- 1Department of Neurology, Xiangya Hospital, Central South University, Changsha, Hunan, China
- 2Clinical Research Center for Cerebrovascular Disease of Hunan Province, Central South University, Changsha, Hunan, China
- 3National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, Hunan, China
Introduction: Patients with Metabolic Syndrome (MetS) are considered at high-risk for incident stroke. An indicator of visceral adiposity dysfunction, the Chinese Visceral Adiposity Index (CVAI) is used to evaluate the dysfunction of visceral fat. Given the impact of visceral adiposity dysfunction on elevating cardiovascular hazards, this study aimed to examine the association between CVAI and stroke risk in MetS patients.
Method: Between November 2017 and December 2018, a total of 18,974 individuals aged ≥40 underwent standardized in-person clinical interviews in Hunan Province, with 6,732 meeting the criteria for MetS. After the baseline survey was completed, subsequent surveys were conducted biennially. The study was split into two stages performed at baseline and after two years. During the former, receiver-operating characteristic curves were used to assess the accuracy of using baseline CVAI in diagnosing MetS. After two years, we examined the association between CVAI and incident stroke in MetS patients using logistic regression, subgroup analysis, and restricted cubic spline (RCS) analysis.
Result: As evidenced by a higher AUC (AUC:0.741), CVAI demonstrated superior diagnostic performance relative to body mass index (AUC:0.631) and waist circumference (AUC:0.627) in diagnosing MetS. After a 2-year follow-up, 72 MetS patients had a stroke event. There was a robust positive correlation between incident stroke and CVAI in patients with MetS. Each 1 SD increase in CVAI was associated with a 1.52-fold higher risk of stroke after adjustment for confounding factors (aOR=1.52, 95%CI: 1.18-1.95). The RCS demonstrated a reduced risk of stroke for MetS patients when the CVAI was below 110.91. However, no significant correlation was detected between CVAI and stroke in non-MetS patients.
Conclusion: Our findings recommend CVAI as a superior screening tool for detecting MetS and suggest that reducing CVAI can mitigate the risk of stroke in patients with MetS.
1 Introduction
Stroke ranks third in terms of morbidity and second in terms of disability-adjusted life-years worldwide (1, 2), resulting in an economic burden greater than 721 billion USD, which is equivalent to 0.66% of the global gross national product (3).
Due to an aging population, the stroke burden in China is escalating. In 2020, a nationwide survey of 676,394 adults aged ≥40 years revealed an estimated incidence and mortality rate for stroke of 502.2 per 100,000 person-years and 343.4 per 100,000 person-years, respectively (4). It is therefore important to identify effective primary prevention and early intervention strategies for stroke (5).
Metabolic syndrome (MetS) is an umbrella term that refers to multiple metabolic abnormalities, including hypertension, central obesity, impaired glucose regulation, and atherogenic dyslipidemia (6). The improvement in the general standard of living has accompanied a significant rise in the incidence of MetS (7), as well as a concomitant elevation in the risk of stroke (8–10).Identifying risk factors for incident stroke in patients with MetS would help to mitigate the anticipated rise in stroke burden resulting from the increased morbidity of MetS. As such, it is essential to identify the risk factors of incident stroke for MetS patients.
Adipose tissue is important not only for storing energy but also regulating endocrine function through the secretion of adipokines (11). Adipose tissue is categorized as either visceral fat tissue (VAT) or subcutaneous fat tissue (SAT) according to its location. The accumulation of VAT is strongly associated with increased cardiometabolic risk (12–15). The study conducted by Huang et al. demonstrated significant correlations between VAT mass and a wide range of CVD outcomes, including but not limited to coronary heart disease, cardiac arrhythmia, vascular diseases, and stroke (16). Additionally, another Mendelian Randomization Study furnished proof of a substantial causal link between VAT and ischemic stroke, as opposed to intracerebral hemorrhage (17). In general, visceral obesity is among the most evident clinical features of MetS (18). Obese patients with MetS have a higher risk of developing cardiovascular disease (CVD) than non-obese patients (19). However, whether a higher degree of visceral adiposity in MetS patients is associated with an increased risk of incident stroke remains poorly characterized.
At present, body mass index (BMI) and waist circumference (WC) are the most common methods for estimating adiposity and assessing central obesity in patients with MetS. However, these metrics are limited: BMI cannot distinguish between the accumulation of fat-free mass and fat, leading to the misdiagnosis of muscular individuals as overweight or obese (20); WC is marred by poor reliability and is inadequate for differentiating between subcutaneous and visceral fat (21). While more technologically advanced alternatives, such as magnetic resonance imaging and computed tomography, are considered gold standards, their technical complexity and high cost prohibit their use in routine clinical practice (22). The need for a reliable and low-cost indicator of visceral adiposity has prompted the development of novel indices based on combining anthropometric and biochemical assessments: e.g., the visceral adiposity index (VAI) and Chinese visceral adiposity index (CVAI) (23). In 2016, a CVAI was established that utilizes clinically available metabolic parameters, including age, BMI, WC, triglycerides (TG), and high-density lipoprotein cholesterol (HDL-C) (24). CVAI is highly correlated with visceral fat area and outperforms BMI, WC, or VAI in the diagnosis of diabetes and hypertension among Chinese population (25, 26). Furthermore, elevated CVAI is significantly associated with increased risks of carotid plaque and CVD (27–29). Few studies, however, have investigated an association between CVAI and incident stroke in patients with MetS. To resolve this dearth in the literature, the present study investigates the relationship between CVAI and incident stroke in a Chinese population.
2 Materials and methods
2.1 Study design and population
The present study used patient data collected by the China Stroke High-risk Population Screening and Intervention Program (CSHPSIP): an ongoing population-based screening project conducted by the China Stroke Prevention Project Committee (30). The program aims to mitigate stroke risk by addressing the prevalence of stroke risk factors through screening, physical examination, and comprehensive interventions. The CSHPSIP enrolled community-dwelling adults who were (1) aged >40 years, (2) resided in the community for >6 months, and (3) provided informed consent (4). The protocol for the program were reviewed and approved by the Institutional Review Board at the Capital Medical University Xuanwu Hospital (No. 2012045).
The present study used data obtained from individuals residing in Hunan Province, a region featuring a relatively elevated incidence of stroke within China (4). 54338 individuals enrolled from twenty-six communities (thirteen urban areas and thirteen rural areas) in the province were administered a baseline survey between January 2017 and December 2018. All eligible participants underwent standardized in-person clinical interviews. After the baseline survey was completed, subsequent surveys were conducted biennially.
A total of 20,487 respondents participated in the follow-up survey two years after completing the baseline survey. Individuals with incomplete sociodemographic information, missing anthropometric measures, or lacking laboratory assay results due to unsuccessful blood collection were excluded from the study.
Of the 18,974 participants included in the final analysis, 6,732 were diagnosed with MetS according to the Chinese Guidelines for the Prevention and Treatment of Type 2 Diabetes (2020 edition). The criteria for defining the detailed components of MetS were as follows (31): (1) abdominal obesity, ascertained by a WC of ≥ 90 cm in males or ≥ 85 cm in females; (2) hypertension, defined as a blood pressure of ≥ 130/85 mmHg or a history of hypertension; (3) hyperglycemia, indicated by a fasting plasma glucose (FBG) level of ≥ 6.10 mmol/L, a 2-h plasma glucose level of ≥7.8 mmol/L, or a diagnosis of type 2 diabetes mellitus; (4) high TG, determined by a fasting TG level of ≥1.70 mmol/L; and (5) low HDL-C, indicated by an HDL-C level of ≤ 1.04 mmol/L. Patients who fulfilled any three of the aforementioned five criteria were diagnosed with MetS.
In parallel with the survey schedule, the study was split into two stages performed at baseline and after two years (Supplemental Figure 1). During the former, we examined the associations between baseline CVAI levels and MetS diagnosis, as well as assessed the diagnostic efficacy of CVAI in detecting MetS. After two years, we investigated the relationship between CVAI and incident stroke risk among patients diagnosed with MetS.
2.2 Baseline data collection and anthropometry measurements
Data on medical, socio-demographic, anthropometric, and lifestyle-related variables were obtained by trained interviewers or medical staff. Demographic information, including age, sex, education level, economic status, lifestyle risk factors (tobacco use, alcohol consumption, and physical activity), medical history (hypertension, diabetes mellitus, dyslipidemia, stroke, and atrial fibrillation), and family medical history of stroke was collected. Education level was classified as “primary school or below,” “middle school,” and “high school or above.” Income was stratified as “<5000 Chinese Yuan (CNY),” “5000–9999 CNY,” “10000–19999 CNY,” and “≥20,000 CNY.” The definition of alcohol consumption in this study was the regular intake of alcoholic beverages at a frequency of three or more times per week, with a minimum of 100 mL per drinking episode. Smoking was defined as the act of smoking continuously or cumulatively for a period exceeding six months. Physical inactivity refers to the absence of moderate-to-vigorous physical activity for >150 minutes/week or vigorous-intensity physical activity for >75 minutes/week (4). Diabetes was defined as a fasting plasma glucose level of ≥ 7.0 mmol/L (126 mg/dL), a previous diagnosis of diabetes mellitus, or the use of antidiabetic medication or insulin (32). Hypertension was defined as a blood pressure of ≥140/90 mmHg, a history of hypertension, or the use of antihypertensive medication (33). Dyslipidemia was defined as serum total cholesterol (TC) concentration ≥ 6.22 mmol/L (240 mg/dL), and/or low-density lipoprotein cholesterol (LDL-C) concentration ≥ 4.14 mmol/L (160 mg/dL), and/or TG concentration ≥ 2.26 mmol/L (200 mg/dL), and/or HDL-C concentration <1.04 mmol/L (40 mg/dL), or previous history of hyperlipidemia and currently taking lipid-lowering drugs (34). Atrial fibrillation was defined as electrocardiographic evidence of atrial fibrillation or treatment for atrial fibrillation.
Each participant underwent a physical examination conducted by a qualified nurse or physician. Height and weight were measured to a precision of 0.1 cm and 0.1 kg, respectively. BMI was calculated by dividing body mass (kilograms) by the height (meters) squared. The WC was measured to a precision of 0.1 cm at the highest point of the iliac crest during minimal respiration. In accordance with previous studies, general obesity was defined as a BMI of ≥25 kg/m2 following Asian-specific criteria (35); abdominal obesity as a WC of ≥90 cm for men and a WC of ≥85 cm for women (31). Blood pressure was measured twice by an examining nurse or physician at an interval of 15 min. The average between the two measurements was used as the final datum.
2.3 Biochemical measurements
Blood samples were collected after an 8-hour fast and analyzed using the HP-AFS/3 automatic immunoassay system A3 Specific Protein Analyzer with supporting reagents (Shijiazhuang Hebo Biotechnology Co., Ltd., Shijiazhuang, China) on the same day of collection. The biochemical indicators assessed included fasting blood glucose (FBG), Hemoglobin A1c (HbA1c), TC, TG, LDL-C, and HDL-C.
2.4 Definition of CVAI
CVAI scores were computed using sex-specific formulas as follows (36):
Males: CVAI = −267.93 + 0.68 × age (years) + 0.03 × BMI (kg/m2) + 4.00 × WC (cm) + 22.00 × log10(TG [mmol/L]) − 16.32 × HDL-C (mmol/L);
Females: CVAI= −187.32 + 1.71 × age (years) + 4.32 × BMI (kg/m2) + 1.12 × WC (cm) + 39.76 × log10(TG [mmol/L]) − 11.66 × HDL-C (mmol/L).
2.5 Definition of outcome incident stroke
All incidents of stroke, including ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage, were documented during the survey period. The diagnosis of stroke was confirmed either through neurological imaging (brain computed tomography or magnetic resonance imaging) or a diagnosis certificate from a secondary or higher medical unit. However, due to limitations in data collection methods, we were unable to record the exact onset time of stroke.
2.6 Statistical analysis
All continuous variables were non-normally distributed and are presented as medians with interquartile ranges (IQR). Categorical variables are presented as percentages. The baseline characteristics of participants without MetS were compared to those of patients with MetS using the Mann-Whitney test for continuous variables and the Chi-square test for categorical variables. The baseline characteristics of MetS patients with and without stroke were analyzed in the same manner. Furthermore, to demonstrate the baseline characteristics of CVAI, we stratified patients into quartiles based on their initial CVAI levels. Differences in baseline variables between groups were assessed using either the Jonckheere-Terpstra trend test or the Chi-square test for trends.
Multivariate logistic regression models were used to assess the correlation between CAVI and MetS diagnosis. Various multivariable models with different levels of adjustment were employed. Model 1 incorporated individual characteristics, such as age, sex, education level, and economic status. Model 2 further included lifestyle risk factors, like smoking, alcohol consumption, and physical activity; medical history of hypertension, diabetes mellitus, dyslipidemia, and stroke; and family medical history of stroke. The variables included in the models all met the criteria of tolerance > 0.1 and variance inflation factor < 10. The diagnostic performance of CVAI in detecting MetS was compared with that of BMI and WC using receiver operating characteristic (ROC) curve analyses. In addition, we employed a restricted cubic spline to evaluate the dose-response relationship between CVAI and MetS (knots on the 5th, 25th, 75th, and 95th percentiles).
After a 2-year follow-up, multivariate logistic regression models were used to evaluate the correlation between stroke risk in MetS patients and CVAI, as well as other adiposity measures. The multivariable models were consistent with those described above. Model 1 contained individual characteristics (age, sex, education, economic status). Model 2 added lifestyle risk factors and medical history (smoking, alcohol drinking and physical activity, hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation, prior stroke, family medical history of stroke on the base of model 1. The odds ratio (OR) was calculated with a 95% confidence interval (CI) for the presence of incident stroke. The dose-response relationship between CVAI or other adiposity measures and stroke risk was evaluated with a restricted cubic spline. Subgroup analyses were further performed to investigate the relationship between CVAI and stroke in various subgroups based on age (≥60, <60 years), sex (male, female), diabetes (yes, no), hypertension (yes, no), dyslipidemia (yes, no), prior stroke (yes, no), current smoking status (yes, no), current drinking habits (yes, no), and physical activity level (lacking or not lacking exercise). The p-value for an interaction between a subgroup variable and CVAI was assessed in each subgroup analysis.
SPSS version 25.0 (IBM SPSS, Armonk, NY, USA) and R version 4.2.3 (R Development Core Team, Vienna, Austria) were used for all statistical analyses. A two-tailed P-value of <0.05 was considered to indicate statistical significance.
3 Results
3.1 Characteristics of the study participants
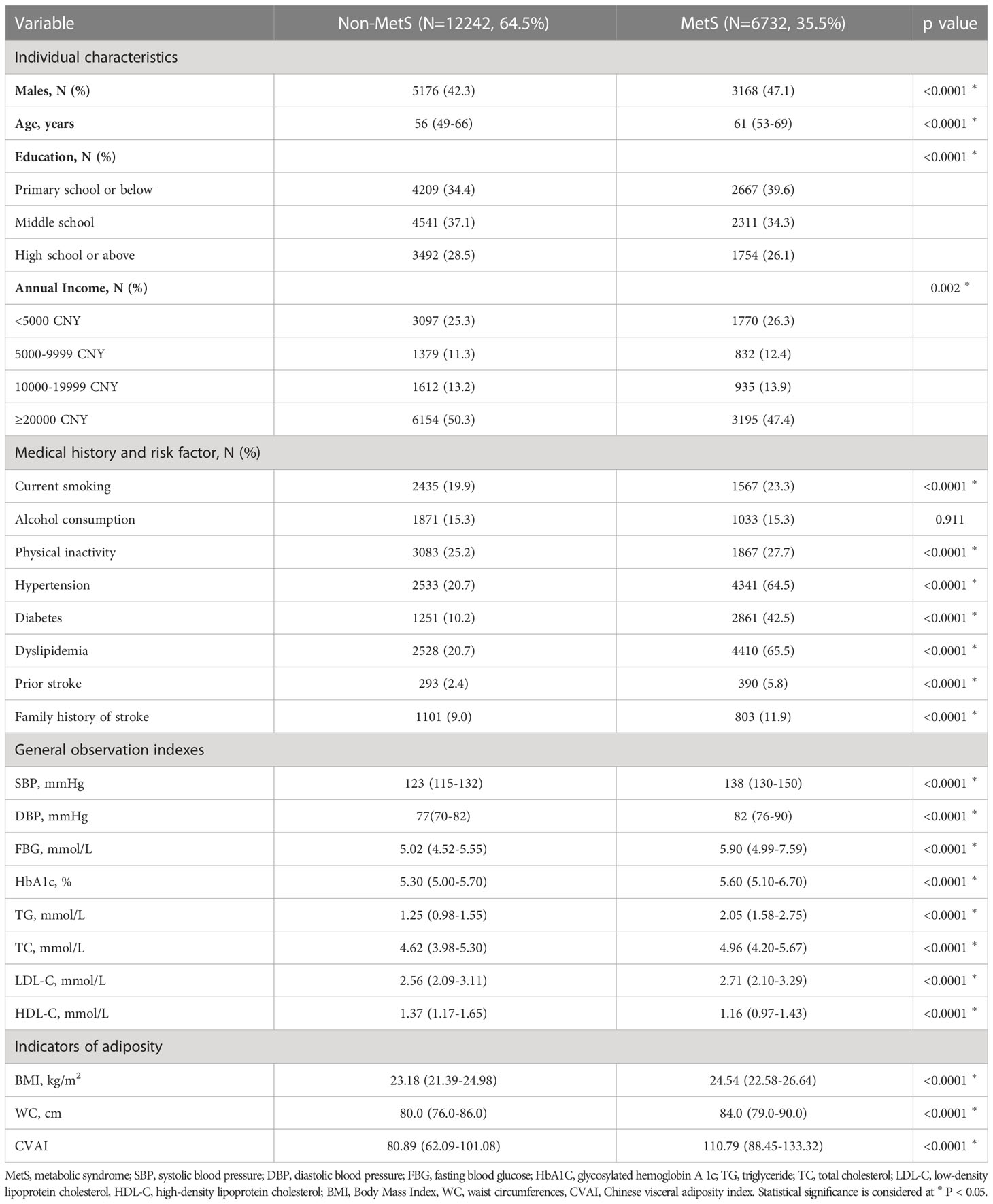
Of the 18,974 participants surveyed at both baseline and the two-year follow-up, 6732 were diagnosed with MetS at baseline. Table 1 outlines the demographic and clinical characteristics of these individuals. Notably, those with MetS had a significantly higher median CVAI compared to their non-MetS counterparts. Supplemental Table 1 presents the characteristics of all study participants stratified by CVAI quartile. The median CVAI for all study participants was 90.49 (IQR, 68.66–114.58).
3.2 Association between CVAI and MetS at baseline
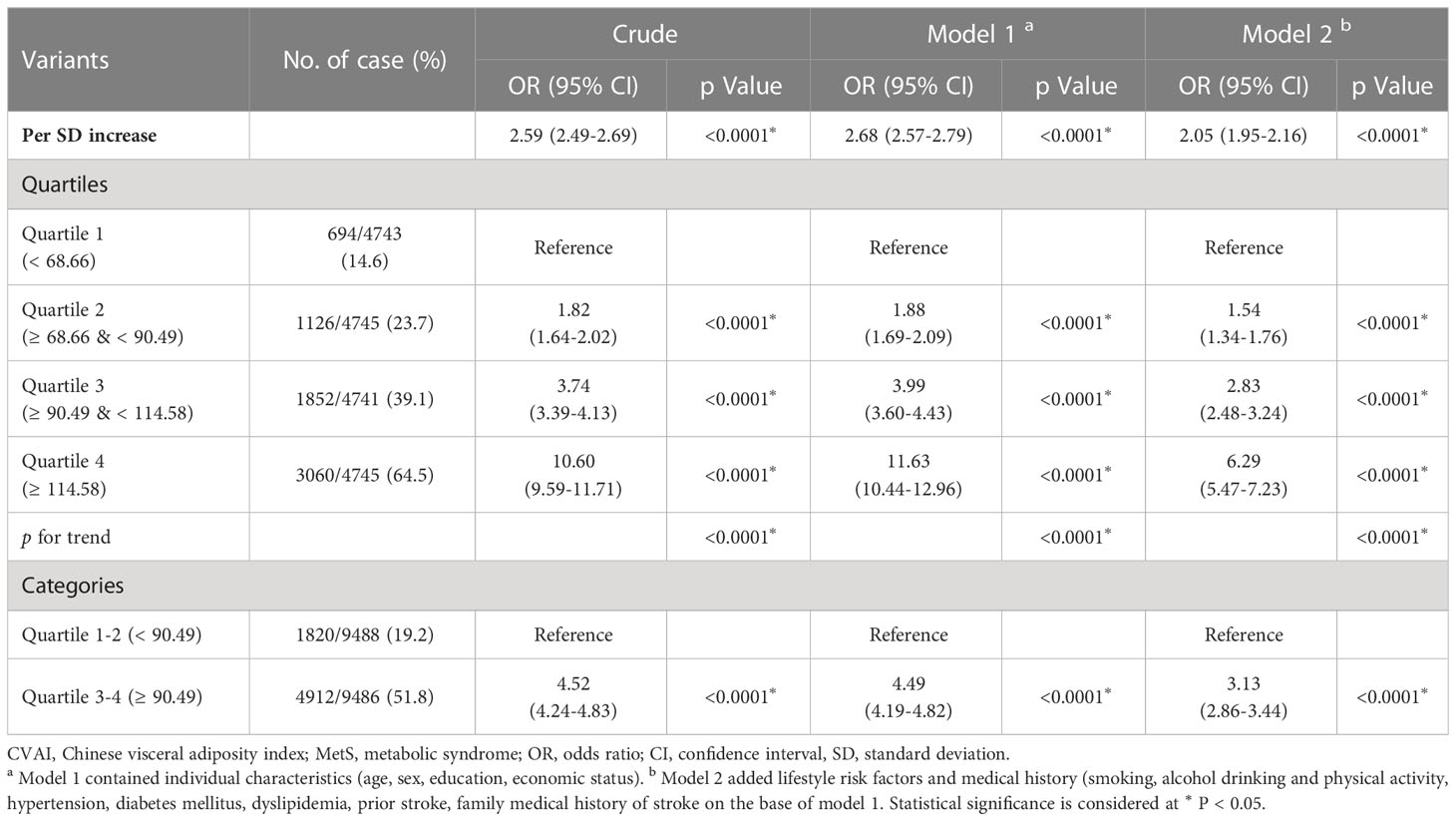
As the CVAI increased, there was a corresponding increase in the prevalence of MetS (Figure 1A). Table 2 shows a correlation between CVAI and MetS. In the unadjusted model, for each additional SD increase in CVAI, the risk of MetS increased by 1.59. In all two adjusted models, CVAI demonstrated an independent association with MetS; the adjusted odds ratios are 2.68 (95% CI: 2.57-2.79), and 2.05 (95% CI: 1.95-2.16), respectively. When assessed as quartiles, CVAI was significantly associated with MetS in the second, third, and fourth quartiles—even after adjustment for all confounding factors (adjusted OR: 1.54, 95% CI: 1.34, 1.76; adjusted OR: 2.83, 95% CI: 2.48, 3.24; adjusted OR: 6.29, 95% CI: 5.47,7.23, respectively). Participants in the third and fourth CVAI quartiles were associated with a significantly higher risk of MetS compared to their counterparts in the first and second quartiles (adjusted OR: 3.13, 95% CI: 2.86, 3.44). Additionally, dose-response relationships between CVAI and MetS were evaluated by restricted cubic splines (Figure 1B). CVAI increases the risk of MetS when higher than 90.94.
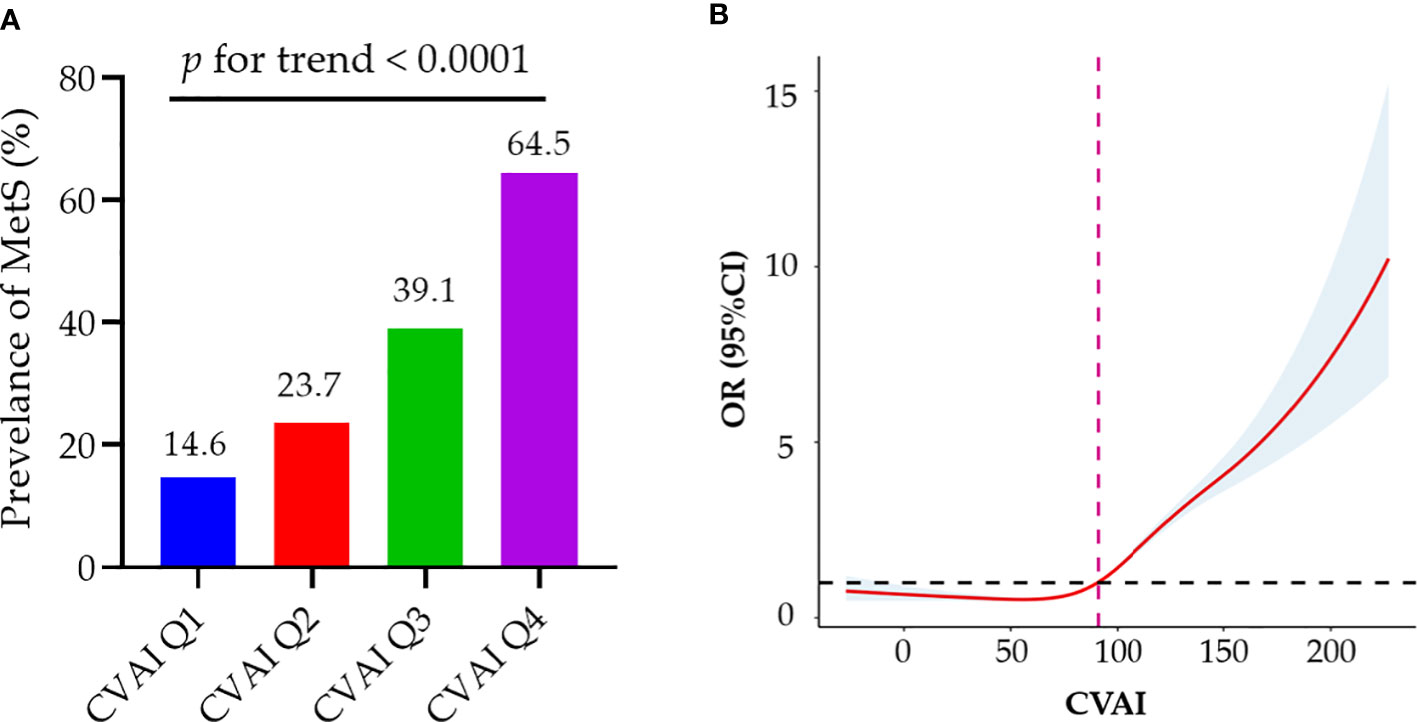
Figure 1 Associations of baseline CVAI with MetS. (A) As the CVAI increased, there was a corresponding rise in the prevalence of MetS; (B) Dose–response relationship of CVAI and MetS risk. CVAI could increase the risk of MetS when higher than 90.94. MetS, metabolic syndrome; CVAI, Chinese Visceral Adiposity Index; OR, odds ratio; CI, confidence interval.
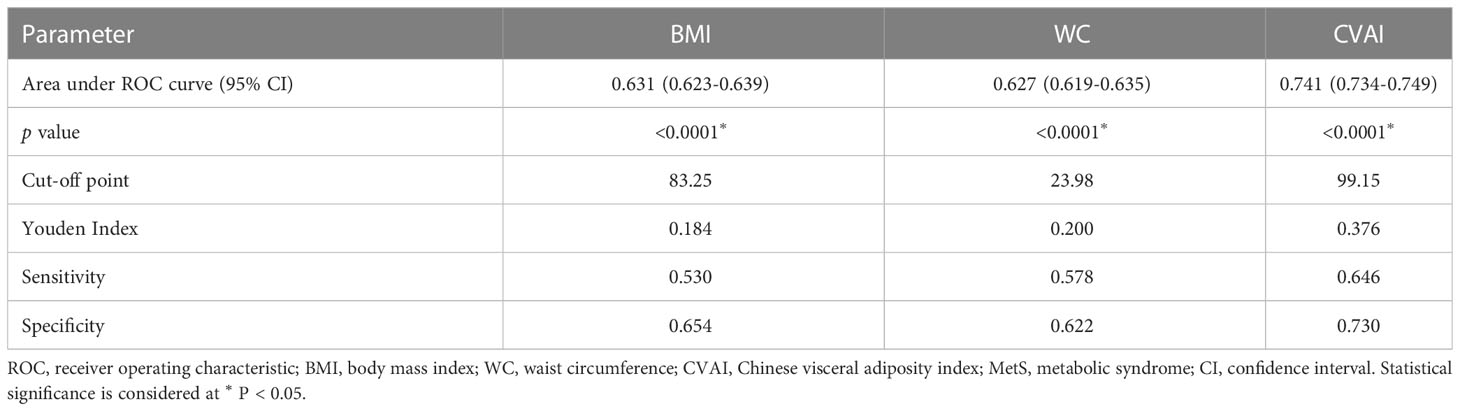
ROC curve analysis was used to compare the diagnostic efficacy of CVAI with that of BMI and WC in detecting MetS (Figure 2). In the diagnosis of MetS, the CVAI demonstrated the highest AUC values (AUC: 0.741, 95% CI: 0.734-0.749), exceeding those of WC (AUC: 0.627, 95% CI: 0.619-0.635) and BMI (AUC: 0.631, 95% CI: 0.623-0.639). Table 3 presents the diagnostic performance of each anthropometric index in identifying MetS, encompassing sensitivity, specificity, and corresponding optimal cut-off values. CVAI exhibited the highest Youden indices (0.376) for identifying MetS, with an optimal cut-off of 99.15.
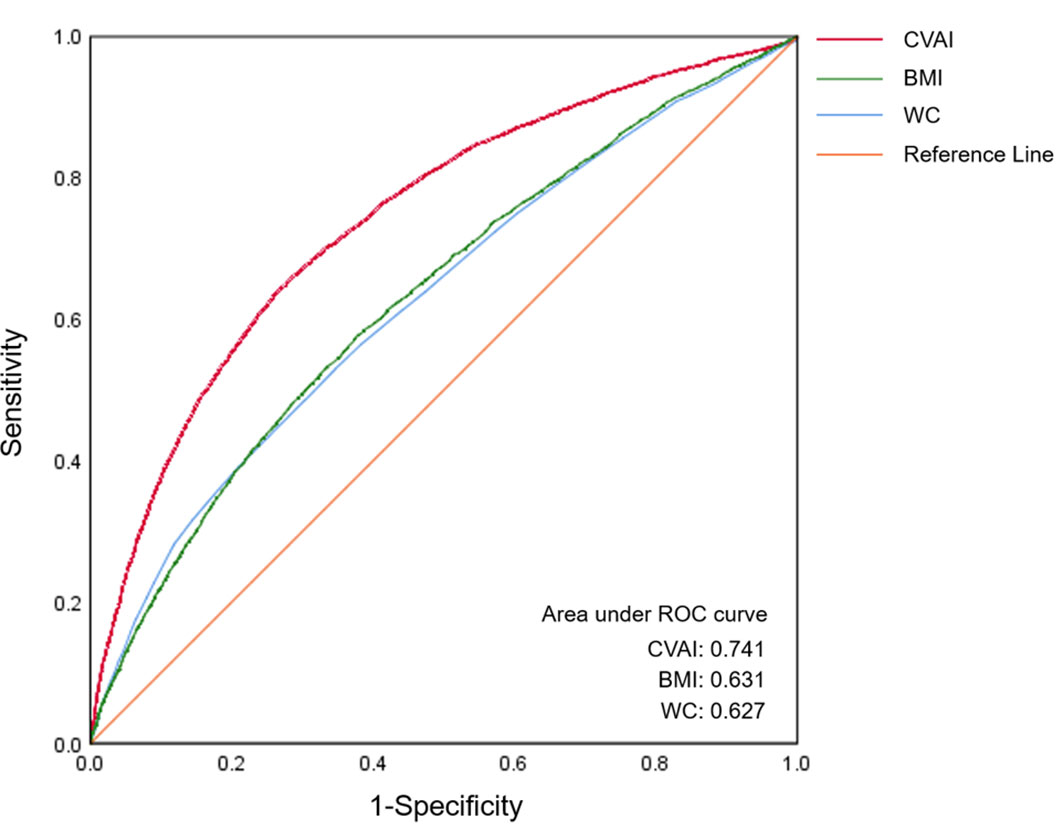
Figure 2 The ROC curves of CVAI, BMI and WC for MetS diagnosis. The area under the ROC curve of CVAI was 0.741 for diagnosing MetS, which is significantly superior to BMI and WC among Chinese adults (all p < 0.05). ROC, receiver-operating characteristic; CVAI, Chinese visceral adiposity index; BMI, body mass index; WC, waist circumference.
3.3 Comparison of baseline characteristics between MetS patients with and without stroke
Of the 6,732 patients with MetS, 72 experienced strokes during the two-year follow-up period; these individuals were more likely to be of advanced age and have a lower income at baseline relative to those without a history of stroke. Hypertension, previous history of stroke, and family history of stroke were more prevalent among individuals with a stroke incident. Additionally, the stroke group exhibited significantly higher SBP levels, BMI, WC, and CVAI at baseline (Supplement Table 2).
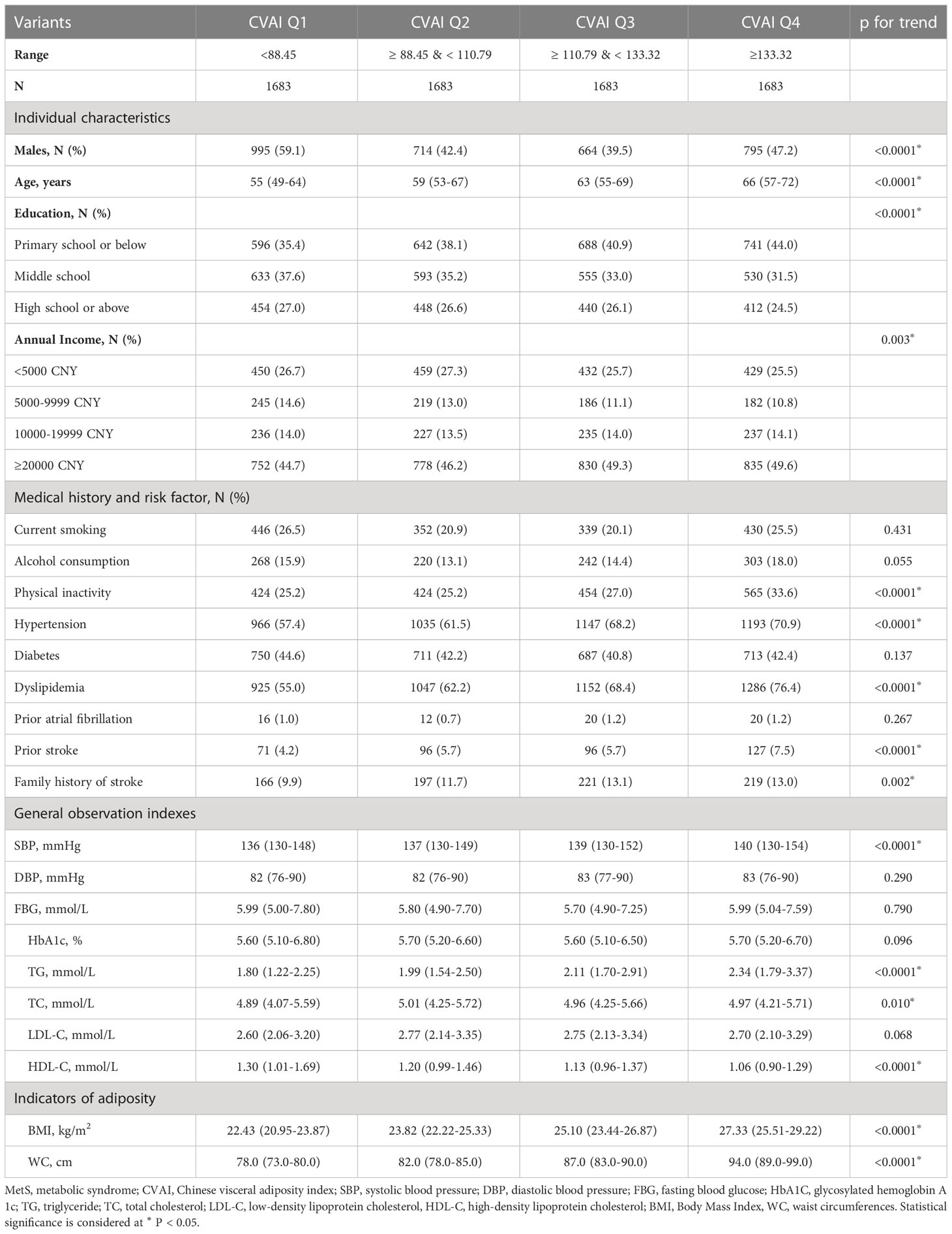
Table 4 shows the characteristics of participants with MetS categorized by CVAI quartile. The median CVAI was 110.79 (IQR, 88.45–133.32). Participants in the upper CVAI quartile tended to be older, female, less educated, and have a higher income. They were also less physically active, and hypertension, dyslipidemia, prior stroke history, and family history of stroke were more prevalent among them. Furthermore, we observed a significant trend towards increasing SBP, TG, and TC levels, as well as decreasing HDL-C levels with increasing CVAI, among the participants who developed MetS.
3.4 Association between CVAI at baseline and incident stroke during the 2-year follow-up of MetS patients
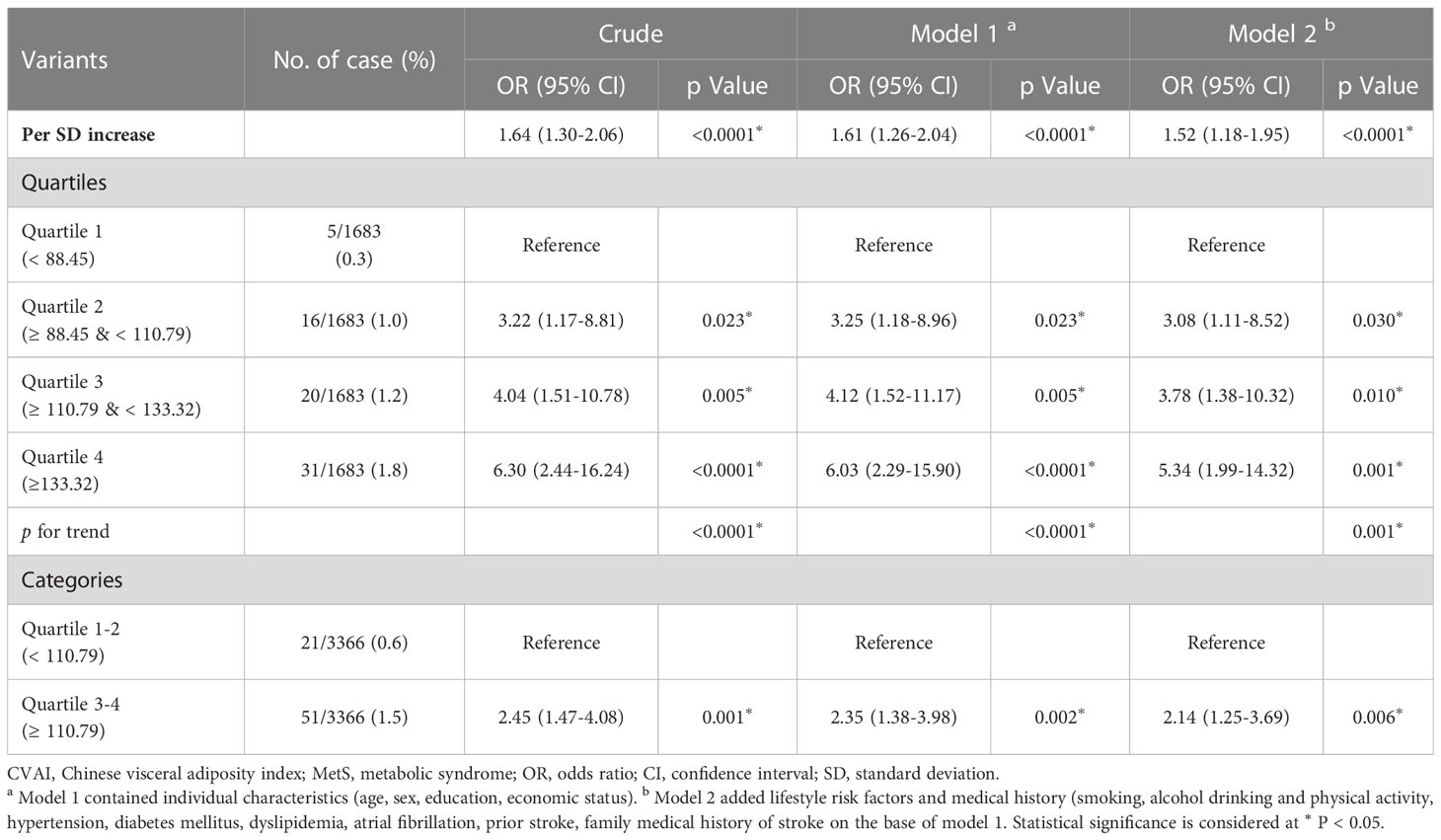
There was a positive association between CVAI and the risk of incident stroke (Figure 3A). Table 5 presents the association between CVAI and incident stroke. In the unadjusted model, the risk of incident stroke increased by 64% (95% CI: 1.30, 2.06) for each SD of CVAI. In both adjusted model 1 and model 2, there was a significant association between the increase in CVAI and the occurrence of stroke, as evidenced by the adjusted ORs of 1.61 (95% CI: 1.26, 2.04) and 1.52 (95% CI: 1.18, 1.95), respectively. When assessing CVAI as quartiles, the associations between incident stroke and the second, third, and fourth CVAI quartiles were 3.22 (95% CI: 1.17-8.81), 4.04 (95% CI: 1.51-10.78), and 6.30 (95% CI: 2.44-16.24), respectively, relative to the first CVAI quartile. These association estimators did not change significantly after additional adjustment for individual characteristics, medical history, and risk factors. Consistently, participants in the third and fourth CVAI quartiles showed a significantly higher risk of incident stroke than those in the first and second quartiles (adjusted OR: 2.14, 95% CI: 1.25, 3.69).
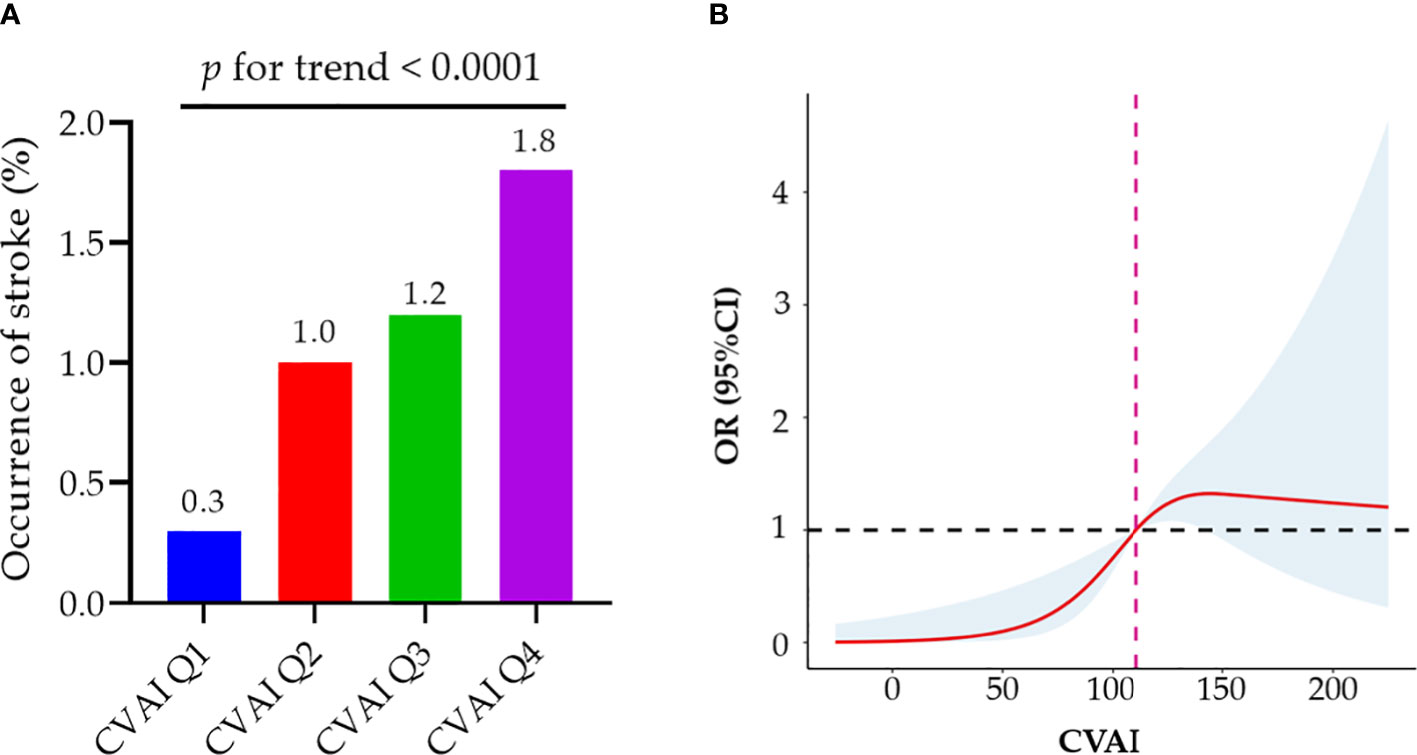
Figure 3 Associations of baseline CVAI with incident stroke among MetS patients. (A) MetS patients in the higher quartile of CVAI demonstrate a significantly elevated risk of stroke; (B) Dose–response relationship of CVAI and stroke risk. A reduced stroke risk was observed when the CVAI was either less than 110.91. MetS, metabolic syndrome; CVAI, Chinese Visceral Adiposity Index; OR, odds ratio; CI, confidence interval.
As demonstrated by the logistic regression models, our findings also indicate a positive correlation between WC and incident stroke in individuals with MetS. Participants exhibiting abdominal obesity had a relative risk of 2.73 (95% CI: 1.67, 4.46) for developing an incident stroke compared to their counterparts without abdominal obesity (Supplement Table 3). The fully adjusted model revealed significant associations between incident stroke and the third and fourth BMI quartiles in patients with MetS, but not the second quartile (Supplement Table 4). Although general obesity may be as-sociated with an elevated risk of stroke, the association did not reach statistical significance after adjustment for confounding factors (adjusted OR: 1.60; 95% CI: 0.99, 2.59). We further used restricted cubic splines to visualize the relation of CVAI, WC, and BMI with incident stroke in patients with MetS. The risk of incident stroke was non-linearly associated with CVAI (Figure 3B) and followed an inverted U-shaped curve with respect to BMI (Supplement Figure 2A). A decreased risk of stroke was observed when the CVAI was below 110.91.
During the two-year follow-up period, 57 strokes occurred among the 12,242 patients without MetS. However, no significant correlation was detected between CVAI and stroke in non-MetS patients (Supplement Table 5).
3.5 Subgroup analyses of stroke risk factors
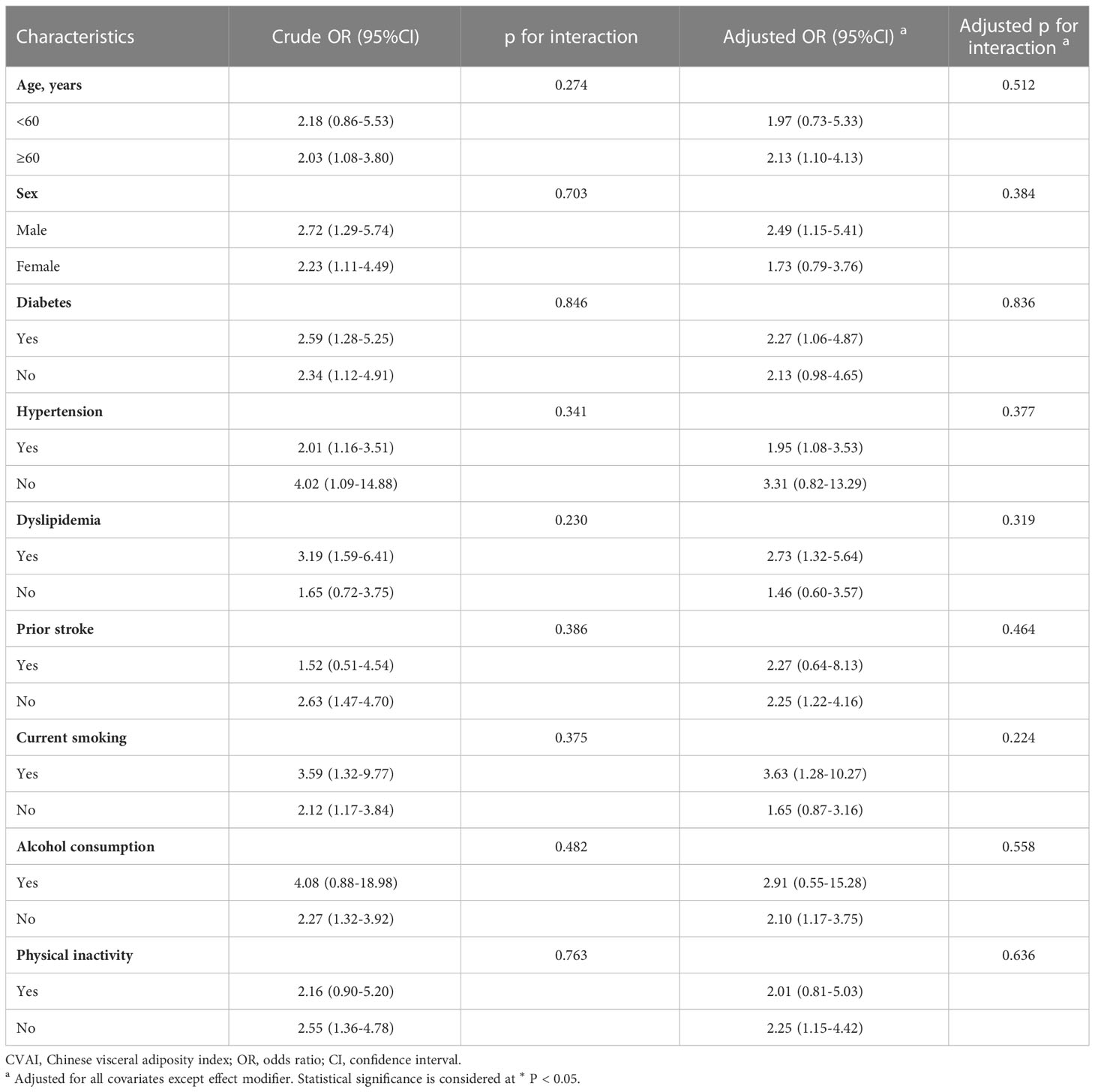
To evaluate any potential modifying effects of stroke risk factors on the association between CVAI (3-4 quartiles vs. 1-2 quartiles) and incident stroke, subgroup analyses were conducted (Table 6). We found no significant interaction between CVAI and covariates in relation to incident stroke.
4 Discussion
The present study confirms the superiority of CVAI over BMI and WC in identifying individuals with MetS in a large Chinese population. Furthermore, this investigation is, to the best of our knowledge, the first to detect a robust positive correlation between incident stroke and CVAI in patients with MetS. Our findings suggest a strong association between elevated levels of CVAI and incident stroke in Chinese adults diagnosed with MetS and that reducing CVAI levels could help to mitigate the risk of stroke in patients with MetS.
Epidemiologic studies have demonstrated that the standardized prevalence of MetS is 31.1% among Chinese individuals aged ≥20 years (37). Furthermore, as the obese population continues to increase, so too does the incidence of MetS. The baseline prevalence of MetS in our sample population (35.5%) was consistent with statistics reported in previous epidemiological research (37). The excessive accumulation of abdominal visceral fat is characteristic of MetS and clinically significant when assessing MetS in individuals of normal body weight (18). In the Chinese population, CVAI has been proposed as a valid, dependable indicator of visceral adiposity dysfunction (38). Our findings indicate that CVAI levels are significantly higher among those with MetS than those without it. Although previous studies have shown that CVAI has a favorable predictive performance for detecting MetS (39, 40), their findings were limited by having been derived from relatively small sample sizes. By contrast, our investigation demonstrates a significant positive association between CVAI levels and MetS in a large study population. CVAI exhibited superior performance in the diagnosis of MetS among Chinese adults, as evidenced by a higher AUC and greater overall discriminative ability than those of BMI and WC. This result is consistent with the findings of the previous research (39, 40), suggesting that CVAI, a quantitative index of visceral fat, can serve as a more reliable indicator of MetS than either BMI or WC.
The present study has also revealed that MetS patients in the higher quartile of CVAI were associated with lower levels of educational attainment. This finding may be attributed to the fact that individuals with higher levels of education are more inclined towards adopting healthier behaviors, which in turn may lead to a greater likelihood of remission from MetS (41). Moreover, it was noted that individuals diagnosed with MetS displayed a significant positive association between their income level and CVAI, signifying a rise in visceral adiposity with an increase in income (42–44). The observed trend is incongruent with the findings in developed nations, where individuals with lower incomes typically exhibit elevated rates of obesity and MetS. One plausible explanation for this disparity may be linked to the inclination of individuals with slightly higher incomes in developing countries to consume highly processed foods that lack nutritional value and contain “empty calories,” which are additional to their daily diet (44). Future studies ought to explore the correlation between visceral adiposity and dietary patterns within developing country populations.
Patients diagnosed with MetS are considered at high-risk for stroke (8–10). The current study demonstrates that the incidence of stroke during 2-year follow-up in patients with MetS is 2.31 times higher than it is for their counterparts without MetS, underscoring the importance of preventing stroke incidence in MetS patients. Mendelian randomization studies have suggested that visceral adiposity exerts a more potent impact on stroke risk than does general adiposity (45). Furthermore, visceral adiposity has been causally linked to the occurrence of stroke (46). While research on the longitudinal associations between visceral adiposity and stroke risk in patients with MetS has been limited, the present large population-based prospective cohort study found a strong association between elevated CVAI and incident stroke in Chinese adults who experienced MetS—even after adjustment for potential confounders. Specifically, each 1 SD increase in CVAI was found to increase the risk of incident stroke by a factor of 1.52 after correction. These findings provide additional support for the potential of reducing CVAI as a strategy to lower the risk of incident stroke among individuals with MetS.
Previous research has indicated that individuals with MetS and a BMI indicative of general obesity are at an increased risk of developing CVD relative to those without general obesity [17]. In our study, participants with general obesity were also found to have a higher risk of incident stroke relative to their non-obese counterparts; however, this trend did not reach statistical significance. The restricted cubic spline analysis revealed a dose-dependent association between CVAI and an increased risk of incident stroke. However, as BMI increases, an inverted U-shaped dose-response relationship was observed. One possible explanation is that BMI is unable to distinguish between the accumulation of fat-free mass and adipose tissue, leading to misidentification of individuals with high muscle mass as overweight or obese. The aforementioned data suggests that CVAI reflects stroke risk better than does BMI. Thus, in the routine practice of stroke prevention, assessing excessive body weight should incorporate an indicator of visceral adiposity rather than BMI alone.
Multiple factors may account for the correlation between CVAI and stroke in patients with MetS. First, CVAI levels are influenced by dyslipidemia, a common vascular risk factor for stroke associated with MetS. Second, the relationship might be explained by a demonstrated correlation between CVAI and the early development of traditional risk factors for stroke: e.g., Han et al. demonstrated a positive correlation between elevated CVAI and heightened risks of diabetes (47), a well-documented risk factor for stroke. The association between elevated CVAI and an increased risk of developing hypertension provides further support for the connection between CVAI and stroke risk factors (48). Finally, visceral adipose tissue not only secretes elevated levels of pro-inflammatory cytokines (49, 50), but also fulfills endocrine functions and plays a pivotal role in the pathogenesis of insulin resistance (51). The pathophysiological alterations prompted by visceral adipose tissue may exacerbate the risk of atherosclerosis, a major contributor to ischemic stroke. Indeed, enhanced levels of CVAI are positively associated with an increased likelihood of carotid atherosclerotic plaque formation (28, 52). Subsequent investigations could further explore the correlation between CVAI and serum concentrations of pro-inflammatory cytokines.
Interestingly, the present investigation did not observe any significant association between CVAI and stroke in non-MetS patients. This finding is consistent with those of Mirzaei et al., who observed no significant elevation in the risk of CVD in either metabolically healthy normal weight and metabolically healthy obese (MHO) groups after a 12-year follow-up period (53). A 14-year population-based prospective study of 5,314 individuals aged ≥55 years in Rotterdam also found that MHO did not augment the risk of cardiovascular disease (54). One possible explanation for this finding is that the incidence of cardiovascular events may be more closely linked to progression towards metabolic syndrome than obesity in middle-aged and older individuals (55). Further investigations are warranted to examine the contribution of CVAI in MetS risk among individuals with metabolically healthy normal weight and MHO.
This study is, to the best of our knowledge, the first study to investigate a correlation between CVAI and incident stroke in a robust sample size of Chinese MetS patients. Specifically, a gradual increase in the risk of stroke was observed when the CVAI surpassed the threshold of 110.91. Therefore, it is recommended that individuals diagnosed with MetS undergo further medical examinations to determine their susceptibility to stroke when their CVAI score exceeds 110.91 and promptly implement preventive measures against stroke. While this study benefitted from meticulous data collection and rigorous adjustment for confounding factors, it is subject to the following limitations. The 2-year follow-up period allowed for only a limited number of events for analysis. For which reason, the logistic regression models did not meet the criterion of 10 events per variable (EPV) in analyzing stroke risk among MetS patients. Nonetheless, it has been recommended that sample sizes of 5 to 10 EPV included in a regression equation could yield fairly stable coefficients in logistic regression models [44,45]. Moreover, our results exhibited a degree of resemblance to analogous previous investigations. Hence, it is suggested that our results are robust to some extent. Additionally, a 2-year follow-up period remains a reasonable timeframe for identifying individuals with an elevated risk of stroke. In addition to long-term risk information, short-term stroke risk information may also be of interest and more persuasive for behavior modification. An additional limitation of this study pertains to its geographical scope, as it was solely carried out in Hunan Province. Therefore, further validation across other provinces or larger populations is necessary to substantiate the universality of the findings. Finally, the impact of diet and medication on incident stroke was not taken into account due to data limitations.
5 Conclusions
CVAI demonstrates an independent and positive correlation with MetS, outperforming BMI and WC in identifying individuals with MetS. These findings recommend CVAI as a superior screening tool for MetS. Furthermore, elevated levels of CVAI are strongly linked to incident stroke in Chinese adults diagnosed with MetS, suggesting that reducing CVAI levels can mitigate the risk of stroke in individuals with MetS.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Xiangya Hospital Ethics Committee. The protocol and informed consent for the study of the China Stroke High-risk Population Screening and Intervention Program were reviewed and approved by the Institutional Review Board at the Capital Medical University Xuanwu Hospital early. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization, JX and ZL. Methodology, QH, BD and ZL. Software, QH and XF. Validation, YD, FY and JF. Formal analysis, ZL and MW. Investigation, XF. Resources and data curation, JX. Writing—original draft preparation, ZL. Writing—review and editing, and project administration, JX. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key Research and Development Projects (2022YFC3602400, 2022YFC3602401), the National Natural Science Foundation of China (82271369), the Provincial Key Plan for Research and Development of Hunan (Grant No. 2020SK2067), the Natural Science Foundation of Hunan Province (Grant No. 2021JJ31109), and the supported by the Fundamental Research Funds for the Central Universities of Central South University (2022ZZTS0821).
Acknowledgments
We thank all the patients, hospitals, and staff involved in the China Stroke High-risk Population Screening and Intervention Program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1218905/full#supplementary-material
References
1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the gbd 2019 study. J Am Coll Cardiol (2020) 76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010
2. GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol (2021) 20(10):795–820. doi: 10.1016/s1474-4422(21)00252-0
3. Feigin VL, Brainin M, Norrving B, Martins S, Sacco RL, Hacke W, et al. World stroke organization (Wso): global stroke fact sheet 2022. Int J stroke (2022) 17(1):18–29. doi: 10.1177/17474930211065917
4. Tu WJ, Hua Y, Yan F, Bian H, Yang Y, Lou M, et al. Prevalence of stroke in China, 2013-2019: a population-based study. Lancet regional Health Western Pacific (2022) 28:100550. doi: 10.1016/j.lanwpc.2022.100550
5. Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res (2017) 120(3):439–48. doi: 10.1161/circresaha.116.308413
6. Samson SL, Garber AJ. Metabolic syndrome. Endocrinol Metab Clinics North America (2014) 43(1):1–23. doi: 10.1016/j.ecl.2013.09.009
7. Ng HY, Kuo WH, Tain YL, Leung FF, Lee WC, Lee CT. Effect of dapagliflozin and magnesium supplementation on renal magnesium handling and magnesium homeostasis in metabolic syndrome. Nutrients (2021) 13(11):4088. doi: 10.3390/nu13114088
8. Zhang WW, Liu CY, Wang YJ, Xu ZQ, Chen Y, Zhou HD. Metabolic syndrome increases the risk of stroke: a 5-year follow-up study in a Chinese population. J Neurol (2009) 256(9):1493–9. doi: 10.1007/s00415-009-5150-2
9. Rodriguez-Colon SM, Mo J, Duan Y, Liu J, Caulfield JE, Jin X, et al. Metabolic syndrome clusters and the risk of incident stroke: the atherosclerosis risk in communities (Aric) study. Stroke (2009) 40(1):200–5. doi: 10.1161/strokeaha.108.523035
10. Liang Y, Yan Z, Hao Y, Wang Q, Zhang Z, She R, et al. Metabolic syndrome in patients with first-ever ischemic stroke: prevalence and association with coronary heart disease. Sci Rep (2022) 12(1):13042. doi: 10.1038/s41598-022-17369-8
11. Ray I, Mahata SK, De RK. Obesity: an immunometabolic perspective. Front Endocrinol (2016) 7:157. doi: 10.3389/fendo.2016.00157
12. Rosenquist KJ, Pedley A, Massaro JM, Therkelsen KE, Murabito JM, Hoffmann U, et al. Visceral and subcutaneous fat quality and cardiometabolic risk. JACC Cardiovasc Imaging (2013) 6(7):762–71. doi: 10.1016/j.jcmg.2012.11.021
13. Koenen M, Hill MA, Cohen P, Sowers JR. Obesity, adipose tissue and vascular dysfunction. Circ Res (2021) 128(7):951–68. doi: 10.1161/circresaha.121.318093
14. Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol (2020) 8(7):616–27. doi: 10.1016/s2213-8587(20)30110-8
15. Sorimachi H, Obokata M, Takahashi N, Reddy YNV, Jain CC, Verbrugge FH, et al. Pathophysiologic importance of visceral adipose tissue in women with heart failure and preserved ejection fraction. Eur Heart J (2021) 42(16):1595–605. doi: 10.1093/eurheartj/ehaa823
16. Huang Y, Liu Y, Ma Y, Tu T, Liu N, Bai F, et al. Associations of visceral adipose tissue, circulating protein biomarkers, and risk of cardiovascular diseases: a mendelian randomization analysis. Front Cell Dev Biol (2022) 10:840866. doi: 10.3389/fcell.2022.840866
17. Xu R, Hu X, Wang T, Yang Y, Jiang N, Luo J, et al. Visceral adiposity and risk of stroke: a mendelian randomization study. Front Neurol (2022) 13:804851. doi: 10.3389/fneur.2022.804851
18. Baek JH, Jin SM, Bae JC, Jee JH, Yu TY, Kim SK, et al. Serum calcium and the risk of incident metabolic syndrome: a 4.3-year retrospective longitudinal study. Diabetes Metab J (2017) 41(1):60–8. doi: 10.4093/dmj.2017.41.1.60
19. Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab (2006) 91(8):2906–12. doi: 10.1210/jc.2006-0594
20. McAuley PA, Blair SN. Obesity paradoxes. J sports Sci (2011) 29(8):773–82. doi: 10.1080/02640414.2011.553965
21. Hou B, Shen X, He Q, Chen Y, Xu Y, Chen M, et al. Is the visceral adiposity index a potential indicator for the risk of kidney stones? Front Endocrinol (2022) 13:1065520. doi: 10.3389/fendo.2022.1065520
22. Ceniccola GD, Castro MG, Piovacari SMF, Horie LM, Corrêa FG, Barrere APN, et al. Current technologies in body composition assessment: advantages and disadvantages. Nutr (Burbank Los Angeles County Calif) (2019) 62:25–31. doi: 10.1016/j.nut.2018.11.028
23. Wan H, Wang Y, Xiang Q, Fang S, Chen Y, Chen C, et al. Associations between abdominal obesity indices and diabetic complications: Chinese visceral adiposity index and neck circumference. Cardiovasc Diabetol (2020) 19(1):118. doi: 10.1186/s12933-020-01095-4
24. Xia MF, Chen Y, Lin HD, Ma H, Li XM, Aleteng Q, et al. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Sci Rep (2016) 6:38214. doi: 10.1038/srep38214
25. Xia MF, Lin HD, Chen LY, Wu L, Ma H, Li Q, et al. Association of visceral adiposity and its longitudinal increase with the risk of diabetes in Chinese adults: a prospective cohort study. Diabetes/metabolism Res Rev (2018) 34(7):e3048. doi: 10.1002/dmrr.3048
26. Li B, Wang J, Zhou X, Liu Y, Wang W, Gao Z, et al. Chinese Visceral adiposity index is more closely associated with hypertension and prehypertension than traditional adiposity indices in Chinese population: results from the reaction study. Front Endocrinol (2022) 13:921997. doi: 10.3389/fendo.2022.921997
27. Wang X, Si Z, Wang H, Meng R, Lu H, Zhao Z, et al. Association of Chinese visceral adiposity index and carotid atherosclerosis in steelworkers: a cross-sectional study. Nutrients (2023) 15(4):1023. doi: 10.3390/nu15041023
28. Bi H, Zhang Y, Qin P, Wang C, Peng X, Chen H, et al. Association of Chinese visceral adiposity index and its dynamic change with risk of carotid plaque in a Large cohort in China. J Am Heart Assoc (2022) 11(1):e022633. doi: 10.1161/jaha.121.022633
29. Wang Y, Zhao X, Chen Y, Yao Y, Zhang Y, Wang N, et al. Visceral adiposity measures are strongly associated with cardiovascular disease among female participants in southwest China: a population-based prospective study. Front Endocrinol (2022) 13:969753. doi: 10.3389/fendo.2022.969753
30. Chao BH, Yan F, Hua Y, Liu JM, Yang Y, Ji XM, et al. Stroke prevention and control system in China: csppc-stroke program. Int J stroke (2021) 16(3):265–72. doi: 10.1177/1747493020913557
31. Liu D, Zhong J, Ruan Y, Zhang Z, Sun J, Chen H. The association between fat-to-Muscle ratio and metabolic disorders in type 2 diabetes. Diabetol Metab syndrome (2021) 13(1):129. doi: 10.1186/s13098-021-00748-y
32. Zhang W, Tang Z, Shi Y, Ji L, Chen X, Chen Y, et al. Association between gamma-glutamyl transferase, total bilirubin and systemic lupus erythematosus in Chinese women. Front Immunol (2021) 12:682400. doi: 10.3389/fimmu.2021.682400
33. Gidding SS, Whelton PK, Carey RM, Levine GN. Writing a trustworthy hypertension guideline. J Am Coll Cardiol (2019) 74(19):2424–7. doi: 10.1016/j.jacc.2019.09.008
34. Zhang H, Kwapong WR, Shao MM, Yan JY, Lin XD, Chen BB, et al. Predictors of the prevalence of dyslipidemia and influencing factors for young health examination cohort: a cross-sectional survey. Front Public Health (2020) 8:400. doi: 10.3389/fpubh.2020.00400
35. Chung GK, Chung RY, Chan DC, Lai FT, Wong H, Lau MK, et al. The independent role of deprivation in abdominal obesity beyond income poverty. a population-based household survey in Chinese adults. J Public Health (Oxford England) (2019) 41(3):476–86. doi: 10.1093/pubmed/fdy161
36. Wei J, Liu X, Xue H, Wang Y, Shi Z. Comparisons of visceral adiposity index, body shape index, body mass index and waist circumference and their associations with diabetes mellitus in adults. Nutrients (2019) 11(7):1580. doi: 10.3390/nu11071580
37. Yao F, Bo Y, Zhao L, Li Y, Ju L, Fang H, et al. Prevalence and influencing factors of metabolic syndrome among adults in China from 2015 to 2017. Nutrients (2021) 13(12):4475. doi: 10.3390/nu13124475
38. Guo L, Wang YY, Wang JH, Zhao HP, Yu Y, Wang GD, et al. Associations of gut microbiota with dyslipidemia based on sex differences in subjects from northwestern China. World J Gastroenterol (2022) 28(27):3455–75. doi: 10.3748/wjg.v28.i27.3455
39. Duan Y, Zhang W, Li Z, Niu Y, Chen Y, Liu X, et al. Predictive ability of obesity- and lipid-related indicators for metabolic syndrome in relatively healthy Chinese adults. Front Endocrinol (2022) 13:1016581. doi: 10.3389/fendo.2022.1016581
40. Gui J, Li Y, Liu H, Guo LL, Li J, Lei Y, et al. Obesity- and lipid-related indices as a predictor of obesity metabolic syndrome in a national cohort study. Front Public Health (2023) 11:1073824. doi: 10.3389/fpubh.2023.1073824
41. Hoveling LA, Liefbroer AC, Bültmann U, Smidt N. Understanding socioeconomic differences in metabolic syndrome remission among adults: what is the mediating role of health behaviors? Int J Behav Nutr Phys activity (2021) 18(1):147. doi: 10.1186/s12966-021-01217-5
42. Riediger ND, Clara I. Prevalence of metabolic syndrome in the Canadian adult population. CMAJ (2011) 183(15):E1127–34. doi: 10.1503/cmaj.110070
43. Kibria GMA, Crispen R, Chowdhury MAB, Rao N, Stennett C. Disparities in absolute cardiovascular risk, metabolic syndrome, hypertension, and other risk factors by income within Racial/Ethnic groups among middle-aged and older us people. J Hum Hypertension (2021) 37(6):480–90. doi: 10.1038/s41371-021-00513-8
44. Żukiewicz-Sobczak W, Wróblewska P, Zwoliński J, Chmielewska-Badora J, Adamczuk P, Krasowska E, et al. Obesity and poverty paradox in developed countries. Ann Agric Environ Med (2014) 21(3):590–4. doi: 10.5604/12321966.1120608
45. Dale CE, Fatemifar G, Palmer TM, White J, Prieto-Merino D, Zabaneh D, et al. Causal associations of adiposity and body fat distribution with coronary heart disease, stroke subtypes, and type 2 diabetes mellitus: a mendelian randomization analysis. Circulation (2017) 135(24):2373–88. doi: 10.1161/circulationaha.116.026560
46. Yan B, Yang J, Qian L, Gao F, Bai L, Wang G, et al. Effect of genetic liability to visceral adiposity on stroke and its subtypes: a mendelian randomization study. Int J stroke (2022) 17(2):172–9. doi: 10.1177/17474930211006285
47. Han M, Qin P, Li Q, Qie R, Liu L, Zhao Y, et al. Chinese Visceral adiposity index: a reliable indicator of visceral fat function associated with risk of type 2 diabetes. Diabetes/metabolism Res Rev (2021) 37(2):e3370. doi: 10.1002/dmrr.3370
48. Han M, Qie R, Li Q, Liu L, Huang S, Wu X, et al. Chinese Visceral adiposity index, a novel indicator of visceral obesity for assessing the risk of incident hypertension in a prospective cohort study. Br J Nutr (2021) 126(4):612–20. doi: 10.1017/s0007114520004298
49. Sam S, Haffner S, Davidson MH, D'Agostino RB, Feinstein S, Kondos G, et al. Hypertriglyceridemic waist phenotype predicts increased visceral fat in subjects with type 2 diabetes. Diabetes Care (2009) 32(10):1916–20. doi: 10.2337/dc09-0412
50. Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine network (2006) 17(1):4–12.
51. Sironi AM, Gastaldelli A, Mari A, Ciociaro D, Positano V, Buzzigoli E, et al. Visceral fat in hypertension: influence on insulin resistance and beta-cell function. Hypertension (Dallas Tex 1979) (2004) 44(2):127–33. doi: 10.1161/01.HYP.0000137982.10191.0a
52. Li B, Lai X, Yan C, Jia X, Li Y. The associations between neutrophil-to-Lymphocyte ratio and the Chinese visceral adiposity index, and carotid atherosclerosis and atherosclerotic cardiovascular disease risk. Exp gerontology (2020) 139:111019. doi: 10.1016/j.exger.2020.111019
53. Mirzaei B, Abdi H, Serahati S, Barzin M, Niroomand M, Azizi F, et al. Cardiovascular risk in different obesity phenotypes over a decade follow-up: Tehran lipid and glucose study. Atherosclerosis (2017) 258:65–71. doi: 10.1016/j.atherosclerosis.2017.02.002
54. Dhana K, Koolhaas CM, van Rossum EF, Ikram MA, Hofman A, Kavousi M, et al. Metabolically healthy obesity and the risk of cardiovascular disease in the elderly population. PLoS One (2016) 11(4):e0154273. doi: 10.1371/journal.pone.0154273
Keywords: Chinese visceral adiposity index, stroke, metabolic syndrome, obesity, visceral fat tissue
Citation: Liu Z, Huang Q, Deng B, Wei M, Feng X, Yu F, Feng J, Du Y and Xia J (2023) Elevated Chinese visceral adiposity index increases the risk of stroke in Chinese patients with metabolic syndrome. Front. Endocrinol. 14:1218905. doi: 10.3389/fendo.2023.1218905
Received: 08 May 2023; Accepted: 15 June 2023;
Published: 29 June 2023.
Edited by:
Lu Cai, University of Louisville, United StatesReviewed by:
Aleksandra Klisic, Primary Health Care Center Podgorica, MontenegroPriyanka Majety, Virginia Commonwealth University Health System, United States
Copyright © 2023 Liu, Huang, Deng, Wei, Feng, Yu, Feng, Du and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Xia, xjian1216@csu.edu.cn
 Zeyu Liu
Zeyu Liu Qin Huang
Qin Huang Bi Deng
Bi Deng Minping Wei
Minping Wei Xianjing Feng
Xianjing Feng Fang Yu
Fang Yu Jie Feng1,2,3
Jie Feng1,2,3 Jian Xia
Jian Xia