- Department of Gastroenterology, The Quzhou Affiliated Hospital of Wenzhou Medical University, Quzhou People’s Hospital, Quzhou, China
Background: Genetic polymorphisms, such as PSCA rs2976392, have been implicated in gastric carcinogenesis, but it is unclear whether there is a direct association. Thus, we conducted a comprehensive meta-analysis to evaluate the association between the PSCA rs2976392 polymorphism and susceptibility to gastric cancer (GC).
Methods: A systematic search of the PubMed, Web of Science, Embase, and Cochrane Library databases was performed up to 13 March 2025. Pooled odds ratios (ORs) with 95% confidence intervals (CIs) were calculated for five genetic models. Subgroup analyses were conducted according to ethnicity and Hardy–Weinberg equilibrium (HWE) status. Heterogeneity and publication bias were assessed, and sensitivity analyses were performed.
Results: A total of 13 studies involving 9,255 patients with gastric cancer and 8,903 controls were included. Overall, a significant association between the PSCA rs2976392 polymorphism and increased GC risk was observed under the allele model (OR = 1.29, 95% CI: 1.19–1.40), dominant model (OR = 1.53, 95% CI: 1.3–1.77), homozygous model (OR = 1.52, 95% CI: 1.18–1.96), and heterozygous model (OR = 1.52, 95% CI: 1.33–1.73), but not under the recessive model. Subgroup analyses revealed a strong association in Asian populations with all genetic models, whereas Caucasians showed a significant association only with the homozygous model. No significant publication bias was detected, and sensitivity analyses confirmed the robustness of the results.
Conclusion: This meta-analysis provides strong evidence that the PSCA rs2976392 AA genotype significantly increases susceptibility to gastric cancer, particularly among Asians.
Introduction
Gastric cancer (GC) is one of the most common malignancies worldwide and represents one of the four leading causes of cancer-related deaths (Siegel et al., 2025). Although the incidence and mortality rates of GC have markedly decreased in some countries, GC remains a major public health challenge because of its late diagnosis and poor prognosis (Thrift et al., 2023). The development of gastric cancer is a multifactorial and multistep process influenced by both environmental and genetic factors. Well-established environmental risk factors include Helicobacter pylori infection, dietary habits, smoking, and alcohol consumption (Maubach et al., 2025; Wizenty and Sigal, 2025). However, there is growing evidence that genetic susceptibility factors contribute to the occurrence and development of GC.
Genome-wide association studies (GWASs) have revealed numerous single nucleotide polymorphisms (SNPs) associated with gastric cancer susceptibility (Wang et al., 2017). Among them, polymorphisms in the prostate stem cell antigen (PSCA) gene have attracted increasing attention (Hu et al., 2016). The PSCA gene, located on chromosome 8q24.2, encodes a glycosylphosphatidylinositol-anchored cell surface protein that is involved in cell adhesion, proliferation, and differentiation (Lao-Sirieix et al., 2010). PSCA expression has been found to be downregulated in gastric cancer tissues compared with adjacent normal tissues, suggesting a potential tumor suppressor role in gastric cancer (Xu et al., 2020).
One particular SNP, rs2976392, which is located in the 5′-UTR of the PSCA gene, has been identified as a possible functional variant affecting PSCA transcriptional activity (Lu et al., 2010). This polymorphism may functionally influence PSCA expression levels, thereby contributing to individual differences in cancer susceptibility (Study Group of Millennium Genome Project for Cancer et al., 2008; Merrell et al., 2011). Several studies indicate that the A allele of rs2976392 is associated with an increased risk of gastric cancer (Matsuo et al., 2009; Ou et al., 2010; Kupcinskas et al., 2014; Cai et al., 2017; Dantas et al., 2020), possibly through altered gene expression and increased susceptibility of gastric mucosal cells to malignant transformation, but the results are conflicting and inconclusive.
Many studies indicate an association between PSCA rs2976392 polymorphism and GC risk (Matsuo et al., 2009; Lu et al., 2010; Ou et al., 2010; Kupcinskas et al., 2014; Qiu et al., 2016; Cai et al., 2017; Dantas et al., 2020). However, this association remains a subject of controversy. Therefore, the aim of the present study is to synthesize data from case-control studies to comprehensively evaluate the association between PSCA rs2976392 polymorphism and GC susceptibility.
Materials and methods
Search strategy
The current study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines (Page et al., 2021). A comprehensive literature search on PubMed, Web of Science, Cochrane Library, and Embase databases to acquire relevant studies about the association of PSCA rs2976392 polymorphism and susceptibility to gastric cancer up to 13 March 2025. The combination of the following keywords and terms was used: “PSCA”, “rs2976392”, “polymorphism”, “SNP”, and “gastric cancer”. Reference lists of retrieved articles were manually searched to identify suitable studies.
Inclusion and exclusion criteria
Available studies were included based on the following criteria: (1) case-control or cohort studies; (2) examined the association of the PSCA rs2976392 polymorphism with gastric cancer risk; (3) provided sufficient data to calculate odds ratio (OR) with a 95% confidence interval. Exclusion criteria included: (1) duplicated or overlapping data; (2) reviews, meta-analyses, case reports, and conference summaries; (3) insufficient data to analyze.
Data extraction and quality assessment
Two investigators independently searched, assessed, and extracted data based on predefined inclusion and exclusion criteria. Disagreements were resolved by discussion or consultation with a third reviewer. The following information was extracted: first author, publication year, country, ethnicity, sample size, genotype frequencies, and Hardy-Weinberg equilibrium (HWE) status in controls. The quality of studies was assessed using the Newcastle-Ottawa Scale (NOS), and studies scoring ≥6 were considered high-quality.
Statistical analysis
The strength of the association of PSCA rs2976392 polymorphism with gastric cancer risk was examined by odd ratios (ORs) with 95% confidence intervals (CIs) under five genetic models: allele model (A vs. G), dominant model (GA + AA vs. GG), recessive model (AA vs. GA + GG), homozygous model (AA vs. GG), and heterozygous model (GA vs. GG). Hardy-Weinberg equilibrium (HWE) assessed by chi-square test. Between-studies heterogeneity was assessed with Cochran’s Q and the I2 statistics. Fixed-effects models (Mantel–Haenszel) were used when I2 < 50%, otherwise random-effects models (DerSimonian–Laird) were applied. Subgroup analyses were conducted by stratification of ethnicity and HWE test to identify potential sources of heterogeneity. Publication bias was evaluated using Begg’s funnel plots and Egger’s test. All the statistical analyses were performed using STATA 12.0 (StataCorp., T.X., United States).
Results
Study selection and study characteristics
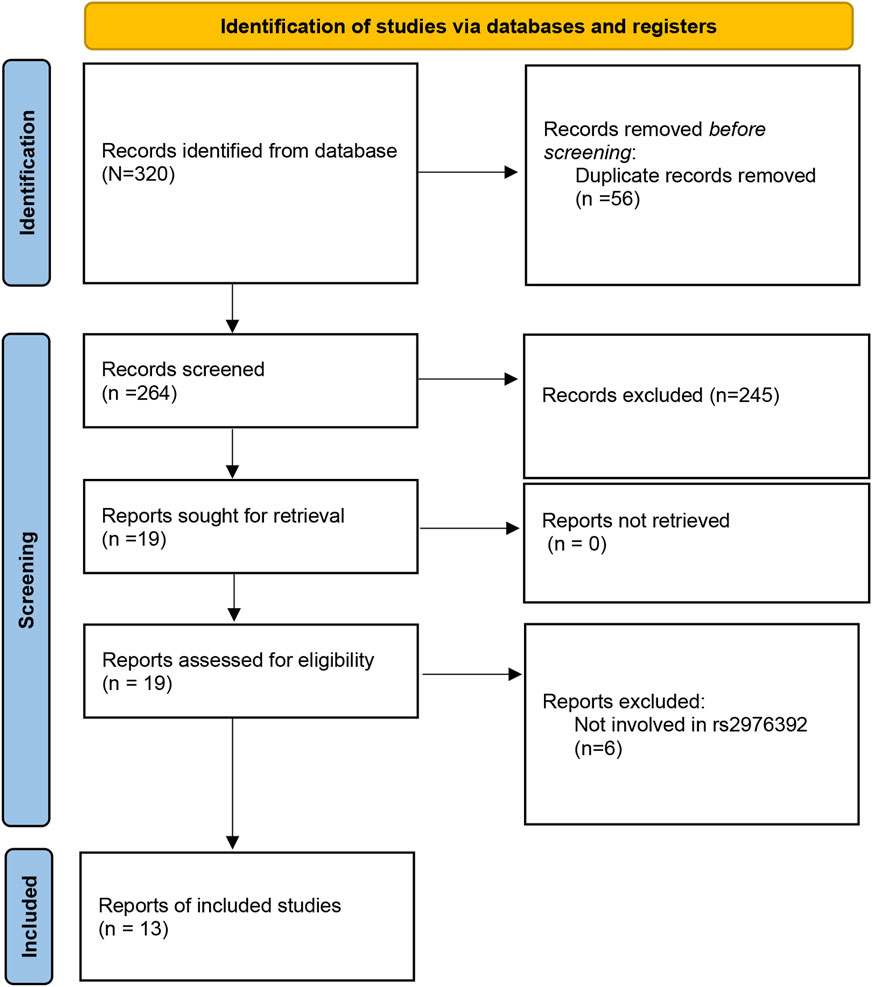
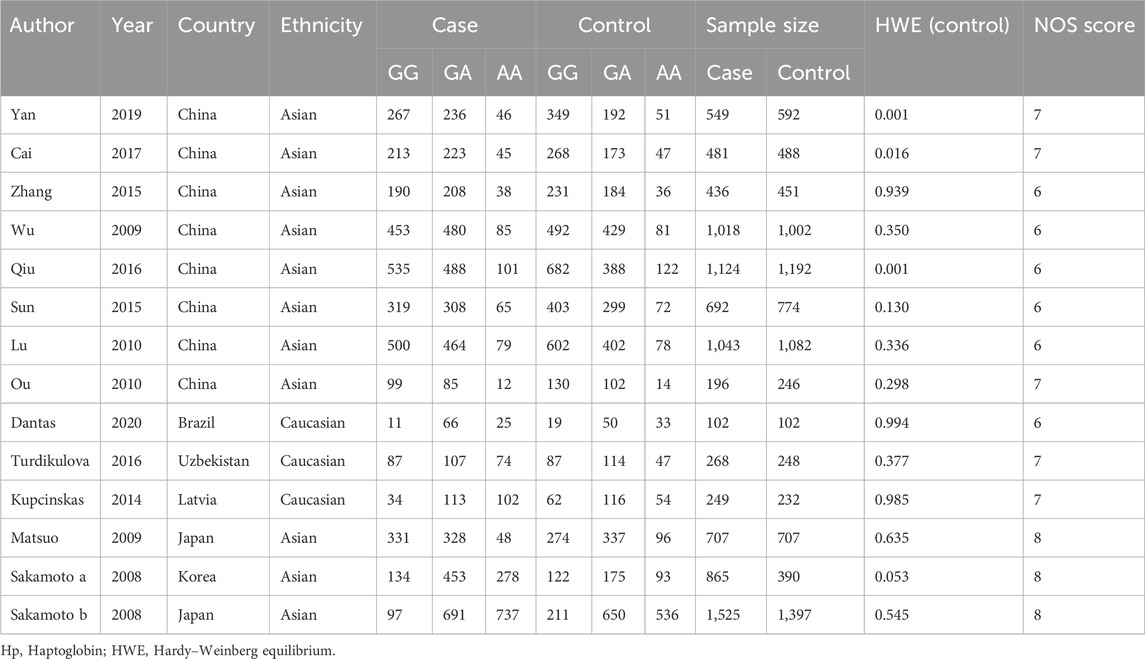
The initial search of the PubMed, Web of Science, Cochrane Library, and Embase databases identified 320 potential studies. Among them, 56 duplicate studies were then removed, leaving 141 studies for further selection. Among these articles, 245 studies were excluded due to screening of the abstract and title. After full-text screening, 6 papers were excluded due to irrelevant reports. Finally, a total of 13 studies (Study Group of Millennium Genome Project for Cancer et al., 2008; Matsuo et al., 2009; Wu et al., 2009; Lu et al., 2010; Ou et al., 2010; Kupcinskas et al., 2014; Sun et al., 2015; Zhang et al., 2015; Qiu et al., 2016; Turdikulova et al., 2016; Cai et al., 2017; Yan et al., 2019; Dantas et al., 2020) involving 9,255 cases and 8,903 controls were included in the meta-analysis (Figure 1). The publication year of the studies ranged from 2008 to 2020. The countries of these studies included Japan, China, Brazil, Uzbekistan, Latvia, Japan, and Korea. The participants included Caucasians and Asians population. The genotype distribution in the control group for 3 studies was not consistent with HWE. The Newcastle-Ottawa Scale (NOS) scores are shown in Table 2, with the scores of the included studies ranged from six to eight (Table 1). The Kappa’s score for data extraction was 0.831, indicating good interobserver agreement between the two investigators.
Quantitative synthesis
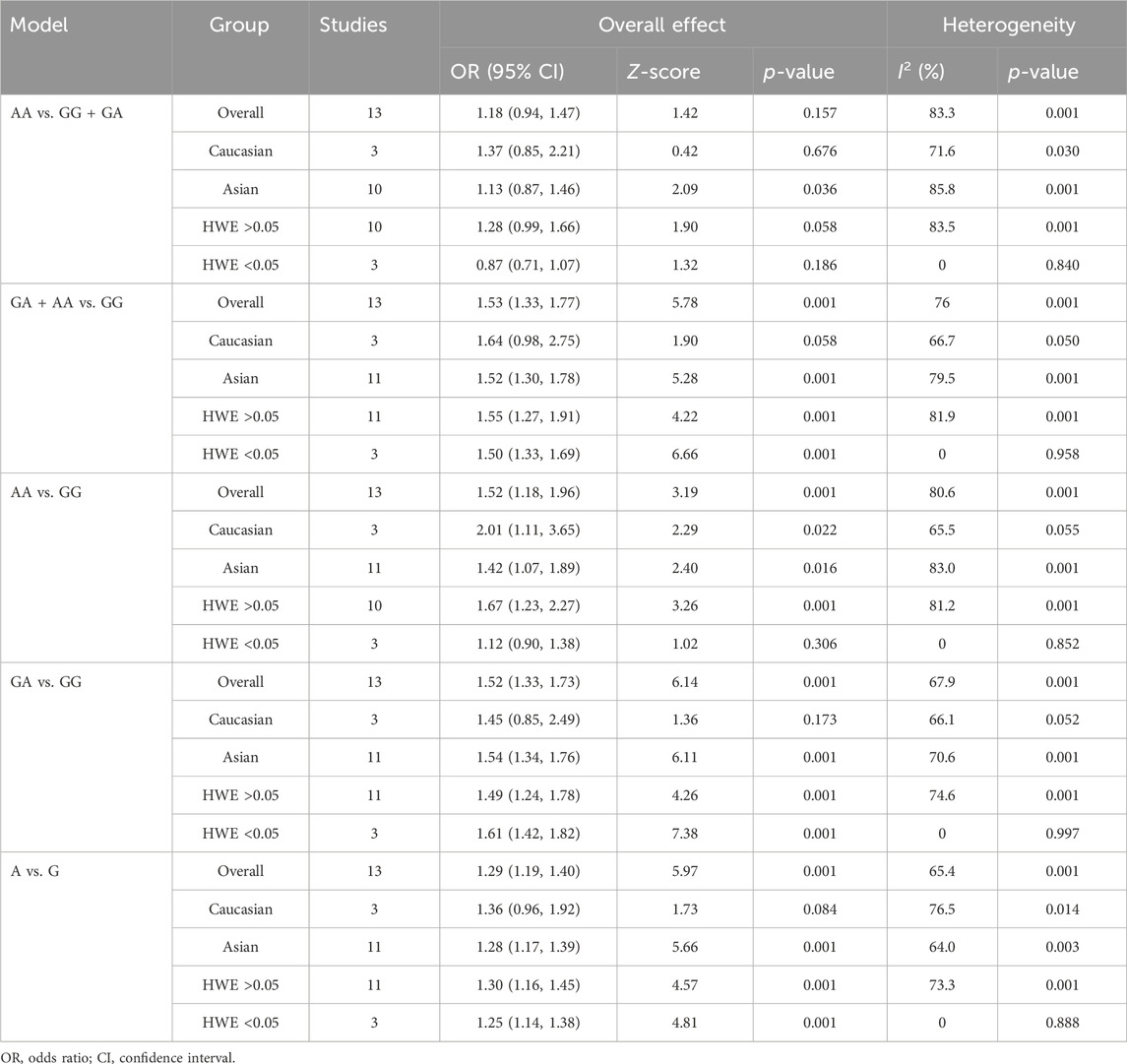
The strength of the association between the PSCA rs2976392 polymorphism and gastric cancer risk is presented in Table 2. A significant association was observed under the allele model (A vs. G), with a pooled OR of 1.29 (95% CI: 1.19–1.40; P = 0.001), indicating that the A allele is associated with increased GC risk (Figure 2). In the dominant model, individuals carrying A allele showed a significantly higher risk of gastric cancer compared to GG homozygotes, with a pooled OR of 1.53 (95% CI: 1.33–1.77, P = 0.001) (Figure 3). A stronger association was detected in the homozygous comparison (AA vs. GG), where the pooled OR was 1.52 (95% CI: 1.18–1.96, P = 0.001) (Figure 4). In the heterozygous model, individuals with the GA genotype also exhibited an increased risk compared to GG homozygotes (OR = 1.52, 95% CI = 1.33–1.73, P = 0.001) (Figure 5), but no association was observed in the recessive model (OR = 1.18, 95% CI = 0.94–1.47, P = 0.157) (Figure 6). Subgroup analyses were carried out according to ethnicity, we found a significant association between PSCA rs2976392 polymorphism and GC risk among the Asian population under the four genetic model (GA + AA vs. GG: OR = 1.52, 95%CI: 1.30–1.78, P = 0.001; AA vs. GG: OR = 1.42, 95%CI: 1.07–1.89, P = 0.016; GA vs. GG: OR = 1.54, 95%CI: 1.34–1.76, P = 0.001; A vs. G: OR = 1.28, 95%CI: 1.17–1.39, P = 0.001), and only associated with the Caucasian population under the homozygous genetic model (AA vs. GG: OR = 2.01, 95%CI: 1.11–3.65, P = 0.022). Moreover, after stratified by HWE test, a significant correlation was observed in the subgroup of HWE >0.05 under allele model, dominant model, homozygous model, and heterozygous model, but not in the recessive model (Table 2).
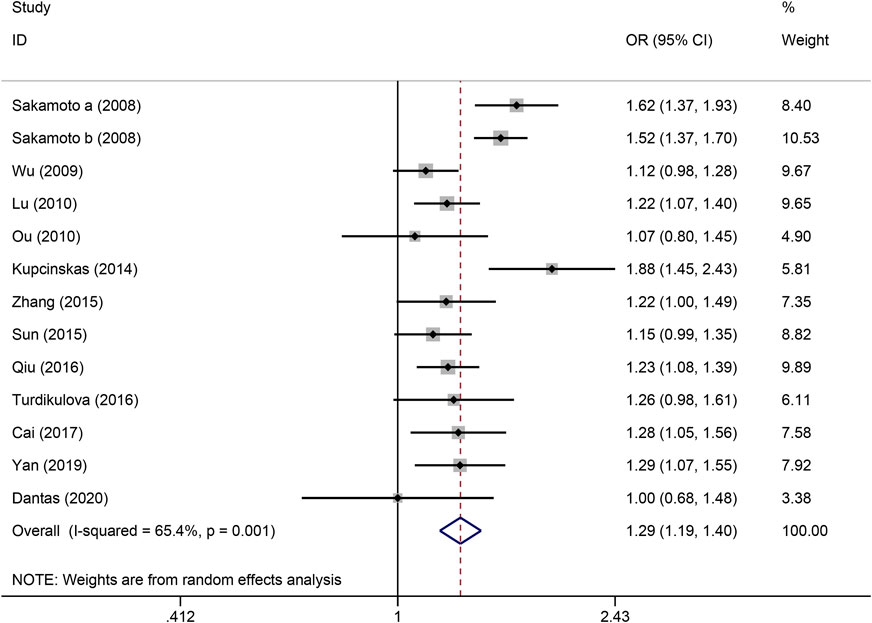
Figure 2. Forest plot of the correlation between PSCA rs2976392 polymorphism and gastric cancer in allele model (A vs. G).
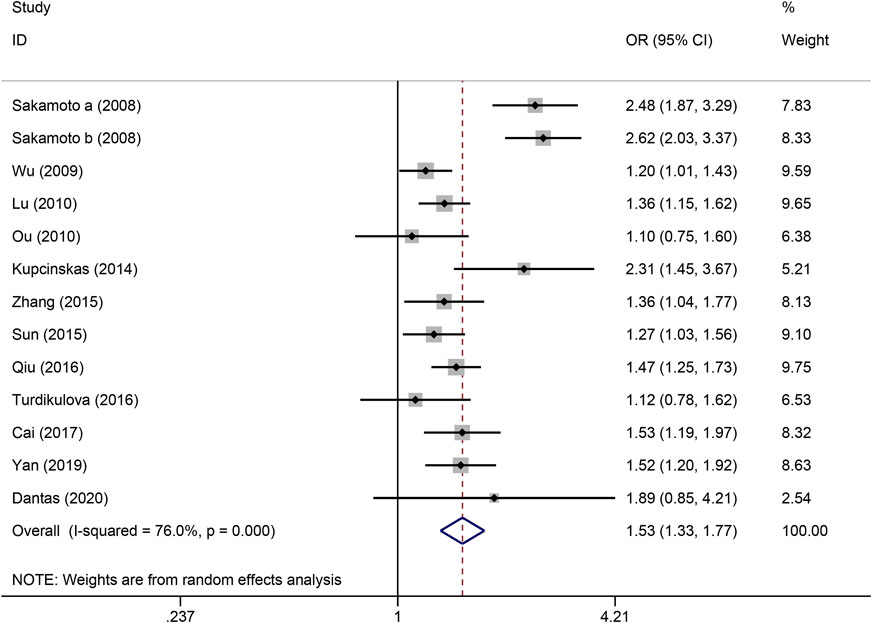
Figure 3. Forest plot of the correlation between PSCA rs2976392 polymorphism and gastric cancer in dominant model (GA + AA vs. GG).
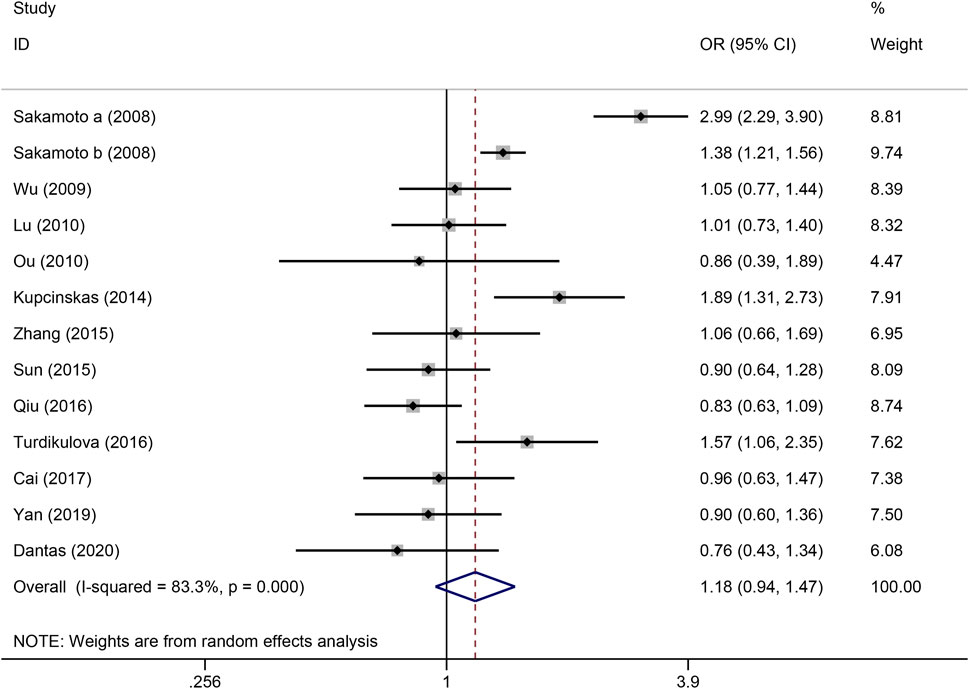
Figure 4. Forest plot of the correlation between PSCA rs2976392 polymorphism and gastric cancer in recessive model (AA vs. GA + GG).
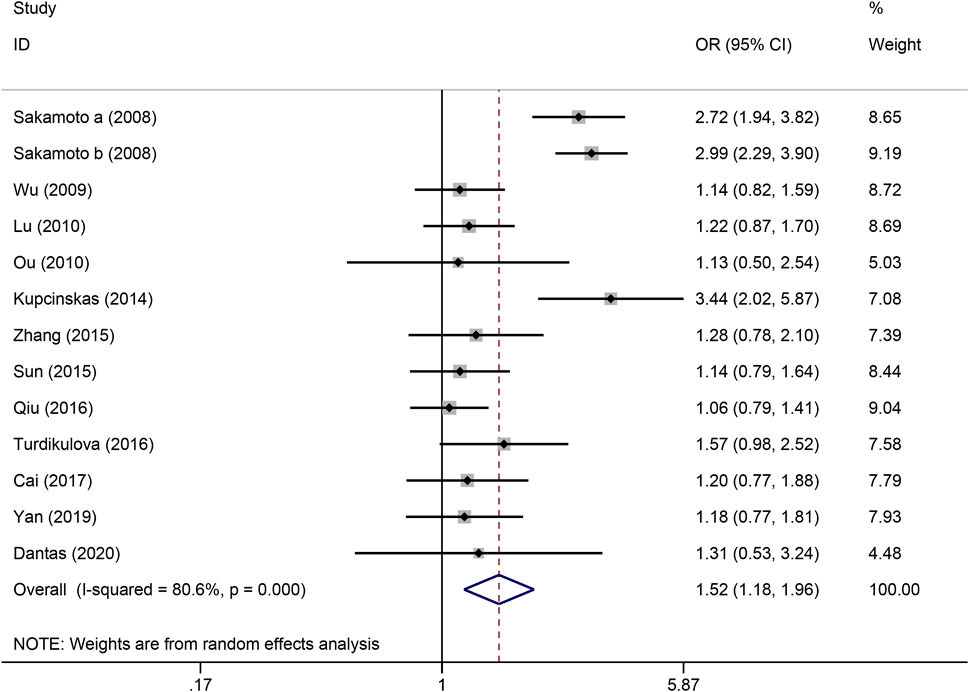
Figure 5. Forest plot of the correlation between PSCA rs2976392 polymorphism and gastric cancer in homozygous model (AA vs. GG).
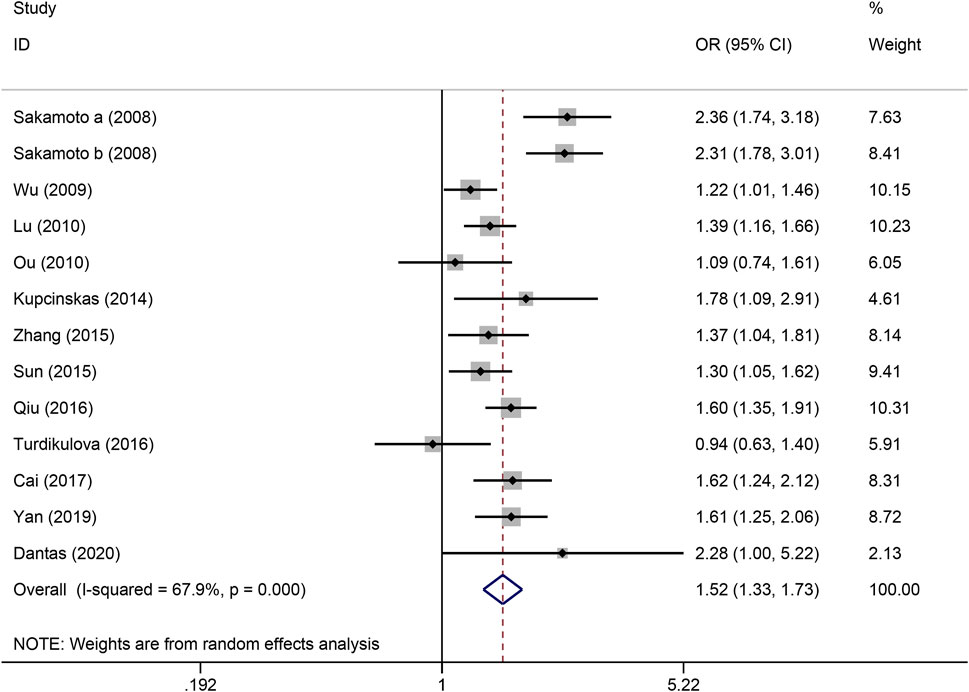
Figure 6. Forest plot of the correlation between PSCA rs2976392 polymorphism and gastric cancer in heterozygous model (GA vs. GG).
Publication bias and sensitivity analysis
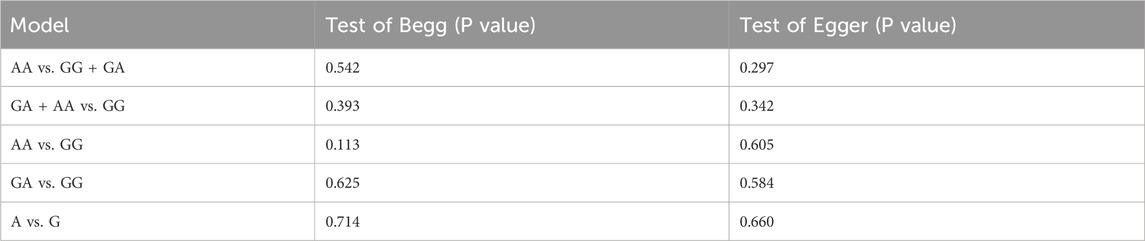
The Begg’s and Egger’s tests were used to assess the publication bias of the studies involved in this meta-analysis. As shown in Table 3, no obvious publication bias was detected in all models. Moreover, we conducted sensitivity analysis to estimate the influence of individual study on pooled results, and none of the single study affected the pooled OR value, which indicated results were robust.
Discussion
In this meta-analysis, we systematically evaluated the association between PSCA rs2976392 polymorphism and the risk of gastric cancer. By combining data from 13 studies, our results provide robust evidence supporting the significant association of the rs2976392 A allele with increased susceptibility to gastric cancer, particularly in Asian populations. These findings help clarify previously conflicting results and offer new insights into the genetic susceptibility underlying gastric carcinogenesis.
Several previous case-control studies have explored the role of rs2976392 in gastric cancer, but the results have been inconsistent (Kupcinskas et al., 2014; Cai et al., 2017; Dantas et al., 2020). For example, a study by Matsuo et al. (2009) in a Japanese cohort reported a strong correlation between the rs2976392 A allele and an increased risk of stomach cancer, whereas Ou et al. (2010) failed to detect any significant association. Such inconsistencies may arise from limited sample sizes, ethnic heterogeneity, or deviations from Hardy-Weinberg equilibrium. By integrating data from multiple studies and conducting subgroup analyses by ethnicity and HWE status, our meta-analysis overcomes these limitations and reveals a more comprehensive and accurate association pattern. Importantly, we confirmed the presence of a significant association in Asians under all five genetic models, whereas the association in Caucasians was observed only under the homozygous model, suggesting that ethnic-specific genetic background and environmental interactions may modulate the functional relevance of rs2976392.
From a mechanistic perspective, rs2976392 is located in the 5′ untranslated region (UTR) of the PSCA gene, which may influence gene transcriptional activity or mRNA stability (Larios-Serrato et al., 2024). Previous functional studies have indicated that the a allele may lead to reduced PSCA expression, impairing its potential tumor suppressor role in gastric epithelial cells. This is supported by transcriptomic and immunohistochemical studies that demonstrate decreased PSCA levels in gastric cancer tissues compared with adjacent normal mucosa (Chandra et al., 2016; Wu et al., 2020). Reduced PSCA expression may promote abnormal proliferation, loss of cell polarity, and immune evasion, all of which are hallmarks of gastric tumorigenesis (Zhang et al., 2015; Xu et al., 2020). In particular, PSCA has been shown to modulate signaling pathways such as the p38/NF-κB and NF-κB/integrin-α4 pathway, which are involved in cytoskeletal remodeling and motility regulation (Liu et al., 2017; Zhao et al., 2020). Moreover, PSCA downregulation may impair immune surveillance. As a membrane-associated antigen, PSCA may facilitate immune recognition by cytotoxic T cells and natural killer (NK) cells. Loss of PSCA expression on the tumor surface could result in decreased antigen visibility, allowing tumor cells to evade immune detection. Additionally, PSCA downregulation may influence the recruitment or polarization of tumor-infiltrating immune cells, contributing to an immunosuppressive microenvironment that favors tumor progression (Zhang et al., 2007; Ahmad et al., 2009; Wang et al., 2024). These mechanisms collectively underscore the functional significance of PSCA loss in cancer development and highlight its potential as both a diagnostic biomarker and a therapeutic target.
Moreover, our stratified analysis based on HWE status further validated the robustness of our findings. The results showed stable and significant associations across most genetic models, implying that the observed associations are unlikely to be artifacts of population stratification or genotyping errors. Sensitivity analyses also confirmed the stability of the results, as no individual study unduly influenced the pooled effect size, and Begg’s and Egger’s tests did not reveal any publication bias, further reinforcing the reliability of the meta-analysis results.
There are limitations to our meta-analysis that should be noted. First, the majority of the included studies were conducted in Asian populations. In contrast, only a limited number of studies involved Caucasian populations, which may limit the generalizability of our findings across different ethnic groups. Second, some confounding factors, such as H. pylori infection status, dietary factors, and histological subtypes of gastric cancer, could not be adjusted for because of a lack of sufficient data. Third, heterogeneity across studies, although reduced through subgroup analysis, may still exist because of uncontrollabl factors, such as geographic distribution, methodological disparities or participant demographics. Fourth, the potential interaction between PSCA rs2976392 and environmental exposure remains insufficiently explored. Elucidating these gene-environment interactions may provide critical insights into the multifactorial nature of GC susceptibility. Future well-designed large-scale studies, including gene environment interaction assessments and adjustments for confounding factors, are needed to further validate our findings. First, large-scale prospective cohort studies involving ethnically diverse populations are needed to validate the association between PSCA rs2976392 and GC risk and to minimize potential biases in case-control designs. Second, functional assays and mechanistic studies should be conducted to elucidate the biological role of PSCA rs2976392 in tumor initiation and progression, including its effects on gene expression, cell signaling, and immune modulation. Finally, given the polygenic characteristics of GC, integrating PSCA rs2976392 and other related SNPs into the polygenic risk model may increase the accuracy of genetic risk prediction and provide a more comprehensive understanding of the genetic factors underlying GC susceptibility.
In conclusion, our meta-analysis provides comprehensive evidence that the PSCA rs2976392 polymorphism is significantly associated with an increased risk of gastric cancer, especially in Asian populations. These results expand our understanding of GC genetics and may provide valuable information for the development of more personalized prevention strategies. Importantly, identification of such risk-associated variants may have important implications for clinical translation. Moreover, PSCA rs2976392 may serve as a biomarker in genetic screening programs, to help identify high-risk individuals.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
X-HL: Methodology, Writing – review and editing, Writing – original draft, Data curation, Software, Conceptualization, Visualization, Validation, Formal Analysis. D-JC: Writing – review and editing, Formal Analysis, Investigation, Methodology, Software, Data curation. L-JW: Formal Analysis, Data curation, Investigation, Writing – review and editing, Methodology. X-PW: Validation, Supervision, Conceptualization, Writing – review and editing, Writing – original draft, Visualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmad, S., Casey, G., Sweeney, P., Tangney, M., and O'Sullivan, G. C. (2009). Prostate stem cell antigen DNA vaccination breaks tolerance to self-antigen and inhibits prostate cancer growth. Mol. Ther. 17 (6), 1101–1108. doi:10.1038/mt.2009.66
Cai, M., Dai, S., Chen, W., Xia, C., Lu, L., Dai, S., et al. (2017). Environmental factors, seven GWAS-identified susceptibility loci, and risk of gastric cancer and its precursors in a Chinese population. Cancer Med. 6 (3), 708–720. doi:10.1002/cam4.1038
Chandra, V., Kim, J. J., Gupta, U., Mittal, B., and Rai, R. (2016). Impact of DCC (rs714) and PSCA (rs2294008 and rs2976392) gene polymorphism in modulating cancer risk in Asian population. Genes (Basel) 7 (2), 9. doi:10.3390/genes7020009
Dantas, R. N., Souza, A. M., Herrero, S. S. T., Kassab, P., Malheiros, C. A., and Lima, E. M. (2020). Association between PSCA, TNF-alpha, PARP1 and TP53 gene polymorphisms and gastric cancer susceptibility in the Brazilian population. Asian Pac J. Cancer Prev. 21 (1), 43–48. doi:10.31557/APJCP.2020.21.1.43
Hu, N., Wang, Z., Song, X., Wei, L., Kim, B. S., Freedman, N. D., et al. (2016). Genome-wide association study of gastric adenocarcinoma in Asia: a comparison of associations between cardia and non-cardia tumours. Gut 65 (10), 1611–1618. doi:10.1136/gutjnl-2015-309340
Kupcinskas, J., Wex, T., Link, A., Bartuseviciute, R., Dedelaite, M., Kevalaite, G., et al. (2014). PSCA and MUC1 gene polymorphisms are associated with gastric cancer and pre-malignant gastric conditions [corrected]. Anticancer Res. 34 (12), 7167–7175.
Lao-Sirieix, P., Caldas, C., and Fitzgerald, R. C. (2010). Genetic predisposition to gastro-oesophageal cancer. Curr. Opin. Genet. Dev. 20 (3), 210–217. doi:10.1016/j.gde.2010.03.002
Larios-Serrato, V., Valdez-Salazar, H. A., and Ruiz-Tachiquin, M. E. (2024). The landscape of 8q24 cytoband in gastric cancer (review). Oncol. Lett. 27 (4), 179. doi:10.3892/ol.2024.14311
Liu, L., Li, E., Luo, L., Zhao, S., Li, F., Wang, J., et al. (2017). PSCA regulates IL-6 expression through p38/NF-κB signaling in prostate cancer. Prostate 77 (14), 1389–1400. doi:10.1002/pros.23399
Lu, Y., Chen, J., Ding, Y., Jin, G., Wu, J., Huang, H., et al. (2010). Genetic variation of PSCA gene is associated with the risk of both diffuse- and intestinal-type gastric cancer in a Chinese population. Int. J. Cancer 127 (9), 2183–2189. doi:10.1002/ijc.25228
Matsuo, K., Tajima, K., Suzuki, T., Kawase, T., Watanabe, M., Shitara, K., et al. (2009). Association of prostate stem cell antigen gene polymorphisms with the risk of stomach cancer in Japanese. Int. J. Cancer 125 (8), 1961–1964. doi:10.1002/ijc.24519
Maubach, G., Kanthasamy, A. K., Gogia, S., and Naumann, M. (2025). The enigma of maladaptation in gastric pathophysiology. Trends Cancer 11, 448–461. doi:10.1016/j.trecan.2025.01.014
Merrell, K. W., Crofts, J. D., Smith, R. L., Sin, J. H., Kmetzsch, K. E., Merrell, A., et al. (2011). Differential recruitment of nuclear receptor coregulators in ligand-dependent transcriptional repression by estrogen receptor-α. Oncogene 30 (13), 1608–1614. doi:10.1038/onc.2010.528
Ou, J., Li, K., Ren, H., Bai, H., Zeng, D., and Zhang, C. (2010). Association and haplotype analysis of prostate stem cell antigen with gastric cancer in tibetans. DNA Cell Biol. 29 (6), 319–323. doi:10.1089/dna.2009.0960
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 18 (3), e1003583. doi:10.1371/journal.pmed.1003583
Qiu, L. X., Cheng, L., He, J., Zhou, Z. R., Wang, M. Y., Zhou, F., et al. (2016). PSCA polymorphisms and gastric cancer susceptibility in an eastern Chinese population. Oncotarget 7 (8), 9420–9428. doi:10.18632/oncotarget.7137
Siegel, R. L., Kratzer, T. B., Giaquinto, A. N., Sung, H., and Jemal, A. (2025). Cancer statistics, 2025. CA Cancer J. Clin. 75 (1), 10–45. doi:10.3322/caac.21871
Study Group of Millennium Genome Project for Cancer, Sakamoto, H., Yoshimura, K., Saeki, N., Katai, H., Shimoda, T., et al. (2008). Genetic variation in PSCA is associated with susceptibility to diffuse-type gastric cancer. Nat. Genet. 40 (6), 730–740. doi:10.1038/ng.152
Sun, H., Wu, X., Wu, F., Li, Y., Yu, Z., Chen, X., et al. (2015). Associations of genetic variants in the PSCA, MUC1 and PLCE1 genes with stomach cancer susceptibility in a Chinese population. PLoS One 10 (2), e0117576. doi:10.1371/journal.pone.0117576
Thrift, A. P., Wenker, T. N., and El-Serag, H. B. (2023). Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention. Nat. Rev. Clin. Oncol. 20 (5), 338–349. doi:10.1038/s41571-023-00747-0
Turdikulova, S., Dalimova, D., Abdurakhimov, A., Adilov, B., Navruzov, S., Yusupbekov, A., et al. (2016). Association of rs2294008 and rs9297976 polymorphisms in PSCA gene with gastric cancer susceptibility in Uzbekistan. Cent. Asian J. Glob. Health 5 (1), 227. doi:10.5195/cajgh.2016.227
Wang, C., Zhu, X., Zheng, M., Chen, Y., Jiang, R., He, X., et al. (2024). Pan-cancer analysis of PSCA that is associated with immune infiltration and affects patient prognosis. PLoS One 19 (6), e0298469. doi:10.1371/journal.pone.0298469
Wang, Z., Dai, J., Hu, N., Miao, X., Abnet, C. C., Yang, M., et al. (2017). Identification of new susceptibility loci for gastric non-cardia adenocarcinoma: pooled results from two Chinese genome-wide association studies. Gut 66 (4), 581–587. doi:10.1136/gutjnl-2015-310612
Wizenty, J., and Sigal, M. (2025). Helicobacter pylori, microbiota and gastric cancer - principles of microorganism-driven carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 22, 296–313. doi:10.1038/s41575-025-01042-2
Wu, C., Wang, G., Yang, M., Huang, L., Yu, D., Tan, W., et al. (2009). Two genetic variants in prostate stem cell antigen and gastric cancer susceptibility in a Chinese population. Mol. Carcinog. 48 (12), 1131–1138. doi:10.1002/mc.20565
Wu, D., Lv, J., Zhao, R., Wu, Z., Zheng, D., Shi, J., et al. (2020). PSCA is a target of chimeric antigen receptor T cells in gastric cancer. Biomark. Res. 8, 3. doi:10.1186/s40364-020-0183-x
Xu, L. P., Qiu, H. B., Yuan, S. Q., Chen, Y. M., Zhou, Z. W., and Chen, Y. B. (2020). Downregulation of PSCA promotes gastric cancer proliferation and is related to poor prognosis. J. Cancer 11 (9), 2708–2715. doi:10.7150/jca.33575
Yan, K., Wu, K., Lin, C., and Jie, Z. (2019). Impact of PSCA gene polymorphisms in modulating gastric cancer risk in the Chinese population. Biosci. Rep. 39 (9). doi:10.1042/BSR20181025
Zhang, W., Liang, P., Wang, W., Dai, P., Wang, Q., Yan, W., et al. (2015). The influence of PSCA gene variation on its expression and gastric adenocarcinoma susceptibility in the northwest Chinese population. Int. J. Mol. Sci. 16 (5), 11648–11658. doi:10.3390/ijms160511648
Zhang, X., Yu, C., Zhao, J., Fu, L., Yi, S., Liu, S., et al. (2007). Vaccination with a DNA vaccine based on human PSCA and HSP70 adjuvant enhances the antigen-specific CD8+ T-cell response and inhibits the PSCA+ tumors growth in mice. J. Gene Med. 9 (8), 715–726. doi:10.1002/jgm.1067
Keywords: PSCA, rs2976392, polymorphism, gastric cancer, meta-analysis
Citation: Lin X-H, Chen D-J, Wang L-J and Wan X-P (2025) Association between the PSCA rs2976392 polymorphism and susceptibility to gastric cancer: a meta-analysis. Front. Genet. 16:1618597. doi: 10.3389/fgene.2025.1618597
Received: 26 April 2025; Accepted: 07 July 2025;
Published: 16 July 2025.
Edited by:
Ashwin Kotnis, All India Institute of Medical Sciences, Bhopal, IndiaReviewed by:
Mohammad Uzzal Hossain, National Institute of Biotechnology (NIB), BangladeshBhawna Bhimte, Gandhi Medical College Bhopal, India
Copyright © 2025 Lin, Chen, Wang and Wan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiu-Ping Wan, bHhoMTM4NjcwMzM2NTdAMTI2LmNvbQ==
 Xiao-Hua Lin
Xiao-Hua Lin Xiu-Ping Wan
Xiu-Ping Wan