- 1Department of Internal Medicine, National Taiwan University College of Medicine, Taipei, Taiwan
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
- 3National Institute of Environmental Health Sciences of the National Health Research Institutes, Zhunan, Taiwan
Background: This study investigated the risk of prostate cancer in ever users and never users of rosiglitazone in diabetes patients in Taiwan.
Methods: The nationwide database of the National Health Insurance was used to enroll male patients who had a new diagnosis of type 2 diabetes mellitus at an age ≥ 25 years from 1999 to 2005. A total of 11,495 ever users and 11,495 never users of rosiglitazone matched on propensity score were selected and they were followed up for the incidence of prostate cancer from January 1, 2006 until December 31, 2011. Cox proportional hazard model incorporated with the inverse probability of treatment weighting using the propensity score was used to estimate hazard ratios.
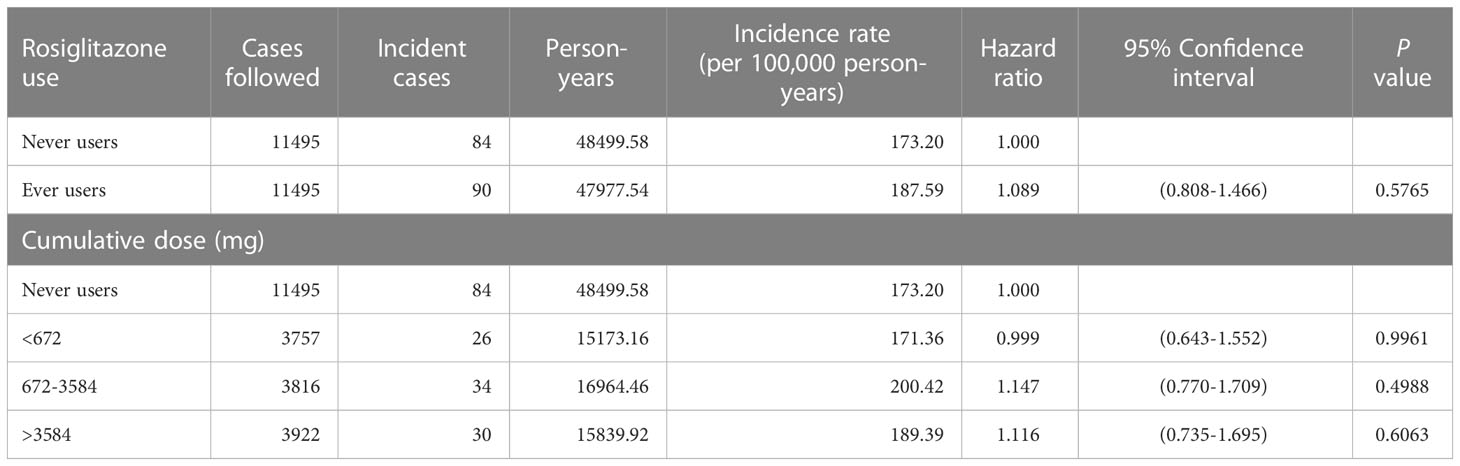
Results: At the end of follow-up, incident cases of prostate cancer were found in 84 never users and 90 ever users of rosiglitazone. The calculated incidence was 173.20 per 100,000 person-years in never users and was 187.59 per 100,000 person-years in ever users. The overall hazard ratio (95% confidence intervals) for ever versus never users was 1.089 (0.808-1.466). The hazard ratios were 0.999 (0.643-1.552) for the first tertile (< 672 mg), 1.147 (0.770-1.709) for the second tertile (672-3584 mg) and 1.116 (0.735-1.695) for the third tertile (> 3584 mg) of cumulative dose. Sensitivity analyses consistently showed a null association between rosiglitazone and prostate cancer risk.
Conclusion: Rosiglitazone has a null effect on the risk of prostate cancer.
Introduction
Prostate cancer is the most common incident cancer in men over the world (1). It was estimated that there were 1.4 million cases of incident prostate cancer and 381,000 deaths ascribed to prostate cancer in 2016 (1). An increase of 40% in prostate cancer cases has been observed within the 10 years following 2006, probably because of the aging and growing population in the world (2). Incidence rates of prostate cancer are highest among the white people and lowest in the Asian populations, and may vary remarkably by 25-fold in different ethnicities (2). Although secular trend of prostate cancer shows a declining rate in the western world, the incidence of prostate cancer is increasing in Asian populations (3–6). Epidemiological data from Taiwan also show a steadily increasing trend in the incidence of (7) and mortality from (8) prostate cancer over the past decades. Although different times of adoption of prostate-specific antigen (PSA) as a screening tool in different countries may partly explain the discrepant trends observed in different ethnicities, genetic variations and changes in the prevalence of risk factors such as population aging and changes in dietary patterns with increasing rates of animal fat consumption and lifestyle changes with less physical activity and lack of exercise leading to obesity etc. are also possible explanations (2).
An increased risk of various types of cancer has been observed in patients with type 2 diabetes mellitus (T2DM). Although the mechanisms are not yet fully known, obesity, glycemic control, hyperinsulinemia, insulin resistance, comorbidities or antidiabetic drugs used to treat the patients are possible contributors (9–14). In contrary to a lower risk of prostate cancer being demonstrated in patients with T2DM in western countries (15, 16), a positive association in terms of mortality (8), incidence (7) and prevalence (17) has been observed in the Taiwanese population and in other Asian populations (18). A meta-analysis that includes 11 cohort studies conducted worldwide also supports that diabetes mellitus is associated with a significantly higher risk of all-cause mortality in patients with prostate cancer, prostate cancer-specific mortality and non-prostate cancer mortality (19).
Peroxisome proliferator-activator receptor gamma (PPARγ) is a nuclear receptor that functions as a transcription factor. Normal prostate and prostate cancer cells express PPARγ (20). Recent in vitro studies suggest that PPARγ agonists may play a dual role in the development and progression of prostate cancer (21). While the development and growth of prostate cancer can be inhibited by PPARγ agonists, stimulation of PPARγ may also directly lead to the carcinogenicity of prostate cancer via androgen receptor-dependent or -independent pathways (21).
Rosiglitazone and pioglitazone belong to a class of thiazolidinedione (TZD) and both have been used as antidiabetic drugs to treat hyperglycemia in patients with T2DM by improving insulin resistance via activation of PPARγ. However, rosiglitazone and pioglitazone may show different results in the association with cardiovascular disease and cancer in patients who use the drugs. For example, a suspicious bladder cancer risk has been reported for pioglitazone (22), but this was not observed for rosiglitazone (23). On the other hand, rosiglitazone has been shown to increase the risk of cardiovascular disease (24), but pioglitazone shows a beneficial effect (25). These discrepant pleiotropic effects of rosiglitazone and pioglitazone can be attributed to the different pathways influenced by different PPARγ agonists and the crosstalk between PPARγ and other signaling pathways (26).
In our previous study, pioglitazone shows a beneficial effect on prostate cancer risk after its prolonged use (27). However, whether rosiglitazone may exert a similar effect in humans has not been previously investigated. In in vitro studies using prostate cancer cell lines, rosiglitazone might inhibit the migration and invasion of prostate cancer cells through its inhibitory effect on the CXCR4/CXCL12 axis (28) and downregulation of vascular endothelial growth factor (29). PPARγ activation by rosiglitazone may also reduce the action of androgen receptor in androgen-dependent prostate cancer cells (30). In prostate cancer cell lines, rosiglitazone may affect cell cycle protein expression (31) and attenuate insulin-like growth factor 1 signaling (32). High rates of fatty acid and protein synthesis are required for the growth of prostate cancer cells, which may be blocked by the activation of fuel-sensing enzyme 5’-adenosine monophosphate-activated protein kinase (AMPK) (33). Although not consistently observed (34), metformin (a well-known activator of AMPK) reduces the risk of prostate cancer in Taiwanese patients with T2DM (35). Rosiglitazone has been shown to inhibit prostate cancer cell growth through its activation of the AMPK in both androgen-independent (DU145 and PC3) and androgen-sensitive (LNCaP) cells (33).
In humans, an early randomized placebo-controlled trial conducted in 106 patients with recurrent prostate cancer after radical prostatectomy and/or radiation therapy did not show any beneficial effect of rosiglitazone (4 mg twice daily) over placebo on the time to disease progression or posttreatment PSA doubling time (36). A meta-analysis suggests that TZD (including rosiglitazone and pioglitazone) has a null association with prostate cancer risk (37). However, another recent meta-analysis shows a null association between TZD use and prostate cancer risk in data derived from observational studies, but a significant risk reduction could be seen in data derived from randomized controlled trials (odds ratio 0.55, P = 0.04) (38).
Because rosiglitazone may show promising effects on prostate cancer cell lines but such a potential beneficial effect has not been extensively investigated in humans, this study was aimed to investigate whether rosiglitazone use might affect the risk of prostate cancer in patients with T2DM.
Materials and methods
The government of Taiwan has implemented a unique, compulsory and universal health care system called the National Health Insurance (NHI) since March 1, 1995. The coverage rate of NHI is very high and includes > 99% of the population. Across Taiwan, all in-hospitals and 93% of all medical settings sign contracts with the Bureau of the NHI to provide healthcare services. According to local regulations, academic researchers can request for the use of the reimbursement database if the research proposal is reviewed and approved by an ethic review board. This study used the database after approval by the Research Ethics Committee of the National Health Research Institutes (approval number: NHIRD-102-175).
All personal data were de-identified for the protection of privacy. The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) was used to code related diagnoses during the study period. Diabetes was coded 250.XX and prostate cancer 185.
The selection procedures of a cohort consisting of 1:1 propensity score (PS) matched-pairs of rosiglitazone ever and never users from the NHI database are shown in Figure 1. The patients were newly diagnosed of diabetes mellitus from 1999 to 2005 and should have received antidiabetic drugs prescribed at the outpatient clinics for 2 or more times (n = 423,949). Patients who had a previous diagnosis of diabetes mellitus within 1996-1998 were not included to assure a new-onset of diabetes mellitus after 1999. The following patients were then excluded step by step: 1) type 1 diabetes mellitus (n = 2400, because rosiglitazone is not indicated for their treatment); 2) missing data (n = 672); 3) patients who had been diagnosed of any cancer (ICD-9-CM 140-208) before entry or within 6 months of diabetes diagnosis (n = 44,587); 4) age <25 (n = 22,061); 5) women (n = 165,445); 6) ever users of pioglitazone (n = 47,309); and 7) follow-up duration < 6 months (n = 4188). A cohort consisting of 1:1 matched-pairs of ever and never users of rosiglitazone was then created by the Greedy 8 ➔ 1 digit match algorithm based on PS (39). Logistic regression was used to create the PS from independent variables that included all characteristics listed in Table 1 and the date of entry.
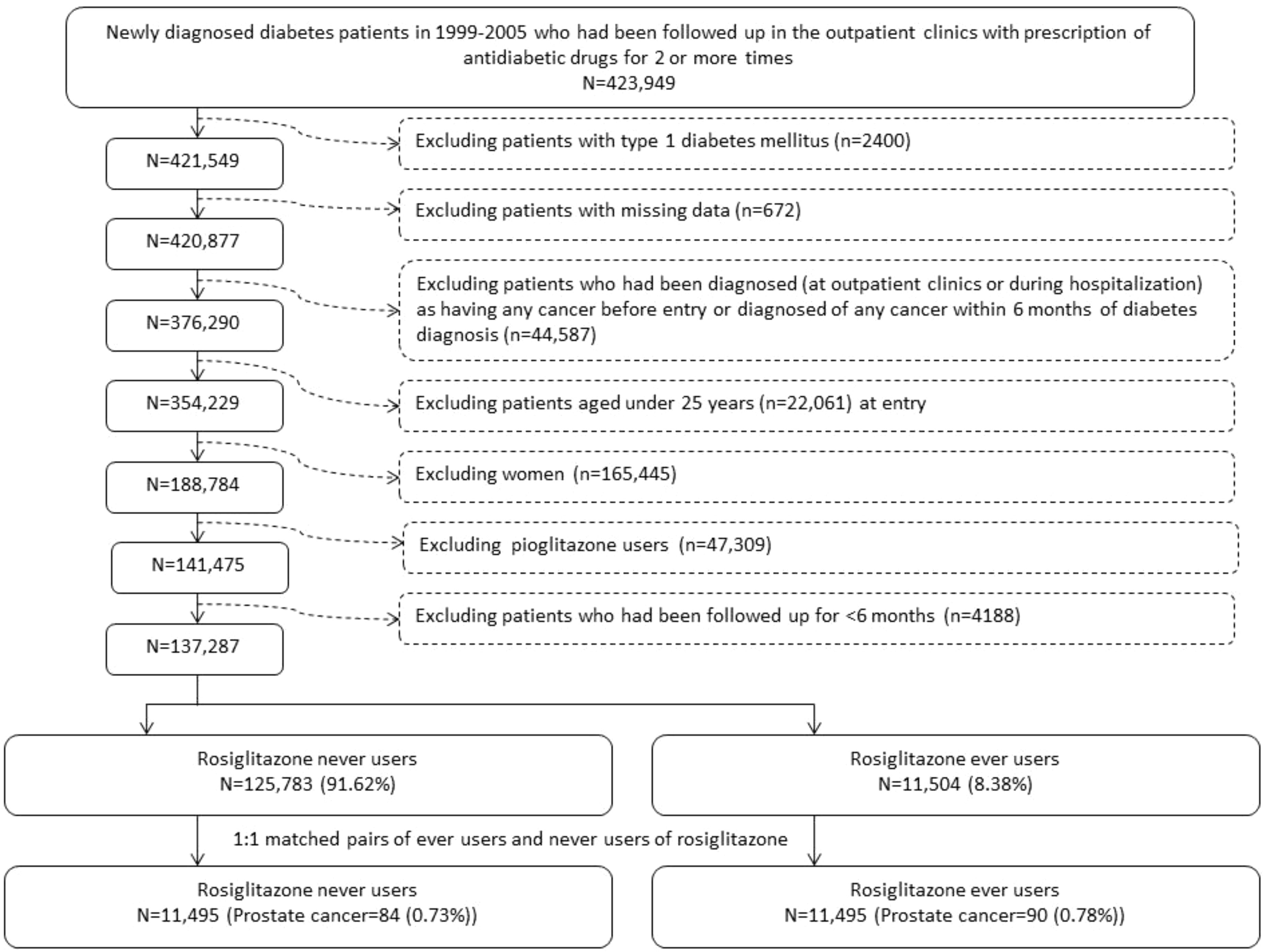
Figure 1 Flowchart showing the procedure in selecting a cohort of 1:1 matched-pairs of ever and never users of rosiglitazone based on propensity score into the study.
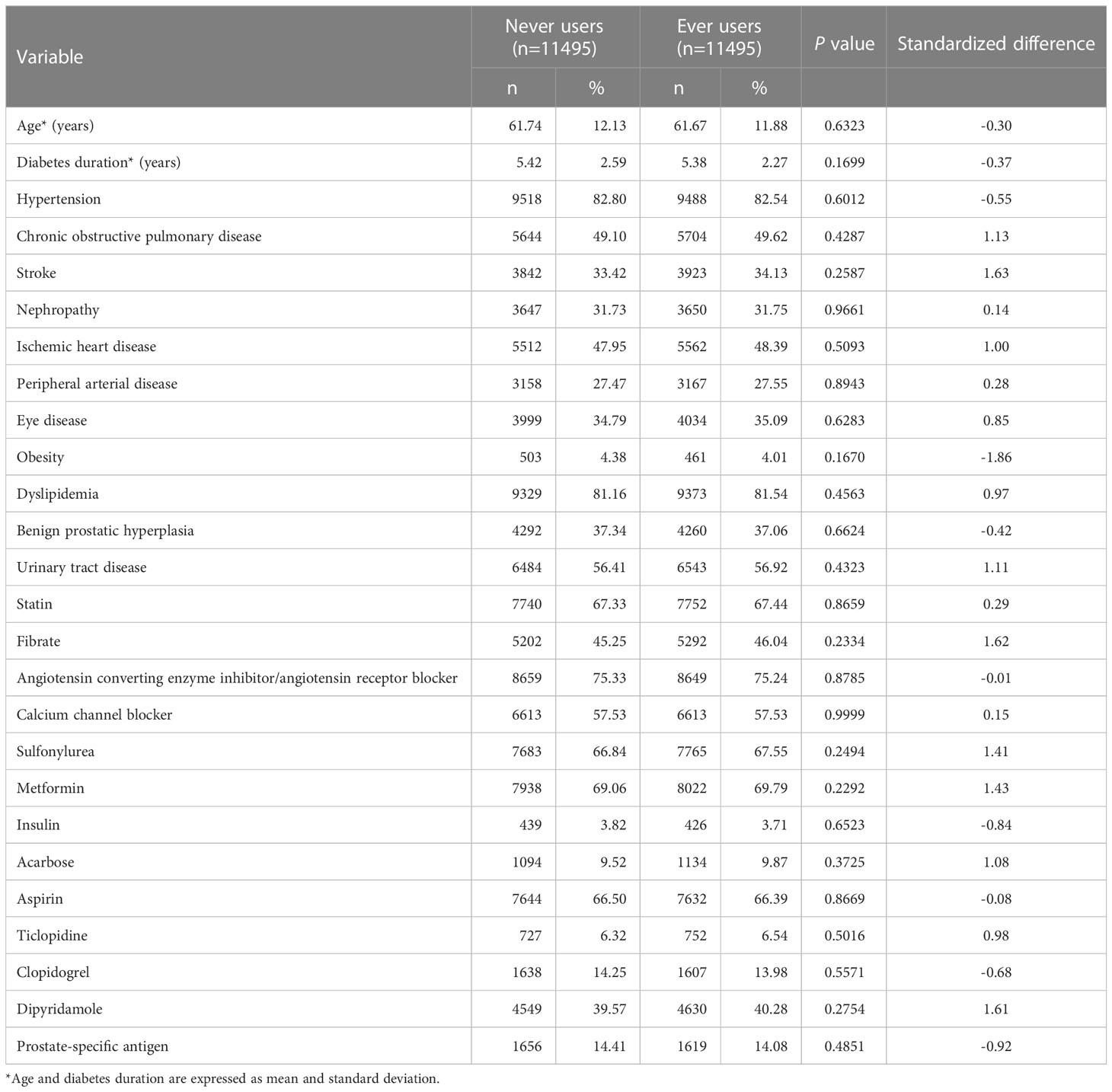
Table 1 Characteristics between never and ever users of rosiglitazone in a propensity score-matched cohort.
In Taiwan, only rosiglitazone and pioglitazone in the class of TZD have ever been marketed. Users of pioglitazone were deliberately excluded in the analyses for the following reasons. Besides their glucose lowering effects, rosiglitazone and pioglitazone show different safety profiles in several clinical aspects. For example, rosiglitazone use has been shown to increase the risk of myocardial infarction and cardiovascular death in a meta-analysis (24). On the contrary, pioglitazone significantly lowers triglycerides and increases high-density lipoprotein cholesterol in a small clinical trial (40). Furthermore, pioglitazone reduces the risk of cardiovascular diseases in patients with T2DM (41) and reduces the risk of stroke and myocardial infarction in non-diabetes patients with ischemic stroke and insulin resistance (25). Our previous observational studies suggest a significantly lower risk of dementia associated with pioglitazone (42) but not with rosiglitazone (43). On the other hand, rosiglitazone significantly reduces the risk of breast cancer (44) and thyroid cancer (45), but pioglitazone shows a null effect on breast cancer (46) and thyroid cancer (47). Therefore, in the analyses of the safety profile and the risk association with cancer or non-cancer diseases, rosiglitazone and pioglitazone should be viewed as two different entities.
Age, diabetes duration, and factors that might be correlated with the exposure (rosiglitazone use) and/or the outcome (prostate cancer) in the study were considered as potential confounders (Table 1). These included hypertension (ICD-9-CM 401-405), chronic obstructive pulmonary disease (a surrogate for smoking; 490-496), stroke (430-438), nephropathy (580-589), ischemic heart disease (410-414), peripheral arterial disease (250.7, 785.4, 443.81 and 440-448), eye disease (250.5, 362.0, 369, 366.41 and 365.44), obesity (278), dyslipidemia (272.0-272.4), benign prostatic hyperplasia (600), urinary tract diseases (590-599), and use of the following drugs: statin, fibrate, angiotensin converting enzyme inhibitor/angiotensin receptor blocker, calcium channel blocker, sulfonylurea, metformin, insulin, acarbose, aspirin, ticlopidine, clopidogrel, and dipyridamole. The use of PSA test was also included because it may affect the detection rate of prostate cancer.
The differences for age and diabetes duration between never users and ever users of rosiglitazone were compared by Student’s t test and the other characteristics of categorical variables by Chi-square test. The value of standardized difference for each covariate was then calculated and a threshold value > 10% was used to indicate a potential confounding from the variable (48).
Cumulative dose of rosiglitazone was calculated in mg and a dose-response relationship was assessed by its tertiles. The incidence density of prostate cancer was calculated with regards to rosiglitazone exposure in subgroups of never users, ever users and the tertiles of cumulative dose of rosiglitazone therapy. The numerator was the number of incident prostate cancer diagnosed during follow-up. The denominator was the person-years of follow-up, which started on January 1, 2006 and ended up to December 31, 2011, at the time of a new diagnosis of prostate cancer, death or on the date of the last reimbursement record.
Hazard ratios that compared ever users to never users and compared the tertile subgroups of cumulative dose of rosiglitazone therapy to never users were estimated by Cox proportion hazard model incorporated with the inverse probability of treatment weighting using PS (49). Overall hazard ratios for ever versus never users of rosiglitazone were also estimated in the following sensitivity analyses after excluding: 1) patients who received a PSA test before the diagnosis of prostate cancer; 2) patients who had a diagnosis of any other cancers during follow-up; 3) patients having a diagnosis of benign prostatic hyperplasia; 4) patients with a diagnosis of nephropathy; 5) patients with a diagnosis of urinary tract disease; 6) patients with a diagnosis of benign prostatic hyperplasia, nephropathy and/or urinary tract disease; and 7) patients aged < 45 years.
SAS statistical software (version 9.4, SAS Institute, Cary, NC) was used as a tool for conducting all the statistical analyses. P < 0.05 was used as a threshold indicator for statistical significance.
Results
The characteristics of the PS matched-pairs consisting of 11,495 never users and 11,495 ever users of rosiglitazone are shown in Table 1. All P values by Student’s t test and Chi-square test were > 0.05 and all variables had values of standardized difference < 10%, suggesting that the two groups were well matched on the covariates and residual confounding was unlikely.
Table 2 shows the incidence of prostate cancer by rosiglitazone exposure and the hazard ratios comparing ever to never users and ever users categorized by the tertiles of cumulative dose to never users. There were 84 incident cases of prostate cancer in never users and 90 incident cases in ever users. The incidence rates in never users and ever users were 173.20 per 100,000 person-years and 187.59 per 100,000 person-years, respectively. The overall hazard ratio of 1.089 (95% confidence interval 0.808-1.466) suggests a null effect of rosiglitazone on prostate cancer. When examining prostate cancer risk by the tertiles of cumulative dose, none of the hazard ratios was statistically significant.
The results of the sensitivity analyses are shown in Table 3. None of the hazard ratios reached statistical significance, supporting the null effect of rosiglitazone as observed in the main analyses (Table 2).

Table 3 Sensitivity analyses estimating overall hazard ratios for ever versus never users of rosiglitazone for prostate cancer.
Discussion
The present study suggests a null effect of rosiglitazone on prostate cancer risk in patients with T2DM (Tables 2, 3).
Unlike what has been observed in a previous study that pioglitazone may exert a beneficial effect on prostate cancer risk after a prolonged use (27), rosiglitazone seemed to have a null effect in the present study (Tables 2, 3). It is interesting that these two drugs in the same class of TZD may exert different effects on cardiovascular disease (24, 25, 41) and on some types of cancer (22, 23, 37, 44–47). The crosstalk between PPARγ and other signaling pathways may probably explain the different clinical effects observed for different PPARγ agonists (26). Another explanation of a lack of protective effect of rosiglitazone is because of the lack of its effect on prostate cell growth at therapeutic levels of rosiglitazone used to treat diabetes. For example, an in vitro study showed that rosiglitazone at the therapeutic level of 1 μM did not affect prostate cell growth in cell cultures derived from normal, transformed or cancerous tissues (50). Even if there could be a minor beneficial effect of rosiglitazone on prostate cancer development, the body weight gain (obesity is a potential risk factor of prostate cancer (51)) commonly associated with rosiglitazone use might have attenuated such a minor beneficial effect (52). Because in vitro studies suggest that PPARγ agonists may exert dual effects on prostate cancer (21), the clinical impact of the use of rosiglitazone depends on the trade-off between these dual effects of PPARγ agonists.
Some in vitro studies suggest that excessive fatty acids may facilitate the malignant progression of prostate cancer promoted by PPARγ (53, 54). While pioglitazone may significantly reduce triglycerides (40, 54), rosiglitazone on the other hand would raise triglycerides (40, 54). Recent human studies suggest an association between triglycerides and prostate cancer risk (55, 56), severity (57) and recurrence (58). A recent in vitro study shows that the synthesis of lipid droplet and the proliferation and migration of prostate cancer cells activated by the PPARγ pathway can be effectively promoted by low-dose rosiglitazone (59). Whether the differential effects on lipid profiles between pioglitazone and rosiglitazone could explain their discrepant effects on prostate cancer risk awaits further confirmation.
The present study has some strengths to render good generalizability of the findings. First, the diagnoses from all claim records found at outpatient visits and hospital admission were included to reduce the possibility of missed diagnoses. Second, bias resulting from differential detection rates of prostate cancer because of different socioeconomic status could be much reduced because patients with a diagnosis of cancer can be waived for most medical co-payments by the NHI. Furthermore, the drug co-payments in patients with low income and in veterans are very low and the co-payments for patients who refill their drug prescriptions for chronic disease can be waived. Third, self-reporting bias could be reduced by the use of objective medical records.
There are some limitations in the study. First, actual measurement data were lacking in the database for potential confounders such as anthropometric factors, lifestyle, dietary factors, physical activity, smoking, alcohol drinking, hormonal profiles, family history and genetic parameters. Second, adhering to a healthy lifestyle including healthy weight, healthy diet, refraining from smoking and vigorous physical activity has been associated with a lower risk of developing prostate cancer in genetically predisposing men (60). However, it is recognized that most of the modifiable risk factors were not available in the database and their potential confounding effects could not be investigated. Third, this study investigated the effect of rosiglitazone on prostate cancer risk in patients with T2DM and without prostate cancer at baseline. Because some in vitro studies suggest an inhibitory effect of rosiglitazone on the growth of prostate cancer cells (28–33), additional research will be needed to look into the usefulness of rosiglitazone for the treatment of prostate cancer. Fourth, because of lack of information, the impact of biochemical data and the pathology, grading and staging of prostate cancer could not be evaluated.
In conclusions, this study suggests a null effect of rosiglitazone on prostate cancer in Taiwanese male patients with T2DM. Even though in vitro and animal studies may suggest a beneficial effect of rosiglitazone on prostate cancer cells, such a benefit cannot be readily applied to humans who use the drug for the treatment of T2DM. However, it is recognized that human studies are still sparse and therefore more studies are required to confirm the findings of the present study. TZD derivatives with more potent anticancer effects on prostate and breast cancer cells are being investigated for the potential development into anticancer drugs for the treatment of prostate cancer and breast cancer (61, 62). Because PPARγ activation may have a dual effect on prostate cancer (21), results derived from cellular studies should be carefully interpreted and clinical trials in humans are pivotal to elucidate the roles of different TZD compounds in the development or prevention of prostate cancer.
Data availability statement
The datasets presented in this article are not readily available because public availability of the dataset is restricted by local regulations to protect privacy. Requests to access the datasets should be directed to C-HT, ccktsh@ms6.hinet.net.
Ethics statement
The studies involving human participants were reviewed and approved by the Research Ethics Committee of the National Health Research Institutes. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
C-HT researched data and wrote manuscript. The author confirms being the sole contributor of this work and has approved it for publication.
Acknowledgments
The author thanks the Ministry of Science and Technology (MOST 103-2314-B-002-187-MY3) of Taiwan for providing financial supports for the study.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: A systematic analysis for the Global Burden of Disease Study. JAMA Oncol (2018) 4:1553–68. doi: 10.1200/JCO.2018.36.15_suppl.1568
2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin (2015) 65:87–108. doi: 10.3322/caac.21262
3. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin (2011) 61:69–90. doi: 10.3322/caac.20107
4. Kimura T, Egawa S. Epidemiology of prostate cancer in Asian countries. Int J Urol (2018) 25:524–31. doi: 10.1111/iju.13593
5. Zhu Y, Wang HK, Qu YY, Ye DW. Prostate cancer in East Asia: Evolving trend over the last decade. Asian J Androl (2015) 17:48–57. doi: 10.4103/1008-682X.132780
6. Van Dong H, Lee AH, Nga NH, Quang N, Le Chuyen V, Binns CW. Epidemiology and prevention of prostate cancer in Vietnam. Asian Pac J Cancer Prev (2014) 15:9747–51. doi: 10.7314/APJCP.2014.15.22.9747
7. Tseng CH. Diabetes and risk of prostate cancer: A study using the National Health Insurance. Diabetes Care (2011) 34:616–21. doi: 10.2337/dc10-1640
8. Tseng CH. Prostate cancer mortality in Taiwanese men: Increasing age-standardized trend in general population and increased risk in diabetic men. Ann Med (2011) 43:142–50. doi: 10.3109/07853890.2010.530683
9. Dong WW, Zhang DL, Wang ZH, Lv CZ, Zhang P, Zhang H. Different types of diabetes mellitus and risk of thyroid cancer: A meta-analysis of cohort studies. Front Endocrinol (Lausanne) (2022) 13:971213. doi: 10.3389/fendo.2022.971213
10. Boubertakh B, Silvestri C, Di Marzo V. Obesity: The fat tissue disease version of cancer. Cells (2022) 11:1872. doi: 10.3390/cells11121872
11. Tseng CH. Metformin may reduce bladder cancer risk in Taiwanese patients with type 2 diabetes. Acta Diabetol (2014) 51:295–303. doi: 10.1007/s00592-014-0562-6
12. Zhu B, Qu S. The relationship between diabetes mellitus and cancers and its underlying mechanisms. Front Endocrinol (Lausanne) (2022) 13:800995. doi: 10.3389/fendo.2022.800995
13. Knura M, Garczorz W, Borek A, Drzymała F, Rachwał K, George K, et al. The influence of anti-diabetic drugs on prostate cancer. Cancers (Basel) (2021) 13:1827. doi: 10.3390/cancers13081827
14. Tseng CH, Lee KY, Tseng FH. An updated review on cancer risk associated with incretin mimetics and enhancers. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev (2015) 33:67–124. doi: 10.1080/10590501.2015.1003496
15. Bonovas S, Filioussi K, Tsantes A. Diabetes mellitus and risk of prostate cancer: a meta-analysis. Diabetologia (2004) 47:1071–8. doi: 10.1007/s00125-004-1415-6
16. Dankner R, Boffetta P, Keinan-Boker L, Balicer RD, Berlin A, Olmer L, et al. Diabetes, prostate cancer screening and risk of low- and high-grade prostate cancer: An 11-year historical population follow-up study of more than 1 million men. Diabetologia (2016) 59:1683–91. doi: 10.1007/s00125-016-3972-x
17. Hsieh MC, Lee TC, Cheng SM, Tu ST, Yen MH, Tseng CH. The influence of type 2 diabetes and glucose-lowering therapies on cancer risk in the Taiwanese. Exp Diabetes Res (2012) 2012:413782. doi: 10.1155/2012/413782
18. Long XJ, Lin S, Sun YN, Zheng ZF. Diabetes mellitus and prostate cancer risk in Asian countries: A meta-analysis. Asian Pac J Cancer Prev (2012) 13:4097–100. doi: 10.7314/APJCP.2012.13.8.4097
19. Cai H, Xu Z, Xu T, Yu B, Zou Q. Diabetes mellitus is associated with elevated risk of mortality amongst patients with prostate cancer: A meta-analysis of 11 cohort studies. Diabetes Metab Res Rev (2015) 31:336–43. doi: 10.1002/dmrr.2582
20. Xu Y, Iyengar S, Roberts RL, Shappell SB, Peehl DM. Primary culture model of peroxisome proliferator-activated receptor gamma activity in prostate cancer cells. J Cell Physiol (2003) 196:131–43. doi: 10.1002/jcp.10281
21. Elix C, Pal SK, Jones JO. The role of peroxisome proliferator-activated receptor gamma in prostate cancer. Asian J Androl (2018) 20:238–43. doi: 10.4103/aja.aja_15_17
22. Lewis JD, Habel LA, Quesenberry CP, Strom BL, Peng T, Hedderson MM, et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA (2015) 314:265–77. doi: 10.1001/jama.2015.7996
23. Tseng CH. Rosiglitazone is not associated with an increased risk of bladder cancer. Cancer Epidemiol (2013) 37:385–9. doi: 10.1016/j.canep.2013.03.013
24. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med (2007) 356:2457–71. doi: 10.1056/NEJMoa072761
25. Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med (2016) 374:1321–31. doi: 10.1056/NEJMoa1506930
26. Fröhlich E, Wahl R. Chemotherapy and chemoprevention by thiazolidinediones. BioMed Res Int (2015) 2015:845340. doi: 10.1155/2015/845340
27. Tseng CH. Pioglitazone and prostate cancer risk in Taiwanese male patients with type 2 diabetes: A retrospective cohort study. World J Mens Health (2023) 41:119–28. doi: 10.5534/wjmh.210157
28. Qin L, Gong C, Chen AM, Guo FJ, Xu F, Ren Y, et al. Peroxisome proliferator-activated receptor γ agonist rosiglitazone inhibits migration and invasion of prostate cancer cells through inhibition of the CXCR4/CXCL12 axis. Mol Med Rep (2014) 10:695–700. doi: 10.3892/mmr.2014.2232
29. Qin L, Ren Y, Chen AM, Guo FJ, Xu F, Gong C, et al. Peroxisome proliferator-activated receptor γ ligands inhibit VEGF-mediated vasculogenic mimicry of prostate cancer through the AKT signaling pathway. Mol Med Rep (2014) 10:276–82. doi: 10.3892/mmr.2014.2198
30. Moss PE, Lyles BE, Stewart LV. The PPARγ ligand ciglitazone regulates androgen receptor activation differently in androgen-dependent versus androgen-independent human prostate cancer cells. Exp Cell Res (2010) 316:3478–88. doi: 10.1016/j.yexcr.2010.09.015
31. Lyles BE, Akinyeke TO, Moss PE, Stewart LV. Thiazolidinediones regulate expression of cell cycle proteins in human prostate cancer cells via PPARgamma-dependent and PPARgamma-independent pathways. Cell Cycle (2009) 8:268–77. doi: 10.4161/cc.8.2.7584
32. Papageorgiou E, Pitulis N, Manoussakis M, Lembessis P, Koutsilieris M. Rosiglitazone attenuates insulin-like growth factor 1 receptor survival signaling in PC-3 cells. Mol Med (2008) 14:403–11. doi: 10.2119/2008-00021.Papageorgiou
33. Xiang X, Saha AK, Wen R, Ruderman NB, Luo Z. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem Biophys Res Commun (2004) 321:161–7. doi: 10.1016/j.bbrc.2004.06.133
34. Chen CB, Eskin M, Eurich DT, Majumdar SR, Johnson JA. Metformin, Asian ethnicity and risk of prostate cancer in type 2 diabetes: A systematic review and meta-analysis. BMC Cancer (2018) 18:65. doi: 10.1186/s12885-017-3934-9
35. Tseng CH. Metformin significantly reduces incident prostate cancer risk in Taiwanese men with type 2 diabetes mellitus. Eur J Cancer (2014) 50:2831–7. doi: 10.1016/j.ejca.2014.08.007
36. Smith MR, Manola J, Kaufman DS, George D, Oh WK, Mueller E, et al. Rosiglitazone versus placebo for men with prostate carcinoma and a rising serum prostate-specific antigen level after radical prostatectomy and/or radiation therapy. Cancer (2004) 101:1569–74. doi: 10.1002/cncr.20493
37. Bosetti C, Rosato V, Buniato D, Zambon A, La Vecchia C, Corrao G. Cancer risk for patients using thiazolidinediones for type 2 diabetes: A meta-analysis. Oncologist (2013) 18:148–56. doi: 10.1634/theoncologist.2012-0302
38. Cui H, Wang Y, Yang S, He G, Jiang Z, Gang X, et al. Antidiabetic medications and the risk of prostate cancer in patients with diabetes mellitus: A systematic review and meta-analysis. Pharmacol Res (2022) 177:106094. doi: 10.1016/j.phrs.2022.106094
39. Parsons LS. Performing a 1:N case-control match on propensity score . Available at: https://support.sas.com/resources/papers/proceedings/proceedings/sugi29/165-29.pdf (Accessed July 4, 2023).
40. Tseng CH, Huang TS. Pioglitazone with sulfonylurea: Glycemic and lipid effects in Taiwanese diabetic patients. Diabetes Res Clin Pract (2005) 70:193–4. doi: 10.1016/j.diabres.2004.11.003
41. Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomised controlled trial. Lancet (2005) 366:1279–89. doi: 10.1016/S0140-6736(05)67528-9
42. Tseng CH. Pioglitazone reduces dementia risk in patients with type 2 diabetes mellitus: A retrospective cohort analysis. J Clin Med (2018) 7:306. doi: 10.3390/jcm7100306
43. Tseng CH. Rosiglitazone has a neutral effect on the risk of dementia in type 2 diabetes patients. Aging (Albany NY) (2019) 11:2724–34. doi: 10.18632/aging.101944
44. Tseng CH. Rosiglitazone reduces breast cancer risk in Taiwanese female patients with type 2 diabetes mellitus. Oncotarget (2017) 8:3042–8. doi: 10.18632/oncotarget.13824
45. Tseng CH. Rosiglitazone may reduce thyroid cancer risk in patients with type 2 diabetes. Ann Med (2013) 45:539–44. doi: 10.3109/07853890.2013.851865
46. Tseng CH. Pioglitazone and breast cancer risk in female patients with type 2 diabetes mellitus: a retrospective cohort analysis. BMC Cancer (2022) 22:559. doi: 10.1186/s12885-022-09660-8
47. Tseng CH. Pioglitazone and thyroid cancer risk in Taiwanese patients with type 2 diabetes. J Diabetes (2014) 6:448–50. doi: 10.1111/1753-0407.12149
48. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med (2015) 34:3661–79. doi: 10.1002/sim.6607
49. Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med (2013) 32:2837–49. doi: 10.1002/sim.5705
50. Murtola TJ, Pennanen P, Syvälä H, Bläuer M, Ylikomi T, Tammela TL. Effects of simvastatin, acetylsalicylic acid, and rosiglitazone on proliferation of normal and cancerous prostate epithelial cells at therapeutic concentrations. Prostate (2009) 69:1017–23. doi: 10.1002/pros.20951
51. Wilson RL, Taaffe DR, Newton RU, Hart NH, Lyons-Wall P, Galvão DA. Obesity and prostate cancer: A narrative review. Crit Rev Oncol Hematol (2022) 169:103543. doi: 10.1016/j.critrevonc.2021.103543
52. Lindberg M, Astrup A. The role of glitazones in management of type 2 diabetes. A dream or a nightmare? Obes Rev (2007) 8:381–4. doi: 10.1111/j.1467-789X.2007.00399.x
53. Forootan FS, Forootan SS, Gou X, Yang J, Liu B, Chen D, et al. Fatty acid activated PPARγ promotes tumorigenicity of prostate cancer cells by up regulating VEGF via PPAR responsive elements of the promoter. Oncotarget (2016) 7:9322–39. doi: 10.18632/oncotarget.6975
54. Goldberg RB, Kendall DM, Deeg MA, Buse JB, Zagar AJ, Pinaire JA, et al. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care (2005) 28:1547–54. doi: 10.2337/diacare.28.7.1547
55. Zhu S, Hu X, Fan Y. Association of triglyceride levels and prostate cancer: A Mendelian randomization study. BMC Urol (2022) 22:167. doi: 10.1186/s12894-022-01120-6
56. Arthur R, Møller H, Garmo H, Holmberg L, Stattin P, Malmstrom H, et al. Association between baseline serum glucose, triglycerides and total cholesterol, and prostate cancer risk categories. Cancer Med (2016) 5:1307–18. doi: 10.1002/cam4.665
57. Salgado-Montilla J, Soto Salgado M, Surillo Trautmann B, Sánchez-Ortiz R, Irizarry-Ramírez M. Association of serum lipid levels and prostate cancer severity among Hispanic Puerto Rican men. Lipids Health Dis (2015) 14:111. doi: 10.1186/s12944-015-0096-0
58. Allott EH, Howard LE, Cooperberg MR, Kane CJ, Aronson WJ, Terris MK, et al. Serum lipid profile and risk of prostate cancer recurrence: Results from the SEARCH database. Cancer Epidemiol Biomarkers Prev (2014) 23:2349–56. doi: 10.1158/1055-9965.EPI-14-0458
59. Zhai Q, Luo M, Zhang Y, Zhang W, Wu C, Lv S, et al. Histone methyltransferase KMT2D mediated lipid metabolism via peroxisome proliferator-activated receptor gamma in prostate cancer. Transl Cancer Res (2022) 11:2607–21. doi: 10.21037/tcr-22-431
60. Plym A, Zhang Y, Stopsack KH, Delcoigne B, Wiklund F, Haiman C, et al. A healthy lifestyle in men at increased genetic risk for prostate cancer. Eur Urol (2023) 83:343–51. doi: 10.1016/j.eururo.2022.05.008
61. Corigliano DM, Syed R, Messineo S, Lupia A, Patel R, Reddy CVR, et al. Indole and 2,4-thiazolidinedione conjugates as potential anticancer modulators. Peer J (2018) 6:e5386. doi: 10.7717/peerj.5386
62. Abdelgawad MA, El-Adl K, El-Hddad SSA, Elhady MM, Saleh NM, Khalifa MM, et al. Design, molecular docking, synthesis, anticancer and anti-hyperglycemic assessments of thiazolidine-2,4-diones bearing sulfonylthiourea moieties as potent VEGFR-2 inhibitors and PPARγ agonists. Pharm (Basel) (2022) 15:226. doi: 10.3390/ph15020226
Keywords: National Health Insurance, peroxisome proliferator-activator receptor gamma, pioglitazone, prostate cancer, rosiglitazone, thiazolidinediones
Citation: Tseng C-H (2023) Rosiglitazone has a null association with the risk of prostate cancer in type 2 diabetes patients. Front. Endocrinol. 14:1185053. doi: 10.3389/fendo.2023.1185053
Received: 13 March 2023; Accepted: 10 July 2023;
Published: 25 July 2023.
Edited by:
Qiang Huo, Nanjing Jiangbei Hospital, ChinaReviewed by:
Prasanna K. Santhekadur, JSS Academy of Higher Education and Research, IndiaMayur Sarangdhar, Cincinnati Children’s Hospital Medical Center, United States
Copyright © 2023 Tseng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chin-Hsiao Tseng, ccktsh@ms6.hinet.net
 Chin-Hsiao Tseng
Chin-Hsiao Tseng