- Department of Pediatrics, Jinhua Municipal Central Hospital, Jinhua, China
Background: Cornelia de Lange syndrome (CdLS) is a genetically heterogeneous disorder involving multi-system organs, causing physical and mental congenital malformation. Nipped-B-like protein (NIPBL) variants are associated with various CdLS phenotypes. Newborns with typical clinical manifestations (intellectual disability, special appearances, and limb malformation) require a diagnosis. However, diagnosing CdLS is challenging on account of its heterogeneity of genotype and phenotype.
Methods: In this study, molecular analysis was applied, containing whole exome sequencing (WES), reverse transcriptase PCR (RT-PCR), and minigene splicing assays.
Results: We identified a novel splice-donor variant (NIPBL c.6343 + 1G>A) by WES. RT-PCR and minigene splicing assays were performed to identify the function of the splice-donor variant on subsequent RNA splicing. The variant caused exon 36 to be skipped. A premature termination codon (PTC) appeared subsequently and a truncated protein with a length of 2088 aa was produced.
Conclusion: A novel pathogenic variant of CdLS is identified, which affects normal mRNA splicing of the NIPBL gene. These findings enrich the knowledge of CdLS gene variants, which may be responsible for developing this rare disease.
1 Introduction
Cornelia de Lange syndrome (CdLS), a rare congenital disease of the multisystem caused by genetic variants, has an extremely low incidence of one estimated CdLS per 10,000 to 30,000 live births (Mannini et al., 2013). Children with CdLS have typical clinical manifestations such as facial features, hypertrichosis, growth restriction, intellectual disability, and upper limb deformity (Kline et al., 2018). Occipital skin thickening was found in nearly 51% of patients, with special appearance features accounting for nearly half of (Clark et al., 2012). Approximately 30% of the patients were reported to have an upper limb deficit (Bağış et al., 2020). Although cardiac abnormalities are not the primary standard and typical clinical manifestation of the disease, approximately 50% of patients with CdLS present with congenital heart defects (Ayerza et al., 2017; Dempsey et al., 2014). An international consensus statement created a scoring system defining CdLS as both classical and non-classical CdLS (Kline et al., 2018). Classical CdLS, due to unique facial features, growth limitations, and limb deformities, can be identified at birth by experienced pediatricians or clinical geneticists. However, due to the heterogeneous genotype and phenotype of CdLS, some non-classical CdLS do not have characteristic clinical manifestations, thus bringing great difficulties for clinical diagnosis.
As currently reported in the literature, CdLS is due to variants in the following genes, NIPBL, SMC1A, SMC3, RAD21, HDAC8, BRD4, and ANKRD11 (Deschamps, 2022). SMC1A variants are found in about 5% of patients with CdLS and the variants in HDAC8, RAD21, and SMC3 totaled about 5%; BRD4 and ANKRD11 gene variants are newly discovered in recent years, being too small to count the percentage; The clinical manifestations of several variants are less typical (Kline et al., 2018). Variants in the NIPBL gene are detected in 60% of patients with CdLS (Nizon et al., 2016; Krantz et al., 2004). The NIPBL gene is located on 5p13.2 and encodes for a triangular protein that plays an important role in the chromatid cohesion process and enhancer-promoter communication (Nizon et al., 2016; Krantz et al., 2004). Potential pathogenic variants in the NIPBL gene have been tightly associated with the typical manifestation of CdLS (Moss et al., 2017; Huisman et al., 2017).
This study reports a classic case of CdLS diagnosed from birth in a child who also had a variant in the NIPBL gene, but we detected a novel intronic splice-donor variant.
2 Materials and methods
2.1 Patient and clinical assessment
The study protocol was approved by the Ethics Committee of Jinhua Central Hospital of Zhejiang Province (approval number No. 2024-43). The study was conducted by the principles of the Declaration of Helsinki. Informed written consent from the proband’s parents was obtained for the publication of this case.
We collected detailed clinical data, including age, sex, birth history (birth size), and characteristic manifestations (including facial features and physical examination). In addition, we collected clinical diagnostic examination results, including ultrasound examination (kidney, testis, hepatobiliary system, cardiac ultrasound examination) and brain magnetic resonance imaging (MRI), and the treatment of the child (medication status and surgery status). Follow-up until 3 years and 5 months of age, the height and weight data were collected. In addition, we also applied a Gesell Developmental Scale to test the patient’s behavior developmental quotient.
2.2 Whole exome sequencing
Qualified whole blood samples from cases of the proband and his parents were collected to extract DNA. Method of Aligent SureSelect was used for DNA sample preparations. Agilent SureSelect XT Human All Exon V6 Kit (Agilent, Santa Clara, CA, United States) was used for targeted region capture and gene library preparation, under the guidance of operating instruction. High-throughput sequencing was fulfilled using an Illumina NovaSeq 6,000 system (Illumina, San Diego, CA, United States), the amount of paired-end reads was 150 bp.
2.3 Prediction of pathogenicity and splicing effects of the variant
The c.6343 + 1G>A variant is on-site +1 of intron36 (G mutated into A) and belongs to a typical site that affected mRNA splicing in all probability. The Human Splicing Finder (HSF, https://hsf.genomnis.com/login), SpliceAI (https://spliceailookup.broadinstitute.org/), and RDDCSC (https://rddc.tsinghua-gd.org/search-middle?to=SplitToolModel) algorithms were used to determine the effects of the splice-donor variant on the mRNA splicing process of NIPBL. Moreover, MutationTaster (https://www.mutationtaster.org/) was used for evaluating the pathogenicity of the splice-donor variant on protein expression.
2.4 Reverse transcription polymerase chain reaction and sanger sequencing
Reverse transcription polymerase chain reaction (RT-PCR) was performed to verify the potential influence on mRNA splicing of the c.6343 + 1G>A variant. The whole blood samples of this proband (variant) and his healthy mother (wild type) were used for extracting total RNA, according to a Total RNA Extraction Kit (TR121-50; GenStone, Littleton, CO, United States). The genomes were digested and synthesis of cDNA was performed by reverse transcription based on HifairTM first Strand cDNA Synthesis SuperMix kit (11123ES70; Yeasen, Shanghai, China). The procedure of this reverse transcription was shown as ‘37 °C for 15 min, then at 85 °C for 5 s’. Primers were designed, and the RT-PCR products were amplified through PCR on the condition of ‘95 °C for 30 s, 60 °C for 30 s, 72 °C for 30 s (35 cycles), then 72 °C for 5 min’. Amplified PCR products were tested using the method of 2% agarose gel electrophoresis, then the electrophoretic bands of PCR products were recovered through gel extraction. Sanger sequencing was then performed.
2.5 Minigene splicing assay
Minigene splicing experiments (in vitro assays) were used to further confirm the function of the variant in NIPBL gene splicing. PCR amplification using designed primers was used to obtain variant and wild-type target gene segments with restriction enzyme cutting sites. Four recombinants were acquired by inserting the obtained target minigene into pcDNA3.1 and pcMINI-N vectors. NIPBL-pcDNA3.1-wt/mut recombinants was successfully constructed by inserting Exon35 (141bp)-Intron35 (148bp)-Exon36 (94bp)-Intron36 (713bp)-Exon37 (155bp) into pcDNA3.1 plasmid. NIPBL-pcMINI-N-wt/-mut recombinants were constructed by inserting Exon35 (141bp)-Intron35 (148bp)-Exon36 (94bp)-partial Intron36 (517bp) into pcMINI-N plasmid. These four recombinants were transfected into HeLa and 293T cells (Rapid Plasmid Mini Kit, 1005250; SIMGEN, Schönefeld, Germany), and the cells were cultured for 48 h. A total of eight RNA extractions were obtained from these cell samples by the application of TRIzol® RNAiso PLUS (9,109; Takara). The Hair first Strand cDNA Synthesis SuperMix for qPCR kit (gDNA digester plus) was used for reverse transcription (11123ES70; Yeasen). PCR amplification was visualized using a method of agarose gel electrophoresis, finally, the product was verified through Sanger sequencing analysis.
3 Results
3.1 Case presentation
The proband was delivered via cesarean section during placental abruption at 36+ weeks of gestation. The patient weighed 2050 g at birth (<10th percentile), had a body length of 40 cm (< third percentile), and with a head circumference indicating 30.8 cm, suggesting fetal growth restriction. The baby had dysmorphic facial features, including a low forehead hairline, a short and up-turn nose, an elongated philtrum, and a small lower jaw (Figure 1A). Other signs or symptoms also indicated a short neck, thickening of the occipital skin, shortened upper limbs, hairiness on the limbs and back, enorchia, and a micropenis (Figure 1B). Ultrasonography revealed an atrial septal defect (ASD), multiple cysts in both kidneys, separation of the left pelvis, and cryptorchidism. Cranial MRI showed an enlarged cisterna magna.
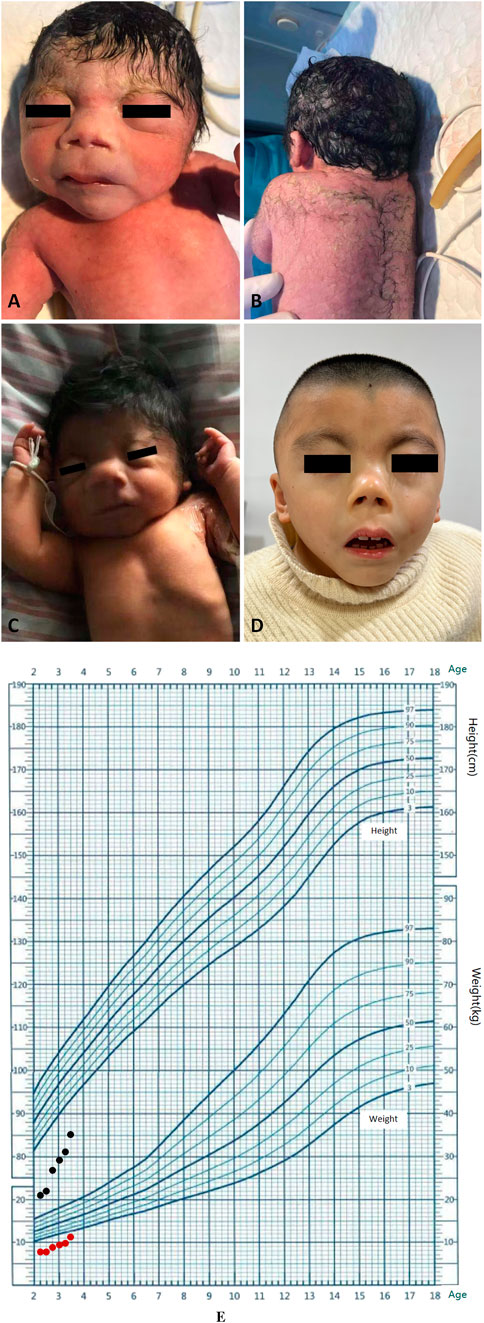
Figure 1. Clinical features of the proband. (A) Facial characteristics at birth. (B) Hypertrichosis at birth. (C) Facial features on 16th day of life. (D) Appearance at age of 3 years and 5 months. (E) Growth curve of children (from the age of 2 years and 2 months up to the age of 3 years and 5 months old, monitored every 3 months). The black dots represent changes in height. The red dots represent changes in weight.
At follow-up, the patient exhibited distinct growth and intellectual disability. We also recorded typical facial characteristics at the age of 16th day of life and 3 years and 5 months of this child (Figures 1C,D). Spontaneous closure of the ASD was observed at 7 months of age. Surgery for cryptorchidism was performed at 2 years of age. He received recombinant human growth hormone (r-hGH) injection and regular rehabilitation training from the age of 2 years and 5 months old. Height and weight were monitored every 3 months Follow-up was performed up to 3 years and 5 months after the birth of this child, the height was only measured as 85 cm (< third percentile), in addition, the weight was merely 10 kg (< third percentile). The growth curve of this child is shown in Figure 1E. Motor assessments showed that the child could take a few steps alone, but the skills were unstable. He could grasp toy bricks and pass objects from one hand to the other. However, he could not put pellets into a bottle accurately. A deficiency in language development was also observed, and he could only unconsciously pronounce words. The Gesell Developmental Scale was used to comprehensively assess the development of the boy. When the proband at age of 3 years and 5 months old, developmental age of gross motorskills and adaptability is only 13 m, fine motor skills is 12.5 m, personal social activity is 13.4 m, and language developmental age is 10.3 m, suggesting a severe developmental delay.
3.2 Identification of NIPBL variant
WES of the proband and his parents was performed to confirm the pathogenic genetic diagnosis in CdLS. The results revealed a novel splice-donor variant in intron 36 of the NIPBL gene (chr5:37044832, NM_133433.3) (Figure 2). Nucleobase guanine(G) changed to adenine(A) in the variant position of the proband. The variant was heterozygous in the proband, whereas the gene in his parents was wild-type, suggesting a de novo variant. The splice-donor variant could not ever be reported in the Human Exon Database (ExAC), Population Genome Mutation Frequency Database (gnomAD), 1,000 Genomes Database, or Human Gene Mutation Database (HGMD), indicating that it was novel and may be the cause of the disorder.
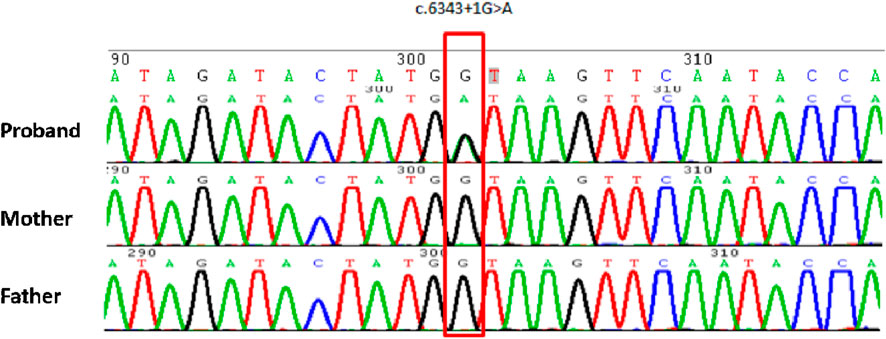
Figure 2. Sanger sequencing schematic diagram of the proband and his parents. The red frame indicates a variant position, which mutates into adenine(A) in proband, and in his parents are normal as guanine(G).
Analysis of the splice-donor variant suggested that the variant position is located in site +1 of intron36 in NIPBL (nucleobase guanine mutate into adenine), which could cause a distinct impact on mRNA splicing. Th splice-donor variant is regarded as a pathogenic variant that may impact the splicing process. We predicted the splicing disturbance and pathogenicity of this variant subsequently. The HSF and SpliceAI algorithms showed that the confidence score of the original donor site decreased in the splice-donor variant, suggesting that the variant may affect NIPBL splicing. The RDDCSC algorithm revealed that the variant may cause exon skipping, frameshift variant, and premature termination codon, indicating that it affected splicing in NIPBL.
3.3 RT-PCR analysis
NIPBL is widely expressed in different tissues and whole blood samples. Bulk tissue gene expression analysis suggests the index of transcripts per million (TPM) is up to 7.818 in whole blood samples, which could be applied to RT-PCR for in vitro splicing analysis. In the control participant, amplified PCR products were visualized as a single longer band “a” on gel electrophoresis, by the normal band size. However, the proband had two different bands, “a” (which was the same as the normal one) and “b” (a shorter band), suggesting a heterozygous variant (Figure 3A). The shorter band was likely caused by exon 36 skipping due to the frameshift variant (Figure 3B). Sanger sequencing confirmed the effect of the variant on RNA splicing. The normal splicing form was presented as Exon34 (137bp)-Exon35 (141bp)-Exon36 (94bp)-Exon37 (155bp)-Exon38 (91bp) in the control, whereas abnormal splicing was observed as Exon34 (137bp)-Exon35 (141bp)-Exon37 (155bp)-Exon38 (91bp) in the variant, confirming the loss of exon 36 (Figure 3C).
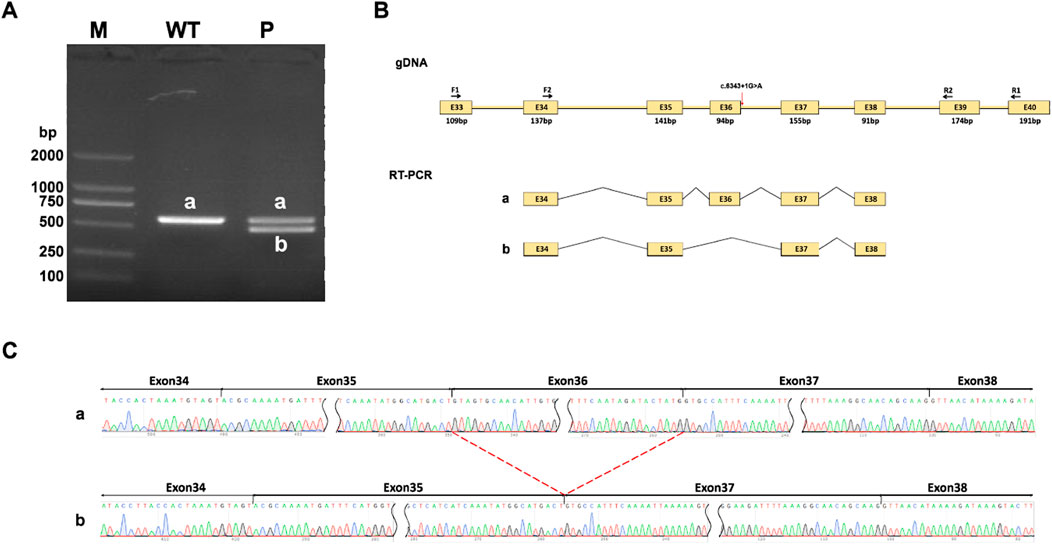
Figure 3. RT-PCR analysis. (A) RT-PCR products are visualized on gel electrophoresis. There exists only one longer band labeled “a” in his healthy mother (WT), while two bands labeled “a” and “b” are found in the proband (P). Bands “a” shows as normal size, Band “b” is shorter. (B) A schematic diagram of primer design and exon 36 skipping splicing, red arrow points variant position. (C) Sanger sequencing analysis of RT-PCR products. Band “b” is shorter than normal size band ‘a’ due to a loss of exon 36.
3.4 Minigene splicing assay results
Minigene splicing assay (in vitro) was also performed to further verify the function of the splice-donor variant on the splicing process. NIPBL-pcDNA3.1-wt/mut and NIPBL-pcMINI-N-wt/mut recombinants were successfully constructed as shown in diagrams (Figure 4A; Figure 5A). Gel electrophoresis showed a larger normal band named “a” in the wild type group, meanwhile, a smaller single band defined as “b” in the variant. In NIPBL-pcDNA3.1-wt/mut constructions, a single 465 bp band was observed in the wild-type, in line with the normal size as expected, whereas a shorter band “b” was produced in the variant (Figure 4B). Similarly, in the constructions of NIPBL-pcMINI-N-wt/mut, the normal band “a” was 315 bp size in the wild-type, revealing normal mRNA splicing, while a smaller band “b” was produced with abnormal splicing in the variant (Figure 5B). Therefore, the splice-donor variant may cause exon 36 skipping (Figures 4C, 5C). Sequencing results confirmed that the splice-donor variant caused the deletion of exon 36 in NIPBL. Sequencing analysis revealed disturbed RNA splicing of Exon35 (141bp)-Exon37 (155bp) in NIPBL-pcDNA3.1-mut (Figure 4D). Similarly, the abnormal splicing caused by the variant in the NIPBL-pcMINI-N-mut constructs was presented as Exon35 (141bp)-ExonB (57bp) (Figure 5D). These results indicate that the splice-donor variant causes the loss of exon 36 and affects normal mRNA splicing.
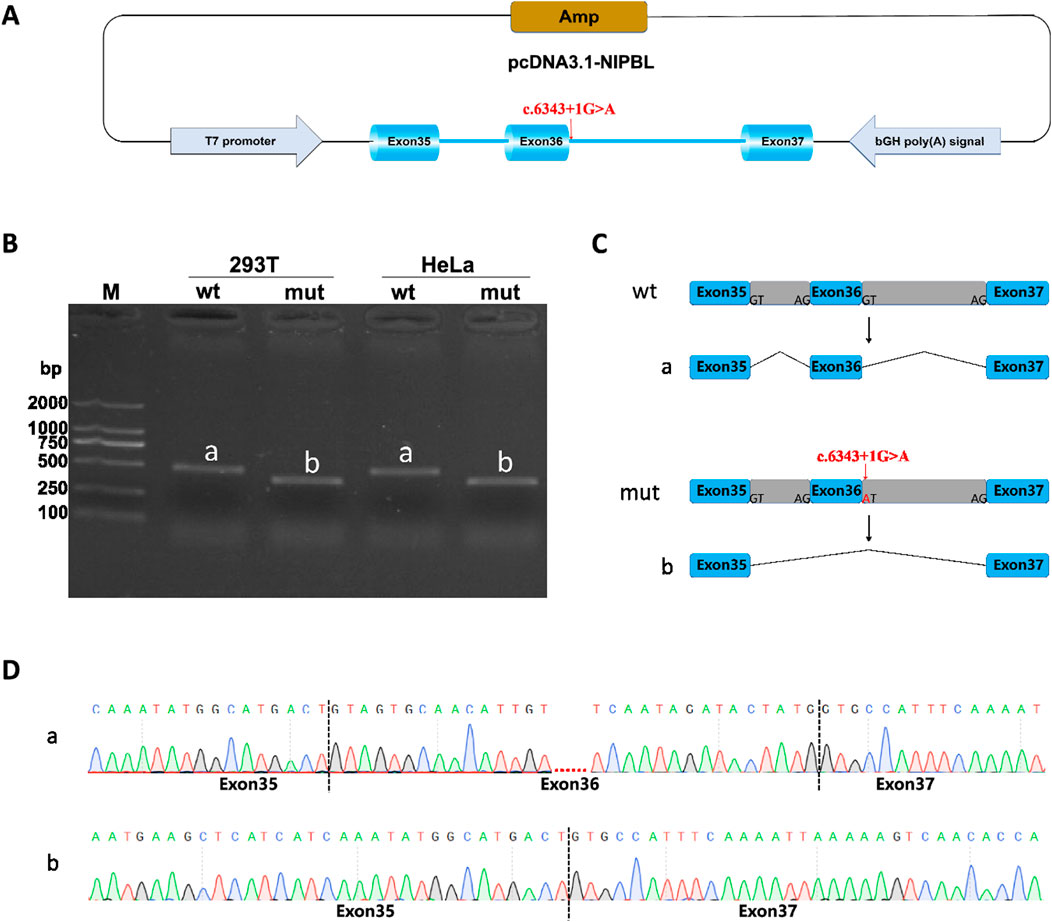
Figure 4. Minigene splicing assay analysis of NIPBL-pcDNA3.1. (A) A schematic diagram of NIPBL-pcDNA3.1 construction. (B) Minigene products of NIPBL-pcDNA3.1 are separated through gel electrophoresis. The longer bands are labeled “a” (wt) and shorted bands are labeled “b” (mut) in HeLa and 293T cells. Bands “a” are normal size, Bands “b” are shorter. (C) Schematic diagram of minigene splicing in NIPBL-pcDNA3.1. (D) Sanger sequencing analysis of minigene products. Bands ‘b’ are shorter than normal size bands ‘a’ due to a deletion of exon 36.
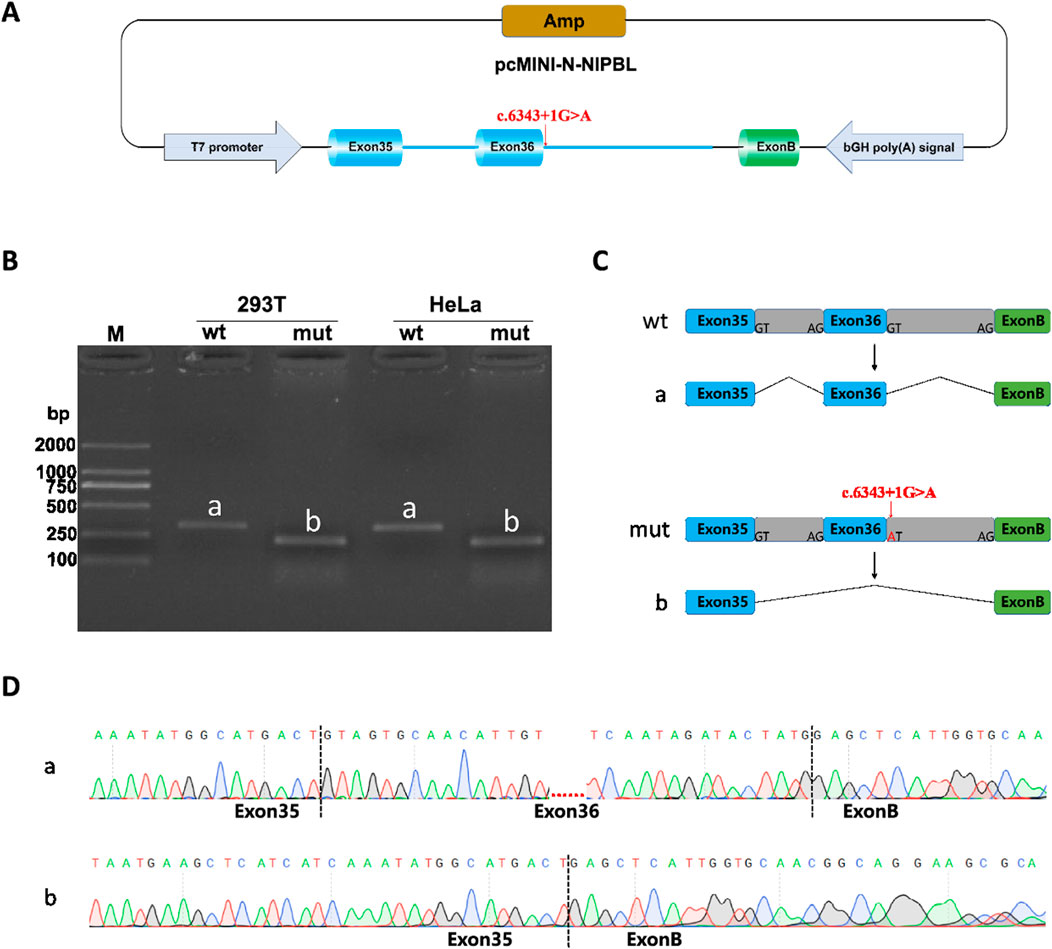
Figure 5. Minigene splicing assay analysis of NIPBL-pcMINI-N. (A) A schematic diagram of NIPBL-pcMINI-N construction. (B) Minigene products of NIPBL-pcMINI-N are separated through gel electrophoresis. The longer bands are labeled “a” (wt) and shorted bands are labeled “b” (mut) in HeLa and 293T cells. Bands “a” are normal size, Bands “b” are shorter. (C) Schematic diagram of minigene splicing in NIPBL-pcMINI-N. (D) Sanger sequencing analysis of minigene products. Bands “b” are shorter than normal size bands “a” due to a deletion of exon 36.
4 Discussion
Many studies have shown that abnormal expression of NIPBL leads to abnormal developmental in the heart, limbs, and nervous system (Garfinkle and Gruber, 2019; Mazzola et al., 2019). Because NIPBL interferes with the function of MAU2 as well as the expression of HOX, participating in craniofacial development and thus resulting in limb growth, NIPBL variants can cause limb malformations (Muto et al., 2014; Smith et al., 2014). Pathogenic variants in the NIPBL gene lead, to varying degrees, to a reduction in normally functional NIPBL or even haploinsufficiency. The alteration of the NIPBL gene leads to the severe clinical features of CdLS, the so-called “classical phenotype. Individuals with pathogenic NIPBL variants always show a classical and more severe in CdLS phenotypic (Krantz et al., 2004). Patients diagnosed with CdLS have been reported to have de novo heterozygous pathogenic variants in most cases, but are not inherited from their parents. CdLS variants result in perturbation of gene expression and thus interfere with global transcription (Sarogni et al., 2020; Yuan et al., 2015).
Missense or nonsense variants, splicing changes, small deletions, and insertions in the NIPBL variants account for approximately 95% of the NIPBL variant reports (HGMD database). Point variants or single-nucleotide variants in NIPBL account for the majority and large-scale genomic rearrangements are rarely reported (Bhuiyan et al., 2007; Ratajska et al., 2010). The clinical severity of CdLS depends on the dosage effect of the gene, which is crucial to influencing the clinical presentation. Truncation, nonsense, splice site, and frameshifts in NIPBL variants contribute to a truncated and possibly non-functional NIPBL protein pathogenic variant associated with a severe CdLS clinical phenotype; however, missense variants generally result in a milder CdLS pattern, and individuals with large deletions associated with CdLS show more severe clinical symptoms; this grading indicates that NIPBL is sensitive to gene dosage variants (Selicorni et al., 2021).
This study investigated a child who was diagnosed with CdLS at birth. WES identified a new NIPBL variant site (c.6343 + 1G > A), but this was not observed in the general population. This was a heterozygous de novo variant that could not be found among his parents. This variant is located in the +1 site of intron 36 and is known as a splicing impact. In our study, RT-PCR and minigene splicing experiments were performed to verify the effect of the splice-donor variant on mRNA splicing. This variant affects the normal splicing of the mRNA of the NIPBL gene, resulting in exon 36 skipping and frameshift variants, creating a premature stop codon (PTC). PTC may lead to non-sense-mediated mRNA degradation or the production of truncated proteins. In this study, a truncated protein with a length of 2088 amino acids (protein with a normal length of 2,804 aa) was produced (c. 6250_6343 delp. Val2085Profs*5). NIPBL is a large protein of more than 2,500 amino acid residues, comprises unstructured regions and several HEAT repeats. The large genomic size and complex domain architectures of NIPBL pose significant challenges for protein-level validation. In our study, the variant results in a substitution of valine to proline at position 2085, occurs in a structurally critical motif. Our next steps will prioritize protein-level validation to elucidate the impact of this variant. This patient had a relatively typical clinical phenotype consistent with the NIPBL genotype: dysmorphic facial features, short neck, occipital skin thickening, upper limb deformities, polytheism, cryptorchidism and micropenis; in addition, the more severe manifestations of the patient may be closely related to nonsense variants caused by the NIPBL gene variant, as was previously described, various gene dosage variants in the gene usually differ in the severity of the CdLS phenotype.
Growth retardation, short stature, and delayed puberty are also relatively common symptoms in children with CdLS (Kline et al., 1993; Kline et al., 2007), recently, a girl with a de novo splicing variant in the NIPBL gene was treated with r-hGH at age 4.3 years. Treatment with r-hGH resulted in a height increase of 1.6 SD score (de Graaf et al., 2017), suggesting that hormonal therapy may be effective in CdLS patients with short stature. In this case, the child was born 40 cm (<third percentile) and the child received r-hGH injection at the age of 2 years and 5 months. From follow-up until 3 years and 5 months after the birth of the child, the height was still only 86 cm (<the third percentile), indicating the effect of growth hormone therapy in this child. Longitudinal growth monitoring demonstrated significant height improvement from 73 cm (−4.5 SD) at 2 years 5 months–86 cm (−2.9 SD) at 3 years 5 months, representing a 13 cm gain over this 1-year treatment period. Although the patient’s height remained below the third percentile, this substantial catch-up growth suggests potential therapeutic efficacy of the growth hormone intervention. Insulin-like Growth Factor 1(IGF-1) levels increased significantly from 96 ng/mL to 228 ng/mL during treatment, demonstrating biochemical responsiveness to growth hormone therapy. In the present case, the short duration of rhGH therapy (1 year) represents a notable limitation, as this timeframe may be insufficient to fully demonstrate the treatment’s growth-promoting effects in severe growth retardation. Previous longitudinal studies have demonstrated that extended growth hormone treatment courses (e.g., 8-year follow-up periods in de Graaf et al. (2017)) are required to fully evaluate therapeutic efficacy, particularly for demonstrating sustained improvements in final adult height. The initial recombinant growth hormone dose in our proband (0.73 mg/m2/day) was 15% lower than the reference standard of 0.86 mg/m2/day established in the positive case in de Graaf et al. (2017). Children with CdLS may demonstrate reduced sensitivity to rhGH therapy, potentially necessitating higher-than-standard dosing regimens to achieve optimal growth outcomes. Based on these findings, dose escalation will be considered for future interventions to optimize growth outcomes. A survey on CdLS showed that most of the children had feeding problems (Sarimski, 1997); combined with the weight gain of 2050 g at birth (<10th percentile), and only 10 kg (<third percentile), due to poor feeding practices, multisystem diseases, and surgical trauma (cryptorchidism). Feeding difficulties and reduced basal metabolic rate may synergistically exacerbate growth retardation in this patient. Moreover, the splice-donor variant in this case is previously unreported, so it remains unknown whether children with variants at this site are sensitive to growth hormone therapy. Mild phenotype characterized by isolated growth retardation with preserved intellectual function in 2017 reported CdLS case, while severe phenotypic manifestation featuring global developmental delay (e.g., growth restriction, cognitive impairment, and facial dysmorphism). The milder case caused an in-frame deletion which typically retains partial protein function, while nonsense and frameshift mutations may cause a sever phenotype. Our proband carries a frameshift variantion, predicted to cause a truncated protein. This genotype-phenotype gradient correlates with observed GH therapy outcomes, better response in mild genotype, attenuated response in severe genotype. This genotypic gradient (mild → severe) parallels both phenotypic severity and GH response, supporting a unified model where variation class dictates clinical trajectory. Precision dosing strategies, informed by genetic profiling, may be warranted for severe genotypes.
Classical children with CdLS also show developmental delays, including motor development, as well as intellectual development. This case was evaluated at the age of 3 years and 5 months of age. The results showed that the developmental quotient of the children was only 31 points, and the developmental age of adaptability, large motor, fine motor, language, and personal-social aspects was only equivalent to the level of 10–13 months of age. This suggests that the child had significant developmental disorders in movement, language, and social interaction. A survey reported that only four out of the 27 children with CdLS were able to communicate using language, but most of the older children expressed their needs (Sarimski, 1997) in non-verbal means. Studies have reported that most children with CdLS have mild to severe intellectual disabilities as well as autistic features (Srivastava et al., 2014; Basile et al., 2007). CdLS is characterized by autistic traits, particularly excessive repetitive behavior, and expressive language deficits. Communication and social deficits are exacerbated with age compared to their neurotypical peers. The results of the current Gesell development scale of the children in this case suggest severe defects. Therefore, in the future follow-up process, attention should be paid to their communication skills and social skills, to early detect whether the children have autism tendencies and intervene in advance.
5 Conclusion
In conclusion, we identified a novel splice-donor variant (NIPBL c.6343+1G > A) that causes a typical CdLS, and revealed its effect on RNA splicing. Providing strong evidence for a definitive diagnosis of this patient. The findings of this study further expand the spectrum of pathogenic variant in CdLS. This study provides insight into genotype–phenotype correlations. Furthermore, the results of this study may lay the foundation for later mechanism research and contribute to future therapeutic approaches of CdLS.
6 Limitation
Currently, there is only one case, demonstrates the correlation between this novel intronic variant of NIPBL gene and Cornelia de Lange syndrome. It is difficult to identify the accurate relationship of genotype and phenotype in absence of more case reports. The functional interpretation of these splicing variants is presently limited to the RNA level, pending subsequent protein characterization studies.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, SRA accession:SRR31906592.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Jinhua Municipal Central Hospital (Approval Number: 2024-43, date of issue: 2024-04-10). A written informed consent was obtained from the participant’ legal guardian of kin (participants’mother) to participate in this research and for the publication of any potentially identifiable images/photographs or data included in this article. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’; legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’; legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
XS: Data curation, Formal Analysis, Writing – original draft. YD: Investigation, Software, Writing – review and editing. YZ: Methodology, Supervision, Writing – review and editing. YC: Supervision, Validation, Visualization, Writing – review and editing. LY: Funding acquisition, Project administration, Resources, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was received financial support by Jinhua science and technology plan project, Grant No. 2023-4-064.
Acknowledgments
The authors are deeply grateful to the participants in this research. This work was supported by Jinhua science and technology plan project, Grant No. 2023-4-064.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2025.1665167/full#supplementary-material
References
Ayerza, C. A., Puisac, U. B., Teresa Rodrigo, M. E., Hernández Marcos, M., Ramos Fuentes, F. J., and Pie Juste, J. (2017). Cornelia de Lange syndrome: congenital heart disease in 149 patients. Med. Clin. Barc. 149 (7), 300–302. doi:10.1016/j.medcli.2017.03.051
Bağış, H., Öztürk, Ö., Bolu, S., and Taşkın, B. (2020). A Novel Mutation in NIPBL Gene with the Cornelia de Lange Syndrome and a 10q11.22-q11.23 Microdeletion in the Same Individual. J. Pediatr. Genet. 11 (3), 245–252. doi:10.1055/s-0040-1718534
Basile, E., Villa, L., Selicorni, A., and Molteni, M. (2007). The behavioural phenotype of Cornelia de Lange Syndrome: a study of 56 individuals. J. Intellect. Disabil. Res. 51 (Pt 9), 671–681. doi:10.1111/j.1365-2788.2007.00977.x
Bhuiyan, Z. A., Stewart, H., Redeker, E. J., Mannens, M. M., and Hennekam, R. C. (2007). Large genomic rearrangements in NIPBL are infrequent in Cornelia de Lange syndrome. Eur. J. Hum. Genet. 15 (4), 505–508. doi:10.1038/sj.ejhg.5201776
Clark, D. M., Sherer, I., Deardorff, M. A., Byrne, J. L. B., Loomes, K. M., Nowaczyk, M. J. M., et al. (2012). Identification of a prenatal profile of Cornelia de Lange syndrome (CdLS): a review of 53 CdLS pregnancies. Am. J. Med. Genet. A 158A (8), 1848–1856. doi:10.1002/ajmg.a.35410
de Graaf, M., Kant, S. G., Wit, J. M., Willem Redeker, E. J., Eduard Santen, G. W., Henriëtta Verkerk, A. J. M., et al. (2017). Successful Growth Hormone Therapy in Cornelia de Lange Syndrome. J. Clin. Res. Pediatr. Endocrinol. 9 (4), 366–370. doi:10.4274/jcrpe.4349
Dempsey, M. A., Knight Johnson, A. E., Swope, B. S., Moldenhauer, J. S., Sroka, H., Chong, K., et al. (2014). Molecular confirmation of nine cases of Cornelia de Lange syndrome diagnosed prenatally. Prenat. Diagn 34 (2), 163–167. doi:10.1002/pd.4279
Deschamps, G. N. (2022). Cornelia de Lange Syndrome. Neonatal Netw. 41 (3), 145–149. doi:10.1891/NN-2021-0011
Garfinkle, E. A. R., and Gruber, T. A. (2019). A tale of two genes: a new connection between NIPBL and NPM1 in acute myeloid leukemia. Haematologica 104 (7), 1289–1291. doi:10.3324/haematol.2019.218867
Huisman, S., Mulder, P. A., Redeker, E., Bader, I., Bisgaard, A. M., Brooks, A., et al. (2017). Phenotypes and genotypes in individuals with SMC1A variants. Am. J. Med. Genet. A 173 (8), 2108–2125. doi:10.1002/ajmg.a.38279
Kline, A. D., Barr, M., and Jackson, L. G. (1993). Growth manifestations in the Brachmann-de lange syndrome. Am. J. Med. Genet. 47 (7), 1042–1049. doi:10.1002/ajmg.1320470722
Kline, A. D., Grados, M., Sponseller, P., Levy, H. P., Blagowidow, N., Schoedel, C., et al. (2007). Natural history of aging in Cornelia de Lange syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 145C (3), 248–260. doi:10.1002/ajmg.c.30137
Kline, A. D., Moss, J. F., Selicorni, A., Bisgaard, A. M., Deardorff, M. A., Gillett, P. M., et al. (2018). Diagnosis and management of Cornelia de Lange syndrome: first international consensus statement. Nat. Rev. Genet. 19 (10), 649–666. doi:10.1038/s41576-018-0031-0
Krantz, I. D., McCallum, J., DeScipio, C., Kaur, M., Gillis, L. A., Yaeger, D., et al. (2004). Cornelia de Lange syndrome is caused by mutations in NIPBL, the human homolog of Drosophila melanogaster Nipped-B. Nat. Genet. 36 (6), 631–635. doi:10.1038/ng1364
Mannini, L., Cucco, F., Quarantotti, V., Krantz, I. D., and Musio, A. (2013). Mutation spectrum and genotype-phenotype correlation in Cornelia de Lange syndrome. Hum. Mutat. 34 (12), 1589–1596. doi:10.1002/humu.22430
Mazzola, M., Deflorian, G., Pezzotta, A., Ferrari, L., Fazio, G., Bresciani, E., et al. (2019). NIPBL: a new player in myeloid cell differentiation. Haematologica 104 (7), 1332–1341. doi:10.3324/haematol.2018.200899
Moss, J., Penhallow, J., Ansari, M., Barton, S., Bourn, D., FitzPatrick, D. R., et al. (2017). Genotype-phenotype correlations in Cornelia de Lange syndrome: behavioral characteristics and changes with age. Am. J. Med. Genet. A 173 (6), 1566–1574. doi:10.1002/ajmg.a.38228
Muto, A., Ikeda, S., Lopez-Burks, M. E., Kikuchi, Y., Calof, A. L., Lander, A. D., et al. (2014). Nipbl and mediator cooperatively regulate gene expression to control limb development. PLoS Genet. 10 (9), e1004671. doi:10.1371/journal.pgen.1004671
Nizon, M., Henry, M., Michot, C., Baumann, C., Bazin, A., Bessières, B., et al. (2016). A series of 38 novel germline and somatic mutations of NIPBL in Cornelia de Lange syndrome. Clin. Genet. 89 (5), 584–589. doi:10.1111/cge.12720
Ratajska, M., Wierzba, J., Pehlivan, D., Xia, Z., Brundage, E. K., Cheung, S. W., et al. (2010). Cornelia de Lange syndrome case due to genomic rearrangements including NIPBL. Eur. J. Med. Genet. 53 (6), 378–382. doi:10.1016/j.ejmg.2010.08.002
Sarimski, K. (1997). Communication, social-emotional development and parenting stress in cornelia-de-lange syndrome. J. Intellect. Disabil. Res. 41 (Pt 1), 70–75. doi:10.1111/j.1365-2788.1997.tb00678.x
Sarogni, P., Pallotta, M. M., and Musio, A. (2020). Cornelia de Lange syndrome: from molecular diagnosis to therapeutic approach. J. Med. Genet. 57 (5), 289–295. doi:10.1136/jmedgenet-2019-106277
Selicorni, A., Mariani, M., Lettieri, A., and Massa, V. (2021). Cornelia de Lange Syndrome: from a Disease to a Broader Spectrum. Genes (Basel) 12 (7), 1075. doi:10.3390/genes12071075
Smith, T. G., Laval, S., Chen, F., Rock, M. J., Strachan, T., and Peters, H. (2014). Neural crest cell-specific inactivation of nipbl or Mau2 during mouse development results in a late onset of craniofacial defects. Genesis 52 (7), 687–694. doi:10.1002/dvg.22780
Srivastava, S., Landy-Schmitt, C., Clark, B., Kline, A. D., Specht, M., and Grados, M. A. (2014). Autism traits in children and adolescents with Cornelia de Lange syndrome. Am. J. Med. Genet. A 164A (6), 1400–1410. doi:10.1002/ajmg.a.36573
Keywords: cornelia de lange syndrome, variant, NIPBL, splicing, minigene
Citation: Shao XT, Dai YX, Zhao YF, Chen YH and Ying LJ (2025) Case Report: A novel intronic variant of NIPBL gene detected in a child with cornelia de lange syndrome. Front. Genet. 16:1665167. doi: 10.3389/fgene.2025.1665167
Received: 21 July 2025; Accepted: 28 August 2025;
Published: 10 September 2025.
Edited by:
Mara Marongiu, National Research Council (CNR), ItalyReviewed by:
Ammar Husami, Cincinnati Children’s Hospital Medical Center, United StatesKarl Pfeifer, National Institute of Child Health and Human Development, Bethesda, Maryland, United States
Copyright © 2025 Shao, Dai, Zhao, Chen and Ying. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Jing Ying, cG1qdGMxNTZAMTYzLmNvbQ==
 Xiao Ting Shao
Xiao Ting Shao Yu Xuan Dai
Yu Xuan Dai Yu Fang Zhao
Yu Fang Zhao Yu Hang Chen
Yu Hang Chen Ling Jing Ying
Ling Jing Ying