Abstract
This review provides an overview of the current data regarding sex- and gender-specific aspects in patients with inflammatory bowel diseases. A particular focus will be on disease course, medical and surgical treatment strategies, psychosocial differences, and special requirements during pregnancy and family planning. The most significant and clinically meaningful gender differences in IBD relate to psychosocial functioning. Although depression, fatigue, anxiety disorders, eating disorders, and sexual dysfunction also occur in male IBD patients, women seem to be affected much more frequently and severely in these areas.
Introduction
In inflammatory bowel disease (IBD), sex- and gender-related differences in genetics, epidemiology, disease phenotype, IBD diagnosis, and treatment modalities are not commonly considered (see also Box 1). However, when looking at the available studies in detail, there are some gender-specific differences. This review provides an overview of the current data regarding sex- and gender specific aspects in patients suffering from IBD. A particular focus is disease course, medical and surgical treatment strategies, psychosocial differences, and special requirements during pregnancy and family planning. Finally, this review provides suggestions for areas for future research to focus on.
Box 1 Sex vs. gender (ChatGPT, 2 November 2022. Prompt: explain the difference between sex and gender in health).
Sex refers to a person’s biological and physiological characteristics, including chromosomes, hormones, and reproductive organs. Typically, individuals are classified as male or female based on their anatomy and genetics.
Gender, on the other hand, refers to the socially constructed roles, behaviors, activities, and attributes that a given society considers appropriate for men and women. Gender identity refers to an individual’s deeply held sense of their own gender, which may or may not align with their assigned sex at birth.
In the context of health, it is important to consider both sex and gender because they can influence health experiences, access to health care, and health outcomes. For example, women and men may experience different symptoms or have different risk factors for certain health conditions. In addition, societal expectations and norms around gender can affect health behaviors and decisions, such as seeking preventive care or engaging in healthy behaviors.
Recognizing and addressing the influence of both sex and gender can help ensure that health care is inclusive and effective for all individuals.
Epidemiology and environmental factors
The gender-specific distributions of Crohn’s disease (CD) and ulcerative colitis (UC) depend, among other things, on ethnicity. Although women in Europe and the USA are affected approximately twice as often as men by Crohn’s disease, in Asia more men are affected than women. In UC, there is no difference in incidence related to sex, nor between continents (1–9). A recently published study representing 16 regions in Europe, North America, Australia, and New Zealand revealed an interesting relationship between age and sex in IBD epidemiology: in this study, women had a lower risk of CD in childhood up to the age of 10–14 years (incidence rate ratio 0.70; 95% confidence interval (CI): 0.53 to 0.93). Thereafter, however, a higher risk of CD was seen, which was statistically significant in the age groups 25–29 years and over 35 years. There was no significant difference in the incidence of UC between female and male patients (except for the 5–9 years age group) up to the age of 45 years; thereafter, a significantly higher incidence of UC was observed for male patients than female patients. Overall, men had a 20% higher incidence rate of UC than women from the age of 45 years (10).
When looking for causes, pediatric studies show that the association between antibiotic exposure and the development of IBD was stronger in boys than in girls (11, 12). Gender-specific associations have also been shown for other risk factors, such as appendectomy, which increases the risk of Crohn’s disease in women (13). Similarly, nicotine abuse increases the risk of Crohn's disease in women in particular. (14). Somewhat surprising is the high proportion of women with Crohn’s disease who smoke, which is significantly higher than that of men (15, 16). Smoking is considered to be a protective factor in UC, but a Dutch study showed that this applies only to men. This study also found that only men who smoke have a lower need for immunosuppressive therapy (17). Women, therefore, have the greatest benefit if they stop smoking and should primarily be supported by smoking cessation programs.
Epidemiological studies have shown that the use of hormones such as oral contraceptives (OC) or post-menopausal hormone replacement therapy (HRT) in women is associated with the occurrence of IBD and complicative courses (18). A meta-analysis by Ortizo et al. found that OC users had a 24% and 30% higher risk of developing CD and UC, respectively (19). A large prospective cohort study also found a positive association between the use of hormone replacement therapy and the risk of developing CD (20). Furthermore, the use of combined OC confers a higher risk of CD-related surgery in long-term users (21). No increased risk for the development of IBD can be derived from the current study situation on HRT. Kane et al. (22) even documented a dose-dependent protective effect of HRT on disease severity in IBD. Other environmental factors, such as nutrition, could also play a gender-specific role, but robust data are lacking.
Genetics
If there is a family history of IBD women are more likely to be affected than their male relatives, this so-called female imprinting is observed, which corresponds to a higher female-to-female transmission rate (23). In addition, some susceptibility gene variants are associated with a modulation of the gender-specific risk for women, including the genes influencing the IL-23 and IL-10 signaling pathways (24, 25). So far, however, these genetic differences have not had any influence on diagnosis, prognosis, or treatment decisions.
Extraintestinal manifestations
The gender-specific occurrence of extraintestinal manifestations is well established (26–31). The best-known gender-specific association is that of primary sclerosing cholangitis (PSC). Here, the male sex is clearly a risk factor [odds ratio (OR) up to 2.771, p = 0.022] (30, 32). Likewise, in men, ankylosing spondylitis and the extremely rare amyloidosis are more common (31, 33), whereas, in women, joint, skin, and eye involvement seem to be more frequent (7, 16, 30, 34–36).
Prognosis and disease activity
A very recently published study reports that there is a longer diagnostic delay in women than in men for both CD and UC due to a drawn-out evaluation of women, with a higher number of misdiagnoses at all levels of the healthcare system (37). It is well known that delayed diagnosis is a risk factor for the need for surgical intervention later in the course of the disease (38). Whether gender alone is a potential risk factor for greater disease activity and a more complicated disease course has been little studied and the results are contradictory. A population-based study by Romberg-Camps et al., in which the data sets of 1,187 patients were examined, could not find any evidence for gender as a prognostic factor for severe disease progression, but a longitudinal study from Israel, in which the data of 269 patients with Crohn’s disease were analyzed over a period of 10 years, identified male gender as a clear risk factor for a complicated course of the disease (OR 2.6, 95% CI: 1.17 to 5.75) (39). However, when looking at remission rates in relation to gender, a large cohort of IBD patients from the Rhine-Main region in Germany showed significantly higher remission rates in male UC patients (Crohn’s disease, 58.5% vs. 53.0%, p=n.s.; and ulcerative colitis, 69.4% vs. 58.0%, p=0.013) than in female UC patients (40). Another study of a German cohort of 1,032 patients with Crohn’s disease came to a similar conclusion; here, women with Crohn’s disease showed increased disease activity compared with men (41). In the inception cohort study iCREST-CD, male sex was significantly associated with the presence of perianal lesions (42).
IBD-related complications
It is particularly interesting to look at the gender differences in the risk of osteoporosis. For example, although women with Crohn’s disease have an increased long-term risk of osteoporosis, in a large retrospective German study the proportion of men with osteopenia or osteoporosis (53.9%) was significantly higher than that of premenopausal women with IBD (29.6%). In this study, the risk is in the same range as in postmenopausal women with IBD (47%) (43, 44).
Male gender thus appears to be an independent risk factor for the development of bone density reduction, an observation that has been confirmed in several studies (16, 45, 46). This phenomenon is certainly underestimated in everyday clinical practice and requires increased attention, screening, and prevention. However, women and patients with stomas have also been shown in some studies to have an increased risk of osteoporosis, which also needs to be considered in daily clinical practice (47, 48).
Compared with the normal population, patients with IBD have an increased risk of colorectal cancer (CRC) regardless of gender (in men, RR 2.6, 95% CI: 2.2 to 3.1; in women, RR 1.9, 95% CI: 1.5 to 2.4) (49). However, there are also clearly gender-specific differences. Men with IBD have an increased risk of developing CRC compared with women with IBD (49, 50). Based on 171,000 person-years of follow-up, men have an approximately 60% increased risk compared with women (RR 1.6, 95% CI: 1.2 to 2.2). The cumulative incidence of CRC 40 years after initial diagnosis of IBD was 8.3% (men) vs. 3.5% (women) (49). The higher risk of CRC in male patients is not exclusive to patients with IBD; the same applies to the general population and can mostly be attributed to a number of risk factors. Male patients are more likely to smoke and consume more alcohol, processed meat, and red meat (51). The mortality of CRC is also higher in men with UC than in women with UC (the standardized mortality ratio (SMR) for men is 1.8, 95% CI: 1.0 to 3.1; for women it is 0.7, 95% CI: 0.3 to 2). In contrast, according to this meta-analysis, women have an increased mortality from pulmonary complications (in women, SMR CD 2.1, 95% CI: 1.4 to 3.0, vs. in men, SMR CD 1.5, 95% CI: 0.9 to 2.6; and, in women, SMR UC 1.6, 95% CI: 1.1 to 2.2, vs. in men, SMR CD 1.1, 95% CI: 0.6 to 2.1) (50).
Drug therapy
For over a decade, gender-specific differences in response to therapy have been the focus of studies in other diseases. These include cardiovascular diseases (52, 53), rheumatoid diseases (54, 55), endocrinological disorders such as diabetes mellitus (56), and cancer (57). For IBD, however, these studies are mostly lacking and only isolated data secondary to studies conducted with a different primary end point are available. In the Dutch IBD Biobank study and the COIN study, no significant differences regarding the use of IBD-specific medication were observed, with the only exception being a more frequent use of prednisone in male CD patients than in female CD patents (6.8% vs. 3.7%, p=0.03) (16). Much higher use of prednisone, also pronounced in men, was found in a large retrospective monocentric study (73.5% vs. 67.4%, p=0.04). The use of immunomodulators was also higher for men than women in this study (86.6% vs. 78.3%, p=0.008) (58). It might be worth mentioning that young male EBV-negative patients treated with thiopurines, especially in combination with TNF-blocker therapy, display an increased risk of hepatosplenic lymphoma (59).
In a separate analysis, it was observed that 13.4% of women with Crohn’s disease, in contrast to 4.6% of men, did not receive IBD-specific drug therapy (p< 0.01) (40). This presumed undertreatment was also reflected in the medications used, with men being treated more frequently with immunosuppressive medications for both Crohn’s disease (men, 46.9%, vs. women, 37.9%) and ulcerative colitis (men, 34.3%, vs. women, 26.3%). This distribution was also found in the biologic therapy used for both Crohn’s disease (men, 6.9%, vs. women, 4.5%) and ulcerative colitis (men, 2.5%, vs. women, 1.4%). No correlation with the age or disease activity of the patients could be found in the examinations. In line with these data, the work of Lesuis et al. also demonstrated an increased use of biologics in men with IBD (54). It is noteworthy that this effect was also observed in patients with psoriasis and rheumatoid arthritis (54). Similarly, this work documented higher subjective, but not objective, disease scores in female patients with CD and rheumatoid arthritis. Based on these findings, justified concerns that too little attention is paid to gender differences in therapy management in IBD were raised (60). Several factors might contribute to the observed undertreatment in women. Many of our patients are in their reproductive years, and hesitance to use proper medication might be associated with the unfounded fear that medication can harm the fetus or impair fertility. For some women, the access to specialized care might be difficult, and this applies especially to patients who have migrated from other countries and do not have adequate health insurance and knowledge regarding the healthcare system.
Treatment response
Predictors of response to a particular therapy have been repeatedly investigated; a systematic review of a total of 11 studies with 995 included CD patients treated with adalimumab identified male gender as an independent risk factor for loss of response and the need for dose escalation (58, 61). However, these data are also controversial, as gender was never the primary end point in the studies. In addition, a monocentric study (n=210 patients) is available that shows both a better and a longer response rate to therapy with infliximab in men with Crohn’s disease (62). In a smaller study in severe ulcerative colitis, where infliximab was used as a rescue therapy, female gender was a prognostic factor for higher remission rate at 1 year and higher cumulative non-colectomy rate (63, 64). In a very recently published individual-participant meta-analysis including data from clinical UC trials only, male patients with active UC receiving TNF-blockers had a significantly lower likelihood of clinical remission, and even mucosal healing, than women (65). In contrast, a small study with a median follow-up of 6 years revealed that drug survival was higher in males than females patients (48.1% vs. 30.8%, p=0.016) (63). It can be argued here that staying on a drug alone does not prove its efficacy on end points such as clinical remission or even mucosal healing.
The reasons for the abovementioned differences remain speculative and are not well explained by the available data. One possible explanation is offered by the work of Zelinkova et al., who demonstrated a significantly higher adverse event rate in women in a retrospective analysis of 1,009 patients. Reduced medication use by female patients with IBD (66). Another study was able to confirm this for adalimumab; this cohort included a total of 107 women and 81 men; here, too, there were significantly more adverse drug reactions in the female IBD patients, which also led more frequently to the termination of therapy (63). In a retrospective study of 529 IBD patients treated with TNF-blockers, female sex was associated with drug discontinuation due to side effects (HR 4.05, 95% CI: 2.36 to 6.98) (67).
Therapy adherence
In all chronic diseases, adherence to therapy is of great importance in strategic considerations, as it is the only way to ensure effective remission-maintaining therapy. Therapy adherence is therefore as problematic in IBD patients as in patients with other chronic diseases (68–70). That it is a key issue is shown by an internet-based cohort from North America, in which 41% of IBD patients self-reported poor adherence (69). In this study, low inflammatory activity correlated with high levels of adherence. The lack of adherence affects both sexes, with older studies showing reduced adherence in men with IBD (68, 70), and more recent work showing that it is more likely to be reduced in young women (71–73). Especially in the setting of a therapy break, it should be noted that men have a higher probability of relapsing.
Surgical therapy
There have been retrospective cohort studies that have shown different gender-specific risk constellations. However, these data cannot be confirmed in more recent analyses, and nowadays much attention is paid to the prevention of postoperative recurrence of Crohn’s disease, and drug therapy is often initiated postoperatively (74).
However, some gender-specific risk factors have been identified. For example, in the Olmsted County cohort, male sex was identified as a risk factor for major abdominal surgery (hazard ratio (HR) 1.6) (75). Another retrospective analysis showed a higher rate of ileocecal resections in women (44% vs. 32%, p=0.004) (7). This study also reported that women had an increased risk of a second resection.
For ulcerative colitis, reference can be made to two population-based studies that identified a higher rate of colectomy in men (HR 2.1, 95% CI: 1.3 to 3.5; and HR 2.6, 95% CI: 1.58 to 4.36) (76, 77). Pouch-specific complications such as ileus, fistulae, and pouch failure appear to be more common in women (78). Men, on the other hand, are more likely to develop antibiotic-refractory pouchitis (16.3% vs. 10.7%, p=0.001) (79).
Psychosocial factors
The most consistent gender differences in IBD relate to psychosocial functioning. Although depression, anxiety disorders, eating disorders, and sexual dysfunction also occur in male IBD patients, these are described far more frequently in women (80), an observation that can also be made in the general population [McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res. 2011 Aug;45(8):1027–35. doi: 10.1016/j.jpsychires.2011.03.006. Epub 2011 Mar 25. PMID: 21439576; PMCID: PMC3135672.]. The first problem is that these partly psychological, partly psychiatric problems are underdiagnosed and undertreated, especially in female IBD patients (80, 81). In a recently published meta-analysis that included 77 studies representing over 30,000 IBD patients, women were more likely to have symptoms of anxiety than men (for women, pooled prevalence 33.8%, 95% CI: 26.5 to 41.5, vs. for men, 22.8%, 95% CI: 18.7 to 27.2; OR 1.7, 95% CI: 1.2 to 2.3). They were also more likely to have symptoms of depression than men (for women, pooled prevalence 21.2%, 95% CI: 15.4 to 27.6, vs. for men, 16.2%, 95% CI: 12.6 to 20.3; OR 1.3, 95% CI 1.0 to 1.8) (82). The authors conclude that encouraging gastroenterologists to screen for and treat these disorders might improve outcomes for patients with IBD. For example, screening for depression is supported by the recommendations provided by the United States Preventive Task Force (PSPSTF) universal screening in all adult populations, including pregnant and postpartum women, and the American College of Gastroenterology (AGA) screen for depression and anxiety in patients with IBD (83, 84).
Bommena et al. have described the effects of depression on IBD-related factors, with the female sex predominant in a recently published review (see Figure 1). The consequences of depression, especially in female IBD patients, are discussed in detail. Only a few should be mentioned here: women with IBD are more likely to experience postpartum depression than women without IBD (85). Over 50% of women with IBD are suffering from sexual dysfunction (SD) compared with 28% in healthy controls (p<0.01), vs. 16.9% in men with IBD compared with 7.4% in healthy controls (p=0.64) (86). Approximately 70% of women with IBD are concerned about their body image (87, 88), a concern that significantly impairs their quality of life (QOL), which is already reduced in female IBD patients (89). Another factor contributing to poor QOL in women with IBD is fatigue. In the Swiss IBD cohort, 672 IBD patients (55.6%) reported significant fatigue compared with 145 (35%) controls (OR 2.71, 95% CI: 2.08 to 3.54; p< 0.001). In IBD, fatigue also significantly affected the daily activities (FSS ≥ 4) of 405 (33.5%) IBD patients vs. 81 (19.6%) controls; p< 0.001). In the MANOVA model, fatigue levels were associated with female gender (coefficient 0.839, 95% CI: 0.556 to 1.123; p< 0.001), younger age at diagnosis (−0.031 per year, 95% CI: −0.042 to −0.019; p< 0.001), shorter disease duration (−0.036 per year, 95% CI: −0.050 to −0.022; p< 0.001), nocturnal diarrhea (0.718, 95% CI: 0.295 to 1.141; p = 0.001), low educational level (p = 0.034), and symptoms of depression and anxiety (90).
Figure 1
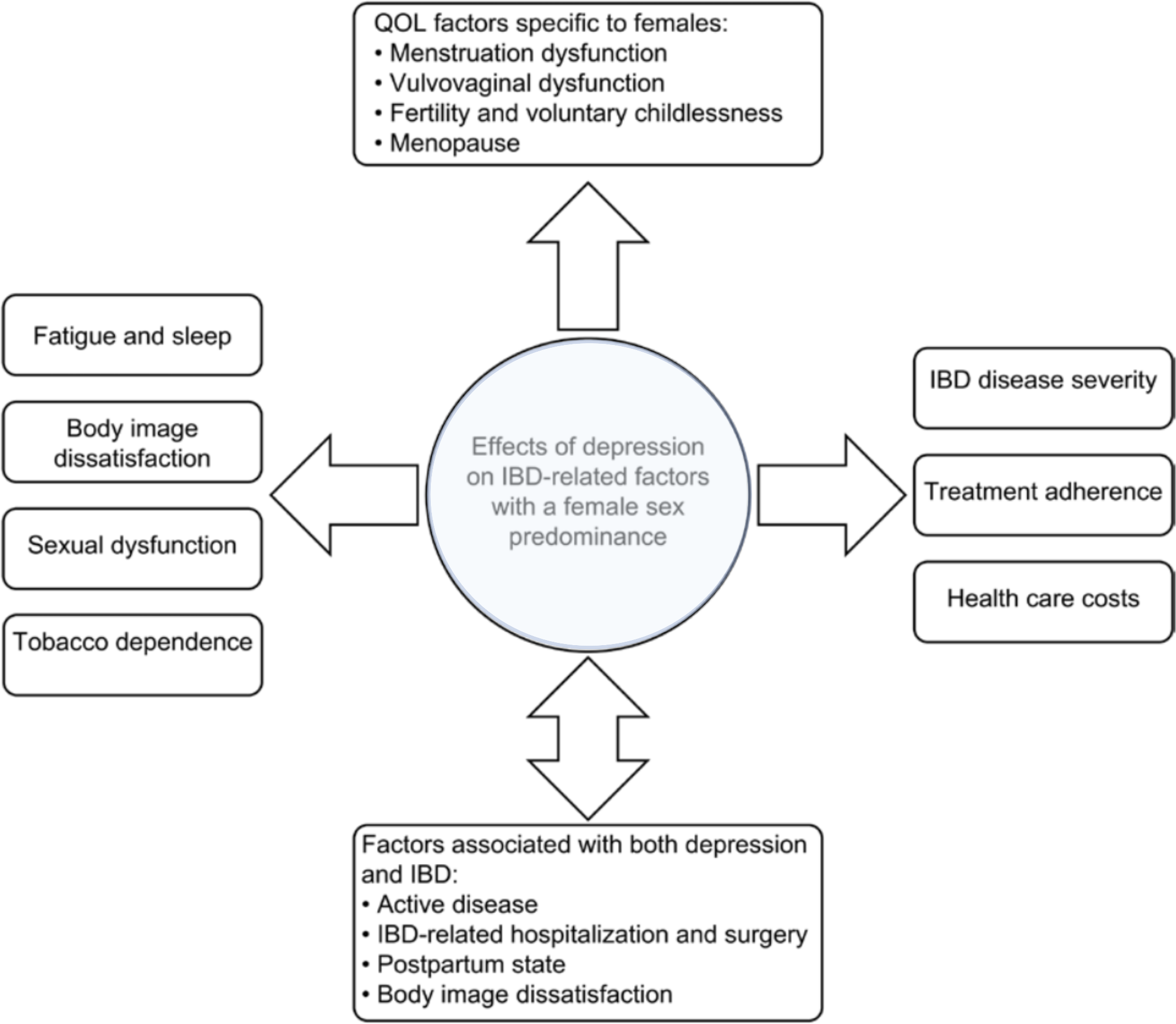
Factors associated with female sex and depression in patients with IBD. (adapted from Bommena et al. (80))
Depression in male IBD patients is less prevalent; however, it also affects their QOL. Depression is related to SD, as mentioned above, which is prevalent in 10%–40% of male patients with IBD (91). Risk factors for male SD in IBD are advanced age, severe disease, post-IBD-related surgery, and smoking (91, 92). Men experience fear of infertility and body image dissatisfaction, which is also strongly correlated to depression and subsequently impairs their QOL (93–95).
Most importantly, a multimodal integrative therapeutic approach is feasible, has been shown to be successful in both male and female IBD patients, and should be implemented more widely (80).
Pregnancy and breastfeeding
Since IBD is often diagnosed in young adults family planning should be considered throughout the treatment. Here the male perspective on fertility and family planning is much less illuminated than the female view (95, 96). Patients with IBD have fewer children than people without an IBD diagnosis. Interestingly, actual reduced fertility due to the disease does not seem to be the cause; rather, it is mostly unfounded, diffuse fears that play the biggest role. In both sexes, there is great uncertainty about the risk of passing on the disease to the child (97). In female patients, the fear of possible complications during pregnancy due to the disease or drug therapy during pregnancy also plays a major role in self-chosen childlessness (98). Another aspect of family planning affecting both sexes is adequate nutrition with a balanced vitamin and mineral content. Not only can malnutrition lead to reduced fertility in men and women (94), but a folic acid deficiency in women can lead to neural tube defects in the embryo. The fact that early comprehensive counseling on the topic of childbearing and pregnancy leads to a better course of pregnancy and healthier newborns has been well studied for women (99). These studies are not yet available for men .
Turning to the aspects of drug therapy in family planning and pregnancy, even greater differences between the sexes are obvious. In one study it was shown that for men a preserved remission of the disease in the procreation phase is of great importance. There is hardly any concern that taking medication could be a problem here or could limit male fertility (100). However, it has been repeatedly shown that this plays a much greater role for woman, especially if they have not received adequate counseling beforehand. Most patients are convinced that drug therapy at the time of conception and further course of pregnancy is significantly more problematic for the unborn child than an increase in disease activity when the previous therapy is discontinued (101, 102).
Summary and outlook
This review provides an up-to-date overview of sex- and gender-specific aspects of IBD in terms of disease phenotype, disease course, and treatment outcome (Figure 2). It should be noted that most of the differences described are based on retrospective data or from studies that did not have the primary end point of identifying these differences.
Figure 2
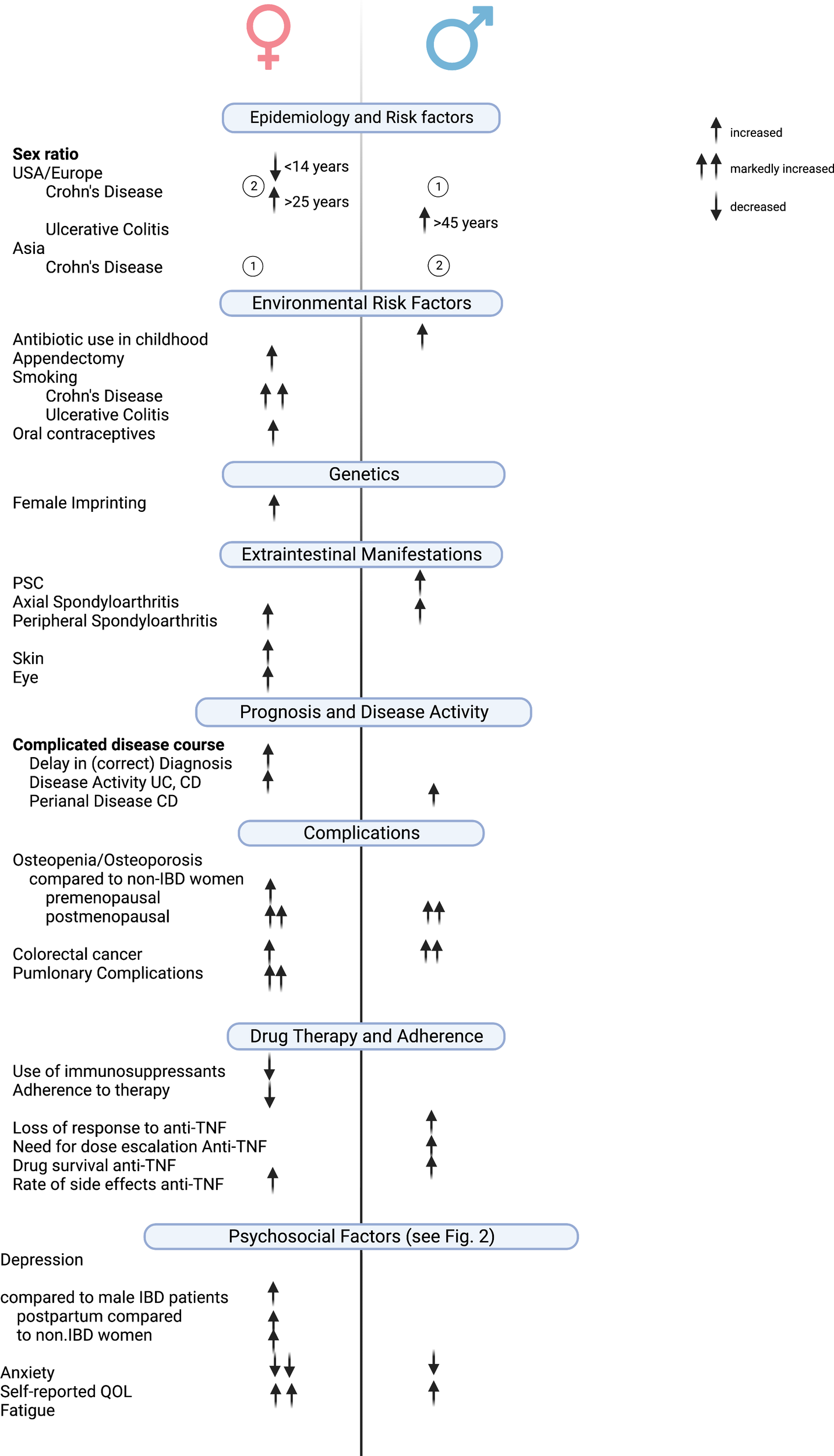
Clinically relevant gender/sex-based differences in IBD. Created with Biorender.com.
The most significant and clinically meaningful sex- and gender-specific differences in IBD relate to psychosocial functioning. Although depression, fatigue, anxiety disorders, eating disorders, and sexual dysfunction also occur in male IBD patients, women seem to be affected much more frequently and severely in these areas.
It seems important that, analogous to other chronic diseases, studies with sex- and gender-specific end points are conducted in the future to enable specialized, personalized medical care for patients with IBD.
Statements
Author contributions
IB and ES drafted the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) IB declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Brahme F Lindström C Wenckert A . Crohn’s disease in a defined population. An epidemiological study of incidence, prevalence, mortality, and secular trends in the city of Malmö, Sweden. Gastroenterology (1975) 69:342–51. doi: 10.1016/S0016-5085(19)32575-2
2
Kyle J . Crohn’s disease in the northeastern and northern Isles of Scotland: An epidemiological review. Gastroenterology (1992) 103:392–9. doi: 10.1016/0016-5085(92)90826-K
3
Latour P Louis E Belaiche J . Incidence of inflammatory bowel disease in the area of Liège: a 3 years prospective study (1993-1996). Acta Gastro-ent Belg (1998) 61:410–3.
4
Leong RWL Lau JY Sung JJY . The Epidemiology and Phenotype of Crohn’s Disease in the Chinese Population. Inflammation Bowel Dis (2004) 10:646–51. doi: 10.1097/00054725-200409000-00022
5
Ng SC Bernstein CN Vatn MH Lakatos PL Loftus EV Tysk C et al . Geographical variability and environmental risk factors in inflammatory bowel disease. Gut (2013) 62:630. doi: 10.1136/gutjnl-2012-303661
6
Shivananda S Pena AS Nap M Weterman IT Mayberry JF Ruitenberg EJ et al . Epidemiology of Crohn’s disease in Regio Leiden, the Netherlands A population study from 1979 to 1983. Gastroenterology (1987) 93:966–74. doi: 10.1016/0016-5085(87)90558-0
7
Wagtmans MJ Verspaget HW Lamers CBHW van Hogezand RA . Gender-Related Differences in the Clinical Course of Crohn’s Disease. Am J Gastroenterol (2001) 96:1541–6. doi: 10.1111/j.1572-0241.2001.03755.x
8
Prideaux L Kamm MA Cruz PPD Chan FKL Ng SC . Inflammatory bowel disease in Asia: A systematic review. J Gastroen Hepatol (2012) 27:1266–80. doi: 10.1111/j.1440-1746.2012.07150.x
9
Yang S-K Loftus EV Sandborn WJ . Epidemiology of Inflammatory Bowel Disease in Asia. Inflammation Bowel Dis (2001) 7:260–70. doi: 10.1097/00054725-200108000-00013
10
Shah SC Khalili H Gower-Rousseau C Olen O Benchimol EI Lynge E et al . Sex-Based Differences in Incidence of Inflammatory Bowel Diseases—Pooled Analysis of Population-Based Studies From Western Countries. Gastroenterology (2018) 155:1079–1089.e3. doi: 10.1053/j.gastro.2018.09.014
11
Virta L Auvinen A Helenius H Huovinen P Kolho K-L . Association of Repeated Exposure to Antibiotics With the Development of Pediatric Crohn’s Disease—A Nationwide, Register-based Finnish Case-Control Study. Am J Epidemiol (2012) 175:775–84. doi: 10.1093/aje/kwr400
12
Shaw SY Blanchard JF Bernstein CN . Association Between the Use of Antibiotics in the First Year of Life and Pediatric Inflammatory Bowel Disease. Am J Gastroenterol (2010) 105:2687–92. doi: 10.1038/ajg.2010.398
13
Andersson RE Olaison G Tysk C Ekbom A . Appendectomy is followed by increased risk of Crohn’s disease. Gastroenterology (2003) 124:40–6. doi: 10.1053/gast.2003.50021
14
Lakatos PL Vegh Z Lovasz BD David G Pandur T Erdelyi Z et al . Is Current Smoking Still an Important Environmental Factor in Inflammatory Bowel Diseases? Results from a Population-based Incident Cohort. Inflammation Bowel Dis (2013) 19:1010–7. doi: 10.1097/MIB.0b013e3182802b3e
15
Biedermann L Fournier N Misselwitz B Frei P Zeitz J Manser CN et al . High Rates of Smoking Especially in Female Crohn’s Disease Patients and Low Use of Supportive Measures to Achieve Smoking Cessation—Data from the Swiss IBD Cohort Study. J Crohn’s Colitis (2015) 9:819–29. doi: 10.1093/ecco-jcc/jjv113
16
Severs M Spekhorst LM Mangen M-JJ Dijkstra G Löwenberg M Hoentjen F et al . Sex-Related Differences in Patients With Inflammatory Bowel Disease: Results of 2 Prospective Cohort Studies. Inflammation Bowel Dis (2018) 24:1298–306. doi: 10.1093/ibd/izy004
17
Cosnes J Nion-larmurier I Afchain P Beaugerie L Gendre J . Gender differences in the response of colitis to smoking. Clin Gastroenterol H (2004) 2:41–8. doi: 10.1016/S1542-3565(03)00290-8
18
Jacenik D Cygankiewicz AI Mokrowiecka A Małecka-Panas E Fichna J Krajewska WM . Sex- and Age-Related Estrogen Signaling Alteration in Inflammatory Bowel Diseases: Modulatory Role of Estrogen Receptors. Int J Mol Sci (2019) 20:3175. doi: 10.3390/ijms20133175
19
Ortizo R Lee SY Nguyen ET Jamal MM Bechtold MM Nguyen DL . Exposure to oral contraceptives increases the risk for development of inflammatory bowel disease. Eur J Gastroen Hepat (2017) 29:1064–70. doi: 10.1097/MEG.0000000000000915
20
Khalili H Higuchi LM Ananthakrishnan AN Richter JM Feskanich D Fuchs CS et al . Oral contraceptives, reproductive factors and risk of inflammatory bowel disease. Gut (2013) 62:1153. doi: 10.1136/gutjnl-2012-302362
21
Khalili H Granath F Smedby KE Ekbom A Neovius M Chan AT et al . Association Between Long-term Oral Contraceptive Use and Risk of Crohn’s Disease Complications in a Nationwide Study. Gastroenterology (2016) 150:1561–1567.e1. doi: 10.1053/j.gastro.2016.02.041
22
Kane SV Reddy D . Hormonal Replacement Therapy After Menopause Is Protective of Disease Activity in Women With Inflammatory Bowel Disease. Am J Gastroenterol (2008) 103:1193–6. doi: 10.1111/j.1572-0241.2007.01700.x
23
Zelinkova Z Stokkers PC van der Linde K Kuipers EJ Peppelenbosch MP Woude CPJ . Maternal imprinting and female predominance in familial Crohn’s disease. J Crohn’s Colitis (2012) 6:771–6. doi: 10.1016/j.crohns.2012.01.002
24
Lin Z Poritz L Franke A Li T-Y Ruether A Byrnes KA et al . Genetic Association of Nonsynonymous Variants of the IL23R with Familial and Sporadic Inflammatory Bowel Disease in Women. Digest Dis Sci (2010) 55:739–46. doi: 10.1007/s10620-009-0782-8
25
Tedde A Putignano AL Bagnoli S Congregati C Milla M Sorbi S et al . Interleukin-10 promoter polymorphisms influence susceptibility to ulcerative colitis in a gender-specific manner. Scand J Gastroentero (2008) 43:712–8. doi: 10.1080/00365520701885507
26
Vadstrup K Alulis S Borsi A Jørgensen TR Nielsen A Munkholm P et al . Extraintestinal Manifestations and Other Comorbidities in Ulcerative Colitis and Crohn Disease: A Danish Nationwide Registry Study 2003–2016. Crohn’s Colitis 360 (2020) 2:otaa070. doi: 10.1093/crocol/otaa070
27
Park S-K Wong Z Park SH Vu KV Bang KB Piyachaturawat P et al . Extraintestinal manifestation of inflammatory bowel disease in Asian patients: A multinational study. Digest Liver Dis (2021) 53:196–201. doi: 10.1016/j.dld.2020.06.046
28
Karmiris K Avgerinos A Tavernaraki A Zeglinas C Karatzas P Koukouratos T et al . Prevalence and Characteristics of Extra-intestinal Manifestations in a Large Cohort of Greek Patients with Inflammatory Bowel Disease. J Crohn’s Colitis (2016) 10:429–36. doi: 10.1093/ecco-jcc/jjv232
29
Vavricka SR Brun L Ballabeni P Pittet V Vavricka BMP Zeitz J et al . Frequency and Risk Factors for Extraintestinal Manifestations in the Swiss Inflammatory Bowel Disease Cohort. Am J Gastroenterol (2011) 106:110–9. doi: 10.1038/ajg.2010.343
30
Algaba A Guerra I Ricart E Iglesias E Mañosa M Gisbert JP et al . Extraintestinal Manifestations in Patients with Inflammatory Bowel Disease: Study Based on the ENEIDA Registry. Digest Dis Sci (2021) 66:2014–23. doi: 10.1007/s10620-020-06424-x
31
Bernstein CN Blanchard JF Rawsthorne P Yu N . The Prevalence of Extraintestinal Diseases in Inflammatory Bowel Disease: A Population-Based Study. Am J Gastroenterol (2001) 96:1116–22. doi: 10.1111/j.1572-0241.2001.03756.x
32
Fraga M Fournier N Safroneeva E Pittet V Godat S Straumann A et al . Primary sclerosing cholangitis in the Swiss Inflammatory Bowel Disease Cohort Study. Eur J Gastroen Hepat (2017) 29:91–7. doi: 10.1097/MEG.0000000000000747
33
Greenstein AJ Sachar DB Panday AKN Dikman SH Meyers S Heimann T et al . Amyloidosis and Inflammatory Bowel Disease A 50-Year Experience with 25 Patients. Medicine (1992) 71:261–70. doi: 10.1097/00005792-199209000-00001
34
Ampuero J Rojas-Feria M Castro-Fernández M Cano C Romero-Gómez M . Erythema nodosum and pyoderma gangrenosum. J Gastroen Hepatol (2014) 29:291–5. doi: 10.1111/jgh.12352
35
Kumar S Pollok R Goldsmith D . Renal and Urological Disorders Associated With Inflammatory Bowel Disease. Inflammation Bowel Dis (2022). doi: 10.1093/ibd/izac140
36
Wu X Mukewar S Kiran RP Remzi FH Hammel J Shen B . Risk factors for peristomal pyoderma gangrenosum complicating inflammatory bowel disease. J Crohn’s Colitis (2013) 7:e171–7. doi: 10.1016/j.crohns.2012.08.001
37
Sempere L Bernabeu P Cameo J Gutiérrez A García MG García MF et al . Gender Biases and Diagnostic Delay in Inflammatory Bowel Disease: Multicenter Observational Study. Inflammation Bowel Dis (2023). doi: 10.1093/ibd/izad001
38
Lee D Koo JS Choe JW Suh SJ Kim SY Hyun JJ et al . Diagnostic delay in inflammatory bowel disease increases the risk of intestinal surgery. World J Gastroentero (2017) 23:6474–81. doi: 10.3748/wjg.v23.i35.6474
39
Mazor Y Maza I Kaufman E Ben-Horin S Karban A Chowers Y et al . Prediction of disease complication occurrence in Crohn’s disease using phenotype and genotype parameters at diagnosis. J Crohn’s Colitis (2011) 5:592–7. doi: 10.1016/j.crohns.2011.06.002
40
Blumenstein I Herrmann E Filmann N Zosel C Tacke W Bock H et al . Female patients suffering from inflammatory bowel diseases are treated less frequently with immunosuppressive medication and have a higher disease activityA subgroup analysis of a large multi-centre, prospective, internet-based study. J Crohn’s Colitis (2011) 5:203–10. doi: 10.1016/j.crohns.2010.12.012
41
Bokemeyer B Hardt J Hüppe D Prenzler A Conrad S Düffelmeyer M et al . Clinical status, psychosocial impairments, medical treatment and health care costs for patients with inflammatory bowel disease (IBD) in Germany: An online IBD registry. J Crohn’s Colitis (2013) 7:355–68. doi: 10.1016/j.crohns.2012.02.014
42
Yamamoto T Nakase H Watanabe K Shinzaki S Takatsu N Fujii T et al . Diagnosis and Clinical Features of Perianal Lesions in Newly Diagnosed Crohn’s Disease: Subgroup Analysis from Inception Cohort Registry Study of Patients with Crohn’s Disease (iCREST-CD). J Crohn’s Colitis (2023). doi: 10.1093/ecco-jcc/jjad038
43
Wada Y Hisamatsu T Naganuma M Matsuoka K Okamoto S Inoue N et al . Risk factors for decreased bone mineral density in inflammatory bowel disease: A cross-sectional study. Clin Nutr (2015) 34:1202–9. doi: 10.1016/j.clnu.2015.01.003
44
Walldorf J Krummenerl A Engler K Busch J Dollinger MM Seufferlein T et al . Health care for osteoporosis in inflammatory bowel disease: Unmet needs in care of male patients? J Crohn’s Colitis (2013) 7:901–7. doi: 10.1016/j.crohns.2012.12.008
45
Bartram SA Peaston RT Rawlings DJ Walshaw D Francis RM Thompson NP . Mutifactorial analysis of risk factors for reduced bone mineral density in patients with Crohn’s disease. World J Gastroentero (2006) 12:5680–6. doi: 10.3748/wjg.v12.i35.5680
46
Robinson RJ Azzawi FA Iqbal SJ Kryswcki T Almond L Abrams K et al . Osteoporosis and Determinants of Bone Density in Patients with Crohn’s Disease. Digest Dis Sci (1998) 43:2500–6. doi: 10.1023/A:1026650719552
47
Haschka J Hirschmann S Kleyer A Englbrecht M Faustini F Simon D et al . High-resolution Quantitative Computed Tomography Demonstrates Structural Defects in Cortical and Trabecular Bone in IBD Patients. J Crohn’s Colitis (2016) 10:532–40. doi: 10.1093/ecco-jcc/jjw012
48
Gupta S Wu X Moore T Shen B . Frequency, Risk Factors, and Adverse Sequelae of Bone Loss in Patients with Ostomy for Inflammatory Bowel Diseases. Inflammation Bowel Dis (2013) 20:259–64. doi: 10.1097/01.MIB.0000439065.92211.d3
49
Söderlund S Granath F Broström O Karlén P Löfberg R Ekbom A et al . Inflammatory Bowel Disease Confers a Lower Risk of Colorectal Cancer to Females Than to Males. Gastroenterology (2010) 138:1697–1703.e2. doi: 10.1053/j.gastro.2010.02.007
50
Jess T Rungoe C Peyrin–Biroulet L . Risk of Colorectal Cancer in Patients With Ulcerative Colitis: A Meta-analysis of Population-Based Cohort Studies. Clin Gastroenterol H (2012) 10:639–45. doi: 10.1016/j.cgh.2012.01.010
51
White A Ironmonger L Steele RJC Ormiston-Smith N Crawford C Seims A . A review of sex-related differences in colorectal cancer incidence, screening uptake, routes to diagnosis, cancer stage and survival in the UK. BMC Cancer (2018) 18:906. doi: 10.1186/s12885-018-4786-7
52
Caso V Paciaroni M Agnelli G Corea F Ageno W Alberti A et al . Gender differences in patients with acute ischemic stroke. Women’s Heal (2010) 6:51–7. doi: 10.2217/WHE.09.82
53
Shaw LJ Shaw RE Merz CNB Brindis RG Klein LW Nallamothu B et al . Impact of Ethnicity and Gender Differences on Angiographic Coronary Artery Disease Prevalence and In-Hospital Mortality in the American College of Cardiology–National Cardiovascular Data Registry. Circulation (2008) 117:1787–801. doi: 10.1161/CIRCULATIONAHA.107.726562
54
Lesuis N Befrits R Nyberg F van Vollenhoven RF . Gender and the treatment of immune-mediated chronic inflammatory diseases: rheumatoid arthritis, inflammatory bowel disease and psoriasis: an observational study. BMC Med (2012) 10:82. doi: 10.1186/1741-7015-10-82
55
Oliver JE Silman AJ . Why are women predisposed to autoimmune rheumatic diseases? Arthritis Res Ther (2009) 11:252. doi: 10.1186/ar2825
56
Szalat A Auryan S Raz I Itamar R . Gender-specific care of diabetes mellitus: particular considerations in the management of diabetic women. Diabetes Obes Metab (2008) 10:1135–56. doi: 10.1111/j.1463-1326.2008.00896.x
57
Schmetzer O Flörcken A . Sex and Gender Differences in Pharmacology. Handb Exp Pharmacol (2012), 411–42. doi: 10.1007/978-3-642-30726-3_19
58
Heath EM Kim RB Wilson A . A Comparative Analysis of Drug Therapy, Disease Phenotype, and Health Care Outcomes for Men and Women with Inflammatory Bowel Disease. Digest Dis Sci (2022) 67:4287–94. doi: 10.1007/s10620-021-07177-x
59
Shah ED Coburn ES Nayyar A Lee KJ Koliani‐Pace JL Siegel CA . Systematic review: hepatosplenic T-cell lymphoma on biologic therapy for inflammatory bowel disease, including data from the Food and Drug Administration Adverse Event Reporting System. Aliment. Pharmacol Ther (2020) 51:527–33. doi: 10.1111/apt.15637
60
Greuter T Manser C Pittet V Vavricka SR Biedermann L Gastroenterology on behalf of SIbd an official working group of the Swiss Society . Gender Differences in Inflammatory Bowel Disease. Digestion (2020) 101:98–104. doi: 10.1159/000504701
61
Billioud V Sandborn WJ Peyrin-Biroulet L . Loss of Response and Need for Adalimumab Dose Intensification in Crohn’s Disease: A Systematic Review. Am J Gastroenterol (2011) 106:674–84. doi: 10.1038/ajg.2011.60
62
Sprakes MB Ford AC Warren L Greer D Hamlin J . Efficacy, tolerability, and predictors of response to infliximab therapy for Crohn’s disease: A large single centre experience. J Crohn’s Colitis (2012) 6:143–53. doi: 10.1016/j.crohns.2011.07.011
63
Lie MRKL Kreijne JE van der Woude CJ . Sex Is Associated with Adalimumab Side Effects and Drug Survival in Patients with Crohn’s Disease. Inflammation Bowel Dis (2016) 23:75–81. doi: 10.1097/MIB.0000000000000981
64
Nasuno M Miyakawa M Tanaka H Motoya S . Short- and Long-Term Outcomes of Infliximab Treatment for Steroid-Refractory Ulcerative Colitis and Related Prognostic Factors: A Single-Center Retrospective Study. Digestion (2017) 95:67–71. doi: 10.1159/000452459
65
Agrawal M Petralia F Tepler A Durbin L Reinisch W Colombel J-F et al . Gender-Based Differences in Response to Tumor Necrosis Factor Inhibitor Therapies for Ulcerative Colitis: Individual Participant Data Meta-Analyses of Clinical Trials. Inflammation Bowel Dis (2022) 29:1–8. doi: 10.1093/ibd/izac067
66
Zelinkova Z Bultman E Vogelaar L Bouziane C Kuipers EJ Woude CJ . Sex-dimorphic adverse drug reactions to immune suppressive agents in inflammatory bowel disease. World J Gastroentero (2012) 18:6967–73. doi: 10.3748/wjg.v18.i47.6967
67
Schultheiss JPD Brand EC Lamers E Berg WCM Schaik FDM Oldenburg B et al . Earlier discontinuation of TNF-α inhibitor therapy in female patients with inflammatory bowel disease is related to a greater risk of side effects. Aliment Pharm Therap (2019) 50:386–96. doi: 10.1111/apt.15380
68
Hawthorne AB Rubin G Ghosh S . Review article: medication non-adherence in ulcerative colitis – strategies to improve adherence with mesalazine and other maintenance therapies. Aliment Pharm Therap (2008) 27:1157–66. doi: 10.1111/j.1365-2036.2008.03698.x
69
Long MD Kappelman MD Martin CF Lewis JD Mayer L Kinneer PM et al . Development of an internet-based cohort of patients with inflammatory bowel diseases (CCFA Partners): Methodology and initial results. Inflammation Bowel Dis (2012) 18:2099–106. doi: 10.1002/ibd.22895
70
Mantzaris GJ Roussos A Kalantzis C Koilakou S Raptis N Kalantzis N . How adherent to treatment with azathioprine are patients with Crohn’s disease in long-term remission? Inflammation Bowel Dis (2007) 13:446–50. doi: 10.1002/ibd.20041
71
Yen L Wu J Hodgkins P Cohen RD Nichol MB . Medication Use Patterns and Predictors of Nonpersistence and Nonadherence with Oral 5-Aminosalicylic Acid Therapy. J Manag Care Pharm (2012) 18:701–12. doi: 10.18553/jmcp.2012.18.9.701
72
Zelante A Giorgi AD Borgoni R Trevisani L Gallerani M . Adherence to medical treatment in inflammatory bowel disease patients. Minerva Gastroenterologica E Dietologica (2014) 60:269–74.
73
Lopez A Billioud V Peyrin-Biroulet C Peyrin-Biroulet L . Adherence to Anti-TNF Therapy in Inflammatory Bowel Diseases. Inflammation Bowel Dis (2013) 19:1528–33. doi: 10.1097/MIB.0b013e31828132cb
74
Cruz PD Kamm MA Hamilton AL Ritchie KJ Krejany EO Gorelik A et al . Efficacy of thiopurines and adalimumab in preventing Crohn’s disease recurrence in high-risk patients – a POCER study analysis. Aliment Pharm Therap (2015) 42:867–79. doi: 10.1111/apt.13353
75
Peyrin-Biroulet L Harmsen SW Tremaine WJ Zinsmeister AR Sandborn WJ Loftus EV . Surgery in a Population-Based Cohort of Crohn’s Disease From Olmsted County, Minnesota (1970–2004). Am J Gastroenterol (2012) 107:1693–701. doi: 10.1038/ajg.2012.298
76
Samuel S Ingle SB Dhillon S Yadav S Harmsen WS Zinsmeister AR et al . Cumulative Incidence and Risk Factors for Hospitalization and Surgery in a Population-based Cohort of Ulcerative Colitis. Inflammation Bowel Dis (2013) 19:1858–66. doi: 10.1097/MIB.0b013e31828c84c5
77
Targownik LE Singh H Nugent Z Bernstein CN . The Epidemiology of Colectomy in Ulcerative Colitis: Results From a Population-Based Cohort. Am J Gastroenterol (2012) 107:1228–35. doi: 10.1038/ajg.2012.127
78
Rottoli M Remzi FH Shen B Kiran RP . Gender of the patient may influence perioperative and long-term complications after restorative proctocolectomy. Colorectal Dis (2012) 14:336–41. doi: 10.1111/j.1463-1318.2011.02634.x
79
Wu X Ashburn J Remzi FH Li Y Fass H Shen B . Male Gender Is Associated with a High Risk for Chronic Antibiotic-Refractory Pouchitis and Ileal Pouch Anastomotic Sinus. J Gastrointest Surg (2016) 20:631–9. doi: 10.1007/s11605-015-2976-z
80
Bommena S Goldberg A Amini M Alishahi Y . Depression in Women With Inflammatory Bowel Disease: A Multifaceted Approach for a Multidimensional Problem. Inflammation Bowel Dis (2022). doi: 10.1093/ibd/izac245
81
Marafini I Longo L Lavasani DM Rossi R Salvatori S Pianigiani F et al . High Frequency of Undiagnosed Psychiatric Disorders in Inflammatory Bowel Diseases. J Clin Med (2020) 9:1387. doi: 10.3390/jcm9051387
82
Barberio B Zamani M Black CJ Savarino EV Ford AC . Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol (2021) 6:359–70. doi: 10.1016/S2468-1253(21)00014-5
83
Siu AL (USPSTF) UPSTF Bibbins-Domingo K Grossman DC Baumann LC Davidson KW et al . Screening for Depression in Adults: US Preventive Services Task Force Recommendation Statement. Jama (2016) 315:380–7. doi: 10.1001/jama.2015.18392
84
Farraye FA Melmed GY Lichtenstein GR Kane SV . ACG Clinical Guideline: Preventive Care in Inflammatory Bowel Disease. Am J Gastroenterol (2017) 112:241–58. doi: 10.1038/ajg.2016.537
85
Vigod SN Kurdyak P Brown HK Nguyen GC Targownik LE Seow CH et al . Inflammatory bowel disease and new-onset psychiatric disorders in pregnancy and post partum: a population-based cohort study. Gut (2019) 68:1597. doi: 10.1136/gutjnl-2018-317610
86
Rivière P Zallot C Desobry P Sabaté JM Vergniol J Zerbib F et al . Frequency of and Factors Associated With Sexual Dysfunction in Patients With Inflammatory Bowel Disease. J Crohn’s Colitis (2017) 11:1347–52. doi: 10.1093/ecco-jcc/jjx100
87
Beese SE Harris IM Moore D Dretzke J . Body image dissatisfaction in patients with inflammatory bowel disease: a systematic review protocol. Syst Rev (2018) 7:184. doi: 10.1186/s13643-018-0844-0
88
McDermott E Mullen G Moloney J Keegan D Byrne K Doherty GA et al . Body Image Dissatisfaction. Inflammation Bowel Dis (2015) 21:353–60. doi: 10.1097/MIB.0000000000000287
89
van der Have M van der Aalst KS Kaptein AdA Leenders M Siersema PD Oldenburg B et al . Determinants of health-related quality of life in Crohn’s disease: A systematic review and meta-analysis. J Crohn’s Colitis (2014) 8:93–106. doi: 10.1016/j.crohns.2013.04.007
90
Schreiner P Rossel J Biedermann L Valko PO Baumann CR Greuter T et al . Fatigue in inflammatory bowel disease and its impact on daily activities. Aliment Pharm Therap (2021) 53:138–49. doi: 10.1111/apt.16145
91
Bel LGJ Vollebregt AM Jong AEVM Fidder HH Hove WRT Vliet‐Vlieland CW et al . Sexual Dysfunctions in Men and Women with Inflammatory Bowel Disease. J Sex Med (2015) 12:1557–67. doi: 10.1111/jsm.12913
92
Timmer A Bauer A Dignass A Rogler G . Sexual Function in Persons With Inflammatory Bowel Disease: A Survey With Matched Controls. Clin Gastroenterol H (2007) 5:87–94. doi: 10.1016/j.cgh.2006.10.018
93
O’Toole A Winter D Friedman S . Review article: the psychosexual impact of inflammatory bowel disease in male patients. Aliment Pharm Therap (2014) 39:1085–94. doi: 10.1111/apt.12720
94
Feagins LA Kane SV . Sexual and Reproductive Issues for Men With Inflammatory Bowel Disease. Am J Gastroenterol (2009) 104:768–73. doi: 10.1038/ajg.2008.90
95
Allocca M Gilardi D Fiorino G Furfaro F Peyrin-Biroulet L Danese S et al . Sexual and reproductive issues and inflammatory bowel disease. Eur J Gastroen Hepat (2018) 30:316–22. doi: 10.1097/MEG.0000000000001074
96
Shin T Okada H . Infertility in men with inflammatory bowel disease. World J Gastrointest Pharmacol Ther (2016) 7:361. doi: 10.4292/wjgpt.v7.i3.361
97
Heetun ZS Byrnes C Neary P O’morain C . Review article: reproduction in the patient with inflammatory bowel disease. Aliment Pharm Therap (2007) 26:513–33. doi: 10.1111/j.1365-2036.2007.03397.x
98
Mountifield R Bampton P Prosser R Muller K Andrews JM . Fear and fertility in inflammatory bowel disease: A mismatch of perception and reality affects family planning decisions. Inflammation Bowel Dis (2009) 15:720–5. doi: 10.1002/ibd.20839
99
Lima A Zelinkova Z Mulders AGMGJ van der Woude CJ . Preconception Care Reduces Relapse of Inflammatory Bowel Disease During Pregnancy. Clin Gastroenterol H (2016) 14:1285–1292.e1. doi: 10.1016/j.cgh.2016.03.018
100
Sands K Jansen R Zaslau S Greenwald D . Review article: the safety of therapeutic drugs in male inflammatory bowel disease patients wishing to conceive. Aliment Pharm Therap (2015) 41:821–34. doi: 10.1111/apt.13142
101
Laube R Yau Y Selinger CP Seow CH Thomas A Chuah SW et al . Knowledge and Attitudes Towards Pregnancy in Females with Inflammatory Bowel Disease: An International, Multi-centre Study. J Crohn’s Colitis (2020) 14:1248–55. doi: 10.1093/ecco-jcc/jjaa047
102
Selinger CP Eaden J Selby W Jones DB Katelaris P Chapman G et al . Inflammatory bowel disease and pregnancy: Lack of knowledge is associated with negative views. J Crohn’s Colitis (2013) 7:e206–13. doi: 10.1016/j.crohns.2012.09.010
Summary
Keywords
IBD, gender and sex, treatment reality, psychological impact, treatment differences
Citation
Blumenstein I and Sonnenberg E (2023) Sex- and gender-related differences in inflammatory bowel diseases. Front. Gastroenterol. 2:1199687. doi: 10.3389/fgstr.2023.1199687
Received
03 April 2023
Accepted
25 July 2023
Published
03 October 2023
Volume
2 - 2023
Edited by
Thomas Klag, University of Tübingen, Germany
Reviewed by
Gabriele Dragoni, University of Florence, Italy; Konstantinos Karmiris, Venizeleio General Hospital, Greece
Updates
Copyright
© 2023 Blumenstein and Sonnenberg.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Irina Blumenstein, Irina.Blumenstein@kgu.de
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.