- Department of Marine Microbiology and Biogeochemistry, NIOZ Royal Netherlands Institute for Sea Research, Utrecht University, Texel, Netherlands
Under natural conditions phytoplankton are often simultaneously subjected to phosphorus (P) limitation and suboptimal light levels. Potential interacting effects of P-limitation and light availability on phytoplankton virus-host interactions have thus far not been reported. We studied the influence of three environmentally relevant light levels (low; 25, medium; 100, and high; 250 μmol quanta m−2 s−1) in combination with P-limitation (vs. P-replete conditions) on virus proliferation in the key phytoplankton species Micromonas pusilla and Phaeocystis globosa. Cultures were acclimated to balanced P-limited growth at 3 light levels by semi-continuous culturing, before one-step infection experiments were carried out in batch mode. Under optimal conditions (medium light, P-replete), the latent period (time until first release of progeny viruses) was 6–9 and 9–12 h, and the burst size (number of viruses released per lysed host cell) was 241 ± 5 and 690 ± 28 for M. pusilla virus MpV and P. globosa virus PgV, respectively. Low light intensity under P-replete conditions prolonged the latent period of PgV (with maximally 3 h). The PgV burst size was 2.8-fold reduced under low light and 2.2-fold reduced under high light. The 10-fold range in light intensity did not affect viral latent period or burst size in P-replete M. pusilla. However, P-limitation (under optimal light) also led to elongated latent periods (with maximally 3 h compared to P-replete) and the viral burst sizes decreased by 2.7-fold for MpV and 3.5-fold for PgV. Finally, infectivity assays showed that PgV progeny from the P-limited high and low light cultures largely lost their infectivity, reducing their infective burst sizes to only 2–4 infective viruses per lysed host cell. Our study demonstrates that the effects of specific light and P-availability on virus-phytoplankton interaction are not only species specific, but can also strengthen each other's effects. Relatively small differences in environmental conditions with depth, geography or time have the potential to drastically affect viral infection of phytoplankton, with consequent effects on host species composition and biogeochemical fluxes.
Introduction
In the heterogeneous natural marine environment phytoplankton is influenced by many environmental factors at the same time (Behrenfeld et al., 2008). Phosphorus (P) limited phytoplankton physiology and growth commonly occurs in marine ecosystems and is, furthermore, expected to become more important in the future ocean due to stratification in combination with diazotroph nitrogen input (Karl et al., 1997; Ruttenberg, 2003; Dyhrman et al., 2007; Moore et al., 2013). Irradiance can either limit phytoplankton growth and production by a reduced availability (turbidity, season, water depth or mixing to deep waters; see Huisman et al., 2002) or by an excess of it, causing photoinhibition (mixing to shallow waters, stratification; Long et al., 1994). The effects of these growth-controling factors can even be cumulative (Cloern, 1999; Colijn and Cadée, 2003; van de Poll et al., 2005; Kulk et al., 2013; Arteaga et al., 2014).
The environment is also a strong determinant on phytoplankton host-virus interactions (Mojica and Brussaard, 2014). Regarding the important role of marine viruses as drivers of biogeochemical cycling (Suttle, 2007; Brussaard et al., 2008), the indirect effects (i.e., via the host) of the environment are underrepresented in our knowledge of marine ecosystem functioning. As viruses are dependent on the host for energy and building blocks, any effects of a suboptimal environment on host physiology might affect virus production as well. Phytoplankton virus proliferation (latent period and burst size) has been shown to be negatively affected by low P-availability or light intensity (Wilson et al., 1996; Bratbak et al., 1998; Clasen and Elser, 2007; Gobler et al., 2007; Baudoux and Brussaard, 2008; Maat et al., 2014). Only very few studies have, however, focused on the combined effect of two environmental factors together on virus-phytoplankton interaction (Cséke and Farkas, 1979; Maat and Brussaard, 2016), and none were performed under light with P-manipulation.
Since the element P and irradiance are both involved in phytoplankton energy metabolism, cumulative effects (whereby one variable strengthens the effect of the other) on the virus can be expected. Where light provides the energy, it is for a large part stored in the energy rich anhydride bonds of P-containing adenosine triphosphate (ATP). Viral replication is dependent on the energy that is delivered by the host for replication, transcription, translation, and genome packaging. This energy is at least partly delivered in the form of ATP (Ando et al., 2012) and is present in the host cell or has to be produced during the lytic cycle by photophosphorylation.
Here we studied the potential co-stressing effects of P-limitation and light intensity on viral proliferation in the key phytoplankton species Micromonas pusilla and Phaeocystis globosa. Phytoplankton cultures were maintained under P-replete and P-limited treatments in combination with three different light conditions. Balanced P-limited growth was realized by semi-continuous culturing (Quinlan, 1986; Nicklisch, 1999), yielding well-acclimated cells at the moment of infection. For clarity, in this study we use (1) “P-limitation” as a general term that describes the effects of low P-availability on phytoplankton growth or physiology (Moore et al., 2013), (2) “P-controlled growth” to describe the balanced nutrient limited semi-continuous culturing phase, and (3) “P-starvation” to describe the situation in the batch cultures (infection experiments; MacIntyre and Cullen, 2005). At steady state, the cultures were subsequently infected with host-specific lytic viruses, whereby the effects of the P-limitation and light conditions on viral latent period (time until first release viral progeny), burst size (number of progeny viruses released per lysed host cell) and infectivity were determined.
Materials and Methods
Culturing and Experimental Set-Up
The prasinophyphyte M. pusilla (2 μm diameter; MP-Lac38; culture collection Marine Research Center, Göteborg University; Butcher; Manton and Parke, 1960) and the prymnesiophyte P. globosa G(A) (5 μm diameter; culture collection University of Groningen, The Netherlands; isolated from the North Sea; T135, Terschelling 135 km off the coast by R. Koeman in 2000) were axenically cultured at 15°C. Both species were grown in Mix-TX medium, a 1:1 mixture of modified f/2 medium (Guillard and Ryther, 1962) and artificial seawater (ESAW; Harrison et al., 1980), enriched with Tris-HCl and Na2SeO3 (Cottrell and Suttle, 1991). However, the potential P-source Na2-glycerophophate was omitted from this medium. Light was supplied by 18W/965 OSRAM daylight spectrum fluorescent tubes (München, Germany) under a light:dark cycle of 16:8 h andat intensities of 25, 100, and 250 μmol quanta m−2 s−1, further referred to as low (LL), medium (ML), and high light (HL). These light conditions are ecologically relevant for the surface waters of the North Sea and Wadden Sea, whereby ML represents a daily average for the spring period (Dring et al., 2001; Ly et al., 2014). We used a 10-fold difference between LL and HL, which is a natural range experienced by phytoplankton in temperate waters (Kulk et al., 2013), and whereby LL represents a level at which the maximum growth rate of natural phytoplankton populations is halved (Cloern, 1999) and HL shows significant inhibiting effects on the photosynthetic efficiency (Fv/Fm) of our model species under P replete conditions. Triplicate 50 ml cultures were grown semi-continuously under P-replete and P-limiting conditions with daily dilution 3 h into the light period. For the P-replete treatments (36 μM Na2HPO4) culturing was done according to the turbidistat principle (dilution based on cell abundance). The P-limited cultures were diluted in a semi-continuous fashion according to the same principle as chemostat culturing (nutrient-limited continuous culture; MacIntyre and Cullen, 2005). Due to the high total number of cultures (72 in total), this type of culturing was a good alternative for chemostat culturing (Quinlan, 1986; Nicklisch, 1999). The concentrations of the limiting nutrients determine the algal abundances (but not the strength of limitation; Quinlan, 1986; MacIntyre and Cullen, 2005) and were 0.25 μM and 1.0 μM Na2HPO4 for M. pusilla and P. globosa, respectively. We aimed for steady state abundances of ± 1.0 and 0.5 × 106 for M. pusilla and P. globosa, respectively, yielding comparable virus-host contact rates (i.e., 1.8 and 1.5 × 109 viruses cm−3 s−1). The contact rates were estimated according to Murray and Jackson (1992), using host cell diameters of 2 and 5 μm. Abundances were however affected by the treatment (light vs. nutrient conditions), leading to small variations in starting abundances for the viral infection experiment (varying maximally 1.9-fold under P-replete and 1.3-fold under P-limiting conditions).
Acclimation Period and Steady State of the Cultures
Maximum phytoplankton growth rates (μmax per day) under the three light conditions were initially determined in replete batch cultures. In the nutrient controlled semi-continuous cultures the algal growth was maximized toward μmax, i.e., highest possible without cells washing out. The growth rate was determined daily according to ln(Xt24/Xt0), whereby X is the algal abundance before and after subculturing and analysis by flow cytometry (FCM). The resulting stable P-controlled steady state constant maximized growth and abundances; reached after a minimum of 5 volume changes) was then maintained for several weeks. During this period, cell abundances, flow cytometry (FCM) mean forward scatter (FSC; a relative measure of cell size), dissolved inorganic P (DIP) and photosynthetic capacity (also used as indicator of nutrient limitation; Beardall et al., 2001) were monitored (typically daily). A day before the viral infection experiments (after 4 weeks of steady state), each of the triplicate cultures (of all treatments) was split yielding one “non-infected control” culture and one “virally infected” culture (Figure 1).
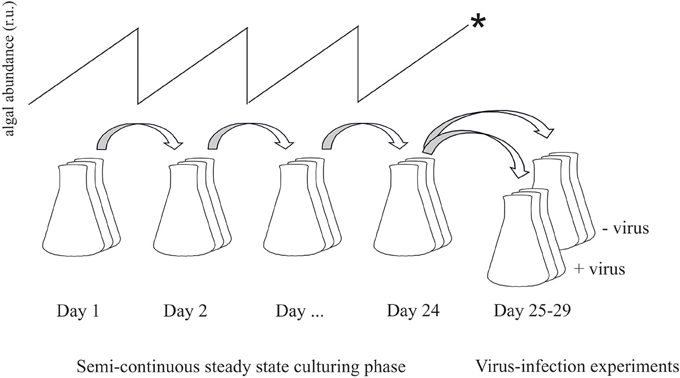
Figure 1. Flow-chart illustrating the experimental set-up. Prior to steady state the cultures were acclimated to the respective P-limitation and light levels by semi-continuous culturing with dilution rates as high as possible without wash-out of cells. Once in steady state, cultures were maintained for several weeks. For the viral infection experiments, dilution was stopped (*) and cultures were split into two new cultures of which one served as non-infected control and the other was infected with viruses.
Viral Infection Experiment
At the day of the infection experiment the semi-continuous dilution was stopped (thus also halting growth of the non-infected P-limited cultures) to allow for proper one-step infection cycles. Continued daily dilution of the cultures after infection would have negatively affected the virus:host ratio, and nutrient input at the moment of infection may affect virus proliferation. Hence, with this set-up we studied viral infection of host cells preconditioned to balanced nutrient limitation and subsequent onset of nutrient starvation. The lytic dsDNA viruses MpV-08T and PgV-07T (NIOZ-culture collection, the Netherlands), infecting M. pusilla and P. globosa, respectively, were pre-cultured under optimal light conditions (ML). The lysates for the P-limited experimental cultures were pre-cultured on P-limited host for at least 3 rounds of infection to assure that no DIP was present in the lysates (also verified by direct measurements). For all infection experiments, the cultures received their respective viruses 3 h into the light period (at the moment at which the cultures would otherwise be diluted) at a virus:host ratio of 10 (100% infectivity, as determined by most probable number endpoint dilution assay, n = 5, 12 10 × dilutions; see below; Suttle, 1993) to ensure a one-step infection cycle. The non-infected control cultures received equal volume of 0.1 μm filtered (polyethersulfone membrane filter; Sartopore Midicap, Sartorius A.G. Goettingen) seawater with macronutrient concentrations below the detection limit (<0.02 μM). The cultures were subsequently sampled for algal and viral abundances (3–6 h) and nutrients (beginning and end). Measurements in all cultures were stopped when cultures fully lysed, but extra samples of the infected cultures were taken to assure no regrowth had taken place.
Analyses
Concentrations of DIP were determined by colorimetry according to Hansen and Koroleff (1999). DIP concentrations during culturing and at the start of the viral infection experiment were always at the detection limit (0.02 μM) and considered 0 μM. Algal cellular P-quotas under P-limitation were calculated as daily added DIP divided by the increase in cell abundance over a 24 h period. Axenic testing was done by epifluorescence microscopy with DAPI (4′,6-diamidino-2-phenylindole, dihydrochloride; Life Technologies Ltd. Paisley, UK) stained cultures (Porter, 1980). At all times during the experiments, the cultures were axenic.
Phytoplankton abundances and mean cellular forward scatter (FSC) were determined on fresh samples using a BD AccuriTM C6 cytometer (BD Biosciences, San Jose, Ca, USA) with the trigger set on red autofluorescence (Marie et al., 1999). The FSC values were used as indicators for relative cell size (Shapiro, 1988; DuRand et al., 2002). A linear relation between BD AccuriTM C6 mean cellular FSC (x) and cell size (y) was confirmed by testing different phytoplankton species from the NIOZ culture collection (y = 0.0075x + 1.2373; r2 = 0.9979).
Algal viruses were fixed and enumerated by FCM according to Brussaard (2004). In short, 0.5% final concentration glutaraldehyde fixed samples (25% stock, EM-grade; Sigma-Aldrich, St. Louis, MO, USA) were flash frozen in liquid nitrogen and stored at −80°C until analysis. After thawing, the samples were diluted 100–1000-fold in 0.2 μm filtered (FP 30/0.2 CA-S Whatman, Dasser, Germany) TE-buffer (Ph = 8) and stained with SYBR green I to a final concentration of 0.5 × 10−4 of the commercial stock (Invitrogen Molecular Probes, Eugene, OR, USA) for 10 min at 80°C, after which they were analyzed using a benchtop BD FacsCalibur (BD Biosciences, San Jose, Ca, USA) with the trigger set on green fluorescence. The algal virus clusters were discriminated based on their green fluorescence and side scatter (Brussaard et al., 2000). All FCM data were analyzed using CYTOWIN 4.31 (Vaulot, 1989). Viral burst size (viruses per lysed host cell) was estimated by dividing the number of produced viruses by the number of lysed host cells.
Viral infectivity was determined by comparing the most probable number of infectious viruses (MPN endpoint dilution assay; Suttle, 1993) with the viral abundances that were determined by flow cytometry. The MPN assays with the progeny lysates of the viral infection experiment were first done on one of the replicates and carried out in triplicate (with 12 times 10-fold dilution). As the results were showing a high reduction in infectivity for two of the treatments the experiment was repeated a year later (giving similar results to the earlier executed experiment), whereby new MPN assays were carried out but this time with 10 replicates). The algal host for the MPN assays was cultured under optimal growth conditions (nutrient replete, ML) and a dilution series without viruses was used as a negative control. The percentage of infective viruses was determined by dividing the number of infectious viruses (MPN) by the number of viral particles (FCM). The infective burst size was calculated by multiplying the fraction infective with the original burst size.
The PAM fluorometry (Water-PAM, Walz, Germany) measurements were placed in the dark for approximately 15 min, after which they were analyzed for minimal (F0) and maximal (Fm) fluorescence. The maximal photosynthetic efficiency Fv/ Fm was calculated from F0 and Fm whereby Fv equals Fm-F0 (see Maxwell and Johnson, 2000).
Statistics were done with the program SigmaplotTM 12.0 (Systat software Inc, Chicago Il, USA). Where applicable, two way ANOVAs were used to simultaneously test for the effects of light and P limitation on steady state parameters and viral burst sizes. Pairwise multiple comparisons of all different treatments were then carried out by a Holm-Šídák test. In the tables, significant differences (p < 0.05) between values are depicted by different letters (superscript), i.e., when values share the same letter they are not significantly different from eachother.
Results
Steady State
Phytoplankton cellular characteristics at steady state were affected by the degree of P-limitation as well as by the light treatments (Table 1). The P-controlled cultures were able to reach (near) P-replete growth rates, but not under all light conditions. For M. pusilla the P-controlled growth rates under ML and LL were similar as under P-replete conditions, but HL led to an approximate 40% growth rate reduction. In contrast, P-controlled P. globosa reached similar growth rates as P-replete under HL, but showed a 10 and 20% reduction under ML and LL respectively. For M. pusilla the highest growth rates were obtained under HL, P-replete conditions, while this was the ML, P-replete treatment for P. globosa.
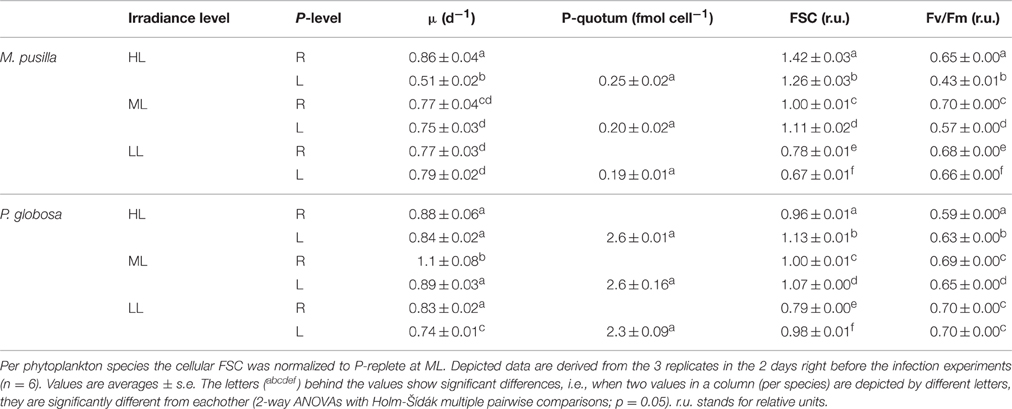
Table 1. Steady state parameters of M. pusilla and P. globosa under P-replete (R) and P-limited (L) growth conditions and 3 different light conditions (250, 100, and 25 μmol quanta m−2 s−1, represented by HL, ML and LL, respectively): Average growth rate (μ d−1), mean forward scatter (FSC), photosynthetic efficiency (Fv/Fm) and P-quotum (fmol cell−1).
The cellular P-quotas per cell under P-controlled growth conditions were not affected by light level (Table 1). However, with average cell diameters of 2 and 5 μm, the volume specific P-quotas were about 2-fold lower for M. pusilla than P. globosa (on average 0.068 and 0.128 fmol μm−3, respectively). The mean cellular FSC was affected by both light and P-control (Table 1). P-controlled conditions led to an increase in FSC for both species under ML. For P. globosa, this increase due to P-controlled growth was also observed under HL and LL, but the opposite (a lower FSC than P-replete under HL and LL) was observed for M. pusilla. Moreover, both species showed a decreased FSC under LL conditions.
The Fv/Fm of both algal species was affected by both factors, i.e., the effect of P-controlled growth on Fv/Fm was strengthened under increasing light (interaction; p < 0.001). P-controlled conditions reduced the algal Fv/Fm as compared to the P-replete growth, more so for M. pusilla than for P. globosa and more so for HL than for LL (Table 1). Only the P. globosa LL cultures showed no significant change compared to the replete cultures. An interaction of both factors was especially clear for the steady state M. pusilla cultures, where P-controlled growth in combination with increasing light level led to a stronger decrease in Fv/Fm than under P-replete conditions.
Viral Infection Experiment
In contrast to the non-infected control cultures, the P-replete M. pusilla cultures showed a rapid growth reduction upon infection with MpV (Figures 2A,B). However, full lysis of the LL infected cultures took up to 2-fold longer than under ML and HL (Figure 2B). The latent period of MpV was 6–9 h, independent of light level (Figures 2C,D). Also the MpV burst sizes under P-replete conditions were not affected by light level (differences in viral yield were due to small differences in maximal algal abundance at LL, ML, and HL; Figure 2C, Table 2). The non-infected P-starved cells ceased growing, while the infected M. pusilla cultures showed similar declines as for the P-replete infected cultures, independent of the light treatments (Figures 3A,B). The viral latent period prolonged with maximally 3 h under P-limitation and the MpV burst size declined 2.5-fold to 94 per lysed host cell (Table 2). Light level did not further impact the viral latent period or burst size in the P-limited treatments (Figures 3C,D, Table 2). Infectivity of the progeny MpV was between 74 and 100%, resulting in infective burst sizes of 78–96 and 143–241 infective viruses per cell for P-limited and P-replete conditions, respectively (Table 2).
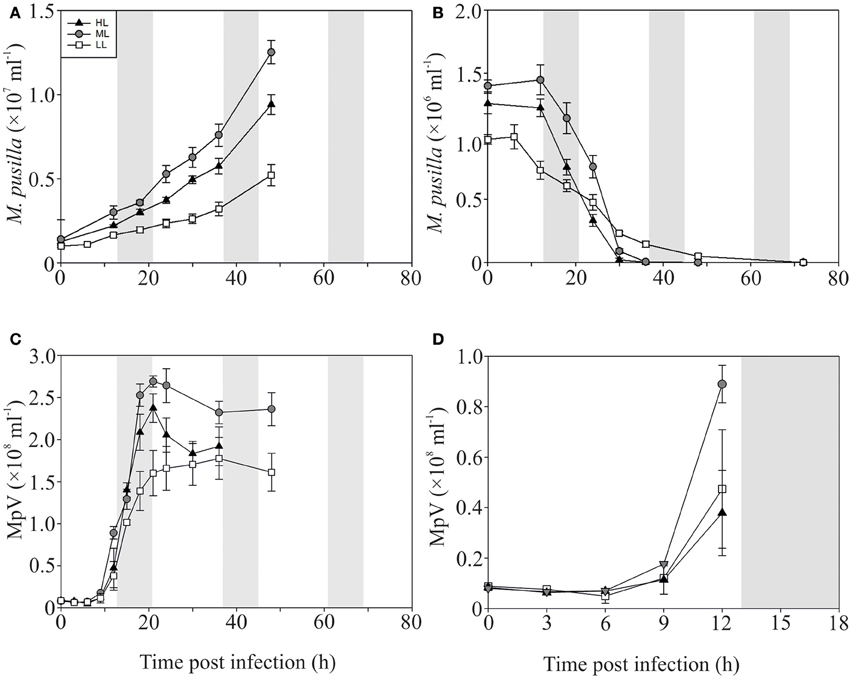
Figure 2. Algal and viral growth curves with abundances of non-infected (A) and infected (B) Micromonas pusilla and MpV (C) under P-replete conditions. (D) Shows MpV abundances in the first 12 h in more detail. Light treatments are depicted by triangles (HL), circles (ML), and squares (LL). Values are averages ± s.d. Gray bar depicts dark (night) period.
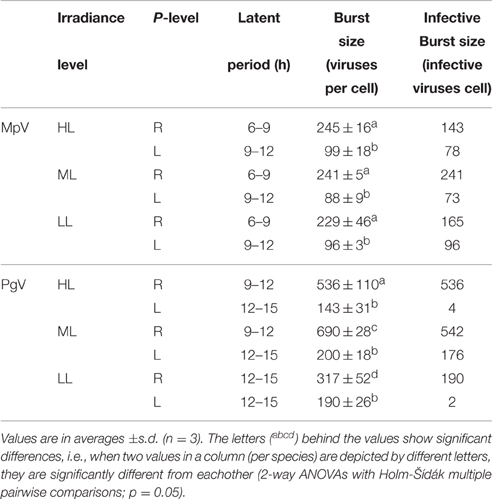
Table 2. Latent periods and burst sizes of M. pusilla virus (MpV) and P. globosa virus (PgV) under P replete (R) and P limited (L) growth conditions and 3 different light conditions (250, 100, and 25 μmol quanta m−2 s−1, represented by HL, ML, and LL, respectively).
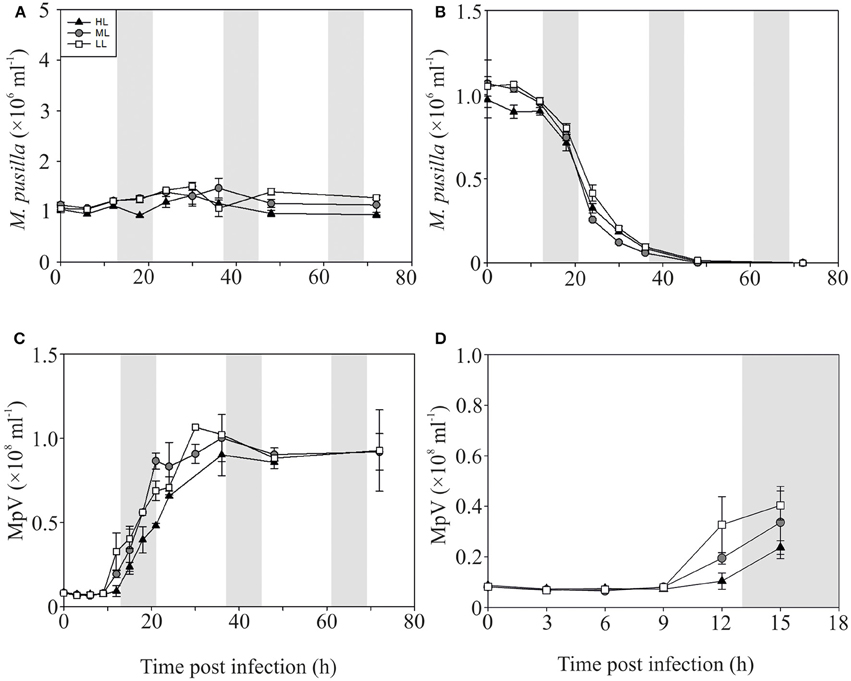
Figure 3. Algal and viral growth curves with abundances of non-infected (A) and infected (B) Micromonas pusilla and MpV (C) under P-limited conditions. (D) Shows MpV abundances in the first 15 h in more detail. Light treatments are depicted by triangles (HL), circles (ML), and squares (LL). Values are averages ± s.d. Gray bar depicts dark (night) period.
The P-replete control cultures of P. globosa showed similar growth dynamics as the replete M. pusilla cultures (Figure 4A). The onset of lysis of the P-replete culture delayed slightly with decreasing light level (Figure 4B). Compared to the latent period at ML (9–12 h), the HL and LL treatment led to a 3 h increase of the latent period (Figures 4C,D; Table 2). Light level under P replete conditions, furthermore, negatively affected the burst size of PgV, i.e., 2.2-fold lower at HL and 1.3-fold at LL (compared to 690 MpV per lysed host cell at ML; Table 2). Light level under P-limitation did not affect the control cultures of P. globosa, but prolonged the time to full lysis under LL (Figures 5A,B). Independent of light level, P-limitation resulted in 1 day delayed lysis of the infected cultures as compared to P-replete cultures. P-limitation prolonged the latent period of PgV with 3 h (to 12–15 h), with the exception of the LL treatment (already 12–15 h under P-replete conditions due to low irradiance; Figures 5C,D; Table 2). Infection of the preconditioned P-limited P. globosa resulted in 3.5-fold reduced PgV burst size at ML (as compared to P-replete). This was further reduced by 2.2 and 2.8-fold at HL and LL, respectively (Table 2). In contrast, under P-limiting conditions there was no significant effect of light level, demonstrating an interaction effect between light and P-limitation (p < 0.001). Infectivity of the PgV progeny under P-replete conditions (all light levels) and P limitation at ML was between 60 and 100%, resulting in infective burst sizes between 176–536 infective viruses per cell (Table 2). However, under P-limitation with HL or LL, the infectivity of the viral progeny was strongly diminished, translating into infective burst sizes of respectively 4 and 2 infectious viruses per lysed host cell.
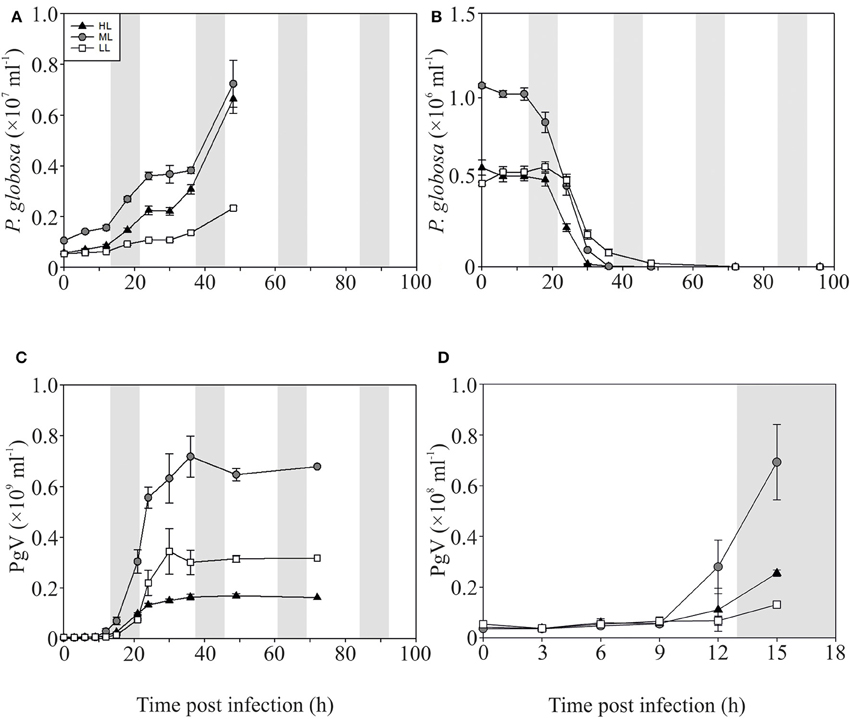
Figure 4. Algal and viral growth curves with abundances of non-infected (A) and infected (B) Phaeocystis globosa and MpV (C) under P-replete conditions. (D) Shows PgV abundances in the first 15 h in more detail. Light treatments are depicted by triangles (HL), circles (ML), and squares (LL). Values are averages ± s.d. Gray bar depicts dark (night) period.
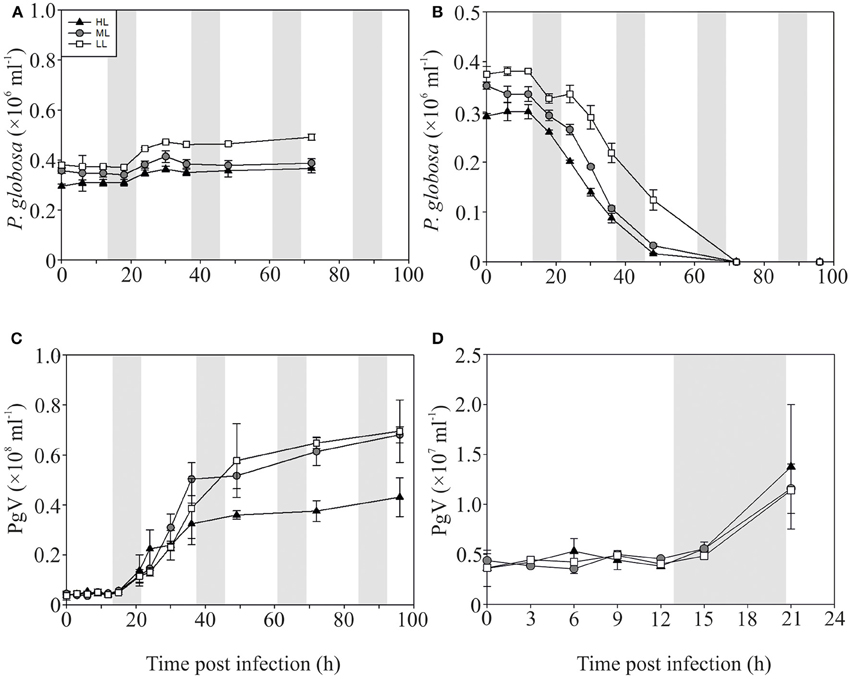
Figure 5. Algal and viral growth curves with abundances of non-infected (A) and infected (B) Phaeocystis globosa and MpV (C) under P-limited conditions. (D) Shows PgV abundances in the first 21 h in more detail. Light treatments are depicted by triangles (HL), circles (ML), and squares (LL). Values are averages ± s.d. Gray bar depicts dark (night) period. Note that the scale of Figure 4C is 10-fold lower than Figure 3C.
Discussion
We showed that P-limitation and light can strongly affect phytoplankton physiology and viral proliferation, whereby both variables strengthened each other's effects, but with differences for both species. Light level as well as P-treatment (P-replete or P-limited) affected the growth rate of the cultures at steady state, with differences for both species. Unfavorable light conditions reduced the growth rates of P. globosa, but they were only further reduced by P-limitation under LL. In contrast, for M. pusilla the growth rates were only negatively affected under HL in combination with P-limitation, demonstrating that both species occupy very different niches. Similar species-specific effects of light on the maximal growth rate were observed for two phytoplankton species in nitrogen (N) limited chemostats (Rhee and Gotham, 1981). The available literature further shows that P-limitation affects photosynthesis and vice versa that photosynthesis affects the cells' P-demand (Lin et al., 2016). However, the exact mechanisms by which unfavorable (HL and LL) light conditions and P-limitation simultaneously affect host growth and physiology in different species need further study. The P-limited state of our cultures was demonstrated by reduced Fv/Fm and cellular P-quotas, the latter respectively 6-fold (1.23 fmol per cell for M. pusilla) and 2-fold (5.68 fmol per cell for P. globosa) lower as under P-replete ML conditions (Maat and Brussaard, 2016).
The FSC, which is a well-accepted indicator for relative cell size (Shapiro, 1988; DuRand et al., 2002), was also affected by P-limitation and light availability in a cumulative way. Our data suggest that cell size for both species increased as a response to P-controlled growth under ML. Such increased phytoplankton cell size under P-limitation has been shown the result of cell cycle arrest in combination with continued accumulation of biochemical compounds, i.e., carbon and nitrogen (see e.g., Lin et al., 2016 and Li et al., 2016). In contrast, M. pusilla (but not P. globosa) FSC decreased under HL and LL. Although the available literature merely shows increased phytoplankton cells sizes under P-limitation (Lin et al., 2016), light intensity might thus counteract those effects. Similar effects were found by Peter and Sommer (2015) who showed that N- and P-limitation led to decreased cell sizes of several phytoplankton species that were grown under the same light levels as our HL treatments. These effects could be merely a physiological response to unfavorable light, but under LL a cell size reduction could also be a strategy to increase the light harvesting capability of the cells, as cell size reductions also decrease intracellular self-shading (Raven, 1998). As changes in cell size (and thus cell surface) would affect the virus-host contact rate (Murray and Jackson, 1992), this could have implications for virus proliferation as well. Further study, using direct cell size measurements, including the determination of intracellular biochemical compounds and with a larger variety of species, can possibly give a more decisive answer.
At the moment of infection, the phytoplankton host cells were in steady state and were thus well acclimated to the P and light conditions, showing constant growth and physiology. Although LL conditions delayed the lysis of the P-replete M. pusilla cultures, it did not significantly affect the latent period or burst size. Hence, MpV proliferation was solely affected by P-limitation and not by light. Quite the opposite, P. globosa showed longer latent periods under LL and lower burst sizes under LL and HL. The effects of P-limitation and light on PgV burst sizes are thus cumulative, as the inhibiting effects of light are dependent on the availability of P. Another ecologically important additive effect was recorded for the progeny PgV that was produced under P-limitation with HL and LL conditions, as less than 3% of the produced viruses under these conditions were found to be able to successfully infect a new host. Despite the relatively large error in the MPN assay (resulting from 10-fold dilution set-up), the differences between infective and total viral abundance was high, i.e., 35 and 120-fold for HL and LL under P-limitation (compared to only 1.1–1.7-fold for all other treatments). Moreover, the MPNs of the repetition experiment showed the same pattern as the first time, we believe that these effects are real. Notably, these results imply that the abiotic environment can almost completely inhibit viral proliferation. Thus far we don't know how infectivity was exactly affected, but if these non-infective viruses are still able to adsorb to the host cell, they potentially may still prevent new infection by viruses that are infective.
A loss of infectivity can be caused by alterations in the ability of a virus to attach, infect (enter host) or transcribe once inside the host (Kapuscinski and Mitchell, 1980). Flow cytometric analysis of the viruses, which is based on DNA-staining, was unaffected demonstrating that the viruses still contained DNA, but genomes might have been incomplete or otherwise affected. It is difficult to explain why P-limitation in combination with LL and HL conditions led to a loss of infectivity, while this was not observed under ML. Possibly the host cell cycle plays a role in this process, as both P-limitation and light limitation have been shown to lead to cell cycle arrest in the G1 phase (Vaulot, 1995; Lin et al., 2016). Although all cells were under the same light:dark regime and all infected at the same time, the cultures experienced P-starvation during the infection experiments. The unfavorable light conditions may have strengthened these effects, leading to cell cycle arrest in G1 and consequently affected production of viral DNA and or proteins. An effect of phytoplankton host cell-cycle on lytic viruses was also found by Thyrhaug et al. (2002) who showed that Pyramimonas orientalis cultures infected with viruses in the beginning of the G1 phase showed 8-times higher virus production than the cultures infected in the S phase. However, in this case no loss of infectivity was observed. The possible role of host cell-cycle in the effects of environmental factors on phytoplankton host-virus interaction remains to be studied. Alternatively, we hypothesize that oxidative stress of the P. globosa host due to unfavorable growth conditions may have affected the infectivity of the PgV progeny. Although we cannot exclude a direct effect of the reactive oxygen species (ROS) on PgV infectivity (under P-limitation with LL or HL), the ROS scavengers dimethylsulfoniopropionate (DMSP) and dimethylsulfide (DMS) are (1) produced by stationary Phaeocystis sp. (likely as a result of P-depletion) and under conditions of low and high light, and (2) shown to affect the infectivity of algal viruses (Stefels and Boekel, 1993; Baumann et al., 1994; Matrai et al., 1995; Stefels and van Leeuwe, 1998; Evans et al., 2006, 2007). Interestingly, an increase in DMS was also observed for virally infected Phaeocystis pouchetii (Malin et al., 1998), but not for infected axenic M. pusilla LB991 (Hill et al., 1998), which may help explain the difference in response to P-limitation with light level we found between the two species. Further study will have to elucidate the mechanisms behind the infectivity loss under these conditions.
It is difficult to pinpoint the underlying mechanisms that led to elongated latent periods and reduced burst sizes under suboptimal light and P-availability. P-limitation could have inhibited viral replication by reducing the availability of P-containing molecules necessary as viral building blocks (nucleotides) or as energy carriers (nucleosides) during replication (Maat et al., 2014). Such an effect on the energy metabolism is supported by the finding that for both species the P-limited treatments, which showed increased latent periods and lowered burst sizes, also showed lower steady state Fv/Fm. This relation was however largely obscured by light conditions and consequently no relation between light intensity, host physiology and virus-host interaction could be demonstrated. Optimal viral replication in P. globosa might depend on both the occurrence of photophosphorylation and the presence of intracellular energy reserves. When light availability during infection is suboptimal, the intracellular energy (ATP) reserves are probably still supporting the production of (a reduced amount of) viruses. However under P-limitation, photophosphorylation (light inhibition and limitation) and energy reserves are both suboptimal, leading to an additional reduction in PgV burst size. Complete darkness during the infection process led to a strong decline in burst size for P-replete ML P. globosa, (Baudoux and Brussaard, 2008) and M. pusilla Lac38 (unpublished data). Intracellular energy reserves can thus sustain viral replication to a similar extent in both species, although for viruses of larger phytoplankton host species this might be the only source of energy during infection when resources are suboptimal. This is supported by the finding that PgV-07T could still replicate in the dark when P. globosa was acclimated to HL and ML, but not when it was acclimated to LL (Baudoux and Brussaard, 2008).
The reason that viral infection in M. pusilla is not affected at all by the same light conditions that strongly affected virus proliferation P. globosa, could be the result of its smaller size, as smaller species are expected to be more efficient in the uptake and utilization of light and nutrients (Raven, 1998). The lower nutrient demand of M. pusilla is supported by the 2-fold lower volume specific P-quota of this species compared to P. globosa during steady state. Likely photophosphorylation during infection would be more effective in the picophytoplankter M. pusilla than in the nanophytoplankter P. globosa and the dependency of viral infection on intracellular energy reserves might thus increase with the size of the phytoplankton host. Similarly, viral proliferation in the small cyanobacterium Anacystis nidulans has been shown to be strictly dependent on photophosphorylation while viruses of the larger-sized Chlorella sp. and Heterosigma akashiwo are thought to be independent on it (Allen and Hutchison, 1976; Van Etten et al., 1983; Juneau et al., 2003).
Ecological Implications
Our results indicate that in a P-limited environment [e.g., at end of P. globosa bloom or open (sub)tropical ocean], the proliferation of phytoplankton viruses would be strongly inhibited, whereby the effects can be intensified by suboptimal (high or low) light conditions. Species-specific differences were particularly distinct in the number of infectious viruses produced by P-limited host at LL and HL. The production of non-infective viral particles by P. globosa under these conditions leads to a higher ratio of non-infective to infective viral particles. Consequently this reduces successful new viral infection of the host, which means lower share of viral lysis (to total mortality). A similar cause may explain the delayed virally induced mortality loss of the bloom-forming phytoplankter Aureococcus anophagefferens under low light conditions (Gobler et al., 2007). In either case, grazing could prevail over viral lysis, which would affect the biogeochemical fate of the produced algal biomass (Brussaard et al., 2008).
Light and P-availability change on a spatial (e.g., vertical distribution in water column and geographical) and temporal (e.g., turbulent waters and seasonal) scale and affect phytoplankton distribution directly (bottom-up) and indirectly (top-down; this study). These effects are expected to play a larger role in the future, as global warming is expected to strengthen vertical stratification and accordingly increased nutrient (P) limitation and prolonged stay at either high (surface ocean) or low (deeper in euphotic zone) irradiance. As phytoplankton viruses play an important role in the marine environment by driving host community dynamics and biogeochemical cycling (Wilhelm and Suttle, 1999; Brussaard, 2004; Suttle, 2007), data on the effects of various co-stressors on phytoplankton host-virus interactions and the underlying mechanisms will further improve our understanding on the marine ecosystem and possible implications of climate change.
Author Contributions
CB and DM designed the project. DM performed culturing work and analyses and Rd performed culturing work. CB provided expertise and supervised the project. DM wrote the manuscript with contributions of all authors.
Funding
This project was funded through grants to CB by the Royal Netherlands Institute for Sea Research (NIOZ), an institute of the Netherlands Organization for Scientific Research (NWO) We acknowledge co-funding by NWO CHARLET (Primary Production in the North Sea: CHAnges in Resource Limitation and Energy Transfer) under contract number 839.10.513.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors want to thank Roos Ruiten and Kristina Mojica for technical assistance.
References
Allen, M. M., and Hutchison, F. (1976). Effect of some environmental factors on cyanophage AS-1 development in Anacystis nidulans. Arch. Microbiol. 110, 55–60. doi: 10.1007/BF00416969
Ando, T., Imamura, H., Suzuki, R., Aizaki, H., Watanabe, T., Wakita, T., et al. (2012). Visualization and measurement of ATP levels in living cells replicating hepatitis C virus genome RNA. PLoS Pathog. 8:e1002561. doi: 10.1371/journal.ppat.1002561
Arteaga, L., Pahlow, M., and Oschlies, A. (2014). Global patterns of phytoplankton nutrient and light colimitation inferred from an optimality-based model. Glob. Biogeochem. Cycles 28, 648–661. doi: 10.1002/2013GB004668
Baudoux, A.-C., and Brussaard, C. P. D. (2008). Influence of irradiance on virus-algal host interactions. J. Phycol. 44, 902–9908. doi: 10.1111/j.1529-8817.2008.00543.x
Baumann, M. E., Brandini, F. P., and Staubes, R. (1994). The influence of light and temperature on carbon-specific DMS release by cultures of Phaeocystis antarctica and three antarctic diatoms. Mar. Chem. 45, 129–1136. doi: 10.1016/0304-4203(94)90097-3
Beardall, J., Young, E., and Roberts, S. (2001). Approaches for determining phytoplankton nutrient limitation. Aquat. Sci. 63, 44–69. doi: 10.1007/PL00001344
Behrenfeld, M. J., Halsey, K. H., and Milligan, A. J. (2008). Evolved physiological responses of phytoplankton to their integrated growth environment. Philos. Trans. R. Soc. B 363, 2687–2703. doi: 10.1098/rstb.2008.0019
Bratbak, G., Jacobsen, A., Heldal, M., Nagasaki, K., and Thingstad, F. (1998). Virus production in Phaeocystis pouchetii and its relation to host cell growth and nutrition. Aquat. Microb. Ecol. 16, 1–9. doi: 10.3354/ame016001
Brussaard, C. P. (2004). Viral control of phytoplankton populations—a Review. J. Eukaryot. Microbiol. 51, 125–138. doi: 10.1111/j.1550-7408.2004.tb00537.x
Brussaard, C. P. D., Wilhelm, S. W., Thingstad, F., Weinbauer, M. G., Bratbak, G., Heldal, M., et al. (2008). Global-scale processes with a nanoscale drive: the role of marine viruses. ISME J. 2, 575–578. doi: 10.1038/ismej.2008.31
Brussaard, C. P., Marie, D., and Bratbak, G. (2000). Flow cytometric detection of viruses. J. Virol. Methods 85, 175–182. doi: 10.1016/S0166-0934(99)00167-6
Clasen, J. L., and Elser, J. J. (2007). The effect of host Chlorella NC64A carbon : phosphorus ratio on the production of Paramecium bursaria Chlorella Virus-1. Freshw. Biol. 52, 112–122. doi: 10.1111/j.1365-2427.2006.01677.x
Cloern, J. E. (1999). The relative importance of light and nutrient limitation of phytoplankton growth: a simple index of coastal ecosystem sensitivity to nutrient enrichment. Aquat. Ecol. 33, 3–15. doi: 10.1023/A:1009952125558
Colijn, F., and Cadée, G. C. (2003). Is phytoplankton growth in the Wadden Sea light or nitrogen limited? J. Sea Res. 49, 83–93. doi: 10.1016/S1385-1101(03)00002-9
Cottrell, M. T., and Suttle, C. A. (1991). Wide-spread occurrence and clonal variation in viruses which cause lysis of a cosmopolitan, eukaryotic marine phytoplankter, Micromonas-pusilla. Mar. Ecol. Prog. Ser. 78, 1–9. doi: 10.3354/meps078001
Cséke, C., and Farkas, G. (1979). Effect of light on the attachment of cyanophage AS-1 to Anacystis nidulans. J. Bacteriol. 137, 667–669.
Dring, M. J., Wagner, A., Franklin, L. A., Kuhlenkamp, R., and Lüning, K. (2001). Seasonal and diurnal variations in ultravioloet-B and ultraviolet-A irradiances at and below the sea surface at Helgoland (North Sea) over a six year period. Helgol. Mar. Res. 55, 3–11. doi: 10.1007/s101520000063
DuRand, M. D., Green, R. E., Sosik, H. M., and Olson, R. J. (2002). Diel variations in optical properties of Micromonas pusilla (prasinophyceae). J. Phycol. 38, 1132–1142. doi: 10.1046/j.1529-8817.2002.02008.x
Dyhrman, S. T., Ammerman, J. W., and Van Mooy, B. A. S. (2007). Microbes and the marine phosphorus cycle. Oceanography 20, 110–116. doi: 10.5670/oceanog.2007.54
Evans, C., Kadner, S. V., Darroch, L. J., Wilson, W. H., Liss, P. S., and Malin, G. (2007). The relative significance of viral lysis and microzooplankton grazing as pathways of dimethylsulfoniopropionate (DMSP) cleavage: an Emiliania huxleyi culture study. Limnol. Oceanogr. 52, 1036–1045. doi: 10.4319/lo.2007.52.3.1036
Evans, C., Malin, G., Wilson, W. H., and Liss, P. S. (2006). Infectious titers of Emiliania huxleyi virus 86 are reduced by exposure to millimolar dimethyl sulfide and acrylic acid. Limnol. Oceanogr. 51, 2468–2471. doi: 10.4319/lo.2006.51.5.2468
Gobler, C. J., Anderson, O., Gastrich, M. D., and Wilhelm, S. W. (2007). Ecological aspects of viral infection and lysis in the harmful brown tide alga Aureococcus anophagefferens. Aquat. Microb. Ecol. 47, 25–36. doi: 10.3354/ame047025
Guillard, R. R., and Ryther, J. H. (1962). Studies of marine planktonic diatoms. I. Cylotella nana hustedt, and Detonula convervacea (cleve) gran. Can. J. Microbiol. 8, 229–239.
Hansen, H. P., and Koroleff, F. (1999). “Determination of nutrients,” in Methods of Seawater Analysis, eds K. Grasshoff, K. Kremling, and M. Erhardt (Weinheim; Wiley), 125–187.
Harrison, P. J., Waters, R. E., and Taylor, F. J. R. (1980). A broad-spectrum artificial seawater medium for coastal and open ocean phytoplankton. J. Phycol. 16, 28–35. doi: 10.1111/j.1529-8817.1980.tb00724.x
Hill, R. W., White, B. A., Cottrell, M. T., and Dacey, J. W. H. (1998). Virus-mediated total release of dimethylsulfoniopropionate from marine phytoplankton: a potential climate process. Aquat. Microb. Ecol. 14, 1–6. doi: 10.3354/ame014001
Huisman, J., Matthijs, H. C., Visser, P. M., Balke, H., Sigon, C. A., Passarge, J., et al. (2002). Principles of the light-limited chemostat: theory and ecological applications. Antonie van Leeuwenhoek 81, 117–133. doi: 10.1023/A:1020537928216
Juneau, P., Lawrence, J. E., Suttle, C. A., and Harrison, P. J. (2003). Effects of viral infection on photosynthetic processes in the bloom-forming alga Heterosigma akashiwo. Aquat. Microb. Ecol. 31, 9–17. doi: 10.3354/ame031009
Kapuscinski, R. B., and Mitchell, R. (1980). Processes controlling virus inactivation in coastal waters. Water Res. 14, 363–371. doi: 10.1016/0043-1354(80)90084-6
Karl, D., Letelier, R., Tupas, L., Dore, J., Christian, J., and Hebel, D. (1997). The role of nitrogen fixation in biogeochemical cycling in the subtropical North Pacific Ocean. Nature 388, 533–538. doi: 10.1038/41474
Kulk, G., van de Poll, W. H., Visser, R. J., and Buma, A. G. (2013). Low nutrient availability reduces high-irradiance–induced viability loss in oceanic phytoplankton. Limnol. Oceanogr. 58, 1747–1760. doi: 10.4319/lo.2013.58.5.1747
Li, M., Shi, X., Guo, C., and Lin, S. (2016). Phosphorus deficiency inhibits cell division but not growth in the dinoflagellate Amphidinium carterae. Front Microbiol. 7:826. doi: 10.3389/fmicb.2016.0082
Lin, S., Litaker, R. W., and Sunda, W. G. (2016). Phosphorus physiological ecology and molecular mechanisms in marine phytoplankton. J. Phycol. 52, 10–36. doi: 10.1111/jpy.12365
Long, S., Humphries, S., and Falkowski, P. G. (1994). Photoinhibition of photosynthesis in nature. Annu. Rev. Plant Biol. 45, 633–662. doi: 10.1146/annurev.pp.45.060194.003221
Ly, J., Philippart, C. J. M., and Kromkamp, J. C. (2014). Phosphorus limitation during a phytoplankton spring bloom in the western Dutch Wadden Sea. J. Sea Res. 88, 109–120. doi: 10.1016/j.seares.2013.12.010
Maat, D. S., and Brussaard, C. P. D. (2016). Both phosphorus and nitrogen limitation constrain viral proliferation in marine phytoplankton. Aquat. Microb. Ecol. 77, 87–97. doi: 10.3354/ame01791
Maat, D. S., Crawfurd, K. J., Timmermans, K. R., and Brussaard, C. P. (2014). Elevated CO2 and Phosphate limitation favor Micromonas pusilla through stimulated growth and reduced viral impact. Appl. Environ. Microbiol. 80, 3119–3127. doi: 10.1128/AEM.03639-13
MacIntyre, H. L., and Cullen, J. J. (2005). “Using cultures to investigate the physiological ecology of microalgae,” in Algal Culturing Techniques, ed R. A. Anderson (Amsterdam: Elsevier Academic press), 287–327.
Malin, G., Wilson, W. H., Bratbak, G., Liss, P. S., and Mann, N. H. (1998). Elevated production of dimethylsulfide resulting from viral infection of cultures of Phaeocystis pouchetii. Limnol. Oceanogr. 43, 1389–1393. doi: 10.4319/lo.1998.43.6.1389
Manton, I., and Parke, M. (1960). Further observations on small green flagellates with special reference to possible relatives of Chromulina pusilla Butcher. J. Mar. Biol. Assoc. U.K. 39, 275–298. doi: 10.1017/S0025315400013321
Marie, D., Partensky, F., Vaulot, D., and Brussaard, C. (1999). “Enumeration of phytoplankton, bacteria, and viruses in marine samples,” in Current Protocols in Cytometry, ed J. P. E. A. Robinson (New York, NY: John Wiley & Sons Inc),11.11.1-11.11.15.
Matrai, P., Vernet, M., Hood, R., Jennings, A., Brody, E., and Saemundsdottir, S. (1995). Light-dependence of carbon and sulfur production by polar clones of the genus Phaeocystis. Mar. Biol. 124, 157–167. doi: 10.1007/BF00349157
Maxwell, K., and Johnson, G. N. (2000). Chlorophyll fluorescence—a practical guide. J. Exp. Bot. 51, 659–668. doi: 10.1093/jexbot/51.345.659
Mojica, K. D., and Brussaard, C. P. (2014). Factors affecting virus dynamics and microbial host–virus interactions in marine environments. FEMS Microbiol. Ecol. 89, 495–515. doi: 10.1111/1574-6941.12343
Moore, C. M., Mills, M. M., Arrigo, K. R., Berman-Frank, I., Bopp, L., Boyd, P. W., et al. (2013). Processes and patterns of oceanic nutrient limitation. Nat. Geosci. 6, 701–710. doi: 10.1038/ngeo1765
Murray, A. G., and Jackson, G. A. (1992). Viral dynamics: a model of the effects of size, shape, motion and abundance of single-celled planktonic organisms and other particles. Mar. Ecol. Prog. Ser. 89, 103–116. doi: 10.3354/meps089103
Nicklisch, A. (1999). Competition between the Cyanobacterium Limnothrix redekei and some spring species of diatoms under P-Limitation. Int. Rev. Hydrobiol. 84, 233–241.
Peter, K. H., and Sommer, U. (2015). Interactive effect of warming, nitrogen and phosphorus limitation on phytoplankton cell size. Ecol. Evol. 5, 1011–1124. doi: 10.1002/ece3.1241
Porter, K. G. (1980). The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 25, 943–948. doi: 10.4319/lo.1980.25.5.0943
Quinlan, A. V. (1986). A semicontinuous culture model that links cell growth to extracellular nutrient concentration. Biotechnol. Bioeng. 28, 1455–1461. doi: 10.1002/bit.260281002
Raven, J. A. (1998). The twelfth Tansley Lecture. Small is beautiful: the picophytoplankton. Funct. Ecol. 12, 503–513.
Rhee, G.-Y., and Gotham, I. J. (1981). The effect of environmental factors on phytoplankton growth: light and the interactions of light with nitrate limitation. Limnol. Oceanogr. 26, 649–659. doi: 10.4319/lo.1981.26.4.0649
Ruttenberg, K. (2003). The global phosphorus cycle. Treatise Geochem. 8, 585–643. doi: 10.1016/B0-08-043751-6/08153-6
Shapiro, H. M. (ed.). (1988). “Parameters and probes,” in Practical Flowcytometry (Hoboken, NJ: John Wiley & Sons, Inc.), 275–276.
Stefels, J., and Boekel, W. V. (1993). Production of DMS from dissolved DMSP in axenic cultures of the marine phytoplankton species Phaeocystis sp. Mar. Ecol. Prog. Ser. 97, 11–18. doi: 10.3354/meps097011
Stefels, J., and van Leeuwe, M. A. (1998). Effects of iron and light stress on the biochemical composition of Antarctic Phaeocystis sp. (Prymnesiophyceae). I. Intracellular DMSP concentrations. J. Phycol. 34, 486–495.
Suttle, C. A. (1993). “Enumeration and isolation of viruses,” in Current Methods in Aquatic Microbial Ecology, eds B. F. KempSherr, E. F. Sherr, and, J. J. Cole Lewis (Florida, FL: Publishers Boca Raton), 121–134.
Suttle, C. A. (2007). Marine viruses - major players in the global ecosystem. Nat. Rev. Microbiol. 5, 801–812. doi: 10.1038/nrmicro1750
Thyrhaug, R., Larsen, A., Brussaard, C. P. D., and Bratbak, G. (2002). Cell cycle dependent virus production in marine phytoplankton. J. Phycol. 38, 338–343. doi: 10.1046/j.1529-8817.2002.01154.x
van de Poll, W. H., Van Leeuwe, M. A., Roggeveld, J., and Buma, A. G. (2005). Nutrient limitation and high irradiance acclimation reduce PAR and UV-induced viability loss in the Antarctic diatom Chaetoceros brevis (Bacillariophyceae). J. Phycol. 41, 840–850. doi: 10.1111/j.1529-8817.2005.00105.x
Van Etten, J. L., Burbank, D. E., Xia, Y., and Meints, R. H. (1983). Growth cycle of a virus, PBCV-1, that infects Chlorella-like algae. Virology 126, 117–125. doi: 10.1016/0042-6822(83)90466-X
Vaulot, D. (1995). “The cell cycle of phytoplankton: coupling cell growth to population growth,” in Molecular Ecology of Aquatic Microbes, ed I. Joint (Berlin; Heidelberg: Springer), 303–322.
Wilhelm, S. W., and Suttle, C. A. (1999). Viruses and Nutrient Cycles in the Sea - Viruses play critical roles in the structure and function of aquatic food webs. Bioscience 49, 781–788. doi: 10.2307/1313569
Keywords: phytoplankton, virus, phosphorus limitation, light intensity, infectivity, burst size, latent period
Citation: Maat DS, de Blok R and Brussaard CPD (2016) Combined Phosphorus Limitation and Light Stress Prevent Viral Proliferation in the Phytoplankton Species Phaeocystis globosa, but Not in Micromonas pusilla. Front. Mar. Sci. 3:160. doi: 10.3389/fmars.2016.00160
Received: 01 June 2016; Accepted: 22 August 2016;
Published: 31 August 2016.
Edited by:
Anton F. Post, Coastal Resource Center, University of Rhode Island, USAReviewed by:
Hélène Montanié, University of La Rochelle, FranceSenjie Lin, University of Connecticut, USA
Copyright © 2016 Maat, de Blok and Brussaard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Douwe S. Maat, ZG91d2UubWFhdEBuaW96Lm5s
 Douwe S. Maat
Douwe S. Maat Reinhoud de Blok
Reinhoud de Blok Corina P. D. Brussaard
Corina P. D. Brussaard