- 1Department of Neurology, The Affiliated Hospital of Kangda College of Nanjing Medical University/ Lianyungang Training Base of Jinzhou Medical University/ The Affiliated Lianyungang Hospital of Xuzhou Medical University, Lianyungang, Jiangsu, China
- 2Department of Neurology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu, China
- 3Department of Clinical Medicine, The Affiliated Hospital of Kangda College of Nanjing Medical University, Lianyungang, China
Background: Migraine, a condition stemming from neurological and vascular irregularities with inflammation involvement, was investigated in a case-control study focusing on the Chinese Han population.
Methods: The research analyzed specific genetic variations—TNF-α −308 G/A, TNF-α −857 C/T, TNF-α −238G/A, IL1B-3953 C/T, and IL1RN −2018T/C SNPs—within 212 migraine patients and 210 healthy controls. Utilizing SNaPshot technology, scientists genotyped these markers related to TNF-α and IL-1 genes.
Results: Findings revealed a significant association between the IL1B−3953 C/T SNP and migraine susceptibility, particularly noting its link with a familial history of the disorder. The presence of the C allele at this location was more prevalent in migraine sufferers. Multivariate analysis reinforced this connection, indicating the C allele under a dominant model as an independent risk factor for migraine (OR = 2.315, 95%CI: 1.053–5.090, P = 0.037). Additionally, the study observed a sex-specific difference regarding the TNF-α −857 C/T SNP among migraine patients.
Conclusion: Overall, this investigation contributes to understanding the genetic underpinnings of migraine in the Han Chinese population, highlighting the IL1B−3953 C/T SNP as a potential biomarker for migraine susceptibility.
1 Introduction
Migraine is a common chronic neurological disorder in clinical practice, with a lifetime prevalence of 33% in women and 18% in men (Charles, 2017; MacGregor, 2017; Ferrari et al., 2022). It is a leading cause of disability worldwide and has a profound effect on people living with the disorder, their families, healthcare systems, and society (Saylor and Steiner, 2018). The estimated prevalence of migraine is 15% in the United States of America and 9.3% in China (Burstein et al., 2015). Migraine is recurrent in nature and classically presents as moderate-to-severe head pain lasting 4–72 h. It is typically unilateral with a pulsating quality, accompanied by nausea, vomiting, photophobia, and/or phonophobia, and may be preceded by an aura that consists of optesthesia, sensory, motor, or language symptoms (i.e., migraine with aura (MA)) or may occur without warning [i.e., migraine without aura (MO)] (Charles, 2017; MacGregor, 2017; Ferrari et al., 2022).
The pathophysiology of migraine is not fully understood but likely involves multiple components of the central and peripheral nervous system. Individual migraine attacks are hypothesized to be initiated by complex interactions between genetically determined lowered triggering threshold, fluctuations in endogenous factors that may further lower the triggering threshold, and external trigger factors (Charles, 2018; Cropper and Pradhan, 2022; Ferrari et al., 2022). Still, migraines have significant genetic susceptibility, and their heritability is estimated to be about 50% (Sutherland et al., 2019). The main theories about the pathogenesis of a migraine attack are the vascular theory, the cortical diffusion inhibition theory, the trigeminal vascular theory, and the inflammatory mediators theory (Schulte et al., 2017; Yamanaka et al., 2023). Injurious stimulation of the trigeminal nerve releases a variety of vasoactive peptides, such as calcitonin gene-related peptide (CGRP) and substance P; additionally, they produce inflammatory mediators such as TNF-α and IL-1, causing a cascade that stimulates cerebral vasodilatation, increases the permeability of the vascular wall, and leads to the extravasation of plasma components, mast cell degranulation, release of histamine, and release of inflammatory factors resulting in an aseptic neurogenic inflammatory response, which in turn contributes to the onset of headache symptoms. At the same time, the inflammatory cytokines, such as interleukin (IL) and tumor necrosis factor-α (TNF-α) can induce the hypothalamus to produce prostaglandin E through the nuclear factor κB signaling pathway, which can induce platelet activation caused by platelet-activating factor and thromboxane A2, which cause platelets to release 5-hydroxytryptamine and other vasoactive substances, which in turn affects the metabolism of norepinephrine, etc., ultimately leading to migraine attacks.
Hence, IL-1 and TNF-α are of great research value as important inflammatory mediators involved in migraine attacks (Fawzi et al., 2015). The TNF-α gene cluster is in the highly polymorphic major histocompatibility complex (MHC) class III region on human chromosome 6p21. It comprises four exons and three introns spanning approximately 12 kilobases (kb) (Dong et al., 2012). TNF-α production is influenced by single-nucleotide polymorphisms (SNPs) in the gene’s promoter and is regulated at the transcriptional and post-transcriptional levels. So far, 43 SNPs in the promoter region of the TNF-α gene have been reported in the literature and are involved in a variety of diseases, including Parkinson’s, schizophrenia, cancer, diabetic complications, tuberculosis, and ischemic stroke (Lara-Gómez et al., 2019; Gao et al., 2021; Aytac et al., 2022; Sun et al., 2022). TNF-α SNPs have been associated with migraine risk in Westerner studies (Schurks et al., 2011; Gu et al., 2012; Sudershan et al., 2023), but few studies have been conducted in the Chinese Han population. IL-1β is an active form of IL-1, and the IL1 receptor antagonist (IL1RN) is an IL-1 receptor binding that inhibits the binding of IL-1β to the receptor (Wang et al., 2023). IL1β and IL1RN, as members of the IL1 gene cluster, are located in the segment 2q12-q14 (Mendoza-Carrera et al., 2019). The IL1B−3953C/T (rs1143634) and IL1RN −2018T/C (rs419598) SNPs are involved in a variety of diseases, such as rheumatoid arthritis, multiple sclerosis, and periodontitis (Asgharzadeh et al., 2020; Gomes da Silva et al., 2020; Brodzikowska et al., 2022). Still, few studies have examined IL-1 SNPs and migraines.
Therefore, this case-control study explored the associations of the TNF-α −308 G/A (rs1800629), TNF-α −857 C/T (rs1799724), TNF-α −238G/A (rs361525), IL1B-3953 C/T (rs1143634), and IL1RN −2018T/C (rs419598) SNPs with migraine in the Chinese Han population. This study aims to establish a foundation for potential targeted therapies in the future.
2 Materials and Methods
2.1 Study design and population
This case-control study enrolled patients with migraine attending the outpatient clinic of the Department of Neurology of The First People’s Hospital of Lianyungang, Jiangsu Province, China, and sex- and age-matched healthy individuals from the medical examination center of the same hospital. The study was approved by the ethics committee of The First People’s Hospital of Lianyungang (approval # LW20171231001/LW20171231002). All participants provided written informed consent before any study procedure.
The inclusion criteria were 1) patient admitted at the neurology outpatient clinic and 2) diagnosis of migraine based on magnetic resonance imaging (MRI), rigorous neurological examination, computed tomography (CT), and detailed questionnaires according to ICHD-II criteria. The exclusion criteria were 1) psychiatric disorders, 2) cardiovascular or cerebrovascular diseases, cancer, asthma, anxiety, or depression, or 3) migraine secondary to another disorder. Healthy individuals with headaches were recruited from the Physical Examination Department as controls. They were matched for age (±5 years) and sex with the patients with migraine. The exclusion criteria were the same as for the patients. All participants were unrelated and Han Chinese.
2.2 Determination of the single nucleotide polymorphisms
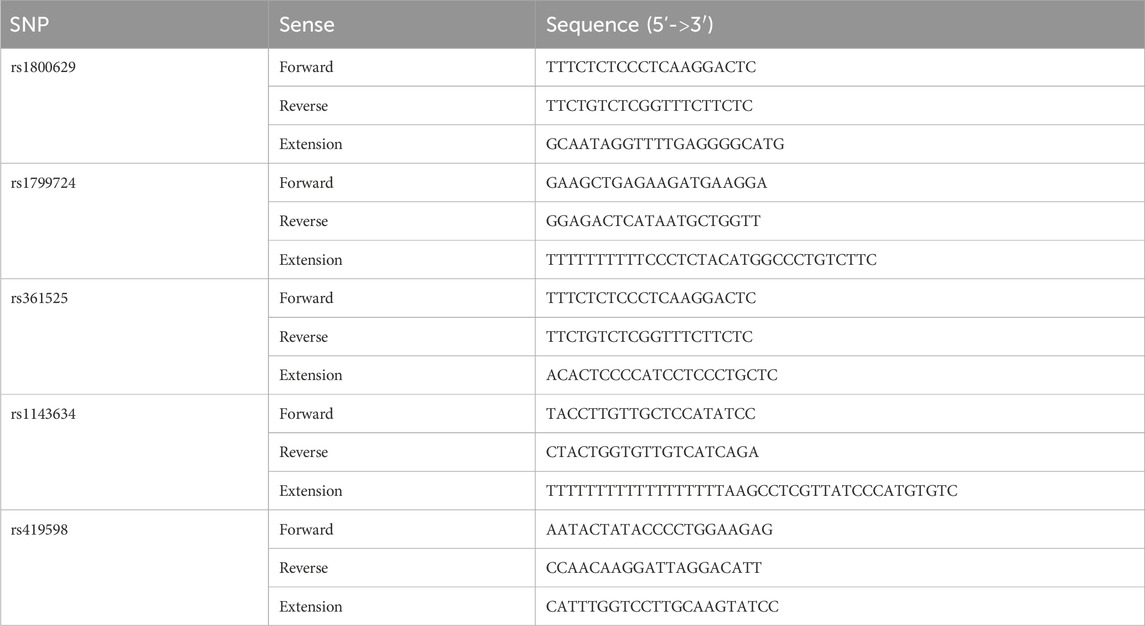
Peripheral venous blood (2 mL) was collected from all participants in EDTA-K2 tubes and stored at −80°C. The samples were processed in batches. The blood was thawed at room temperature, and DNA was extracted using the TIANGEN Blood Genomic DNA Extraction Kit (Shanghai Jierui Bioengineering Co., Ltd.) according to the manufacturer’s instructions. The primers (Table 1) were synthesized by Shanghai Jierui Bioengineering Co., Ltd. (Shanghai, China). After amplification, 1 µL of the extension product was added to 9 µL of HIDI, denatured at 95°C for 3 min, immediately immersed in ice water, and loaded on the SNaPshot system. The sample’s genotype was determined according to the color of the peak, and the corresponding SNP site of the extension product was determined according to the position of the peak. Some samples were selected for validation by Sanger sequencing (Supplementary Figures S1–S5).
2.3 Sample size
According to the relevant literature, the incidence of SNPs in migraine patients and healthy people was estimated at pA = 91.7% and pB = 79.3%, respectively. Using the equation below and α = 0.05, κ = 1, β = 0.9, z1−α, and z1−β (the standard normal distribution quantile expressed in phase with α and β values, respectively), the minimal sample size for each group was determined as 140. Considering a possible loss of 10% due to consent withdrawal or technical failure, 154 cases were needed for each group.
2.4 Statistical analysis
The results were analyzed using SPSS 26.0 (IBM, Armonk, NY, United States). The continuous data were expressed as medians (25th and 75th percentile). Age comparisons were made using the independent samples t-test. Categorical data were expressed as n (%). The comparisons of genotypes and alleles between groups and strata were made using the chi-square test or Fisher’s exact test. Hardy-Weinberg’s law of equilibrium was used to assess whether the samples complied with the law of genetic equilibrium. Univariable and multivariable logistic regression analyses were used to examine the associations of the SNPs with migraine. The results were presented as odds ratios (ORs) and 95% confidence intervals (CIs). Two-sided P-values <0.05 were considered statistically significant.
A multivariable logistic regression model was constructed to examine the relationship between SNPs and migraine susceptibility. Adjustments were made for migraine type (MO vs MA), family history of migraine, age, and sex, based on their potential biological relevance and previous studies (Dong et al., 2012; Fawzi et al., 2015; Khosravi et al., 2015; Dahash and Kusrat Hussein, 2023). The model was fitted using a step-by-step selection process to minimize overfitting and ensure robustness.
Additionally, a multifactor dimensionality reduction (MDR) analysis was conducted to address gene-gene and gene-environment interactions. MDR aggregates multi-locus genotypes into “high-risk” and “low-risk” categories, reducing dimensionality from N to a single dimension. Cross-validation and permutation testing were used to assess the predictive efficacy of the derived variables.
3 Results
3.1 Characteristics of the participants
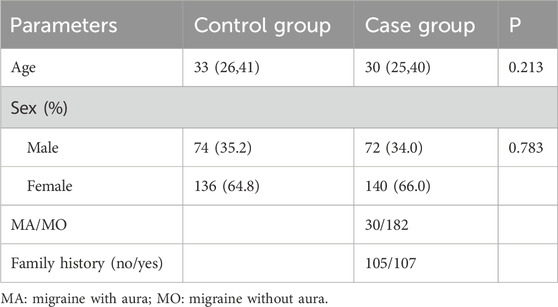
The study enrolled 422 participants: 212 patients with migraine and 210 healthy controls. There were no statistically significant differences in age (P = 0.213) and sex (P = 0.783) (Table 2).
3.2 Hardy-weinberg equilibrium
The genotypes and alleles of the included SNPs were tested using the Hardy-Weinberg balanced test (Supplementary Table S1), and all conformed to Mendel’s law of inheritance (all P > 0.05).
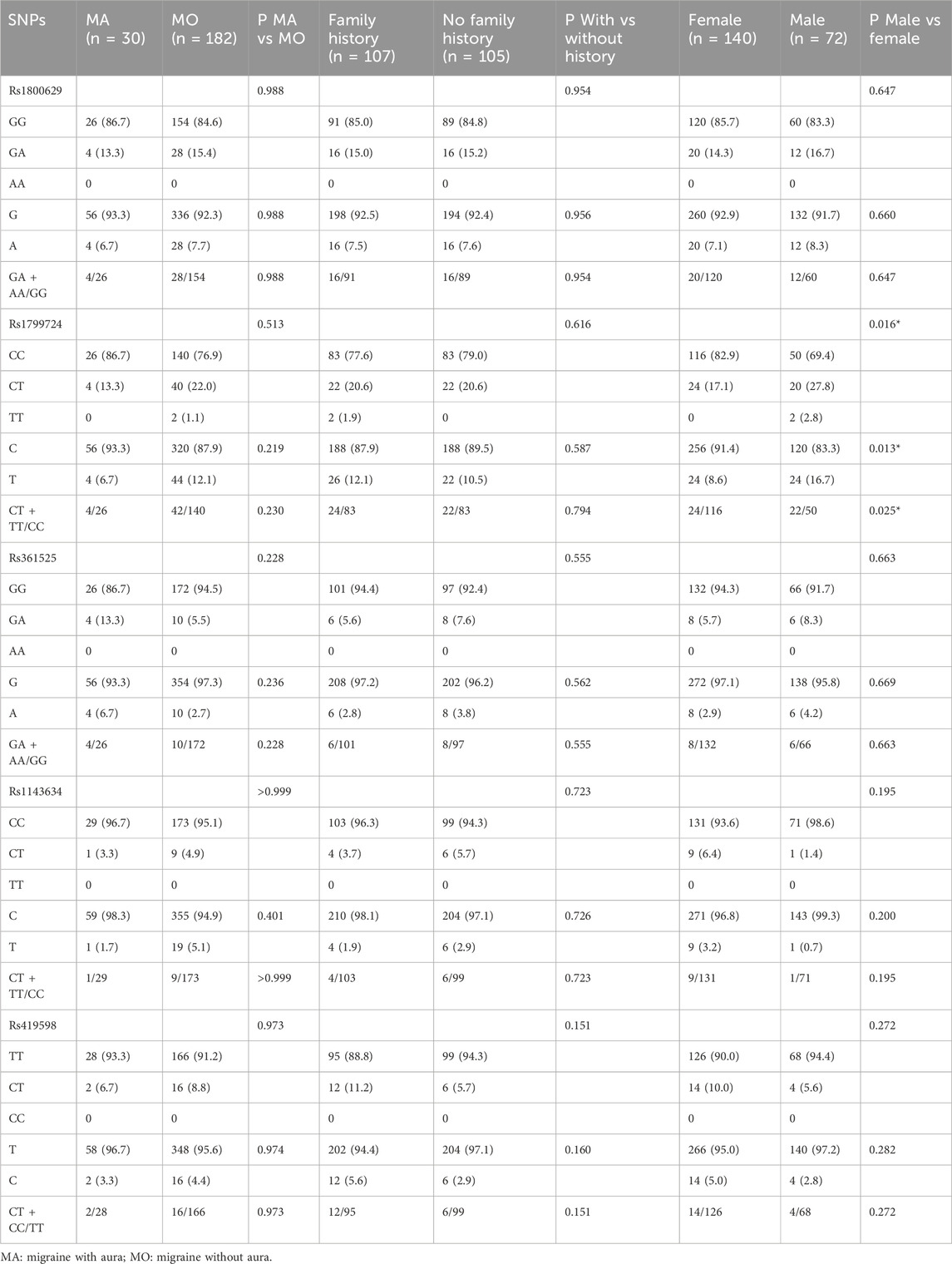
Genotype and allele associations of controls with migraine and migraine subtypesThe IL1B−3953 C/T (rs1143634) genotype was associated with migraine (P = 0.038) and with a family history of migraine (P = 0.050). The C allele frequency was higher in the migraine group than in the control group (P = 0.041). Based on the dominant model, the C allele was associated with migraine (P = 0.038) and with a family history of migraine (P = 0.050). The other SNPs analyzed in this study were not associated with migraine, migraine type, or a family history of migraine (all P > 0.05) (Table 3). The multivariable analysis showed that after adjustment for migraine type (MA/MO), family history, age, and sex, the C allele of the IL1B−3953 C/T (rs1143634) locus (dominant model) was independently associated with migraine (OR = 2.315, 95%CI: 1.053–5.090, P = 0.037) (Table 4).

Table 3. Analysis of genotypes and alleles at different loci in the control and migraine groups and subtypes.
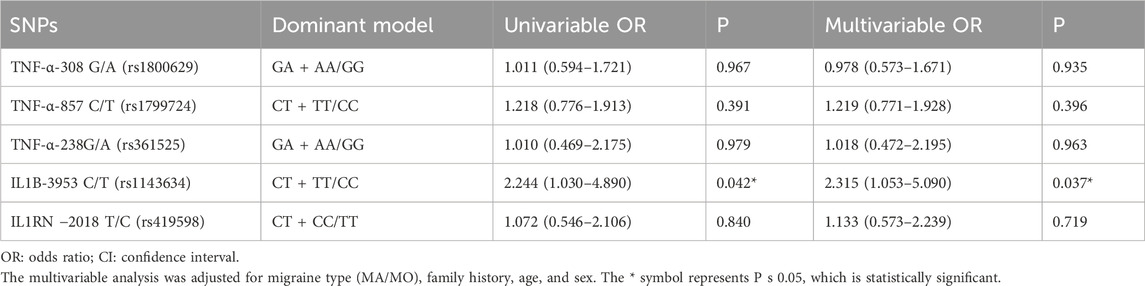
Table 4. Univariable and multivariable analyses of the polymorphic loci with migraine susceptibility.
3.3 Multifactor dimensionality reduction
The optimal interaction model identified is the second-order interaction between rs361525 and rs1143634. This model demonstrates the highest validation accuracy of 50.07% and a cross-validation consistency of 8 out of 10. The linkage coefficient between rs1143634 and rs361525 is 0.19% (Table 5). Figure 1 illustrates the high-order interaction entropy among three genetic loci: rs1143634, rs1800629, and rs361525. The information gain entropy of rs1143634 is the highest (0.75%), indicating its greatest contribution to disease prediction, while rs361525 has the smallest contribution (0.24%). Figure 2 displays high-risk and low-risk genotypes within the optimal interaction model. Dark gray cells indicate high risk (case-to-control ratio of the genotype combination ≥1.0), while light Gy cells denote low risk (case-to-control ratio of the genotype combination ≤1.0). In the figure, the combination of rs361525 = 0 and rs1143634 = 0 (case-to-control ratio = 192.0) exhibits the highest risk, whereas the combination of rs361525 = 2 and rs1143634 = 0 (case-to-control ratio = 0.0) shows the lowest risk. These results reveal key genetic loci and their interactions, providing important clues for disease prediction and mechanistic research.
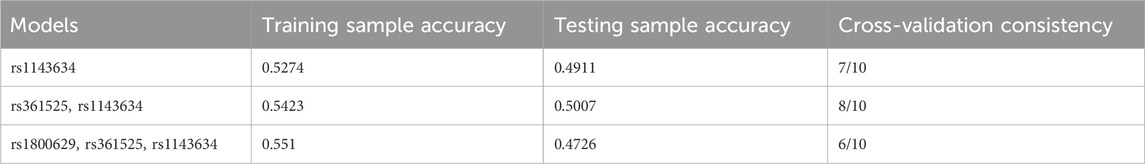
Table 5. Multifactor interaction model for MDR analysis of influencing factors of migraine in population.
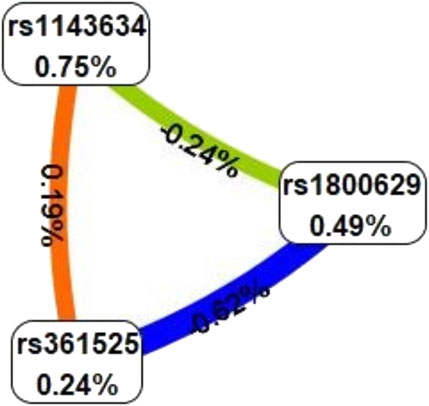
Figure 1. High order interaction entropy diagram of genes. The color gradient corresponds to the entropy of information gain, spanning from red (denoting the maximum information gain) through yellow-green to blue (representing the greatest information redundancy).
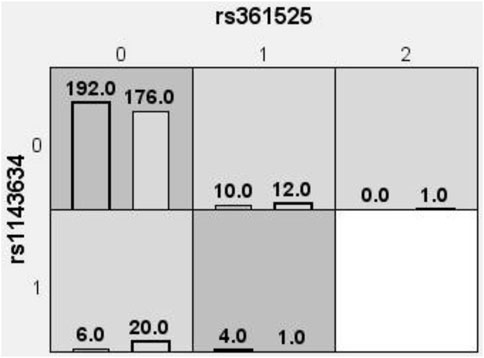
Figure 2. High-risk and low-risk genotypes within the optimal interaction model. Dark gray cells signify high risk (with a case-to-control ratio of the genotype combination ≥1.0), whereas light Gy cells denote low risk (with a case-to-control ratio of the genotype combination ≤1.0). 0 = wild-type homozygote, 1 = heterozygote, 2 = mutant homozygote.
3.4 Associations of genotypes and alleles with patient characteristics among patients with migraine
Among the patients with migraine, significant differences between sexes were observed at the TNF-α −857 C/T (rs1799724) locus at the genotype (P = 0.016), T allele frequency (P = 0.013), and T allele in the dominant model (P = 0.025), with the T alleles being more common in males. No other associations were observed (all P > 0.05) (Table 6).
4 Discussion
Migraine is a disease caused by neurovascular dysfunction, and inflammatory factors play an important role in migraine pathogenesis. This case-control study explored the associations of SNPs with migraine in the Chinese Han population. The results showed that the IL1B−3953 C/T (rs1143634) SNP is associated with susceptibility to migraine in Han Chinese. These results could have significance in managing patients with migraine and their families.
IL1B−3953 C/T (rs1143634) is in the exon five coding region, and the nucleotide change from C to T causes a synonymous mutation. Nevertheless, this study found that the frequency of the C allele in the migraine group was significantly higher than in the healthy control group, and the CT + TT genotype has a certain protective effect on migraine in the Han population. On the other hand, in a previous study in the United States, Yilmaz et al. (2010) found that the IL1B−3953 T allele was higher in migraine patients than in healthy controls. This discrepancy was also observed for other diseases among different populations. In the Iraqi population, it was shown that the TT genotype was significantly reduced in periodontitis cases (Dahash and Kusrat Hussein, 2023), while that association was not observed in the Polish population (Brodzikowska et al., 2022). In Brazil, the C/T genotype was reported to have a protective effect on rheumatoid arthritis (Gomes da Silva et al., 2020), while no differences in distribution between rheumatoid patients and controls were found in the Algerian population (Allam et al., 2013). The IL1B+3953 T allele and T/T genotype increase the risk of multiple sclerosis in Iranians (Khosravi et al., 2015). Another study in the Iranian Azerbaijani population found no significant correlation between the multiple system sclerosis group and the control group (Asgharzadeh et al., 2020). Hence, the IL1B−3,953 (rs1143634) SNP shows different effects in different diseases or across different populations. The IL-1 family members are first synthesized into inactive precursors that cannot bind to their receptors and then activated by the inflammasome (Dinarello, 2018). There are two types of IL-1 receptors in humans (Dinarello, 2018; Mailhot et al., 2020). Studies have shown that IL-1R1 ligation can lead to a variety of inflammatory responses, while IL-1R2 has also been shown to be a decoy receptor that provides a negative regulatory mechanism for inflammation (Peters et al., 2013). Therefore, different SNPs and mutations may also lead to selective binding to different receptors and effects. The allele C of Il-1β changed into allele T, which changed the ability of its competitive binding with IL-1RN to the IL-1 receptor, broke the balance between anti-inflammatory and pro-inflammatory, and led to the difference in disease among people (Tahmasebi et al., 2013). The scope of the heterogeneity of IL1B−3,953 (rs1143634) in the worldwide population is still unknown, but the present study strongly suggests that IL1B−3,953 (rs1143634) is associated with migraine in the Han Chinese population. On the other hand, the stratified analysis showed no statistically significant sex-specific differences in IL1B-3953 C/T polymorphisms in migraine patients (P = 0.195). However, the IL1B-3953 C/T SNP was significantly associated with total migraine susceptibility in the Han population. Therefore, although this SNP is associated with migraine risk, its effect does not appear to be dependent on sex in the study population.
The present study did not find significant differences in the three SNPs in the promoter region of TNF-α between migraine and control subjects. However, the differences of TNF-α −308G/A (rs1800629) and −857 (rs1799724) between migraine and control subjects were previously found in the Jordanian (Hamad et al., 2021) and Egyptian populations (Fawzi et al., 2015), which may be related to the heterogeneity of migraine. On the other hand, the present study showed sex differences in the genotype, allele, and dominant model of the TNF-α −857 (rs1799724) polymorphism in migraine patients. Since there is an important difference in migraine prevalence between men and women (Charles, 2017; MacGregor, 2017; Ferrari et al., 2022; Zhang and Robbins, 2023), the TNF-α −857 (rs1799724) genotype could play a role. However, this present study did not control the age of the enrolled patients. A Greek study in children (Pappa et al., 2010) found a significant difference in the genotype, allele, and dominant model of the TNF-α −857 (rs1799724) polymorphism in migraine patients. The difference in the results may be related to estrogen content because the difference between men and women is small before puberty. Still, the content of estrogen in women increases after puberty and peaks in the reproductive age, consistent with the peak of migraine attack frequency in the young and middle-aged and decreases after menopause (Nappi et al., 2022). It is also possible that the immune inflammatory mechanism underlying migraine has not been formed in childhood, thus affecting the expression of TNF-α (Boćkowski et al., 2010). The multivariable logistic regression analysis showed no significant associations between the TNF-α-857 C/T SNP and migraine risk, but sex-specific differences were observed in the distribution of the TNF-α-857 C/T SNP in migraine patients. Specifically, the T allele was more prevalent in males. Although this SNP does not directly increase overall migraine risk, its sex-specific distribution suggests a potential role in the differential migraine mechanisms between sexes.
The analysis showed no significant differences in genotype or allele frequencies between MA and MO for TNF-α-857 C/T (rs1799724) or IL1B−3953 C/T (rs1143634) (all P > 0.05). Therefore, these SNPs do not appear to affect migraine subtype differentiation in the study population.
This study has limitations. First, migraine, except for familial hemiplegic migraine that is caused by a single gene, is mostly considered to be a polygenic multifactorial disease, and the results of the associations will vary among different populations with different genetics and environmental exposures. Hence, future studies on migraine will have to consider environmental and genetic factors. In this paper, only the local population was included, limiting generalizability.
The IL1B−3953 C/T (rs1143634) SNP is associated with migraine susceptibility in the Han Chinese population. There are sex differences at the TNF-α −857 C/T (rs1799724) locus in migraine patients. No significant associations were found between TNF-α −308 G/A (rs1800629), TNF-α −857 C/T (rs1799724), TNF-α −238G/A (rs361525), and IL1RN −2018 T/C (rs419598) and migraine.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of First People’s Hospital of Lianyungang (LW-20171231001/LW-20171231002). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
GH: Data curation, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review and editing. XD: Data curation, Investigation, Project administration, Resources, Software, Visualization, Writing – original draft, Writing – review and editing. XS: Conceptualization, Data curation, Methodology, Project administration, Resources, Writing – original draft, Writing – review and editing. MW: Data curation, Formal Analysis, Funding acquisition, Investigation, Project administration, Software, Writing – original draft, Writing – review and editing. SG: Formal Analysis, Methodology, Resources, Software, Writing – original draft, Writing – review and editing. YW: Data curation, Formal Analysis, Methodology, Project administration, Resources, Software, Writing – original draft, Writing – review and editing. XG: Data curation, Investigation, Project administration, Resources, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. 1. National Natural Science Foundation of China Youth Project (82001177). 2. Jiangsu University “Blue Project.” 3. Lianyungang City Science and Technology Plan (Social Development) [Project KJSKJJZC24013].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2025.1556498/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Sanger sequencing confirmation of rs1800629.GAGGCAATAGGTTTTGAGGGGCATG[A/G]GGACGGGGTTCAGCCTCCAGGGTCC.
SUPPLEMENTARY FIGURE S2 | Sanger sequencing confirmation of rs1799724. GTCGAGTATGGGGACCCCCCCTTAA[C/T]GAAGACAGGGCCATGTAGAGGGCCC.
SUPPLEMENTARY FIGURE S3 | Sanger sequencing confirmation of rs361525. GGCCCAGAAGACCCCCCTCGGAATC[A/G]GAGCAGGGAGGATGGGGAGTGTGAG.
SUPPLEMENTARY FIGURE S4 | Sanger sequencing confirmation of rs1143634. CATAAGCCTCGTTATCCCATGTGTC[A/G]AAGAAGATAGGTTCTGAAATGTGGA.
SUPPLEMENTARY FIGURE S5 | Sanger sequencing confirmation of rs419598. ATCTGAGGAACAACCAACTAGTTGC[C/T]GGATACTTGCAAGGACCAAATGTCA.
References
Allam, I., Djidjik, R., Ouikhlef, N., Louahchi, S., Raaf, N., Behaz, N., et al. (2013). Interleukin-1 and the interleukin-1 receptor antagonist gene polymorphisms study in patients with rheumatoid arthritis. Pathol. Biol. Paris. 61 (6), 264–268. doi:10.1016/j.patbio.2013.05.005
Asgharzadeh, M., Jigheh, Z. A., Kafil, H. S., Farhoudi, M., Oskouei, D. S., Khaki-Khatibi, F., et al. (2020). Association of interleukin-1 and inteleukin-1 receptor antagonist gene polymorphisms with multiple sclerosis in azeri population of Iran. Endocr. Metab. Immune Disord. Drug Targets 20 (7), 1110–1116. doi:10.2174/1871530320666200309142541
Aytac, H. M., Ozdilli, K., Tuncel, F. C., Pehlivan, M., and Pehlivan, S. (2022). Tumor necrosis factor-alpha (TNF-α) -238 G/A polymorphism is associated with the treatment resistance and attempted suicide in schizophrenia. Immunol. Invest. 51 (2), 368–380. doi:10.1080/08820139.2020.1832115
Boćkowski, L., Smigielska-Kuzia, J., Sobaniec, W., Zelazowska-Rutkowska, B., Kułak, W., and Sendrowski, K. (2010). Anti-inflammatory plasma cytokines in children and adolescents with migraine headaches. Pharmacol. Rep. 62 (2), 287–291. doi:10.1016/s1734-1140(10)70268-1
Brodzikowska, A., Górski, B., and Bogusławska-Kapała, A. (2022). Association between IL-1 gene polymorphisms and stage III grade B periodontitis in polish population. Int. J. Environ. Res. Public Health 19 (22), 14687. doi:10.3390/ijerph192214687
Burstein, R., Noseda, R., and Borsook, D. (2015). Migraine: multiple processes, complex pathophysiology. J. Neurosci. 35 (17), 6619–6629. doi:10.1523/jneurosci.0373-15.2015
Charles, A. (2018). The pathophysiology of migraine: implications for clinical management. Lancet Neurol. 17 (2), 174–182. doi:10.1016/s1474-4422(17)30435-0
Cropper, H. C., and Pradhan, A. A. (2022). Seq-ing the mechanisms of migraine. Neuron 110 (11), 1745–1746. doi:10.1016/j.neuron.2022.04.028
Dahash, S. A., and Kusrat Hussein, L. (2023). Interleukin-1β rs1143634 polymorphism and susceptibility to periodontitis in the Iraqi population. Arch. Razi Inst. 78 (2), 751–756. doi:10.22092/ari.2022.359864.2491
Dinarello, C. A. (2018). Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 281 (1), 8–27. doi:10.1111/imr.12621
Dong, W., Jia, S., Ye, X., and Ni, J. (2012). Association analysis of TNFRSF1B polymorphism with susceptibility for migraine in the Chinese Han population. J. Clin. Neurosci. 19 (5), 750–752. doi:10.1016/j.jocn.2011.08.033
Fawzi, M. S., El-Shal, A. S., Rashad, N. M., and Fathy, H. A. (2015). Influence of tumor necrosis factor alpha gene promoter polymorphisms and its serum level on migraine susceptibility in Egyptian patients. J. Neurol. Sci. 348 (1-2), 74–80. doi:10.1016/j.jns.2014.11.009
Ferrari, M. D., Goadsby, P. J., Burstein, R., Kurth, T., Ayata, C., Charles, A., et al. (2022). Migraine. Nat. Rev. Dis. Prim. 8 (1), 2. doi:10.1038/s41572-021-00328-4
Gao, W., Zhu, R., and Yang, L. (2021). Association of tumor necrosis factor-alpha-308 G/A and -238 G/A polymorphism with diabetic retinopathy: a systematic review and updated meta-analysis. Ophthalmic Res. 64 (6), 903–915. doi:10.1159/000513586
Gomes da Silva, I. I. F., Lima, C. A. D., Monteiro, M. L. A., Barboza, D., Rushansky, E., Mariano, M., et al. (2020). IL1β, IL18, NFKB1 and IFNG gene interactions are associated with severity of rheumatoid arthritis: a pilot study. Autoimmunity 53 (2), 95–101. doi:10.1080/08916934.2019.1710831
Gu, L., Yan, Y., Long, J., Su, L., Hu, Y., Chen, Q., et al. (2012). The TNF-alpha-308G/A polymorphism is associated with migraine risk: a meta-analysis. Exp. Ther. Med. 3 (6), 1082–1086. doi:10.3892/etm.2012.533
Hamad, N., Alzoubi, K. H., Swedan, S. F., Khabour, O. F., and El-Salem, K. (2021). Association between tumor necrosis factor alpha and lymphotoxin alpha gene polymorphisms and migraine occurrence among Jordanians. Neurol. Sci. 42 (9), 3625–3630. doi:10.1007/s10072-020-04967-5
Khosravi, A., Javan, B., Tabatabaiefar, M. A., Ebadi, H., Fathi, D., and Shahbazi, M. (2015). Association of interleukin-1 gene cluster polymorphisms and haplotypes with multiple sclerosis in an Iranian population. J. Neuroimmunol. 288, 114–119. doi:10.1016/j.jneuroim.2015.09.009
Lara-Gómez, R. E., Moreno-Cortes, M. L., Muñiz-Salazar, R., and Zenteno-Cuevas, R. (2019). Association of polymorphisms at -174 in IL-6, and -308 and -238 in TNF-α, in the development of tuberculosis and type 2 diabetes mellitus in the Mexican population. Gene 702, 1–7. doi:10.1016/j.gene.2019.03.050
MacGregor, E. A. (2017). Migraine. Ann. Intern Med. 166 (7), ITC49-ITC64–ITC64. doi:10.7326/AITC201704040
Mailhot, B., Christin, M., Tessandier, N., Sotoudeh, C., Bretheau, F., Turmel, R., et al. (2020). Neuronal interleukin-1 receptors mediate pain in chronic inflammatory diseases. J. Exp. Med. 217 (9), e20191430. doi:10.1084/jem.20191430
Mendoza-Carrera, F., Ramírez-López, G., Hernández-Ramos, L. E., Leal-Cortés, C., Portilla-de-Buen, E., Castro-Martínez, X. H., et al. (2019). Interleukin-1 alpha polymorphisms are associated with body mass index in male but not in female adolescents. Arch. Med. Res. 50 (3), 151–157. doi:10.1016/j.arcmed.2019.07.006
Nappi, R. E., Tiranini, L., Sacco, S., De Matteis, E., De Icco, R., and Tassorelli, C. (2022). Role of estrogens in menstrual migraine. Cells 11 (8), 1355. doi:10.3390/cells11081355
Pappa, S., Hatzistilianou, M., Kouvatsi, A., Pantzartzi, C., Sakellaropoulou, A., Pavlou, E., et al. (2010). Tumour necrosis factor gene polymorphisms and migraine in Greek children. Arch. Med. Sci. 6 (3), 430–437. doi:10.5114/aoms.2010.14267
Peters, V. A., Joesting, J. J., and Freund, G. G. (2013). IL-1 receptor 2 (IL-1R2) and its role in immune regulation. Brain Behav. Immun. 32, 1–8. doi:10.1016/j.bbi.2012.11.006
Saylor, D., and Steiner, T. J. (2018). The global burden of headache. Semin. Neurol. 38 (2), 182–190. doi:10.1055/s-0038-1646946
Schulte, L. H., Allers, A., and May, A. (2017). Hypothalamus as a mediator of chronic migraine: evidence from high-resolution fMRI. Neurology 88 (21), 2011–2016. doi:10.1212/wnl.0000000000003963
Schurks, M., Rist, P. M., Zee, R. Y., Chasman, D. I., and Kurth, T. (2011). Tumour necrosis factor gene polymorphisms and migraine: a systematic review and meta-analysis. Cephalalgia 31 (13), 1381–1404. doi:10.1177/0333102411419022
Sudershan, A., Sudershan, S., Younis, M., Bhagat, M., Pushap, A. C., Kumar, H., et al. (2023). Enlightening the association between TNF-alpha -308 G > A and migraine: a meta-analysis with meta-regression and trial sequential analysis. BMC Neurol. 23 (1), 159. doi:10.1186/s12883-023-03174-x
Sun, X., He, Y., Shen, Z., and Chen, L. (2022). TNF-Α (G308A) polymorphism and vascular Parkinson complicated with pulmonary infection. J. Coll. Physicians Surg. Pak 32 (7), 860–863. doi:10.29271/jcpsp.2022.07.860
Sutherland, H. G., Albury, C. L., and Griffiths, L. R. (2019). Advances in genetics of migraine. J. Headache Pain 20 (1), 72. doi:10.1186/s10194-019-1017-9
Tahmasebi, Z., Akbarian, M., Mirkazemi, S., Shahlaee, A., Alizadeh, Z., Amirzargar, A. A., et al. (2013). Interleukin-1 gene cluster and IL-1 receptor polymorphisms in Iranian patients with systemic lupus erythematosus. Rheumatol. Int. 33 (10), 2591–2596. doi:10.1007/s00296-013-2784-2
Wang, Y., Wang, J., Zheng, W., Zhang, J., Wang, J., Jin, T., et al. (2023). Identification of an IL-1 receptor mutation driving autoinflammation directs IL-1-targeted drug design. Immunity 56 (7), 1485–1501.e7. doi:10.1016/j.immuni.2023.05.014
Yamanaka, G., Hayashi, K., Morishita, N., Takeshita, M., Ishii, C., Suzuki, S., et al. (2023). Experimental and clinical investigation of cytokines in migraine: a narrative review. Int. J. Mol. Sci. 24 (9), 8343. doi:10.3390/ijms24098343
Yilmaz, I. A., Ozge, A., Erdal, M. E., Edgünlü, T. G., Cakmak, S. E., and Yalin, O. O. (2010). Cytokine polymorphism in patients with migraine: some suggestive clues of migraine and inflammation. Pain Med. 11 (4), 492–497. doi:10.1111/j.1526-4637.2009.00791.x
Keywords: inflammation, interleukins-1, migraine, tumor necrosis factor-alpha, single nucleotide polymorphisms
Citation: Huang G, Dong X, Shao X, Wu M, Gao S, Wang Y and Guan X (2025) Correlation of tumor necrosis factor-α and interleukin-1 single-nucleotide polymorphisms with the risk of migraine development. Front. Genet. 16:1556498. doi: 10.3389/fgene.2025.1556498
Received: 07 January 2025; Accepted: 03 April 2025;
Published: 25 April 2025.
Edited by:
Salvatore Gallone, University of Turin, ItalyReviewed by:
Nancy Lucero Martínez Rodríguez, Federico Gómez Children’s Hospital, MexicoSivasamy Ramasamy, Bharathiar University, India
Copyright © 2025 Huang, Dong, Shao, Wu, Gao, Wang and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinying Guan, Z3h5MTIzMTAwN0Bob3RtYWlsLmNvbQ==
†These authors share first authorship
 Guijiao Huang1†
Guijiao Huang1† Xinying Guan
Xinying Guan