- 1Department of Anatomy, Institute of Biomedical Sciences, University of Sao Paulo, São Paulo, Brazil
- 2Faculty of Environmental Engineering, Wrocław University of Science and Technology, Wrocław, Poland
- 3Department of Pathology, Universidade de Sao Paulo Medical School (FMUSP), São Paulo, Brazil
Traditional methods of air pollution monitoring require substantial investment in equipment and infrastructure. However, efficient and cost-effective alternatives offer promising solutions for region-specific pollution assessments and understanding their impact on local populations. This review explores examples of low-cost monitoring methods, focusing on natural bioindicators, human interaction-based techniques, and the outcomes associated with air pollution exposure. Bioindicators such as spider webs, lichens, mosses, and Tradescantia pallida (T. pallida) are discussed as potential tools for air pollution monitoring. Human biomonitoring techniques, including the micronucleus assay and the assessment of pulmonary anthracosis, are examined for their ability to provide valuable insights into genotoxic effects and long-term exposure. The advantages and limitations of each method are highlighted. The review advocates for continued research and development to refine these approaches, with the aim of mitigating the adverse health impacts of air pollution on both individuals and communities.
1 Introduction
Air pollution poses a significant threat to human health, causing approximately 4.2 million premature deaths in 2019, as reported by the World Health Organization (World Health Organization, 2022). Assessing human exposure to air pollution is critical for understanding the potential health risks associated with airborne particles. Conventional methods for air pollution monitoring and estimating human exposure are widely used by environmental agencies, research institutions, and industries for regulatory compliance, public health assessment, and scientific research. They involve a range of established techniques and equipment, and assessing air quality requires a significant investment in the appropriate infrastructure for such measurements (Knox et al., 2012; World Health Organization, 2023).
In response to these challenges, efficient, cost-effective, and innovative solutions for assessing pollutants in specific regions and their impact on local populations have continuously emerged. Alternative methods for monitoring air pollution and assessing human exposure or the effects of air pollution often complement traditional techniques. They provide innovative perspectives to deepen our understanding and devise effective strategies to address air quality concerns.
This review provides an overview of selected monitoring methods that focus on natural bioindicators commonly used for environmental air pollution monitoring. Additionally, it covers individualized health-related monitoring methods and health outcomes associated with air pollution exposure. We explore examples of alternative and cost-effective methods used to monitor air pollution and measure exposure to airborne particles, as well as the effects caused by such exposure. Aspects of their methodologies, strengths, and limitations are summarized.
2 Bioindicators
Different living organisms can serve as bioindicators. This approach uses plants and animals with the capacity to monitor environmental conditions. What is important, all the bioindicators can be used in a defined time of exposure. Certain bioindicators exhibit changes or responses to immediate or short-lived fluctuations in air pollutant levels. They might reflect acute exposure or fluctuations over hours, days, or few weeks. On the other hand, changes or effects that occur over an extended period due to chronic exposure to air pollutants reflect the cumulative impact of pollutants on biological systems over months, years, or even decades.
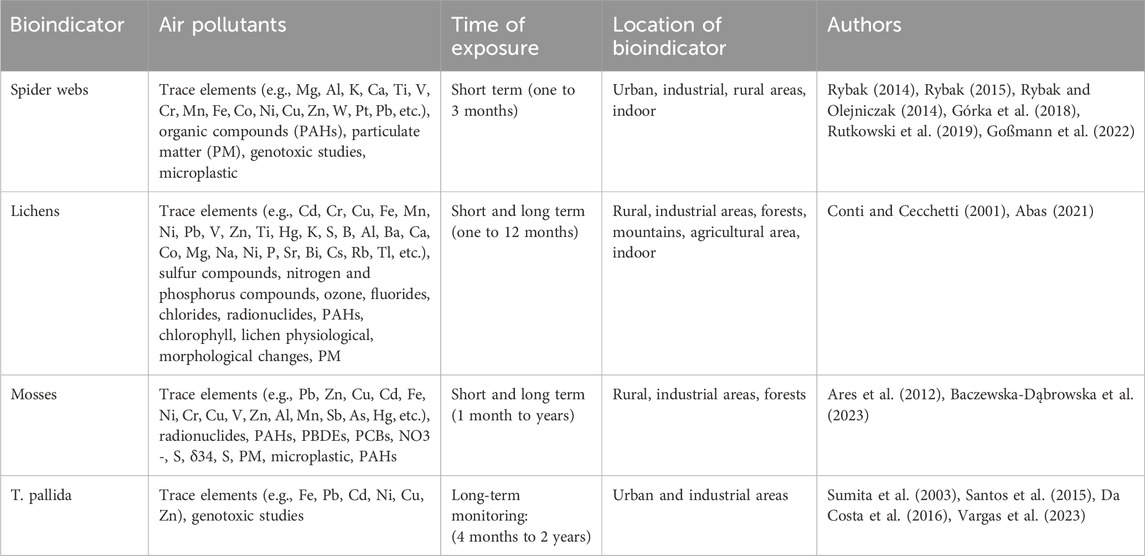
Biological monitoring complements the chemical analyses by providing biological information about the effect and the interaction with pollutants. In the sections that follow, we will explore an assortment of interesting examples of these environmental sentinels. Summarized information about the examples of bioindicators mentioned in this review is described in Table 1.
2.1 Spider webs
Recently spider webs seem to be a reliable and cheap air monitoring method. Webs are made of silk, a protein that is produced by the spider’s spinnerets. The feature that allows using it for air pollution monitoring is that dust particles can be trapped inside the web as prey. The first studies were conducted in Australia (Hose et al., 2002). The scientists found that spider webs can be a useful indicator of environmental pollution. Since then, this method has become increasingly popular and researchers have used it for monitoring various pollutants such as metals and metalloids, trace elements, polycyclic aromatic compounds, dioxins, magnetic susceptibility, and even for the assessment of pollutants’ origin (Rybak, 2014; Rybak and Olejniczak, 2014; Rybak, 2015; Rachwał et al., 2018; Rutkowski et al., 2019; Stojanowska et al., 2020; van Laaten et al., 2020). This tool can be used in a defined time of exposure, usually from two to 3 months. This bioindicator is not specific for different air pollutants, although according to studies, spider webs are probably more suitable for the indication of organic compounds such as PAHs due to their chemical affinity to the fibroin which is the basic component of web. Organic pollutants penetrate webs more deeply than other air pollutants (Stojanowska et al., 2020). Although, the mechanism of accumulation of organic compounds is still unknown. What is interesting, it has been found that only compounds with high molecular weight were trapped in webs, suggesting that more volatile compounds can be lost and are not detected. Therefore, applying webs in closed polluted areas, i.e., car parks, homes can remove this problem (Stojanowska et al., 2020). The control quality is possible and relies on obtaining the clean web woven under laboratory conditions.
Spider webs have many advantages as they are cheap, organic, widely available, and non-selective i.e., they can accumulate various pollutants. They can be applied in both long-term and short-term monitoring (Rutkowski et al., 2018). The web can also be obtained from laboratory-bred spiders and then applied to indoor and outdoor studies with a defined time of exposure (Rachwał et al., 2018). The main obstacle is the tiny structure of the web, which makes it difficult to collect and analyze them. Therefore, not all spider families can be used for bioindication. Spider webs have been considered a biomonitoring tool that can be used to determine air quality, especially in preliminary studies, used to indicate the most polluted areas “hotspots” for later application, more detailed and accessible monitoring using conventional methods.
2.2 Lichens
Lichens have a long history as bioindicators (Adamo et al., 2003; Kłos et al., 2018; Stojanowska et al., 2020). They are applied to assess various types of contaminants, similar to spider webs (Shukla et al., 2010). As lichens do not have cuticles and roots to absorb water and minerals, they are dependent on atmospheric deposition. What is important, the presence of lichen species alone in defined area can indicate a good air quality. Another method relies on transplanting lichens from the pristine area to a polluted one and measuring the morphological changes in the thallus along with assessing the physiological parameters and level of bioaccumulation of the contaminants. The physiological parameters such as photosynthesis, chlorophyll level and biodegradation of chlorophyll, respiration, etc., are used for monitoring of environmental pollution with lichens (Conti and Cecchetti, 2001). Various studies found a positive correlation between the sulphur, ozone and nitrogen content of lichens and SO2, NOx, O3 in the air. Furthermore, lichens are very good bioaccumulators of trace elements. For other atmospheric contaminants, such as fluorides, chlorides, polychlorinated dibenzodioxins and polychlorinated dibenzofurans (PCDDs/PCDFs), the application of lichens are very scarce. The concentration of trace elements in lichens is usually correlated with their content in air. To evaluate the pollution level it is necessary to assess the background level of the contaminants in the air and in the lichens (Conti and Cecchetti, 2001). Lichens are usually exposed to pollutants from two to 3 months. They are not specific for different air pollutants, although their application for the assessment of SO2, NOx, O3 content in the air as well as the level of bioaccumulation of trace metals seems to be the most popular.
However, this method also has its limitations as lichens can reach a saturation point at which pollutants cannot be absorbed anymore (Garty et al., 1993). The other problem with applying this method is that the use of lichens could be restricted to unfavorable weather conditions. Lichens’ sensitivity to sulfur dioxide can be an additional obstacle as their application could be limited to some places (Nash, 2008).
2.3 Mosses
Mosses are also commonly used in biomonitoring (Bargagli et al., 2002; Liu et al., 2009). Similar to lichens, mosses do not have roots, epidermis, and cuticles which makes them dependent on atmospheric deposition too. They are abundant worldwide, and present in different environments which makes them a very good bioindicator (Szczepaniak and Biziuk, 2003). Mosses are applied in elements and organic pollutants assessment (Holoubek et al., 2000). Although, they are mainly used in assessment of metals and nitrogen deposition (Díaz-Álvarez et al., 2018). The methods are three: the application of native moses, the use of naturally growing mosses and the transplantation of mosses into the study area (Baczewska-Dąbrowska et al., 2023). Świsłowski et al. (2022) studied two methods of mosses application (moss bags and native mosses). It was found that moss bags accumulated more pollutants. In order to evaluate the level of contamination, it becomes essential to determine the baseline levels of the pollutants in both the air and in the mosses. Mosses the same as other biomonitors are usually exposed to pollutants from two to 3 months.
However, they can also have limitations. Sometimes it is difficult to obtain mosses from a clean area for transplantation as they can be hidden by snow in wintertime (Lodenius, 2014). Another problem is, that when using ‘moss bags’ they can dry out and thus could lose their efficiency in accumulating pollutants (Szczepaniak and Biziuk, 2003). According to Bargagli (2016) the assessment of some elements, e.g., Hg content in mosses does not exactly reflect its atmospheric deposition. The biomonitoring with mosses defines Hg hot spots and changes in its spatial distribution only.
2.4 Others
The use of higher plants, including trees, as passive samplers in biomonitoring gives advantages due to the excellent availability of plants and the low cost of sample collection. Many higher plants have been used for monitoring air pollution. Although trees are not as good indicators as lower plants such as lichens or mosses, they are widely distributed across many countries and have long lifecycles, so the studies can be repeated after few decades for a comparative air monitoring and for the assessment a time-trend distribution of trace elements (Sawidis et al., 2011). While some plant bioindicators can be sensitive to only one type of pollutant, others respond to a wide range of stressors.
Plants can also be utilized for pollution monitoring through active methods, which involve transplanting them into the study area for a specified period of time. In this context, T. pallida (Tradescantia pallida), commonly known as Purple Heart, has been recognized for its sensitivity as a passive and active biomonitoring of air pollution (Sumita et al., 2003; Santos et al., 2015; Da Costa et al., 2016). These studies have demonstrated that elements associated with air pollution from vehicle emissions and anthropogenic sources, such as iron (Fe), lead (Pb), cadmium (Cd), nickel (Ni), copper (Cu), and zinc (Zn), exhibit higher concentrations in leaf samples collected in highly trafficked areas compared to those in low-traffic or soil samples. The selection of monitoring sites is typically guided by data on air pollution levels, including measurements of CO, NO2, SO2, and PM10 provided by monitoring agencies. Based on the amount of pollutant elements measured in the plants, it is possible to distinguish the polluted areas with good precision. Its ability to accumulate and reflect the presence of trace elements in the air makes it a valuable tool for assessing air quality without the need for very complex laboratory equipment.
Given its wide availability, easy cultivation, and cost-effectiveness, T. pallida is a suitable choice for long-term monitoring projects that last for months (Santos et al., 2015) and years (Da Costa et al., 2016). To ensure quality assurance when using T. pallida as an air pollution bioindicator, it is important to cultivate samples in uniform, uncontaminated soil with controlled water supply enriched by a nutritive solution before exposure to pollution, while maintaining validated standard operating procedures (SOPs) consistently throughout the study. Utilizing conditions considered as reference standards and replicating extracted samples serve as examples of quality control measures for this alternative method of assessing air pollutants. Then, through proper study design and data analysis, T. pallida can serve as a valuable complementary tool for assessing air pollution levels in urban areas with different traffic intensities and under varying environmental conditions, as well as in industrial settings. It is interesting to note that T. pallida (and other plants) can act as early warning indicators, and because they are located near people, they can offer a true representation of exposure scenarios.
Despite its advantages, T. pallida-based air pollution monitoring has some limitations. The plant’s sensitivity to pollutants can also be influenced by environmental factors such as weather conditions and seasonal variations (Santos et al., 2015), potentially affecting the accuracy of results. Moreover, the plant’s response to specific pollutants may vary, making it essential to consider the local context when interpreting data. However, research efforts have identified T. pallida as a reference for biomonitoring air genotoxicity. These studies have revealed a significant correlation between the levels of air pollutants and the genetic damage observed when using this remarkable natural tool (Da Costa et al., 2016; Amato-Lourenco et al., 2017; Rocha et al., 2018; Vargas et al., 2023).
3 Human biomarkers and monitoring
3.1 Micronucleus assay
Numerous approaches exist for assessing the biomarkers of the effects of air pollution through the collection of human samples. Within this context, a plethora of studies have contributed valuable insights into the genetic damage induced by exposure to environmental pollutants. An interesting method for measuring the levels of potential genotoxic effects of air pollutants on exposed populations is the micronucleus (MN) assay. This is a cytogenetic technique used to assess particularly DNA damage and chromosome breakage in cells (Fenech, 1993; Sommer et al., 2020). It involves the detection of micronuclei, which are small additional nuclei formed during cell division when chromosome fragments or whole chromosomes are not incorporated into the daughter nuclei. The methodology for assessing MN is notably simple and it can be conducted on various cell types, including human peripheral blood lymphocytes and buccal cells.
A summary of the steps for conducting the MN assay in buccal cells (cells from the inner lining of the mouth) includes sample collection, fixation, preparation of slides, staining, microscopic examination, scoring and analysis, and data interpretation (Thomas et al., 2009; Kashyap and Reddy, 2012). It is important to note that the MN assay requires careful handling, standardization, and appropriate controls to ensure reliable and meaningful results. Additionally, variations in the procedure may exist depending on the specific laboratory protocols and research objectives.
Several studies have explored the association between air pollution exposure and MN frequencies, yielding interesting findings. O’Callaghan-Gordo et al., 2015 demonstrated the genotoxic repercussions of air pollution on pregnant women. The study revealed that exposure to air pollution, particularly particulate matter (PM), resulted in increased MN frequencies in mothers, especially those with lower vitamin C intake and who smoked during pregnancy. Ishikawa et al. (2006) observed higher MN frequencies in women residing in industrial areas of Shenyang City compared to those in rural areas, highlighting the influence of industrial air pollution on MN formation. In parallel, the frequency of MN also demonstrated a positive association with quasi-ultrafine particulate matter (PM0.5) and traffic proximity near the homes of children residing in an industrialized area, in contrast to that observed in children living in a control area without significant anthropogenic influences (Panico et al., 2020). The study conducted by Zhao et al. (1998), which compared MN frequencies between traffic police and household register police, revealed that traffic police exhibited significantly higher MN frequencies. This finding underscores the role of intense automobile exhaust exposure in inducing genotoxic effects. Additionally, another scenario demonstrating an association between the MN test and trace element concentrations found in the blood and urine was observed in workers from areas of artisanal gem mining in the state of Minas Gerais (Brazil) (Santos et al., 2023).
While many studies have investigated the association between PM exposure and MN frequency (Da Silva Junior et al., 2023), the results of the MN frequency usually cannot be linked to a particular compound. Instead, they are associated with the intricate blend of pollutants in the atmosphere, originating from sources such as vehicular emissions, industrial activities, and occupational exposure (Da Costa et al., 2016). Other limitations associated with this technique include the variability in baseline values, the influence of inter-individual differences, and the potential confounding effects of environmental factors (Thomas et al., 2009; Kirsch-Volders et al., 2011; Bolognesi and Fenech, 2013; Luzhna et al., 2013). Employing questionnaires for the population under study can be instrumental in gaining insights into their background information, thereby enhancing the interpretation of the results.
3.2 Pulmonary anthracosis
Developing innovative approaches to accurately estimate individual exposure is also extremely necessary. Methods for assessing individual air pollution exposure by focusing on the utilization of anthracosis or black carbon deposition within the lung are considered promising. Decades ago, Zeidberg and Prindle (1963) proposed that pleural anthracosis could serve as an indicator of air pollution exposure. Their study involved the histopathological examination of autopsied lungs from Nashville residents, a city known for coal-derived pollution. The lungs were categorized based on the extent of anthracosis. Their findings revealed a positive correlation between the amount of anthracosis deposited in the lungs and the duration of exposure, measured as the time residents had lived in the city. Given the robust supporting evidence from subsequent studies (Brauer et al., 2001; Tsuda et al., 2013), which demonstrated that human lungs retain ambient particles, further research was undertaken to explore whether exposure to urban air pollution correlates with the degree of pleural anthracosis. This investigation considered potential modifying factors, such as personal habits, mobility patterns, and occupational activities, obtained through a questionnaire administered to the relatives of the study subjects (Takano et al., 2019; Da Motta Singer et al., 2023).
To summarize, the methodology involves the following steps during post-mortem examinations: removal of the lungs and thorough cleaning of the pleural surface; placement of a Petri dish strategically on the anterior surfaces of lung lobes to create a flat observation area; capture of high-resolution images of the pleura surface using a camera; image processing using ImageJ software; calculation and estimation of the proportion of black spots in each lobe; aggregation of these individual lobe values to calculate the mean proportion of anthracosis for the entire lung (Takano et al., 2019).
The primary limitation of this method is its reliance on an autopsy service, coupled with the requirement for a relatively substantial sample size. Furthermore, it overlooks the analysis of the composition of pigments deposited in the lungs. Although previous studies have already identified and measured the chemical element profile of anthracosis in the lungs of residents in Sao Paulo (Saieg et al., 2011; Dos Santos et al., 2022), it is not possible to relate the health effects associated to anthracosis deposition with a specific compound. Establishing a suitable control group is particularly intricate; however, collected data can be analyzed effectively with statistical support.
Nonetheless, when evaluating the entire procedure of data collection and analysis, it emerges as a remarkably cost-effective approach. The method for assessing individual exposure based on pleural anthracosis is significant, as it can aid in evaluating long-term exposure to urban air pollution and its impact on health.
4 Conclusion and further perspectives
This review explores bioindicators such as spider webs, lichens, mosses, and higher plants as potential methods for air pollution monitoring due to their organic nature, widespread availability, and non-selective accumulation of various pollutants. However, each bioindicator method has limitations related to practical challenges and environmental dependencies, necessitating careful consideration in their application, which does not diminish the fact that they can be successfully used in the initial, low-cost phase of studies (screening tests), which can quickly give the information about the scale of the problem. Within the scope of human biomonitoring, techniques like the micronucleus assay and assessing pulmonary anthracosis offer valuable insights into biological consequences after contaminants have crossed one of the body’s surfaces and entered tissues or fluids. While these methods provide cost-effective means for evaluating individual exposure, they also have limitations related to variations in baseline values, inter-individual differences, and dependency on post-mortem services.
The significance of employing combined methods for measuring air pollutants must be emphasized. The cost-effective alternative methods mentioned in the manuscript have the potential to complement conventional methods, offering a valuable approach for local areas, cities, and countries. It is important to note that no single method is perfect, and multiple methods are used, as models require measurements for calibration and validation. A concerted focus on refining and developing these alternative methods holds the promise of revolutionizing air pollution monitoring, facilitating a better understanding, and aiding in the implementation of targeted intervention strategies to mitigate the adverse health effects of air pollution on individuals and communities.
Author contributions
AT: Conceptualization, Writing–original draft, Writing–review and editing. JR: Writing–review and editing. MV: Conceptualization, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abas, A. (2021). A systematic review on biomonitoring using lichen as the biological indicator: a decade of practices, progress and challenges. Ecol. Indic. 121, 107197. doi:10.1016/j.ecolind.2020.107197
Adamo, P., Giordano, S., Vingiani, S., Castaldo Cobianchi, R., and Violante, P. (2003). Trace element accumulation by moss and lichen exposed in bags in the city of Naples (Italy). Environ. Pollut. 122 (1), 91–103. doi:10.1016/S0269-7491(02)00277-4
Amato-Lourenco, L. F., Lobo, D. J. A., Guimarães, E. T., Moreira, T. C. L., Carvalho-Oliveira, R., Saiki, M., et al. (2017). Biomonitoring of genotoxic effects and elemental accumulation derived from air pollution in community urban gardens. Sci. Total Environ. 575, 1438–1444. doi:10.1016/j.scitotenv.2016.09.221
Ares, A., Aboal, J. R., Carballeira, A., Giordano, S., Adamo, P., and Fernández, J. A. (2012). Moss bag biomonitoring: a methodological review. Sci. Total Environ. 432, 143–158. doi:10.1016/j.scitotenv.2012.05.087
Baczewska-Dąbrowska, A. H., Gworek, B., and Dmuchowski, W. (2023). The use of mosses in biomonitoring of air pollution in the terrestrial environment. A review. Environ. Prot. Nat. Resour. 34 (2), 19–30. doi:10.2478/oszn-2023-0005
Balchum, E., Sussman, S., Toyama, T., Zeidberg, L. D., and Prindle, R. A. (1963). The Nashville air pollution study. II. Pulmonary anthracosis as an index of air pollution. Am. J. Public Health Nations Health 53 (2), 185–199. doi:10.2105/ajph.53.2.185
Bargagli, R. (2016). Moss and lichen biomonitoring of atmospheric mercury: a review. Sci. Total Environ. 572, 216–231. doi:10.1016/j.scitotenv.2016.07.202
Bargagli, R., Monaci, F., Borghini, F., Bravi, F., and Agnorelli, C. (2002). Mosses and lichens as biomonitors of trace metals. A comparison study on Hypnum cupressiforme and Parmelia caperata in a former mining district in Italy. Environ. Pollut. 116 (2), 279–287. doi:10.1016/S0269-7491(01)00125-7
Bolognesi, C., and Fenech, M. (2013). Micronucleus assay in human cells: lymphocytes and buccal cells. Methods Mol. Biol. 1044, 191–207. doi:10.1007/978-1-62703-529-3_10
Brauer, M., Avila-Casado, C., Fortoul, T. I., Vedal, S., Stevens, B., and Churg, A. (2001). Air pollution and retained particles in the lung. Environ. Health Perspect. 109 (10), 1039–1043. doi:10.1289/ehp.011091039
Conti, M., and Cecchetti, G. (2001). Biological monitoring: lichens as bioindicators of air pollution assessment — a review. Environ. Pollut. 114 (3), 471–492. doi:10.1016/S0269-7491(00)00224-4
Da Costa, G. M., Petry, C. T., and Droste, A. (2016). Active versus passive biomonitoring of air quality: genetic damage and bioaccumulation of trace elements in flower buds of Tradescantia pallida var. purpurea. Water Air Soil Pollut. 227, 229. doi:10.1007/s11270-016-2923-y
Da Motta Singer, J., Saldiva de André, C. D., Afonso de André, P., Monteiro Rocha, F. M., Waked, D., Vaz, A. M., et al. (2023). Assessing socioeconomic bias of exposure to urban air pollution: an autopsy-based study in São Paulo, Brazil. Lancet Regional Health - Am. 22, 100500. doi:10.1016/j.lana.2023.100500
Da Silva Junior, F. C., de Araújo, L. P., Freitas, J. P. d. M., de Oliveira Alves, N., Bonassi, S., and Batistuzzo de Medeiros, S. R. (2023). Empirical relationship between chromosomal damage and airborne particulate matter: a systematic review and meta-analysis of studies in exposed populations. Mutat. Research/Reviews Mutat. Res. 791, 108454. doi:10.1016/j.mrrev.2023.108454
Díaz-Álvarez, E. A., Lindig-Cisneros, R., and De La Barrera, E. (2018). Biomonitors of atmospheric nitrogen deposition: potential uses and limitations. Conserv. Physiol. 6 (1), coy011. doi:10.1093/conphys/coy011
Dos Santos, N. V., Vieira, C. L. Z., Saldiva, P. H. N., De André, C. D. S., Mazzilli, B. P., de Fátima Andrade, M., et al. (2022). Accumulation of trace element content in the lungs of Sao Paulo city residents and its correlation to lifetime exposure to air pollution. Sci. Rep. 12 (1), 11083. doi:10.1038/s41598-022-15048-2
Fenech, M. (1993). The cytokinesis-block micronucleus technique and its application to genotoxicity studies in human populations. Environ. Health Perspect. 101 (3), 101–107. doi:10.1289/ehp.93101s3101
Garty, J., Karary, Y., and Harel, J. (1993). The impact of air pollution on the integrity of cell membranes and chlorophyll in the lichen Ramalina duriaei (de not.) bagl. transplanted to industrial sites in Israel. Arch. Environ. Contam. Toxicol. 24, 455–460. doi:10.1007/BF01146161
Górka, M., Bartz, W., and Rybak, J. (2018). The mineralogical interpretation of particulate matter deposited on Agelenidae and Pholcidae spider webs in the city of Wrocław (SW Poland): a preliminary case study. J. Aerosol Sci. 123, 63–75. doi:10.1016/j.jaerosci.2018.06.008
Goßmann, I., Süßmuth, R., and Scholz-Böttcher, B. M. (2022). Plastic in the air?! - spider webs as spatial and temporal mirror for microplastics including tire wear particles in urban air. Sci. Total Environ. 832, 155008. doi:10.1016/j.scitotenv.2022.155008
Holoubek, I., Kořınek, P., Šeda, Z., Schneiderová, E., Holoubková, I., Pacl, A., et al. (2000). The use of mosses and pine needles to detect persistent organic pollutants at local and regional scales. Environ. Pollut. 109, 283–292. doi:10.1016/S0269-7491(99)00260-2
Hose, G. C., James, J. M., and Gray, M. R. (2002). Spider webs as environmental indicators. Environ. Pollut. 120 (3), 725–733. doi:10.1016/S0269-7491(02)00171-9
Ishikawa, H., Tian, Y., Piao, F., Duan, Z., Zhang, Y., Ma, M., et al. (2006). Genotoxic damage in female residents exposed to environmental air pollution in Shenyang city, China. Cancer Lett. 240 (1), 29–35. doi:10.1016/j.canlet.2005.08.023
Kashyap, B., and Reddy, P. S. (2012). Micronuclei assay of exfoliated oral buccal cells: means to assess the nuclear abnormalities in different diseases. J. Cancer Res. Ther. 8 (2), 184–191. doi:10.4103/0973-1482.98968
Kirsch-Volders, M., Plas, G., Elhajouji, A., Lukamowicz, M., Gonzalez, L., Vande Loock, K., et al. (2011). The in vitro MN assay in 2011: origin and fate, biological significance, protocols, high throughput methodologies and toxicological relevance. Arch. Toxicol. 85 (8), 873–899. doi:10.1007/s00204-011-0691-4
Kłos, A., Ziembik, Z., Rajfur, M., Dołhańczuk-Śródka, A., Bochenek, Z., Bjerke, J. W., et al. (2018). Using moss and lichens in biomonitoring of heavy-metal contamination of forest areas in southern and north-eastern Poland. Sci. Total Environ. 627, 438–449. doi:10.1016/j.scitotenv.2018.01.211
Knox, A., Evans, G. J., Lee, C. J., and Brook, J. R. (2012). “Air pollution monitoring and sustainability,” in Encyclopedia of sustainability science and technology. Editor R. A. Meyers (New York, NY: Springer). doi:10.1007/978-1-4419-0851-3_373
Liu, X. Y., Xiao, H. Y., Liu, C. Q., Xiao, H. W., and Wang, Y. L. (2009). Assessment of atmospheric sulfur with the epilithic moss Haplocladium microphyllum: evidences from tissue sulfur and δ34S analysis. Environ. Pollut. 157 (7), 2066–2071. doi:10.1016/j.envpol.2009.02.020
Lodenius, M. (2014). Biomonitoring of airborne metal pollution. WIT Trans. Ecol. Environ. 183, 75–85. doi:10.2495/AIR140071
Luzhna, L., Kathiria, P., and Kovalchuk, O. (2013). Micronuclei in genotoxicity assessment: from genetics to epigenetics and beyond. Front. Genet. 4, 131. doi:10.3389/fgene.2013.00131
Nash, T. H. (2008). “Lichen sensitivity to air pollution” in Lichen Biology. Second Edition. Chennai: Cambridge University Press, 299–314. doi:10.1017/CBO9780511790478.016
O’Callaghan-Gordo, C., Fthenou, E., Pedersen, M., Espinosa, A., Chatzi, L., Beelen, R., et al. (2015). Outdoor air pollution exposures and micronuclei frequencies in lymphocytes from pregnant women and newborns in Crete, Greece (Rhea cohort). Environ. Res. 143, 170–176. doi:10.1016/j.envres.2015.10.011
Panico, A., Grassi, T., Bagordo, F., Idolo, A., Serio, F., Tumolo, M. R., et al. (2020). Micronucleus frequency in exfoliated buccal cells of children living in an industrialized area of apulia (Italy). Int. J. Environ. Res. Public Health 17 (4), 1208. doi:10.3390/ijerph17041208
Rachwał, M., Rybak, J., and Rogula-Kozłowska, W. (2018). Magnetic susceptibility of spider webs as a proxy of airborne metal pollution. Environ. Pollut. 234, 543–551. doi:10.1016/j.envpol.2017.11.088
Rocha, A. D. N., Candido, L. S., Pereira, J. G., Silva, C. A. M., da Silva, S. V., and Mussury, R. M. (2018). Evaluation of vehicular pollution using the TRAD-MCN mutagenic bioassay with Tradescantia pallida (Commelinaceae). Environ. Pollut. 240, 440–447. doi:10.1016/j.envpol.2018.04.091
Rutkowski, R., Rybak, J., Mach, T., and Rogula-Kozłowska, W. (2018). Spider webs in monitoring of air pollution. SHS Web Conf. 57, 02011. doi:10.1051/shsconf/20185702011
Rutkowski, R., Rybak, J., Rogula-Kozłowska, W., Bełcik, M., Piekarska, K., and Jureczko, I. (2019). Mutagenicity of indoor air pollutants adsorbed on spider webs. Ecotoxicol. Environ. Saf. 171, 549–557. doi:10.1016/j.ecoenv.2019.01.019
Rybak, J. (2014). Possible use of spider webs for the indication of organic road pollutants. J. Ecol. Eng. 15, 39–45. doi:10.12911/22998993.1109121
Rybak, J. (2015). Accumulation of major and trace elements in spider webs. Water Air Soil Pollut. 226, 105. doi:10.1007/s11270-015-2369-7
Rybak, J., and Olejniczak, T. (2014). Accumulation of polycyclic aromatic hydrocarbons (PAHs) on the spider webs in the vicinity of road traffic emissions. Environ. Sci. Pollut. Res. 21 (3), 2313–2324. doi:10.1007/s11356-013-2092-0
Saieg, M. A., Cury, P. M., Godleski, J. J., Stearns, R., Duarte, L. G., D'Agostino, L., et al. (2011). Differential elemental distribution of retained particles along the respiratory tract. Inhal. Toxicol. 23, 459–467. doi:10.3109/08958378.2011.582895
Santos, A. P., Segura-Muñoz, S. I., Nadal, M., Schuhmacher, M., Domingo, J. L., Martinez, C. A., et al. (2015). Traffic-related air pollution biomonitoring with Tradescantia pallida (Rose) Hunt. cv. purpurea Boom in Brazil. Environ. Monit. Assess. 187 (2), 39. doi:10.1007/s10661-014-4234-3
Santos, A. P. R., Silva, L. Z., Freire, B. M., da Silva Faria, M. C., Batista, B. L., Rocha, B. A., et al. (2023). Artisanal gem mining in Brazil: a source of genotoxicity and exposure to toxic elements. Int. J. Environ. Res. Public Health 20 (3), 2510. doi:10.3390/ijerph20032510
Sawidis, T., Breuste, J., Mitrovic, M., Pavlovic, P., and Tsigaridas, K. (2011). Trees as bioindicator of heavy metal pollution in three European cities. Environ. Pollut. 159 (12), 3560–3570. doi:10.1016/j.envpol.2011.08.008
Shukla, V., Joshi, G. P., and Rawat, M. S. M. (2010). Lichens as a potential natural source of bioactive compounds: a review. Phytochem. Rev. 9, 303–314. doi:10.1007/s11101-010-9189-6
Sommer, S., Buraczewska, I., and Kruszewski, M. (2020). Micronucleus Assay: the state of art, and future directions. Int. J. Mol. Sci. 21 (4), 1534. doi:10.3390/ijms21041534
Stojanowska, A., Rybak, J., Bożym, M., Olszowski, T., and Bihałowicz, J. S. (2020). Spider webs and lichens as bioindicators of heavy metals: a comparison study in the vicinity of a copper smelter (Poland). Sustainability 12 (19), 8066. doi:10.3390/su12198066
Sumita, N. M., Mendes, M. E., Macchione, M., Guimarães, E. T., Lichtenfels, A. J. d. F. C., Lobo, D. J. d. A., et al. (2003). Tradescantia pallida cv. purpurea boom in the characterization of air pollution by accumulation of trace elements. J. Air and Waste Manag. Assoc. 53 (5), 574–579. doi:10.1080/10473289.2003.10466197
Świsłowski, P., Nowak, A., and Rajfur, M. (2022). Comparison of exposure techniques and vitality assessment of mosses in active biomonitoring for their suitability in assessing heavy metal pollution in atmospheric aerosol. Environ. Toxicol. Chem. 41 (6), 1429–1438. doi:10.1002/etc.5321
Szczepaniak, K., and Biziuk, M. (2003). Aspects of the biomonitoring studies using mosses and lichens as indicators of metal pollution. Environ. Res. 93 (3), 221–230. doi:10.1016/S0013-9351(03)00141-5
Takano, A. P. C., Justo, L. T., Dos Santos, N. V., Marquezini, M. V., de André, P. A., da Rocha, F. M. M., et al. (2019). Pleural anthracosis as an indicator of lifetime exposure to urban air pollution: an autopsy-based study in Sao Paulo. Environ. Res. 173, 23–32. doi:10.1016/j.envres.2019.03.006
Thomas, P., Holland, N., Bolognesi, C., Kirsch-Volders, M., Bonassi, S., Zeiger, E., et al. (2009). Buccal micronucleus cytome assay. Nat. Protoc. 4 (6), 825–837. doi:10.1038/nprot.2009.53
Tsuda, A., Henry, F. S., and Butler, J. P. (2013). Particle transport and deposition: basic physics of particle kinetics. Compr. Physiol. 3, 1437–1471. doi:10.1002/cphy.c100085
van Laaten, N., Merten, D., von Tümpling, W., Schäfer, T., and Pirrung, M. (2020). Comparison of spider web and moss bag biomonitoring to detect sources of airborne trace elements. Water Air Soil Pollut. 231, 512. doi:10.1007/s11270-020-04881-8
Vargas, V. M. F., da Silva Júnior, F. M. R., Silva Pereira, T. D., Silva, C. S. D., and Coronas, M. V. (2023). A comprehensive overview of genotoxicity and mutagenicity associated with outdoor air pollution exposure in Brazil. J. Toxicol. Environ. Health, Part B 26 (3), 172–199. doi:10.1080/10937404.2023.2175092
World Health Organization (2022). Fact sheets - ambient (outdoor) air pollution. Available at: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (Accessed January 6, 2024).
World Health Organization (2023). Overview of methods to assess population exposure to ambient air pollution. Geneva: World Health Organization. Available at: https://iris.who.int/bitstream/handle/10665/373014/9789240073494-eng.pdf?sequence=1 (Accessed January 6, 2024).
Keywords: air pollution monitoring, bioindicators, human biomarkers, alternative monitoring methods, cost-effective
Citation: Takano APC, Rybak J and Veras MM (2024) Bioindicators and human biomarkers as alternative approaches for cost-effective assessment of air pollution exposure. Front. Environ. Eng. 3:1346863. doi: 10.3389/fenve.2024.1346863
Received: 30 November 2023; Accepted: 19 January 2024;
Published: 31 January 2024.
Edited by:
Isidro A. Pérez, University of Valladolid, SpainReviewed by:
Burcu Onat, Istanbul University-Cerrahpasa, TürkiyeCopyright © 2024 Takano, Rybak and Veras. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ana Paula Cremasco Takano, YW5hdGFrYW5vQHVzcC5icg==
 Ana Paula Cremasco Takano
Ana Paula Cremasco Takano Justyna Rybak
Justyna Rybak Mariana Matera Veras
Mariana Matera Veras