- Noguchi Memorial Institute for Medical Research, University of Ghana, Accra, Ghana
The Noguchi Memorial Institute for Medical Research (NMIMR) is a semi-autonomous institute of the College of Health Sciences, University of Ghana, Legon. Founded in 1979, the Institute was built with a grant aid from the Government of Japan as a gift to the people of Ghana in memory of the renowned Japanese medical scientist, Dr. Hideyo Noguchi, who died from Yellow fever infection while conducting research on the disease in Ghana. The Institute has a three-pronged mandate to conduct health related research, build human capacity and provide specialized diagnostic and disease monitoring services in support of the Ghana Health Service. Over the past 40 years, the Institute has grown to be a leading biomedical research institute in the African region. It has strong and long-standing collaborations with scientists and institutions in Africa, Japan, Europe, Australia and North America on several projects on diseases of public health importance. The Institute also hosts several regional and national centres such as Regional Influenza laboratory. The Institute’s research activities are relevant to the control and prevention of infectious diseases in Ghana, particularly, HIV/AIDS, Tuberculosis, Buruli ulcer, Polio, Malaria and emerging infectious diseases. The Institute also plays a technical/advisory role to government through collaborations with disease control programmes and has since inception provided the country with needed critical evidence in support of health policy as well as laboratory diagnostic services among others. Going forward, the Institute seeks to expand and consolidate its activities in areas of antimicrobial resistance (AMR), clinical trials, genomic surveillance and academic programs and in the next 25 years, NMIMR hopes to approach every research area using the one health approach.
1 Introduction
The Noguchi Memorial Institute for Medical Research (NMIMR) is a semi-autonomous institute of the College of Health Sciences, University of Ghana, Legon. Founded in 1979, the Institute was built with a grant aid from the Government of Japan as a gift to the people of Ghana following the friendship of Prof. Kenji Honda (Fukushima Medical University, Japan) and the renowned Ghanaian Scientist and then Dean of the University of Ghana Medical School, Prof. Charles Odamtten Easmon. This was in memory of the renowned Japanese medical scientist, Dr. Hideyo Noguchi, who died from a Yellow fever infection while conducting research on Yellow Fever in Ghana. The Noguchi Memorial Institute for Medical Research is mandated to: (a) conduct research into diseases of public health importance in Ghana, (b) provide training opportunities for postgraduate students in medical research and manpower training of health professionals and (c) support public health programmes of the Ministry of Health with specialized laboratory diagnostic and monitoring services.
The Institute has grown into a centre of excellence in biomedical research in the African region, leading global health and biomedical research in Ghana. It has strong and long-standing collaborations with scientists and institutions in Africa, Japan, Europe, Australia and North America working on several projects covering diseases of public health importance. In addition to the central administration headed by the Director, The NMIMR is organized into nine scientific departments: Animal Experimentation, Bacteriology, Clinical Pathology, Electron Microscopy & Histopathology, Epidemiology, Immunology, Nutrition, Parasitology and Virology. The Institute also hosts several regional and national centres such as 1) Lymphatic Filariasis Support Centre for Africa, 2) West African Centre for International Parasite Control 3) National Influenza Centre and 4) SARS-CoV-2 Regional Africa Pathogen Genomic Initiative (APGI) Hub and National Genomic Sequencing Centre; as well as Regional Reference laboratories including:1) WHO Reference Laboratory for Polio Diagnosis 2) WHO Regional Rotavirus Reference Laboratory 3) WHO Buruli ulcer Reference Laboratory and 4) National TB Reference Laboratory.
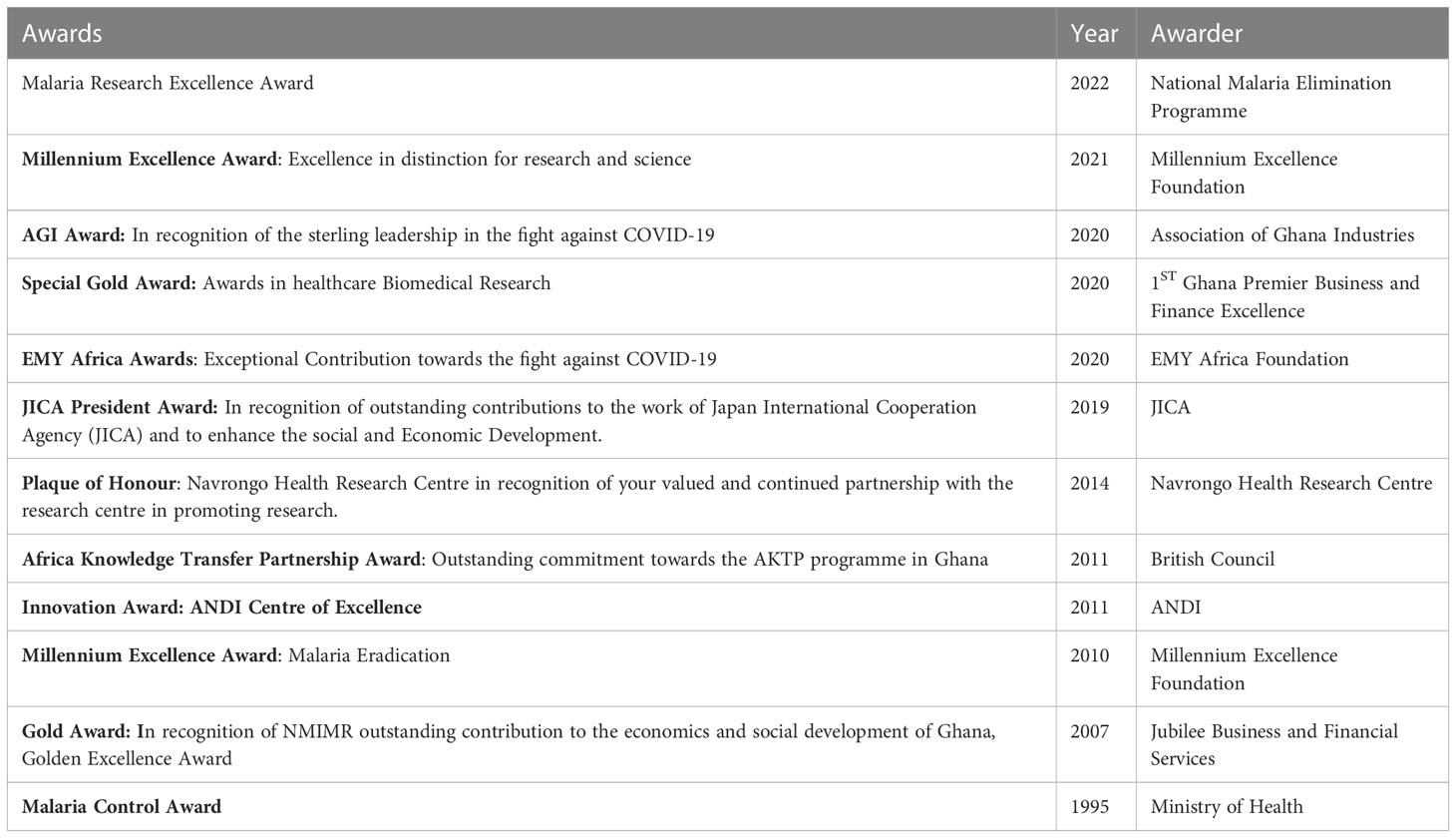
In addition, there is the Institutional Review Board (IRB) that oversees research to ensure the rights, dignity and protection of human subjects who participate in the research activities conducted by the Institute and its partners. The Institute’s research activities, although largely funded by international agencies (such as NIH, JICA, WHO, NIHR, Wellcome Trust, EDCTP, US Navy etc.), are relevant to the control and prevention of infectious diseases in Ghana, particularly, HIV/AIDS, Tuberculosis, Buruli ulcer, Polio, Malaria and emerging infectious diseases. The Institute also plays a technical/advisory role to government through collaborations with disease control programmes and has since inception provided the country with needed critical evidence in support of health policy as well as laboratory diagnostic services among others. The Table 1 below are examples of awards won by the NMIMR in recognition of its excellent activities by national and international organizations.
2 Infrastructure
The Institute is located on the University of Ghana campus at Legon, approximately 5km from the Kotoka International Airport, Accra. The Institute is a member of the College of Health Sciences, University of Ghana. The College is headed by a Provost who in turn is responsible to the Vice Chancellor of the University. The administrative head of the Institute, the Director, therefore is responsible to the Provost of the College. The Institute boasts of state-of-the art equipment and facilities for research, including Biosafety level 3 (BSL3) laboratories, animal experimentation facility, a flow cytometry core, well-equipped cell and molecular biology laboratories, a transmission electron microscope and a clinical trial facility.
2.1 Strengthening research facilities – The Noguchi advanced research laboratories
NMIMR commissioned the Noguchi Advanced Research Laboratories (NARL) in September 2018 as a donation by the Government of Japan for research into infectious diseases. It is an almost $30 million ultra-modern, 2-storey, fully furnished facility that covers a 4500 square meter space, with seating capacity for about 100 research scientists. It has about 30 biosafety level (BSL) 2 and 2 large BSL3 facilities. These include sterilization rooms, media preparation laboratory, cell culture, specimen handling (receipt, documentation and treatment), separate parasite culturing and virus isolation laboratories, serology, flow cytometry, imaging, molecular laboratory ensuring unidirectional workflow with sequencing capabilities (NGS and Sanger) and a bioinformatics laboratory. All laboratories are equipped with biosafety cabinets, autoclaves, and incubators. An equipment calibration laboratory has been set up to give technical validity to our quest to use only calibrated equipment in the Institute.
The institute also has a dedicated facility and capacity for early phase (Phases 1 and 2) clinical studies. The facility has hosted several clinical studies for vaccines against diseases such as Lassa fever, Measles, Rotavirus, Polio and COVID-19. There are on-going clinical trials for Lassa fever (Phase 1b), COVID-19 (innate and adaptive responses to COVID-19 vaccines) and Rotavirus (Phase 3) with additional three Phase 2/3 COVID-19 trials upcoming.
The Institute has a local area network and VSAT system that provides 24/7 internet connectivity and network services, and dedicated servers for data storage and archiving. Power supply is from the national grid which is backed up by four 500kVA generators that switches on automatically in the event of power outage, and also a back-up 30,000-gallon capacity water storage tank. The Institute has supplemented solar-system power supply with the capacity to supply 40% of the Institute’s daily power needs.
3 Research management at NMIMR
The Institute, in 2006, noted the growing need for an office to manage its ever-increasing research and project grants in a more professional and efficient manner. As a result, the Office for Research Support (ORS) was established that year to streamline grants application and awards, and the project implementation process. The office has skilled staff who have received international training and have the capacity to manage grants from various funders, especially US and European funders. The unit is made up of the following sub-units: finance, database management, monitoring and evaluation, research link unit, documentation and compliances.
The ORS provides pre-award administration by identifying eligible research funding opportunities and encouraging the Institute’s researchers to apply. The office assists in proposal development by providing support with budget preparation, letters of support, biosketches and other relevant administrative documents required for submission, final review and submission of proposal. Overall coordination of the grant application process is done by the Office. It also ensures that the Institute and key persons are following funding agencies’ policies and regulations. The office also effectively negotiates and recommends resolutions for discrepancies and disagreements that may arise between NMIMR and funding agencies. The office serves as a bridge between the funders and the Principal Investigators (PI) at the Institute, and addresses inquiries on behalf of the Institute.
The ORS also performs post-award functions which include setting up of project account after receiving the notice of award, review of contracts, agreements and MoUs, processing of requisitions and facilitation of communication between PI and funders, and among key persons on the project. Timely submission of technical and financial reports is facilitated through email alerts and reminders to PIs. The Office liaises with NMIMR Accounts Office on the financial management of projects and plays a significant role in account close out which includes preparing and submitting final reports to funding agencies.
These activities have yielded major successes and during the first half of 2022, the office had successfully facilitated the submission of 35 grants to agencies such as National Institute of Health (NIH), Center for Disease Control and Prevention (CDC), Bill and Melinda Gates Foundation (BMGF), Wellcome Trust, L’Initiative, and European Union (EU). Currently the Office is effectively coordinating 90 active projects including Sustaining and Improving Surveillance of human and animal influenza and other respiratory pathogens in Ghana (SHARPEN-FLU)- CDC, Capacity Building for Enhanced Research Administration (CaBERA-II) in Africa- NIH, Developing a Drug Discovery Hub in Ghana -BMGF, among others. In addition to these accomplishments, the ORS has successfully completed two Yellow Book audit by NIH and two site visits by CDC and NIH. The office also facilitated the institution of the Noguchi COVID-19 fund which awarded grants to five young fellows in the Institute to conduct research related to COVID-19 in 2021/2022.
As the Institute increases its research portfolio through active grant-writing and submission of award-winning proposals, the Office is regularly updating on emerging trends in grants management to be well-equipped in providing current and top standard services to PIs.
4 Research areas
The institute, with total staff strength of 450 including scientists, technical staff and administrative support staff, also has over 200 short-term staff, interns and National Service Personnel who support the work of the Institute. The Institute has a mandate of conducting high-quality cutting-edge research into both communicable and non-communicable diseases. Communicable disease research conducted in NMIMR includes viral diseases such as Influenza, Poliomyelitis, HIV/AIDS, SARS-CoV-2, Rotavirus, Norovirus with special attention for viral haemorrhagic fevers such as Marburg, Dengue, Lassa fever, Yellow fever and Ebola virus disease. Parasitic diseases of interest include Malaria, Toxoplasmosis, Cryptosporidiosis, Amoebiasis and neglected tropical diseases such as Helminthes, Filariasis, Leishmaniasis, Schistosomiasis, Onchocerciasis, Buruli ulcer and Trypanosomiasis. The NMIMR also carries out research into bacterial diseases such tuberculosis, diarrheal diseases, meningitis, cholera, anthrax and syphilis. There is also in-depth research into vector control of several diseases and drug discovery through plant medicine research.
There is a growing trend in non-communicable diseases in sub-Saharan Africa and the Institute is championing the research of such diseases: diabetes, cancer, mental-health challenges, mal-nutrition, cardiovascular diseases, chronic/acute kidney diseases, Hemoglobinopathies (Sickle Cell Disease etc.), allergies, snake bites and preeclampsia. Research at NMIMR is transdisciplinary covering several research themes like basic sciences (genomics, proteomics, host-pathogen interactions etc.) social sciences (Socio-cultural determinants of diseases), environmental sciences (pollution and climate change), clinical trials/studies (various clinical trials for vaccines and new drugs), surveillance studies, translational studies and animal experimentation. There are also co-morbidity studies such TB-diabetes, malaria-preeclampsia, hepatitis-malaria and malaria-sickle cell disease.
5 Medical/health research capacity in Ghana
The Institute has over the years supported the building of health research capacity in Ghana. Building capacity to conduct multidisciplinary health research is key to the realization of the Sustainable Development Goals (SDGs). Health research capacity involves the ability to define problems, set objectives and priorities, build sustainable health system institutions and organizations, and identify solutions to key national health problems.
The Institute can boast of a strong collaboration with the Ministry of Health (MOH) and the Public Health Division of the Ghana Health Service (GHS) as well as the specific program managements within the GHS, such as the National Malaria Elimination Programme (NMEP), National AIDs Control Programme (NACP), National Tuberculosis Control Programme (NTP), Non-Communicable Disease Control Programme (NCD), National Viral Hepatitis Control Programme (NVHCP), Neglected Tropical Diseases Programme (NTDs), National Buruli Ulcer Control and Yaws Eradication Programme, and the Expanded Programme on Immunization (EPI). The strong collaboration with the MOH and the GHS provides the leverage for defining general and program-specific problems and setting objectives and priorities for research to help address health challenges in Ghana.
Scientists in the Institute have developed health research capacities and are at the forefront of training the next generation graduate and post-graduate researchers in Ghana and Africa. Health research expertise acquired by Scientists are shared with undergraduate and graduate students through teaching, thesis supervision, student internships as well as post-doctoral project supervision. Training of undergraduate and graduate students cover institutions within Africa (including Ghana), Europe and the USA. Between 2012 and 2018, the Institute implemented a postdoctoral training program with support from the Bill and Melinda Gates Foundation. This was amongst the first structured postdoctoral training opportunities on the continent, with the aim to facilitate the retain of Western-trained researchers of African descent to the continent, and a total of 18 postdoctoral fellows from 8 African countries were trained on this program at the Institute.
6 The impact of NMIMR research on health delivery in Ghana and Global health
The Institute has over the years contributed to health policy in Ghana and globally. The contributions have covered mainly infectious diseases. In combating malaria, NMIMR supports the Global Health agenda by hosting several national, regional and international reference laboratories including those for Influenza, Polio, HIV drug resistance, Buruli Ulcer, Tuberculosis and diarrheal diseases caused by rotaviruses. The Institute is therefore very well placed within Ghana’s health system and works closely with almost all disease control programs under the GHS, impacting health policy and healthcare practice and delivery in Ghana. The Institute also works closely with several international bodies including the World Health Organization (WHO), the Africa Centres for Disease Control and Prevention (Africa CDC), the West African Health Organization (WAHO) and the Centre for Diseases Control and Prevention (CDC), USA, to conduct regionally relevant research, develop tools for disease monitoring and establish diagnostic capacity across the African continent.
6.1 Malaria drug efficacy studies
Scientists in the Institute were able to show the declining efficacy of chloroquine, which had been Ghana’s first-line antimalarial for treating uncomplicated malaria for decades, from 91.1% in 1989 to 38.2% in 2003 (1). In 2003, Scientists in the Institute also showed the superiority of Artemisinin-based Combination Therapies (ACTs) over monotherapy with chloroquine and sulfadoxine-pyrimethamine, which was Ghana’s second-line antimalarial for treating uncomplicated malaria (2). These two major findings necessitated the establishment of a taskforce on the review of Ghana’s antimalarial medicines policy in 2004, chaired by a Scientist from the Institute. Artemisinin-based Combination Therapies were therefore adopted in 2004 in Ghana to replace chloroquine and sulfadoxine-pyrimethamine as antimalarials for treating uncomplicated malaria in the country. The Institute continues to support the National Malaria Elimination Programme by providing continuous data on the efficacy of ACTs to inform treatment policy reviews as well as malaria case management guidelines.
6.2 Lymphatic filariasis
Research conducted at the Institute was very key in the decision by the WHO to launch the Global Programme to Eliminate Lymphatic Filariasis (GPELF) in 2000 with the aim of eliminating the disease as a public health problem by 2025 (3). Researchers at the Institute conducted research on the efficacy and safety of single-dose ivermectin (150–200 μg/kg) and albendazole (400 mg) treatment administered separately or in combination for Wuchereria bancrofti infections in 1996–1998 in a randomized double-blind placebo-controlled field trial in Ghana: 1425 individuals from 4 lymphatic filariasis-endemic villages, 340 of whom were microfilaria (mf)-positive before treatment, were randomized into 4 groups to receive albendazole alone, ivermectin alone, combination of albendazole and ivermectin, or placebo, respectively (4).
6.3 Polio surveillance towards eradication
NMIMR’s effort to implement polio surveillance in support of the polio eradication initiative started in the mid-1980s when polio cell lines and seed viruses were brought in from Germany by Dr. Mubarak Osei-Kwasi to commence tissue culture and virus isolation activities. The initial poliovirus isolates could however not be characterized as wild or Sabin as a result of a lack of appropriate reagents. This however improved in the years following, and the polio laboratory of the Institute was designated the first World Health Organization Regional Reference Polio Laboratory (WHO-RRPL) in the African region in 1992. The laboratory subsequently helped to build the Polio Laboratory Network in the Africa region from 1992-2000 and became a Centre of Excellence for training programs on the laboratory diagnosis of polio, measles and yellow fever. The Institute confirmed the first two documented and well-characterized poliovirus isolates in Ghana from the Volta and Greater Accra regions in 1995. In 1996, Ghana established an active acute flaccid paralysis (AFP) surveillance for poliovirus, to identify geographical areas of Ghana that had wild-type poliovirus transmission and refine immunization strategies to eradicate poliomyelitis. As the National Polio Laboratory, all the indigenous wild polioviruses that circulated in the country until 1999 as well as the wild imported polioviruses that circulated between 2003 and 2008 were isolated and characterized. The Polio Laboratory of the Institute has since remained the National Polio Laboratory in Ghana and continues to support polio surveillance activities.
The last wild poliovirus was detected in Ghana in 2008 and until 2018 no wild poliovirus nor vaccine-derived poliovirus was seen apart from non-polio enteroviruses (NPEVs). Through the initiative of NMIMR and funding support from the WHO Country Office, sewage/wastewater surveillance for poliovirus was piloted in the country with four sites in Greater Accra and two from the Eastern region. Following the successful pilot, wastewater surveillance was rolled out in 2018 with four additional sites; two each in the Northern and Volta regions to detect poliovirus from wastewater. In June 2019, the Polio Laboratory reported the first confirmed vaccine-derived poliovirus type 2 (VDPV2) from the environmental surveillance sites in Tamale in the Northern region, and this served as an early warning to the surveillance program. The VDPV2 was classified with the support of CDC as a circulating vaccine-derived poliovirus type 2 (cVDPV2) which was linked to a cVDPV2 that circulated in Nigeria in 2018. The virus was later found in seven of the then 12 environmental surveillance sites in the country and in several AFP cases and their contacts. The cVDPV2 outbreak in northern Ghana lasted until June 2020. The outbreak led to mass vaccination of children under 5 years with the monovalent polio vaccine type 2 (mOPV2). Two rounds of mOPV2 vaccination in 3 phases helped to interrupt the transmission. No cVDPV2 was seen until May 2022 when another VDPV2 outbreak was detected through wastewater surveillance in the Tamale Metro in Northern Ghana. This also informed two rounds of mass vaccination with the novel polio vaccine type 2 (nOPV2) and the outbreak was interrupted. The wastewater surveillance was expanded to detect SARS-CoV-2 from the environment, and SARS-CoV-2 has been found in all 14 wastewater sites in the country. From 2023, this environmental surveillance program will further be expanded to detect enteric viruses and bacteria. The Polio Laboratory at NMIMR, in its capacity as a Regional Reference Polio Laboratory, has in the past supported laboratories in other countries including Nigeria, Uganda, Benin, Togo and Sierra Leone by isolating and characterizing their polioviruses. Currently, we support polio laboratories in Benin, Ghana, Liberia, Niger, Sierra Leone and Togo to process their samples through virus isolation and characterization.
6.4 Influenza surveillance and characterization
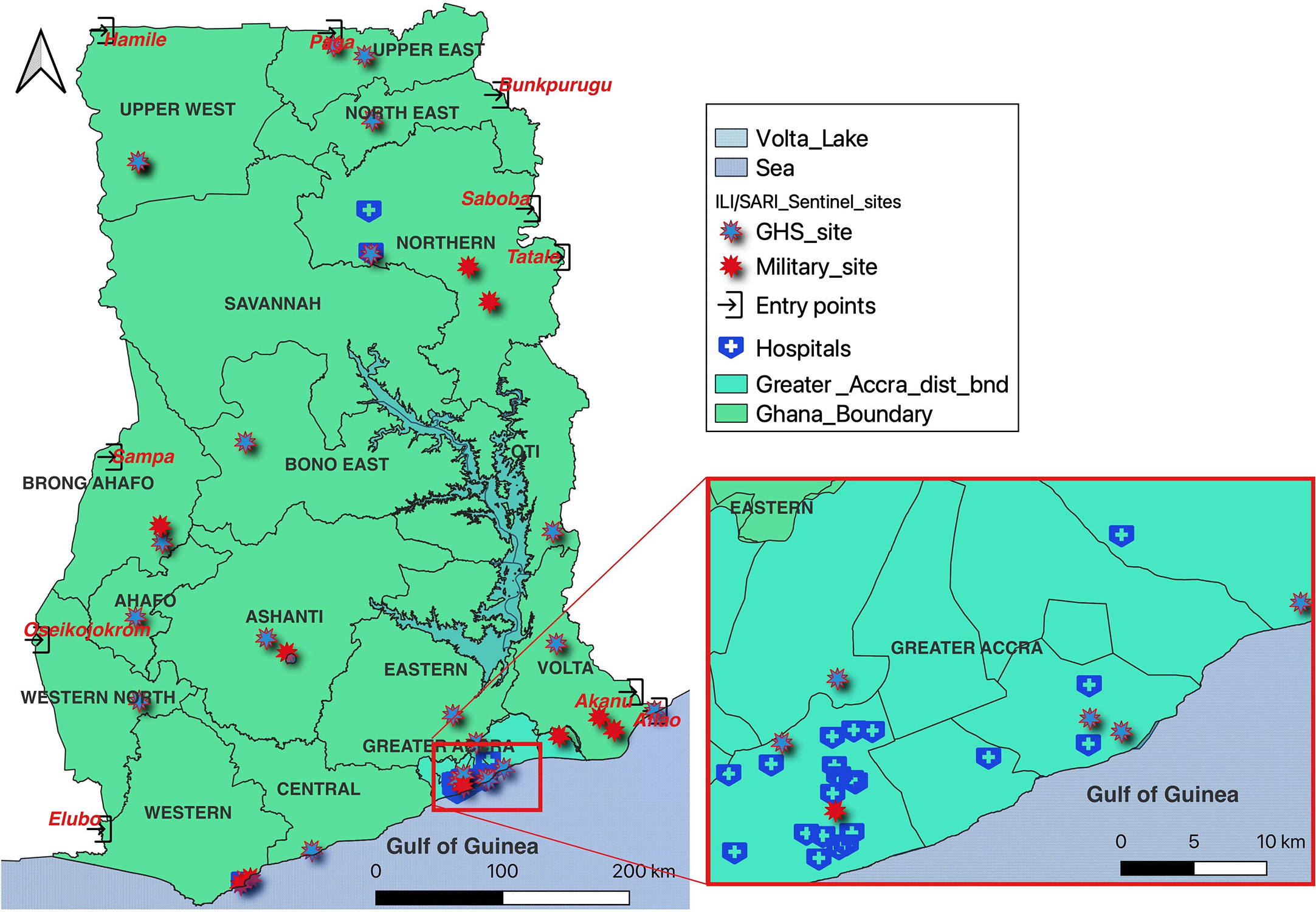
The Institute houses the only influenza laboratory in the country. The influenza laboratory was established in 2007 to detect and confirm influenza cases from both humans and birds. Following its establishment, the influenza laboratory investigated the avian flu outbreak in Akatamanso (Greater Accra region) and supported in culling of infected birds as a control measure. The laboratory was instrumental in the diagnosis of influenza cases during the influenza A H1N1 outbreak in 2009. The country currently has 35 influenza sentinel sites across all 16 regions: 24 established by Ghana Health Service and 11 by the Military (Ghana Armed Forces). Influenza samples from all the sentinel sites are brought to NMIMR for diagnosis and the results are shared with the Disease Surveillance Department of the Ghana Health Service and the facilities where the samples come from. Weekly results are also shared with the Global Influenza Surveillance and Response Systems (GISRS) platform and selected positive isolates are sent to the WHO Collaborative Centre in London, UK monthly.
The Influenza Laboratory was designated as the National Influenza Centre (NIC) in 2010. Since then, the Centre has been providing training for influenza laboratories within the sub-region. In 2011, the Centre established influenza sentinel sites in all the Military Barracks in Ghana with the support of the Naval Medical Research Unit Three (NAMRU-3) of the United States. Military staff were trained to detect influenza cases and collect samples for transport to NMIMR. An avian influenza surveillance program was also instituted to educate the troops and their families on influenza disease and how to maintain proper biosecurity measures in their backyard poultry to minimize the risk of avian influenza outbreaks. Annual review meetings are also conducted for the sites to review the work done, share good practices and find solutions for identified challenges. In 2020 during the COVID-19 pandemic, the NIC integrated SARS-CoV-2 surveillance into the existing influenza surveillance to screen all suspected influenza samples for SARS-CoV-2 (Figure 1). The NIC continues to expand by the integration of other respiratory viral diseases into its surveillance activities. The NIC is therefore advancing global health by timely diagnoses of cases received in the laboratory and sharing of results with the Ministry of Health for decision-making and intervention.
6.5 Viral hemorrhagic fever surveillance
NMIMR has established molecular testing capacity for viral hemorrhagic fevers (VHF) which includes Lassa fever, Yellow fever, Dengue fever, Chikungunya, Zika, Marburg and Ebola. With this capacity, all suspected VHF samples in Ghana and some of its neighboring countries are diagnosed at NMIMR. In 2014 during the West African Ebola outbreak, all the suspected samples from within Ghana and some West African neighboring countries were processed at NMIMR. The role played by NMIMR in the fight against Ebola encouraged the Japanese Government to build the Advanced Research Laboratories at NMIMR to be used as a center for capacity building in the West African sub-region. Since its inauguration in 2018, a post-Ebola annual training course, dubbed Third Country Training, has been organized annually at the center. An average of 12 participants are trained annually, initially from the West Africa sub-region but this has currently been expanded to include participants from the Central Africa region.
NMIMR has become the first point of call during outbreaks. About 80% of all the samples from the Yellow fever outbreak in 2021 in Ghana were tested at NMIMR. Some proportions of negative Yellow fever samples from the National Public Health and Reference Laboratory were sent to NMIMR to be investigated for VHFs. The first two Lassa fever cases were investigated and confirmed at NMIMR between 2011 and 2015. Seroprevalence research conducted at NMIMR has also shown that some individuals have had exposure to the Zika virus. In July 2022, NMIMR acted rapidly on samples from suspected VHFs to confirm the first outbreak of Marburg virus disease in the country. The report helped the Ghana Health Service to implement measures to interrupt what would otherwise have been a fatal disease outbreak. The Institute is also spearheading testing and diagnosis of the recent Monkey pox outbreak from May 2022 to date in the country.
6.6 HIV surveillance and drug resistance monitoring
The Institute continues to play a role in the surveillance of HIV infection in Ghana. NMIMR scientists were the first to diagnose HIV in Ghana in 1982 and the Institute has since served as a reference centre, supporting the monitoring of antiretroviral therapy for HIV/AIDS and as the confirmatory HIV laboratory in the country. The Institute continues to support the genomic and antigenic analysis and characterization of HIV 1 and HIV 2 strains as part of its contribution towards future vaccine development as well as drug resistance monitoring (5–9).
The Lab has optimized protocols for running samples for HIV drug resistance and subtype diversity using Sanger sequencing protocols. This has been used mainly for research purposes. Over the period, we have analyzed specimens from patients referred to us by their clinicians as failing treatment based on their clinical symptoms and viral loads. The Lab conducted the first HIVDR Threshold Survey for Ghana and other drug-resistance-related projects among pregnant women, mother-baby pairs, children, ART-Naïve and ART-experienced adults. The lab has trained a number of postgraduate students. Research activities undertaken in the lab in the past 3 years include: the impact of low level viraemia on HIV drug resistance, the emergence of HIV-1 drug resistance mutations in patients after 12 months of antiretroviral therapy and the transmitted drug resistance and circulating HIV-1 subtypes in ART-naïve HIV patients.
6.7 COVID-19 response
The NMIMR is the first institution in Ghana to set up a COVID-19 testing facility and started testing for the SARS-CoV-2 virus as early as January 2020. The Institute supports and coordinates the national COVID-19 laboratory diagnosis and has been the backbone of the fight against COVID-19 in Ghana since the report of the first case in March, 2020 (10, 11). To expand the in-country capacity for COVID-19 testing, the Institute rolled out a capacity building program with the support of the Ghana Government to set up additional COVID-19 testing centers in Ghana. Through this arrangement, institutions such as the Veterinary Services Department of the Ministry of Food and Agriculture, the Public Reference Laboratory of the Korle-Bu Teaching hospital as well as over 40 private laboratories across the country have received training and built the needed capacity for COVID-19 testing.
The Institute remains the largest single entity for COVID-19 testing and diagnosis and processes over 40% of samples collected from suspected cases by the health system in Ghana. At the peak of the pandemic, we provided 24/7 diagnostic support to the Ghana Health Service and travellers. Our sample pooling technique was shared globally and has been responsible for building capacity within both public and private sector for establishment of new COVID-19 testing centres around the country to decentralise diagnosis. The Institute also conducted an eight-week training programme in partnership with the Japan International Cooperation Agency (JICA) for health service personnel across West-African countries Nigeria, Sierra Leone, Liberia, Niger, Senegal, Guinea, La Cote d’Ivoire, Burkina Faso, Benin and Togo (12). The institute provides laboratory supports to FDA/GH to evaluate several rapid antibody and antigen-based diagnostics for COVID-19.
The NMIMR is one of the Africa CDC Pathogen Genomics Initiative Centres of Excellence responsible for Ghana, Togo, Benin, Sierra Leone and Liberia. In collaboration with the West Africa Centre for Cell Biology of Infectious Pathogens, we carried out the first in-country whole genome sequencing (WGS) analysis of SARS-CoV-2 in Ghana (13). This report presented the first most in-depth analysis of multiple SARS-CoV-2 whole genome analysis in Africa and hence, demonstrated the progress made in building local capacity for performing high quality molecular epidemiology studies. NMIMR as a sequencing hub, has sequenced more than 4,000 SARS-CoV-2 genomes from the respective countries, contributing to the global surveillance of emerging variants of concern.
The Institute hosts the WHO Regional Rotavirus Reference Laboratory (RRL) which has been providing training for clinicians and laboratorians from West African countries on rotavirus detection and characterization for the past 15 years. As a founding member of the African Rotavirus Surveillance Network, the RRL team has provided technical support and advocacy which has resulted in the roll out of surveillance programmes in more than 30 countries in Africa, establishing the country-specific burden of rotavirus-associated gastroenteritis. The RRL has successfully carried out three vaccine trials, results of which culminated in the WHO recommendation for the global adoption of rotavirus vaccines (14–25). Vaccine efficacy studies and vaccine advocacy by the RRL has resulted in a rapid roll out of rotavirus vaccine programmes in African countries (over 74% of countries, 2009 to 2020) (14–25), resulting in significant reduction in the burden of rotavirus disease on the continent (26–30). The RRL remains a key partner of the Global Paediatric Diarrhoea Surveillance Network (GRPDNet-report).
6.8 Buruli ulcer and other neglected tropical diseases
Collaborative work between NMIMR and Ghana Health Service in Buruli ulcer diagnostic and epidemiology helped in the control and reduction in the disease burden by establishing a BU diagnostic lab at NMIMR (31, 32). This together with community engagement activities (33–37) improved early case detection for prompt and appropriate diagnosis, greater understanding of disease epidemiology and gradually reduced the number of cases in the affected communities. The Institute worked with other global partners in the establishment of the first drug treatment for BU which was adopted by the WHO as the standard treatment regimen. The Institute, in addition to developing an animal model for Buruli ulcer (38, 39), has developed a point-of-care diagnostic (LAMP) (40) for early diagnosis of the disease which is undergoing a FIND-WHO evaluation. The Institute presently is a member of the WHO-recognized laboratories for confirmation of BU and provides laboratory support for the control and elimination of Leprosy and Yaws.
6.9 Tuberculosis
The NMIMR has collaborated with the National TB control programme (NTP) since 2002 in the development of laboratory manuals, training of laboratory personnel across Ghana on TB microscopy and quality assurance (41, 42) and in 2013 assisted the NTP to conduct the first nationwide TB survey (43). Having understood the genotypic distribution of members of the causative organism, Mycobacterium tuberculosis complex (MTBC) (44, 45), novel in Ghana, the Institute has performed population-based molecular epidemiological studies to identify hotspots of TB transmission (46) detect factors that drive recurrence of the disease (47) as well as identifying other predisposing factors such as ethnicity (48, 49) and diabetes (50). The results have been used by the NTP to direct control measures to help curb the disease burden. As a national reference lab for TB, the NMIMR has established a program offering specialized diagnostic services (51) to support the NTP especially in the area of drug resistance monitoring (52, 53) and have followed up over 800 difficult-to-treat cases within the last 3 years. In 2018 we detected the first case of XDR-TB (54), and currently have detected additional 2 XDRs. Within the COVID-19 pandemic, the Institute took advantage of the sputum samples used for COVID-19 diagnosis to test for TB and was able to detect 7 TB cases from suspected COVID-19 samples who were referred for management.
6.10 Antimicrobial resistance surveillance
The institute collaborates with Ghana Health Service/Ministry of Health as well as international organisations such as JICA, WHO, CDC to equip laboratory staff (locally and within the West-African region) with skills for correct identification and standard antimicrobial susceptibility testing (AST) of commonly isolated bacteria species. In a recent training, we collaborated with African Society for Laboratory Medicine (ASLM) to organise the Qualifying the workforce on antimicrobial resistance surveillance in Africa and Asia (Qwars) through theoretical and hands-on training sessions for microbiology laboratory staff from Ghana Health Service and the Veterinary Services Directorate (55).
7 The future outlook of NMIMR
As per its mandate, the Institute has contributed immensely to the understanding of infectious diseases of public health importance to Ghana and the sub-region. The Institute has contributed immensely to training of research personnel within academia and the needed middle-level manpower for health delivery institutions, as well as providing high-end laboratory services to support health care delivery within Ghana and West-Africa. The dynamics of the day, including climate change, human migration, deforestation, emergence of new infections, re-emergence and presentation of old infections with new challenges such as anti-microbial resistance, including drug-resistant tuberculosis, the resurgence of non-communicable diseases such as diabetes, which also affects the epidemiology of some infectious diseases (e.g. interaction between TB and diabetes), requires that the Institute repositions itself by developing new research programs and expanding some of its ongoing research activities into holistic programs.
Going forward, areas that require expansion and consolidation will include One Health, AMR, clinical trials, genomic surveillance and academic programs. One health research is necessary for the full control of diseases through detection, preparedness, response and management (56). One health includes a focus on zoonotic and vector-borne diseases, antimicrobial resistance, food safety, food security, environmental contamination, and other health threats. Interest in zoonotic diseases has increased among scientists in the Institute and studies investigating the impact of the ecosystem have been initiated. In the next 25 years, NMIMR hopes to approach every research area using the one health approach.
Antimicrobial resistance is emerging at an alarming rate due to overuse and inappropriate use of antibiotics. One key solution is active surveillance to curb it through early warning systems. There is active surveillance of methicillin resistant S. aureus (MRSA) and Extended spectrum beta-lactamase (ESBL) producing bacteria at NMIMR. Surprisingly, AMR of bacteria species recovered from children with malaria, animal products and droppings and foodborne bacterial pathogens have been reported. There is the need for whole genome sequencing of bacteria species and capacity building for bioinformatics and continuous AMR testing. Antibiotic residues in animal products would be a major focus for the Institute. Future formative studies (Socio-behavioral studies) will be carried out to gain more insight on human behavioral changes that may lead to AMR.
The Institute will seek to expand its facilities and capacity to conduct drug and vaccine pre-clinical and clinical trials as part of bringing new products to the market. NMIMR has a dedicated animal facility in a two-story building; BSL-1/BSL-2, HEPA-filtered 4-room barrier suite for specific pathogen-free (SPF) animals, clean air racks with both negative and positive pressure in four animal holding rooms, connected to study and procedure rooms. Irrespective of these pre-clinical facilities, we seek to expand our facilities for both clinical and pre-clinical trials to accommodate several studies at a time. The expansion, maintenance and operation of these facilities require huge funds, hence, in the next 25 years, the Institute will rely on internally generated funds from research grants from funding agencies and the Government of Ghana through strong advocacy.
The Institute will strive to establish more surveillance programs and sentinel sites for a significant number of the emerging and re-emerging infectious diseases of pandemic potential. Due to lack of appropriate resources at these sentinel sites, preliminary research cannot be carried out at these centres, however, personnel at these centres will be trained on identification of cases, sampling, packaging and transportation of samples to NMIMR. We will harness the resourcefulness of genomics in monitoring these diseases. Through genomic sequencing, the Institute will establish genomic surveillance of emerging and re-emerging diseases in Africa to detect and manage their trends.
There is the need to establish strong and sustainable research programs into NCDs, which are an emerging pandemic on the African continent. Although the Institute is already involved in research into a number of NCDs, we need to strengthen our research programs in this area. Future studies on co-morbidities of infectious diseases and NCDs will be prioritized without neglecting co-morbidities of communicable diseases.
Moreover, we seek to enhance the Institute’s capabilities for translational research, product development and the generation of intellectual property through technology transfer. These will include the discovery and early development of diagnostics, drugs, vaccines and animal models for human disease research. This vision requires the set-up of a biotechnology facility in the Institute which will lead product development. Before our next 25 years as an Institute, we hope to establish a well-equipped biotechnology facility with state-of-the-art design to meet international standards which will enhance the preparedness of the Institute to response to pandemics through production of vaccines and diagnostics in a short time.
The Institute will expand upon our national, regional and international networks in order to make more impactful contribution to the global health agenda. Although we have research networks in most parts of the world, the Institute is yet to establish networks in places like South America and the Middle East. In the next 25 years, the Institute shall expand its network to all parts of the world and with all major disease coordinating agencies. Locally, we will expand our influence to cover all sixteen regions and all districts of Ghana through diagnostic support, training and capacity building for health personnel, conferences and seminars and continuous community engagements.
The Institute will also seek to establish a well-structured biobank with well archived biospecimen and accompanying data to support future research. Currently, biospecimen banks are mostly kept on a project basis, and as some of the diseases for which these biospecimen were collected become rarer, it will be necessary to fall back on archived samples to do scientific investigations. The Institute will therefore seek for opportunities and resources to set up a well-structured biobank that will serve researchers both locally and abroad. On the basis of this vision, the NMIMR will continue to maintain its relevance as a flagship health research institution in the Africa region and contribute significantly to improving health globally.
Author contributions
DY-M contributed to conception of manuscript. DY-M, SO-W contributed to the design. DY-M, JO, SO-W, AA-B, GO-A, AA-P, JA, BA, KK, CA wrote the first draft. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Koram K. Mapping response of plasmodium falciparum to chloroquine and other antimalarial drugs in Ghana, project ID 980034. Final Rep submitted to MIM/TDR (2003).
2. Koram KA, Abuaku B, Duah N, Quashie N. Comparative efficacy of antimalarial drugs including ACTs in the treatment of uncomplicated malaria among children under 5 years in Ghana. Acta Tropica (2005) 95(3):194–203. doi: 10.1016/j.actatropica.2005.06.018
3. Ottesen EA. The global programme to eliminate lymphatic filariasis. Wiley Online Library (2000) 5:591–4. doi: 10.1046/j.1365-3156.2000.00620.x
4. Dunyo SK, Nkrumah FK, Simonsen PE. A randomized double-blind placebo-controlled field trial of ivermectin and albendazole alone and in combination for the treatment of lymphatic filariasis in Ghana. Trans R Soc Trop Med Hygiene (2000) 94(2):205–11. doi: 10.1016/S0035-9203(00)90278-5
5. Abana CZ, Sagoe KWC, Bonney EY, Maina EK, Aziati ID, Agbosu E, et al. Drug resistance mutations and viral load in human immunodeficiency virus type 2 and dual HIV-1/HIV-2 infected patients in Ghana. Med (Baltimore) (2019) 98(6):e14313. doi: 10.1097/MD.0000000000014313
6. Bonney EY, Addo NA, Ntim NA, Addo-Yobo F, Bondzie P, Aryee KE, et al. Low level of transmitted HIV drug resistance at two HIV care centres in Ghana: a threshold survey. Ghana Med J (2013) 47(2):82–6.
7. Martin-Odoom A, Adiku T, Delgado E, Lartey M, Ampofo WK. Occurrence of transmitted HIV-1 drug resistance among drug-naive pregnant women in selected HIV-care centres in Ghana. Ghana Med J (2017) 51(1):20–3. doi: 10.4314/gmj.v51i1.4
8. Nii-Trebi NI, Ibe S, Barnor JS, Ishikawa K, Brandful JA, Ofori SB, et al. HIV-1 drug-resistance surveillance among treatment-experienced and -naive patients after the implementation of antiretroviral therapy in Ghana. PLoS One (2013) 8(8):e71972. doi: 10.1371/journal.pone.0071972
9. Obeng BM, Bonney EY, Asamoah-Akuoko L, Nii-Trebi NI, Mawuli G, Abana CZ, et al. Transmitted drug resistance mutations and subtype diversity amongst HIV-1 sero-positive voluntary blood donors in Accra, Ghana. Virol J (2020) 17(1):114. doi: 10.1186/s12985-020-01386-y
10. NMIMR-Media-Report_COVID-19_2. COVID-19: Noguchi memorial institute tests 100,000 samples - prof annan discloses. (Ghana: Modern Ghana). (2020) https://www.modernghana.com/news/999039/covid-19-noguchi-memorial-institute-tests-100000.html
11. NMIMR-Media-Report_COVID-19_5. Covid-19: Ghana conducts over 80,000 individual tests — noguchi clarifies. (Ghana: Modern Ghana). (2020) https://www.modernghana.com/news/997271/covid-19-ghana-conducts-over-80000-individual.html
12. NMIMR-Media-Report_COVID-19_6. Noguchi trains African scientists on coronavirus. (Ghana: Modern Ghana). (2020) https://www.modernghana.com/news/988074/noguchi-trains-african-scientists-on-coronavirus.html
13. Ngoi JM, Quashie PK, Morang'a CM, Bonney JH, Amuzu DS, Kumordjie S, et al. Genomic analysis of SARS-CoV-2 reveals local viral evolution in Ghana. Exp Biol Med (Maywood) (2021) 246(8):960–70. doi: 10.1177/1535370220975351
14. Tate JE, Mwenda JM, Armah G, Jani B, Omore R, Ademe A, et al. Evaluation of intussusception after monovalent rotavirus vaccination in Africa. N Engl J Med (2018) 378(16):1521–8. doi: 10.1056/NEJMoa1713909
15. Armah GE, Cortese MM, Dennis FE, Yu Y, Morrow AL, McNeal MM, et al. Rotavirus vaccine take in infants is associated with secretor status. J Infect Dis (2019) 219(5):746–9. doi: 10.1093/infdis/jiy573
16. Nonvignon J, Atherly D, Pecenka C, Aikins M, Gazley L, Groman D, et al. Cost-effectiveness of rotavirus vaccination in Ghana: Examining impacts from 2012 to 2031. Vaccine (2017) 36(47):7215–21. doi: 10.1016/j.vaccine.2017.11.080
17. Armah G, Pringle K, Enweronu-Laryea CC, Ansong D, Mwenda JM, Diamenu SK, et al. Impact and effectiveness of monovalent rotavirus vaccine against severe rotavirus diarrhea in Ghana. Clin Infect Dis (2016) 62 Suppl 2:S200–7. doi: 10.1093/cid/ciw014
18. Armah G, Lewis KD, Cortese MM, Parashar UD, Ansah A, Gazley L, et al. A randomized, controlled trial of the impact of alternative dosing schedules on the immune response to human rotavirus vaccine in rural ghanaian infants. J Infect Dis (2016) 213(11):1678–85. doi: 10.1093/infdis/jiw023
19. Armah GE, Kapikian AZ, Vesikari T, Cunliffe N, Jacobson RM, Burlington DB, et al. Efficacy, immunogenicity, and safety of two doses of a tetravalent rotavirus vaccine RRV-TV in Ghana with the first dose administered during the neonatal period. J Infect Dis (2013) 208(3):423–31. doi: 10.1093/infdis/jit174
20. Armah GE, Breiman RF, Tapia MD, Dallas MJ, Neuzil KM, Binka FN, et al. Immunogenicity of the pentavalent rotavirus vaccine in African infants. Vaccine (2012) 30 Suppl 1:A86–93. doi: 10.1016/j.vaccine.2011.10.006
21. Breiman RF, Zaman K, Armah G, Sow SO, Anh DD, Victor JC, et al. Analyses of health outcomes from the 5 sites participating in the Africa and Asia clinical efficacy trials of the oral pentavalent rotavirus vaccine. Vaccine (2012) 30 Suppl 1:A24–9. doi: 10.1016/j.vaccine.2011.08.124
22. Tapia MD, Armah G, Breiman RF, Dallas MJ, Lewis KD, Sow SO, et al. Secondary efficacy endpoints of the pentavalent rotavirus vaccine against gastroenteritis in sub-Saharan Africa. Vaccine (2012) 30 Suppl 1:A79–85. doi: 10.1016/j.vaccine.2012.01.022
23. Lewis KD, Dallas MJ, Victor JC, Ciarlet M, Mast TC, Ji M, et al. Comparison of two clinical severity scoring systems in two multi-center, developing country rotavirus vaccine trials in Africa and Asia. Vaccine (2012) 30 Suppl 1:A159–66. doi: 10.1016/j.vaccine.2011.07.126
24. Abbott C, Tiede B, Armah G, Mahmoud A. Evaluation of cost-effectiveness of live oral pentavalent reassortant rotavirus vaccine introduction in Ghana. Vaccine (2012) 30(15):2582–7. doi: 10.1016/j.vaccine.2012.01.076
25. Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet (2010) 376(9741):606–14. doi: 10.1016/S0140-6736(10)60889-6
26. Enweronu-Laryea CC, Boamah I, Sifah E, Diamenu SK, Armah G. Decline in severe diarrhea hospitalizations after the introduction of rotavirus vaccination in Ghana: a prevalence study. BMC Infect Dis (2014) 14:431. doi: 10.1186/1471-2334-14-431
27. Enweronu-Laryea CC, Armah G, Sagoe KW, Ansong D, Addo-Yobo E, Diamenu SK, et al. Sustained impact of rotavirus vaccine introduction on rotavirus gastroenteritis hospitalizations in children <5years of age, Ghana 2009-2016. Vaccine (2018) 36(47):7131–34. doi: 10.1016/j.vaccine.2018.02.058
28. Enweronu-Laryea CC, Sagoe KW, Mwenda JM, Armah GE. Severe acute rotavirus gastroenteritis in children less than 5 years in southern Ghana: 2006-2011. Pediatr Infect Dis J (2014) 33 Suppl 1:S9–S13. doi: 10.1097/INF.0000000000000045
29. Enweronu-Laryea CC, Sagoe KW, Glover-Addy H, Asmah RH, Mingle JA, Armah GE. Prevalence of severe acute rotavirus gastroenteritis and intussusceptions in ghanaian children under 5 years of age. J Infect Dev Ctries (2012) 6(2):148–55.
30. Mwenda JM, Ntoto KM, Abebe A, Enweronu-Laryea C, Amina I, McHomvu J, et al. Burden and epidemiology of rotavirus diarrhea in selected African countries: preliminary results from the African rotavirus surveillance network. J Infect Dis (2010) 202 Suppl:S5–S11. doi: 10.1086/653557
31. Mensah-Quainoo E, Yeboah-Manu D, Asebi C, Patafuor F, Ofori-Adjei D, Junghanss T, et al. Diagnosis of mycobacterium ulcerans infection (Buruli ulcer) at a treatment centre in Ghana: a retrospective analysis of laboratory results of clinically diagnosed cases. Trop Med Int Health (2008) 13(2):191–8. doi: 10.1111/j.1365-3156.2007.01990.x
32. Yeboah-Manu D, Aboagye SY, Asare P, Asante-Poku A, Ampah K, Danso E, et al. Laboratory confirmation of buruli ulcer cases in Ghana 2008-2016. PloS Negl Trop Dis (2018) 12(6):e0006560. doi: 10.1371/journal.pntd.0006560
33. Ahorlu CK, Koka E, Kumordzi S, Yeboah-Manu D, Ampadu E. Social and economic factors influencing buruli ulcer health seeking decision making in the Ga West and south municipalities. Adv Appl Sociology (2013) 03(04):187–92. doi: 10.4236/aasoci.2013.34025
34. Ahorlu CK, Koka E, Yeboah-Manu D, Lamptey I, Ampadu E. Enhancing buruli ulcer control in Ghana through social interventions: a case study from the obom sub-district. BMC Public Health (2013) 13:59. doi: 10.1186/1471-2458-13-59
35. Iddrisah FN, Yeboah-Manu D, Nortey PA, Nyarko KM, Anim J, Antara SN, et al. Outcome of streptomycin-rifampicin treatment of buruli ulcer in two ghanaian districts. Pan Afr Med J (2016) 25(Suppl 1):13. doi: 10.11604/pamj.supp.2016.25.1.6203
36. Koka E, Yeboah-Manu D, Okyere D, Adongo PB, Ahorlu CK. Cultural understanding of wounds, buruli ulcers and their management at the obom Sub-district of the Ga south municipality of the greater Accra region of Ghana. PloS Negl Trop Dis (2016) 10(7):e0004825. doi: 10.1371/journal.pntd.0004825
37. Yeboah-Manu D, Asante-Poku A, Asan-Ampah K, Ampadu ED, Pluschke G. Combining PCR with microscopy to reduce costs of laboratory diagnosis of buruli ulcer. Am J Trop Med Hyg (2011) 85(5):900–4. doi: 10.4269/ajtmh.2011.11-0362
38. Addo P, Owusu E, Adu-Addai B, Quartey M, Abbas M, Dodoo A, et al. Findings from a buruli ulcer mouse model study. Ghana Med J (2005) 39(3):86–93.
39. Addo P, Adu-Addai B, Quartey M, Abbas M, Okang I, Owusu E, et al. Clinical and histopathological presentation of buruli ulcer in experimentally infected grasscutters (Thryonomys swinderianus). Internet J Trop Med (2006) 3. doi: 10.5580/df7
40. Beissner M, Phillips RO, Battke F, Bauer M, Badziklou K, Sarfo FS, et al. Loop-mediated isothermal amplification for laboratory confirmation of buruli ulcer disease-towards a point-of-Care test. PLoS Negl Trop Dis (2015) 9(11):e0004219. doi: 10.1371/journal.pntd.0004219
41. Addo K, Dan-Dzide M, Yeboah-Manu D, Owusu-Darko K, Caulley P, Minamikawa M, et al. Improving the laboratory diagnosis of TB in Ghana: the impact of a quality assurance system. Int J Tuberculosis Lung Disease (2006) 10(7):812–7.
42. Addo K, Owusu-Darko K, Dan-Dzide M, Yeboah-Manu D, Ablordey A, Caulley P, et al. Situation analysis of TB microscopy centres in Ghana. Int J Tuberculosis Lung Disease (2006) 10(8):870–5.
43. Bonsu F, Addo KK, Alebachew Z, Gyapong J, Badu-Peprah A, Gockah R, et al. National population-based tuberculosis prevalence survey in Ghana 2013. Int J Tuberc Lung Dis (2020) 24(3):321–8. doi: 10.5588/ijtld.19.0163
44. Yeboah-Manu D, Asare P, Asante-Poku A, Otchere ID, Osei-Wusu S, Danso E, et al. Spatio-temporal distribution of mycobacterium tuberculosis complex strains in Ghana. PLoS One (2016) 11(8):e0161892. doi: 10.1371/journal.pone.0161892
45. Yeboah-Manu D, Asante-Poku A, Bodmer T, Stucki D, Koram K, Bonsu F, et al. Genotypic diversity and drug susceptibility patterns among m. tuberculosis Complex isolates South-Western Ghana. PloS One (2011) 6(7):e21906. doi: 10.1371/journal.pone.0021906
46. Asare P, Otchere ID, Bedeley E, Brites D, Loiseau C, Baddoo NA, et al. Whole genome sequencing and spatial analysis identifies recent tuberculosis transmission hotspots in Ghana. Front Med (2020) 7(161):161. doi: 10.3389/fmed.2020.00161
47. Asare P, Osei-Wusu S, Baddoo NA, Bedeley E, Otchere ID, Brites D, et al. Genomic epidemiological analysis identifies high relapse among individuals with recurring tuberculosis and provides evidence of recent household-related transmission of tuberculosis in Ghana. Int J Infect Dis (2021) 106:13–22. doi: 10.1016/j.ijid.2021.02.110
48. Asante-Poku A, Otchere ID, Osei-Wusu S, Sarpong E, Baddoo A, Forson A, et al. Molecular epidemiology of mycobacterium africanum in Ghana. BMC Infect Diseases (2016) 16:385. doi: 10.1186/s12879-016-1725-6
49. Asante-Poku A, Yeboah-Manu D, Otchere ID, Aboagye SY, Stucki D, Hattendorf J, et al. Mycobacterium africanum is associated with patient ethnicity in Ghana. PLoS Negl Trop Diseases (2015) 9(1):e3370. doi: 10.1371/journal.pntd.0003370
50. Asante-Poku A, Asare P, Baddoo NA, Forson A, Klevor P, Otchere ID, et al. TB-diabetes co-morbidity in Ghana: The importance of mycobacterium africanum infection. PLoS One (2019) 14(2):e0211822. doi: 10.1371/journal.pone.0211822
51. Mensah GI, Sowah SA, Yeboah NY, Addo KK, Jackson-Sillah D. Utility of QuantiFERON tuberculosis gold-in-tube test for detecting latent tuberculosis infection among close household contacts of confirmed tuberculosis patients in Accra, Ghana. Int J Mycobacteriol (2017) 6(1):27–33. doi: 10.4103/2212-5531.201891
52. Addo KK, Addo SO, Mensah GI, Mosi L, Bonsu FA. Genotyping and drug susceptibility testing of mycobacterial isolates from population-based tuberculosis prevalence survey in Ghana. BMC Infect Dis (2017) 17(1):743. doi: 10.1186/s12879-017-2853-3
53. Otchere ID, Asante-Poku A, Osei-Wusu S, Baddoo A, Sarpong E, Ganiyu AH, et al. Detection and characterization of drug-resistant conferring genes in mycobacterium tuberculosis complex strains: A prospective study in two distant regions of Ghana. Tuberculosis (Edinb) (2016) 99:147–54. doi: 10.1016/j.tube.2016.05.014
54. Osei-Wusu S, Amo Omari M, Asante-Poku A, Darko Otchere I, Asare P, Forson A, et al. Second-line anti-tuberculosis drug resistance testing in Ghana identifies the first extensively drug-resistant tuberculosis case. Infection Drug Resistance (2018) 11:239–46. doi: 10.2147/IDR.S152720
55. NMIMR-Media-Report_AMR1. NMIMR & QWArS collaborates for a training workshop on AMR. (Ghana: NMIMR). (2021). Available at: https://twitter.com/NMIMR_UG/status/1410981600427454464
56. WHO. (2017). World Health Organization. Available at: https://www.who.int/health-topics/one-health#tab=tab_1.
Keywords: Noguchi, health policy, biomedical research, Ghana, West Africa, Africa, NMIMR
Citation: Yeboah-Manu D, Odoom JK, Osei-Wusu S, Adoma-Boakye A, Osae-Amoako G, Asante-Poku A, Akorli J, Abuaku B, Kusi KA and Ahorlu CS (2023) From research to health policy: The Noguchi story in the past, present and next 25 years. Front. Trop. Dis 4:1135354. doi: 10.3389/fitd.2023.1135354
Received: 31 December 2022; Accepted: 27 March 2023;
Published: 06 April 2023.
Edited by:
Moses Bockarie, European & Developing Countries Clinical Trials Partnership, NetherlandsReviewed by:
Basu Dev Pandey, Everest International Clinic and Research Center, NepalThomas Nyirenda, European & Developing Countries Clinical Trials Partnership, Netherlands
Copyright © 2023 Yeboah-Manu, Odoom, Osei-Wusu, Adoma-Boakye, Osae-Amoako, Asante-Poku, Akorli, Abuaku, Kusi and Ahorlu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dorothy Yeboah-Manu, dyeboah-manu@noguchi.ug.edu.gh
 Dorothy Yeboah-Manu
Dorothy Yeboah-Manu John Kofi Odoom
John Kofi Odoom Stephen Osei-Wusu
Stephen Osei-Wusu Afia Adoma-Boakye
Afia Adoma-Boakye Adwoa Asante-Poku
Adwoa Asante-Poku Jewelna Akorli
Jewelna Akorli Benjamin Abuaku
Benjamin Abuaku Kwadwo Asamoah Kusi
Kwadwo Asamoah Kusi