- 1Department of Endocrinology, The First Medical Center of Chinese PLA General Hospital, Beijing, China
- 2Department of Pediatrics, The First Medical Center of Chinese PLA General Hospital, Beijing, China
Object: Eldecalcitol (ED-71) is a vitamin D analog for the treatment of osteoporosis. However, inconsistent results have been reported in this regard. Hence, this meta-analysis of randomized controlled trials (RCTs) aimed to assess the efficacy and safety of ED-71 for osteoporosis.
Methods: The PubMed, Embase, and the Cochrane Library databases were systematically searched to identify potential trials from inception until April 2021. The investigated outcomes included bone mineral density and fractures at various sites, and potential adverse events. The pooled effect estimates were calculated using weighted mean difference (WMD) and relative risk (RR) with 95% confidence interval (CI) using the random-effects model.
Results: Eight RCTs involving 2368 patients were selected for the final meta-analysis. The pooled results showed that ED-71 were associated with a higher level of femoral neck (FN) bone mineral density (BMD) (WMD: 0.92; 95% CI: 0.24–1.60; P = 0.008), while it had no significant effect on lumbar spine BMD (WMD: 1.09; 95% CI: –0.11 to 2.30; P = 0.076) and hip BMD (WMD: 1.12; 95% CI: –0.16 to 2.40; P = 0.088). Moreover, the use of ED-71 could protect against the risk of all osteoporotic fracture (RR: 0.70; 95% CI: 0.55–0.88; P = 0.003) and vertebral fracture (RR: 0.74; 95% CI: 0.55–0.98; P = 0.038), while it did not affect the risk of nonvertebral fracture (RR: 0.53; 95%CI: 0.23–1.23; P = 0.140). The subgroup analyses found that the effects of ED-71 were superior to those of alfacalcidol on both BMD and fracture results. Moreover, the use of ED-71 plus bisphosphonate was associated with a greater improvement in BMD at various sites compared with bisphosphonate alone. Finally, ED-71 was associated with an increased risk of increased urine calcium level (RR: 1.69; 95% CI: 1.33–2.15; P < 0.001).
Conclusion: This study found that the use of ED-71 could improve BMD and fractures at various sites, especially compared with alfacalcidol or a combination with bisphosphonate for patients with osteoporosis.
Systematic Review Registration: [http://www.crd.york.ac.uk/prospero], identifier [CRD42021270536].
Introduction
Osteoporosis is a chronic, progressive condition characterized by decreased bone mass and damaged microstructure of the bone, thus increasing the risk of skeletal fractures and accounting for more concerns in nowadays aging society (1). It has been reported that about 30% of all postmenopausal women are diagnosed as osteoporosis. And approximately 40% of these patients will go through skeletal fractures (2). More than 8.9 million fractures occurred due to osteoporosis worldwide (3). Osteoporosis can be divided into primary osteoporosis and secondary osteoporosis (4). Primary osteoporosis is an age-related metabolic disease. Its incidence increases with age, and it presents with many atypical clinical symptoms (5). The prevalence of primary osteoporosis in elderly individuals (> 60.0 years) was 36% in China; the incidence in women was higher than that in men (6). A previous study found that osteoporosis could cause serious consequences in terms of osteoporotic fractures, and the most common site was the vertebral body (7).
Currently, bisphosphonates are widely used for osteoporosis to prevent the risk of skeletal fractures (8). However, the bioavailability of bisphosphonates is lower, with less than 1% absorption in the gut. Moreover, after administering bisphosphonates, the patients cannot lie down within 30 min to reduce the topical effect on the gastric mucosa and esophageal regurgitation (9). These factors caused lower compliance and balanced the treatment effects of bisphosphonates. Eldecalcitol (ED-71) was developed in the early 1980s, which has already been approved for osteoporosis in Japan. In 2020, ED-71 was approved for osteoporosis of postmenopausal women in China. ED-71 could reduce the levels of biochemical and histological parameters for bone resorption (10). Numerous studies reported the treatment effectiveness of ED-71 for osteoporosis; however, inconsistent results were obtained (11–18). Therefore, the current study was performed based on randomized controlled trials (RCTs) to determine the efficacy and safety of ED-71 for patients with osteoporosis.
Methods
Protocol and Registration
Our meta-analysis was performed according to the Preferred Reporting Items for Systematic reviews and meta-analyses (PRISMA) recommendations. A protocol for this meta-analysis has been registered on PROSPERO (http://www.crd.york.ac.uk/prospero) and the registration number is CRD42021270536.
Data Sources, Search Strategy, and Selection Criteria
This study was performed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (19). Studies designed as RCTs and reporting the efficacy and safety of ED-71 for patients with osteoporosis were selected for meta-analysis. The publication language was restricted to English, while no restriction was placed on the publication status. The potential studies were identified using the following steps: (1) The PubMed, Embase, and the Cochrane Central Register of Controlled Trials were systematically searched throughout April 2021 using the following search terms: (eldecalcitol OR ED-71) AND (randomized controlled trials). The website http://clinicaltrials.gov/(US NIH) was applied to search trials that were already completed but not yet published. Then, the reference lists of relevant reviews or original articles were manually searched for further new eligible trials.
The literature search and study selection process were independently performed by two reviewers, and conflicts between reviewers were settled by mutual discussion until a consensus was reached. The inclusion criteria were as follows: (1) patients: osteoporosis or low bone mineral density (BMD)/osteopenia. Dual energy X-ray absorptiometry (DXA) was used to measure BMD. Osteoporosis is defined as lumbar spine (L1-4) BMD T-score was below -2.5 SD. Osteopenia is defined as L1-4 BMD T-score between -1.0 and -2.5 SD; (2) intervention: ED-71, irrespective of being used as monotherapy or combined with bisphosphonate; (3) control: placebo, alfacalcidol, or bisphosphonate; (4) outcomes: BMD at the lumbar spine, femoral neck (FN), or hip, all osteoporotic fractures, vertebral fractures, nonvertebral fractures, and potential adverse events; and (5) study design: all included studies having RCT design.
Data Collection and Quality Assessment
Two reviewers independently abstracted the data from included trials following a standardized protocol. The collected information included first authors’ name, publication year, country, sample size, mean age, male proportion, body mass index, intervention, control, co-intervention, follow-up duration, and reported outcomes. The methodological quality of included trials was evaluated using the Cochrane Collaboration risk-of-bias tool, which included seven domains; each domain was assigned as low risk, unclear risk, and high risk (20). The data abstraction and quality assessment were independently performed by two reviewers, and inconsistencies between reviewers were settled by an additional reviewer referring to the original article.
Statistical Analysis
The BMD at various sites was defined as continuous data, while fractures at various sites and adverse events were assigned as categorical data. Then, the weighted mean difference (WMD) and relative risk (RR) with 95% confidence interval (CI) were calculated in each trial before data pooling. After this, the random-effects model was used to calculate the pooled results, which considered the underlying variations across included trials (21, 22). The heterogeneity across included trials for each outcome was assessed using I2 and Q statistic; significant heterogeneity was defined as I2 > 50.0% or P < 0.10 (23, 24). The robustness of pooled results was assessed using sensitivity analysis by sequentially removing a single trial (25). The subgroup analyses for BMD and fractures at various sites were performed according to the intervention and control, and the difference among subgroups was assessed using the interaction P test (26). The publication bias for BMD and fractures at various sites was evaluated using funnel plots and Egger and Begg tests (27, 28). All reported P values were two sided, and the inspection level was 0.05. The quality of included trials was assessed using Review Manager (version 5.3), while quantitative analyses were performed using software Stata (Version 10.0; StataCorp, TX, USA).
Results
Literature Search
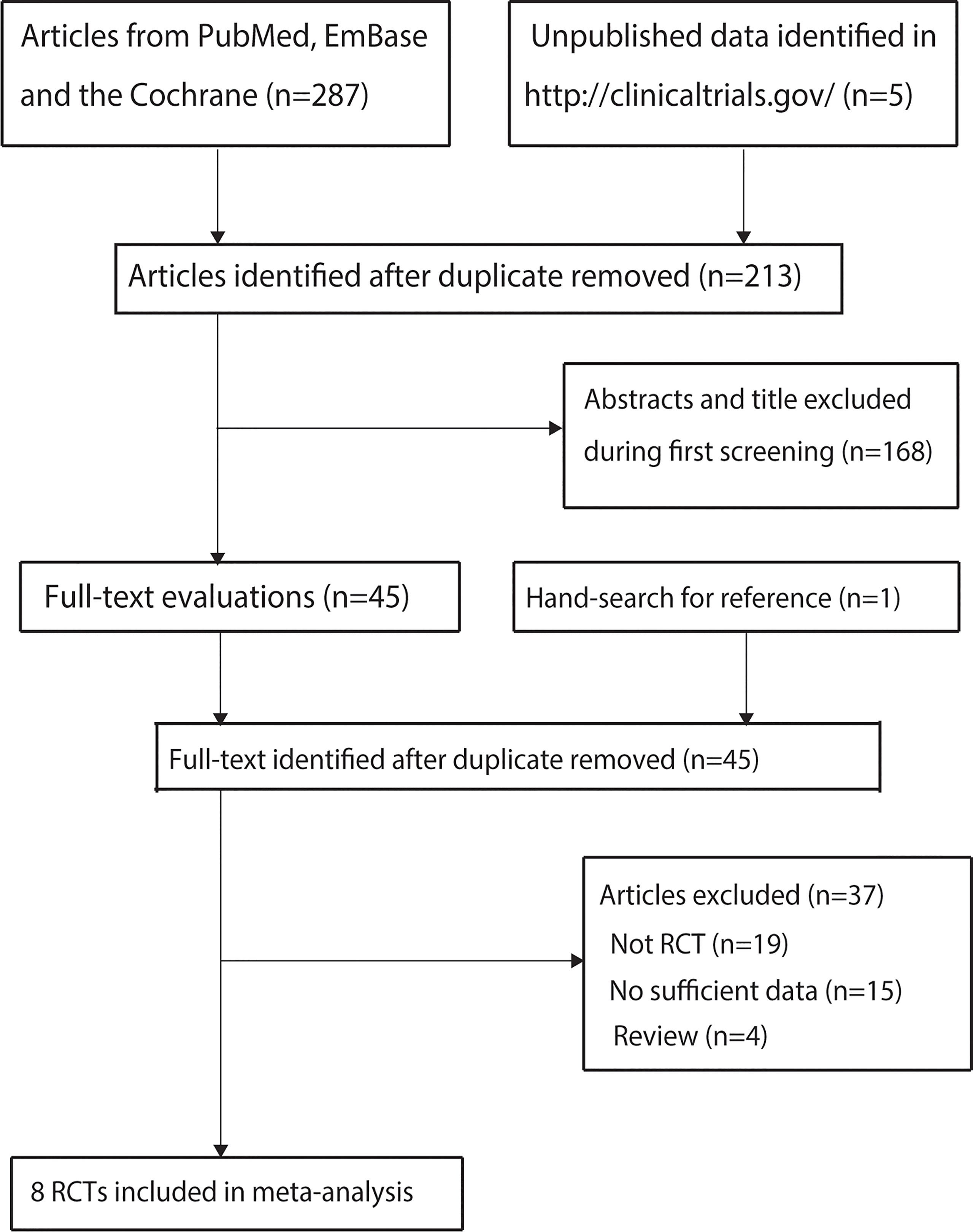
A total of 287 studies were identified from initial electronic searches, and 213 studies were retained after duplicate studies were removed. Further, 168 studies were excluded during title and abstract review because these studies reported irrelevant topics. The remaining 45 studies were retrieved for full-text evaluations, and 37 were removed because of the following reasons: not RCT (n = 19), no sufficient data (n = 15), and review (n = 4). The studies from other sources did not provide any new eligible trial. Finally, eight RCTs were selected for the final meta-analysis (9–16). The details of the study selection process are shown in Figure 1.
Study Characteristics
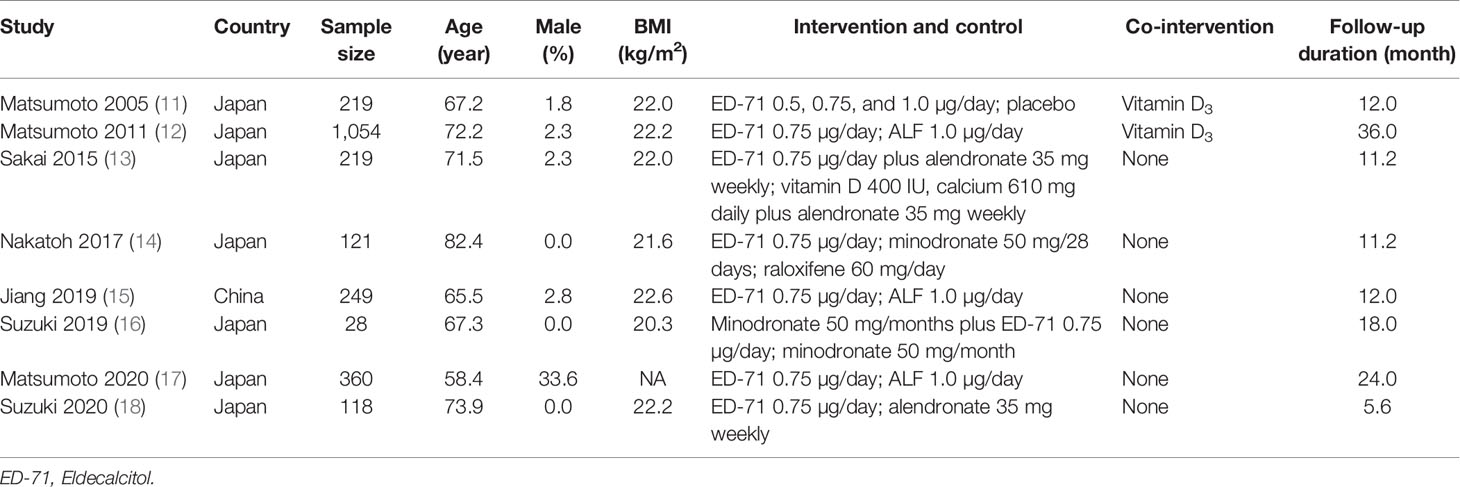
The baseline characteristics of included studies are shown in Table 1. Seven trials were performed in Japan, and the remaining one trial was conducted in China. A total of 2368 patients were involved, and the sample size in an individual trial ranged from 28 to 1054. Three trials included only female patients, while the remaining five trials comprised both male and female patients. Three trials compared ED-71 with alfacalcidol, two trials compared ED-71 plus bisphosphonate with bisphosphonate alone, two trials compared ED-71 monotherapy with bisphosphonate, and the remaining one trial compared ED-71 with placebo. The follow-up duration for included trials ranged from 5.6 months to 36.0 months.
Quality of Included Trials
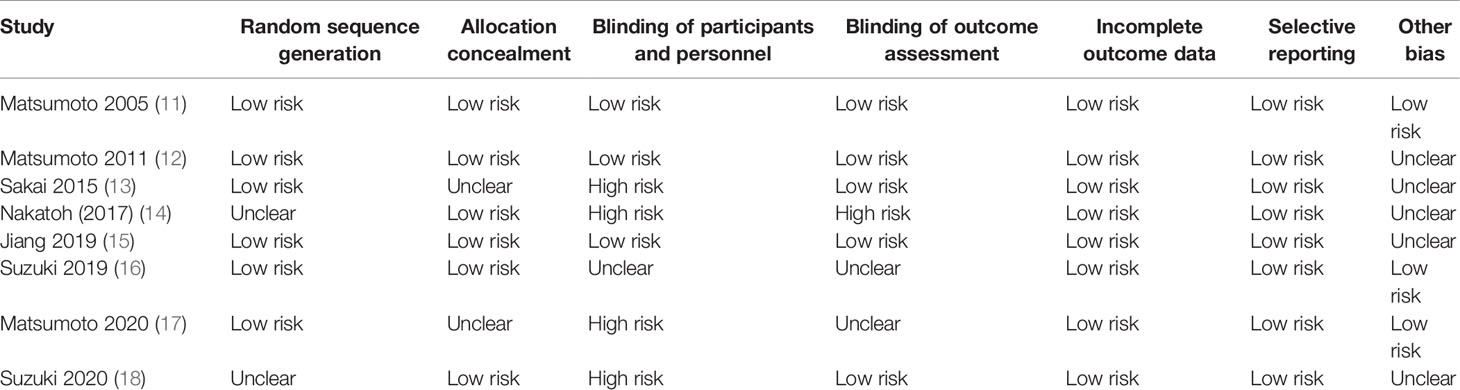
The risk of bias for included studies are summarized in Table 2. All included trials were of moderate to high quality. Six trials reported a low risk of bias for random sequence generation, and allocation concealment. All trials reported a low risk of bias for incomplete outcome data, and selective reporting. Five trials reported a low risk of bias for blinding of outcome assessment, and three trials reported a low risk of bias for blinding of participants and personnel, and other biases.
Bone Mineral Density
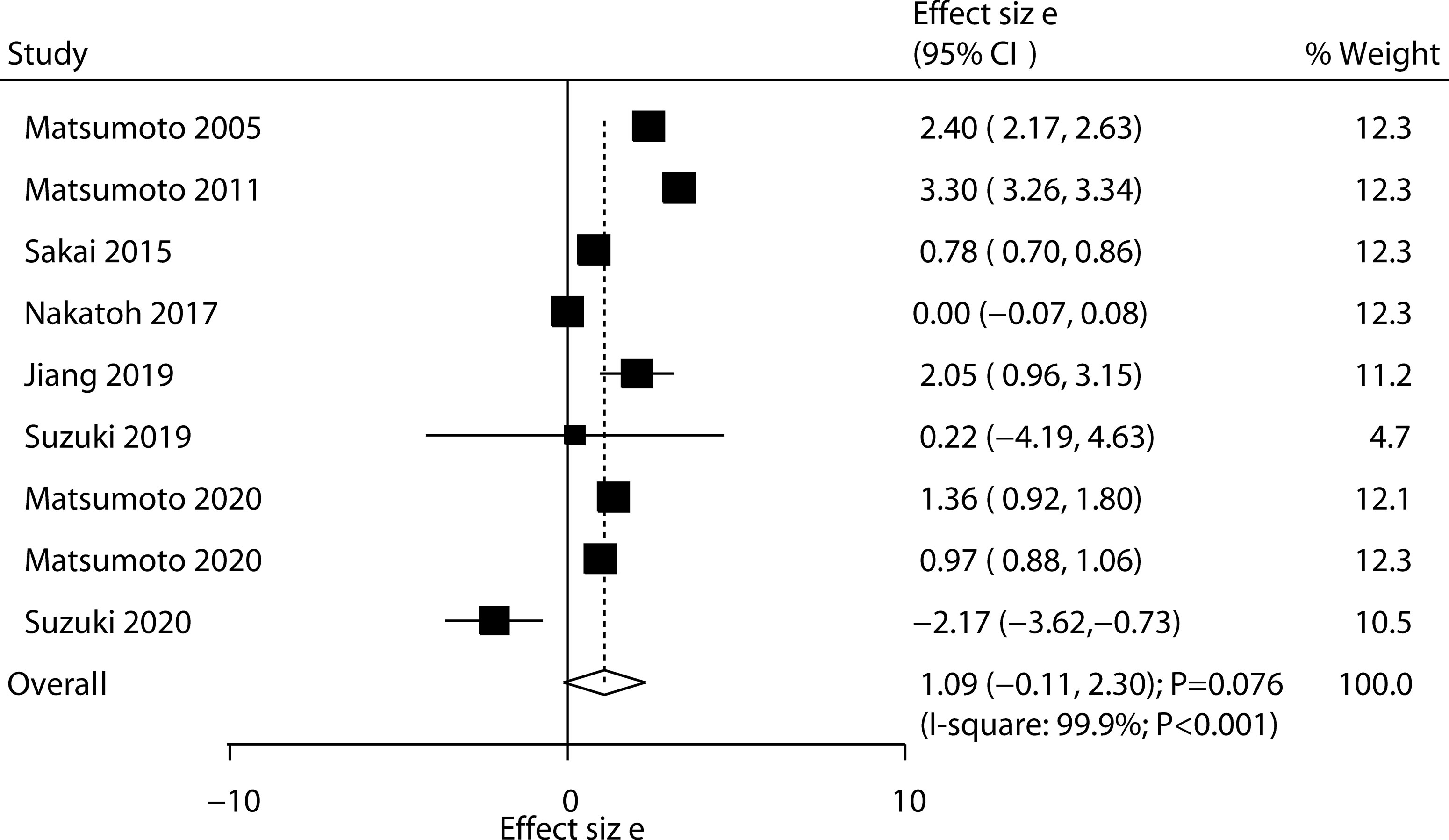
All included trials reported the effect of ED-71 on the change in lumbar spine BMD. After pooling all trials, we noted that ED-71 was not associated with the change in lumbar spine BMD (WMD: 1.09; 95% CI: –0.11 to 2.30; P = 0.076; Figure 2), and significant heterogeneity was observed across included trials (I2 = 99.9%; P < 0.001). The sensitivity analysis indicated that the pooled conclusion was variable because of marginal 95% CI (Supplementary 1). The subgroup analysis found that ED-71 was associated with high lumbar spine BMD compared with ED-71 versus placebo, ED-71 versus alfacalcidol, and ED-71 plus bisphosphonate versus bisphosphonate alone (Table 3). No significant publication bias for lumbar spine BMD was observed (P value for Egger: 0.379; P value for Begg: 0.602; Supplementary 2).
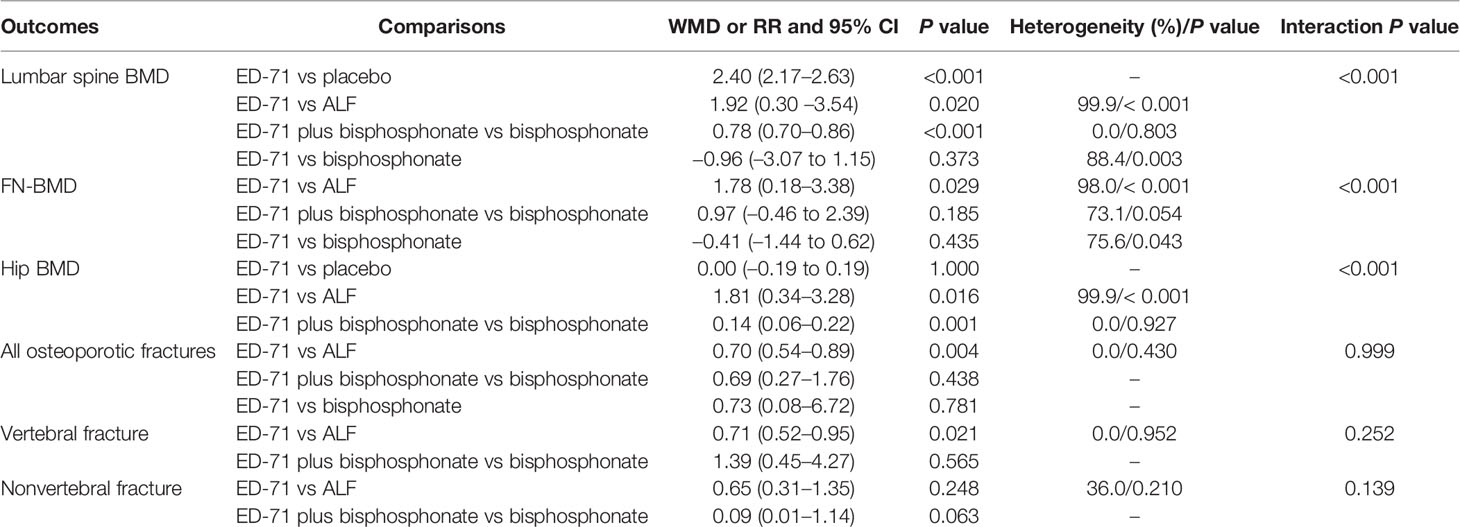
Six trials reported the effect of ED-71 on the change in FN-BMD. The pooled result indicated that ED-71 was associated with a high level of FN-BMD (WMD: 0.92; 95% CI: 0.24–1.60; P = 0.008; Figure 3), and significant heterogeneity was detected among included trials (I2 = 99.2%; P < 0.001). The pooled conclusion was not stable because the lower limit was close to 0 (Supplementary 1). The subgroup analysis found that ED-71 significantly increased FN-BMD level compared with alfacalcidol (Table 3). No significant publication bias was found for FN-BMD (P value for Egger: 0.202; P value for Begg: 0.764; Supplementary 2).
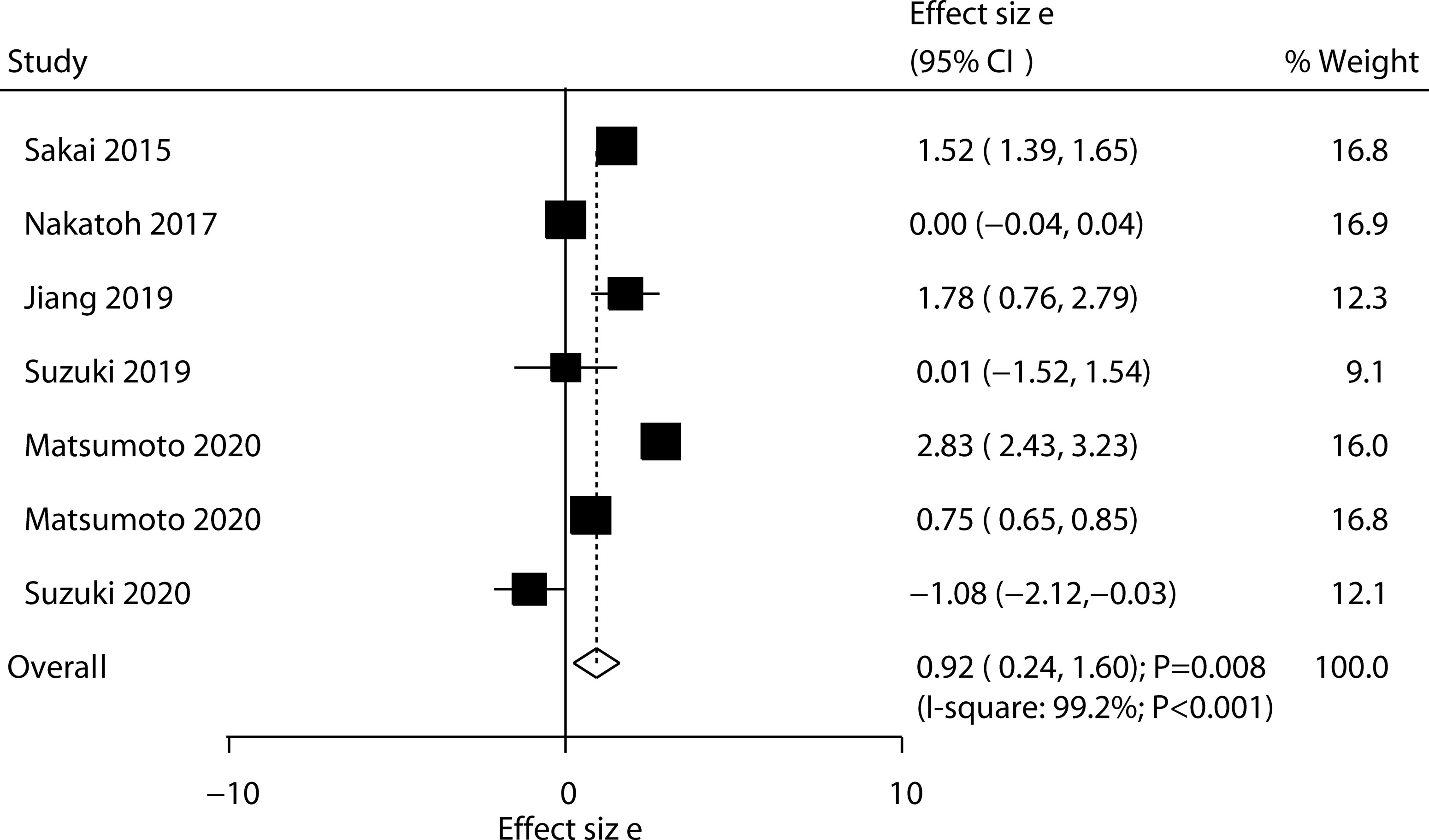
Six trials reported the effect of ED-71 on the change in hip BMD. No significant difference was observed between ED-71 and control for the change in hip BMD (WMD: 1.12; 95% CI: –0.16 to 2.40; P = 0.088; Figure 4), and significant heterogeneity was observed (I2 = 99.9%; P < 0.001). The sensitivity analysis suggested that ED-71 might exert a beneficial effect on hip BMD (Supplementary 1). The subgroup analysis found that ED-71 significantly increased hip BMD compared with a combination of ED-71 with alfacalcidol, and ED-71 plus bisphosphonate versus bisphosphonate alone (Table 3). No significant publication bias for hip BMD was observed (P value for Egger: 0.222; P value for Begg: 0.548; Supplementary 2).
Fractures
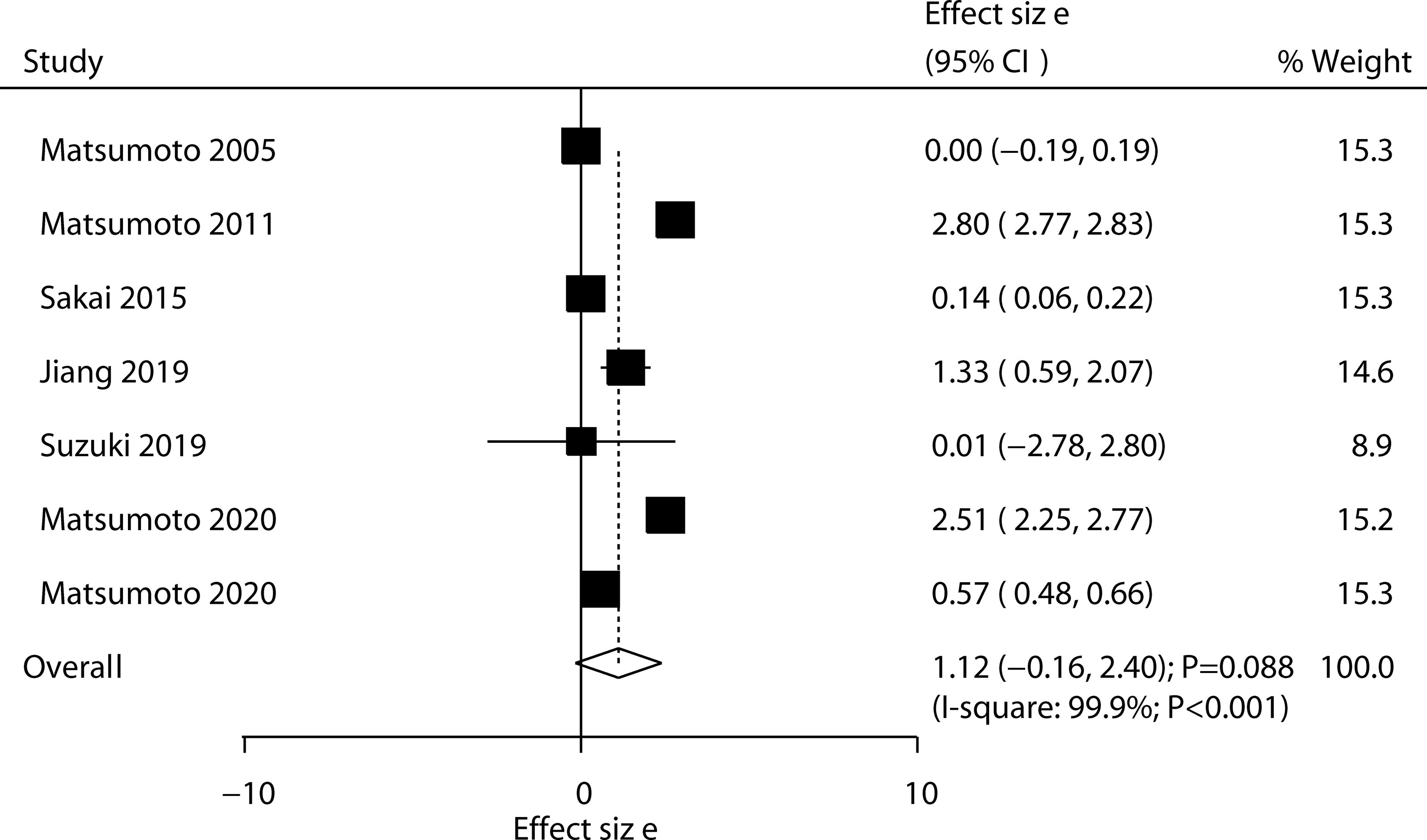
Five trials reported the effect of ED-71 on the risk of all osteoporotic fractures; the use of ED-71 was associated with a reduced risk of all osteoporotic fractures (RR: 0.70; 95% CI: 0.55–0.88; P = 0.003; Figure 5). No evidence of heterogeneity was observed for all osteoporotic fractures (I2 = 0.0%; P = 0.792). The beneficial role of ED-71 in the risk of all osteoporotic fractures was not observed when removing the trial conducted by Matsumoto 2011, which specifically reported greater weight from the overall analysis (Supplementary 1). The subgroup analysis found that ED-71 significantly reduced the risk of osteoporotic fractures compared with alfacalcidol (Table 3). No significant publication bias was observed for all osteoporotic fractures (P value for Egger: 0.505; P value for Begg: 0.462; Supplementary 2).
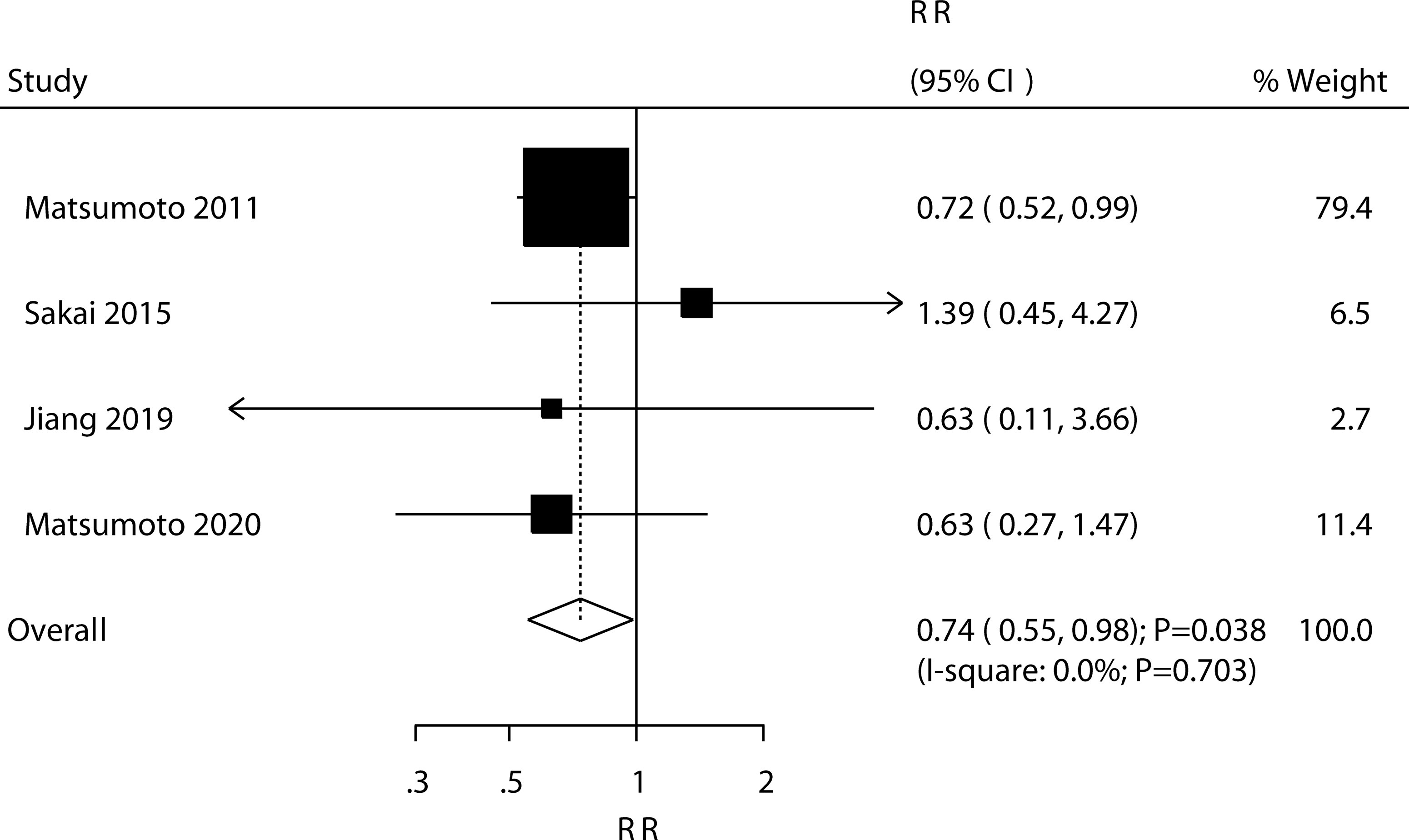
Four trials reported the effect of ED-71 on the risk of vertebral fractures. ED-71 could protect against the risk of vertebral fractures (RR: 0.74; 95% CI: 0.55–0.98; P = 0.038; Figure 6), and no evidence of heterogeneity was observed (I2 = 0.0%; P = 0.703). The pooled result for vertebral fractures was not stable after sequentially removing a single study (Supplementary 1). The subgroup analysis suggested that ED-71 significantly reduced the risk of vertebral fractures compared with alfacalcidol (Table 3). No significant publication bias for vertebral fractures was observed (P value for Egger: 0.689; P value for Begg: 0.734; Supplementary 2).
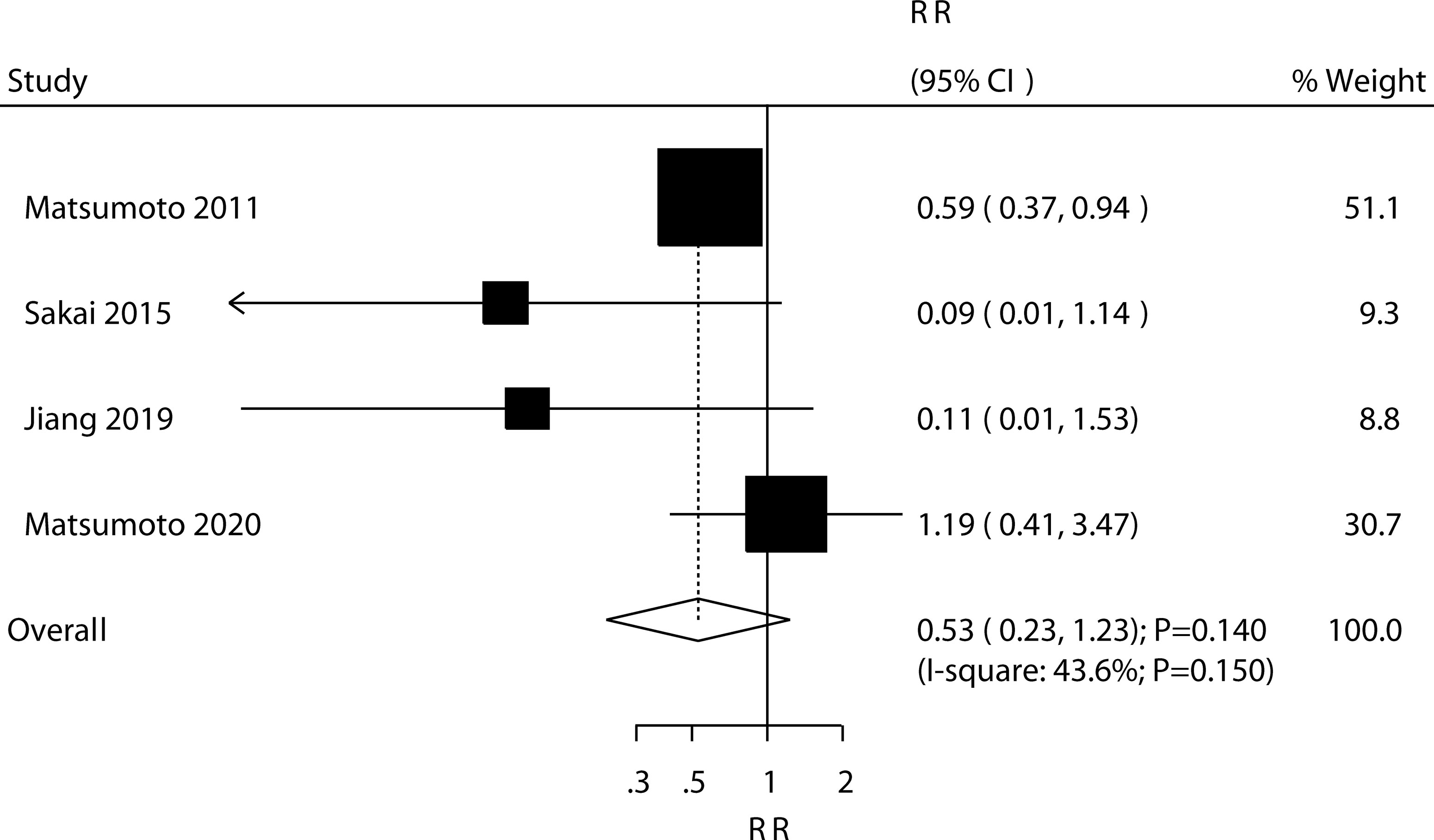
Four trials reported the effect of ED-71 on the risk of nonvertebral fractures. The pooled result found that ED-71 was not associated with the risk of nonvertebral fractures (RR: 0.53; 95% CI: 0.23–1.23; P = 0.140; Figure 7). No significant heterogeneity for nonvertebral fractures was detected (I2 = 43.6%; P = 0.150). The sensitivity analysis indicated that the pooled conclusion was robust and not altered after excluding any particular trial (Supplementary 1). The subgroup analysis indicated that ED-71 was not associated with the risk of nonvertebral fractures, irrespective of the comparisons of ED-71 versus alfacalcidol, or ED-71 plus bisphosphonate versus bisphosphonate alone (Table 3).
Adverse Events
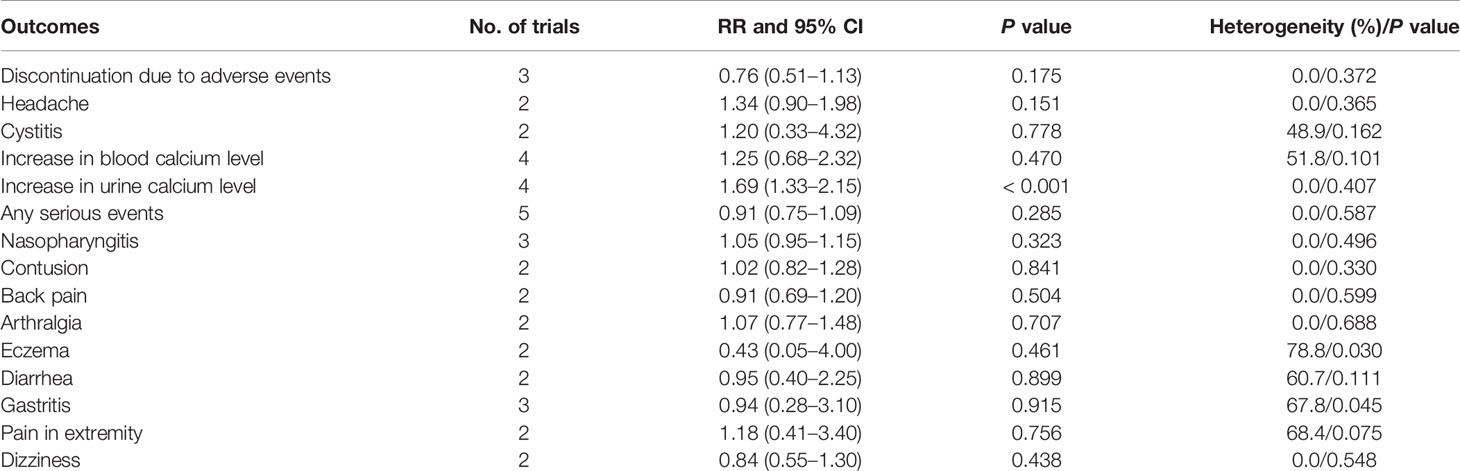
The pooled results for adverse events are summarized in Table 4. ED-71 significantly increased the risk of the increase in urine calcium level (RR: 1.69; 95% CI: 1.33–2.15; P < 0.001). However, significant differences were found between ED-71 and control for the risk of discontinuation due to adverse events, headache, cystitis, increase in blood calcium level, nasopharyngitis, contusion, back pain, arthralgia, eczema, diarrhea, gastritis, pain in extremity, and dizziness.
Discussion
The present study was an updated meta-analysis to assess the efficacy and safety of ED-71 for osteoporosis based on RCTs comprehensively. A total of 2368 patients across a broad range of characteristics from eight RCTs were identified. This study found that ED-71 significantly increased FN-BMD and reduced the risk of all osteoporotic fractures and vertebral fractures. Moreover, the beneficial effects were mainly observed in the comparisons of ED-71 versus alfacalcidol, and ED-71 plus bisphosphonate versus bisphosphonate alone. Furthermore, the risk of the increase in urine calcium level significantly increased when using ED-71.
Several systematic reviews and meta-analyses already addressed the use of ED-71 for osteoporosis. Xu et al. performed a meta-analysis of three trials and found that ED-71 was associated with a high level of lumbar spine BMD and reduced the risk of vertebral fractures (29). However, the analysis was based on three RCTs, and several other efficacy outcomes were not addressed. Zheng et al. performed a meta-analysis of four studies and found that ED-71 plus bisphosphonate was associated with high FN-BMD and hip BMD after 6 months compared with bisphosphonate alone. Moreover, these effects were not observed after 12 months, while ED-71 plus bisphosphonate was associated with high lumbar spine BMD after 12 months compared with bisphosphonate alone (30). However, the analysis of this study was based on two RCTs and two observational studies, and the pooled results might be affected by uncontrolled biases. Therefore, we performed a meta-analysis of RCTs to evaluate the treatment effectiveness of ED-71 for osteoporosis.
The summary results indicated that ED-71 was superior to alfacalcidol for improving lumbar spine BMD, FN-BMD, or hip BMD, and reduced the risk of all osteoporotic fractures and vertebral fractures. The reason for these could be that ED-71 could reduce the number of preosteoblastic cells, which resulted in a reduction in the number and activity of osteoclasts on the bone surface through interacting with osteoclast precursors (31). Moreover, the “improvement in hip geometry and/or biomechanics in ED-71 was superior to that in alfacalcidol through increasing the cross-sectional area, volumetric BMD, and cortical thickness by mitigating endocortical bone resorption (32). However, ED-71 had no significant effect on the risk of nonvertebral fractures. This was because the number of events was lower than expected, and the power was not enough to detect a potential difference between ED-71 and alfacalcidol.
Compared with bisphosphonate alone, ED-71 plus bisphosphonate was associated with a greater increase in lumbar spine BMD and hip BMD. The use of bisphosphonate could improve the process of osteoporosis, while BMD continued to decline in nearly 15% of patients using bisphosphonate (33). Moreover, bisphosphonate could slow down the speed of bone gain with time (34). ED-71 could reduce the re-absorption of blood calcium in the intestine and improve the BMD for patients with osteoporosis (15). The use of ED-71 could decrease the bone resorption level for patients treated with bisphosphonate (35). Furthermore, superior effects of ED-71 plus bisphosphonate on the risk of fractures at various sites were not detected. This could be explained by the smaller number of trials included; also, the number of events was lower than expected. Finally, no significant differences were found between ED-71 and control for mostly reported adverse events. However, ED-71 was associated with an increased risk of the increase in urine calcium level, probably because it could reduce the re-absorption of blood calcium and the urine calcium level relatively increased.
Several shortcomings of this study should be acknowledged. (1) The heterogeneity for BMD at various sites were not fully explained by sensitivity and subgroup analyses. (2) The co-intervention of vitamin D and calcium were not consistent among included studies, which could affect the change in BMD and the risk of fractures. (3) The cause and severity of osteoporosis were different, and the improvement in BMD and fracture risk was affected. (4) The analysis was based on pooled data from published articles, the detailed analysis was restricted, and publication bias was inevitable.
In conclusion, this study found that ED-71 was superior to alfacalcidol in improving BMD at various sites and reduced the risk of all osteoporotic fractures and vertebral fractures. Moreover, ED-71 plus bisphosphonate was associated with a greater improvement in lumbar spine BMD and hip BMD compared with bisphosphonate alone. Further large-scale RCTs should be performed to assess the long-term treatment effects of BMD on fractures for patients with specific characteristics.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
HL and WG designed the study and edited the manuscript. HL, GW, and TW analyzed the data, performed the statistical analysis, and interpreted the data. YM edited and provided critical revision to the article. WG had primary responsibility for the final content. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.854439/full#supplementary-material
References
1. Klibanski A, Adams-Campbell L, Bassford T, Blair SN, Boden SD, Dickersin K. Osteoporosis Prevention, Diagnosis, and Therapy. JAMA (2001) 285:785–95. doi: 10.1001/jama.285.6.785
2. Johnell O, Kanis JA. An Estimate of the Worldwide Prevalence and Disability Associated With Osteoporotic Fractures. Osteoporos Int (2006) 17:1726–33. doi: 10.1007/s00198-006-0172-4
3. Johnston CB, Dagar M. Osteoporosis in Older Adults. Med Clin North Am (2020) 104:873–84. doi: 10.1016/j.mcna.2020.06.004
4. Tarantino U, Iolascon G, Cianferotti L, Masi L, Marcucci G, Giusti F, et al. Clinical Guidelines for the Prevention and Treatment of Osteoporosis: Summary Statements and Recommendations From the Italian Society for Orthopaedics and Traumatology. J Orthop Traumatol (2017) 18:3–36. doi: 10.1007/s10195-017-0474-7
5. Baccaro LF, Conde DM, Costa-Paiva L, Pinto-Neto AM. The Epidemiology and Management of Postmenopausal Osteoporosis: A Viewpoint From Brazil. Clin Interv Aging (2015) 10:583–91. doi: 10.2147/CIA.S54614
6. Lin X, Xiong D, Peng YQ, Sheng ZF, Wu XY, Wu XP, et al. Epidemiology and Management of Osteoporosis in the People's Republic of China: Current Perspectives. Clin Interv Aging (2015) 10:1017–33. doi: 10.2147/cia.S54613
7. Daffner SD, Karnes JM, Watkins CM. Surgeon Specialty Influences Referral Rate for Osteoporosis Management Following Vertebral Compression Fractures. Global Spine J (2016) 6:524–8. doi: 10.1055/s-0035-1569057
8. Maraka S, Kennel KA. Bisphosphonates for the Prevention and Treatment of Osteoporosis. BMJ (2015) 351:h3783. doi: 10.1136/bmj.h3783
9. Rakel A, Boucher A, Ste-Marie LG. Role of Zoledronic Acid in the Prevention and Treatment of Osteoporosis. Clin Interv Aging (2011) 6:89–99. doi: 10.2147/cia.S7282
10. Uchiyama Y, Higuchi Y, Takeda S, Masaki T, Shira-Ishi A, Sato K, et al. ED-71, A Vitamin D Analog, Is a More Potent Inhibitor of Bone Resorption Than Alfacalcidol in an Estrogen-Deficient Rat Model of Osteoporosis. Bone (2002) 30:582–8. doi: 10.1016/S8756-3282(02)00682-8
11. Matsumoto T, Miki T, Hagino H, Sugimoto T, Okamoto S, Hirota T, et al. A New Active Vitamin D, ED-71, Increases Bone Mass in Osteoporotic Patients Under Vitamin D Supplementation: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J Clin Endocrinol Metab (2005) 90:5031–6. doi: 10.1210/jc.2004-2552
12. Matsumoto T, Ito M, Hayashi Y, Hirota T, Tanigawara Y, Sone T, et al. A New Active Vitamin D3 Analog, Eldecalcitol, Prevents the Risk of Osteoporotic Fractures-A Randomized, Active Comparator, Double-Blind Study. Bone (2011) 49:605–12. doi: 10.1016/j.bone.2011.07.011
13. Sakai A, Ito M, Tomomitsu T, Tsurukami H, Ikeda S, Tomomitsu T, Fukuda F, et al. Efficacy of Combined Treatment With Alendronate (ALN) and Eldecalcitol, A New Active Vitamin D Analog, Compared to That of Concomitant ALN, Vitamin D Plus Calcium Treatment in Japanese Patients With Primary Osteoporosis. Osteoporos Int (2015) 26:1193–202. doi: 10.1007/s00198-014-2991-z
14. Nakatoh S. Effect of Osteoporosis Medication on Changes in Bone Mineral Density and Bone Turnover Markers After 24-Month Administration of Daily Teriparatide: Comparison Among Minodronate, Raloxifene, and Eldecalcitol. J Bone Miner Metab (2018) 36:221–8. doi: 10.1007/s00774-017-0829-4
15. Jiang Y, Tang H, Ma X, Cheng Q, Lin H, Jin X, et al. Eldecalcitol Increases Bone Mineral Density in Chinese Osteoporotic Patients Without Vitamin D or Calcium Supplementation. J Bone Miner Metab (2019) 37:1036–47. doi: 10.1007/s00774-019-01009-9
16. Suzuki T, Nakamura Y, Kato H. Effects of Monthly Minodronate With or Without Eldecalcitol Addition in Osteoporosis Patients With Rheumatoid Arthritis: An 18-Month Prospective Study. Osteoporos Sarcopenia (2019) 5:122–7. doi: 10.1016/j.afos.2019.11.004
17. Matsumoto T, Yamamoto K, Takeuchi T, Tanaka Y, Tanaka S, Nakano T, et al. Eldecalcitol Is Superior to Alfacalcidol in Maintaining Bone Mineral Density in Glucocorticoid-Induced Osteoporosis Patients (E-GLORIA). J Bone Miner Metab (2020) 38:522–32. doi: 10.1007/s00774-020-01091-4
18. Suzuki T, Harada A, Shimada H, Hosoi T, Kawata Y, Inoue T, et al. Assessment of Eldecalcitol and Alendronate Effect on Postural Balance Control in Aged Women With Osteoporosis. J Bone Miner Metab (2020) 38:859–67. doi: 10.1007/s00774-020-01118-w
19. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PloS Med (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
20. Higgins J, Altman DG. Assessing Risk of Bias in Included Studies. In: Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions, vol 8. Oxford, England: Cochrane Collaboration (2008).
21. DerSimonian R, Laird N. Meta-Analysis in Clinical Trials. Control Clin Trials (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
22. Ades AE, Lu G, Higgins JP. The Interpretation of Random-Effects Meta-Analysis in Decision Models. Med Decis Making (2005) 25:646–54. doi: 10.1177/0272989X05282643
23. Deeks JJ HJ, Altman DG. Analysing Data and Undertaking Meta-Analyses. In: Higgins JGS, editor. Cochrane Handbook for Systematic Reviews of Interventions. Oxford: The Cochrane Collaboration (2008).
24. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring Inconsistency in Meta-Analyses. BMJ (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
25. Tobias A. Assessing the Influence of a Single Study in the Meta-Analysis. Stata Tech Bull (1999) 47:15–7.
26. Altman DG, Bland JM. Interaction Revisited: The Difference Between Two Estimates. BMJ (2003) 326:219. doi: 10.1136/bmj.326.7382.219
27. Egger M, Davey Smith G, Schneider M, Minder C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
28. Begg CB, Mazumdar M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics (1994) 50:1088–101. doi: 10.2307/2533446
29. Xu Z, Fan C, Zhao X, Tao H. Treatment of Osteoporosis With Eldecalcitol, a New Vitamin D Analog: A Comprehensive Review and Meta-Analysis of Randomized Clinical Trials. Drug Des Devel Ther (2016) 10:509–17. doi: 10.2147/dddt.S84264
30. Zheng Z, Luo J. The Therapeutic Effect to Eldecalcitol + Bisphosphonate Is Superior to Bisphosphonate Alone in the Treatment of Osteoporosis: A Meta-Analysis. J Orthop Surg Res (2020) 15:390. doi: 10.1186/s13018-020-01896-z
31. de Freitas PH, Hasegawa T, Takeda S, Sasaki M, Tabata C, Oda K, et al. Eldecalcitol, A Second-Generation Vitamin D Analog, Drives Bone Minimodeling and Reduces Osteoclastic Number in the Trabecular Bone of Ovariectomized Rats. Bone (2011) 49:335–42. doi: 10.1016/j.bone.2011.05.022
32. Ito M, Nakamura T, Fukunaga M, Shiraki M, Matsumoto T. Effect of Eldecalcitol, an Active Vitamin D Analog, on Hip Structure and Biomechanical Properties: 3D Assessment by Clinical CT. Bone (2011) 49:328–34. doi: 10.1016/j.bone.2011.05.002
33. Heckman GA, Papaioannou A, Sebaldt RJ, Ioannidis G, Petrie A, Goldsmith C, et al. Effect of Vitamin D on Bone Mineral Density of Elderly Patients With Osteoporosis Responding Poorly to Bisphosphonates. BMC Musculoskelet Disord (2002) 3:6. doi: 10.1186/1471-2474-3-6
34. Orr-Walker B, Wattie DJ, Evans MC, Reid IR. Effects of Prolonged Bisphosphonate Therapy and Its Discontinuation on Bone Mineral Density in Post-Menopausal Osteoporosis. Clin Endocrinol (1997) 46:87–92. doi: 10.1046/j.1365-2265.1997.d01-1741.x
35. Iba K, Sonoda T, Takada J, Dohke T, Yamashita T. Further Significant Effects of Eldecalcitol on Bone Resorption Markers and Bone Mineral Density in Postmenopausal Osteoporosis Patients Having Undergone Long-Term Bisphosphonate Treatment. J Bone Miner Metab (2017) 35:171–6. doi: 10.1007/s00774-016-0738-y
Keywords: eldecalcitol, osteoporosis, randomized controlled trials (RCT), meta-analysis, vitamin D analog
Citation: Liu H, Wang G, Wu T, Mu Y and Gu W (2022) Efficacy and Safety of Eldecalcitol for Osteoporosis: A Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 13:854439. doi: 10.3389/fendo.2022.854439
Received: 17 January 2022; Accepted: 15 March 2022;
Published: 19 April 2022.
Edited by:
Thomas Baum, Technical University of Munich, GermanyReviewed by:
Michael Dieckmeyer, Technical University of Munich, GermanyJanina Maria Patsch, Medical University of Vienna, Austria
Copyright © 2022 Liu, Wang, Wu, Mu and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weijun Gu, guweijun301@163.com
†These authors have contributed equally to this work
 Hongyan Liu
Hongyan Liu Guoqi Wang
Guoqi Wang Ting Wu1
Ting Wu1 Weijun Gu
Weijun Gu