- 1Department of Dermatology of First Affiliated Hospital, Institute of Dermatology, Anhui Medical University, Hefei, China
- 2Key Laboratory of Dermatology, Anhui Medical University, Ministry of Education, Hefei, China
- 3The Center for Scientific Research of Anhui Medical University, Hefei, China
Forty-nine susceptible loci have been reported to be significantly associated with vitiligo by genome-wide association studies (GWASs) in European-derived whites. To date, some of these reported susceptibility loci have not yet been validated in the Chinese Han population. The purpose of this study was to examine whether the 16 reported susceptible loci in European-derived whites were associated with vitiligo in the Chinese Han population. Imputation was performed using our previous GWAS dataset by IMPUTE v2.2.2. The 16 imputed top single-nucleotide polymorphisms (SNPs) with suggestive signals, together with the reported SNPs, were genotyped in a total of 2581 patients and 2579 controls by the Sequenom MassARRAY system. PLINK 2.0 software was used to perform association analysis. The dbSNP database, HaploReg, and eQTL data were adopted to annotate the biological function of the SNPs. Finally, four SNPs from three loci were significantly associated with vitiligo, including rs3747517 (P = 1.29 × 10–3, OR = 0.87) in 2q24.2, rs4807000 (P = 7.78 × 10–24, OR = 0.66) and rs6510827 (P = 3.65 × 10–5, OR = 1.19) in 19p13.3, and rs4822024 (P = 6.37 × 10–10, OR = 0.67) in 22q13.2. According to the dbSNP database, rs3747517 is a missense variant of IFIH1, rs4807000 and rs6510827 are located in TICAM1, and rs4822024 is located 6 kb upstream of TEF. Further bioinformatics analysis by HaploReg and eQTL found that rs4807000, rs6510827, and rs4822024 are involved in regulating gene expression. Our study revealed the strong association of 2q24.2 (rs3747517), 19p13.3 (rs4807000, rs6510827), and 22q13.2 (rs4822024) with the risk of vitiligo in the Chinese Han population, which implicates common factors for vitiligo across different ethnicities, and helps expand the understanding of the genetic basis of this disease.
Introduction
Vitiligo is an organ-specific autoimmune disease directed against melanocytes. It is characterized by whitish patches on the skin (Jin et al., 2019). The disease occurs in approximately 0.5–1% of the world population (Ezzedine et al., 2012). Most patients develop vitiligo before 40 years of age (Ezzedine et al., 2015). Vitiligo is also related to many other autoimmune diseases, in particular autoimmune thyroid disease, type 1 diabetes, and rheumatoid arthritis (Jin et al., 2019). The etiology of vitiligo remains elusive. There are several hypotheses, but an autoimmune etiology associated with specific genetic variants is still considered the leading theory (Iannella et al., 2016). It is believed that genetic components contribute to the onset of this disease (Spritz, 2012).
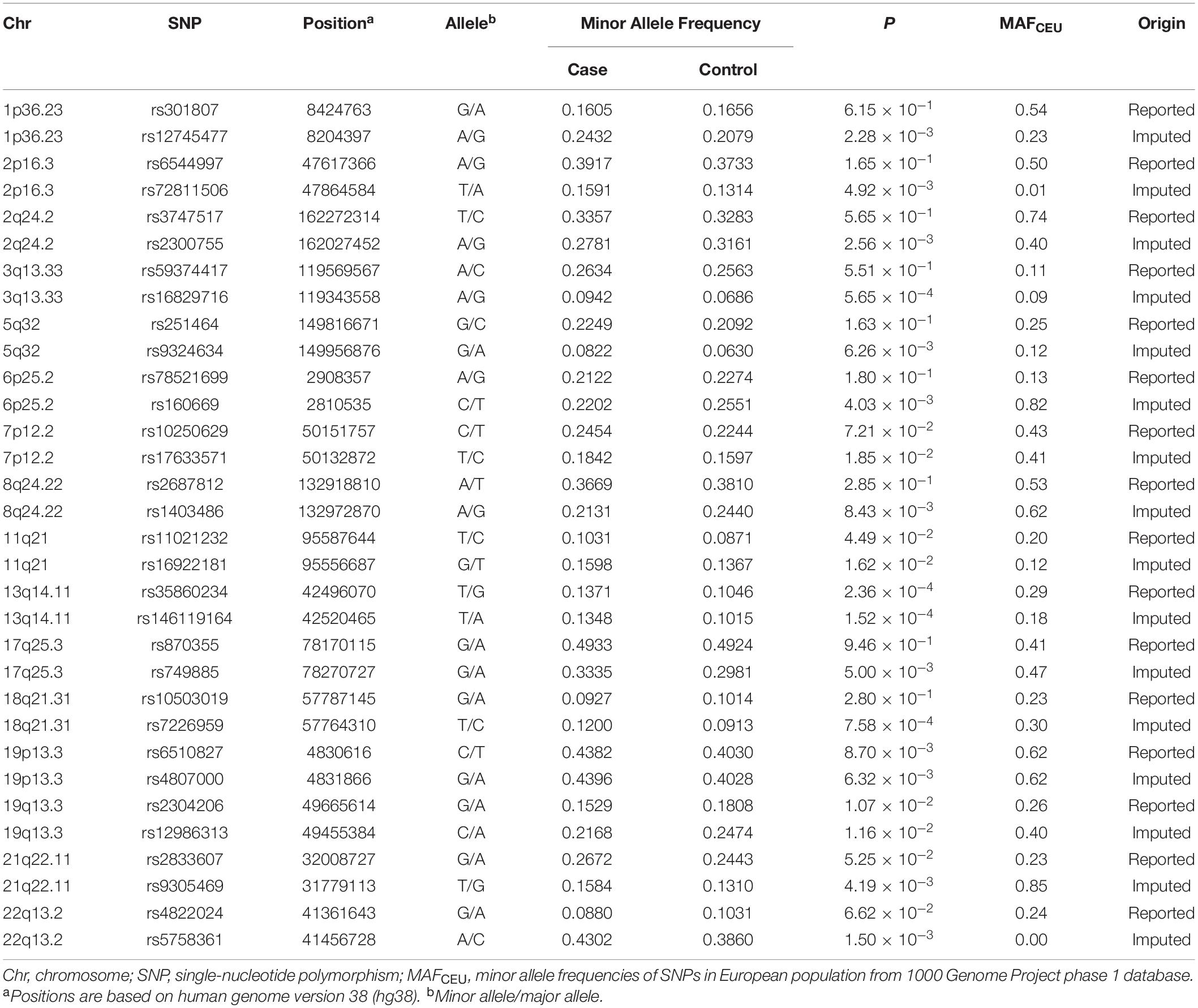
In 2010, we performed a genome-wide association study (GWAS) of vitiligo in the Chinese population, identifying 6p21.33, 6q27, and 10q22.3 as susceptible loci (Quan et al., 2010). Further two-stage replication studies identified three additional susceptibility loci at 10q22.1, 11q23.3, and 12q13.2 (Tang et al., 2013). From 2010 to 2018, the Spritz group carried out several large-scale GWASs in European-derived whites and discovered 49 novel susceptibility loci that contributed to vitiligo risk (Jin et al., 2010a,b, 2012, 2016; Ben et al., 2018). However, due to the genetic heterogeneity across different ethnicities, only some of these loci have been validated in the Chinese Han population, whereas others apparently have not been (Li et al., 2015; Xu et al., 2018; Tang et al., 2019). Of the 49 reported new susceptibility loci, we selected 16 single-nucleotide polymorphisms (SNPs) from 16 loci for validation after excluding those loci validated previously (Li et al., 2015; Xu et al., 2018; Tang et al., 2019). To investigate the association of the 16 loci with the risk of vitiligo, imputation of the 16 SNPs was conducted using our previous GWAS data on vitiligo, and the 16 imputed top SNPs together with the reported SNPs were genotyped in the Chinese Han population. Biological function annotation was performed to explain potential regulatory functions.
Materials and Methods
Study Subjects
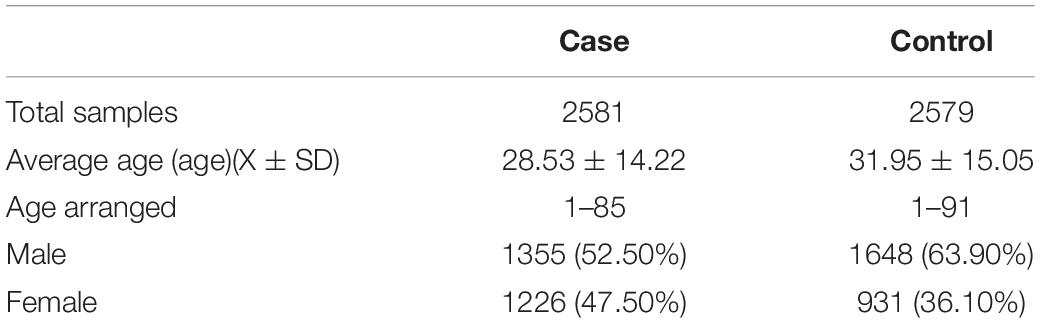
All samples were recruited in collaboration with multiple hospitals in China. A total of 2581 unrelated patients (1335 men and 1226 women) and 2579 matched controls (1648 men and 931 women) were recruited for this study (Table 1). The patients were all of ethnic Chinese Han origin and diagnosed by at least two experienced dermatologists. Recruited controls who did not meet the following criteria were excluded: (a) healthy individual without vitiligo, any other autoimmune diseases, or systemic disorders; (b) Han nationality; (c) no family history of vitiligo (including of first-, second- and third-degree relatives). Written informed consent was obtained from all subjects before collecting their clinical information and blood samples. The Ethics Committee of Anhui Medical University approved this study protocol, and the study was carried out according to the ethical standards of the Declaration of Helsinki.
SNP Selection for Replication
The genotype data of the 16 SNPs in adjacent upstream/downstream 250 kb regions were imputed from our previous vitiligo GWAS dataset (Quan et al., 2010). IMPUTE v2.2.2 was used to perform genotype imputation with the reference panel of the 1000 Genome Project phase 1 database (Howie et al., 2009). For quality control, SNPs that met the following criteria were included: (1) P-value of <10–3; (2) call rates >90%; (3) minor allele frequency (MAF) > 0.05 in controls; (4) P-value for Hardy–Weinberg equilibrium (PHWE) > 0.01 in controls. Then we selected the reported SNPs and the most significant SNPs in the16 loci (Table 2). Of those, the designs of the PCR primers for six SNPs failed. Finally, 26 SNPs within 16 loci were enrolled in the genotyping.
DNA Extraction and Genotyping
Genomic DNA was extracted from blood samples using FlexiGene DNA kits (QIAGEN, Hilden, Germany) according to the manufacturer’s protocol. After the concentration and purity were measured using a spectrophotometer, the 26 SNPs were genotyped using the Sequenom MassARRAY platform (Sequenom, San Diego, CA, United States). The procedures used for genotyping were presented in a previous study (Cai et al., 2019).
Statistical Analysis
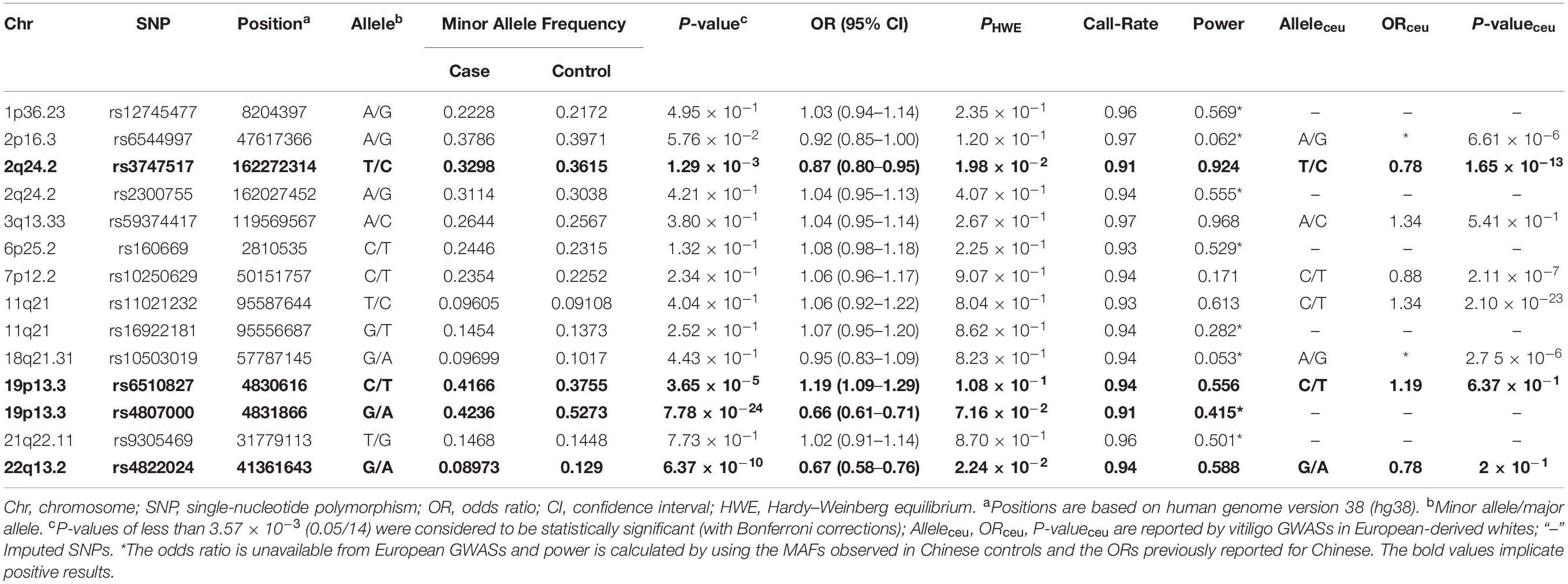
PLINK 2.0 software was used to calculate the allele frequency, P-values, odds ratios (OR), and Hardy–Weinberg equilibriums (HWE) for each SNP. The association of vitiligo with the SNPs was analyzed by comparing the MAF between patients and controls. The statistical power was calculated using Power and Sample Size Calculation software using either the sample size, the MAFs observed in the Chinese controls, and the ORs previously reported for European-derived whites, or the ORs in our GWAS and the imputed results for the Chinese Han when they were not provided for the white European population. For quality control, SNPs with a call rate < 90% and PHWE < 0.01 were excluded. A P-value after Bonferroni corrections of less than 3.57 × 10–3 (0.05/14) was regarded as statistically significant.
Bioinformatics Analysis
Several bioinformatics tools were utilized in this study. The Single Nucleotide Polymorphism database (dbSNP) was used for gene mapping.1 HaploReg4.12 was adopted to select the strongly linked SNPs and evaluate the potential biological significance for targeted SNPs. In addition, expression quantitative trait loci (eQTL) study data based on the GTEx database3 were adopted (GTEx Consortium, 2013).
Results
Association Between SNPs and Vitiligo
In this study, 26 selected SNPs from 16 loci were genotyped in 2581 vitiligo patients and 2579 healthy controls. Twelve SNPs were eliminated for not meeting the inclusion criteria (call rate < 90%, PHWE < 0.01), leaving 14 SNPs for analysis. Four SNPs showed significant associations with vitiligo in the independent Chinese Han cohort, including rs3747517 (P = 1.29 × 10–3, OR = 0.87, 95% CI: 0.80–0.95) at 2q24.2, rs4807000 (P = 7.78 × 10–24, OR = 0.66, 95% CI: 0.61–0.71) and rs6510827 (P = 3.65 × 10–5, OR = 1.19, 95% CI: 1.09–1.29) at 19p13.3, and rs4822024 (P = 6.37 × 10–10, OR = 0.67, 95% CI: 0.58–0.76) at 22q13.2 (Table 3). However, no evidence of a significant association was observed for any other SNPs.
Functional Annotation via Bioinformatics Analysis
The positive vitiligo SNPs rs4807000 and rs6510827 are in the strong linkage disequilibrium (LD) region (r2 = 0.99) of 19p13.3 (Supplementary Figure 1), separately in 140 bp upstream and intronic region of TICAM1. According to Haploreg v4.1, these two SNPs are in the area of enhancer histone H3K4mel in primary melanocyte cells and primary keratinocyte cells. The eQTL data extracted from GTEx show that they are associated with the expression of TICAM1 in whole blood (P = 1.70 × 10–36; P = 2.13 × 10–36, respectively). Moreover, rs4807000 is in the binding site of the NF-κB transcription factor from the ENCODE project.
Rs4822024 is located 1.5 kb downstream of ZC3H76, 6 kb upstream of thyrotroph embryonic factor (TEF), and 15 kb upstream of RNU6-495P in 22q13.2 (Supplementary Figure 2). This SNP shows a significant QTL effect on the expression level of TEF in the blood (P = 1.45 × 10–14) (GTEx Consortium, 2015) and is in the region of enhancer histone H3K4me1 in primary melanocyte cells according to Haploreg v4.1. Furthermore, the SNP also contains enrichment enhancer and promoter histone marks in epithelial cells, which suggests a transcriptional regulation function.
Gene mapping showed that rs3747517 is a missense variant in the interferon-induced helicase C domain 1 (IFIH1) gene by dbSNP (Supplementary Figure 3). As a vitiligo-protective variant, the functional analysis of rs3747517 has been addressed previously (Jin et al., 2017).
Discussion
Vitiligo is a common depigmenting skin disorder influenced by genetic and environmental factors. Our team conducted a series of genetic studies of vitiligo in the Chinese Han population. A GWAS of vitiligo was carried out in 2010. The 34 most promising SNPs in the MHC region were replicated, and one new risk locus was identified at 6q27, which contains three genes, RNASET2, FGFR1OP, and CCR6 (Quan et al., 2010). In 2013, the previous vitiligo GWAS was extended with a two-stage replication study, and 50 SNPs at 44 loci showing a suggestive association (Pinitial < 1 × 10–4 in GWAS) were selected for replication testing, which identified three susceptibility loci (12q13.2, 11q23.3, and 10q22.1) for vitiligo (Tang et al., 2013). In 2015, 12q13.2 was verified to be significantly associated with vitiligo accompanying immune-related diseases by testing 10 immune susceptibility SNPs (Li et al., 2015). In 2017, two newly identified SNPs (rs613791 and rs523604) showed independent signals in the associated locus 11q23.3 for vitiligo (Zhao et al., 2017). In 2019, 3q29 was demonstrated to be associated with vitiligo after genotyping 14 reported loci identified from a meta-analysis of GWAS in European-derived whites (Tang et al., 2019). In this study, 16 reported susceptible loci (1 × 10–3 > all Pinitial > 1 × 10–4 in our GWAS, located in non-MHC regions) in European-derived whites by GWAS were first tested to be associated with vitiligo in the Chinese Han population (Table 2), which revealed the strong association of 2q24.2 (rs3747517), 19p13.3 (rs4807000 and rs6510827), and 22q13.2 (rs4822024) with vitiligo.
SNP rs3747517 in 2q24.2 is a missense variant of IFIH1 encoding the IFIH1 protein, which can activate innate immune reaction to bind to damage-associated molecular patterns (Looney et al., 2015). Dysfunction of IFIH impairs the activation of downstream innate immune responses (Jin et al., 2017). SNPs rs4807000 and rs6510827 in 19p13.3 are located separately in 140 bp upstream and the intron of TICAM1 with significant LD (r2 = 0.99). TICAM1 regulates the innate immune reaction to viruses by inducing pattern recognition receptor–mediated IFN production (Seya et al., 2009). SNP rs4822024 in 22q13.2 is located 6 kb upstream of TEF, which encodes the TEF protein, and is associated with the expression level of TEF in blood (GTEx Consortium, 2015). TEF is a member of the proline and acidic amino-acid-rich basic leucine zipper (PAR bZip) transcription factor family, which plays a pivotal role in regulating circadian rhythm. The above loci may be involved in the pathogenesis of vitiligo through immune or other mechanisms.
The current findings revealed four SNPs in three loci that contribute to vitiligo susceptibility in Chinese Han individuals. However, we were unable to obtain evidence of an association for the other 10 SNPs. Aside from these 14 SNPs, 6 SNPs failed in the assay design, and 12 SNPs were eliminated for not meeting the quality-control criteria. The MAFs of these SNPs exhibited some difference between the Chinese Han (CHB) and European (CEU) cohorts (Table 2), and the correlation identified in the European population may not be well present in this study. Power analysis showed that the sample of 2581 patients and 2579 controls provided insufficient statistical power (<80%) for these negative SNPs except for rs59374417 in 3q13.33, which was not sufficient for detecting an association (Table 3), so larger sample sizes are needed in future studies. In addition, the difference in association evidence for rs59374417 between the Chinese Han and European-derived whites may implicate a genetic heterogeneity of vitiligo susceptibility between ethnic populations.
In this study, we tested the association of 16 susceptible SNPs for vitiligo identified in European-derived whites and the respective top imputed SNPs of candidate regions in a Chinese Han population. The negative results only indicated that these SNPs, not their LD region, were not related to vitiligo in the Chinese Han population. The differences in LD structure around the causal variants might result in distinct observations of associations in different populations. A more in-depth comparison of the LD structures in the candidate regions in Chinese Han versus European populations is necessary, either by targeted sequencing or fine genotyping in future studies.
In conclusion, the strong association of 2q24.2 (rs3747517), 19p13.3 (rs4807000 and rs6510827), and 22q13.2 (rs4822024) with the risk of vitiligo was demonstrated in a Chinese Han population, which implicates common factors for vitiligo across different ethnicities, and helps expand the understanding of the genetic basis of this disease.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Anhui Medical University. Written informed consent to participate in this study was provided by the participants.
Author Contributions
F-LX designed and supervised the study. LC wrote and revised the manuscript. X-YC helped to analyze the data. X-FT helped to select the SNPs. BL and HC enrolled the patients. LC, JiZ, MW-W, and JuZ conducted the experiments. X-DZ processed the data and performed statistical analysis. F-SZ and PL helped with genotyping. All authors contributed to the article and approved the submitted version.
Funding
The study was funded by Natural Science Foundation of Anhui Province (No. 1708085MH217), the National Natural Science Foundation of China (Nos. 81172838 and 81972926), and scientific research activities of academic and technological leaders of Anhui Province (No. 2017D141).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all the individuals and their families who participated in this project.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2020.542275/full#supplementary-material
Footnotes
- ^ http://www.ncbi.nlm.nih.gov/snp
- ^ https://pubs.broadinstitute.org/mammals/haploreg/haploreg.php
- ^ http://www.gtexportal.org/home/
References
Ben, S., Jin, Y., Santorico, S. A., and Spritz, R. A. (2018). Genome-wide association of PVT1 with vitiligo. J. Invest. Dermatol. 138, 1884–1886. doi: 10.1016/j.jid.2018.02.025
Cai, X. Y., Cheng, L., Yu, C. X., Wu, Y. Y., Fang, L., Zheng, X. D., et al. (2019). GWAS follow-up study discovers a novel genetic signal on 10q21.2 for atopic dermatitis in Chinese Han population. Front. Genet. 10:174. doi: 10.3389/fgene.2019.00174
Ezzedine, K., Eleftheriadou, V., Whitton, M., and van Geel, N. (2015). Vitiligo. Lancet 386, 74–84. doi: 10.1016/S0140-6736(14)60763-7
Ezzedine, K., Lim, H. W., Suzuki, T., Katayama, I., Hamzavi, I., Lan, C. C., et al. (2012). Revised classification/nomenclature of vitiligo and related issues: the Vitiligo Global Issues Consensus Conference. Pigment Cell Melanoma Res. 25, E1–E13. doi: 10.1111/j.1755-148X.2012.00997.x
GTEx Consortium (2013). The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 45, 580–585. doi: 10.1038/ng.2653
GTEx Consortium (2015). Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 348, 648–660. doi: 10.1126/science.1262110
Howie, B. N., Donnelly, P., and Marchini, J. (2009). A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 5:e1000529. doi: 10.1371/journal.pgen.1000529
Iannella, G., Greco, A., Didona, D., Didona, B., Granata, G., Manno, A., et al. (2016). Vitiligo: pathogenesis, clinical variants and treatment approaches. Autoimmun. Rev. 15, 335–343. doi: 10.1016/j.autrev.2015.12.006
Jin, Y., Andersen, G. H. L., Santorico, S. A., and Spritz, R. A. (2017). Multiple functional variants of IFIH1, a gene involved in triggering innate immune responses, protect against Vitiligo. J. Invest. Dermatol. 137, 522–524. doi: 10.1016/j.jid.2016.09.021
Jin, Y., Andersen, G., Yorgov, D., Ferrara, T. M., Ben, S., Brownson, K. M., et al. (2016). Genome-wide association studies of autoimmune vitiligo identify 23 new risk loci and highlight key pathways and regulatory variants. Nat. Genet. 48, 1418–1424. doi: 10.1038/ng.3680
Jin, Y., Birlea, S. A., Fain, P. R., Ferrara, T. M., Ben, S., Riccardi, S. L., et al. (2012). Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat. Genet. 44, 676–680. doi: 10.1038/ng.2272
Jin, Y., Birlea, S. A., Fain, P. R., Gowan, K., Riccardi, S. L., Holland, P. J., et al. (2010a). Variant of TYR and autoimmunity susceptibility loci in generalized vitiligo. N. Engl. J. Med. 362, 1686–1697. doi: 10.1056/NEJMoa0908547
Jin, Y., Birlea, S. A., Fain, P. R., Mailloux, C. M., Riccardi, S. L., Gowan, K., et al. (2010b). Common variants in FOXP1 are associated with generalized vitiligo. Nat. Genet. 42, 576–578. doi: 10.1038/ng.602
Jin, Y., Roberts, G. H. L., Ferrara, T. M., Ben, S., van Geel, N., Wolkerstorfer, A., et al. (2019). Early-onset autoimmune vitiligo associated with an enhancer variant haplotype that upregulates class II HLA expression. Nat. Commun. 10:391. doi: 10.1038/s41467-019-08337-4
Li, S., Yao, W., Pan, Q., Tang, X., Zhao, S., Wang, W., et al. (2015). Association analysis revealed one susceptibility locus for vitiligo with immune-related diseases in the Chinese Han population. Immunogenetics 67, 347–354. doi: 10.1007/s00251-015-0843-4
Looney, B. M., Xia, C. Q., Concannon, P., Ostrov, D. A., and Clare-Salzler, M. J. (2015). Effects of type 1 diabetes-associated IFIH1 polymorphisms on MDA5 function and expression. Curr. Diab. Rep. 15:96. doi: 10.1007/s11892-015-0656-8
Quan, C., Ren, Y. Q., Xiang, L. H., Sun, L. D., Xu, A. E., Gao, X. H., et al. (2010). Genome-wide association study for vitiligo identifies susceptibility loci at 6q27 and the MHC. Nat. Genet. 42, 614–618. doi: 10.1038/ng.603
Seya, T., Matsumoto, M., Ebihara, T., and Oshiumi, H. (2009). Functional evolution of the TICAM-1 pathway for extrinsic RNA sensing. Immunol. Rev. 227, 44–53. doi: 10.1111/j.1600-065X.2008.00723.x
Spritz, R. A. (2012). Six decades of vitiligo genetics: genome-wide studies provide insights into autoimmune pathogenesis. J. Invest. Dermatol. 132, 268–273. doi: 10.1038/jid.2011.321
Tang, X. F., Zhang, Z., Hu, D. Y., Xu, A. E., Zhou, H. S., Sun, L. D., et al. (2013). Association analyses identify three susceptibility loci for vitiligo in the Chinese Han population. J. Invest. Dermatol. 133, 403–410. doi: 10.1038/jid.2012.320
Tang, X., Fang, F., Yang, J., Zheng, X., Fan, M., Wang, L., et al. (2019). Association study reveals one susceptibility locus with vitiligo in the Chinese Han population. Genet. Test. Mol. Biomarkers 23, 791–796. doi: 10.1089/gtmb.2019.0129
Xu, M., Liu, Y., Liu, Y., Li, X., Chen, G., Dong, W., et al. (2018). Genetic polymorphisms of GZMB and vitiligo: a genetic association study based on Chinese Han population. Sci. Rep. 8:13001. doi: 10.1038/s41598-018-31233-8
Keywords: IFIH1, TEF, TICAM1, association study, single-nucleotide polymorphism, vitiligo
Citation: Cheng L, Liang B, Tang X-F, Cai X-Y, Cheng H, Zheng X-D, Zheng J, Wang M-W, Zhu J, Zhou F-S, Li P and Xiao F-L (2020) Validation of Susceptibility Loci for Vitiligo Identified by GWAS in the Chinese Han Population. Front. Genet. 11:542275. doi: 10.3389/fgene.2020.542275
Received: 12 March 2020; Accepted: 12 October 2020;
Published: 03 December 2020.
Edited by:
Robert Klein, Icahn School of Medicine at Mount Sinai, United StatesReviewed by:
Richard Spritz, University of Colorado, United StatesSolomon K. Musani, University of Mississippi Medical Center, United States
Copyright © 2020 Cheng, Liang, Tang, Cai, Cheng, Zheng, Zheng, Wang, Zhu, Zhou, Li and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng-Li Xiao, eGlhb2ZlbmdsaUAxMjYuY29t
†These authors have contributed equally to this work
 Lu Cheng
Lu Cheng Bo Liang1,2†
Bo Liang1,2† Xin-Ying Cai
Xin-Ying Cai Xiao-Dong Zheng
Xiao-Dong Zheng Fu-Sheng Zhou
Fu-Sheng Zhou Feng-Li Xiao
Feng-Li Xiao