- 1Department of Research, Sorlandet Hospital, Kristiansand, Norway
- 2Department of Nutrition and Public Health, Faculty of Health and Sport Sciences, University of Agder, Kristiansand, Norway
- 3Department of Pediatric and Adolescents Medicine, Akershus University Hospital, Akershus, Norway
- 4General Practice Research Unit (AFE), Department of General Medicine, Institute of Health and Society, University of Oslo, Oslo, Norway
- 5Department of Public Health and Nursing, Norwegian University of Science and Technology (NTNU), Trondheim, Norway
- 6Department of Clinical Services, St.Olavs Hospital Trondheim University Hospital, Trondheim, Norway
- 7Institute of Clinical Medicine, University of Oslo, Oslo, Norway
- 8Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway
- 9Oslo Centre of Biostatistics and Epidemiology, Research Support Services, Oslo University Hospital, Oslo, Norway
- 10Department of Obstetrics and Gynaecology, Sorlandet Hospital, Kristiansand, Norway
Background/objective: There is no international consensus about the optimal approach to screening and diagnosis of gestational diabetes mellitus (GDM). Fasting plasma glucose (FPG) has been proposed as an alternative universal screening test to simplify the diagnosis of GDM. We investigate the ability of the FPG to predict a 2-hour glucose value below the cut-off for GDM, thereby “ruling out” the necessity of a full OGTT and assess the proportion of GDM-related complications associated with the identified FPG level.
Materials and methods: This study included secondary data from four Norwegian pregnancy cohorts (2002-2013), encompassing 2960 women universally screened with late mid-pregnancy 75g OGTT measuring FPG and 2-hour glucose. For a range of FPG thresholds, we calculated sensitivity to predict elevated 2-hour glucose, number of OGTTs needed and percentage of GDM cases missed, applying modified World Health Organization (WHO) 2013 criteria (2013WHO) and 2017 Norwegian criteria (2017Norwegian). We analyzed pregnancy outcomes for women above and below our selected threshold.
Results: The prevalence of GDM was 16.6% (2013WHO) and 10.1% (2017Norwegian). A FPG threshold of 4.7 mmol/L had a sensitivity of 76% (2013WHO) and 80% (2017Norwegian) for detecting elevated 2-hour glucose, with few missed GDM cases (2.0% of those ruled out and 7.5% of all GDM cases for 2013WHO, and 1.1% of those ruled out and 7% of all GDM cases for 2017Norwegian). When excluding women with FPG <4.7mmol/l and those with GDM based on FPG, only 24% (2013WHO) and 29% (2017Norwegian) would require OGTT. Women with FPG <4.7mmol/l, including missed GDM cases, had low risk of large-for-gestational-age newborns, cesarean section and operative vaginal delivery.
Conclusion: A FPG threshold of 4.7mmol/l as a first step when screening for GDM could potentially eliminate the need for OGTT in 70-77% of pregnancies. Women with FPG below this threshold appear to carry low risk of GDM-associated adverse pregnancy outcomes.
Introduction
Gestational diabetes mellitus (GDM) is one of the most common disorders of pregnancy, responsible for several adverse outcomes in both mother and child during gestation and in the longer term (1). Despite extensive research over the past decades, there is still no consensus about the optimal approach to screening strategies and diagnostic criteria for GDM, reflected by substantial variations in clinical recommendations throughout the world (2, 3).
Although different diagnostic criteria for the identification of GDM are used, the oral glucose tolerance test (OGTT) is endorsed by all diabetes and health organizations as the “gold standard” diagnostic test for GDM. The use of OGTT in a clinical setting, however, poses several challenges. The test is poorly reproducible (4), time-consuming, and not user-friendly (5, 6), leading to a significant burden on the healthcare system in terms of infrastructure and cost. While the International Federation of Gynecology and Obstetrics strongly recommends universal testing (7) several European countries, including Norway, practice risk-factor based selective screening with the intention to identify the most severe cases of GDM and, concurrently, limit the number of OGTT’s. However, this selection process is also demanding for healthcare providers, requiring screening of about 70% of the pregnant population (8).
Fasting plasma glucose (FPG) has been proposed as an alternative universal screening test for GDM (9), as it is easy to administer, less time-consuming for patients and healthcare providers, and inexpensive (10). During the Covid-19 pandemic, in order to minimize transmission of the virus and reduce use of medical resources, several health authorities and professional bodies suggested limiting the OGTT to women with FPG above a certain threshold value, “ruling out” those with lower FPG values where the GDM risk was considered low (11). If still recommended today, these new strategies should, however, be balanced by the need to ensure the best possible pregnancy outcomes for women and their infants. To date, a number of studies have proposed FPG cut-offs to accurately rule in and rule out GDM, with wide variation amongst different geographical regions in the world (12–16), but few have evaluated pregnancy complications potentially detected or missed (13, 17).
In light of this context, we aimed to explore the use of FPG to identify women at low risk for GDM and GDM-related adverse outcomes, limiting the need for an oral glucose tolerance test (OGTT). Our primary aim was to investigate the ability of the FPG to predict a 2-hour glucose value below the cut-off for GDM, thereby “ruling out” the necessity of a full OGTT, based on two different diagnostic GDM criteria in a Norwegian pregnant population. The secondary aim was to assess the proportion of GDM-associated complications for the identified FPG level in order to evaluate whether pregnancies ruled out can be safely excluded from further post-load glucose testing.
Material and methods
Study population and setting
We used secondary data from four Norwegian population-based birth cohort studies with a special focus on GDM. The criteria for the selection of studies have been previously described in detail (8). Participant characteristics for all studies are summarized in Table S1. The merged dataset consisted of two cohort studies (18, 19) and two randomized controlled trials (RCT) (20, 21) collecting data between 2002 and 2013. The interventions in the two trials consisted of either an exercise program (20) or a combination of a physical activity component and dietary counselling (21), but these interventions demonstrated no effect on the incidence of GDM or the outcomes of LGA and caesarean section. We excluded women with multiple pregnancies, those lacking glucose values, and infants with missing birthweight. We also excluded fetal deaths, as all except one had missing OGTT and/or outcome data (Flow chart, Figure S1). The Norwegian Regional Ethics committees (REC) had approved each study, and the current study was approved by the REC South East. All participants provided written informed consent.
Data collection
All women were offered a 75 g OGTT measuring fasting and 2-hour (2-h) glucose levels. In the STORK Groruddalen study (22) venous blood samples were collected in tubes containing ethylenediaminetetraacetic acid according to standardized protocols, and glucose was analyzed on site in fresh, whole EDTA blood, using HemoCue 201+ glucose analyser (Angelholm, Sweden) calibrated for plasma. In two studies (19, 23), glucose levels were measured in serum by the routine methods used at the participating hospital laboratories, and blood samples were stored at -80°C. The Fit for Delivery study measured glucose in plasma using a Cobas 6000 c501 chemistry analyzer (Roche Diagnostics) (24). Inter-essay coefficients for each study are reported in Table S1 (CV 2.0-3.6%), and further details about the laboratory measurements can be found in the original studies.
During data collection, the diagnosis of GDM was made according to the 1999 World Health Organization (WHO) criteria (1999WHO) (FPG ≥7.0 mmol/l or 2-h glucose ≥7.8 mmol/l). We retrospectively applied modified 2013 WHO (2013WHO) diagnostic cut-offs (FPG ≥5.1 mmol/l or 2-h glucose ≥8.5 mmol/L, as 1-hour glucose was not measured in the respective studies) and the 2017 Norwegian (2017Norwegian) cut-offs (FPG ≥ 5.3 mmol/l or 2-h glucose ≥ 9.0 mmol/L) to the same OGTT. Women with GDM by 1999-WHO criteria were informed and referred to their general practitioner or specialist care according to protocol. Women received standard GDM treatment in accordance with either global guidelines in place at the time (25) or local guidelines specific to each hospital (treatment targets provided in Table S1). However, we lack specific information about the treatment provided to each woman, including whether the clinicians adhered to the guidelines. Only 12 women have been documented as receiving pharmacological treatment.
All participants provided questionnaire data, self-reported (19–21) or through interviews (18), with information on maternal age, parity, smoking status and their highest level of education. Ethnic origin was defined by the pregnant woman’s mother’s country of birth and further merged into three groups in the current study: European (predominantly Scandinavian), Middle Eastern/African, and Asian (primarily South and East Asian ethnicity). Height was measured directly on site while weight prior to pregnancy was self-reported. Pre-pregnancy body mass index (BMI) was defined as weight (kg) divided by height (m)2 and categorized as normal weight (≤24.9 kg/m2), overweight (25-29.9 kg/m2) and obesity (≥30 kg/m2).
Pregnancy and delivery outcome data
Outcome data collected at the time of birth were birthweight (grams), gestational age at birth, delivery method (normal vaginal delivery, cesarean section (planned or emergency), operative vaginal delivery (vacuum extraction or forceps)), preeclampsia or severe hypertensive disorder, and preterm delivery (<37 weeks). As in clinical practice in Norway, LGA (sex and gestational age-specific birthweight >90th percentile) was calculated using Norwegian national references (26), while macrosomia for the present study was defined as birthweight >4000 g.
Statistical analyses
The area (AUC) under the receiver operating characteristic (ROC) curve was used to analyze the discriminative power of FPG to predict an elevated 2-h glucose value, using the modified 2013WHO criteria and the 2017Norwegian criteria. Elevated 2-h glucose was used instead of the diagnosis of GDM since the latter also includes those diagnosed based on FPG. Using standard definitions, we assessed diagnostic accuracy measures such as sensitivity, specificity, and negative predictive value (NPV) of a range of threshold values of FPG (varying from 4.4 to 5.0 mmol/l). The number of OGTTs needed was analyzed after excluding women who had GDM based on FPG alone (FPG≥5.3 mmol/l or ≥5.1 mmol/l, depending on the diagnostic criteria). In addition, we calculated the proportion of missed GDM cases (women with GDM according to the 2-hour glucose but “ruled out” and excluded from the OGTT because of the specified FPG threshold). In the process of selecting the “optimal” FPG threshold, options that demonstrated good/acceptable sensitivity were considered (27). The thresholds were then reviewed individually according to diagnostic needs and clinical usefulness, with particular emphasis on the number of required OGTTs and missed GDM cases.
Characteristics of the women were categorized by FPG-status, and the groups were compared using X2 statistic for categorical data and ANOVA for continuous variables. Data are presented as frequencies and percentages for categorical variables and mean and standard deviations (SD) for continuous variables.
To examine the risk of pregnancy complications among missed GDM cases (GDM according to the 2-hour glucose, but potentially excluded from the OGTT based on low FPG), we stratified women further into two groups: FPG below or FPG at/above the proposed threshold (4.7 mmol/l). For both strata, multivariable logistic regression models were performed for the pregnancy outcomes LGA, cesarean section and operative vaginal delivery, with elevated 2-h glucose (categorized as less than or at/above 9.0 mmol/l) as the main exposure. We adjusted for pre-specified confounders such as maternal age, pre-pregnancy BMI, ethnicity, parity, cohort, smoking and gestational age at birth. The effect estimates for 2-h glucose (less than or at/above 9.0 mmol/l) is presented as odds ratios (OR) with 95% confidence intervals (CI). The level of significance was set as 0.05. Statistical analyses were performed using statistical package IBM SPSS (version 23.0. Armonk, NY: IBM Corp).
Results
Of the 2970 women included in the present study, 16.6% fulfilled the modified 2013WHO criteria for GDM, while 10.1% met the 2017Norwegian criteria. More than 80% of all GDM cases were identified by elevated FPG, both by the modified 2013WHO (≥5.1 mmol/L) and the 2017Norwegian (≥5.3 mmol/L) criteria, while 16.0% and 17.6% were identified by elevated 2-h glucose alone [2013WHO (≥8.5 mmol/L) and 2017Norwegian criteria (≥9.0 mmol/L)] (Table S2).
The ability of FPG to ‘rule-out’ the need for a full OGTT
The ROC curves along with the AUC quantifying the performance of the FPG to predict an elevated 2-h glucose (diagnostic for GDM) were assessed graphically (Figure 1). The AUC was 0.81 (95% CI 0.76-0.85) using the 2017Norwegian criteria and slightly lower (0.78, 95% CI 0.75-0.82) when the modified 2013WHO criteria were used to define GDM. A separate ROC analysis for women with non-European background gave an AUC of 0.70 (95% CI 0.6-0.8) using the 2017Norwegian criteria (Figure S2).
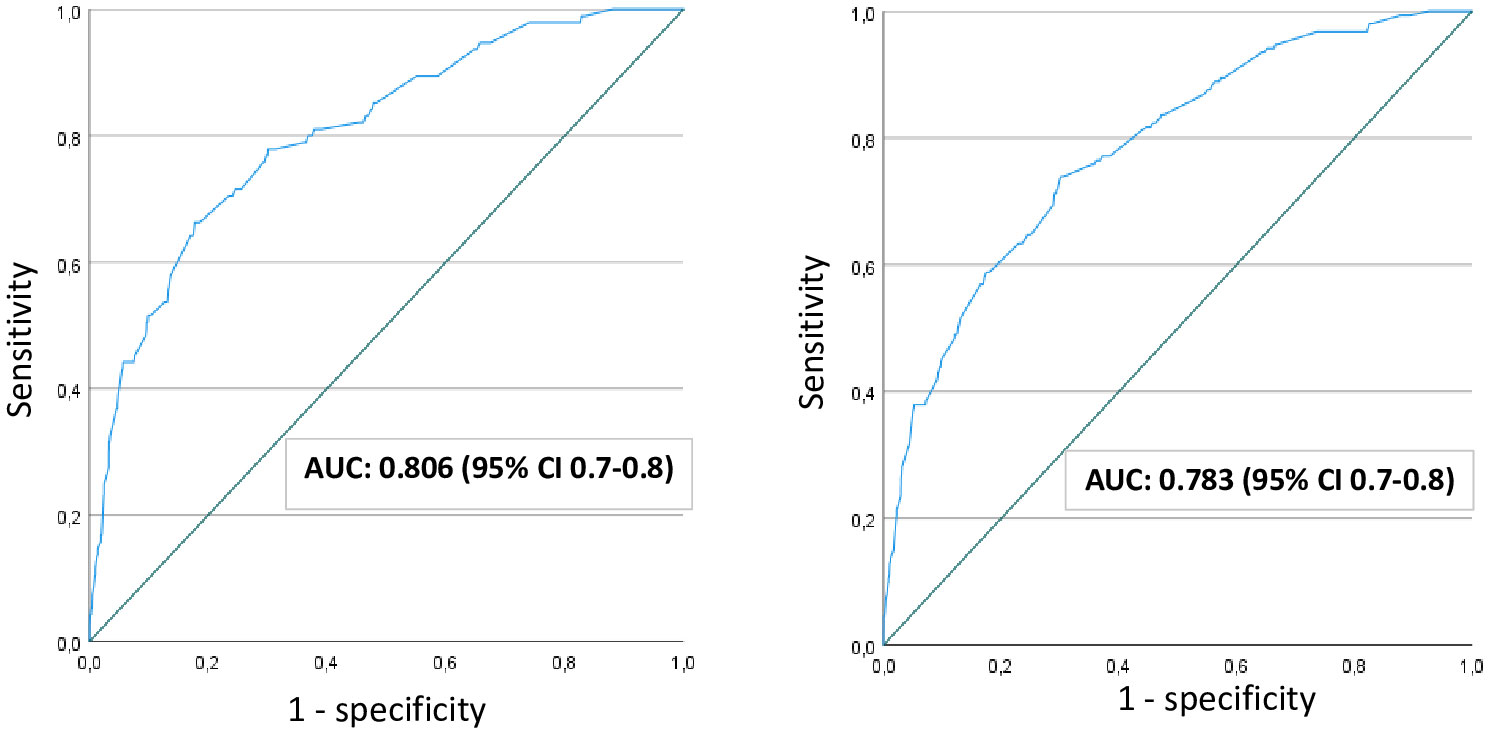
Figure 1 ROC curve to assess the performance of fasting plasma glucose to predict elevated 2-hour glucose, applying 2017-Norwegian criteria (left) and applying modified 2013-WHO criteria (right). AUC, area under the curve; CI, confidence interval; WHO, World Health Organization.
Table 1A lists a range of threshold values for FPG with the associated sensitivity, specificity, and negative predictive value (NPV) using the Norwegian criteria. As the cut-off value rises, the sensitivity of the screening test decreases and the specificity increases. Conversely, a lower FPG threshold has high sensitivity and identifies most women with GDM but an excessive number of women without GDM will need to undergo the OGTT due to the corresponding poor specificity. Based on test properties (27) and careful clinical judgment the threshold value 4.7 mmol/L for FPG was selected, as this threshold had an acceptable sensitivity (78.9%) to predict elevated 2-hour glucose and appeared to offer the best trade-off to limit the number of missed GDM cases while avoiding unnecessary OGTTs. In total, 1855 women (62.5%) had FPG below this threshold and could potentially be ‘ruled-out’ as non-GDM. Of these women, 20 (1.1% of those ruled out and 6.6% of all GDM cases) had an elevated 2-hour glucose value, and would hence be “misclassified” as non-GDM, with the NPV being 98.9%. Of the remaining 1111 women with FPG above the 4.7mmol/l threshold, 248 (8.4% of the entire cohort) had FPG ≥5.3 mmol/l, i.e. GDM according to the 2017Norwegian diagnostic criteria (Table 2). Thus, only the remaining 864 (29.1% of the entire cohort) would have to undergo the complete OGTT.
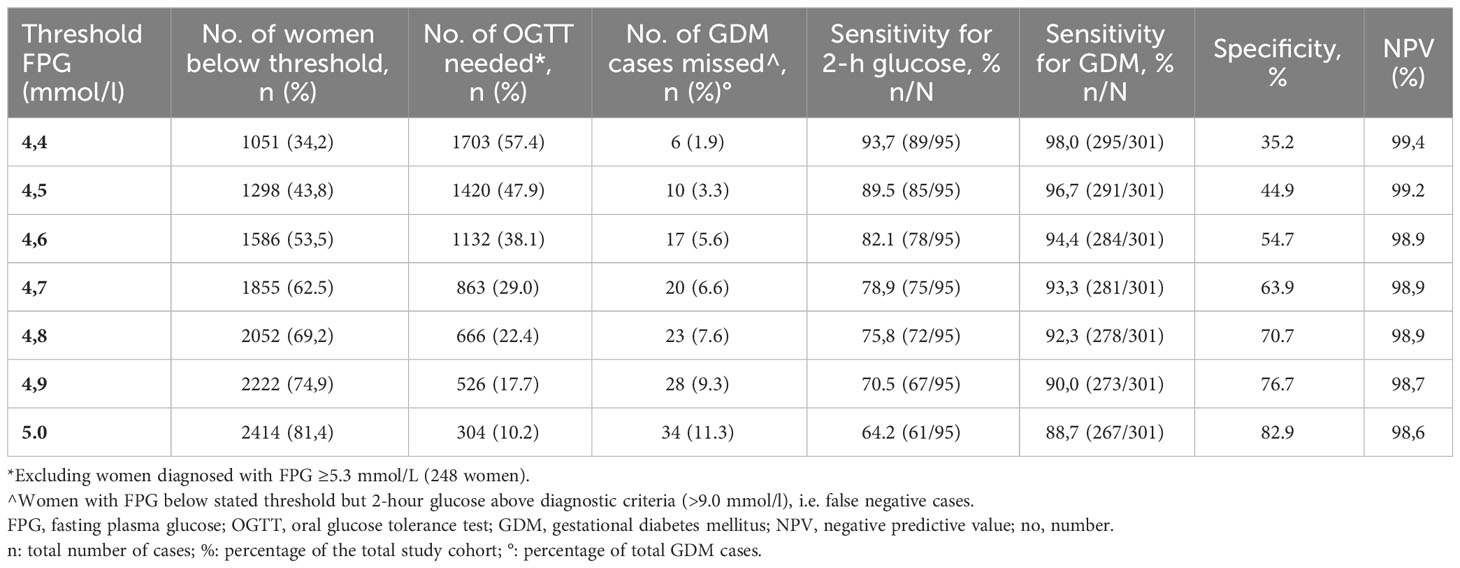
Table 1A Overview of the sensitivity of different thresholds of FPG to the need for an OGTT to screen for GDM (Norwegian 2017 criteria).
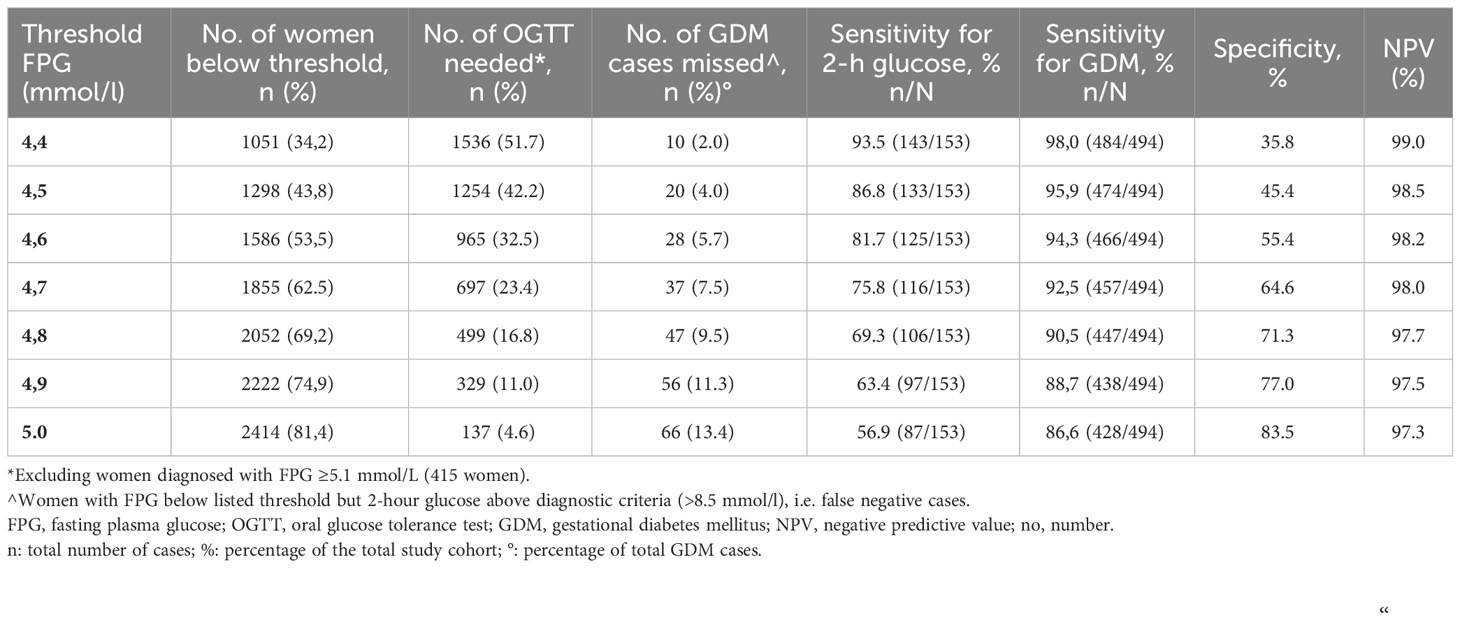
Table 1B Overview of the sensitivity of different thresholds of FPG to the need for an OGTT to screen for GDM 2013 WHO criteria.
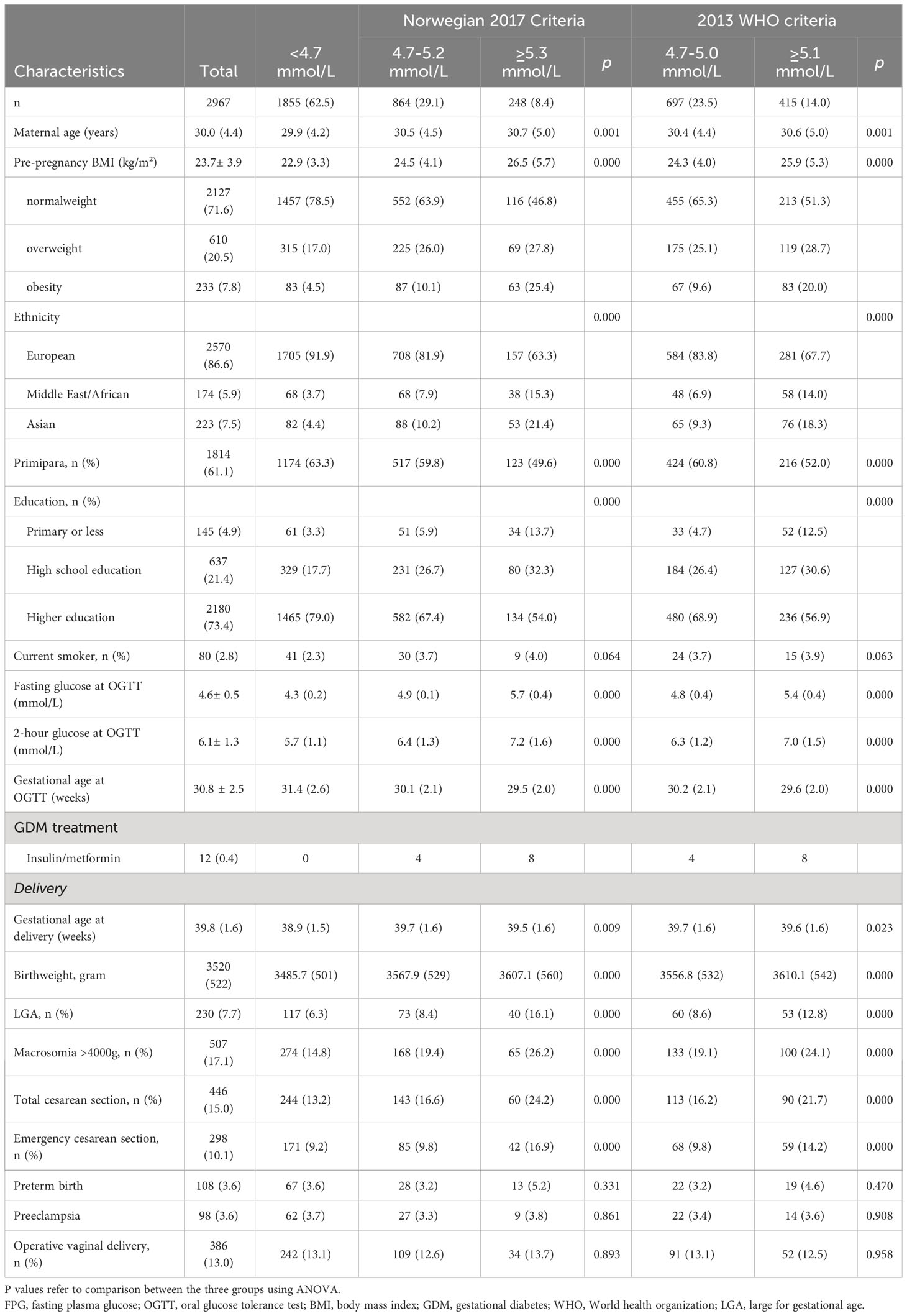
Table 2 Comparison of characteristics and pregnancy outcomes between women with fasting plasma glucose below and at/above 4.7 and ≥5.3/5.1 mmol/l (2017 Norwegian cut offs and 2013 WHO cut offs).
Similar results were found for the modified 2013WHO criteria (Table 1B), although sensitivity to predict elevated 2-hour glucose was slightly reduced (75.8%), and the number of missed GDMs cases was slightly higher (2.0% of those ruled out and 7.5% of all GDM cases) for the same FPG threshold. The proportion of women requiring further evaluation to define their GDM status on the basis of FPG 4.7-5.0 mmol/L (number of OGTT needed) was, on the other hand, slightly lower using these criteria (23.5%).
Thus, if the FPG was offered to all women and a FPG threshold of 4.7 mmol/l was used to decide whether the OGTT was needed or not, 70.9% of women in the cohort would not require further testing when using the 2017Norwegian diagnostic thresholds, and 76.5% when using the modified 2013WHO criteria.
Comparison of women below or above the selected FPG threshold
Table 2 presents maternal characteristics and the proportion of pregnancy outcomes found among women classified as low FPG (<4.7 mmol/l), indeterminate FPG (4.7-5.2/5.0 mmol/l) and elevated FPG (FPG ≥5.3/5.1 mmol/l) according to the 2017Norwegian criteria and 2013WHO criteria, respectively. Women in the low FPG group had the lowest pre-pregnancy BMI. They also had the highest proportion of primiparas and the lowest proportion of women with a non-European ethnicity. Furthermore, the lowest proportion of LGA, macrosomia (>4000g) and total cesarean section was observed in women with FPG <4.7 mmol/L and the highest proportion in the elevated FPG groups. None of the women with FPG <4.7 mmol/l and GDM by 1999WHO criteria received insulin or other antidiabetic medication.
Table 3 reports the proportion of pregnancy complications amongst women with GDM based on an elevated 2-hour glucose value (≥9.0 mmol/L), after dividing the sample into those below or at/above the FPG threshold of 4.7 mmol/l. For women with FPG ≥4.7 mmol/l [including those meeting current GDM criteria (FPG ≥5.3 mmol/L)], 2-h glucose ≥9.0 mmol/l was associated with higher risk for LGA (OR 2.61; 95%CI 1.37-4.95) but not for cesarean section and operative vaginal delivery. For women with FPG <4.7 mmol/l, who would not be offered an OGTT according to the proposed strategy, 2-h glucose ≥9.0 mmol/l was not associated with an increased risk for any of these outcomes.
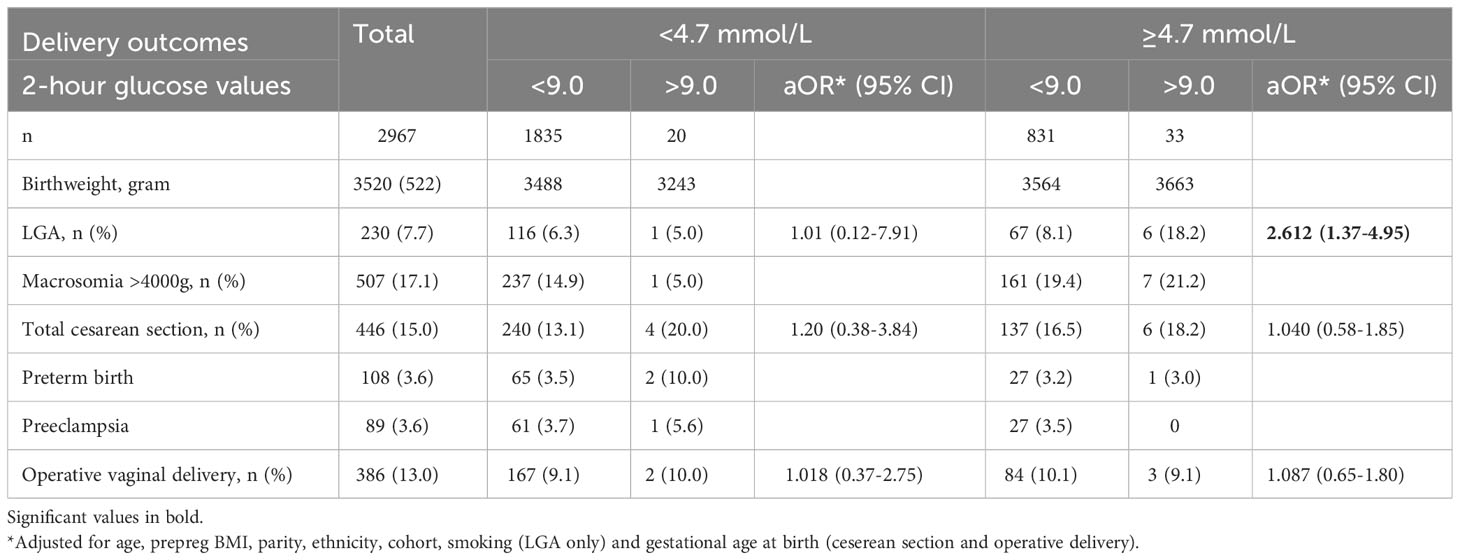
Table 3 Pregnancy outcomes stratified according to fasting plasma glucose (2017 Norwegian criteria).
Discussion
Main findings
Using data from a Norwegian sample offered universal mid-pregnancy GDM screening, we found that a FPG threshold of 4.7 mmol/L demonstrated an acceptable sensitivity of 76-80% to predict an elevated 2-hour glucose value (using modified 2013WHO and 2017Norwegian criteria respectively); 63% of participants had FPG below this threshold and could be “ruled-out” from further testing regardless of criteria used. Importantly, this group appears to carry a low risk of a range of pregnancy complications commonly associated with GDM. Furthermore, because FPG is included in the diagnostic criteria, we could identify (“rule-in”) over 80% of GDM cases using FPG alone in our sample. This implies that if a rule-in/rule-out approach was used, OGTT would be needed in only 24-29% of our population, i.e. only those with FPG in the range 4.7-5.0/5.2 mmol/L.
Interpretation
FPG thresholds previously suggested as the preferred cut-off to avoid unnecessary OGTT’s include 5.0 mmol/l in Mexican adolescents (14), 4.8 mmol/l in Swedish women (16), 4.4 mmol/l in both an Arab (12) and Chinese population (15), and 4.3 mmol/l in studies from South Asia (28) and Belgium (13). The diagnostic performance of FPG as a screening test is dependent on the population tested, GDM prevalence and GDM criteria used (29, 30). In addition, the determination of ideal test sensitivity and specificity requires judicious assessment of harms related to missed diagnosis as well as burdens associated with large-scale testing. A low FPG threshold will have high sensitivity and identify most women with GDM but an excessive number of women without GDM will need to undergo the OGTT due to corresponding poor specificity, putting pressure on health services and medicalizing low-risk pregnancies. Similar to our findings, a recent Australian study concluded that FPG ≥4.7 mmol/l had the best sensitivity and specificity for abnormal OGTT results (31), and this preliminary test was employed in Australia during the Covid-19 pandemic.
Although more than 70% of OGTT’s could be avoided by the proposed strategy in our study, applying either 2013WHO or 2017Norwegian criteria, 7-8% of GDMs identified with universal OGTT would be missed. Others have reported higher rates of “missed GDM” for the same threshold. Van Gemert et al. compared the use of a preliminary FPG ≥4.7 mmol/l to universal OGTT, reporting that 29% of women who would otherwise be diagnosed with GDM by 2013WHO criteria could be missed (11). The contrasting finding in this study may at least to some degree be explained by additional measurements of 1-hour glucose values which we lacked. Nevertheless, recognizing and diagnosing GDM is essential, as management of GDM has been associated with reduced maternal, fetal and newborn complications (32–34). Furthermore, the identification of GDM provides a valuable opportunity to assess the women’s future risk of diabetes and implement preventive measures, a possibility that would remain beyond reach without proper identification.
In our cohort, 82-84% of all GDM cases were identified based on FPG, making the idea of entirely abandoning an assessment of post-load glycemia rather appealing. A single FPG test offered to all pregnant women is a simple and low-cost option to diagnose GDM. However, the proportion of women diagnosed by FPG in our study was much higher than reported by others, including the multinational HAPO study, where 55% were diagnosed with GDM by FPG, using 2013WHO criteria (35). Given the wide variability in the percentage of women diagnosed exclusively by FPG, probably explained by factors such as ethnicity and varying rates of obesity (35, 36) continued use of OGTT seems indicated.
Few previous studies have addressed whether pregnancies with FPG below a proposed threshold are in fact associated with low rates of GDM-associated complications. McIntyre et al. examined the outcomes associated with the Australian Covid-19 model of limiting GDM testing to those with FPG ≥4.7 mmol/l, using a subset of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study (37). Broadly consistent with our findings, participants with FPG <4.7 mmol/L had lower rates of pregnancy complications than those above this threshold. A recent Belgian study assessed pregnancy outcomes for a FPG threshold of 4.3 mmol/l, finding a better metabolic profile and low incidence of adverse outcomes below this cut-off (13), but the clinical relevance may be limited as this threshold excludes few women from testing. Our findings indicate that women with FPG <4.7 mmol/l had a better metabolic profile, with less overweight/obesity, compared to women with higher FPG. In addition, pregnancies with FPG <4.7 mmol/l had low rates of LGA, macrosomia and total cesarean section, indicating that these women can safely continue routine care. However, long-term health risks in these women and their children, particularly related to type 2 diabetes, are unknown.
We have previously reported that selection criteria for BMI and age currently used in Norway would result in recommending OGTT to about 70% of women with European ethnicity in our sample (8). The results from the current study lend support to the universal use of FPG as an alternative to risk-profiling for selectively offering the OGTT, with the potential to limit the use of OGTT to less than 30% of all pregnancies and achieve similar sensitivity of about 80%. Additionally, it may avoid potentially stigmatizing selection based on age, BMI and ethnicity.
However, the proposed screening strategy requires certain logistics to be in place in order to make the implementation successful. Ideally, the fasting venous sample would have to be analyzed without delay by a measure with acceptable validity and reliability and at the same facility. This should be followed by an immediate decision as to whether a full OGTT is required, thereby avoiding prolonged waiting time and enabling women to complete the test on the same day as the fasting blood test. Moreover, our study is centered on GDM diagnosis made late in pregnancy. In light of a recent RCT indicating potential benefits of early screening (38), the matter of early versus late screening also warrants further consideration and exploration.
Strengths and weaknesses
Our study has several strengths. We merged previously collected maternal and offspring data from four cohorts, allowing more powerful and flexible analyses. Moreover, there is no pre-selection bias as an OGTT was offered to all included women. Importantly, most previous studies that have explored the performance of FPG provide limited or no information on adverse pregnancy outcomes.
The main limitation of our study is that glucose results were not blinded and women with GDM diagnosed by 1999WHO criteria were routinely treated. This implies that conclusions drawn about likely clinical outcomes for women classified as “missed GDMs” may be inaccurate, as patients had a known diagnosis and received care, although none of these women received pharmacological treatment. Nonetheless, our results are comparable to those of McIntyre et al., which used a population blinded to OGTT results in their retrospective analysis of FPG and pregnancy outcomes (17). Additionally, our sample had slightly lower rates of obesity than our background population (7.8% vs. 12-12.5% nationally in 2007-2013) (39). This may affect the prevalence of GDM and its associated outcomes, and the proportion of GDM identified by FPG. The pre-analytical processing and measurement of glucose is critical for accuracy in GDM diagnosis. Differences in sampling and analytical procedures across studies (one study used point-of-care whole blood glucose and two studies used serum) is another weakness of the current study, potentially affecting the uniformity of GDM diagnosis. Despite high precision for glucose measurement in all studies (small CV’s), we cannot rule out that minor bias may have been introduced. Furthermore, the 1-hour glucose was not measured in any of the four cohorts. In the HAPO study population, 5.7% additional GDMs were identified by the 1-hour values when using the 2013WHO criteria (40), and a higher prevalence of GDM in our study could be expected if such data were available. Finally, very few women were diagnosed based on 2-hour glucose alone (16-18% of GDM cases) which makes analysis of women in this category difficult due to power limitations. Importantly, the proposed approach may not circumvent as many OGTTs in other populations as indicated by our study and such differences may need to be considered when extrapolating our results to other settings (i.e. to more high-risk populations). If implemented, this screening procedure should be followed by careful assessment of any potential increase in unwanted pregnancy outcomes. Further studies are needed to compare current risk-factor based screening strategies with a “rule-in, rule-out” procedure with focus on birth outcomes and cost-effectiveness.
Conclusion
Our study suggests that a two-step approach to GDM screening, with an initial universal FPG and exclusion of low-risk women from further testing, could potentially limit the use of OGTT to less than 30% of all pregnancies. A FPG threshold of 4.7 mmol/l appears to identify women at low risk of both elevated 2-hour glucose and GDM-associated adverse pregnancy outcomes. Additional studies are needed to validate our findings and confirm the safety of this screening approach, including long-term health outcomes, especially in populations where a higher proportion of women are diagnosed with GDM from post-load values.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The datasets analyzed during the current study are not publicly available due to the dataset containing clinical data which cannot be shared publicly, and as the study is part of a PhD work. The data are available from the corresponding author on reasonable request. Requests to access these datasets should be directed to Line Sletner, line.sletner@medisin.uio.no.
Ethics statement
The studies involving humans were approved by Norwegian Regional Ethics Committee South East. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AR: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. LS: Supervision, Data curation, Writing – review & editing. AJ: Conceptualization, Investigation, Data curation, Supervision, Writing – review & editing. NØ: Supervision, Data curation, Writing – review & editing. SS: Data curation, Writing – review & editing. EQ: Data curation, Writing – review & editing. AP: Formal analysis. LRS: Supervision, Data curation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was made possible by a grant from the South-Eastern Norway Regional Health Authority, project number 2019056.
Acknowledgments
The authors would like to thank the following members of the Norwegian Hyperglycemia in Pregnancy consortium: Marie Cecilie Paasche-Roland (Oslo University Hospital, Rikshospitalet, Oslo, Norway) for her contribution in the STORK Rikshospitalet study, Siv Mørkved (Norwegian University of Science and Technology, Trondheim, Norway) for her contribution in the TRIP study, and Ingvild Vistad (University of Oslo, Oslo, Norway) for her contribution in the Fit for Delivery study. We also thank Jens Petter Berg (Oslo University Hospital, Ullevål, Oslo, Norway, and University of Oslo) for providing valuable advice and suggestions regarding the sampling and analytical procedures.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1278523/full#supplementary-material
Supplementary Figure 1 | Flowchart of included studies and excluded participants from each study.
Supplementary Figure 2 | ROC curve to assess the performance of fasting plasma glucose to predict elevated 2-hour glucose in women with non-European background (A) applying 2017Norwegian criteria (B) applying modified 2013WHO criteria AUC, area under the curve; CI, confidence interval; WHO, World Health Organization.
References
1. Hod M, Kapur A, Sacks DA, Hadar E, Agarwal M, Di Renzo GC, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. (2015) 131(Suppl 3):S173–211. doi: 10.1016/S0020-7292(15)30007-2
2. Benhalima K, Mathieu C, Van Assche A, Damm P, Devlieger R, Mahmood T, et al. Survey by the European Board and College of Obstetrics and Gynaecology on screening for gestational diabetes in Europe. Eur J Obstet Gynecol Reprod Biol (2016) 201:197–202. doi: 10.1016/j.ejogrb.2016.04.003
3. White SL, Ayman G, Bakhai C, Hillier TA, Magee LA. Screening and diagnosis of gestational diabetes. BMJ. (2023) 381:e071920. doi: 10.1136/bmj-2022-071920
4. Catalano PM, Avallone DA, Drago NM, Amini SB. Reproducibility of the oral glucose tolerance test in pregnant women. Am J Obstet Gynecol. (1993) 169(4):874–81. doi: 10.1016/0002-9378(93)90019-F
5. Davidson MB. Counterpoint: the oral glucose tolerance test is superfluous. Diabetes Care (2002) 25(10):1883–5. doi: 10.2337/diacare.25.10.1883
6. Agarwal MM, Punnose J, Dhatt GS. Gestational diabetes: problems associated with the oral glucose tolerance test. Diabetes Res Clin Pract (2004) 63(1):73–4. doi: 10.1016/j.diabres.2003.08.005
7. Hod M, Pretty M, Mahmood T. Joint position statement on universal screening for GDM in Europe by FIGO, EBCOG and EAPM. Eur J Obstet Gynecol Reprod Biol (2018) 228:329–30. doi: 10.1016/j.ejogrb.2018.05.037
8. Rai AS, Sletner L, Jenum AK, Øverby NC, Stafne SN, Lekva T, et al. Identifying women with gestational diabetes based on maternal characteristics: an analysis of four Norwegian prospective studies. BMC Pregnancy Childbirth. (2021) 21(1):615. doi: 10.1186/s12884-021-04086-9
9. Agarwal MM, Dhatt GS, Shah SM. Gestational diabetes mellitus: simplifying the international association of diabetes and pregnancy diagnostic algorithm using fasting plasma glucose. Diabetes Care (2010) 33(9):2018–20. doi: 10.2337/dc10-0572
10. Poomalar GK, Rangaswamy V. A comparison of fasting plasma glucose and glucose challenge test for screening of gestational diabetes mellitus. J Obstet Gynaecol. (2013) 33(5):447–50. doi: 10.3109/01443615.2013.771156
11. van Gemert TE, Moses RG, Pape AV, Morris GJ. Gestational diabetes mellitus testing in the COVID-19 pandemic: The problems with simplifying the diagnostic process. Aust N Z J Obstet Gynaecol. (2020) 60(5):671–4. doi: 10.1111/ajo.13203
12. Babaniamansour S, Aliniagerdroudbari E, Afrakhteh M, Hosseinpanah F, Farzaneh F, Niroomand M. Can fasting plasma glucose replace oral glucose-tolerance test for diagnosis of gestational diabetes mellitus? Diabetol Int (2021) 12(3):277–85. doi: 10.1007/s13340-020-00484-0
13. Beunen K, Neys A, Van Crombrugge P, Moyson C, Verhaeghe J, Vandeginste S, et al. Fasting plasma glucose level to guide the need for an OGTT to screen for gestational diabetes mellitus. Acta Diabetol (2022) 59(3):381–94. doi: 10.1007/s00592-021-01812-9
14. Reyes-Muñoz E, Sandoval-Osuna NL, Reyes-Mayoral C, Ortega-González C, Martínez-Cruz N, Ramírez-Torres MA, et al. Sensitivity of fasting glucose for gestational diabetes mellitus screening in Mexican adolescents based on International Association of Diabetes and Pregnancy Study Groups criteria: a diagnostic accuracy study based on retrospective data analysis. BMJ Open (2018) 8(4):e021617. doi: 10.1136/bmjopen-2018-021617
15. Zhu WW, Fan L, Yang HX, Kong LY, Su SP, Wang ZL, et al. Fasting plasma glucose at 24-28 weeks to screen for gestational diabetes mellitus: new evidence from China. Diabetes Care (2013) 36(7):2038–40. doi: 10.2337/dc12-2465
16. Saeedi M, Hanson U, Simmons D, Fadl H. Characteristics of different risk factors and fasting plasma glucose for identifying GDM when using IADPSG criteria: a cross-sectional study. BMC Pregnancy Childbirth. (2018) 18(1):225. doi: 10.1186/s12884-018-1875-1
17. McIntyre HD, Gibbons KS, Ma RCW, Tam WH, Sacks DA, Lowe J, et al. Testing for gestational diabetes during the COVID-19 pandemic. An evaluation of proposed protocols for the United Kingdom, Canada and Australia. Diabetes Res Clin Pract (2020) 167:108353. doi: 10.1016/j.diabres.2020.108353
18. Jenum AK, Sletner L, Voldner N, Vangen S, Mørkrid K, Andersen LF, et al. The STORK Groruddalen research programme: A population-based cohort study of gestational diabetes, physical activity, and obesity in pregnancy in a multiethnic population. Rationale, methods, study population, and participation rates. Scand J Public Health (2010) 38(5 Suppl):60–70. doi: 10.1177/1403494810378921
19. Lekva T, Bollerslev J, Godang K, Roland MC, Friis CM, Voldner N, et al. β-cell dysfunction in women with previous gestational diabetes is associated with visceral adipose tissue distribution. Eur J Endocrinol (2015) 173(1):63–70. doi: 10.1530/EJE-15-0153
20. Stafne SN, Salvesen K, Romundstad PR, Eggebø TM, Carlsen SM, Mørkved S. Regular exercise during pregnancy to prevent gestational diabetes: a randomized controlled trial. Obstet Gynecol. (2012) 119(1):29–36. doi: 10.1097/AOG.0b013e3182393f86
21. Sagedal LR, Øverby NC, Bere E, Torstveit MK, Lohne-Seiler H, Småstuen M, et al. Lifestyle intervention to limit gestational weight gain: the Norwegian Fit for Delivery randomised controlled trial. BJOG. (2017) 124(1):97–109. doi: 10.1111/1471-0528.13862
22. Jenum AK, Mørkrid K, Sletner L, Vangen S, Torper JL, Nakstad B, et al. Impact of ethnicity on gestational diabetes identified with the WHO and the modified International Association of Diabetes and Pregnancy Study Groups criteria: a population-based cohort study. Eur J Endocrinol (2012) 166(2):317–24. doi: 10.1530/EJE-11-0866
23. Odsæter IH, Åsberg A, Vanky E, Mørkved S, Stafne SN, Salvesen KÅ, et al. Hemoglobin A1c as screening for gestational diabetes mellitus in Nordic Caucasian women. Diabetol Metab Syndr (2016) 8(1):43. doi: 10.1186/s13098-016-0168-y
24. Sagedal LR, Vistad I, Øverby NC, Bere E, Torstveit MK, Lohne-Seiler H, et al. The effect of a prenatal lifestyle intervention on glucose metabolism: results of the Norwegian Fit for Delivery randomized controlled trial. BMC Pregnancy Childbirth. (2017) 17(1):167. doi: 10.1186/s12884-017-1340-6
25. Norsk gynekologisk forening. Veilder i fødselshjelp (2014). Available at: https://www.legeforeningen.no/foreningsledd/fagmed/norsk-gynekologisk-forening/veiledere/arkiv-utgatte-veiledere/veileder-i-fodselshjelp-2014/8-b.-svangerskapsdiabetes-ny/.
26. Skjaerven R, Gjessing HK, Bakketeig LS. Birthweight by gestational age in Norway. Acta Obstet Gynecol Scand (2000) 79(6):440–9. doi: 10.1034/j.1600-0412.2000.079006440.x
27. Power M, Fell G, Wright M. Principles for high-quality, high-value testing. Evid Based Med (2013) 18(1):5–10. doi: 10.1136/eb-2012-100645
28. Agarwal MM, Punnose J, Sukhija K, Sharma A, Choudhary NK. Gestational diabetes mellitus: using the fasting plasma glucose level to simplify the international association of diabetes and pregnancy study groups diagnostic algorithm in an adult south asian population. Can J Diabetes (2018) 42(5):500–4. doi: 10.1016/j.jcjd.2017.12.009
29. Agarwal MM. Gestational diabetes mellitus: Screening with fasting plasma glucose. World J Diabetes. (2016) 7(14):279–89. doi: 10.4239/wjd.v7.i14.279
30. Agarwal MM, Dhatt GS, Punnose J. Gestational diabetes: utility of fasting plasma glucose as a screening test depends on the diagnostic criteria. Diabetes Med (2006) 23(12):1319–26. doi: 10.1111/j.1464-5491.2006.01987.x
31. d’Emden M, McLeod D, Ungerer J, Appleton C, Kanowski D. Development of a fasting blood glucose-based strategy to diagnose women with gestational diabetes mellitus at increased risk of adverse outcomes in a COVID-19 environment. PloS One (2020) 15(12):e0243192. doi: 10.1371/journal.pone.0243192
32. Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med (2009) 361(14):1339–48. doi: 10.1056/NEJMoa0902430
33. Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med (2005) 352(24):2477–86. doi: 10.1056/NEJMoa042973
34. Committee ADAPP. 15. Management of diabetes in pregnancy: standards of medical care in diabetes-2022. Diabetes Care (2022) 45(Suppl 1):S232–s43. doi: 10.2337/dc22-S015
35. Sacks DA, Hadden DR, Maresh M, Deerochanawong C, Dyer AR, Metzger BE, et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel–recommended criteria: the hyperglycemia and adverse pregnancy outcome (HAPO) study. Diabetes Care (2012) 35(3):526–8. doi: 10.2337/dc11-1641
36. Wong VW. Gestational diabetes mellitus in five ethnic groups: a comparison of their clinical characteristics. Diabetes Med (2012) 29(3):366–71. doi: 10.1111/j.1464-5491.2011.03439.x
37. McIntyre HD, Moses RG. The diagnosis and management of gestational diabetes mellitus in the context of the COVID-19 pandemic. Diabetes Care (2020) 43(7):1433–4. doi: 10.2337/dci20-0026
38. Simmons D, Immanuel J, Hague WM, Teede H, Nolan CJ, Peek MJ, et al. Treatment of gestational diabetes mellitus diagnosed early in pregnancy. N Engl J Med (2023) 388(23):2132–44. doi: 10.1056/NEJMoa2214956
39. Mors kroppsmasseindeks før svangerskap (2007-2013). Available at: http://statistikkbank.fhi.no/mfr/.
Keywords: gestational diabetes, screening, pregnancy outcomes, fasting plasma glucose, OGTT 3
Citation: Rai AS, Sletner L, Jenum AK, Øverby NC, Stafne SN, Qvigstad E, Pripp AH and Sagedal LR (2023) Employing fasting plasma glucose to safely limit the use of oral glucose tolerance tests in pregnancy: a pooled analysis of four Norwegian studies. Front. Endocrinol. 14:1278523. doi: 10.3389/fendo.2023.1278523
Received: 16 August 2023; Accepted: 10 November 2023;
Published: 30 November 2023.
Edited by:
Zhanhui Du, Qingdao Women and Infants Hospital, ChinaReviewed by:
Gehanath Baral, Nobel Medical College Teaching Hospital (NMCTH), NepalHelena Elisabeth Backman, Örebro University, Sweden
Copyright © 2023 Rai, Sletner, Jenum, Øverby, Stafne, Qvigstad, Pripp and Sagedal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anam Shakil Rai, anam.s.rai@uia.no
 Anam Shakil Rai
Anam Shakil Rai Line Sletner3
Line Sletner3