- 1Center for Reproductive Medicine, Henan Key Laboratory of Reproduction and Genetics, Henan Provincial Obstetrical and Gynecological Diseases (Reproductive Medicine) Clinical Research Center, Henan Engineering Laboratory of Preimplantation Genetic Diagnosis and Screening, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Department of Obstetrics, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Objective: This study aimed to evaluate potential predictors for recovery time in pregnant patients with moderate to severe ovarian hyperstimulation syndrome (OHSS).
Methods: A total of 424 pregnant patients with moderate to severe OHSS who underwent in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) were retrospectively identified. The clinical features and laboratory findings within 24 h after admission were collected. Treatment for OHSS was carried out according to standard procedures, including fluid replacement therapy, human albumin, aspirin, low-molecular-weight heparin, and paracentesis, when necessary. Patients were discharged from the hospital when the tmorning hematocrit was <40% and no obvious clinically relevant symptoms existed, such as abdominal distension, abdominal pain, and shortness of breath. Meanwhile, ultrasound indicating little pleural or abdominal effusion and biochemical abnormalities returning to normal were required. Spearman’s correlation analysis was used to assess the association between the blood-related parameters and recovery time. Multiple linear regression models were used to assess the relationship between the clinical or laboratory parameters and recovery time.
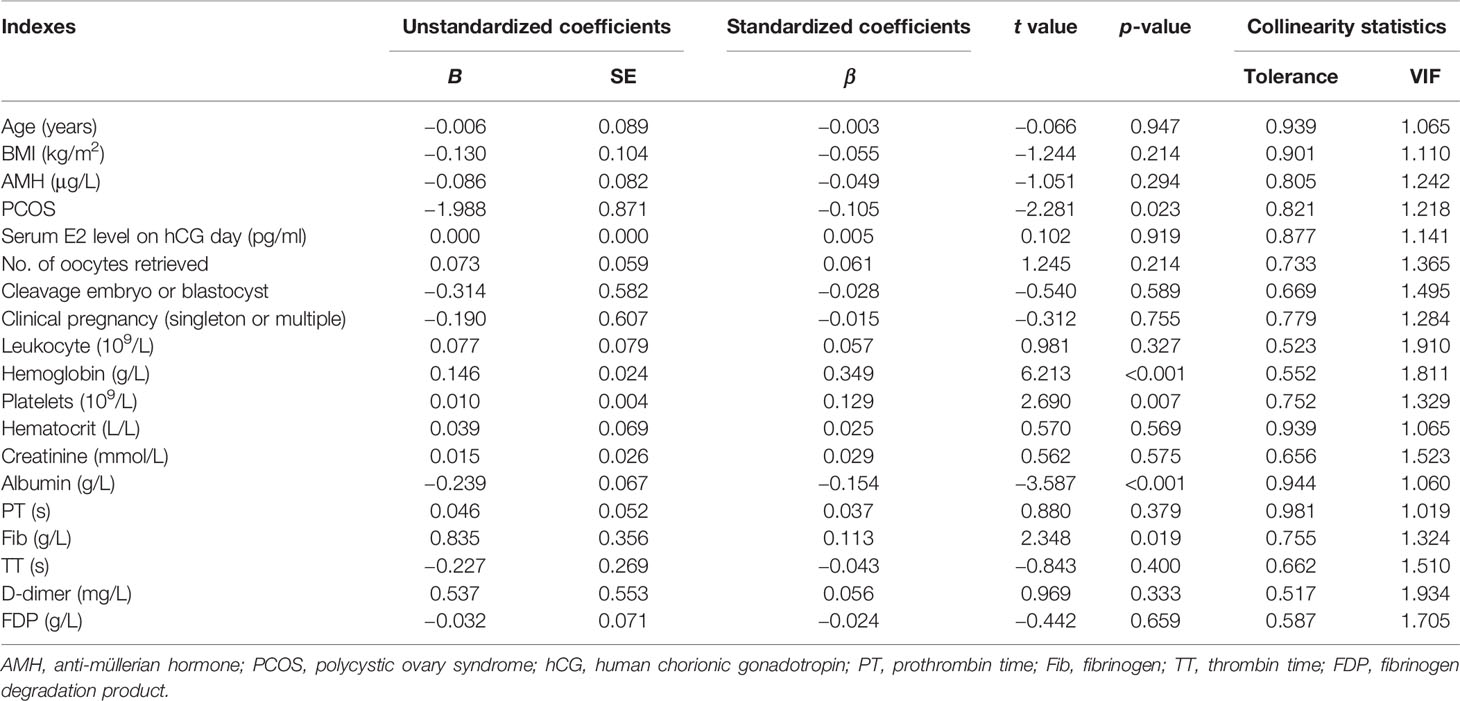
Results: The median recovery time of these patients was 11 days. In Spearman’s correlation test, leukocytes, hemoglobin, platelets, hematocrit, creatinine, prothrombin time (PT), fibrinogen (Fib), D-dimer, and fibrinogen degradation products (FDPs) were positively correlated with recovery time. On the other hand, albumin and thrombin time (TT) were negatively correlated with recovery time. Multiple linear regression analysis showed that polycystic ovary syndrome (PCOS), hemoglobin, platelets, albumin, and Fib were significantly associated with the recovery time of patients with OHSS (p = 0.023, p < 0.001, p = 0.007, p < 0.001, and p = 0.019, respectively).
Conclusions: In pregnant patients with OHSS, PCOS and hypoalbuminemia were associated with a significantly longer recovery time. Meanwhile, the recovery time was longer when patients have high levels of hemoglobin, platelets, and Fib.
Introduction
Ovarian hyperstimulation syndrome (OHSS) is a self-limiting disease classically encountered in patients who undergo controlled ovarian hyperstimulation (COH) cycles. The mild manifestations of OHSS include nausea, vomiting, abdominal distension, and shortness of breath. In severe cases, ascites and pleural fluid may occur, causing respiratory, circulatory, and coagulation dysfunction, and especially thrombosis may endanger a patient’s life (1).
Evidence from initial investigations indicates that some women are at increased risk of OHSS. The risk factors include young age (2, 3), body mass index (BMI) (4, 5), diagnosis of polycystic ovary syndrome (PCOS) (3, 6) high anti-müllerian hormone (AMH) levels (7, 8), large number and size of follicles in the ovary (8, 9), high serum estradiol (E2) concentrations (3, 9, 10) high number of retrieved oocytes (3, 9, 11), pregnancy following fresh embryo transfer (12), and a history of OHSS (13). The exact cause of OHSS is currently complex and remains subject to controversy. Latest research has demonstrated that OHSS is related to age, BMI, ovarian function, and the ovulation stimulation protocol.
Evidence has shown that OHSS occurs only after exposure to human chorionic gonadotropin (hCG), which has a significantly longer half-life than that of luteinizing hormone (LH) and a higher receptor affinity, thus causing extensive luteinization in the granulosa cells within the corpus luteum (14). This, in turn, leads to the production of vasoactive substances, including vascular endothelial growth factor (VEGF), renin–angiotensin system, interleukin 6, interleukin 1b, angiotensin II, insulin-like growth factor 1, and transforming growth factor b, of which VEGF is the most important in causing increased vascular permeability and hemoconcentration (15–17). VEGF stimulates endothelial cell mitogenesis and renders capillaries highly permeable to high-molecular-weight proteins (15). The pathophysiology of OHSS is characterized by arteriolar vasodilation and an increase in capillary permeability, leading to the leakage of fluid from the vascular compartment, with third space fluid accumulation and intravascular dehydration, causing intravascular volume depletion, hemoconcentration, hypoalbuminemia, electrolyte imbalance, and even thrombosis.
Whereas treatment for OHSS is largely supportive, prevention is crucial. Most current studies have been devoted to prevention and treatment strategies for OHSS, with relatively little attention paid to its clinical prognosis. This study provides clinicians with potential predictors of time to cure by describing some clinical features and laboratory findings in pregnant patients with OHSS.
Materials and Methods
Study Population
This retrospective study was performed in the Reproductive Medical Center of The First Affiliated Hospital of Zhengzhou University, Henan Province, China. Patients who underwent in vitro fertilization (IVF)/intracytoplasmic single sperm injection (ICSI) after assisted conception with late-onset moderate to severe OHSS between January 2018 and December 2020 were selected. The access and processing of patient data was approved by the ethics committee under a protocol for retrospective studies. The inclusion criteria were as follows: 1) diagnosis of OHSS according to the Golan criteria; 2) patients with IVF/ICSI-assisted pregnancy in the first cycle; 3) patients given a long-acting gonadotropin-releasing hormone (GnRH) agonist by subcutaneous injection; 4) patients with a positive pregnancy test; and 5) age <35 years. The exclusion criteria included: 1) women treated with antithrombotic drugs; 2) women with known coagulopathies; and 3) uncertain laboratory results and missing laboratory data. To analyze the relationship between the recovery time of patients with OHSS and the blood parameters such as leukocyte, hemoglobin, platelet, hematocrit, creatinine, total protein, albumin, prothrombin time (PT), activated partial thromboplastin time (APTT), thrombin time (TT), fibrinogen (Fib), D-dimer, and fibrinogen degradation products (FDPs) within 24 h after admission.
Treatment of OHSS usually involves fluid replacement to maintain intravascular perfusion and supportive care, such as low-molecular-weight dextrose and hydroxyethyl starch. The patient’s blood count, coagulation profile, electrolytes, creatinine, and albumin were observed. Depending on the patient’s condition, albumin was given intravenously, and anticoagulant drugs were given to patients with thrombotic tendency and hypercoagulable state to prevent thrombosis (18). Moreover, when the patient has large quantities of pleural and ascites, puncture and drainage were performed under ultrasound guidance. The details of the patients’ treatments are shown in Table 1.
A patient is clinically cured when the morning hematocrit was <40% and no obvious clinically relevant symptoms existed, such as abdominal distension, abdominal pain, and shortness of breath (19). On the other hand, ultrasound should indicate no pleural and abdominal effusion or a small amount of effusion, and the leukocyte count, creatinine, albumin, alanine transaminase (ALT), aspartate transaminase (AST), electrolytes, and other biochemical indicators should return to normal. The discharge criteria were considered the patients’ cure criteria.
Laboratory Variables
The patients underwent basic blood routine, liver and kidney function, blood coagulation function, D-dimer, FDP, and other tests 24 h after admission. The levels of these parameters were measured using the Roche HP800 automatic biochemical analyzer and Sysmex series automatic blood analyzer.
Controlled Ovarian Hyperstimulation Protocol
On the second to the third day of menstruation, the patients were given a long-acting GnRH agonist (Diphereline, 3.75 mg; Beaufour-Ipsen, Dreux, France) by subcutaneous injection. After 30 days, when the follicle-stimulating hormone (FSH) level was <5 IU/L, the LH level was <3 IU/L, and the antral follicle was nearly 5 mm in diameter, COH was initiated. We determined the individualized dosage of gonadotropin (Gn) (GONAL-f; Merck Serono, Darmstadt, Germany) according to the patient’s age, BMI, and ovarian reserve. The Gn dosage was maintained or adjusted according to the follicle growth and serum hormone levels during the course of the drug administration. When one dominant follicle was ≥20 mm in diameter and at least three dominant follicles were ≥17 mm in diameter, a trigger injection of hCG (recombinant hCG alpha for injection; Merck Serono) was administered on the same night. After 36–37 h of the trigger injection, we performed transvaginal oocyte retrieval; the luteal phase support was routinely given approximately 14 days after oocyte retrieval.
Two fresh cleavage embryos or one blastocyst was transferred on day 3 or 5 after egg retrieval. The transplant was cancelled if the patient was deemed at high risk of OHSS, the P level on the day of hCG was >3 ng/ml, or a uterine effusion was demonstrated.
Statistical Analysis
All statistical analyses were conducted using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, NY, USA). Descriptive variables were expressed as the mean and standard deviation (SD) if the data were normally distributed, as median and interquartile range (IQR) if the data were not normally distributed, or as frequency and percentage for nominal data. Spearman‘s correlation analysis was used to evaluate the associations between the variables of interest and the clinical outcomes. Multiple linear regression analysis was used when the outcomes were continuous variables. A bilateral p-value <0.05 was considered as significant.
Results
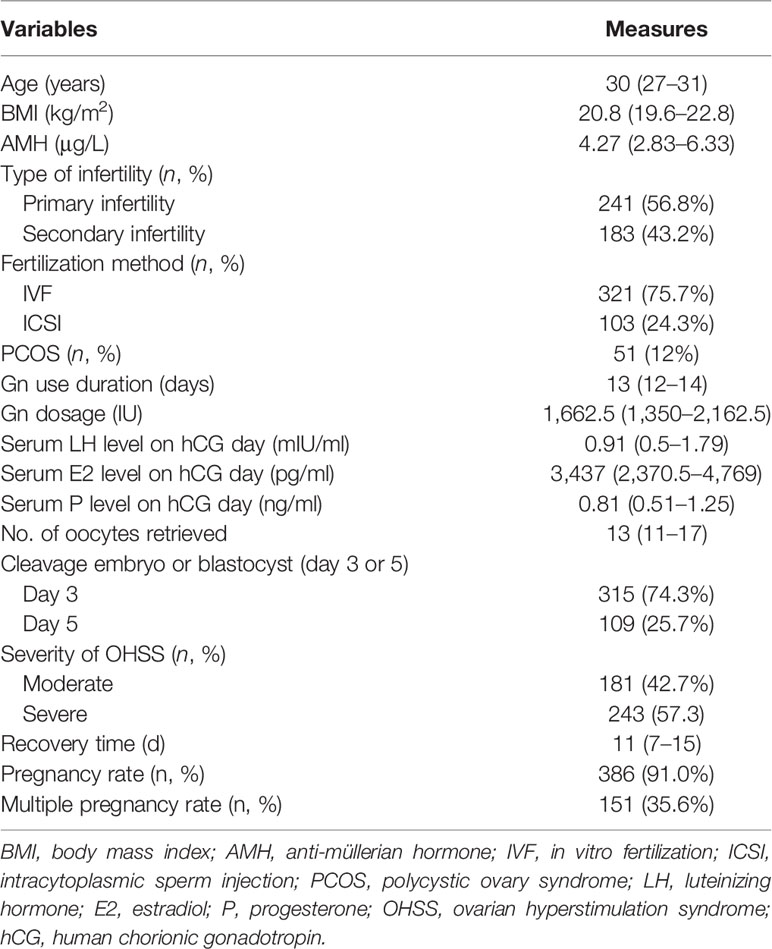
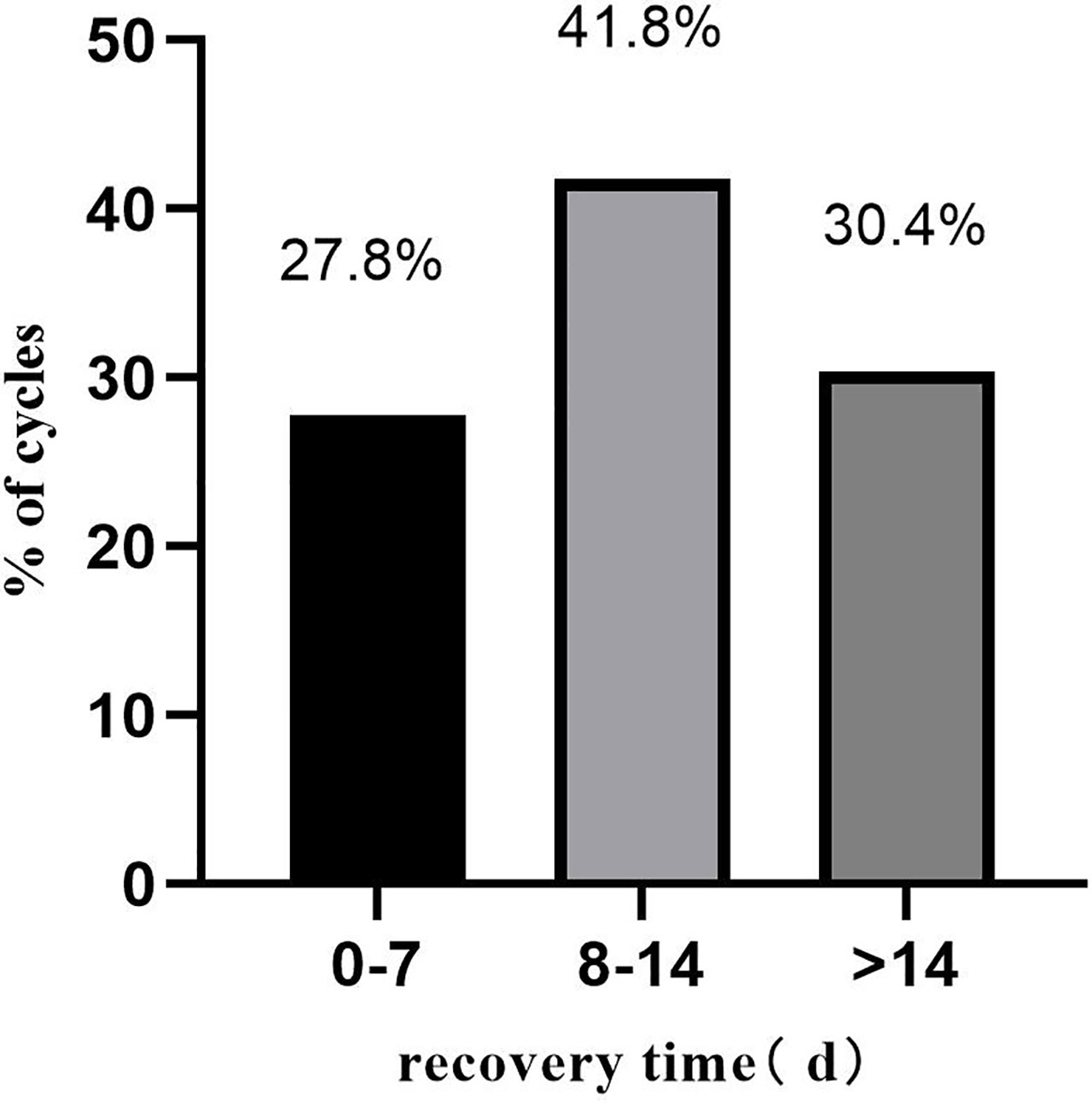
A total of 424 pregnant patients who developed moderate to severe OHSS after IVF/ICSI treatment were included in this study. Table 2 summarizes the basic information of these patients. The median recovery time of these patients was 11 days. A detailed distribution of the recovery times is shown in Figure 1.
The patients’ primary laboratory findings are shown in Table 3. Spearman‘s correlation coefficients were calculated between the patients’ recovery times and the laboratory indices examined on the day of their admission to the hospital. The levels of leukocytes, hemoglobin, platelets, hematocrit, creatinine, albumin, PT, Fib, TT, D-dimer, and FDPs were correlated with the time to healing. Leukocytes, hemoglobin, platelets, hematocrit, creatinine, PT, Fib, D-dimer, and FDP were positively correlated with recovery time. On the contrary, albumin and TT were negatively correlated with recovery time.
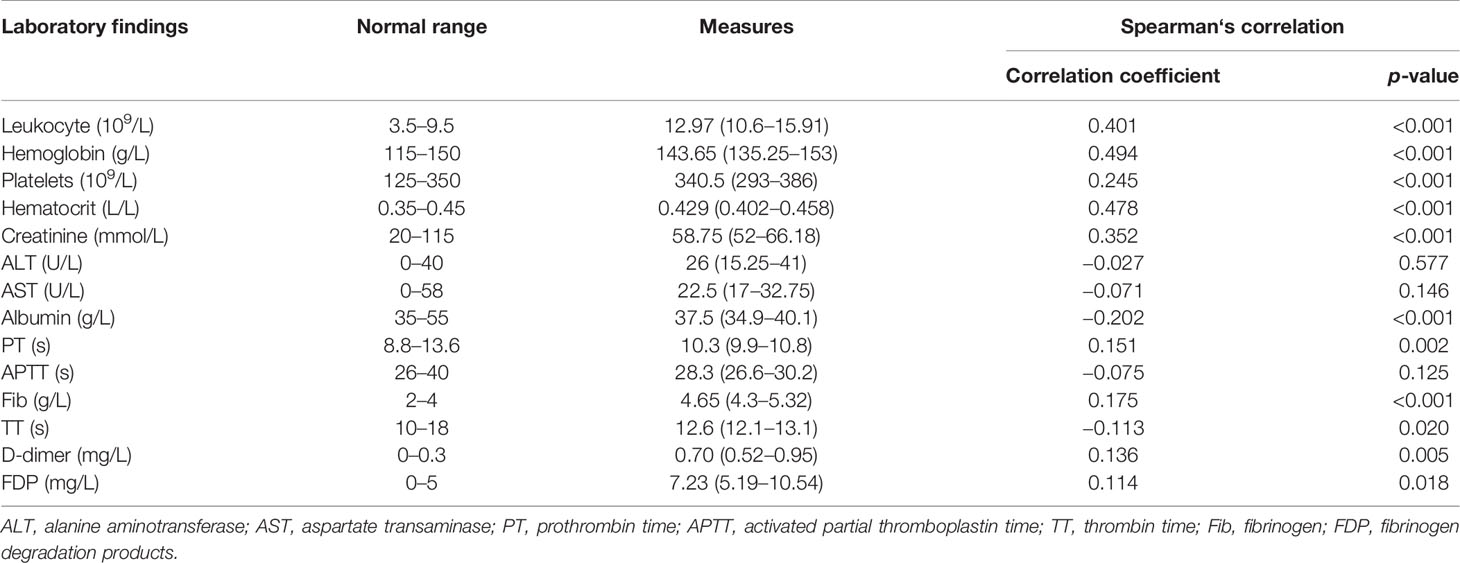
Table 3 Relationship between the laboratory findings and recovery time of patients with ovarian hyperstimulation syndrome (OHSS).
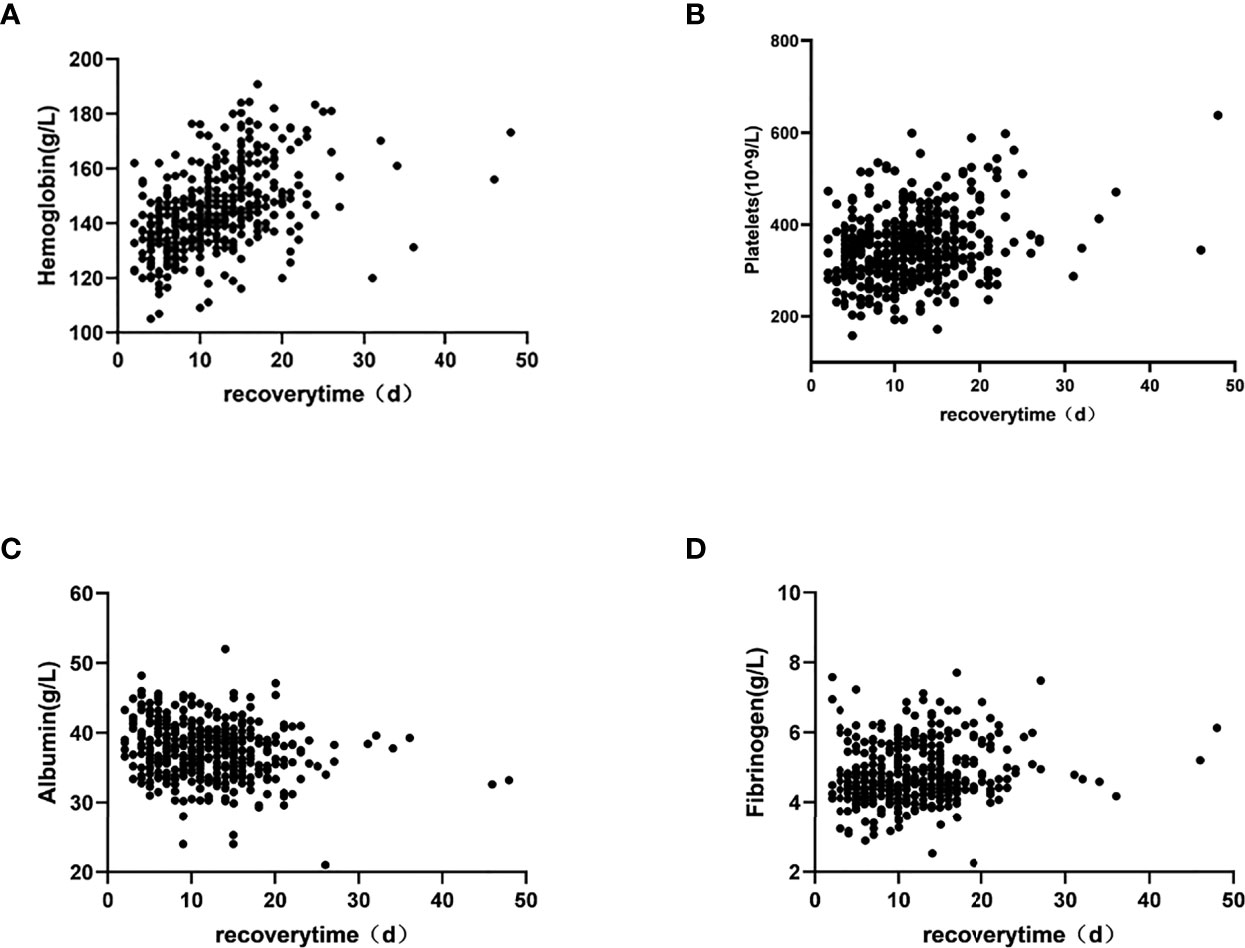
All data with p < 0.05 in the above-mentioned correlation analysis and the high-risk factors affecting OHSS reported in the literature were included in the multiple linear regression. The results showed that PCOS, hemoglobin, platelets, albumin, and Fib significantly influenced the patients’ recovery times (p = 0.023, p < 0.001, p = 0.007, p < 0.001, and p = 0.019, respectively) (Table 4). Moreover, scatter diagrams were used to clearly describe the relationship between the recovery time and the levels of hemoglobin, platelets, albumin, and Fib (Figure 2). The remaining indicators were not highly correlated with the recovery time of patients.
Discussion
OHSS is a major iatrogenic complication that arises during the process of assisted reproductive technology (ART), affecting 0.5%–2% of IVF cycles. There are two distinct types of OHSS: early-onset OHSS occurs in response to the hCG trigger within 7 days of ovulation, while late-onset OHSS is caused by the rising hCG hormone levels produced by the placenta in conception cycles (10). The present study focused on the recovery time of conceived women with OHSS requiring hospitalization.
To date, there is no universally accepted definition of OHSS recovery. The definition used in this research was based on a previous study (19). Patients were discharged from the hospital when the morning hematocrit was <40% and no obvious clinically relevant symptoms existed, such as abdominal distension, abdominal pain, and shortness of breath. On the other hand, ultrasound indicating little pleural or abdominal effusion and biochemical abnormalities returning to normal were required. The median recovery time in our study population was 11 days. This is much longer than that reported in the literature, partly due to the particular population and our strict release criteria.
Current studies have revealed that the incidence of OHSS was associated with numerous clinical and laboratory parameters. The concentration of E2 on the day of hCG >4,500 pg/ml and the number of oocytes retrieved >15 are commonly proposed to be risk factors for OHSS (3). However, our results showed that the median concentration of E2 on the day of hCG was 3,437 pg/ml and that the median number of oocytes retrieved was 13. This is consistent with the study of Wiser et al. because the freeze-all strategy was carried out in women at high risk of OHSS after retrieval of oocytes (20). In addition, the concentration of E2 on the day of hCG is not the main reason for the late-onset OHSS: pregnancy remains the main cause (21). Despite scholars having proposed multiple pregnancy as a predictive factor for recovery time from OHSS, our research found no differences between patients with singleton and multiple pregnancies.
As demonstrated in Table 4, the presence of PCOS was negatively correlated with the recovery time of pregnant OHSS patients, which is in accordance with the result of Nouri et al. (19). PCOS appeared to be the major predisposing factor for OHSS in a large number of studies (2). The explanation might be that PCOS cases are known to produce three times more follicles and oocytes than do normal ovulation patients when stimulated according to similar protocols (6). However, the reason for the prolonged recovery time of patients with PCOS remains unknown. VEGF is thought to be the major mediator of OHSS (22). An increased expression of VEGF mRNA in women with PCOS has been reported, and this may be responsible for the prolonged recovery time (23). Nevertheless, this is completely unproven. Further investigations are necessary to confirm this hypothesis. It is our suggestion that more stringent embryo transfer criteria should be administered or that patients with PCOS undergo whole embryo cryopreservation.
The second predictive factor for recovery time is serum albumin. Hypoalbuminemia is associated with a significantly longer recovery time. The mechanisms underlying the potential effect of serum albumin on OHSS are unknown. Some studies have suggested that the binding properties of albumin are beneficial in neutralizing vascular permeability mediators (24). Other studies have shown that serum albumin could maintain the intravascular volume in the event of capillary leakage, thus avoiding hypovolemia and hemoconcentration (25). Therefore, the level of serum albumin may reflect the severity of OHSS.
Studies have demonstrated that the levels of leukocytes, platelets, hematocrit, Fib, D-dimer, and FDP in OHSS patients were higher in routine laboratory tests (26). As shown in Table 3, this is consistent with our study. Moreover, in multiple linear regression analysis, hemoglobin, platelets, and Fib were positively correlated with the recovery time, and the difference was statistically significant. There are two reasons for the changes in these blood-related parameters: stress and hemoconcentration (27). OHSS is a potentially lethal disease, the pathophysiological hallmark of which is massive extravascular exudate accumulation combined with profound intravascular volume depletion and hemoconcentration (28). The degree of hemoconcentration seems to have the best correlation with the severity of OHSS (29). The levels of hemoglobin, platelets, and Fib are the earliest and most sensitive indicators of changes in the blood and are also ideal predictors for recovery time from OHSS.
It seems that an elevation in circulating estrogens during ovulation induction causes a shift in the hemostatic balance in the direction of a procoagulable state. Fib is a conventional coagulation indicator that indicates the activation of the coagulation system. Coagulation causes an increased Fib consumption and promotes Fib synthesis in the body, resulting in increased plasma Fib levels. Thrombosis is the most serious complication of OHSS, leading to dysfunction of coagulation and fibrinolysis in vivo. Thrombosis causes secondary hyperfibrinolysis; as plasma D-dimer and FDP are degradation products of fibrinogen, their levels will therefore increase rapidly. Therefore, monitoring the levels of plasma Fib, D-dimer, and FDP can prompt clinical correction of hypercoagulable blood concentration. Heparin ameliorates the risk of thrombotic complications associated with OHSS and has become the recommended treatment protocol (30). VEGF plays a leading role in increasing vascular permeability, and a 5-kDa heparin fragment can inhibit VEGF-A-mediated angiogenesis (31). Tissue factor (TF) is also important in angiogenesis because it enhances the expression of VEGF-A, and heparin reduces this by inhibiting the release of TF (32).
This is the first study to comprehensively assess the coagulation function and recovery time of conceived women with moderate to severe OHSS. As a result of OHSS-specific symptoms and the costs of the treatment program, decreased quality of life and economic losses are inevitable. Therefore, recovery time should be a clinically important parameter, and our study is of great value. Simultaneously, this study has some limitations. We conducted this retrospective study without considering all confounding factors. Only patients receiving a particular ovulation induction program were included in our study. Moreover, differences in the dietary habits of patients during hospitalization have a certain impact on the recovery time from OHSS.
In general, the existence of PCOS and the levels of hemoglobin, platelets, albumin, and Fib may contribute to the prognostic evaluation of OHSS. In consideration of the particular study population and limitations, large-scale, multicenter, prospective studies are necessary to confirm our results.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by The First Affiliated Hospital of Zhengzhou University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
KH and JZ designed the research and guided the writing. YS collected and analyzed the data. KH and YS drafted the manuscript. G-ZC helped to collect and analyze data. HS contributed to the data analysis. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (nos. 81701448 and 82071649).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the medical staff and patients in The First Affiliated Hospital of Zhengzhou University for recording the data and cooperating with the treatment.
References
1. ASRM. Prevention and Treatment of Moderate and Severe Ovarian Hyperstimulation Syndrome: A Guideline. Fertil Steril (2016) 106(7):1634–47. doi: 10.1016/j.fertnstert.2016.08.048
2. Luke B, Brown MB, Morbeck DE, Hudson SB. Factors Associated With Ovarian Hyperstimulation Syndrome (OHSS) and its Effect on Assisted Reproductive Technology (ART) Treatment and Outcome. Fertil Steril (2010) 94(4):1399–404. doi: 10.1016/j.fertnstert.2009.05.092
3. Sousa M, Cunha M, Teixeira da Silva J, Oliveira C, Silva J, Viana P, et al. Ovarian Hyperstimulation Syndrome: A Clinical Report on 4894 Consecutive ART Treatment Cycles. Reprod Biol Endocrinol (2015) 13:66. doi: 10.1186/s12958-015-0067-3
4. Fauser BC, Diedrich K, Devroey P. Evian Annual Reproduction Workshop Group 2007. Predictors of Ovarian Response: Progress Towards Individualized Treatment in Ovulation Induction and Ovarian Stimulation. Hum Reprod Update (2008) 14(1):1–14. doi: 10.1093/humupd/dmm034
5. Courbiere B, Oborski V, Braunstein D, Desparoir A, Noizet A, Gamerre M. Obstetric Outcome of Women With In Vitro Fertilization Pregnancies Hospitalized for Ovarian Hyperstimulation Syndrome: A Case-Control Study. Fertil Steril (2011) 95(5):1629–32. doi: 10.1016/j.fertnstert.2010.12.015
6. Delvigne A, Rozenberg S. Epidemiology and Prevention of Ovarian Hyperstimulation Syndrome (OHSS): A Review. Hum Reprod Update (2002) 8(6):559–77. doi: 10.1093/humupd/8.6.559
7. Nakhuda GS, Chu MC, Wang JG, Sauer MV, Lobo RA. Elevated Serum Müllerian-Inhibiting Substance may be a Marker for Ovarian Hyperstimulation Syndrome in Normal Women Undergoing In Vitro Fertilization. Fertil steril (2006) 85(5):1541–3. doi: 10.1016/j.fertnstert.2005.10.052
8. Ocal P, Sahmay S, Cetin M, Irez T, Guralp O, Cepni I. Serum Anti-Mullerian Hormone and Antral Follicle Count as Predictive Markers of OHSS in ART Cycles. J Assist Reprod Genet (2011) 28(12):1197–203. doi: 10.1007/s10815-011-9627-4
9. Ashrafi M, Bahmanabadi A, Akhond MR, Arabipoor A. Predictive Factors of Early Moderate/Severe Ovarian Hyperstimulation Syndrome in non-Polycystic Ovarian Syndrome Patients: A Statistical Model. Arch Gynecol Obstet (2015) 292(5):1145–52. doi: 10.1007/s00404-015-3723-0
10. Mathur RS, Akande AV, Keay SD, Hunt LP, Jenkins JM. Distinction Between Early and Late Ovarian Hyperstimulation Syndrome. Fertil Steril (2000) 73(5):901–7. doi: 10.1016/s0015-0282(00)00492-1
11. Steward RG, Lan L, Shah AA, Yeh JS, Price TM, Goldfarb JM, et al. Oocyte Number as a Predictor for Ovarian Hyperstimulation Syndrome and Live Birth: An Analysis of 256,381 In Vitro Fertilization Cycles. Fertil Steril (2014) 101(4):967–73. doi: 10.1016/j.fertnstert.2013.12.026
12. Enskog A, Henriksson M, Unander M, Nilsson L, Brannstrom M. Prospective Study of the Clinical and Laboratory Parameters of Patients in Whom Ovarian Hyperstimulation Syndrome Developed During Controlled Ovarian Hyperstimulation for In Vitro Fertilization. Fertil Steril (1999) 71(5):808–14. doi: 10.1016/s0015-0282(99)00090-4
13. Delvigne A, Dubois M, Battheu B, Bassil S, Meuleman C, De Sutter P, et al. The Ovarian Hyperstimulation Syndrome in in-Vitro Fertilization: A Belgian Multicentric Study. II. Multiple Discriminant Analysis for Risk Prediction. Hum Reprod (1993) 8(9):1361–6. doi: 10.1093/oxfordjournals.humrep.a138261
14. Aboulghar MA, Mansour RT. Ovarian Hyperstimulation Syndrome: Classifications and Critical Analysis of Preventive Measures. Hum Reprod Update (2003) 9(3):275–89. doi: 10.1093/humupd/dmg018
15. McClure N, Healy DL, Rogers PA, Sullivan J, Beaton L, Haning RV Jr, et al. Vascular Endothelial Growth Factor as Capillary Permeability Agent in Ovarian Hyperstimulation Syndrome. Lancet (1994) 344(8917):235–6. doi: 10.1016/s0140-6736(94)93001-5
16. Soares SR, Gómez R, Simón C, García-Velasco JA, Pellicer A. Targeting the Vascular Endothelial Growth Factor System to Prevent Ovarian Hyperstimulation Syndrome. Hum Reprod Update (2008) 14(4):321–33. doi: 10.1093/humupd/dmn008
17. Geva E, Jaffe RB. Role of Vascular Endothelial Growth Factor in Ovarian Physiology and Pathology. Fertil Steril (2000) 74(3):429–38. doi: 10.1016/s0015-0282(00)00670-1
18. Choux C, Barberet J, Ginod P, Cottenet J, Bruno C, Benzénine E, et al. Severe Ovarian Hyperstimulation Syndrome Modifies Early Maternal Serum Beta-Human Chorionic Gonadotropin Kinetics, But Obstetrical and Neonatal Outcomes are Not Impacted. Fertil Steril (2017) 108(4):650–8. doi: 10.1016/j.fertnstert.2017.07.1160
19. Nouri K, Tempfer CB, Lenart C, Windischbauer L, Walch K, Promberger R, et al. Predictive Factors for Recovery Time in Patients Suffering From Severe OHSS. Reprod Biol Endocrinol (2014) 5(12):59. doi: 10.1186/1477-7827-12-59
20. Wiser A, Levron J, Kreizer D, Achiron R, Shrim A, Schiff E, et al. Outcome of Pregnancies Complicated by Severe Ovarian Hyperstimulation Syndrome (OHSS): A Follow-Up Beyond the Second Trimester. Hum Reprod (2005) 20(4):910–4. doi: 10.1093/humrep/deh713
21. Salama KM, Abo Ragab HM, El Sherbiny MF, Morsi AA, Souidan II. Sequential E2 Levels Not Ovarian Maximal Diameter Estimates Were Correlated With Outcome of Cetrotide Therapy for Management of Women at High-Risk of Ovarian Hyperstimulation Syndrome: A Randomized Controlled Study. BMC Womens Health (2017) 17(1):108. doi: 10.1186/s12905-017-0466-z
22. Fiedler K, Ezcurra D. Predicting and Preventing Ovarian Hyperstimulation Syndrome (OHSS): The Need for Individualized Not Standardized Treatment. Reprod Biol Endocrinol (2012) 10(1):32. doi: 10.1186/1477-7827-10-32
23. Kamat BR, Brown LF, Manseau EJ, Senger DR, Dvorak HF. Expression of Vascular Permeability Factor/Vascular Endothelial Growth Factor by Human Granulosa and Theca Lutein Cells. Role in Corpus Luteum Development. Am J Pathol (1995) 146(1):157–65. doi: 10.1097/00002093-199509020-00009
24. Youssef MA, Al-Inany HG, Evers JL, Aboulghar M. Intra-Venous Fluids for the Prevention of Severe Ovarian Hyperstimulation Syndrome. Cochrane Database Syst Rev (2011) 2(2):CD001302. doi: 10.1002/14651858.CD001302.pub2
25. Nastri CO, Teixeira DM, Moroni RM, Leitão VM, Martins WP. Ovarian Hyperstimulation Syndrome: Pathophysiology, Staging, Prediction and Prevention. Ultrasound Obstet Gynecol (2015) 45(4):377–93. doi: 10.1002/uog.14684
26. Levin I, Gamzu R, Hasson Y, Lessing JB, Amit A, Shapira I, et al. Increased Erythrocyte Aggregation in Ovarian Hyperstimulation Syndrome: A Possible Contributing Factor in the Pathophysiology of This Disease. Hum Reprod (2004) 19(5):1076–80. doi: 10.1093/humrep/deh210
27. Navot D, Bergh PA, Laufer N. Ovarian Hyperstimulation Syndrome in Novel Reproductive Technologies: Prevention and Treatment. Fertil Steril (1992) 58(2):249–61. doi: 10.1016/s0015-0282(16)55188-7
28. Schenker JG, Weinstein D. Ovarian Hyperstimulation Syndrome: A Current Survey. Fertil Steril (1978) 30(3):255–68. doi: 10.1016/s0015-0282(16)43508-9
29. Borenstein R, Elhalah U, Lunenfeld B, Schwartz ZS. Severe Ovarian Hyperstimulation Syndrome: A Reevaluated Therapeutic Approach. Fertil Steril (1989) 51(5):791–5. doi: 10.1016/s0015-0282(16)60668-4
30. Dourron NE, Williams DB. Prevention and Treatment of Ovarian Hyperstimulation Syndrome. Semin Reprod Endocrinol (1996) 14(4):355–65. doi: 10.1055/s-2008-1067980
31. Norrby K. 2.5 kDa and 5.0 kDa Heparin Fragments Specifically Inhibit Microvessel Sprouting and Network Formation in VEGF165-Mediated Mammalian Angiogenesis. Int J Exp Pathol (2000) 81(3):191–8. doi: 10.1046/j.1365-2613.2000.00150.x
Keywords: ovarian hyperstimulation syndrome (OHSS), clinical features, recovery time, potential predictors, coagulation function
Citation: Huang K, Shi Y, Chen G, Shi H and Zhai J (2022) Predictive Factors for Recovery Time in Conceived Women Suffering From Moderate to Severe Ovarian Hyperstimulation Syndrome. Front. Endocrinol. 13:870008. doi: 10.3389/fendo.2022.870008
Received: 05 February 2022; Accepted: 11 April 2022;
Published: 15 June 2022.
Edited by:
Richard Ivell, University of Nottingham, United KingdomCopyright © 2022 Huang, Shi, Chen, Shi and Zhai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Zhai, bestzhai2005@163.com
†These authors have contributed equally to this work and share first authorship
 Kai Huang
Kai Huang Ying Shi
Ying Shi Gezi Chen2
Gezi Chen2 Hao Shi
Hao Shi Jun Zhai
Jun Zhai