- 1Department of Obstetrics & Gynecology, Division of Family Planning, Indiana University School of Medicine, Indianapolis, IN, United States
- 2Moi Teaching & Referral Hospital/Moi University & Academic Model Providing Access to Healthcare (AMPATH), Eldoret, Kenya
- 3Department of Biostatistics, Indiana University School of Medicine, Indianapolis, IN, United States
- 4Bixby Center for Global Reproductive Health, Department of Obstetrics, Gynecology & Reproductive Health, University of California San Francisco, CA, United States
- 5Centre for Microbiology Research, Kenya Medical Research Institute, Nairobi, Kenya
- 6Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, United States
- 7Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, WA, United States
Introduction: Understanding interests in and preferences for multipurpose technology (MPT) for the co-administration of contraception and antiretroviral therapy (ART) and alternative, non-oral ART methods among women living with HIV (WLHIV) is vital to successful implementation of future treatment options, such as long-acting injectable ART.
Methods: Between May 2016 and March 2017 we conducted a cross-sectional telephone survey of 1,132 WLHIV of reproductive potential with prior experience using intermediate- or long-acting contraceptive methods in western Kenya. We present descriptive statistics and multinomial logistic regression to evaluate predictors of interest in specific MPT and non-oral ART methods.
Results: Two-thirds (67%) reported interest in MPT, with the most common reason for interest being ease of using a single medication for both purposes of HIV treatment and pregnancy prevention (26%). Main reasons for lack of interest in MPT were need to stop/not use contraception while continuing ART (21%) and risk of side effects (16%). Important characteristics of MPT were effectiveness for pregnancy prevention (26%) and HIV treatment (24%) and less than daily dosing (19%). Important characteristics of non-oral ART methods were less than daily dosing (47%), saving time accessing ART (16%), and effectiveness of HIV treatment (15%). The leading preferred methods for both MPT and non-oral ART were injectables (50 and 54%) and implants (32 and 31%). Prior use of a contraceptive implant or injectable predicted interest in similar methods for both MPT and non-oral ART methods, while this relationship did not appear to vary between younger vs. older WLHIV.
Discussion: Most WLHIV in western Kenya are interested in MPT for HIV treatment and contraception. Prior exposure to contraceptive implants or injectables appears to predict interest in similar methods of MPT and non-oral ART. Developers of MPT and non-oral ART methods should strongly consider WLHIV's preferences, including their changing reproductive desires.
Introduction
Women account for greater than half of the estimated 36 million people living with HIV and 2 million new infections annually (1). Strides have been made in achieving universal access to antiretroviral therapy (ART), improving the quality of life and years of reproductive potential for women living with HIV (WLHIV) (2). Similar strides remain to be realized for family planning, especially for WLHIV, who are less likely to use any contraception, more likely to rely on condoms alone, and more likely to experience unintended pregnancy, compared with HIV-negative women (3–5).
Progress has been made in the development of multipurpose technology (MPT) for the delivery of antiretrovirals and contraception for the prevention of HIV and pregnancy (6). Similarly, MPT that combines contraception with ART for HIV treatment may improve access to and effectiveness of both ART and contraceptive use. Such technology could decrease morbidity and mortality related to both HIV and pregnancy, two of the greatest health threats to women of reproductive age (7). User-independent long-acting reversible contraception (LARC), including intrauterine devices (IUDs) and implants, and intermediate-acting injectable methods have higher effectiveness and continuation rates than methods requiring use daily or at every act of intercourse (8, 9). Oral pill ART has similar difficulties (10), thus longer-acting, non-oral ART methods may improve ART adherence and HIV outcomes. As a parallel, injectable pre-exposure HIV prophylaxis shows nine times greater efficacy than oral methods (11).
The HIV treatment field is at an exciting cusp of offering long-acting injectable ART delivery alternatives (12–18), and additional long-acting methods show promising results. Providing an array of options for HIV treatment and pregnancy prevention has the potential to revolutionize personal decision-making and improve long-term HIV and reproductive health outcomes for WLHIV, leading to greater gender equity and women's empowerment.
However, little is known about users' interest in and preferences for MPT and non-oral ART, especially for WLHIV in resource-limited settings, who will be the majority users. Given this gap, our primary objective was to determine the interest in and preferences for future MPT and non-oral ART among WLHIV with prior contraceptive experience in western Kenya. Our secondary objective was to evaluate whether prior use of a specific contraceptive method was associated with preferences for similar MPT and non-oral ART methods; we hypothesized that prior use would positively effect preference for a similar method for MPT and non-oral ART.
Materials and Methods
Design
We conducted a cross-sectional telephone survey from May 2016 to March 2017 among WLHIV age 15–45 years at the time of enrollment in care (January 2011–December 2015), whose electronic medical records (EMR) indicated use of an intermediate- or long-acting contraceptive method and who were followed at two large HIV treatment programs in western Kenya affiliated with the East Africa International Epidemiology Databases to Evaluate AIDS (EA-IeDEA). These President's Emergency Plan for AIDS Relief-sponsored HIV treatment programs, the Academic Model Providing Access to Healthcare (AMPATH) and Family AIDS Care & Education Services (FACES), support care for approximately 65,000 and 43,000 HIV-positive individuals in western Kenya, respectively (19, 20).
Study Population and Sampling
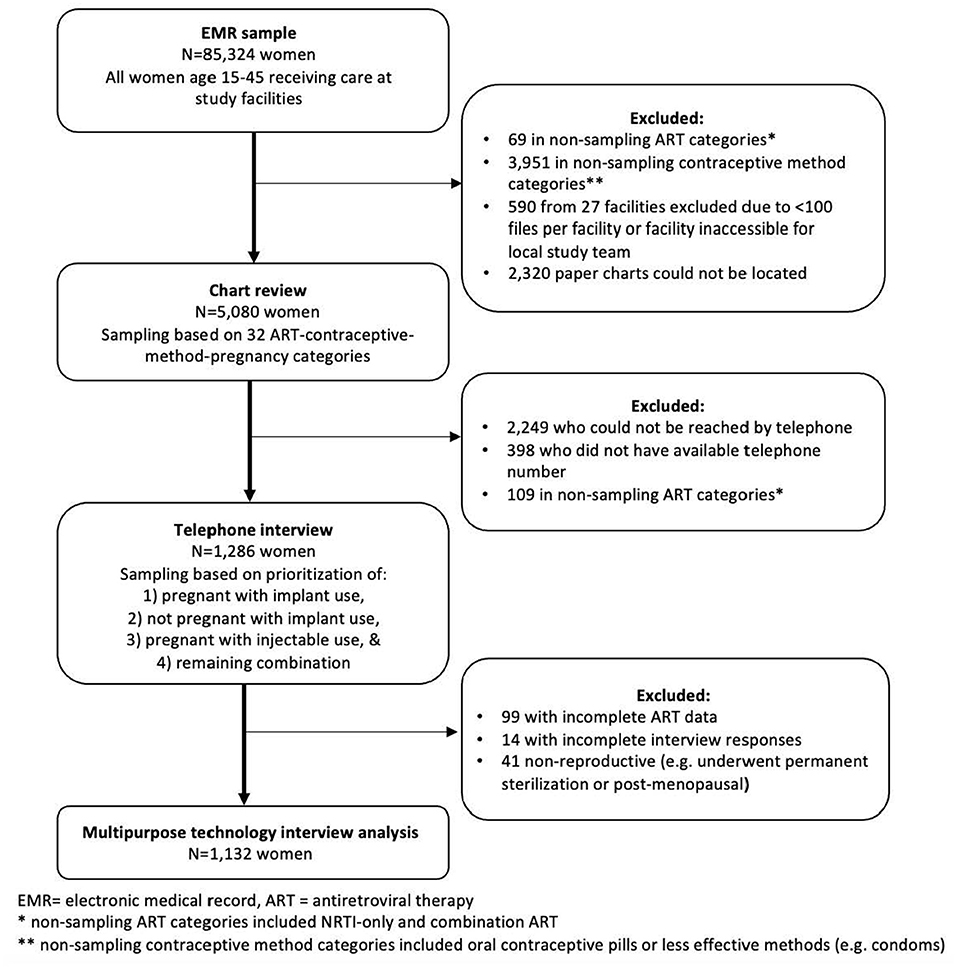
This telephone survey was part of a larger, 3-phase sampling study to validate exposures of contraceptive use and ART and outcome of incident pregnancy at AMPATH and FACES (Figure 1). Details of the sampling methods have been published elsewhere (21). Briefly, the initial study cohort of 85,324 participants consisted of all women age 15–45 receiving care at study facilities. Routine clinical and demographic data were collected at the program-supported health facilities at enrollment and follow-up visits utilizing standardized, paper instruments, which trained data clerks transcribed into EMR systems supported by an OpenMRS platform. From the first-phase sample, we sampled a random subset of observations for the second phase for the manual chart review based on a combination of 32 exposure and outcome categories. Our second-phase sample consisted of ~7% and 12% of the women contributing data to the first phase from AMPATH and FACES, respectively. We successfully conducted a chart review for 2,455 of 3,643 (67.4%) and 2,625 of 3,757 (69.9%) of the index observations, i.e., the combination exposure and outcome category that was chosen for any given woman, sampled for the second phase for AMPATH and FACES, respectively, for a total of 5,080 charts reviewed. From the chart reviews that were successfully completed for the second phase, we conducted telephone interviews with a non-random subset of the women. These women were selected for telephone interview based on the index observation in the first sample. The index observations where a woman was noted to be pregnant while using an implant, regardless of ART, were given the first priority in attempting phone calls. The second priority group was the index observations where a woman was noted not to be pregnant while using an implant, regardless of ART. The third and fourth priority groups were the index observations where the woman was noted to be using injectable contraception and pregnant or not pregnant, respectively. The study team generated periodic lists of priority second-phase sample participants to call for the telephone interviews as the chart reviews progressed based on these contraceptive-ART-pregnancy categories. In total we were able to reach and interview 1,286 women. We excluded 99 due to incomplete ART regimen information, 14 due to incomplete responses, and 41 due to being non-reproductive (i.e., postmenopausal or underwent permanent sterilization), leaving 1,132 women for inclusion in this analysis.
Telephone Interview
Both male and female research assistants with a higher education diploma or degree and additional training specific to this interview guide performed the interviews. They were bi- or trilingual native speakers and conducted the interviews in English, Kiswahili, or Dholuo, based on the participant's preference. The interview questions and responses were translated from English to Kiswahili and Dholuo and back-translated to confirm accuracy. Interviewers identified themselves as research staff associated with the clinical care program in which the client was enrolled. Interview participants underwent verbal informed consent. The interview consisted of a series of questions to confirm the contraceptive method, ART, and pregnancy history during the study observation period. Baseline reproductive and socio-demographic data collected during the EMR and chart reviewed were not confirmed. Next, the interviewer asked a series of questions about MPT prefaced by the statement: “Someday HIV medication and family planning methods may be combined into one medication to both treat HIV and prevent pregnancy.” Participants were asked whether they would be interested in such a medication and reasons why or why not. Similarly, the interviewer asked a series of questions about non-oral methods of ART prefaced by the statement: “Someday HIV medication may come in a different form, such as an injection you take every few weeks, instead of a pill you take every day.” Preferences for MPT and non-oral ART methods were elicited with respondents given options including injectable, implant, transdermal patch, intrauterine device (IUD, also known as “coil”), vaginal ring, pill, and other, and asked to provide their first, second and third choices. Least preferred MPT methods were also elicited along with reasons for lack of interest in the method(s).
Participants were then asked about the most important aspect of a future MPT or non-oral ART method, and asked to rank their first, second and third choices. If they did not provide an unprompted answer, they were prompted with example characteristics from the following list: dosing less frequently than daily, effectiveness of HIV treatment, effectiveness of pregnancy prevention (for MPT), privacy/concealability, safety, and lack of side effects. Lastly, participants were queried regarding their preferences for integration of HIV and contraception care, and disclosure of HIV status to contraception providers and contraceptive use to HIV providers when care is not integrated.
Analytic Framework
After data collection, prior to analysis, we interpreted and characterized reasons for interest/lack of interest in MPT and important characteristics of MPT and non-oral ART based on the analytic framework developed by Wyatt et al. (22) This framework is based on a thorough review of patients' values and preferences for important attributes of contraceptive methods and categorizes potential attributes influencing contraceptive choice in order to guide contraceptive decision aid development. We hypothesize that these values and preferences will be important to consider in MPT product development to ensure successful implementation and use. Major categories within this framework are: (1) method effect, (2) mechanistic, (3) practical, and (4) social/normative characteristics. Method effects include efficacy for HIV treatment and pregnancy prevention, non-contraceptive health benefits, and side effects. Mechanistic characteristics include how the method is used, including required user action. Practical characteristics include availability and cost. Social/normative characteristics include internal and external influences on use.
Statistical Analysis
We described socio-demographic and reproductive characteristics of study participants using descriptive statistics. We use frequency tables to describe the reasons for interest/lack of interest in MPT, importance of characteristics of MPT and non-oral ART methods, and method preferences. To determine whether previous implant and/or injectable contraceptive use (binary variable) was associated with implant and/or injectable method preference for the first choice of MPT and non-oral ART (binary variable), we used multinomial logistic regression to calculate adjusted odds ratios of stated method choice. Each model was adjusted for HIV care program, age, education level, marital status, number of living children, most recent ART regimen, and World Health Organization (WHO) clinical stage of HIV disease. We chose these covariates based on an a priori assessment of their potential as confounders (3, 23, 24) and their availability within our dataset. To make the coefficients in the models estimable, we combined preferences with low numbers into larger categories. To account for bias created by the missing data for education level, marital status, and number of living children, we conducted multiple imputations by iterative chain estimates. To better understand any differences between younger (ages 15–24) and older (ages > 25) WLHIV, we conducted subgroup analyses with our regression models comparing younger vs. older study participants. All analyses were performed using R version 3.3.3. (25).
Results
Baseline Characteristics
The median age at time of interview was 35 years (Table 1). The majority were married/cohabitating (56%), had at least one living child (69%), and reported previous or current contraceptive use (94%), including implants (67%), injectables (48%), pills (7%), and IUDs (2%). Most women were taking either efavirenz or nevirapine-containing ART regimens (85%) and had a recent WHO Stage 1 or 2 disease status (72%).
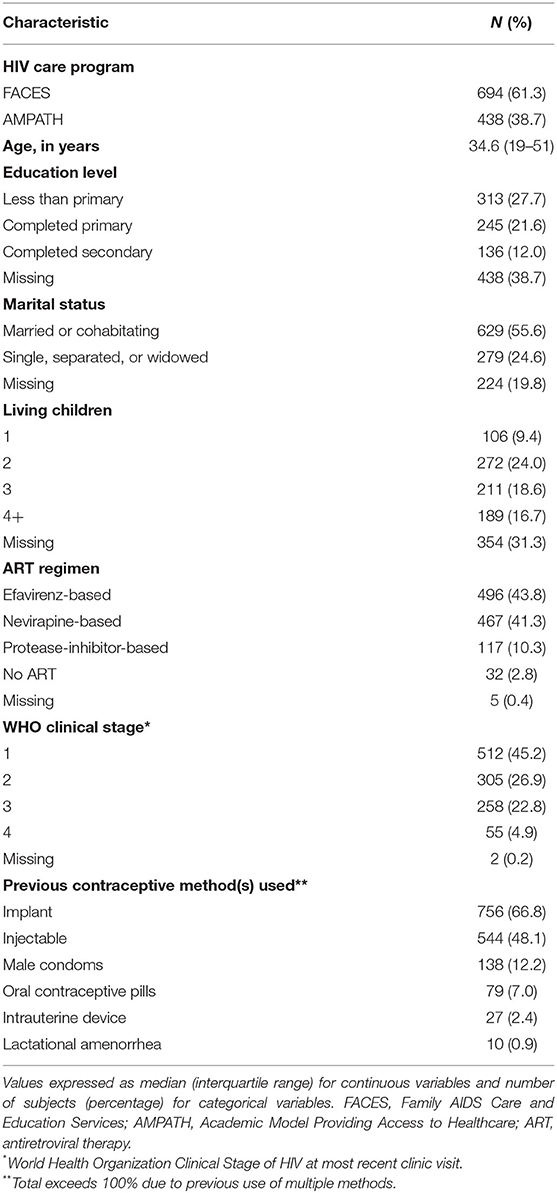
Table 1. Socio-demographic and reproductive characteristics of women of reproductive potential living with HIV with prior experience using effective contraceptive methods who participated in a telephone interview regarding interest in and preferences for multipurpose technology (MPT) and non-oral antiretroviral therapy (ART) methods (N = 1,132).
Multipurpose Technology (MPT)
Interest in MPT and Reasons for Interest or Disinterest
Two-thirds of participants (760, 67%) reported interest in MPT for combined ART and contraception. All responses to reasons for interest, disinterest, or “unsure” interest were classified according to Wyatt et al.'s analytic framework (Figure 2). The most commonly cited positive method effect characteristics were for pregnancy prevention (122, 16%) and to both treat HIV and prevent pregnancy (combined effects, 114, 15%). The most commonly cited positive mechanistic characteristic was ease of use of one medication for both purposes (194, 26%). The most commonly cited positive practical reason was to receive HIV and contraceptive care at the same site (service integration, 92, 12%).
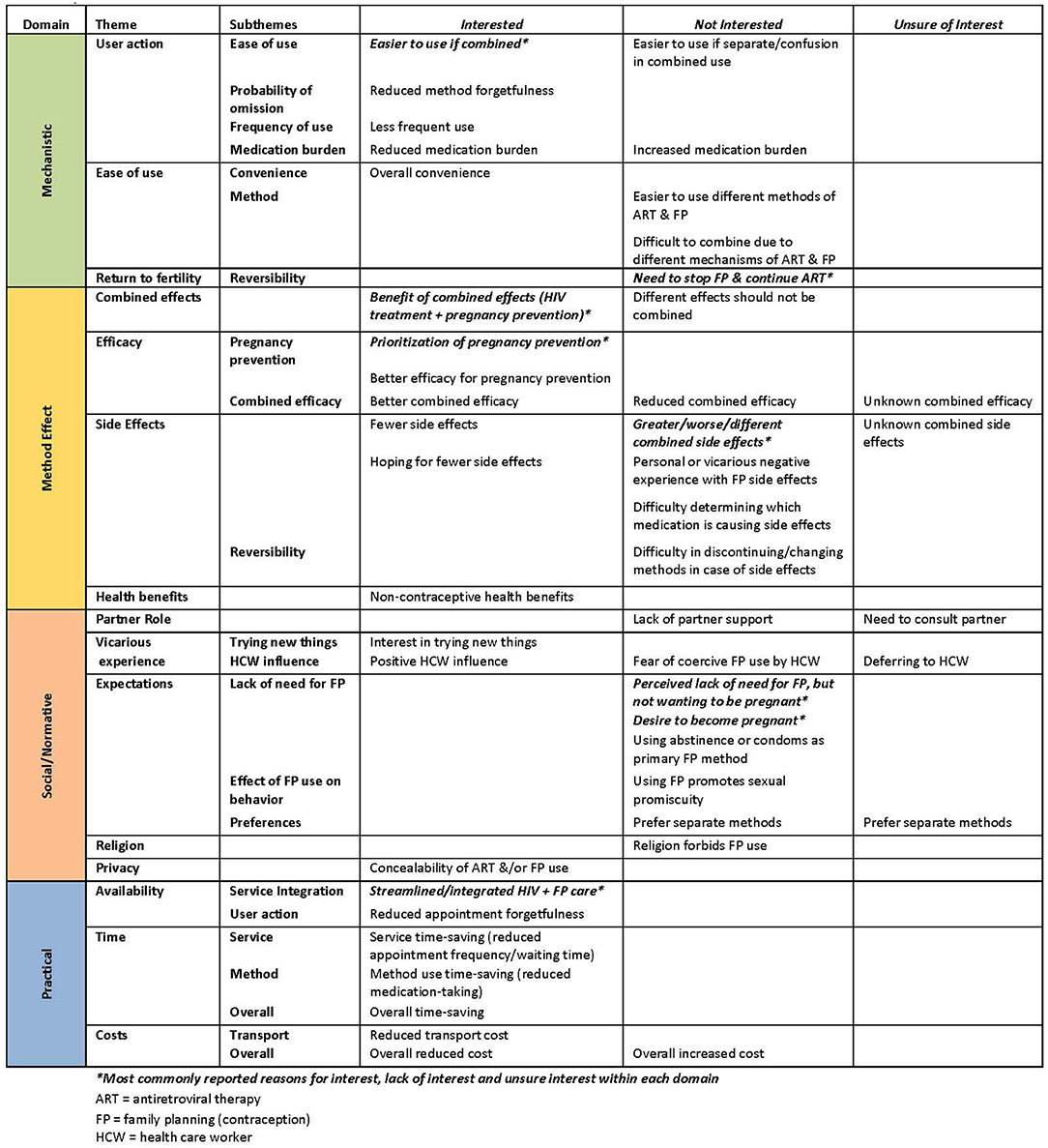
Figure 2. Reported reasons for interest, lack of interest, and unsure interest in the use of multipurpose technology (MPT) for contraception and antiretroviral (ART) therapy among women of reproductive potential living with HIV (N = 1132). Qualitative interpretation classified based on the analytic framework of Wyatt, et al. for the categorization of attributes influencing contraceptive choice.
Of the 350 women not interested in MPT, the most commonly cited negative effect characteristic was risk of increased, worse, or different side effects related to a combined medication (55, 16%). The most commonly cited negative mechanistic characteristic was the potential need to stop or not use contraception while continuing ART (reversibility, 75, 21%). The most commonly cited negative social/normative characteristics were perceived lack of need for contraception despite not desiring pregnancy (43, 12%) and desiring pregnancy (29, 8%)
Preferences for MPT Methods
The most important reported characteristics of a potential MPT method were effectiveness of pregnancy prevention (289, 26%), effectiveness of HIV treatment (274, 24%) and less than daily dosing (218, 19%) (Table 2). Privacy/concealability, method safety, lack of side effects, and time-saving were lower priority characteristics. The most commonly cited first and second choice MPT methods were injectables (560, 50%; 159, 14%) and implants (364, 32%; 280, 25%) (Table 3). Least preferred MPT methods were the intrauterine device (354, 31%), pills (31, 28%), and vaginal ring (228, 20%). Common reasons cited for lack of interest in all methods were fear of side effects/complications and previous negative personal or vicarious experiences, including bleeding, pain, and contraceptive method failure.
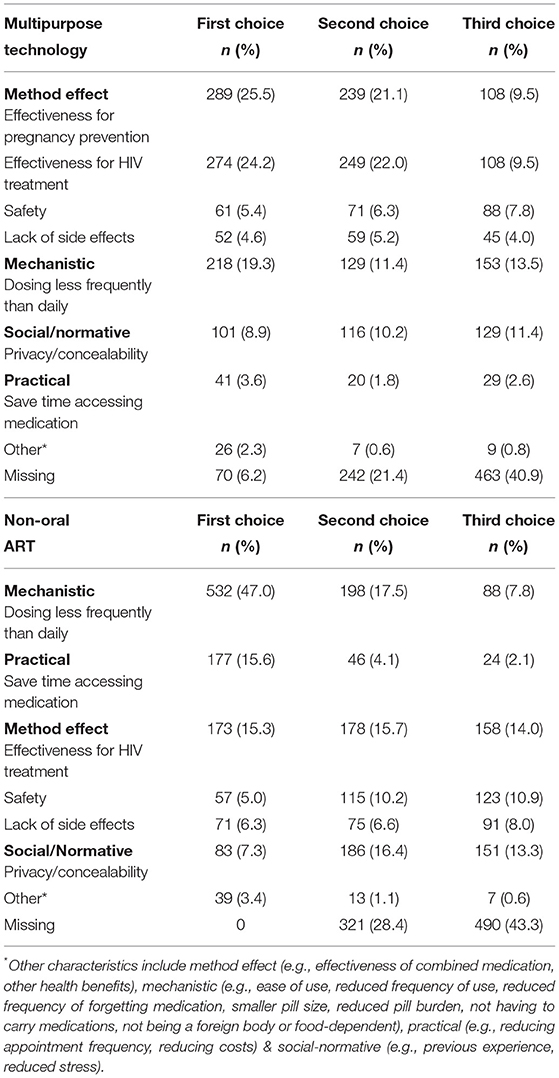
Table 2. Importance of characteristics of methods of multipurpose technology (MPT) for contraception and antiretroviral therapy (ART) and non-oral ART reported among women of reproductive potential living with HIV (N = 1,132).
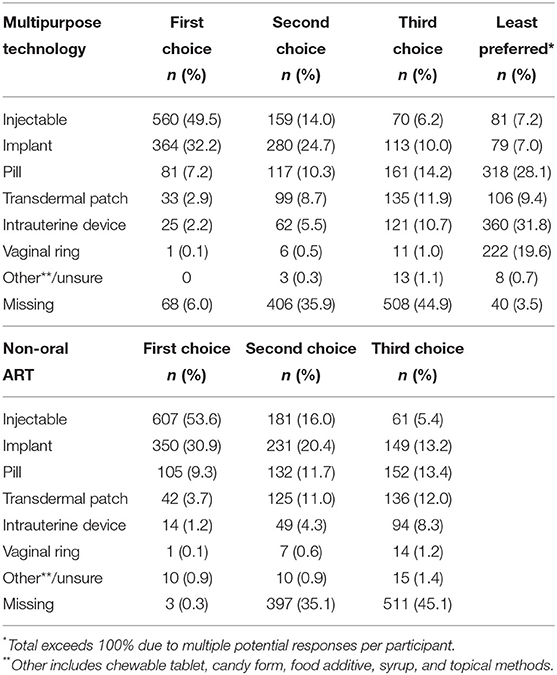
Table 3. Most and least preferred methods of multipurpose technology (MPT) for contraception and antiretroviral therapy (ART) and non-oral ART reported among women of reproductive potential living with HIV (N = 1,132).
Predictors of MPT Preferences
Women who previously used a contraceptive implant were more likely to be interested in an implant for MPT compared to both injectables (aOR 2.65, 95% CI 1.74–4.04) and pills (aOR 3.16, 95% CI 1.48–6.71) (Table 4). Similarly, women who previously used injectable contraception were more likely to be interested in an injectable for MPT compared to an implant (aOR 1.69, 95% CI 1.20–2.44). There was no significant difference in preference for implant compared to injectables or pills by younger vs. older age (aOR 1.67, 95% CI 0.69–4.03 and aOR 0.62, 95% CI 0.15–2.51, respectively). Women enrolled in AMPATH and those who completed secondary education were more likely to prefer implants over injectables (aOR 1.99, 95% CI 1.37–2.88 and aOR 2.89, 95% CI 1.58–5.27, respectively). There was no statistically significant relationship between other measured covariates and preferred method for MPT.
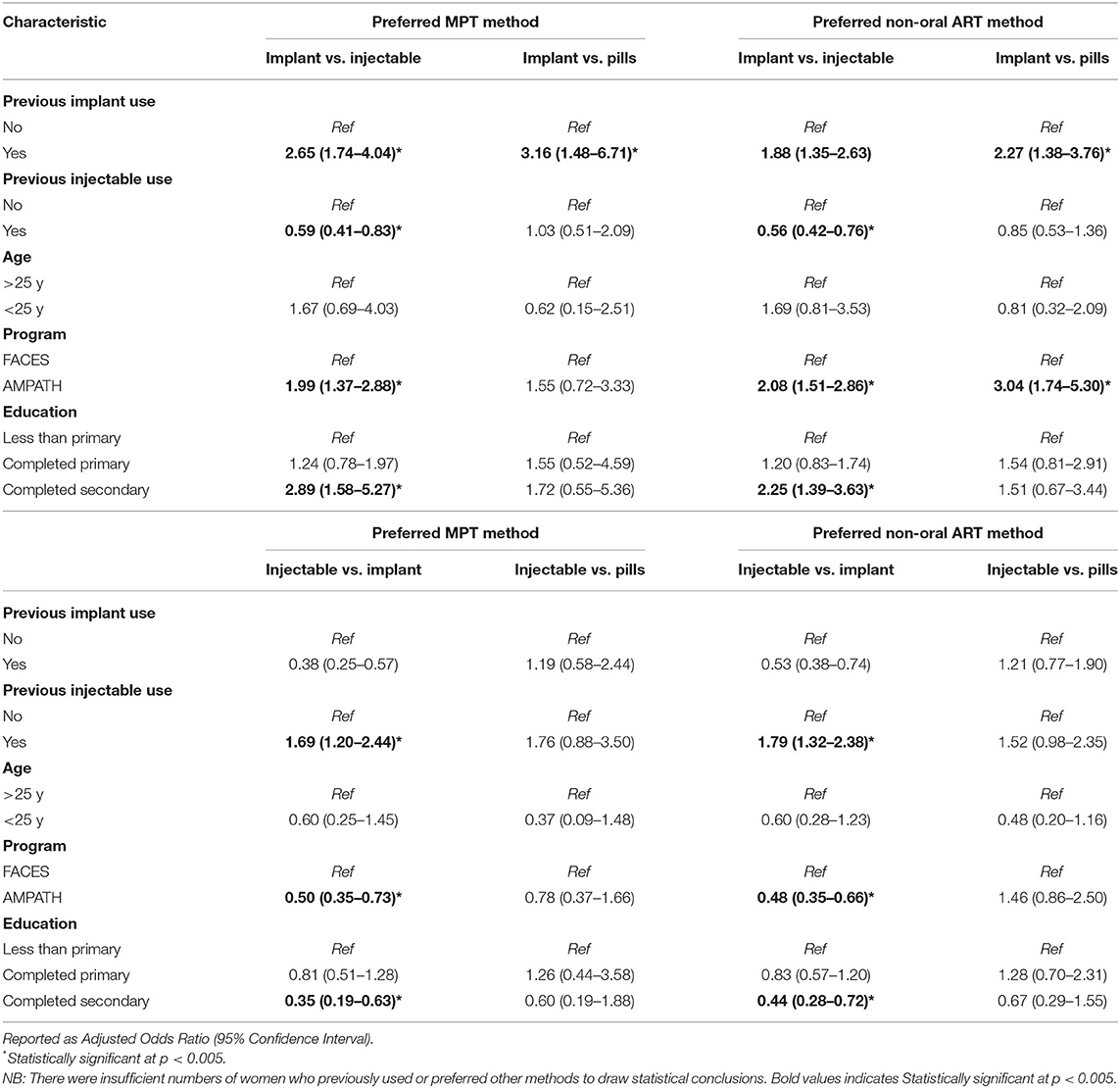
Table 4. Results of multinomial logistic regression to determine factors associated with preferred method of multipurpose technology (MPT) for contraception and antiretroviral therapy (ART) and method of non-oral ART (N = 1,132).
Non-oral Methods of Antiretroviral Therapy (ART)
Interest in Non-oral Methods of ART and Reasons for Interest
The most common first and second choices for non-oral ART methods were injectables (607, 54%; 181, 16%) and implants (350, 31%; 231, 20%) (Table 3). The most important characteristics of non-oral ART methods were less than daily dosing (532, 47%), saving time accessing it (177, 16%), and effectiveness of HIV treatment (173, 15%). Privacy/concealability, method safety, and lack of side effects were lower priorities.
Predictors of Non-oral ART Method Preference
Women who previously used an implant for contraception were also more likely to be interested in an implant for non-oral ART compared to both injectables (aOR 1.88, 95% CI 1.35–2.63) and pills (aOR 2.27, 95% CI 1.38–3.76) (Table 4). Similarly, women who previously used injectable contraception were more likely to be interested in an injectable for non-oral ART compared to an implant (aOR 1.79, 95% CI 1.32–2.38). There was no significant difference in preference for implant compared to injectables or pills by younger vs. older age (aOR 1.69, 95% CI 0.81–3.53 and aOR 0.81, 95% CI 0.32–2.09, respectively). Women enrolled in AMPATH and those who completed secondary education were more likely to prefer implants over injectables (aOR 2.08, 95% CI 1.51–2.86 and aOR 2.25, 95% CI 1.39–3.63, respectively). There was no statistically significant relationship between other measured covariates and preferred method for non-oral ART.
Dosing Frequency and Preference for Site of HIV and Contraception Care
Most participants preferred annual or semi-annual dosing for both MPT and ART (610, 54%; and 680, 60%, respectively). Most participants preferred to receive integrated contraceptive and HIV services at the same site (865, 76%). The majority of participants reported disclosing contraceptive use to their HIV care provider (1,111, 98%), while fewer reported disclosing their HIV status to their contraception provider (925, 82%). Of the 171 women who reported not disclosing HIV status to a contraception provider, the most commonly cited reasons were not using contraception (31, 18%), feeling that it was unnecessary to disclose HIV status when obtaining contraception (22, 13%), and discomfort in disclosing HIV status (22, 13%).
Discussion
Main Findings
Our study is the first to elicit values and preferences regarding potential multipurpose technology for ART and contraception and non-oral ART among WLHIV in a resource-limited setting. We find that the majority of WLHIV who have some experience with injectable or long-acting reversible contraception in western Kenya are interested in the use of such MPT, which is promising for the advancement of the MPT field for those already living with HIV, and not just for women at risk of acquiring HIV (26). Additionally, the high interest in implants and injectables for both MPT and non-oral ART are important findings to guide product development and scale-up, especially now that injectable ART has been approved in resource-rich settings and is on the cusp of approval in resource-limited settings (27).
Interpretation
The development of a target product profile (TPP) that identifies desired method parameters, characteristics of the user population, and the environment influencing acceptability are important to guide product development and implementation (28). The Wyatt et al. framework we use offers an approach that prioritizes user values and preferences for contraceptive methods that are vital to incorporate into the TPP for MPT and non-oral ART in order to achieve sustainability of these technologies. Our findings highlight similarities in the TPP for both non-oral ART and MPT, including the effectiveness of pregnancy prevention and HIV treatment, less than daily dosing, and methods that save time for users. Our data also indicates the importance of emphasizing the co-benefits and the ease of use of one product for both pregnancy prevention and HIV treatment, including service integration to improve simultaneous access to HIV and family planning services. This reinforces previous work that has shown high client satisfaction and improved contraceptive access with integrated services (29). At the same time, attention needs to be paid to user concerns about contraceptive reversibility while maintaining ART effectiveness. Implementation plans will need to emphasize respect for women who choose not to use contraception or MPT. The development of MPT and non-oral ART can be conceptualized within the contraceptive method mix framework, with multiple options allowing women to choose the best fit based on their values and preferences, thus improving uptake, continuation, and effectiveness (30). Additionally, preferences vary over time, as reproductive intentions change, and both across and within regions (31), stressing the importance of understanding the preferences and values of the target population early in the TPP process.
We found that prior use of an implant or injectable contraception was associated with interest in a similar method for both MPT and non-oral ART, consistent with recent findings that women's prior contraceptive method use was predictive of preference for a preventive MPT method (32). Notably, most women in our study preferred implants or injectables regardless of previous method use, indicating the opportunity for successful implementation of long-acting ART. We did not find any significant differences in method preference by age, indicating that there may be wide appeal for long-acting MPT and non-oral ART. Similar to findings about preferred preventative MPT (33), the vaginal ring was one of the least preferred methods. This may be reflective of a lack of knowledge or experience with this method, including potential transfer of concerns about IUDs to vaginal rings. This is an important finding given the significant investment and research to-date into vaginal rings for MPT (34). While we advocate for a wide method mix for MPT, questions remain about the acceptability of vaginal rings and how implementation strategies may improve acceptability, and greater emphasis should focus on the development of other methods, including injectables and implants.
Strengths and Limitations
While this is the first survey of its kind, our study has several limitations. Our study sample was largely WLHIV with experience with intermediate- or long-acting contraceptives due to the nature of the parent study sampling design and comes from HIV treatment programs that have made considerable efforts in integrating contraception into HIV services, limiting the generalizability of our findings other settings. Our data set was missing a relatively large percentage of key sociodemographic confounders, e.g., pregnancy intentions, that may impact the outcomes assessed. The study utilized multiple interviewers and we did not assess for potential inter-interviewer variability that may have contributed to participant responses. Finally, the analytic framework we utilized may not capture all preferences and values of all potential populations, and we hope this provides impetus for the development of new analytic frameworks.
Conclusion
This study furthers our understanding about the preferred characteristics of methods for MPT and non-oral ART to inform the development and implementation of this important technology for WLHIV. The majority of WLHIV in western Kenya appear interested in MPT and non-oral ART injectable and implantable methods, and want them to be easily reversible. This interest, especially for women experienced with similar methods, signals opportunities for improved adherence to ART and use of effective contraception through longer-acting methods compared to daily pills. Importantly, combining HIV treatment and contraception in one delivery mechanism mirrors the benefits seen for integration of HIV and contraceptive service access. Prioritizing the values and preferences of WLHIV must be central to the product development and implementation for MPT and non-oral ART to maximize uptake and integration into care.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Moi Teaching and Referral Hospital/Moi University Institutional Research and Ethics Committee (IREC). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
CB assisted with research conception and study design and led implementation at AMPATH. BJ provided assistance with study development and implementation at AMPATH. WF provided assistance with data analysis. AM provided assistance with study development and implementation and data management. MO provided assistance with study development and implementation at FACES. EB provided assisted with study development and implementation at FACES. KW-K assisted with early study conceptualization and reviewed and edited the manuscript. CC helped conceive of and implement this research, and reviewed and edited this manuscript. RP conceived of the research question and study design, led implementation of the study, including data collection, and data analysis. All listed authors made substantial contributions to the conception or design of the work or the acquisition, analysis, or interpretation of data for the work drafted or critically revised the work, submitted final approval of the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
CB received support for this research from the Clinical and Translational Science Award (CTSA) program of the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) under Award# UL1 TR000448 and TL1 TR000449 and NIH Reproductive Epidemiology Training Grant# T32HD055172. RP received support for this research from the National Institute of Health National Institute of Allergy and Infectious Diseases (K23AI120855). Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases (NIAID), Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), National Institute on Drug Abuse (NIDA), National Cancer Institute (NCI) and the National Institute of Mental Health (NIMH), in accordance with the regulatory requirements of the National Institutes of Health for the EA-IeDEA Consortium (U01AI069911). This grant included external peer review for scientific quality; the funder was not involved in the conduct of research or manuscript writing.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgwh.2022.869623/full#supplementary-material
References
1. UNAIDS 2018 estimates. Available online at: http://www.unwomen.org/en/what-we-do/hiv-and-aids/facts-and-figures (accessed: February 28, 2019).
2. Global report: UNAIDS report on the global AIDS epidemic (2013). World Health Organization, Geneva (2013).
3. Schwartz SR, Rees H, Mehta S, Venter WDF, Taha TE, Black V. High incidence of unplanned pregnancy after antiretroviral therapy initiation: findings from a prospective cohort study in South Africa. PLoS ONE. (2012) 7:e36039. doi: 10.1371/journal.pone.0036039
4. Harrington EK, Newmann SJ, Onono M, Schwartz KD, Bukusi EA, Cohen CR, et al. Fertility intentions and interest in integrated family planning services among women living with HIV in Nyanza Province, Kenya: a qualitative study. Infect Dis Obstet Gynecol. (2012) 2012:809682. doi: 10.1155/2012/809682
5. Todd CS, Anderman TC, Long S, Myer L, Bekker LG, Petro GA, et al. A systematic review of contraceptive continuation among women living with HIV. Contraception. (2018) 98:8–24. doi: 10.1016/j.contraception.2018.02.002
6. Friend DR, Clark JT, Kiser PF, Clark MR. Multipurpose prevention technologies: products in development. Antiviral Res. (2013) 100:S39–47. doi: 10.1016/j.antiviral.2013.09.030
7. Institute for Health Metrics and Evaluation (IHME). Findings From the Global Burden of Disease Study. (2017). Seattle, WA: IHME (2018).
8. Winner B, Peipert JF, Zhao Q, Buckel C, Madden T, Allsworth JE, et al. Effectiveness of long-acting reversible contraception. N Engl J Med. (2012) 366:1998–2007. doi: 10.1056/NEJMoa1110855
9. Patel R, Onono M, Gandhi M, Hagey J, Blat C, Shade S, et al. Pregnancy rates among HIV-positive women using various forms of antiretroviral therapy and contraceptives in Kenya. Lancet HIV. (2015) 2:e474–82. doi: 10.1016/S2352-3018(15)00184-8
10. Patel RC, Odoyo J, Anand K, Stanford-Moore G, Wakhungu I, Bukusi EA, et al. Facilitators and barriers of antiretroviral therapy initiation among HIV discordant couples in Kenya: qualitative insights from a pre-exposure prophylaxis implementation study. PLoS ONE. (2016) 11:e0168057. doi: 10.1371/journal.pone.0168057
11. HIV Prevention Trials Network. HPTN 084 Study Demonstrates Superiority of CAB LA to Oral FTC/TDF for the Prevention of HIV [press release]. Available online at: https://www.hptn.org/news-and-events/press-releases/hptn-084-study-demonstrates-superiority-of-cab-la-to-oral-ftctdf-for (accessed: November 9, 2020).
12. April 29. ViiV Healthcare Submits New Drug Application to US FDA for the First Monthly, Injectable, Two-Drug Regimen of Cabotegravir Rilpivirine for Treatment of HIV. Available online at: https://www.viivhealthcare.com/en-gb/media/press-releases/2019/april/viiv-healthcare-submits-new-drug-application-to-us-fda-for-the-first-monthly-injectable-two-drug-regimen-of-cabotegravir-and-rilpivirine-for-treatment-of-hiv/
13. Swindells S, Andrade-Villanueva J-F, Richmond GJ, Rizzardini G, Baumgarten A, Del Mar Masia M, et al. Long-acting Cabotegravir + Rilpivirine as maintenance therapy: ATLAS Week 48 results. In: Conference on Retroviruses and Opportunistic Infections (CROI) (2020).
14. Orkin C, Arastéh K, Hernández-Mora MG, Pokrovsky V, Overton ET, Girard P-M, et al. Long-Acting Cabotegravir + Rilpivirine For HIV Maintenance: FLAIR Week 48 results. In: Conference on Retroviruses and Opportunistic Infections (CROI) (2020).
15. Kovarova M, Benhabbour SR, Massud I, Spagnuolo RA, Skinner B, Baker CE, et al. Ultra-long-acting removable drug delivery system for HIV treatment and prevention. Nat Commun. (2018) 9:4156. doi: 10.1038/s41467-018-06490-w
16. Gatto GJ, Brand RM, Girouard N, Li LA, Johnson L, Marzinke MA, et al. Development of an End-user Informed Tenofovir Alafenamide (TAF) Implant for Long-acting (LA)-HIV Pre-exposure Prophylaxis (PrEP). In: HIV Research for Prevention Conference (HIVR4P) (Madrid, Spain) (2018).
17. August, 19,. Novel Nano-Engineered ‘Microneedles' to Help HIV Drug Delivery. Available online at: https://news.liverpool.ac.uk/2019/08/19/novel-nano-engineered-microneedles-to-help-hiv-drug-delivery/
18. Matthews RP, Barrett SE, Patel M, Zhu W, Fillgrove KL, Haspeslagh L, et al. First-in-Human Trial of MK-8591-Eluting Implants Demonstrates Concentrations Suitable for HIV Prophylaxis for at Least One year. In: International AIDS Society Conference on HIV Science (IAS) Mexico City, Mexico (2019).
19. Lewis-Kulzer J, Penner JA, Marima R, Oyaro P, Oyanga AO, Shade SB, et al. Family model of HIV care and treatment: a retrospective study in Kenya. J Int AIDS Soc. (2012) 15:8. doi: 10.1186/1758-2652-15-8
20. Mercer T, Gardner A, Andama B, Chesoli C, Christoffersen-Deb A, Dick J, et al. Leveraging the power of partnerships: spreading the vision for a population health care delivery model in western Kenya. Global Health. (2018) 14:44. doi: 10.1186/s12992-018-0366-5
21. Patel RC, Amorim G, Jakait B, Shepherd BE, Mocello AR, Musick B, et al. Pregnancies among women living with HIV using contraceptives and antiretroviral therapy in western Kenya: a retrospective, cohort study. BMC Med. (2021)19:178. doi: 10.1186/s12916-021-02043-z
22. Wyatt KD, Anderson RT, Creedon D, Montori VM, Bachman J, Erwin P, et al. Women's values in contraceptive choice: a systematic review of relevant attributes included in decision aids. BMC Women's Health. (2014) 14:28. doi: 10.1186/1472-6874-14-28
23. Kaida A, Matthews LT, Kanters S, Kabakyenga J, Muzoora C, Mocello AR, et al. Incidence and predictors of pregnancy among a cohort of HIV-positive women initiating antiretroviral therapy in Mbarara, Uganda. PLoS ONE. (2013) 8:e63411. doi: 10.1371/annotation/17310bbb-e5bf-4901-8b6e-529577a280db
24. Homsy J, Bunnell R, Moore D, King R, Malamba S, Nakityo R, et al. Reproductive intentions and outcomes among women on antiretroviral therapy in rural Uganda: a prospective cohort study. PLoS ONE. (2009) 4:e4149. doi: 10.1371/journal.pone.0004149
25. R Core Team. R: A Language Environment for Statistical Computing. (2017). R Foundation for Statistical Computing, Vienna, Austria. Available online at: https://www.R-project.org/.
26. Initiative for MPTs (IMPT) for the USAID Office of HIV/AIDS Research Division. Toward a Roadmap for Biomedical HIV Prevention Investment Standards: Strategic Insights from Key Industry Stakeholders. (2019). Available online at: https://theimpt.org/documents/reports/Biomedical-HIV-Prevention-Report_01272020.pdf.
27. Feb 1. ViiV Healthcare Announces US FDA of Cabenuva (Cabotegravir, Rilpivirine) for Use Every Two Months, Expanding the Label of the First and Only Complete Long-Acting HIV Treatment. Available online at: https://viivhealthcare.com/hiv-news-and-media/news/press-releases/2022/january/viiv-healthcare-announces-fda-approval-of-cabenuva-for-use-every-two-months/#:~:text=Cabenuva%20is%20the%20first%20and,1%20in%20virologically%20suppressed%20adults.
28. Brady M, Tolley E. Aligning product development and user perspectives: social-behavioral dimensions of multipurpose prevention technologies. BJOG. (2014) 121:70–8. doi: 10.1111/1471-0528.12844
29. Ayieko J, Ti A, Hagey J, Akama E, Bukusi EA, Cohen CR, et al. HIV status and treatment influence on fertility desires among women newly becoming eligible for antiretroviral therapy in western Kenya: insights from a qualitative study. Reprod Health. (2017) 14:93. doi: 10.1186/s12978-017-0355-9
30. Gray AL, Smit JA, Manzini N, Beksinska M. (2006) Systematic review of contraceptive medicines “Does choice make a difference?” Reproductive Health & HIV Research Unit of the University of the Witwatersrand, South Africa.
31. Montgomery E, Beksinska M, Mgodi N, Schwartz J, Weinrib R, Browne E, et al. Preference and Choice of Four Vaginal HIV Prevention Placebo Dosage Forms Among Young African Women: Results of the Quatro Randomized Crossover Trial. HIV Research for Prevention conference (HIVR4P 2018) 21–25 October (2018).
32. Cohen CR, Grossman D, Onono M, Blat C, Newmann SJ, Burger RL, et al. Integration of family planning services into HIV care clinics: results one year after a cluster randomized controlled trial in Kenya. PLoS ONE. (2017) 12:e0172992. doi: 10.1371/journal.pone.0172992
33. Weinrib R, Minnis A, Agot K, Ahmed K, Owino F, Manenzhe K, et al. End-users' product preference across three multipurpose prevention technology delivery forms: baseline results from young women in Kenya and South Africa. AIDS Behav. (2018) 22:133–45. doi: 10.1007/s10461-017-1911-6
Keywords: contraception, HIV, multipurpose technology, women, antiretroviral therapy
Citation: Bernard C, Jakait B, Fadel WF, Mocello AR, Onono MA, Bukusi EA, Wools-Kaloustian KK, Cohen CR and Patel RC (2022) Preferences for Multipurpose Technology and Non-oral Methods of Antiretroviral Therapy Among Women Living With HIV in Western Kenya: A Survey Study. Front. Glob. Womens Health 3:869623. doi: 10.3389/fgwh.2022.869623
Received: 04 February 2022; Accepted: 27 April 2022;
Published: 19 May 2022.
Edited by:
Chelsea Morroni, University of Edinburgh, United KingdomReviewed by:
Esaú Custódio João Filho, Hospital Federal dos Servidores do Estado, BrazilErnest Kenu, University of Ghana, Ghana
Copyright © 2022 Bernard, Jakait, Fadel, Mocello, Onono, Bukusi, Wools-Kaloustian, Cohen and Patel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caitlin Bernard, Q2FpdGxpbmJAaXUuZWR1
 Caitlin Bernard
Caitlin Bernard Beatrice Jakait2
Beatrice Jakait2 A. Rain Mocello
A. Rain Mocello Maricianah A. Onono
Maricianah A. Onono