- 1College of Forest Resources and Environmental Science, Michigan Technological University, Houghton, MI, United States
- 2College of Veterinary Medicine, Oklahoma State University, Stillwater, OK, United States
Due to their semi-aquatic lifestyles, otters are exposed to pathogens from both terrestrial and aquatic systems. Although parasites are a natural part of any system, they are a source of environmental stress for Lutrinae of which twelve of the fourteen species are currently listed as threatened or endangered. Climate change, fragmentation, habitat loss, and increasing interactions with humans are directly affecting otter populations and increasing the risk of exposure to diseases. Identifying parasitic diseases and the potential threat to otters is the first step in minimizing this stressor. In this paper, we systematically reviewed the literature and summarized parasitic diseases reported for Lutrinae. We analyzed the overall prevalence of the parasites reported and noted major emerging parasites of concern for otter species. Overall, there were 146 genera representing 164 parasite species listed for 10 otter species. No parasite studies were found for Smooth-coated Otters (Lutrogale perspicillata), Hairy-nosed Otters (Lutra sumatrana), and Congo Clawless Otters (Aonyx congicus). Published studies were also limited for 7 additional otter species indicating a need for surveys of parasites in otters.
1 Introduction
Parasites are eukaryotic organisms that live on or within another living organism to obtain food resources without causing direct death to the host (Poulin, 2007). Parasites are part of any ecological system and contribute to the overall biodiversity and function of the food web (Thompson et al., 2010). Mild infections or infestations of parasites particularly in young animals may help the host to develop immunity and in turn improve the overall lifetime health of an individual (Spencer and Zuk, 2016). However, many parasites are pathogenic and may cause further declines in species that are vulnerable or endangered (McCallum and Dobson, 1995). The effects of parasites are often overlooked in wildlife populations (Chretien et al., 2023).
Parasites directly affect populations by causing deaths of individuals or indirectly through declines in birth rate and life span (Mathews, 2009). A more immediate effect of parasitic infections or infestations are changes to individual host behavior that could lead to trophic and community changes (Selbach et al., 2022; Sures et al., 2017). Parasitic infections or infestations may also compound the effect of impaired immunological response to other environmental stressors such as pollution (Grabner et al., 2023). In aquatic environments, increases in these environmental stressors are mostly linked to human disturbances (Adlard et al., 2015). Human disturbance may introduce new diseases or increase the virulence of existing diseases via evolutionary processes (Adlard et al., 2015). Environmental stressors may also cause the loss of the parasite species themselves or decouple the host-parasite relationship (Sures et al., 2023). Parasite species with multiple hosts as part of their life cycle are more susceptible to environmental stressors whereas parasites with single hosts are more likely to be successful in stressed environments particularly if the host is tolerant of the altered conditions (Johnson and Paull, 2011; Sures et al., 2023). Considering several recent zoonotic outbreaks, there are growing concerns about wildlife as reservoirs for parasites that cause disease (Kruse et al., 2004; Salvarani et al., 2025; Winter and Landaeta-Aqueveque, 2025). Not only is it important to determine parasites that may transfer from wildlife to humans but also the parasites that may be moving into natural systems from humans and domestic animals (Thompson et al., 2010). Parasites of domestic animals may enter wildlife populations through spillovers and new parasites are introduced to ecosystems through human translocation of host species (Daszak et al., 2001).
Freshwater systems often act as points of transmission for disease due to the need for drinking water by host organisms, the reliance on water for parasite mobility for at least part of their life cycles, and the limited availability of freshwater in many ecosystems creating ecological hotspots (Johnson and Paull, 2011; Titcomb et al., 2021). As water is needed for humans as well for drinking, agriculture, and transportation, there is a natural draw for human development to occur along both freshwater systems (Wang and He, 2022) and marine coastal environments (Davis, 2005).
Otters are a group of species that live in aquatic systems that also serve as indicators of overall watershed health. The members of Lutrinae, a subfamily of Mustelidae, include 14 species of otters of which 12 are listed as near-threatened, vulnerable, or endangered by the IUCN (IUCN, 2025). One species, Lontra annectens, has not been evaluated by the IUCN due to its recent taxonomic split from Lontra longicaudis (de Ferran et al., 2024). All the members of this sub-family are semi-aquatic with 12 species occurring in freshwater systems and 2 species found exclusively in marine systems (Enhydra lutris and Lontra felina). They feed mainly on fish, amphibians, and aquatic invertebrates such as mussels. Most of the otter species declines have been caused by overharvest and loss of habitat (Duplaix and Savage, 2018). However, the Global Otter Conservation Strategy listed diseases transmitted mainly from domestic animals as major threats for five species of otter (Enhydra lutris, Pteronura brasiliensis, Lontra longicaudis, Lontra felina, and Lontra provocax) including diseases caused by parasites (Duplaix and Savage, 2018). In the Order Carnivora, the Mustelidae family has the most host species for zoonotic parasites although the pathway of transmission is lacking for many mustelid host species (Han et al., 2021).
For most wildlife species, a comprehensive list of parasites does not exist (Mathews, 2009). Little is also known about which parasites are pathogenic or the susceptivity of species to these pathogens (Thompson, 2013). This includes the otter species of the world. The purpose of this paper is to 1) compile a list of the parasite species that have been found in otter species around the globe 2) estimate prevalence for each parasite species and 3) identify parasite species that are currently a threat to otter populations or have the potential to become a concern.
2 Methods
We conducted a systematic literature review following the PRISMA format (Page et al., 2021). Literature searches were conducted using the words “otter + parasite” and “otter + disease” in the Google Scholar, ProQuest Dissertation and Thesis, and Web of Science databases. For our definition of parasite, we included any eukaryotic organism that lives on or within another living species. We also included pathogens that may be transmitted by parasites such as ticks that carry Rickettsia spp. Lists were compiled, and any duplicates were removed. Then each title was reviewed by both researchers for relevance. Any unrelated papers were removed at this step. Abstracts were collected for all remaining papers and reviewed by both researchers. Again, any paper that did not seem relevant was removed from the list. All remaining papers were read completely and if the paper met the requirements, data were collected. Papers needed to include at least one parasite species that had infected one or more otter species and reported the prevalence of the parasitic infection. Basic paper information including author, publication date, study dates, and location was also recorded. Literature cited sections for each paper were also considered and any papers not on the original list were added to the final list. Papers that were reviews or summaries of other studies were read, and citations were compiled, however, data were not recorded as the reviews repeated information already reported in empirical papers.
Parasites, otter species, number of otters sampled, and number of positive cases were recorded for each paper. When possible, data were collected on the sex of the otters, age of the otters, weight of otters, whether otter were alive or dead, method of parasite detection, anatomical locations of the parasites, and whether the infections or infestations were subclinical or clinical.
Data collected were sorted by parasite species and otter species. Samples from the same parasite and otter species were combined to generate a total number of positive cases and total number of individuals sampled. From these values, prevalence was calculated for each parasite and otter species. For each percentage, a 95% confidence interval (95% CI) was calculated using a modified Wald method for proportions.
3 Results
3.1 Literature review results
Literature searches occurred on December 14, 2024. A total of 4515 sources were compiled from the database searches with 2000 hits from Google Scholar, 990 from Web of Science and 1525 from ProQuest Dissertation and Theses. After removal of duplicates, 2861 papers remained (Figure 1). Papers were reviewed by title by both authors and any papers that were not relevant were removed which left a total of 1134. Both authors reviewed the abstracts and again removed any irrelevant papers which left 325 papers for full review (Supplementary 1 (S1). In the full paper review additional papers were removed 1) if the paper was a review paper that summarized other studies, 2) if the paper did not report on otter species or parasites, and/or 3) if the paper duplicated what had been published in a thesis or dissertation. There were also 9 abstracts where the full paper could not be located. Any citations from the read papers that were relevant and not already included were also added to the list (n=18). The final list of papers from which data were collected was 240 papers. The earliest paper on parasites in otters was by Viana in 1924. The number of publications has steadily increased each decade with 76 publications in 2010–2019 and the 2020’s on track to at least meet or exceed that number (Figure 2). Figure 3 shows the distribution of the study areas with a majority of the studies occurring in Europe and North America.
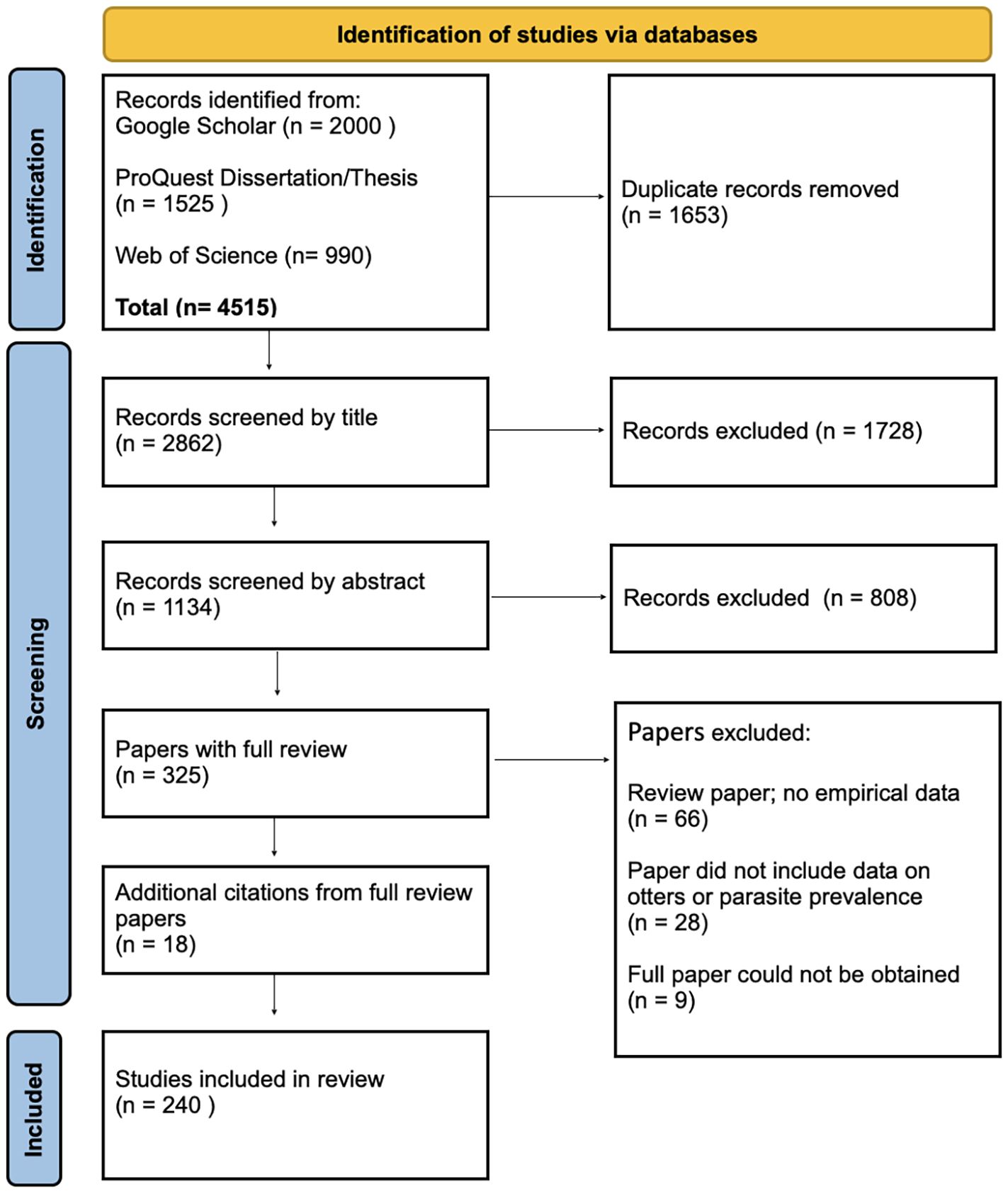
Figure 1. PRISMA 2020 flow diagram for systemic review of parasites in otters. Chart modified from Page MJ, et al. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. This work is licensed under CC BY 4.0. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.
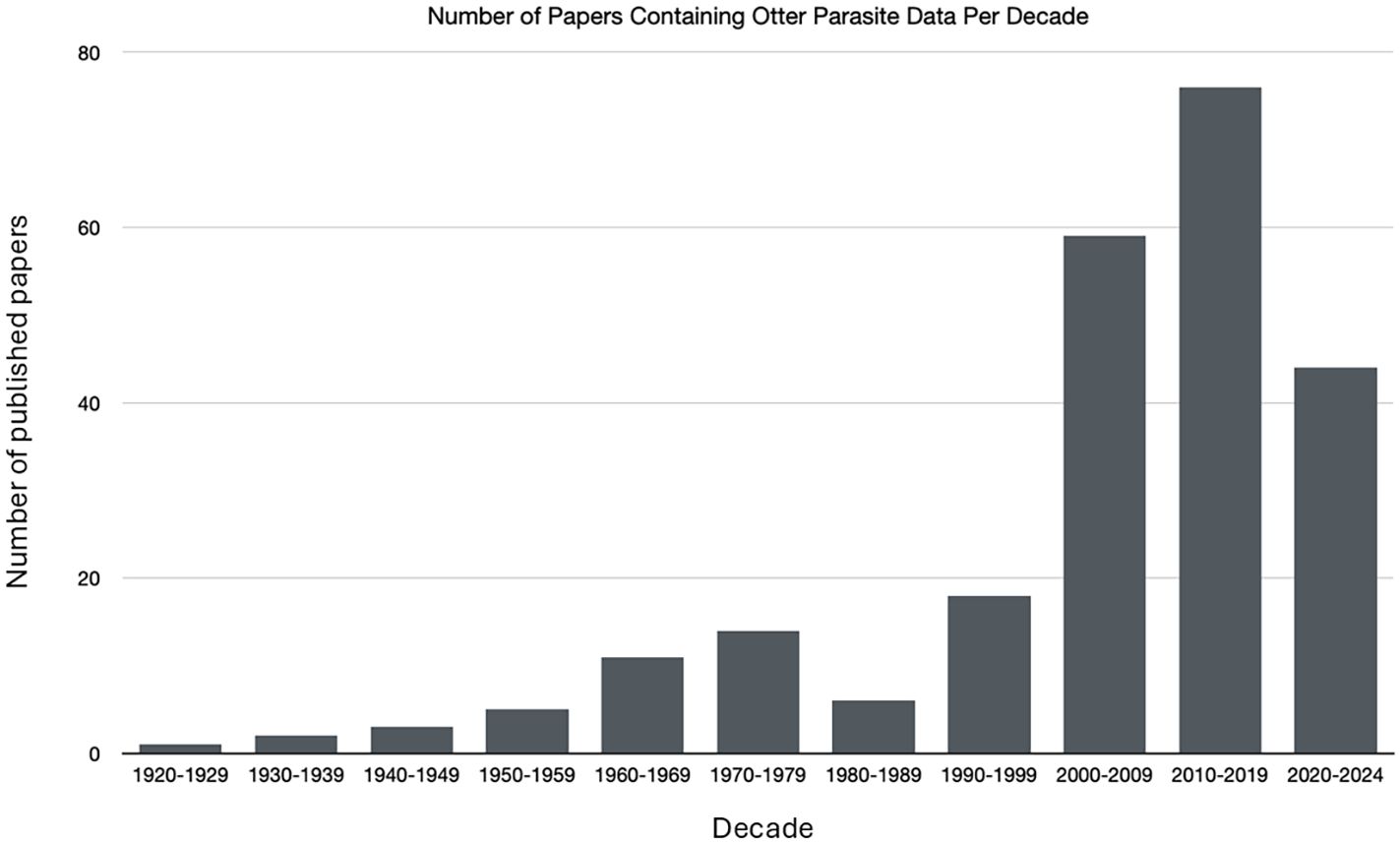
Figure 2. Number of published papers containing otter parasite data by decade. Note that the 2020 decade only contains four years.
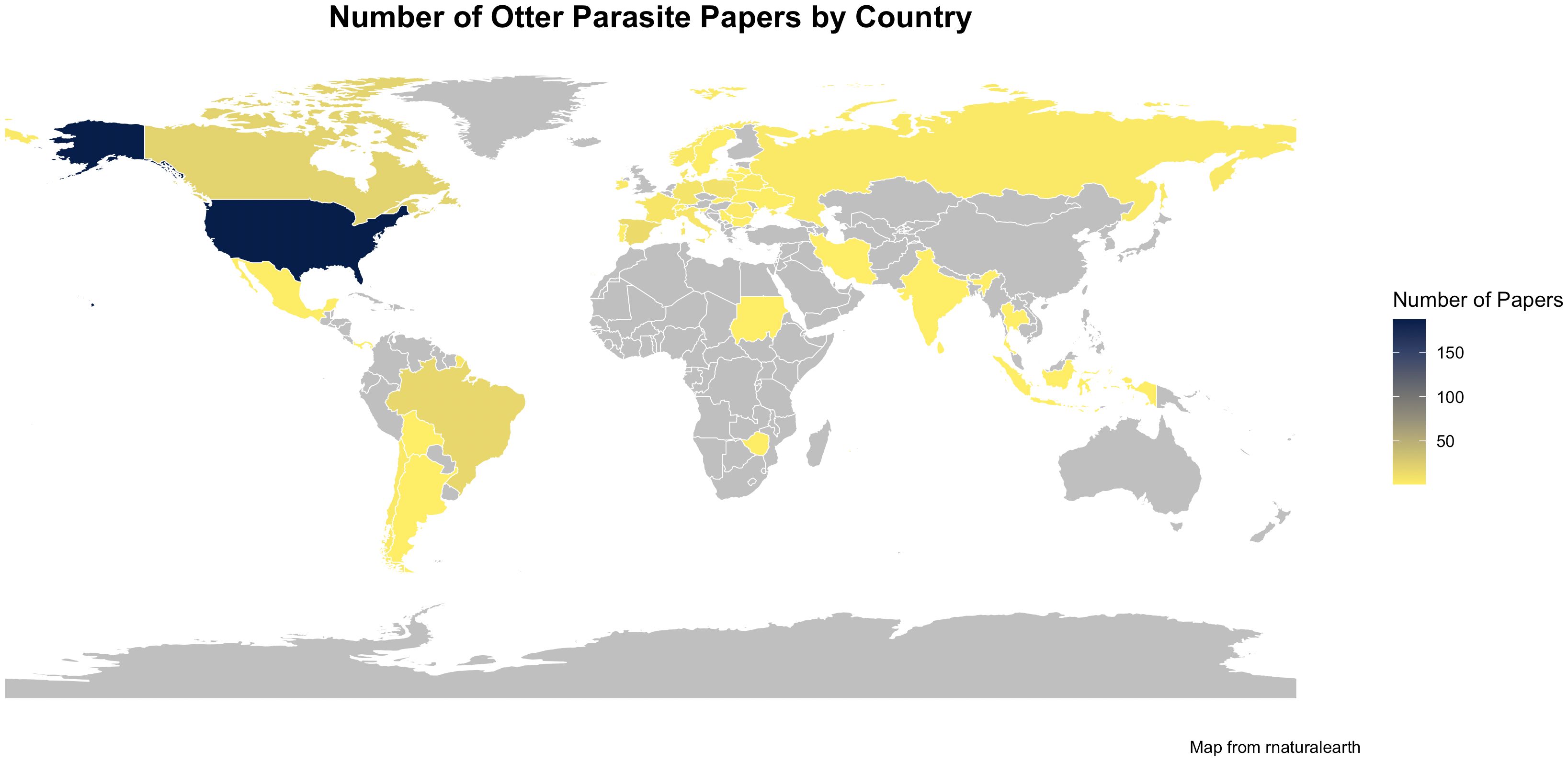
Figure 3. Map of the number of published otter parasite papers by country. No papers were found in countries in gray.
3.2 Otter results
There are 14 species of otters worldwide, but parasites were not reported for all species. Supplementary 2 (S2) lists the otter species and number of papers that were recorded. Most studies centered on the Eurasian otter (Lutra lutra) (n= 89) followed by the Sea Otter (Enhydra lutris) (n=79) and North American otter (Lontra canadensis) (n= 47). Three species of otter, Smooth-coated Otter (Lutrogale perspicillata), Hairy-nosed Otter (Lutra sumatrana), and Congo Clawless Otter (Aonyx congicus) did not have any published literature on parasites in the databases that were reviewed. Note that de Ferran et al. (2024) proposed that Lontra longicaudis should be split into a fourteenth species (Lontra annectens), however, for the purpose of this paper, we did not split the species since there was not time before our analysis for parasites to be reported for L. annectens in the literature. There was also not a method to accurately determine whether any of the parasites previously reported for L. longicaudis were found in L. annectens.
The Eurasian Otter (Lutra lutra) had the highest number of parasite species listed (n=184). The North American River Otter (Lontra canadensis) had 66 parasite species reported, and the Sea Otter (Enhydra lutris) had 41 species reported. Only one species has been recorded in both the Marine Otter (Lontra felina) and Southern River Otter (Lontra provocax). S2 lists each otter species and the number of species recorded for each phylum of parasite as well. The Phylum Apicomplexa had the highest number of parasite species for the Marine Otter (Lontra felina) and Southern River Otter (Lontra provocax). Phylum Nemotoda contained the most species for Eurasian Otter, North American River Otter, Giant Otter, and Neotropical Otter. Phylum Platyhelminthes held the most parasite species for the Spotted-necked Otter, African Clawless Otter, and Sea Otter. The Asian Small-clawed Otter had two species in each of the Phylum Apicomplexa and Phylum Nematoda.
3.3 Specific phyla results
Parasites of otters fell into five major phyla of Protozoa (Apicomplexa), Thorny-headed Worms (Acanthocephala), Roundworms (Nematoda), Flatworms (Platyhelminthes), ticks, mites, and insects (Arthropoda), and the remaining species were categorized as “tick-borne bacteria and other parasites” with 9 phyla represented. Overall, there were 146 genera representing 164 species. The following sections describe further details for each group including parasites that are specific to otters as well as highly pathogenic parasites that may affect otter populations.
3.4 Phylum Apicomplexa (protozoa)
Apicomplexans reported in otters comprised 9 genera and 15 species. Supplementary 3 (S3) Apicomplexa sheet contains the full list of parasites, otter species, and prevalence. Otters are considered the definitive hosts for Cystoisospora gottschalki and Sarcocystis lutrae. Definitive hosts for Hepatozoon martis are mustelids. All Apicomplexa in otters are pathogenic. However, three parasites are considered highly pathogenic including Neospora caninum (Dubey, 2003), Sarcocystis neurona (Dubey et al., 2015), and Toxoplasma gondii (Sanchez and Besteiro, 2021). Otters are accidental hosts of these three parasites and infections may be fatal (Dubey et al., 2003). Neospora caninum was found in the Sea Otter with overall prevalence of 19.4% (n=278; 95% CI 15.2-24.5%) and in the Eurasian Otter with prevalence of 1.1% (n= 91; 95% CI 0.01-6.6%). Sarcocystis neurona in Sea Otter had an overall prevalence of 18.5% (n=1612; 95% CI 16.7-20.5%). Five species of otter were positive for Toxoplasma gondii and had the highest number of samples in Phylum Apicomplexa. The Asian-clawed Otter had the highest prevalence at 100% (95% CI 51.1-100%) but also the smallest sample size of 5. The prevalence of T. gondii was 76.9% (n=13; 95% CI 49.1-92.5%) in the Southern River Otter, 29.2% (n=489; 95% CI 25.4-33.4%) in the North America River Otter, 14.3% (n=1008; 95% CI 12.3-16.6%) in the Eurasian Otter, 12.6% (n=5307; 95% CI 11.7-13.5%) in the Sea Otter, and 5.3%(n=19; 95% CI 0.01-26.5%) in the Marine Otter. Five papers also identified Sea Otters with co-infections of S. neurona and T. gondii. Prevalence of co-infection was 5.1% (n=1350; 95% CI 4.1-6.4%). Apicomplexa were located in the spleen, liver, blood, gastric mucosa, intestines, diaphragm, tongue, skeletal muscle, heart, central nervous system, and brain.
3.5 Phylum Acanthocephala (thorny-headed worms)
Acanthocephalans reported for otters included 6 genera and 10 species. S3 Acanthocephala sheet lists specific Acanthocephala and prevalences in otter species. Thorny-headed worms in otters are considered pathogenic but none are considered highly pathogenic. Corynosoma enhydri is a specific parasite of the Sea Otter (Mayer et al., 2003). Corynosoma enhydri had a prevalence of 84.4% (95% CI 80.5-87.6%) in the 404 otters sampled in 5 studies (Hennessy and Morejohn, 1977; Kikuchi and Nakajima, 1993; Margolis, 1997; Mayer et al., 2003; Shanebeck et al., 2020). Thorny-headed worms were found in the gastrointestinal tract including the stomach, intestines, and alimentary canal of infected otters.
3.6 Phylum Nematoda (roundworms)
Nematodes were the most common group of parasites found in otters with 58 genera and 58 species represented. S3 Nematoda sheet provides the full list of roundworms, otter hosts, and prevalence. Most of the listed species are considered pathogenic.
Dirofilaria lutrae, Dracunculus lutrae, Skrjabingylus lutrae, and Strongyloides lutrae are specific parasites of otters. Prevalence of Dirofilaria lutrae in North American River Otters was 16.5% (n=243; 95% CI 12.3-21.7%). Dracunculus lutrae prevalence was 87.7% (n=203; 95% CI 82.4-91.6%) in North American River Otters. Also in North American River Otters, Skrjabingylus lutrae prevalence was 7.6% (n=118; 95% CI 3.9-14.0%) and Strongyloides lutrae prevalence was 29.2% (n=48; 95% CI 18.2-43.3%). Strongyloides lutrae was also reported in Eurasian otters with prevalence of 7.7% (n=516; 95% CI 5.6-10.4%). Otters are the definitive hosts of Eucoleus schvalovoj with a prevalence of 36.7% (n=188; 95% CI 29.8-44.0%) and Globocephalis lutrae with a prevalence of 7.7% (n=13; 95% CI 0.01-35.4%) reported in Eurasian Otters. Capillaria mucronata, Capillaria mustelae, and Capillaria putori primarily infest mustelids. Prevalence of C. mucronata was 18.7% in Eurasian Otters (n=123; 95% CI 12.7-26.6%) and C. putori prevalence was 10.5% in Eurasian Otters (n=76; 95% CI 5.2-19.7%). Aonchotheca putorii is typically found in terrestrial mustelids and was recorded in the Eurasian Otter with a prevalence of 6.4% (n=109; 95% CI 2.93-12.88%)Ancylostoma spp. and Hystichis spp. are all considered highly pathogenic in otters. Ancylostoma spp. are generalist parasites in mammals and Hystichis spp. is a generalist parasite of waterfowl. Ascaris spp. was also reported for otters, but this genus has only two identified species that have humans and swine hosts. It is likely that Ascaris spp. reported is another ascarid nematode that has a similar egg. Capilliaria hepatica, Dirofilaria immitis, Trichinella spiralis, and Trichinella britovi are classified as generalist parasites of mammals. Skrjabingylus nasicola is specific to mustelids and is considered highly pathogenic to otters. Accidental infestations of Anisakis simplex (marine fish and invertebrate hosts), Pseudoterranova decipiens (marine fish hosts), Toxocara cati (feline hosts) and Trichurus vulpis (canid hosts) occurred, however, otters are not considered normal hosts for these parasites. Toxocara canis and Heterakis gallinarum were screened in 38 and 7 Eurasian Otters, respectively, but no otters were found with the parasites. In samples with more than 10 animals, prevalence was below 10% for most highly pathogenic parasites except for Ascaris spp. and Capillaria spp. Ascaris spp. prevalence was 13.9% in 36 North American River Otter (95% CI 5.8-29.1%). Capillaria spp. prevalence was 30% in 20 North America River Otter (95% CI 14.3-52.1%).
Roundworms are found in many locations in the otters including the heart, lungs, intestines, stomach, urinary bladder, liver, kidneys, subcutaneous layers, connective tissues, and muscle tissue.
Free-living soil nematode species, Aphelenchus avenae, Diploscapter spp., and Prionchulus spp., were found in 7 Eurasian Otters. Aphelenchus avenue was identified in one Eurasian Otter (Woo et al., 2023).
3.7 Phylum Platyhelminthes (flatworms)
Flatworms were also commonly found in otter species with a total of 47 genera and 52 species described in the literature. A full list of the parasites, otter species, and prevalence for each is in S3 Platyhelminthes sheet. All the members of this group are considered pathogenic or potentially pathogenic.
Sea Otters are the definitive host for Microphallus enhydrae but the parasite has only been observed in one study with a prevalence of 33.3% (n=3; 95% CI 5.6-79.8%) (Rausch and Locker, 1951). Diplogonoporus tetrapterus is a specialist parasite of marine mammals including the Sea Otter. Total prevalence of 15.9% (n=69; 95% CI 9.0-26.5%) was calculated from three studies (Kenyon, 1969; Margolis et al., 1997; Rausch, 1964). Freshwater otters are the definitive hosts for Plagiorchis lutrae were reported in Eurasian otters with prevalences of 50.0% (n=2; 95% CI 9.5-90.6%).
Four species (Diphyllobothrium latum, Nanophyetus salmincola, Opisthorchis felineus, Paragonimus kellicotti) are generalist parasites of fish-eating mammals. Diphyllobothrium latum was found in Eurasian Otters in two studies with an overall prevalence of 2.1% (n=144; 95% CI 0.4-6.2%) (Gorski et al., 2006; Gorski et al., 2010). An unidentified Diphyllobothrium spp. was also present in 18.3% of North American River Otters (n=180; 95% CI 13.3-24.7%). Nanophyetus salmincola and P. kellicotti were both reported in North American River Otters with a prevalence of 66.7% (n=3; 95% CI 20.2-94.4%) and 3.3% (n=30; 95% CI 0.01-18.1%), respectively. Other highly pathogenic parasites included Fasciola hepatica (ruminant and human hosts) was found in 4% of Eurasian Otter (n=25; 95% CI 0.01-21.1%) sampled and Schistocephalus solidus (fish-eating bird hosts) in 10.9% (n=46; 95% CI 4.3-23.5%) of North American River Otters and 8.6% (n=35; 95% CI 2.2-23.1%) in Eurasian Otters.
Platyhelminths were located in the gastro-intestinal tracts, bile ducts of the liver, gall bladder, and lungs of otters.
3.8 Phylum Arthropoda (insects, ticks, mites)
Fifteen genera and 21 species of Arthropods were identified in the literature review studies (S3 Arthropoda sheet). Most arthropods of otters are not severely pathogenic but can vector pathogens such as Anaplasma spp., Babesia spp., and Rickettsia spp. mentioned in the other single-celled parasite section. The exceptions are the Cochliomyia hominivorax, New World Screwworm Fly, larvae that feed on tissue of living animals and Halarachne halichoteri, a nasal mite which causes respiratory disease. Only one Giant Otter was found to have a C. hominivorax infestation (Foerster et al., 2022). Halarachne halichoteri prevalence in Sea Otters was 25.6% (n=156; 95% CI 19.4-33.1%).
Four species of arthropods are primary parasites of otters or mustelids. One mite species, Lutracarus canadensis, is specific to the North American River Otter but has only been reported for one animal (Fain and Yunker 1980). Lutridia exilis is a louse found on Eurasian Otters. It was reported in two studies with 100% prevalence (n=10; 95% CI 67.9-100%) (Haitlinger and Lupicki 2009; Rohner et al., 2023). Demodex lutrae are mites also found on Eurasian Otters with two individuals infested in one study (n=2; 95% CI 29.0-100.0%) (Izdebska and Rolbiecki 2014). Lynxacarus mustelae, a fur mite of mustelids, was discovered on one North American River Otter (Fain and Yunker 1980).
The majority of arthropod parasites were located in the fur or skin of otters except for Halarachne spp. which were found in the nasal passages, trachea and bronchi.
3.9 Tick-borne bacteria and other parasites
There were several additional species sampled in otters that are transmitted by ticks or lice that cause disease in wildlife or that did not fit into the more common parasite phyla. This category included 8 phyla, 15 genera and 8 species (S3 Tick borne sheet). Three parasites were only identified to genus including Entamoeba spp., Myxobolus spp., and Enterocytozoon spp. and all are pathogenic species. Leishmania infantum, Giardia duodenalis, and Encephalitozoon cuniculi are species recorded in otters that are also considered highly pathogenic. Tick-borne bacteria reported in otters include Ehrlichia canis, Rickettsia spp. and Anaplasma spp. Except for Leishmania infantum and Ehrlichia canis which are specialist parasites of canids (Day, 2011), these other pathogens are generalists and can infect an array of mammals and vertebrates. Giardia spp. was the most commonly reported in four species of otters with a prevalence of 1% in Sea Otters (n=103; 95% CI 0.01-5.8%), 9.2% in Neotropical Otters (n=314; 95% CI 6.5-13.0%), 20.8% in Giant Otters (n=24, 95% CI 8.8-40.9%) and 6.5% in Eurasian otters (n=475; 95% CI 4.6-9.1%). Co-infections of Giardia spp. and Cryptosporidium were found in Neotropical Otters (4.5%; n=314; 95% CI 2.6-7.4%) and Giant Otters (20.8%; n= 24; 95% CI 8.8-40.9%). Single-celled parasites were found in the spleen, intestines, tongue, diaphragm and brain of otters.
4 Discussion
4.1 Biases
Although databases and citations were extensively searched there are biases that need to be addressed. The first bias is the large number of studies conducted in North American and European studies even though parasites have higher diversity in sub-tropical and tropical regions (Chretien et al., 2023). This may be due to publication bias for databases that cover non-English publications. It also may reflect the ability to access species to collect data. No parasite species were reported for the Smooth-coated Otter and the Hairy-nosed Otter from southeast Asia and the Congo Clawless Otter from the Congo Basin in Africa. Again, these species occur in areas with high prevalence of parasites due to climatic conditions and yet the studies are lacking due to access to study areas, funding for research, and/or publication bias for English publications. In general, surveys for parasite diversity are conducted more frequently in temperate regions and therefore the number of known species for temperate regions is higher than tropical regions (Poulin, 2010).
Prevalence estimates are affected by the methods used for parasite identification, sampling methods, and sample size. Variation in diagnostic methods could cause differences in reported prevalence as some tests detect previous infections through antibodies while others test for active infections (dissections and DNA). Vigneault et al. (2025) found that the use of multiple sampling methods produced different prevalence estimates within the same population. Low sample size or few sampling locations resulted in overestimation of prevalence (Vigneault et al., 2025). Small sample sizes also mean lower accuracy in prevalence estimates (Jovani and Tella, 2006).
Another major bias to the data is that most studies surveyed sick or dead animals. When sampling only occurs with animals that are already sick or found dead, this potentially skews the prevalence data to higher levels than systematic surveillance (Mathews, 2009). Opportunistic sampling of carcasses may also create bias for certain portions of the otter population such as dispersing young (Mathews, 2009). It also means that parasites which create clinical symptoms tend to be detected more frequently than parasites that do not create outward signs of infection or infestation (Mathews, 2009). Opportunistic samples also likely occur near human development where additional stressors affect the immunity of otters. Compounding the issue is that opportunistic sampling meant small sample sizes for a large portion of the studies in this review. Many only reported parasites on samples with less than 10 otters. The same otters were also tested for multiple parasites so the parasite prevalences were not independent of each other. To gain a better understanding of parasite distribution and otter hosts, a more systematic system of sampling needs to be developed.
Along with systematic sampling is the need for more detailed information about the otters themselves. Susceptibility to parasites and infection rates could naturally vary among otter species, among populations, or even individuals and this is an area that needs to be further investigated. Individual behaviors of otters could affect infection risk (Lopes et al., 2022).
Characteristics such as sex and age have been found to be important factors for parasite infections or infestations. Yet many studies did not report this basic information or if it was reported, it was as a ratio for the entire study and did not include prevalence rates for each sex. This lack of information prevented more detailed analysis of these factors from the reviewed literature. However, differences in parasite prevalence between sex and age have been reported in individual studies (Burgess et al., 2018; Crait et al., 2015; Miller et al., 2002; Sanders et al., 2020; Smallbone et al., 2017). The effects of parasites on otter hosts have also been found to differ among sexes (Sherrard-Smith et al., 2013). Understanding if the parasite is more common in one sex or age helps to determine the ecology of the parasite and determine mechanisms of infection or infestation.
Through time, the number of papers that have identified otter parasites has increased. This may reflect the effects of environmental changes on the presence of parasites and prevalence. However, these increases could also be partially caused by improvements in sampling techniques and reporting through time. The development of DNA amplification through PCR has allowed for more broad surveillance of parasites from more diverse sources such as environmental DNA and feces (Thompson et al., 2010). Parasite classification through traditional morphological characteristics may be confirmed or revised through DNA analysis (Rojas et al., 2024). DNA analysis has also delineated more species which were previously lumped together into one species classification due to morphology (Sures et al., 2017). DNA analysis also includes identification of species that may not be parasites of otters but simply organisms that were accidentally eaten with prey either through uptake of soil or from something the prey had consumed. This likely explains the occurrence of free-living species such as soil nematode species, Aphelenchus avenae, Diploscapter spp., and Prionchulus spp.
Host density also has a positive correlation with parasite diversity (Kamiya et al., 2014). Since most of the studies surveyed animals that were ill or dead, the animals may reflect otter populations with high densities. Those samples that were collected opportunistically were likely collected near human development where other stressors may make the otters more susceptible to infection or infestation. Detailed spatial information is also something that is lacking in most of the studies with many reporting only the country or region of the country where the animals were sampled. As most parasites are not evenly distributed across the landscape, adding more specific spatial information can help to identify parasite hotspots and better understand exposure risk and infection rates (Cotey et al., 2022).
4.2 General parasite trends
There is considerable research on parasites of the Eurasian Otter, Sea Otter, and North American River Otter. The Eurasian Otter had the highest number of different parasite genera and species. It may reflect publication bias; however, it also may reflect the wide range of the Eurasian Otter that covers Europe, Asia and North Africa. The Sea Otter and North American River Otter ranges are also extensive. Host species with a larger range typically have a greater diversity of parasites due to more variability in habitats and overlapping ranges with other host species (Kamiya et al., 2014; Poulin, 2014). Despite the number of studies on these three otter species, little information is known about the incidence or etiology of parasites (Kimber and Kollias, 2000). More details about parasite diversity as well as incidence and etiology are needed for the remaining 11 otter species.
Fifteen parasites from all phyla were specific to otters and eight parasites were specific to mustelids. However, the majority of parasites listed are considered generalist parasites for mammals or vertebrates, particularly the parasites that are classified as highly pathogenic. Generalist parasites infect or infest a broader range of hosts which allows the parasite to be maintained in more tolerant host species over a wider range and increases the overall pool of infected or infested stages (Hudson et al., 2006). Sensitive host species may therefore be more frequently exposed to virulent parasites (Hudson et al., 2006). This may be a concern for otters, particularly those that are listed as endangered.
In addition to the parasite specificity, environmental factors need to be considered. In degraded environments, parasites with complex life cycles may decline but parasites with a single-host or monoxenous life cycle may become more successful in these stressed environments particularly if the stress makes the host more susceptible to parasitism (Sures et al., 2023). Monoxenous parasites include Eimeria spp (Bangoura and Daugschies, 2018), Giardia spp., Cryptosporidium spp. (Nichols and Smith, 2002) and some intestinal nematodes including Ascaris spp., Toxocara spp. and Trichuris spp. (Mas-Coma et al., 2008) all of which have been found in otters. Changes to ranges in both otters and parasites, due to climate change or human alterations to the landscapes could result in new community structures and species interactions (Morales-Montor et al., 2022).
4.3 Phylum Apicomplexa (protozoa)
Phylum Apicomplexa is a diverse group of protists that parasitize almost all invertebrate and vertebrate species. Most members of Apicomplexa have high host specificity and many are highly pathogenic (Votýpka et al., 2016).
Toxoplasma gondii and Sarcocystis neurona are significant in that they cause encephalitis and mortality in Sea Otters (Miller et al., 2001, 2020: Shapiro et al., 2019). Both parasites are generalists with wide distributions that potentially impact all species of otters although mortality rates due to infection in other otter species are unknown. The amount of genetic diversity that has been discovered in T. gondii is a concern as this could lead to recombination and development of more virulent strains that could have impacts on populations and conservation (Thompson et al., 2010).
4.4 Phylum Acanthocephala (thorny-headed worms)
Acanthocephalan species are found more frequently in aquatic environments than terrestrial (Poulin and Morand, 2004). Many of the Acanthocephala species reported in otters may be incidental infestations from fish in the otters’ diets (Kimber and Kollias, 2000). However, Shanebeck et al. (2022), found that Corynosoma enhydri parasitized Sea Otter pups at an early age which potentially suppressed the immunoresponse and increased susceptibility to other parasites or bacterial infections that led to mortalities. Heavy infestations of Profilicollis altmani and P. kenti have directly caused mortalities in Sea Otters (Mayer et al., 2003).
4.5 Phylum Nematoda (roundworms)
The diversity of nematode parasites is mainly a reflection of the otter’s piscivorous diet (Torres et al., 2004). Nematoda parasites, Truttaedacnitis truttae and Contracaecum spp. were likely detected from the fish hosts and not a reflection of otter parasitism (Crait et al., 2015). Otter prey diversity and low densities of otters may also contribute to the low species richness of Nematoda in otters (Crait et al., 2015). Although the members of Nematoda rarely cause death of the host, infestations may still alter individual behavior and population dynamics of the host species (Morand et al., 2006). The exception to this is Capillaria hepatica which requires the death of the host for transmission (Morand et al., 2006). The presence of the mustelid specialist Aonchotheca putorii in Eurasian Otter was unusual as the intermediate host of A. putorii is earthworms which are not usually part of otter diets (Torres et al., 2004). Environmental conditions and human disturbance also play a role in otter infestations. Dirofilaria immitis occurred more often in Eurasian otters near irrigated fields likely due to the standing water conditions that support the mosquito vector (Torres et al., 2004).
4.6 Phylum Platyhelminthes (flatworms)
Much like the nematodes, many of the platyhelminths found in otters are obtained through their diet. Diphyllobothrium spp. have fish as intermediate hosts and definitive bird and mammal hosts including otters (Melquist et al., 2003; Weber, 1991). Paragonimus kellicotti has a broad range in North America and mammals are the definitive host, especially mink (Huffman, 2014). The intermediate host is crustaceans mainly crayfish (Huffman, 2014). The disease can cause lesions in the bile ducts and gall bladder (Huffman, 2014). Both D. latum and P. kellicotti have zoonotic potential as well (Huffman, 2014). In addition to otter diets, climate conditions may increase the prevalence of certain Platyhelminthes. Sherrard-Smith et al. (2013) found warmer temperatures, reduced rainfall and fewer frost-days increased prevalence of Pseudamphistomum truncatum and Metorchis albidus in the Eurasian Otter.
4.7 Phylum Arthropoda (insects, ticks, mites)
Ticks are relatively common on otters, and this reflects their semi-aquatic lifestyle. In general, ticks can survive approximately 3-days or more underwater (Honzáková 1971). Some ticks have been shown to survive much longer underwater including Dermacentor reticulatus (up to 65 days for adults; Honzáková 1971), Ixodes ricinus (up to 44 days for adults; Honzáková 1971), and Amblyomma americanum (up to 70 days for adults; Bidder et al., 2019), all which have been found to naturally infest otters. Bites from infected ticks are the route of transmission for tick-borne Apicomplexa. Tick bites are also the most common form of transmission for the piroplasmids which include Babesia spp. and Cytauxzoon spp (Alvarado-Rybak et al., 2016). Species of Babesia are relatively common in surveyed populations of North American River Otters and Eurasian otters (Garrett et al., 2022; Santoro et al., 2019; Birkenheuer et al., 2006).
4.8 Tick-borne bacteria and other parasites
Cryptosporidium and Giardia are found in many species of wildlife with strains specific to certain species or families (Appelbee et al., 2005). Agricultural runoff and untreated sewage are ways that both Cryptosporidium and Giardia from domestic animals and humans may be introduced into aquatic systems (Appelbee et al., 2005; Heitman et al., 2002) potentially exposing otter populations to infection. Giardia duodenalis which causes giardiasis in humans, has been often attributed to zoonotic transmission from aquatic mammals (Thompson, 2004). However, there is growing support for the hypothesis that G. duodenalis from human wastewater causes infections in aquatic mammals such as otters which then provides more hosts for the parasite (Appelbee et al., 2005; Thompson, 2004).
4.9 Conclusions
A diverse number of parasites have been reported mainly for three otter species, Eurasian Otter, North American River Otter, and Sea Otter. However, for seven otter species there were less than 15 papers published in the databases we searched and for three species there were no publications related to parasites. No parasite data has been reported for the newly listed species Lontra annectens either. Given that most otter species are also considered vulnerable or endangered by the IUCN, identifying the parasites that may have an impact on otter populations is of utmost importance. Gaining a better understanding of how different species of otters become infected or infested, spatial patterns of pathogens, and otter immunity to parasites, will also lead to better management actions to counteract highly pathogenic parasites. In conclusion, parasites need to be considered as a factor when evaluating otter populations and developing conservation plans.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
SC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. MR: Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Assistance with publication fees was provided by the College of Forest Resources and Environmental Science at Michigan Technological University.
Acknowledgments
Thanks to the library staff at Michigan Technological University for assistance in locating papers for review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmamm.2025.1673488/full#supplementary-material
References
Adlard R. D., Miller T. L., and Smit N. J. (2015). The butterfly effect: parasite diversity, environment, and emerging disease in aquatic wildlife. Trends Parasitol. 31, 160–166. doi: 10.1016/j.pt.2014.11.001
Alvarado-Rybak M., Solano-Gallego L., and Millán J. (2016). A review of piroplasmid infections in wild carnivores worldwide: importance for domestic animal health and wildlife conservation. Parasites Vectors 9, 538. doi: 10.1186/s13071-016-1808-7
Appelbee A. J., Thompson R. A., and Olson M. E. (2005). Giardia and Cryptosporidium in mammalian wildlife–current status and future needs. Trends Parasitol. 21, 370–376. doi: 10.1016/j.pt.2005.06.004
Bangoura B. and Daugschies A. (2018). “Eimeria,” in Parasitic Protozoa of Farm Animals and Pets. Eds. Florin-Christensen M. and Schnittger L. (Springer, Cham). doi: 10.1007/978-3-319-70132-5_3
Bidder L. A., Asmussen K. M., Campbell S. E., Goffigan K. A., and Gaff. H. D. (2019). Assessing the underwater survival of two tick species, Amblyomma americanum and Amblyomma maculatum. Ticks Tick-Borne Pathog. 10, 18–22. doi: 10.1016/j.ttbdis.2018.08.013
Birkenheuer A., Harms C., Neel J., Marr H., Tucker M., Acton A., et al. (2006). The identification of a genetically unique piroplasma in North American river otters (Lontra canadensis). Parasitology 134, 631–635. doi: 10.1017/S0031182006002095
Burgess T. L., Tim Tinker M., Miller M. A., Bodkin J. L., Murray M. J., Saarinen J. A., et al. (2018). Defining the risk landscape in the context of pathogen pollution: Toxoplasma gondii in sea otters along the Pacific Rim. R. Soc. Open Sci. 5, 171178. doi: 10.1098/rsos.171178
Chretien E., De Bonville J., Guitard J., Binning S. A., Melis E., Kack A., et al. (2023). Few studies of wild animal performance account for parasite infections: A systematic review. J. Anim. Ecol. 92, 794–806. doi: 10.1111/1365-2656.13864
Cotey S. R., Scimeca R., Chang L., Carpenter A. L., Will E. E., Ott-Conn C., et al. (2022). Toxoplasma gondii prevalence, partial genotypes, and Spatial variation in North American river otters (Lontra canadensis) in the Upper Peninsula of Michigan, USA. J. Wildlife Dis. 58, 869–881. doi: 10.7589/JWD-D-22-00021
Crait J. R., McIntosh A. D., Greiner E. C., and Ben-David M. (2015). The influence of changing prey availability on the prevalence of Diphyllobothrium in river otters from Yellowstone National Park. J. Parasitol. 101, 240–243. doi: 10.1645/14-546.1
Daszak P., Cunningham A. A., and Hyatt A. D. (2001). Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Tropica 78, 103–116. doi: 10.1016/S0001-706X(00)00179-0
Davis R. A. (2005). “Human impact on coasts,” in Encyclopedia of Coastal Science (Springer, Dordrecht), 530–535.
Day M. J. (2011). The immunopathology of canine vector-borne diseases. Parasites Vectors 4, 48. doi: 10.1186/1756-3305-4-48
de Ferran V., Vieira Figueiró H., Trinca C. S., Hernández-Romero P. C., Lorenzana G. P., Gutiérrez-Rodríguez C., et al. (2024). Genome-wide data support recognition of an additional species of Neotropical river otter (Mammalia, Mustelidae, Lutrinae). J. Mammalogy 105, 534–542. doi: 10.1093/jmammal/gyae009
Dubey J. P. (2003). Review of Neospora caninum and neosporosis in animals. Korean J. Parasitol. 41, 1. doi: 10.3347/kjp.2003.41.1.1
Dubey J. P., Howe D. K., Furr M., Saville W. J., Marsh A. E., Reed S. M., et al. (2015). An update on Sarcocystis neurona infections in animals and equine protozoal myeloencephalitis (EPM). Veterinary Parasitol. 209, 1–42. doi: 10.1016/j.vetpar.2015.01.026
Dubey J. P., Zarnke R., Thomas N. J., Wong S. K., Van Bonn W., Briggs M., et al. (2003). Toxoplasma gondii, Neospora caninum, Sarcocystis neurona, and Sarcocystis canis-like infections in marine mammals. Veterinary Parasitol. 116, 275–296. doi: 10.1016/S0304-4017(03)00263-2
Duplaix N. and Savage M. (2018). The global otter conservation strategy (Salem, Oregon, USA: eScholarship, University of California). 166 pp.
Fain A. and Yunker C. E. (1980). Lutracarus canadensis, ng, n. sp.(Acari: Listrophoridae) from the river otter, Lutra canadensis. J. Med. Entomology 17, 424–426.
Foerster N., Soresini G., Paiva F., Silva F. A. D., Leuchtenberger C., and Mourão G. (2022). First report of myiasis caused by Cochliomyia hominivorax in free-ranging giant otter (Pteronura brasiliensis). Rev. Bras. Parasitologia Veterinária. 31(4), e009522.
Garrett K., Halseth A., Ruder M. G., Beasley J., Shock B., Birkenheuer A.J., et al. (2022). “Prevalence and genetic characterization of a Babesia microti-like species in the North American River Otter (Lontra canadensis),” in Veterinary Parasitology: Regional Studies and Reports, vol. 29, 100696.
Górski P., Zalewski A., Kazimierczak K., and Kotomski G. (2010). Coproscopical investigations of the European otter (Lutra lutra) from Białowieża Primeval Forest. Wiadomości Parazytologiczne 56, 179–180.
Górski P., Zalewski A., and Lakomy M. (2006). Parasites of carnivorous mammals in Bialowieza Primeval Forest. Ann. Parasitol. 52, 49–53.
Grabner D., Rothe L. E., and Sures B. (2023). Parasites and pollutants: effects of multiple stressors on aquatic organisms. Environ. Toxicol. Chem. 42, 1946–1959. doi: 10.1002/etc.5689
Haitlinger R. and İupicki D. (2009). “First records of arthropods (Phthiraptera: Trichodectidae, Acari: Ixodidae) from Lutra lutra (Linnaeu)(Carnivora: Mustelidae) in Poland,”, vol. 59. (Biologia i Hodowla Zwierzat, Zeszyty Naukowe Uniwersytetu Przyrodniczego we Wroclawiu), 39–41.
Han B. A., Castellanos A. A., Schmidt J. P., Fischhoff I. R., and Drake J. M. (2021). The ecology of zoonotic parasites in the Carnivora. Trends Parasitol. 37, 1096–1110. doi: 10.1016/j.pt.2021.08.006
Heitman T. L., Frederick L. M., Viste J. R., Guselle N. J., Morgan U. M., Thompson R. C., et al. (2002). Prevalence of Giardia and Cryptosporidium and characterization of Cryptosporidium spp. isolated from wildlife, human, and agricultural sources in the North Saskatchewan River Basin in Alberta, Canada. Can. J. Microbiol. 48, 530–541. doi: 10.1139/w02-047
Hennessy S. L. and Morejohn G. V. (1977). Acanthocephalan parasites of the sea otter, Enhydra lutris, off coastal California. California Fish Game 1977, 268–272.
Honzáková E. (1971). Survival of some ixodid tick species submerged in water in laboratory experiments. Folia Parasitologica 18, 155–159. Available online at: https://www.cabidigitallibrary.org/action/doSearch?do=Folia+Parasitologica.
Hudson P. J., Dobson A. P., and Lafferty K. D. (2006). Is a healthy ecosystem one that is rich in parasites? Trends Ecol. Evol. 21, 381–385. doi: 10.1016/j.tree.2006.04.007
Huffman J. E. (2014). “Selected wildlife trematodiasis,” in Digenetic Trematodes. Advances in Experimental Medicine and Biology, vol. 766 . Eds. Toledo R. and Fried B. (Springer, New York), 429–456.
International Union for Conservation of Nature and Natural Resources (IUCN) (2025). The IUCN Red List of Threatened Species. Version 2025-1. Available online at: https://www.iucnredlist.org (Accessed July 10, 2025).
Izdebska J. N. and Rolbiecki L. (2014). Demodex lutrae n. sp.(Acari) in European otter Lutra lutra (Carnivora: Mustelidae) with data from other demodecid mites in carnivores. J. Parasitol. 100, 784–789.
Johnson P. T. and Paull S. H. (2011). The ecology and emergence of diseases in fresh waters. Freshw. Biol. 56, 638–657. doi: 10.1111/j.1365-2427.2010.02546.x
Jovani R. and Tella J. L. (2006). Parasite prevalence and sample size: misconceptions and solutions. Trends Parasitol. 22, 214–218. doi: 10.1016/j.pt.2006.02.011
Kamiya T., O’Dwyer K., Nakagawa S., and Poulin R. (2014). What determines species richness of parasitic organisms? A meta-analysis across animal, plant and fungal hosts. Biol. Rev. 89, 123–134. doi: 10.1111/brv.12046
Kenyon K. W. (1969). The sea otter in the eastern Pacific Ocean. North Am. Fauna No. 68 (Washington, D.C), 366.
Kimber K. R. and Kollias G. V. (2000). Infectious and parasitic diseases and contaminant-related problems of North American river otters (Lontra canadensis): a review. J. Zoo Wildlife Med. 31, 452–472. doi: 10.1638/1042-7260(2000)031[0452:IAPDAC]2.0.CO;2
Kikuchi S. and Nakajima M. (1993). Corynosoma enhydri (Acanthocephala) from sea otters Enhydra lutris. Japanese J. Parasitol. 42, 331–339.
Kruse H., Kirkemo A. M., and Handeland K. (2004). Wildlife as source of zoonotic infections. Emerging Infect. Dis. 10, 2067. doi: 10.3201/eid1012.040707
Lopes P. C., French S. S., Woodhams D. C., Binning S. A., Ezenwa V., Altizer S., et al. (2022). Infection avoidance behaviors across vertebrate taxa: patterns, processes, and future directions. Anim. Behav. parasitism Ezenwa V. O., Altizer S., and Hall R. J. Oxford University Press, 237. doi: 10.1093/oso/9780192895561.003.0014
Morales-Montor J., Hugo Del Río-Araiza V., and Hernandéz-Bello R. (eds) (2022). “Parasitic Helminths and Zoonoses - From Basic to Applied Research. IntechOpen. doi: 10.5772/intechopen.98178
Margolis L., Groff J. M., Johnson S. C., McDonald T. E., Kent M. L., and Blaylock R. B. (1997). Helminth parasites of sea otters (Enhydra lutris) from Prince William Sound, Alaska: comparisons with other populations of sea otters and comments on the origin of their parasites. J. Helminthological Soc. Washington 64, 161–168.
Mas-Coma S., Valero M. A., and Bargues M. D. (2008). Effects of climate change on animal and zoonotic helminthiases. Rev. Sci. Tech 27, 443–457. doi: 10.20506/rst.27.2.1822
Mathews F. (2009). Zoonoses in wildlife: integrating ecology into management. Adv. Parasitol. 68, 185–209. doi: 10.1016/S0065-308X(08)00608-8
Mayer K. A., Dailey M. D., and Miller M. A. (2003). Helminth parasites of the southern sea otter Enhydra lutris nereis in central California: abundance, distribution and pathology. Dis. Aquat Org 53, 77–88. doi: 10.3354/dao053077
McCallum H. and Dobson A. (1995). Detecting disease and parasite threats to endangered species and ecosystems. Trends Ecol. Evol. 10, 190–194. doi: 10.1016/S0169-5347(00)89050-3
Melquist W. E., Polechla J. P. J., and Toweill D. (2003). “River otter: Lontra canadensis,” in Wild mammals of North America: Biology, management, and conservation. Eds. Feldhamer G. A., Thompson B. C., and Chapman J. A. (Johns Hopkins University Press, Baltimore, Maryland), 708–734.
Miller M. A., Crosbie P. R., Sverlow K., Hanni K., Barr B.C., Kock N., et al. (2001). Isolation and characterization of Sarcocystis from brain tissue of a free-living southern sea otter (Enhydra lutris nereis) with fatal meningoencephalitis. Parasitol. Res. 87, 252–257. doi: 10.1007/s004360000340
Miller M. A., Gardner I. A., Kreuder C., Paradies D. M., Worcester K. R., Jessup D. A., et al. (2002). Coastal freshwater runoff is a risk factor for Toxoplasma gondii infection of southern sea otters (Enhydra lutris nereis). Int. J. Parasitol. 32, 997–1006. doi: 10.1016/S0020-7519(02)00069-3
Miller M. A., Moriarty M. E., Henkel L., et al. (2020). Predators, disease, and environmental change in the nearshore ecosystem: mortality in southern sea otters (Enhydra lutris nereis) from 1998–2012. Front. Mar. Sci. 7, 582. doi: 10.3389/fmars.2020.00582
Morand S., Bouamer S., and Hugot J. P. (2006). “Nematodes,” in Micromammals and macroparasites: From evolutionary ecology to management (Springer Japan, Tokyo), 63–79.
Nichols R. and Smith H. (2002). “Parasites: Cryptosporidium, Giardia and Cyclospora as foodborne pathogens,” in Foodborne Pathogens (CRC Press, Boca Raton, Florida), 453–478.
Page M. J., McKenzie J. E., Bossuyt P. M., Boutron I., Hoffmann T. C., Mulrow C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 18, e1003583. doi: 10.1371/journal.pmed.1003583
Poulin R. (2007). Evolutionary ecology of parasites (Princeton, New Jersey: Princeton University Press).
Poulin R. (2010). Latitudinal gradients in parasite diversity: bridging the gap between temperate and tropical areas. Neotropical Helminthology 4, 169–177.
Poulin R. (2014). Parasite biodiversity revisited: frontiers and constraints. Int. J. Parasitol. 44, 581–589. doi: 10.1016/j.ijpara.2014.02.003
Rausch R. L. (1964). Studies on the Helminth Fauna of Alaska: XLI Observations on Cestodes of the Genus Diplogonoporus Lonnber (Diphyllobothriidae). Can. J. Zool. 42, 1049–1069.
Rausch R. and Lçcker B. (1951). Studies on the helminth fauna of Alaska II. On some helminths parasitic in the sea otter, Enhydra lutris (L.). Proc. Helminthological Soc. Washington 18, 77–81.
Rohner S., Boyi J. O., Artemeva V., Zinke O., Kiendl A., Siebert U., et al. (2023). Back from exile? First records of chewing lice (Lutridia exilis; Ischnocera; Mallophaga) in growing Eurasian otter (Lutra lutra) populations from Northern Germany. Pathogens 12, 587.
Rojas A., Germitsch N., Oren S., Sazmand A., and Deak G. (2024). Wildlife parasitology: sample collection and processing, diagnostic constraints, and methodological challenges in terrestrial carnivores. Parasites Vectors 17, 127. doi: 10.1186/s13071-024-06226-4
Salvarani F. M., Oliveira H. G. D. S., Correa L. Y. S., Soares A. A. L., and Ferreira B. C. (2025). The importance of studying infectious and parasitic diseases of wild animals in the Amazon biome with a focus on One Health. Veterinary Sci. 12, 100. doi: 10.3390/vetsci12020100
Sanchez S. G. and Besteiro S. (2021). The pathogenicity and virulence of Toxoplasma gondii. Virulence 12, 3095–3114. doi: 10.1080/21505594.2021.2012346
Sanders C. W., Olfenbuttel C., Pacifici K., Hess G. R., Livingston R. S., and DePerno C. S. (2020). Leptospira, parvovirus, and toxoplasma in the North American river otter (Lontra canadensis) in North Carolina, USA. J. Wildlife Dis. 56, 791–802. doi: 10.7589/2019-05-129
Santoro M., Auriemma C., Lucibelli M. G., Borriello G., D'Alessio N., Sgroi G., et al. (2019). Molecular detection of Babesia spp. (Apicomplexa: Piroplasma) in free-ranging canids and mustelids from southern Italy. Front. Veterinary Sci. 6, 269. doi: 10.3389/fvets.2019.00269
Selbach C., Mouritsen K. N., Poulin R., Sures B., and Smit N. J. (2022). Bridging the gap: aquatic parasites in the One Health concept. Trends Parasitol. 38, 109–111. doi: 10.1016/j.pt.2021.10.007
Shanebeck K. M., Lakemeyer J., Siebert U., and Lehnert K. (2020). Novel infections of Corynosoma enhydri and Profilicollis sp. (Acanthocephala: Polymorphidae) identified in sea otters Enhydra lutris. Dis. Aquat. Organisms 137, 239–246. doi: 10.3354/dao03442
Shanebeck K. M., Thacker C., and Lagrue C. (2022). Corynosoma strumosum (Acanthocephala) infection in marine foraging mink (Neogale vison) and river otter (Lontra canadensis) and associated peritonitis in a juvenile mink. Parasitol. Int. 89, 02579.
Shapiro K., VanWormer E., Packham A., Dodd E., Conrad P. A., and Miller M. (2019). Type X strains of Toxoplasma gondii are virulent for southern sea otters (Enhydra lutris nereis) and present in felids from nearby watersheds. Proc. R. Soc. B 286, 20191334. doi: 10.1098/rspb.2019.1334
Sherrard-Smith E., Chadwick E. A., and Cable J. (2013). Climatic variables are associated with the prevalence of biliary trematodes in otters. Int. J. Parasitol. 43, 729–737. doi: 10.1016/j.ijpara.2013.04.006
Smallbone W. A., Chadwick E. A., Francis J., Guy E., Perkins S. E., Sherrard-Smith E., et al. (2017). East-West Divide: Temperature and land cover drive spatial variation of Toxoplasma gondii infection in Eurasian otters (Lutra lutra) from England and Wales. Parasitology 144, 1433–1440. doi: 10.1017/S0031182017000865
Spencer H. G. and Zuk M. (2016). For host’s sake: the pluses of parasite preservation. Trends Ecol. Evol. 31, 341–343. doi: 10.1016/j.tree.2016.02.021
Sures B., Nachev M., Pahl M., Grabner D., and Selbach C. (2017). Parasites as drivers of key processes in aquatic ecosystems: Facts and future directions. Exp. Parasitol. 180, 141–147. doi: 10.1016/j.exppara.2017.03.011
Sures B., Nachev M., Schwelm J., Grabner D., and Selbach C. (2023). Environmental parasitology: stressor effects on aquatic parasites. Trends Parasitol. 39, 461–474. doi: 10.1016/j.pt.2023.03.005
Thompson R. C. A. (2004). The zoonotic significance and molecular epidemiology of Giardia and giardiasis. Veterinary Parasitol. 126, 15–35. doi: 10.1016/j.vetpar.2004.09.008
Thompson R. C. A. (2013). Parasite zoonoses and wildlife: one health, spillover and human activity. Int. J. Parasitol. 43, 1079–1088. doi: 10.1016/j.ijpara.2013.06.007
Thompson R. C. A., Lymbery A. J., and Smith A. (2010). Parasites, emerging disease and wildlife conservation. Int. J. Parasitol. 40, 1163–1170. doi: 10.1016/j.ijpara.2010.04.009
Titcomb G., Mantas J. N., Hulke J., Rodriguez I., Branch D., and Young H. (2021). Water sources aggregate parasites with increasing effects in more arid conditions. Nat. Commun. 12, 7066. doi: 10.1038/s41467-021-27352-y
Torres J., Feliu C., Fernández-Morán J., Ruíz-Olmo J., Rosoux R., Santos-Reis M., et al. (2004). Helminth parasites of the Eurasian otter Lutra lutra in southwest Europe. J. Helminthology 78, 353–359. doi: 10.1079/JOH2004253
Vigneault J., Binning S. A., and Harvey E. (2025). Local environment and sampling bias drive parasite prevalence estimates in freshwater fish communities. Oikos 2025, e11196. doi: 10.1002/oik.11196
Votýpka J., Modrý D., Oborník M., Šlapeta J., and Lukeš J. (2016). “Apicomplexa,” in Handbook of the Protists (Springer, Cham), 1–58.
Wang H. and He G. (2022). Rivers: Linking nature, life, and civilization. River 1, 25–36. doi: 10.1002/rvr2.7
Weber J. M. (1991). Gastrointestinal helminths of the otter, Lutra lutra, in Shetland. J. Zoology 224, 341–346. doi: 10.1111/j.1469-7998.1991.tb04814.x
Winter M. and Landaeta-Aqueveque C. (2025). Foodborne zoonotic parasites and parasitoses. Front. Trop. Dis. 6, 1568734. doi: 10.3389/fitd.2025.1568734
Woo C., Bhuiyan M. I. U., Eo K. Y., Lee W. S., Kimura J., and Yamamoto N. (2023). Diversity of fecal parasitomes of wild carnivores inhabiting Korea, including zoonotic parasites and parasites of their prey animals, as revealed by 18S rRNA gene sequencing. Int. J. Parasitology: Parasites Wildlife 21, 179–184.
Keywords: otters, parasites, disease, pathogens, Lutrinae
Citation: Cotey SR and Reichard MV (2025) A systematic review of parasitic diseases of otters. Front. Mamm. Sci. 4:1673488. doi: 10.3389/fmamm.2025.1673488
Received: 25 July 2025; Accepted: 06 October 2025;
Published: 13 November 2025.
Edited by:
Caroline Leuchtenberger, Instituto Federal Farroupilha, BrazilReviewed by:
Estevam G. Lux Hoppe, São Paulo State University, BrazilGonzalo Medina-Vogel, Andres Bello University, Chile
Copyright © 2025 Cotey and Reichard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stacy R. Cotey, c3Jjb3RleUBtdHUuZWR1
 Stacy R. Cotey
Stacy R. Cotey Mason V. Reichard2
Mason V. Reichard2