- 1Department of Infectious Diseases, Imperial College Healthcare NHS Trust, London, United Kingdom
- 2UCL Centre for Digital Public Health in Emergencies, Institute for Risk and Disaster Reduction, University College London (UCL), London, United Kingdom
- 3Wits Reproductive Health and HIV Institute, University of Witwatersrand, Johannesburg, South Africa
- 4Desmond Tutu TB Centre, Department of Paediatrics and Child Health, University of Stellenbosch, Stellenbosch, South Africa
- 5Wellcome Centre for Infectious Diseases Research in Africa, Institute of Infectious Disease and Molecular Medicine, University of Cape Town, Cape Town, South Africa
- 6Institute for Global Health, University College London, London, United Kingdom
- 7MRC Clinical Trials Unit, University College London, London, United Kingdom
Background: Drug-resistant tuberculosis (DR-TB) is a major contributor to antimicrobial resistance (AMR) globally and is projected to be responsible for up to a quarter of AMR-associated deaths in the future. Management of DR-TB is increasingly decentralised to primary healthcare settings, and simultaneously becoming more complex due to a growing range of treatment options (e.g. novel agents, shorter regimens). This is reflected in the numerous recent updates to international guidelines and as such understanding the barriers and enablers to how healthcare workers access and use guidelines is vital.
Materials and Methods: We used an established psychological framework – the theoretical domains framework (TDF) – to construct and analyse an online survey and focus groups to explore healthcare workers current use of DR-TB guidelines in South Africa. We aimed to identify barriers and enablers with which to direct future attempts at improving guideline use.
Results: There were 19 responses to the online survey and 14 participants in two focus groups. 28% used the most up-to-date national guidelines, 79% accessed guidelines primarily on electronic devices. The TDF domains of ‘Social Influences’ (mean Likert score = 4.3) and ‘Beliefs about Consequences’ (4.2) were key enablers, with healthcare workers encouraged to use guidelines and also recognising the value in doing so. ‘Environmental Resources’ (3.7) and ‘Knowledge’ (3.3) were key barriers with limited, or variable access to guidelines and lack of confidence using them being notable issues. This was most noted for certain subgroups: children, HIV co-infected, pregnant women (2.7).
Discussion: Current use of DR-TB guidelines in South Africa is suboptimal. Planned interventions should focus on overcoming the identified key barriers and might include an increased use of digital tools.
Introduction
Globally, in 2018 there were approximately 500,000 new cases of drug-resistant tuberculosis (DR-TB). Only a third of these were estimated to have been enrolled in treatment and treatment success rates remain low at 56% (1). DR-TB is anticipated to remain a significant contributor to antimicrobial resistance (AMR), causing up to a 25% of AMR associated deaths in the future (2).
DR-TB management is becoming increasingly decentralised, a model of care particularly relevant to high burden countries, such as South Africa, where the number of decentralised centres has increased from 17 in 2011 to 658 in 2019, and is now available in the majority of sub-districts (3). Decentralised care has been shown to improve patient outcomes (4, 5), and to reduce costs (6), however there are challenges. These surround patient expectations (7) and the different skillset of the non-expert provider (8), resulting in the need for high level support (9), training (10) and improved provider-provider communications (11).
Simultaneously, there has been a substantial increase in the evidence base for and complexity of DR-TB management guidelines, in South Africa (12) and globally (13). Advances include the introduction of the shorter course, the removal of injectable agents, and the increasing use of bedaquiline and other novel and repurposed agents. As a result, the WHO guidelines have had 12 iterations or amendments over the last ten years and the South African guidelines have also seen numerous recent changes (14). Further modifications are expected as a number of trials are due to report results over the next 2-3 years (15). Whilst some patients are infected with an already resistant strain of tuberculosis, others acquire resistance during treatment. The latter can broadly be avoided if the correct drug regimen is prescribed and subsequently taken. Much work has focused on improving patient adherence, but the relatively recent increase in potential prescribing complexity may create an emerging role for ensuring the correct drugs are prescribed.
Understanding how best to support clinicians with DR-TB management requires a consideration of potential barriers and enablers to best practice; which should include clinician attitudes, clinical context and practice. Using theoretical frameworks developed within behavioural science, to understand influences on behaviour, mechanisms of change and thus how to inform effective development of behaviour change interventions, have widely known benefits (16, 17). The Theoretical Domains Framework (TDF) is a synthesis of 33 behaviour change theories organised into 14 (originally 12) domains (18), with proven effectiveness in identifying barriers and enablers for a wide-range of healthcare worker behaviours and healthcare implementation challenges (19–21), including application of clinical guidelines (22–24).
The TDF maps onto a broader framework - the COM-B model (25) - to help move barrier identification onto systematic design of tailored interventions to successfully reach behaviour targets. The COM-B model posits that the interaction between an individual’s Capability, Opportunity and Motivation is necessary for any given Behaviour to occur. The COM-B model forms the hub of the Behaviour Change Wheel framework; a practical tool to guide selection of functions for an intervention (e.g. training, incentivisation etc.) and policy categories for how an intervention may be implemented (e.g. through legislation, guidelines etc.). See Table 1 for a guide as to how the models map onto one another.
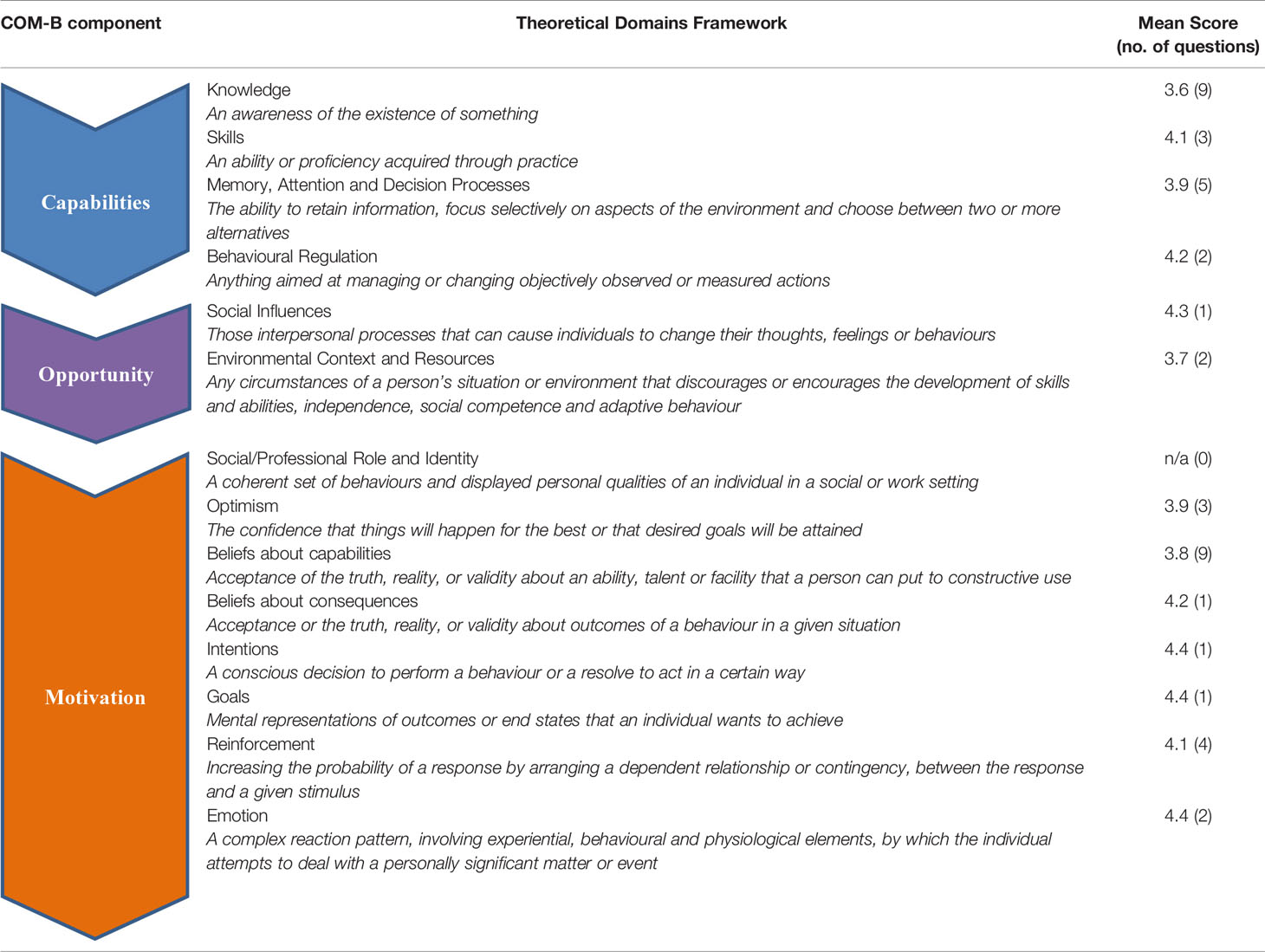
Table 1 Mean scores on Likert scale for online survey responses summarised and sorted by TDF (theoretical domain framework) domains.
Given the increasing complexity, length, and update frequency of guidelines, in the context of wider decentralised care in South Africa, the TDF provides a systematic theoretical approach to help understand challenges related to DR-TB management. Our aim therefore, was to use the TDF to explore how healthcare workers in South Africa use DR-TB guidelines in day-to-day practice, to help identify the difficulties they face, and importantly, what might facilitate more effective use. We discuss strategies for strengthening implementation of best practice guidelines using the Behaviour Change Wheel as a framework. The Consolidated Criteria for REporting Qualitative research (COREQ) checklist was used for reporting of methodology and results.
Materials and Methods
Design and Theoretical Framework
We used a mixed-methods design to ensure an adequate breadth of responses. Using both an online survey and focus groups allowed comparison and discrepancy interrogation across the qualitative and quantitative data collected. The TDF was used as an overarching framework to inform survey development, schedules for the focus groups and to guide analysis.
Participants and Recruitment
Healthcare workers (HCWs) with management responsibilities for patients with DR-TB in South Africa were invited to participate in an online survey (surveymonkey.com). An invitation was shared to South African TB communication networks and via the personal professional networks of the project team. At the end of the survey, HCWs were invited to leave their email address if they were interested in taking part in focus groups, which would seek to further explore guideline implementation challenges. There are no good estimates of how many healthcare workers are at least in part responsible for managing DR-TB in South Africa to inform the sample size calculation. We initially aimed to recruit 50 participants for the online survey and 20 for the focus groups. However, we also wanted to ensure recruitment of a diverse group of participants with respect to job role and practice setting. As such we determined that if despite repeated attempts at encouraging participation we would stop recruitment despite sample size not being reached, if participation appeared saturated and there was sufficient participant heterogeneity as determined by the researchers.
Procedure
Online Survey
The invitation to the online survey contained a URL link which took participants directly to information about the research aims, and a consent statement which participants needed to complete before moving onto the survey. The survey was open between 16 Jan 2020 and 22 Feb 2020. Participants were asked to respond to each item and, where appropriate, rate items using a Likert scale: 1 (strongly disagree) to 5 (strongly agree).
Focus Groups
Two focus groups were hosted in non-healthcare locations, one rural and one urban, in two provinces in South Africa. Carried out in February 2020, each lasted approximately two hours and was led by one of the researchers (HE), whose role was clearly explained at the beginning of each session. HE led in a minimal way in order to encourage honest and open responses and to capture group norms. Groups were audio recorded following consent, and the researchers made field notes. Participants initially sat in a circle and later broke out into smaller groups to facilitate more in-depth discussions. In order to thank them for their time, light refreshments were provided.
Materials
Online Survey
A sixty-question survey was designed to assess how and why HCWs used DR-TB guidelines in their practice. The survey items represented 13 domains from the TDF and were developed through a process of consultation between project leads and the senior behavioural researcher on the project (CW) with expertise in the topic and in question creation. Pilot testing or use of further validation methodology was not possible. Additional questions in the survey asked participants where they sought information about DR-TB management from, which national, regional or international guidelines they used and how they typically chose to access them (e.g. hard copy or electronic), with additional space for any other comments.
Focus Groups
The focus groups were semi-structured and designed to explore areas revealed as key barriers from the survey data. The focus group schedule asked participants about the decision-making process they went through when managing patients with DR-TB and referring to guidance. Given the increasing demand for mobile, readily accessible guidelines and best practice at the point of care, we also asked participants about their previous experience with digital technologies and smartphone apps.
Analysis
Online Survey
Data was exported from surveymonkey.com into SPSS v22.0 for analysis. Distribution of participant responses across the five-point Likert scale for each of the TDF items were visually examined, negatively worded items (e.g. ‘I am less likely to follow guidelines if I am tired’) were reversed coded and mean scores were calculated for each of the 13 domains. Items where the majority of participants rated the extremes of the scale (i.e. either strongly disagree or strongly agree) were marked as potential key barriers or enablers to guideline implementation.
Focus Groups
Audio recordings from the two focus groups were transcribed and any participant identifiable information removed. Transcripts were coded independently by two researchers (SL, CW). Coding involved identifying statements throughout the transcript and assigning them as either barriers or enablers to guideline implementation. Statements were then grouped according to TDF domains. In instances when statements were considered to fit into more than one domain, they were included under both/all domains. Coding continued until all statements had been assigned. Consensus about assignment of statements to barriers and enablers, and to TDF domains was reached through a process of discussion between coders. There were no instances where consensus could not be reached.
Ethics
The project was funded by a Global Challenges Research Fund grant from University College London (UCL). The funder did not play any role in design, analysis or write-up of the project. This project involved an anonymous, survey with no questions on sensitive topics and focus groups with healthcare workers considered to be “non-vulnerable” participants. Additionally all recruitment and project procedures were conducted outside of healthcare or institutional settings. Therefore, in line with guidance from the UCL ethics committee, specific ethics approval was not required or sought.
Results
A total of 19 HCWs completed the online survey in full; two partially completed it. Responses came from five provinces across South Africa (Western Cape = 13, Eastern Cape = 5, Guateng = 1, Freestate = 1, KwaZulu-Natal = 1). 38% were 25-34 years old, 28% were 35-44 years old and 34% were >45 years old. 76% were female. The majority (62%) were medical officers (physicians without post-graduate specialist training) working in decentralised settings, with a median of six years’ experience managing patients with DR-TB. Eighty percent of participants reported making treatment decisions on a frequent (e.g. often, but not daily) or very frequent (e.g. daily) basis. Twenty-one percent reported making decisions on a frequent/very frequent basis for paediatric patients. Further demographics of the online survey respondents are available (Appendix 1). A total of 14 HCWs (71% female), eight (57%) of whom were medical officers, participated in two focus groups.
The online survey first asked participants about how they got information to guide their management of patients with DR-TB. When asked where they most often chose to get information, the most popular response was the National Department of Health (NDoH) guidelines (70%). However, few (28%) reported using only the most up-to-date national guidelines (November 2019), with the remainder reporting using out-of-date editions (2018 or earlier), regional or other guidelines. The majority (79%) reported accessing these guidelines on electronic sources (i.e. viewable on computer or mobile device). The next two most popular responses participants selected were ‘I speak face to face with local TB experts’ and ‘I seek information via a social messaging group’.
The online survey next asked participants about their use of the current national DR-TB guidelines. These results have been supplemented with data from the focus groups and sorted into TDF and COM-B domains for both key barriers and enablers to guideline use. These are shown in Table 1.
Key Barriers to Implementation of DR-TB Management Guidelines
Capability
Survey data indicated ‘Knowledge’ as a key barrier to implementation, with the majority of participants selecting a mid-point score on the rating scale for the associated domain items (mean score on Likert scale = 3.6). Whilst they reported finding it easy to find relevant information in the guidelines when they were initiating, monitoring or stopping treatment, they were less confident managing complications (3.3), vulnerable populations (2.7), and HIV co-infected patients (2.7). Overall, less than a third felt confident in finding and applying the guidelines to children, pregnant women or patients with HIV co-infection. A similar theme emerged from the focus groups, mostly with respect to managing children.
“starting kids on treatment is difficult, as there are no formulations for the kids, and doses have to be extensively calculated and tablets need splitting.” - Medical Officer
‘Memory, Attention and Decision Processes’ was also identified as a barrier (3.9) with 74% of survey respondents finding the guidelines too long, and focus group participants reporting difficulties memorizing and recalling the more complex areas of the guidelines. Although few in the survey reported not having enough time to use the guidelines effectively, this was a concern raised in the focus groups:
“… Also, nursing staff don’t always have time to go through the guidelines as there are time constraints because of the caseload and not enough staff to manage/monitor and counsel these patients.” - Nurse
Opportunity
Another key barrier indicated through the survey data was ‘Environmental Context and Resources’ (3.7). There were a number of examples of this, including the rapid rotation of doctors through clinics, resulting in limited time to gain the necessary experience to become independent:
“…high turn-over of doctors, no chance to get expertise” - Medical Officer
This resulted in a reliance on provider-provider communications, which some found time-consuming, also noting potential concerns regarding patient confidentiality. Focus group participants noted that access to hard copies of the guidelines was limited and a significant proportion of survey respondents felt they were not made aware of the importance of following the guidelines by senior colleagues. When participants did report that photocopies were available, they were sometimes incomplete or out of date, and whilst some reported steps had been taken to improve access, including using electronic versions, this appeared to be patchy in uptake:
“…yes, it’s a challenge – there are no hard copies lying around, pages are missing, they are old…” - Medical Officer
Motivation
A further barrier identified was ‘Beliefs about Capabilities’ (mean score = 3.8). Whilst this was mostly explained by respondents’ beliefs about using the guidelines for previously mentioned specific scenarios (e.g. children, pregnant women), there were also concerns that in many clinics not all patients were managed in-line with the guidelines.
Key Enablers to Implementation of DR-TB Management Guidelines
Capability
A key enabler for guideline use was ‘Skills’ (mean score = 4.1), with almost all comfortable that they have the skill-set to effectively use the guidelines and the majority having had training to this purpose.
Opportunity
Another enabler realised from the survey data was ‘Social Influences’ (mean score = 4.3) with the vast majority of respondents feeling that other team members encouraged them to follow guidelines and that with the exception of a few more senior colleagues, most colleagues whom they respected considered the guidelines to be a good idea:
“… was always told – you have to read the guidelines.” - Medical Officer
Motivation
There were a number of key enablers noted within this category. This included ‘Intentions’ (4.4), ‘Goals’ (4.4) and ‘Beliefs about Consequences’ (4.2), with 95% intending to use the guidelines in their practice, with the belief that if followed patients would have improved health outcomes. This was echoed in focus groups with participants consistently stating they felt guidelines improved patient care, something intrinsically tied in with ‘Social/Professional Role’ and ‘Identity’.
When asked to consider what would most likely support decision making in the future, 95% of respondents cited they would find a combination of a smartphone app and an e-learning tool most helpful:
“I think most helpful would be a smartphone app… …A dedicated app should probably only be undertaken if it works well and is aesthetically (relatively) pleasing, otherwise in my opinion, it might feel more frustrating than helpful!” - Specialist Registrar
Discussion
To our knowledge this is the first study to specifically investigate provider attitudes to use of guidelines for DR-TB. This study shows a range of barriers and enablers that may influence use of DR-TB guidelines amongst HCWs in South Africa. It is expected that non-expert providers rely more on guidelines, and so in the current context of increasingly decentralised care, recognising and understanding what influences guideline use is vital for enabling improvement.
Our online survey and focus groups identified a number of significant enablers to guideline use. The most substantial were attributed to the ‘Social Influences’ and ‘Beliefs about Consequences’ domains, mapping respectively onto Opportunity and Motivation in the COM-B model. The participants clearly recognised the importance of using guidelines both as part of their role, but also to facilitate good clinical care and appeared to generally be working in contexts where use of guidelines was encouraged. Recognition and celebration of these enablers should ensure that they continue to support appropriate guideline use.
However, we’ve found evidence that HCWs information seeking behaviours are at times sub-optimal (e.g. using out of date guidance or social messaging groups). Potential barriers contributing to this identified in our study were the domains of ‘Knowledge’ and ‘Environmental Context and Resources’, mapping onto Capability and Opportunity in the COM-B model. Our survey showed that despite significant experience managing patients with DR-TB there are still a number of areas where HCWs lack confidence, particularly centred on applying the guidelines to children, pregnant women and patients with HIV co-infection. Given the prevalence and vulnerability of the aforementioned groups, this is of concern.
Efforts to improve guideline use might attend first to ensuring guidelines are available for use; in the context of rapid guideline update and increasing number of decentralised clinics, the focus of this effort should be on provision of electronic versions. This would need to be coupled with appropriate teaching and support. The management of children, pregnant women and HIV co-infection should be specifically focused on. Given the increasing complexity of DR-TB guidelines and the growing use of non-expert care providers, the use of clinical decision support should be considered. This is vital as a decentralised workforce will by default have other clinical priorities, and adherence to national guidelines for management of DR-TB is central to maintaining good clinical outcomes.
A notable outcome from our study is the importance and potential role for digital health, with many participants already using their phones both to access guidelines, and to get advice from others. The use of digital health in TB has been reviewed (26) and given a formal structure by the WHO (27). Clinical decision support systems (CDSS) are a subset of digital health and one of the nine digital products the WHO has suggested should align with their END TB strategy (28) and a target product profile has been developed (29). CDSS and digital tools have been shown to improve guideline adherence in asthma (30), anti-microbial prescribing (31–33), infection control (34), and maternal health (35), and can interestingly have ‘carry over’ benefits when not used anymore (36). We suggest there is a current demand and space for a digital health intervention to further improve use of the national DR-TB guidelines in South Africa. Such a tool might perform as a CDSS, with a focus on vulnerable or complex populations (e.g. HIV co-infected, paediatrics), but should also be educational. Utilising identified facilitators to guideline use, such as ‘Beliefs about Consequences’, and using co-creation and co-authorship participatory methods (37) during development of such a tool might help to maximise end-user uptake. We could not find any mHealth applications or CDSS that supported regimen decision making for DR-TB beyond creating an electronic version of existing guidelines. This is possibly because up until recently regimen creation has been relatively straightforward, with complex cases being managed by a centralised expert committee. In order for a mHealth application to progress beyond pilot phase and change user behaviours, it should be well designed in a user-centred manner (38), utilise user engagement techniques (39), and be grounded in behavioural change theory (40).
The strengths of our study include the mixed methods used, which were designed and analysed using an appropriate theoretical framework. This has resulted in a broad, nuanced and structured dataset. We acknowledge that there are limitations to this work. In particular with regard to the sample size of the online survey, and the use of convenience sampling for the focus groups meaning they may not be a true or complete representation. In general, participants of our survey frequently managed patients with DR-TB, but despite this we were able to identify a number of barriers to the use of guidelines. It is possible, therefore, that in those with less experience, challenges may be more pronounced. Another limitation is the inability to analyse our results by profession as only 14% of participants were nurses. This is relevant as nurse-led, decentralised management of DR-TB is expanding. Finally, whilst the survey and the focus groups were conducted in both urban and rural setting across multiple provinces in South Africa, our findings may not be generalisable to other countries.
Participants expressed an interest in smartphone apps and e-learning resources to support decision making. In South Africa, guidelines are updated frequently as new evidence becomes available, which paradoxically may contribute to challenges associated with adherence. Our survey was conducted three months after the change in guidelines but 28% reported using only the most up-to-date version. Mobile technologies are fast becoming a key part of the healthcare professional’s toolkit and could significantly increase access to and accurate use of the latest guidelines at the point-of-care (41). Such tools could provide a valuable platform for training, easily accessible at a time most convenient for the user and capable of upskilling the healthcare workforce at scale.
We have identified that DR-TB guideline use in South Africa may currently be suboptimal, and that there are a number of barriers and enablers present. Given the increasing complexity of DR-TB management and current decentralised model of care, this needs to be urgently addressed. In line with WHO suggestions, a digital health intervention may be an effective solution.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
AG, CW, and HE prepared the manuscript, with all other authors reviewing and agreeing for its submission for publication. All authors contributed to the article and approved the submitted version.
Funding
Funding for the study was through a University College London (UCL) Global Challenges Research Fund (GCRF) award from UKRI with additional support through a James Maxwell Grant Prophit Fellowship from the Royal College of Physicians (HE).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank all the respondents and participants for their participation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2021.645933/full#supplementary-material
References
1. World Health Organization. Global tuberculosis report 2019. Geneva: World Health Organization (2019).
2. O’Neill J. Tackling drug-resistant infections globally: final report and recommendations . Available at: https://apo.org.au/node/63983 (Accessed 22 Dec 2020).
3. South African Department of Health. Multi-drug resistant tuberculosis: A policy framework on decentralised and deinstitutionalised management for South Africa. Pretoria, South Africa: Department of Health (2019).
4. Cox H, Hughes J, Daniels J, Azevedo V, McDermid C, Poolman M, et al. Community-based treatment of drug-resistant tuberculosis in Khayelitsha, South Africa. Int J Tuberc Lung Dis (2014) 18(4):441–8. doi: 10.5588/ijtld.13.0742
5. Loveday M, Wallengren K, Voce A, Margot B, Reddy T, Master I, et al. Comparing early treatment outcomes of MDR-TB in decentralised and centralised settings in KwaZulu-Natal, South Africa. Int J Tuberc Lung Dis (2012) 16(2):209–15. doi: 10.5588/ijtld.11.0401
6. van Rensburg C, Berhanu R, Hirasen K, Evans D, Rosen S, Long L. Cost outcome analysis of decentralized care for drug-resistant tuberculosis in Johannesburg, South Africa. PLoS One (2019) 14(6):e0217820. doi: 10.1371/journal.pone.0217820
7. Kassa GM, Teferra AS, Wolde HF, Muluneh AG, Merid MW. Incidence and predictors of lost to follow-up among drug-resistant tuberculosis patients at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia: a retrospective follow-up study. BMC Infect Dis (2019) 19(1):817. doi: 10.1186/s12879-019-4447-8
8. Nansera D, Bajunirwe F, Kabakyenga J, Asiimwe PKJ, Mayanja-Kizza H. Opportunities and barriers for implementation of integrated TB and HIV care in lower level health units: experiences from a rural western Ugandan district. Afr Health Sci (2010) 10(4):312–9.
9. Vanleeuw L, Atkins S, Zembe-Mkabile W, Loveday M. Provider perspectives of the introduction and implementation of care for drug-resistant tuberculosis patients in district-level facilities in South Africa: a qualitative study. BMJ Open (2020) 10(2):e032591. doi: 10.1136/bmjopen-2019-032591
10. Escott S, Walley J. Listening to those on the frontline: lessons for community-based tuberculosis programmes from a qualitative study in Swaziland. Soc Sci Med (2005) 61(8):1701–10. doi: 10.1016/j.socscimed.2005.03.040
11. Watermeyer J, Penn C, Scott M, Seabi T. Bench, bed and beyond: Communication and responsibility in decentralised tuberculosis care. Health SA (2019) 24:1208. doi: 10.4102/hsag.v24i0.1208
12. South African Department of Health. Management of rifampicin-resistant tuberculosis: a clinical reference guide. Pretoria, South Africa: Department of Health (2019).
13. World Health Organization. Consolidated guidelines on tuberculosis. Geneva: World Health Organization (2020).
14. Gray AT, Boyles T, Luedtke S, Sossen B, Birjovanu G, Kostkova P, et al. A threat to decentralised care for drug-resistant tuberculosis. Lancet Respir Med (2020) 8(10):950–2. doi: 10.1016/S2213-2600(20)30392-1
15. Lee A, Xie YL, Barry CE, Chen RY. Current and future treatments for tuberculosis. BMJ (2020) 368:m216. doi: 10.1136/bmj.m216
16. Davis R, Campbell R, Hildon Z, Hobbs L, Michie S. Theories of behaviour and behaviour change across the social and behavioural sciences: a scoping review. Health Psychol Rev (2015) 9(3):323–44. doi: 10.1080/17437199.2014.941722
17. Freedland KE. The Behavioural Medicine Research Council: Its origins, mission, and methods. Health Psychol (2019) 38(4):277–89. doi: 10.1037/hea0000731
18. Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci (2012) 7:37. doi: 10.1186/1748-5908-7-37
19. Duncan EM, Francis JJ, Johnston M, Davey P, Maxwell S, McKay GA, et al. Learning curves, taking instructions, and patient safety: using a theoretical domains framework in an interview study to investigate prescribing errors among trainee doctors. Implement Sci (2012) 7:86. doi: 10.1186/1748-5908-7-86
20. McSherry LA, Dombrowski SU, Francis JJ, Murphy J, Martin CM, O’Leary JJ, et al. ‘It’s a can of worms’: understanding primary care practitioners’ behaviours in relation to HPV using the Theoretical Domains Framework. Implement Sci (2012) 7:73. doi: 10.1186/1748-5908-7-73
21. Murphy K, O’Connor DA, Browning CJ, French SD, Michie S, Francis JJ, et al. Understanding diagnosis and management of dementia and guideline implementation in general practice: a qualitative study using the theoretical domains framework. Implement Sci (2014) 9:31. doi: 10.1186/1748-5908-9-31
22. Kredo T, Cooper S, Abrams A, Muller J, Volmink J, Atkins S. Using the behaviour change wheel to identify barriers to and potential solutions for primary care clinical guideline use in four provinces in South Africa. BMC Health Serv Res (2018) 18:965. doi: 10.1186/s12913-018-3778-2
23. Backman R, Foy R, Michael BD, Defres S, Kneen R, Solomon T. The development of an intervention to promote adherence to national guidelines for suspected viral encephalitis. Implement Sci (2015) 10:37. doi: 10.1186/s13012-015-0224-2
24. Taylor N, Lawton R, Moore S, Craig J, Slater B, Cracknell A, et al. Collaborating with front-line healthcare professionals: the clinical and cost effectiveness of a theory based approach to the implementation of a national guideline. BMC Health Serv Res (2014) 14:648. doi: 10.1186/s12913-014-0648-4
25. Michie S, van Stralen MM, West R. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implement Sci (2011) 6:42. doi: 10.1186/1748-5908-6-42
26. Lee Y, Raviglione MC, Flahault A. Use of Digital Technology to Enhance Tuberculosis Control: Scoping Review. J Med Internet Res (2020) 22(2):e15727. doi: 10.2196/15727
27. World Health Organization. Recommendations on digital interventions for health system strengthening. Geneva: World Health Organisation (2019).
28. World Health Organization. Digital health for the END TB strategy - an agenda for action. Geneva: World Health Organization (2015).
29. Pellé KG, Rambaud-Althaus C, D’Acremont V, Moran G, Sampath R, Katz Z, et al. Electronic clinical decision support algorithms incorporating point-of-care diagnostic tests in low-resource settings: a target product profile. BMJ Glob Health (2020) 5(2):e002067. doi: 10.1136/bmjgh-2019-002067
30. Kwok R, Dinh M, Dinh D, Chu M. Improving adherence to asthma clinical guidelines and discharge documentation from emergency departments: Implementation of a dynamic and integrated electronic decision support system. Emerg Med Australas (2009) 21(1):31–7. doi: 10.1111/j.1742-6723.2008.01149.x
31. Yoon CH, Ritchie SR, Duffy EJ, Thomas MG, McBride S, Read K, et al. Impact of a smartphone app on prescriber adherence to antibiotic guidelines in adult patients with community acquired pneumonia or urinary tract infections. PLoS One (2019) 14(1):e0211157. doi: 10.1371/journal.pone.0211157
32. Curtis CE, Al Bahar F, Marriott JF. The effectiveness of computerised decision support on antibiotic use in hospitals: A systematic review. PLoS One (2017) 12(8):e0183062. doi: 10.1371/journal.pone.0183062
33. Birjovanu G, Wood C, Olufemi O, Ogunsola F, Okonji P, Kpokiri E, et al. GADSA: Decision Support App for Antibiotics Prescribing in Nigeria. In: Proceedings of the 9th International Conference on Digital Public Health ACM Digital Library (2019). p. 9–10. doi: 10.1145/3357729.3357734
34. Wiseman S, Jawaheer G, Kostkova P, Madle G. Specialist Digital Libraries—National Resource for Infection Control (NRIC)—Information overload or underload? (www.nric.org.uk). Br J Infect Control (2008) 9(5):4–9. doi: 10.1177/1469044608093959
35. Mueller S, Soriano D, Boscor A, Saville N, Arjyal A, Baral S, et al. MANTRA: development and localization of a mobile educational health game targeting low literacy players in low and middle income countries. BMC Public Health (2020) 20:1171. doi: 10.1186/s12889-020-09246-8
36. Wyatt J, Spiegelhalter D. Field trials of medical decision-aids: potential problems and solutions. Proc Annu Symp Comput Appl Med Care (1991) 3–7.
37. Borda A, Molnar A, Kostkova P. Serious Games and Participatory Research in Public Health. Proc 9th Int Conf Digit Public Health (2019) 133. doi: 10.1145/3357729.3357762
38. Curtis KE, Lahiri S, Brown KE. Targeting parents for childhood weight management: Development of a theory-driven and user-centered healthy eating app. JMIR Mhealth Uhealth (2015) 3(2):e69. doi: 10.2196/mhealth.3857
39. O’Brien H, Cairns P. Why engagement matters: Cross-disciplinary perspectives of user engagement in digital media Vol. 1. Switzerland: Springer (2016) p. 1–222. doi: 10.1007/978-3-319-27446-1
40. Korpershoek YJG, Hermsen S, Schoonhoven L, Schuurmans MJ, Trappenburg JCA. User-centered design of a mobile health intervention to enhance exacerbation-related self-management in patients with chronic obstructive pulmonary disease (Copilot): Mixed methods study. J Med Internet Res (2020) 22(6):e15449. doi: 10.2196/15449
Keywords: drug-resistant tuberculosis, decentralised, theoretical domains framework (TDF), guidelines, clinical decision support (CDS)
Citation: Gray AT, Wood CE, Boyles T, Luedtke S, Birjovanu G, Hughes J, Kostkova P and Esmail H (2021) Following Guidelines for Drug-Resistant Tuberculosis: “Yes, it’s a challenge”. Front. Trop. Dis 2:645933. doi: 10.3389/fitd.2021.645933
Received: 24 December 2020; Accepted: 19 February 2021;
Published: 11 March 2021.
Edited by:
Eric Sampane-Donkor, University of Ghana, GhanaReviewed by:
Gerald Mboowa, Makerere University, UgandaDorothy Yeboah-Manu, University of Ghana, Ghana
Copyright © 2021 Gray, Wood, Boyles, Luedtke, Birjovanu, Hughes, Kostkova and Esmail. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hanif Esmail, h.esmail@ucl.ac.uk
†These authors share last authorship
 Adam T. Gray
Adam T. Gray Caroline E. Wood
Caroline E. Wood Tom Boyles3
Tom Boyles3 Patty Kostkova
Patty Kostkova Hanif Esmail
Hanif Esmail