- 1Department of Geriatric Cardiology, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, China
- 2Department of Operations Management, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, China
In recent years, the relationship between Cyp2C19*2 gene polymorphism and clopidogrel resistance reflected by platelet function assay has been studied extensively, but there is no clear conclusion yet. In order to evaluate the relationship between Cyp2C19*2 gene polymorphism and clopidogrel resistance more accurately, meta-analysis was conducted in this study. The I2 value taking 50% as the limit, the heterogeneity is judged as high or low, and then a random effect model or a fixed effect model is selected for statistical analysis. PubMed, EMBASE, Web of Science, CNKI, and China Wanfang database were searched, and the related literatures from the establishment of the database to May 2020 were collected and analyzed by STATA 15.0 software. A total of 3,073 patients were involved in 12 studies, including 1,174 patients with clopidogrel resistance and 1,899 patients with non-clopidogrel resistance. The results of this study showed that allele model (A vs. G): OR = 2.42 (95%CI: 1.97–2.98); dominant model (AA+GA vs. GG): OR = 2.74 (95%CI: 2.09–3.59); recessive model (AA vs. GA+GG): OR = 4.07 (95%CI: 3.06–5.41); homozygous model (AA vs. GG): OR = 5.70 (95%CI: 4.22–7.71); heterozygote model (GA vs. GG): OR = 2.32 (95%CI: 1.76–3.07), the differences were statistically significant. Also, the analysis of the Ethnicity subgroup indicated that the Asian allele model and the other four gene models were statistically significant. In conclusion, Cyp2C19*2 gene polymorphism is strongly associated with clopidogrel resistance. Allele A, genotype GA, AA, and GG + GA can increase clopidogrel resistance, especially in the Asian population.
Introduction
Coronary Atherosclerotic Herat Disease (CAHD), hereinafter referred to as Coronary Heart Disease (CHD), is a kind of heart disease caused by coronary atherosclerosis, which could induce vascular stenosis or obstruction, coronary circulation disturbance, myocardial ischemia, hypoxia and even necrosis. With the improvement of living standards, the incidence of coronary heart disease is increasing year by year. CHD has become one of the common diseases seriously affecting human health (Brown et al., 2016). At present, Percutaneous Coronary Intervention (PCI) is the main method for the treatment of CHD, but post-operative patients may develop stent thrombosis (ST), which is a stubborn problem of PCI. Some studies have indicated that 12 months after PCI, the incidence of ST is about 1.5% (Harnek et al., 2019). Antiplatelet therapy is an important treatment for CHD to reduce ST after PCI. Dual antiplatelet therapy with clopidogrel and aspirin is the standard therapy for PCI, which can greatly reduce the incidence of subacute thrombosis after PCI (Siasos et al., 2015). The mechanism of thrombosis is so complex that thrombotic events still occur in many post-PCI patients who receive clopidogrel combined with aspirin. As the methods of evaluating platelet function and the definition of antiplatelet drug resistance are different in different studies, the incidence of antiplatelet drug response variability also varies from study to study. Currently, most studies define clopidogrel resistance (CR) as a <10% ADP-induced decrease in the maximum platelet aggregation from the baseline level (Patel et al., 2019). There are many factors affecting the antiplatelet effect of clopidogrel, including age, diabetes, smoking and proton pump inhibitors (Nakagawa et al., 2016; Desai et al., 2017). Moreover, many studies suggest that the polymorphism of genes encoding related functional proteins is also an important factor leading to clopidogrel response variability. The different genotypes lead to different clopidogrel reactivity, hence resulting in different clinical events (Hokimoto et al., 2014; Xiao et al., 2017; Wu et al., 2018). Furthermore, the research on the gene polymorphism of clopidogrel response variability mainly focuses on the coding genes of related functional proteins in absorption, metabolic transformation and binding to the P2Y12 receptor. Among the genes researched, the study of gene polymorphisms affecting the metabolic transformation of clopidogrel has attracted the most attention. The relationship between CYP2C19 gene polymorphism and clopidogrel response variability is consistent in different studies, which may suggest of the important role of CYP2C19 in the two-step metabolic transformation of clopidogrel. The metabolic transformation of clopidogrel in the liver mainly goes through two cytochrome P.450 (CYP)-dependent steps: the first step produces 2.OXO. Clopidogrel is catalyzed by cytochrome Cyp2C19, CYPlA2, and CYP286 in different proportions, and the second step produces active metabolites catalyzed by cytochrome CYP3A4/5, CYP286, Cyp2C19, and CYP2C9 (Ford, 2016). Gene polymorphism amongst different individuals would vary in changes at the level of functional proteins, which thus influence the degree of active metabolites of clopidogrel, and eventually lead to differences in clopidogrel reactions. The variation in clopidogrel response caused by altering Cyp2C19*2 site and *3 site is the most concerned. It is now agreed that clopidogrel resistance occurs when drugs fail to achieve their desired pharmacological effects, which can be analyzed in the laboratory through a variety of platelet functions. So far, some meta-analyses have been conducted to determine the relationship between Cyp2C19*2 gene polymorphism and clinical outcomes, such as thrombosis or stroke (Jin et al., 2011; Pan et al., 2017). However, the researches on the relationship between Cyp2C19*2 gene polymorphism and clopidogrel resistance reflected by platelet function measurement are ongoing, and there is no clear conclusion. Most studies tend to believe that the variant of Cyp2C19*2, allele G → A, could increase clopidogrel resistance (Chen et al., 2010; Cuisset et al., 2011), but some new studies suggest that the variant of allele G → A has nothing to do with clopidogrel resistance (Amin et al., 2017). Therefore, in this study, a meta-analysis was conducted to evaluate the association between clopidogrel resistance and Cyp2C19*2 polymorphism in patients with coronary heart disease.
Materials and Methods
Literature Search
We performed this meta-analysis based on Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) (Moher et al., 2009) (Supplementary Table 1). The databases of PubMed, Excerpt Medica Database (EMBASE), Web of Science, National Knowledge Infrastructure (CNKI), China Wanfang were searched to collect relevant literature up to May 2020. The retrieval strategy is as follows: (“cytochrome P450 2C19” OR “Cyp2C19”) AND (“genetic polymorphism” OR “allele” OR “genotype” OR “polymorphism”) AND clopidogrel AND (“resistance” OR “platelet reactivity” OR “platelet response”). For studies with overlapping data or the same population, only the most recent group of subjects were included. There are no language restrictions. Literature retrieval was carried out independently, cross-checked by two researchers in each database. If there were differences, they could be resolved through discussion, or decided by the third researcher.
Inclusion and Exclusion Criteria
Inclusion Criteria
(1) The relationship between Cyp2C19*2 gene polymorphism and clopidogrel resistance; (2) The study was a cohort study or a case-control study; (3) Patients were diagnosed with coronary heart disease; (4) All patients received clopidogrel antiplatelet therapy; (5) The study should provide the number of patients with clopidogrel resistance and non-resistance of each genotype; (6) Diagnostic criteria of clopidogrel resistance: percentage of adenosine diphosphate inhibition ≤ 10%, MPA induced by adenosine diphosphate ≥ 50%, angiotensin converting enzyme index ≥ 50%, PRI ≤ 50%, PRU > 208, IPA < 30%.
Exclusion Criteria
(1) Studies belong to reviews, letters, or case reports; (2) Studies involve other definitions of clopidogrel resistance; (3) Repeatedly published data from the same study; (4) Non-coronary heart disease studies, such as ischemic stroke; (5) The control group does not meet the Hardy-Weinberg equilibrium (HWE); (6) NOS(Newcastle-Ottawa Scale) score is <6.
Data Extraction
The information was extracted as follows: first author, publication years, age, country, diagnostic criteria of clopidogrel resistance, clopidogrel load, genotype, and number of patients with clopidogrel resistance. The data were extracted independently by two researchers and discussed and solved by the third researcher when there was disagreement. When the included research information was insufficient, contacting author if possible.
Literature Quality Evaluation
After reading the literature carefully, the quality of the literature was evaluated according to The NOS (Stang, 2010). The literature with <6 stars was of low quality, and those with 6 stars or more were high quality literature. Only those with an evaluation of 6 stars and above were included in this study.
Statistical Analysis
Meta-analysis was performed with Stata 15.0 statistical software. Q-test was used to test the heterogeneity of the research results. If I2 ≥ 50%, or P ≤ 0.05 were considered heterogeneity, Random-effects model (REM) was used (Welton et al., 2007). If I2 < 50% and P > 0.05 were considered no heterogeneity, fixed-effects model (FEM) should be used for data merging (Leonard and Duffy, 2010). The significance of OR value was performed by Z test. We also made a subgroup analysis of Ethnicity, the year of publication, and the definition of clopidogrel resistance. This Meta-analysis included the evaluation of publication bias, using the funnel plot to determine whether it was symmetrical or not. If the funnel chart was asymmetric, publication bias may exist. Egger's Test was used to test the publication bias. Finally, sensitivity analysis was performed for the robust of results.
Results
Basic Information of Research Data
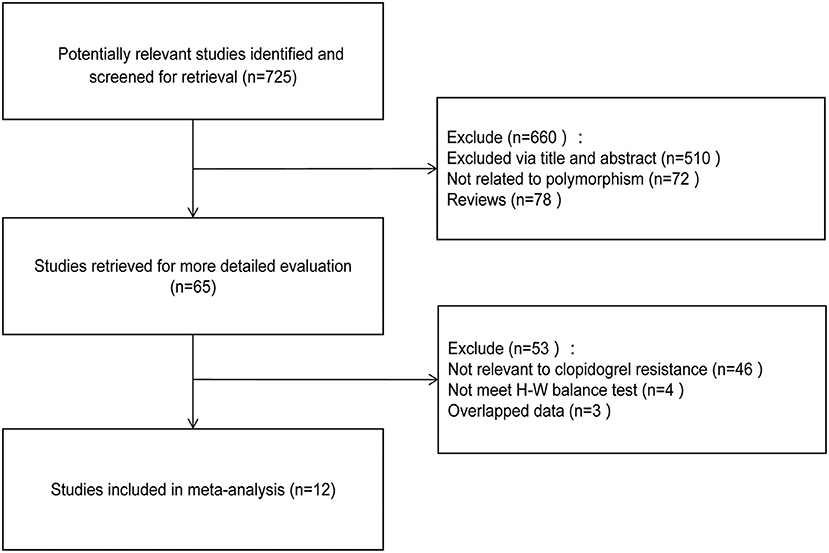
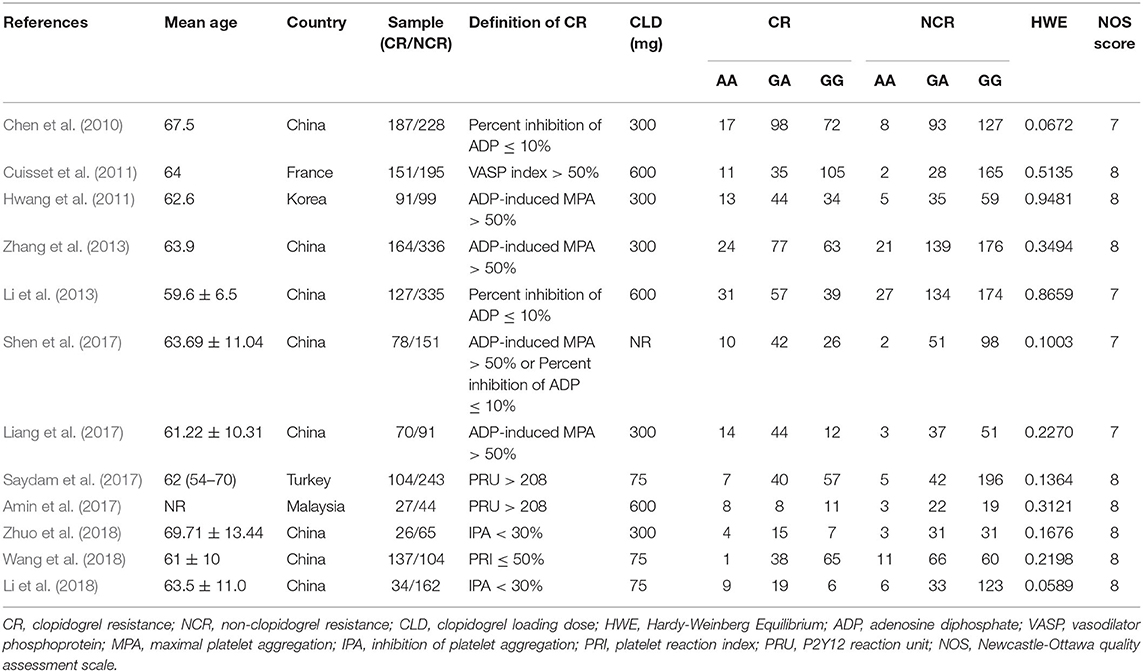
A total of 12 trials were selected according to the criteria (Chen et al., 2010; Cuisset et al., 2011; Hwang et al., 2011; Li et al., 2013, 2018; Zhang et al., 2013; Amin et al., 2017; Liang et al., 2017; Saydam et al., 2017; Shen et al., 2017; Wang et al., 2018; Zhuo et al., 2018), including 10 from Asian population and 2 from Caucasian population. A total of 3,073 subjects were involved, including 1,174 patients with clopidogrel resistance and 1,899 patients with non-clopidogrel resistance. The specific screening process can be found in Figure 1. The characteristics of each study and the genotype distribution reported in the study can be found in Table 1. The results of NOS quality evaluation of the literature were shown in Supplementary Table 2. Thus, it can be seen that the NOS scores of the studies included in this study were all above 6, which belonged to high-quality research.
Meta-Analysis Results
Allele Contrast
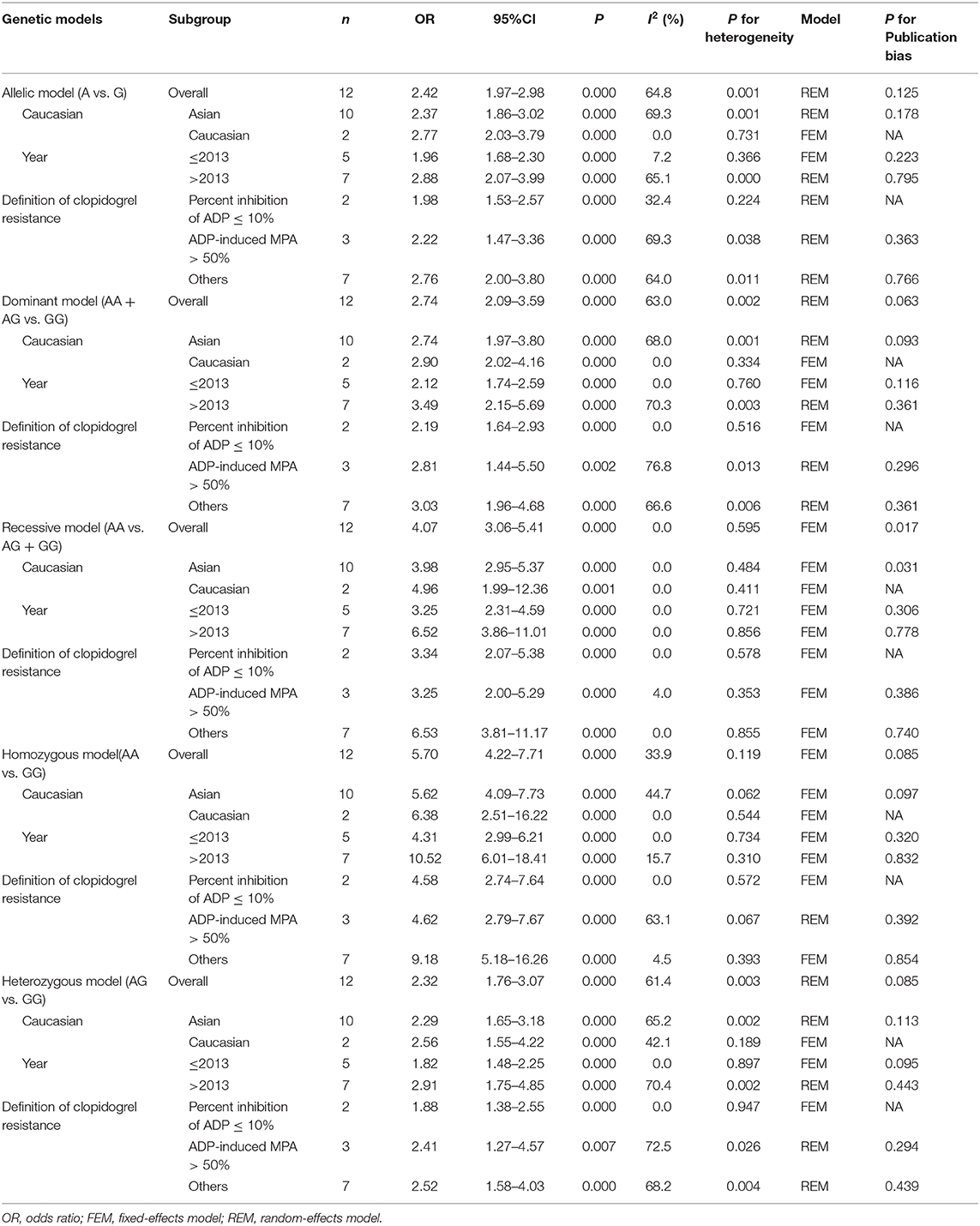
The main results of meta-analysis were shown in Table 2 and Figure 2. Compared A allele with G allele, I2 = 64.8%, P < 0.05, which indicated that the heterogeneity among studies was statistically significant, and random effect model was used. The final results showed that OR = 2.42 (95%CI: 1.97–2.98, P < 0.01), the difference was statistically significant. According to the Ethnicity subgroup analysis, the results showed that there was a significant difference in Asian population with OR = 2.37 (95%CI: 1.86–3.02, P < 0.01), and there was also statistical significance in Caucasian population shown in Figure 2A. This suggested that there was a correlation between Cyp2C19*2 polymorphism and clopidogrel resistance. The funnel plot in Figure 3A was basically symmetrical, and Egger's Test showed that P-value was >0.05, which indicated that there was no publication bias.
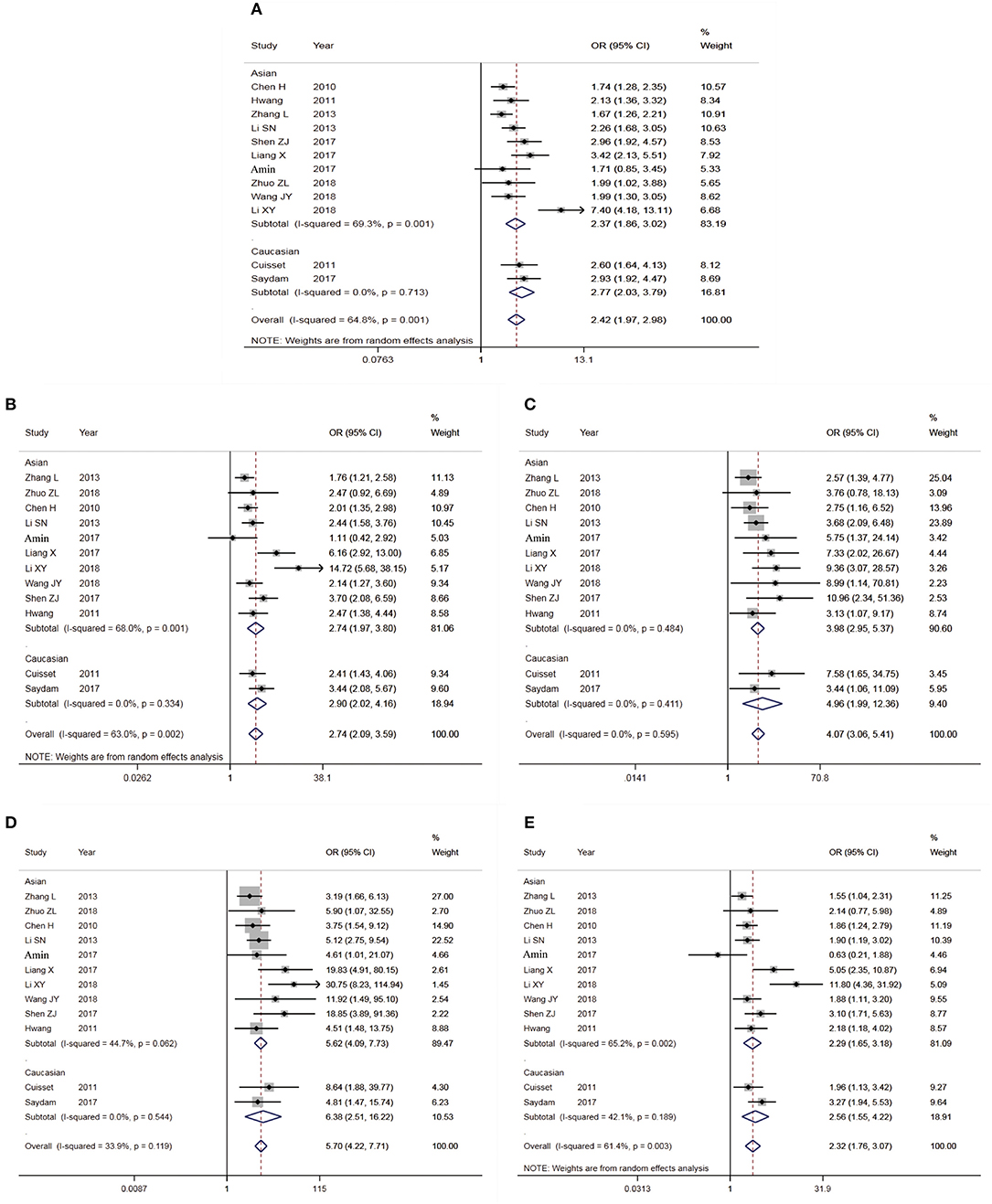
Figure 2. Forest plot for the association between cyp2c19*2 Polymorphism and Clopidogrel resistance (A: Allelic model; B: Dominant model; C: Recessive model; D: Homozygous model; E: Heterozygote model).
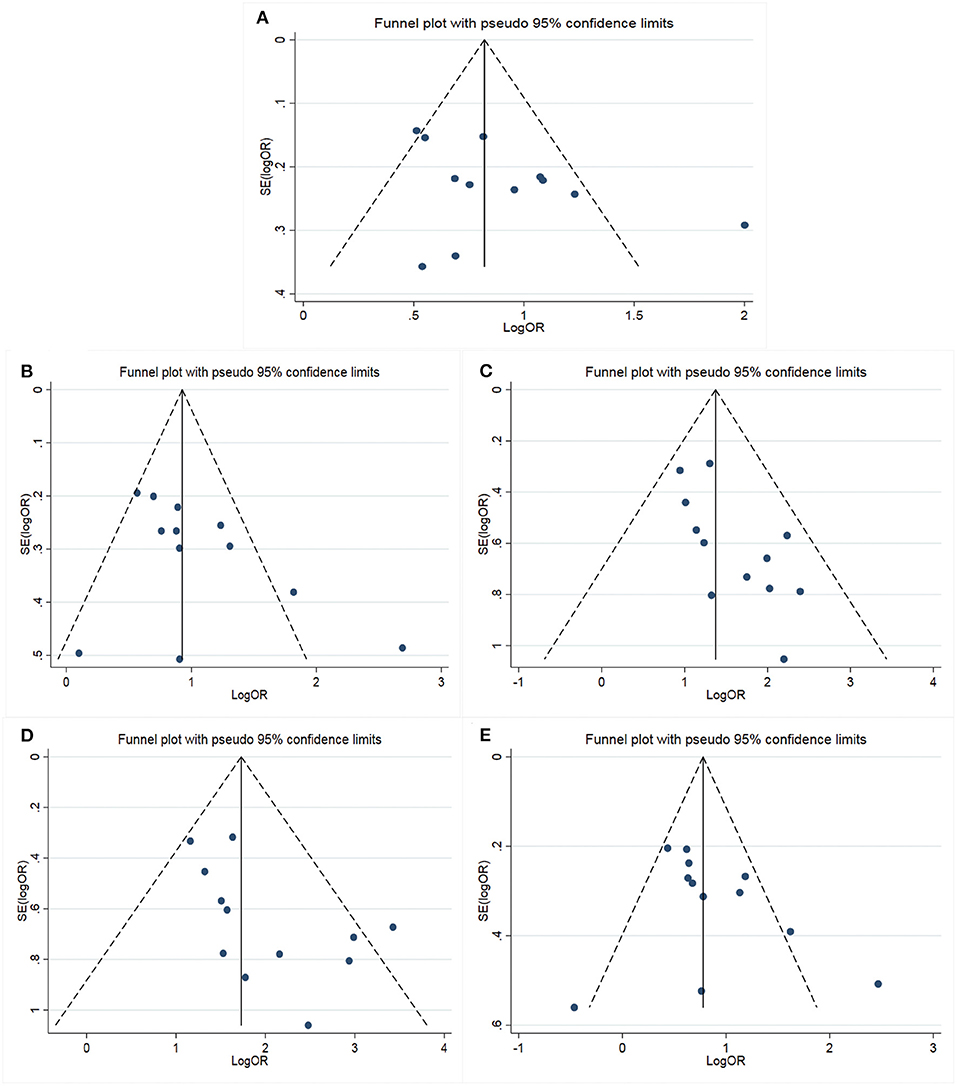
Figure 3. Funnel plot for the assessment of publication bias (A: Allelic model; B: Dominant model; C: Recessive model; D: Homozygous model; E: Heterozygote model).
Dominant Genetic Model
The genotype AA + GA vs. the genotype GG, I2 = 63.0%, P < 0.05, which indicated that the heterogeneity among the studies was statistically significant, and the random effect model was used. The results showed that there was a significant difference with OR = 2.74 (95%CI: 2.09–3.59, P < 0.01). The ethnic subgroup analysis showed the same results, the comparison of Caucasian and Asian dominant genetic models was statistically significant in Figure 2B. The funnel plot was basically symmetrical shown in Figure 3B, Egger's Test suggested that P-value was >0.05, which meant that the publication bias was well-controlled.
Recessive Genetic Model
The genotype AA was compared with genotype GA + GG, I2 = 0.0%, P > 0.05, which suggested that there was no statistical significance in the heterogeneity among the studies, and the fixed effect model was used. The results showed that there was a significant difference with OR = 4.07 (95%CI: 3.06–5.41, P > 0.05). The ethnic subgroup analysis indicated the same results, the comparison of Caucasian and Asian recessive genetic models was statistically significant, and was shown in Figure 2C. The symmetry of funnel plot showed bias (Figure 3C), Egger's Test showed P < 0.05, which indicated that there was a certain bias.
Homozygous Model
The genotype AA vs. GG, I2 = 33.9%, P > 0.05, which showed that there was no statistical significance in the heterogeneity among the studies, and the fixed effect model was used. The final results showed that there was a significant difference with OR = 5.70 (95%CI: 4.22–7.71, P < 0.01). The ethnic subgroup analysis indicated the same results, the comparison of Caucasian and Asian homozygous genetic models was statistically significant shown in Figure 2D. The funnel plot was basically symmetrical (Figure 3D), Egger's Test showed that P > 0.05, which suggested that the publication bias was well-controlled.
Heterozygote Model
The genotype GA compared with GG, I2 = 61.4%, P < 0.05, which showed that the heterogeneity among the studies was statistically significant, and the random effect model was used. The final results showed that there was a significant difference with OR = 2.32 (95%CI: 1.76–3.07, P > 0.05). The ethnic subgroup analysis suggested the same results, the comparison of Caucasian and Asian heterozygous genetic models was statistically significant, and the forest plot was shown in Figure 2E. The funnel plot was basically symmetrical (Figure 3E), Egger's Test showed that P > 0.05, the difference was not statistically significant, which indicated that the publication bias was well-controlled.
Subgroup Analysis of Year and CR Definition
The results of subgroup analysis of the published year (Table 2) showed that the differences were statistically significant in all genetic models. Moreover, the heterogeneity of studies before 2013 decreased significantly.
The results of subgroup analysis defined by CR (Table 2) showed that the differences were statistically significant in all genetic models. Moreover, when CR was defined as Percent inhibition of ADP ≤ 10%, the heterogeneity of the study decreased significantly.
Sensitivity Analysis
The results of the sensitivity analysis were shown in Supplementary Figures 1A–E. Each study was excluded one by one and meta-analysis was used. The results of the meta-analysis showed that the combined effect of the allele model and the other four gene models did not change significantly after the removal of a single study, indicating that the results were robust.
Discussion
The polymorphism of Cyp2C19 gene determines the difference of its enzymes among individuals, which affects the concentration of the metabolically active products and anti-platelet aggregation of clopidogrel (Watala et al., 2004). Brandt et al. (2007) found that the level of active metabolites of clopidogrel in people with defective Cyp2C19*2 gene was significantly lower than that in non-carriers after taking clopidogrel. Geisler et al. (2008) found that after the first administration of loading dose of clopidogrel, the degree of platelet aggregation induced by ADP was closely related to Cyp2C19 genotype, and the residual platelet aggregation (RPA) was significantly increased in patients with Cyp2C19*2 genotype. Pettersen et al. (2011) measured the platelet function of patients taking clopidogrel. By measuring P2Y12-PRU and VASP-PRI, it was observed that Cyp2C19*2 carriers had higher platelet activity than non-carriers. Lee et al. (2011) also obtained the same conclusion by testing P2Y12-PRU in his study. Previous studies have different definition of clopidogrel resistance, and there are no recognized indicators at home and abroad to define clopidogrel resistance. Currently, the definition of clopidogrel resistance is that the ADP-induced decrease in maximal platelet aggregation rate is <10% compared with baseline (Chen et al., 2010). Clopidogrel resistance used by Ma et al. (2010) was defined by flow cytometry with the platelet reactivity index of vasodilator-stimulated phosphoprotein (VASP-PRI), VASP-PRI >50%. Parodi et al. (2011) detected ADP-induced platelet aggregation in all patients, defined platelet aggregation rate >70% as high residual platelet reactivity (HRPR), platelet aggregation rate <70% as low residual platelet reactivity (LRPR), followed up for 2 years, compared with the LRPR group, the HRPR group had a higher incidence of ST and major end point events. In this study, multiple studies on the definition of clopidogrel resistance were integrated, and domestic and foreign studies on the correlation between Cyp2C19*2 gene polymorphism and clopidogrel resistance in coronary heart disease were collected to comprehensively analyze the relationship between them, so as to provide more in-depth evidence-based medicine for clinical practice.
According to the strict inclusion and exclusion criteria, this study included 12 high-quality literatures with a total of 3,073 subjects. The results showed that the allele model (A vs. G): OR = 2.42 (95%CI: 1.97–2.98), the difference was statistically significant. Dominant gene model, recessive gene model, homozygote model, and heterozygote model were also statistically significant. The funnel chart and Egger's Test results of publication bias showed that there was no publication bias. The results of subgroup analysis showed that the results of the Asian population were consistent with those of the total population, while the Caucasian population was inconclusive because only two articles were included. The results of subgroup analysis of different definitions of clopidogrel resistance and the year of publication showed that the differences were statistically significant in each genetic model of each subgroup. Sensitivity analysis results indicated that after excluding a single study, the meta-analysis results of the allele model and the other four gene models did not have a statistically significant change, indicating that the results were robust. Therefore, it could be considered that there is a strong association between Cyp2P19*2 polymorphism and clopidogrel resistance in patients with CHD, and the variant of allele G could increase clopidogrel resistance in antiplatelet therapy. This is basically consistent with the conclusion of a previous meta-analysis by Hou et al. (2014) including eight studies. Furthermore, compared with previous studies, this study is more stringent in the literature inclusion criteria, such as excluding the study that the control group does not conform to HWE, and including more latest studies. From the publication bias and stability results, the conclusions of this study are reliable and valuable.
In this study, several limitations should be noted: (1) Since there is no standard definition of clopidogrel resistance at present, multiple definitions of clopidogrel resistance were used in this study, which may weaken the comparability of the data; (2) In the subgroup analysis, the sample size is relatively small, and there are only 2 studies in the Caucasian population, so the conclusion need to be carefully adapted to the Caucasian population; (3) The lack of raw data also limits further assessment of potential gene-gene or gene-environment interactions.
In conclusion, Cyp2C19*2 gene polymorphism is associated with clopidogrel resistance reflected by platelet function assay, and the allele (A vs. G) increases clopidogrel resistance, which is reflected in the other four models, especially in the Asian population. This conclusion can be used to guide the individualized antiplatelet therapy of clopidogrel. Due to the limitations of this study, such as multiple definitions of clopidogrel resistance, gene-gene interaction, it is necessary to do more and more in-depth studies on Cyp2C19*2 gene polymorphism and antiplatelet therapy.
Author Contributions
YS, GY, and QL have given substantial contributions to the conception or the design of the manuscript, GY and YS to acquisition, analysis, and interpretation of the data. All authors have participated to drafting the manuscript, BC and GY revised it critically. All authors read and approved the final version of the manuscript.
Funding
This research was funded by a grant from the Key project of Science and Technology of Sichuan Province (Grant Number: 20ZDYF1567).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2020.576046/full#supplementary-material
Supplementary Figure 1. Sensitivity analysis results (A: Allelic model; B: Dominant model; C: Recessive model; D: Homozygous model; E: Heterozygote model).
Supplementary Table 1. PRISMA checklist.
Supplementary Table 2. Quality assessment of the studies included in this meta-analysis (NOS).
Abbreviations
CAHD, coronary atherosclerotic heart disease; CHD, coronary heart disease; CNKI, National Knowledge Infrastructure; CR, clopidogrel resistance; EMBASE, Excerpt Medica Database; FEM, fixed-effects model; HRPR, high residual platelet reactivity; HWE, Hardy-Weinberg equilibrium; LRPR, low residual platelet reactivity; NOS, Newcastle-Ottawa Scale; PCI, percutaneous coronary intervention; REM, random-effects model; ST, stent thrombosis; RPA, residual platelet aggregation; VASP-PRI, vasodilator-stimulated phosphoprotein.
References
Amin, A. M., Sheau Chin, L., Mohamed Noor, D. A., Mostafa, H., Abdul Kader, M. A. S. K., Kah Hay, Y., et al. (2017). The effect of CYP2C19 genetic polymorphism and non-genetic factors on clopidogrel platelets inhibition in East Asian coronary artery disease patients. Thromb. Res. 158, 22–24. doi: 10.1016/j.thromres.2017.07.032
Brandt, J. T., Close, S. L., Iturria, S. J., Payne, C. D., Farid, N. A., Ernest, C. S., et al. (2007). Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J. Thromb. Haemost. 5, 2429–2436. doi: 10.1111/j.1538-7836.2007.02775.x
Brown, R. A., Shantsila, E., Varma, C., and Lip, G. Y. (2016). Epidemiology and pathogenesis of diffuse obstructive coronary artery disease: the role of arterial stiffness, shear stress, monocyte subsets and circulating microparticles. Ann. Med. 48, 444–455. doi: 10.1080/07853890.2016.1190861
Chen, H., Yan, W., and Wu, X. Y. (2010). Relationships of blood stasis syndrome, cyp2c19 gene polymorphism with clopidogrel resistance and post-PCI prognosis. Chin. J. Integr. Trad. West Med. 30, 1245–1249.
Cuisset, T., Quilici, J., Cohen, W., Fourcade, L., Saut, N., Pankert, M., et al. (2011). Usefulness of high clopidogrel maintenance dose according to CYP2C19 genotypes in clopidogrel low responders undergoing coronary stenting for non ST elevation acute coronary syndrome. Am. J. Cardiol. 108, 760–765. doi: 10.1016/j.amjcard.2011.05.045
Desai, R. J., Spoendlin, J., Mogun, H., and Gagne, J. J. (2017). Contemporary time trends in use of antiplatelet agents among patients with acute coronary syndrome and comorbid diabetes mellitus or chronic kidney disease. Pharmacotherapy 37, 1322–1327. doi: 10.1002/phar.2018
Ford, N. F. (2016). The metabolism of clopidogrel: CYP2C19 is a minor pathway. J. Clin. Pharmacol. 56, 1474–1483. doi: 10.1002/jcph.769
Geisler, T., Sehaefeler, E., and Dippon, J. (2008). CYP2C19 and nongenetic factors predict poor responsiveness to clopidogrel loading dose after coronary stent implantation. Pharmacogenomics 9, 1251–1259. doi: 10.2217/14622416.9.9.1251
Harnek, J., James, S., and Lagerqvist, B. (2019). Coronary artery perforation and tamponade-incidence, risk factors, predictors and outcomes from 12 years' data of the SCAAR registry. Circ. J. 84, 43–53. doi: 10.1253/circj.CJ-19-0757
Hokimoto, S., Chitose, T., Mizobe, M., Akasaka, T., Arima, Y., Kaikita, K., et al. (2014). Impact of CYP3A5 polymorphism on platelet reactivity at percutaneous coronary intervention and after 9 months of aspirin and clopidogrel therapy in Japanese patients with coronary artery disease. Eur. J. Clin. Pharmacol. 70, 667–673. doi: 10.1007/s00228-014-1672-3
Hou, X., Shi, J., and Sun, H. (2014). Gene polymorphism of cytochrome P450 2C19*2 and clopidogrel resistance reflected by platelet function assays: a meta-analysis. Eur. J. Clin. Pharmacol. 70, 1041–1047. doi: 10.1007/s00228-014-1714-x
Hwang, S. J., Jeong, Y. H., Kim, I. S., Koh, J. S., Kang, M. K., and Park, Y. (2011). The cytochrome 2C19*2 and *3 alleles attenuate response to clopidogrel similarly in East Asian patients undergoing elective percutaneous coronary interven-tion. Thromb. Res. 127, 23–28. doi: 10.1016/j.thromres.2010.10.021
Jin, B., Ni, H. C., Shen, W., Li, J., Shi, H. M., and Li, Y. (2011). Cytochrome P450 2C19 polymorphism is associated with poor clinical outcomes in coronary artery disease patients treated with clopidogrel. Mol. Biol. Rep. 38, 1697–1702. doi: 10.1007/s11033-010-0282-0
Lee, J. B., Lee, K., and Lee, K. (2011). Cytochrome P450 2C19 polymorphism is associated with reduced clopidogrel response in cerebrovascular disease. Yonsei Med. J. 52, 734–738. doi: 10.3349/ymj.2011.52.5.734
Leonard, T., and Duffy, J. C. (2010). A Bayesian fixed effects analysis of the Mantel-Haenszel model applied to meta-analysis. Stats. Med. 21, 2295–2312. doi: 10.1002/sim.1048
Li, S. N., Liu, Z., Luo, Y., Chen, P. A., Lei, X. M., and Li, G. L. (2013). The effects of cytochrome P4502C19 genetic polymorphism on clopidogrel resistance and recent prognosis of patients with acute coronary syndrome. Chin. J. Intern. Med. 52, 961–965.
Li, X. Y., Wang, Z., Wang, Q. B., Xu, Q., and Lv, Q. Z. (2018). Clopidogrel-associated genetic variants on inhibition of platelet activity and clinical outcome for acute coronary syndrome patients. Basic Clin. Pharmacol. Toxicol. 124, 84–93. doi: 10.1111/bcpt.13110
Liang, X., Chen, S. C., and Xia, B. B. (2017). Study on the association between cytochrome P450 2 C19, PONl and ABCBl gene polymorphism and clopidogrel resistance. Chin. J. Clin. Pharmacol. 33, 585–588.
Ma, T. K. W., Lam, Y., Tan, V. P., Kiernan, T. J., and Yan, B. P. (2010). Impact of genetic and acquired alteration in cytochrome P450 system on pharmacologic and clinical response to clopidogrel. Pharmacol. Ther. 125, 249–259. doi: 10.1016/j.pharmthera.2009.10.008
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and PRISMA Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J. Clin. Epidemiol. 151, 264–269. doi: 10.7326/0003-4819-151-4-200908180-00135
Nakagawa, I., Park, H. S., Yokoyama, S., Wada, T., Hironaka, Y., Motoyama, Y., et al. (2016). Influence of diabetes mellitus and cigarette smoking on variability of the clopidogrel-induced antiplatelet effect and efficacy of active management of the target P2Y12 reaction unit range in patients undergoing neurointerventional procedures. J. Stroke Cerebrovasc. Dis. 25, 163–171. doi: 10.1016/j.jstrokecerebrovasdis.2015.09.010
Pan, Y., Chen, W., Xu, Y., Yi, X., Han, Y., Yang, Q., et al. (2017). Genetic Polymorphisms and clopidogrel efficacy for acute ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Circulation 135, 21–33. doi: 10.1161/CIRCULATIONAHA.116.024913
Parodi, G., Marcucci, R., Valenti, R., Gori, A. M., Migliorini, A., Giusti, B., et al. (2011). High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA 306, 1215–1223. doi: 10.1001/jama.2011.1332
Patel, S., Arya, V., Saraf, A., Bhargava, M., and Agrawal, C. S. (2019). Aspirin and clopidogrel resistance in indian patients with ischemic stroke and its associations with gene polymorphisms: a pilot study. Ann. Indian Acad. Neur. 22, 147–152.
Pettersen, A. A. R., Arnesen, H., Opstad, T. B., and Seljeflot, I. (2011). The influence of CYP 2C19*2 polymorphism on platelet function testing during single antiplatelet treatment with clopidogrel. Thromb. J. 9:4. doi: 10.1186/1477-9560-9-4
Saydam, F., Degirmenci, I., Birdane, A., Özdemir, M., Ulus, T, Özbayer, C, Çolak, E., Ata, N., et al. (2017). The CYP2C19*2 and CYP2C19*17 polymorphisms play a vital role in clopidogrel responsiveness after percutaneous coronary intervention: a pharmacogenomics study. Basic Clin. Pharmacol. 37:e48. doi: 10.1016/j.clinthera.2015.05.146
Shen, Z. J., Chen, X. K., Wang, Y. J., Guo, W., Chen, T. J., and Wang, X. L. (2017). Correlation between cyp2c19 gene polymorphism with clopidogrel resistance and distribution of chinese medicine syndrome in 229 acute coronary syndrome patients. Chin. J. Integr. Trad. West Med. 37, 291–296.
Siasos, G., Oikonomou, E., Zaromitidou, M., Kioufis, S., Kokkou, E., Mourouzis, K., et al. (2015). Clopidogrel response variability is associated with endothelial dysfunction in coronary artery disease patients receiving dual antiplatelet therapy. Atherosclerosis 242, 102–108. doi: 10.1016/j.atherosclerosis.2015.07.009
Stang, A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605. doi: 10.1007/s10654-010-9491-z
Wang, J. Y., Zhang, Y. J., Li, H., Hu, X. L., Li, M. P., Song, P. Y., et al. (2018). CRISPLD1 rs12115090 polymorphisms alters antiplatelet potency of clopidogrel in coronary artery disease patients in Chinese Han. Gene 678, 226–232. doi: 10.1016/j.gene.2018.08.027
Watala, C., Golanski, J., Pluta, J., Boncler, M., Rozalski, M., Luzak, B. A., et al. (2004). Reduced sensitivity of platelets from type 2 diabetic patients to acetylsalicylic acid (aspirin)—its relation to metabolic control. Thromb. Res. 113, 101–113. doi: 10.1016/j.thromres.2003.12.016
Welton, N., White, I., Lu, G., Higgins, J., Hilden, J., and Ades, A. (2007). The interpretation of random-effects meta-analysis in decision models. Med. Decis. Making 27:212. doi: 10.1177/0272989X07300428
Wu, Y., Zhou, Y., Pan, Y., Zhao, X., Liu, L., Wang, D., et al. (2018). Impact of CYP2C19 polymorphism in prognosis of minor stroke or TIA patients with declined eGFR on dual antiplatelet therapy: CHANCE substudy. Pharmacogenomics J. 18, 713–720. doi: 10.1038/s41397-018-0018-4
Xiao, F. Y., Liu, M., Chen, B. L., Cao, S., Fan, L., Liu, Z. Q., et al. (2017). Effects of four novel genetic polymorphisms on clopidogrel efficacy in Chinese acute coronary syndromes patients. Gene 623, 63–71. doi: 10.1016/j.gene.2017.04.029
Zhang, L., Chen, Y., Jin, Y., Qu, F., and Yin, T. (2013). Genetic determinants of high on-treatment platelet reactivity in clopidogrel treated Chinese patients. Thromb. Res. 132, 81–87. doi: 10.1016/j.thromres.2013.05.006
Keywords: Cyp2C19, polymorphism, clopidogrel resistance, meta-analysis, coronary heart disease
Citation: Sun Y, Lu Q, Tao X, Cheng B and Yang G (2020) Cyp2C19*2 Polymorphism Related to Clopidogrel Resistance in Patients With Coronary Heart Disease, Especially in the Asian Population: A Systematic Review and Meta-Analysis. Front. Genet. 11:576046. doi: 10.3389/fgene.2020.576046
Received: 25 June 2020; Accepted: 30 November 2020;
Published: 22 December 2020.
Edited by:
Chonlaphat Sukasem, Mahidol University, ThailandReviewed by:
Chakradhara Rao Satyanarayana Uppugunduri, Université de Genève, SwitzerlandSurasak Saokaew, University of Phayao, Thailand
Copyright © 2020 Sun, Lu, Tao, Cheng and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guoxing Yang, c3BwaHlhbmdndW94aW5nQDE2My5jb20=; Biao Cheng, c3BwaGNoZW5nYmlhb0AxNjMuY29t
†These authors have contributed equally to this work
 Ying Sun
Ying Sun Qing Lu1†
Qing Lu1† Guoxing Yang
Guoxing Yang