- 1Department of Rheumatology and Clinical Immunology, Faculty of Medicine, University of Thessaly, Larissa, Greece
- 2Department of Neurology, Faculty of Medicine, University of Thessaly, Larissa, Greece
- 3Department of Dermatology, Faculty of Medicine, University of Thessaly, Larissa, Greece
Introduction: Circulating T follicular helper (cTfh) cells and circulating T peripheral helper (cTph) cells (which share common characteristics with the cTfh population) are implicated in the pathogenesis of immune-mediated and autoimmune diseases such as psoriasis (Ps). Their close interplay with the interleukin 17 (IL-17) axis and the ex vivo effect of IL-17-targeting biologic agents used to treat Ps on them are elusive. This study aimed to investigate the effect of biologics targeting IL-17 on cTfh and cTph cell subpopulations isolated from the blood of patients with Ps.
Methods: Peripheral blood mononuclear cells (PBMCs) were isolated from patients with Ps at treatment initiation and three months later. Samples were also collected from controls. Cells were stained using monoclonal antibodies. Flow cytometry assessed the fraction of cTfh (CD3+CD4+CXCR5+) and cTph (CD3+CD4+CXCR5-PD-1hi) cells..
Results: Flow cytometric analysis showed increased fractions of activated cTfh subsets including ICOS+ and ICOS+PD-1+ expressing cells, in patients compared to controls. Biologic blocking of IL-17A diminished the cTfh population. Furthermore, ICOS+ and ICOS+PD-1+ sub-populations were also inhibited. Finally, the cTph cell fraction significantly decreased after three months of successful treatment with biologics.
Conclusion: Early anti-IL-17-mediated clinical remission in Ps is associated with decreased cTfh and cTph cell subpopulations.
1 Introduction
Psoriasis (Ps) is an inflammatory disease of the skin. The role of effector T cell populations in the development of Ps has been extensively studied. However, the involvement of follicular helper T (Tfh) cells has not yet been explored in detail. Tfh cells constitute a distinct CD4+ T helper (Th) population, identified by B-cell lymphoma 6 (Bcl6) and surface markers such as CXC chemokine receptor 5 (CXCR5) (1), inducible co-stimulator (ICOS) and programmed death 1 (PD-1) protein (2), that supports the proliferation of B cells, and the production of antibodies (3, 4).
Another cell population of relevance, the T peripheral helper (Tph) cell subset, constitutes a CD4+ subset with properties and characteristics that resemble those of Tfh-cells. Tph cells are not characterized by CXCR5 expression; on the contrary, PD-1, ICOS, interleukin (IL-) 21 and CXC motif chemokine ligand 13 (CXCL13) molecules are highly expressed (5). Circulating Tph (cTph) cells, phenotypically defined as CD4+CXCR5-PD-1hi cells (6), specialize in supporting B cells within non-lymphoid tissues. Their participation in the induction of autoimmunity has been supported (7, 8).
Sampling constraints in humans have hindered attempts to understand better the exemplary contribution of Tfh and Tph cells in inflammatory diseases. Researchers have turned to the investigation of circulating Tfh (cTfh) cells, a CXCR5+ Tfh-like cell population that may not express Bcl6 but shares immunophenotyping properties and functions with tissue Tfh populations (1). cTph cells have also been studied in peripheral blood in the context of autoimmunity due to restraints of exploring them into inflamed tissues (5).
Recent evidence has implicated both cell subsets in psoriasis. Firstly, cTfh sub-populations and cTph cells increase in the periphery of patients with Ps (9–11). Secondly, expanded populations are accompanied by elevated activation levels as defined by the expression of ICOS or PD-1 (12–14). Thirdly, cTfh subpopulations correlate with Ps severity (10, 11, 13). Finally, cTfh have been observed to format ectopic lymphoid-like structures, which serve as starting points for various immune interactions and advancement of pathogenic disease-specific cell populations (4, 15). In psoriatic skin, such clusters of dendritic and T-cell subsets have been recognized in developing pro-inflammatory conditions that mediate skewing towards Th17 and keratinocyte proliferation (16–18). Interestingly, cTfh cells have been identified in increased numbers in psoriatic skin (13).
Recent studies have identified IL-17 as a critical molecule for the Tfh population. IL-17 has been found to arrest B cell migration, thus promoting the formation of germinal centers in which Tfh and B cell interactions occur (19). Significantly, in vitro IL-17 blockade has been found to suppress plasmablast differentiation (20). In a murine model of experimental autoimmune encephalomyelitis, myelin-specific Tfh could not induce disease when transferred into recipient mice; however, anti-CXCL13 treatment (CXCL13: chemokine that binds to CXCR5, which is highly expressed on Tfh) attenuated Th17-mediated disease severity (21).
Since patients with Ps are frequently treated with IL-17-neutralizing agents, and an association between increased expression of molecules within the IL-23/IL-17 pathway and enhanced Tfh-mediated functions in mice was recently reported (20), we explored the effect of anti-IL-17 biologic treatment on human cTfh cells. The current study aimed to assess by multicolor flow cytometry the impact of biologics specifically targeting the IL-17 axis (secukinumab or brodalumab) on the fractions of cTfh and cTph cell subsets in the blood of patients with Ps.
2 Patients and methods
2.1 Patients
Thirty consecutive individuals with a diagnosis of moderate-severe Ps vulgaris, as defined by a Psoriasis Area and Severity Index (PASI) of more than 7, who attended the Department of Dermatology of University General Hospital of Larissa in Central Greece were characterized as eligible for enrollment. Patients with no other autoimmune or autoinflammatory comorbidity were enrolled. Blood collection from Ps patients was attained at baseline and three months of therapy and prior to therapy initiation, a 3-month washout period had preceded. During treatment, patients did not receive any other systemic drug for the management of Ps. Ten demographically matched HCs were also enrolled. Clinical descriptions of the cohorts enrolled are presented in Table 1. PASI improvement at three months determined response to therapy. A ≥50% PASI improvement was defined as PASI50, a ≥90% PASI improvement as PASI90, and a 100% improvement as PASI100.
From each study participant, a peripheral blood sample of 30 mL was collected and used to obtain peripheral blood mononuclear cells (PBMCs) utilizing a standard protocol as previously described (22). Briefly, blood layering on the surface of a LymphoPrep gradient (Axis-Shield, Oslo, Norway), centrifugation, and washing (twice) with serum-free RPMI 1640 (Pan Biotech, Aidenbach, Germany) were followed to accomplish PBMC isolation. A hemocytometer was used for cell counting. We then assessed cell viability using trypan blue, which consistently exceeded 95%. Afterward, we resuspended PBMCs in a solution containing cell cryoprotectants [dimethyl sulfoxide (10%) and fetal bovine serum (90%)]. PBMCs were subsequently portioned into cryogenic tubes, placed at -80°C for 24 hours, and finally reserved in tanks containing liquid nitrogen until experimental use.
2.2 Leukocyte immunophenotyping by multicolor flow cytometry
Leukocyte immunophenotyping was executed as previously described (23). In brief, cryovials containing cells were transferred in the water bath at 37°C and held on the water’s surface for approximately 1 min. until a small portion of ice remained frozen. Then, vials were immediately transferred to the biosafety hood, where pre-warmed RPMI 1640 was added dropwise in each cryotube. Consequently, the content of each cryotube was transferred dropwise in a centrifuge tube. PBMCs that were thawed were centrifuged for 5 min. Moreover, the supernatant was then discharged. Finally, PBMCs were resuspended in a culture medium containing 10% fetal calf serum (FCS) and 90% RPMI 1640. After PBMC thawing and washing, cells were resuspended using a solution containing phosphate buffered saline (PBS) and FCS (2%) (staining buffer). To assess surface phenotypes of isolated PBMCs, we utilized anti-human monoclonal antibodies (mAbs): fluorescein isothiocyanate (FITC)-conjugated anti-CXCR5 (clone J252D4), phycoerythrin (PE)-conjugated anti-PD-1 (clone EH12.2H7), PE-conjugated anti-CCR6 (clone G034E3), peridinin chlorophyll protein (PerCP)-conjugated anti-CD3 (clone HIT3a), PE-Cy7-conjugated anti-CD4 (clone RPA-T4) and APC-Cyanine7-conjugated anti-ICOS (clone C398.4A) (BioLegend, San Diego, USA) (Supplementary Table 1). Subsequently, a 30-minute incubation with mAbs on ice was performed, and fixation of PBMCs using a paraformaldehyde solution (2%) was achieved. A supplemental panel was utilized to assess an intracellular marker. Specifically, PBMCs were stained with PE-conjugated anti-human Bcl-6 (clone 7D1), FITC-conjugated anti-CXCR5 (clone J252D4), PerCP-conjugated anti-CD3 (clone HIT3a), and PE-Cy7-conjugated anti-CD4 (clone RPA-T4), as described above. Intracellular staining was carried out following surface staining and fixation. Per the manufacturer’s instructions, a transcription factor buffer set was used (BioLegend, San Diego, USA). To assess apoptosis of PBMCs after thawing for each sample included, we further utilized FITC-conjugated Annexin V using the manufacturer’s protocol that includes Annexin V Binding Buffer (BioLegend, San Diego, USA). A benchtop flow cytometer was used to analyze (Guava EasyCyte 8, Merck-Millipore, Missouri, USA). Supplementary Table 2 shows the flow cytometer setup used to perform experiments. Logarithmic amplification was applied. Total lymphocytes were gated based on forward and side light scatter. Proper quantification of scarce cell subsets was ensured by collecting ≥3x105 events in each total lymphocyte gate. Isotype controls were utilized to exclude false positive or high background readouts (Supplementary Figure 1). To assess the reproducibility of the results, we conducted experiments in duplicate. Data were acquired using guavaSoft software (Merck-Millipore, Missouri, USA) and analyzed in FlowJo (BD Biosciences).
2.3 Statistical analysis
Study sample characteristics were described using descriptive statistics. Epitope expression on cell surfaces was reported as a percentage of cells for each epitope. Cell fractions expressing each epitope were presented by mean and standard deviation. Cell-frequency differences between Ps patients and healthy controls (HCs) were assessed using an unpaired t-test or Mann-Whitney U test. Comparisons between two time points for each cell group were executed by paired t-test or Wilcoxon signed-rank test, whichever was appropriate. Shapiro-Wilk test was performed to assess normality. Differences in mean cell percentage changes between various responding groups were explored using one-way ANOVA. Correlations were investigated using the Pearson correlation coefficient. Mean value and 95% confidence interval or median value of difference are reported to describe differences between each participant group or time points of each cell subset. Significance was defined as p ≤0.05. GraphPad Prism software was utilized to perform statistical calculations.
3 Results
3.1 Clinical characteristics
Lymphocytes and sub-populations were gated as depicted in Supplementary Figure 2. Supplementary Table 3 summarizes cell subset identification—based on immunophenotyping characteristics—used in our research.
After three months of biologic therapy, all Ps patients (30 of 30, 100%) were at least partially responders and, as such, were included in the analyses (21 of 30 Ps patients [70%] were complete responders). There was no significant difference in lymphocyte populations between baseline and post-treatment assessment, as indicated by routine laboratory values (Supplementary Table 4). Furthermore, changes in CD3+CD4+ cell percentages were not observed between baseline and post-treatment (Supplementary Figure 3).
3.2 Increased fractions of ICOS+ and ICOS+PD-1+ cTfh cells in patients with Ps compared to controls
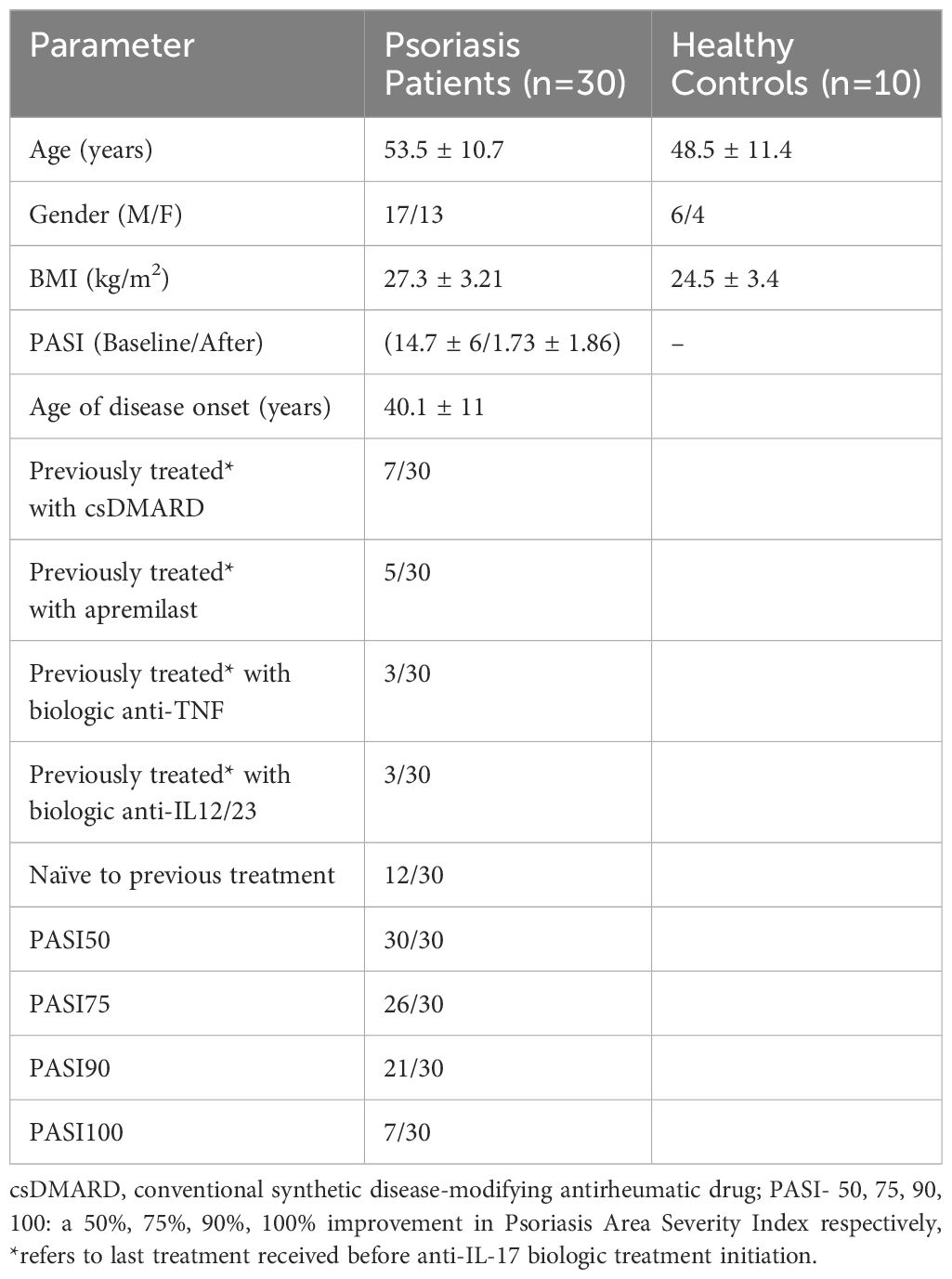
We initially assessed differences between Ps patients and HCs. To determine the immune-silencing capacity of each subset, we investigated surface PD-1. PD-1 expression was lower in the CD4+ compartment in Ps patients (difference between means = -5.4, 95% CI -8.65 to -2.1, p = 0.0020) (Figure 1). The percentage of cells expressing ICOS in the same compartment did not differ between the two groups.
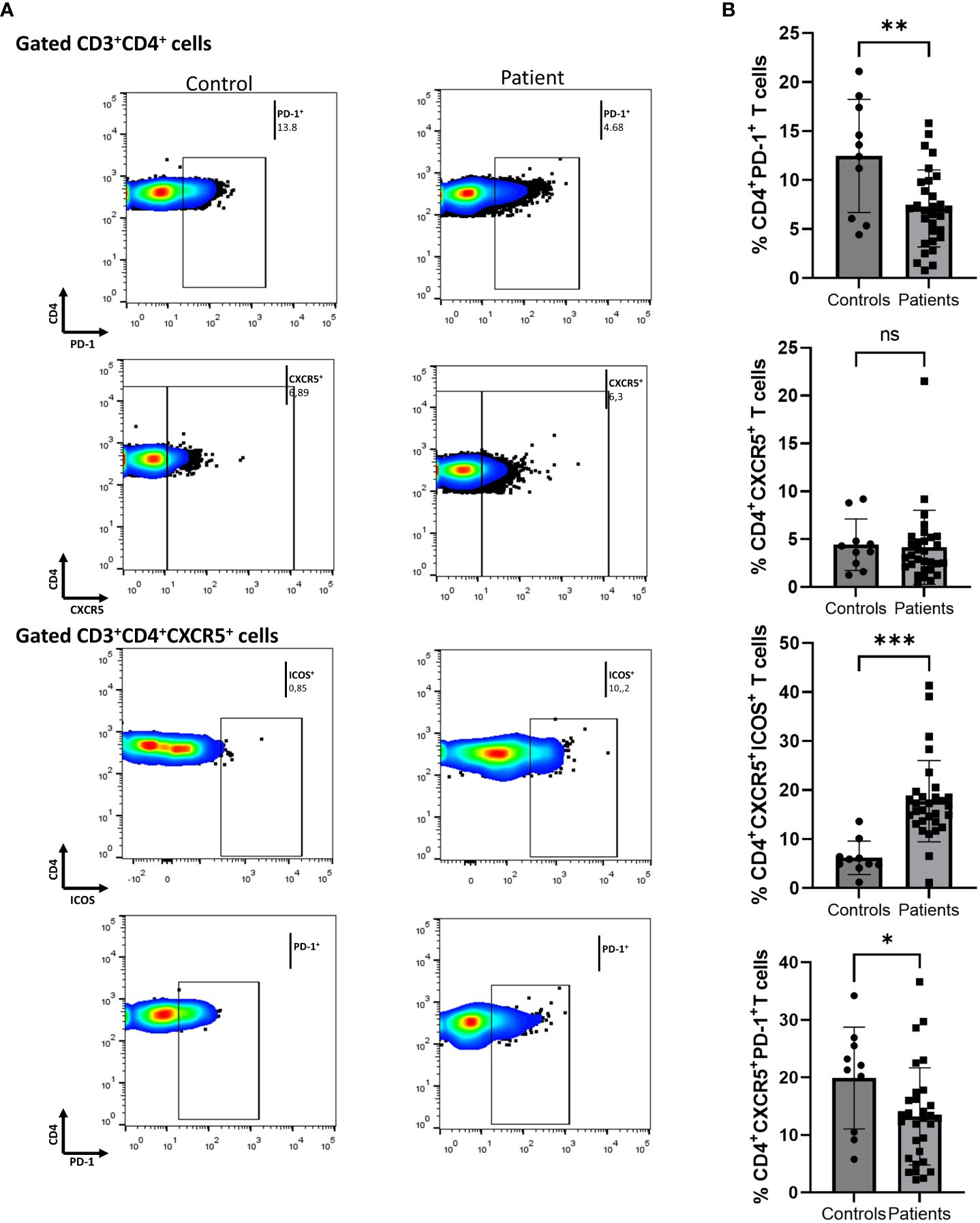
Figure 1 Comparison of CD4+PD-1+, CD4+CXCR5+, CD4+CXCR5+ICOS+, and CD4+CXCR5+PD-1+ cell populations between psoriasis (Ps) patients and healthy controls (HCs). A statistical analysis of data derived from plots generated by flow cytometry of PBMCs was performed. Characterization of sub-populations was executed based on CD3, CD4, CXCR5, ICOS and PD-1 surface markers expression. (A) Differences in frequencies of CD4+PD-1+, CD4+CXCR5+, CD4+CXCR5+ICOS+, and CD4+CXCR5+PD-1+ cell populations between Ps patients and HCs, as presented in representative plots. (B) Graphical representation of significant differences in percentages of CD4+PD-1+, CD4+CXCR5+ICOS+, and CD4+CXCR5+PD-1+ cell subsets between Ps patients (n=30) and HCs (n=10). Graphs, mean ± SD; ns p >0.05; *p ≤0.05; **p ≤ 0.01; ***p ≤0.001.
Next, we evaluated the differences within the cTfh cell population. We found an increase in the fraction of ICOS+ cells in Ps patients compared to controls (difference between means = 11.55, 95% CI 6.06 to 17.04, p = 0.0001) (Figure 1). Since an increase in cell activation in disease was observed, we also explored the double positive cTfh subset. The percentage of CD4+CXCR5+PD-1+ICOS+ cells was also significantly increased in Ps (difference between means = 3.81, 95% CI 1.56 to 6.07, p = 0.0016). On the contrary, we observed a smaller percentage of PD-1+ cells within the cTfh compartment in Ps patients (difference between means = -6.66, 95% CI -12.98 to -0.34).
3.3 Biologic blocking of IL-17A diminished the cTfh population in Ps patients
In Figure 2, we demonstrate that secukinumab or brodalumab administration, at three months of treatment, significantly inhibited fractions of cTfh (mean of differences = -1.84, 95% CI -3.1 to -0.57, p = 0.0059) and ICOS-producing CD4+ T (mean of differences = -2.52, 95% CI -3.78 to -1.27, p = 0.0003) but did not alter the PD-1-producing CD4+ cells.
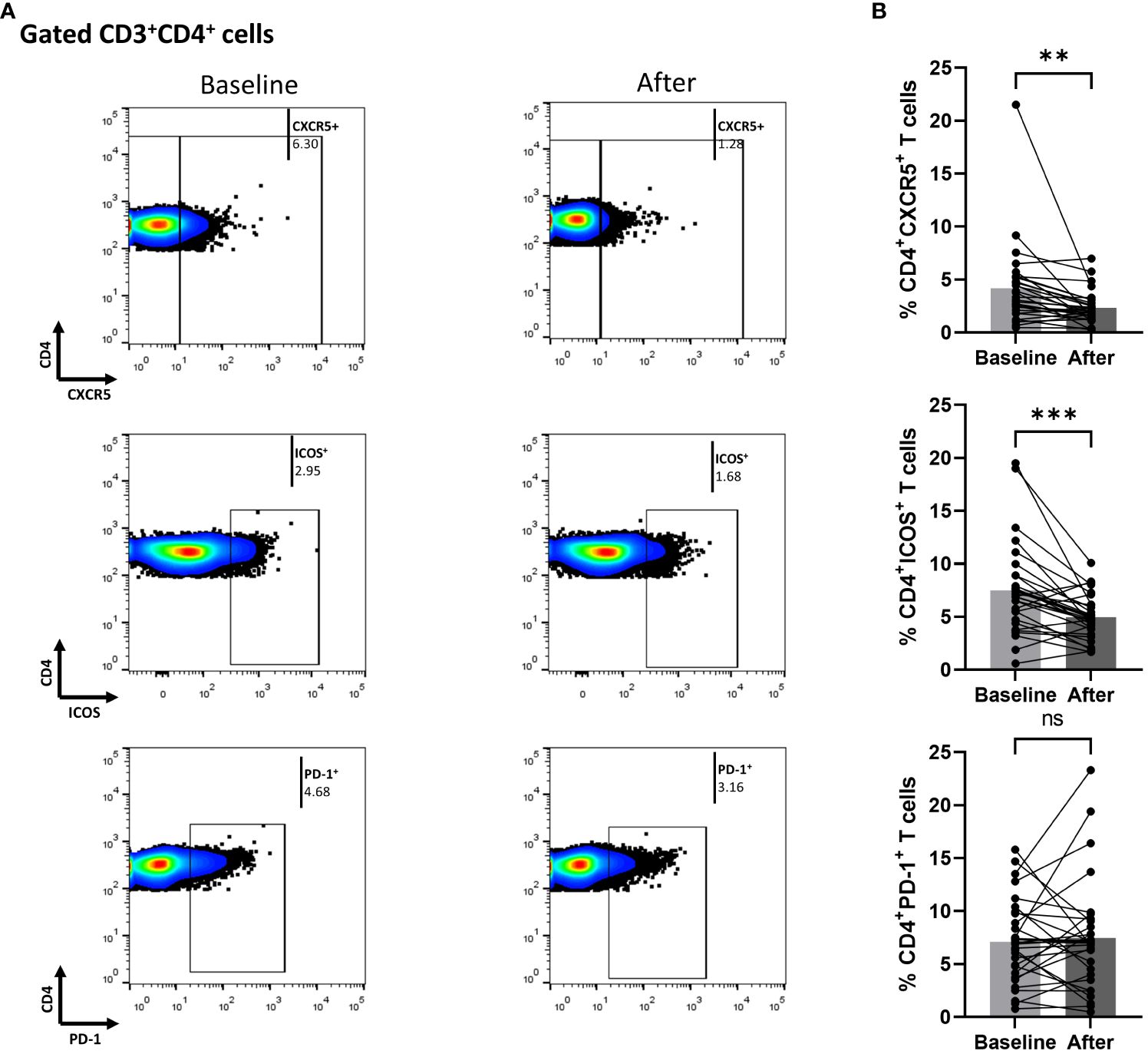
Figure 2 Anti-IL17 treatment significantly decreases percentages of CD3+CD4+CXCR5+ and CD3+CD4+ICOS+ cell populations in psoriasis (Ps) patients. A statistical analysis of data was performed for patients with Ps at IL-17A biologic treatment initiation (baseline) and three months afterwards. Characterization of sub-populations was executed based on expression of CD3, CD4, CXCR5, ICOS and PD-1 surface markers. (A) Decreased percentages of CD3+CD4+CXCR5+ and CD3+CD4+ICOS+ cell populations in Ps patients after treatment, as presented in representative plots. (B) Graphical representation of significant inhibition of the percentages of CD3+CD4+CXCR5+ and CD3+CD4+ICOS+ cells in Ps patients (n=30) after anti-IL17A biologic therapy. Graphs, mean ± SD; ns p >0.05; **p ≤ 0.01; ***p ≤0.001.
3.4 Biologic blocking of IL-17A diminished activated cTfh cells in Ps patients
We also measured biological treatment-induced changes within the cTfh cell population at three months. As shown in Figure 3, the fraction of ICOS-producing cells significantly decreased [mean of differences = -5.5, 95% CI -7.76 to -3.26, p < 0.0001). Interestingly, biological therapy also considerably reduced the proportion of CD4+CXCR5+PD-1+ICOS+ T cells (mean of differences = -1.43, 95% CI -2.28 to -0.58, p = 0.0018)]. The proportion of cells expressing PD-1 in the CD4+CXCR5+ T compartment was also assessed, but no significant difference was observed between the baseline and the post-treatment timepoints. Differences in mean changes between complete and partial responders were not observed for any T cell subpopulation (Supplementary Figure 4).
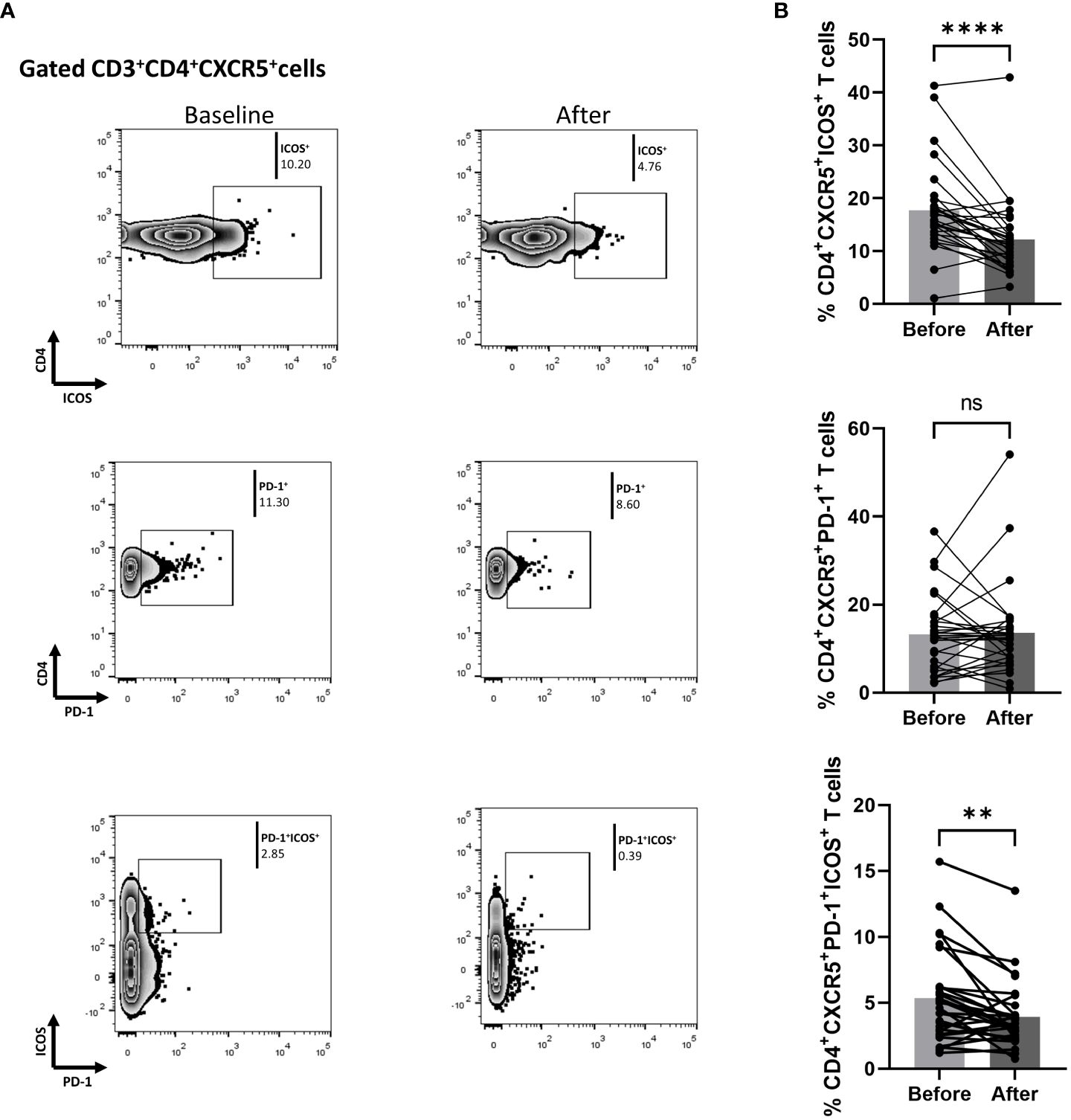
Figure 3 Biologic treatment with anti-IL-17 blocking agents diminishes the frequency of activated cTfh cell populations in psoriasis (Ps) patients. We performed statistical analysis of data for patients with Ps at anti-IL-17A biologic treatment initiation (baseline) and three months afterwards. Characterization of sub-populations was executed based on CD3, CD4, CXCR5, ICOS, and PD-1 surface markers expression. (A) Decreased frequency of the CD3+CD4+CXCR5+ICOS+ and CD3+CD4+CXCR5+PD-1+ICOS+ cell populations in Ps patients after treatment, as presented in representative plots. (B) Graphical representation of significant inhibition of percentage of the CD3+CD4+CXCR5+ICOS+ and CD3+CD4+CXCR5+PD-1+ICOS+ cell populations in Ps patients (n=30) after anti-IL-17A biologic therapy. Graphs, mean ± SD; ns p >0.05; **p ≤ 0.01; ****p ≤ 0.0001.
Given the central role of Th17 in the pathogenesis of Ps, we also investigated whether biologic therapy affected the proportion of CD4+CXCR5+CCR6+ cells, but no significant difference was observed (n = 5, mean of differences = 1.15, 95% CI -4.53 to 7.60).
3.5 Biologic blocking of IL-17A diminished cTph cells in Ps patients
Since biologics reduced the fraction of blood cTfh cells in Ps patients, we explored whether the percentage of cTph cells was also affected. Indeed, the proportion of Tph in the periphery of Ps patients was significantly decreased (mean of differences = -0.25, 95% CI -0.40 to -0.11, p = 0.0016) at three months of treatment (Figure 4).
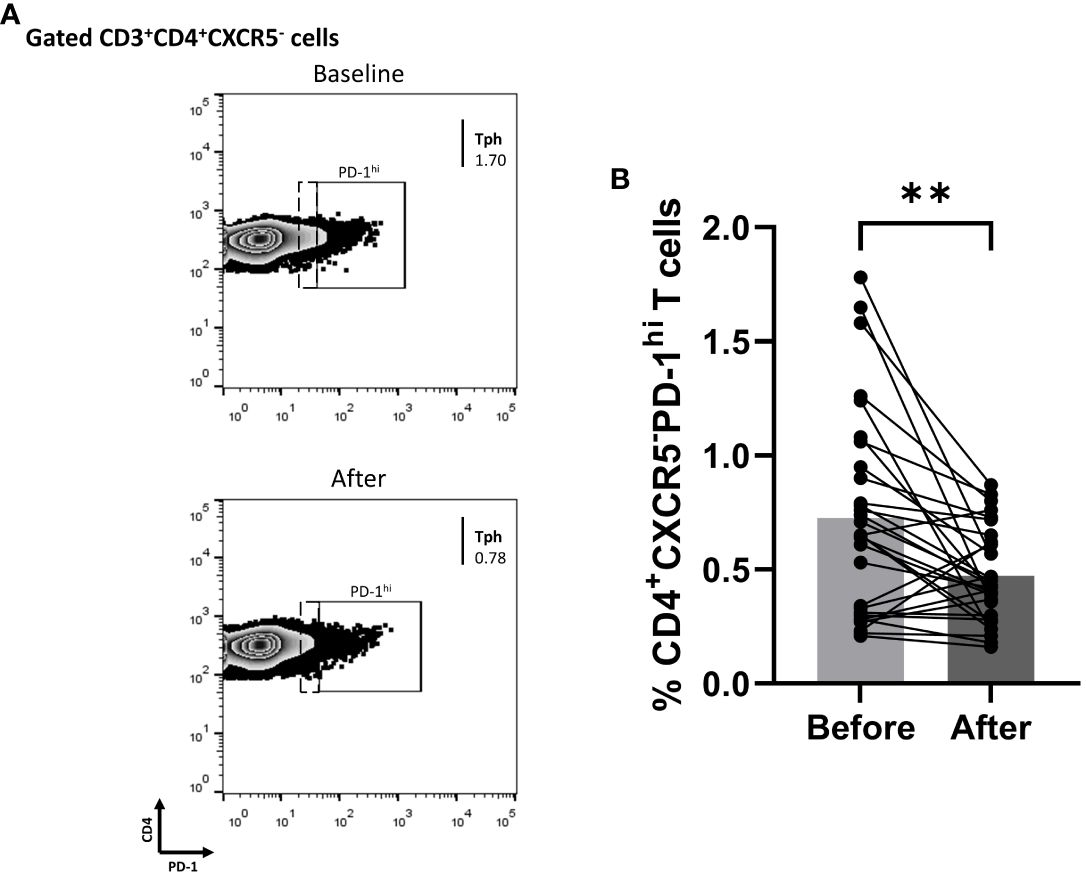
Figure 4 Anti-IL-17 treatment significantly decreases the percentage of CD3+CD4+CXCR5-PD-1hi (Tph) cell population in successfully treated patients with psoriasis (Ps). We performed statistical analysis of data for patients with Ps at IL-17A biologic treatment initiation (baseline) and three months post-treatment. Characterization of sub-populations was executed based on CD3, CD4, CXCR5, and PD-1 surface markers expression. (A) Decreased frequency of CD3+CD4+CXCR5-PD-1hi (Tph) cell population in Ps patients after treatment, as presented in representative plots. (B) Graphical representation of significant inhibition of the percentage of CD3+CD4+CXCR5-PD-1hi (Tph) cells in Ps patients (n=30) after anti-IL-17A biologic therapy. Graphs, mean ± SD; **p ≤ 0.01.
3.6 Significant correlations between clinical improvement and changes in mean percentages of cTfh cell sub-populations were not observed
Significant correlations were not observed between mean changes in PASI scores (baseline versus three months timepoint) and changes in mean percentages of T cell populations (in the corresponding timepoints). Furthermore, significant correlations between PASI scores and the percentages of CD3+CD4+CXCR5+ cells were also not observed in either baseline or the three-month post-treatment timepoint. Of the 30 patients, 17 were treated with secukinumab, and 13 were treated with brodalumab. No significant differences in mean cell percentage changes were observed between secukinumab- or brodalumab-induced effects (Supplementary Figure 5). In agreement with our previous findings [22], our cell-thawing protocol did not influence cell viability. Thus, the percentage of apoptotic cells was insignificant (Supplementary Figure 6). Assessment of Bcl-6 expression on cell sub-populations showed an 8-fold higher expression of Bcl-6 in the CD4+CXCR5+ T compartment compared to the CD4+CXCR5- (n=5, mean of differences = -1.90, 95% CI -2.67 to -1.12, p = 0.0024, Supplementary Figure 7). It should be noted that even in the CD4+CXCR5+ sub-population, Bcl-6 expression was shallow (n=5). Anti-IL-17 mediated changes in Bcl-6 expression in the CD4+CXCR5+ compartment were not significant.
4 Discussion
In this study, we assessed differences between Ps patients and HCs in the circulating frequencies of cTfh cells, of PD-1-expressing Th and cTfh cells, ICOS-expressing Th and cTfh cells, and ICOS+PD-1+ cTfh cells.
While some data in the past indicate increased percentages of CD4+CXCR5+ T cells in Ps (11, 13) our study failed to report them. This is consistent with another study that also reported no distinction between the two groups (12). Such discrepancies could be attributed to differences in patient characteristics, patient ethnic origins, overall PASIs, and previous exposure to therapeutic agents between cohorts included in different studies.
We report that PD-1 expression within the CD4+CXCR5+ T compartment is reduced in Ps. Inhibited expression of PD-1 in cTfh of Ps patients is likely associated with decreased immune-silencing properties. The concept of PD-1-PDL-1 pathway disruption in Ps has been studied in murine models. For instance, PD-1 knock-out mice presented more significant epidermal hyperplasia and Th17-cytokine expression when exposed to imiquimod compared to wild-type mice (24). Interestingly, the eruption of psoriasiform lesions has been reported in patients with malignancies treated with nivolumab, a human anti-PD-1 antibody (25, 26). Our data are in accordance with previous findings in Ps (12). Regarding the expression of PD-1 in the overall CD4+ T cell population, it should be noted that PD-1 expression can often be an activation marker rather than a marker of cell exhaustion. Accordingly, contradictory results have been shown for the frequency of PD-1+ CD4+ T cells in patients with psoriatic arthritis compared to HCs (27–29), with some of them reporting decreased PD-1+ T-cell percentages (14). All studies, including our own, included a relatively limited number of patients. We also explored levels of cell activation. The ICOS molecule has been known to increase in activated CXCR5+ T cells (30) and has been used as a marker for cell activation (13). Interestingly, blood cTfh cells remain in a non-ICOS-expressing resting state; however, in disease, they are activated and produce ICOS (31). Our results clearly document that ICOS+-CD4+CXCR5+ T cells increase in the blood of patients, which is consistent with published data (11). We also observed an increase in the percentage of double-positive activated cTfh cells (CXCR5+PD-1+ICOS+) in patients with Ps.
After studying the differences between patients with Ps and HCs, we aimed to investigate the effects of anti-IL-17 biologic treatment. Brodalumab and secukinumab significantly reduced the number of cTfh population in the periphery of responding patients with Ps. They also hindered the proportion of activated cTfh cell sub-populations. Specifically, ICOS+PD-1+-cTfh and ICOS+-cTfh cells inhibited post-treatment. In addition, ICOS expression also decreased in total CD4+ T cells. Our results clearly indicate post-treatment attenuation of pro-inflammatory stimulatory cell sub-populations, which may contribute to the improvement of the clinical manifestations.
The question of whether the decrease of activated cTfh subsets observed after therapy is a result of the gradual resolution of inflammation or a direct effect of the antibodies on the cTfh cell development pathway (or potentially both) remains. An answer is challenging to obtain in translation studies based on biological material. However, the immunomodulatory properties of IL-17 on Tfh cells have recently been explored in murine models. Indeed, in IL-17 deficient Roquinsan/san mice, activated Tfh (CD4+CXCR5+ICOS+PD-1+) cells within germinal centers significantly decreased compared to non-deficient mice. Moreover, IL-17 deficiency affected germinal center formation in murine spleens and hindered differentiation towards plasma cells in Roquinsan/san mice (32). The addition of neutralizing antibodies against IL-17A in co-cultures of Tfh and B cells has been found to suppress cell differentiation and autoantibody secretion (20). Our data suggest that direct associations between the IL-17 pathway and cTfh cell function exist in humans and in particular in patients with Ps.
In addition, we report that cTph cells decreased after secukinumab or brodalumab treatment. While Ps has been suggested to implicate autoimmune-related mechanisms, few autoantigens, such as cathelicidin, LL37, ADAMTSL5, lipid antigen PLA2G4D, and keratin 17 have been reported (33), but their relevance still remains obscure. The recent identification of Ps-related autoantigens has raised the expectation that B cells may also participate in disease pathogenesis (34, 35), but the extent of such involvement and the relevance to disease progression still remains incomplete. Although antigen-specific T cells have been found in Ps patients, B cell participation and antibody production have also been suggested to engage as potent mechanisms (35). Tph cells have been reported to induce B cell functions in peripheral inflamed tissue, partially in an IL-21-mediated manner. Interestingly, IL-21 belongs to the key molecules driving keratinocyte proliferation and T effector cell differentiation in Ps (36). Interestingly, cTph cells have been reported to correlate with disease severity in Ps (10). Whether the cTph subset participates in disease development through IL-21 production or B cell promotion needs meticulous investigation.
To the best of our knowledge, our study is the first to demonstrate that IL-17 therapeutic biologic blockade modulates cTfh and cTph cell populations and expression of activation markers in Ps patients. Our data document significant differences between patients and controls, as well as substantial changes in cell percentages before and after biological treatment. However, due to the endogenous limitations of our study’s design, we urge caution in interpreting our findings. Our experiments were performed on cryopreserved PBMCs. While it has been reported that functional T cell populations remain stable after long-term cryopreservation (37) and assessment of the percentages of Tfh cells in patients has previously been reported in such cells (38), that kind of manipulation may fundamentally affect cell activation. The findings must be explored further in more extended cohorts for longer periods of time. Nevertheless, this first set of results suggesting that a clinical remission mediated by anti-IL-17 biologics is modulating cTfh cells in the periphery of Ps patients can be the impetus for subsequent investigations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the University General Hospital of Larissa, University of Thessaly (#20931-11/06/2021). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ST: Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft. AM: Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. ED: Writing – review & editing, Methodology, Supervision. EZ: Writing – review & editing, Supervision, Conceptualization, Data curation, Investigation. DB: Conceptualization, Supervision, Writing – review & editing, Data curation, Investigation, Funding acquisition, Project administration, Validation, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The current research was supported in part by grants #5847 and #6606, University of Thessaly Research Committee (grant holder: DB).
Acknowledgments
We gratefully acknowledge Professor Eirini I. Rigopoulou for her critical comments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1325356/full#supplementary-material
References
1. Morita R, Schmitt N, Bentebibel S-E, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. (2011) 34:108–21. doi: 10.1016/j.immuni.2010.12.012
2. Crotty S. T follicular helper cell biology: A decade of discovery and diseases. Immunity. (2019) 50:1132–48. doi: 10.1016/j.immuni.2019.04.011
3. Marsman C, Verstegen NJM, Streutker M, Jorritsma T, Boon L, ten Brinke A, et al. Termination of CD40L co-stimulation promotes human B cell differentiation into antibody-secreting cells. Eur J Immunol. (2022) 52:1662–75. doi: 10.1002/eji.202249972
4. Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. (2014) 41:529–42. doi: 10.1016/j.immuni.2014.10.004
5. Yoshitomi H, Ueno H. Shared and distinct roles of T peripheral helper and T follicular helper cells in human diseases. Cell Mol Immunol. (2021) 18:523–7. doi: 10.1038/s41423-020-00529-z
6. Rao DA, Gurish MF, Marshall JL, Slowikowski K, Fonseka CY, Liu Y, et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature. (2017) 542:110–4. doi: 10.1038/nature20810
7. Makiyama A, Chiba A, Noto D, Murayama G, Yamaji K, Tamura N, et al. Expanded circulating peripheral helper T cells in systemic lupus erythematosus: association with disease activity and B cell differentiation. Rheumatol (Oxford). (2019) 58:1861–9. doi: 10.1093/rheumatology/kez077
8. Kamekura R, Yamamoto M, Takano K, Yabe H, Ito F, Ikegami I, et al. Circulating PD-1(+)CXCR5(-)CD4(+) T cells underlying the immunological mechanisms of IgG4-related disease. Rheumatol Adv Pract. (2018) 2:rky043. doi: 10.1093/rap/rky043
9. Wang Y, Wang L, Shi Y, Wang F, Yang H, Han S, et al. Altered circulating T follicular helper cell subsets in patients with psoriasis vulgaris. Immunol Lett. (2017) 181:101–8. doi: 10.1016/j.imlet.2016.09.008
10. Liu W, Zhou X, Wang A, Ma J, Bai Y. Increased peripheral helper T cells type 17 subset correlates with the severity of psoriasis vulgaris. Immunol Lett. (2021) 229:48–54. doi: 10.1016/j.imlet.2020.11.005
11. Niu J, Song Z, Yang X, Zhai Z, Zhong H, Hao F. Increased circulating follicular helper T cells and activated B cells correlate with disease severity in patients with psoriasis. J Eur Acad Dermatol Venereol. (2015) 29:1791–6. doi: 10.1111/jdv.13027
12. Shin D, Kim DS, Kim SH, Je JH, Kim HJ, Young Kim D, et al. Decreased PD-1 positive blood follicular helper T cells in patients with psoriasis. Arch Dermatol Res. (2016) 308:593–9. doi: 10.1007/s00403-016-1679-y
13. Wang Y, Wang L, Yang H, Yuan W, Ren J, Bai Y. Activated circulating T follicular helper cells are associated with disease severity in patients with psoriasis. J Immunol Res. (2016) 2016:7346030. doi: 10.1155/2016/7346030
14. Bartosińska J, Zakrzewska E, Raczkiewicz D, Purkot J, Michalak-Stoma A, Kowal M, et al. Suppressed programmed death 1 expression on CD4+ and CD8+ T cells in psoriatic patients. Mediators Inflammation. (2017) 2017:1–8. doi: 10.1155/2017/5385102
15. Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol. (2012) 8:337–47. doi: 10.1038/nrrheum.2012.58
16. Chiricozzi A, Romanelli P, Volpe E, Borsellino G, Romanelli M. Scanning the immunopathogenesis of psoriasis. Int J Mol Sci. (2018) 19. doi: 10.3390/ijms19010179
17. Kim T-G, Jee H, Fuentes-Duculan J, Wu WH, Byamba D, Kim D-S, et al. Dermal clusters of mature dendritic cells and T cells are associated with the CCL20/CCR6 chemokine system in chronic psoriasis. J Invest Dermatol. (2014) 134:1462–5. doi: 10.1038/jid.2013.534
18. Mitsui H, Suárez-Fariñas M, Belkin DA, Levenkova N, Fuentes-Duculan J, Coats I, et al. Combined use of laser capture microdissection and cDNA microarray analysis identifies locally expressed disease-related genes in focal regions of psoriasis vulgaris skin lesions. J Invest Dermatol. (2012) 132:1615–26. doi: 10.1038/jid.2012.33
19. Hsu H, Yang P, Wang J, Wu Q, Myers R, Chen J, et al. Interleukin 17 – producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. (2008) 9:166–75. doi: 10.1038/ni1552
20. Kim V, Lee K, Tian H, Jang SH, Diamond B, Kim SJ. IL-17–producing follicular Th cells enhance plasma cell differentiation in lupus-prone mice. JCI Insight. (2022) 7:1–13. doi: 10.1172/jci.insight.157332
21. Quinn JL, Kumar G, Agasing A, Ko RM, Axtell RC. Role of TFH cells in promoting T helper 17-induced neuroinflammation. Front Immunol. (2018) 9:382. doi: 10.3389/fimmu.2018.00382
22. Tsiogkas SG, Mavropoulos A, Skyvalidas DN, Patrikiou E, Ntavari N, Daponte AI, et al. Delphinidin diminishes in vitro interferon-γ and interleukin-17 producing cells in patients with psoriatic disease. Immunol Res. (2022) 70:161–73. doi: 10.1007/s12026-021-09251-y
23. Skyvalidas D, Mavropoulos A, Tsiogkas S, Dardiotis E, Liaskos C, Mamuris Z, et al. Curcumin mediates attenuation of pro-inflammatory interferon γ and interleukin 17 cytokine responses in psoriatic disease, strengthening its role as a dietary immunosuppressant. Nutr Res. (2020) 75:95–108. doi: 10.1016/j.nutres.2020.01.005
24. Imai Y, Ayithan N, Wu X, Yuan Y, Wang L, Hwang ST. Cutting edge: PD-1 regulates imiquimod-induced psoriasiform dermatitis through inhibition of IL-17A expression by innate γδ-low T cells. J Immunol. (2015) 195:421–5. doi: 10.4049/jimmunol.1500448
25. Mullangi S, Ponnam S, Lekkala MR, Koya S. A case of de novo psoriasis secondary to nivolumab in a patient with metastatic renal cell carcinoma. Cureus. (2021) 13:e15703. doi: 10.7759/cureus.15703
26. Ohtsuka M, Miura T, Mori T, Ishikawa M, Yamamoto T. Occurrence of psoriasiform eruption during nivolumab therapy for primary oral mucosal melanoma. JAMA Dermatol. (2015) 151:797–9. doi: 10.1001/jamadermatol.2015.0249
27. Adamczyk M, Krasowska D. PD1/PD-L1 pathway in psoriasis and psoriatic arthritis: a review. Postep dermatologii i Alergol. (2021) 38:925–30. doi: 10.5114/ada.2021.112274
28. Bommarito D, Hall C, Taams LS, Corrigall VM. Inflammatory cytokines compromise programmed cell death-1 (PD-1)-mediated T cell suppression in inflammatory arthritis through up-regulation of soluble PD-1. Clin Exp Immunol. (2017) 188:455–66. doi: 10.1111/cei.12949
29. Peled M, Strazza M, Azoulay-Alfaguter I, Silverman GJ, Scher JU, Mor A. Analysis of programmed death-1 in patients with psoriatic arthritis. Inflammation. (2015) 38:1573–9. doi: 10.1007/s10753–015-0132–2
30. Chevalier N, Jarrossay D, Ho E, Avery DT, Ma CS, Yu D, et al. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J Immunol. (2011) 186:5556–68. doi: 10.4049/jimmunol.1002828
31. Bentebibel S-E, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med. (2013) 5:176ra32. doi: 10.1126/scitranslmed.3005191
32. Lee S, Lee SH, Seo HB, Ryu JG, Jung KA, Choi JW, et al. Inhibition of IL-17 ameliorates systemic lupus erythematosus in Roquin san/san mice through regulating the balance of TFH cells, GC B cells, Treg and Breg. Sci Rep. (2019) 9:1–8. doi: 10.1038/s41598–019-41534–1
33. Rendon A, Schäkel K. Psoriasis pathogenesis and treatment. Int J Mol Sci. (2019) 20:1–28. doi: 10.3390/ijms20061475
34. Gál B, Dulic S, Kiss M, Groma G, Kovács L, Kemény L, et al. Increased circulating anti-α6-integrin autoantibodies in psoriasis and psoriatic arthritis but not in rheumatoid arthritis. J Dermatol. (2017) 44:370–4. doi: 10.1111/1346–8138.13667
35. ten Bergen LL, Petrovic A, Aarebrot AK, Appel S. Current knowledge on autoantigens and autoantibodies in psoriasis. Scand J Immunol. (2020) 92:e12945. doi: 10.1111/sji.12945
36. Wang Y, Wang LL, Yang HY, Wang FF, Zhang XX, Bai YP. Interleukin-21 is associated with the severity of psoriasis vulgaris through promoting CD4+ T cells to differentiate into Th17 cells. Am J Transl Res. (2016) 8:3188–96.
37. Li B, Yang C, Jia G, Liu Y, Wang N, Yang F, et al. Comprehensive evaluation of the effects of long-term cryopreservation on peripheral blood mononuclear cells using flow cytometry. BMC Immunol. (2022) 23:1–14. doi: 10.1186/s12865–022-00505–4
Keywords: psoriasis, cTfh, cTph, anti-IL-17, PBMCs, secukinumab, brodalumab, psoriatic disease
Citation: Tsiogkas SG, Mavropoulos A, Dardiotis E, Zafiriou E and Bogdanos DP (2024) Biologics targeting IL-17 sharply reduce circulating T follicular helper and T peripheral helper cell sub-populations in psoriasis. Front. Immunol. 15:1325356. doi: 10.3389/fimmu.2024.1325356
Received: 21 October 2023; Accepted: 07 May 2024;
Published: 21 May 2024.
Edited by:
Ciro Romano, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Sun Jung Kim, Northwell Health, United StatesAngelo Ruggiero, University of Naples Federico II, Italy
Copyright © 2024 Tsiogkas, Mavropoulos, Dardiotis, Zafiriou and Bogdanos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dimitrios P. Bogdanos, bogdanos@med.uth.gr
†These authors have contributed equally to this work
 Sotirios G. Tsiogkas
Sotirios G. Tsiogkas Athanasios Mavropoulos1
Athanasios Mavropoulos1 Efthimios Dardiotis
Efthimios Dardiotis Dimitrios P. Bogdanos
Dimitrios P. Bogdanos