- 1Inner Mongolia Preschool Education College for the Nationalities, Ordos, China
- 2Jungar Banner Agriculture and Animal Husbandry Bureau, Ordos, China
- 3Department of Entomology, Faculty of Physical and Applied Sciences, The University of Haripur, Haripur, Khyber Pakhtunkhwa, Pakistan
Termites are eusocial and economically important insects which are found in the world’s tropical regions as a harmful or beneficial organism. They play a dual role, both as pests damaging crops and urban structure and as an ecological engineer sustaining the ecosystem. Pakistan is part of the Indomalayan realm hosting diverse flora and fauna including termites; however, the status (diversity, distribution, feeding hosts, pest and non-pest) of the genus Angulitermes in the northwestern region (Khyber Pakhtunkhwa, Pakistan) has been largely neglected. Termite cultures were collected from diverse ecosystems, cleaned, and preserved in alcohol-filled vials for subsequent morphometric identification and DNA barcoding. Coordinates with relevant ecological data were also recorded. Soldiers were used for capturing refined images and morphometric identification through available literature, which resulted as an Angulitermes dehraensis and a new locality record. A revised and updated world’s species list for the genus was made along with the distribution map of this study via ArcGIS. The identified representative soldier’s leg was processed for mtDNA extraction followed by amplification and sequencing. The received sequence was subjected to BLASTn search, and only top 15 sequences via BLASTn search and then via manual search for taxon Angulitermes were retrieved from GenBank. Aligned and trimmed sequences were processed for phylogenetic tree (neighbor-joining and maximum-likelihood) construction and validation of understudy species sequence analogy. A novel sequence was submitted to GenBank for accession number (PX423737). Based on the available and recorded feeding host substrate data, it is a pest species which needs management.
1 Introduction
Termites are eusocial insects which are known for causing serious damages (1, 2) around the world. They are divided into seven families, and ~3% of the known species are responsible for the damages in buildings and crops (3). They live in colonies which consist of workers, soldiers, and reproductive caste. Workers are in abundance and are the only damage-causing caste. Soldiers supply defense to the colony and are fed by workers through trophallaxis (1). They are also used for the identification of species (4) including this study. The reproductive caste (King; Queen aided by primary or secondary reproductive) maintain the colony population.
Naturally, termites are present in diverse terrestrial ecosystems of the tropical regions and playing an important role of being either harmful (pests) to buildings, forests, and agriculture (5) or beneficial (ecosystem engineers) to the ecosystem (6). They attack humans’ belongings in urban structures, trees, and saplings in forests whereas they attack crops in the agroecosystem. There are also some species of low ecological interest in the subtropical environment which are causing losses in the urban environment (1), agriculture, and forestry. Losses caused by termites vary by region, species, and available food sources, with reported figures including worldwide losses of $40 billion, and regional losses such as $11 billion in the USA, $10–12 billion in Malaysia, $1.5 billion in Australia, $1 billion in China, $1 billion in Indonesia, $0.8–1 billion in Japan, $0.5 billion in France, $0.5 billion in Thailand, $100s of millions in the Philippines, $35.12 billion in India, $4 billion in Taiwan, $1 billion in China, and $0.5 billion in Fiji Islands (7). Despite of the damages caused, termites are also the ecological engineers and help in the decomposition of wood and animal dung, recycling of nutrients, improvement of soil’s stability, water-holding and absorbing capacity, vegetation and tree diversity, etc. They are also a source of food for the wildlife and help in pollination although rarely (8). The presence of termites in each habitat defines their ecological role; while they are considered pests in urban areas due to structural damage, they play a beneficial role in natural habitats by decomposing wood, dung, and other organic matter.
Termites are generally controlled by insecticide (dusts or solutions) application (2), but often management fails and damages are caused. Failure of control strategies may also include incorrect identification of the present harmful species leading to the application of hazardous insecticides. Correct identification is important for understanding the role of termites (economic and ecological) (9), but it always requires experts. Taxa having similar phenotypic traits (10, 11) and poor records (12) make identification more challenging. Addition of universally accepted molecular studies (13, 14) including DNA barcoding has revolutionized the insect’s systematics (15, 16) by providing a quick way of identification as compared with traditional taxonomy (17).
The Indomalayan/Oriental region sprawls to the south of Himalayas in Southeast Asia, as shown in Figure 1a (18), having hot and humid climatic conditions. Pakistan (excluding Baluchistan) is part of the Indomalayan region with rich flora and fauna including termites, which is further divided into 10 agroecological zones by the Pakistan Agriculture Research Council (PARC) (19). According to Iqbal and Saeed (20), 11 out of 54 species (21) are economically important and belong to genera Heterotermes, Coptotermes (Rhinotermitidae), Odontotermes, and Microtermes (Termitidae) in Pakistan. Termites of Pakistan have been studied by Desneux (22, 23), Holmgren and Holmgren (24), Ahmad (25), Akhtar (26), Chaudry and Ahmad (27), and Nasir (28), excluding the northern Malakand (Buner), lower Hazara (Haripur), and eastern Mardan (Swabi) regions. This area belongs to three different agro-zones of Khyber Pakhtunkhwa (29), which was previously ignored for the termite’s faunal studies. No information was available on the species occurrence, distribution, and status (as a pest or non-pest) of termites from the genus Angulitermes, which could be utilized for designing any management or conservation strategy. Genus Angulitermes composed of 29 recorded species till date in which more than 70% species belong to the Indomalaya region (India, Myanmar, Pakistan, and Bangladesh) (Table 1). Therefore, the present study was conducted for studying the morphometrics, mapping, and distributional and feeding host substrate data with DNA barcoding of the termites from the genus Angulitermes in the northern Malakand (Buner), lower Hazara (Haripur), and eastern Mardan (Swabi) of Khyber Pakhtunkhwa-Pakistan, a part of the Northwestern Indomalaya region.
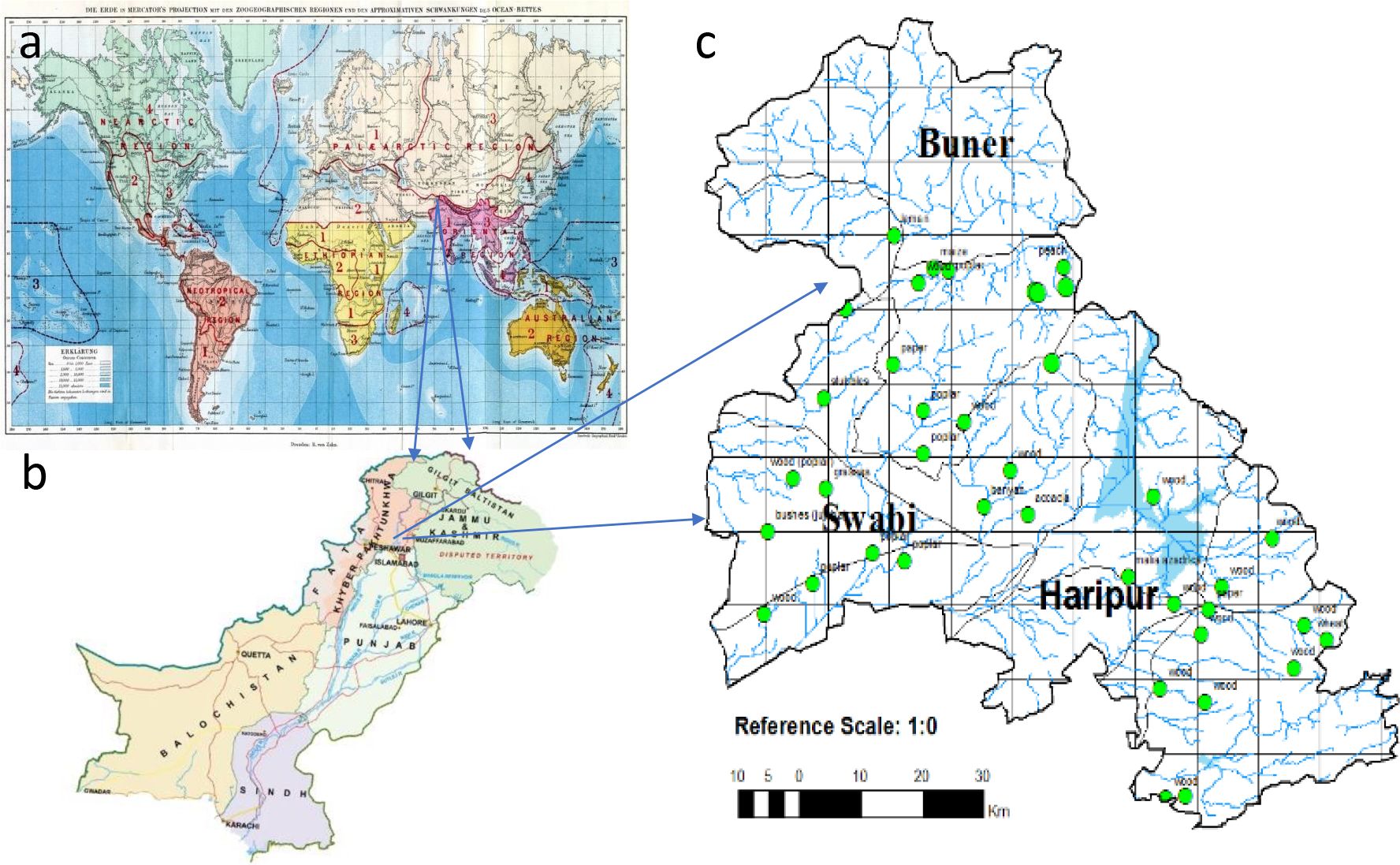
Figure 1. Genus Angulitermes sample’s collection from the selected study region of the Northwestern Indomalayan region (Pakistan; Khyber Pakhtunkhwa; Swabi, Buner and Haripur) (a) Wallace zoogeographical map; (b) map of Pakistan; (c) focused map of the study area.
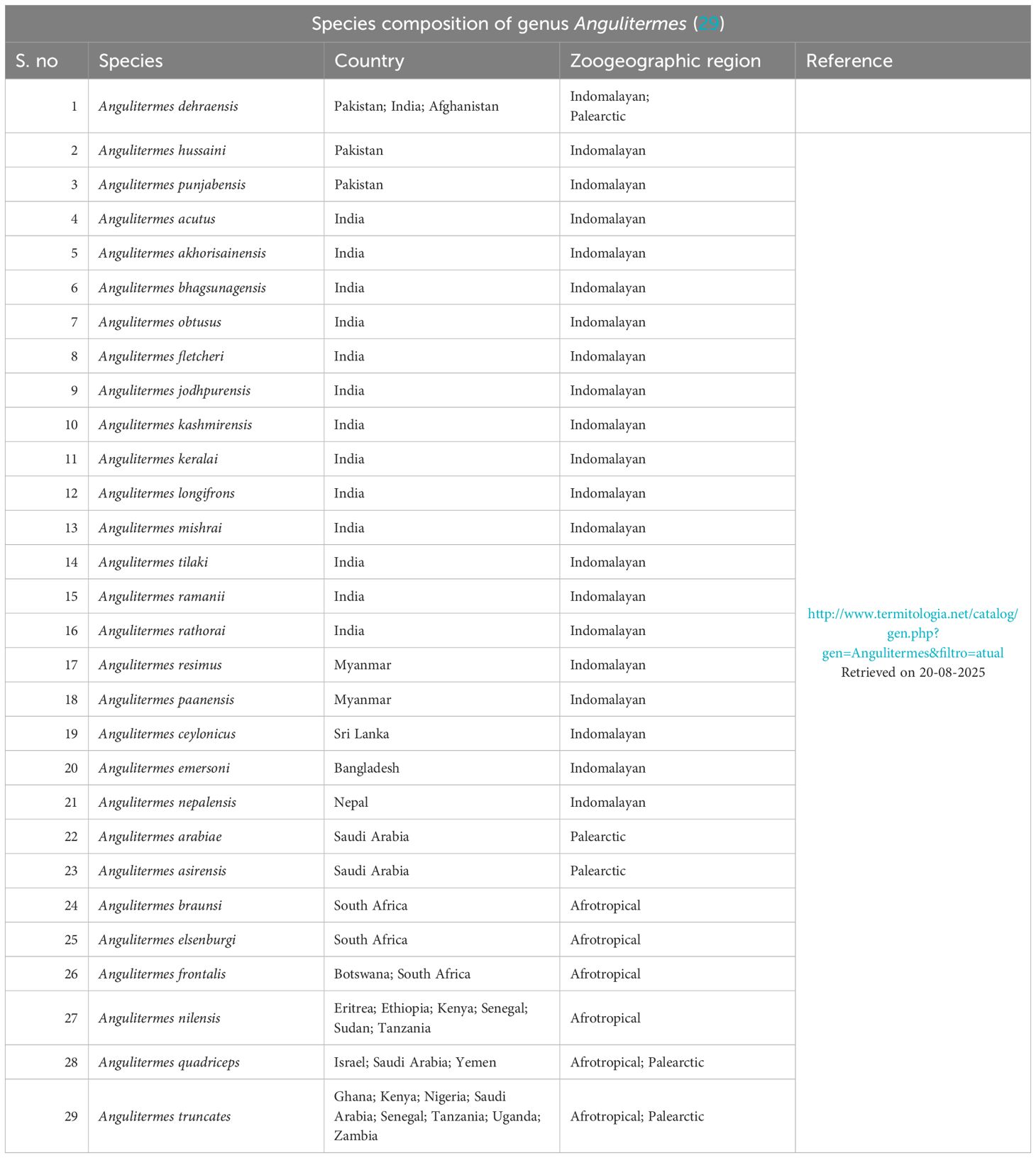
Table 1. Species composition of genus Angulitermes (30) (Termitidae: Promirotermitinae) from three major zoogeographic regions.
2 Materials and methods
2.1 Study area and survey for culture collection
An intensive termite's culture collection survey was conducted for 3 years (in the summer season) in the Northwestern Indomalayan region (districts Swabi, Buner, and Haripur) (Figures 1a–c, Supplementary Figure S1) by following the modified method of Kaakeh (31) and Saha et al. (32). Visible mud galleries on the tree trunks (in forests/orchards) and walls (in buildings) were broken just above the ground, and termites running in the broken galleries were collected in a paper funnel with a gentle swipe of a camel brush (Figures 2a–c). The funnel was attached to either a collecting vial (containing alcohol 75% or 99%) or a wet blotting paper in case of any attached dirt. The termites became stuck to the wet blotting paper, and dirt was removed (Figure 2d) by a gentle tap. Cleaned and sorted specimens were stored in the tagged vials for morphometric identification and DNA barcoding. Sampling point coordinates (via GPS device eTrex 10), feeding host substrate, and other relevant ecological data (habitat type, elevation, canopy covered) were also recorded.

Figure 2. Termite’s collection protocol from the selected study region of Northwestern Indomalaya (a) termite galleries on a stem; (b) inspecting mud chunk for termite presence; (c) breaking mud galleries on the trunk for on spot collection; (d) termites with dirt after breaking mud galleries.
2.2 Termite’s morphometric identification
Randomly selected soldiers were used for the identification of 13 morphological characters and five indices (Table 2) by following Chaudry and Ahmad (27) and Chhotani (33). All measurements were taken (in millimeters) under a trinocular stereo-zoom microscope (Nikon 745T) with a mounted Nikon FSi2 digital camera for capturing pictures. The captured pictures were stacked by Helicon Focus 6.0 (Helicon Soft Ltd, Ukraine, 2000-2022) and further refined by Adobe Photoshop (Version CS6 13.1.2 San Jose, CA, USA, 2017). Means for all characters and indices were calculated in Microsoft Excel 365 (34).

Table 2. List of morphological characters and indices for the morphometric identification of Angulitermes species.
2.3 Distributional data
The sampling site’s (via GPS device eTrex 10) coordinates were recorded during surveys, which were then used for the construction of maps in ArcGIS 10.0 (35).
2.4 Molecular analysis
An identified representative soldier was washed with distilled water and allowed to air-dry. Then, the broken hind leg was ground with the pipette tip for DNA extraction followed by amplification (36) (Supplementary Table S1). For sequencing, 15µl of the extracted DNA was added to washed and sterile tubes having 2µl reverse and 2µl forward primers. The prepared samples were sent for Sanger sequencing (to Eurofins, Denmark; https://www.eurofins.com/).
For validating sequence analogy, received sequences were processed in BLASTn (NCBI) search for retrieving correct matches (37). Based on percent query covered, percent identity, bit cover, and relevant taxon match, only top-matched BLASTn (NCBI) sequences were retrieved for phylogenetic tree construction (Supplementary Table S4). Reference sequences were also searched manually for taxon “Angulitermes” (38). Then, all retrieved sequences were aligned in MEGA 6.0 by using ClustalW (39) and both ends were trimmed for removing any ambiguities. Neighbor-joining and maximum likelihood methods were employed for phylogenetic tree construction in MEGA 6.0 (40). Resulted novel sequences were submitted to GenBank for accession number.
3 Results and discussion
Termites from genus Angulitermes (30) are distinct and easily identified by the soldier’s frontal projection as compared with other termites. It is recorded from the Indomalayan, Afrotropical, and Palearctic zoogeographic regions with 29 known species in which 21 species are reported for the Indomalayan region (India, Nepal, Myanmar, Pakistan, and Bangladesh), making it a diverse region for this genus (Table 1). There are only three species, namely, Angulitermes dehraensis (Gardner) (41), A. hussaini (Ahmad), and A. punjabensis (Akhtar) reported from Pakistan (27) with unknown status (pest or non pest). In this study, A. dehraensis is recorded as a new locality record from a sub-hilly terrain (Supplementary Table S3).
3.1 Angulitermes dehraensis
3.1.1 Diagnostic characters of soldier caste
Head’s capsule, antenna, legs, body, and mandibles are of yellowish, fawn, straw yellow, and reddish-brown in color, respectively. Relatively the head is less hairy as compared with the body and frontal projection which are hairier. Antennae are of 14 segments in which segment 3 is longer than segment 2 whereas segment 4 is the shortest (Figures 3a–e). Other measurements include the following:
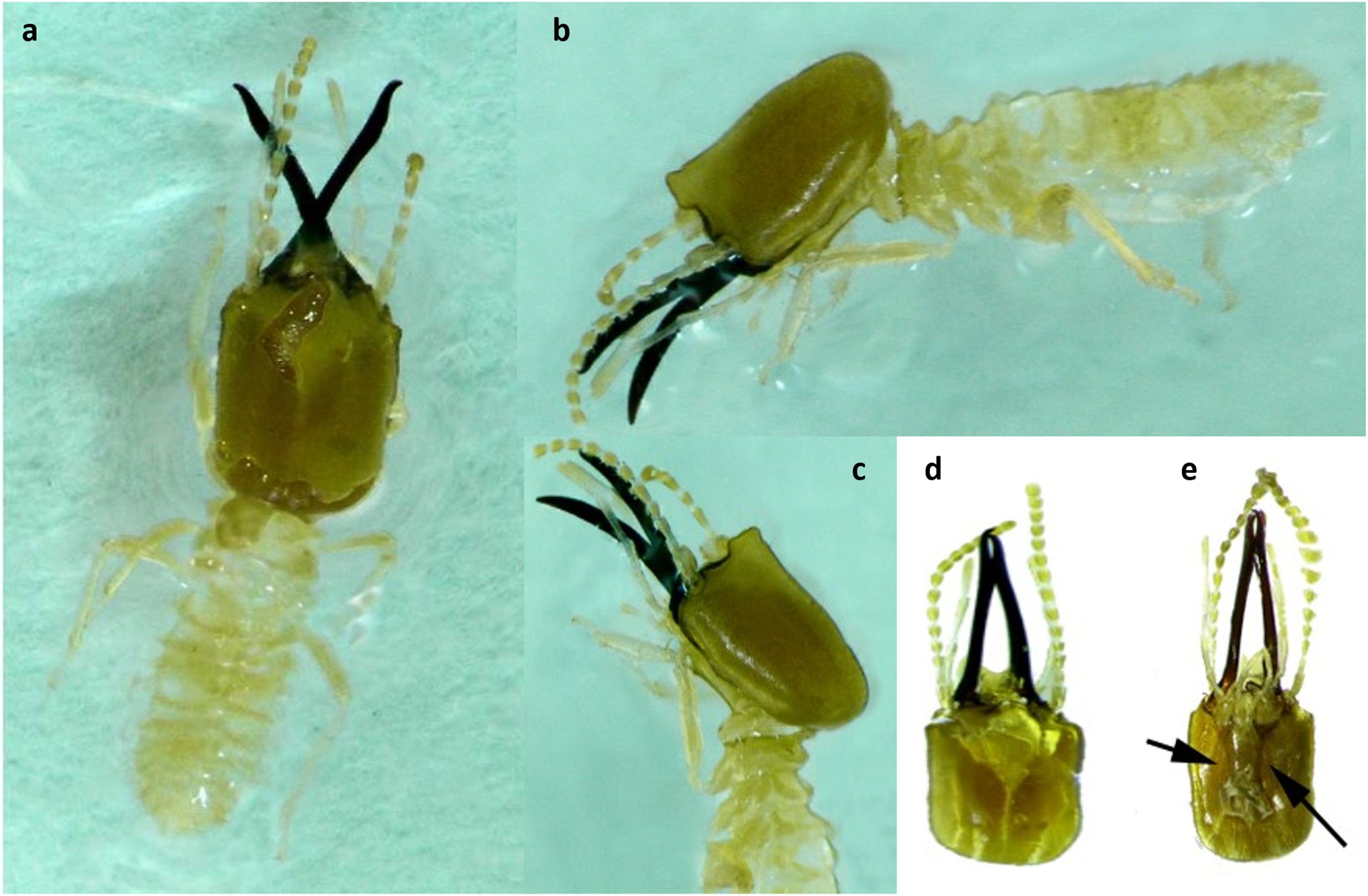
Figure 3. (a–e) Different diagnostic characters of Angulitermes dehraensis soldiers (a) dorsal view of the soldier; (b) side view of the soldier; (c) head region (fontanelle) of the soldier; (d) dorsal view of the head; (e) ventral view of the head (arrow = postmentum).
Mandibles = 1.34-1.45
Total body length= 3.90-4.60
Labrum; length= 0.33, width= 0.30
Pronotum; length = 0.23, width = 0.50
Length of frontal projection= 0.10-0.13
Postmentum; length= 0.43-0.40, max. width = 0.30, width at waist = 0.25
Index: Left mandible-length/head-length to base of mandibles= 1.16-1.25
All measurements are in millimeters. Details on characters are also available at Chhotani (33).
3.1.1.1 Morphometrics
Measurements for the 13 characters and five indices were recorded and then compared with the ranges in literature (33) for the confirmation of understudy species morphometrics (Figure 4). Top four indices are adopted from other species and reported for the first time for A. dehraensis with no available measurement ranges in the literature (Supplementary Table S2). All the observed measurements are falling within the available morphometric ranges, which confirms that the specimens are of A. dehraensis, a species of genus Angulitermes (Figure 4).
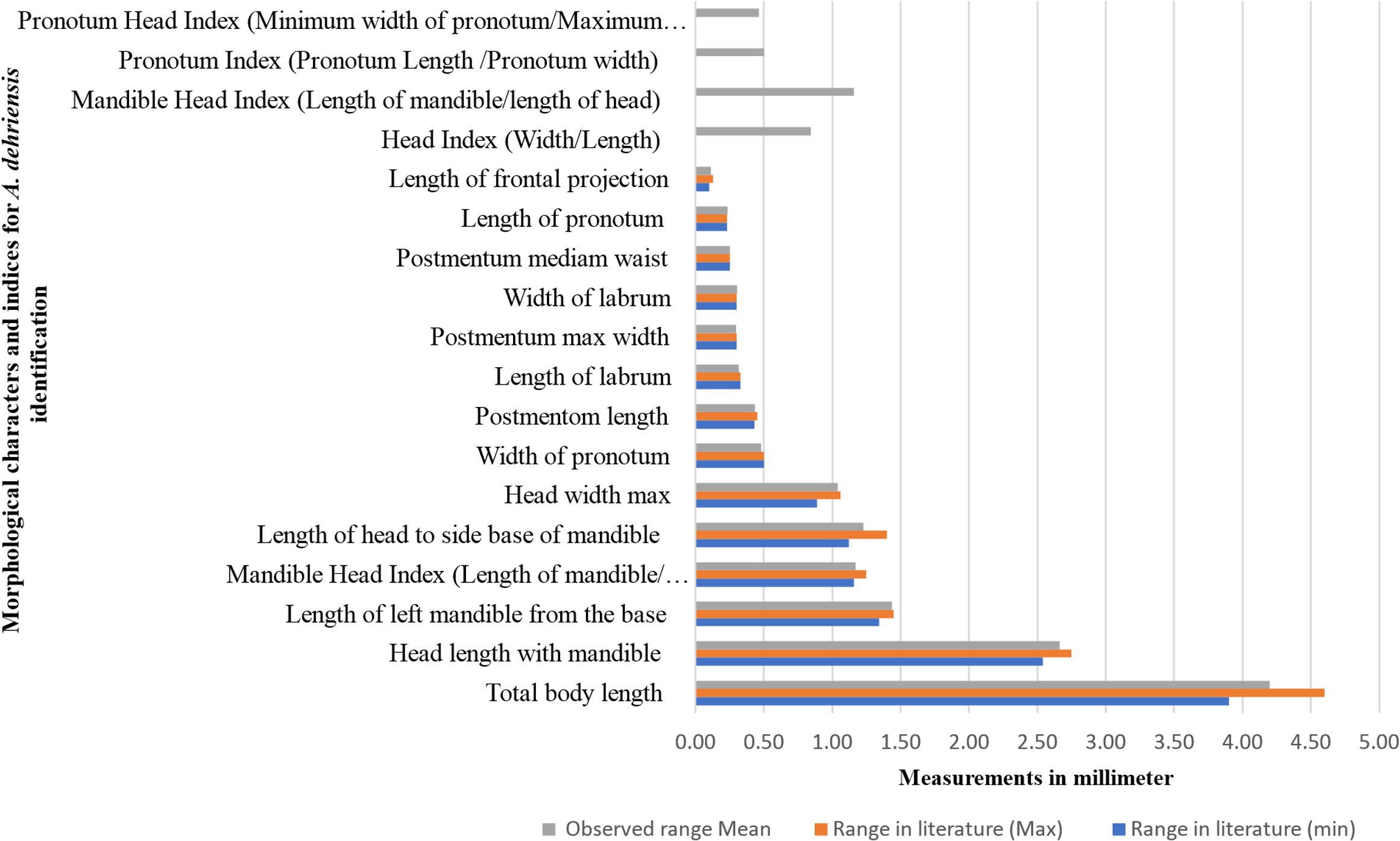
Figure 4. Morphometrics comparison of Angulitermes dehraensis with the measurement ranges available in the literature for species confirmation.
3.2 Distribution and habitat preference
Chhotani (33) Rathore and Bhattacharyya (42); and Krishna et al. (43) reported A. dehraensis from India (Indomalayan region) and Afghanistan (Palearctic region) whereas Chaudry and Ahmad (27) and Ahmad and Akhtar (44) reported it from Pirwala forest, Chattarbagh, Chinari, Hangu, Parachinar, and Bara in Pakistan (Indomalayan region).
During this study, culture collection was done from agroecosystem, forest, and urban ecosystem. However, A. dehraensis was only reported from guava orchard (agricultural ecosystem) as a new locality record for Haripur (Figure 5, Supplementary Table S3) and was found absent in structures/buildings and forests. This is a rare species which is found in the hilly areas of Pakistan.
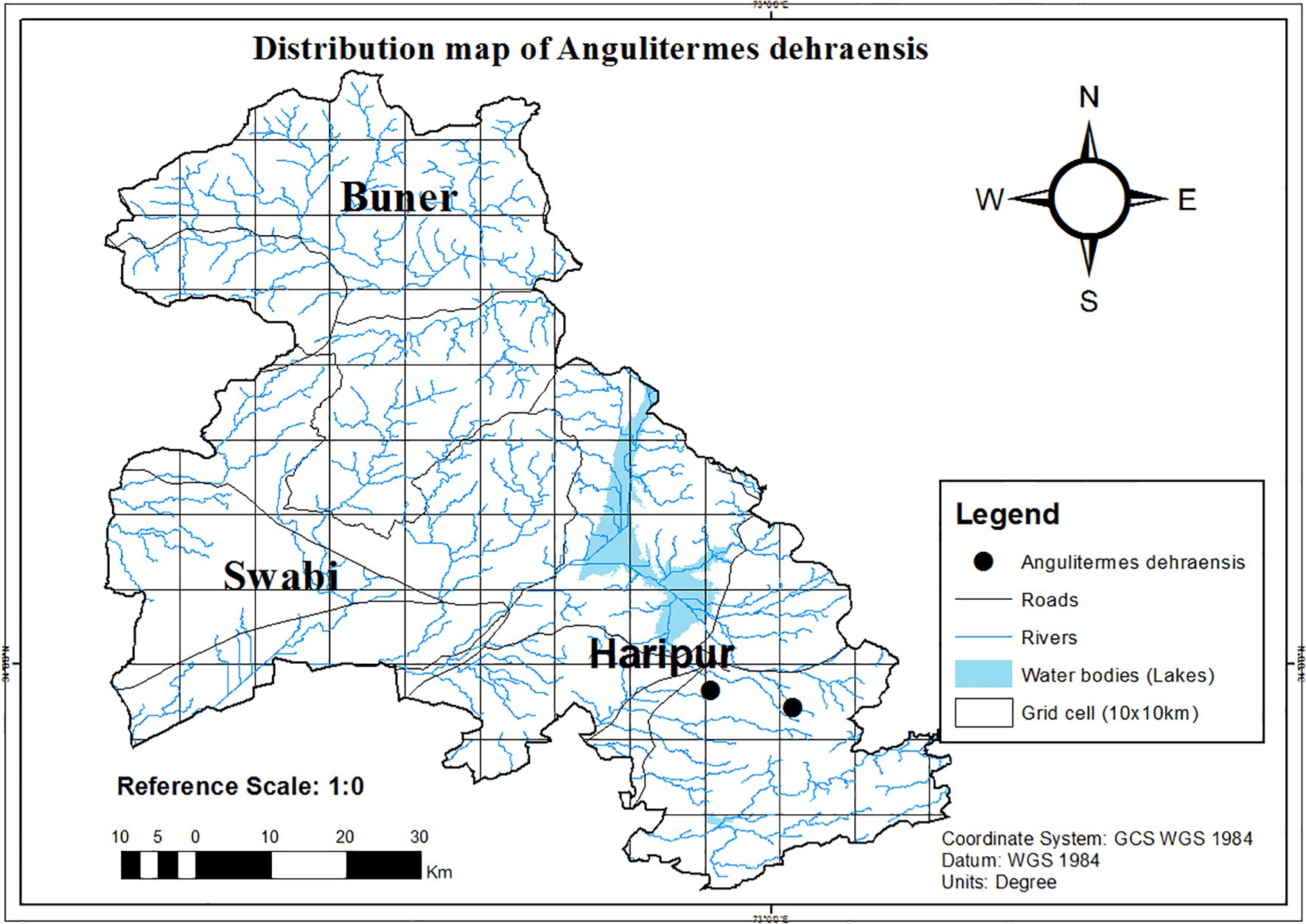
Figure 5. Distribution map of Angulitermes dehraensis in the selected study region of Khyber Pakhtunkhwa-Pakistan, a part of the Northwestern Indomalayan region.
3.3 Feeding host substrate
A. dehraensis was found feeding on the twigs, stumps, stem, date tree, and cow dung (27, 42, 44). However, in this study, it was feeding on the humus inside a hollow guava tree, making the tree survival at risk. Keeping this in view, this is a pest species of trees (dead/alive) which needs management.
3.4 DNA barcoding
For phylogenetic analysis, only top 15 sequences based on percent query covered, percent identity, bit cover, and relevant taxon match from GenBank were selected (Table 3). Then, GenBank was manually searched for the taxon/species name “Angulitermes dehraensis” and reference sequence, resulting DQ442073.1 as a relevant sequence with no reference sequence. Taxonomically, all the matched-up sequences were from four genera of family Termitidae. In the absence of a reference sequence, DQ442073.1 was used as a standard for analogy validation (Supplementary Figure S2) showing 99.02% homology with the understudy sequence.
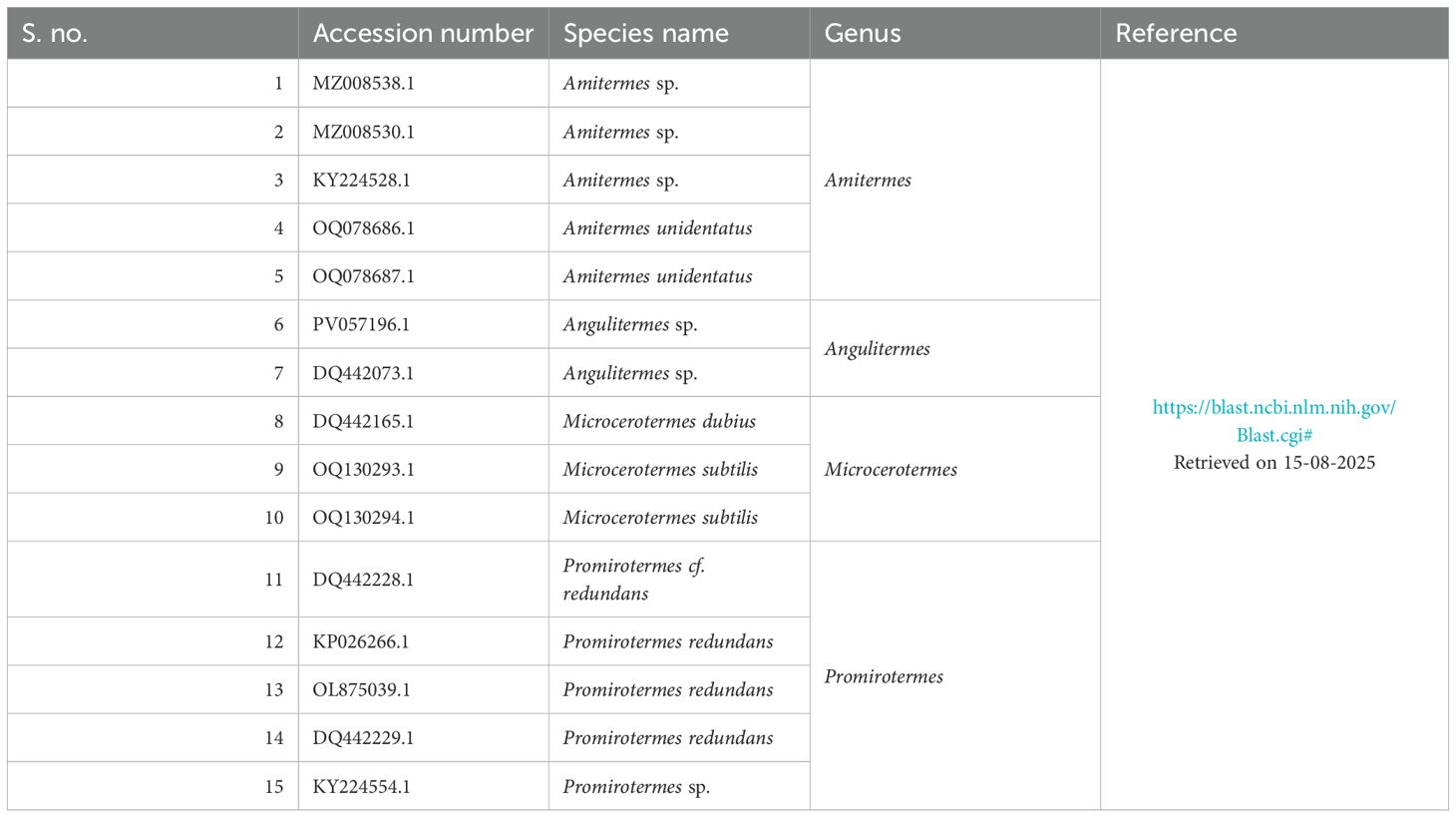
Table 3. List of the top 15 GenBank retrieved sequences for Angulitermes species analogy validation.
3.4.1 Neighbor-joining (N-J) tree
An N-J tree was constructed in MEGA (45) via the neighbor-joining method (46) and resulted into an unrooted tree consisting of two main branches. Branch 1 consists of 11 sequences, which is further branched for the sister species of the genera Angulitermes, Promirotermes, and Amitermes, whereas branch 2 consists of five sequences for the remaining sister species of the genera Angulitermes and Microcerotermes, which is further branched. Subbranch 1 shows the understudy sequence denoted by “●” with DQ442073.1, whereas subbranch 2 shows the remaining three sequences as shown in Figure 6 (details on analysis are available at supplementary data N-J tree).
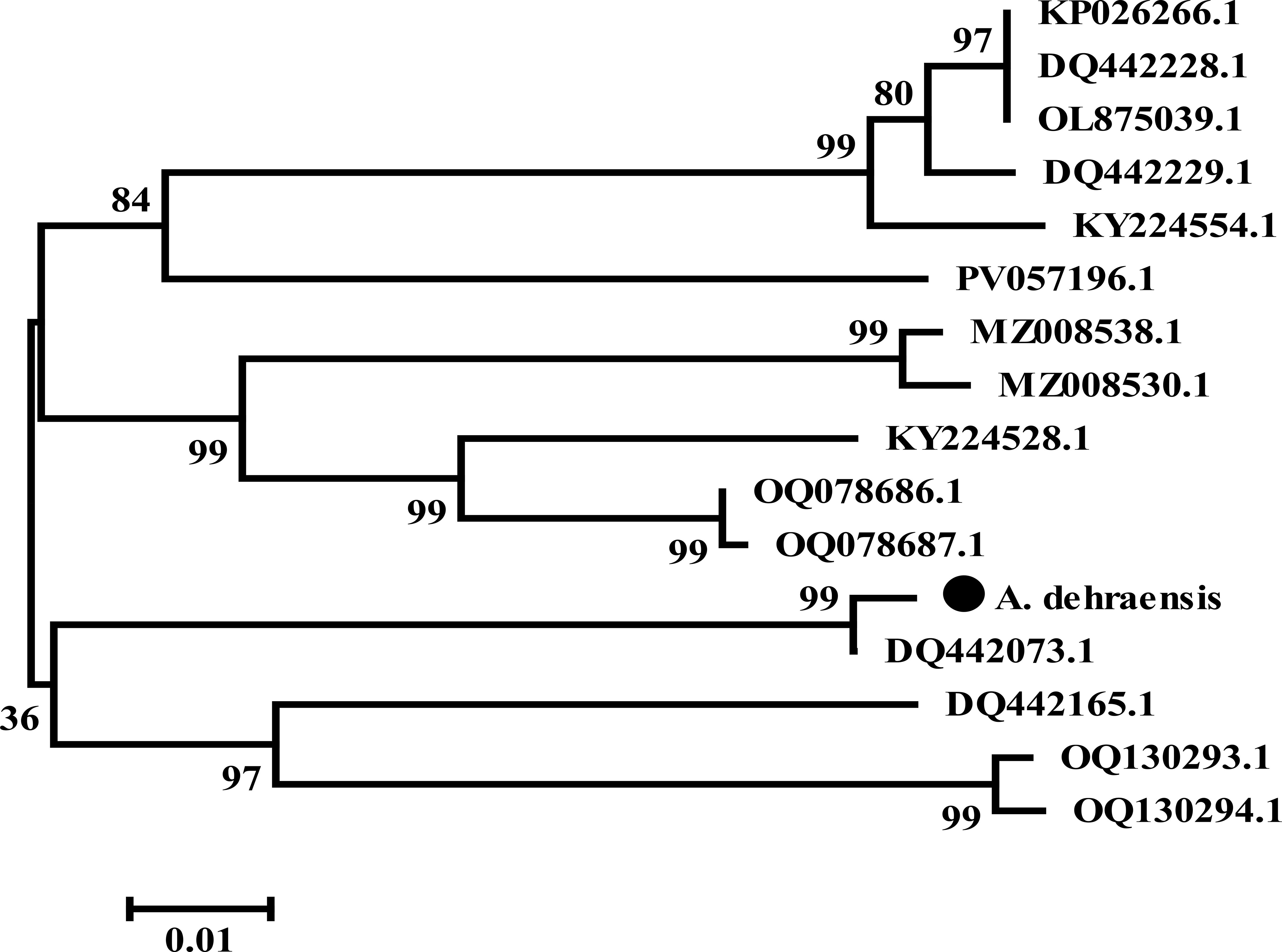
Figure 6. Unrooted neighbor-joining tree of Angulitermes dehraensis collected from the selected studied area of Khyber-Pakhtunkhwa, Pakistan, a part of the northwestern Indomalaya region. (● = under study sequence).
3.4.2 Maximum likelihood (M-L) tree
An M-L tree was constructed in MEGA (45) via the Tamura-Nei model (47) and resulted into an unrooted tree consisting of two main branches. Main branch 1 consists of 13 sequences of genera Angulitermes, Promirotermes, and Amitermes with two subbranches. Subbranch 1 is further divided into two more smaller branches; one contains eight accessions of the sister species along with the understudy sequence denoted by “●” with DQ442073.1, whereas the other contains the remaining five accessions. Main branch 2 represents the three sequences of genera Microcerotermes as shown in the Figure 7 (details on analysis are available at supplementary data M-L tree).
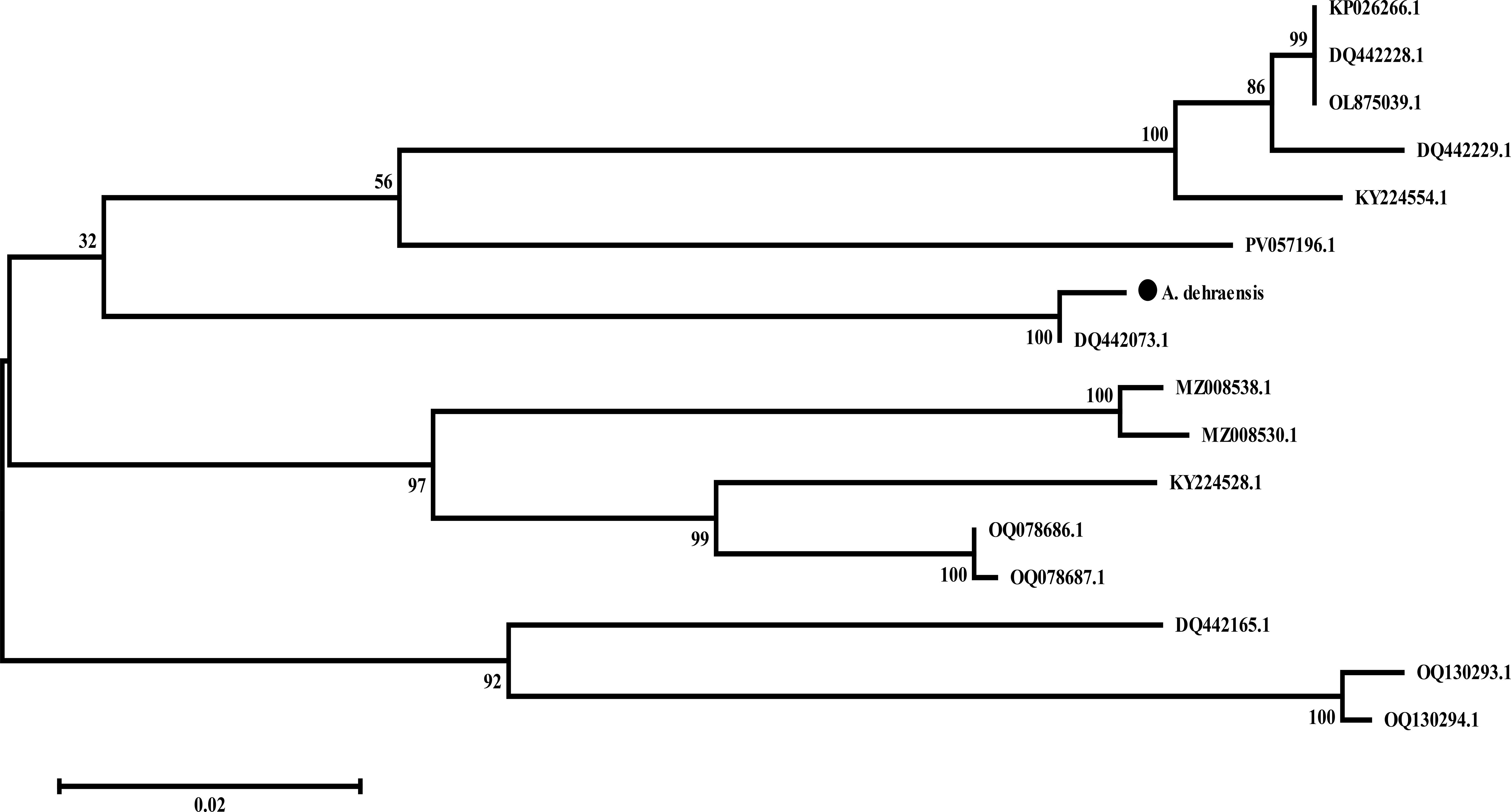
Figure 7. Unrooted maximum-likelihood tree of Angulitermes dehraensis collected from the selected studied area of Khyber-Pakhtunkhwa-Pakistan, a part of the Northwestern Indomalayan region. (● = under study sequence).
Hebert et al. (48), Austen et al. (49), Firouzabadi et al. (50), and Adetitun (51) stated that there could be genetic diversity of 0.0%–0.51% among intraspecies whereas 97% among interspecies. In this study, the understudy sequence matched 99.02% (Supplementary Figure S2) with sequence DQ442073.1 (Angulitermes sp.), which is an incomplete taxon. Divergence of 0.98% with the understudy sequence is greater than the range 0.0%–0.51%, which indicates the novelty of understudy sequence, also supported by N-J and M-L tree analyses as shown in Figures 6, 7. The reference sequence curated by NCBI is absent for the genus Angulitermes, making it a poorly recorded taxon. Clustering of sequence PV057196.1 (Angulitermes sp.) with other sister species and homology of 88% (Supplementary Figure S3) with the understudy sequence reveals wrong taxon naming.
4 Remark on specie status
A. dehraensis is a valid species and reported by several researchers including Chaudry and Ahmad (27), and Ahmad and Akhtar (44) for Indomalaya and Palearctic regions. It is a rare species which was collected from two localities of a sub-hilly terrain in a 3-year study. In this study, it was found feeding on the humus in a hollow guava tree trunk sharing the living space with the Odontotermes obesus species (33, 36). Based on the available and collected data of various feeding host substrate in horticultural ecosystems of the Indomalayan region, it is declared as a pest species of termites (43, Vol. 1, p. 145), which needs management.
Genus Angulitermes is poorly recorded in GenBank with no COII sequence data for A. dehraensis or any other species of the genus. Thus, a novel COII sequence of A. dehraensis is submitted to GenBank for accession number. It will provide a baseline for molecular studies on this genus. Integrated Termite Management (7) practices are recommended for the control of this pest species in the orchards.
4.1 Data availability and specimen deposition
In response to submitted sequence of A. dehraensis, accession number PX423737 was received from the GenBank and specimens are submitted to Insect’s Museum at the Department of Entomology, The University of Agriculture, Peshawar, Pakistan.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The animal study was approved by Offices of Research, Innovation and Commercialization, University of Agriculture, Peshawar, Pakistan. The study was conducted in accordance with the local legislation and institutional requirements. Experimental research and field studies on insects, including the collection of insects, complied with relevant institutional, national, and international guidelines and legislation. A prior approval under letter no. F. no. (3)/AUP/Ento/2019/201 was undertaken from the Offices of Research, Innovation and Commercialization (ORIC), The University of Agriculture, Peshawar, Pakistan. We provide confirmation that during collection and execution of the experiment, the authors have complied with the IUCN (https://iucn.org/) Statement on “Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora". All methods were performed in accordance with the relevant guidelines and regulations of scientific justification, human euthanasia, and ethical review.
Author contributions
XL: Conceptualization, Funding acquisition, Writing – review & editing. XZ: Funding acquisition, Writing – review & editing. RA: Resources, Writing – review & editing. MZ: Conceptualization, Software, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Ordos Municipal Key Research and Development Program Project (YF20240042) and Inner Mongolia higher education institutions to strengthen the construction of important ecological security barrier in northern China (STAQZX202312) supported this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/finsc.2025.1695789/full#supplementary-material
References
1. Hickey CD. Effect of disodium octaborate tetrahydrate in ethylene glycol on consumption and mortality of eastern subterranean termites. University of Florida, Florida (2006). p. 57.
2. Khan IA, Zaman M, Akbar R, Ali I, Alam M, Saeed M, et al. Efficacy of single and mixed particles sand size as physical barrier against Heterotermes indicola under laboratory conditions. JEZS. (2015) 3:106–9.
3. Jones DT, Robert HJV, and Eggleton P. Methods for sampling termites. In: Leather SR, editor. Insect Sampling in Forest Ecosystems. Blackwell Science Ltd, United Kingdom (2005). p. 222.
4. Ahmed BM and French JRJ. An overview of termites control methods in Australia and their links to aspects of termite biology and ecology. Pak Entomol. (2008) 30:101–18.
5. Kirton LG. (2005). The importance of accurate termite taxonomy in the broader perspective of termite management, in: Proceedings of the Fifth International Conference on Urban Pest, Perniagaan Ph’ng, Malaysia. 10–13 July 2005. pp. 1–7.
6. Eggleton P. Global patterns of termite distribution. In: Abe T, Bignell DE, and Higashi M, editors. Termites: Evolution, Sociality, Symbiosis, Ecology. Kluwer Academic Publishers, Dordrecht (2000). p. 25–51.
7. Ahmad F, Fouad H, Liang S, Hu Y, and Mo J. Termites and Chinese agricultural system: applications and advances in integrated termite management and chemical control. Insect Sci. (2021) 28:2–20. doi: 10.1111/1744-7917.12726
8. Govorushko S. Economic and ecological importance of termites: A global review. Entomological Sci. (2019) 22:21–35. doi: 10.1111/ens.12328
9. Lim SY and Forschler BT. Reticulitermes nelsonae, a new species of subterranean termite (Rhinotermitidae) from the Southeastern United State. Insects. (2012) 3:62–90. doi: 10.3390/insects3010062s
10. Bahder BW. Taxonomy of the soldier less termites (Isoptera: termitidae: apicotermitinae) of the Dominican republic based on morphological characters and genetic analysis of mtDNA and nuclear DNA. M. Sc. Thesis. Univ Florida. (2009), 1–95.
11. Jarman SN and Elliott NG. DNA evidence for morphological and cryptic Cenozoic speciations in the Anaspididae, ‘living fossils’ from the Triassic. J Evol Biol. (2000) 13:624–33. doi: 10.1046/j.1420-9101.2000.00207.x
12. Blaxter M and Floyd R. Molecular taxonomics for biodiversity surveys: already a reality. Trends Ecol Evol. (2003) 18:268–9. doi: 10.1016/S0169-5347(03)00102-2
13. Tautz D, Aractander P, Minelli A, Thomas RH, and Vogler AP. DNA points the way ahead in taxonomy. Nature. (2002) 418:479. doi: 10.1038/418479a
14. Tautz D, Aractander P, Minelli A, Thomas RH, and Vogler AP. A plea for DNA taxonomy. Trends Ecol Evol. (2003) 18:70–4. doi: 10.1016/S0169-5347(02)00041-1
15. Caterino MS, ChoCho S, and Sperling FAH. The current state of insect molecular systematics: a thriving tower of Babel. Ann Rev Entomol. (2000) 45:1–54. doi: 10.1146/annurev.ento.45.1.1
16. Sobti RC, Kumari M, Sharma VL, Sodhi M, Mukesh M, and Shouche Y. Sequence analysis of a few species of termites (Order: Isoptera) on the basis of partial characterization of COII gene. Mol Cell Biochem. (2009) 331:145–51. doi: 10.1007/s11010-009-0152-z
17. Leigh AN, Wallman JF, and Dowton M. Identification of forensically important Chrysomya (Diptera: Calliphoridae) species using the second ribosomal internal transcribed spacer (ITS2). Foren. Sci Int. (2008) 177:238–47. doi: 10.1016/j.forsciint.2008.01.009
18. Ben GH, Lessard J, Borregaard MK, Fritz SA, Araújo MB, Dimitrov D, et al. An update of Wallace’s zoogeographic regions of the world. Science. (2013) 339:74–8. doi: 10.1126/science.1228282
19. PARC. Agro-ecological Zones of Pakistan. Islamabad, Pakistan: Pakistan Agricultural Research Council (1980).
20. Iqbal NN and Saeed S. Toxicity of six new chemical insecticides against the termite, Microtermes mycophagus D. (Isoptera: Termitidae: Macrotermitinae). Pak J Zool. (2013) 45:709–13.
21. Zaman M, Faraz A, Azad R, Khan IA, Faheem B, Rafiq N, et al. Updating the systematic status of genus Heterotermes (Rhinotermitidae: Isoptera: Blattodea) by combining morphometric analysis, distribution mapping, and DNA barcoding approaches. Ecol Evol. (2025) 15(8):e71993. doi: 10.1002/ece3.71993
24. Holmgren K and Holmgrem N. Report on a collection of termites from India. Mem Dep. Agric India (Ent. Ser.). (1917) 5:138–71.
26. Akhtar MS. Studies on the taxonomy and zoogeography of termites of Pakistan. University of Punjab, Lahore (1972).
27. Chaudry IM and Ahmad M. Termites of Pakistan, identity, distribution and ecological relationships. Pakistan Forest Institute, Peshawar-Pakistan (1972). p. 1–72. Final technical report. Project no. A17-FS-12.
28. Nasir S. Diversity and Swarming pattern of termites of district Mianwali. Depart. Of Zoology, University of Punjab, Lahore, Pakistan (2003) p. 1–267.
29. Inamullah and Khan AA. Agro-ecological zones of Pakistan. In: Agriculture the Basics. Ikhwan Publisher, Qissa Khwani Bazar, Peshawar (2015). p. 97–9. Mohallah Jangi.
30. Sjostedt Y. Neue arten und gattungen afrikanischer termiten (vorlaufige mitteilung). Rev Zoologique Africaine (Bruxelles). (1924) 12:253–7.
31. Kaakeh W. Identification, geographical distribution and hosts of subterranean termites in the United Arab Emirates arid ecosystem. J Agric Mar Sci. (2005) 10:33–40. doi: 10.24200/jams.vol10iss1pp33-40
32. Saha N, Mazumdar PC, Basak J, Raha A, Majumder A, and Chandra K. Subterranean termite genus Odontotermes (Blattaria: Isoptera: Termitidae) from Chhattisgarh, India with its annotated checklist and revised key. J Threatened Taxa. (2016) 8:8602–10. doi: 10.11609/jott.2654.8.3.8602-8610
34. Manzoor F. Monograph on Morphometric Studies on the termite genus Odontotermes. Pakistan: Higher Education Commission (2006). p. 154.
35. ESRI. ArcGIS Desktop: Release 10. Redlands, CA: Environmental Systems Research Institute (2010).
36. Zaman M, Khan IA, Schmidt S, Murphy R, and Poulsen M. Morphometrics, distribution, and DNA barcoding: integrative identification approach to the genus odontotermes (Termitidae: blattodea) of khyber pakhtunkhwa, Pakistan. Forests;. (2022) 13:674. doi: 10.3390/f13050674
37. Trinh VH, Nguyen TH, Nguyen HY, and Huyen TT. Applying molecular technology to identify termite species of the genus Coptotermes in the Hanoi Old Quarter (2020). Available online at: http://www.prtrg.org/pdfs/S1%201%20Van%20Hanh.pdf (Accessed December 23, 2020).
38. Meiklejohn KA, Damaso N, and Robertson JM. Assessment of BOLD and GenBank – Their accuracy and reliability for the identification of biological materials. PloS One. (2019) 14:e0217084. doi: 10.1371/journal.pone.0217084
39. Thompson JD, HigginsHiggins DG, and Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. (1994) 22:4673–80. doi: 10.1093/nar/22.22.4673
40. Alotaibi N, Mashaly A, Alajmi R, Ahmed A, and Ayaad T. Genetic diversity of termites from Ta’if City, Saudi Arabia. J Environ Biol. (2019) 40:29–35. doi: 10.22438/jeb/40/1/MRN-843
41. Gardner JCM. New termitidae from India and Burma (Isoptera). Indian J Entomology. (1945) 6:103–10.
42. Rathore NS and Bhattacharyya AK. Termite (Insecta: Isoptera) Fauna of Gujarat and Rajasthan-Present state of knowledge. Rec. Zool Surv India. Occ. Paper. No. (2004) 223:1–77.
43. Krishna K, Grimaldi DA, Krishna V, and Engel MS. Neoisoptera excluding termitidae. In: Isoptera of the world, American Museum of Natural History: NY, USA. vol. 1. (2013). p. 145. 758; Vol. (4); 1141; 1196; 1233; 1246.
44. Ahmad MM and Akhtar MS. Catalogue of the termites (Isoptera) of the oriental region. Pak J Zool. (2002), 1–86.
45. Tamura K, Stecher G, Peterson D, Filipski A, and Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Bio. Evol. (2013) 30:2725–9. doi: 10.1093/molbev/mst197
46. Saitou NN and Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Bio. Evol. (1987) 4:406–25. doi: 10.1093/oxfordjournals.molbev.a040454
47. Tamura K and Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Bio. Evol. (1993) 10:512–26. doi: 10.1093/oxfordjournals.molbev.a040023
48. Hebert PDN, Cywinska A, Ball SL, and DeWaard JR. Biological identifications through DNA barcodes. Proc R Soc Lond B Biol Sci. (2003) 270:313–21. doi: 10.1098/rspb.2002.2218
49. Austen JW, Szalanski A,L, Solorzano C, Magnus R, and Rudolf SH. Mitochondrial DNA Genetic Diversity of the Dry wood Termites Incisitermes minor and I. snyderi (Isoptera: Kalotermitidae). Florida Entomologist. (2012) 95:75–81. doi: 10.1653/024.095.0112
50. Firouzabadi EA, Habibpour B, Galehdari H, and Shishehbor P. Genetic diversity and morphometric study of thirteen populations of Microcerotermes diversus (Silvestri)(Isoptera: Termitidae) in southern Iran. In: Proceedings IRG Annual Meeting (ISSN 2000-8953). IRG, sweden (2012).
51. Adetitun D. Re: What is the acceptable percentage similarity of BLAST nucleotide sequence that could suggest a novel organism? (2016). Available online at: https://www.researchgate.net/post/What_is_the_acceptable_percentage_similarity_of_BLAST_nucleotide_sequence_that_could_suggest_a_novel_organism/57070e6593553b217d3cfdf7/citation/download (Accessed December 30, 2020).
Keywords: termites, Angulitermes dehraensis, morphometrics, distribution, DNA barcoding, COII
Citation: Lv X, Zhang X, Azad R and Zaman M (2025) Biosystematics of Angulitermes dehraensis in the Northwestern Indomalayan region by integrating morphometrics and distributional data with DNA barcoding. Front. Insect Sci. 5:1695789. doi: 10.3389/finsc.2025.1695789
Received: 30 August 2025; Accepted: 24 September 2025;
Published: 24 October 2025.
Edited by:
Chenyang Cai, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Habib Ali, Khwaja Fareed University of Engineering and Information Technology (KFUEIT), PakistanKashif Kamran, University of Balochistan, Pakistan
Copyright © 2025 Lv, Zhang, Azad and Zaman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiuhua Lv, bHhoNTg1OEBzaW5hLmNvbQ==; Maid Zaman, TWFpZHphbWFuQHVvaC5lZHUucGs=
 Xiuhua Lv1*
Xiuhua Lv1* Maid Zaman
Maid Zaman