and Insulin Resistance in Obese Postmenopausal Women. J Clin Endocrinol Metab (1998) 83:4002–5. doi: 10.1210/jc.83.11.4002
46. Urhammer SA, Clausen JO, Hansen T, Pedersen O. Insulin Sensitivity and Body Weight Changes in Young White Carriers of the Codon 64 Amino Acid Polymorphism of the Beta 3-Adrenergic Receptor Gene. Diabetes (1996) 45:1115–20. doi: 10.2337/diabetes.45.8.1115
47. Jesus ÍC, Alle LF, Munhoz EC, Silva LRD, Lopes WA, Tureck LV, et al. Trp64Arg Polymorphism of the ADRB3 Gene Associated With Maximal Fat Oxidation and LDL-C Levels in Non-Obese Adolescents. J Pediatr (Rio J) (2018) 94:425–31. doi: 10.1016/j.jped.2017.07.010
48. Szendrei B, González-Lamuño D, Amigo T, Wang G, Pitsiladis Y, Benito P, et al. Influence of ADRB2 Gln27Glu and ADRB3 Trp64Arg Polymorphisms on Body Weight and Body Composition Changes After a Controlled Weight-Loss Intervention. Diabetes Obes Metab (2016) 41:307–14. doi: 10.1139/apnm-2015-0425
49. Højlund K, Christiansen C, Bjørnsbo KS, Poulsen P, Bathum L, Henriksen JE, et al. Energy Body Composition and Insulin Response to Glucose in Male Twins Discordant for the Trp64Arg Polymorphism of the β3 -Adrenergic Receptor Gene. Diabetes Obes Metab (2006) 8:322–30. doi: 10.1111/j.1463-1326.2005.00509.x
Keywords: Trp64Arg, insulin resistance, β3-adrenergic receptor gene, meta-analysis, subgroup analysis
Citation: Wang H-D, Zhang C-S, Li M-W, Lin Q, Zhang Q, Liu D-F, Ma Z-Y and Dong J (2021) The Association of Trp64Arg Polymorphism in the Beta-Adrenergic Receptor With Insulin Resistance: Meta-Analysis. Front. Endocrinol. 12:708139. doi: 10.3389/fendo.2021.708139
Received: 11 May 2021; Accepted: 27 July 2021;
Published: 26 August 2021.
Edited by:
Gaetano Santulli, Columbia University, United StatesReviewed by:
Dorota Latek, University of Warsaw, PolandSandro Soares Almeida, Hospital Israelita Albert Einstein, Brazil
Copyright © 2021 Wang, Zhang, Li, Lin, Zhang, Liu, Ma and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Dong, ZG9uZ2ppbmc2QGhvdG1haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Hai-Dan Wang
Hai-Dan Wang Cai-Shun Zhang
Cai-Shun Zhang Man-Wen Li
Man-Wen Li Qian Lin
Qian Lin Qing Zhang1
Qing Zhang1 De-Feng Liu
De-Feng Liu Zheng-Ye Ma
Zheng-Ye Ma Jing Dong
Jing Dong