- 1Department of Reproductive Medicine Center, The Third Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
- 2State Key Laboratory of Reproductive Medicine (Henan Centre), The Third Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
- 3State Key Laboratory of Reproductive Medicine, Nanjing Medical University, Nanjing, Jiangsu, China
- 4Department of Epidemiology, Center for Global Health, School of Public Health, Nanjing Medical University, Nanjing, Jiangsu, China
Objective: To evaluate whether singleton live births achieved following in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) in women with late moderate-to-severe ovarian hyperstimulation syndrome (OHSS) is associated with adverse perinatal outcomes.
Methods: This was a single-center retrospective cohort study conducted from January 2016 to June 2021. A total of 4,012 IVF/ICSI-fresh embryo transfer cycles that achieved singleton live births were included. According to the diagnosis of OHSS, the cycles were divided into two groups: late moderate-to-severe OHSS (MS-OHSS) group (n = 114) and non-OHSS group (n = 3,898). Multiple baseline covariates were controlled by propensity score matching, yielding 114 late MS-OHSS singleton live births matched to 337 non-OHSS singleton live births. The primary outcome of the study was normal term infant. The secondary outcomes were perinatal complications, gestational age at birth, birth weight, and birth height.
Result(s): Before propensity score matching, no significant difference in perinatal outcomes was identified between late MS-OHSS group and non-OHSS group. After matching maternal age, BMI, basal serum FSH level, basal serum AMH level, basal antral follicle count, type of stimulation protocol, day of embryo development for embryo transfer, number of embryo transfer, and number of oocytes retrieved, there was still no significant difference in obstetric outcomes and neonatal outcomes between the two groups.
Conclusion(s): The findings demonstrate that the perinatal outcomes were similar between the two groups. However, because the sample size of patients with late MS-OHSS was limited in this study, further investigations are warranted using a larger sample size.
Introduction
Ovarian hyperstimulation syndrome (OHSS) is an iatrogenic complication of assisted reproductive technology (ART) and can occur in any woman who undergoes ovulation induction (1). The incidence of moderate-to-severe (MS) OHSS in women undergoing ART is 3% to 8% (2). MS-OHSS is characterized by enlarged ovaries, high concentrations of sex steroid hormones and the accumulation of extravascular exudate (2, 3). Additionally, abdominal bloating, abdominal pain, ascites, hydrothorax, oliguria and anuria are common clinical manifestations (3). Potential complications, such as renal failure and oliguria, hypovolemic shock, thromboembolic episodes, adult respiratory distress syndrome, and even death, mean MS-OHSS is potentially life-threatening (2, 4, 5).
OHSS not only increases the financial burden of patients but also causes them physical and mental suffering (6). Mild OHSS does not need any treatment except for rest and routine follow-up. Patients with MS-OHSS are generally admitted to hospital and treated with fluid resuscitation, supportive care, paracentesis and prophylactic anticoagulation (7). However, the best course of action in women undergoing ART is to prevent the development of OHSS, which will requires a greater understanding of the pathophysiology of OHSS. It is believed that the key factor in the pathogenesis of OHSS is human chorionic gonadotrophin (HCG), which induces the secretion of vasoactive substances (8–10), such as vascular endothelial growth factor, leading to increased systemic vascular permeability (11, 12) and consequent massive transudation of protein-rich fluid from the vascular space into the peritoneal cavity and, to a lesser extent, the pleural and pericardial cavities (13).
Numerous studies on the prevention of OHSS have been published. However, few studies have shown whether pregnancies conceived after OHSS are associated with an increased risk of adverse perinatal outcomes. Three recent studies have reported that perinatal outcomes are comparable between women with and without OHSS (14–16). Nevertheless, several studies have found that OHSS may be correlated with an increased risk of preterm birth or low birth weight (LBW) (17–21). Recently, Hu et al. found that the incidence rates of thrombosis, gestational diabetes mellitus (GDM) and neonatal intensive care unit (NICU) admission were significantly higher in patients with MS-OHSS than in those without OHSS (22). However, these conclusions were based on limited data, and some studies have not ruled out multiple pregnancies and other factors that could be responsible. Therefore, the effects of late MS-OHSS on the perinatal outcomes of singleton live births warrant further investigation.
The aim of this retrospective cohort study was to investigate the effects of late MS-OHSS on obstetric and neonatal outcomes of single live births conceived following in vitro fertilization (IVF)-/intracytoplasmic sperm injection (ICSI)-fresh embryo transfer (ET) cycles.
Materials and methods
Study design and patients
In this study, we reviewed the medical records of all patients who underwent IVF-/ICSI-fresh ET cycles in the Center of Reproductive Medicine, the Third Affiliated Hospital of Zhengzhou University, from January 1, 2016, to June 30, 2021. All of the patients had undergone controlled ovarian hyperstimulation. Only singleton live births conceived after IVF-/ICSI-fresh ET cycles were included. The diagnosis and severity grading of OHSS were based on the criteria reported by Golan et al. (6). The classification of OHSS symptoms were as follows: mild OHSS: abdominal distension and discomfort, nausea, vomiting, enlarged ovaries, diarrhea; moderate OHSS: mild features, ultrasonic evidence of ascites; severe OHSS: moderate features, clinical evidence of ascites, hydrothorax or breathing difficulties, change in blood volume, increased blood viscosity due to hemoconcentration, coagulation abnormalities, diminished renal perfusion and function. The exclusion criteria were as follows: involved donated oocytes or donated sperm, mild OHSS, and patients with abnormal uterine anatomy, multiple pregnancies, recurrent spontaneous abortion, or recurrent implantation failure. Patients with moderate-to-severe OHSS were included in the late MS-OHSS group, and patients without OHSS were included in the non-OHSS group.
Clinical protocols
All patients involved in this study were subjected a long protocol of gonadotropin -releasing hormone (GnRH)-agonists or GnRH-antagonists. The ovulation induction drug was recombinant and/or urinary gonadotrophins (Gonal-F, Merck, Serono, Germany) with or without luteinizing hormone (Luveris, Merck Serono, Germany). Experienced clinicians individually adjusted the starting dose of medication according to the patient’s age, body mass index (BMI), medical history and ovarian reserve. Recombinant human chorionic gonadotropin (r-HCG, Ovidrel, Merck Serono, Germany) at a dose of 5000 to 10000 IU was administrated after the dominant follicle reached 20 mm or at least three follicles reached 18 mm. Oocytes were retrieved under the transvaginal ultrasound monitoring 36-38 hours later (23). Then routine IVF or ICSI and embryo culture were performed (24). One or 2 cleavage-stage embryos were transferred on the third day or one blastocyst was transferred on the fifth day after fertilization. Oral dydrogesterone (Abbott Co. America) and progesterone sustained-release vaginal gel (Merck Co. Germany) were provided for luteal support. Beta-HCG serum concentrations were measured at 14 days after embryo transfer to determine whether biochemical pregnancy had been achieved (25). Clinical pregnancy was defined as the presence of an intrauterine gestational sac on transvaginal ultrasound at 4 weeks after transfer.
Perinatal outcomes and their definitions
We obtained all patients’ characteristic data and IVF/ICSI treatment cycles data from the electronic medical record system of our hospital. The perinatal outcomes of pregnant patients were obtained through electronic medical record system and telephone follow-up. The primary outcome of this study was delivery of a normal term infant, defined as an infant born at the gestational age of ≥ 37 weeks to < 42 weeks (260 to 293 days), with weight > 2,500 g to < 4,000 g, and without any malformation or disease (26). The secondary outcomes were placenta previa, placental abruption (PA), premature rupture of membranes (PROM), hypertensive disorders of pregnancy (HDP), GDM, cesarean delivery, gestational age (weeks), preterm delivery (<37 weeks), birth weight (grams) [including macrosomia (>4,000 g), LBW (<2,500 g), very LBW (<1,500 g), small for gestational age (SGA), and large for gestational age (LGA) (27)], birth height (centimeters), admission to the pediatrics department, and birth defect (28, 29).
Statistical analysis
SPSS 26.0 (IBM, Chicago, IL, USA) was used for statistical analyses. Continuous variables are presented as means ± standard deviations, and Student’s t-test was used to compare the differences between the two groups. Categorical data are presented as frequencies and corresponding percentages, and chi-square tests and Fisher’s exact tests were performed to assess the differences between the two groups. Logistic and linear regression analyses were used to assess perinatal outcomes. Odds ratios and adjusted odds ratios are reported with 95% confidence intervals. Two-tailed P values < 0.05 were considered statistically significant.
Maternal age, BMI, basal serum FSH level, basal serum AMH level, basal antral follicle count, type of stimulation protocol, day of embryo development for embryo transfer, number of embryo transfer, and number of oocytes retrieved were matched with propensity score matching (PSM) to control for confounding factors. To optimize the precision of the study, patients with late MS-OHSS were matched to patients without OHSS in a 1:3 ratio.
Results
Study population
As shown in Figure 1, screening of the medical records revealed that 13,691 IVF-/ICSI-fresh ET cycles were performed from January 2016 to June 2021 in our center. Of these, 114 late MS-OHSS cycles and 3,898 non-OHSS cycles that met the inclusion criteria were included in this study. After PSM, 114 late MS-OHSS singleton live births and 337 non-OHSS singleton live births were included.
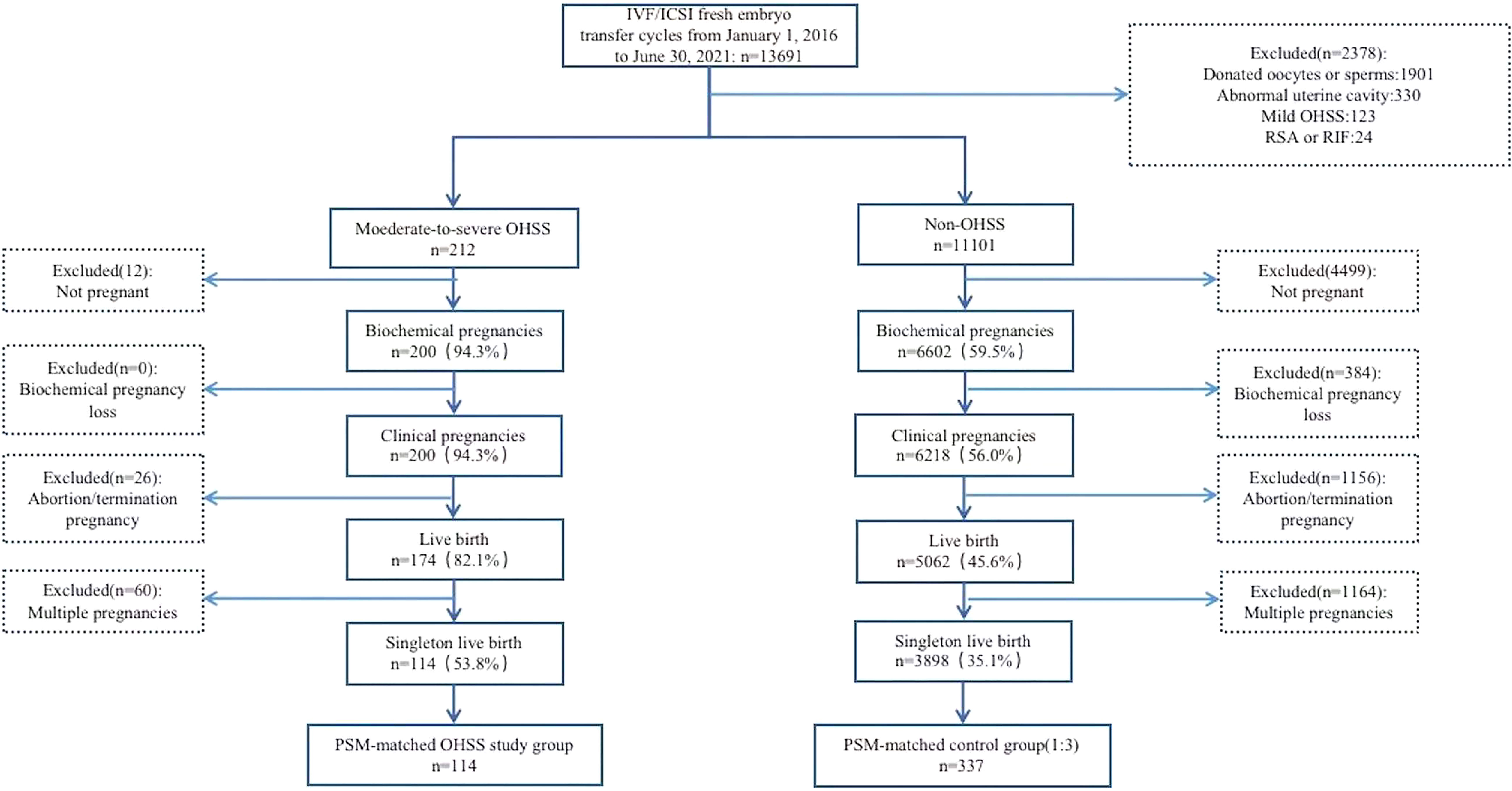
Figure 1 Flow diagram of study participants IVF, in vitro fertilization; ICSI, intracytoplasmic sperm injection; OHSS, ovarian hyperstimulation syndrome; RSA, recurrent spontaneous abortion; RIF, repeated implantation failure; PSM, prospensity score matching.
Participant characteristics
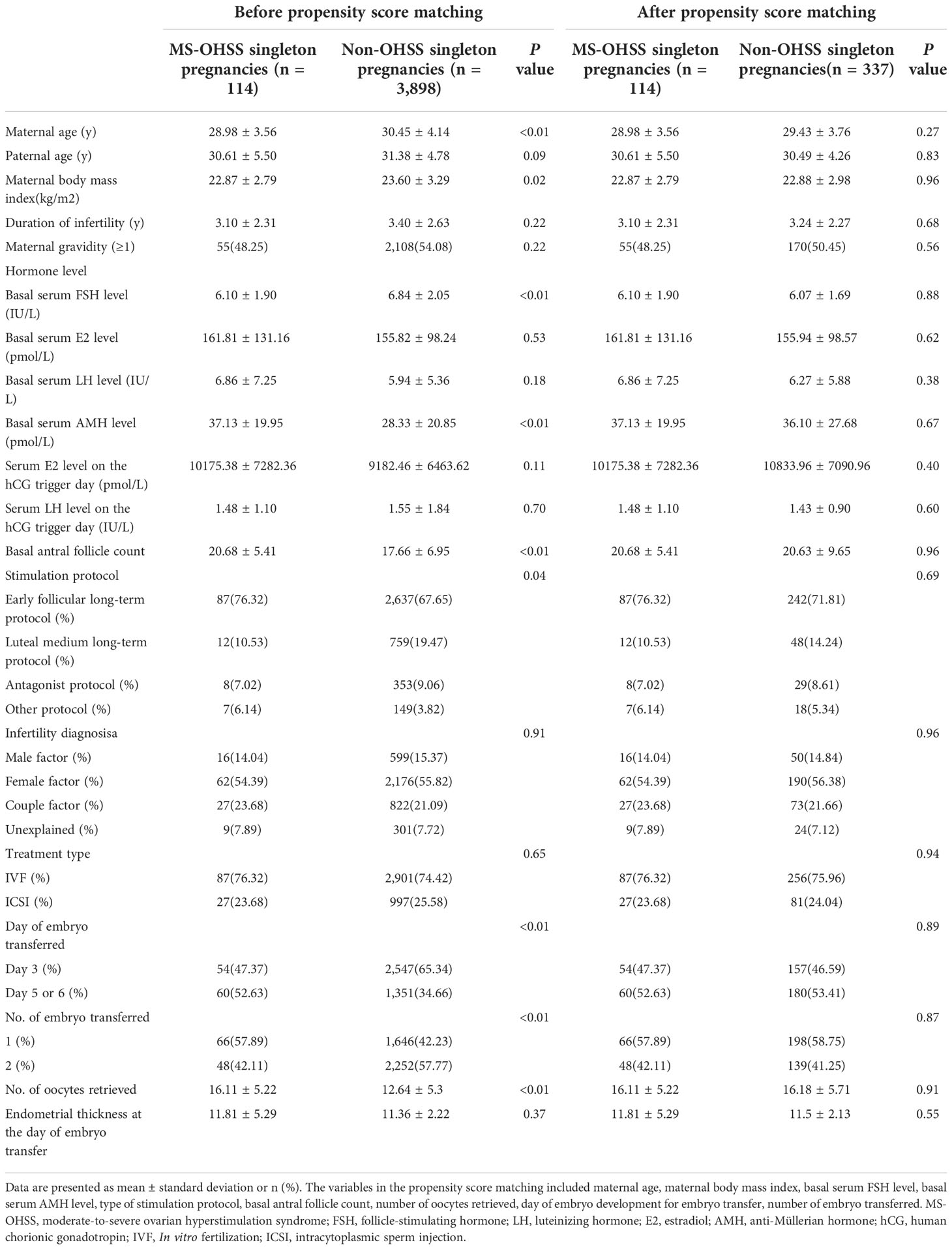
Table 1 shows that, compared with patients without OHSS, those with late MS-OHSS in our center were younger (P < 0.01) and had a lower body mass index (P = 0.02), lower basal serum follicle-stimulating hormone concentrations (P < 0.01), higher basal serum anti-Mullerian hormone concentrations (P < 0.01), and higher antral follicle counts (P < 0.01). Furthermore, there were significant differences between the late MS-OHSS and non-OHSS group in terms of the stimulation protocol (P = 0.04), day of ET (P < 0.01), number of embryos transferred (P < 0.01), and number of oocytes retrieved (P < 0.01). However, after PSM, all of the baseline characteristics were similar between the two groups.
Perinatal outcomes
The perinatal outcomes of the late MS-OHSS and non-OHSS group are presented in Table 2. After univariate and multivariate regression, the rates of normal term infant, placenta previa, PA, PROM, HDP, GDM, cesarean delivery, preterm birth, post-term birth, macrosomia, LBW, LGA, admission to the pediatrics department, and birth defect were not significantly different between the late MS-OHSS and non-OHSS groups. In addition, the other perinatal outcomes examined in this study (gestational age, birth weight, birth height, and 1-minute Apgar scores) did not differ significantly between the late MS-OHSS and non-OHSS groups. After PSM, as shown in Table 3, the risk of perinatal outcomes was not significantly different between the late MS-OHSS and matched non-OHSS groups.
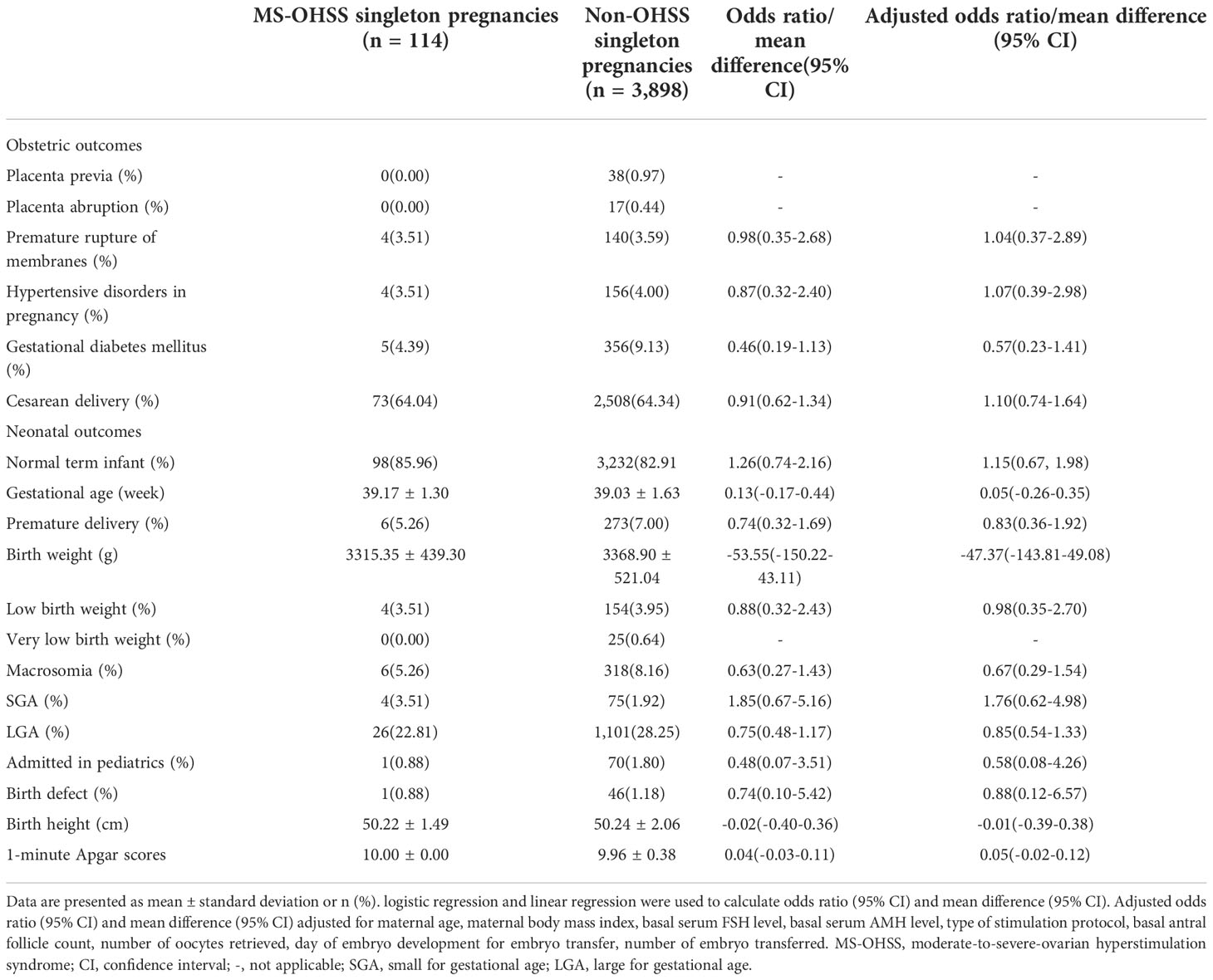
Table 2 Perinatal outcomes among MS-OHSS patients and Non-OHSS patients before propensity score matching.
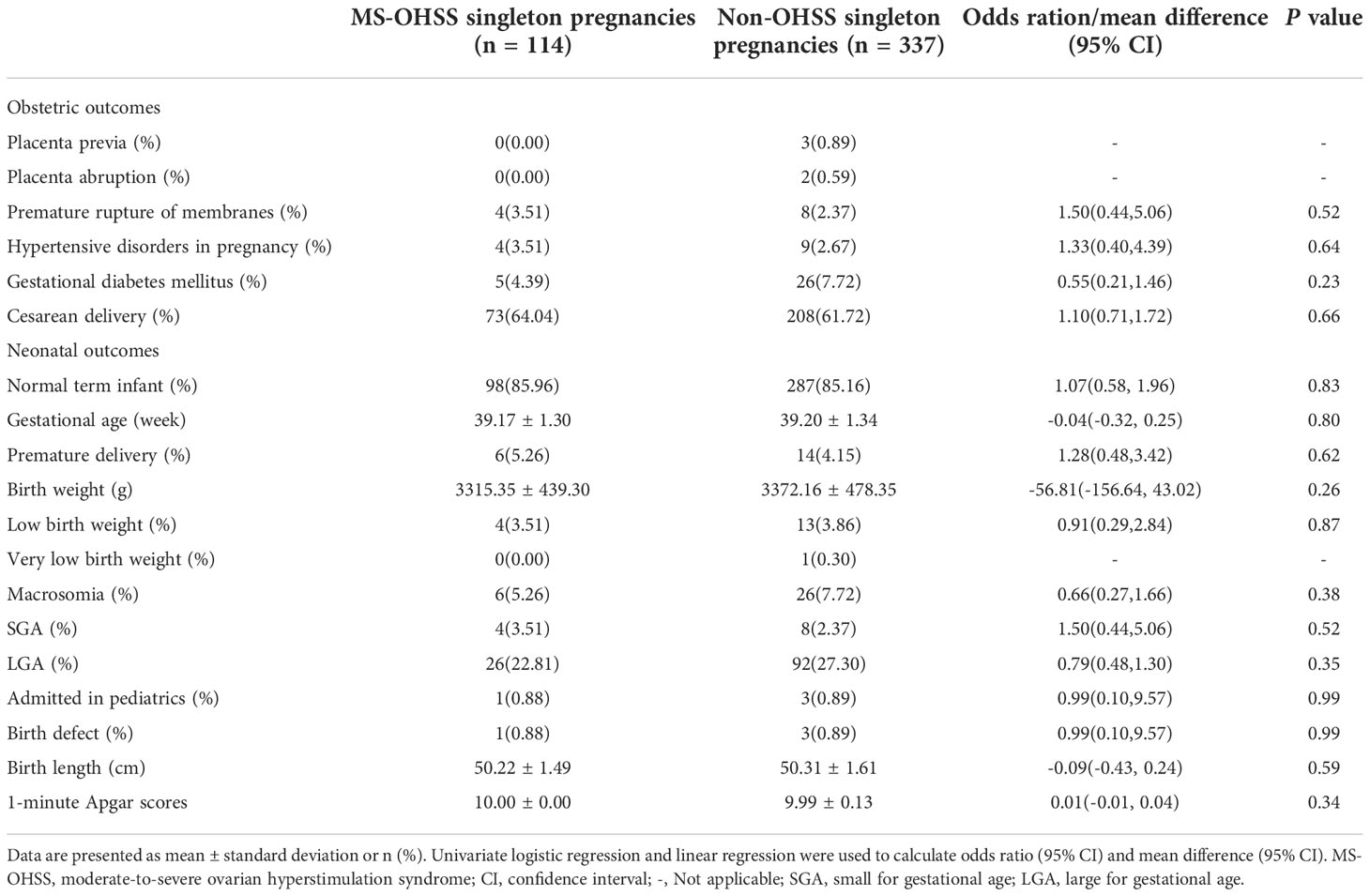
Table 3 Perinatal outcomes among MS-OHSS patients and Non-OHSS patients after propensity score matching.
Discussion
The results of our data showed that the perinatal outcomes were similar between MS-OHSS group and non-OHSS group. The perinatal outcomes of pregnancies complicated by MS-OHSS have not yet been thoroughly investigated. Our results were similar to several previous reports (15, 16, 30). The study by Choux et al. (16) included 77 patients hospitalized for severe OHSS and 231 matched patients without OHSS, only 55 OHSS patients achieved singleton live births. They found the perinatal outcomes were similar between the OHSS group and non-OHSS group. A retrospective cohort study of 190 patients with OHSS in China showed that OHSS did not exert any obviously adverse effect on subsequent pregnancies (15). The authors further analyzed the differences in the rates of multiple pregnancy. But they did not separately compare the perinatal outcomes of patients who achieved singleton live births. Jia et al. (30) also demonstrated similar outcomes to our study. However, they only included patients with mild and moderate OHSS and did not assess the effects of OHSS on obstetric complications. As patients with mild OHSS only have minor clinical symptoms and the disorder is self-limiting (6, 31), few patients with mild OHSS require admission to hospital for treatment. Some patients may only experience mild bloating and are unaware of having OHSS. Therefore, it is challenging to estimate the actual sample size of patients with mild OHSS. Thus, only patients with MS-OHSS were included in our study. Hu et al. (22) used a study design similar to ours but obtained conflicting results. They used PSM to match the rate of multiple gestations rather than analyzed the obstetric and neonatal outcomes in OHSS patients who achieved single live births separately. They found that late MS-OHSS increased the risk of GDM, thrombosis, and NICU hospitalization. Schirmer et al. (21) analyzed National Assisted Reproductive Technology Surveillance System data and found that patients with OHSS had higher risks of preterm delivery and LBW than those without OHSS.
OHSS is a self-limiting disorder associated with hormones (6, 9). The occurrence of OHSS is correlated with administration of HCG and concentrations of estradiol (E2) (16, 32). HCG promotes the fusion of cytotrophoblasts to create syncytiotrophoblasts and helps to remodel spiral arteries, thus improving the propensity of implantation and playing a role in the success of pregnancies in the presence of OHSS (16). Depending on the time of occurrence, OHSS is classified as early OHSS (occurring 9 or fewer days after oocyte retrieval) or late OHSS (occurring no sooner than 10 days after oocyte retrieval) (33). However, the pathogeneses of these two types of OHSS are distinct. Early OHSS is an acute response of multiple follicles to exogenous HCG given in a short period of time, while late OHSS is usually caused by endogenous HCG secreted by trophoblast cells after achieving pregnancy (8, 33, 34). Compared with early OHSS, the stimulation caused by endogenous HCG in late OHSS was slower (33). There may be adaptive processes in the organism. This hypothesis need to be confirmed in future studies. The MS-OHSS patients included in our study were all late onset. Furthermore, OHSS with abnormal transient hemodynamics usually occurs only in the first trimester of pregnancy. This implies that OHSS may have less impact on subsequent pregnancies.
It was proposed that serum concentrations of E2 are a predictor of OHSS in women undergoing ART (32). Some studies have shown that supraphysiological concentrations of E2 after controlled ovarian hyperstimulation negatively affect embryo quality, extravillous trophoblast invasion, endometrial receptivity, and placental development (35–37). Moreover, studies by researchers at our center have demonstrated that supraphysiological E2 concentrations (≥ 4,000 pg/ml) on the day of HCG injection increase the risks of singleton SGA, LBW, and full-term LBW (38). However, in our study, the serum E2 concentrations on the day of HCG injection or the basal serum E2 concentrations were similar between the MS-OHSS and non-OHSS groups. This may be related to the treatment strategy adopted for these patients. To prevent severe overstimulation, we recommend patients whose ovaries are enlarged before transplantation or whose serum E2 concentrations are excessively high on the day of HCG injection to cancel the transfer and freeze all the embryos. Transferring the frozen embryos later may guarantee an acceptable reproductive outcome. This may also be the reason why there was no difference in perinatal outcomes between MS-OHSS group and non-OHSS group.
Strengths and limitations
We analyzed the effects of late MS-OHSS on the perinatal outcomes of patients who achieved single live births after IVF-/ICSI-fresh ET cycles. Moreover, the influence of various confounding factors on the perinatal outcomes were controlled by PSM and regression. Therefore, our study results provide useful insights for both clinicians and patients. However, our study has some limitations. First, the sample size of patients with MS-OHSS was limited. Therefore, our observations should be interpreted with caution, and a larger sample size will be used in our future study. Second, we did not compare the perinatal outcomes of patients with different severity levels of OHSS. Third, we did not analyze the hospitalization information of patients, such as the length of hospitalization and specific treatment received. Last, this study was a retrospective study. Therefore, the possibility of potential recall bias cannot be ignored.
Conclusion
The perinatal outcomes of singleton live births in patients with MS-OHSS were similar to those in patients without OHSS. To confirm this conclusion, we will adopt a larger sample size in a future study. In addition, further studies are warranted to evaluate the impact of OHSS on long-term prognosis by conducting long-term follow-up of patients and neonates, and to further explore the pathogenesis of OHSS through basic experiments.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
This retrospective cohort study was approved by the ethics committee of the Third Affiliated Hospital of Zhengzhou University (ethics approval number: 2022-164-01).
Author contributions
YG and SR designed the study, selected the population to be included and excluded, extracted and analyzed data and drafted of the manuscript. RZ, HW, BR, WenZ and JD were involved in the data collection and statistical analysis. WZ, CY, SY, and JD contributed to the revising of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by State Key Laboratory of Reproductive Medicine, Nanjing Medical University (SKLRM-K201903), Henan young and middle-aged health science and technology innovation leading talent training project (YXKC2021020) and National Health Commission Scientific Research Foundation Henan Medical Science and Technology Research Program Provincial Joint Construction Project (SBGJ202102180).
Acknowledgments
The authors acknowledge all the patients participated in the study and the participants contributed to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ovarian hyperstimulation syndrome. Lancet (1991) 338(8775):1111–2. doi: 10.1016/0140-6736(91)91967-y
2. Mourad S, Brown J, Farquhar C. Interventions for the prevention of ohss in art cycles: An overview of cochrane reviews. Cochrane Database Syst Rev (2017) 1:CD012103. doi: 10.1002/14651858.CD012103.pub2
3. Blumenfeld Z. The ovarian hyperstimulation syndrome. Vitam Horm (2018) 107:423–51. doi: 10.1016/bs.vh.2018.01.018.4
4. Gera PS, Tatpati LL, Allemand MC, Wentworth MA, Coddington CC. Ovarian hyperstimulation syndrome: Steps to maximize success and minimize effect for assisted reproductive outcome. Fertil Steril (2010) 94(1):173–8. doi: 10.1016/j.fertnstert.2009.02.049
5. Zosmer A, Katz Z, Lancet M, Konichezky S, Schwartz-Shoham Z. Adult respiratory distress syndrome complicating ovarian hyperstimulation syndrome. Fertil Steril (1987) 47(3):524–6. doi: 10.1016/s0015-0282(16)59069-4
6. Golan A, Ron-el R, Herman A, Soffer Y, Weinraub Z, Caspi E. Ovarian hyperstimulation syndrome: An update review. Obstet Gynecol Survey (1989) 44(6):430–40. doi: 10.1097/00006254-198906000-00004
7. Practice committee of the American society for reproductive medicine. electronic address aao, practice committee of the American society for reproductive m. prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: A guideline. Fertil Steril (2016) 106(7):1634–47. doi: 10.1016/j.fertnstert.2016.08.048
8. Lyons C, Wheeler C, Frishman G, Hackett R, Seifer D, Haning R. Early and late presentation of the ovarian hyperstimulation syndrome: Two distinct entities with different risk factors. Hum Reprod (1994) 9(5):792–9. doi: 10.1093/oxfordjournals.humrep.a138598
9. Tsirigotis M, Craft I. Ovarian hyperstimulation syndrome (Ohss): How much do we really know about it? Eur J obstet gynecol Reprod Biol (1994) 55(3):151–5. doi: 10.1016/0028-2243(94)90030-2
10. Wallach EE, Navot D, Bergh PA, Laufer N. Ovarian hyperstimulation syndrome in novel reproductive technologies: Prevention and treatment. Fertil Steril (1992) 58(2):249–61. doi: 10.1016/s0015-0282(16)55188-7
11. Soares SR, Gomez R, Simon C, Garcia-Velasco JA, Pellicer A. Targeting the vascular endothelial growth factor system to prevent ovarian hyperstimulation syndrome. Hum Reprod Update (2008) 14(4):321–33. doi: 10.1093/humupd/dmn008
12. McClure N, Healy D, Rogers P, Sullivan J, Beaton L, Haning R, et al. Vascular endothelial growth factor as capillary permeability agent in ovarian hyperstimulation syndrome. Lancet (1994) 344(8917):235–6. doi: 10.1016/s0140-6736(94)93001-5
13. Delvigne A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (Ohss): A review. Hum Reprod Update (2002) 8(6):559–77. doi: 10.1093/humupd/8.6.559
14. Wiser A, Levron J, Kreizer D, Achiron R, Shrim A, Schiff E, et al. Outcome of pregnancies complicated by severe ovarian hyperstimulation syndrome (Ohss): A follow-up beyond the second trimester. Hum Reprod (2005) 20(4):910–4. doi: 10.1093/humrep/deh713
15. Jiang X, Deng CY, Sun ZY, Chen WL, Wang HB, Zhou YZ, et al. Pregnancy outcomes of in vitro fertilization with or without ovarian hyperstimulation syndrome: A retrospective cohort study in Chinese patients. Chin Med J (Engl) (2015) 128(23):3167–72. doi: 10.4103/0366-6999.170280
16. Choux C, Barberet J, Ginod P, Cottenet J, Bruno C, Benzenine E, et al. Severe ovarian hyperstimulation syndrome modifies early maternal serum beta-human chorionic gonadotropin kinetics, but obstetrical and neonatal outcomes are not impacted. Fertil Steril (2017) 108(4):650–8.e2. doi: 10.1016/j.fertnstert.2017.07.1160
17. Courbiere B, Oborski V, Braunstein D, Desparoir A, Noizet A, Gamerre M. Obstetric outcome of women with in vitro fertilization pregnancies hospitalized for ovarian hyperstimulation syndrome: A case-control study. Fertil Steril (2011) 95(5):1629–32. doi: 10.1016/j.fertnstert.2010.12.015
18. Haas J, Baum M, Meridor K, Hershko-Klement A, Elizur S, Hourvitz A, et al. Is severe ohss associated with adverse pregnancy outcomes? evidence from a case-control study. Reprod BioMed Online (2014) 29(2):216–21. doi: 10.1016/j.rbmo.2014.04.015
19. Luke B, Brown MB, Morbeck DE, Hudson SB, Coddington CC 3rd, Stern JE. Factors associated with ovarian hyperstimulation syndrome (Ohss) and its effect on assisted reproductive technology (Art) treatment and outcome. Fertil Steril (2010) 94(4):1399–404. doi: 10.1016/j.fertnstert.2009.05.092
20. Dobrosavljevic A, Rakic S, Mihajlovic S. Risk of spontaneous preterm labor in pregnancies achieved by in vitro fertilization and complicated with severe form of ovarian hyperstimulation syndrome: A case control study. Pak J Med Sci (2019) 35(4):923–8. doi: 10.12669/pjms.35.4.145
21. Schirmer DA 3rd, Kulkarni AD, Zhang Y, Kawwass JF, Boulet SL, Kissin DM. Ovarian hyperstimulation syndrome after assisted reproductive technologies: Trends, predictors, and pregnancy outcomes. Fertil Steril (2020) 114(3):567–78. doi: 10.1016/j.fertnstert.2020.04.004
22. Hu L, Xie R, Wang M, Sun Y. Patients with ivf complicated by moderate-to-Critical ohss experience increased thrombosis, gdm and neonatal nicu admission but slightly shorter gestation compared with matched ivf counterparts: A retrospective Chinese cohort study. Reprod Biol Endocrinol (2021) 19(1):8. doi: 10.1186/s12958-020-00678-w
23. Lou H, Li N, Zhang X, Sun L, Wang X, Hao D, et al. Does the sex ratio of singleton births after frozen single blastocyst transfer differ in relation to blastocyst development? Reprod Biol Endocrinol (2020) 18(1):72. doi: 10.1186/s12958-020-00623-x
24. Liu J, Wang XL, Zhang X, Shen CY, Zhang Z. Live births resulting from 0pn-derived embryos in conventional ivf cycles. J Assist Reprod Genet (2016) 33(3):373–8. doi: 10.1007/s10815-015-0644-6
25. Du M, Zhang J, Yu X, Guan Y. Elevated anti-mullerian hormone is an independent risk factor for preterm birth among patients with overweight polycystic ovary syndrome. Front Endocrinol (2021) 12:788000. doi: 10.3389/fendo.2021.788000
26. Styer AK, Luke B, Vitek W, Christianson MS, Baker VL, Christy AY, et al. Factors associated with the use of elective single-embryo transfer and pregnancy outcomes in the united states, 2004-2012. Fertil Steril (2016) 106(1):80–9. doi: 10.1016/j.fertnstert.2016.02.034
27. Dai L, Deng C, Li Y, Zhu J, Mu Y, Deng Y, et al. Birth weight reference percentiles for Chinese. PloS One (2014) 9(8):e104779. doi: 10.1371/journal.pone.0104779
28. Bonduelle M, Liebaers I, Deketelaere V, Derde M, Camus M, Devroey P, et al. Neonatal data on a cohort of 2889 infants born after icsi (1991-1999) and of 2995 infants born after ivf (1983-1999). Hum Reprod (2002) 17(3):671–94. doi: 10.1093/humrep/17.3.671
29. Bonduelle M, Wilikens A, Buysse A, Van Assche E, Wisanto A, Devroey P, et al. Prospective follow-up study of 877 children born after intracytoplasmic sperm injection (Icsi), with ejaculated epididymal and testicular spermatozoa and after replacement of cryopreserved embryos obtained after icsi. Hum Reprod (1996) 11(suppl 4):131–55. doi: 10.1093/humrep/11.suppl_4.131
30. Jia Q, Fang L, Wang Z, Wu Z, Yan Y, Liu B, et al. Ovarian hyperstimulation syndrome is associated with a high secondary sex ratio in fresh ivf cycles with cleavage-stage embryo transfer: Results for a cohort study. Reprod Sci (2021) 28(12):3341–51. doi: 10.1007/s43032-021-00637-9
31. Rizk B, Aboulghar M. Modern management of ovarian hyperstimulation syndrome. Hum Reprod (1991) 6(8):1082–7. doi: 10.1093/oxfordjournals.humrep.a137488
32. Lee TH, Liu CH, Huang CC, Wu YL, Shih YT, Ho HN, et al. Serum anti-mullerian hormone and estradiol levels as predictors of ovarian hyperstimulation syndrome in assisted reproduction technology cycles. Hum Reprod (2008) 23(1):160–7. doi: 10.1093/humrep/dem254
33. Mathur R, Akande A, Keay S, Hunt L, Jenkins J. Distinction between early and late ovarian hyperstimulation syndrome. Fertil Steril (2000) 73(5):901–7. doi: 10.1016/s0015-0282(00)00492-1
34. Papanikolaou EG, Tournaye H, Verpoest W, Camus M, Vernaeve V, Van Steirteghem A, et al. Early and late ovarian hyperstimulation syndrome: Early pregnancy outcome and profile. Hum Reprod (2005) 20(3):636–41. doi: 10.1093/humrep/deh638
35. Bonagura TW, Babischkin JS, Aberdeen GW, Pepe GJ, Albrecht ED. Prematurely elevating estradiol in early baboon pregnancy suppresses uterine artery remodeling and expression of extravillous placental vascular endothelial growth factor and Alpha1beta1 and Alpha5beta1 integrins. Endocrinology (2012) 153(6):2897–906. doi: 10.1210/en.2012-1141
36. Albrecht ED, Bonagura TW, Burleigh DW, Enders AC, Aberdeen GW, Pepe GJ. Suppression of extravillous trophoblast invasion of uterine spiral arteries by estrogen during early baboon pregnancy. Placenta (2006) 27(4-5):483–90. doi: 10.1016/j.placenta.2005.04.005
37. Ertzeid G, Storeng R. The impact of ovarian stimulation on implantation and fetal development in mice. Hum Reprod (2001) 16(2):221–5. doi: 10.1093/humrep/16.2.221
Keywords: ovarian hyperstimulation syndrome, in vitro fertilization, intracytoplasmic sperm injection, perinatal outcomes, normal term infant
Citation: Ran S, Zu R, Wu H, Zheng W, Yang C, Yang S, Ren B, Zhang W, Du J and Guan Y (2022) Perinatal outcomes of singleton live births after late moderate-to-severe ovarian hyperstimulation syndrome: A propensity score-matched study. Front. Endocrinol. 13:1063066. doi: 10.3389/fendo.2022.1063066
Received: 06 October 2022; Accepted: 15 November 2022;
Published: 01 December 2022.
Edited by:
Da Li, ShengJing Hospital of China Medical University, ChinaReviewed by:
Gang Li, First Affiliated Hospital of Zhengzhou University, ChinaAlan Decherney, Clinical Center (NIH), United States
Copyright © 2022 Ran, Zu, Wu, Zheng, Yang, Yang, Ren, Zhang, Du and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yichun Guan, lisamayguan@163.com
 Shiyu Ran
Shiyu Ran Ruowen Zu1
Ruowen Zu1 Huan Wu
Huan Wu Wei Zheng
Wei Zheng Chen Yang
Chen Yang Shuheng Yang
Shuheng Yang Wen Zhang
Wen Zhang Yichun Guan
Yichun Guan