- 1Department of Human Anatomy, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, Serdang, Malaysia
- 2Department of Biomedical Sciences, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, Serdang, Malaysia
- 3Department of Emergency Medicine, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
- 4Advanced Medical and Dental Institute, Universiti Sains Malaysia, Penang, Malaysia
- 5Department of Medical Microbiology and Parasitology, Faculty of Medicine and Health Science, Universiti Putra Malaysia (UPM) , Serdang, Malaysia
- 6Centre for Tissue Engineering and Regenerative Medicine, Faculty of Medicine, Universiti Kebangsaan, Kuala Lumpur, Malaysia
Maternal obesity is the key predictor for childhood obesity and neurodevelopmental delay in the offspring. Medicinal plants are considered to be the safe and best option, and at the same time, probiotic consumption during pregnancy provides beneficial effects for both the mother and the child. Current research has shown that Elateriospermum tapos (E. tapos) yoghurt is safe to consume and consists of many bioactive compounds that can exert an anti-obesity effect. Thus, this study has been designed to study the role of E. tapos yoghurt in mitigating maternal obesity. In this study, a total of 48 female Sprague Dawley (SD) rats were assigned to six groups, with eight rats per group, and obesity was induced over 16 weeks with a high-fat diet (HFD) pellet. On the 17th week, the rats were allowed to mate and pregnancy was confirmed through vaginal smear. The obese induced group was further divided into negative and positive control groups, followed by E. tapos yoghurt treatment groups with three different concentrations (5, 50, and 500 mg/kg). The changes in body weight, calorie intake, lipid profile, liver profile, renal profile, and histopathological analysis were measured on postnatal day (PND) 21. The results show that the group with the highest concentration of E. tapos yoghurt (HYT500) supplementation shows gradual reduction in body weight and calorie intake on PND 21 and modulates the lipid level, liver, and renal enzymes to a normal level similar to the normal group. In histological analysis, HYT500 reverses the damage caused by HFD in liver and colon, and reverses the adipocytes’ hypertrophy in retroperitoneal white adipose tissue and visceral fat. In conclusion, supplementation of E. tapos yoghurt during the gestational period up to weaning is effective in the gradual weight loss of maternal obese dams from the 500-mg/kg-supplemented group in this study.
1 Introduction
Maternal obesity is currently alarming and often ignored due to lack of knowledge on its long-term effects for both mothers and the fetus. A pre-pregnancy body mass index of more than ≥30 kg/m2 is termed maternal obesity (1). Maternal obesity is the key predictor for childhood obesity and often associated with neurocognitive development of the fetus. This could be due to the shared genetic polymorphism between the obese mother and the child or changes of epigenome in the child (2). The prevalence rate for obesity has tripled over the past few decades and more than 41 million children aged less than 5 years have been classified as obese globally (3). Studies show that the prevalence rate is increasing mostly in low- and middle-income countries while Asia leads the list with the highest documented cases for child obesity. The rising trends show that at least 85% of the adults will be obese by the year 2030. The cost of treating obesity-related comorbidity is distressing and requires urgent medical attention (4). Although countless measures have been taken to manage obesity, it is impossible to curb obesity completely since the root cause lies within the maternal subjects. Current available treatments such as metformin (5) are known to cause adverse gastrointestinal effects such as nausea, vomiting, and diarrhea (6) while moderate to vigorous exercise during pregnancy could induce hypoxia, hyperthermia, or even abnormal heart rate in the growing fetus (7).
Therefore, medicinal plants have been documented as the best option to treat maternal obesity through a pharmacological approach. Among these, Elateriospermum tapos (E. tapos), a tropical plant, has been proven to exhibit an anti-obesity effect due to its natural antioxidant property that is able to inhibit lipid peroxidation (8). E. tapos, also locally known as buah perah, commonly found in the deep forest of Southeast Asia, contains a wide range of bioactive compounds that has the potential to prevent accumulations of fats. Some of the identified bioactive compounds include tannins, alkaloids, linolenic acids, polyunsaturated fats, saponins, and sterols (9). Concomitantly, we found that consumption of probiotic during pregnancy improves the metabolism in the pregnant mother and reduces the risk of maternal obesity complications (10). Thus, we speculate that the consumption of medicinal plant-integrated yoghurt will give out more beneficial effects for both obese mothers and the child by mitigating maternal obesity and its complication. A recent study proves that it is safe to consume up to 2000 mg/kg of E. tapos yoghurt daily (9). Hence, this study has been designed to investigate the effect of E. tapos yoghurt in the mitigation of maternal obesity through various parameters.
2 Materials and methods
2.1 Collection and confirmation of E. tapos seeds
The fresh E. tapos seeds were obtained from the Forest Research Institute of Malaysia (FRIM), Pahang and were sent to the Herbarium Biodiversity Unit at the Institute of Bioscience, Universiti Putra Malaysia for purity check. The approved voucher code is UPM SK 3154/17.
2.2 Ethanol extraction of E. tapos seeds
About 500 g of E. tapos seeds was cleaned with running tap water and were soaked in 2 L of 95% ethanol for 7 days in room temperature. On the 7th day, it was filtered using filter paper (Whatman No. 1) to obtain the crude extract and was filtered again using a rotary evaporator (11). The precipitate collected is then mixed with maltodextrin powder in the ratio of 1:1 before proceeding with an overnight oven-drying process (12).
2.3 Formulation of E. tapos yoghurt
The yoghurt was prepared according to the guideline described elsewhere (13). Full cream milk (100 ml; Dutch Lady Purefarm UHT) was boiled at 75°C followed by cooling down at room temperature for 15–20 min. The starter culture consisting of Streptococcus thermophilus APC151 and Lactobacillus delbrueckiisubsp. Bulgaricus ATCC 11842 was added to the milk and was incubated in a Pensonic PYM-700 yoghurt maker for 7–8 h. The final produced yoghurt was refrigerated overnight at 4°C and E. tapos powder was added to the yoghurt in the ratio of 2 g/100 ml (13).
2.4 Active compounds screening for E. tapos yoghurt using UHPLC-QTOF-MS
The E. tapos yoghurt fraction was evaluated using UHPLC. UHPLC was performed on an ACQUITY UPLC I-Class system from Waters, consisting of a binary pump, a vacuum degasser, an auto sampler, and a column oven. Phenolic compounds were chromatographically separated using a column ACQUITY UPLC HSS T3 (100 mm × 2.1 mm × 1.8 μm) also from Waters, maintained at 40°C. A linear binary gradient of water (0.1% formic acid) and acetonitrile (mobile phase B) was used as mobile phase A and B, respectively. The mobile phase composition was changed during the run as follows: 0 min, 1% B; 0.5 min, 1% B; 16.00 min, 35% B; 18.00 min, 100% B; and 20.00 min, 1% B. The flow rate was set to 0.6 ml/min and the injection volume was 1 μl (14).
2.5 TWIMS-QTOFMS analysis for E. tapos yoghurt
The UHPLC system was coupled to a Vion IMS QTOF hybrid mass spectrometer from Waters, equipped with a Lock Spray ion source. The ion source was operated in negative electrospray ionization (ESI) mode under the following specific conditions: capillary voltage, 1.50 kV; reference capillary voltage, 3.00 kV; source temperature, 120°C; desolvation gas temperature, 550°C; desolvation gas flow, 800 L/h, and cone gas flow, 50 L/h. Nitrogen (>99.5%) was employed as desolvation and cone gas. Data were acquired in high-definition MSE (HDMSE) mode in the range m/z 50–1500 at 0.1 s/scan. Thus, two independent scans with different collision energies (CE) were alternatively acquired during the run: a low-energy (LE) scan at a fixed CE of 4 eV, and a high- energy (HE) scan where the CE was ramped from 10 to 40 eV. Argon (99.999%) was used as collision-induced dissociation (CID) gas (14).
2.6 High-fat diet
The high-fat diet was prepared with 17% protein, 40% fat, and 43% carbohydrate, containing 414 kcal/100 g. All ingredients were then blended with 6% corn oil (Vecorn, Yee Lee Corporation Berhad, Kuala Lumpur, Malaysia), 6% ghee [Crispo, Crispo-Tato (M) Sdn Bhd, Kuala Lumpur, Malaysia], 20% milk powder (Dutch lady, Dutch Lady Milk Industries Berhad, Selangor, Malaysia), and 68% standard chow pellet [Gold Coin Feedmills (M) Sdn Bhd, Selangor, Malaysia]. Standard chow pellet contains 306.2 kcal/100 g with 21% protein, 3% fat, and 48.8% carbohydrate (15, 16).
2.7 Experimental animals
All animal studies were performed upon obtaining approval from the Institutional Animal Care and Use Committee (IACUC), Universiti Putra Malaysia. The animal ethics committee granted approval for this study under the code UPM/IACUC/AUP-R025/2022. In this study, 48 female Sprague Dawley (SD) rats weighing between 200 and 250 g were used. All rats were acclimatized for 1 week at 22 ± 3°C at 12/12 h light/dark. All rats were supplemented with standard rat chow for the first week with free access to water.
2.8 Obesity induction
A total of 40 rats were supplemented with HFD for 16 weeks and 8 rats were fed with standard chow pellet ad libitum. Body weight and 24-h food intake (kJ) were recorded weekly for all rats. After 16 weeks, obesity was confirmed by measuring the difference of mean body weight between HFD- and standard chow pellet-supplemented groups. A significant increase in mean body weight of 14% in the HFD group is considered obese (17).
2.9 Mating
Upon confirmation of obesity in HFD groups, all rats in both normal chow-fed rats and HFD-fed groups were allowed to mate. This is done by placing female rats with fertile male rats in the ratio of 1:1 until successful copulation. Each day, vaginal smear was performed in female rats at approximately 8–10 a.m. to confirm pregnancy, and the smears were observed under a microscope with a ×100 magnification (KF2; Carl Zeiss, Hamburg, Germany). Detection of sperm indicates successful mating. The first sperm detection has been recorded as 0 post-coitum (18).
2.10 Gestation and weaning
The treatment with E. tapos yoghurt initiated from the first day of gestation until postnatal day (PND) 21. The obese dam’s treatment groups are as follows: normal chow and saline (NS), HFD and saline (HS), HFD and yoghurt (HY), HFD and 5 mg/kg of E. tapos yoghurt (HYT5), HFD and 50 mg/kg of E. tapos yoghurt (HYT50), and HFD and 500 mg/kg of E. tapos yoghurt (HYT500).
2.11 Body weight and calorie intake
Changes in body weight were documented weekly for all dams and 24-h food intake (kJ) and calorie intake were measured weekly according to the method describe elsewhere (17).
2.12 Plasma lipid profile, renal profile, liver profile, and leptin analysis
At the end of the study, on PND 21, all rats were fasted overnight prior to blood taking. For the blood-taking procedure, 5 ml of blood was collected in heparin tubes via cardiac puncture. The tubes were then centrifuged at 3,500 rpm for 15 min to obtain the plasma. Plasma was collected into a plain tube and stored at −20°C. Plasma lipid profiles [total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDH), and high-density lipoprotein (HDL)] were analyzed with a diagnostic reagent test kit (Roche, Germany) using Hitachi Automatic Analyzer 902 (Tokyo, Japan) (19). The serum leptin levels were measured based on the rat leptin ELISA kit provided by MyBioSource. The assays were performed according to the guideline provided with the sensitivity maintained at 1.0 ng/ml (20). Plasma was further analyzed for renal and liver profile using Alere Cholestech LDX® Analyzer (Alere, UK).
2.13 Histopathological analysis
Organs such as liver, kidney, colon, and fat tissue (visceral, retroperitoneal white adipose tissue) were collected after euthanizing all rats with carbon dioxide overdose. Tissue processing and sectioning were done on all preserved organs until a thin layer of paraffin ribbon is obtained, which was then placed in the water bath before being transferred to glass slides. All slides were then stained with hematoxylin and eosin (H&E) stain prior to histological viewing under the microscope (21). The presence of lesions and any form of abnormalities has been verified by a certified histopathologist from Universiti Putra Malaysia.
2.14 Statistical analysis
Statistical analyses were performed using SPSS 27.0, and the results were expressed as mean ± SEM. All data were tested for normality and compared using one-way analysis of variance followed by post-hoc least significant difference. p-value < 0.05 was considered significant. Comparison data between genders were analyzed by Student’s independent t-test while Pearson correlation test was used to find the correlation between body weight and calorie intake.
3 Results
3.1 Analysis of bioactive compounds of E. tapos yoghurt
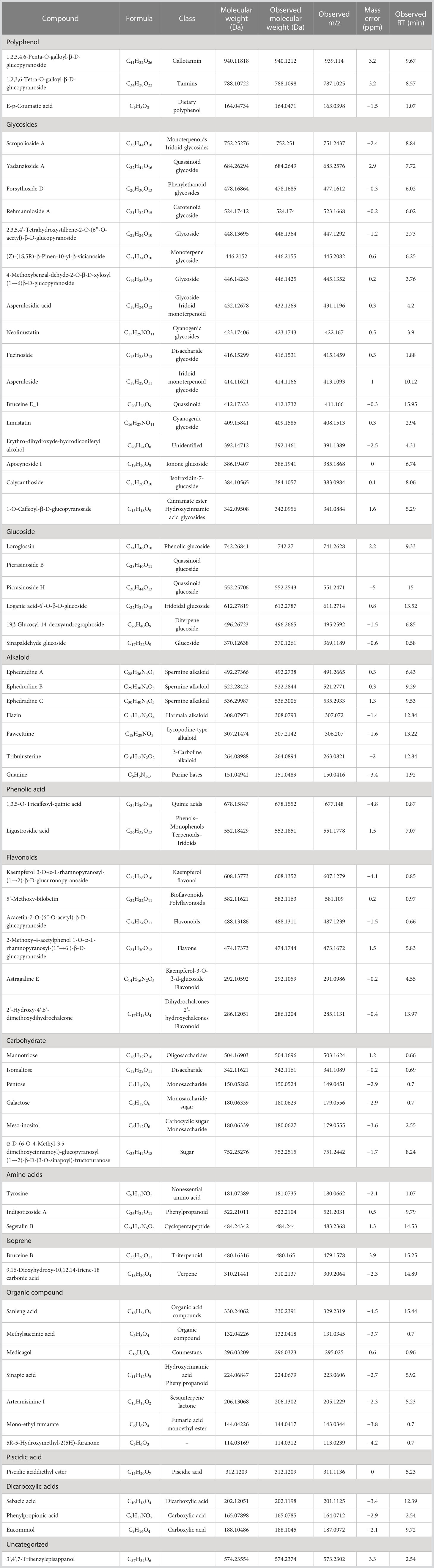
Figure 1 shows the chromatograms of bioactive compound quantification from 1 ml of E. tapos yoghurt and its peak maxima. The peak maxima were observed at 16.69 s followed by 18.58 s. Table 1 shows the isolated bioactive compounds from E. tapos yoghurt. All identified bioactive compounds from E. tapos yoghurt have been classified according to the class they belong to in Table 1. There are more than 12 classes of bioactive compounds identified from E. tapos yoghurt. The classes identified include polyphenols, glycosides, glucosides, alkaloids, flavonoids, phenolic acids, carbohydrates, amino acids, isoprenes, organic compounds, piscidic acids, and dicarboxylic acids. According to the TWIMS-QTOFMS analysis, E. tapos yoghurt as in Figure 1 shows various peaks at different time intervals. The highest peak was recorded at a retention time of 16.69 min for isomaltose, a disaccharide.
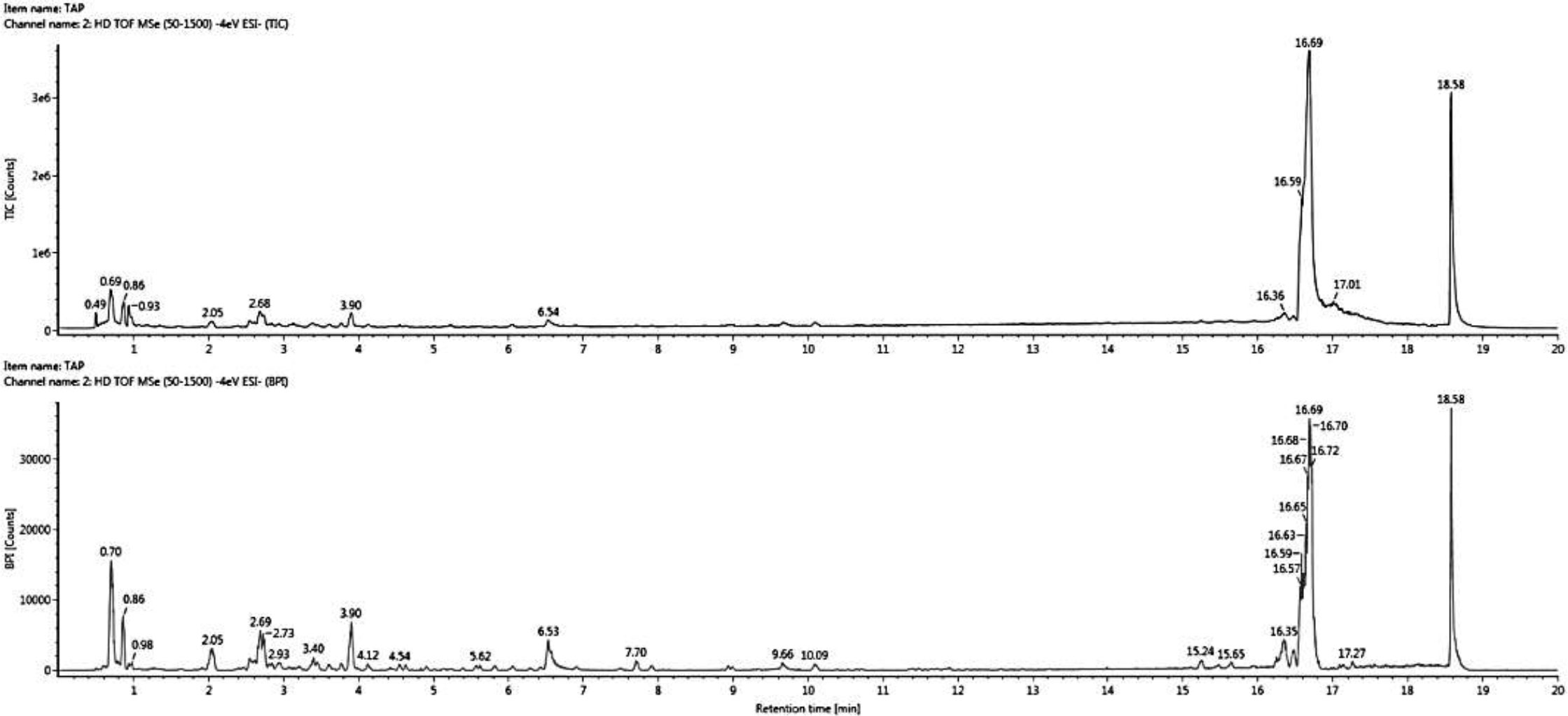
Figure 1 Comprehensive peak characterization trace showing bioactive compounds present in E. tapos yoghurt from TWIMS-QTOFMS analysis.
3.2 Changes in body weight and total calorie intake in dams after obesity induction prior to mating
Figure 2A shows the changes in body weight before and after obesity induction. The HS groups show significantly (p < 0.05) higher body weight (14%) compared to NS. Figure 2B shows the total calorie intake after obesity induction prior to mating. The result shows that the calorie intake in HS groups is significantly high (p < 0.05) compared to the NS group. The Pearson correlation shows a strong positive correlation (r = 0.92) between calorie intake and body weight.
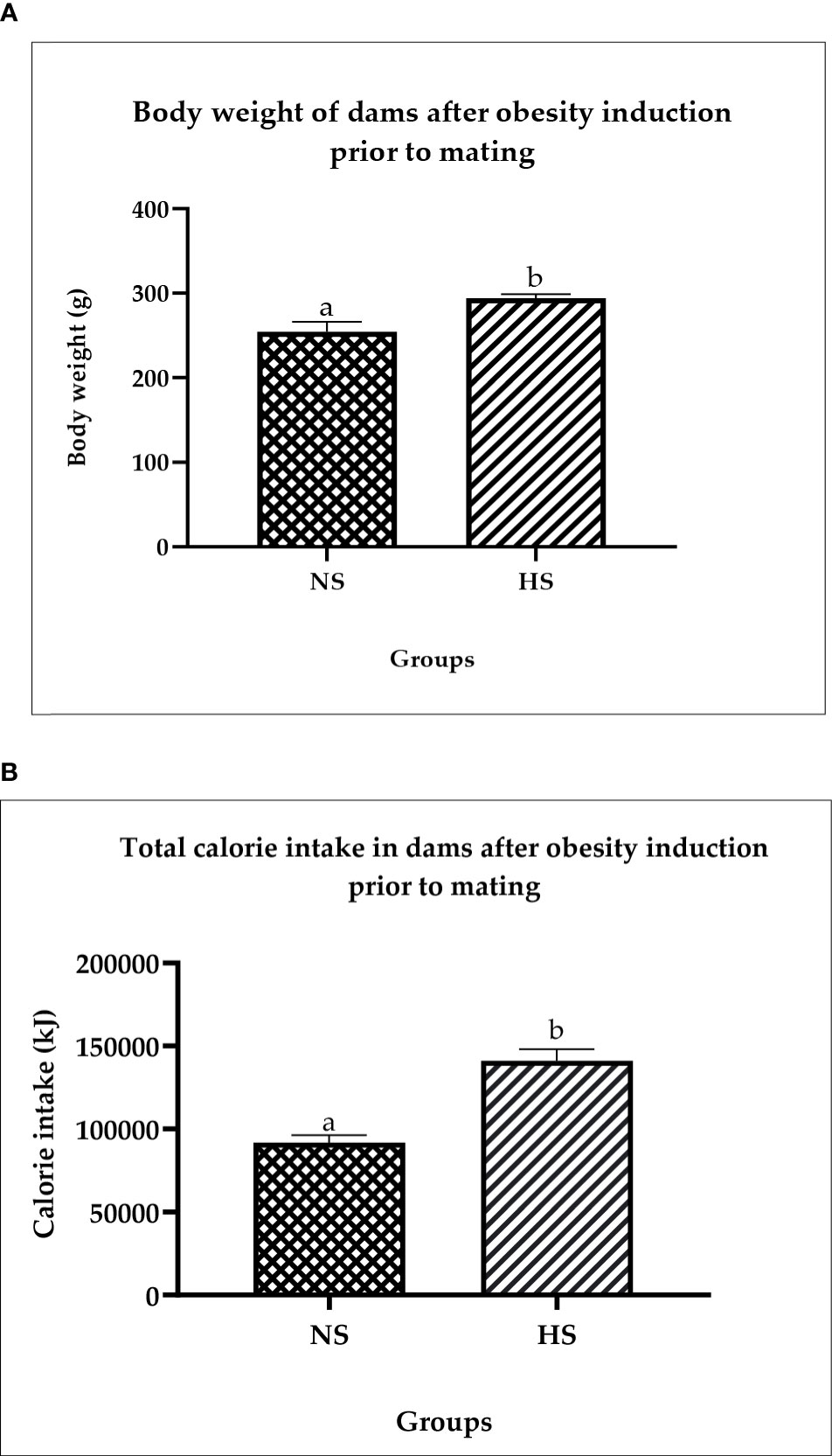
Figure 2 (A) Changes in body weight in dams after obesity induction prior to mating. NS: normal chow and saline; HS: HFD and saline. Values are expressed as mean ± SEM. Different letters indicate a significant difference at p < 0.05. (B) Total calorie in dams after obesity induction prior to mating. NS, normal chow and saline; HS, HFD and saline. Values are expressed as mean ± SEM. Different letters indicate a significant difference at p < 0.05.
3.3 Changes in body weight and total calorie intake in dams during weaning
Figure 3A shows the changes in obese dam’s body weight during weaning. The results show that the body weight of HS on PND 1, 7, 14, and 21 is significantly higher (p < 0.05) compared to NS. However, on PND 1, 7, 14, and 21, the HYT500-treated group shows significant (p < 0.05) reduction in body weight compared to HS. The mean value of HYT500 is similar to NS. There is no significant (p > 0.05) difference between the HY-, HY5-, and HYT50-treated groups on PND 1, 7, and 14. However, on PND 21, the body weight in HYT50 shows significant (p < 0.05) reduction compared to the HS group and the mean value of HYT50 is similar to NS. Figure 3B shows the total calorie intake during weaning. The data show that there is a significant increase (p < 0.05) of calorie intake in HS, HY, HYT5, and HYT50 groups compared to the NS, while there is a significant decrease (p < 0.05) of calorie intake in the HYT500 group compared to HS. There is a significant decrease (p < 0.05) of calorie intake in the HYT500 group compared to HY. The mean value for total calorie intake in HYT500 is similar to the NS group. Pearson correlation shows a strong positive correlation (r = 0.89) between total calorie intake and body weight in obese dams during weaning up to PND 21.
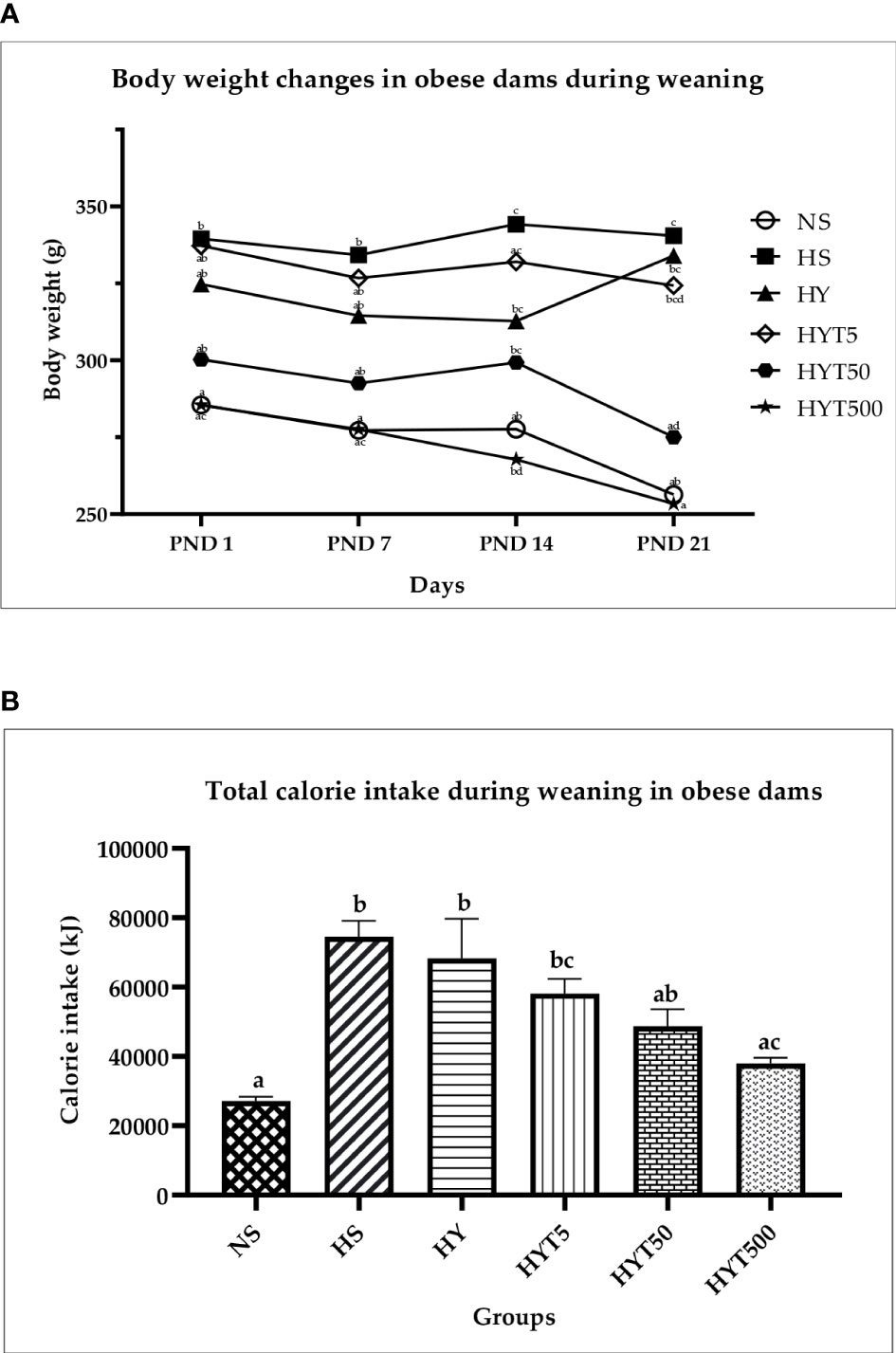
Figure 3 (A) Changes in body weight of dams during weaning. NS: normal chow and saline; HS: HFD and saline; HY: HFD and yoghurt; HYT5: HFD and 5 mg/kg of E tapos yoghurt; HYT50: HFD and 50 mg/kg of E tapos yoghurt; HYT500: HFD and 500 mg/kg of E tapos yoghurt. Values are expressed as mean ± SEM. Different letters indicate a significant difference at p < 0.05. (B) Total calorie intake in obese dams during weaning. NS, normal chow and saline; HS, HFD and saline; HY, HFD and yoghurt; HYT5, HFD and 5 mg/kg of E tapos yoghurt; HYT50, HFD and 50 mg/kg of E tapos yoghurt; HYT500, HFD and 500 mg/kg of E tapos yoghurt. Values are expressed as mean ± SEM. Different letters indicate a significant difference at p < 0.05.
3.4 The effect of E. tapos yoghurt on plasma leptin of obese dams on PND 21
Figure 4 shows the plasma leptin level of obese dams. The data show that the plasma leptin level of HS is significantly high (p < 0.05) compared to the NS group. There is no significant (p > 0.05) difference among HY, HYT5, and HYT50 groups on plasma leptin level. However, there is a significant (p < 0.05) reduction in plasma leptin level of the highest E. tapos yoghurt supplemented group (HYT500), and the mean value of HYT500 is comparable to that of the NS group on PND 21.
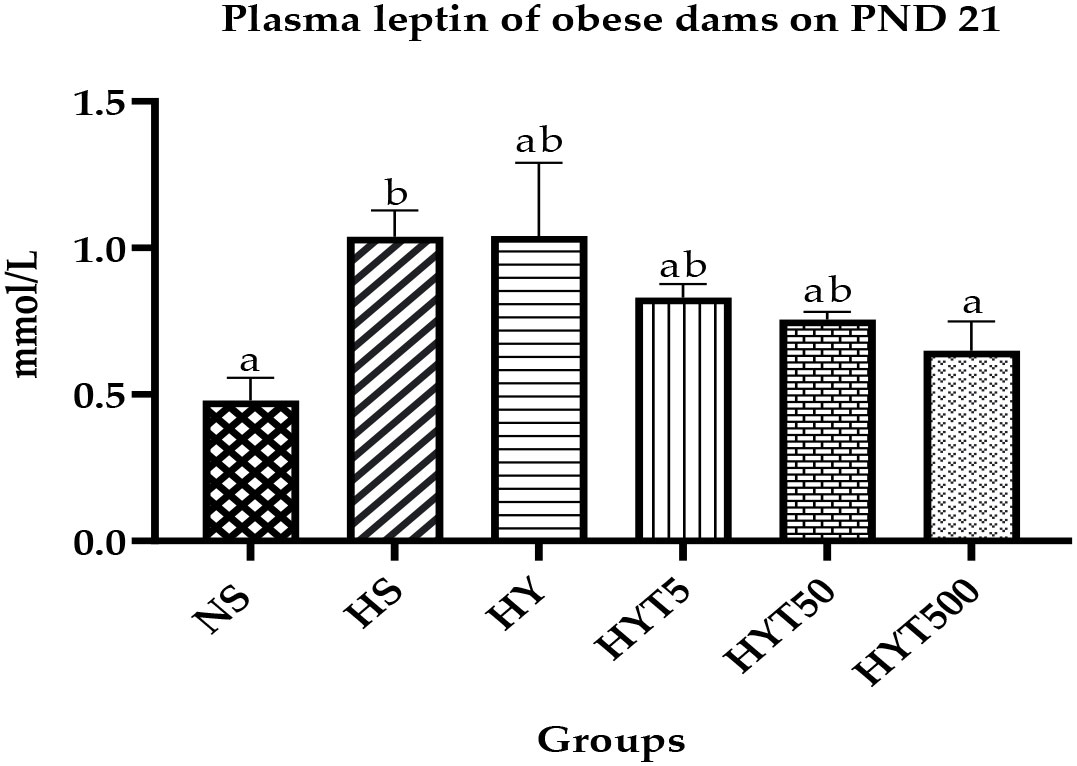
Figure 4 Plasma leptin level in obese dams on PND 21. NS, normal chow and saline; HS, HFD and saline; HY, HFD and yoghurt; HYT5, HFD and 5 mg/kg of E. tapos yoghurt; HYT50, HFD and 50 mg/kg of E. tapos yoghurt; HYT500, HFD and 500 mg/kg of E. tapos yoghurt. Values are expressed as mean ± SEM. Different letters indicate a significant difference at p < 0.05.
3.5 The effect of E. tapos yoghurt on obese dam’s renal profile on PND 21
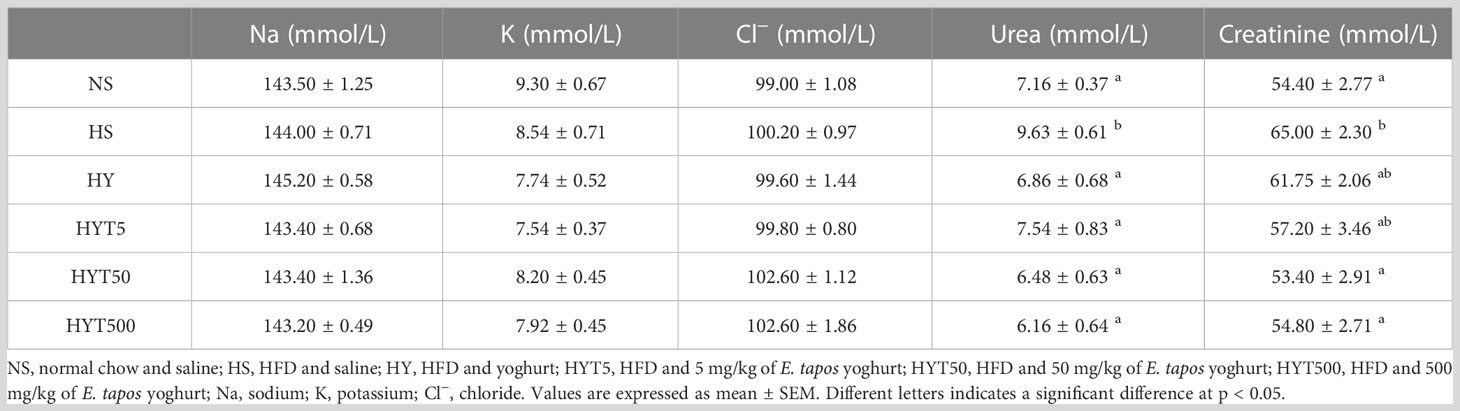
Table 2 shows the effect of E. tapos yoghurt on the obese dam’s renal profile. The data show that the plasma level of urea and creatinine is significantly high (p < 0.05) in the HS group compared to the NS group. However, the treatment with E. tapos yoghurt with all three different concentrations restores the plasma level of urea and creatinine in obese dams. In this setting, the HYT5, HYT50, and HYT500 groups show a significantly low (p < 0.05) level of urea compared to the HS group. The mean value of HYT5, HYT50, and HYT500 for plasma urea level correlates with the NS group’s mean value. Meanwhile, the HYT50 and HYT500 groups show a significantly low (p < 0.05) level of creatinine in plasma compared to the HS group. The mean value for plasma creatinine level for the HYT50 and HYT500 groups was almost similar to that of the NS group. However, there is no significant (p > 0.05) difference between NS, HS, HY, HYT5, and HYT50 on sodium (Na), potassium (K), and chloride (Cl−) levels in plasma of obese dams.
3.6 The effect of E. tapos yoghurt on obese dam’s liver enzymes on PND 21
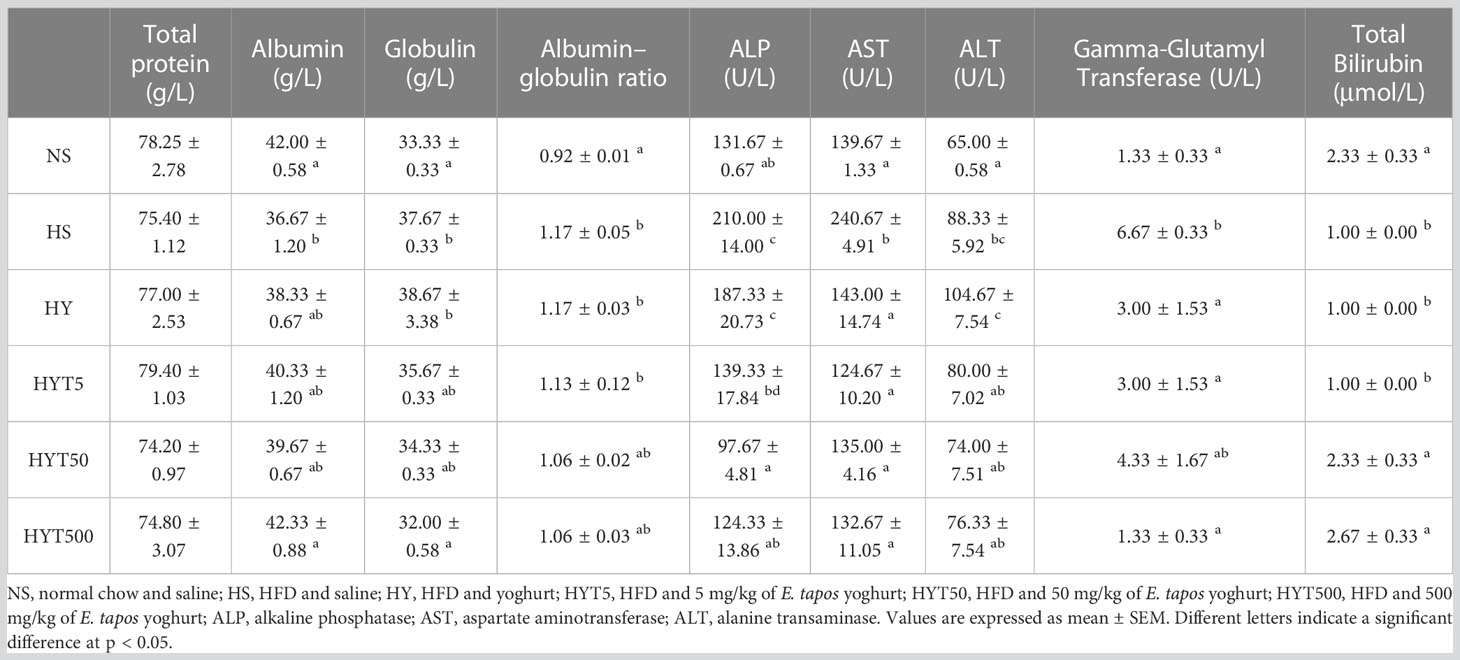
Table 3 shows the effect of E. tapos yoghurt on obese dam’s liver enzymes on different groups. The data show no significant difference (p > 0.05) on total protein between NS, HS, HY, HYT5, HYT50, and HYT500 groups. The level of albumin and total bilirubin is significantly low (p < 0.05) in the HS group compared to the NS group. However, the high E. tapos concentration (HYT500) shows a significant increase in albumin level while the HYT50 and HYT500 groups show a significant increase (p < 0.05) in total bilirubin compared to the HS group. The mean value of HYT500 for albumin level and that of HYT50 and HYT500 for total bilirubin level are almost similar to the NS group. There is no significant (p > 0.05) difference among HY, HYT5, and HYT50 compared to the HS group for plasma albumin level. Meanwhile, the levels of globulin, albumin/globulin ratio, alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine transaminase (ALT), and gamma-glutamyl transferase are significantly (p < 0.05) high in the HS group compared to the NS group. For globulin, the high concentration of E. tapos yoghurt (HYT500) significantly reduced the level of plasma globulin with an identical mean value to the NS group. There is no significant difference among HYT5 and HYT50 compared to the HS group for plasma globulin level. The HY group shows a significantly (p < 0.05) high level of globulin compared to the NS group and no significant (p > 0.05) difference compared to the HS group. For the albumin/globulin ratio, the mean value of HY and HYT5 is significantly (p < 0.05) high compared to the HS group. However, HYT50 and HYT500 show gradual reduction in plasma albumin/globulin ratio, yet there is no significant (p > 0.05) difference among HYT50 and HYT500 compared to the HS and NS groups. The level of plasma ALP is significantly (p < 0.05) low in the HY group compared to the HS group. However, the ALP level remains significantly (p < 0.05) high compared to the NS group. All three different concentrations of E. tapos yoghurt (HYT5, HYT50, and HYT500) significantly (p < 0.05) decrease the plasma ALP level compared to the HS group with a mean value similar to the NS group. The plasma level of AST is significantly (p < 0.05) low in the HY, HYT5, HYT50, and HYT500 groups compared to the HS group, with an identical mean value to the NS group. There is no significant (p > 0.05) difference among HY, HYT5, HYT50, and HYT500 in plasma AST level. Meanwhile, the HY group shows a significantly (p < 0.05) high level of ALT compared to the NS group. However, all three different concentrations of E. tapos yoghurt (HYT5, HYT50, and HYT500) significantly (p < 0.05) decrease the plasma ALT level compared to the HY group. There is no significant difference (p > 0.05) between HYT5, HYT50, and HYT500 compared to the HS or NS group. The level of gamma-glutamyl transferase is significantly (p < 0.05) decreased in HY, HYT5, and HYT500 compared to the HS group, with a mean value almost similar to the NS group. However, there is no significant difference (p > 0.05) recorded in the HYT50 group in comparison with the HY or NS group. The data for total bilirubin were significantly (p < 0.05) low for both HY and HYT5 compared to the HS group. However, HYT50 and HYT500 show a significant (p < 0.05) increase in total bilirubin level of plasma compared to the HS group. The mean value of HYT50 and HYT500 is similar to the NS group.
3.7 The effect of E. tapos yoghurt on obese dam’s lipid profile on PND 21
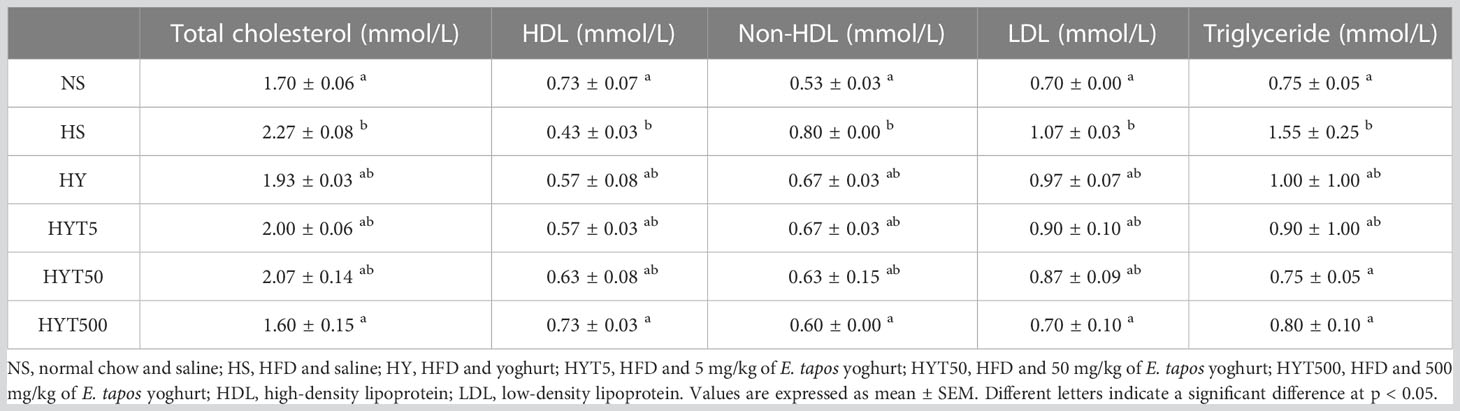
Table 4 shows the effect of E. tapos yoghurt on the obese dam’s lipid profile. The data show that the level of HDL is significantly low (p < 0.05) while the levels of non-HDL, LDL, and triglyceride are significantly high (p < 0.05) in the HS group compared to the NS group. However, the treatment with E. tapos yoghurt with all three different concentrations restores the lipid profile level in obese dams. The HYT500 group shows a significantly high level of HDL and low level of LDL, non-HDL, and triglyceride compared to the HS group. The mean value of the HYT500 group is almost similar to the NS group. However, there is no significant (p > 0.05) difference between HS, HY, HYT5, and HYT50 in lipid analyses.
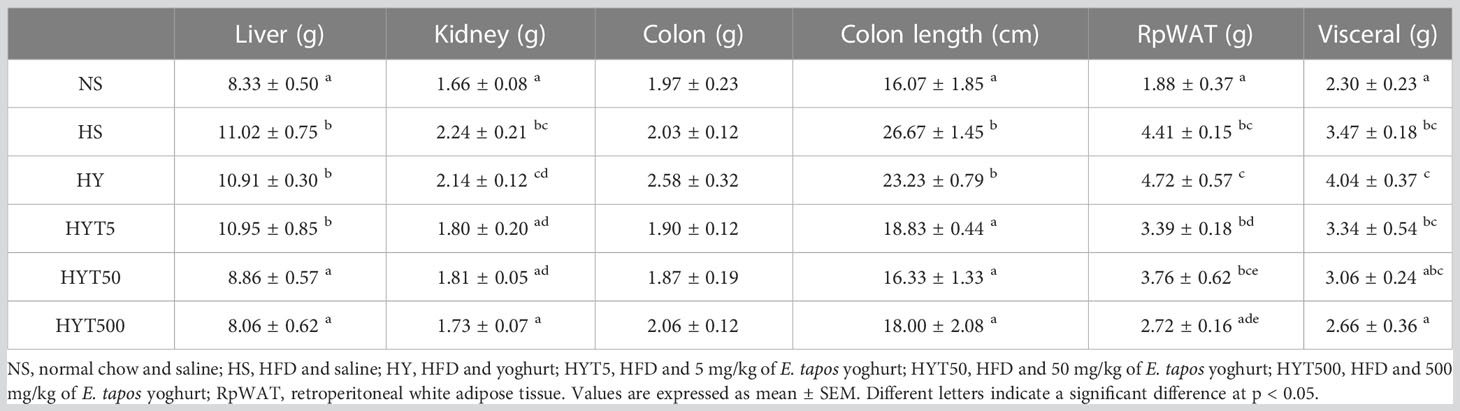
3.8 The effect of E. tapos yoghurt on obese dam’s organs on PND 21
Table 5 shows the effect of E. tapos yoghurt on obese dam’s organs up to PND 21. The data show a significant increase (p < 0.05) in the liver, kidney, retroperitoneal white adipose tissue (RpWAT), visceral weight, and the length of colon in the HS group compared to the NS group. E. tapos yoghurt treatment of HYT50 and HYT500 shows a significant decrease (p < 0.05) in liver weight compared to HS group. The mean value of HYT50 and HYT500 for liver weight is almost similar to the NS group. However, liver weight in the HY and HYT5 groups remains significantly high compared to the NS group with a mean value similar to the HS group. The kidney weight shows a significant decrease (p < 0.05) in the HYT5, HYT50, and HYT500 groups compared to the HS group. There is no significant difference (p > 0.05) in the HYT5, HYT50, and HYT500 groups compared to the NS group for kidney weight. However, the HY group’s kidney is significantly higher (p < 0.05) than NS and shows no significant (p > 0.05) difference compared to the HS group. Colon weight shows no significant (p > 0.05) difference between the HS, HY, HYT5, HYT50, and HYT500 groups compared to the NS group. However, colon length is significantly (p < 0.05) higher in the HS and HY groups compared to the NS group. Meanwhile, colon length is significantly (p < 0.05) shorter in the HYT5, HYT50, and HYT500 groups compared to the HS and HY groups. There is no significant (p > 0.05) difference in colon length of HYT5, HYT50, and HYT500 compared to the NS group. Both retroperitoneal and visceral fat tissue are significantly (p < 0.05) heavier in the HS group compared to the NS group. There is a significant (p < 0.05) reduction in RpWAT and visceral fat in the HYT500 group compared to the HS group and the mean value of the HYT500 group is similar to that of the NS group. However, in HY5 and HY50, the weight of RpWAT and visceral remains significantly high (p < 0.05) compared to the NS group and there is no significant (p > 0.05) difference between HY5 and HY50 in RpWAT weight compared to the HS group.
3.9 The effect of E. tapos yoghurt on histological changes of obese dams on PND 21
Figure 5 shows the effect of E. tapos yoghurt on the histological changes of kidney, liver, colon, RpWAT, and visceral of obese dams on PND 21. The histological analysis of kidney shows no abnormal lesion or tubular dilation. The glomerulus (G), distal convoluted tubule (DC), and renal corpuscle show normal appearance. Thus, no abnormalities were observed in kidney histology section in HS, HY, HYT5, HYT50, and HYT500 compared to the NS group. Meanwhile, the histological section of the liver in HS shows abnormal hepatocytes (H), sinusoids (s), and central vein (CV). The grading score for the HS group’s liver is recorded as 2 due to the presence of cell ballooning, more than 30% of cell steatosis, and lobular inflammation. The histological section of the HY group shows abnormal hepatocytes, sinusoids, and central vein. The grading score for the HY group liver is recorded as 1 due to the presence of cell ballooning and lobular inflammation and the absence of cell steatosis. However, the histological section of liver in NS, HYT5, HYT50, and HYT500 shows standard strands of hepatocytes, sinusoids, and central vein. It showed that 0% of biopsied hepatocytes were affected. The liver grading score for NS, HYT5, HYT50, and HYT500 was documented as 0 due to the absence of steatosis, lobular inflammation, and hepatocyte ballooning or lipid droplet. The histological section of the colon in HS shows severe detachment of epithelial cells, reduced mucosa content in the colonic wall, severe infiltration of lamina propria, severe inflammation, and the presence of fat deposition in the muscle layer, while submucosa and muscularis mucosa appear intact. The colonic tissue of the HY and HYT5 groups shows moderate level of infiltration in the lamina propria, moderate level of inflammation, and the presence of fat deposition in the muscle layer, while submucosa and muscularis mucosa appear intact. The colonic tissue of the HYT50 group shows a reduced level of infiltration in the lamina propria and inflammation compared to the HS group. Meanwhile, the colonic section of HYT500 shows a reduced level of infiltration in the lamina propria and fat deposition in the muscle layer compared to the HS group. The epithelial cell, mucosal content, and the architecture of submucosa and muscularis mucosa appear normal. The colonic tissue of the NS group shows normal architecture with normal appearance of epithelial cell, mucosal content, submucosa, and muscularis mucosa; the absence of infiltration in lamina propria; and no fat deposition in the muscle layer. The histological section of RpWAT and visceral of the HS group shows adipocyte expansion/hypertrophy of fat cells compared to NS. There are no abnormalities or hypertrophy of fat cells documented in RpWAT and visceral of HY, HYT5, HYT50, and HYT500 compared to NS. The histological structure of RpWAT and visceral of HY, HYT5, HYT50, and HYT500 appears similar to the NS group.
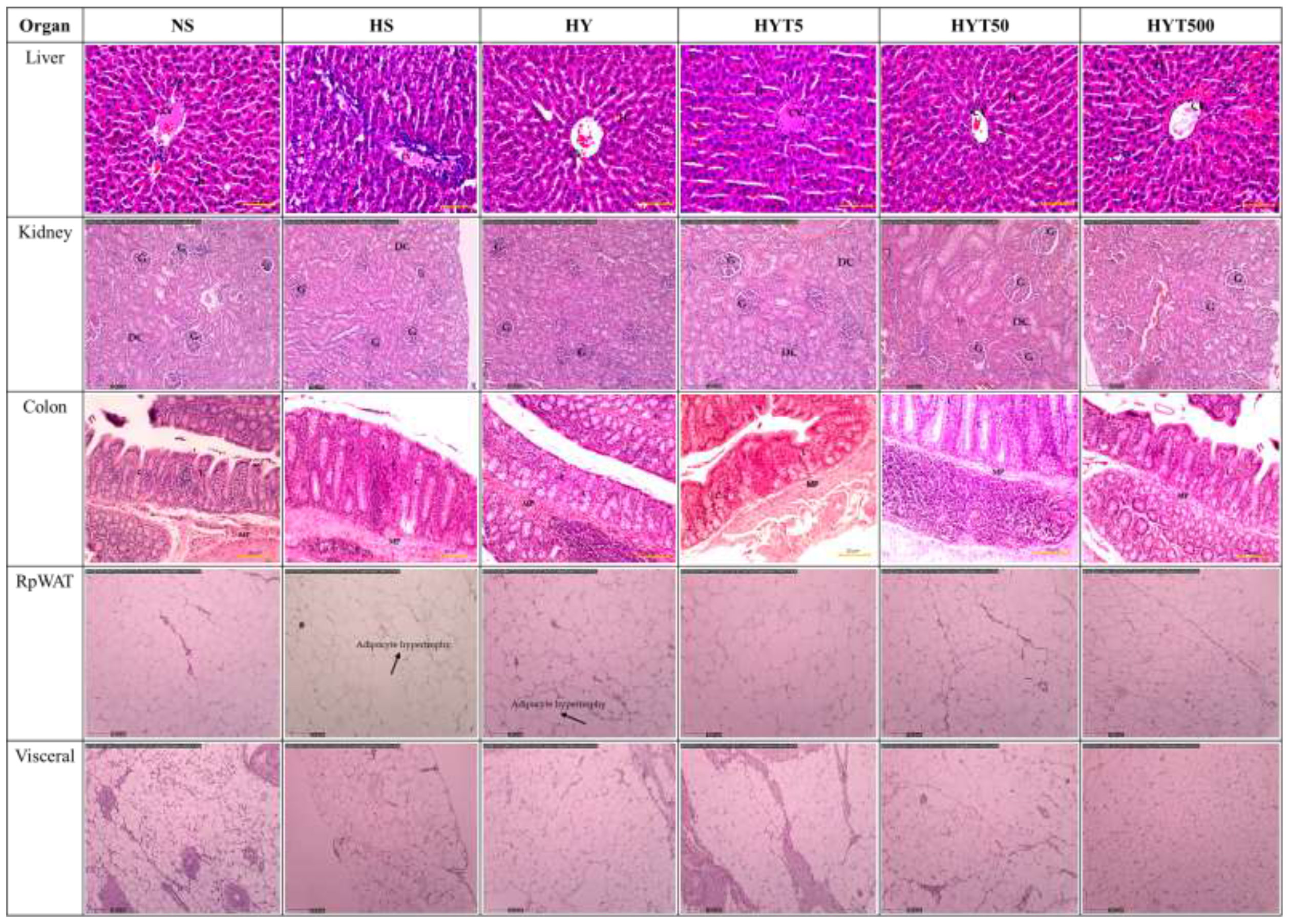
Figure 5 The effect of E. tapos yoghurt on the histological changes of kidney, liver, colon, RpWAT, and visceral of obese dams on PND 21. H, hepatocyte; CV, central vein; S, sinusoids; G, glomerulus; DC, distal convulated tubule; L, lamina propria; MP, mucosa; C, crypts of mucosa; RpWAT, retriperitoneal white adipose tissue.
4 Discussion
Maternal obesity is the key predictor for many health conditions and the emergence of childhood obesity. Studies claim that maternal obesity is associated with the child’s adiposity level, and in turn might affect the neurodevelopmental progress. HFD consumption is one of the main reasons for obesity (22). Reportedly, a study proves that HFD intake increases the obese gene product known as leptin, total body fat, and calorie intake (23). This, in turn, manifests in the changes in histological and morphological changes of organs, liver, and renal markers (24). Thus, HFD is considered to be one of the most studied methods to induce obesity (22). In relevance to this claim, the difference of mean value of 14% in the HS group compared to the NS group proves the successful obesity induction in female SD rats prior to mating in the first phase of this study (17). In contrast, a recent study shows that E. tapos extract naturally possesses the anti-obesity effect in obese dams (25). However, the specific mechanism of how E. tapos yoghurt acts as an anti-obesity agent has not been discovered and yet to be revealed in this study. Hence, this study summarizes the specific effects of E. tapos yoghurt on total calorie intake, liver enzymes, renal enzymes, obese gene products, lipid profile, morphological and histological changes in liver, kidney, colon, and fat tissues.
In the second phase of this study, the HS group shows the highest level of calorie intake, body weight retention, and increased concentration of plasma leptin on PND 21. Studies shows that maternal obesity induces hypothalamic leptin resistance, and it is directly proportional to the calorie intake and body fat (26). In such a condition, satiety will be suppressed through the brain–gut axis (27), triggering the over-intake of nutrients, eventually leading to an increase in body weight, which often manifests as increased body weight or body mass index (28). Meanwhile, obese dams supplemented with the highest dosage of E. tapos concentration show gradual reduction in total calorie intake, body weight, and plasma leptin concentration on PND 21 in the HYT500 group. This could be due to the presence of 5′-methoxy-bilobetin, a bioflavonoid compound that is commonly used to treat hyperlipidemic condition. Likewise, bioflavonoid compounds have a vital role in suppressing lipid production, triglycerides, and total cholesterol level (29). Moreover, the presence of polyphenols in E. tapos further modulates adipohormones and the expression of gut peptide hormone, thereby suppressing the appetite (27), leading to decreased calorie intake and reduced level of body weight.
Furthermore, obese dams without intervention in this study show a high level of urea and creatinine in the plasma while E. tapos yoghurt-supplemented obese dams have shown reduction in plasma urea and creatinine levels. These results are similar to those of the previous study that proves that the HFD-supplemented group stimulates changes in renal function, by reducing glomerular filtration rate (GFR), thereby causing creatinine and urea level to rise in plasma (30). The main reason for this is the increased lipid level in HFD-supplemented dams, which, in turn, affects the renal function (31). In addition, HFD consumption could activate the lipogenic molecular pathways that may increase the level of triglycerides and the total cholesterol level as seen in the HS group’s lipid profile, causing lipid accumulation, which will lead to the reduced level of GFR in the end (32). On the flipside, the presence of high concentration of glycosides such as Asperuloside in E. tapos yoghurt is proven to regulate metabolic homeostasis by reversing HFD-induced obesity via the modulation of gut dysbiosis. A recent study further proves the effect of Asperuloside in treating HFD-induced obesity by improving metabolism in adipose tissue (33). Therefore, the high concentration of Asperuloside in E. tapos yoghurt could be one of the major reasons for the gradual reduction of triglycerides, non-HDL, total cholesterol, and LDL as shown in the lipid analysis of obese dams.
Besides, the increase of triglycerides, non-HDL, total cholesterol, and LDL in obese dams further influences the dysregulation of liver enzymes. As seen in the HS group, the level of albumin is significantly reduced while the level of globulin is increased compared to the NS group. This is because obesity is classified as a chronic inflammatory condition where the hypertrophy of adipocytes and hypoxia is a common clinical manifestation. In such condition, excessive level of tumor necrosis factor alpha (TNFα) will be produced, causing the albumin level to reduce (34) and the globulin level to increase (35). Henceforth, the imbalance of albumin/globulin ratio as seen in the HS group is one of the key predictors for the presence of inflammation due to fat tissue hypertrophy. Similarly, ALP is commonly expressed in adipocytes and ALP plays a vital role in the regulation of intracellular deposition of fats. As such, hypertrophy of fat tissue will stimulate the release of ALP into the blood circulation, causing the plasma ALP to rise in obese subjects (36). Comparably, obesity correlates positively with the plasma concentration of AST, ALT, and GGT (37), and inversely correlates with bilirubin (38). This could probably be due to the fact that obesity stimulates the release of excessive oxidative stress due to the presence of steatosis, ballooning, or inflammation in liver (38). As a result, DNA methylation in the liver will increase, indicating liver damage (39). In addition, excessive visceral fat secretes leptins and TNFα, which may further increase inflammation in the liver (40). Therefore, elevation of AST, ALT, and GGT is a common indicator in obesity (37) as seen in the HS group.
Conversely, treatment with E. tapos yoghurt (HYT500) in HFD-induced obesity dams propitiously increases the level of total serum bilirubin and albumin, and decreases the level of globulin, albumin–globulin ratio, ALT, AST, ALP, and GGT. The effectiveness of E. tapos yoghurt in regulating liver enzymes may be due to the presence of bioactive compounds that naturally possess anti-inflammatory properties such as Scropolioside A (41), Picrasinoside B (42), Ephedradine B (43), Indigoticoside A (44), Bruceine B (45), Asperulosidic acid (46), 2,3,5,4’-Tetrahydroxystilbene-2-O-(6’’-O-acetyl)-β-D-glucopyranoside (47), Astragaline E (48), and Sinapic acid (49). For instance, 2,3,5,4’-Tetrahydroxystilbene-2-O-(6’’-O-acetyl)-β-D-glucopyranoside could induce autophagy in liver via the activation of PI3K/Akt and Erk signaling pathway (50). In obesity, the supply of hypernutrition will deactivate AMPK, causing the activation of mTORC1, leading to the inactivation or suppression of autophagy. In such condition, lipid droplets will accumulate in the liver (51). As such, stimulation of autophagy will enhance lipid droplet removal and reduce the level of oxidative stress production as well as lipid peroxidation, thereby protecting the hepatocytes (52).
Furthermore, an increased level of triglyceride will cause fat deposition in the liver, leading to the development of abnormal hepatocytes, cell ballooning, inflammation, and scarring (53). This will eventually increase the weight of the liver as seen in the HS group’s morphological and histological analysis in this study. Lipid accumulation in the kidney may cause kidney weight to increase as well (30). In addition, studies show that the length of the intestine is longer in obese subjects compared to lean subjects (54). In fact, hypernutrition due to HFD intake compromises uncontrolled cell proliferation and length and depth of intestine villi, causing the length of the colon to increase (55). This is the main reason for the increase in colon length in the HS group in this study, which is further characterized by the detachment of epithelial cells and infiltration of fat in the submucosa. Hypertrophy of fat tissue in RpWAT and visceral in the HS group further defines the characteristics of obesity model in this study. In contrast, the oral administration of E. tapos yoghurt in HFD-induced maternal obese dams successfully reverses the abnormal condition in most of the organs. The presence of non-essential amino acids in E. tapos yoghurt such as tyrosine enhances lipid and carbohydrate metabolism, thereby promoting fat loss (56) in HFD-supplemented obese dams. As a result, HYT5-, HYT50-, and HYT500-supplemented groups’ organ histology appears to have an almost similar architecture to the NS group.
5 Conclusions
Maternal obesity is the root cause of childhood obesity. Thus, curbing maternal obesity is the best option to prevent the emerging critical cases of childhood obesity and its consequences. This study discovers the therapeutic effects of E. tapos yoghurt as an anti-obesity agent in maternal obese dams, specifically focusing into each possible parameters based on physiological, biochemical, and histological analysis. The bioactive compounds present in E. tapos yoghurt naturally possess the ability to suppress inflammation and appetite, thereby stimulating fat loss, and eventually causing gradual weight loss. From this study, we conclude that E. tapos yoghurt possesses no side effect. Hence, supplementation of E. tapos yoghurt during the gestational period up to weaning has been effective in the gradual weight loss of maternal obese dams from the 500-mg/kg-supplemented group in this study. As such, this study proves the effectiveness of the first medicinal plant-integrated yoghurt in curbing maternal obesity, and these data provide useful insights into considering natural probiotics such as yoghurt as a therapeutic agent to treat maternal obesity instead of modern drugs.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by All animal study was performed upon obtaining approval from Institutional Animal Care and Use Committee (IACUC), Universiti Putra Malaysia. The animal ethics committee granted approval for this study under the code UPM/IACUC/AUP-R025/2022.
Author contributions
Conceptualization, RN and RR. Methodology, FO. Software, SB. Validation, HB, MY, and ST. Formal analysis, RN. Investigation, RN. Resources, AJ. Data curation, AA. Writing—original draft preparation, RN. Writing—review and editing, RN. Visualization, HE. Supervision, HB and MY. Project administration, SJ. Funding acquisition, HB and MY. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Ministry of Higher Education, Malaysia, through the Fundamental Research Grant Scheme, with the reference number Universiti Putra Malaysia 04-0L-20-2274FR and the project code FRGS/1/2020/SKK0/UPM/02/4.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Forno AE, Young OM. Maternal obesity in pregnancy , gestational weight gain , and risk of childhood asthma. Pediatrics (2014) 134:535–46. doi: 10.1542/peds.2014-0439
2. Rhee KE, Phelan S, Mccaffery J. Early determinants of Obesity : genetic , epigenetic , and In utero influences. Int J Pediatr (2012) 2012. doi: 10.1155/2012/463850
3. Heslehurst N, Vieira R, Akhter Z, Bailey H, Slack E, Ngongalah L, et al. The association between maternal body mass index and child obesity : a systematic review and meta-analysis. PloS Med (2019) 16:e1002817. doi: 10.1371/journal.pmed.1002817
4. Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics (2014) 33:673–89. doi: 10.1007/s40273-014-0243-x
5. Dodd JM, Grivell RM, Deussen AR, Hague WM. Metformin for women who are overweight or obese during pregnancy for improving maternal and infant outcomes. Cochrane Database Syst Rev (2018) 7:CD010564. doi: 10.1002/14651858.CD010564.pub2
6. Jorquera G, Echiburú B, Crisosto N, Sotomayor-Zárate R, Maliqueo M, Cruz G. Metformin during pregnancy: effects on offspring development and metabolic function. Front Pharmacol (2020) 11:653/BIBTEX. doi: 10.3389/FPHAR.2020.00653/BIBTEX
7. Jarski RW, Trippett DL. The risks and benefits of exercise during pregnancy. J Fam Pract (1990) 30:185–9.
8. Perumal KV, Ja’afar NL, Taib CNM, Shafie NH, Bahari H. Antiobesity activity of Elateriospermum tapos shell extract in obesity-induced sprague dawley rats. Molecules (2021) 26:1–10. doi: 10.3390/molecules26020321
9. Naomi R, Rusli RNM, Balan SS, Othman F, Jasni AS, Jumidil SH, et al. E. tapos yoghurt–a view from nutritional composition and toxicological evaluation. Foods (2022) 11:1–19. doi: 10.3390/foods11131903
10. He A, Chin J, Lomiguen CM. Benefits of probiotic yogurt consumption on maternal health and pregnancy outcomes: a systematic review. Cureus (2020) 12:e9408. doi: 10.7759/cureus.9408
11. Tisadondilok S, Senawong T, Swatsitang P, Rattanasing A. Antioxidant and antiproliferative activities of ethanolic extracts of Elateriospermum tapos blume (Euphorbiaceae). J Med Plants Res (2018) 12:474–82. doi: 10.5897/jmpr2018.6666
12. Siccama JW, Pegiou E, Zhang L, Mumm R, Hall RD, Boom RM, et al. Maltodextrin improves physical properties and volatile compound retention of spray-dried asparagus concentrate. LWT-Food Sci Technol (2021) 142:111058. doi: 10.1016/j.lwt.2021.111058
13. Aril-Dela Cruz JV, Bungihan ME, Dela Cruz TEE, Sagum RS. Canarium ovatum engl. (Pili) exocarp crude extract as functional food colorant incorporated in yogurt developed product. Food Res (2017) 2:89–98. doi: 10.26656/fr.2017.2(1).173
14. Farooq MU, Mumtaz MW, Mukhtar H, Rashid U, Akhtar MT, Raza SA, et al. UHPLC-QTOF-MS/MS based phytochemical characterization and anti-hyperglycemic prospective of hydro-ethanolic leaf extract of butea monosperma. Sci Rep (2020) 10:1–14. doi: 10.1038/s41598-020-60076-5
15. Perumal KV, Ja’afar NL, Balan SS, Zainal Abidin A, Arapoc DJ, Shafie NH, et al. Preventive effect of Elateriospermum tapos seed extract against obese sprague dawley rats. Orient Pharm Exp Med (2019) 20:1–7. doi: 10.1007/S13596-019-00394-W
16. Balan SS, Abidin AZ, Perumal KV, Lotafi AHA, Danabala S, Manimaran M, et al. Effect of Elateriospermum tapos extract as coadjuvant in ameliorating maternal obesity on female offspring at weaning. Malaysian J Microsc (2019) 15:111–28.
17. Kadir NAA, Rahmat A, Jaafar HZE. Protective effects of tamarillo extract against high fat diet induced obesity in sprague dawley rats. J Obes (2015) 2015:1–8. doi: 10.1155/2015/846041
18. Ypsilantis P, Somalou P, Panidou E, Simopoulos C. Laparoscopic early pregnancy diagnosis in the laboratory rat. Lab Anim (2018) 52:265–70. doi: 10.1177/0023677217723933
19. Aberare OL, Okuonghae P, Mukoro N, Dirisu JO, Osazuwa F, Odigie E, et al. Triglycerides, total cholesterol, high density lipoprotein cholesterol and low density lipoprotein cholesterol in rats exposed to premium motor spirit fumes. N Am J Med Sci (2011) 3:277–80. doi: 10.4297/najms.2011.3277
20. Labban RSM, Alfawaz HA, Amina M, Bhat RS, Hassan WM, El-Ansary A. Synergism between extracts of garcinia mangostana pericarp and curcuma in ameliorating altered brain neurotransmitters, systemic inflammation, and leptin levels in high-fat diet-induced obesity in Male wistar albino rats. Nutrients (2022) 14:4630. doi: 10.3390/nu14214630
21. Balan SS, Abidin AZ, Perumal KV, Shafie NH, Abdullah MA, Jasni AS, et al. Transgenerational evaluation of Elateriospermum tapos extracts on the male offspring of obesity-induced sprague dawley rats. Sains Malaysiana (2021) 50:3045–57. doi: 10.17576/jsm-2021-5010-17
22. Sullivan EL, Nousen EK, Chamlou KA, Grove KL. The impact of maternal high-fat diet consumption on neural development and behavior of offspring. Int J Obes Suppl (2012) 2:S7–S13. doi: 10.1038/ijosup.2012.15
23. Ainslie DA, Proietto J, Fam BC, Thorburn AW. Short-term, high-fat diets lower circulating leptin concentrations in rats. Am J Clin Nutr (2000) 71:438–42. doi: 10.1093/ajcn/71.2.438
24. Sucedaram Y, Johns EJ, Husain R, Sattar MA, Abdulla MH, Nelli G, et al. Exposure to high-fat style diet induced renal and liver structural changes, lipid accumulation and inflammation in intact and ovariectomized female rats. J Inflamm Res (2021) 14:689–710. doi: 10.2147/JIR.S299083
25. Bahari H, Abidin AZ, Balan SS, Perumal KV, Rosli NS, Lotafi AHA, et al. The effects of elateriospermum tapos against obese maternal rat in mitigating obesity development among their adult female offspring. Pharmacogn -Magazine (2020) 16:706–12. doi: 10.4103/pm.pm_142_20
26. Jara A, Dreher M, Porter K, Christian LM. The association of maternal obesity and race with serum adipokines in pregnancy and postpartum: implications for gestational weight gain and infant birth weight. Brain Behav Immun - Heal (2020) 3:100053. doi: 10.1016/J.BBIH.2020.100053
27. Singh M, Thrimawithana T, Shukla R, Adhikari B. Managing obesity through natural polyphenols: a review. Futur Foods (2020) 1–2:100002. doi: 10.1016/j.fufo.2020.100002
28. Obradovic M, Sudar-Milovanovic E, Soskic S, Essack M, Arya S, Stewart AJ, et al. Leptin and obesity: role and clinical implication. Front Endocrinol (Lausanne) (2021) 12:585887. doi: 10.3389/fendo.2021.585887
29. Šamec D, Karalija E, Dahija S, Hassan STS. Biflavonoids: important contributions to the health benefits of ginkgo (Ginkgo biloba l.). Plants (2022) 11:1381. doi: 10.3390/PLANTS11101381
30. Muller CR, Leite APO, Yokota R, Pereira RO, Americo ALV, Nascimento NRF, et al. Post-weaning exposure to high-fat diet induces kidney lipid accumulation and function impairment in adult rats. Front Nutr (2019) 6:60. doi: 10.3389/FNUT.2019.00060
31. Martins AR, Más S. Lipotoxicity and kidney. Port J Nephrol Hypertens (2015) 29:306–15. doi: 10.32932/pjnh
32. Sun Y, Ge X, Li X, He J, Wei X, Du J, et al. High-fat diet promotes renal injury by inducing oxidative stress and mitochondrial dysfunction. Cell Death Dis 2020 1110 (2020) 11:1–14. doi: 10.1038/s41419-020-03122-4
33. Nakamura A, Yokoyama Y, Tanaka K, Benegiamo G, Hirayama A, Zhu Q, et al. Asperuloside improves obesity and type 2 diabetes through modulation of gut microbiota and metabolic signaling. iScience (2020) 23:101522. doi: 10.1016/J.ISCI.2020.101522
34. Mosli HH. Obesity and morbid obesity associated with higher odds of hypoalbuminemia in adults without liver disease or renal failure. Dovepress (2017) 2017:467–72. doi: 10.2147/DMSO.S149832
35. Teitelbaum JI, Cooperberg AA, Kalant N. Effect of a high-fat diet on the concentration of antihemophilic globulin and factor V. Can Med Assoc J (1962) 87:1001.
36. Khan AR, Awan FR, Najam SS, Islam M, Siddique T, Zain M. Elevated serum level of human alkaline phosphatase in obesity. J Pak Med Assoc (2015) 65:1182–5.
37. Jalili V, Poorahmadi Z, Hasanpour Ardekanizadeh N, Gholamalizadeh M, Ajami M, Houshiarrad A, et al. The association between obesity with serum levels of liver enzymes, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase and gamma-glutamyl transferase in adult women. Endocrinol Diabetes Metab (2022) 5:1–6. doi: 10.1002/edm2.367
38. Žiberna L, Jenko-Pražnikar Z, Petelin A. Serum bilirubin levels in overweight and obese individuals: the importance of anti-inflammatory and antioxidant responses. Antioxidants (2021) 10:1352. doi: 10.3390/antiox10091352
39. Horvath S, Erhart W, Brosch M, Ammerpohl O, Von Schönfels W, Ahrens M, et al. Obesity accelerates epigenetic aging of human liver. Proc Natl Acad Sci USA (2014) 111:15538–43. doi: 10.1073/pnas.1412759111
40. Wree A, Kahraman A, Gerken G, Canbay A. Obesity affects the liver – the link between adipocytes and hepatocytes. Digestion (2011) 83:124–33. doi: 10.1159/000318741
41. Zhu T, Zhang L, Ling S, Qian F, Li Y, Xu JW. Anti-inflammatory activity comparison among scropoliosides-catalpol derivatives with 6-o-substituted cinnamyl moieties. Molecules (2015) 20:19823–36. doi: 10.3390/molecules201119659
42. Lee J, Gong Y-X, Jeong H, Seo H, Xie D-P, Sun H-N, et al. Pharmacological effects of picrasma quassioides (D. don) benn for inflammation, cancer and neuroprotection (Review). Exp Ther Med (2021) 22:1–16. doi: 10.3892/etm.2021.10792
43. Khattabi L, Boudiar T, Bouhenna MM, Chettoum A, Chebrouk F, Chader H, et al. RP-HPLC-ESI-QTOF-MS qualitative profiling, antioxidant, anti-enzymatic, anti-inflammatory and non-cytotoxic properties of ephedra alata monjauzeana. Foods (2022) 11:1–18. doi: 10.3390/foods11020145
44. Xiao P, Huang H, Chen J, Li X. In vitro antioxidant and anti-inflammatory activities of radix isatidis extract and bioaccessibility of six bioactive compounds after simulated gastro-intestinal digestion. J Ethnopharmacol (2014) 157:55–61. doi: 10.1016/J.JEP.2014.09.005
45. Utoguchi N, Nakata T, Cheng HH, Ikeda K, Makimoto H, Mu Y, et al. A potent inhibitor of leukocyte-endothelial cell adhesion. Inflammation (1997) 21:223–33. doi: 10.1023/A:1027374321718
46. He J, Lu X, Wei T, Dong Y, Cai Z, Tang L, et al. Asperuloside and asperulosidic acid exert an anti-inflammatory effect via suppression of the NF-κB and MAPK signaling pathways in LPS-induced RAW 264.7 macrophages. Int J Mol Sci (2018) 19:2027. doi: 10.3390/IJMS19072027
47. Chen YH, Chen YC, Chin YT, Wang CC, Hwang LL, Yang LY, et al. 2, 3, 5, 4’-tetrahydroxystilbene-2-O-beta-D-glucoside protects against neuronal cell death and traumatic brain injury-induced pathophysiology. Aging (Albany NY) (2022) 14:2607–27. doi: 10.18632/aging.203958
48. Hu X, Wang M, Pan Y, Xie Y, Han J, Zhang X, et al. Anti-inflammatory effect of astragalin and chlorogenic acid on escherichia coli-induced inflammation of sheep endometrial epithelium cells. Front Vet Sci (2020) 7:201/BIBTEX. doi: 10.3389/FVETS.2020.00201/BIBTEX
49. Yun KJ, Koh DJ, Kim SH, Park SJ, Ryu JH, Kim DG, et al. Anti-inflammatory effects of sinapic acid through the suppression of inducible nitric oxide synthase, cyclooxygase-2, and proinflammatory cytokines expressions via nuclear factor-kappaB inactivation. J Agric Food Chem (2008) 56:10265–72. doi: 10.1021/JF802095G
50. Wang X, Zeng J, Wang X, Li J, Chen J, Wang N, et al. 2,3,5,4’-tetrahydroxystilbene-2-O-β-D-glucoside induces autophagy of liver by activating PI3K/Akt and erk pathway in prediabetic rats. BMC Complement Med Ther (2020) 20:177. doi: 10.1186/s12906-020-02949-w
51. Namkoong S, Cho C, Semple I, Lee JH. Molecules and cells autophagy dysregulation and obesity-associated pathologies. Mol Cells (2018) 41:3–10. doi: 10.14348/molcells.2018.2213
52. Czaja MJ, Ding WX, Donohue TM, Friedman SL, Kim JS, Komatsu M, et al. Functions of autophagy in normal and diseased liver. Autophagy (2013) 9:1131–58. doi: 10.4161/auto.25063
53. Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic and clinical implications. Hepatology (2010) 51:679–89. doi: 10.1002/hep.23280
54. Al Mushref M, Srinivasan S. Effect of high fat-diet and obesity on gastrointestinal motility. Ann Transl Med (2012) 1:14. doi: 10.3978/j.issn.2305-5839.2012.11.01
55. Dailey MJ. Nutrient-induced intestinal adaption and its effect in obesity. Physiol Behav (2014) 78:74–8. doi: 10.1016/J.PHYSBEH.2014.03.026
Keywords: probiotic, maternal programming, obesity, natural food product, inflammation
Citation: Naomi R, Rusli RNM, Othman F, Balan SS, Abidin AZ, Embong H, Teoh SH, Jasni AS, Jumidil SH, Bahari H and Yazid MD (2023) The role of Elateriospermum tapos yoghurt in mitigating high-fat dietary cause of maternal obesity—an experimental study. Front. Endocrinol. 14:1131830. doi: 10.3389/fendo.2023.1131830
Received: 26 December 2022; Accepted: 31 May 2023;
Published: 21 June 2023.
Edited by:
Elena Succurro, University of Magna Graecia, ItalyReviewed by:
Zubaidah Hasain, National Defence University of Malaysia, MalaysiaElaine De Oliveira, Rio de Janeiro State University, Brazil
Copyright © 2023 Naomi, Rusli, Othman, Balan, Abidin, Embong, Teoh, Jasni, Jumidil, Bahari and Yazid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hasnah Bahari, haba@upm.edu.my; Muhammad Dain Yazid, dain@ukm.edu.my
 Ruth Naomi
Ruth Naomi Rusydatul Nabila Mahmad Rusli1
Rusydatul Nabila Mahmad Rusli1 Fezah Othman
Fezah Othman Santhra Segaran Balan
Santhra Segaran Balan Hashim Embong
Hashim Embong Soo Huat Teoh
Soo Huat Teoh Azmiza Syawani Jasni
Azmiza Syawani Jasni Hasnah Bahari
Hasnah Bahari Muhammad Dain Yazid
Muhammad Dain Yazid