- 1Department of Neurology, First Hospital of Shanxi Medical University, Taiyuan, Shanxi, China
- 2Clinical College, Shanxi Medical University, Taiyuan, Shanxi, China
- 3Department of Cardiology, Second Hospital of Shanxi Medical University, Taiyuan, Shanxi, China
- 4Department of Neurology, The Bethune Hospital of Shanxi Province, Taiyuan, Shanxi, China
- 5Department of Neurology, Sixth Hospital of Shanxi Medical University (General Hospital of Tisco), Taiyuan, Shanxi, China
Background: Stress hyperglycemia has served as a reliable biomarker to predict poor outcomes after ischemic stroke. However, recent studies have reported some contrary conclusions. Different stroke subtypes may respond inconsistently to stress hyperglycemia. The progression of intracranial atherosclerotic stenosis (ICAS) is tightly related to hyperglycemia. Thus, this study aims to determine the relationship between stress hyperglycemia and recurrent stroke in ischemic stroke patients with or without intracranial atherosclerotic stenosis.
Methods: This is a multicenter retrospective observational cohort study. Patients with acute minor ischemic stroke and eligible computed tomography and magnetic resonance imaging data were enrolled. The severity of stress hyperglycemia is measured by the stress hyperglycemia ratio (SHR). SHR was calculated based on fasting plasma glucose (FPG) and hemoglobin A1c (HbA1c) levels. The primary outcome was stroke recurrence during hospitalization. The interaction of SHR levels with the presence of ICAS on the primary outcome was investigated using univariable and multivariable Cox proportional hazards models. Restricted cubic splines were applied to determine the nonlinear relationship between SHR and primary outcome. A two-piecewise linear regression model was used to identify the threshold of SHR.
Results: A total of 610 participants were included in the study. The average age of the patients was 61.4 ± 12.9 years old, and approximately 70% of participants were males. A total of 189 (30.98%) patients had ICAS. The patients were categorized into 3 groups based on the tertiles of SHR. Compared with the group with a lower SHR, a higher SHR was significantly associated with the risk of stroke recurrence in the ICAS group (hazard ratio [HR], 8.52, 95% confidence interval [CI], 3.16-22.96, P<0.001). When SHR was treated as a continuous variable, each 0.1-unit increase in SHR in the ICAS group was associated with a 1.63-fold increase in the risk of recurrence (HR, 1.63, 95% CI, 1.39-1.9, P<0.001) with a threshold of 0.75. FPG but not HbA1c was associated with stroke recurrence in ICAS patients (HR, 1.17, 95% CI, 1.08-1.26, P<0.001). Sensitive analyses showed consistent results after adjusting for previous diabetes mellitus, oral hypoglycemic agents and insulin injection.
Conclusions: SHR represents a better biomarker to predict the risk of stroke recurrence in patients with ICAS than FPG and HbA1c regardless of previous diabetes mellitus.
Trial registration: https://www.chictr.org.cn/showproj.aspx?proj=125817; Identifier, [ChiCTR2100046958].
1 Introduction
Ischemic stroke is a leading cause of death and long-term disability worldwide (1), and the economic costs of treatment and poststroke care are substantial. Intracranial atherosclerotic stenosis (ICAS) of a major intracranial artery is one of the most common causes of minor stroke worldwide and is more prevalent in Asian, black and Hispanic patients. In contrast, extracranial carotid atherosclerotic stenosis (ECAS) is more common in white patients (2). ICAS is associated with a high risk of recurrent stroke compared with other stroke subtypes (3) because arterial stenosis makes blood flow more turbulent and can induce rupture of atherosclerotic plaques or thrombus formation, causing subsequent embolism (4). The Chinese Intracranial Atherosclerosis (CICAS) study showed that ICAS is present in 46.6% of stroke patients in China and confirmed that ICAS is an independent risk factor for stroke recurrence (2). Secondary analysis of two large randomized controlled trials, Warfarin–Aspirin Symptomatic Intracranial Disease (WASID) and the Stenting and Aggressive Medical Management for Preventing Recurrent stroke in Intracranial Stenosis (SAMMPRIS) study, illustrated that symptomatic intracranial stenosis > 70% significantly increased the risk of stroke recurrence (5, 6). This conclusion has also been confirmed in minor stroke or transient ischemic attack (TIA) patients characterized by a higher rate of recurrence and early neurologic deterioration during hospitalization (7).
Previous studies have shown that stress hyperglycemia predicts increased risks of in-hospital mortality and poor functional recovery after ischemic stroke (8, 9). Stress hyperglycemia is defined as hyperglycemia occurring in the acute phase of critical disease, such as stroke, myocardial infarction and hemorrhagic shock. Moreover, stress hyperglycemia occurs regardless of preexisting diabetes mellitus. In contrast to chronic hyperglycemia, stress hyperglycemia reflects the inflammatory and neurohormonal dysregulations that occur during a major illness and is strongly associated with adverse outcomes (10). The direct impact of stress hyperglycemia on endothelial cells, which is characterized by increased the activation of prothrombotic factors, such as fibrinopeptide A and factor VII, and decreased plasma fibrinolytic activity, represents a plausible mechanism (11–13). Moreover, evidence that lowering glucose with insulin reduces ischemic brain damage in animal models of stroke suggests that stress-induced hyperglycemia may be a risk factor for brain damage (8). Previous studies have shown that chronic hyperglycemia is one of the most important contributors to ICAS (14). Hyperglycemia arises in 30–40% of people with acute ischemic stroke (15). In the acute phase of ischemic stroke caused by ICAS, the narrowed vessel on the infarcted side is more vulnerable to hemodynamic fluctuation and elevated blood viscosity induced by stress hyperglycemia, which may lead to an increased risk of stroke recurrence. There have been several parameters, such as fasting glucose, glycemic gap and stress hyperglycemia ratio (SHR) to measure the extent of stress hyperglycemia, among which SHR provided the best prognostic insight at admission to assess the relationship between stress hyperglycemia and ischemic stroke outcome (16). SHR was calculated based on hemoglobin A1c (HbA1c) and fasting plasma glucose (FPG). HbA1c was used to estimate the average blood glucose before stroke onset, based on the equation derived by Nathan et al. as follows: Estimated average glucose = [1.59 * HbA1c (%)] – 2.59. SHR was then calculated as FPG divided by the estimated average glucose. Several clinical studies have confirmed the relationship between stress hyperglycemia and stroke recurrence, especially for minor ischemic stroke patients (The National Institutes of Health Stroke Scale [NIHSS] ≤5) (17, 18). However, the interaction or combined effects of stress hyperglycemia and ICAS on patients with acute minor stroke remain uncertain.
Thus, in this study, we assessed the association between stress hyperglycemia level and recurrence risk in acute ischemic minor stroke patients with or without ICAS.
2 Materials and methods
2.1 Study design
This study is a multicenter retrospective observational cohort study based on real-world research conducted at three advanced stroke centers in Shanxi Province (First Hospital of Shanxi Medical University, Bethune Hospital of Shanxi Province, Sixth Hospital of Shanxi Medical University (General Hospital of Tisco). The study included patients with acute minor ischemic stroke (NIHSS score ≤ 5) treated with antiplatelet therapy within 72 h of symptom onset. Diagnosis of the index stroke was confirmed by the attending neurologist according to the World Health Organization definition and was based on medical history, clinical presentation, and findings in neuroimaging (computed tomography or magnetic resonance imaging). We retrieved the electronic medical records of all patients hospitalized for minor ischemic stroke during four months each year (March, June, September, December) from 2010 to 2018.
2.2 Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) patients with ischemic stroke within 72 hours of onset; (2) NIHSS score ≤ 5 at onset or NIHSS score > 5 at onset, but symptoms were relieved before antiplatelet therapy; (3) three-dimensional time-of-flight magnetic resonance angiography (MRA) or computed tomography angiography (CTA) was performed on admission. The exclusion criteria were as follows: (1) modified Rankin score (MRS) > 2, (2) TIA, (3) recombinant tissue plasminogen activator (rt-PA) treatment, (4) mechanical thrombectomy or stent-assisted angioplasty, (5) participation in other clinical trials, and (6) cardiogenic stroke. Cardiogenic stroke is defined according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria as arterial occlusions presumably due to single or multiple emboli from the heart. At least one cardiac source for the occlusion, such as atrial fibrillation, mechanical prosthetic valve or left ventricular thrombus, must be identified for the diagnosis of cardioembolic stroke. Potential large-artery atherosclerotic sources and any other causes of stroke do not support the above diagnosis (19). Patients who are diagnosed with bleeding or other pathological diseases, such as vascular malformations, tumors, abscesses or other major nonischemic brain diseases (such as multiple sclerosis), on baseline head computed tomography (CT) or magnetic resonance imaging (MRI) are not eligible for the study. Glucose and glycosylated hemoglobin measurements that were not recorded in the hospital were also excluded.
2.3 Baseline data collection
Baseline data included demographics, stroke features, and vascular risk factors. All the data were directly obtained from a prespecified anonymous case report form (CRF) completed by trained physicians and nurses after searching every hospital’s electronic medical record system. Demographics included age, sex, body mass index, systolic pressure, and diastolic pressure. Stroke features included hours from symptom onset to admission, stroke subtype, classified according to TOAST criteria, and baseline NIHSS score. Vascular risk factors, such as hypertension (HTN), history of stroke, TIA, myocardial infarction (MI), coronary atherosclerotic heart disease (CHD), peripheral arterial disease (PAD), diabetes mellitus, hyperlipidemia, history of cerebral hemorrhage and smoking, were also collected. Smoking was defined as current smokers who had smoked more than 100 cigarettes in their lifetime and had smoked in the 30 days preceding the stroke. Laboratory findings, including homocysteine (HCY), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides (TGs), total cholesterol (TC), hemoglobin A1c (HbA1c), and fasting plasma glucose (FPG), were collected. Medication information included data on antiplatelet medication at admission, statin therapy, insulin injection and oral hypoglycemic agents. All patients received antiplatelet and statin medication within 72 hours of stroke onset.
2.4 Assessment of stress hyperglycemia
All patients had venous blood drawn within the first 24 hours after admission. FPG and HbA1c were then measured. HbA1c was used to estimate the average blood glucose prior to admission based on the equation derived by Nathan et al. as follows: Estimated average glucose = [1.59 * HbA1c (%)] – 2.59 (20). SHR was then calculated as FPG (mmol/L) divided by the estimated average glucose. The patients were then categorized into 3 groups based on tertiles of SHR (low, middle and high) for further comparisons.
2.5 Imaging collection and analysis
All eligible CT and MRI scans of individual patients were collected from centers participating in the imaging subgroup analysis. For MRI, T1-weighted imaging, T2-weighted imaging, diffusion-weighted imaging, and 3D time-of-flight MRA were needed for the analysis. ICAS was determined by the presence of 50%–99% stenosis according to Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) trial criteria or occlusion of at least one of the following arterial segments: intracranial internal carotid artery, A1 or A2 of anterior cerebral artery, M1 or M2 of middle cerebral artery, P1 or P2 of posterior cerebral artery, posterior inferior cerebellar artery, vertebral artery, and basilar artery (21). If MRI is not available, CT angiography can be used instead to assess the degree of cerebral vascular stenosis. For any potential ICAS lesion, disagreements regarding the degree of stenosis were resolved by consulting with a third experienced physician. We retrospectively collected all data described above from the hospital’s electronic medical record system. The study subjects were divided into two groups according to the presence of ICAS.
2.6 Study outcome
The primary outcome was stroke recurrence, including new ischemic stroke, hemorrhagic stroke, and TIA, during hospitalization. Due to the long inclusion span, most patients lack follow-up information after discharge. Thus, this paper only studied the in-hospital recurrence. Recurrence of ischemic stroke was defined as acute focal cerebral or retinal infarction as follows: an increase in the NIHSS score of four or more, new infarction or enlargement of the original focus on MRI or CT. Hemorrhagic stroke was defined as acute infiltration of blood into the brain parenchyma or subarachnoid space with associated neurological symptoms and imaging findings (22). TIA was defined as a transient episode of neurological dysfunction without acute cerebral infarction, as described previously (23).
2.7 Statistical analysis
Categorical variables are presented as frequencies and percentages, and continuous variables are presented as the means (standard deviations) or medians (interquartile ranges). Intergroup differences for categorical variables were analyzed using Pearson’s chi-squared test or Fisher’s exact test, and continuous variables were analyzed using Student’s t test, Analysis of Variance (ANOVA), Kruskal−Wallis test or the Wilcoxon rank-sum test, as appropriate. For the chi-squared test, at least 80% of the cells in the contingency table must have more than 5 counts. Otherwise, the test is unreliable, and Fisher’s exact test was employed. Between the included and excluded groups, Student’s t test was applied when the distributions of the two independent samples were normal; otherwise, the Wilcoxon rank-sum test was used. To compare continuous variables among different tertiles of SHR, ANOVA (parametric) or the Kruskal−Wallis (nonparametric) test were used. The interaction of SHR levels with ICAS on the study outcome was investigated using crude and multivariable Cox proportional hazards models. Confounders were identified based on univariable analysis and previous reports. Model I was adjusted for age and sex. Model II was adjusted for age, sex, smoking, NIHSS, stroke history, antiplatelet therapy. Model III was adjusted for age, sex, systolic pressure, diastolic pressure, smoking, body mass index (BMI), NIHSS, history of stroke, hyperlipoidemia, antiplatelet medication at admission and statin therapy. Models IV and V further adjusted for diabetes mellitus and hypoglycemic treatment as sensitivity analyses. Subgroup and interaction analyses according to history of diabetes mellitus were performed. The nonlinear relationship between SHR levels and recurrent stroke was assessed by restricted cubic splines, and a two-piecewise linear regression model was applied to identify the threshold of the smoothing curve. All tests were two-sided, and a P value of 0.05 was considered to indicate statistical significance. A Kaplan−Meier curve was drawn and compared by the log-rank test. To maximize statistical power and minimize bias that might occur, we used multiple imputation with chained equations to impute missing values. We repeated all analyses with the complete data cohort for comparison. The results were consistent with the present analysis. A post hoc power analysis was performed to see the sampling variation, by simulating the power of set parameters of regression and interaction analysis. Statistical analysis was performed using the statistical software package R (http://www.R-project.org, The R Foundation).
3 Results
3.1 Clinical characteristics among SHR groups
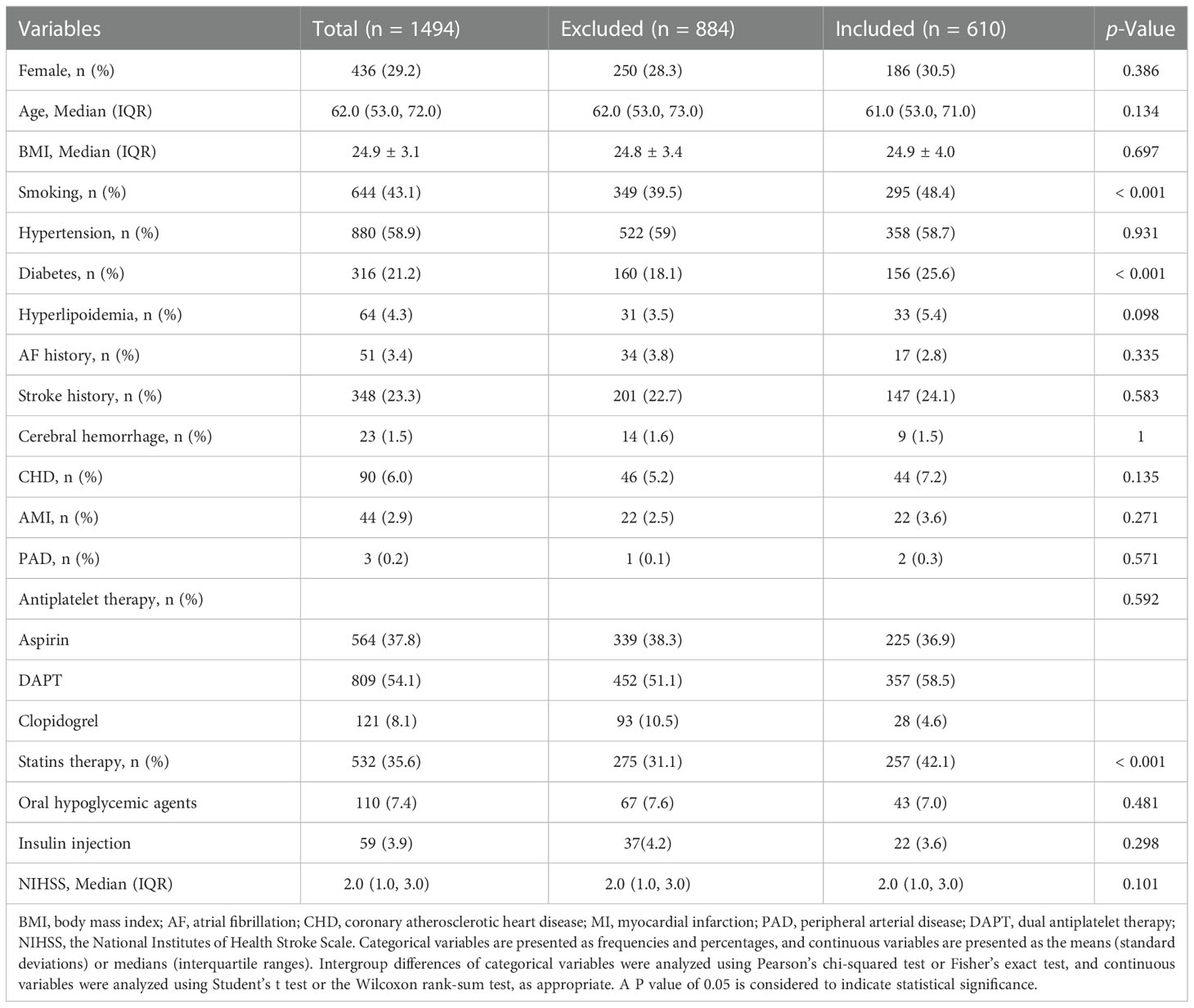
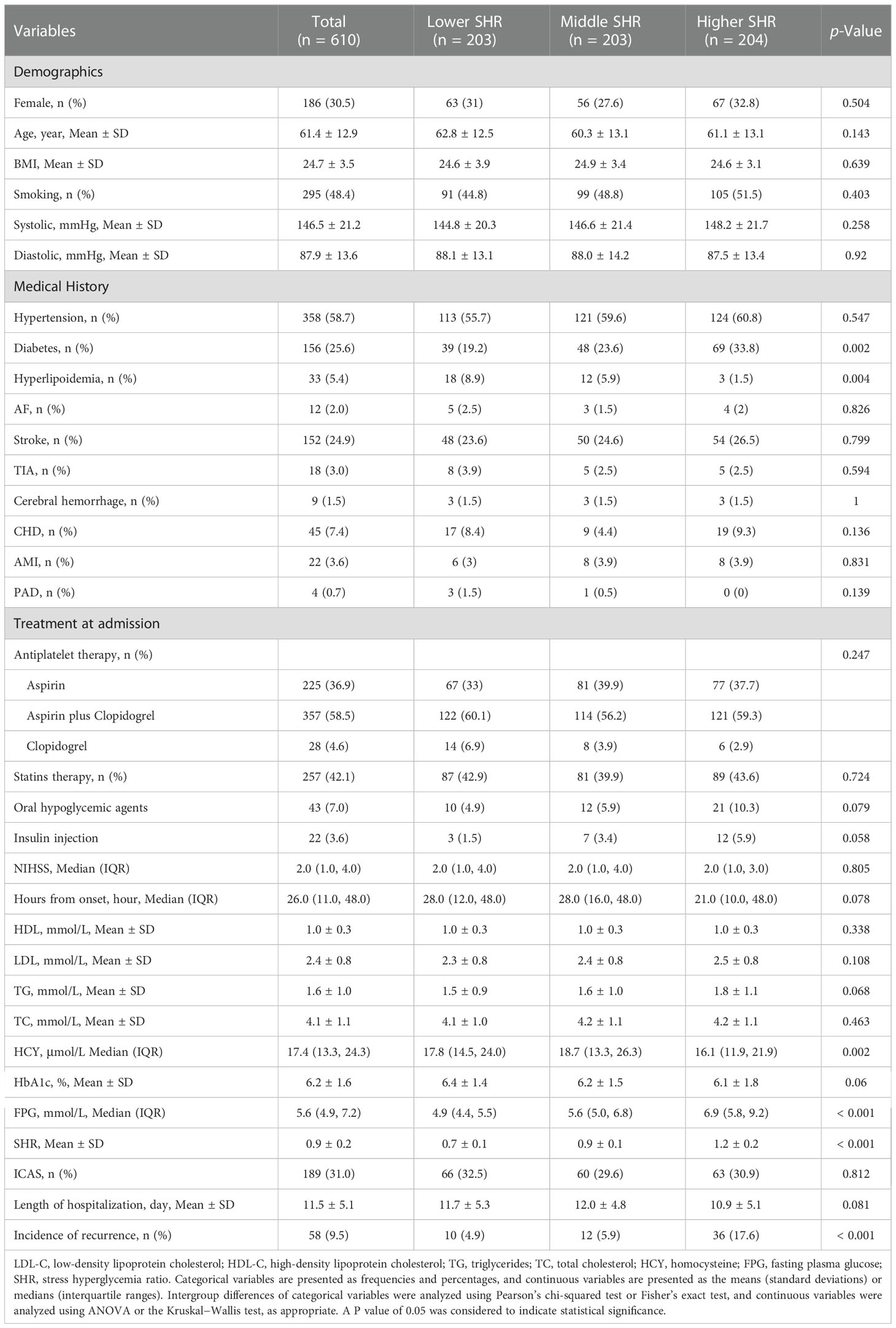
A total of 610 patients were included in the study, and the specific flowchart is shown in Figure 1. The clinical characteristics of the patients included and those excluded were well balanced except for a higher proportion of smoking, diabetes mellitus, and statin use in the included group (Table 1). The baseline characteristics of all the participants are shown in Table 2. The mean age was 61.4 ± 12.9 years, and approximately 70% were male. The study included 189 (30.89%) patients with ICAS. Patients with higher SHR were more likely to have previous diabetes mellitus, higher FPG, and lower HCY but less likely to have hyperlipoidemia. No significant difference in the incidence of ICAS was noted among the different SHR groups. During in-hospital follow-up, a total of 58 (9.5%) patients experienced stroke recurrence. Patients in the higher SHR group endured more recurrence than those in the lower SHR group.
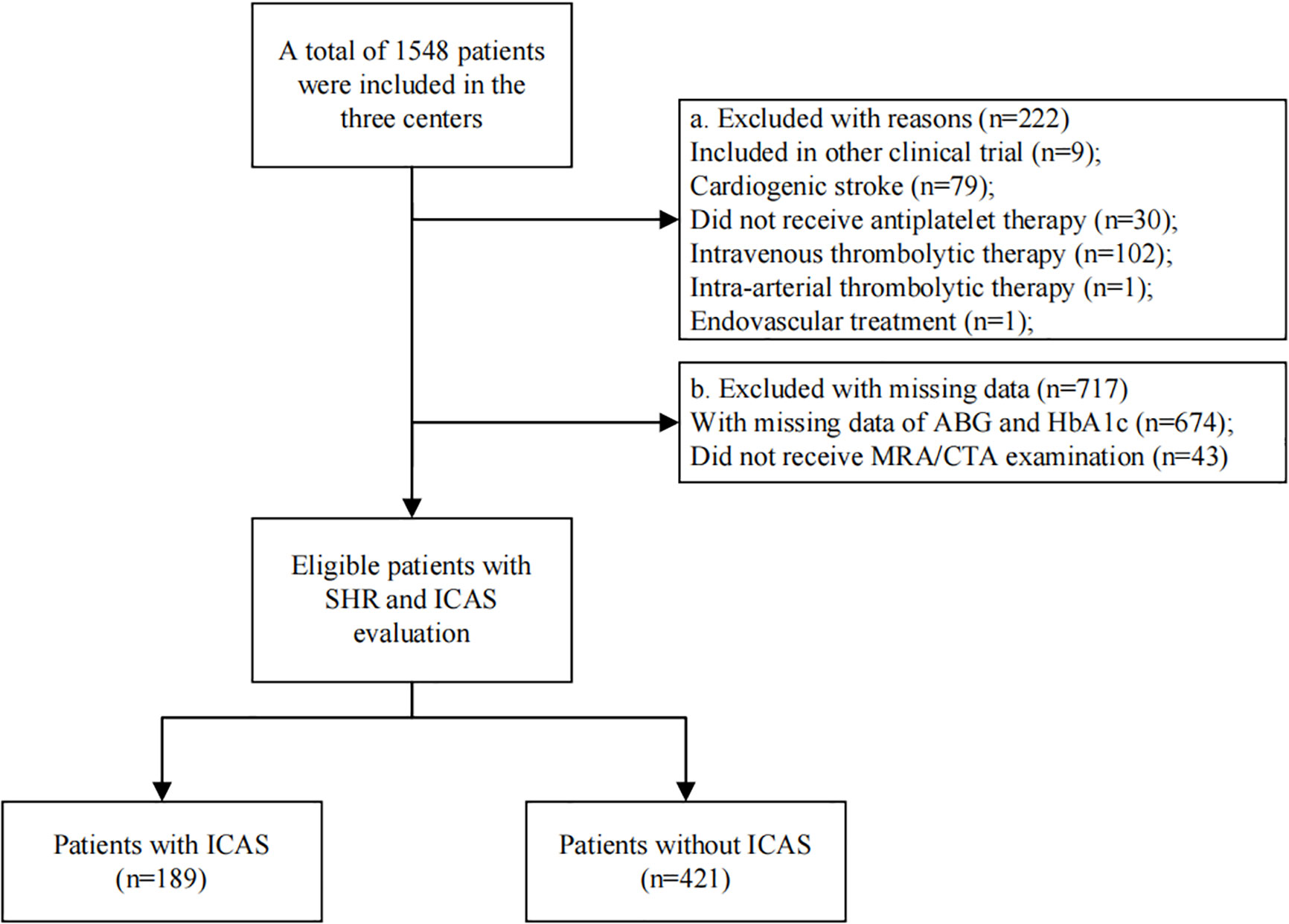
Figure 1 Flowchart of patient selection. There were 610 eligible patients included in the final analysis. Patients were grouped according to whether they have ICAS or not.
3.2 Univariate and multivariate analyses of factors associated with stroke recurrence
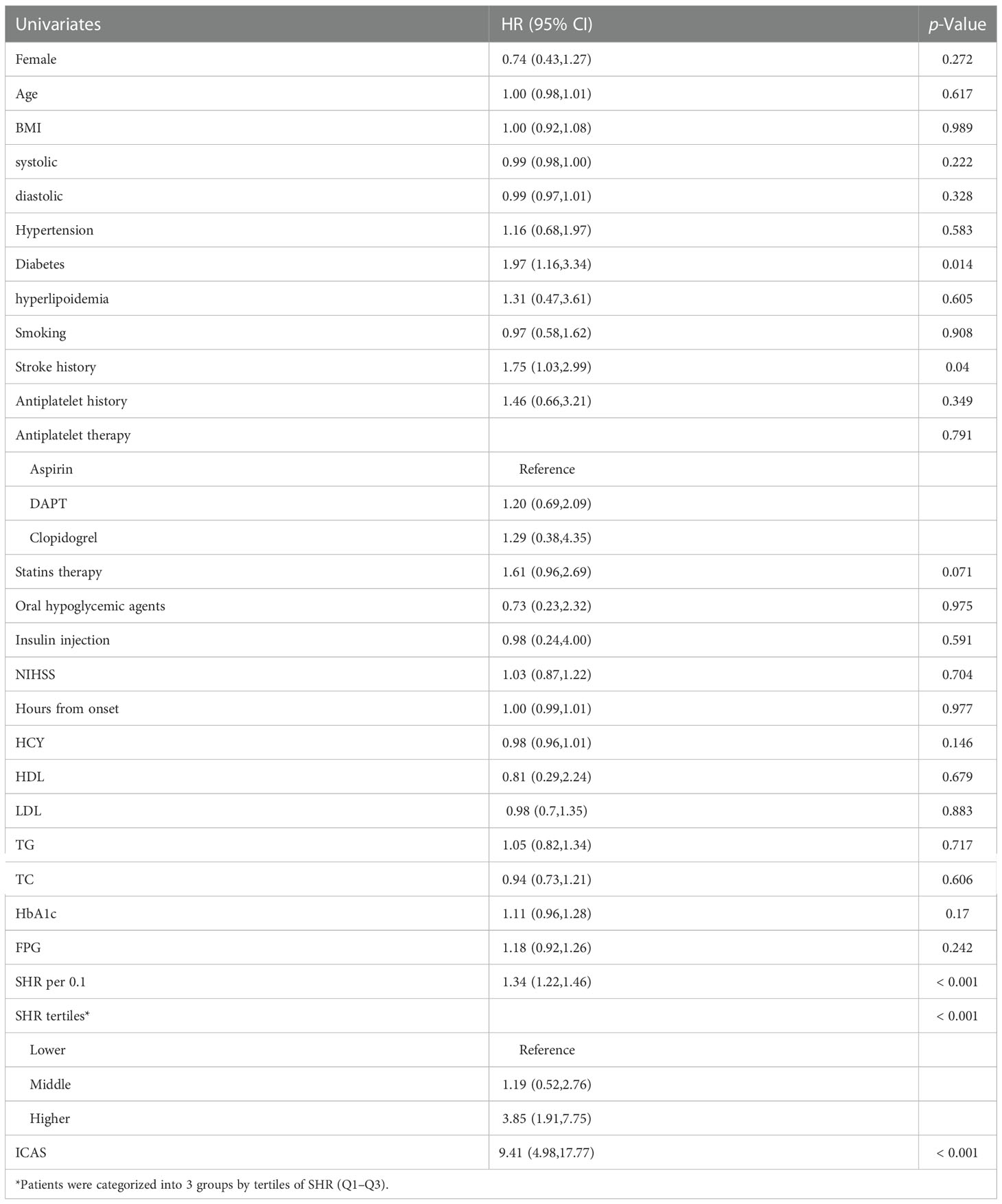
Univariate and multivariate Cox regression analyses were performed to determine factors associated with stroke recurrence. Univariate Cox regression analysis is shown in Table 3. Diabetes mellitus (HR, 1.97; 95% CI, 1.16-3.34, P=0.011), stroke history (HR, 1.75; 95% CI, 1.03-2.99, P=0.04), higher SHR (HR, 3.85; 95% CI, 1.91-7.75, P<0.001), and ICAS (HR, 9.41; 95% CI, 4.98-17.77, P<0.001) were associated with in-hospital stroke recurrence (P<0.05). The univariate results tentatively suggested that ICAS and SHR levels were associated with an increased risk of in-hospital stroke recurrence.
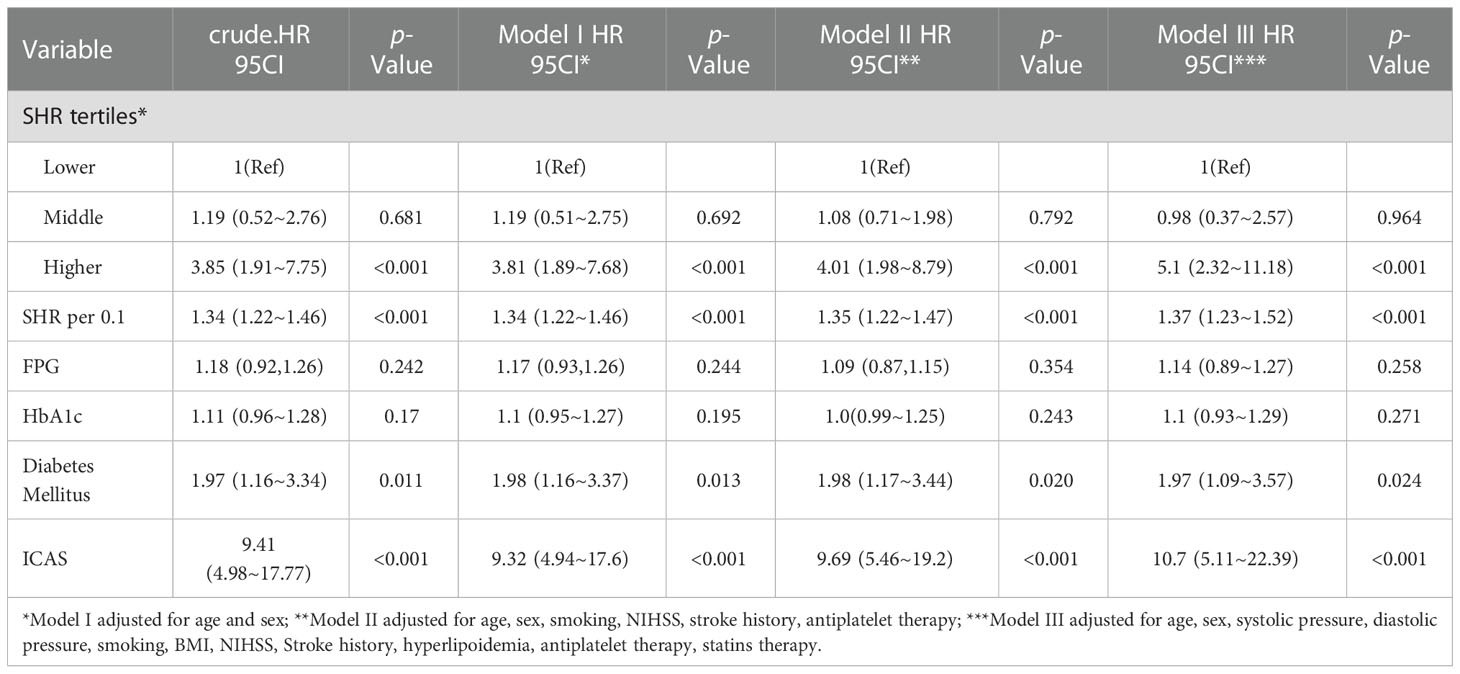
In multivariate Cox regression analyses, to control for potential confounders, Model I was adjusted for age and sex, Model II was adjusted for age, sex, smoking, NIHSS, stroke history, antiplatelet therapy, and Model III was adjusted for age, sex, systolic pressure, diastolic pressure, BMI, NIHSS, history of stroke, hyperlipoidemia, antiplatelet medication at admission and statin therapy. Confounders were determined based on univariate analyses and previous literature reports. We first verified the effects of different glucose metrics (FPG, HbA1c, SHR and diabetes mellitus) and ICAS on stroke recurrence, as shown in Table 4. Higher SHR, ICAS and the presence of diabetes mellitus were all associated with stroke recurrence (HR, 5.1, 95% CI, 2.32-11.18, P<0.001; HR, 10.7, 95% CI, 5.11-22.39, P<0.001; HR, 1.97, 95% CI, 1.09-3.57, P=0.024; respectively). To further determine the combined effect of ICAS and SHR, an interaction analysis was conducted, as shown in Table 5. In the ICAS group, a higher SHR was an independent risk factor for stroke recurrence (HR, 8.52; 95% CI, 3.16-22.96, P<0.001). However, the effect was not observed in the non-ICAS group. We further treated SHR as a continuous variable, and each 0.1-unit increase in SHR in the ICAS group was associated with a 1.63-fold increase in stroke recurrence (HR, 1.63, 95% CI, 1.39-1.9, P<0.001). A significant interactive effect of SHR levels with or without ICAS (P for interaction was 0.002) was noted. FPG was also associated with recurrence only in the ICAS group (HR, 1.17, 95% CI, 1.08-1.26, P<0.001). In the missing data analysis, there were 4.9%, 3.4%, 3.1%, 3.1%, 3.0%, 0.33%, 0.33% missing for HCY, TC, HDL, LDL, TG, systolic pressure, diastolic pressure respectively. To avoid differences in statistical test efficacy from direct exclusion of missing values, multiple imputation was used to treat the missing values, and the results of the subsequent analysis before and after processing were consistent.
The power simulation result is shown in Figure 1S and Table 4S. When the HR is set as 1.6, a sample of 600 reaches a statistical power of greater than 0.8. This result indicates that a sample of 610 in the analysis reaches sufficient power and has the ability to detect the significant regression effect.
3.3 Sensitivity analysis
Sensitivity analyses were performed to clarify the impact of previous diabetes mellitus and hypoglycemic treatment, including insulin injection and oral agents (Tables 1S, 2S). After further adjusting for diabetes mellitus (Model IV), each 0.1-unit increase in SHR in the ICAS group was associated with a 1.61-fold increase in the risk of an outcome event (HR, 1.61, 95% CI, 1.37-1.89, P<0.001). The significant interactive effect of SHR levels with ICAS on the primary outcome remained (P for interaction 0.002) (Table 1S). When further adjusting for hypoglycemic treatment (Model V), the results obtained were similar to those of Model II, III and IV (Table 2S). We then performed a subgroup analysis regarding whether the presence of diabetes interacts with SHR in patients with or without ICAS. No significant interaction was noted between diabetes mellitus and SHR (Figure 2).
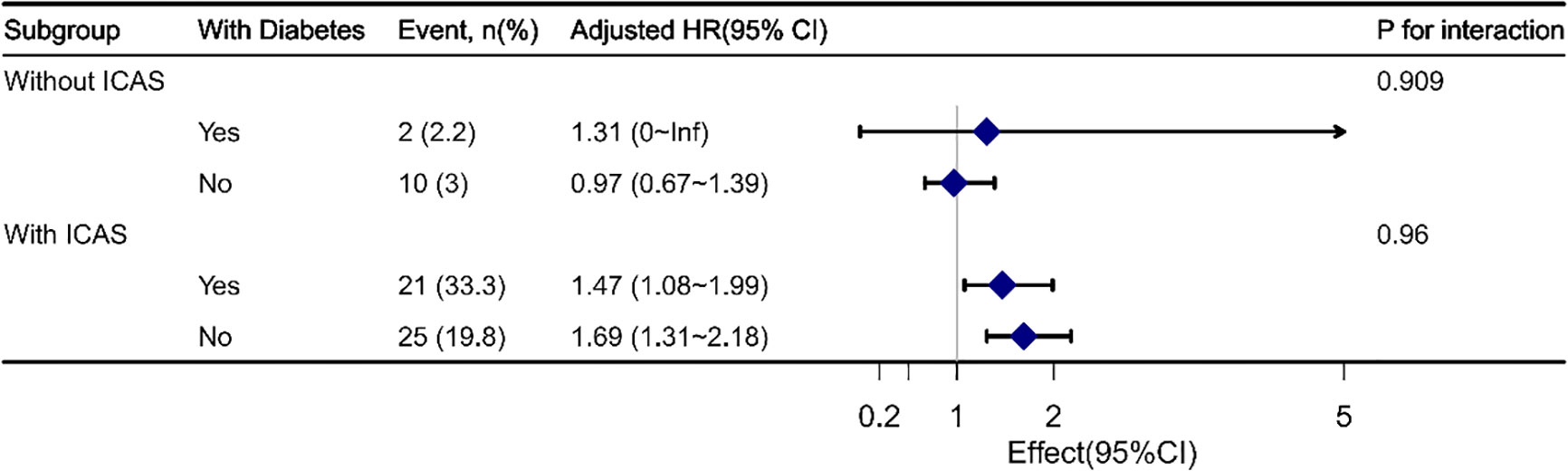
Figure 2 Interaction analyses between SHR and diabetes mellitus in ICAS and non-ICAS groups. No significant interaction was found between diabetes mellitus and SHR.
Using a Cox regression model with restricted cubic spline, we found that a higher level of SHR was associated with an increased risk of stroke with a threshold of 0.75 in the ICAS population (Figure 3; Table 3S). The association was not statistically significant in the population without ICAS.
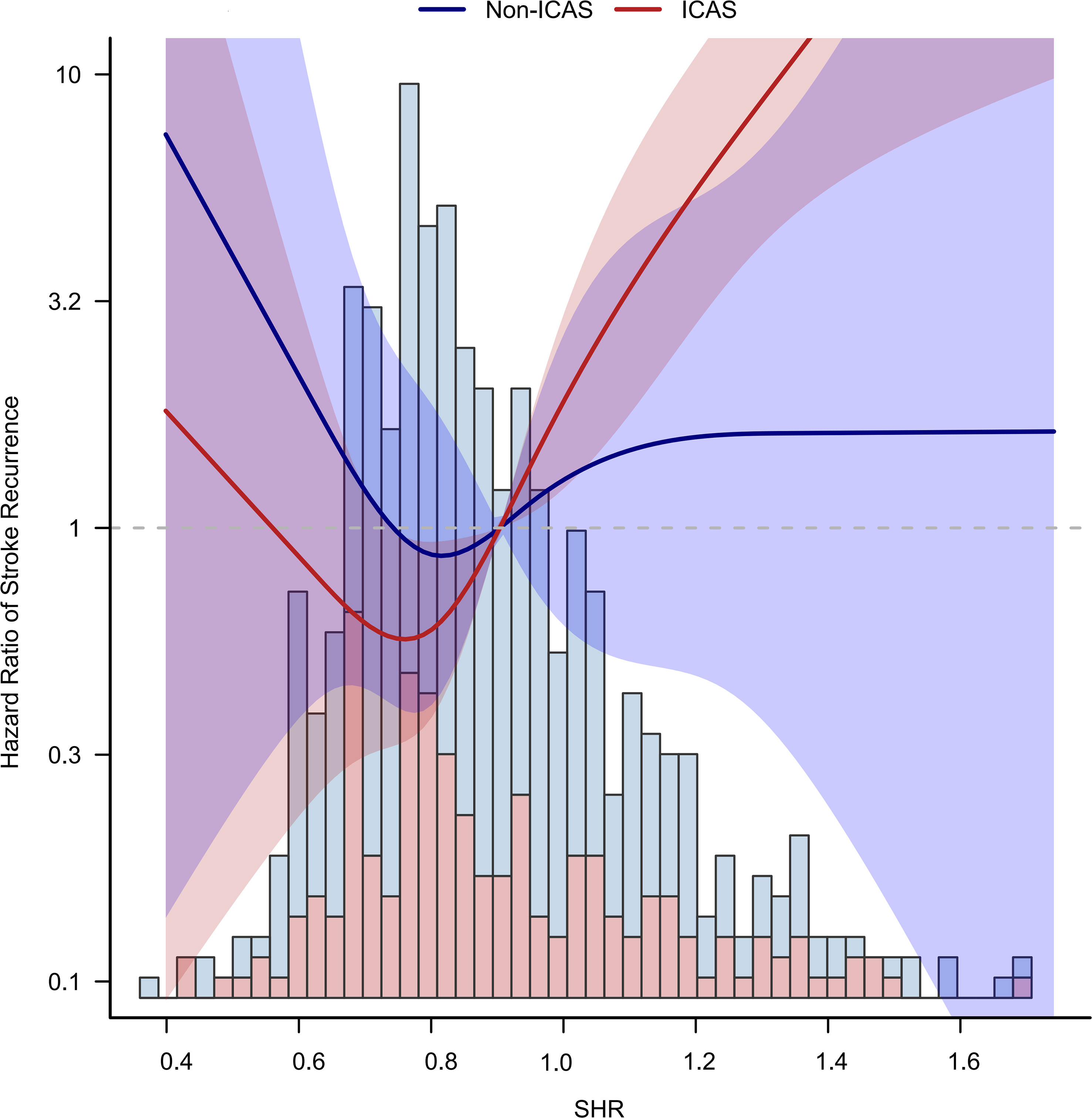
Figure 3 Non-liner relationship between SHR and stroke recurrence in ICAS and non-ICAS groups. A higher level of SHR over 0.75 was associated with an increased risk of stroke in the ICAS patients.
Patients were divided into four groups based on the SHR threshold and ICAS status: low SHR level (<0.75) without ICAS, high SHR level (≥0.75) without ICAS, low SHR level with ICAS, and high SHR level with ICAS. We used low SHR without ICAS as a control group and performed a Cox regression analysis of the combined grouping of ICAS and SHR on the risk of stroke recurrence. The risk of stroke recurrence was increased 3.67-fold for low SHR with ICAS (HR, 3.67, 95% CI, 0.86-15.67, P=0.079). The risk of stroke recurrence was increased 9.89-fold for high SHR with ICAS (HR, 9.89, 95% CI, 2.96-33.92, P<0.001). The survival curve illustrated that for patients with ICAS, recurrence mainly occurred in the first 5 days. SHR levels had minimal influence on recurrence among patients without ICAS (Figure 4).
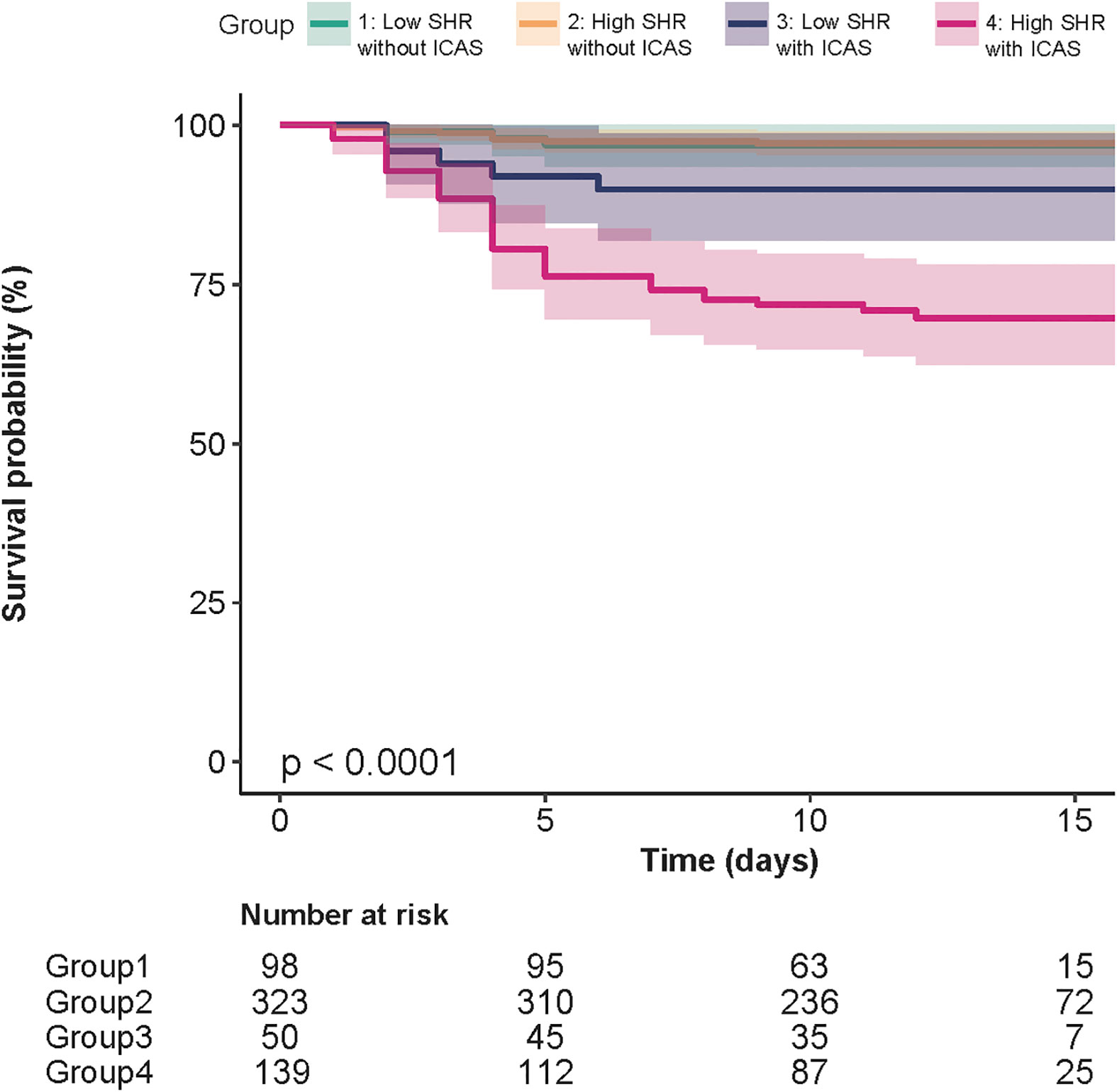
Figure 4 Kaplan−Meier curves of groups divided by the presence of ICAS and SHR threshold of 0.75. For patients with ICAS, recurrence mainly occurred in the first 5 days. SHR levels had minimal influence on recurrence among patients without ICAS.
In order to determine whether the different time of onset will affect the conclusions of the study, we performed a sensitivity analysis according to the time of onset (within 24 hours of onset and within 24-72 hours of onset), and found that the time of onset did not affect the previous results (Table 5S).
4 Discussion
The results suggest that the increase in stress hyperglycemia and the presence of ICAS have a combined effect on increasing the recurrence risk during hospitalization for ischemic stroke patients.
FPG has long been considered to be a risk factor for poor prognosis of stroke. The results of our study also confirmed this finding. Traditionally, stress hyperglycemia is defined as elevated FPG in the acute phase of severe diseases, especially for nondiabetic patients, and it represents acute inflammatory and neurohormonal dysregulation, which is strongly associated with adverse outcomes and poor prognosis (8). Many studies have shown that stress hyperglycemia is independently associated with mortality and worse functional outcomes following stroke (1, 17, 18, 24). However, it is not appropriate to use elevated FPG as a biomarker of stress hyperglycemia because diabetic patients with chronic poor glucose control may be incorrectly considered to have stress hyperglycemia without taking into account background or baseline glycemia levels (10). Moreover, the presence of diabetes mellitus may also influence the power of FPG to evaluate stress hyperglycemia. Stress hyperglycemia calculated based on background blood glucose levels can better reflect the poor prognosis of severe diseases. SHR, which was first proposed by Roberts (10) and defined as admission glucose divided by estimated average glucose derived from HbA1c, helps identify the risk of critical illness better than absolute hyperglycemia, and it has been introduced to cardiovascular and stroke studies. For ST-elevation myocardial infarction patients, SHR helps predict major adverse cardiac and cerebrovascular events after percutaneous coronary intervention (25). SHR is also associated with new-onset atrial fibrillation after acute myocardial infarction (26). In a retrospective cohort study including 300 hospitalized patients with ischemic stroke, SHR at admission provided the best prognostic insight into poor outcomes compared to glycemic gap and fasting glucose (16). For stroke patients who received intravenous thrombolysis, SHR also has a better predictive value for poor functional outcome than random glucose or FPG (24). However, few studies have investigated whether stress hyperglycemia could be applied to predict the recurrence risk of ischemic minor stroke. In a secondary analysis of clopidogrel with aspirin in the Acute Minor Stroke or Transient Ischemic Attack (CHANCE) trial, researchers revealed that stress hyperglycemia was associated with a 3-month risk of recurrent stroke in minor ischemic stroke patients (17). However, their study did not take into account specific pathophysiological differences in stroke types, which may have a great impact on the relationship between stress hyperglycemia and stroke recurrence. Thus, our study hypothesized that the adverse effect of stress hyperglycemia may be more prominent in stroke patients with ICAS. The results of our study were consistent with previous reports. Our study further added evidence that stress hyperglycemia was only associated with stroke recurrence in patients with ICAS.
Our results also suggested that the SHR could estimate the risk of stroke recurrence in ICAS patients regardless of previously diagnosed diabetes mellitus. This finding is consistent with a secondary analysis of the CHANCE trial, which proved that stress hyperglycemia may have a higher risk of stroke recurrence compared to previously diagnosed diabetes mellitus or nondiabetes mellitus groups (18). However, another study conducted in ischemic stroke patients showed that the value of the SHR to predict recurrence was only significant in patients without preexisting diabetes mellitus (27). This finding seems to be inconsistent with our results. Stress hyperglycemia was traditionally defined as absolute hyperglycemia without evidence of previously diagnosed diabetes, but the limitation of the diagnostic criterion is that it did not take into account the background blood glucose level and cannot distinguish between stress hyperglycemia and diabetes mellitus. Thus, more attention should be given to the immediate severity of stress hyperglycemia rather than previously diagnosed diabetes. Another reason why the study conclusions are controversial is the different recruiting criteria among the studies. ICAS, which reflects the abnormal structure of large blood vessels, may be more sensitive to glucose stress levels.
Large artery atherosclerotic stroke (LAA) is the most common stroke subtype in the TOAST classification. In this condition, plaque rupture caused by atherosclerosis leads to platelet aggregation, which leads to thrombosis and finally to the clinical manifestation of vascular blockage (28). The CICAS study in China reported that severe stenosis and multiple stenoses represent risk factors for recurrent stroke. The risk of recurrent stroke was significantly higher in patients with ICAS (29). Mu et al. (30) found that arterial stenosis and systolic/diastolic dysfunction in the early stage of diabetes mellitus can lead to worse prognosis after ischemic stroke. We hypothesize that ICAS can be used as an indirect factor of stress hyperglycemia in stroke patients with poor prognosis. In our study, we found that the existence of ICAS is a prerequisite for stress hyperglycemia to influence stroke recurrence. This finding may be explained by the adverse consequences caused by stress hyperglycemia exaggerating the process of thrombosis after atherosclerotic rupture.
Hyperglycemia can aggravate the enlargement and rupture of atherosclerotic plaques in intracranial arteries, coronary arteries and peripheral arteries by affecting the function of brain endothelial cells, which is more likely to lead to thrombosis (31). The acute fluctuation of blood glucose levels is related to an increase in plaque instability and infarction area (32). Flynn et al. (33) found that an abnormal instantaneous increase in circulating glucose concentration promotes bone marrow formation, leads to an increase in circulating monocytes and accelerates the occurrence of atherosclerosis. Yang et al. (34) noted that the increase in the stress hyperglycemia plays an important role in predicting poor prognosis of patients with coronary heart disease undergoing percutaneous coronary intervention. For angina patients diagnosed as ischemia with nonobstructive coronary arteries, SHR at admission can significantly increase the risk of rehospitalization for chest pain (35). Given that the essence of coronary heart disease is atherosclerosis, it further suggests that the increase in the stress hyperglycemia may be involved in the rupture of atherosclerotic plaques and lead to hospital reinfarction for stroke patients. Interestingly, Dong et al. found that after middle cerebral artery occlusion, cerebral ischemia−reperfusion injury is worsened in hyperglycemic rats. Inhibition of microglial cells by stress hyperglycemia might partly explain poor outcomes after ischemic stroke (36).
This article suggested using simple calculations to evaluate the level of stress hyperglycemia for minor stroke at admission and combining imaging indicators to help further identify the risk of recurrence (23). For patients with higher SHR and severe intracranial atherosclerosis, more aggressive antithrombotic strategies, lipid-lowering treatment to stabilize the atherosclerotic plaque and intensive glucose monitoring may be needed (37).
In future research, we can further quantify the degree of intracranial artery stenosis to conduct more accurate and individualized research to help us better understand the relationship between stress hyperglycemia and intracranial artery stenosis. Furthermore, we can carry out more glucose-lowering strategies and antithrombotic strategies for this group of people to reduce the in-hospital recurrence rate of minor stroke patients with severe intracranial artery stenosis and stress hyperglycemia.
Some limitations of our study should be noted. First, as a design limitation, the results are not defined by time but vary according to the length of hospital stay. Thus, different follow-ups may bias the research results to determine the events of patients with longer lengths of stay rather than patients with shorter lengths of stay. Second, our study did not clearly define the artery responsible for the infarction but simply divided the degree of artery stenosis into less than 50% and greater than 50%. It is necessary and feasible to determine the relationship between the artery responsible for stenosis and reinfarction. We will quantify this index in future research. Third, compared with digital subtraction angiography, MRA limits spatial resolution and may increase the insensitivity to accurate assessment of arterial stenosis. Fourth, this study only included 610 patients from three provincial capital hospitals, and limited statistical ability was inevitable. Due to the high prevalence of ICAS in Asians, the universality of subgroup analysis needs to be further verified. With this in mind, research on other races is necessary to present the scalability of results on a global scale. Fifth, the impact of stress hyperglycemia on the rheological and hemodynamic characteristics of ICAS requires further investigation (4). Stress hyperglycemia may further influence key biochemical factors, such as the endothelial function and filtration rate of low-density lipoprotein, which deserve further exploration (38). By integrating the SHR into the existing stroke risk stratification tools derived from cerebral hemodynamic parameters, the effectiveness of predicting stroke recurrence will be improved (39).
5 Conclusion
SHR represents a better biomarker to predict the risk of stroke recurrence in patients with ICAS than FPG and HbA1c regardless of previous diabetes mellitus.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by The Ethics Committee of First Hospital of Shanxi Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
YW was a major contributor to the writing of the manuscript. YW and HongF performed the statistical analysis. WD, ZR, and XuL drafted the manuscript. XN designed and supervised the study. YL, KZ, HongF, JR and JL performed data collection. TL revised the manuscript. XL and XW supervised each stroke center. All authors contributed to the article and approved the submitted version.
Acknowledgments
This is a multicenter study. We thank the hospitals of First Hospital of Shanxi Medical University, Bethune Hospital of Shanxi Province, and Sixth Hospital of Shanxi Medical University (General Hospital of Tisco).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.954916/full#supplementary-material
Supplementary Figure 1 | Post hoc power simulation analysis. A sample of 600 reaches a statistical power of greater than 0.8.
Supplementary Figure 2 | Distribution of missing data. A. Histogram of missing data distribution; B. Heatmap of missing data. There were 4.9%, 3.4%, 3.1%, 3.1%, 3.0%, 0.33%, 0.33% missing for HCY, TC, HDL, LDL, TG, systolic pressure, diastolic pressure respectively.
Abbreviations
SHR, stress hyperglycemia ratio; ICAS, intracranial atherosclerotic stenosis; ECAS, extracranial carotid atherosclerotic stenosis; CICAS, the Chinese Intracranial Atherosclerosis study; BMI, body mass index; MRI, magnetic resonance imaging; CT, computed tomography; NIHSS, the National Institutes of Health Stroke Scale; TIA, transient ischemic attack; rt-PA, recombinant tissue plasminogen activator; MRA, magnetic resonance angiography; FPG, fasting plasma glucose; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, Triglycerides; TC, total cholesterol; HCY, homocysteine; FPG, fasting plasma glucose; MACCE, major adverse cardiac and cerebrovascular events; STEMI, ST-elevation myocardial infarction; HTN, hypertension; AF, atrial fibrillation; MI, myocardial infarction; CHD, coronary atherosclerotic heart disease; PAD, peripheral arterial disease; DAPT, dual antiplatelet therapy; WASID, Warfarin-Aspirin Symptomatic Intracranial Disease; CHANCE, Clopidogrel with Aspirin in Acute Minor Stroke or Transient Ischemic Attack.
References
1. Johnson CO, Nguyen M, Roth GA, Nichols E, Alam T, Abate D, et al. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurol (2019) 18:439–58. doi: 10.1016/S1474-4422(19)30034-1
2. Wang Y, Zhao X, Liu L, Soo YOY, Pu Y, Pan Y, et al. Prevalence and outcomes of symptomatic intracranial Large artery stenoses and occlusions in China: The Chinese intracranial atherosclerosis (CICAS) study. Stroke (2014) 45:663–9. doi: 10.1161/STROKEAHA.113.003508
3. Gorelick PB, Wong KS, Bae H-J, Pandey DK. Large Artery intracranial occlusive disease: A Large worldwide burden but a relatively neglected frontier. Stroke (2008) 39:2396–9. doi: 10.1161/STROKEAHA.107.505776
4. Liu H, Lan L, Abrigo J, Ip HL, Soo Y, Zheng D, et al. Comparison of Newtonian and non-newtonian fluid models in blood flow simulation in patients with intracranial arterial stenosis. Front Physiol (2021) 12:718540. doi: 10.3389/fphys.2021.718540
5. Kasner SE, Lynn MJ, Chimowitz MI, Frankel MR, Howlett-Smith H, Hertzberg VS, et al. Warfarin vs aspirin for symptomatic intracranial stenosis: Subgroup analyses from WASID. Neurology (2006) 67:1275–8. doi: 10.1212/01.wnl.0000238506.76873.2f
6. Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol (2013) 12:1106–14. doi: 10.1016/S1474-4422(13)70195-9
7. Pan Y, Meng X, Jing J, Li H, Zhao X, Liu L, et al. Association of multiple infarctions and ICAS with outcomes of minor stroke and TIA. Neurology (2017) 88:1081–8. doi: 10.1212/WNL.0000000000003719
8. Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: A systematic overview. Stroke (2001) 32:2426–32. doi: 10.1161/hs1001.096194
9. Douketis J. Review: Stress hyperglycemia after ischemic stroke indicates a greater risk for death in patients without diabetes. ACP J Club (2002) 136:114. doi: 10.7326/ACPJC-2002-136-3-114
10. Roberts GW, Quinn SJ, Valentine N, Alhawassi T, O’Dea H, Stranks SN, et al. Relative hyperglycemia, a marker of critical illness: Introducing the stress hyperglycemia ratio. J Clin Endocrinol Metab (2015) 100:4490–7. doi: 10.1210/jc.2015-2660
11. Ceriello A, Giugliano D, Quatraro A, Russo PD, Marchi E, Torella R. Hyperglycemia may determine fibrinopeptide a plasma level increase in humans. Metabolism (1989) 38:1162–3. doi: 10.1016/0026-0495(89)90152-2
12. Ceriello A, Giugliano D, Quatraro A, Russo PD, Torella R. Blood glucose may condition factor VII levels in diabetic and normal subjects. Diabetologia (1988) 31(12):889–91. doi: 10.1007/BF00265372
13. Pandolfi A, Giaccari A, Cilli C, Alberta MM, Morviducci L, De Filippis EA, et al. Acute hyperglycemia and acute hyperinsulinemia decrease plasma fibrinolytic activity and increase plasminogen activator inhibitor type 1 in the rat. Acta Diabetol (2001) 38:71–6. doi: 10.1007/s005920170016
14. Fabris F, Zanocchi M, Bo M, Fonte G, Poli L, Bergoglio I, et al. Carotid plaque, aging, and risk factors. a study of 457 subjects. Stroke (1994) 25:1133–40. doi: 10.1161/01.STR.25.6.1133
15. Uyttenboogaart M, Koch MW, Stewart RE, Vroomen PC, Luijckx G-J, De Keyser J. Moderate hyperglycaemia is associated with favourable outcome in acute lacunar stroke. Brain (2007) 130:1626–30. doi: 10.1093/brain/awm087
16. Roberts G, Sires J, Chen A, Thynne T, Sullivan C, Quinn S, et al. A comparison of the stress hyperglycemia ratio, glycemic gap, and glucose to assess the impact of stress-induced hyperglycemia on ischemic stroke outcome. J Diabetes (2021) 13:1034–42. doi: 10.1111/1753-0407.13223
17. Pan Y, Cai X, Jing J, Meng X, Li H, Wang Y, et al. Stress hyperglycemia and prognosis of minor ischemic stroke and transient ischemic attack: The CHANCE study (Clopidogrel in high-risk patients with acute nondisabling cerebrovascular events). Stroke (2017) 48:3006–11. doi: 10.1161/STROKEAHA.117.019081
18. Guo Y, Wang G, Jing J, Wang A, Zhang X, Meng X, et al. Stress hyperglycemia may have higher risk of stroke recurrence than previously diagnosed diabetes mellitus. Aging (2021) 13:9108–18. doi: 10.18632/aging.202797
19. Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. definitions for use in a multicenter clinical trial. TOAST. trial of org 10172 in acute stroke treatment. Stroke (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
20. Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ. For the A1c-derived average glucose (ADAG) study group. Translating the A1C assay into estimated average glucose values. Diabetes Care (2008) 31:1473–8. doi: 10.2337/dc08-0545
21. Chimowitz MI, Kokkinos J, Strong J, Brown MB, Levine SR, Silliman S, et al. The warfarin-aspirin symptomatic intracranial disease study. Neurology (1995) 45:1488. doi: 10.1212/WNL.45.8.1488
22. Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med (2013) 369:11–9. doi: 10.1056/NEJMoa1215340
23. Kennedy J, Hill MD, Ryckborst KJ, Eliasziw M, Demchuk AM, Buchan AM. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol (2007) 6:961–9. doi: 10.1016/S1474-4422(07)70250-8
24. Chen G, Ren J, Huang H, Shen J, Yang C, Hu J, et al. Admission random blood glucose, fasting blood glucose, stress hyperglycemia ratio, and functional outcomes in patients with acute ischemic stroke treated with intravenous thrombolysis. Front Aging Neurosci (2022) 14:782282. doi: 10.3389/fnagi.2022.782282
25. Djordjevic-Radojkovic D, Koracevic G, Stanojevic D, Damjanovic M, Apostolovic S, Pavlovic M. Stress hyperglycemia in acute ST-segment elevation myocardial infarction is a marker of left ventricular remodeling. Acute Cardiac Care (2013) 15:38–43. doi: 10.3109/17482941.2013.781190
26. Pan L, Li Z, Li C, Dong X, Hidru TH, Liu F, et al. Stress hyperglycemia ratio and neutrophil to lymphocyte ratio are reliable predictors of new-onset atrial fibrillation in patients with acute myocardial infarction. Front Cardiovasc Med (2022) 9:1051078. doi: 10.3389/fcvm.2022.1051078
27. Zhu B, Pan Y, Jing J, Meng X, Zhao X, Liu L, et al. Stress hyperglycemia and outcome of non-diabetic patients after acute ischemic stroke. Front Neurol (2019) 10:1003. doi: 10.3389/fneur.2019.01003
28. Derdeyn CP. Mechanisms of ischemic stroke secondary to Large artery atherosclerotic disease. Neuroimaging Clinics North America (2007) 17:303–11. doi: 10.1016/j.nic.2007.03.001
29. Sun P, Liu L, Pan Y, Wang X, Mi D, Pu Y, et al. Intracranial atherosclerosis burden and stroke recurrence for symptomatic intracranial artery stenosis (sICAS). Aging Dis (2018) 9:1096. doi: 10.14336/AD.2018.0301
30. Mu Z-H, Jiang Z, Lin X-J, Wang L-P, Xi Y, Zhang Z-J, et al. Vessel dilation attenuates endothelial dysfunction following middle cerebral artery occlusion in hyperglycemic rats. CNS Neurosci Ther (2016) 22:316–24. doi: 10.1111/cns.12500
31. Yuan C, Chen S, Ruan Y, Liu Y, Cheng H, Zeng Y, et al. The stress hyperglycemia ratio is associated with hemorrhagic transformation in patients with acute ischemic stroke. CIA (2021) 16:431–42. doi: 10.2147/CIA.S280808
32. Ujueta F, Weiss EN, Sedlis SP, Shah B. Glycemic control in coronary revascularization. Curr Treat Options Cardio Med (2016) 18:12. doi: 10.1007/s11936-015-0434-6
33. Flynn MC, Kraakman MJ, Tikellis C, Lee MKS, Hanssen NMJ, Kammoun HL, et al. Transient intermittent hyperglycemia accelerates atherosclerosis by promoting myelopoiesis. Circ Res (2020) 127:877–92. doi: 10.1161/CIRCRESAHA.120.316653
34. Yang Y, Kim T-H, Yoon K-H, Chung WS, Ahn Y, Jeong M-H, et al. The stress hyperglycemia ratio, an index of relative hyperglycemia, as a predictor of clinical outcomes after percutaneous coronary intervention. Int J Cardiol (2017) 241:57–63. doi: 10.1016/j.ijcard.2017.02.065
35. Mone P, Lombardi A, Salemme L, Cioppa A, Popusoi G, Varzideh F, et al. Stress hyperglycemia drives the risk of hospitalization for chest pain in patients with ischemia and nonobstructive coronary arteries (INOCA). Diabetes Care (2022), dc220783. doi: 10.2337/dc22-0783
36. Dong L, Ma Y, Xu J, Guo Y, Yang L, Guo F-Y, et al. Effect of hyperglycemia on microglial polarization after cerebral ischemia-reperfusion injury in rats. Life Sci (2021) 279:119660. doi: 10.1016/j.lfs.2021.119660
37. Wijngaard IR, Holswilder G, Walderveen MAA, Algra A, Wermer MJH, Zaidat OO, et al. Treatment and imaging of intracranial atherosclerotic stenosis: current perspectives and future directions. Brain Behav (2016) 6(11):e00536. doi: 10.1002/brb3.536
38. Liu H, Gong Y, Leng X, Xia L, Wong KS, Ou S, et al. Estimating current and long-term risks of coronary artery in silico by fractional flow reserve, wall shear stress and low-density lipoprotein filtration rate. BioMed Phys Eng Express (2018) 4:025006. doi: 10.1088/2057-1976/aa9a09
Keywords: stress hyperglycemia, intracranial atherosclerotic stenosis, ischemic stroke, stroke recurrence, fasting blood glucose
Citation: Wang Y, Fan H, Duan W, Ren Z, Liu X, Liu T, Li Y, Zhang K, Fan H, Ren J, Li J, Li X, Wu X and Niu X (2023) Elevated stress hyperglycemia and the presence of intracranial artery stenosis increase the risk of recurrent stroke. Front. Endocrinol. 13:954916. doi: 10.3389/fendo.2022.954916
Received: 27 May 2022; Accepted: 12 December 2022;
Published: 09 January 2023.
Edited by:
Gaetano Santulli, Albert Einstein College of Medicine, United StatesReviewed by:
Hung Yi Chiou, Taipei Medical University, TaiwanHaipeng Liu, Coventry University, United Kingdom
Ka Lung Chan, The Chinese University of Hong Kong, China
Copyright © 2023 Wang, Fan, Duan, Ren, Liu, Liu, Li, Zhang, Fan, Ren, Li, Li, Wu and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyuan Niu, niuxiaoyuan1958@163.com
 Yongle Wang
Yongle Wang Hongxuan Fan
Hongxuan Fan Weiying Duan2
Weiying Duan2 Kaili Zhang
Kaili Zhang Xiaoyuan Niu
Xiaoyuan Niu