- 1College of Earth, Ocean, and Atmospheric Sciences, Oregon State University, Corvallis, OR, USA
- 2National Oceanography Centre, Liverpool, UK
- 3North Pacific Marine Science Organization, Sidney, BC, Canada
Most coral reef organisms have a bipartite life-cycle; they are site attached to reefs as adults but have pelagic larval stages that allow them to disperse to other reefs. Connectivity among coral reef patches is critical to the survival of local populations of reef organisms, and requires movement across gaps that are not suitable habitat for recruitment. Knowledge of population connectivity among individual reef habitats within a broader geographic region of coral reefs has been identified as key to developing efficient spatial management strategies to protect marine ecosystems. The study of larval connectivity of marine organisms is a complex multidisciplinary challenge that is difficult to address by direct observation alone. An approach that couples ocean circulation models with individual based models (IBMs) of larvae with different degrees of life-history complexity has been previously used to assess connectivity patterns in several coral reef regions [e.g., the Great Barrier Reef (GBR) and the Caribbean]. We applied the IBM particle tracking approach to the Kenya-Tanzania region, which exhibits strong seasonality in the alongshore currents due to the influence of the monsoon. A 3-dimensional (3D) ocean circulation model with 2 km horizontal resolution was coupled to IBMs that track virtual larvae released from each of 661 reef habitats, associated with 15 distinct regions. Given that reefs provide homes to numerous species, each with distinctive, and in aggregate very diverse life-histories, several life-history scenarios were modeled to examine the variety of dispersal and connectivity patterns possible. We characterize virtual larvae of Acropora corals and Acanthurus surgeonfish, two coral reef inhabitants with greatly differing pelagic life-histories, to examine the effects of short (<12 days) and long (>50 days) pelagic larval durations (PLD), differences in swimming abilities (implemented as reef perception distances), and active depth keeping in reef connectivity. Acropora virtual larvae were modeled as 3D passive particles with a precompetency period of 4 days, a total PLD of 12 days and a perception distance of 10 m. Acanthurus virtual larvae were characterized by 50 days precompetency period, a total PLD of 72 days and a perception distance of 4 km. Acanthurus virtual larvae were modeled in two ways—as 3D passive particles and including an idealized ontogenetic vertical migration behavior. A range of distances within which larvae were able to perceive reefs and directionally swim to settle on them during the competency period were evaluated. The influence of interannual environmental variations was assessed for 2 years (2000, 2005) of contrasting physics. The spatial scale of connectivity is much smaller for the short PLD coral, with successful connections restricted to a 1° radius (~100 km) around source reefs. In contrast, long distance connections from the southern to the northernmost reefs (~950 km) are common for virtual Acanthurids. Successful settlement for virtual Acropora larvae was <0.3%, and within region settlement (local retention) was 0.38%, substantially greater than inter-region settlement (ca. 0.2%). Settlement of Acanthurus virtual larvae was >20% overall, with cross-region recruitment much increased compared to the coral larvae. Approximately 8% of Acropora larvae that successfully settled, recruited to their source reef (self-recruitment), an important proportion compared to only 1–2% self-recruitment for Acanthurus. These rates and dispersal distances are similar to previous modeling studies of similar species in other coral reef regions and agree well with the few observational studies within the Kenya-Tanzania region.
Introduction
Tropical coral reef ecosystems are very important from both the ecological and economical points of view (Spalding et al., 2001). However, they are also particularly fragile, and have been declining in recent years in most regions of the world (Hughes et al., 2003; Pandolfi et al., 2003; Melbourne-Thomas et al., 2011), since they are highly susceptible to anthropogenic stressors operating at global scales (e.g., global warming and ocean acidification) and local scales (e.g., pollution/eutrophication, fishing, over-commercialization for recreation). Coral reef ecosystems are complex communities with very high species diversity. Most reef species have bipartite life histories with a planktonic larval stage and a benthos associated adult life. As adults, coral reef organisms exhibit various degrees of site attachment ranging from completely sessile, like corals and sponges, to highly mobile, like fish and crustaceans. Generally, even fish capable of swimming several kilometers in a few hours have restricted home ranges, since they are relatively territorial and are associated with specific reef habitats that are patchily distributed (Sale, 2006). Most adult reef organisms are distributed in metapopulations connected by pelagic larvae that disperse subject to the ocean currents (Bode et al., 2006; Cowen and Sponaugle, 2009).
Coral reefs extend along the coast of East Africa from the equator to ~14°S, being absent only at major river outflows or Pleistocene river valleys. Fringing reefs are the most common type, but complex formations occur around islands and other regions where the continental shelf extends more than a few kilometers from shore. Reefs are absent on the Somali coast north of the equator due to seasonal upwelling of cold water associated with the monsoon winds. The southernmost reef is found in Mozambique at 26°S; but scattered colonies of scleractinian corals occur as far south as 34°S, in South Africa (Day, 1974 cited in Hamilton and Brakel, 1984). Western Indian Ocean coral reef communities are characterized by high levels of species diversity and may be centers of biodiversity (Spalding et al., 2001). Coastal communities of Kenya and Tanzania depend on the reef for food. Since there is little regulation on the use of these resources through formal resource management strategies, reef areas in Kenya and Tanzania have been degraded due to overfishing, destructive fishing techniques, coastal pollution and other activities affecting the coastal environment (Hamilton and Brakel, 1984; Spalding et al., 2001). Increasing interest in coral reef tourism is simultaneously leading to increased pressure on some coral reefs while providing a powerful local incentive for conservation (Spalding et al., 2001). There are 26 Marine Protected Areas (MPAs) in Kenya and Tanzania reported in the Protected Planet Database (http://www.protectedplanet.net/; accessed July 2016) that encompass coral reef habitat; some of these were established as recently as 2010. Eight of the 26 MPAs are no-take areas, while 18 of them allow extraction using traditional fishing methods like handlines and traps (Muthiga et al., 2008). The benefits of MPAs for biodiversity conservation and fisheries management are well known (McClanahan and Mangi, 2000; Gell and Roberts, 2003; Roberts et al., 2005; Lester et al., 2009; Micheli et al., 2012); however, the design (spacing, size and separation distance) of effective MPA networks is not trivial (e.g., Botsford et al., 2003; McLeod et al., 2009; Edgar et al., 2014). Many studies (e.g., Botsford et al., 2009; McCook et al., 2009; Hogan et al., 2011; Rossi et al., 2014) emphasize the importance of larval connectivity on the performance of MPA spatial management for meeting conservation and fisheries yield objectives.
Larval connectivity is vital to the survival of marine metapopulations, both at ecological and evolutionary time scales (James et al., 2002; Cowen and Sponaugle, 2009; Burgess et al., 2014). Population connectivity plays a fundamental role in local and metapopulation dynamics, community dynamics and structure, genetic diversity, ecosystem responses to environmental changes, and the resiliency of populations to human exploitation (Cowen et al., 2007). Connectivity among marine metapopulations is controlled by physical transport and dispersion, temperature, and biological processes such as the timing of spawning, pelagic larval duration (PLD), larval behavior, and mortality. The net combined effect of these processes determines the spatial scales over which a population is connected (Gawarkiewicz et al., 2007). Connectivity is therefore a function of several interacting variables including species, geographical area, and ocean conditions, and is highly variable in both time and space (e.g., Cowen and Sponaugle, 2009; Christie et al., 2010b; Domingues et al., 2012). Often, little is known about the connections among different coral reef regions (Cowen et al., 2000; Mora and Sale, 2002; Sponaugle et al., 2002) and the degree to which local populations are open (dependent on recruits from external sources) (e.g., Saenz-Agudelo et al., 2011) or closed (self-replenishing) (e.g., Schultz and Cowen, 1994).
Observational approaches for studying connectivity use genetic techniques (Baums et al., 2005; Jones et al., 2005; Christie et al., 2010a,b; Hogan et al., 2011; Harrison et al., 2012), spatially varying natural bio-markers leaving a geochemical signature in calcified structures (i.e., otoliths and statoliths) (Thorrold et al., 1998, 2007) or tagging otoliths of larvae (Jones et al., 1999; Almany et al., 2007). These techniques are limited in the spatio-temporal scales they can resolve and some of them are restricted to specific species or environments (Gawarkiewicz et al., 2007; Hedgecock et al., 2007; Thorrold et al., 2007). Challenges of applying observational techniques to larval connectivity in the Kenya-Tanzania (KT) region are that these methods are expensive, time-consuming and require highly specialized equipment and expertise (Thorrold et al., 2007). Individual based Lagrangian particle tracking models (IBM) coupled to realistic ocean circulation models are a less limiting method to study potential connectivity among East-African coral reefs. So long as the ocean circulation model reasonably depicts the time-varying flows, IBMs can resolve time varying 3-dimensional (3D) potential dispersion of planktonic larvae over large spatial scales with high spatio-temporal resolution (Werner et al., 2007). Results of numerical simulations can only provide estimates of potential connectivity, that need to be validated with empirical measurements (i.e., Foster et al., 2012; Soria et al., 2012) and scaled by observed reproductive input (Watson et al., 2010) and settlement (i.e., Sponaugle et al., 2012). Even in the absence of empirical confirmations, estimates of potential connectivity from modeling studies provide a comprehensive understanding of the spatial-temporal dynamics of marine populations, that inform the design of more efficient MPAs (Willis et al., 2003; Sale et al., 2005). With the exception of a few studies (McClanahan, 1994; McClanahan et al., 1994; Mangubhai, 2008; Yahya et al., 2011; Kruse et al., 2016), knowledge of reef biota ecology is lacking for much of the Western Indian Ocean region, due to the lack of infrastructure and local expertise, combined with problems of national security in some areas (Spalding et al., 2001). Few studies have examined larval supply and connectivity in coral reefs in the Western Indian Ocean (Kaunda-Arara et al., 2009; Crochelet et al., 2013, 2016). Genetic techniques have been used to study connectivity at evolutionary time scales of several reef fish (Dorenbosch et al., 2006, Lutjanus fulviamma; Visram et al., 2010, Scarus ghobban; and Muths et al., 2012, Lutjanus kasmira). High gene flow and weak genetic structure were found in these fish, even among sites as distant as 4000 km (Muths et al., 2012). Recently, van der Ven et al. (2016) used genetic techniques to examine connectivity at evolutionary time scales of the branching coral Acropora tenuis in the Kenya-Tanzania region. They report high but variable connectivity among sample sites spanning 900 km along the coast. These studies do not address ecologically significant timescales of a few generations, and are in general concerned with large spatial scales (~1000 s of km), therefore they cannot provide insights on population demography at temporal and spatial scales relevant to the implementation of management and conservation strategies at national and regional levels. Only Souter et al. (2009) used genetic techniques to examine both evolutionary and ecological connectivity of the coral Pocillopora damicornis in the MPAs of the KT region. They identified the Mnemba Conservation area northeast of Zanzibar Island as a potential source for the P. damicornis population, and Malindi Marine National Park and Reserve in north Kenya as a genetically isolated reef.
For decades the spatial connectivity of larval fish and invertebrates was thought to be a passive process governed primarily by the ocean physics and the duration of the larval period (e.g., Shanks, 2009). The PLD of coral reef organisms varies greatly; from a few hours for some coral species to a few months for some fish and crustaceans (Shanks, 2009). Recent studies (i.e., Leis and Carson-Ewart, 2003; Paris and Cowen, 2004; Shanks, 2009; Pineda et al., 2010) have shown that larval transport of most marine organisms is not strictly passive and that there is an uncoupling between dispersal distance and PLD due to larval behavior, such as active depth selection and directional swimming. Discrepancies between the passive transport hypothesis and observed patterns of recruitment point to the importance of biological factors (i.e., behavior, predation, starvation, etc.) in the control of larval dispersal and connectivity (Paris and Cowen, 2004; Cowen et al., 2006; Leis et al., 2007; Cowen and Sponaugle, 2009; Sponaugle et al., 2012). Even excluding mortality, the degree to which biological factors influence connectivity is greater than originally hypothesized (Shanks, 2009). Recent studies have shown the importance of physiological and behavioral characteristics of larvae on influencing the connectivity and dispersal of species with a planktonic larval stage (i.e., Kingsford et al., 2002). Growth rates (e.g., Bergenius et al., 2002), ontogenetic and diel vertical migrations (Paris et al., 2007; Drake et al., 2013), swimming ability (e.g., Stobutzki and Bellwood, 1997; Wolanski et al., 1997; Leis and Carson-Ewart, 2003; Leis et al., 2007), orientation through olfaction (Atema et al., 2002, 2015; Gerlach et al., 2007; Paris et al., 2013) and audition (Tolimieri et al., 2000; Leis et al., 2003; Simpson et al., 2005; Heenan et al., 2009; Vermeij et al., 2010), and settlement strategies (Leis and Carson-Ewart, 1999; Lecchini, 2005) are important in controlling connectivity of coral reef organisms. Observational studies suggest that marked ontogenetic vertical zonation is important for larval transport (Boehlert and Mundy, 1993; Cowen and Castro, 1994). In modeling studies, vertical migration often promotes local retention and recruitment of pelagic larvae to suitable habitat. A modeling study of the California Current System (CCS) by Drake et al. (2013) showed that larvae that remained below the surface boundary layer were 500 times more likely to be retained within 5 km of the coast after 30 days than larvae that remained near the surface. Settlement in the CCS increased by an order of magnitude when larvae remained at 30 m depth. Similarly, settlement success in different regions of the Caribbean increased when a shallow ontogenetic vertical migration (OVM) behavior was added to the virtual larvae (Paris et al., 2007). Potential settlement estimates increased up to 190% in the southern Florida Keys with the OVM (Paris et al., 2007). The influence of larval physiology and behavior on connectivity and dispersal of coral reef species is now well established (Kingsford et al., 2002; Paris et al., 2007; Wolanski and Kingsford, 2014). However, biological characteristics are known with certainty only for a handful of species.
The hydrodynamics of the KT coastal ocean is highly variable at seasonal and subseasonal time scales, due to the influence of the monsoons and complex tidal interactions. The coastal circulation is mainly influenced by: (1) the northward flowing East African Coastal Current (EACC) fed by (2) the regionally westward flowing North East Madagascar Current (NEMC), (3) the seasonally reversing Somali Current (SC), (4) tides and (5) local winds (see Figure 1 of Mayorga-Adame et al., 2016). SW monsoon conditions are characterized by strong continuous northward flow along the coast and relatively cool (~24°C) sea surface temperatures (SST) that prevail from May to October. During the NE monsoon, from January to March, a strong north-south SST gradient is caused by the intrusion of the shallow, southward flowing, cold and salty Somali Current that meets the slow northward flowing, warm and fresh EACC. The convergence of the two currents forms the eastward flowing South Equatorial Counter Current. The inter-monsoon seasons, in between these two periods, are characterized by strong mixing and slow currents.
The relative lack of physiological and behavioral data for larvae of coral reef species in the Kenya-Tanzania region led us to examine connectivity among coral reefs using idealized particle tracking experiments that simulate larvae with characteristics of two ubiquitous and ecologically important species groups: the Acropora branching corals with short PLD (ca. 12 days; Babcock and Heyward, 1986; Nishikawa et al., 2003; Nozawa and Harrison, 2008) and the Acanthurus surgeon fish with long PLD (72 days; Rocha et al., 2002) (See Mayorga-Adame, 2015 for a review of the genus life histories). Particle tracking of individual organisms using the output of ocean circulation models is a suitable, cost effective tool to examine larval connectivity among coral reefs in large areas and at fine spatio-temporal scales relevant to the population ecology of coral reef species (Werner et al., 2001, 2007; Cowen and Sponaugle, 2009). Insight developed from connectivity matrices generated from this study could aid local managers and decision makers tasked with regulating the use of marine resources in the Kenya-Tanzania region. Hindcasting the connections among reefs in the strongly dynamical Kenya-Tanzania region is challenging and the level of uncertainty is high. The results presented are a first attempt at assessing connectivity in the region and should be treated as a regional result suitable for comparison with similar studies in other coral reef regions [Great Barrier Reef (GBR); Mesoamerican Caribbean Reef]. In addition, these model results should be useful for developing hypotheses and designing observational campaigns aimed at validating or improving the described connectivity patterns.
Methods
Hydrodynamic Model
A 2 km horizontal resolution Regional Ocean Model System (ROMS) (Haidvogel et al., 2008) that includes tides, the 2 km Kenyan-Tanzanian Coastal Model (hereafter 2KTCM), was used to generate (3D) ocean velocity fields. This model is an enhanced resolution version of the 4 km Kenyan-Tanzanian Coastal Model (KTCM) (Mayorga-Adame et al., 2016). The model domain is a rectangular grid extending from 38° to 47°E and from the equator to 10°S (Figure 1). It has 31 terrain following vertical levels. The model bathymetry comes from the 30 s global GEBCO product (http://www.gebco.net/data_and_products/gridded_bathymetry_data/; accessed April 2011). The model coastline was manually edited to retain as many features as the 2 km resolution allowed. Only Pate Island in north Kenya, and Pemba, Zanzibar and Mafia Islands in Tanzania are included as dry cells in the land mask. The atmospheric forcing (wind stress, heat and freshwater fluxes) is calculated by ROMS bulk formulation using atmospheric variables from the daily NCEP/NCAR reanalysis (Kalnay et al., 1996). The model is initialized and forced at the boundaries by monthly fields of T, S and velocity from the KTCM (Mayorga-Adame et al., 2016) and tides are provided by the TPXO6 global tidal model (Egbert et al., 1994; Egbert and Erofeeva, 2002). Freshwater runoff and diurnal wind variability are not included in the model. The ocean model was run continuously for 8.25 years from October 1999 to December 2007. Three-hourly averages of the velocity fields for 2000 and 2005 were stored and used for the particle tracking experiments.
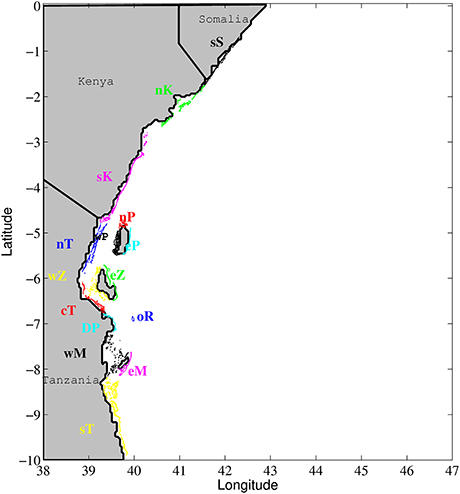
Figure 1. Study area with coral reefs grouped by color into 15 regions. sS, south Somalia; nK, north Kenya; sK, south Kenya; wP, west Pemba; eP, east Pemba; nP, north Pemba; nT, north Tanzania; wZ, west Zanzibar; eZ, east Zanzibar; cT, central Tanzania; DP, Dar es Salaam Peninsula; oR, offshore Reef; wM, west Mafia; eM, east Mafia; sT, south Tanzania.
Lagrangian Particle Tracking
An Individual Based Model (IBM) (Batchelder, 2006) was run offline using previously stored 3-h averages of the 3D-2KTCM velocity fields. The IBM interpolates tri-linearly in space and linearly in time the velocity fields from the ROMS simulation. Particle trajectories are computed using a 4th order Runge-Kutta algorithm. No explicit diffusion (e.g., random walk applied to the individual's position) is invoked since the 2 km horizontal resolution of the ocean circulation model is enough for significant eddy formation and horizontal mixing to occur around reefs, and the terrain following coordinates provide very high vertical resolution (<15 cm) in the shallow regions. A 3D advection-only version of the IBM was used to track forward in time the dispersal of particles (virtual larvae) originating from all reef polygons. The tracking was done using a 30 min time step. Coral larvae were tracked using the 3D passive advection scenario only. For surgeonfish, with longer PLDs and greater ability to control depth in the water column, an idealized ontogenetic vertical migration scenario was implemented.
Biological Assumptions
In the model experiments all reefs were seeded randomly with a density of 50 particles per square kilometer of reef. Reefs smaller than 1 km2 were seeded with 50 particles. A total of 129 184 particles were released for each modeled spawning day, using identical seeding locations for all simulations. Spawning was assumed to take place at 5:30 p.m. local time (~sunset) during February and March, the months of peak spawning for coral reef species in the Western Indian Ocean (Mangubhai, 2008; Mangubhai and Harrison, 2008). All particles were released at 3 m depth. For the 3D passive experiments (reference experiments) virtual larvae were spawned at the release locations at 3 day intervals starting on February 2nd for a total of 20 releases. PLD for Caribbean species of Acanthurus range from 45 to 70 days (Rocha et al., 2002). The Indo-Pacific species A. triostegus has a mean PLD of 54 days (range of 44–83 days) (Randall, 1961; McCormick, 1999; Longenecker et al., 2008). Acanthurus virtual larva were tracked for 72 days and considered competent to settle 50 days after their release, giving them a competency period of 22 days. Acropora virtual larvae were tracked for 12 days and considered competent after 4 days giving them a competency period of 8 days. These assumptions were made considering the results of laboratory rearing studies that reported a minimum pre-competency period of 3 to 4 days for A. muricata, A. valida (Nozawa and Harrison, 2008), and A. tenuis (Nishikawa et al., 2003), and up to 97% settlement 10 days after spawning (Babcock and Heyward, 1986; Nishikawa et al., 2003; Nozawa and Harrison, 2008). The ability of reef larvae to sense nearby reefs and swim toward settlement habitat is often represented in models as a sensory zone based on perception distance (Paris et al., 2007; Sponaugle et al., 2012), a buffer distance around suitable habitat that defines how far away from a reef larvae are able to successfully settle. Based on observational studies of sensing, swimming and settling ability (Leis and Carson-Ewart, 1999; Leis and Fisher, 2006; Atema et al., 2015), perception distance for competent Acanthurus larvae was assumed to be 4 km, which is consistent with the distance used to model other coral reef fish (Paris et al., 2007; Sponaugle et al., 2012). Perception distance for competent Acropora larvae was assumed to be much shorter, only 10 m, because despite their ability to perceive sounds (Vermeij et al., 2010) and chemical cues (Dixson et al., 2014) emanating from reefs they have very limited swimming ability and are unlikely to overcome water speeds (Baird et al., 2014). Virtual larvae were evaluated each night during their competency period to determine if reefs were within their perception distance. If so, they were assumed to settle on the first reef they encountered. Sensitivity analysis to evaluate whether the destination reef of settled larvae was affected by the time of evaluation during the dark hours indicated little temporal variation within a night. Therefore, settlement of virtual larvae was evaluated once per night at 11:30 p.m. local time. The Acanthurus ontogenetic vertical migration (OVM) experiment included passive dispersal for 20 days, then larvae were shifted to 50 m (or 3 m above the bottom at locations shallower than 50 m). Virtual larvae at 50 m continued to be passive in their horizontal movement but were kept at fixed depth for 20 days. At day 40, the larvae migrated back to 3 m depth to find suitable reef habitat when reaching competency (50 days after spawning). After the upward migration, larvae are advected passively in three dimensions until day 72, when the trajectory was terminated. Successful settlement was assessed as described in the reference experiment. OVM experiments were run for the February to March period as the passive experiments, but with larvae released only every sixth day (for a total of 10 release dates).
Seascape Analysis
Kenya and Tanzania have very narrow continental shelves, with the 200 m isobath only 12 km offshore, except at the Mafia and Zanzibar Channels. The shores of Kenya and Tanzania are bordered by a virtually continuous chain of fringing coral reefs that stretches along the coast, only breaking at river mouths and estuaries. The coral reef polygons in the model domain were extracted from the Global Distribution of Coral Reefs 2010 database available at the Ocean Data Viewer webpage (http://data.unep-wcmc.org/). After simplifying the polygons using ArcGIS, by merging adjacent reefs (separated by <20 m), and discarding individual reefs smaller than 25 m2, a total of 661 individual reef polygons identified reef habitat for larval settlement (Figure 1). A connectivity matrix showing the origin locations on one axis and destination locations on the other axis is used to visualize the geographic connections among habitat patches for simple alongshore linear systems. However, the two dimensional nature of the reef systems bordering East Africa, with multiple reefs at the same latitude (e.g., mainland fringing reefs, atolls or patch reefs in the channels between the islands and mainland, fringing reefs on the west and east coast of the islands), make the reef to reef connectivity matrices organized by the latitude of the centroid of the reef polygons insufficiently informative regarding inshore-offshore connections. Due to the spatial complexity of the reef habitat, we simplified the connectivity matrices by assigning individual reefs to one of fifteen geographic subregions (Figure 1). Geographic regions considered mainland continuity of reefs, but also national borders and offshore island masses, many of which have both shoreward facing and offshore facing fringing reefs (Figure 1). This allowed a more meaningful visualization of the results. Based on the number of particles released within a region, the percentage of particles that successfully connect from region to region was calculated. Summing the percentages in the horizontal direction (all destination regions) on the connectivity matrices shows the percent of successful recruits from each region of origin.
The term local retention refers to the ratio of virtual larvae settling at their released location and the total number of virtual larvae released at that location, while self-recruitment is the ratio of virtual larvae settling at their released location and the total number of larvae settling at that location. In the results section the comparatives “weaker” and “stronger” are used to refer to the magnitude of connections between two specific sites, indicating the proportion of particles connecting from one reef or region to another. Strong connections appear as large color-coded circles in the connectivity matrices, while weak connections are small black circles. Conversely “few” and “more/lots” are used to refer to the number of sites that are connecting to a reef or region. The number of connections for a region will be represented by the number of circles on each row or column for origin and destination regions, respectively.
We use the terms “source” and “origin” interchangeably to refer to reefs or regions from which virtual larvae are released. Similarly, we use the terms “sinks” and “destinations” interchangeably to refer to reefs or regions into which virtual larvae successfully settle. We are not referring to population source/sinks according to the classical population ecology definition, since we do not consider spatially variable reproductive input nor variable mortality during the settlement phase. In this case we are referring only to source/sinks of the planktonic pool of successful virtual larvae, and therefore the terms only refer to the diversity of origins/destinations of the virtual larvae that are assumed to successfully settle.
Sensitivity Analysis
Complementary analysis and “in silico” experiments were carried out to determine the sensitivity of the resulting connectivity matrices linking origins and destinations to the perception distance assumption and to the inclusion of vertical diffusion. To investigate the sensitivity of settlement success to perception distance, the coral and surgeonfish reference runs of 2000 were re-analyzed with perception distances of 10, 500, and 4000 m. This analysis was performed for particle releases every sixth day for a total of 10 spawning days within February and March. The percent of larvae that successfully settled on reef habitat and the standard deviation among the 10 release dates was calculated.
In order to assess the effects of vertical diffusion on connectivity, and to investigate if an important proportion of reef-to-reef connections is being missed by considering advective only experiments, additional experiments similar to the advective only Acanthurus reference runs, but with the addition of vertical diffusion processes, were carried out for 26 selected reefs (Supplementary Figure 5). In order to perform these simulations with the same computer resources used for the advective only simulations the number of release locations had to be greatly reduced; we subsampled 10% (a total of 2694 release locations) from the 26938 release locations used for the advection only scenario for these 26 reefs. For each advection-diffusion release location 100 replicate particles were released on each of 3 release dates; at the beginning, middle and end of the presumed spawning season (February 2nd, March 1st, and March 31st, respectively) of 2000 and 2005. Vertical diffusion was implemented as a vertical random walk scaled by the vertical viscosity coefficient of the ocean model according to the model detailed by Batchelder et al. (2002). The connectivity provided by the advection-diffusion simulations was compared to advection only results from the same 26 reefs to investigate the potential role of vertical diffusion in either enhancing or reducing connectivity and modifying the general patterns observed in the advective only experiments. Comparisons were done at the reef scale. Magnitude of the connections was calculated as the percentage of particles released per reef that settled. The number of particles released was an order of magnitude higher in the experiments with diffusion, while the number of release locations was 10 times greater in the advective only experiments (e.g., 1 particle from each of 50 different locations from a 1 km2 reef in the advection only case vs. 500 particles from each of only 5 release locations within the same reef in experiments including vertical diffusion). The results of all 6 release dates (three in each of 2000 and 2005) were aggregated on a binary reef to reef connectivity matrix, which neglects the magnitude of the connections, and compared to the advective only counterpart. The subtraction of the matrices eliminates connections present in both scenarios and provides an estimate of how many connections were missed by one or the other experimental set ups. Trajectories and connectivity patterns were also visualized and compared to gain understanding of the observed differences, but are not shown.
Results
Acanthurus and Acropora 3-D Passive Advective Experiments
Settlement Success
Larvae that find a reef within their perception distance during their competency period are assumed to successfully settle. The percentage of successful settlers differs greatly between the two modeled genera. For virtual larvae characterized as Acanthurus surgeonfish the mean settlement success of the 40 releases during February and March of 2000 and 2005 is 24.4 ± 4.7% while for the Acropora coral virtual larvae the mean is 0.28 ± 0.04% (Figure 2). Settlement success variability around the mean is similar between the two species groups. Changes in yearly mean settlement success are opposite for the two modeled genera (Acanthurus 25.5% in 2000 and 23.3% in 2005, Acropora 0.27% in 2000 and 0.28% in 2005).
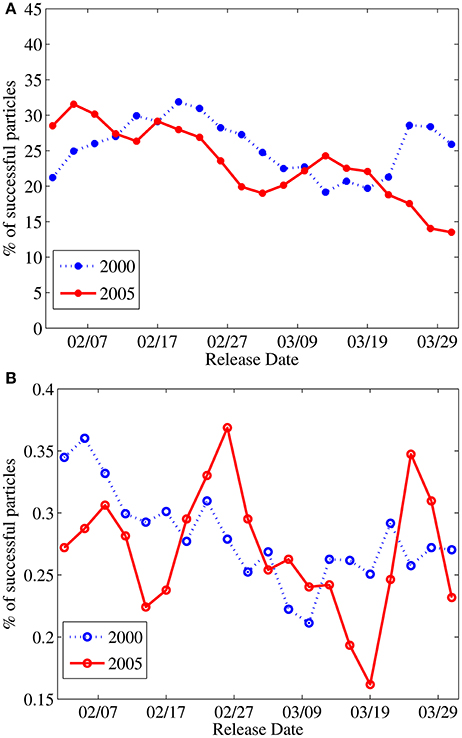
Figure 2. Percentage of settlement success per each simulated release date during 2000 and 2005 for the (A) Acanthurus and (B) Acropora genera.
Region to Region Connectivity Matrices
The region to region connectivity matrices allow an easier visualization of the main connectivity patterns, synthesizing the information of reef to reef connectivity matrices (Supplementary Figure 6). Regional connectivity matrices with reefs grouped into 15 regions (Figure 1) show a dominant South to North connectivity pattern along the Kenya-Tanzania coast (Figure 3), as represented by the predominance of circles below the 1:1 diagonal line, which indicates sites where local retention occurred (larvae released in a region settled in the same region). This pattern is prevalent in both modeled years (2000 and 2005) for both larvae types (Acanthurus and Acropora) (Figure 3), and reflects the strong south to north flows that prevail along most of the Kenya-Tanzania region, which is influenced by the northward flowing EACC year round. Most of the small number of circles above the 1:1 line of the connectivity matrices indicate north to south connections [a few of them represent west to east connections, for example west Zanzibar (wZ) to east Zanzibar (eZ)], which are much less common but occur in the northern part of the domain due to the influence of the southward flowing Somali Current during the Northeast monsoon (December-March), or to small scale recirculation features, such as eddies, in a few other locations. The magnitude and location of north to south connections is particularly variable interannually. For the 2000 Acanthurus simulation (Figure 3A) small proportions of north to south connections occur in most regions, but mainly at the northern-most (Somalia [sS], Kenya regions [nK, sK]) and southern-most regions (east Mafia [eM]) and in some central regions (Dar es Salaam Peninsula [DP] and central Tanzania [cT]). In 2005 (Figure 3C) north to south connections are weak at the northern and southern limits of the domain, but somewhat stronger in the central region (Dar es Salaam Peninsula [DP], western Zanzibar [wZ] and central Tanzania [cT]).
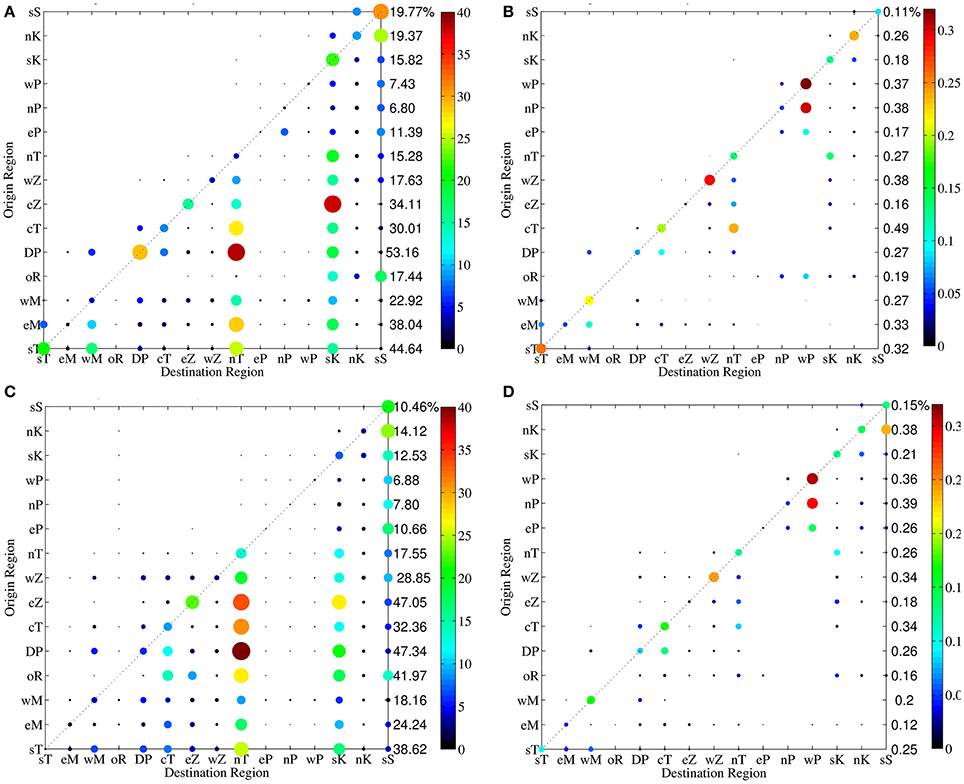
Figure 3. Region to region connectivity matrices for Acanthurus in 2000 (A), 2005 (C), Acropora in 2000 (B), 2005 (D), with reefs grouped into 15 regions marked with different colors in Figure 1 and identified as sS, south Somalia; nK, north Kenya; sK, south Kenya; wP, west Pemba; nP, north Pemba; eP, east Pemba; nT, north Tanzania; wZ, west Zanzibar; eZ, east Zanzibar; cT, central Tanzania; DP, Dar es Salaam Peninsula; oR, offshore Reef; wM, west Mafia; eM, east Mafia, and sT, south Tanzania. Color and size of the circles are proportional to the percentage of successful connections from source (origin) to sink (destination) reefs according to the colorbars to the right of the panels.
In Acropora corals (Figures 3B,D) most of the connections are due to within region recruitment, and strong connections are restricted to a 1 degree (~100 km) radius around the reef of origin. The reef offshore of Dar es Salaam (oR), east Mafia (eM) and south Tanzania (sT) regions show the longest distance connections. Interannual variability in north to south connections is similar to that of Acanthurus virtual larvae.
Regional connectivity matrices enable differentiation among across-shore reefs at the same latitude, and yield insights about well-connected and isolated reef regions. At a regional scale, the Kenya (sK, nK) and Somali (SS) reefs receive Acanthurus virtual larvae from all other reef regions in both modeled years. In contrast reef regions adjacent to Pemba Island (eP, nP, wP) receive very few Acanthurus larvae from any other reef habitats within the model domain (Figures 3A,C). The reef offshore of the Dar es Salaam peninsula (oR at ca. 7°S), due to its oceanic location and exposure to the strong northward flowing EACC, has potential for long distance connections. Settlement of Acanthurus from oR was very different in the two modeled years. In 2000 it was a source for larvae settling on distant Kenyan (sK, nK) and Somali (sS) reefs only; in 2005 it exported larvae to Kenyan (sK, nK) and Somali (sS) reefs, but also to some nearer Tanzania regions (central and north Tanzania and east Zanzibar regions; cT, nT, eZ). This offshore reef (oR) is not a large sink reef for Acanthurus larvae, but the origin of its arriving larvae is diverse, coming from reefs to the south of it in 2000 and from all regions except east Zanzibar (eZ) in 2005. The Somalia (sS) and Kenya (sK, nK) regions are the sink regions with the greatest diversity of source reefs, followed by the north Tanzania (nT) region. Larvae from Dar es Salaam Peninsula (DP) region settling in the north Tanzania region represented the strongest connection in 2000, followed in magnitude by the connection from the east Zanzibar (eZ) region to the south Kenya (sK) region. In 2005 the strongest connection remains the same but the second strongest connection was between east Zanzibar (eZ) and the north Tanzania (nT) region. During 2005 source reefs around Zanzibar Island (eZ, wZ) and the central Tanzania (cT) region had more connections to southern destination reefs. However, local retention of Acanthurus surgeonfish virtual larvae at the regional scale was larger in 2000.
For Acropora corals (Figures 3B,D) the highest proportion of recruitment is due to local within region recruitment at the west Zanzibar (wZ) and west Pemba (wP) regions in both 2000 and 2005. In all regions except east Pemba (eP) and the offshore reef (oR), the probability of recruiting locally is higher than the probability of connecting to another reef region. Similar to Acanthurus, more north to south connections of Acropora are observed in the regions between north Tanzania (nT) and the Dar es Salaam Peninsula (DP) in 2005 than in 2000, when substantial north to south connection occurred only between Dar es Salaam (DP) and west Mafia (wM). In 2000, the offshore reef (oR) connects to all Pemba regions (eP, wP, nP) and north Kenya (nK), while in 2005 it connects to all regions north of Dar es Salam except north and west Pemba (nP, wP). This offshore reef is the only source of Acropora larvae for the east Pemba (eP) region. North and west Pemba get recruits from all Pemba regions in both years.
The variable number of north to south connections between the two modeled years is explained by the influence of the mesoscale circulation on the shelf circulation pattern. In 2000 the northward flowing East African Coastal Current (EACC) was weak during the spawning months (<0.5 m s−1) as is typical during the NE monsoon season. The Somali Current (SC) that flows southward at this time of the year was strong in February (~0.68 m s−1) and its subsurface influence prevailed until April (Supplementary Figure 1). The strong influence of the southward flowing SC current in the northern part of the domain is responsible for the north to south connections in that region. In the rest of the domain the weak EACC generates slower northward velocities on the shelf during the spawning months, especially February (Supplementary Figure 2), allowing for some north to south connections at most latitudes, more evident in the reef to reef connectivity matrices (Supplementary Figure 6).
In contrast, in 2005, the SC was weaker and only present during February and March since the transition to SW monsoon conditions happened very early in the year, with strong northward flow (>1 m s−1) established in March and already re-established in the upper 300 m by April (Supplementary Figure 3). The weak SC only promotes a few north to south connections in the northern part of the domain. The strong EACC intensifies the flow reversal north of the Mafia and Zanzibar Channels, which is generated as the northward flow overshoots and turns southward into the channels when trying to follow the curved bathymetric contours past the islands (Supplementary Figure 4). This small scale circulation pattern is responsible for the north to south connections observed on regions around the north and south entrances of the Zanzibar Channel in 2000 for both Acanthurus and Acropora virtual larvae (Supplementary Figure 6).
Acanthurus Ontogenetic Vertical Migration (OVM) Experiments
Acanthurus OVM Settlement Success
Experiments that include an idealized OVM exhibit greater variability in Acanthurus settlement success among release dates within a year and between the two modeled years compared to the passive larvae experiments (Figures 2A, 4). Mean settlement success is 34.5% ± 14.6 for 2000 and 17.7% ± 8.3 in 2005, but due to the large variability among release dates, the year to year difference is not statistically significant. There is a marked decrease in settlement success from earlier to later spawning dates in the OVM scenario, going from 46.8% for particles released in February 2nd to 7.2% for those released on March 31th in 2000 and from 34.0 to 15.9% for those same dates in 2005. The decrease in settlement success occurred earlier in 2005 than during 2000, associated with the rapid transition to SW monsoon conditions in 2005 (Supplementary Figure 4). In the northern part of the model domain the core of the northward flowing EACC is subsurface (below 70 m depth) at the beginning of the spawning season (NE monsoon), but it re-establishes in the upper 300 m by May in 2000 and April in 2005 (Supplementary Figures 2, 4). This implied that 2005 larvae migrating down to 50 m are affected longer by the strong northward flow than the 2000 larvae. Three-dimensional passive larvae tend to stay near the surface and are therefore less likely to be carried away from suitable habitat by the strong northward flowing EACC core transitioning from deep to shallow waters during the second half of the spawning season.
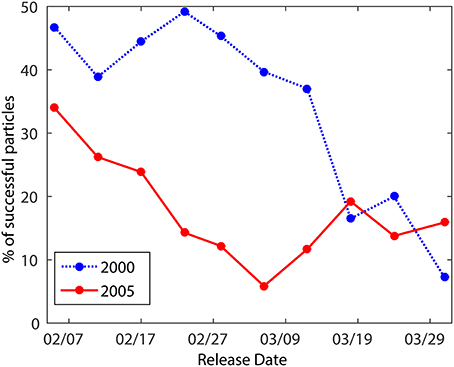
Figure 4. Percentage of settlement success per each simulated release date during 2000 and 2005 for Acanthurus virtual larvae with an idealized Ontogenetic Vertical Migration (OVM).
Acanthurus OVM Region to Region Connectivity Matrices
When grouped at the regional level the Acanthurus OVM numerical experiments showed that in 2000 (Figure 5A) the strongest connection was between central Tanzania (cT) and north Tanzania (nT). The north Tanzania (nT) region received virtual larvae from all southward reefs. The south and north Kenya regions (sK, nK) and the south Somalia (sS) region receive recruitments from all other regions. Southern Tanzania (sT), east and west Mafia (eM, wM) and the Dar es Salaam Peninsula (DP) regions provide larvae to most other regions except the offshore reef (oR), but their probabilities of connecting to the Pemba regions are very low. The main sinks for larvae coming from the offshore reef (oR) in 2000 are the distant Kenya (sK, nK) and Somalia (sS) regions. Minimal north to south connections occur with larvae originating at east and west Mafia (eM, wM) and the Dar es Salam Peninsula regions (DP) in Tanzania, the south and north Kenya (sK, nK) regions and south Somalia (sS), connecting to southward regions. Across shore connections are observed mainly from west Zanzibar (wZ) to north Tanzania (nT) and from east to west Mafia (eM to wM).
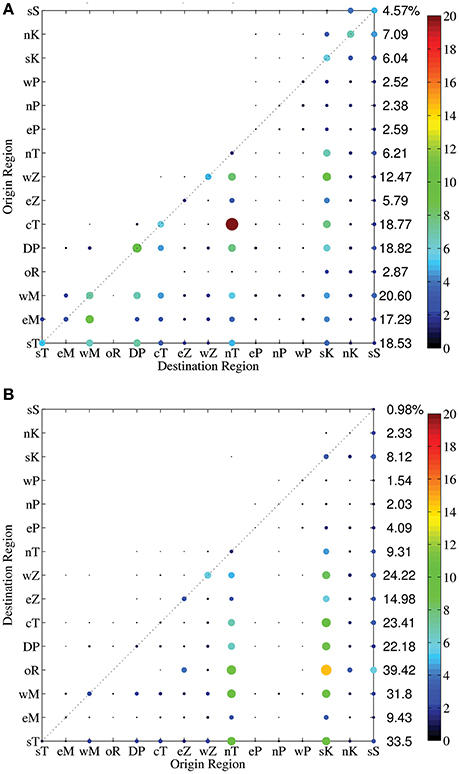
Figure 5. Region to region connectivity matrices for Acanthurus virtual larvae with OVM for 2000 (A) and 2005 (B). Reefs were grouped into 15 regions identified by two letters in Figure 1. Color and size of the circles are proportional to the percentage of successful connections from source (origin) to sink (destination) reefs according to the colorbar.
In 2005 (Figure 5B), the offshore Dar es Salaam reef (oR) has strong connections with both Zanzibar (eZ, wZ) and north Tanzania (nT) regions as well as distant Kenya and Somalia regions. The strongest connection of 2005 occurred between the offshore reef (oR) and the south Kenya (sK) region. Most regions successfully connect to northward regions, except to the three Pemba Island reef regions, which get few recruits in both years modeled. Small proportions of the larvae spawned at the Dar es Salaam Peninsula (DP) and north Tanzania (nT) regions connect southward to the west Mafia and west Zanzibar regions, respectively. Across shore connections are weaker.
Interannual variability in the general patterns of reef connectivity for Acanthurus is enhanced when the ontogenetic vertical migration behavior is included, especially regarding the magnitude of the connections. Weaker and fewer connections are observed in 2005 in comparison to 2000 (Figure 5). As in the passive scenario, a strong interannual difference is observed in the number and location of south to north connections, with stronger north to south connectivity in the southern and northern most regions in 2000, and uniformly weak north to south connections in 2005. Overall, spatial connectivity of OVM Acanthurus in each of 2000 and 2005 are remarkably similar to the patterns observed for 3D passive Acanthurus, although the overall connectivity is lower with OVM than the passive, particularly in 2005.
Sensitivity Analysis
Sensitivity to Perception Distance
The analysis with increased (reduced) perception distance for Acropora (Acanthurus) is presented to provide insight on one of the processes responsible for the large difference in settlement success between the modeled species groups, and to illustrate the variability that might be expected among coral reef species with different life-history strategies. The percentage of settlement success increased with greater perception distance for both the Acanthurus surgeonfish and the Acropora coral simulations (Figure 6). However, the increase in settlement success for the short PLD coral was always much higher than that of the surgeonfish for the same increase in perception distance, with the coral reaching 95% settlement success with a 4 km perception distance. The difference in settlement success between the two genera was significant for all perception distance scenarios (Figure 6), indicating that with the same perception distance short PLD virtual larvae will always be more successful. Perception distance scenarios alternative to the reference run experiments are not appropriate for the specific genera used here, but might be appropriate for other coral reef organisms with different life-history traits. Acropora coral larvae perceive reefs through sound (Visram et al., 2010) and chemical cues (Heyward and Negri, 1999; Dixson et al., 2014), but their perception capabilities are unlikely to exceed 100 m. Laboratory experiments have shown that coral larvae are able to detect reef sounds 0–1 m from the source and move toward them (Visram et al., 2010). If planulae are capable of detecting particle motions anticipated perception distances are on the 10–100 m range (Visram et al., 2010). Despite their sensing abilities, the swimming ability of coral larvae is very limited and usually negligible in comparison to ocean currents (Kingsford et al., 2002; Baird et al., 2014). To the contrary, Acanthurus late larvae are one of the strongest swimmers among coral reef fish larvae, with reported in situ swimming speeds ranging from 8.7 to 65.3 cm s−1 (Leis and Carson-Ewart, 1999; Leis and Fisher, 2006). They have been observed to navigate in situ disregarding current direction, perhaps guided by a sun compass (Leis and Carson-Ewart, 2003). Navigational capabilities exceeding 1 km are therefore expected for Acanthurus.
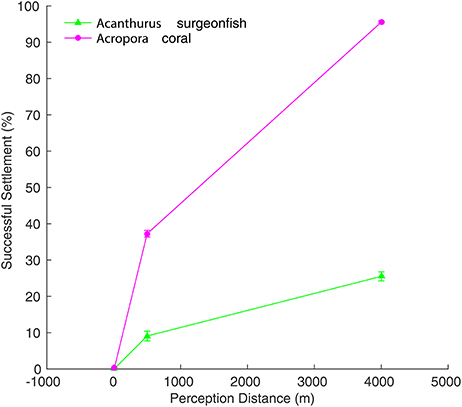
Figure 6. Settlement success for Acropora (magenta circles) and Acanthurus (green triangles) larvae with different perception distances (10, 500, and 4000 m). Shown is the mean (±1 standard error) from 10 release dates during 2000.
Diffusion Effect
The inclusion of vertical diffusion greatly increased the vertical spread of the virtual larvae and distributed them throughout the water column over the shelf. This, in turn, increased the horizontal spread of virtual larvae. The main connectivity patterns of the Acanthurus advective only experiments were also represented in the experiments with diffusion, including the interannual differences (not shown). The strength of the connections on the Acanthurus diffusive scenario was, however, an order of magnitude smaller than in the advective only scenario, indicating that the vertical spread of particles released at the same location leads to the majority of them dispersing away from suitable habitat. The extent to which the inclusion of vertical diffusion generated new connections, not represented in the advective only scenario was modest (Figure 7). From the 100% represented by the total number of connections found only in one of the two scenarios, 12.4% came from the scenario that included vertical diffusion, whereas 87.6% came from the advective only scenario. Thus, it is clear that including diffusion to the advection scenario produces relatively few new connections to the matrix, and that, for this particular case, the inclusion of a vertical random walk component may not be essential to providing a representative connectivity matrix.
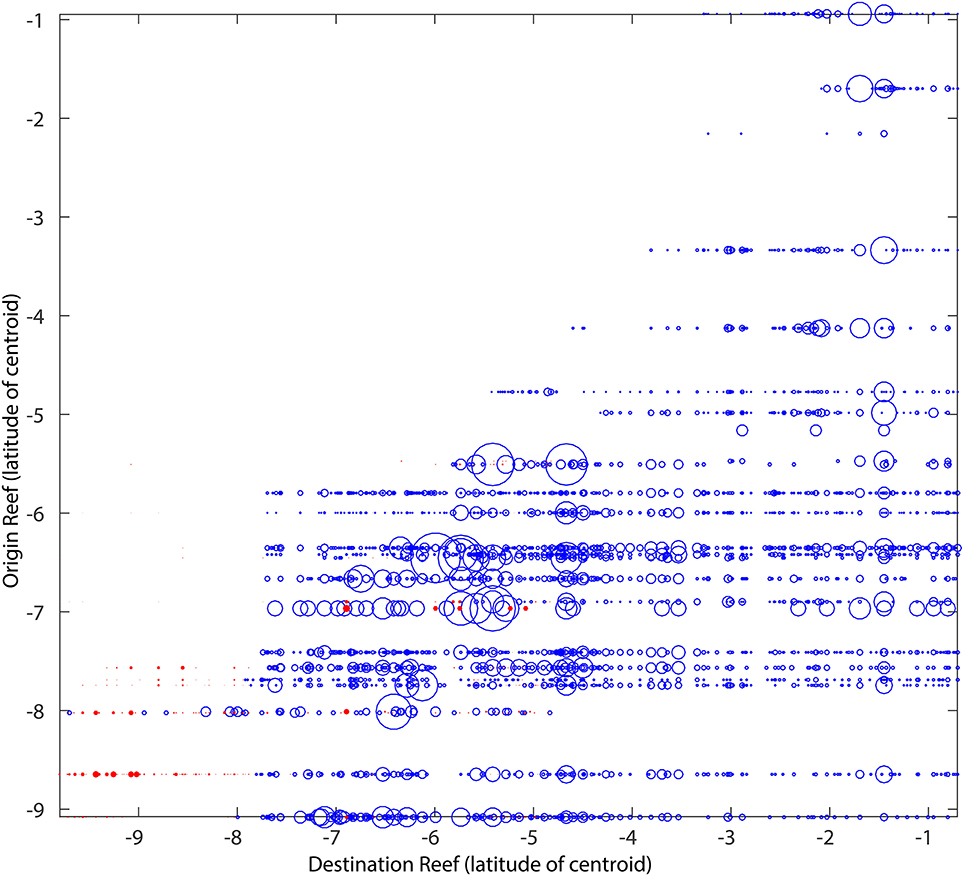
Figure 7. Acanthurus connectivity matrix for the 26 origin reefs selected for the experiments including vertical diffusion (Supplementary Figure 5), showing only the unique connections present in either the advective only experiments (blue) or the advection-diffusion experiments (red). The figure is the cumulative result of 3 release dates in each modeled year (2000 and 2005). The size of the bubbles is proportional to the percentage of particles exchanged with the biggest bubble representing 11%.
Discussion
The dominant pattern of connectivity for both Acanthurus and Acropora in the KT region is southern reefs providing virtual larvae to northern reefs. The spatial scale of connectivity is much smaller for the short PLD coral group; successful connections are restricted to a 1° radius (~100 km) around source reefs. 8.2% of Acropora larvae that successfully settle, recruited to their source reef (self-), an important proportion compared to only 1–2% for Acanthurus. Some Acropora were capable of long distance dispersal, particularly larvae spawned at the reef offshore of Dar es Salaam peninsula. This indicates that they can take advantage of the strong offshore EACC to reach distant northern reefs, and that even for short PLD, latitudinal isolation may be minimal, especially at longer (i.e., evolutionary) timescales.
In contrast to the generally short dispersal distances of Acropora, long distance connections from the southern to the northern most reefs (~950 km) are common for virtual Acanthurus. Their longer pelagic durations lead to greater transport distances and reduced local retention. Overall settlement success was significantly greater in Acanthurus (24%) than in Acropora (<0.5%). This is due to several factors that enhance Acanthurus successful settlement probabilities: longer competency period, greater reef perception distance and swimming ability.
While south to north connections predominate in the connectivity matrices, some north to south connections occur, mostly in inshore regions that experience substantial eddy flows and topographically steered flow reversals. Examples of these are (1) the northern region that is seasonally influenced by the southward flowing Somali current (SC) (Figure 3), and (2) the northern entrance of the Zanzibar Channel and the region south of the Dar es Salaam peninsula where nearshore flow reversal is promoted by strong northward offshore currents (Mayorga-Adame et al., 2016). Therefore, there is strong interannual variability in the amount and location of north to south connections depending on the strength of the offshore mesoscale currents. When the Somali Current is strong (e.g., in 2000), short distance north to south connections are common at most latitudes, but are most prevalent near the northern and southern edges of the study region. When northward offshore flow is strong and the SC disappears early in the year (e.g., 2005), north to south connections are restricted to reefs in the wider shelf region between Pemba and Mafia Islands (due to enhanced small scale flow reversals) and the region north of 3°S where the Somali Current has a direct effect.
Interannual variability is also evident in the strength of the connections among reefs and the proportion of local retention. Thirteen reef regions (all but oR, eP) experience local retention in 2000, however in 2005 five regions (sT, oR, eP, nP, wP) had no or minimal local retention. In several cases the strong connections among regions are not consistent between 2000 and 2005; analysis of simulations for other years is needed to assess the persistence of connectivity patterns. Multi-year simulations [e.g., James et al. (2002), 20 years' model of reef fish connectivity in a section of the (GBR); Dorman et al. (2015), 46 years' model of Acropora millepora connectivity in the South China Sea] would give further insight on the variability and robustness of the connectivity patterns and help to identify connections that are vital to maintaining regional metapopulations of different species groups.
The different connectivity patterns and scales of dispersal for the two genera characterized in these modeling experiments show that it is important to consider interspecies life-history variability when implementing conservation strategies to ecosystems as diverse as coral reefs, since ideal spatial management strategies would enhance settlement success for a wide suite of species with different perception and dispersal capabilities. Our results indicate, for example, that Acanthurus virtual larvae settling to coral reefs around Pemba Island come from relatively few source reefs, which highlights the need for strong local protection since the resilience, (e.g., potential recolonization of Pemba's Acanthurus populations from more distant reefs), is minimal, despite their relatively lengthy larval pelagic phase. Pemba Island Acropora coral populations are less vulnerable since they show stronger and more variable connections. This seems counter intuitive given the smaller scale of connectivity and higher local retention rates of Acropora, however the local oceanographic regime around Pemba Island, including a strong return flow on the western side of the island, promotes retention at short time scales, favoring Acropora connections, while the much longer PLD of Acanthurus favors transport away from Pemba's suitable habitat.
The level of connectivity of a reef is a component of its resilience and the extent of its ecological impact in the region. For example, strong sink reefs, those receiving settlers from many different source reefs, are more resilient to local and global stresses, since having multiple sources increases the probability of receiving recruits in any given year. The diversity of sources providing potential recruits would enhance resilience to short term local detrimental phenomena such as bleaching events and overfishing. On the other hand, important source reefs, those with potential of providing settlers to many other reefs, could have a disproportionately large ecological impact for many other reefs. Reefs that provide larvae to many other sites are important to protect from a larger ecosystem conservation perspective, since an increase of the local spawning population would likely impact recruitment to a large number of reefs elsewhere, therefore increasing the impacts of spatially limited conservation measures (e.g., MPA) beyond their boundaries (Bode et al., 2006; Figueira, 2009). “Source and sink” maps are useful for identifying ecologically important areas based on the number and type of connections present. Source and sink maps for Acanthurus are shown in Figures 8A,C, and for Acropora virtual larvae in Figures 8B,D. The passive and OVM scenarios (not shown) for Acanthurus yielded similar source and sink maps. In general reefs south of Mafia Island (8°S) provide larvae of both species groups to the greatest number of reefs. In the case of Acanthurus larvae (Figure 8A) these reefs connect to more than 350 different reefs, and for Acropora (Figure 8B) to more than 70 reefs. Reefs in the northern half of Tanzania are good sources of Acropora larvae, connecting to more than 50 reefs, while Kenyan reefs connect to ~30 different reefs. Local conservation efforts in these areas are likely to have an important ecological impact beyond their local ecosystem, since they can help maintain and replenish multiple other metapopulations at various destination reefs. Somali reefs to the contrary provide Acanthurus virtual larvae to <50 reefs and Acropora virtual larvae to <10 different reefs. While this may be an artifact of these reefs being near the northern border outflow, the role of Somali reefs in providing recruits of reef species further north is minimal as there are few coral reefs within the immediate north region. Kenyan and Somali reefs are however the most common sink reefs, receiving larvae from many reefs to their south (Figure 8D). These northern reefs may be more resilient to local threats. The source and sink patterns reflect the strong, mostly unidirectional south to north flow along the coast. For Acropora larvae, the source and sink maps are much more patchy (Figures 8B,D, respectively), reflecting the effects of the smaller dispersal scale of Acropora larvae.
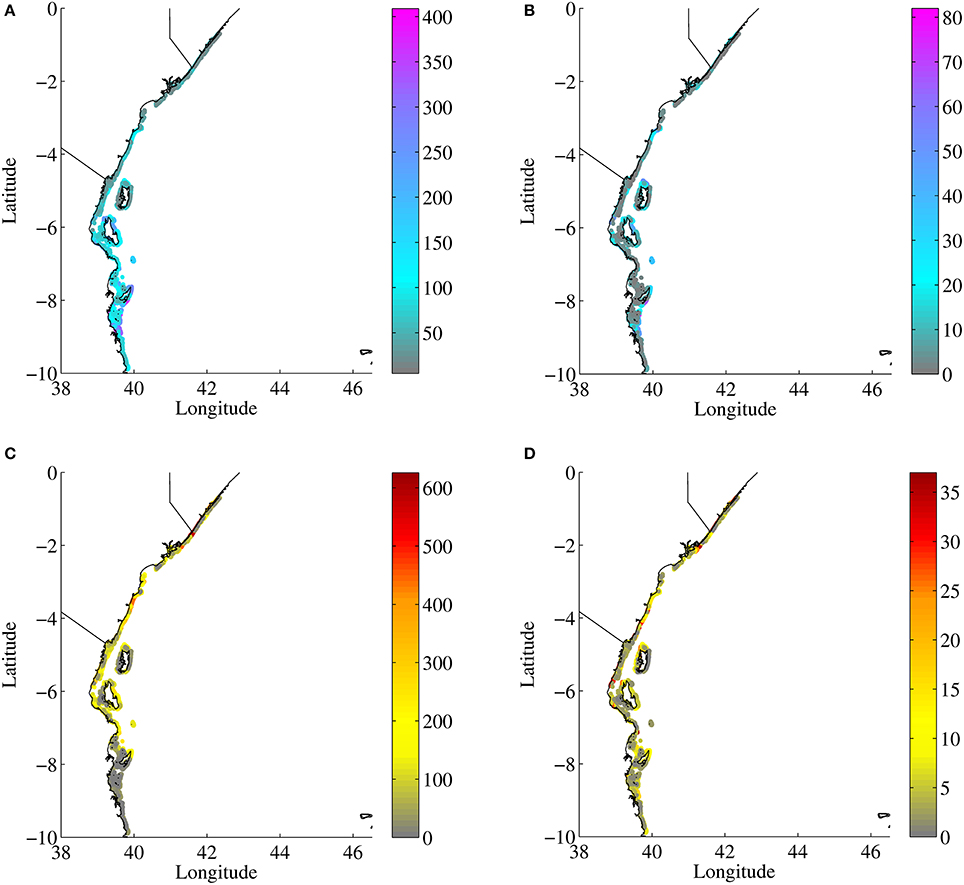
Figure 8. Top row, reefs color coded by the number of different destination reefs reached by virtual larvae originated from them, in both simulated years, to identify the best source reefs for Acanthurus (A) and Acropora (B). Bottom row, reefs color coded by the number of different source reefs it received virtual larvae from, in both simulated years, to identify the best sink reefs for Acanthurus (C) and Acropora (D).
A recent genetic study of Acropora tenuis connectivity in the KT region, reports high but variable connectivity between sample sites, which cluster in 3 different groups: (1) Kenya and northern-Tanzania, (2) southern Tanzania, and (3) sample sites located in the Zanzibar and Pemba channels (van der Ven et al., 2016). No clear genetic break on samples collected along 900 km of coral reefs was observed. Also, no genetic differentiation with increasing geographical separation was found, in contrast to similar studies in the (GBR) and Japan. They associate the genetic uniformity in the KT region with uniform oceanographic conditions promoted by the continuous south to north linear flow of the EACC. The highest differentiation observed in group 3 is associated with local oceanographic conditions causing larval retention. The connectivity patterns of our modeling study agree with their findings, despite the different time scales assessed. The north to south connectivity we report is expected to minimize genetic differences along the KT coast. Our model also corroborates the isolation they infer for their highly differentiated Pemba and Zanzibar Channel sites. Our model results indicate that west Pemba (wP) and west Zanzibar regions, receive the majority of their Acropora virtual larvae through within region recruitment. The circulation patterns depicted by our ROMS model included flow reversals at the northern entrance of the channels and eddy blockage in the southern entrance of the Zanzibar Channel (Mayorga-Adame et al., 2016); these might provide sufficient isolation to upstream sources to produce genetically different Acropora populations in the channel sites.
Genetic connectivity patterns for Pocillopora damicornis (a brooding coral species) based on contemporary gene flow (Souter et al., 2009) can be compared with our modeling results for the longer PLD, broadcast spawner Acropora corals. Souter et al. (2009) identified first generation migrants of P. damicornis at 29 reefs sites in the Kenya-Tanzania region, and therefore determined the degree of isolation of the different reefs sampled. They found patchiness in the degree of isolation at very small scales, with marked differences even between lagoon and fringing reefs within the Malindi Marine National Park and Reserve in south Kenya. The patchiness observed in our Acropora source and sink maps is consistent with their results, indicating strong small-scale spatial variability in the number of connections (degree of isolation) among nearby reefs. Souter et al. (2009) identified isolated reefs, highly dependent on local retention for population renewal, in south Kenya, west Pemba, and south Mafia. In the Acropora simulations these regions receive virtual larvae from <10 different source reefs (Figure 8D). The regional Acropora connectivity matrices (Figures 3B,D) show that local retention is important for these regions. In our regional connectivity results, however, only west Pemba, east Mafia and south Tanzania show relative isolation, receiving Acropora larvae from only three and two other regions respectively; south Kenya in contrast receives settlers from many regions further south. This discrepancy between our model results and Souter et al.'s (2009) genetic study may reflect the different spatial scales considered, since our regional grouping aggregates connections for several reefs that might have different degrees of isolation. Souter et al. (2009) identify Mnemba Conservation Area (in the east Zanzibar region) as a strong source for other sampled sites. This site shows the highest genetic diversity and is similar only to one site in the Dar es Salaam Peninsula and one site in southeast Mafia Island. In the simulation Acropora larvae that settle in the east Zanzibar region, which includes Mnemba Island, come from few origin reefs (mainly Dar es Salaam Peninsula and east Mafia regions; Figures 3B,D). The number of regions that receive Acropora larvae from east Zanzibar ranges between 3 and 5 in the 2000 and 2005 simulations. The source map for Acropora (Figure 8B) shows that most reefs around Mnemba (east Zanzibar) provide larvae to ~30 to 60 different reefs. Our model results identify specific reefs in the west Mafia and southern Tanzania regions as the main providers of Acropora larvae while the genetic results of Souter et al. (2009) do not identify their south Mafia and Mtwara (south of our model domain) sites as important sources. This could be due to the high reef to reef patchiness on the level of isolation identified by both their observational and our modeling study, the uncertainty of which specific reefs were actually sampled for the genetic study, and the shorter PLD of Pocillopora damicornis. To the extent allowed by the comparison of this model with the genetic sampling of specific reefs results of Souter et al. (2009), the main connectivity patterns elucidated by their genetic study for an ecologically similar coral species are well represented in the connectivity results provided by the coupled biophysical model for Acropora. This comparison is limited to the regional level, since the exact location of the reefs sampled by Souter et al. (2009) is not reported.
Only at the beginning of the spawning season did the ontogenetic vertical migration “in silico” experiments of Acanthurus virtual larvae generate more successful settlers than the 3D passive scenario (Figures 2A, 5). This is inconsistent with prior reports in the literature for various larvae in the Caribbean (Paris et al., 2007) and the California Current System (Drake et al., 2013), where OVM consistently increased settlement success. Differences in shear and stratification of the water column of each region may be responsible for this marked difference among oceanographically distinct regions. The shelf circulation of Kenya and Tanzania is dominated by strong alongshore flows with an increased magnitude offshore, a shallow (i.e., <50–100 m) wind driven mixed layer is not common. Northward flow velocities dominate the coastal circulation off Kenya and Tanzania down to 300 m depth during most of the year. Therefore, a vertical migration down to 50 m depth would have little effect on transport pathways. During the SE monsoon (Dec-Mar) the Somali Current flows southward in the upper 100 m north of 3°S. During this period, which encompasses part of the spawning period, staying near the surface, instead of migrating to deeper waters, would facilitate north to south connections and retention if the duration of the pelagic phase includes the seasonal reversal to northward flow. Only during the transition between NE to SE monsoon conditions would a shallow migration result in significantly shorter horizontal displacements for Acanthurus larvae. Intraseasonal and interannual variability increased when the simple ontogenetic vertical migration behavior was implemented because the evolution of the vertical structure of alongshore velocities was markedly different in the two modeled years (Supplementary Figures 2, 4). The ontogenetic vertical migration pattern modeled here is based on the increased depth of the Acanthurus larvae during ontogeny observed by Irisson et al. (2010). The depth of the migration is not well defined and could be site dependent. For example, Irisson et al. (2010) reported post-flexion Acanthurus larvae in the 25–60 m depth range near reefs in French Polynesia, while Oxenford et al. (2008) found aggregations of late Acanthurus larvae to be more abundant at 120 m in the eastern Caribbean Sea. No observations for the East-African coast exist. Observations of vertical distribution and abundance of pelagic larvae concurrent with hydrographic conditions are needed to design more realistic vertical migration experiments, and to assess larval fish responses to temperature, light, or velocity. The implementation of vertical migration in these numerical experiments was highly idealized, shifting all particles to 50 m depth 20 days after release, ignoring their vertical position at that time. This meant that some larvae that had passively advected deeper than the 50 m fixed migration depth were actually displaced upward with this vertical migrating behavior. The number of particles that advected below 50 m depth was not an important fraction of the successful larvae since most larvae stayed in the upper 5 m when passively advected; a small proportion, however, reached depths below 100 m. Migrating only shallow particles downward would be a more realistic scenario as well as distributing the particles within a broader depth range rather than fixing them to a single specific depth. Many other, perhaps more realistic scenarios are possible next steps. However, in-situ data of larvae depth distributions would be required to properly parameterize more realistic scenarios. The aim of the simple scenario modeled here was to illustrate the potential effects on connectivity of an ontogenetic vertical migration to 50 m (with depth keeping) in a rapidly evolving water column with deep stratification and strong shear.
The numerical experiments presented here are deterministic and represent a population where all larvae develop and behave identically, without actively responding to its environment. Real larvae are complex organisms, with strong inter-specific and potentially intra-individual variability in physiology and behavior, constantly reacting to their environment. Complex models with behavior cueing on the environmental conditions experienced by the virtual larvae have been developed (e.g., Armsworth, 2001; Staaterman et al., 2012; Wolanski and Kingsford, 2014). Assuming that larvae are well adapted to the pelagic phase, larval behavior, particularly sensing, orientation and swimming abilities would enhance their probability of finding suitable settlement habitat, which might reduce interannual variability in settlement success. However, when challenged by increased environmental variability due to climate change effects, their strategies may not be guaranteed to work. The numerical experiments presented here, although idealized, serve as an initial effort to develop hypotheses that might be examined using more complex models and empirical studies. Monitoring recruitment of coral reef organisms is basic to assessing the effects of environmental variability on settlement success. Having long term time series of recruitment of coral reef dependent species in the Kenya-Tanzania region would be valuable for “tuning” models, as has been done for other coral reef regions (i.e., Sponaugle et al., 2012).
The fraction of released larvae that settle on suitable habitat is highly sensitive to the individual's habitat perception and swimming abilities; further knowledge regarding the capabilities of coral reef larvae to perceive, navigate and settle on suitable habitat is a very important and a challenging piece of information to obtain. Both in-situ and laboratory observations of larval development and behavior are needed to further increase the realism of modeling experiments. The dependence of PLD on temperature is well established for aquatic organisms (O'Connor et al., 2007) but observations for the studied genera are insufficient to adequately parameterize the functional response between temperature and PLD. The inclusion of temperature dependent PLD in bio-physical models is essential for examining climate change effects on connectivity and settlement success of marine larvae (Lett et al., 2010; Figueiredo et al., 2014). Changes in ocean circulation will alter connectivity patterns, but physiological effects due to the increased temperature will also have an important effect (Munday et al., 2009; Lett et al., 2010; Kendall et al., 2016). Reduced pelagic larval durations are expected under faster developmental rates, which could lead to a reduction in dispersal distances and the spatial scale of connectivity (Munday et al., 2009; Lett et al., 2010). Bio-physical modeling connectivity studies including temperature dependent PLD report increased local retention (Figueiredo et al., 2014; Andrello et al., 2015) and significant changes in Marine Protected Area network interconnectivity (Andrello et al., 2015) under climate change scenarios. Well-informed idealized experiments that include temperature dependent PLD of virtual larvae are a future direction for assessing the effects of climate change scenarios on connectivity and recruitment of coral reef organisms in the East-African coast.
Larvae in the ocean are subject to mixing at scales smaller than those represented in the ocean circulation model. In particle tracking models these unresolved motions are often implemented as a random walk scaled by the model diffusivity. Simulations that implement a random walk to mimic diffusion are considered more realistic but computationally expensive. We conducted a few sensitivity experiments that included (3D) variable vertical diffusion. Simulations that included vertical diffusion (not shown) reproduced the main connectivity patterns produced by the 3D advective only experiments, but with smaller connectivities—mostly due to greater vertical dispersion that subjected larvae to greater horizontal flow variation. These results are probably more realistic for early or weakly swimming larvae (e.g., coral species) that are unable to maintain their vertical position in the water column in the presence of vigorous vertical mixing.
While reef-to-reef connectivity is important in metapopulation ecology, regional connectivity is expected to be more robust to the uncertainty introduced by the oceanographic and biological assumptions made in these models. Region to region connectivity matrices synthesize the information of reef to reef connectivity matrices, making it more manageable and easier to interpret. The regional summary could assist managers, policy makers and the general public to understand the interconnections among coral reef regions due to pelagic larval dispersion of their local populations. Previous bio-physical connectivity studies highlight the importance of considering larval connectivity at regional levels when trying to prioritize the implementation of management strategies for both conservation and fisheries enhancement goals. One of the insights of examining connectivity at a regional scale is that the importance of international connections becomes obvious, as has been shown by Kough et al. (2013) for the Mesoamerican reefs and by Rossi et al. (2014) for the Mediterranean Sea. In all numerical experiments Tanzanian reefs were an important source of settlers to Kenyan reefs; this provides insight and guidance on the spatial scale at which management strategies are required and points to the need for regional international collaborations in order to provide enduring conservation measures and protection to the east African coral reef ecosystem.
This modeling study is a first approach to understanding the connectivity among coral reef populations in a data poor region. The information provided, even though preliminary, presents a general pattern of the potential regional connectivity and identifies particularly resilient and vulnerable areas as well as the hydrodynamic features driving the connections. Spatial scales of connectivity and settlement success rates are within the ranges reported by other bio-physical modeling studies for similar genera in other coral reef regions (Paris et al., 2007; Dorman et al., 2015). However, the robustness of the connectivity patterns presented needs to be further evaluated by performing experiments for more years and longer spawning seasons, and carrying out more extensive sensitivity analysis to the model assumptions. After gaining more confidence in the modeled connectivity patterns, the information provided by this modeling study could be carefully and critically evaluated, in order to be applied to optimize the effectiveness of marine protected area management and other marine protection efforts. Further modeling experiments similar to those presented here, but better informed by empirical data, and including the capability of larvae to respond to the ocean conditions will provide greater detail on the complex biophysical interactions that occur in the sea, and will provide a more realistic, and less uncertain, representation of connectivity patterns. These results will aid in understanding how a range of species specific individual responses influence the distribution and connectivity patterns and should enable more specific guidelines for spatial management that provide better resource resiliency and protection throughout the Kenya-Tanzania coastal region.
Author Contributions
CGMA, HB, and YS designed the experiments, analysis and paper. CGMA Carried out the numerical modeling experiments and analysis and wrote initial draft of the paper. HB and YS revised and improved the initial manuscript.
Funding
CGMA was partially funded by CONACYT Mexico.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thanks to Dr. Ted Strub for access to computing resources. To CEOAS-OSU technicians Eric Beals and Tom Leach for technical support. To CONACYT Mexico for scholarship funding for CGMA, and to the UK National Oceanography Centre (NOC) for allowing her time to finalize and review this paper. HB thanks the North Pacific Marine Science Organization (PICES) for allowing time for him to contribute to this paper, and for payment of the article processing fee.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmars.2017.00092/full#supplementary-material
References
Almany, G. R., Berumen, L. M., Thorrold, R. S., Planes, S., and Jones, G. P. (2007). Local replenishment of coral reef fish populations in a marine reserve. Science 316, 742–744. doi: 10.1126/science.1140597
Andrello, M., Mouillot, D., Somot, S., Thuiller, W., and Manel, S. (2015). Additive effects of climate change on connectivity between marine protected areas and larval supply to fished areas. Divers. Distributions 21, 139–150. doi: 10.1111/ddi.12250
Armsworth, P. R. (2001). Directed motion in the sea: efficient swimming by reef fish larvae. J. Theor. Biol. 210, 81–91. doi: 10.1006/jtbi.2001.2299
Atema, J., Gerlach, G., and Paris, C. B. (2015). “Sensory biology and navigation behavior of reef fish larvae,” in Ecology of Fishes on Coral Reefs, ed C. Mora (Cambridge: Cambridge University Press), 3–15.
Atema, J., Kingsford, M. J., and Gerlach, G. (2002). Larval reef fish could use odour for detection, retention and orientation to reefs. Mar. Ecol. Prog. Ser. 241, 151–160. doi: 10.3354/meps241151
Babcock, R. C., and Heyward, A. J. (1986). Larval development of certain gamete-spawning scleractinian corals. Coral Reefs 5, 111–116. doi: 10.1007/BF00298178
Baird, A. H., Cumbo, V. R., Figueiredo, J., Harii, S., Hata, T., and Madin, J. S. (2014). Comment on “Chemically mediated behavior of recruiting corals and fishes: a tipping point that may limit reef recovery.” PeerJ PrePrints 2:e628v1. doi: 10.7287/peerj.preprints.628v1
Batchelder, H. P. (2006). Forward-in-Time-/Backward-in-Time-Trajectory (FITT/BITT) modeling of particles and organisms in the coastal ocean*. J. Atmos. Oceanic Technol. 23, 727–741. doi: 10.1175/JTECH1874.1
Batchelder, H. P., Edwards, C. A., and Powell, T. M. (2002). Individual-based models of copepod populations in coastal upwelling regions: implications of physiologically and environmentally influenced diel vertical migration on demographic success and nearshore retention. Progr. Oceanogr. 53, 307–333. doi: 10.1016/S0079-6611(02)00035-6
Baums, I. B., Miller, M. W., and Hellberg, M. E. (2005). Regionally isolated populations of an imperiled caribbean coral, Acropora Palmata. Mol. Ecol. 14, 1377–1390. doi: 10.1111/j.1365-294X.2005.02489.x
Bergenius, M. A., Meekan, M. G., Robertson, R. D., and McCormick, M. I. (2002). Larval growth predicts the recruitment success of a coral reef fish. Oecologia 131, 521–525. doi: 10.1007/s00442-002-0918-4
Bode, M., Lance, B., and Paul Armsworth, R. (2006). Larval dispersal reveals regional sources and sinks in the great barrier reef. Mar. Ecol. Prog. Ser. 308, 17–25. doi: 10.3354/meps308017
Boehlert, G. W., and Mundy, B. C. (1993). Ichthyoplankton assemblages at seamounts and oceanic islands. Bull. Mar. Sci. 53, 336–361.
Botsford, L. W., Micheli, F., and Hastings, A. (2003). Principles for the design of marine reserves. Ecol. Appl. 13, 25–31. doi: 10.1890/1051-0761(2003)013[0025:PFTDOM]2.0.CO;2
Botsford, L. W., White, J. W., Coffroth, M. A., Paris, C. B., Planes, S., Shearer, T. L., et al. (2009). Connectivity and resilience of coral reef metapopulations in marine protected areas: matching empirical efforts to predictive needs. Coral Reefs 28, 327–337. doi: 10.1007/s00338-009-0466-z
Burgess, S. C., Nickols, K. J., Griesemer, C. D., Barnett, L. A. K., Dedrick, A. G., Satterthwaite, E. V., et al. (2014). Beyond connectivity: how empirical methods can quantify population persistence to improve marine protected-area design. Ecol. Appl. 24, 257–270. doi: 10.1890/13-0710.1
Christie, M. R., Johnson, D. W., Stallings, C. D., and Hixon, M. A. (2010a). Self-recruitment and sweepstakes reproduction amid extensive gene flow in a coral-reef fish. Mol. Ecol. 19, 1042–1057. doi: 10.1111/j.1365-294X.2010.04524.x
Christie, M. R., Tissot, B. N., Albins, A. A., Beets, P., Jia, Y., Ortiz, D. M., et al. (2010b). Larval connectivity in an effective network of marine protected areas. PLoS ONE 5:e15715. doi: 10.1371/journal.pone.0015715
Cowen, R. K., and Castro, L. R. (1994). Relation of coral reef fish larval distributions to island scale circulation around barbados, west indies. Bull. Mar. Sci. 54, 228–244.
Cowen, R., Gawarkiewicz, G., Pineda, J., Thorrold, S., and Werner, F. (2007). Population connectivity in marine systems: an overview. Oceanography 20, 14–21. doi: 10.5670/oceanog.2007.26
Cowen, R. K., Lwiza, K. M. M., Sponaugle, S., Paris, C. B., and Olson, D. B. (2000). Connectivity of marine populations: open or closed? Science 287, 857–859. doi: 10.1126/science.287.5454.857
Cowen, R. K., Paris, C. B., and Srinivasan, A. (2006). Scaling of connectivity in marine populations. Science 311, 522–527. doi: 10.1126/science.1122039
Cowen, R. K., and Sponaugle, S. (2009). Larval dispersal and marine population connectivity. Ann. Rev. Mar. Sci. 1, 443–466. doi: 10.1146/annurev.marine.010908.163757
Crochelet, E., Chabanet, P., Pothin, K., Lagabrielle, E., Roberts, J., Pennober, G., et al. (2013). Validation of a fish larvae dispersal model with otolith data in the Western Indian Ocean and implications for marine spatial planning in data-poor regions. Ocean Coast. Manage. 86, 13–21. doi: 10.1016/j.ocecoaman.2013.10.002
Crochelet, E., Roberts, J., Lagabrielle, E., Obura, D., Petit, M., and Chabanet, P. (2016). A model-based assessment of reef larvae dispersal in the Western Indian Ocean reveals regional connectivity patterns—potential implications for conservation policies. Reg. Stud. Mar. Sci. 7, 159–167. doi: 10.1016/j.rsma.2016.06.007
Dixson, D. L., Abrego, D., and Hay, M. E. (2014). Chemically mediated behavior of recruiting corals and fishes: a tipping point that may limit reef recovery. Science 345, 892–897. doi: 10.1126/science.1255057
Domingues, C. P., Nolasco, R., Dubert, J., and Queiroga, H. (2012). Model-derived dispersal pathways from multiple source populations explain variability of invertebrate larval supply. PLoS ONE 7:e35794. doi: 10.1371/journal.pone.0035794
Dorenbosch, M., Pollux, B. J. A., Pustjens, A., Rajagopal, Z., Nagelkerken, S., van der Velde, G., et al. (2006). Population structure of the dory snapper, lutjanus fulviflamma, in the Western Indian Ocean revealed by means of AFLP fingerprinting. Hydrobiologia 568, 43–53. doi: 10.1007/s10750-006-0020-8
Dorman, J. G., Frederic Castruccio, S., Enrique Curchitser, N., Joan Kleypas, A., and Thomas Powell, M. (2015). Modeled connectivity of acropora millepora populations from reefs of the spratly islands and the greater South China Sea. Coral Reefs 35, 169–179. doi: 10.1007/s00338-015-1354-3
Drake, P. T., Edwards, C. A., Morgan, S. G., and Dever, E. P. (2013). Influence of larval behavior on transport and population connectivity in a realistic simulation of the california current system. J. Mar. Res. 71, 317–350. doi: 10.1357/002224013808877099
Edgar, G. J., Stuart-Smith, R. D., Willis, T. J., Kininmonth, S., Baker, S. C., Banks, S., et al. (2014). Global conservation outcomes depend on marine protected areas with five key features. Nature 506, 216–220. doi: 10.1038/nature13022
Egbert, G. D., Bennett, A. F., and Foreman, M. G. G. (1994). TOPEX/POSEIDON tides estimated using a global inverse model. J. Geophys. Res. Oceans 99, 24821–24852. doi: 10.1029/94JC01894
Egbert, G. D., and Erofeeva, S. Y. (2002). Efficient inverse modeling of barotropic ocean tides. J. Atmos. Oceanic Technol. 19, 183–204. doi: 10.1175/1520-0426(2002)019<0183:EIMOBO>2.0.CO;2
Figueira, W. F. (2009). Connectivity or demography: defining sources and sinks in coral reef fish metapopulations. Ecol. Modell. 220, 1126–1137. doi: 10.1016/j.ecolmodel.2009.01.021
Figueiredo, J., Baird, A. H., Harii, S., and Connolly, S. R. (2014). Increased local retention of reef coral larvae as a result of ocean warming. Nat. Clim. Chang 4, 498–502. doi: 10.1038/nclimate2210
Foster, N. L., Paris, C. B., Kool, J. T., Baums, I. B., Stevens, J. R., Sanchez, J. A., et al. (2012). Connectivity of caribbean coral populations: complementary insights from empirical and modelled gene flow. Mol. Ecol. 21, 1143–1157. doi: 10.1111/j.1365-294X.2012.05455.x
Gawarkiewicz, G., Monismith, S., and Largier, J. (2007). Observing larval transport processes affecting population connectivity: progress and challenges. Oceanography 20, 40–53. doi: 10.5670/oceanog.2007.28
Gell, F. R., and Roberts, C. M. (2003). Benefits beyond boundaries: the fishery effects of marine reserves. Trends Ecol. Evol. 18, 448–455. doi: 10.1016/S0169-5347(03)00189-7
Gerlach, G., Atema, J., Kingsford, M. J., Black, K. P., and Miller-Sims, V. (2007). Smelling home can prevent dispersal of reef fish larvae. Proc. Natl. Acad. Sci. U.S.A. 104, 858–863. doi: 10.1073/pnas.0606777104
Haidvogel, D. B., Arango, H., Budgell, W. P., Cornuelle, B. D., Curchitser, E., Di Lorenzo, E., et al. (2008). Ocean forecasting in terrain-following coordinates: formulation and skill assessment of the regional ocean modeling system. J. Comput. Phys. 227, 3595–3624. doi: 10.1016/j.jcp.2007.06.016
Hamilton, H. G. H., and Brakel, W. H. (1984). Structure and Coral Fauna of East African Reefs. Bull. Mar. Sci. 34, 248–266.
Harrison, H. B., Williamson, D. H., Evans, R. D., Almany, G. R., Thorrold, S. R., Russ, G. R., et al. (2012). Larval export from marine reserves and the recruitment benefit for fish and fisheries. Curr. Biol. 22, 1023–1028. doi: 10.1016/j.cub.2012.04.008
Heenan, A., Simpson, S. D., and Braithwaite, V. A. (2009). “Testing the generality of acoustic cue use at settlement in larval coral reef fish,” in Proceedings of the 11th International Coral Reef Symposium, International Society for Reef Studies. Available online at: http://research-information.bristol.ac.uk/en/publications/testing-the-generality-of-acoustic-cue-use-at-settlement-in-larval-coral-reef-fish(a6fce20c-2ee6-4898-a72c-766263aea453).html
Hedgecock, D., Barber, P. H., and Edmands, S. (2007). Genetic approaches to measuring connectivity. Oceanography 20, 70–79. doi: 10.5670/oceanog.2007.30
Heyward, A. J., and Negri, A. P. (1999). Natural inducers for coral larval metamorphosis. Coral Reefs 18, 273–279. doi: 10.1007/s003380050193
Hogan, J. D., Thiessen, R. J., Sale, P. F., and Heath, D. D. (2011). Local retention, dispersal and fluctuating connectivity among populations of a coral reef fish. Oecologia 168, 61–71. doi: 10.1007/s00442-011-2058-1
Hughes, T. P., Baird, A. H., Bellwood, D. R., Card, M., Connolly, S. R., Folke, C., et al. (2003). Climate change, human impacts, and the resilience of coral reefs. Science 301, 929–933. doi: 10.1126/science.1085046
Irisson, J., Paris, C. B., Guigand, C., and Planes, S. (2010). Vertical distribution and ontogenetic ‘migration’ in coral reef fish larvae. Limnol. Oceanogr. 55, 909–919. doi: 10.4319/lo.2009.55.2.0909
James, M. K., Armsworth, P. R., Mason, L. B., and Bode, L. (2002). The structure of reef fish metapopulations: modelling larval dispersal and retention patterns. Proc. R. Soc. Lond. B Biol. Sci. 269, 2079–2086. doi: 10.1098/rspb.2002.2128
Jones, G. P., Milicich, M. J., Emslie, M. J., and Lunow, C. (1999). Self-Recruitment in a coral reef fish population. Nature 402, 802–804. doi: 10.1038/45538
Jones, G. P., Planes, S., and Thorrold, S. R. (2005). Coral reef fish larvae settle close to home. Curr. Biol. 15, 1314–1318. doi: 10.1016/j.cub.2005.06.061
Kalnay, E., Kanamitsu, M., Kistler, R., Collins, W., Deaven, D., Gandin, L., et al. (1996). The NCEP/NCAR 40-Year reanalysis project. Bull. Am. Meteorol. Soc. 77, 437–471. doi: 10.1175/1520-0477(1996)077<0437:TNYRP>2.0.CO;2
Kaunda-Arara, B., James Mwaluma, M., Gamoe Locham, A., and Vidar Øresland Melckzedeck Osore, K. (2009). Temporal variability in fish larval supply to malindi marine park, coastal Kenya. Aquat. Conserv. Mar. Freshwater Ecosyst. 19, S10–S18. doi: 10.1002/aqc.1038
Kendall, M. S., Poti, M., and Karnauskas, K. B. (2016). Climate change and larval transport in the ocean: fractional effects from physical and physiological factors. Glob. Chang. Biol. 22, 1532–1547. doi: 10.1111/gcb.13159
Kingsford, M. J., Leis, J. M., Shanks, A., Lindeman, K. C., Morgan, S. G., and Pineda, J. (2002). Sensory environments, larval abilities and local self-recruitment. Bull. Mar. Sci. 70, 309–340.
Kough, A. S., Paris, C. B., and Butler, M. J. IV. (2013). Larval connectivity and the international management of fisheries. PLoS ONE 8:e64970. doi: 10.1371/journal.pone.0064970
Kruse, M., Taylor, M., Muhando, C. A., and Reuter, H. (2016). Lunar, diel, and tidal changes in fish assemblages in an East African marine reserve. Reg. Stud. Mar. Sci. 3, 49–57. doi: 10.1016/j.rsma.2015.05.001
Lecchini, D. (2005). Spatial and behavioural patterns of reef habitat settlement by fish larvae. Mar. Ecol. Prog. Ser. 301, 247–252. doi: 10.3354/meps301247
Leis, J. M., and Carson-Ewart, B. M. (1999). In situ swimming and settlement behaviour of larvae of an indo-pacific coral-reef fish, the coral trout plectropomus leopardus (Pisces: Serranidae). Mar. Biol. 134, 51–64. doi: 10.1007/s002270050524
Leis, J. M., and Carson-Ewart, B. M. (2003). Orientation of pelagic larvae of coral-reef fishes in the ocean. Mar. Ecol. Prog. Ser. 252, 239–253. doi: 10.3354/meps252239
Leis, J. M., Carson-Ewart, B. M., Hay, A. C., and Cato, D. H. (2003). Coral-reef sounds enable nocturnal navigation by some reef-fish larvae in some places and at some times. J. Fish Biol. 63, 724–737. doi: 10.1046/j.1095-8649.2003.00182.x
Leis, J. M., and Fisher, R. (2006). “Swimming speed of settlement-stage reef-fish larvae measured in the laboratory and in the field: a comparison of critical speed and in situ speed,” in Proceedings of the 10th International Coral Reef Symposium (Okinawa).
Leis, J. M., Wright, K. J., and Johnson, R. N. (2007). Behaviour that influences dispersal and connectivity in the small, young larvae of a reef fish. Mar. Biol. 153, 103–117. doi: 10.1007/s00227-007-0794-x
Lester, S., Halpern, B., Grorud-Colvert, K., Lubchenco, J., Ruttenberg, B., Gaines, S., et al. (2009). Biological effects within no-take marine reserves: a global synthesis. Mar. Ecol. Prog. Ser. 384, 33–46. doi: 10.3354/meps08029
Lett, C., Ayata, S., Huret, M., and Irisson, J. (2010). Biophysical modelling to investigate the effects of climate change on marine population dispersal and connectivity. Prog. Oceanogr. 87, 106–113. doi: 10.1016/j.pocean.2010.09.005
Longenecker, K., Langston, R., and Barrett, B. (2008). A Compendium of Live History Information for some Exploited Hawaiian Reef Fishes. Bishop Museum Technical Report (No. 44). Fisheries Local Action Strategy, Honolulu, HI.
Mangubhai, S. (2008). “Spawning patterns of Acropora species in the Mombasa lagoon in Kenya,” in Ten Years after Bleaching - Facing the Consequences of Climate Change in the Indian Ocean, CORDIO Status Report 2008, eds D. O. Obura, J. Tamelander, and O. Linden (Mombasa: Coastal Ocean Research and Development in the Indian Ocean/Sida-SAREC), 213–221.
Mangubhai, S., and Harrison, P. (2008). Asynchronous coral spawning patterns on equatorial reefs in Kenya. Mar. Ecol. Prog. Ser. 360, 85–96. doi: 10.3354/meps07385
Mayorga-Adame, C. G. (2015). Modeled Larval Connectivity Patterns in Two Coral Reef Regions : The Western Caribbean and the Kenyan-Tanzanian Shelf. PhD thesis, Oregon State University, Corvallis, OR. Available online at: http://ir.library.oregonstate.edu/xmlui/handle/1957/57502
Mayorga-Adame, C. G., Strub, P. T., Batchelder, H. P., and Spitz, Y. H. (2016). Characterizing the circulation off the kenyan-tanzanian coast using an ocean model. J. Geophys. Res. Oceans 121, 1377–1399. doi: 10.1002/2015jc010860
McClanahan, T. R. (1994). Kenyan coral reef lagoon fish: effects of fishing, substrate complexity, and sea urchins. Coral Reefs 13, 231–241. doi: 10.1007/BF00303637
McClanahan, T. R., and Mangi, S. (2000). Spillover of exploitable fishes from a marine park and its effect on the adjacent fishery. Ecol. Appl. 10, 1792–1805. doi: 10.1890/1051-0761(2000)010[1792:SOEFFA]2.0.CO;2
McClanahan, T. R., Nugues, M., and Mwachireya, S. (1994). Fish and sea urchin herbivory and competition in kenyan coral reef lagoons: the role of reef management. J. Exp. Mar. Biol. Ecol. 184, 237–254. doi: 10.1016/0022-0981(94)90007-8
McCook, L. J., Almany, G. R., Berumen, M. L., Day, J. C., Green, A. L., Jones, G. P., et al. (2009). Management under uncertainty: guide-lines for incorporating connectivity into the protection of coral reefs. Coral Reefs 28, 353–366. doi: 10.1007/s00338-008-0463-7
McCormick, M. I. (1999). Delayed metamorphosis of a tropical reef fish (Acanthurus Triostegus): a field experiment. Mar. Ecol. Prog. Ser. 176, 25–38. doi: 10.3354/meps176025
McLeod, E., Salm, R., Green, A., and Almany, J. (2009). Designing marine protected area networks to address the impacts of climate change. Front. Ecol. Environ. 7, 362–370. doi: 10.1890/070211
Melbourne-Thomas, J., Johnson, C. R., Aliño, P. M., Geronimo, R. C., Villanoy, C. L., and Gurney, G. G. (2011). A multi-scale biophysical model to inform regional management of coral reefs in the western Philippines and South China Sea. Environ. Modell. Softw. 26, 66–82. doi: 10.1016/j.envsoft.2010.03.033
Micheli, F., Saenz-Arroyo, A., Greenley, A., Vazquez, L., Espinoza Montes, J. A., Rossetto, M., et al. (2012). Evidence that marine reserves enhance resilience to climatic impacts. PLoS ONE 7:e40832. doi: 10.1371/journal.pone.0040832
Mora, C., and Sale, P. F. (2002). Are populations of coral reef fish open or closed? Trends Ecol. Evol. 17, 422–428. doi: 10.1016/S0169-5347(02)02584-3
Munday, P. L., Leis, J. M., Lough, J. M., Paris, C. B., Kingsford, M. J., Berumen, M. L., et al. (2009). Climate change and coral reef connectivity. Coral Reefs 28, 379–395. doi: 10.1007/s00338-008-0461-9
Muthiga, N., Costa, A., Motta, H., Muhando, C., Mwaipopo, R., and Schleyer, M. (2008). “Status of coral reefs in East Africa: Kenya, Tanzania, Mozambique and South Africa,” in Status of Coral Reefs of the World: 2008, ed C. Wilkinson (Townsville, AU: Global Coral Reef Monitoring Network and Reef and Rainforest Research Centre), 91–104.
Muths, D., Gouws, G., Mwale, M., Tessier, E., and Bourjea, J. (2012). Genetic connectivity of the reef fish lutjanus kasmira at the scale of the Western Indian Ocean. Can. J. Fish. Aquat. Sci. 69, 842–853. doi: 10.1139/f2012-012
Nishikawa, A., Masaya, K., and Sakai, K. (2003). Larval settlement rates and gene flow of broadcast-spawning (Acropora Tenuis) and Planula-Brooding (Stylophora Pistillata) corals. Mar. Ecol. Prog. Ser. 256, 87–97. doi: 10.3354/meps256087
Nozawa, Y., and Harrison, P. L. (2008). Temporal patterns of larval settlement and survivorship of two broadcast-spawning acroporid corals. Mar. Biol. 155, 347–351. doi: 10.1007/s00227-008-1034-8
O'Connor, M. I., Bruno, J. F., Gaines, S. D., Halpern, B. S., Lester, S. E., Kinlan, B. P., et al. (2007). Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proc. Natl. Acad. Sci. U.S.A. 104, 1266–1271. doi: 10.1073/pnas.0603422104
Oxenford, H. A., Fanning, P., and Cowen, R. K. (2008). “Spatial distribution of surgeonfish (Acanthuridae) pelagic larvae in the eastern caribbean,” in Proceedings of a Special Symposium, 9–11 November 2006, 59th Annual Meeting of the Gulf and Caribbean Fisheries Institute, Caribbean Connectivity: Implications Fo R Marine Protected Area Management, Vol. 195, eds Rikki Grober-Dunsmore and D. Brian Keller (Belize City, Belize; Silver Spring, MD: Marine Sanctuaries Conservation Series ONMS-08-07. U.S. Department of Commerce, National Oceanic and Atmospheric Administration, Office of National Marine Sanctuaries), 42–51.
Pandolfi, J. M., Bradbury, R. H., Sala, E., Hughes, T. P., Bjorndal, K. A., Cooke, R. G., et al. (2003). Global trajectories of the long-term decline of coral reef ecosystems. Science 301, 955–958. doi: 10.1126/science.1085706
Paris, C. B., Atema, J., Irisson, J. O., Kingsford, M., Gerlach, G., and Guigand, C. M. (2013). Reef odor: a wake up call for navigation in reef fish larvae. PLoS ONE 8:e72808. doi: 10.1371/journal.pone.0072808
Paris, C. B., Chérubin, L., and Cowen, R. K. (2007). Surfing, spinning, or diving from reef to reef: effects on population connectivity. Mar. Ecol. Prog. Ser. 347, 285–300. doi: 10.3354/meps06985
Paris, C. B., and Cowen, R. K. (2004). Direct evidence of a biophysical retention mechanism for coral reef fish larvae. Limnol. Oceanogr. 49, 1964–1979. doi: 10.4319/lo.2004.49.6.1964
Pineda, J., Porri, F., Starczak, V., and Blythe, J. (2010). Causes of decoupling between larval supply and settlement and consequences for understanding recruitment and population connectivity. J. Exp. Mar. Biol. Ecol. 392, 9–21. doi: 10.1016/j.jembe.2010.04.008
Randall, J. E. (1961). A contribution to the biology of the convict surgeonfish of the hawaiian islands, Acanthurus triostegus sandvicensis. Pac. Sci. 15, 215–272.
Roberts, C. M., Hawkins, J. P., and Gell, F. R. (2005). The role of marine reserves in achieving sustainable fisheries. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360, 123–132. doi: 10.1098/rstb.2004.1578
Rocha, L. A., Bass, A. L., Robertson, D. R., and Bowen, B. W. (2002). Adult habitat preferences, larval dispersal, and the comparative phylogeography of three Atlantic surgeonfishes (Teleostei: Acanthuridae). Mol. Ecol. 11, 243–251. doi: 10.1046/j.0962-1083.2001.01431.x
Rossi, V., Ser-Giacomi, E., López, C., and Hernández-García, E. (2014). Hydrodynamic provinces and oceanic connectivity from a transport network help designing marine reserves. Geophys. Res. Lett. 41, 2883–2891. doi: 10.1002/2014GL059540
Saenz-Agudelo, P., Jones, G. P., Thorrold, S. R., and Planes, S. (2011). Connectivity dominates larval replenishment in a coastal reef fish metapopulation. Proc. R. Soc. Lond. B Biol. Sci. 278, 2954–2961. doi: 10.1098/rspb.2010.2780
Sale, P. F. (2006). Coral Reef Fishes: Dynamics and Diversity in a Complex Ecosystem. San Diego, CA: Gulf Professional Publishing.
Sale, P. F., Cowen, R. K., Danilowicz, B. S., Jones, G. P., Kritzer, J. P., Lindeman, K. C., et al. (2005). Critical science gaps impede use of no-take fishery reserves. Trends Ecol. Evol. 20, 74–80. doi: 10.1016/j.tree.2004.11.007
Schultz, E. T., and Cowen, R. K. (1994). Recruitment of coral reef fishes to bermuda: local retention or long-distance transport? Mar. Ecol. Prog. Ser. 109, 15–28.
Shanks, A. L. (2009). Pelagic larval duration and dispersal distance revisited. Biol. Bull. 216, 373–385. doi: 10.1086/BBLv216n3p373
Simpson, S. D., Meekan, M., Montgomery, J., McCauley, R., and Jeffs, A. (2005). Homeward sound. Science 308, 221–221. doi: 10.1126/science.1107406
Soria, G., MunguaVega, A., Marinone, S. G., MorenoBez, M., MartnezTovar, I., and CudneyBueno, R. (2012). Linking bio-oceanography and population genetics to assess larval connectivity. Mar. Ecol. Prog. Ser. 463, 159–175. doi: 10.3354/meps09866
Souter, P., Henriksson, O., Olsson, N., and Grahn, M. (2009). Patterns of genetic structuring in the coral pocillopora damicornis on reefs in East Africa. BMC Ecol. 9:19. doi: 10.1186/1472-6785-9-19
Spalding, M., Ravilious, C., and Green, E. P. (2001). World Atlas of Coral Reefs. Bekeley, CA: University of California Press.
Sponaugle, S., Cowen, R. K., Shanks, A., Morgan, S. G., Leis, J. M., Pineda, J., et al. (2002). Predicting self-recruitment in marine populations: biophysical correlates and mechanisms. Bull. Mar. Sci. 70(Suppl. 1), 341–375.
Sponaugle, S., Paris, C., Walter, K., Kourafalou, V., and D'Alessandro, E. (2012). Observed and modeled larval settlement of a reef fish to the florida keys. Mar. Ecol. Prog. Ser. 453, 201–212. doi: 10.3354/meps09641
Staaterman, E., Paris, C. B., and Helgers, J. (2012). Orientation behavior in fish larvae: a missing piece to Hjort's critical period hypothesis. J. Theor. Biol. 304, 188–196. doi: 10.1016/j.jtbi.2012.03.016
Stobutzki, I. C., and Bellwood, D. R. (1997). Sustained swimming abilities of the late pelagic stages of coral reef fishes. Oceanogr. Lit. Rev. 9:986. doi: 10.3354/meps149035
Thorrold, S. R., Jones, C. M., Campana, S. E., McLaren, J. W., and Lam, J. W. H. (1998). Trace element signatures in otoliths record natal river of juvenile American Shad (Alosa Sapidissima). Limnol. Oceanogr. 43, 1826–1835. doi: 10.4319/lo.1998.43.8.1826
Thorrold, S. R., Zacherl, D., and Levin, L. (2007). Population connectivity and larval dispersal using geochemical signatures in calcified structures. Oceanography 20, 80–89. doi: 10.5670/oceanog.2007.31
Tolimieri, N., Jeffs, A., and Montgomery, J. (2000). Ambient sound as a cue for navigation by the pelagic larvae of reef fishes. Mar. Ecol. Prog. Ser. 207, 219–224. doi: 10.3354/meps207219
van der Ven, R. M., Triest, L., De Ryck, D. J. R., Mwaura, J. M., Mohammed, M. S., and Kochzius, M. (2016). Population genetic structure of the stony coral Acropora Tenuis shows high but variable connectivity in East Africa. J. Biogeogr. 43, 510–519. doi: 10.1111/jbi.12643
Vermeij, M. J., Marhaver, K. L., Huijbers, C. M., Nagelkerken, I., and Simpson, S. D. (2010). Coral larvae move toward reef sounds. PLoS ONE 5:e10660. doi: 10.1371/journal.pone.0010660
Visram, S., Yang, M.-C., Pillay, R. M., Said, S., Henriksson, O., Grahn, M., et al. (2010). Genetic connectivity and historical demography of the blue barred parrotfish (Scarus ghobban) in the Western Indian Ocean. Mar. Biol. 157, 1475–1487. doi: 10.1007/s00227-010-1422-8
Watson, J. R., Mitarai, S., Siegel, D. A., Caselle, E., Dong, C., and McWilliams, J. C. (2010). Realized and Potential Larval Connectivity in the Southern California Bight. Mar. Ecol. Prog. Ser. 401, 31–48. doi: 10.3354/meps08376
Werner, F., Cowen, R. K., and Paris, C. (2007). Coupled biological and physical models: present capabilities and necessary developments for future studies of population connectivity. Oceanography 20, 54–69. doi: 10.5670/oceanog.2007.29
Werner, F. E., Quinlan, J. A., Lough, R. G., and Lynch, D. R. (2001). Spatially-explicit individual based modeling of marine populations: a review of the advances in the 1990s. Sarsia 86, 411–421. doi: 10.1080/00364827.2001.10420483
Willis, T. J., Millar, R. B., Babcock, R. C., and Tolimieri, N. (2003). Burdens of evidence and the benefits of marine reserves: putting descartes before des horse? Environ. Conserv. 97–103. doi: 10.1017/S0376892903000092
Wolanski, E., Doherty, P., and Carleton, J. (1997). Directional swimming of fish larvae determines connectivity of fish populations on the great barrier reef. Naturwissenschaften 84, 262–268. doi: 10.1007/s001140050394
Wolanski, E., and Kingsford, M. J. (2014). Oceanographic and behavioural assumptions in models of the fate of coral and coral reef fish larvae. J. R. Soc. Interface 11:20140209. doi: 10.1098/rsif.2014.0209
Yahya, S. A. S., Christopher, M., and Martin, G. (2011). Fish and Sea Urchin Community Patterns and Habitat Effects on Tanzanian Coral Reefs. Available online at: http://www.diva-portal.org/smash/record.jsf;jsessionid=nipyf9_yNdz7jOSnL4fD0CFn7-kTc3Qeqsy5MQ7I.diva2-search7-vm?pid=diva2%3A440148&dswid=-8234
Keywords: larval connectivity, coral reefs, Western Indian Ocean, individual based modeling, particle tracking, ocean modeling
Citation: Mayorga-Adame CG, Batchelder HP and Spitz YH (2017) Modeling Larval Connectivity of Coral Reef Organisms in the Kenya-Tanzania Region. Front. Mar. Sci. 4:92. doi: 10.3389/fmars.2017.00092
Received: 05 August 2016; Accepted: 15 March 2017;
Published: 13 April 2017.
Edited by:
Christian Lindemann, University of Bergen, NorwayReviewed by:
Louis Worth Botsford, University of California, Davis, USAAnne Chenuil, Centre National de la Recherche Scientifique (CNRS), France
Guillem Chust, AZTI-Tecnalia, Spain
Copyright © 2017 Mayorga-Adame, Batchelder and Spitz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: C. Gabriela Mayorga-Adame, Z21heWFAbm9jLmFjLnVr
 C. Gabriela Mayorga-Adame
C. Gabriela Mayorga-Adame Harold P. Batchelder
Harold P. Batchelder Yvette. H. Spitz1
Yvette. H. Spitz1