- 1Oncology and Chemotherapy Department, Lishui People’s Hospital, Lishui, China
- 2Department of Orthopedics, Yunhe People’s Hospital, Lishui, China
- 3Department of General Surgery, Lishui People’s Hospital, Lishui, China
- 4Department of General Practice, Lishui Central Hospital, Lishui, China
Purpose: The optimal resuscitative fluid for patients with diabetic ketoacidosis (DKA) remains controversial. Therefore, our objective was to assess the effect of balanced crystalloids in contrast to normal saline on clinical outcomes among patients with DKA.
Methods: We searched electronic databases for randomized controlled trials comparing balanced crystalloids versus normal saline in patients with DKA, the search period was from inception through October 20th, 2023. The outcomes were the time to resolution of DKA, major adverse kidney events, post-resuscitation chloride, and incidence of hypokalemia.
Results: Our meta-analysis encompassed 11 trials, incorporating a total of 753 patients with DKA. There was no significant difference between balanced crystalloids and normal saline group for the time to resolution of DKA (MD -1.49, 95%CI -4.29 to 1.31, P=0.30, I2 = 65%), major adverse kidney events (RR 0.88, 95%CI 0.58 to 1.34, P=0.56, I2 = 0%), and incidence of hypokalemia (RR 0.80, 95%CI 0.43 to 1.46, P=0.46, I2 = 56%). However, there was a significant reduction in the post-resuscitation chloride (MD -3.16, 95%CI -5.82 to -0.49, P=0.02, I2 = 73%) among patients received balanced crystalloids.
Conclusion: Among patients with DKA, the use of balanced crystalloids as compared to normal saline has no effect on the time to resolution of DKA, major adverse kidney events, and incidence of hypokalemia. However, the use of balanced crystalloids could reduce the post-resuscitation chloride.
Systematic review registration: https://osf.io, identifier c8f3d.
Introduction
Diabetic ketoacidosis (DKA) is a life-threatening complication of diabetes mellitus described in patients with both type 1 and type 2 diabetes (1). The pivotal facets of acute DKA management involve the administration of intravenous fluids and insulin therapy (2, 3). Despite a consensus on the appropriate insulin dosage and administration route, the selection of fluid therapy remains a subject of contention, particularly in the context of both DKA and critical illness (4, 5).
For decades, the normal saline (0.9% sodium chloride solution) has been the most commonly administered crystalloid solution worldwide (5, 6). The current international guidelines also recommend normal saline as the replacement fluid of choice for DKA (7, 8). However, recent findings raise concerns regarding the potential consequences of normal saline administration, including heightened acidemia, diminished renal blood flow, and reduced urine output (9). Such alterations may culminate in acute kidney injury (AKI) (10–12) and mortality (13, 14). Since the high chloride content of normal saline may lead to possible adverse effects, balanced crystalloids, in which chloride anions are replaced by lactate or acetate, are increasingly used alternatives (15). These alternatives, wherein chloride anions are substituted with lactate or acetate, present a chemical composition more akin to human plasma than normal saline. This compositional alignment involves reduced chloride levels and an augmented in vivo strong ion difference (16).
Currently, the preferred choice between normal saline and balanced crystalloids in patients with DKA remains a subject of debate. In view of the widespread use of intravenous fluids therapy worldwide, even minor distinctions in fluid selection and their implications for clinical outcomes carry substantial clinical significance. Consequently, the aim of this meta-analysis was to scrutinize the repercussions of utilizing balanced solutions in contrast to 0.9% saline solutions on clinical outcomes in patients with DKA.
Methods
We performed our meta-analysis following the guidelines of updated PRISMA statement (17) (Supplementary Material 1). The protocol of this meta-analysis has been registered in the Open Science Framework (https://osf.io/c8f3d). A systematic exploration for eligible randomized controlled trials (RCTs) in the English language was conducted through an extensive literature search across four electronic databases (PubMed, Embase, Scopus, and Cochrane Library), spanning from their inception through October 20th, 2023. The literature search was conducted through the utilization of keywords comprising “balanced crystalloids”, “normal saline”, “DKA”, and “randomized”. The comprehensive search methodologies are detailed in Supplementary Material 2.
Eligibility criteria
The inclusion criteria were shown as follows: 1. Design: randomized trials; 2. Population: patients with DKA, the definition of DKA was according to the current international guidelines (7, 8); 3. Intervention: balanced crystalloids characterized by a chloride concentration closely approximating physiological levels (e.g., Lactated Ringer’s, Plasma-Lyte); 4. Comparison: normal saline, specified as 0.9% saline with a chloride content of 154 mmol/L; 5. Outcomes: primary outcome were the time to resolution of DKA, defined by the original authors, major adverse kidney events (defined as KDIGO stage II or higher (18), or receipt of renal-replacement therapy, or defined by the original authors), post-resuscitation chloride, and incidence of hypokalemia (potassium < 3.0 mmol/L).
Data extraction and quality assessment
Two authors (Yuting Liu, Jianfeng Zhang) conducted the retrieval of relevant studies. Reports considered potential for inclusion were screened in full text. Differences in this process were resolved by consensus. When no consensus was reached, a third co-author (Xiaoya Xu) would resolve the issue. Standardized form from the Cochrane Data Collection template was adapted and used to create a study-specific data abstraction form, data including the first author, publication years, study design, sample size, population characteristics, and details pertaining to the intervention and control agents were independently extracted by two authors (Yuting Liu, Jianfeng Zhang). Predefined outcomes from the included studies were also extracted. If data were not available in the trial report or data collection, we contacted the corresponding authors to provide important missing data. In instances where studies reported continuous outcomes in the form of median and interquartile range, we used the median, interquartile range, and sample size to estimate the approximate mean value and standard deviation. The calculating formula were proposed by Wan et al. (19), they also developed a software to estimate the mean value and standard deviation.
The Cochrane risk of bias tool (20) was used for assessing the methodological quality by two authors (Yuting Liu, Jianfeng Zhang). Discrepancies in assessments were resolved through consultation with a third co-author (Xiaoya Xu).
Statistical synthesis and analysis
The risk ratios (RRs) with corresponding 95% confidence intervals (CI) were calculated by using a Mantel and Haenszel model for dichotomous outcomes. The mean difference (MD) with 95% CI were calculated by using an inverse variance model for continuous outcomes. Pooled estimates are displayed in forest plots. The evaluation of heterogeneity among studies relied on Higgins inconsistency (I2) statistics (21), with substantial heterogeneity identified when the I2 value exceeded 50%. In the absence of significant heterogeneity, a fixed-effects model was employed for analysis; otherwise, a random-effects model was applied. Furthermore, an assessment of publication bias was conducted using the funnel plot. Additionally, a sensitivity analysis was executed to investigate the impact of individual studies through the successive exclusion of each study.
In order to control the type-I and II errors, we performed post hoc trial sequential analyses (TSA) by using the TSA software (0.9.5.10 Beta, The Copenhagen Trial Unit, Denmark). The parameters used in TSA were detailed in Supplementary Material 2.
Results
Study identification and characteristics
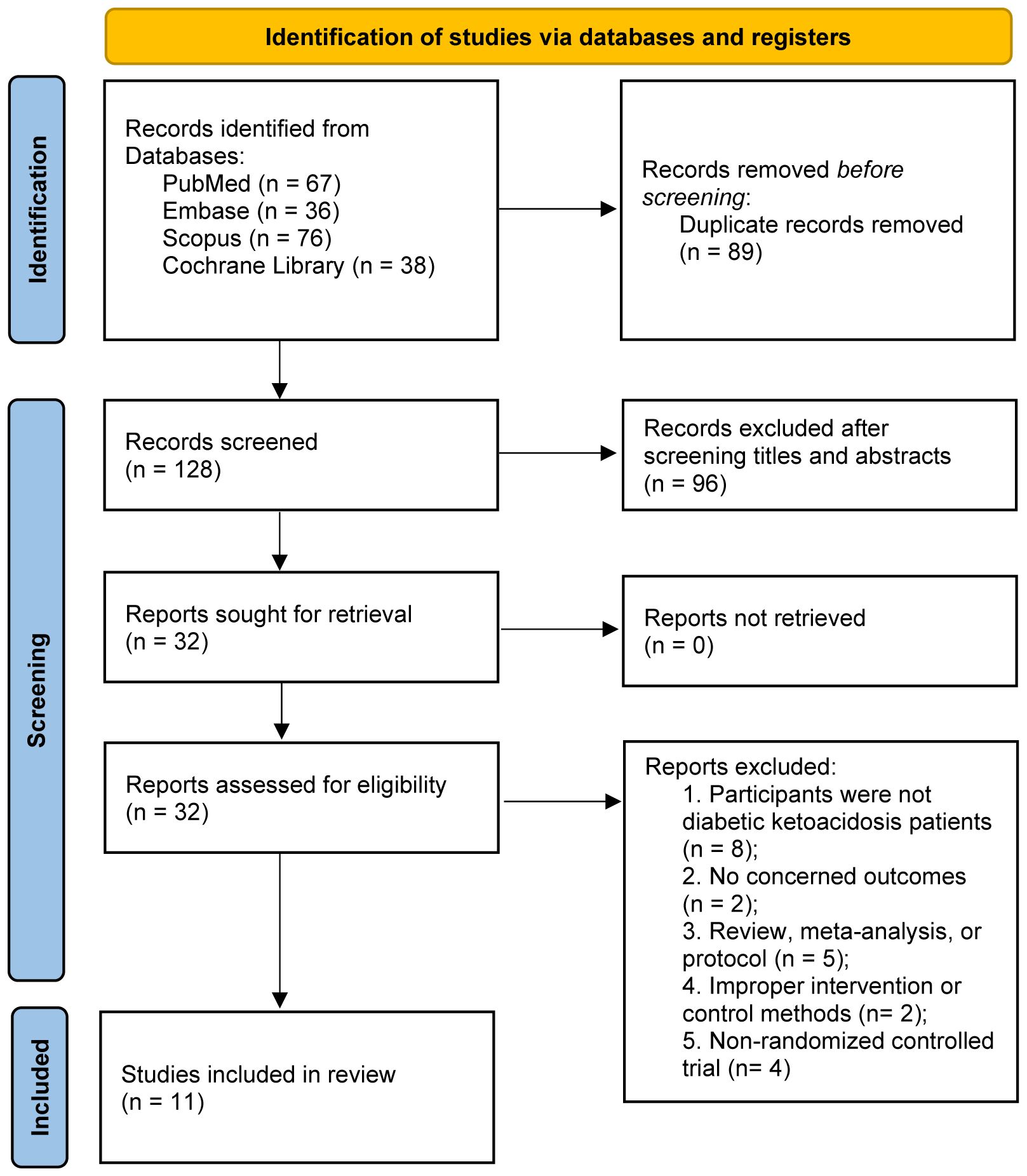
In the initial phase of the search process, 217 articles were identified. First, all records were imported into a document management software, and 89 duplicated literatures were electronically removed. After reading the titles and abstracts, 96 studies were further excluded. During the evaluation of the full text, 21 studies were excluded for specific reasons (Supplementary Material 2 recorded the list of excluded studies with reasons). Finally, our study included a total of 11 RCTs (22–32), (flow chart presented in Figure 1).
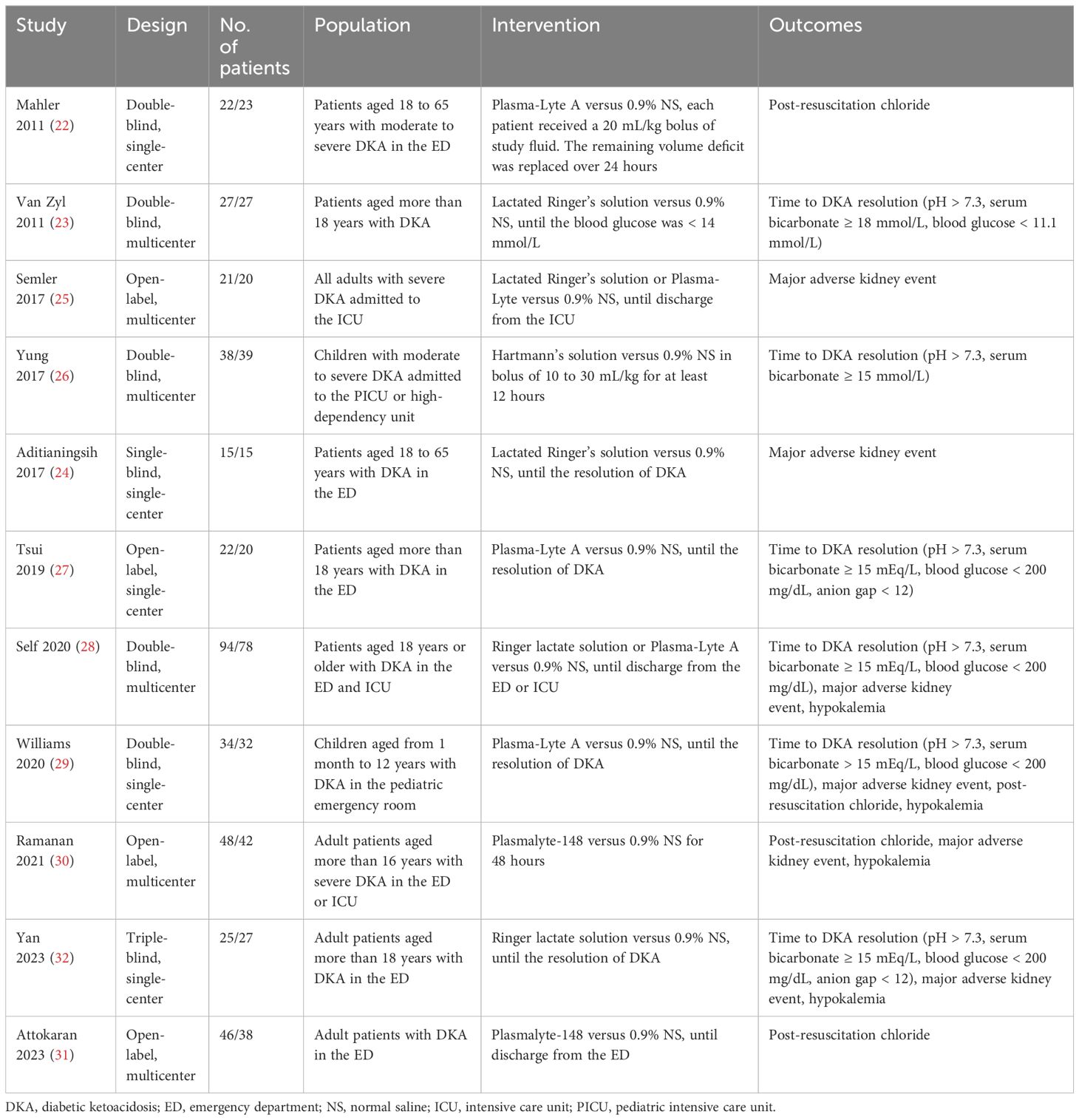
Table 1 delineates the attributes of the studies incorporated in our analysis. The analysis encompassed a total of 753 patients, with 392 patients subjected to balanced crystalloids and 361 patients administered normal saline throughout the study duration. The patient count across individual studies exhibited variability, ranging from a minimum of 30 to a maximum of 172. Notably, the studies included in our analysis exhibited diversity in terms of study populations: nine trials (22–25, 27, 28, 30–32) included adult patients with DKA, and two trial (26, 29) included patients in medical ICU. Different intervention drugs were also identified: Plasma-Lyte in six trials (22, 27–30, 32), Lactated Ringer’s solution in four trials (23–25, 32), and Hartmann’s solution in one trial (26). Apart from trial fluid administered, there were no other changes to the standard DKA treatments including insulin, electrolyte replacement, and/or supportive management.
Quality assessment
Figure 2 presents the quality assessment by the Cochrane risk of bias tool. Notably, five trials (24, 25, 27, 30, 31) were single-blind or open-label trials, they had high risk of bias. Additionally, four studies (22, 24, 27, 32) did not report the details of random sequence generation or allocation concealment. Regarding the blinding method for outcome assessment, two trials (24, 27) exhibited an unclear risk of bias, introducing potential variability in the size of the observed effect. The detailed description of quality assessment was reported in Supplementary Material 2. The evaluation of publication bias, as illustrated by the funnel plot (Supplementary Material 2), did not indicate a significant risk of publication bias.
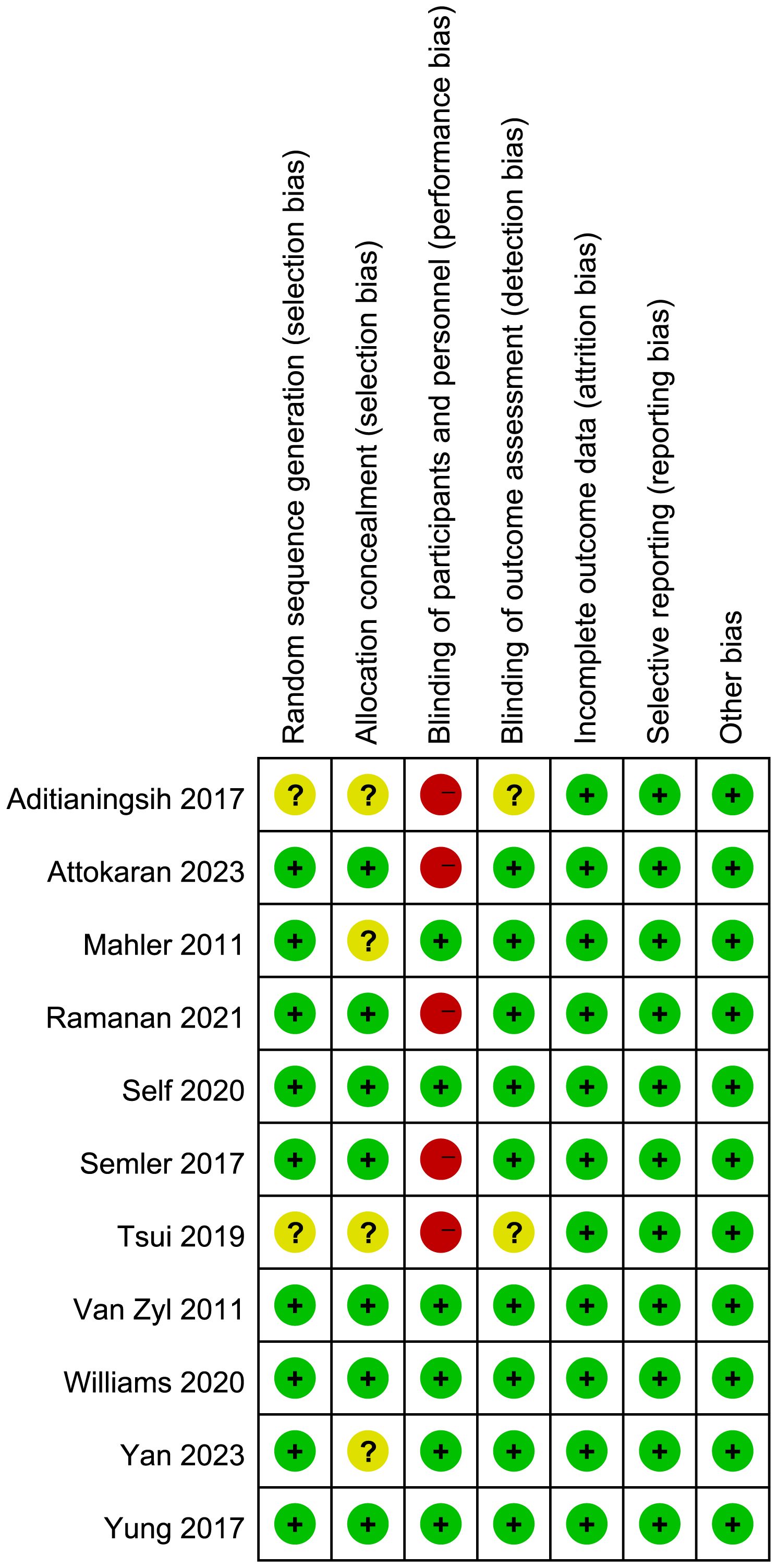
Figure 2 Assesment of quality by the Cochrane risk of bias tool. Red denotes high risk, yellow unclear risk and green low rsik.
Outcomes
Six trials reported the time to resolution of DKA and no significant difference was identified between patients receiving balanced crystalloids and normal saline (MD -1.49, 95%CI -4.29 to 1.31, P=0.30, I2 = 65%, Figure 3). Six trials reported the major adverse kidney events, four reported the incidence of hypokalemia, there was no significant difference between balanced crystalloids and normal saline groups for the major adverse kidney events (RR 0.88, 95%CI 0.58 to 1.34, P=0.56, I2 = 0%, Figure 4A) and incidence of hypokalemia (RR 0.80, 95%CI 0.43 to 1.46, P=0.46, I2 = 56%, Figure 4B). Four trials reported the post-resuscitation chloride and the use of balanced crystalloids could reduce the post-resuscitation chloride (MD -3.16, 95%CI -5.82 to -0.49, P=0.02, I2 = 73%, Figure 5).
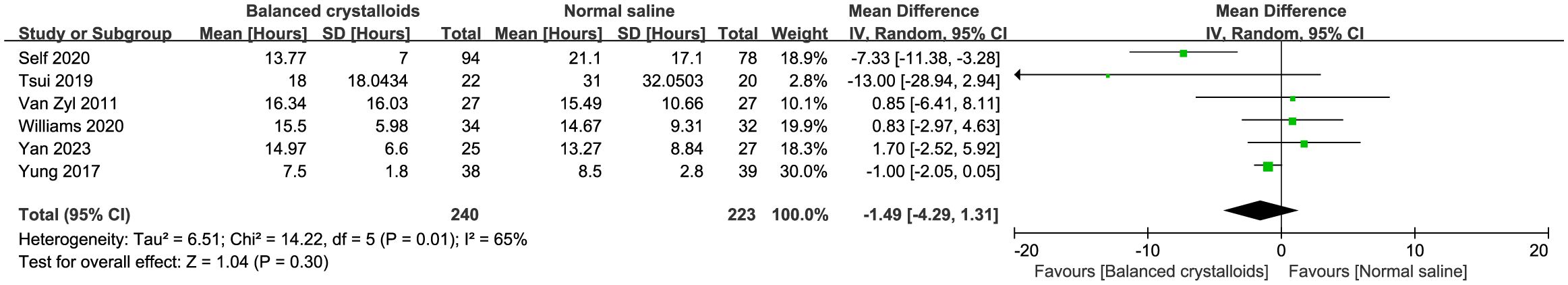
Figure 3 Forest plot showing the effect of balanced crystalloids versus normal saline on the time of resolution to DKA.
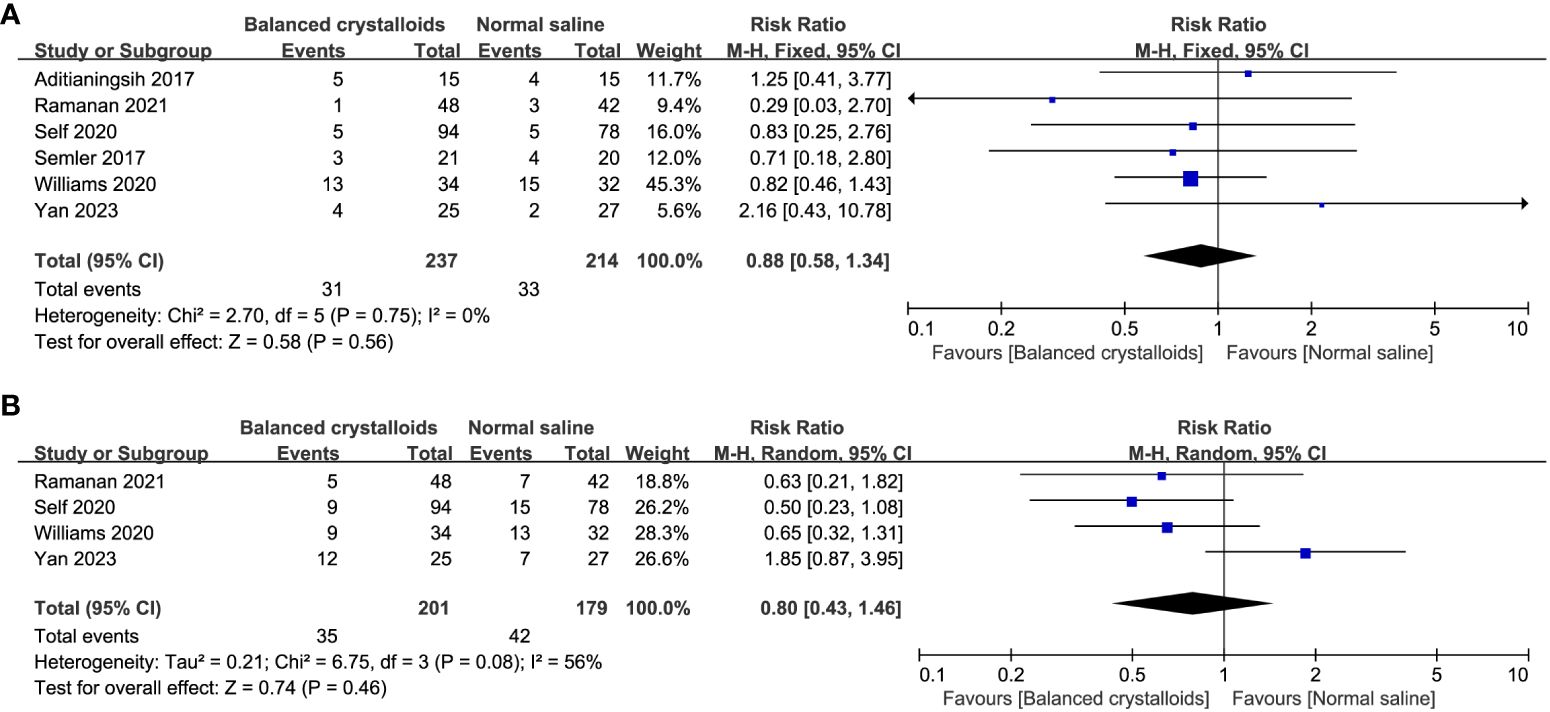
Figure 4 Forest plot showing the effect of balanced crystalloids versus normal saline on the (A) major adverse kidney events, (B) incidence of hypokalemia.
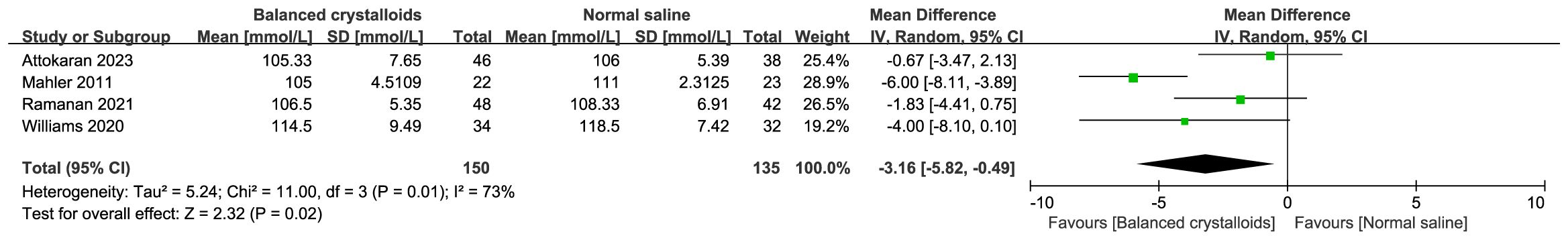
Figure 5 Forest plot showing the effect of balanced crystalloids versus normal saline on the post-resuscitation chloride.
Furthermore, we analyzed the effect of every single trial on the pooled result by omitting each study. The use of balanced crystalloids was relevant to the obvious decreasing in incidence of hypokalemia (RR 0.59, 95%CI 0.37 to 0.93, P=0.02, I2 = 0%) after omitting the study by Yan et al. (32) (Supplementary Material 3). Moreover, sensitivity analysis by excluding each study showed no significant difference for other outcomes, indicating the good robustness (Supplementary Material 3).
Results of TSA are presented in Figure 6, showing that the current systematic review did not achieve the required information sizes to detect the pre-specified effect sizes for time to resolution of DKA, major adverse kidney events, and incidence of hypokalemia, indicating that more trials are required for a definitive conclusion for these outcomes. Furthermore, the TSA confirmed the use of balanced crystalloids was associated a significant reduction in the post-resuscitation chloride with high certainty.
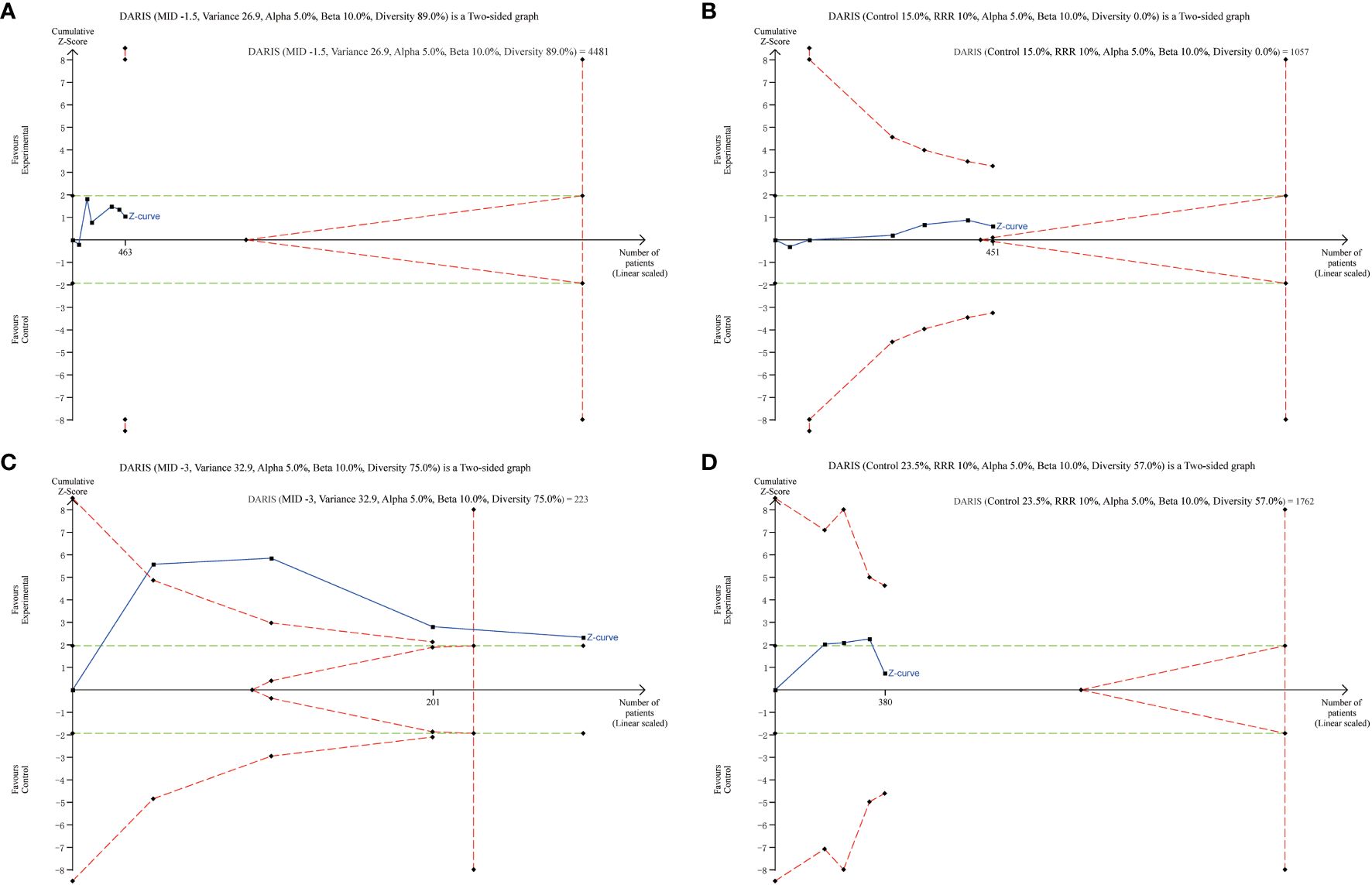
Figure 6 Trial Sequential Analysis of Clinical Outcomes. (A) time to resolution of DKA (6 studies, n=463); (B) major adverse kidney events (6 studies, n=451); (C) post-resuscitation chloride (4 studies, n=285); (D) incidence of hypokalemia (4 studies, n=380). The Z curve in blue measures the treatment effect (pooled relative risk). The parallel lines in green are the boundaries of conventional meta-analysis (alpha 5%), and the boundaries of benefit and harm are boundaries of conventional meta-analysis adjusted for between-trial heterogeneity and multiple statistical testing (TSA boundaries). A treatment effect outside the TSA boundaries of benefit/harm indicates reliable evidence for a treatment effect, and a treatment effect within the futility zone (the triangle between the parallel lines) indicates reliable evidence of no treatment effect.
Discussion
Although there have been growing meta-analyses compares the effect of balanced crystalloids with normal saline among emergency and critical patients (9, 33–40). There is relatively limited evidence for patients with DKA. Therefore, in this meta-analysis, we comprehensively reviewed 11 RCTs to directly compare the balanced crystalloids versus normal saline in patients with DKA. Overall, compared with normal saline, the use of balanced crystalloids has no effect on the time to resolution of DKA, major adverse kidney events, and incidence of hypokalemia. However, we found that the treatment of DKA with balanced crystalloids compared to normal saline may reduce the post-resuscitation chloride. Furthermore, TSA indicated that more trials are needed to further confirm these findings.
To the best of our knowledge, this study is the most comprehensive meta-analysis of RCTs to compare the effect of balanced crystalloids with normal saline as fluid therapy in patients with DKA. Some of the findings of our meta-analysis are consist with the most recent meta-analysis by Tamzil et al. (41) that there was no significant difference in the duration of DKA resolution and acute renal failure. Conversely, Catahay et al. (42) analyzed 3 RCTs and indicated that the use of balanced crystalloids was associated with faster rates of DKA resolution compared to normal saline. However, Catahay et al. (42) only focused on the adult population and one single outcome, thus excluding other eligible trials and affecting the results.
Furthermore, our investigation revealed that the utilization of balanced crystalloids could reduce post-resuscitation chloride levels in patients with DKA. This suggests that opting for balanced crystalloids as resuscitation fluids may decrease the incidence of hyperchloremia in DKA patients. The diminished serum chloride levels observed in the balanced crystalloids group align with their lower chloride content, as these solutions emulate the plasma concentration of electrolytes. In contrast, the elevated serum chloride levels associated with normal saline could pose a risk of exacerbating hyperchloremia, thereby worsening metabolic acidosis and increasing the likelihood of acute kidney injury. Such complications may contribute to prolonged hospital stays, attributed to the deterioration of acidosis in DKA (43).
Although our meta−analysis did not reveal any noteworthy difference in the time to resolution of DKA or the major adverse kidney events between balanced crystalloids and normal saline groups, it is imperative to acknowledge potential contributing factors. These may include limitations associated with the relatively small sample size, variations in criteria for DKA resolution and adverse kidney events, or the absence of measurements related to ketone or anion gap resolution.
Our discoveries stem from an exhaustive and methodical exploration of the literature, wherein studies were rigorously identified via an exhaustive search methodology. Our inclusion criteria encompassed patients of diverse ages exhibiting varying comorbidities or etiologies of DKA at baseline. Two studies (26, 29) included the children with DKA, four studies (22, 25, 26, 30) included adult patients with moderate to severe DKA, while others included mild to severe DKA patients. Our findings should be taken with caution, even though the inclusion of all these studies allowed us to cover a wide range of patients. Criteria for the resolution of DKA varied substantially between studies: three studies (27, 28, 32) adhered to the criteria outlined in the American Diabetes Association (ADA) Consensus Statement on hyperglycemic crises 2009 criteria (44), one (23) adhered to the ADA Consensus Statement 2006 criteria (45), and another study (29) adopted criteria in accordance with the International Society of Pediatric and Adolescent Diabetes Guidelines 2014 (46). Hence, the variability in criteria employed is poised to exert an impact on the observed outcomes. Regrettably, the limited quantity of studies at our disposal precluded the execution of subgroup analyses. Such analyses would have been instrumental in elucidating the translational potential of our findings across a broader spectrum of patients. In addition, when we analyzed the effect of every single trial on the pooled results, we found that after omitting the study by Yan et al. (32), the use of balanced crystalloids was relevant to the obvious decreasing in incidence of hypokalemia. Since the patients in balanced crystalloids group had higher rate of comorbidities and co-diagnoses to DKA, the difference in the baseline disease severity between groups might led to this result.
Strength and limitations
The strength of our work lies in the comprehensive search and analysis and the predefined analysis plan for meta-analysis, all of which increase the transparency of information. Furthermore, the use of TSA enabled us to detect the risk of type-I or type-II errors in our findings. The DARIS estimated from TSA will also inform the sample size needed for adequately powered future trials.
The current study had certain limitations as well. Primarily, it is noteworthy that all incorporated trials are inherently characterized as small-sample studies (each with fewer than 100 patients per arm), thereby introducing the potential for bias associated with the small study effect (47). There should ideally be more large-scale RCTs as the majority of included studies had limited sample sizes, which might affect the power of these analyses. Secondly, various elements contribute to the heterogeneity inherent in our analysis, encompassing divergent population characteristics, diverse etiologies of DKA, and variations in the criteria employed for DKA resolution across the studies. Additionally, a broad spectrum of major adverse kidney events exhibited considerable variability, underscoring challenges in the uniformity of definitions and the inherent difficulties in the detection and reporting of adverse events. Thirdly, some important clinical outcomes such as length of hospital stay, time-to-discontinuation of insulin, and total insulin infusion were rarely recorded and analyzed in included trials, future studies should focus on these indicators of recovery. Last but not the least, the need for additional investigation persists regarding whether various facets of fluid composition and administration, such as osmolarity, temperature, and infusion speed, exert a modifying influence on the impact of crystalloid composition on clinical outcomes (48, 49). Further clinical trials on whether there is a difference in outcome between the types of balanced crystalloids used on DKA patients would also be a good addition in future studies to show whether the difference in composition between balanced crystalloids types would exhibit superiority or non-inferiority in patient outcomes when compared.
Conclusion
In this meta-analysis, the use of balanced crystalloids among patients with DKA has no significant different effect on the time to resolution of DKA, major adverse kidney events, and incidence of hypokalemia. However, its administration might decrease the post-resuscitation chloride. More large-scale RCTs with relatively large fluid exposure among patients with DKA are needed to guide the choice of the type of fluid resuscitation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
YL: Writing – original draft, Formal analysis, Data curation. JZ: Writing – review & editing, Software, Methodology, Formal analysis. XX: Writing – review & editing, Project administration, Methodology, Data curation. XZ: Writing – original draft, Project administration.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1367916/full#supplementary-material
References
1. Virdi N, Poon Y, Abaniel R, Bergenstal RM. Prevalence, cost, and burden of diabetic ketoacidosis. Diabetes Technol Ther. (2023) 25:S75–s84. doi: 10.1089/dia.2023.0149
2. Barski L, Golbets E, Jotkowitz A, Schwarzfuchs D. Management of diabetic ketoacidosis. Eur J Intern Med. (2023) 117:38–44. doi: 10.1016/j.ejim.2023.07.005
3. Long B, Willis GC, Lentz S, Koyfman A, Gottlieb M. Evaluation and management of the critically ill adult with diabetic ketoacidosis. J Emerg Med. (2020) 59:371–83. doi: 10.1016/j.jemermed.2020.06.059
4. Perner A, Hjortrup PB, Arabi Y. Focus on fluid therapy in critically ill patients. Intensive Care Med. (2019) 45:1469–71. doi: 10.1007/s00134-019-05703-0
5. Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med. (2013) 369:1243–51. doi: 10.1056/NEJMra1208627
6. Finfer S, Liu B, Taylor C, Bellomo R, Billot L, Cook D, et al. Resuscitation fluid use in critically ill adults: an international cross-sectional study in 391 intensive care units. Crit Care. (2010) 14:R185. doi: 10.1186/cc9293
7. Dhatariya KK. The management of diabetic ketoacidosis in adults-An updated guideline from the Joint British Diabetes Society for Inpatient Care. Diabetes Med. (2022) 39:e14788. doi: 10.1111/dme.14788
8. Glaser N, Fritsch M, Priyambada L, Rewers A, Cherubini V, Estrada S, et al. ISPAD clinical practice consensus guidelines 2022: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes. (2022) 23:835–56. doi: 10.1111/pedi.13406
9. Semler MW, Kellum JA. Balanced crystalloid solutions. Am J Respir Crit Care Med. (2019) 199:952–60. doi: 10.1164/rccm.201809-1677CI
10. Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. Jama. (2012) 308:1566–72. doi: 10.1001/jama.2012.13356
11. Zampieri FG, Ranzani OT, Azevedo LC, Martins ID, Kellum JA, Libório AB. Lactated ringer is associated with reduced mortality and less acute kidney injury in critically ill patients: A retrospective cohort analysis. Crit Care Med. (2016) 44:2163–70. doi: 10.1097/CCM.0000000000001948
12. Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. (2018) 378:829–39. doi: 10.1056/NEJMoa1711584
13. Raghunathan K, Shaw A, Nathanson B, Stürmer T, Brookhart A, Stefan MS, et al. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis*. Crit Care Med. (2014) 42:1585–91. doi: 10.1097/CCM.0000000000000305
14. Brown RM, Wang L, Coston TD, Krishnan NI, Casey JD, Wanderer JP, et al. Balanced crystalloids versus saline in sepsis. A secondary analysis of the SMART clinical trial. Am J Respir Crit Care Med. (2019) 200:1487–95. doi: 10.1164/rccm.201903-0557OC
15. Hayes W. Ab-normal saline in abnormal kidney function: risks and alternatives. Pediatr Nephrol (Berlin Germany). (2019) 34:1191–9. doi: 10.1007/s00467-018-4008-1
16. Finfer S, Myburgh J, Bellomo R. Intravenous fluid therapy in critically ill adults. Nat Rev Nephrol. (2018) 14:541–57. doi: 10.1038/s41581-018-0044-0
17. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
18. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. (2012) 120:c179–184. doi: 10.1159/000339789
19. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
20. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj. (2011) 343:d5928. doi: 10.1136/bmj.d5928
21. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
22. Mahler SA, Conrad SA, Wang H, Arnold TC. Resuscitation with balanced electrolyte solution prevents hyperchloremic metabolic acidosis in patients with diabetic ketoacidosis. Am J Emerg Med. (2011) 29:670–4. doi: 10.1016/j.ajem.2010.02.004
23. Van Zyl DG, Rheeder P, Delport E. Fluid management in diabetic-acidosis–Ringer’s lactate versus normal saline: a randomized controlled trial. Qjm. (2012) 105:337–43. doi: 10.1093/qjmed/hcr226
24. Aditianingsih D, Djaja AS, George YWH. The effect of balanced electrolyte solution versus normal saline in the prevention of hyperchloremic metabolic acidosis in diabetic ketoacidosis patients: a randomized controlled trial. Med J Indonesia. (2017) 26:(2). doi: 10.13181/mji.v26i2
25. Semler MW, Wanderer JP, Ehrenfeld JM, Stollings JL, Self WH, Siew ED, et al. Balanced crystalloids versus saline in the intensive care unit. The SALT randomized trial. Am J Respir Crit Care Med. (2017) 195:1362–72. doi: 10.1164/rccm.201607-1345OC
26. Yung M, Letton G, Keeley S. Controlled trial of Hartmann’s solution versus 0.9% saline for diabetic ketoacidosis. J Paediatr Child Health. (2017) 53(1):12–7. doi: 10.1111/jpc.13436
27. Tsui TY. Using Balanced Electrolyte Solution Versus Normal Saline for Fluid Resuscitation in Diabetic Ketoacidosis – A Randomized Clinical Trial. Michigan(USA: ProQuest LLC: University of Oklahoma (2019).
28. Self WH, Evans CS, Jenkins CA, Brown RM, Casey JD, Collins SP, et al. Clinical effects of balanced crystalloids vs saline in adults with diabetic ketoacidosis: A subgroup analysis of cluster randomized clinical trials. JAMA Netw Open. (2020) 3(11):e2024596. doi: 10.1001/jamanetworkopen.2020.24596
29. Williams V, Jayashree M, Nallasamy K, Dayal D, Rawat A. 0.9% saline versus Plasma-Lyte as initial fluid in children with diabetic ketoacidosis (SPinK trial): a double-blind randomized controlled trial. Crit Care. (2020) 24(1):1. doi: 10.1186/s13054-019-2683-3
30. Ramanan M, Attokaran A, Murray L, Bhadange N, Stewart D, Rajendran G, et al. Sodium chloride or Plasmalyte-148 evaluation in severe diabetic ketoacidosis (SCOPE-DKA): a cluster, crossover, randomized, controlled trial. Intensive Care Med. (2021) 47(11):1248–57. doi: 10.1007/s00134-021-06480-5
31. Attokaran AG, Ramanan M, Hunt L, Chandra K, Sandha R, Watts S, et al. Sodium chloride or plasmalyte-148 for patients presenting to emergency departments with diabetic ketoacidosis: A nested cohort study within a multicentre, cluster, crossover, randomised, controlled trial. Emerg Med Australas. (2023) 35:657–63. doi: 10.1111/1742-6723.14198
32. Yan JW, Slim A, Van Aarsen K, Choi YH, Byrne C, Poonai N, et al. Balanced crystalloids (RInger’s lactate) versus normal Saline in adults with diabetic Ketoacidosis in the Emergency Department (BRISK-ED): a pilot randomised controlled trial. Emerg Med J. (2023) 9(1):121. doi: 10.1186/s40814-023-01356-5
33. Zayed YZM, Aburahma AMY, Barbarawi MO, Hamid K, Banifadel MRN, Rashdan L, et al. Balanced crystalloids versus isotonic saline in critically ill patients: systematic review and meta-analysis. J Intensive Care. (2018) 6:51. doi: 10.1186/s40560-018-0320-x
34. Xue M, Zhang X, Liu F, Chang W, Xie J, Xu J, et al. Effects of chloride content of intravenous crystalloid solutions in critically ill adult patients: a meta-analysis with trial sequential analysis of randomized trials. Ann Intensive Care. (2019) 9:30. doi: 10.1186/s13613-019-0506-y
35. Zwager CL, Tuinman PR, de Grooth HJ, Kooter J, Ket H, Fleuren LM, et al. Why physiology will continue to guide the choice between balanced crystalloids and normal saline: a systematic review and meta-analysis. Crit Care. (2019) 23:366. doi: 10.1186/s13054-019-2658-4
36. Hammond DA, Lam SW, Rech MA, Smith MN, Westrick J, Trivedi AP, et al. Balanced crystalloids versus saline in critically ill adults: A systematic review and meta-analysis. Ann Pharmacother. (2020) 54:5–13. doi: 10.1177/1060028019866420
37. Tseng CH, Chen TT, Wu MY, Chan MC, Shih MC, Tu YK. Resuscitation fluid types in sepsis, surgical, and trauma patients: a systematic review and sequential network meta-analyses. Crit Care. (2020) 24:693. doi: 10.1186/s13054-020-03419-y
38. Zhu Y, Guo N, Song M, Xia F, Wu Y, Wang X, et al. Balanced crystalloids versus saline in critically ill patients: The PRISMA study of a meta-analysis. Med (Baltimore). (2021) 100:e27203. doi: 10.1097/MD.0000000000027203
39. Dong WH, Yan WQ, Song X, Zhou WQ, Chen Z. Fluid resuscitation with balanced crystalloids versus normal saline in critically ill patients: a systematic review and meta-analysis. Scand J Trauma Resusc Emerg Med. (2022) 30:28. doi: 10.1186/s13049-022-01015-3
40. Florez ID, Sierra J, Pérez-Gaxiola G. Balanced crystalloid solutions versus 0.9% saline for treating acute diarrhoea and severe dehydration in children. Cochrane Database Syst Rev. (2023) 5(5):Cd013640. doi: 10.1002/14651858.CD013640.pub2
41. Tamzil R, Yaacob N, Noor NM, Baharuddin KA. Comparing the clinical effects of balanced electrolyte solutions versus normal saline in managing diabetic ketoacidosis: A systematic review and meta-analyses. Turk J Emerg Med. (2023) 23(3):131–8. doi: 10.4103/tjem.tjem_355_22
42. Catahay JA, Polintan ET, Casimiro M, Notarte KI, Velasco JV, Ver AT, et al. Balanced electrolyte solutions versus isotonic saline in adult patients with diabetic ketoacidosis: A systematic review and meta-analysis. Heart Lung. (2022) 54:74–9. doi: 10.1016/j.hrtlng.2022.03.014
43. Maharjan J, Pandit S, Arne Johansson K, Khanal P, Karmacharya B, Kaur G, et al. Effectiveness of interventions for emergency care of hypoglycaemia and diabetic ketoacidosis: A systematic review. Diabetes Res Clin Pract. (2023) 207:111078. doi: 10.1016/j.diabres.2023.111078
44. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. (2009) 32:1335–43. doi: 10.2337/dc09-9032
45. Kitabchi AE, Umpierrez GE, Murphy MB, Kreisberg RA. Hyperglycemic crises in adult patients with diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. (2006) 29:2739–48. doi: 10.2337/dc06-9916
46. Wolfsdorf JI. The International Society of Pediatric and Adolescent Diabetes guidelines for management of diabetic ketoacidosis: Do the guidelines need to be modified? Pediatr Diabetes. (2014) 15:277–86. doi: 10.1111/pedi.2014.15.issue-4
47. Zhang Z, Xu X, Ni H. Small studies may overestimate the effect sizes in critical care meta-analyses: a meta-epidemiological study. Crit Care. (2013) 17:R2. doi: 10.1186/cc11919
48. Zampieri FG, MaChado FR, Biondi RS, Freitas FGR, Veiga VC, Figueiredo RC, et al. Effect of slower vs faster intravenous fluid bolus rates on mortality in critically ill patients: the baSICS randomized clinical trial. Jama. (2021) 326:830–8. doi: 10.1001/jama.2021.18545
Keywords: balanced crystalloids, normal saline, diabetic ketoacidosis, meta-analysis, resuscitation
Citation: Liu Y, Zhang J, Xu X and Zou X (2024) Comparison of balanced crystalloids versus normal saline in patients with diabetic ketoacidosis: a meta-analysis of randomized controlled trials. Front. Endocrinol. 15:1367916. doi: 10.3389/fendo.2024.1367916
Received: 09 January 2024; Accepted: 03 May 2024;
Published: 21 May 2024.
Edited by:
Chunjie Jiang, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Brittany Bruggeman, University of Florida, United StatesXi Jin, University of Pennsylvania, United States
Copyright © 2024 Liu, Zhang, Xu and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyun Zou, xyzou3589@163.com
 Yuting Liu1
Yuting Liu1 Xiaoya Xu
Xiaoya Xu