Abstract
Background: Melanoma genetic testing reportedly increases preventative behaviour without causing psychological harm. Genetic testing for familial melanoma risk is now available, yet little is known about dermatologists’ perceptions regarding the utility of testing and genetic testing ordering behaviours.
Objectives: To survey Australasian Dermatologists on the perceived utility of genetic testing, current use in practice, as well as their confidence and preferences for the delivery of genomics education.
Methods: A 37-item survey, based on previously validated instruments, was sent to accredited members of the Australasian College of Dermatologists in March 2021. Quantitative items were analysed statistically, with one open-ended question analysed qualitatively. Results: The response rate was 56% (256/461), with 60% (153/253) of respondents between 11 and 30 years post-graduation. While 44% (112/252) of respondents agreed, or strongly agreed, that genetic testing was relevant to their practice today, relevance to future practice was reported significantly higher at 84% (212/251) (t = -9.82, p < 0.001). Ninety three percent (235/254) of respondents reported rarely or never ordering genetic testing. Dermatologists who viewed genetic testing as relevant to current practice were more likely to have discussed (p < 0.001) and/or offered testing (p < 0.001). Respondents indicated high confidence in discussing family history of melanoma, but lower confidence in ordering genetic tests and interpreting results. Eighty four percent (207/247) believed that genetic testing could negatively impact life insurance, while only 26% (63/244) were aware of the moratorium on using genetic test results in underwriting in Australia. A minority (22%, 55/254) reported prior continuing education in genetics. Face-to-face courses were the preferred learning modality for upskilling.
Conclusion: Australian Dermatologists widely recognise the relevance of genetic testing to future practice, yet few currently order genetic tests. Future educational interventions could focus on how to order appropriate genetic tests and interpret results, as well as potential implications on insurance.
1 Introduction
Availability of genetic testing for hereditary cancers has increased in the last 2 decades (Collins and McKusick, 2001; Brittain et al., 2017; Williams, 2019). However, the integration of genetic testing into routine clinical practice remains low (Cornel, 2019; Johnson et al., 2020; Best et al., 2021). Traditionally, clinical genetic testing is offered by a qualified genetic counsellor or clinical geneticist (Badenas et al., 2012). Recently, given the rising demand for genomic services (Frost et al., 2019), and the limited clinical genetic workforce (Hoskovec et al., 2018; McCuaig et al., 2018), genetic care has been integrated into non-genetics clinics in a process known as “mainstreaming”. This has increasingly occurred in oncology care for hereditary breast, ovarian, colorectal and endometrial cancer (McCuaig et al., 2018; O'Shea et al., 2021). Previous mainstreaming interventions have demonstrated significant positive outcomes such as increased access to genetic testing and identification of hereditary cancer cases (Heald et al., 2013; Miesfeldt et al., 2018), as well as decreased health care costs (George et al., 2016; Plaskocinska et al., 2016; Kemp et al., 2019) and wait times for appointments and results (Heald et al., 2013; George et al., 2016; Plaskocinska et al., 2016; Kentwell et al., 2017; Rahman et al., 2019; Richardson et al., 2020; Rumford et al., 2020). This suggests that non-genetics practitioners are capable of understanding and ordering genetic tests for their patients.
We propose that there is an opportunity for mainstreaming familial melanoma genetic testing into dermatology practice as the genetics are well understood (Leachman et al., 2017; Abdo et al., 2020; Toussi et al., 2020). CDKN2A, accounts for 20%–40% of hereditary melanoma cases and 90% of positive results (Aoude et al., 2015; Potrony et al., 2015; Read et al., 2016). However, several other genes are associated with familial melanoma including BAP1, BRCA2, CDK4, MC1R, MITF, POLE, POT1, PTEN, RB1, TERT and TP53 (Ribeiro Moura Brasil Arnaut et al., 2021; Maas et al., 2022). A pathogenic mutation in these genes can incur a lifetime risk of melanoma of up to 84% (Box et al., 2001), compared to the national Australian average of approximately 5% (Duffy et al., 2019). Recent reviews on the impact of familial melanoma genetic testing have shown a positive effect on protective behaviours (Primiero et al., 2021a), without adverse psychological outcomes (Primiero et al., 2021b). Longitudinal studies have reported improved Sun protection including decreased sunburns and Sun exposure, and increased adherence to regular clinical skin examinations, for up to 2 years after receiving genetic testing for CDKN2A mutations (Aspinwall et al., 2008; Aspinwall et al., 2014; Stump et al., 2020). Not only is increased screening/surveillance in high-risk individuals cost affective (Guitera et al., 2021) but it is associated with earlier detection and improved outcomes i.e., morbidity and mortality (Gordon and Rowell, 2015). Dermatologists are well placed to identify patients who may benefit from genetic testing (Zhou et al., 2021) and customise subsequent screening recommendations accordingly (Zhou et al., 2021). Given the success of previous mainstreaming efforts in other specialist settings (Talwar et al., 2017; Kohut et al., 2019), we envision that genetic testing for familial melanoma could feasibly become standard dermatological practice. We note that a pilot program to upskill clinicians to provide genetic testing for familial melanoma is currently being trailed in a small cohort of clinicians (Primiero et al., 2022). Future integration of a mainstreaming intervention should be guided by an implementation theory framework, such as Procter’s implementation outcome framework (Proctor et al., 2011), or the Consolidated Framework for Implementation Research (CFIR) (Damschroder et al., 2009). Both frameworks describe the importance of engaging key stakeholders to gauge receptibility, attitudes and experience of a new intervention. They can also be used to evaluate the efficacy and sustainability of the intervention.
Previous studies investigating attitudes and preparedness for genetic testing in other settings have largely targeted general (family) practitioners (Carroll et al., 2009; Nippert et al., 2011a; Bouhnik et al., 2017; Diamonstein et al., 2018; Carroll et al., 2019; Haga et al., 2019; Harding et al., 2019; DeLuca et al., 2020), obstetricians-gynaecologists (Nippert et al., 2011a; Douma et al., 2016; Bouhnik et al., 2017; Diamonstein et al., 2018; Kathrens-Gallardo et al., 2021), oncologists (Douma et al., 2016; Bouhnik et al., 2017; Chow-White et al., 2017) and paediatricians (Nippert et al., 2011a; Johnson et al., 2017; Diamonstein et al., 2018). To date, evaluation of dermatologists’ views on genomic medicine has been limited to paediatric dermatologists (Shagalov et al., 2017), trainees (Murphy, 2015), and fellowship program directors (Torre et al., 2018). These studies report an increasing recognition of the relevance of genetic testing to their speciality, but also a deficit in training and education (Chow-White et al., 2017; Johnson et al., 2017; Diamonstein et al., 2018; Torre et al., 2018; Crellin et al., 2019; Harding et al., 2019). The current study evaluates the present perception, use, and confidence in using genetic testing in dermatology practice in Australia, including preferences for the delivery of future education interventions. In this survey we refer to both “genetic/genomic” testing. However, in this manuscript we will refer to both as “genetic testing”.
2 Methods
A cross-sectional survey was posted to all Australian Dermatologists to capture current confidence and attitudes regarding genetic testing, and guide future education interventions. Specifically, we were interested in predictors of genetic confidence, and the relationship between attitudes towards genetic testing and current practice.
The capability, opportunity, and motivation model of behaviour (COM-B) was the guiding framework in the survey design. The COM-B model considers three interacting constructs relating to the uptake of a new practice including; 1. Capability (knowledge and competence), 2. Opportunity (resources), and 3. Motivation (perceived benefit of genetic testing) (Michie et al., 2011; McClaren et al., 2020). This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
2.1 Survey development
The survey was structured into four sections, using previously validated scales and purpose-designed items. Section 1 measured attitudes regarding the relevance of genetic testing to current and future dermatology practice, using a five-point Likert scale. These questions were adapted from an original 4-item instrument developed for community-based physicians, by using only the first two items, and replacing “genomic medicine” with “genetic testing” (Reed et al., 2016). Section 2 captured genetic experience and confidence domains with three validated scales: an 8-item scale, adapted from a 14-item validated scale (Bouhnik et al., 2017) by referring to “genetic testing for melanoma”, instead of BRCA1 or BRCA2 testing, and selecting questions relevant to the potential benefits and limitations of genetic testing for familial melanoma (Agree/Disagree); a 5-item scale (Culver et al., 2001) assessing whether respondents had previously performed certain genetic skills in practice (Yes/No); and a 11-item, five-point Likert Scale (Not at all confident/Very confident) (Reed et al., 2016) with one additional question regarding patient consent, required respondents to rate their confidence in performing specific genetic skills (Cronbach’s Alpha = 0.90). Section 3 used purpose-designed questions to capture education preferences (rank 1–5), previous continuing education in genetics/genomics (Yes/No), and perceived usefulness of previous training (Scale 1–10, where 1 was “Not at all useful” and 10 was “Very useful”). Section 4 collected demographics, frequency of ordering genetic testing, and patient enquiries about genetic testing. Finally, an open-ended question invited qualitive feedback regarding their attitudes towards genetic testing in dermatology (see Supplementary Material S1 for survey). The final survey was piloted in two dermatologists for feedback prior to wider distribution.
2.2 Study population
In March 2021 a survey was posted to all Australian members of the Australasian College of Dermatologists (ACD), excluding trainees as no site of employment was available for them. Contact details were accessible for only one member in New Zealand, therefore only Australian members were included. A paper-based survey was administered, to allow the inclusion of an AUS$10 cash incentive, as incentives have previously been shown to increase physician response rates (James et al., 2011; Noel and Huang, 2019; Demeshko et al., 2020). The ACD included a notification in their e-newsletter prior to the survey mailing. Two weeks after survey mailing, the ACD emailed the membership to remind them to complete the survey and provided a link to an online version of the survey. The online survey tool was hosted by The University of Queensland using Checkbox®. A waiver of consent was obtained as consent was considered implied if participants chose to complete the survey. Human Research Ethics was approved by The University of Queensland HREC (ref: 2020002658) and ratified by the University of Technology Sydney HREC (ref: ETH20-5671).
2.3 Data analysis
Surveys were transcribed in a text-delimited format and analysed using IBM SPSS Statistical Package (Corp, 2020). Descriptive statistics were used to summarise participant characteristics. Confidence ratings for individual genetic skills were consolidated to create a cumulative average genetic confidence score (out of 5). Simple linear regression identified variables associated with overall genetic confidence. An unpaired Student t-test compared attitudes towards relevancy of genetic testing in current and future practice. Chi-squared analysis (or Fisher’s Exact Test if the “n” was <5 in any of the cells) compared current and future attitudes towards the relevancy of genetic testing and the likelihood of having previously discussed, offered, ordered, or referred for genetic tests. p-values of <0.05 were considered significant and reported with 95% confidence intervals (CI). Qualitative responses to the open-ended question were evaluated using thematic content analysis methods (Bengtsson, 2016) employing NVivo 12 Plus to manage transcripts, as well as code and compile inferences from responses.
3 Results
3.1 Sample demographics
A total of 494 surveys were posted; 34 were returned unopened and deemed ineligible. Of the 461 eligible surveys, 247 were returned, and a further nine were completed online, giving a response rate of 56%. Demographic characteristics of respondents are displayed in Table 1. Most respondents were from either New South Wales (35%), Victoria (22%), or Queensland (20%), which encompass Australia’s three most populous cities. Over half the respondents were either 11–20 years (33%), or 21–30 years post-graduation (27%). A third of respondents reported having a sub-specialty.
TABLE 1
| Variable | n (%) |
|---|---|
| Survey format | 256 |
| Paper | 247 (96) |
| Online | 9 (4) |
| Location (within Australia/New Zealand) | 255 |
| New South Wales | 87 (34) |
| Victoria | 59 (23) |
| Queensland | 54 (21) |
| Western Australia | 28 (11) |
| South Australia | 21 (8) |
| Australian Capital Territory | 3 (<1) |
| Tasmania | 2 (<1) |
| Northern Territory | 1 (<1) |
| Years since graduated from medical school | 253 |
| 1–10 years | 15 (6) |
| 11–20 years | 86 (34) |
| 21–30 years | 67 (26) |
| 31–40 years | 47 (19) |
| 40 > years | 38 (15) |
| Reported Sub-Speciality in Dermatology | 250 |
| Yes | 84 (34) |
| Paediatric Dermatology | 26 (31) |
| Mohs and Dermatologic Surgery | 21 (25) |
| Oncology Dermatology | 8 (10) |
| Other | 29 (35) |
Participant demographic characteristics (n = 256).
Bold values represent the n that provided a response to that subset of questions.
3.2 Current role of genetic testing in dermatology
When asked if they have ever discussed, offered, or referred people for genetic testing, the majority (96%, n = 246) reported having previously discussed it; 91% had offered it, 89% had referred patients for it, and 60% had previously ordered tests. When asked how frequently genetic testing was ordered, 29% reported never ordering, 63% ordered rarely (≤5 times a year), 6% often (≥ once a month), and only 1% (n = 3) routinely (≥ once a week). Only 19% indicated they had the “necessary services and staff” to offer genetic testing. Respondents who indicated they “never” or “rarely” ordered genetic testing, were subsequently asked, “why not?”, and provided with a list of common reasons to select from, including an open field for “other”. Of note, 22% of respondents reported that their lack of confidence contributed to their infrequent ordering. Results are displayed in Table 2.
TABLE 2
| Previous performed genetic tasks | n = 256 (%) |
|---|---|
| I have discussed genetic testing with my patients | 246 (96) |
| I have offered genetic testing to my patients | 232 (91) |
| I have ordered genetic testing for my patients | 153 (60) |
| I have referred my patients to clinical genetic services | 226 (89) |
| I have the necessary resources to offer genetic testing to patients | 49 (19) |
| Frequency of ordering genetic/genomic testing | n = 254 (%) |
| Never | 74 (29) |
| Rarely (≤5 times a year) | 161 (63) |
| Often (≥ once a month | 16 (6) |
| Routinely (≥ once a week) | 3 (1) |
| Reasons for not ordering genetic testing more frequently | n = 235 (%) |
| Not relevant to my practice | 81 (34) |
| I do not feel confident | 51 (22) |
| It is not my role | 20 (9) |
| I do not have access to a genetic service | 28 (12) |
| I do not have time | 2 (<1) |
| Other (categorised below) | 65 (28) |
| Refer patients to genetic services | 29 (12) |
| Genetic testing irrelevant for my patients | 23 (10) |
| Lack of information/knowledge on available genetic testing | 6 (3) |
| Concerns regarding costs | 5 (2) |
| Lack of time to discuss/order genetic testing | 2 (<1) |
| Concerns about impact on insurance | 1 (<1) |
Current role of genomic medicine for any dermatological condition, including previously-performed genetic tasks, frequency of ordering genetic/genomic testing, and reasons for infrequent ordering.
Bold values represent the n that provided a response to that subset of questions.
3.3 Attitudes towards genetic testing in dermatology
While 44% of respondents agreed/strongly agreed that genetic testing was relevant to current practice, 85% agreed/strongly agreed that genetic testing will be relevant to future practice (t = -9.82, p < 0.001).
Participants were asked whether they agreed or disagreed with possible benefits and limitations of genetic testing for melanoma. All statements relating to benefits of genetic testing had very strong agreeance, with at least 90% agreeing that genetic testing for melanoma could be of value to the patient, their family, for management decisions, and/or to improve primary/secondary prevention (Table 3).
TABLE 3
| Perceptions on genetic testing for melanoma in dermatology | “Yes”/total (%)a |
|---|---|
| Benefits of Genetic testing | |
| Genetic testing for melanoma could be of value to the patient | 235/252 (93%) |
| Genetic testing for melanoma could be of value to the patient’s family | 242/253 (96%) |
| Genetic testing for melanoma could be of value in informing management decisions | 226/252 (90%) |
| Genetic testing for melanoma could be of value in improving primary/secondary prevention | 224/250 (90%) |
| Limitations of Genetic testing | “Agree”/total (%)b |
| Genetic testing for melanoma could limit the patient’s private health insurance coverage | 196/247 (79%) |
| Genetic testing for melanoma could limit the patient’s life insurance coverage | 207/247 (84%) |
| Awareness that a moratorium was introduced on the use of genetic testing in life insurance underwriting in July 2019 | 63/244 (26%) |
| Genetic testing for melanoma could stigmatise the patients as a “worried well” person | 145/252 (58%) |
Respondent’s perceptions on benefits, and limitations of genetic testing in dermatology.
Yes/No options. Number of those answering “Yes” divided by total number who answered the question.
Agree/Disagree options. Number of those answering “Agree” divided by total number who answered the question.
Most respondents also agreed with the statements on perceived limitations of genetic testing; 79% believed it could limit access to private health insurance, 84% agreed that it could limit access to life insurance, and 58% felt that melanoma genetic testing could stigmatise individuals as a “worried well”.
Participants who viewed genetic testing as relevant to their current practice were significantly more likely to have discussed (p < 0.001, Fisher’s Exact Test) or offered it to patients, X2 (1, N = 256) = 17.5, p < 0.001, ordered testing, X2 (1, N = 256) = 6.8, p = 0.009, and referred patients to genetic services, X2 (1, N = 256) = 9.3, p = 0.002.
3.4 Confidence in basic genetic tasks
High confidence was reported for collecting family history (mean score 4.3/5; CI 4.2–4.4), and identifying patients at risk of a hereditary condition (4.2/5; CI 4.1–4.3). Moderate confidence was reported for identifying patients who could benefit from genetic testing (3.5/5; CI 3.4–3.7), educating patients on genetic aetiology of disease (3.4/5; CI 3.3–3.5), obtaining informed consent (3.3/5; CI 3.1–3.4), discussing possible outcomes of genetic testing (3.2/5; CI 3.0–3.3), and using genetic information in management decisions (3.0/5; CI 2.9–3.2). Lower confidence was reported for identifying appropriate tests (2.6/5; CI 2.5–2.8), test ordering (2.5/5; CI 2.4–2.7), and interpreting results (2.5/5; CI 2.3–2.6). A cumulative average was calculated to provide an overall genetic skills confidence score (3.3/5; CI 3.2–3.3). Confidence results are displayed in Figure 1.
FIGURE 1
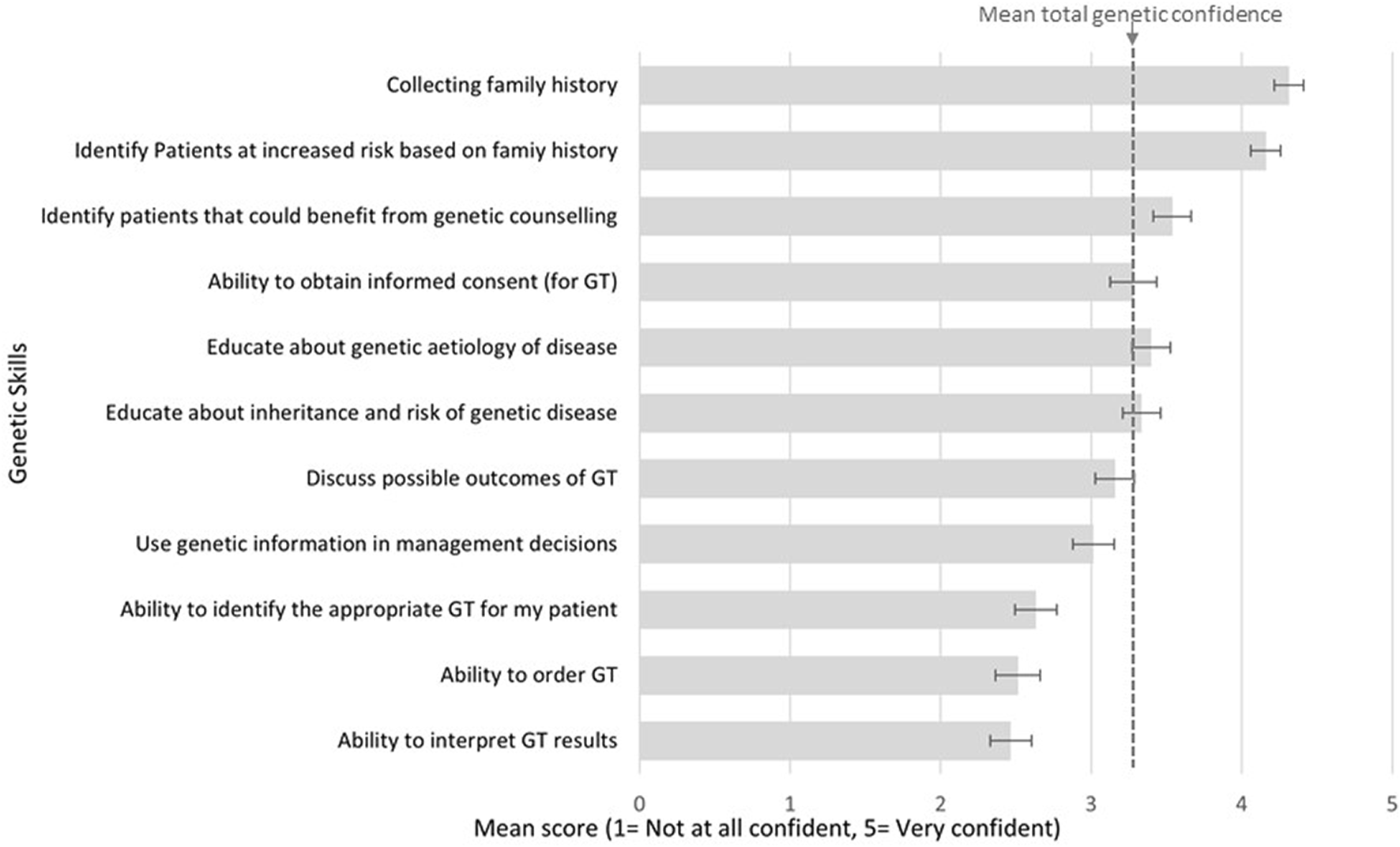
Respondents reported confidence in basic genetic skills.
3.5 Previous education and preferences for future training
Prior experience with continuing education in genetics, and its relative usefulness are displayed in Table 4. Less than a quarter of respondents reported previous education in genetics, but the usefulness of these educational interventions was rated highly (6.9–7.3 out of 10).
TABLE 4
| Education in genetics/genomicsa | n = 253 (%) | Usefulness meanb (CI) |
|---|---|---|
| Completed a unit of study as part of an award course (degree/certificate/diploma) | 55 (22) | 7.1 (6.5–7.6) |
| Completed a short course on genetics/genomics | 30 (12) | 7.3 (6.8–7.9) |
| Completed training regarding the ethical considerations of genetics/genomics testing or research | 54 (21) | 6.9 (6.2–7.6) |
| Preferences for future learning modalities in geneticsc | n = 141 d (%) | Average score e |
| Face-to-face courses/tutorials | 42 (30) | 2.4 |
| A “hotline” to talk to genetics professional | 38 (27) | 2.9 |
| Online courses/tutorials | 34 (24) | 2.6 |
| Experiential (observation/immersion in cases) | 17 (12) | 3.5 |
| Printed material | 10 (7) | 3.6 |
Prior experience with genetic/genomic education and training, and preferences for future learning.
The number and percent of respondents who have completed previous education in genomics, and its perceived usefulness (scored 1 to 10, with 10 indicating “very useful”).
The mean score from 1 to 10, selected by respondents (with 10 indicating “very useful”).
The number and percent of respondent’s highest ranked preference for future learning activities used to upskill in genomic medicine.
Unclear wording of question meant that only 141 individuals accurately completed this question.
The average cumulative score, a lower score indicative of most preferred (calculated by the sum of ranked values, divided by the number of values).
Bold values represent the n that provided a response to that subset of questions.
Respondents were asked to rank their preference from 1 to 5 (with one being the most preferred option) for the delivery of future education initiatives in genomic medicine. For the 141 who completed this question correctly, the most preferred learning modality was face-to-face courses (42/141), followed by a hotline for genetics advice (38/141), online courses (34/141), experiential (17/141), and lastly, printed material (10/141). When averaging the ranked score for each modality the order of preference changed only for online courses (third to second preference) and hotline for advice (second to third preference) (Table 4).
3.6 Variables associated with dermatologists’ confidence
Linear regression was used to identify independent variables associated with dermatologists’ overall confidence in their genetic skills (Table 5).
TABLE 5
| B | 95% CI | β | p-value | R 2 | F | |
|---|---|---|---|---|---|---|
| Characteristics and Attitude towards genetic testing | ||||||
| Years since graduating medical school | -0.05 | -0.13 – 0.03 | -0.07 | 0.251 | 0.01 | 1.32 |
| Having a sub-speciality | 0.27 | 0.08–0.46 | 0.17 | 0.007 | 0.03 | 7.50 |
| Relevance of genetic testing to current practice | 0.12 | 0.04–0.20 | 0.19 | 0.003 | 0.04 | 9.09 |
| Relevance of genetic testing to future practice | 0.06 | -0.3 – 0.16 | 0.09 | 0.18 | 0.01 | 1.85 |
| Genetic Skills Performed | ||||||
| Discussed genetic testing with patients | 0.74 | 0.48–1.00 | 0.33 | <0.001 | 0.11 | 31.73 |
| Offered genetic testing to patients | 0.55 | 0.34–0.76 | 0.31 | <0.001 | 0.09 | 26.28 |
| Ordered genetic testing for at least one patient | 0.50 | 0.32–0.68 | 0.33 | <0.001 | 0.12 | 30.27 |
| Referred to genetic services for genetic testing | 0.35 | 0.06–0.65 | 0.15 | 0.02 | 0.02 | 5.46 |
| Continued Genetic Education | ||||||
| Award unit of study on genomics | 0.17 | -0.06 – 0.39 | 0.09 | 0.149 | 0.01 | 2.09 |
| Short course on genomics | 0.43 | 0.15–0.72 | 0.18 | 0.003 | 0.04 | 9.05 |
| Training on ethical considerations of genomic research | 0.52 | 0.30–0.74 | 0.28 | <0.001 | 0.08 | 21.34 |
Simple linear regression assessing independent predictors of dermatologists’ overall confidence in genetic skills. The dependent variable is the overall average confidence score.
β = standardised regression coefficient.
B = unstandardised regression coefficient.
Bold values highlight significant results.
No significant relationship was found between years since graduating from medical school, however having a sub-speciality significantly predicted genetic confidence (β = 0.17, p = 0.007). Perceived relevance of genetic testing to current practice significantly predicted confidence (β = 0.19, p = 0.003), however perceived relevance to future practice was not significant. Genetic confidence was significantly predicted by having previously discussed (β = 0.33, p < 0.001), offered (β = 0.31, p < 0.001), ordered (β = 0.33, p < 0.001), or referred patients for genetic testing (β = 0.15, p = 0.02). When continued education variables were assessed, an “award unit of study on genomics’ was not associated with overall confidence, but completing a short course (β = 0.18, p = 0.003) or receiving training on ethical considerations (β = 0.28, p < 0.001) significantly predicted genetic confidence.
3.7 Qualitative feedback
Almost a third of participants (n = 81) provided a response to open-ended question “Do you have any additional thoughts regarding genetic testing in dermatology, which you would like to share?” Half (n = 41) identified the need for further training, education, and/or resources for providing genetic testing in clinical practice. Specific suggestions included requests for the College (ACD) to provide/advertise a short course on genetics, presentation updates at the Annual Scientific Meeting, and online Continuing Professional Development modules. There were several recommendations for a “cheat sheet”, or “flip book” providing clinicians with core information on tests, testing criteria, genetic counselling discussion points, test ordering and result interpretation. A few respondents expressed a need for information on relevant costs and Medicare rebates for genetic tests. Twelve individuals indicated that they are already referring patients for genetic testing, with three respondents expressing a preference to continue to refer patients rather than provide genetic testing directly. Ten comments stated the need for greater access to genetic services. Just five individuals commented that genetic testing was not useful or needed in dermatology. Five further respondents expressed concerns about costs, genetic discrimination regarding insurance, and concerns of patient anxiety.
4 Discussion
To our knowledge, this is the first cross-sectional survey to comprehensively examine how dermatologists are currently using genetic testing in practice, including confidence in ordering genetic tests, attitudes towards the utility of testing, and preferences for education and training. Respondents were more likely to believe genetic testing would be relevant to future practice than it was currently. This finding is consistent with a recent study of Australian non-genetic clinicians which reported that genetic testing would become increasingly important in the future (McClaren et al., 2020; Nisselle et al., 2021). Furthermore, respondents in this study were significantly more likely to have previously discussed, offered or ordered genetic testing for patients when they viewed genetic testing as relevant to their current practice. When combined with qualitative feedback, which expressed a high demand for further training in genetic medicine, these findings offer a strong indicator of motivation, a key component of the COM-B (capability, opportunity and motivation) model for behaviour change (Michie et al., 2011).
Questions exploring perceptions regarding genetic testing identified a knowledge gap regarding the potential impact on insurance in Australia. Less than a third of respondents were aware of the 2019 Australian moratorium on the use of genetic testing results in insurance underwriting (Tiller et al., 2021a). Furthermore, 79% incorrectly considered that genetic testing could impact a patient’s ability to access private health insurance, as this is not based on a risk assessment in Australia (Tiller et al., 2020). In contrast, life insurance can incorporate genetic test results in an underwriting process (Tiller et al., 2017). The moratorium prohibits life insurers from requesting genetic test results on new policies < AUS$500,000 (Tiller et al., 2021b). While this protection falls short of international standards, where some countries explicitly prohibit the use of genetic information by life insurers (Rothstein, 2018), this is still important information for dermatologists to be aware of when offering testing.
The first construct in the COM-B model relates to capability, that is, possessing the necessary knowledge and skills to perform the task (Michie et al., 2011). This survey has been valuable in illustrating which genetic tasks dermatologists already feel confident in, so as to inform the focus of future educational interventions. It is not surprising that confidence was high for identifying individuals at increased risk of a hereditary condition and recording family history. Such processes have been relevant to medical practice generally (Scheuner et al., 1997), and dermatology specifically (Rees, 1992) for an extended period. Lowest confidence was reported for tasks related directly to genetic testing and counselling, such as ordering tests and interpreting the results. These findings are congruent with similar surveys of GPs, oncologists (Demeshko et al., 2020) and obstetricians/gynaecologists (Nippert et al., 2011b). According to the COM-B model, failure to address the low confidence in genetic tasks would negatively affect successful uptake of providing genetic testing by clinicians.
Any training initiatives for dermatologists to provide genetic testing would need to cover the challenges of informed consent and interpreting and explaining test results. If a dermatologist has a patient test positive for a genetic test, they are advised to report this result to the patient and then refer them to clinical genetic services for further discussion regarding appropriate screening for non-cutaneous cancers and to arrange testing for at-risk family members. Any individuals found to carry mutations through this testing process would then be referred back to the dermatologist for appropriate screening.
An important objective of this survey was to understand the impact of past genomic education/training to guide future interventions. It was encouraging to find that completing a short course in genomics or receiving training on ethical considerations of genomic research positively predicted overall confidence in performing genetic tasks. While completing a unit/subject as part of an award course was not associated with confidence, this is not unexpected, as such genomic units are usually undertaken during early undergraduate degrees, and therefore were more than 2 decades ago for most respondents. Preferred learning modalities for future genetic education included face-to-face training, a hotline to a genetic professional, and an online course. Face-to-face courses provide opportunity for group training in an interactive setting and have previously demonstrated positive impacts on attitudes and behaviours (Cornel, 2019). A genetic professional hotline is a “just in time” method which has recently been implemented for non-genetic clinicians as an informal education strategy (Carroll et al., 2019). Online courses provide a fast and accessible educational tools, that cater to physicians unable to commit to extensive programs and democratise access to clinicians in rural/remote settings (Casebeer et al., 2010; Freeley, 2020).
The three intertwining factors of the COM-B model (Capability, Opportunity, and Motivation) are imperative to the successful implementation of new interventions. The survey results have clearly demonstrated motivation for upskilling in this area by dermatologists. Furthermore, results on confidence in core genetic testing tasks are useful in guiding emphasis in training materials to boost clinician capability. However, training initiatives are only useful if there is a similar focus on opportunity—this may be difficult given the majority of respondents report having insufficient resources to offer patients genetic testing. Future education and training interventions for mainstreaming genetic testing into dermatological practice would benefit from comprehensive consideration of factors affecting opportunity (Damschroder et al., 2009; Proctor et al., 2011).
This report has described genetic testing for familial melanoma as a practical example of testing that could be provided by dermatologists. Provision of guidelines regarding eligibility criteria, consenting process, test ordering and interpretation of test results would be beneficial to increase clinician confidence and uptake.
4.1 Strengths and limitations
The generalisability of the survey results is strengthened by the fact that all Australians dermatologists were surveyed, and a high response rate (56%). This is comparably higher than some recent genomic medicine surveys to non-genetic health professionals (11%–26%) (Douma et al., 2016; Carroll et al., 2019). However, the study is still subject to response bias, as those with previous experience/interest in genetics would be more likely to respond. The survey benefited from using previously validated items which allowed comparison with similar studies. However, purpose-designed survey questions on preferences for learning modalities presents decreased generalisability as only 55% of respondents correctly completed the question. The results reported for variables associated with dermatologists’ confidence did not include multiple regression due to small sample size of subgroups and therefore vulnerable to confounders. Furthermore, the study evaluated confidence as opposed to objectively measuring dermatologists’ knowledge and competence. Another limitation to the survey was restricting the population to fully-accredited members of the ACD only. Including trainees would have provided insights into recent changes in education in genetics and any resulting impact on attitudes. Furthermore, including GPs who work full time in skin cancer screening clinics would have been valuable. This is particularly relevant in Australia where, unlike other countries, it is not uncommon for melanomas to be managed entirely within the primary care setting (Smith et al., 2020).
5 Conclusion
Australian dermatologists perceive genetic testing as increasingly relevant to practice. However, they currently report low confidence in ordering tests and interpreting results. Having received continuing education on genetics/genomics was found to positively predict genetic confidence. We also found a strong demand for additional resources to enable the upskilling of dermatologists in how to provide genetic testing. This study has generated new evidence to help inform the future implementation of genetic testing into routine dermatological practice in Australia.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by The University of Queensland HREC and ratified by the University of Technology Sydney HREC. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
CP and AB contributed equally to the manuscript. All co-authors contributed to writing, provided intellectual input and approved the final version of the manuscript.
Funding
AM-L is funded by a National Health and Medical Research Council (NHMRC) Early Career Fellowship (ID 1158111). HS holds an NHMRC MRFF Next Generation Clinical Researchers Program Practitioner Fellowship (APP1137127). This research was made possible by a bequest from the estate of Bernard Alan Corfield. This research was carried out at the Translational Research Institute, Woolloongabba, Qld 4102, Australia. The Translational Research Institute is supported by a grant from the Australian Government. CW, EM, and CP are supported by an Australian Government Research Training Program Scholarship. PL is supported by a National Heart Foundation Future Leader Fellowship (102604).
Acknowledgments
We are very grateful to the individuals who participated in this study and to the Australasian College of Dermatologists for their collaboration.
Conflict of interest
HS is a shareholder of MoleMap NZ Limited and e-derm consult GmbH, and undertakes regular teledermatological reporting for both companies. HS is a Medical Consultant for Canfield Scientific Inc., MoleMap Australia Pty Ltd., Blaze Bioscience Inc., Revenio Research Oy and a Medical Advisor for First Derm.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.919134/full#supplementary-material
References
1
Abdo J. F. Sharma A. Sharma R. (2020). Role of heredity in melanoma susceptibility: A primer for the practicing surgeon. Surg. Clin. North Am.100 (1), 13–28. 10.1016/j.suc.2019.09.006
2
Aoude L. G. Wadt K. A. Pritchard A. L. Hayward N. K. (2015). Genetics of familial melanoma: 20 years after CDKN2A. Pigment. Cell Melanoma Res.28 (2), 148–160. 10.1111/pcmr.12333
3
Aspinwall L. G. Leaf S. L. Dola E. R. Kohlmann W. Leachman S. A. (2008). CDKN2A/p16 genetic test reporting improves early detection intentions and practices in high-risk melanoma families. Cancer Epidemiol. Biomarkers Prev.17 (6), 1510–1519. 10.1158/1055-9965.EPI-08-0010
4
Aspinwall L. G. Taber J. M. Kohlmann W. Leaf S. L. Leachman S. A. (2014). Unaffected family members report improvements in daily routine sun protection 2 years following melanoma genetic testing. Genet. Med.16 (11), 846–853. 10.1038/gim.2014.37
5
Badenas C. Aguilera P. Puig-Butillé J. A. Carrera C. Malvehy J. Puig S. (2012). Genetic counseling in melanoma. Dermatol. Ther.25 (5), 397–402. 10.1111/j.1529-8019.2012.01499.x
6
Bengtsson M. (2016). How to plan and perform a qualitative study using content analysis. NursingPlus Open2, 8–14. 10.1016/j.npls.2016.01.001
7
Best S. Long J. C. Gaff C. Braithwaite J. Taylor N. (2021). Investigating the adoption of clinical genomics in Australia. An implementation science case study. Genes (Basel)12 (2), 317. 10.3390/genes12020317
8
Bouhnik A. D. N'Diaye K. Evans D. G. Harris H. Tibben A. van Asperen C. et al (2017). Validation of a scale for assessing attitudes towards outcomes of genetic cancer testing among primary care providers and breast specialists. PLoS One12 (6), e0178447. 10.1371/journal.pone.0178447
9
Box N. F. Duffy D. L. Chen W. Stark M. Martin N. G. Sturm R. A. et al (2001). MC1R genotype modifies risk of melanoma in families segregating CDKN2A mutations. Am. J. Hum. Genet.69 (4), 765–773. 10.1086/323412
10
Brittain H. K. Scott R. Thomas E. (2017). The rise of the genome and personalised medicine. Clin. Med.17 (6), 545–551. 10.7861/clinmedicine.17-6-545
11
Carroll J. C. Allanson J. Morrison S. Miller F. A. Wilson B. J. Permaul J. A. et al (2019). Informing integration of genomic medicine into primary care: An assessment of current practice, attitudes, and desired resources. Front. Genet.10 (1189), 1189. 10.3389/fgene.2019.01189
12
Carroll J. C. Rideout A. L. Wilson B. J. Allanson J. M. Blaine S. M. Esplen M. J. et al (2009). Genetic education for primary care providers: Improving attitudes, knowledge, and confidence. Can. Fam. Physician55 (12), e92–e99.
13
Casebeer L. Brown J. Roepke N. Grimes C. Henson B. Palmore R. et al (2010). Evidence-based choices of physicians: A comparative analysis of physicians participating in internet CME and non-participants. BMC Med. Educ.10, 42. 10.1186/1472-6920-10-42
14
Chow-White P. Ha D. Laskin J. (2017). Knowledge, attitudes, and values among physicians working with clinical genomics: A survey of medical oncologists. Hum. Resour. Health15 (1), 42. 10.1186/s12960-017-0218-z
15
Collins F. S. McKusick V. A. (2001). Implications of the human genome project for medical science. Jama285 (5), 540–544. 10.1001/jama.285.5.540
16
Cornel M. C. (2019). Evidence-based genetic education of non-genetic-expert physicians: Experiences over three decades in amsterdam. Front. Genet.10 (712), 712. 10.3389/fgene.2019.00712
17
Corp I. (2020). IBM SPSS statistics for macintosh. 0 edn.NY: Armonk, 27. In.,
18
Crellin E. McClaren B. Nisselle A. Best S. Gaff C. Metcalfe S. (2019). Preparing medical specialists to practice genomic medicine: Education an essential part of a broader strategy. Front. Genet.10, 789. 10.3389/fgene.2019.00789
19
Culver J. O. Hull J. L. Dunne D. F. Burke W. (2001). Oncologists' opinions on genetic testing for breast and ovarian cancer. Genet. Med.3 (2), 120–125. 10.1097/00125817-200103000-00006
20
Damschroder L. J. Aron D. C. Keith R. E. Kirsh S. R. Alexander J. A. Lowery J. C. (2009). Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implement. Sci.4, 50. 10.1186/1748-5908-4-50
21
DeLuca J. Selig D. Poon L. Livezey J. Oliver T. Barrett J. et al (2020). Toward personalized medicine implementation: Survey of military medicine providers in the area of pharmacogenomics. Mil. Med.185 (3-4), 336–340. 10.1093/milmed/usz419
22
Demeshko A. Pennisi D. J. Narayan S. Gray S. W. Brown M. A. McInerney-Leo A. M. (2020). Factors influencing cancer genetic somatic mutation test ordering by cancer physician. J. Transl. Med.18 (1), 431. 10.1186/s12967-020-02610-7
23
Diamonstein C. Stevens B. Shahrukh Hashmi S. Refuerzo J. Sullivan C. Hoskovec J. (2018). Physicians’ awareness and utilization of genetic services in Texas. J. Genet. Couns.27 (4), 968–977. 10.1007/s10897-017-0199-z
24
Douma K. F. L. Smets E. M. A. Allain D. C. (2016). Non-genetic health professionals’ attitude towards, knowledge of and skills in discussing and ordering genetic testing for hereditary cancer. Fam. Cancer15 (2), 341–350. 10.1007/s10689-015-9852-6
25
Duffy D. L. Lee K. J. Jagirdar K. Pflugfelder A. Stark M. S. McMeniman E. K. et al (2019). High naevus count and MC1R red hair alleles contribute synergistically to increased melanoma risk. Br. J. Dermatol.181 (5), 1009–1016. 10.1111/bjd.17833
26
Freeley M. (2020). Current postgraduate training programs and online courses in precision medicine. Expert Rev. Mol. diagn.20 (6), 569–574. 10.1080/14737159.2020.1709826
27
Frost C. J. Andrulis I. L. Buys S. S. Hopper J. L. John E. M. Terry M. B. et al (2019). Assessing patient readiness for personalized genomic medicine. J. Community Genet.10 (1), 109–120. 10.1007/s12687-018-0365-5
28
George A. Riddell D. Seal S. Talukdar S. Mahamdallie S. Ruark E. et al (2016). Implementing rapid, robust, cost-effective, patient-centred, routine genetic testing in ovarian cancer patients. Sci. Rep.6, 29506. 10.1038/srep29506
29
Gordon L. G. Rowell D. (2015). Health system costs of skin cancer and cost-effectiveness of skin cancer prevention and screening: A systematic review. Eur. J. Cancer Prev.24 (2), 141–149. 10.1097/CEJ.0000000000000056
30
Guitera P. Menzies S. W. Coates E. Azzi A. Fernandez-Penas P. Lilleyman A. et al (2021). Efficiency of detecting new primary melanoma among individuals treated in a high-risk clinic for skin surveillance. JAMA Dermatol.157 (5), 521–530. 10.1001/jamadermatol.2020.5651
31
Haga S. B. Kim E. Myers R. A. Ginsburg G. S. (2019). Primary care physicians' knowledge, attitudes, and experience with personal genetic testing. J. Pers. Med.9 (2), E29. 10.3390/jpm9020029
32
Harding B. Webber C. Rühland L. Dalgarno N. Armour C. Birtwhistle R. et al (2019). Bridging the gap in genetics: A progressive model for primary to specialist care. BMC Med. Educ.19 (1), 195. 10.1186/s12909-019-1622-y
33
Heald B. Plesec T. Liu X. Pai R. Patil D. Moline J. et al (2013). Implementation of universal microsatellite instability and immunohistochemistry screening for diagnosing lynch syndrome in a large academic medical center. J. Clin. Oncol.31 (10), 1336–1340. 10.1200/JCO.2012.45.1674
34
Hoskovec J. M. Bennett R. L. Carey M. E. DaVanzo J. E. Dougherty M. Hahn S. E. et al (2018). Projecting the supply and demand for certified genetic counselors: A workforce study. J. Genet. Couns.27 (1), 16–20. 10.1007/s10897-017-0158-8
35
James K. M. Ziegenfuss J. Y. Tilburt J. C. Harris A. M. Beebe T. J. (2011). Getting physicians to respond: The impact of incentive type and timing on physician survey response rates. Health Serv. Res.46, 232–242. 10.1111/j.1475-6773.2010.01181.x
36
Johnson K. B. Clayton E. W. Starren J. Peterson J. (2020). The implementation chasm hindering genome-informed health care. J. Law Med. Ethics48 (1), 119–125. 10.1177/1073110520916999
37
Johnson L-M. Valdez J. M. Quinn E. A. Sykes A. D. McGee R. B. Nuccio R. et al (2017). Integrating next-generation sequencing into pediatric oncology practice: An assessment of physician confidence and understanding of clinical genomics. Cancer123 (12), 2352–2359. 10.1002/cncr.30581
38
Kathrens-Gallardo A. Propst L. Linn E. Pothast R. Wicklund C. Arjunan A. (2021). OB/GYN residents' training, attitudes, and comfort level regarding genetics. J. Assist. Reprod. Genet.38, 2871–2880. 10.1007/s10815-021-02310-1
39
Kemp Z. Turnbull A. Yost S. Seal S. Mahamdallie S. Poyastro-Pearson E. et al (2019). Evaluation of cancer-based criteria for use in mainstream BRCA1 and BRCA2 genetic testing in patients with breast cancer. JAMA Netw. Open2 (5), e194428. 10.1001/jamanetworkopen.2019.4428
40
Kentwell M. Dow E. Antill Y. Wrede C. D. McNally O. Higgs E. et al (2017). Mainstreaming cancer genetics: A model integrating germline BRCA testing into routine ovarian cancer clinics. Gynecol. Oncol.145 (1), 130–136. 10.1016/j.ygyno.2017.01.030
41
Kohut K. Limb S. Crawford G. (2019). The changing role of the genetic counsellor in the genomics era. Curr. Genet. Med. Rep.7 (2), 75–84. 10.1007/s40142-019-00163-w
42
Leachman S. A. Lucero O. M. Sampson J. E. Cassidy P. Bruno W. Queirolo P. et al (2017). Identification, genetic testing, and management of hereditary melanoma. Cancer Metastasis Rev.36 (1), 77–90. 10.1007/s10555-017-9661-5
43
Maas E. J. Betz-Stablein B. Aoude L. G. Soyer H. P. McInerney-Leo A. M. (2022). Unusual suspects in hereditary melanoma: POT1, POLE, BAP1. Trends Genet.S0168-9525 (22), online ahead of print. 10.1016/j.tig.2022.06.007
44
McClaren B. J. King E. A. Crellin E. Gaff C. Metcalfe S. A. Nisselle A. (2020). Development of an evidence-based, theory-informed national survey of physician preparedness for genomic medicine and preferences for genomics continuing education. Front. Genet.11 (59), 59. 10.3389/fgene.2020.00059
45
McCuaig J. M. Armel S. R. Care M. Volenik A. Kim R. H. Metcalfe K. A. (2018). Next-Generation service delivery: A scoping review of patient outcomes associated with alternative models of genetic counseling and genetic testing for hereditary cancer. Cancers (Basel)10 (11), E435. 10.3390/cancers10110435
46
Michie S. van Stralen M. M. West R. (2011). The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implement. Sci.6 (1), 42. 10.1186/1748-5908-6-42
47
Miesfeldt S. Feero W. G. Lucas F. L. Rasmussen K. (2018). Association of patient navigation with care coordination in an Lynch syndrome screening program. Transl. Behav. Med.8 (3), 450–455. 10.1093/tbm/ibx078
48
Murphy M. J. (2015). Attitudes concerning clinical molecular testing among dermatology trainees at a single institution. Am. J. Dermatopathol.37 (7), 590. 10.1097/DAD.0000000000000136
49
Nippert I. Harris H. J. Julian-Reynier C. Kristoffersson U. Ten Kate L. P. Anionwu E. et al (2011). Confidence of primary care physicians in their ability to carry out basic medical genetic tasks-a European survey in five countries-Part 1. J. Community Genet.2 (1), 1–11. 10.1007/s12687-010-0030-0
50
Nippert I. Harris H. J. Julian-Reynier C. Kristoffersson U. ten Kate L. P. Anionwu E. et al (2011). Confidence of primary care physicians in their ability to carry out basic medical genetic tasks—A European survey in five countries—Part 1. J. Community Genet.2 (1), 1–11. 10.1007/s12687-010-0030-0
51
Nisselle A. King E. A. McClaren B. Janinski M. Metcalfe S. Gaff C. et al (2021). Measuring physician practice, preparedness and preferences for genomic medicine: A national survey. BMJ Open11 (7), e044408. 10.1136/bmjopen-2020-044408
52
Noel H. Huang A. R. (2019). The effect of varying incentive amounts on physician survey response. Eval. Health Prof.42 (1), 71–81. 10.1177/0163278718809844
53
O'Shea R. Taylor N. Crook A. Jacobs C. Jung Kang Y. Lewis S. et al (2021). Health system interventions to integrate genetic testing in routine oncology services: A systematic review. PLoS One16 (5), e0250379. 10.1371/journal.pone.0250379
54
Plaskocinska I. Shipman H. Drummond J. Thompson E. Buchanan V. Newcombe B. et al (2016). New paradigms for BRCA1/BRCA2 testing in women with ovarian cancer: Results of the genetic testing in epithelial ovarian cancer (GTEOC) study. J. Med. Genet.53 (10), 655–661. 10.1136/jmedgenet-2016-103902
55
Potrony M. Badenas C. Aguilera P. Puig-Butille J. A. Carrera C. Malvehy J. et al (2015). Update in genetic susceptibility in melanoma. Ann. Transl. Med.3 (15), 210. 10.3978/j.issn.2305-5839.2015.08.11
56
Primiero C. A. Finnane A. Yanes T. Peach B. Soyer H. P. (2022). Protocol to evaluate a pilot program to upskill clinicians in providing genetic testing for familial melanoma. PLOS One. in press/forthcoming.
57
Primiero C. A. Yanes T. Finnane A. Soyer H. P. McInerney-Leo A. M. (2021). A systematic review on the impact of genetic testing for familial melanoma I: Primary and secondary preventative behaviours. Dermatology237 (5), 806–815. 10.1159/000513919
58
Primiero C. A. Yanes T. Finnane A. Soyer H. P. McInerney-Leo A. M. (2021). A systematic review on the impact of genetic testing for familial melanoma II: Psychosocial outcomes and attitudes. Dermatology237 (5), 816–826. 10.1159/000513576
59
Proctor E. Silmere H. Raghavan R. Hovmand P. Aarons G. Bunger A. et al (2011). Outcomes for implementation research: Conceptual distinctions, measurement challenges, and research agenda. Adm. Policy Ment. Health38 (2), 65–76. 10.1007/s10488-010-0319-7
60
Rahman B. Lanceley A. Kristeleit R. S. Ledermann J. A. Lockley M. McCormack M. et al (2019). Mainstreamed genetic testing for women with ovarian cancer: First-year experience. J. Med. Genet.56 (3), 195–198. 10.1136/jmedgenet-2017-105140
61
Read J. Wadt K. A. Hayward N. K. (2016). Melanoma genetics. J. Med. Genet.53 (1), 1–14. 10.1136/jmedgenet-2015-103150
62
Reed E. K. Johansen Taber K. A. Ingram Nissen T. Schott S. Dowling L. O. O'Leary J. C. et al (2016). What works in genomics education: Outcomes of an evidenced-based instructional model for community-based physicians. Genet. Med.18 (7), 737–745. 10.1038/gim.2015.144
63
Rees J. (1992). Forward dermatology. Bmj304 (6827), 590. 10.1136/bmj.304.6827.590
64
Ribeiro Moura Brasil Arnaut J. Dos Santos Guimarães I. Evangelista Dos Santos A. C. de Moraes Lino da Silva F. Machado J. R. de Melo A. C. (2021). Molecular landscape of hereditary melanoma. Crit. Rev. Oncol. Hematol.164, 103425. 10.1016/j.critrevonc.2021.103425
65
Richardson M. Min H. J. Hong Q. Compton K. Mung S. W. Lohn Z. et al (2020). Oncology clinic-based hereditary cancer genetic testing in a population-based health care system. Cancers (Basel)12 (2), E338. 10.3390/cancers12020338
66
Rothstein M. A. (2018). Time to end the use of genetic test results in life insurance underwriting. J. Law Med. Ethics46 (3), 794–801. 10.1177/1073110518804243
67
Rumford M. Lythgoe M. McNeish I. Gabra H. Tookman L. Rahman N. et al (2020). Oncologist-led BRCA 'mainstreaming' in the ovarian cancer clinic: A study of 255 patients and its impact on their management. Sci. Rep.10 (1), 3390. 10.1038/s41598-020-60149-5
68
Scheuner M. T. Wang S. J. Raffel L. J. Larabell S. K. Rotter J. I. (1997). Family history: A comprehensive genetic risk assessment method for the chronic conditions of adulthood. Am. J. Med. Genet.71 (3), 315–324. 10.1002/(sici)1096-8628(19970822)71:3<315:aid-ajmg12>3.0.co;2-n
69
Shagalov D. R. Ferzli G. M. Wildman T. Glick S. A. (2017). Genetic testing in dermatology: A survey analyzing obstacles to appropriate care. Pediatr. Dermatol.34 (1), 33–38. 10.1111/pde.12981
70
Smith A. L. Watts C. G. Robinson S. Schmid H. Chang C. H. Thompson J. F. et al (2020). GPs' involvement in diagnosing, treating, and referring patients with suspected or confirmed primary cutaneous melanoma: A qualitative study. BJGP Open4 (2), bjgpopen20X101028. 10.3399/bjgpopen20X101028
71
Stump T. K. Aspinwall L. G. Drummond D. M. Taber J. M. Kohlmann W. Champine M. et al (2020). CDKN2A testing and genetic counseling promote reductions in objectively measured sun exposure one year later. Genet. Med.22 (1), 26–34. 10.1038/s41436-019-0608-9
72
Talwar D. Tseng T-S. Foster M. Xu L. Chen L-S. (2017). Genetics/genomics education for nongenetic health professionals: A systematic literature review. Genet. Med.19 (7), 725–732. 10.1038/gim.2016.156
73
Tiller J. McInerney-Leo A. Belcher A. Boughtwood T. Gleeson P. Delatycki M. et al (2021). Study protocol: The Australian genetics and life insurance moratorium-monitoring the effectiveness and response (A-GLIMMER) project. BMC Med. Ethics22 (1), 63. 10.1186/s12910-021-00634-2
74
Tiller J. Morris S. Rice T. Barter K. Riaz M. Keogh L. et al (2020). Genetic discrimination by Australian insurance companies: A survey of consumer experiences. Eur. J. Hum. Genet.28 (1), 108–113. 10.1038/s41431-019-0426-1
75
Tiller J. Otlowski M. Lacaze P. (2017). Should Australia ban the use of genetic test results in life insurance?Front. Public Health5 (330), 330. 10.3389/fpubh.2017.00330
76
Tiller J. M. Keogh L. A. McInerney-Leo A. M. Belcher A. Barlow-Stewart K. Boughtwood T. et al (2021). A step forward, but still inadequate: Australian health professionals' views on the genetics and life insurance moratorium. J. Med. Genet.59, 817–826. 10.1136/jmedgenet-2021-107989
77
Torre K. Russomanno K. Ferringer T. Elston D. Murphy M. J. (2018). Educational gaps in molecular diagnostics, genomics, and personalized medicine in dermatopathology training: A survey of U.S. Dermatopathology fellowship program directors. Am. J. Dermatopathol.40 (1), 43–48. 10.1097/DAD.0000000000000909
78
Toussi A. Mans N. Welborn J. Kiuru M. (2020). Germline mutations predisposing to melanoma. J. Cutan. Pathol.47 (7), 606–616. 10.1111/cup.13689
79
Williams M. S. (2019). Early lessons from the implementation of genomic medicine programs. Annu. Rev. Genomics Hum. Genet.20, 389–411. 10.1146/annurev-genom-083118-014924
80
Zhou A. E. Hoegler K. M. Solimine J. F. (2021). Genetic counseling and testing for hereditary causes of melanoma can lead to earlier detection of skin cancer and other malignancies. Int. J. Dermatol.61, e233–e234. 10.1111/ijd.15716
Summary
Keywords
genetics, genomics, dermatology, mainstreaming, familial melanoma
Citation
Primiero CA, Baker AM, Wallingford CK, Maas EJ, Yanes T, Fowles L, Janda M, Young M-A, Nisselle A, Terrill B, Lodge JM, Tiller JM, Lacaze P, Andersen H, McErlean G, Turbitt E, Soyer HP and McInerney-Leo AM (2022) Attitudes of Australian dermatologists on the use of genetic testing: A cross-sectional survey with a focus on melanoma. Front. Genet. 13:919134. doi: 10.3389/fgene.2022.919134
Received
13 April 2022
Accepted
10 October 2022
Published
24 October 2022
Volume
13 - 2022
Edited by
Maritha J. Kotze, Stellenbosch University, South Africa
Reviewed by
Rosie O. Shea, The University of Sydney, Australia
Elouise Elizabeth Kroon, Stellenbosch University, South Africa
Updates
Copyright
© 2022 Primiero, Baker, Wallingford, Maas, Yanes, Fowles, Janda, Young, Nisselle, Terrill, Lodge, Tiller, Lacaze, Andersen, McErlean, Turbitt, Soyer and McInerney-Leo.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aideen M. McInerney-Leo, a.mcinerney@uq.edu.au
†These authors have contributed equally to this work
This article was submitted to Human and Medical Genomics, a section of the journal Frontiers in Genetics
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.