- Animal Models and Vaccine Section, Vaccine Branch, Center for Cancer Research, National Cancer Institute, Bethesda, MD, United States
Germinal centers (GCs) are organized lymphoid tissue microstructures where B cells proliferate and differentiate into memory B cells and plasma cells. A few distinctive subsets of highly specialized T cells gain access to the GCs by expressing the B cell zone–homing C-X-C chemokine receptor type 5 (CXCR5) while losing the T cell zone–homing chemokine receptor CCR7. Help from T cells is critical to induce B cell proliferation and somatic hyper mutation and to limit GC reactions. CD4+ T follicular helper (TFH) cells required for the formation of GCs and for the generation of long-lived, high-affinity B cells. Regulatory CD4+ (TFR) and CD8+ T cells co-localize with TFH cells and keep their expansion in check, thus limiting GC reactions. A cytotoxic CXCR5pos CD8+ T cell subset has been described in GCs in humans: although low in number, GC CD8+ T cells can expand rapidly during certain viral infections. Because these subsets find their home in secondary lymphoid tissues (lymph nodes and spleen) that are difficult to obtain in humans, GC–homing T cells have been extensively studied in mice. Nevertheless, significant limitations in using this model, such as evolutionary divergences between mice and humans and the lack of an optimal mouse model for certain human diseases, have prompted investigators to characterize GC–homing T cells in macaques instead. This review will focus on discoveries made in macaques, particularly in the non-human primate models of simian immunodeficiency virus and simian–human immunodeficiency virus infection. Indeed, experimental studies in these models have allowed researchers to gain insight into the relative role of follicular T cell subsets in HIV progression, virus persistence, and specific B cell responses induced by HIV vaccines. These discoveries have prompted the testing of novel approaches aimed to manipulate follicular T cells to increase the efficacy of HIV vaccines and to eliminate HIV reservoirs.
Introduction
Effective antibody responses are crucial for preventing viral infections and are the basis for the majority of successful vaccination strategies (1). The quality of such antibodies is largely dependent on T cell–B cell interactions. In physiological conditions, T and B cells are subcompartmentalized within lymphoid lobules of lymph nodes (LNs). B cells reside within the outer cortex areas of the lobules enriched for the B cell-attracting CXCL13, while T cells express the (C-C motif) receptor 7 (CCR7) and recirculate through the paracortex and interfollicular cortex enriched in CCR7 ligands (CCL21 and CCL19). Following antigenic stimulation, a small number of activated T cells lose CCR7 expression and upregulate CXCR5, the receptor for CXCL13 (2–4). CXCR5pos T cells travel toward the B cell-rich follicles in the outer cortex areas, where they interact with B cells (5–7). Two highly specialized CXCR5pos CD4+ T cell subsets, T follicular helper (TFH) and T regulatory (TFR) cells, have been identified in B cell follicles. Activated TFH cells migrate to the T–B borders and B cell follicles where they are required for the formation and maintenance of germinal centers (GCs) [reviewed in Ref. (8, 9)]. GCs are organized lymphoid tissue microstructures where B cells expand and differentiate during immune responses to appropriate pathogens or antigens (10, 11). In the GCs, TFH cells support B cells class switching recombination and somatic hypermutation (SHM) (10, 12). GC reactions ultimately result in the selection of resting B cell memory cells and long-lived plasma cells producing antibodies with high affinity for the encountered antigen (13). The strong reaction occurring in the GCs needs to be tightly regulated to avoid the generation of autoantibodies and excessive inflammation (14–17). T follicular regulatory cells have recently been described as a subset of CXCR5pos T regulatory cells that co-localize with TFH cells, control their expansion, and modulate TFH cell-driven B cell maturation, antibody class switching, and affinity maturation (14, 16, 17). CD8+ T cells are also part of the follicular T cell population (18–25). Recent studies have started to shed light on the role of these cells in regulating GC reactions and their interaction with TFH and B cells in certain infections (19, 20, 23–25).
Because of their critical role in every step of B cell differentiation, TFH cells have been the focus of intense interest in HIV infection. Indeed, aberrant B cell responses and B cell dysfunction are characteristics of chronic HIV infection (26). TFH cells are infected with HIV (27–29), they accumulate in lymphoid tissues of some individuals during chronic infection (27, 28), and their ability to provide B cell help is impaired (30). Hence, HIV-associated changes in TFH cells most likely affect the generation of effective B cell responses against the virus. Moreover, by homing to the GCs, TFH cells escape immunological control and establish a persistent reservoir (21, 22, 31). The quest for an effective vaccine against HIV has also fueled intense interest in the biology of CXCR5pos T cells and their role in GC reactions. Ideally, an HIV vaccine would induce high affinity broadly neutralizing HIV-1 antibodies capable of neutralizing multiple HIV-1 viral strains. These antibodies show remarkable levels of somatic mutation (32); henceforth, their generation is most likely highly dependent on effective TFH–B cell interactions in GCs.
Much of the current knowledge on the role of GC-resident T cells during HIV infection has been attained by studies performed in non-human primate (NHP) models. Macaques can be infected with the simian immunodeficiency virus (SIV) that closely mimics many aspects of HIV infection (33), giving the NHP model advantages by comparison to both rodents and humans. This review will focus on discoveries made in macaques, on how GC–homing T cells are affected during HIV/SIV infection, and on how HIV-associated changes in these cells may alter antibody responses. Strategies tested in NHP models aimed to target TFH cells to eliminate HIV reservoir from GCs and to increase the effectiveness of HIV vaccine responses will also be discussed.
Characterization of GCs in NHPs
Studies in mice have been fundamental in revealing the phenotype and function of GC-resident T cells and studying their key lineage-specific transcription factors (14, 16, 34). However, the similarity between humans and NHPs makes NHPs optimal for research on complex immunological interactions. Macaques have several advantages over rodents, and the first is that their genetic evolution more closely resembles those of humans (35, 36). For example, evolutionary divergence between the signaling pathways that shape TFH cell differentiation in humans and mice has recently been discovered (37). Second, their immune system also resembles those of humans. Indeed, NHPs have been used to study fundamental aspects of immunology, including the development and maintenance of T cell memory (38), immunodominance (39), and the aging immune system (40). Third, macaques LN’s structure is more similar to humans than rodents LN’ structure (41). In macaque lobes, T cell zones and B cell follicles can be identified with equal function and cell distribution as in humans. Finally, lymphoid cells and a number of their different subtypes are also identifiable with equivalent markers and methodologies used in humans.
Germinal centers are typically few in LNs of naive animals, with very little TFH cell number (31). Upon vaccination or infection, selected follicles are activated and develop into GCs. The interactions between cognate B and T cells have been reported to occur 1–2 days after antigen exposure (42–44). Studies in macaques have shown that GCs are formed in draining LNs a few days after intramuscular immunization at the same site of the delivery, while they are absent in contralateral LNs (45, 46). GCs in macaques can be readily identified as positive for the B cell marker CD20 and express high levels of the proliferation marker Ki67 (CD20pos Ki67hi). Alternatively, Hoechst staining of nuclei is used to discern GCs from the adjacent marginal zone by less intense staining in immunohistochemistry analyses (47). Marginal zone B cells, responsible for an early antibody response to blood-borne pathogens, have been identified in cynomolgus and rhesus monkeys as B cells (CD19+, CD20+) expressing high levels of complement receptor 2 (CD21) and low levels of CD27 (47, 48). CD4, CD20, PD-1, and Ki67 markers were simultaneously used to study B follicles and TFH cells in rhesus macaques (48).
Compared to human subjects, the use of NHPs allows researchers to conduct controlled challenge experiments, multiple live surgeries, and invasive and terminal experiments, ultimately granting access to different tissues to an extent that is not feasible in humans (49). NHP models have also been used to test sampling techniques aimed to study the cellular composition of GCs. While in macaques it is possible to surgically remove the draining LNs at different time points after an immunization or an experimental infection, this procedure is invasive and may disrupt ongoing immune responses. Two studies have used fine-needle aspirations (FNAs) technique to collect cells from LNs of pigtail and rhesus macaques (50, 51). In both models, TFH cell where readably measurable, suggesting that FNA may be an interesting alternative to collect small numbers of GC cells for further analyses, while maintaining ongoing immune responses.
Characterization of TFH Cells in Macaques
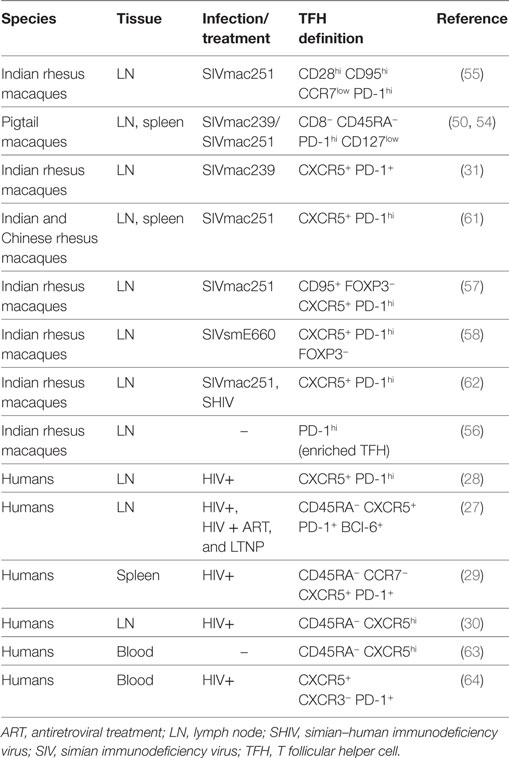
TFH cells in macaques have a phenotype comparable to that of humans. TFH cells are considered a distinct cell subset with a specialized function and a specific transcription factor that differs from other T helper cell subsets. The master regulator of TFH cell differentiation is the transcription factor Bcl-6 (52, 53). While Bcl-6 is the unique marker of TFH cells, other canonical markers used to identify them are CXCR5, PD-1, and the inducible T cell costimulator, ICOS. High expression of PD-1 has been considered an effective way to identify GC–TFH cells in intact tissues (31) when GCs are co-stained. In healthy macaques, PD-1hi cells within the GCs are almost exclusively CD4+ T cells (31). Different combinations of markers have been used to define TFH cell in cell suspensions by flow cytometry, in human and macaques (Table 1). The percentage of TFH cells frequency in LNs depends on the choice of the markers used to define and the gating strategy. Because of the unavailability of a cross-reactive antibody for CXCR5, TFH cells were originally identified in secondary lymphoid tissues of pigtail macaques as CD4+ T cells-expressing programmed cell death 1 of PD-1hi and low levels of interleukin-7 receptor alpha (IL-7Rα) chain (CD127) (50, 54). This cell population was only present in spleen and LNs, but not in blood, and expressed high levels of ICOS and Bcl-6. In rhesus macaques, TFH cells were first identified in cell suspension from LNs as central memory (CD28hi CD95hi) CD4+ T cells expressing low levels of CCR7lo and high levels of PD-1 and ICOS (55). When the cross-reactive anti-CXCR5 antibody clone MU5UBEE became available, co-expression of CXCR5, coupled with high levels of PD-1 expression, has been widely used to identify and sort TFH cells (56–59). However, others have reported changes in both markers, and particularly in PD-1 following ex vivo HIV infection, warning against using only these two markers to define TFH cells (60).
Macaques have been a useful model for validating circulating biomarkers of GC responses that can be easily translated to humans. One example is the measurement of the level of plasma CXCL13. In macaques, CXCL13 is detectable in plasma, it increases following immunization, and its levels are associated with the frequency of TFH cells in LNs (65). Importantly, a substantial frequency of CD4+ T cells expressing CXCR5 is also present in the blood of rhesus macaques, as is the case in humans (63). Phenotypically, circulating TFH (cTFH) cells share common markers with GC-resident TFH cells and can be identified as CXCR5pos PD-1pos CD4+ T cells. However, cTFH cells express lower levels of ICOS and of the activation marker CD69 than GC TFH cells, suggesting that they are present in a resting phase (66). While the origin of cTFH cells is still unclear, the marker expression and ability to interact with B cells and promote B cell responses in vitro suggest that they may be circulating counterparts of TFH cells in LNs. In mice, humans, and macaques, circulating CXCR5pos PD-1hi CD4+ T cells are heterogenic and can be divided into subsets based on their expression on (C-X-C motif) chemokine receptor 3 (CXCR3), a marker for CD4+ T helper type 1 (Th1) cells, alone or together with CCR6. CXCR5pos CXCR3neg PD-1pos TFH cells present the most genetic and functional similarities to TFH cells in LNs (64). When the expression of CCR6 is considered, cTFH cells can be further divided into three subpopulations that mirror the unique phenotype and cytokine signature of lineages of non-TFH CD4+ T cells in blood: TFH type 1 (CXCR3pos CCR6neg), type 2 (CXCR3neg CCR6neg), and type 17 (CXCR3neg CCR6pos). More studies are needed to identify the role of these cell subsets in generating or maintaining antibody responses to pathogens.
Functionally, TFH cells help B cells by secreting cytokines and expressing surface molecules and providing survival, proliferation, and differentiation signals [reviewed in Ref. (9, 67).]. In macaques, as in humans, GC-resident TFH cells express the costimulatory receptor ICOS, the costimulatory protein CD40L required for B cell survival, and they produce the B cell helper cytokines IL-21 and IL-4 although TFH cells can also produce other cytokines depending on the stimulus they receive (9). IL-21 signaling is pivotal for B cell differentiation and for the development of B cell memory. In vitro IL-21 production is often used as a means to measure antigen-specific responses, particularly following immunization in humans (68) and macaques (69). However, TFH and cTFH cells produce limited quantities of IL-21. As a result, the tracking of antigen-specific responses by intracellular staining is challenging. A recent study has used the macaque model to develop a cytokine-independent technique aimed improve the quantification of antigen-specific TFH cells. Havenar-Daughton et al. have shown that the co-expression of OX40 and CD25 surface markers is sufficient to identify antigen-specific GC TFH and pTFH cells in the LNs and blood of immunized animals (70). Importantly, this technique offers the possibility to isolate antigen-specific TFH cells by cell sorting, which is not possible with intracellular cytokine detection.
HIV-/SIV-Associated Changes in TFH Cells
HIV infection is associated with numerous B cell anomalies (26). Untreated HIV and AIDS patients develop profound B cell dysfunction, characterized by hypergammaglobulinemia, and polyclonal B cell activation (26, 71–73). The majority of HIV-infected individuals and SIV-infected macaques fail to produce protective antibodies against HIV/SIV and low-affinity B cells mature inappropriately into plasma cells (74). Because TFH cells are required for the induction of high-affinity antibody responses and the generation of long-lived B cell memory (75), several groups have investigated HIV/SIV-associated changes in TFH cells and their possible effect on B cell abnormalities.
Recent data suggest that GC–CXCR5+ PD-1hi TFH cells are susceptible to HIV-1/SIV infection (27, 28, 54, 55, 60). Interestingly, unlike non-TFH CD4+ T cells, TFH cell frequency and number increase in chronic HIV/SIV infection in the LNs of some humans (27, 28) and macaques (31, 54, 55, 57, 58, 60). In both macaques and humans, the increase in TFH cell frequency in chronic infection is approximately 10 times compared to non-infected levels (28, 55). In humans, a median of 60% of HIV-1 RNA-producing cells was found within lymphoid follicles by in situ hybridization in chronically infected untreated patients with a median of 17% of follicles tissues per inguinal LN (22).
Remarkably, in all the studies reported, TFH cell expansion is observed only during chronic infection, but not in acute infection. Although the reason for the increase in TFH cell levels during chronic HIV/SIV infection is not clear, different hypotheses have been proposed. The accumulation of TFH cells during chronic SIV and HIV has been associated with immune activation (55) and plasma viremia (57, 58) in some studies. Other studies suggest that this expansion may be driven by prolonged T cell receptor stimulation (62). In mice, LCMV infection redirects CD4+ T cell development away from the Th1 cell responses induced during an acute infection toward TFH cells (76). Others have shown that HIV-specific GC–TFH cells, particularly against the gag, also expanded in chronic infection in humans (27, 28). Finally, effective antiretroviral treatment (ART) decreases the number of TFH cells in humans and macaques, suggesting that active HIV replication is necessary for TFH cell expansion (27, 28).
The increase in TFH cells levels is also associated with increased frequency of activated GC B cells and SIV-specific antibodies (55) in macaques, and plasma cells and immunoglobulin levels in HIV infection (28). Moreover, broadly neutralizing antibodies (bNabs) are present in HIV patients with high levels of circulating CXCR5pos CXCR3neg PD-1hi CD4+ T cells (64). These results suggest that TFH cells may be highly functional during HIV/SIV infection; however, other studies have revealed that they provide inadequate help to B cells (30, 77). GC-resident TFH cells isolated from HIV-infected patients produce less IL-21, a cytokine pivotal for GC formation, GC B cell proliferation, and B cell maturation (9). The replenishment of exogenous IL-21 in vitro to TFH/B cell co-cultures or the in vivo administration to SIV-infected macaques significantly improves memory B cell levels (30, 78), suggesting that lost IL-21 production may be a contributing factor to the generation of defective memory B cell responses. TFH cells express a number of molecules that restrain them from excessive proliferation such as PD-1 (53). The PD-1 expression is highly increased in HIV-infected CD4+ T cells (31), and the level of its ligand PDL-1 on B cells increases in HIV patients. Interestingly, by blocking the PD-1–PD-L1 interaction, IL-21 production by TFH cells is recovered and B cell functions are restored. Therefore, it is possible that TFH cell impairments may be, at least in part, mediated by HIV-associated changes affecting B cells (30).
Studies in monkeys have reported that during SIV infection, TFH cells express non-characteristic transcriptional factors together with canonical ones and that gene and cytokine expressions are skewed toward CD4+ Th1 cells and interferon (IFN)-γ (78). During chronic SIV infection, IFN-γ-induced genes are upregulated while the expression of the IL-4 gene is downmodulated (55). Accordingly, the majority of GC–TFH cells in chronically infected macaques are positive for CXCR3+ and produce IFN-γ (Th1-type cytokine) alongside IL-21. While these cells are capable of helping B cells in vitro, they express higher levels of CCR5 and harbor more SIV-DNA than CXCR3neg GC–TFH cells (79). T-bet, the transcriptional regulator of Th1, is also increased in TFH cells isolated from SIV-infected macaques’ spleens (61). Importantly, an association between IFN-γlow IL-21hi GC resident TFH cells and the broad neutralization activity against the envelope was found in simian–human immunodeficiency virus (SHIV)-infected macaques (80). Taken together, these studies suggest that, while functional, TFH cells may undergo changes in levels and function that may affect their ability to help B cells induce high-quality antibodies. These conclusions are corroborated by the lack of responsiveness to other infections or vaccines observed during late HIV/SIV infection.
TFR Cells and SIV-Associated Changes in Macaques
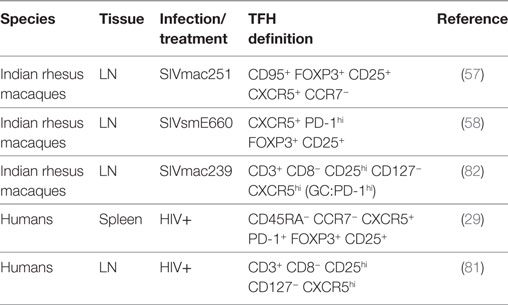
T follicular regulatory cells control the magnitude of GC reactions and avoid the onset of some autoimmune diseases (14–17). The frequency of TFR cells is low in mice, humans, and monkeys compared to other CD4+ T cells subsets, and as for the TFH cells, the percentage varies depending on the markers used to identify this subset. Phenotypically, they share the canonical markers of TFH cells (CXCR5, ICOS, PD-1) and TREGS [FOXP3, CD25, cytotoxic T-lymphocyte-associated protein 4 (CTLA4) positive and CD127 negative] (Table 2). Functionally, TFR cells produce IL-10 and TGF-β and express the inhibitory molecule CTLA4 (16). TFR cells have been characterized in the LNs of rhesus macaques as FOXP3pos CD25pos CXCR5pos CCR7neg as FOXP3pos CD25pos PD-1hi CD127neg CXCR5pos CD4+ T cells or, alternatively, as CD25pos CD127neg CD3+ CD8− T cells (57, 58, 81, 82). Depending on the markers used, their frequency ranges between 2 and 5% of CD4+ T cells or CD8− CD3+ T cells. We showed that an enriched population of TFR cells, obtained from the LNs of macaques by isolating sorted CD25pos CD4+ T cells migrating toward CXCL13, was capable of suppressing autologous GC–TFH cell proliferation in vitro (58).
TFR cells are essential to the control of TFH cell numbers in mice (14, 16). TFR cell decrease or stagnation during chronic SIV infection may contribute to the TFH cell dynamic seen in HIV infection. Two macaque studies have shown that TFR cells are susceptible to infection by different SIV strains: SIVmac251 and SIVsmE660 (57, 58). A recent study expanded this knowledge to humans by showing that TFR cells are highly permissive to infection both ex vivo and in vivo in chronic HIV-untreated patients (81). In a longitudinal study in SIVmac251-infected macaques, we showed that the frequency and number of TFR cells significantly decreased in LNs during the chronic phase and that the reduction was associated with an increase in TFH cell levels (58). These findings were corroborated by the parallel independent study by Chowdhury et al., showing changes in the ratio of TFH to TFR cells in favor of TFH during chronic infection with SIVsmE660 (59). Interestingly, in a cross-sectional study, Miles et al. showed an increase in the number of GC-resident TFR cells in HIV-infected humans and SIVmac239-infected macaques. In humans, an increase in the CD4+ Foxp3+ cell count was observed when the LN area was considered to account for LN enlargement that occurs during chronic HIV infection (81). Differences in the study design (longitudinal versus cross-sectional), TFR cell definition, and analyses may have contributed to the inconsistent findings in these studies.
The increase of TFH cells in chronic SIV infection has been previously associated with an increase in the titers of gp120-specific antibodies with high avidity (55). Interestingly, we observed an antithetical role of TFR and TFH cells in the avidity of antibodies to the SIV-gp120 protein throughout the infection. TFR cell levels were associated with a reduction of binding high-avidity antibodies to SIV-gp120 in all the infected animals (58). The role of TFR cells in the impairment of humoral immunity during HIV infection remains to be determined. Finally, TFR cells are a relatively newly discovered population, and many of the studies performed on TFH cells in humans and macaques did not include markers for TFR cells exclusion (Table 1). Given their changes in frequency, susceptibility to infection and function, discriminating markers for TFR should be included when studying TFH cells, particularly in HIV vaccine studies.
GC-Resident CD8+ T Cells in Macaques
GC-resident or CXCR5pos CD8+ T cells are present in lymphoid tissues of humans (20, 83–85) and macaques (19, 31). In fact, three decades ago, high frequencies of CD8+ T cells were found in inflamed lymphoid follicles in heroin addicts and HIV-related lymphadenopathy (83, 84). However, compared to TFH cells, current research on the CXCR5+ CD8+ T cells is relatively scarce. Several studies suggest that CXCR5+ CD8+ T cells represent a subset of follicular cytotoxic CD8+ T cells and may contribute to virus control in B cell follicles (23). Indeed, follicular cytotoxic CD8+ T cells express granzyme A and B and perforin at higher levels than their CXCR5neg counterpart (85). Interestingly, a study identified a subset of CD8+ T cells with the suppressive activity on TFH cells in rhesus macaques’ LNs and humans’ tonsils (19). CD8+ with a regulatory function produce IL-10 and express high levels of CXCR5 and the homing cell adhesion molecule CD44. Together these studies suggest that GC-resident CD8+ T cells may be a heterogeneous cell population.
The accumulation of infected TFH cells in LNs during chronic infection is a major obstacle toward eradication. Cytotoxic CD8+ T cells are critical for the clearance of virus-infected CD4+ T cells; thus, studies focused on understanding the phenotype and function of HIV/SIV-specific CD8+ T cells have been performed in humans and macaques. To date, there are conflicting data on the quantity of specific CXCR5pos CD8+ T cells and their ability to clear virus-infected TFH cells. Virus-specific CD8+ T cells are present in GCs of humans and macaques, but they may not be enough to clear the increasing population of infected TFH cells (21, 22), may be functionally impaired/exhausted (25), may exert regulatory instead of cytotoxic function, or be predisposed to provide B cell help once they enter the B cell follicles (18, 19, 25). Two in vivo CD8 cell depletion studies have been performed in SIV-infected macaques (24, 25). Fukazawa et al. showed that SIV is restricted to CD4+ T cells in the B cell follicles (with a median of 95% of productively SIV-infected cells) in macaques that are naturally controlling infection (elite controller or EC), but not in animals with normal disease progression. In vivo depletion of CD8+ cells in EC macaques resulted in a temporal redistribution of productive CD4+ T cells in the extrafollicular area, until CD8+ T cells absolute count returned to normal levels (24). Li et al. showed higher levels of both follicular and extrafollicular SIV-producing cells after CD8+ cell depletion in normal disease progression macaques, with the greatest increase in the extrafollicular areas (8.9 versus 3.8 cells/mm2 average change in the follicles) (25). Although these two studies differed in the CD8 depletion protocol (repeated low dose administrations, one single high-dose bolus), both showed profound depletion in the LNs. It should be noted that in vivo CD8 cell depletion may have eliminated other CD8-expressing cell populations (for example, NKs).
Some CXCR5pos CD8+ T cells with the ability to contain LCMV have been found in GCs in mice and in blood of HIV-infected patients (86), where their levels correlated with viral load. In patients with HIV, the number of virus-specific CXCR5pos CD8+ T cell subset is inversely correlated with viral load in LNs (86). Peripheral and GC CXCR5pos CD8+ T cells are also present in SIV-infected macaques, where their levels increase after immunization, and it is higher in macaques controlling infection than ones who do not (87). CD8+ T cells can still contain viral replication in chronic infections although the mechanism of this containment is largely unknown (86). Recent work by Petrovas et al. show that CD8+ T cell in the GC had better killing activity than non-follicular CD8+ T cells, despite being less polyfunctional (20). Taken together, these results suggest that CD8+ T cells could be an effective component of an HIV cure strategy.
TFH Cells as Privileged Latent Reservoir
Lymphoid organs constitute the first established reservoir of HIV infection. In untreated HIV patients, viral replication is found in GCs soon after and all through the duration of infection (88–90), and the free virus can be detected even during clinical latency asymptomatic phase (91, 92). Viral replication is never completely curtailed from the LNs, and it is detected in the GCs till they involute with advancing disease (93). The macaque model of HIV-1 infection has been fundamental to study B cell follicles as immune privileged sites and for extending these observations to gut-associated lymphoid tissue (21, 94, 95). Antiretroviral therapy contains viral replication; however, it fails to eliminate the virus from lymphoid tissues. A steady-state level of very low viremia has also been described among those on ART, but the exact mechanism for persistent viremia during ART is not completely understood (96). Upon ART discontinuation, viral replication rebounds, resulting in titers similar to those observed prior to treatment. Thus, it is possible that some cellular sanctuary may exist, which allow the virus to persist.
Recent data suggest that GC–CXCR5+PD-1hi TFH cells are highly susceptible to HIV-1 infection (27, 28, 60). Some studies report that TFH cells are a preferentially infected by HIV/SIV (27, 57), while others report that the permissiveness of TFH cells is comparable to other subsets of memory CD4+ T cells (54, 55). The levels of the virus entry co-receptor CCR5 expressed by TFH cells varies in different studies (54, 60, 62, 97), possibly depending on the definition used to identify TFH cells. The role of levels of CCR5 expression of TFH cells and susceptibility to HIV/SIV infection is also not clear. While a study reports no association between the levels of co-receptor and susceptibility (60), Xu et al. used NHP to explain the apparent discrepancy between low levels of the HIV co-receptor and the heightened permissiveness to infection (97). The group identified a subset of LN-resident TFH cell precursors expressing intermediate levels of PD-1 and higher levels of CCR5 than fully differentiated PD-1hi TFH cells and showed that in their precursor state TFH cells are highly susceptible to in vitro SIV infection. Other parameters may also contribute to the high susceptibility to HIV/SIV infection of TFH cells described in certain studies. Recent work by Ruffin et al. showed that GC–TFH cells from LNs and tonsil obtained from chronically infected patients express low levels of the HIV-1 restriction factor SAMHD1 (98, 99).
Localization of TFH cells within the GCs most likely contributes to their high susceptibility to HIV/SIV infection and their expansion (22, 31). The increase in TFH cell permissiveness, when compared to other memory CD4+ T cell subsets, does not associate with their activation status or levels of HIV co-receptor expression (60). It is possible that their unique localization in the GC may play a role in the heightened susceptibility. Indeed, it has been shown that CD4+ T cells located in the follicles are 40 times more likely to be infected by HIV than those located outside the follicles (22). In the GCs, the virus can be transmitted by follicular dendritic cells (FDCs) that are capable of long-term antigen retention (100). FDCs trap multiple intact viral particles on the surface and efficient transmission to GC-resident CD4+ T cells (101–104). Indeed, FDCs act as HIV “archives” by retaining ART-resistant virus variants that are not present elsewhere (104). While this is an interesting theory, it is also expected that other molecules may be bound together with the virus, such as antibody and complement, and it is not clear how this trapped virus would serve as a source for CCR5-expressing TFH cell precursors (97). Other HIV-/SIV-associated changes in cellular composition within the GCs, such as changes in TFH/TFR cell ratio, may contribute to the accumulation of TFH cells, at least in macaques (57, 58). Moreover, the paucity of virus-specific CD8+ T cells in the B cell follicles, when compared to extrafollicular areas in both HIV (22) and SIV infection (21), as well as cell exhaustion or malfunction, may account for the lack of clearance of infected TFH cells as previously described.
Improving the ability of potent antiviral CD8+ T cells to traffic into B cell follicles may result in the elimination of virus reservoirs. To determine whether the expression of CXCR5 may be sufficient for CD8+ T cells to enter the follicles, Ayala et al. infused six SIV-infected macaques with autologous CD8+ T cells genetically modified to express CXCR5 (105). The engineered T cells were found in abundance within the B follicles, with some cells localized in the proximity of infected TFH cells. While CD8+ T cells used in this study were circulating T cells that were not selected by their specificity to HIV, this study is an important step forward in the further development of strategies aimed to eliminate virus persistence in treated patients (105). Finally, a better understanding of immune cell type localization in the GCs, particularly those with the ability to eliminate infected TFH cells, will be a key for designing new eradication strategies.
Vaccine-Induced TFH Cells
The goal of a vaccine is to induce long-lasting memory responses to the pathogen. HIV presents a greater challenge than other viruses, in part because it replicates in CD4+ T cells and induces profound deregulation of the overall immune system. Neutralizing antibodies against the autologous virus are detectable only after years from seroconversion and only 20% of infected patients develop cross-react antibodies against different gp120 regions (106–108). Although these antibodies have shown protection in non-human macaques’ models using SHIV (109, 110), the most desirable response for an HIV vaccine would be the induction of bNabs. bNabs can act against a wide spectrum of viruses by targeting relatively conserved regions on the surface HIV envelope trimer spike (111). Because of the striking amount of SHM in HIV bNabs (112–114), it is conceivable that T follicular helper cells and GCs play a critical role in generating such antibodies. However, the elicitation of bNabs trough vaccination is challenging. These antibodies are uncommon (produced by 10% of HIV-infected individuals) (115), and conventional HIV vaccines are unable to induce the number of mutations observed in bNabs. Some studies have been performed in macaques to test the ability of different adjuvants to stimulate TFH differentiation, SHMs, and affinity maturation to neutralizing HIV epitopes. Importantly, in this model, the levels of GC-resident TFH cells are associated with the generation of neutralizing antibody breadth during SIV/SHIV infection (80). A study in macaques revealed scarce differences in the mean SHM levels or CDR H3 lengths using eight different adjuvants in combination with a gp140 protein vaccine to immunize macaques (116). PLGA, a toll-like receptor ligand containing nanoparticles adjuvant, induces strong GCs reactions in monkeys (117, 118). A native-like Env trimer, given twice intramuscularly together with a strong adjuvant ISCOMATRIX induced neutralizing antibodies against Tier 2 (difficult to neutralize) viruses (118). Potent TFH cell responses were found in LNs of rhesus macaques after immunization, and no changes were observed in the levels of TFR cells. The effectiveness of this immunization was not tested in challenge experiments.
While the search for a strategy capable of bestowing protection via the induction of neutralizing antibodies or even bNAbs continues, non-neutralizing antibodies may also be increased during vaccination by increasing TFH cell differentiation. Of note, the only vaccine to provide low, but significant protection from HIV acquisition in humans induced binding non-neutralizing antibodies to the variable region of the gp120 V2 loop (119, 120). In a retrospective study, it was shown that volunteers vaccinated with an ALVAC-SIV + gp120 alum vaccine had higher levels of IL-21-producing cTFH cells than individuals immunized with strategies that failed to protect (68). Therefore, it is possible that an increase in binding antibodies to gp120, and particularly to the V2 loop, via TFH cells may increase the efficacy of an HIV vaccine, despite the absence of neutralization. Studies in macaques have revealed that conventional vectored vaccines indeed stimulate TFH cells in combination with gp120 or gp140 protein boosts. Codelivery of MVA-SIV and gp120 protein in alum increased the levels of CXCR3pos CXCR5pos CD4+ T cells in the blood and LNs of rhesus macaques measured at the peak of immune responses after vaccination. Interestingly, while CXCR3POS cTFH cells favored antibody responses, they were also associated with increased peak viremia upon infection with SIVmac251 (121). An Adenovirus 5- based vector vaccine, encoding for Env, Gag, and Nef, followed by a gp120 or gp140 protein boost induced IL-21-producing TFH cells in rhesus macaques’ LNs. TFH cell levels measured after 2 weeks from the last immunization were associated with the titers of binding antibodies to the gp120 (69). While this vaccine only protected female macaques from SIVmac251 infection, it is noteworthy that only small differences in the levels of IL-21-producing TFH cells were found when animals were stratified by sex. Taken together, these studies suggest that TFH cells are induced by different vaccination strategies, and their induction results in potentially protective antibody responses that are measurable in LNs and blood. Studies comparing different strategies side by side should be performed to shed light on the association between the levels and function of TFH cell induction and vaccine efficacy.
Conclusion
The NHP model has played a fundamental role in understanding the dynamics of TFH cells during HIV infection and their role as major sites for viral replication and the establishment of viral reservoirs. This model has been a key to the development of new techniques to study TFH cells and GC responses that can be translated to humans, and it makes it possible to conduct preclinical studies aimed at eradicating HIV. Undoubtedly a fuller appreciation for the range of cells participating in meaningful cellular reservoirs could result in a rational attack on latent HIV-1 and may provide inroads into creating an effective vaccine designed to generate HIV neutralizing antibodies. However, the obvious limitation is that NHPs are not humans. Much of what is learned from non-human primates, especially at the preclinical level, must be validate in humans.
Author Contributions
MV drafted the manuscript. MV and GF critically revised the manuscript.
Conflict of Interest Statement
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or conflict with the subject matter or materials discussed in the manuscript.
Funding
No funding for writing assistance was utilized in the production of this manuscript.
References
1. Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis (2008) 47:401–9. doi:10.1086/589862
2. Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt’s lymphoma receptor-1. Nature (1998) 391:799–803. doi:10.1038/35876
3. Ansel KM, McHeyzer-Williams LJ, Ngo VN, McHeyzer-Williams MG, Cyster JG. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J Exp Med (1999) 190:1123–34. doi:10.1084/jem.190.8.1123
4. Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med (2000) 192:1545–52. doi:10.1084/jem.192.11.1545
5. Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med (2001) 193:1373–81. doi:10.1084/jem.193.12.1373
6. Hardtke S, Ohl L, Forster R. Balanced expression of CXCR5 and CCR7 on follicular T helper cells determines their transient positioning to lymph node follicles and is essential for efficient B-cell help. Blood (2005) 106:1924–31. doi:10.1182/blood-2004-11-4494
7. Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med (2000) 192:1553–62. doi:10.1084/jem.192.11.1553
8. de Vinuesa CG, Cook MC, Ball J, Drew M, Sunners Y, Cascalho M, et al. Germinal centers without T cells. J Exp Med (2000) 191:485–94. doi:10.1084/jem.191.3.485
9. Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol (2011) 29:621–63. doi:10.1146/annurev-immunol-031210-101400
10. Toellner KM, Jenkinson WE, Taylor DR, Khan M, Sze DM, Sansom DM, et al. Low-level hypermutation in T cell-independent germinal centers compared with high mutation rates associated with T cell-dependent germinal centers. J Exp Med (2002) 195:383–9. doi:10.1084/jem.20011112
11. MacLennan IC. Germinal centers. Annu Rev Immunol (1994) 12:117–39. doi:10.1146/annurev.iy.12.040194.001001
12. Goodnow CC, Vinuesa CG, Randall KL, Mackay F, Brink R. Control systems and decision making for antibody production. Nat Immunol (2010) 11:681–8. doi:10.1038/ni.1900
13. De Silva NS, Klein U. Dynamics of B cells in germinal centres. Nat Rev Immunol (2015) 15:137–48. doi:10.1038/nri3804
14. Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med (2011) 17:983–8. doi:10.1038/nm.2426
15. Wollenberg I, Agua-Doce A, Hernández A, Almeida C, Oliveira VG, Faro J, et al. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J Immunol (2011) 187:4553–60. doi:10.4049/jimmunol.1101328
16. Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med (2011) 17:975–82. doi:10.1038/nm.2425
17. Alexander CM, Tygrett LT, Boyden AW, Wolniak KL, Legge KL, Waldschmidt TJ. T regulatory cells participate in the control of germinal centre reactions. Immunology (2011) 133:452–68. doi:10.1111/j.1365-2567.2011.03456.x
18. Quigley MF, Gonzalez VD, Granath A, Andersson J, Sandberg JK. CXCR5+ CCR7- CD8 T cells are early effector memory cells that infiltrate tonsil B cell follicles. Eur J Immunol (2007) 37:3352–62. doi:10.1002/eji.200636746
19. Miles B, Miller SM, Folkvord JM, Levy DN, Rakasz EG, Skinner PJ, et al. Follicular regulatory CD8 T cells impair the germinal center response in SIV and ex vivo HIV infection. PLoS Pathog (2016) 12:e1005924. doi:10.1371/journal.ppat.1005924
20. Petrovas C, Ferrando-Martinez S, Gerner MY, Casazza JP, Pegu A, Deleage C, et al. Follicular CD8 T cells accumulate in HIV infection and can kill infected cells in vitro via bispecific antibodies. Sci Transl Med (2017) 9:eaag2285. doi:10.1126/scitranslmed.aag2285
21. Connick E, Folkvord JM, Lind KT, Rakasz EG, Miles B, Wilson NA, et al. Compartmentalization of simian immunodeficiency virus replication within secondary lymphoid tissues of rhesus macaques is linked to disease stage and inversely related to localization of virus-specific CTL. J Immunol (2014) 193:5613–25. doi:10.4049/jimmunol.1401161
22. Connick E, Mattila T, Folkvord JM, Schlichtemeier R, Meditz AL, Ray MG, et al. CTL fail to accumulate at sites of HIV-1 replication in lymphoid tissue. J Immunol (2007) 178:6975–83. doi:10.4049/jimmunol.178.11.6975
23. Leong YA, Chen Y, Ong HS, Wu D, Man K, Deleage C, et al. CXCR5(+) follicular cytotoxic T cells control viral infection in B cell follicles. Nat Immunol (2016) 17:1187–96. doi:10.1038/ni.3543
24. Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med (2015) 21:132–9. doi:10.1038/nm.3781
25. Li S, Folkvord JM, Rakasz EG, Abdelaal HM, Wagstaff RK, Kovacs KJ, et al. Simian immunodeficiency virus-producing cells in follicles are partially suppressed by CD8+ cells in vivo. J Virol (2016) 90:11168–80. doi:10.1128/JVI.01332-16
26. Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol (2009) 9:235–45. doi:10.1038/nri2524
27. Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med (2013) 210:143–56. doi:10.1084/jem.20121932
28. Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest (2012) 122:3271–80. doi:10.1172/JCI64314
29. Colineau L, Rouers A, Yamamoto T, Xu Y, Urrutia A, Pham HP, et al. HIV-infected spleens present altered follicular helper T cell (Tfh) subsets and skewed B cell maturation. PLoS One (2015) 10:e0140978. doi:10.1371/journal.pone.0140978
30. Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med (2013) 19:494–9. doi:10.1038/nm.3109
31. Hong JJ, Amancha PK, Rogers K, Ansari AA, Villinger F. Spatial alterations between CD4(+) T follicular helper, B, and CD8(+) T cells during simian immunodeficiency virus infection: T/B cell homeostasis, activation, and potential mechanism for viral escape. J Immunol (2012) 188:3247–56. doi:10.4049/jimmunol.1103138
32. Klein F, Mouquet H, Dosenovic P, Scheid JF, Scharf L, Nussenzweig MC. Antibodies in HIV-1 vaccine development and therapy. Science (2013) 341:1199–204. doi:10.1126/science.1241144
33. Chalifoux LV, Ringler DJ, King NW, Sehgal PK, Desrosiers RC, Daniel MD, et al. Lymphadenopathy in macaques experimentally infected with the simian immunodeficiency virus (SIV). Am J Pathol (1987) 128:104–10.
34. Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity (2008) 29:138–49. doi:10.1016/j.immuni.2008.05.009
35. Rhesus Macaque Genome Sequencing and Analysis Consortium, Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, et al. Evolutionary and biomedical insights from the rhesus macaque genome. Science (2007) 316:222–34. doi:10.1126/science.1139247
36. Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A (2013) 110:3507–12. doi:10.1073/pnas.1222878110
37. Locci M, Wu JE, Arumemi F, Mikulski Z, Dahlberg C, Miller AT, et al. Activin A programs the differentiation of human TFH cells. Nat Immunol (2016) 17:976–84. doi:10.1038/ni.3494
38. Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, et al. Development and homeostasis of T cell memory in rhesus macaque. J Immunol (2002) 168:29–43. doi:10.4049/jimmunol.168.1.29
39. Hasegawa A, Moriya C, Liu H, Charini WA, Vinet HC, Subbramanian RA, et al. Analysis of TCRalphabeta combinations used by simian immunodeficiency virus-specific CD8+ T cells in rhesus monkeys: implications for CTL immunodominance. J Immunol (2007) 178:3409–17. doi:10.4049/jimmunol.178.6.3409
40. Cicin-Sain L, Messaoudi I, Park B, Currier N, Planer S, Fischer M, et al. Dramatic increase in naive T cell turnover is linked to loss of naive T cells from old primates. Proc Natl Acad Sci U S A (2007) 104:19960–5. doi:10.1073/pnas.0705905104
41. Haley PJ. The lymphoid system: a review of species differences. J Toxicol Pathol (2017) 30:111–23. doi:10.1293/tox.2016-0075
42. Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science (1998) 281:96–9. doi:10.1126/science.281.5373.96
43. Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, et al. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol (2005) 3:e150. doi:10.1371/journal.pbio.0030150
44. Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature (2008) 455:764–9. doi:10.1038/nature07345
45. Liang F, Lindgren G, Sandgren KJ, Thompson EA, Francica JR, Seubert A, et al. Vaccine priming is restricted to draining lymph nodes and controlled by adjuvant-mediated antigen uptake. Sci Transl Med (2017) 9:eaal2094. doi:10.1126/scitranslmed.aal2094
46. Hong JJ, Silveira ELDV, Amancha PK, Byrareddy SN, Gumber S, Chang KT, et al. Early initiation of antiretroviral treatment postSIV infection does not resolve lymphoid tissue activation. AIDS (2017) 31:1819–24. doi:10.1097/QAD.0000000000001576
47. Peruchon S, Chaoul N, Burelout C, Delache B, Brochard P, Laurent P, et al. Tissue-specific B-cell dysfunction and generalized memory B-cell loss during acute SIV infection. PLoS One (2009) 4:e5966. doi:10.1371/journal.pone.0005966
48. Demberg T, Mohanram V, Musich T, Brocca-Cofano E, McKinnon KM, Venzon D, et al. Loss of marginal zone B-cells in SHIVSF162P4 challenged rhesus macaques despite control of viremia to low or undetectable levels in chronic infection. Virology (2015) 484:323–33. doi:10.1016/j.virol.2015.06.022
49. Gardner MB, Luciw PA. Macaque models of human infectious disease. ILAR J (2008) 49:220–55. doi:10.1093/ilar.49.2.220
50. Xu Y, Fernandez C, Alcantara S, Bailey M, De Rose R, Kelleher AD, et al. Serial study of lymph node cell subsets using fine needle aspiration in pigtail macaques. J Immunol Methods (2013) 394:73–83. doi:10.1016/j.jim.2013.05.005
51. Klippert A, Stolte-Leeb N, Neumann B, Sauermann U, Daskalaki M, Gawanbacht A, et al. Frequencies of lymphoid T-follicular helper cells obtained longitudinally by lymph node fine-needle aspiration correlate significantly with viral load in SIV-infected rhesus monkeys. J Med Primatol (2015) 44:253–62. doi:10.1111/jmp.12186
52. Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 mediates the development of T follicular helper cells. Science (2009) 325:1001–5. doi:10.1126/science.1176676
53. Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity (2014) 41:529–42. doi:10.1016/j.immuni.2014.10.004
54. Xu Y, Weatherall C, Bailey M, Alcantara S, De Rose R, Estaquier J, et al. Simian immunodeficiency virus infects follicular helper CD4 T cells in lymphoid tissues during pathogenic infection of pigtail macaques. J Virol (2013) 87:3760–73. doi:10.1128/JVI.02497-12
55. Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, et al. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest (2012) 122:3281–94. doi:10.1172/JCI63039
56. Onabajo OO, George J, Lewis MG, Mattapallil JJ. Rhesus macaque lymph node PD-1(hi)CD4+ T cells express high levels of CXCR5 and IL-21 and display a CCR7(lo)ICOS+Bcl6+ T-follicular helper (Tfh) cell phenotype. PLoS One (2013) 8:e59758. doi:10.1371/journal.pone.0059758
57. Blackburn MJ, Zhong-Min M, Caccuri F, McKinnon K, Schifanella L, Guan Y, et al. Regulatory and helper follicular T cells and antibody avidity to simian immunodeficiency virus glycoprotein 120. J Immunol (2015) 195:3227–36. doi:10.4049/jimmunol.1402699
58. Chowdhury A, Del Rio Estrada PM, Tharp GK, Trible RP, Amara RR, Chahroudi A, et al. Decreased T follicular regulatory cell/T follicular helper cell (TFH) in simian immunodeficiency virus-infected rhesus macaques may contribute to accumulation of TFH in chronic infection. J Immunol (2015) 195:3237–47. doi:10.4049/jimmunol.1402701
59. Xu H, Wang X, Lackner AA, Veazey RS. PD-1(HIGH) follicular CD4 T helper cell subsets residing in lymph node germinal centers correlate with B cell maturation and IgG production in rhesus macaques. Front Immunol (2014) 5:85. doi:10.3389/fimmu.2014.00085
60. Kohler SL, Pham MN, Folkvord JM, Arends T, Miller SM, Miles B, et al. Germinal center T follicular helper cells are highly permissive to HIV-1 and alter their phenotype during virus replication. J Immunol (2016) 196:2711–22. doi:10.4049/jimmunol.1502174
61. Moukambi F, Rabezanahary H, Rodrigues V, Racine G, Robitaille L, Krust B, et al. Early loss of splenic Tfh cells in SIV-infected rhesus macaques. PLoS Pathog (2016) 12:e1005393. doi:10.1371/journal.ppat.1005393
62. Xu H, Wang X, Malam N, Aye PP, Alvarez X, Lackner AA, et al. Persistent simian immunodeficiency virus infection drives differentiation, aberrant accumulation, and latent infection of germinal center follicular T helper cells. J Virol (2015) 90:1578–87. doi:10.1128/JVI.02471-15
63. Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity (2011) 34:108–21. doi:10.1016/j.immuni.2010.12.012
64. Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, et al. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity (2013) 39:758–69. doi:10.1016/j.immuni.2013.08.031
65. Havenar-Daughton C, Lindqvist M, Heit A, Wu JE, Reiss SM, Kendric K, et al. CXCL13 is a plasma biomarker of germinal center activity. Proc Natl Acad Sci U S A (2016) 113:2702–7. doi:10.1073/pnas.1520112113
66. Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol (2014) 35:436–42. doi:10.1016/j.it.2014.06.002
67. Vinuesa CG, Cyster JG. How T cells earn the follicular rite of passage. Immunity (2011) 35:671–80. doi:10.1016/j.immuni.2011.11.001
68. Schultz BT, Teigler JE, Pissani F, Oster AF, Kranias G, Alter G, et al. Circulating HIV-specific interleukin-21(+)CD4(+) T cells represent peripheral Tfh cells with antigen-dependent helper functions. Immunity (2016) 44:167–78. doi:10.1016/j.immuni.2015.12.011
69. Vargas-Inchaustegui DA, Demers A, Shaw JM, Kang G, Ball D, Tuero I, et al. Vaccine induction of lymph node-resident simian immunodeficiency virus Env-specific T follicular helper cells in rhesus macaques. J Immunol (2016) 196:1700–10. doi:10.4049/jimmunol.1502137
70. Havenar-Daughton C, Reiss SM, Carnathan DG, Wu JE, Kendric K, Torrents de la Peña A, et al. Cytokine-independent detection of antigen-specific germinal center T follicular helper cells in immunized nonhuman primates using a live cell activation-induced marker technique. J Immunol (2016) 197:994–1002. doi:10.4049/jimmunol.1600320
71. Lane HC, Masur H, Edgar LC, Whalen G, Rook AH, Fauci AS. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med (1983) 309:453–8. doi:10.1056/NEJM198308253090803
72. Schnittman SM, Lane HC, Higgins SE, Folks T, Fauci AS. Direct polyclonal activation of human B lymphocytes by the acquired immune deficiency syndrome virus. Science (1986) 233:1084–6. doi:10.1126/science.3016902
73. Metroka CE, Cunningham-Rundles S, Pollack MS, Sonnabend JA, Davis JM, Gordon B, et al. Generalized lymphadenopathy in homosexual men. Ann Intern Med (1983) 99:585–91. doi:10.7326/0003-4819-99-5-585
74. De Milito A, Nilsson A, Titanji K, Thorstensson R, Reizenstein E, Narita M, et al. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood (2004) 103:2180–6. doi:10.1182/blood-2003-07-2375
75. McHeyzer-Williams M, Okitsu S, Wang N, McHeyzer-Williams L. Molecular programming of B cell memory. Nat Rev Immunol (2011) 12:24–34. doi:10.1038/nri3128
76. Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, McGavern DB, Brooks DG. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med (2011) 208:987–99. doi:10.1084/jem.20101773
77. Cubas R, Perreau M. The dysfunction of T follicular helper cells. Curr Opin HIV AIDS (2014) 9:485–91. doi:10.1097/COH.0000000000000095
78. Pallikkuth S, Rogers K, Villinger F, Dosterii M, Vaccari M, Franchini G, et al. Interleukin-21 administration to rhesus macaques chronically infected with simian immunodeficiency virus increases cytotoxic effector molecules in T cells and NK cells and enhances B cell function without increasing immune activation or viral replication. Vaccine (2011) 29:9229–38. doi:10.1016/j.vaccine.2011.09.118
79. Velu V, Mylvaganam GH, Gangadhara S, Hong JJ, Iyer SS, Gumber S, et al. Induction of Th1-biased T follicular helper (Tfh) cells in lymphoid tissues during chronic simian immunodeficiency virus infection defines functionally distinct germinal center Tfh cells. J Immunol (2016) 197:1832–42. doi:10.4049/jimmunol.1600143
80. Yamamoto T, Lynch RM, Gautam R, Matus-Nicodemos R, Schmidt SD, Boswell KL, et al. Quality and quantity of TFH cells are critical for broad antibody development in SHIVAD8 infection. Sci Transl Med (2015) 7:298ra120. doi:10.1126/scitranslmed.aab3964
81. Miles B, Miller SM, Folkvord JM, Kimball A, Chamanian M, Meditz AL, et al. Follicular regulatory T cells impair follicular T helper cells in HIV and SIV infection. Nat Commun (2015) 6:8608. doi:10.1038/ncomms9608
82. Miller SM, Miles B, Guo K, Folkvord J, Meditz AL, McCarter MD, et al. Follicular regulatory T cells are highly permissive to R5-tropic HIV-1. J Virol (2017) 91:e430–417. doi:10.1128/JVI.00430-17
83. Toccanier MF, Kapanci Y. Lymphadenopathy in drug addicts. A study of the distribution of T lymphocyte subsets in the lymph nodes. Virchows Arch A Pathol Anat Histopathol (1985) 406:149–63. doi:10.1007/BF00737082
84. Brask S, Hager H, Pallesen G, Porwit A, Biberfeld P, Gerstoft J. Quantification of CD8-positive lymphocytes in lymph node follicles from HIV-infected male homosexuals and controls. Acta Pathol Microbiol Immunol Scand A (1987) 95:155–7.
85. Tang J, Zha J, Guo X, Shi P, Xu B. CXCR5+CD8+ T cells present elevated capacity in mediating cytotoxicity toward autologous tumor cells through interleukin 10 in diffuse large B-cell lymphoma. Int Immunopharmacol (2017) 50:146–51. doi:10.1016/j.intimp.2017.06.020
86. He R, Hou S, Liu C, Zhang A, Bai Q, Han M, et al. Follicular CXCR5-expressing CD8(+) T cells curtail chronic viral infection. Nature (2016) 537:412–28. doi:10.1038/nature19317
87. Mylvaganam GH, Velu V, Hong JJ, Sadagopal S, Kwa S, Basu R, et al. Diminished viral control during simian immunodeficiency virus infection is associated with aberrant PD-1hi CD4 T cell enrichment in the lymphoid follicles of the rectal mucosa. J Immunol (2014) 193:4527–36. doi:10.4049/jimmunol.1401222
88. Tenner-Racz K, Racz P, Bofill M, Schulz-Meyer A, Dietrich M, Kern P, et al. HTLV-III/LAV viral antigens in lymph nodes of homosexual men with persistent generalized lymphadenopathy and AIDS. Am J Pathol (1986) 123:9–15.
89. Biberfeld P, Ost A, Porwit A, Sandstedt B, Pallesen G, Böttiger B, et al. Histopathology and immunohistology of HTLV-III/LAV related lymphadenopathy and AIDS. Acta Pathol Microbiol Immunol Scand A (1987) 95:47–65.
90. Fox CH, Tenner-Rácz K, Rácz P, Firpo A, Pizzo PA, Fauci AS. Lymphoid germinal centers are reservoirs of human immunodeficiency virus type 1 RNA. J Infect Dis (1991) 164:1051–7. doi:10.1093/infdis/164.6.1051
91. Pantaleo G, Graziosi C, Demarest JF, Butini L, Montroni M, Fox CH, et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature (1993) 362:355–8. doi:10.1038/362355a0
92. Piatak M Jr, Saag MS, Yang LC, Clark SJ, Kappes JC, Luk KC, et al. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science (1993) 259:1749–54. doi:10.1126/science.8096089
93. Graziosi C, Pantaleo G, Demarest JF, Cohen OJ, Vaccarezza M, Butini L, et al. HIV-1 infection in the lymphoid organs. AIDS (1993) 7(Suppl 2):S53–8. doi:10.1097/00002030-199311002-00012
94. Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science (1998) 280:427–31. doi:10.1126/science.280.5362.427
95. Brenchley JM, Vinton C, Tabb B, Hao XP, Connick E, Paiardini M, et al. Differential infection patterns of CD4+ T cells and lymphoid tissue viral burden distinguish progressive and nonprogressive lentiviral infections. Blood (2012) 120:4172–81. doi:10.1182/blood-2012-06-437608
96. Maldarelli F, Palmer S, King MS, Wiegand A, Polis MA, Mican J, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog (2007) 3:e46. doi:10.1371/journal.ppat.0030046
97. Xu Y, Phetsouphanh C, Suzuki K, Aggrawal A, Graff-Dubois S, Roche M, et al. HIV-1 and SIV predominantly use CCR5 expressed on a precursor population to establish infection in T follicular helper cells. Front Immunol (2017) 8:376. doi:10.3389/fimmu.2017.00376
98. Ruffin N, Brezar V, Ayinde D, Lefebvre C, Schulze Zur Wiesch J, van Lunzen J, et al. Low SAMHD1 expression following T-cell activation and proliferation renders CD4+ T cells susceptible to HIV-1. AIDS (2015) 29:519–30. doi:10.1097/QAD.0000000000000594
99. Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Ségéral E, et al. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature (2011) 474:654–7. doi:10.1038/nature10117
100. Mandel TE, Phipps RP, Abbot AP, Tew JG. Long-term antigen retention by dendritic cells in the popliteal lymph node of immunized mice. Immunology (1981) 43:353–62.
101. Aguzzi A, Kranich J, Krautler NJ. Follicular dendritic cells: origin, phenotype, and function in health and disease. Trends Immunol (2014) 35:105–13. doi:10.1016/j.it.2013.11.001
102. Spiegel H, Herbst H, Niedobitek G, Foss HD, Stein H. Follicular dendritic cells are a major reservoir for human immunodeficiency virus type 1 in lymphoid tissues facilitating infection of CD4+ T-helper cells. Am J Pathol (1992) 140:15–22.
103. Heath SL, Tew JG, Tew JG, Szakal AK, Burton GF. Follicular dendritic cells and human immunodeficiency virus infectivity. Nature (1995) 377(6551):740–4. doi:10.1038/377740a0
104. Keele BF, Tazi L, Gartner S, Liu Y, Burgon TB, Estes JD, et al. Characterization of the follicular dendritic cell reservoir of human immunodeficiency virus type 1. J Virol (2008) 82:5548–61. doi:10.1128/JVI.00124-08
105. Ayala VI, Deleage C, Trivett MT, Jain S, Coren LV, Breed MW, et al. CXCR5-dependent entry of CD8 T cells into rhesus macaque B-cell follicles achieved through T-cell engineering. J Virol (2017) 91:e2507–16. doi:10.1128/JVI.02507-16
106. Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol (2008) 82:12449–63. doi:10.1128/JVI.01708-08
107. Gray ES, Madiga MC, Hermanus T, Moore PL, Wibmer CK, Tumba NL, et al. The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J Virol (2011) 85:4828–40. doi:10.1128/JVI.00198-11
108. Dhillon AK, Donners H, Pantophlet R, Johnson WE, Decker JM, Shaw GM, et al. Dissecting the neutralizing antibody specificities of broadly neutralizing sera from human immunodeficiency virus type 1-infected donors. J Virol (2007) 81:6548–62. doi:10.1128/JVI.02749-06
109. Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med (2000) 6:207–10. doi:10.1038/72318
110. Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, et al. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med (1999) 5:204–10. doi:10.1038/5568
111. Burton DR, Hangartner L. Broadly neutralizing antibodies to HIV and their role in vaccine design. Annu Rev Immunol (2016) 34:635–59. doi:10.1146/annurev-immunol-041015-055515
112. Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature (2009) 458:636–40. doi:10.1038/nature07930
113. Xiao X, Chen W, Feng Y, Dimitrov DS. Maturation pathways of cross-reactive HIV-1 neutralizing antibodies. Viruses (2009) 1:802–17. doi:10.3390/v1030802
114. Klein F, Diskin R, Scheid JF, Gaebler C, Mouquet H, Georgiev IS, et al. Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell (2013) 153:126–38. doi:10.1016/j.cell.2013.03.018
115. Simek MD, Rida W, Priddy FH, Pung P, Carrow E, Laufer DS, et al. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol (2009) 83:7337–48. doi:10.1128/JVI.00110-09
116. Francica JR, Sheng Z, Zhang Z, Nishimura Y, Shingai M, Ramesh A, et al. Analysis of immunoglobulin transcripts and hypermutation following SHIV(AD8) infection and protein-plus-adjuvant immunization. Nat Commun (2015) 6:6565. doi:10.1038/ncomms7565
117. Kasturi SP, Kozlowskib PA, Nakayaa HI, Burgerc MC, Russoc P, Phama M, et al. Adjuvanting a simian immunodeficiency virus vaccine with toll-like receptor ligands encapsulated in nanoparticles induces persistent antibody responses and enhanced protection in TRIM5alpha restrictive macaques. J Virol (2017) 91:e1844–1816. doi:10.1128/JVI.01844-16
118. Havenar-Daughton C, Carnathan DG, Torrents de la Peña A, Pauthner M, Briney B, Reiss SM, et al. Direct probing of germinal center responses reveals immunological features and bottlenecks for neutralizing antibody responses to HIV Env trimer. Cell Rep (2016) 17:2195–209. doi:10.1016/j.celrep.2016.10.085
119. Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med (2012) 366:1275–86. doi:10.1056/NEJMoa1113425
120. Vaccari M, Gordon SN, Fourati S, Schifanella L, Liyanage NP, Cameron M, et al. Adjuvant-dependent innate and adaptive immune signatures of risk of SIVmac251 acquisition. Nat Med (2016) 22:762–70. doi:10.1038/nm.4105
Keywords: T follicular helper cell, T follicular regulatory cells, non-human primate, HIV infections, simian immunodeficiency virus, germinal center
Citation: Vaccari M and Franchini G (2018) T Cell Subsets in the Germinal Center: Lessons from the Macaque Model. Front. Immunol. 9:348. doi: 10.3389/fimmu.2018.00348
Received: 31 October 2017; Accepted: 07 February 2018;
Published: 26 February 2018
Edited by:
Vijayakumar Velu, Emory University, United StatesReviewed by:
Matthieu Perreau, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandHuanbin Xu, Tulane University, United States
Elizabeth Connick, University of Arizona, United States
Copyright: © 2018 Vaccari and Franchini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Monica Vaccari, vaccarim@mail.nih.gov
 Monica Vaccari
Monica Vaccari Genoveffa Franchini
Genoveffa Franchini