- 1Department of Archaeology, Max Planck Institute of Geoanthropology, Jena, Germany
- 2Interdisciplinary Center for Archaeology and the Evolution of Human Behavior, Universidade do Algarve, Faro, Portugal
- 3Department of Evolutionary Anthropology, Faculty of Life Sciences, University of Vienna, Vienna, Austria
- 4Human Evolution and Archaeological Sciences (HEAS) Research Network, University of Vienna, Vienna, Austria
- 5Department of Archaeology and Natural History, School of Culture, History & Language, Australian National University, Canberra, ACT, Australia
- 6School of Social Science, The University of Queensland, Brisbane, QLD, Australia
- 7Griffith Sciences, Griffith University, Nathan, QLD, Australia
Recent advancements in biomolecular archaeology, such as stable isotope and ancient DNA research, have expanded our understanding of megafauna extinction processes and dynamics. The rise of palaeoproteomics, specifically Zooarchaeology by Mass Spectrometry (ZooMS), has added yet another method to this toolkit, as it can be used to taxonomically identify megafauna remains amongst highly fragmented bone assemblages. However, taxonomic identifications with ZooMS are reliant on the availability of collagen peptide markers for the regional fauna of interest. In the absence of a global reference database, most studies to date have been restricted to Eurasian contexts. Here, we report ZooMS peptide markers for three extinct Australian megafauna species: Zygomaturus trilobus, Palorchestes azael, and Protemnodon mamkurra. We show that these taxa can be differentiated from extant Australian fauna with these peptide markers. This foundational work represents an important step in establishing ZooMS as a method that can be used to identify new megafauna specimens in Australia’s highly fragmented fossil record and ultimately help resolve fundamental questions related to human–fauna–environment interactions.
1 Introduction
The emergence of novel analytical methodologies in archaeology and palaeontology has helped shed new light on long-standing research questions within the discipline. Amongst the topics such methods have helped to address is the timing and nature of the global megafauna extinctions in the late Quaternary. In most cases, the application of chronometric dating and modelling approaches are seen as key to understanding the timing and demise of megafauna species at a global, continental, and local level (e.g., Brook and Bowman, 2002; Stuart and Lister, 2012; Prescott et al., 2012; Stuart, 2014). More recently, the application of biomolecular methods has led to a deeper understanding of extinction processes and dynamics (see also Swift et al., 2019). The application of stable isotope analysis, for example, has allowed for improved reconstruction of megafauna diet (e.g., Bocherens et al., 2017; Ma et al., 2019; Koutamanis et al., 2023; Varela et al., 2023), mobility (e.g., Price et al., 2017; Wooller et al., 2021; Heddell-Stevens et al., 2024), and ecology (e.g., Trayler et al., 2015; González-Guarda et al., 2017; Rabanus-Wallace et al., 2017). Similarly, the application of ancient DNA has revealed new insights into the demography and population dynamics of megafauna species (e.g., Llamas et al., 2014; Fellows Yates et al., 2017; Pečnerová et al., 2017), as well as their migration and geographic range shifts (e.g., Haile et al., 2009; Lorenzen et al., 2011; Seersholm et al., 2020; Canteri et al., 2022).
One biomolecular technique that has not yet been extensively applied to research questions related to late Quaternary megafaunal extinctions is palaeoproteomics, and specifically Zooarchaeology by Mass Spectrometry (ZooMS). ZooMS is a type of peptide mass fingerprinting in which differences in collagen type I between (sub)families, genera, and sometimes species are used to taxonomically identify collagen-bearing materials, such as bone and ivory (Buckley et al., 2009). The method offers several key advantages, such as its ability to provide taxonomic information from fragmentary and otherwise unidentifiable zooarchaeological and paleontological remains (Brown et al., 2021b; Sinet-Mathiot et al., 2023), its scalability to screen large fragmentary assemblages for a targeted species of interest (Douka et al., 2019), and its applicability to material coming from a wide range of environments, including sub-tropical and tropical ranges (Peters et al., 2023; Wang et al., 2023). Yet, an important prerequisite for the successful application of ZooMS is the presence of a comprehensive reference database of collagen peptide markers to make these taxonomic identifications possible. Thus far, studies that have used ZooMS to identify megafauna remains have mostly been restricted to Eurasia (e.g., Buckley et al., 2017b; Brown et al., 2021b; Smith et al., 2024; Xia et al., 2024) and North America (e.g., Kubiak et al., 2023; Antonosyan et al., 2024). This geographical bias can largely be attributed to the absence of collagen peptide markers for extinct megafauna from other continents.
To address this lacuna and build upon reference libraries recently created for extant Australian fauna (Buckley et al., 2017a; Peters et al., 2021), we sought to begin to develop peptide markers for extinct megafauna species in Australia, a country for which peptide markers are currently only available for a single extinct megafaunal taxon, Simosthenurus occidentalis (Buckley et al., 2017a). While the age of megafaunal reference specimens poses challenges to collagen preservation, especially in the warmer climates found in much of Australia, recent research suggests that collagen preservation in the continent extend back to over 50,000 years ago, even in warmer and more humid regions of Australia (Peters et al., 2023). Nonetheless, the poorly delineated age of many megafauna reference specimens, often attributed only to broad geological periods, poses further challenges to the selection of suitable material for peptide marker development.
Here, we report ZooMS peptide markers for three megafauna species from southern Australia and Tasmania that went extinct in the Late Pleistocene, namely, Zygomaturus trilobus, Palorchestes azael, and Protemnodon mamkurra. The targeted specimens were all directly dated previously using AMS radiocarbon dating (Gillespie et al., 2012, 2015). The Protemnodon mamkurra specimen was accurately dated to 42.2–43.1 ka cal BP, the other two specimens extend beyond the limits of radiocarbon dating (Gillespie et al., 2012, 2015). Species of Zygomaturus and Palorchestes were amongst the largest-bodied mammalian megafauna of Australia during the Pleistocene (Figure 1; Johnson, 2006), and both represent families (Diprotodontidae and Palorchestidae, respectively) that went completely extinct in the late Quaternary (Koch and Barnosky, 2006). Remains of Zygomaturus trilobus have been recovered from fossil sites across mainland Australia (Long et al., 2002; Webb, 2008). It was adapted to forested environments (Black et al., 2012), feeding on both C3 and C4 plants (DeSantis et al., 2017). Palorchestes azael, on the other hand, was a highly specialized browser with distinct, powerful forelimbs and sharp claws (Richards et al., 2019) that was widely distributed across eastern Australia and Tasmania (Pledge, 1991; Long et al., 2002). Protemnodon represents a clade of extinct giant kangaroos, of which all species are now extinct. Protemnodon mamkurra was widespread across the forested environments of southern Australia and Tasmania (Kerr et al., 2024). The development of collagen peptide markers for these species will enable future ZooMS research into the extinction of megafauna in Australia.
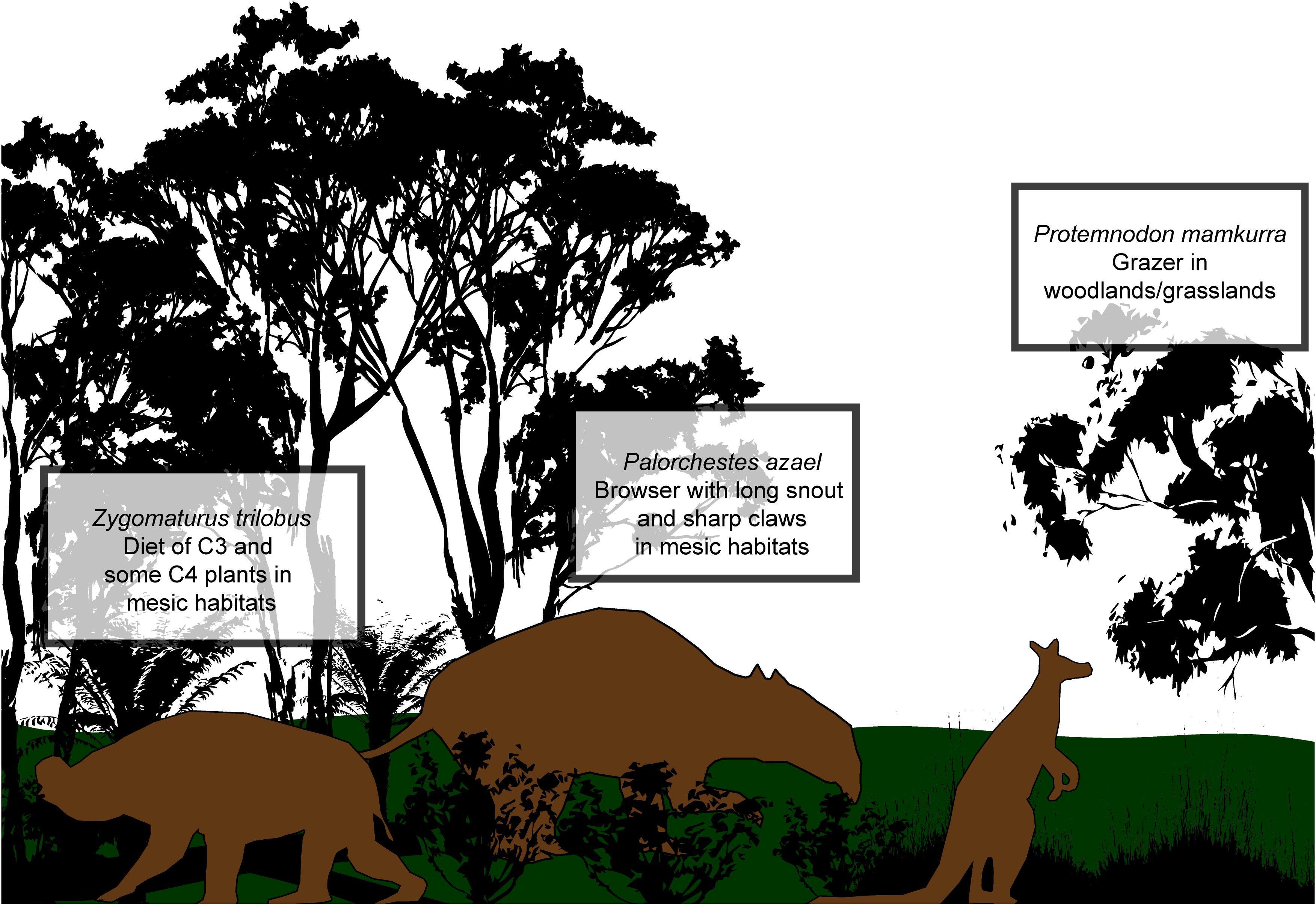
Figure 1. Diagram showing the three extinct megafauna species studied with imagined reconstruction and associated habitats, based on Prideaux et al. (2009), Gillespie et al. (2012, 2015), and Richards et al. (2019). Zygomaturus trilobus probably weighed ~500–700 kg, was quadrupedal, and was found in mesic habitats near the continental margin. Palorchestes azael was likely ~1000 kg, a mostly quadrupedal browser but able to stand on two legs to browse higher bushes and trees, while Protemnodon mamkurra was a browser/mixed-feeder of ~100–150 kg (Kerr et al., 2024). All three species were found in southern Australia and Tasmania.
2 Materials and methods
2.1 Material
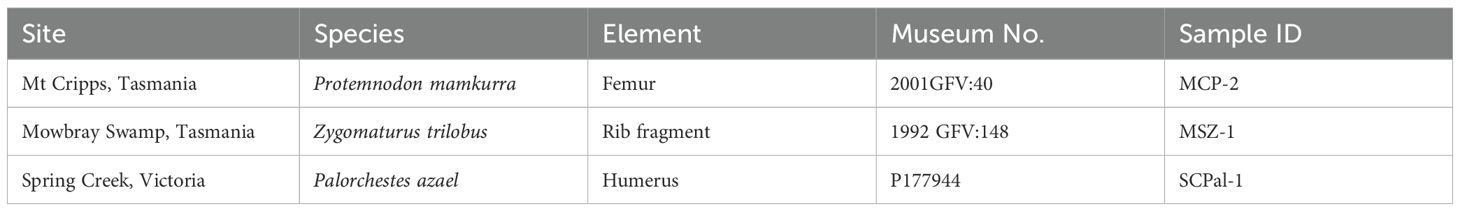
The samples that were analyzed for this study consist of three megafauna specimens from which collagen was previously extracted for radiocarbon and stable isotope analysis (Figure 2; Table 1). This includes a rib fragment of Zygomaturus trilobus from Mowbray Swamp, Tasmania (MSZ-1), a humerus of Palorchestes azael from Spring Creek, Victoria (SCPal-1), and a femur of Protemnodon mamkurra sp. nov. from Mt. Cripps, Tasmania (MCP-2) (Gillespie et al., 2012, 2015). The six dates reported for the highly contaminated Zygomaturus trilobus form a curve approaching an asymptote of >50,500 BP, while the three dates of the less-contaminated Spring Creek Palorchestes azael reach an asymptote of >53,500 BP (Gillespie et al., 2012, 2015). Both results are near the maximum age possible using the applied chemistry and radiocarbon method, and, as also suggested by the geology (Gill and Banks, 1956; Banks et al., 1976), they should be treated as minimum ages for these two specimens (Chappell et al., 1996). The Protemnodon mamkurra specimen from Mt. Cripps yielded an age of 42.4–43.1 cal BP (Gillespie et al., 2012, 2015). This specimen is one of the youngest extinct marsupial megafauna reported from Australia to date, and covers a small period of overlap with the first humans in Tasmania, who could have entered Tasmania via the earliest pedestrian land crossing available at ~43 ± 4 ka (Lambeck and Chappell, 2001). All megafauna reference specimens were originally sampled by RG from collections at the Queen Victoria Museum and Art Gallery (Launceston, Tasmania) and Museums Victoria (Melbourne, Victoria).
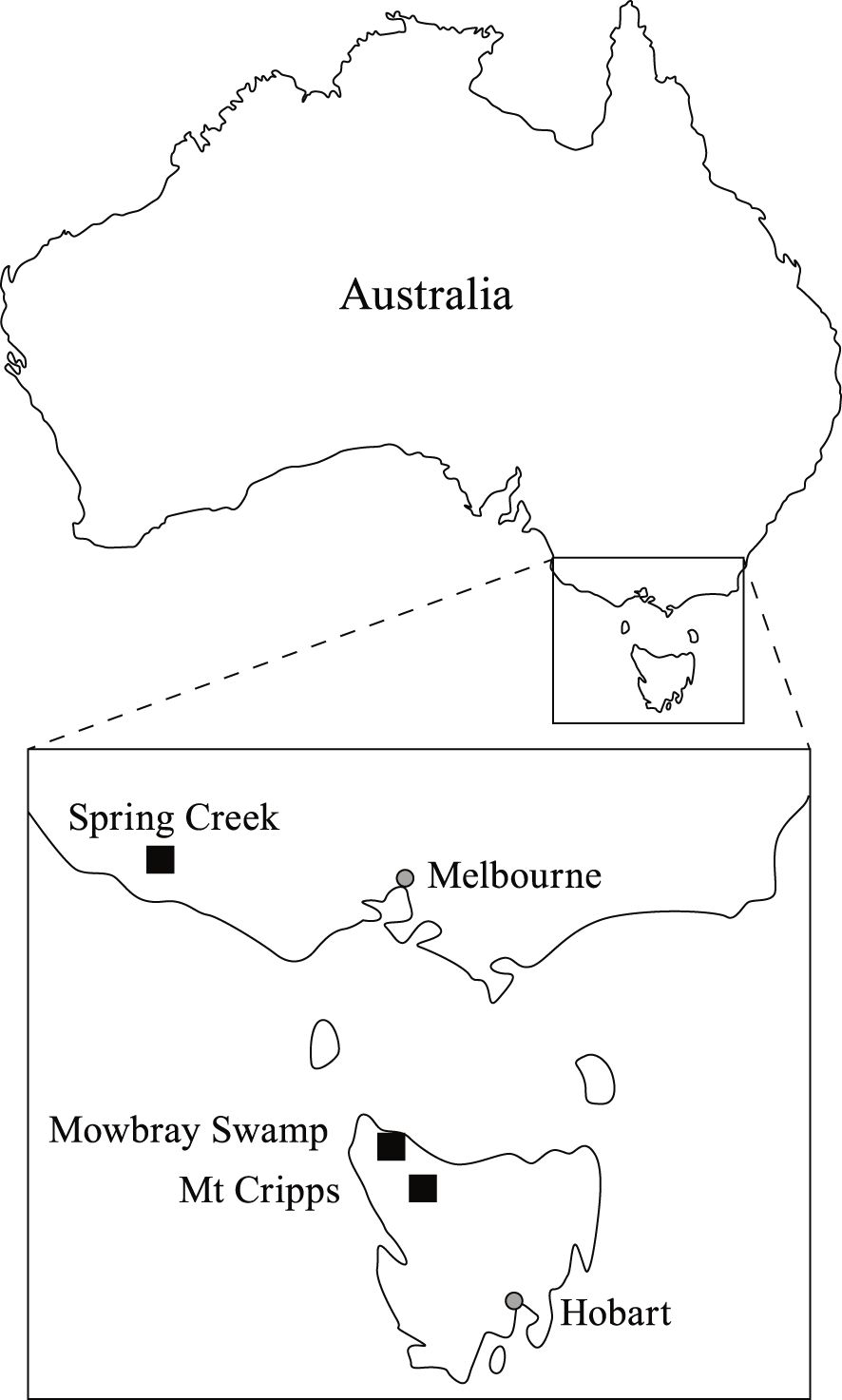
Figure 2. Location of sites containing megafauna reference specimens included in this study (modified after Gillespie et al., 2015).
2.2 Zooarchaeology by Mass Spectrometry
An acid insoluble protocol was used for the Protemnodon mamkurra and Palorchestes azael bones to extract collagen (Buckley et al., 2009; Welker et al., 2015). Approximately 20 mg of bone was demineralized in 500μl of 0.6 M hydrochloric acid (HCl) for 3 days and washed 3 × with 200 μl of 50 mM ammonium bicarbonate (AmBic). 100 μl of AmBic was added and the sample gelatinized at 65°C for 1 hour. 50 μl of the resulting supernatant was removed and 1 μl of 0.4 μg/μl trypsin solution (Pierce™ Trypsin Protease, Thermo Scientific) was added for digestion at 37°C for ~18 h. The following day, 1 µl of 5% trifluoracetic acid (TFA) was added to the supernatant, which was then purified and concentrated using C18 ZipTips (Pierce™ C18 Tips, Thermo Scientific), spotted in triplicate with matrix solution (α-cyano-4-hydroxycinnamic acid of 10 mg/ml in 50% acetonitrile/0.1% TFA) and analyzed with a MALDI-TOF-MS (Autoflex, Bruker Daltonics). The Z. trilobus sample consisted of ultrafiltered gelatin that was previously prepared for radiocarbon dating using the Oxford protocol (Higham et al., 2006). About 2 mg of this pretreated gelatin was separated and 50 μl AmBic was added. Then, the same steps of digestion, purification, and spotting were undertaken as described for the other two samples. The resulting MALDI spectra were processed in mMass v5.5.0 (Strohalm et al., 2010) with smoothing (Method = Savitzky-Golay, Window size = 0.3, Cycles = 2), baseline correction (Precision = 15, Relative offset = 25), and peak picking (S/N = 3.0, Picking height = 75%).
2.3 Liquid chromatography tandem mass spectrometry
Following ZooMS analysis, 20 μl of the collagen extract was dried down for further LC-MS/MS analysis at the Functional Genomics Center Zurich using a Q-Exactive HF mass spectrometer (Thermo Scientific) coupled with an ACQUITY UPLC M-Class system (Waters, AG). Solvent composition was 0.1% formic acid for channel A and 0.1% formic acid in 99.9% ACN for channel B. The column temperature was 50°C. For every sample, 4 μl of peptides were loaded on a commercial MZ Symmetry C18 Trap Columns (Å, 5 μm, 180 μm × 20 mm, Waters) followed by a nanoEase MZ C18 HSS T3 Column (100 Å, 1.8 μm, 75 μm × 250 mm, Waters). The peptides were eluted at a flow rate of 300 nl min−1 by a gradient from 5 to 40% B in 120 min and 98% B in 5 min. After each run, the column was cleaned with 98% solvent B for 5 min and holding 98% B for 8 min prior to re-establishing loading condition. The mass spectrometers were operated in data-dependent mode (DDA) performing higher energy collision dissociation (HCD) fragmentation on the 12 most intense signals per cycle. Full-scan MS spectra (300–1500 m/z) were acquired at a resolution of 120,000 at 200 m/z after accumulation to a target value (AGC) of 3,000,000, while HCD spectra were acquired at a resolution of 30,000 using a normalized collision energy of 28 (maximum injection time: 50 ms; AGC: 10,000 ions). Unassigned singly charged ions were excluded. Precursor masses previously selected for MS/MS measurement were excluded from further selection for 30 s, and the exclusion window was set at 10 ppm. The samples were acquired using internal lock mass calibration on m/z 371.1012 and 445.1200.
2.4 Peptide marker development
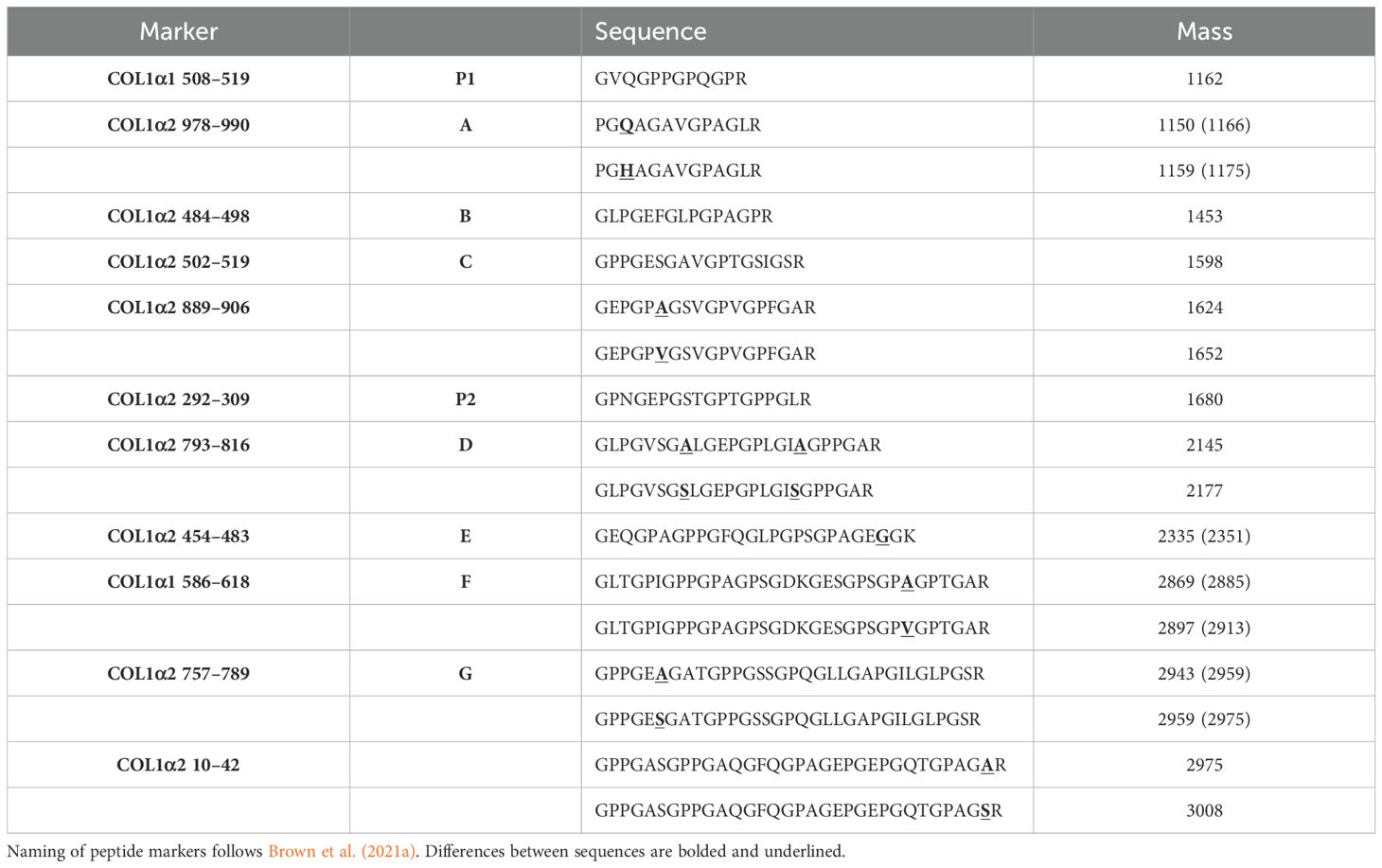
The identification and confirmation of collagen peptide markers followed multiple steps. First, candidate collagen peptide markers were identified. For this, MALDI spectra were visually inspected using mMass v. 5.5.0. (Strohalm et al., 2010) and compared to a list of published collagen markers (Buckley et al., 2017a; Peters et al., 2021). To confirm candidate peptide markers, the MS/MS data was analyzed using Byonic v. 3.2.0. (Protein Metrics Inc., Bern et al., 2012), following a multi-stage approach first introduced by Richter et al. (2020).
Initially, the MS/MS spectra were searched against a reference database including all collagen type I (COL1α1 and COL1α2) sequences available for marsupials from NCBI and UniProt, collagen peptide sequences for marsupials reported in Peters et al. (2021), and common contaminants. The taxa for which complete collagen sequences were available are koala (Phascolarctos cinereus, XP_020853290.1 & XP_020855640.1), common wombat (Vombatus ursinus, A0A4X2KF99 & A0A4X2M815), Tasmanian devil (Sarcophilus harrisii, G3WK23 & G3VSR0), and kangaroo (Macropus sp., Buckley et al., 2017b). The search parameters were set to: cleavage sites fully specific on C-term arginine (R) and lysine (K); 3 missed cleavages allowed; 6 common mass changes and no rare mass changes allowed; common mass changes: oxidation of lysine (K), methionine (M) and proline (P), deamidation of asparagine (N) and glutamine (Q); protein FDR 2%. Peptide sequences (with PEP2D scores <0.01) corresponding to candidate peptide markers were recorded.
Candidate peptide markers for which the peptide sequence could not be identified in the initial search were re-analyzed using an error-tolerant search strategy. Here, the same database was used, but with different search parameters to allow for the identification of novel sequence variants. Parameter settings that were altered are: 2 missed cleavages allowed; 5 common and 1 rare mass change allowed; rare mass changes: all sequence variants allowed. All other parameter settings were identical to those listed for the initial search. All possible sequence variants were noted down and their corresponding masses recorded.
The samples were then searched against a database with the proteomes of V. ursinus (UP000314987) and S. harrisii (UP000007648), as well as all sequence data available in Swissprot. The parameter settings for this search were: cleavage sites fully specific on C-term arginine (R) and lysine (K); 3 missed cleavages allowed; 2 common and 1 rare mass change allowed; common mass changes: oxidation of lysine (K), methionine (M) and proline (P), deamidation of asparagine (N) and glutamine (Q); rare mass changes: pyro-Glu on N-term glutamic acid (E) and glutamine (Q), ammonia-loss on N-term cysteine (C); protein FDR 2%. The results were checked for other identified bone proteins and (common) contaminants to confirm the authenticity of the samples.
A new database was created using the output of the first three searches. This database includes the collagen type I sequences of the original reference database, all sequence variants identified in the error tolerant search, the bone proteins identified in the proteome-wide search, and common contaminants. The MS/MS spectra were searched once more against this database, using the same parameter setting as in the first database search. Only peptides recurring at least three times and with a PEP2D score <0.01 were considered confirmed.
3 Results
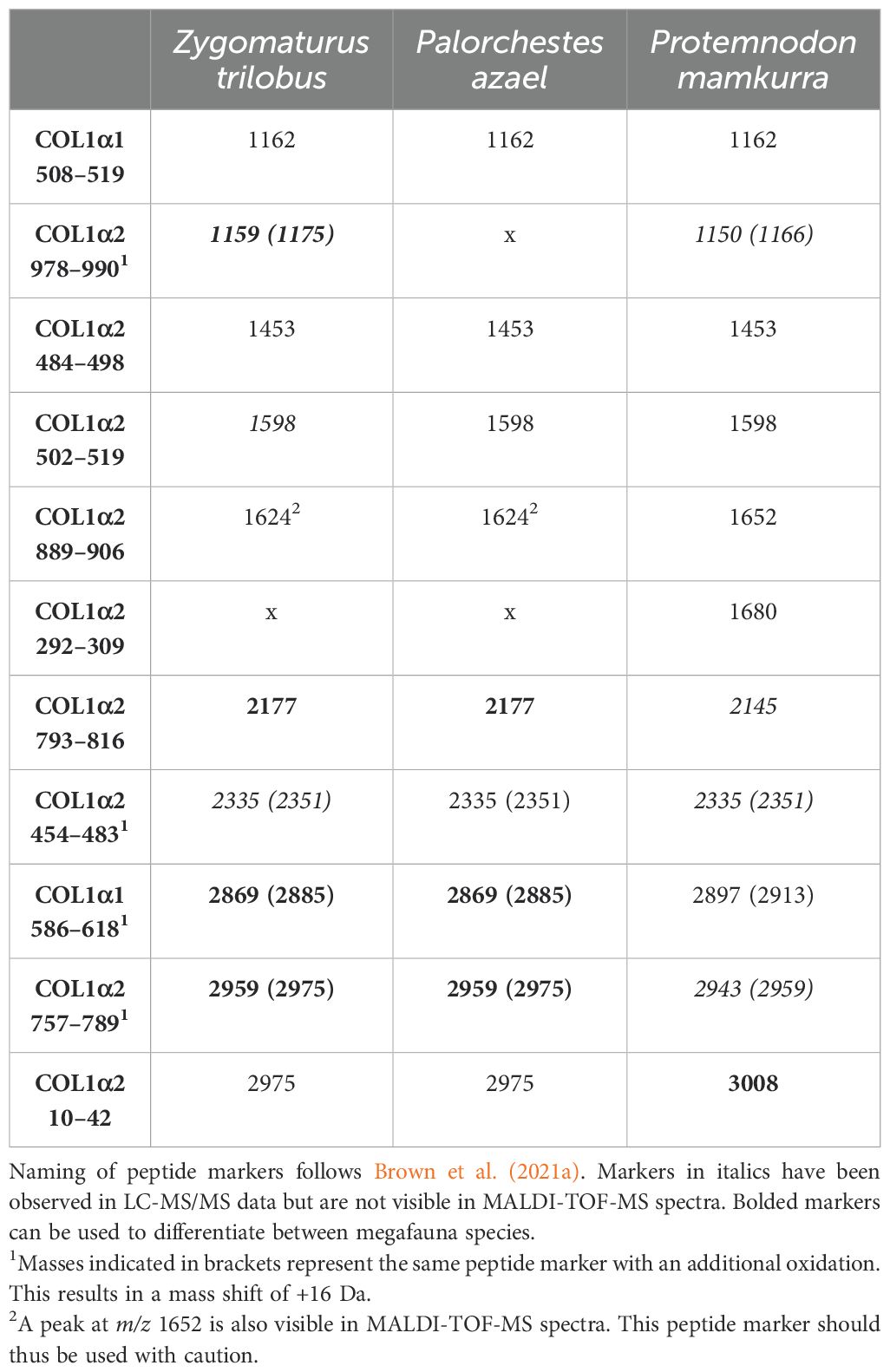
The three megafauna specimens all showed good collagen preservation, enabling the development of collagen peptide markers for all three species. An overview of the identified peptide markers can be found in Table 2, and associated sequence data is reported in Table 3 (see also Supplementary Figures S1-S8).
ZooMS allows for the unique identification of Protemnodon compared to extant kangaroo genera (Macropus, Notamacropus, Osphranter, Lagorchestes and Lagostrophus) for which peptide markers were previously developed (Peters et al., 2021) through the identification of peptide marker COL1α2 10–42 (m/z 3008 in Protemnodon). Similarly, Protemnodon can be differentiated from another genus of extinct kangaroo, Simosthenurus (Buckley et al., 2017a), using peptide markers COL1α1 586–618 (m/z 2897/2913 and m/z 2881/2897, respectively) and COL1α2 10–42 (m/z 3008 and m/z 2975, respectively).
Zygomaturus trilobus and Palorchestes azael can be differentiated from other extant and extinct large-bodied marsupials using a combination of peptide markers, most notably COL1α2 793–816 (Figure 3, m/z 2177), COL1α1 586–618 (m/z 2869/2885), and COL1α2 757–789 (m/z 2959/2975). It should be noted, however, that it is not possible to distinguish between the two species using ZooMS. The only observed difference between them is at peptide marker COL1α2 978–990, but since no peptide sequence could be confirmed for P. azael at this location, this peptide marker should not be used to make identifications. The absence of collagen peptide markers to differentiate between the two species does not necessarily reflect a phylogenetic signal. COL1 is a highly constrained protein with sequence mutations accumulating at a slow rate (Stover and Verrelli, 2011). As such, ZooMS can in many cases only be used to make genus- or family-level identifications (Richter et al., 2022).
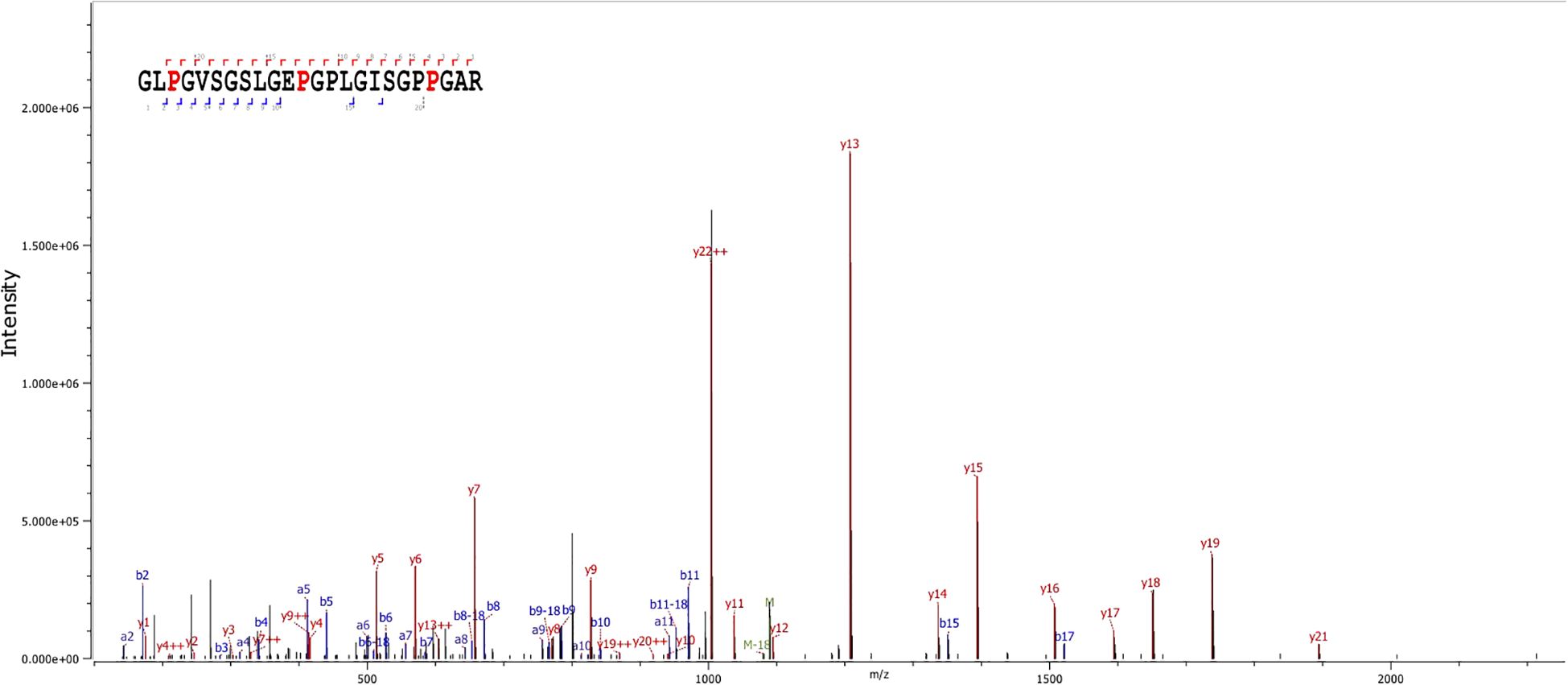
Figure 3. Example of MS/MS spectrum of peptide marker COL1α2 793–816 at m/z 2177 for Zygomaturus trilobus (MSB01; LC-MS/MS analysis code: DA1075).
4 Discussion and conclusion
We report collagen peptide markers for three extinct Australian marsupial megafauna taxa, Zygomaturus trilobus, Palorchestes azael, and Protemnodon mamkurra. The samples used in this study also further showcase the value of using leftover collagen or gelatin from radiocarbon dating and stable isotope analysis for palaeoproteomic analysis (e.g. Charlton et al., 2016; Mylopotamitaki et al., 2024; Smith et al., 2024). Here, this leftover gelatin was specifically used as reference samples for ZooMS peptide marker development, and, in doing so, minimizing the need for additional destructive sampling of valuable reference specimens of extinct taxa (Pálsdóttir et al., 2019).
With the addition of reference data for Zygomaturus trilobus, Palorchestes azael, and Protemnodon mamkurra, ZooMS can now be used to support the identification of four extinct Australian megafauna taxa. All of these taxa can be differentiated from extant marsupial species. However, collagen peptide markers were only developed for a single species per genus. This means that there is a reasonable possibility that other species within these genera will have identical peptide marker sets. For example, P. anak, another species of Protemnodon with a geographic range spanning eastern Australia (Kerr et al., 2024), and P. tumbuna, a species specific to New Guinea (Prideaux et al., 2022), are likely to have an identical set of peptide markers to Protemnodon mamkurra. Therefore, the peptide markers reported in this study can optimally be used to make genus- rather than species-level identifications.
Importantly, all of the specimens analyzed as part of this study, as well as those from Buckley et al. (2017a), originated from Tasmania or southern Australia, reflecting regions with cooler temperatures. This means that there is a regional bias in extinct megafauna species for which collagen peptide markers have been developed, as markers are currently only available for species from these more temperate regions of Australia. Regardless of this geographical bias, the fact that ZooMS peptide markers are genus- or (sub)family-specific in most instances (Janzen et al., 2021; Peters et al., 2021; Richter et al., 2022) means that the newly reported megafauna peptide markers can still be applied to identify possible megafauna specimens in fragmented assemblages across the country, even expanding into Papua New Guinea, which was formerly part of the palaeocontinent of Sahul prior to sea level rise at the end of the Last Glacial Maximum (Lambeck and Chappell, 2001) and accordingly closely related megafaunal taxa can be found there. For example, as mentioned previously, P. tumbuna can likely be identified with the Protemnodon peptide markers reported in this study. In addition, extinct Papua New Guinean species in the subfamily Zygomaturinae (e.g., Hulitherium, Kolopsoides, and Kolopsis) may have similar peptide markers to Zygomaturus. However, as is the case for Australia, issues with collagen preservation in the tropical and humid environment of Papua New Guinea may impact the success of ZooMS as a method for the identification of these extinct megafauna species. Nevertheless, these new ZooMS markers can be used to explore the possible presence of late surviving megafauna in the New Guinea Highlands where climatic conditions are more amenable to collagen preservation (Prideaux et al., 2022).
The development of collagen peptide markers for extinct Australian megafauna species represents a significant step in the establishment of ZooMS as a useful technique in addressing archaeological and paleontological research questions on the continent. Future work will be critical in expanding this reference library, as well as in applying the markers to the identification of bone assemblages from archaeological and paleontological sites. By expanding the body of identifiable megafauna specimens for Australia, these markers have the potential to play a key role in improving understanding of megafauna palaeobiology and palaeodemography, and in identifying megafauna specimens with collagen preservation suitable for subsequent stable isotope analysis and radiocarbon dating. Ultimately, we expect that the palaeoproteomic identification and analysis of megafauna specimens from localities across Sahul will provide important new insights into the long-debated extinction of megafauna in the late Quaternary.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: http://www.proteomexchange.org/, PXD053101; http://doi.org/10.52891/zenodo.14418148, Zenodo record 14418148; http://doi.org/10.25345/C5XW4872S, MSV000095033.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
CP: Conceptualization, Data curation, Formal Analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. AO: Investigation, Visualization, Writing – original draft, Writing – review & editing. RG: Resources, Writing – review & editing. NB: Conceptualization, Funding acquisition, Resources, Writing – review & editing. KD: Conceptualization, Funding acquisition, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research received funding from the Max Planck Society and the European Research Council (ERC) under the European Union's Horizon 2020 Research and Innovation Program (FINDER-StG-715069) awarded to Katerina Douka. Annette Oertle is funded by a Marie-Skłodowska-Curie postdoctoral fellowship (project DENI-CESTOR #101059683).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmamm.2025.1564287/full#supplementary-material
References
Antonosyan M., Hill E., Jodry M., Amano N., Brown S., Rick T., et al. (2024). A new legacy: Potential of Zooarchaeology by Mass Spectrometry in the analysis of North American megafaunal remains. Front. Mamm. Sci. 3. doi: 10.3389/fmamm.2024.1399358
Banks M. R., Colhoun E. A., van der Geer G. (1976). Late Quaternary Palorchestes azael (Mammalia, Diprotodontidae) from northwestern Tasmania. Alcheringia 1, 159–166. doi: 10.1080/03115517608619067
Bern M., Kil Y. J., Becker C. (2012). Byonic: Advanced peptide and protein identification software. Curr. Protoc. Bioinform. 13, 13.20.1–13.20.14. doi: 10.1002/0471250953.bi1320s40
Black K. H., Archer M., Hand S. J., Godthelp H. (2012). “The rise of Australian marsupials: A synopsis of biostratigraphic, phylogenetic, palaeoecologic and palaeobiogeographic understanding,” in Earth and Life (Springer Netherlands, Dordrecht), 983–1078. doi: 10.1007/978-90-481-3428-1_35
Bocherens H., Cotte M., Bonini R. A., Straccia P., Scian D., Soibelzon L., et al. (2017). Isotopic insight on paleodiet of extinct Pleistocene megafaunal Xenarthrans from Argentina. Gondwana Res. Int. Geosci. J. 48, 7–14. doi: 10.1016/j.gr.2017.04.003
Brook B. W., Bowman D. M. J. S. (2002). Explaining the Pleistocene megafaunal extinctions: Models, chronologies and assumptions. PNAS 99, 14624–14627. doi: 10.1073/pnas.232126899
Brown S., Douka K., Collins M. J., Richter K. K. (2021a). On the standardization of ZooMS nomenclature. J. Prot. 235, 104041. doi: 10.1016/j.jprot.2020.104041
Brown S., Wang N., Oertle A., Kozlikin M. B., Shunkov M. V., Derevianko A. P., et al. (2021b). Zooarchaeology through the lens of collagen fingerprinting at Denisova Cave. Sci. Rep. 11, 15457. doi: 10.1038/s41598-021-94731-2
Buckley M., Collins M. J., Thomas-Oates J., Wilson J. C. (2009). Species identification by analysis of bone collagen using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 23, 3843–3854. doi: 10.1002/rcm.4316
Buckley M., Cosgrove R., Garvey J., Prideaux G. J. (2017a). Identifying remains of extinct kangaroos in Late Pleistocene deposits using collagen fingerprinting. J. Quat. Sci. 32, 653–660. doi: 10.1002/jqs.2964
Buckley M., Harvey V. L., Chamberlain A. T. (2017b). Species identification and decay assessment of Late Pleistocene fragmentary vertebrate remains from Pin Hole Cave (Creswell Crags, UK) using collagen fingerprinting. Boreas 46, 402–411. doi: 10.1111/bor.12225
Canteri E., Brown S. C., Schmidt N. M., Heller R., Nogués-Bravo D., Fordham D. A. (2022). Spatiotemporal influences of climate and humans on muskox range dynamics over multiple millennia. Glob. Change Biol. 28, 6602–6617. doi: 10.1111/gcb.16375
Chappell J., Head J., Magee J. (1996). Beyond the radiocarbon limit in Australian archaeology and Quaternary research. Antiquity 70, 543–552. doi: 10.1017/S0003598X00083708
Charlton M., Alexander M., Collins M., Milner N., Mellars P., O´Connell T. C., et al. (2016). Finding Britain´s last hunter-gatherers: a new biomolecular approach to ´unidentifiable´ bone fragments utilizing bone collagen. J. Archaeol. Sci. 73, 55–61. doi: 10.1016/j.jas.2016.07.014
DeSantis L. R. G., Field J. H., Wroe S., Dodson J. R. (2017). Dietary responses of Sahul (Pleistocene Australia–New Guinea) megafauna to climate and environmental change. Paleobiology 43, 181–195. doi: 10.1017/pab.2016.50
Douka K., Brown S., Higham T., Pääbo S., Derevianko A., Shunkov M. (2019). FINDER project: Collagen fingerprinting (ZooMS) for the identification of new human fossils. Antiquity 93, e1. doi: 10.15184/aqy.2019.3
Fellows Yates J. A., Drucker D. D., Reiter E., Heumos S., Welker F., Münzel S. C., et al. (2017). Central European woolly mammoth population dynamics: Insights from Late Pleistocene mitochondrial genomes. Sci. Rep. 7, 17714. doi: 10.1038/s41598-017-17723-1
Gill E. D., Banks M. R. (1956). Cainozoic history of the Mowbray Swamp and other areas of north-western Tasmania. Rec. Queen Victoria Museum (Launceston) 6, 1–14.
Gillespie R., Camens A. B., Worthy T. H., Rawlence N. J., Reid C., Bertuch F., et al. (2012). Man and megafauna in Tasmania: Closing the gap. Quat. Sci. Rev. 37, 38–47. doi: 10.1016/j.quascirev.2012.01.013
Gillespie R., Wood R., Fallon S., Stafford T. W. Jr., Southon J. (2015). New 14C dates for Spring Creek and Mowbray Swamp megafauna: XAD-2 processing. Archaeol. Oceania 50, 43–48. doi: 10.1002/arco.5045
González-Guarda E., Domingo L., Tornero C., Pino M., Hernández Fernández M., Sevilla P., et al. (2017). Late Pleistocene ecological, environmental and climatic reconstruction based on megafauna stable isotopes from northwestern Chilean Patagonia. Quat. Sci. Rev. 170, 188–202. doi: 10.1016/j.quascirev.2017.06.035
Haile J., Froese D. G., Macphee R. D. E., Roberts R. G., Arnold L. J., Reyes A. V., et al. (2009). Ancient DNA reveals late survival of mammoth and horse in interior Alaska. PNAS 106, 22352–22357. doi: 10.1073/pnas.0912510106
Heddell-Stevens P., Jöris O., Britton K., Matthies T., Lucas M., Scott E., et al. (2024). Multi-isotope reconstruction of Late Pleistocene large-herbivore biogeography and mobility patterns in Central Europe. Commun. Biol. 7, 568. doi: 10.1038/s42003-024-06233-2
Higham T. F. G., Jacobi R. M., Bronk Ramsay C. (2006). AMS radiocarbon dating of ancient bone using ultrafiltration. Radiocarbon 48, 179–195. doi: 10.1017/S0033822200066388
Janzen A., Richter K. K., Mwebi O., Brown S., Onduso V., Gatwiri F., et al. (2021). Distinguishing African bovids using Zooarchaeology by Mass Spectrometry (ZooMS): New peptide markers and insights into Iron Age economies in Zambia. PLoS One 16, e0251061. doi: 10.1371/journal.pone.0251061
Johnson C. (2006). Australia’s mammal extinctions: A 50,000-year history (Cambridge, England: Cambridge University Press).
Kerr I. A. R., Camens A. B., Van Zoelen J. D., Worthy T. H., Prideaux G. J. (2024). Systematics and palaeobiology of kangaroos of the Late Cenozoic genus Protemnodon (Marsupialia, Macropodidae). Megataxa 11, 1–261. doi: 10.11646/megataxa.11.1.1
Koch P. L., Barnosky A. D. (2006). Late Quaternary extinctions: State of the debate. Ann. Rev. Ecol. Evol. System. 37, 215–250. doi: 10.1146/annurev.ecolsys.34.011802.132415
Koutamanis D., McCurry M., Tacail T., Dosseto A. (2023). Reconstructing Pleistocene Australian herbivore megafauna diet using calcium and strontium isotopes. R. Soc Open Sci. 10, 230991. doi: 10.1098/rsos.230991
Kubiak C., Grimes V., Van Biesen G., Keddie G., Buckley M., Macdonald R., et al. (2023). Dietary niche separation of three Late Pleistocene bear species from Vancouver Island, on the Pacific northwest coast of North America. J. Quat. Sci. 38, 8–20. doi: 10.1002/jqs.3451
Lambeck K., Chappell J. (2001). Sea level change through the Last Glacial Cycle. Science 292, 679–686. doi: 10.1126/science.1059549
Llamas B., Brotherton P., Mitchell K. J., Templeton J. E. L., Thomson V. A., Metcalf J. L., et al. (2014). Late Pleistocene Australian marsupial DNA clarifies the affinities of extinct megafaunal kangaroos and wallabies. Mol. Bio. Evol. 32, 574–584. doi: 10.1093/molbev/msu338
Long J., Archer M., Flannery T. F., Hand S. J. (2002). Prehistoric mammals of Australia and New Guinea: one hundred million years of evolution (Baltimore: John Hopkins University Press).
Lorenzen E. D., Nogués-Bravo D., Orlando L., Weinstock J., Binladen J., Marske K. A., et al. (2011). Species-specific responses of Late Quaternary megafauna to climate and humans. Nature 479, 359–364. doi: 10.1038/nature10574
Ma J., Wang Y., Jin C., Hu Y., Bocherens H. (2019). Ecological flexibility and differential survival of Pleistocene Stegodon orientalis and Elephas maximus in mainland Southeast Asia revealed by stable isotope (C, O) analysis. Quat. Sci. Rev. 212, 33–44. doi: 10.1016/j.quascirev.2019.03.021
Mylopotamitaki D., Fewlass H., Zavala E. I., Rougier H., Sümer A., Hajdinjak M., et al. (2024). Homo sapiens reached the higher latitudes of Europe by 45,000 years ago. Nature 626, 341–346. doi: 10.1038/s41586-023-06923-7
Pálsdóttir A. H., Bläuer A., Rannamäe E., Boessenkool S., Hallsson J. H. (2019). Not a limitless resource: ethics and guidelines for destructive sampling of archaeofaunal remains. R. Soc Open Sci. 6, 191059. doi: 10.1098/rsos.191059
Pečnerová P., Palkopoulou E., Wheat C. W., Skoglund P., Vartanyan S., Tikhonov A., et al. (2017). Mitogenome evolution in the last surviving woolly mammoth population reveals neutral and functional consequences of small population size. Evol. Lett. 1, 292–303. doi: 10.1002/evl3.33
Peters C., Richter K. K., Manne T., Dortch J., Paterson A., Travouillon K., et al. (2021). Species identification of Australian marsupials using collagen fingerprinting. R. Soc Open Sci. 8, 211229. doi: 10.1098/rsos.211229
Peters C., Wang Y., Vakil V., Cramb J., Dortch J., Hocknull S., et al. (2023). Bone collagen from subtropical Australia is preserved for more than 50,000 years. Commun. Earth Environ. 4, 1–8. doi: 10.1038/s43247-023-01114-8
Pledge N. S. (1991). Occurrences of palorchestes species (Marsupialia: palorchestidae) in South Australia. Rec. S. Aust. Mus. 25, 161–174.
Prescott G. W., Williams D. R., Balmford A., Green R. E., Manica A. (2012). Quantitative global analysis of the role of climate and people in explaining late Quaternary megafaunal extinctions. PNAS 109, 4527–4531. doi: 10.1073/pnas.1113875109
Price G. J., Ferguson K. J., Webb G. E., Feng Y.-F., Higgins P., Nguyen A. D., et al. (2017). Seasonal migration of marsupial megafauna in Pleistocene Sahul (Australia-New Guinea). Proc. R. Soc B. Biol. Sci. 284, (1863). doi: 10.1098/rspb.2017.0785
Prideaux G. J., Ayliffe L. K., DeSantis L. R. G., Schubert B. W., Murray P. F., Gagan M. K., et al. (2009). Extinction implications of a chenopod browse diet for a giant Pleistocene kangaroo. PNAS 106, 11646–11650. doi: 10.1073/pnas.0900956106
Prideaux G. J., Kerr I. A. R., van Zoelen J. D., Grün R., van der Kaars S., Oertle A., et al. (2022). Re-evaluating the evidence for late-surviving megafauna at Nombe Rockshelter in the New Guinea Highlands. Archaeol. Oceania 57, 223–248. doi: 10.1002/arco.5274
Rabanus-Wallace M. T., Wooller M. J., Zazula G. D., Shute E., Jahren A. H., Kosintsev P., et al. (2017). Megafaunal isotopes reveal role of increased moisture on rangeland during Late Pleistocene extinctions. Nat. Ecol. Evol. 1, 125. doi: 10.1038/s41559-017-0125
Richards H. L., Wells R. T., Evans A. R., Fitzgerald E. M. G., Adams J. W. (2019). The extraordinary osteology and functional morphology of the limbs in Palorchestidae, a family of strange extinct marsupial giants. PLoS One 14, e0221824. doi: 10.1371/journal.pone.0221824
Richter K. K., Codlin M. C., Seabrook M., Warinner C. (2022). A primer for ZooMS applications in archaeology. PNAS 119, e2109323119. doi: 10.1073/pnas.2109323119
Richter K. K., McGrath K., Masson-MacLean E., Hickinbotham E., Tedder A., Britton K., et al. (2020). What’s the catch? Archaeological application of rapid collagen-based species identification for Pacific salmon. J. Archaeol. Sci. 116, 105116. doi: 10.1016/j.jas.2020.105116
Seersholm F. V., Werndly D. J., Grealy A., Johnson T., Keenan Early E. M., Lundelius E. L. Jr., et al. (2020). Rapid range shifts and megafaunal extinctions associated with Late Pleistocene climate change. Nat. Commun. 11, 2770. doi: 10.1038/s41467-020-16502-3
Sinet-Mathiot V., Rendu W., Steele T. E., Spasov R., Madelaine S., Renou S., et al. (2023). Identifying the unidentified fauna enhances insights into hominin subsistence strategies during the Middle to Upper Palaeolithic transition. Archaeol. Anthropol. Sci. 15, 139. doi: 10.1007/s12520-023-01830-4
Smith G. M., Ruebens K., Zavala E. I., Sinet-Mathiot V., Fewlass H., Pederzani S., et al. (2024). The ecology, subsistence and diet of ~45,000-year-old Homo sapiens at Ilsenhöhle in Ranis, Germany. Nat. Ecol. Evol. 8, 564–577. doi: 10.1038/s41559-023-02303-6
Stover D. A., Verrelli B. C. (2011). Comparative vertebrate evolutionary analyses of type I collagen: Potential of COL1a1 gene structure and intron variation for common bone-related diseases. Mol. Biol. Evol. 28, 533–542. doi: 10.1093/molbev/msq221
Strohalm M., Kavan D., Novák P., Volný M., Havlícek V. (2010). mMass 3: A cross-platform software environment for precise analysis of mass spectrometric data. Anal. Chem. 82, 4648–4651. doi: 10.1021/ac100818g
Stuart A. J. (2014). Late Quaternary megafaunal extinctions on the continents: a short review. Geol. J. 50, 338–363. doi: 10.1002/gj.2633
Stuart A. J., Lister A. M. (2012). Extinction chronology of the woolly rhinoceros Coelondonta antiquitatis in the context of late Quaternary megafaunal extinctions in northern Eurasia. Quat. Sci. Rev. 51, 1–17. doi: 10.1016/j.quascirev.2012.06.007
Swift J. A., Bunce M., Dortch J., Douglass K., Tyler Faith J., Fellows Yates J. A., et al. (2019). Micro methods for megafauna: Novel approaches to Late Quaternary extinctions and their contributions to faunal conservation in the Anthropocene. Bioscience 69, 877–887. doi: 10.1093/biosci/biz105
Trayler R. B., Dundas R. G., Fox-Dobbs K., Van De Water P. K. (2015). Inland California during the Pleistocene-Megafaunal stable isotope records reveal new paleoecological and paleoenvironmental insights. Palaeogeogr. Palaeoclimatol. Palaeoecol. 437, 132–140. doi: 10.1016/j.palaeo.2015.07.034
Varela L., Clavijo L., Tambusso P. S., Fariña R. A. (2023). A window into a Late Pleistocene megafauna community: Stable isotopes show niche partitioning among herbivorous taxa at the Arroyo Del Vizcaíno site (Uruguay). Quat. Sci. Rev. 317, 108286. doi: 10.1016/j.quascirev.2023.108286
Wang N., Xu Y., Tang Z., He C., Hu X., Cui Y., et al. (2023). Large-scale application of palaeoproteomics (Zooarchaeology by Mass Spectrometry; ZooMS) in two Palaeolithic faunal assemblages from China. Proc. R. Soc B Biol. Sci. 290, 20231129. doi: 10.1098/rspb.2023.1129
Webb S. (2008). Megafauna demography and late Quaternary climatic change in Australia: a predisposition to extinction. Boreas 37, 329–345. doi: 10.111/j.1502-3885.2008.00026.x
Welker F., Soressi M., Rendu W., Hublin J.-J., Collins M. J. (2015). Using ZooMS to identify fragmentary bone from the Late Middle/Early Upper Palaeolithic sequence of Les Cottés, France. J. Archaeol. Sci. 54, 279–286. doi: 10.1016/j.jas.2014.12.010
Wooller M. J., Bataille C., Druckenmiller P., Erickson G. M., Groves P., Haubenstock N., et al. (2021). Lifetime mobility of an Arctic woolly mammoth. Science 373, 806–808. doi: 10.1126/science.abg1134
Keywords: Zooarchaeology by Mass Spectrometry, Diprotodontidae, Palorchestidae, Macropodidae, late Quaternary
Citation: Peters C, Oertle A, Gillespie R, Boivin N and Douka K (2025) Collagen peptide markers for three extinct Australian megafauna species. Front. Mamm. Sci. 4:1564287. doi: 10.3389/fmamm.2025.1564287
Received: 21 January 2025; Accepted: 04 April 2025;
Published: 03 June 2025.
Edited by:
Larisa R. G. DeSantis, Vanderbilt University, United StatesReviewed by:
Youri Van Den Hurk, Norwegian University of Science and Technology, NorwayJose Luis Prado, National University of Central Buenos Aires, Argentina
Copyright © 2025 Peters, Oertle, Gillespie, Boivin and Douka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carli Peters, cGV0ZXJzQGdlYS5tcGcuZGU=; Katerina Douka, a2F0ZXJpbmEuZG91a2FAdW5pdmllLmFjLmF0
 Carli Peters
Carli Peters Annette Oertle3,4
Annette Oertle3,4 Richard Gillespie
Richard Gillespie