- Research and Technology Centre, University of Kiel, Büsum, Germany
Ship traffic in Northwestern European seas is intense and continuing to increase, posing a threat to vulnerable seabird species as a result of disturbance. However, information on species-specific effects of ship traffic on seabirds at sea is limited, and tools are needed to prioritize species and areas to support the integration of conservation needs in Marine Spatial Planning. In this study, we investigated the responses of 26 characteristic seabird species in the German North and Baltic Seas to experimental ship disturbance using large datasets collected as part of the Seabirds at Sea counts. We developed a Disturbance Vulnerability Index (DVI) for ship traffic combining indicators for species’ shyness, escape costs, and compensatory potential, and analyzed the relationships among shyness, escape costs, and vulnerability. The DVI was calculated using the following eight indicators: escape distance, proportion of escaping birds, proportion of birds swimming prior to disturbance, wing loading, habitat use flexibility, biogeographic population size, adult survival rate, European threat and conservation status. Species-specific disturbance responses differed considerably, with common scoters (Melanitta nigra) and red-throated loons (Gavia stellata) showing the longest escape distances and highest proportions of escaping individuals. Red-throated loon, black guillemot (Cepphus grylle), Arctic loon (Gavia arctica), velvet scoter (Melanitta fusca), and red-breasted merganser (Mergus serrator) had the highest DVI values, and gulls and terns had the lowest. Contrary to theoretical considerations, shyness correlated positively with escape costs, with the shyest species also being the most vulnerable among the species studied. The strong reactions of several species to disturbance by ships suggest the need for areas with little or no disturbance in some marine protected areas, to act as a refuge for vulnerable species. This DVI can be used in combination with distribution data to identify the areas most vulnerable to disturbance.
Introduction
The German North Sea and Baltic Sea are heavily impacted by ship traffic (OSPAR, 2010; Bahlke, 2017; HELCOM, 2018). A growing maritime economy in general and the construction and maintenance of offshore wind farms in particular will lead to further increases in ship traffic, including outside designated shipping lanes (Ecorys et al., 2012; Fridell et al., 2015; Bahlke, 2017; Matczak, 2018). Ship traffic is known to be associated with various negative environmental impacts as a result of emissions into the water and air (OSPAR, 2010; HELCOM, 2018). In addition, approaching vessels may present a threatening stimulus to marine birds, with subsequent risk-avoidance behavior reducing the time available for other activities such as feeding, resting, or mating (Gill et al., 1996; Frid and Dill, 2002; Beale and Monaghan, 2004b). Observable responses by seabirds include flying off, escape diving, and increased alertness, which can result in loss of energy and opportunities, displacement, and net habitat loss (Bélanger and Bédard, 1990; Madsen and Fox, 1995; Béchet et al., 2004). Disturbance by ships may thus reduce survival and reproductive success and affect population dynamics (Goss-Custard et al., 1995a; Madsen, 1995; Carney and Sydeman, 1999; Sutherland, 1998).
The German North and Baltic Seas are important wintering sites for a large number of seabirds (Mendel et al., 2008; Markones et al., 2015). Several species are listed in Annex 1 of the European Union Birds Directive, which obliges member states to conserve their “most suitable territories” as Special Protection Areas. Winter and spring are considered to be the most critical times for accumulating body fat and establishing pair bonds in most waterbirds (Madsen and Fox, 1995; Knapton et al., 2000). However, despite the importance of the area and the high frequency of vessel traffic, disturbance of seabirds as a result of ship traffic is often neglected in (cumulative) impact assessments and planning processes. This can be partly attributed to the lack of detailed information and tools to identify and prioritize vulnerable species and areas.
Vulnerability indices have been established as tools to estimate levels of concern for species and areas, and have been developed for several human activities in the marine environment (surface pollutants: Williams et al., 1995; oil pollution: Camphuysen, 1998; traffic disturbance: Camphuysen et al., 1999; set-net fishery: Sonntag et al., 2012; wind energy: e.g., Kelsey et al., 2018). When combined with distribution data, they can be used to identify the most vulnerable areas (e.g., Garthe and Hüppop, 2004; Sonntag et al., 2012; Bradbury et al., 2014). An extensive literature has documented the effects of disturbance on breeding and non-breeding waterbirds in coastal and freshwater habitats (Carney and Sydeman, 1999; Rodgers and Schwikert, 2002; Steven et al., 2011; Glover et al., 2015; Krüger, 2016; McFadden et al., 2017), but information on disturbance responses of seabirds at sea to vessel traffic is limited to a few species (Bellebaum et al., 2006; Kaiser et al., 2006; Schwemmer et al., 2011).
Disturbance responses differ among species, with some species being more sensitive than others. Responses are measurable as escape distances, the proportions of escaping birds, and as physiological responses such as heart rate and corticosterone levels. Given that the physiological responses of free-ranging seabirds at sea are extremely difficult to measure, most studies of disturbance effects have reported escape distances as a measure of effect (Blumstein et al., 2005). However, a species’ vulnerability to disturbance cannot be assessed based on escape distance alone, given that the decision of when to take flight represents a trade-off between safety and fitness-enhancing activities (Ydenberg and Dill, 1986; Lima and Dill, 1990; Gill et al., 2001; Frid and Dill, 2002; Gill, 2007). A bird in good body condition and with sufficient feeding alternatives might flush earlier than a bird short of resources, as demonstrated in an experimental study with waders (Beale and Monaghan, 2004a). Visible disturbance responses alone are thus generally not considered to be a good indicator of vulnerability (Gill et al., 2001; Frid and Dill, 2002; Beale and Monaghan, 2004a; Beale, 2007). Vulnerability analysis should therefore consider the total costs of disturbance events including the ability to compensate for losses at the individual and population levels.
This study aimed to further our knowledge of species-specific behavioral disturbance responses at sea for all common and characteristic seabirds in German waters using experimental disturbance. We also aimed to develop a Disturbance Vulnerability Index (DVI) for ship traffic, combining indicators for species’ shyness, escape costs, and compensatory potential, which can be used as a management tool to assess different vulnerabilities of a given sea area with respect to disturbance by ships. Finally, we aimed to investigate the general relationships among shyness, escape costs, and vulnerability in seabirds by cross-species comparisons of disturbance-related factors.
Materials And Methods
Behavioral Observations
All behavioral observations were carried out during ship-based Seabirds at Sea counts in the coastal and offshore zones of the German North Sea (Figure 1) and Baltic Sea (Figure 2), following internationally standardized methods (Tasker et al., 1984; Webb and Durinck, 1992; Garthe et al., 2002; Camphuysen and Garthe, 2004). All birds were classified as either swimming or flying. Swimming birds were counted continuously within a 300 m wide transect parallel to the ship’s keel line. Flying birds were only counted at full minutes and within a distance of 300 m to the side and to the front of the vessel, to avoid overestimation. We analyzed three different datasets collected within this framework from all seasons combined, described in the following paragraphs.
Figure 1. Study area and locations of data collection in the years 2016/2017 in the German North Sea. Purple locations, data collected on proportions of responding birds; orange locations, additionally measured escape distances.
Figure 2. Study area and locations of data collection in the years 2016/2017 in the German Baltic Sea. Purple locations, data collected on proportions of responding birds; orange locations, additionally measured escape distances.
Proportion of Swimming Birds
We calculated the proportion of swimming birds for each species from data collected on more than 1,200 survey days in the years 2000 to 2017. Distance-correction factors (Garthe et al., 2007, 2009; Markones et al., 2013) were applied to the number of swimming birds to account for overlooked birds, resulting in a dataset of over 1.1 million birds. In our context ‘swimming’ encompassed all activities performed on the water surface, including resting, preening, active swimming, or others, each of which implies slightly different, but low energetic costs compared with flying (Norberg, 1996). Because birds reacted to the approaching vessels, we looked far ahead to detect swimming birds before flushing, and always recorded flushed birds as swimming birds prior to disturbance.
Proportion of Escaping Birds
Species-specific disturbance responses were recorded on 139 survey days in 2016 and 2017 (Figures 1, 2). We distinguished between two types of disturbance responses: flying off and escape diving. Common murres (Uria aalge) with young were excluded from the analysis, because we assumed that adult birds escaped less often to stay in the vicinity of their offspring. We further excluded species with fewer than 15 observations from the analysis of the proportion of escaping birds. The total dataset comprised 221,071 individuals from 25 species and species groups (loons and auks).
Escape Distance
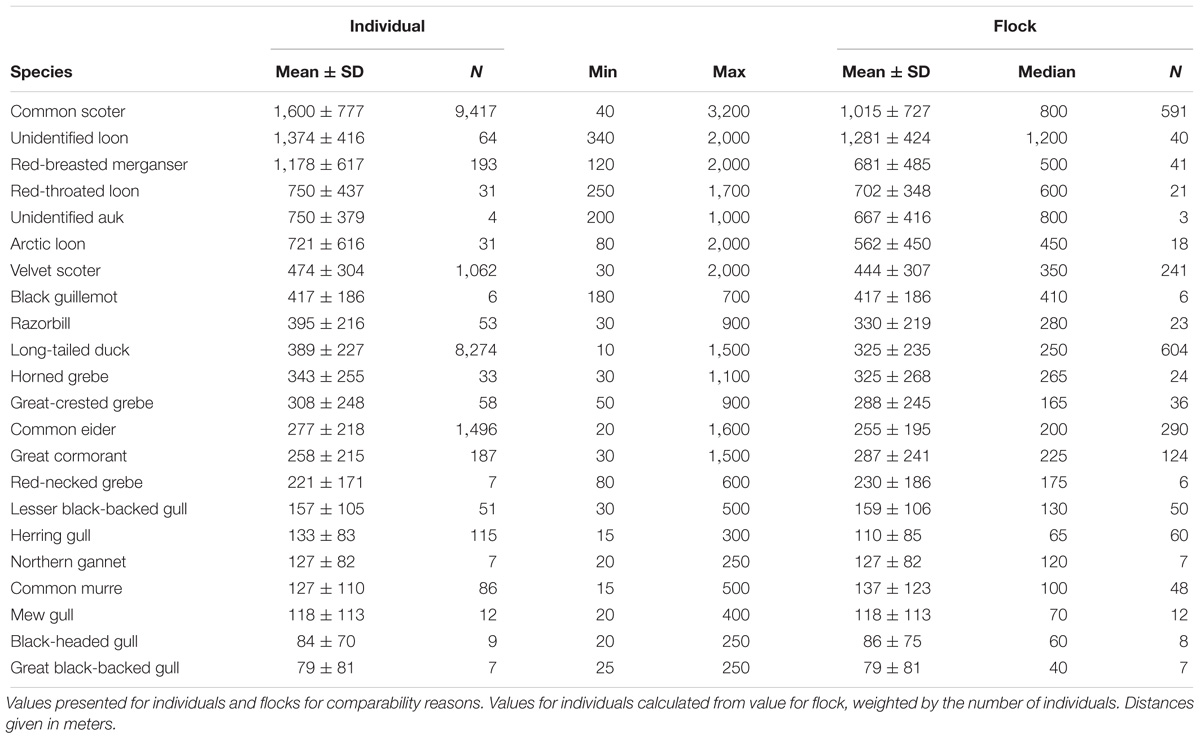
We additionally measured the escape distances of disturbed birds, also called flight initiation distance (e.g., Bonenfant and Kramer, 1996; Blumstein, 2006) or flush distance (e.g., Rodgers and Smith, 1995; Schwemmer et al., 2011), on 51 of the 139 survey days in the years 2016 and 2017. Our method was based on the distance estimation using geometrical functions, as described by Heinemann (1981). We recorded escape distances following the same principle as Schwemmer et al. (2011), but using individualized rulers instead of calipers or binoculars with reticles. To keep a consistent method, we refrained from measuring distances with radar or rangefinder, which are not consistently feasible under conditions at sea (Schwemmer et al., 2011; Borkenhagen et al., 2017). We randomly selected flocks of different sizes and measured the distance between the observer vessel and the first escaping bird in a flock at the moment of flushing or escape diving. Measurements were taken in directions between 90° and 0° of the course of the ship. Five research vessels were used: MS Haithabu (39 m long, n = 465 measurements); FS Heincke (55 m long, n = 42 measurements); MS Odin (32 m long, n = 161 measurements); MS Prandtl (31 m long, n = 1575 measurements); and MS Skoven (42 m long, n = 17 measurements). The measurements were taken at an average speed of 18.5 ± 1.8 km/h. We scanned the water surface constantly using binoculars to ensure that birds further away were not missed. Because escape distance generally increases with flock size (Burger and Gochfeld, 1991; Mori et al., 2001; Kaiser et al., 2006; Schwemmer et al., 2011), we calculated the mean escape distance per individual for later use in the DVI. Escape distance per flock was also presented to allow comparisons with other studies. A considerable proportion of auks and loons cannot be identified to species level, especially at greater distances. If differences between species (e.g., red-throated loon) and their respective species group (loons) occurred, we presented both values separately to ensure that all behavioral observations were included. Only species with at least five observations were included in the analysis of escape distances. We calculated mean escape distances for a total of 22 species and species groups (loons and auks) based on 2,260 measurements. Statistical analysis was performed in R 3.2.4 using simple summary statistics (R Core Team, 2016; RStudio Team, 2016).
Disturbance Vulnerability Index
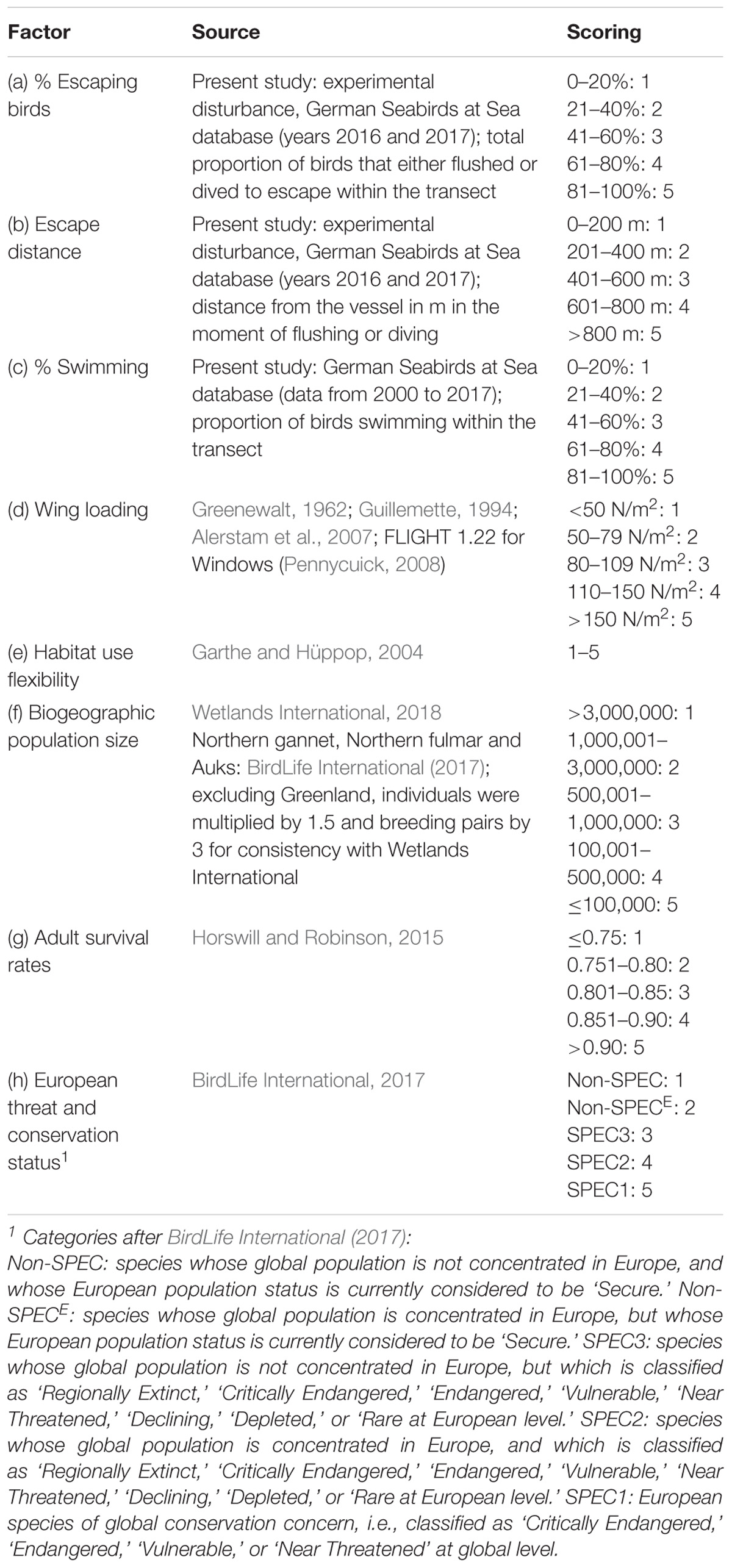
We constructed the DVI for ship traffic to reflect the total costs of disturbance, defined by three components: (1) the probability of a disturbance event based on species’ shyness; (2) the energetic costs of escape of each disturbance event; and (3) the costs on the population level based on status factors. We chose eight factors as indicators of the described components:
(1) Shyness:
(a) Proportion of escaping birds: species with a high proportion of escaping birds flush or dive more often to escape from ships.
(b) Escape distance: the affected area is larger for species with a long escape distance.
(2) Escape costs:
(c) Proportion of swimming birds: flying is energetically much more costly than swimming (floating), and a flushing event is thus proportionally more costly for birds that seldom fly than for frequently flying birds.
(d) Wing loading: species differ in the energy expenditure required for reactions such as flushing or diving because of morphological and physiological differences. Birds with a higher wing loading have higher energetic costs for flushing (Norberg, 1996).
(e) Habitat use flexibility: disturbance may displace birds from suitable habitat. Species relying on specific habitat features thus have higher costs than species with less specific habitat preferences.
(3) Population status:
(f) Biogeographic population size: energetic losses, displacement, and habitat loss may increase mortality and reduce reproduction. Species with small biogeographic populations are considered more vulnerable to additional losses.
(g) Adult survival rate: species with high adult survival rates are more affected by additional adult mortality than species with low adult survival rates (Sæther and Bakke, 2000).
(h) European threat and conservation status: species with a high conservation status are considered more vulnerable to any additional pressures.
Each factor was scored on a 5-point scale from 1 (low) to 5 (high). Factors (a–c) above were based on data collected in the present study. We used the weighted mean value of species and species group for auks and loons in factor (b), because birds could often not be identified to species level at longer distances. Factor (e) was assessed by subjective considerations based on at-sea experience by Garthe and Hüppop (2004). If data for one species was missing, we used scores for closely related species. The sources of the values for each factor and their scores are given in Table 1. An average score was calculated for each component and subsequently multiplied by each other to produce the DVI for each species, following the methodology of the wind farm sensitivity index developed by Garthe and Hüppop (2004):
The relationships among the three components of the DVI were investigated by Spearman’s rank-order correlations in R 3.2.4 (R Core Team, 2016; RStudio Team, 2016; Wickham, 2016).
Results
Behavioral Observations
Proportion of Birds Swimming
The proportion of time the birds spent swimming differed strongly among species (Figure 3). Grebes, seaducks, auks, and loons were detected swimming most often, with proportions ranging from 91% (red-throated loon) to 100% [red-necked grebe (Podiceps grisegena) and horned grebe (Podiceps auritus)]. Moderate proportions of great cormorants (Phalacrocorax carbo), gull species, northern fulmars (Fulmarus glacialis), and northern gannets (Morus bassanus) were seen swimming, while terns had the lowest proportions of swimming birds (16–23%).
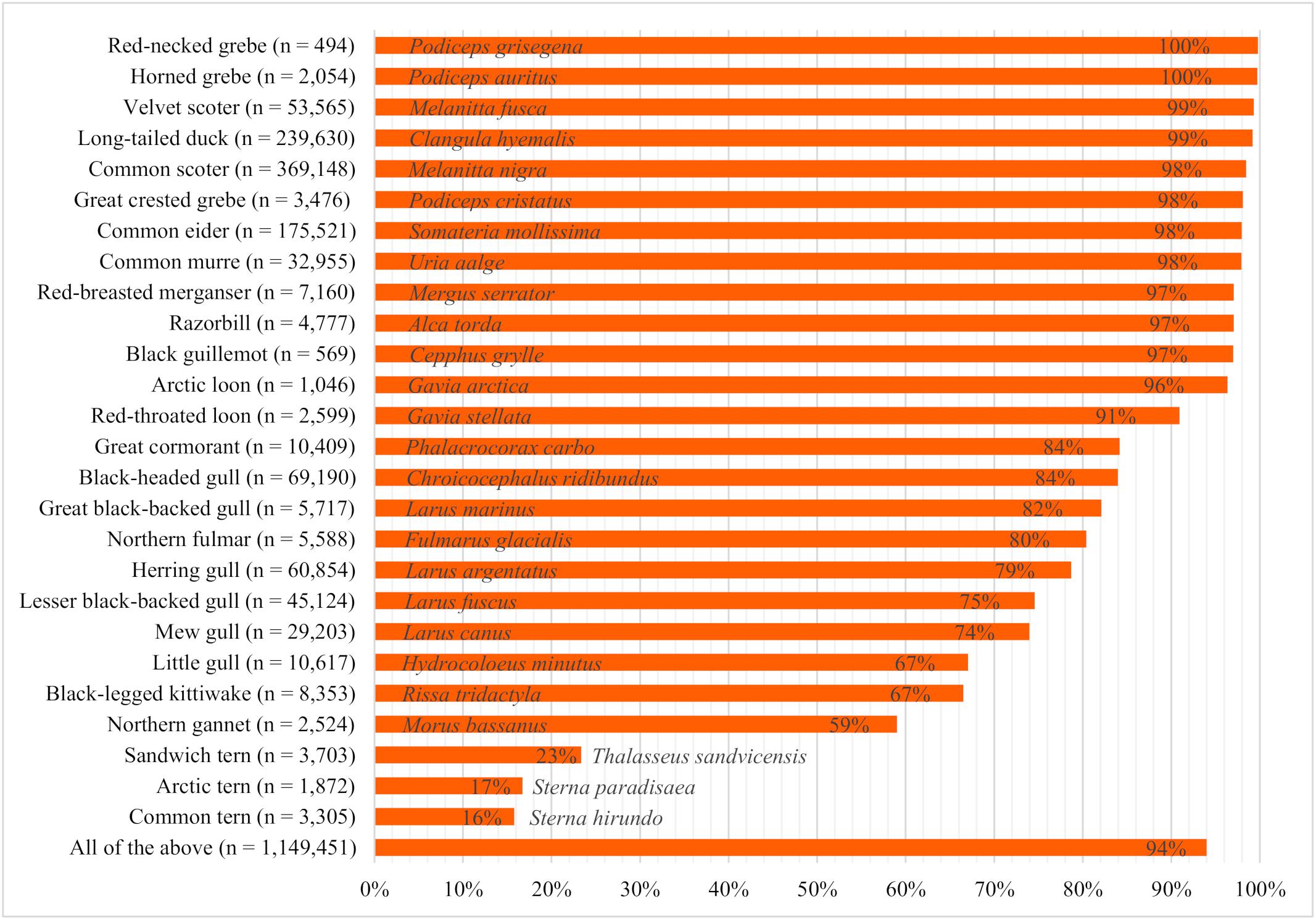
Figure 3. Proportion of swimming birds observed during ship-based surveys in the German North and Baltic Seas in the years 2000 to 2017 (n = total number of individuals considered).
Proportion of Escaping Birds
The proportion of individuals showing disturbance responses such as flushing or escape diving differed greatly between species (Figure 4). Overall, flushing was the most common disturbance response, with 73% of all recorded birds flushing in front of the vessel, compared with 1% that escaped by diving. Even among species capable of diving, only a small proportion of birds dived, except for Arctic loons, common murres, and red-necked grebes.
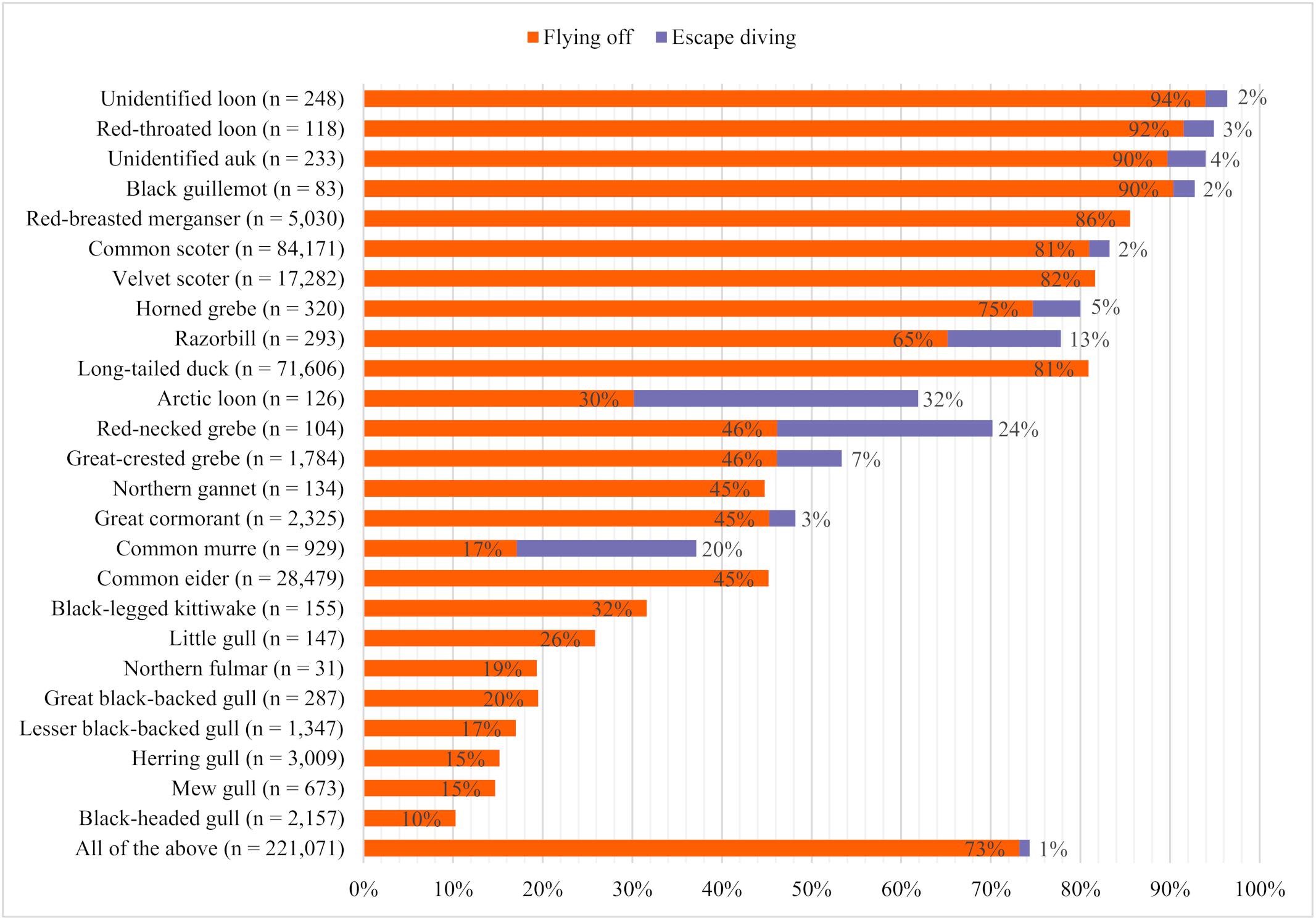
Figure 4. Species-specific proportions of birds showing different disturbance responses in front of approaching research vessels in 2016 and 2017 (n = total number of individuals considered).
The highest total proportions of birds with observed disturbance responses were calculated for unidentified loons (96%), red-throated loons (95%), unidentified auks (94%), and black guillemots (92%), followed by red-breasted mergansers (Mergus serrator; 86%), common scoters (83%), velvet scoters (82%), horned grebes (80%), razorbills (Alca torda; 78%), and long-tailed ducks (Clangula hyemalis; 81%). The lowest proportions of disturbance responses were found in gull species and northern fulmars, among which black-legged kittiwakes (Rissa tridactyla) had the highest (32%) and black-headed gulls (Croicocephalus ridibundus) the lowest (10%) proportions.
There were sometimes large differences in the proportions of individuals of closely related species displaying certain behaviors; 92% of red-throated loons took flight in front of the vessel and only 3% dived to escape, while only 30% of Arctic loons took flight, and 32% dived to escape. The proportion of flushed individuals was very high among black guillemots (90%), compared with razorbills (65%) and common murres (17%). In contrast, the proportion of individuals that dived was considerably higher among common murres (20%) compared with razorbills (13%) and black guillemots (2%). Horned grebes (75%) flushed more often than red-necked grebes and great crested grebes (Podiceps cristatus; each 46%), but red-necked grebes dived more often (24%). Velvet scoters, common scoters, and long-tailed ducks showed similar proportions of flushed individuals (82%, 81%, and 81%, respectively), while the proportion of common eiders that flushed was only about half (Somateria mollissima; 45%).
Escape Distance
Escape distances differed widely among species. The mean escape distance per individual was higher than the mean escape distance per flock in most species (Table 2). This effect was most pronounced in red-breasted mergansers (1,178 m per individual vs. 681 m per flock) and common scoters (1,600 m per individual vs. 1,015 m per flock). Of all species, common scoters had the highest mean escape distance per individual (1,600 ± 777 m), followed by unidentified loons (1,374 ± 416 m), red-breasted mergansers (1,178 ± 617 m), red-throated loons (750 ± 437 m), unidentified auks (750 ± 379 m), and Arctic loons (721 ± 616 m). The escape distances of the remaining seaduck species, razorbills, black guillemots, grebes, and great cormorants were considerably lower, with mean values between 474 ± 304 m (velvet scoter) and 221 ± 171 m (red-necked grebe). The lowest mean escape distances were calculated for gull species (lesser black-backed gull (Larus fuscus): 157 ± 105 m, herring gull (Larus argentatus): 133 ± 83 m, mew gull (Larus canus): 118 ± 113 m, black-headed gull: 84 ± 70 m, great black-backed gull (Larus marinus): 79 ± 81 m), northern gannets (127 ± 82 m), and common murres (127 ± 110 m) (Table 2). The maximum escape distance was observed in common scoters (3,200 m). Other seaduck species and loons also had high maximum escape distances between 1,500 m and 2,000 m. Seaducks sometimes flushed at a distance of around 3,000 m, but could not be identified to species level and were not included in the analysis.
Disturbance Vulnerability Index
The species differed strongly in their DVI values (Table 3). The highest values were calculated for red-throated loon, black guillemot, and Arctic loon, followed by velvet scoter, red-breasted merganser, razorbill and horned grebe. Mew gull, black-headed gull, common tern (Sterna hirundo) and Arctic tern (Sterna paradisaea) were the least vulnerable species. Rankings based on behavioral sensitivity (shyness × escape costs) and population sensitivity diverged in some cases, and we therefore presented both values separately. Behavioral sensitivity was highest in red-throated loon, common scoter, red-breasted merganser and Arctic loon and lowest in Sandwich tern (Thalasseus sandvicensis), lesser black-backed gull, common tern and Arctic tern. Common eider and black guillemot ranked highest in terms of population sensitivity, and common scoter, great-crested grebe, black-headed gull and Arctic tern lowest. In the DVI, common scoter thus ranked lower and common eider ranked higher than suggested based on behavioral sensitivity alone.
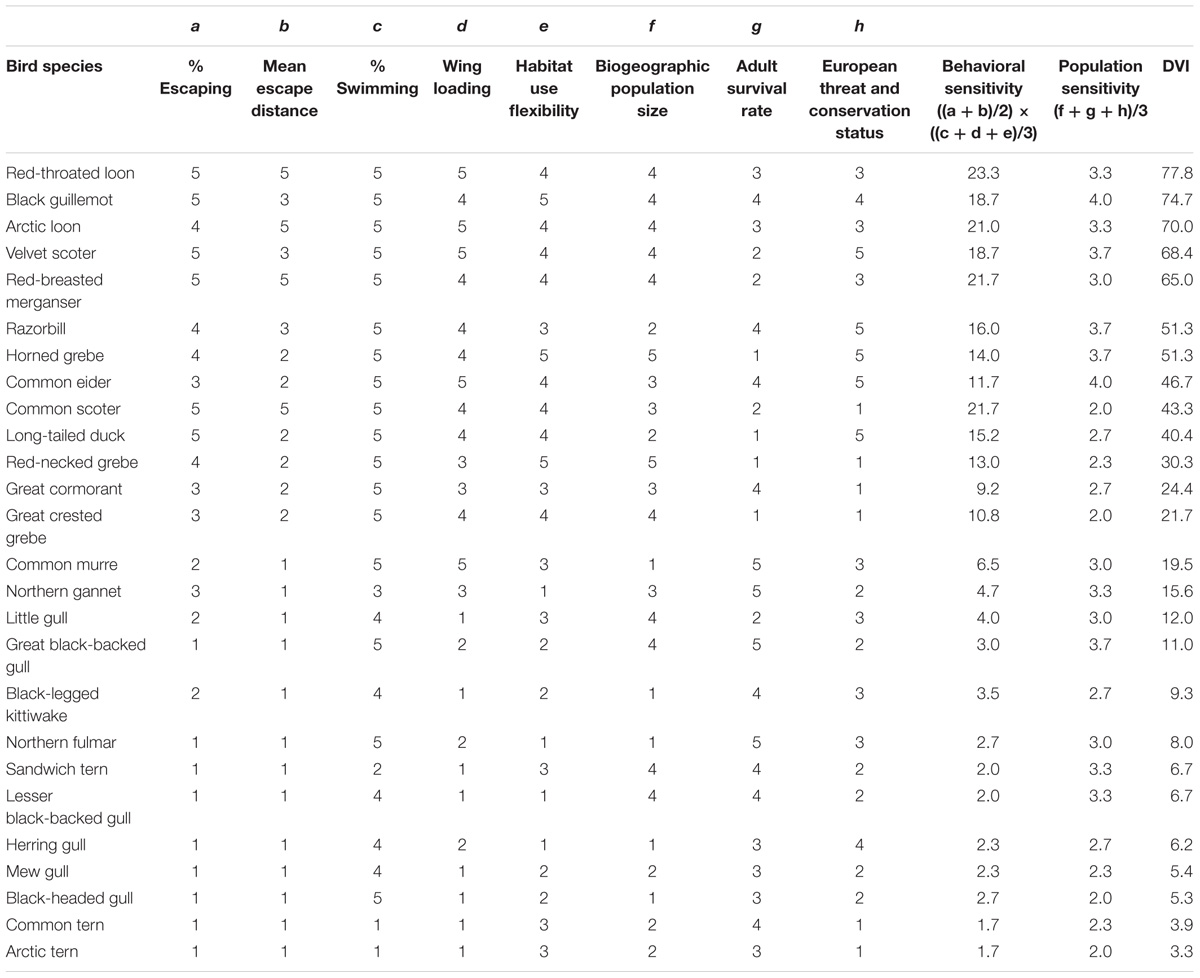
Table 3. Factor scores and resulting Disturbance Vulnerability Index (DVI) values for 26 common European seabird species.
The scores for the three components of the index (shyness, escape costs, population status) correlated positively with each other (Figure 5). There was a strong significant correlation between shyness (mean of factors a and b) and escape costs (mean of factors c–e) (r = 0.81, p ≤ 0.001, n = 26). Escape costs also correlated significantly with population status (mean of factors f–h) (r = 0.43, p ≤ 0.05, n = 26), but the positive correlation coefficient between shyness and population status was not significant (r = 0.27, p = 0.189, n = 26).
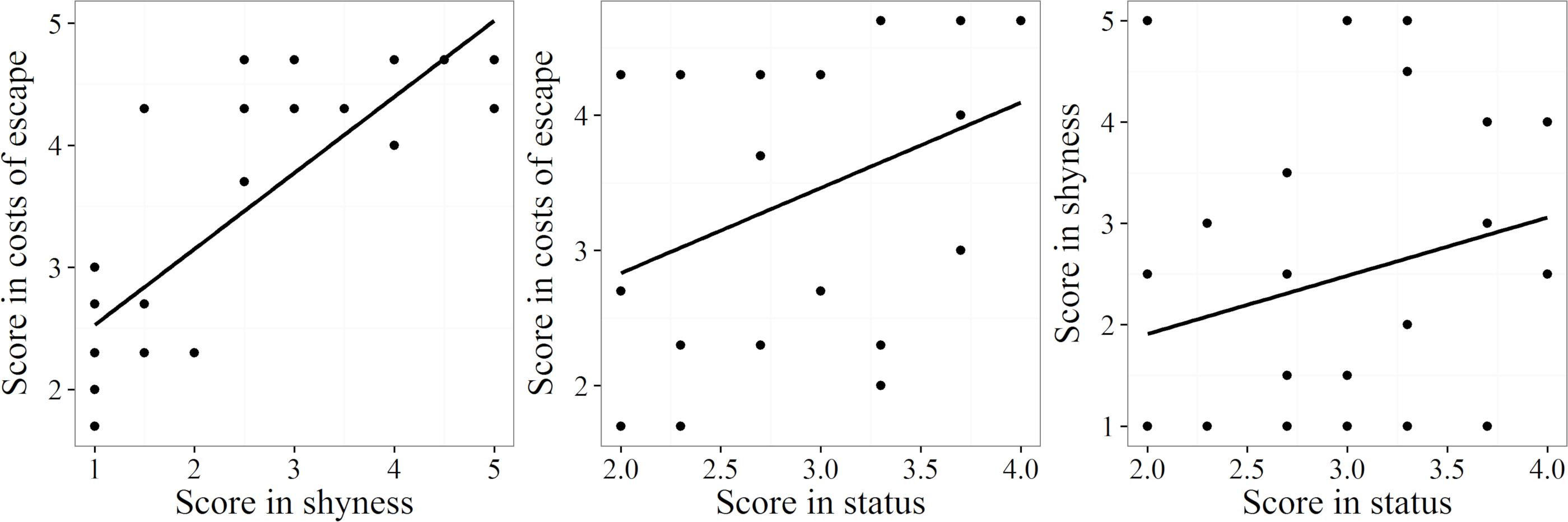
Figure 5. Associations of the three components, shyness, escape costs, and population status, used to compute the DVI. Trend lines were calculated using linear regression.
Discussion
Behavioral Observations
The current study detected large interspecific differences in the proportions of swimming birds prior to disturbance, associated with species’ ecology. Species adapted to diving for (relatively) stationary prey exhibited the highest percentages of swimming individuals. Flying is the energetically most demanding form of locomotion per unit time (Norberg, 1996), and can be up to 31 times more costly than the basal metabolic rate (see Elliott et al., 2013 for an example in thick-billed murres, Uria lomvia). Short flights are especially costly per unit time, because take-off, ascent, and descent form a large part of the total flight time (Nudds and Bryant, 2000). Birds that rarely fly under normal circumstances thus suffer proportionally higher flight costs due to disturbance than birds that fly more frequently.
Disturbance responses to ships differed strongly among species. Species with long escape distances were also among the species with the highest proportions of escaping birds, reflecting the fact that these parameters are related and measure the same trait (shyness). However, differences between the shyest species were much more pronounced in terms of escape distances than in the proportions of escaping birds. Investigating both factors thus gave a more detailed picture of interspecies differences in disturbance behavior, and both were included as indicators for species’ shyness in the vulnerability index.
The detected differences in disturbance responses among species could largely be explained by different perceptions of predation risk, and less by the cost of escape. The decision on when to take flight is the result of a trade-off between safety and feeding or other fitness-enhancing activities (Ydenberg and Dill, 1986; Lima and Dill, 1990; Frid and Dill, 2002). Birds will thus flush when the costs of remaining and escape are equal (Ydenberg and Dill, 1986). In theory, escape distance should increase with higher flight costs (high wing loadings) and higher costs due to lost opportunities (low habitat flexibility). In our study, however, species with higher escape costs were overall also those with stronger disturbance responses (except for common eider and common murre), suggesting that these species must have highly different predation risks, i.e., costs of remaining (Ydenberg and Dill, 1986), which dominated the influence of escape costs on escape distances. Birds with high wing loadings are generally less maneuverable in flight, need a longer time to take off, and thus have more difficulty escaping from predators. Many of those species are also subject to hunting by humans (Hirschfeld and Heyd, 2005; Hirschfeld and Attard, 2017), which might have increased their shyness toward human activities. In contrast, purely pelagic seabirds such as northern gannets, northern fulmars, and terns have a lower predation risk, and like gulls, often benefit from increased feeding opportunities in the presence of ships by using discards (Garthe and Hüppop, 1994; Garthe et al., 2016).
Wing loading, as an escape cost, might partially explain differences in disturbance behavior between closely related species. Common murres and Arctic loons each have approximately 30% higher wing loading than the closely related razorbills and red-throated loons (Thaxter et al., 2010; Alerstam et al., 2007; Pennycuick, 2008). Common murres had a much lower escape distance than razorbills, while Arctic loons seemed to escape slightly later than red-throated loons. We also observed fewer common murres and Arctic loons escaping compared with razorbills and red-throated loons, and a much higher percentage dived to escape instead of flying off. Similarly, the wing loading of common eiders is higher than in other seaduck species (Guillemette, 1994; Alerstam et al., 2007), and the mean escape distance and proportion of birds taking flight was considerably lower (see also Schwemmer et al., 2011). However, differences among the other seaduck species cannot be explained by wing loading. The positive relationship between body mass and escape distance reported in several other studies (Blumstein, 2006; Fernández-Juricic et al., 2006; Weston et al., 2012) did also not seem to exist in the seabird species studied here. A detailed knowledge of the costs, habitat and resource uses, and predation risks for each species would be required to understand the interspecific differences fully.
We also detected high intraspecific variability in escape distance. Escape distances can be influenced by various environmental (season, weather condition, location, habitat quality, size, speed, and noise of approaching vessels, angle of approach) and individual parameters (body condition, body size, flock size, previous experiences, molting stage, state, personality; e.g., Madsen, 1985; Burger and Gochfeld, 1991; Schwemmer et al., 2011). Notably, habitat quality and the state of the individual are likely to have strong effects on escape distances, leading to differences between populations and between different life cycle stages for the same individual. For example, high habitat quality and few habitat alternatives may lead to lower escape distances because birds should avoid leaving profitable areas (Frid and Dill, 2002); however, birds feeding in a high-quality habitat might be in better body condition and thus be able to maximize their safety by flushing earlier (Beale and Monaghan, 2004a). Some species undergo a flightless period during molting, leading to reduced escape distances (own observations); however, the energy demand during this period is high and individuals are thought to be more vulnerable to disturbance (Thiel et al., 1992). During courtship, males are less prepared to leave females and breaking of pair bonds due to disturbance might have a direct negative effect on reproduction. These examples illustrate the facts that escape distances are context-dependent and thus highly variable within species, and that escape distances alone do not translate into vulnerability (Beale, 2007). Although the above parameters generated variation in our study, a recapitulation of their effects was beyond the scope of the current study. Our data were collected over a large study area and at different seasons to represent birds in different states and different habitats to allow the calculation of a mean escape distance per species, which could serve as an indicator of the probability of disturbance responses for that species.
Common scoters and loons are known to escape at long distances in front of ships or low flying planes and helicopters (Camphuysen et al., 1999; Garthe and Hüppop, 2004; Kaiser et al., 2006; Thaxter and Burton, 2009; Schwemmer et al., 2011), but documented information on the disturbance behaviors of other species at sea is limited. The flush distances of seaduck flocks presented by Schwemmer et al. (2011) were similar to the current results, in terms of both absolute values and proportions between species, suggesting consistency of our method and a negligible observer effect (see also Guay et al., 2013). Kaiser et al. (2006) measured flush distances of common scoter flocks of 1,000–2,000 m using radar, which also falls within the range of our observations. The values for velvet scoters, long-tailed ducks, and loons given by Bellebaum et al. (2006) were not directly comparable because of the different methods used, but the ratios between species were similar to those in the present study. Flush distances at sea appear to be considerably higher than in estuarine areas (Ronconi and Clair, 2002; McFadden et al., 2017). This could be explained by habituation to vessel traffic, which is usually high in estuarine areas. Another explanation might be longer sighting distances in the open seascape. Differences in escape distances between habitats highlight the importance of studying the responses within the area of interest to define setback distances (Rodgers and Schwikert, 2002; Blumstein et al., 2003; McFadden et al., 2017) for a specific habitat.
Notably, use of the mean escape distances calculated here must take account of the fact that they were based on ships of a specific size and speed, and bigger and/or faster ships might induce longer escape distances and higher proportions of flushed birds. Furthermore, the mean escape distances were still likely to be underestimated, because birds flushing at longer distances were less likely to be detected or the species was less likely to be identified. Finally, we only measured visible disturbance responses, which are energetically the most costly reactions to disturbance. However, physiological stress responses, measurable as corticosterone levels (Cockrem, 2007; Fowler, 1999) and increased heart rate (e.g., Nimon et al., 1995), which is linked to an elevated metabolic rate (Green, 2011), commence well before behavioral changes become visible (Weimerskirch et al., 2017). Similarly, individuals might still experience stress despite behavioral habituation, as shown in penguins habituating to human disturbance (Walker et al., 2006). Thus a reduced escape distance in certain areas, which could be interpreted as indicating habituation or lowered vulnerability, might also be a consequence of limited alternative habitat. Setback distances should thus be considerably higher than mean escape distances to minimize the physiological effects of disturbance.
Disturbance Vulnerability Index
Ship traffic disturbance has been the subject of a few vulnerability indices in the past. Camphuysen et al. (1999) evaluated the behavioral sensitivity of seabird species to traffic disturbance, but did not include conservation status. In Garthe and Hüppop (2004), sensitivity to disturbance by ship and helicopter traffic was ranked by expert judgment as one factor for assessing seabird vulnerability to offshore wind energy developments. Both these indices ordered species similarly to the current index with respect to behavioral sensitivity, highlighting the consistency and reliability of expert-based evaluations, even when different methodologies are used.
Similar to other vulnerability indices, the current DVI depends on the selected factors, the scoring system, and the relative grouping and weighting of the factors. Another limitation of our index is that it only covers species occurring in German waters, and therefore does not include some especially sensitive species such as common loon (Gavia immer). We therefore suggest including measurements of escape distances and records of disturbance responses from other European monitoring programs to broaden the set of species. This might also provide more information on intra-specific behavioral differences and allow adjustment of population-specific vulnerability indices to account for variations in the selected factors between populations.
For the DVI, we only chose those factors that we considered to be most relevant for estimating the total costs of disturbance, and thus gave all the chosen factors the same weight. We included behavioral factors as well as population status factors, and combined them in a systematic way to estimate the total costs of disturbance. All but one factor, habitat use flexibility (evaluated by Garthe and Hüppop, 2004), were based on real data. Habitat use flexibility was used to indicate how well a bird can compensate for the cost of lost opportunities. Feeding time needed to meet energetic requirements would be a meaningful additional indicator for the ability to compensate for losses (Mayhew, 1988; Madsen and Fox, 1995; Wisniewska et al., 2016). However, integration of this factor in the index would require a detailed knowledge of the species’ activity budgets, including activity at night, and time needed for digestion and recuperation from diving. Although such information can be obtained from telemetry studies, it is not yet available for most species. The application of telemetry data in behavior-based and individual-based models also helps to improve predictions regarding the impacts of disturbance on habitat use, survival, and reproduction (Goss-Custard et al., 2006a). However, even sophisticated modeling techniques may not be able to describe the whole complexity of the interactions between wildlife and humans (May et al., 2019). We therefore consider that vulnerability indices like the one presented here provide the best available solution for assessing the potential vulnerabilities of a large set of species.
Relationship Between Shyness and Vulnerability in Seabirds
Cross-species comparisons and the systematic combination of disturbance-related factors within the DVI also allowed us to draw conclusions about the general relationships among shyness, escape costs, and disturbance vulnerability in seabirds. Theoretical considerations and experimental evidence suggest that visible disturbance responses alone are generally not a good indicator of vulnerability, because birds in good body condition and with sufficient feeding alternatives should flush earlier than birds short of resources (Gill et al., 2001; Frid and Dill, 2002; Beale and Monaghan, 2004a). In contrast to the expected situation however, shyness and escape costs were positively correlated in the present study. Escape costs also correlated positively with population status, such that several species ranked high in all three factor groups of our index. Among the species studied here, the shyest thus also seemed to be the most vulnerable. We propose that, while escape distance is not a good indicator of vulnerability of individuals within the same species, it may serve as an indicator of vulnerability within a range of species. This is in line with Møller (2008) and Møller et al. (2014), who found that long escape distances were associated with population declines among European and Australian birds. A better understanding of the relationships among shyness, escape costs, and population status might have important implications for conservation management. The next step should thus be to carry out further investigations based on the emerging patterns for seabirds using an extended set of species and possibly populations.
The Dimension of the Problem
Escape costs do not comprise only direct energetic costs and reduced energy uptake through lost time for feeding (Owens, 1977; Bélanger and Bédard, 1989); flushed birds might also be displaced from the best feeding resources (Madsen, 1998; Madsen and Fox, 1995). Altered distribution patterns within shipping lanes (Schwemmer et al., 2011) and in relation to vessel traffic to and from offshore wind farms (Mendel et al., 2019) have already been demonstrated in loons. We observed many common scoter flocks flying so far away after flushing that they could not be seen resettling before moving out of sight. Schwemmer et al. (2011) found that most common scoters did not return within 3 h after disturbance by a vessel, while common eiders and long-tailed ducks returned to pre-disturbance numbers within one to 3 h after disturbance. This suggests that very shy species may abandon an area completely, while others may suffer temporary habitat loss.
If birds cannot compensate for energetic losses, disturbance will affect body condition, reproduction, and survival (Madsen, 1985; Goss-Custard et al., 1995a,b, 2006a,b; McHuron et al., 2018). Ducks and geese have been observed feeding at night to compensate for being disturbed during the day (Bélanger and Bédard, 1990; Knapton et al., 2000; Merkel et al., 2009) and shorebirds were shown to increase feeding rates to compensate for lost feeding time (Swennen et al., 1989; Urfi et al., 1996). However, feeding rates and times cannot be extended limitlessly. The time needed to meet energetic requirements determines by how much feeding rates can be increased.
Seabirds might be able to habituate and even adapt to disturbance by ship traffic, if they were able to identify vessels as non-threatening objects. Habituation of birds to particular types of disturbance and within certain areas has been documented before (Smit and Visser, 1993; Samia et al., 2015). For example, among waterbirds, snow geese became accustomed to gunfire (Bélanger and Bédard, 1989) and common eiders and long-tailed ducks showed reduced flush distances within shipping lanes (Schwemmer et al., 2011). However, ships differ greatly in size, shape, speed, and engine noise, making recognizing them as non-threatening objects difficult. Furthermore, waterbirds are hunted using motorboats in some parts of Europe (Laursen and Frikke, 2008). In an environment where predation risk exists, either from natural predators or human activity, birds are thus likely to regard big moving objects as potential threats, and the potential for habituation among sensitive species seems very limited under the current conditions. Notably, even after decades of intense ship traffic in European waters, most species still reacted strongly to our experimental disturbance.
Conclusions and Recommendations
This study provides further evidence for the disturbance effects of ship traffic on seabirds, and additional information on the species-specific responses. We present the first comprehensive set of data on escape distances and relative numbers of escaping birds, covering most seabird species found in Northwest European waters. Our findings are based on extensive field experience and data sets from up to 17 years of behavioral observations of seabirds at sea. We show that species differ strongly in their disturbance responses. Our DVI is based on a comprehensive set of variables and addresses a wide range of disturbance-related aspects of bird ecology. It allows for objective quantification of species’ vulnerability to ship traffic disturbance. It identified red-throated loon, black guillemot, Arctic loon and velvet scoter as the most vulnerable species to ship disturbance, and common and Arctic tern as the least vulnerable. The shyest seabird species also had the highest escape costs and seemed to be the most vulnerable.
The strong reactions of several species to disturbance by ships have important management implications. Because most human activity at sea involves vessel movements, their effects should be considered more comprehensively in environmental impact assessments and conservation planning. Our DVI can be used to inform marine spatial planning, conservation management, and impact assessments to identify and prioritize the most vulnerable areas in a practical way: The values given here can be multiplied by rasterized species abundances and then summed over all species in a raster cell to produce vulnerability maps (see Garthe and Hüppop, 2004; Sonntag et al., 2012 for examples). Following this, a possible management solution would be to implement low-disturbance or disturbance-free zones in some marine protected areas (von Nordheim et al., 2006). An alternative option in other vulnerable areas could involve the spatial and temporal coordination of ship traffic, which might be especially relevant in areas where ship traffic has increased dramatically as a result of the construction and maintenance of offshore wind turbines. The displacement of sensitive seabirds from wind farm areas has been shown to be a combined effect of the wind farm itself and the associated ship traffic, but these effects are difficult to separate (Mendel et al., 2019). Our study demonstrated strong reactions to ships alone, emphasizing the importance of minimizing wind farm-associated ship traffic in order to achieve an environmentally friendly transition to the use of renewable energies. Vulnerability maps produced using our DVI can be applied in this context to identify the most suitable corridors.
Ship traffic might also have to be included in single-species action plans as a relevant threat to declining seabird species identified as most vulnerable to ship traffic disturbance (see Wildfowl and Wetlands Trust, 2015 for an example of a species action plan under AEWA). The mean flush distances for such species reported in this study can be used to develop species-specific conservation measures, such as setback distances from important feeding and resting sites. In this context, the setback distances should be longer than the mean escape distances in order to minimize the behavioral and physiological effects of disturbance. Setback distances might also be necessary with respect to human activities in the vicinity of marine protected areas to conserve their proposed function as refuges for vulnerable species.
Clearly, the effects of disturbance events by ships are cumulative and equate to net habitat loss (see also Madsen and Fox, 1995). If regulation of ship traffic is applied as a management tool, threshold levels are needed above which species’ abundance will be significantly reduced. The results of this study highlight the fact that these thresholds will be species-specific, and need to be investigated further.
Ethics Statement
Data collection was carried out within the German Marine Biodiversity Monitoring and did thus not require approval of an ethics committee.
Author Contributions
KF and SG contributed to conception and design of the study. PS developed the methodology for measuring escape distances. NM and SG organized the database. KB, KF, NG, and NM conducted field work and recorded data. KF performed the analysis and wrote the first draft of the manuscript. KB, NG, NM, PS, and SG provided critical revision and discussions, and corrected the manuscript. All authors contributed to manuscript revision, and read and approved the submitted version.
Funding
Data collection and parts of the manuscript preparation were carried out within the German Marine Biodiversity Monitoring and the Project “Fachbeitrag Naturschutz zur maritimen Raumordnung” (FABENA; FKZ 3515 82 0600), both funded by the Federal Agency for Nature Conservation (BfN). We acknowledge financial support by Land Schleswig-Holstein within the funding programme Open Access Publikationsfonds.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank our colleagues and collaborators R. Borrmann, J. Buddemeier, D. Cimiotti, F. Güpner, J. Kottsieper, H. Lemke, and E. Rickert for support with data collection during Seabirds at Sea counts. We are grateful to the Alfred-Wegener-Institut (AWI), the University of Hamburg, and the Helmholtz-Zentrum Geesthacht (HZG) for the opportunity to participate in research cruises for data collection. We thank all the crew members of the research vessels used. We also thank S. Furness for providing language support and the reviewers for improving the manuscript.
References
Alerstam, T., Rosén, M., Bäckman, J., Ericson, P. G. P., and Hellgren, O. (2007). Flight speeds among bird species: allometric and phylogenetic effects. PLoS Biol. 5:e197. doi: 10.1371/journal.pbio.0050197
Bahlke, C. (2017). Wadden Sea Quality Status Report 2017: Harbours and Shipping. Last Updated 21.12.2017. Wilhelmshaven: Common Wadden Sea Secretariat.
Beale, C. M. (2007). The behavioral ecology of disturbance responses. Int. J. Comp. Psychol. 20, 111–120.
Beale, C. M., and Monaghan, P. (2004a). Behavioural responses to human disturbance: a matter of choice? Anim. Behav. 68, 1065–1069. doi: 10.1016/j.anbehav.2004.07.002
Beale, C. M., and Monaghan, P. (2004b). Human disturbance: people as predation-free predators? J. Appl. Ecol. 41, 335–343. doi: 10.1111/j.0021-8901.2004.00900.x
Béchet, A., Giroux, J.-F., and Gauthier, G. (2004). The effects of disturbance on behaviour, habitat use and energy of spring staging snow geese. J. Appl. Ecol. 41, 689–700. doi: 10.1111/j.0021-8901.2004.00928.x
Bélanger, L., and Bédard, J. (1989). Responses of staging greater snow geese to human disturbance. J. Wildl. Manag. 53, 713–719. doi: 10.2307/3809202
Bélanger, L., and Bédard, J. (1990). Energetic cost of man-induced disturbance to staging snow geese. J. Wildl. Manag. 54, 36–41. doi: 10.2307/3808897
Bellebaum, J., Diederichs, A., Kube, J., Schulz, A., and Nehls, G. (2006). Flucht-und meidedistanzen überwinternder seetaucher und meeresenten gegenüber Schiffen auf see. Ornithol. Rundbr. Mecklenbg. 45, 86–90.
BirdLife International (2017). European Birds of Conservation Concern: Populations, Trends and National Responsibilities. Cambridge: BirdLife International.
Blumstein, D. T. (2006). Developing an evolutionary ecology of fear: how life history and natural history traits affect disturbance tolerance in birds. Anim. Behav. 71, 389–399. doi: 10.1016/j.anbehav.2005.05.010
Blumstein, D. T., Anthony, L. L., Harcourt, R., and Ross, G. (2003). Testing a key assumption of wildlife buffer zones: is flight initiation distance a species-specific trait? Biol. Conserv. 110, 97–100. doi: 10.1016/S0006-3207(02)00180-5
Blumstein, D. T., Fernández-Juricic, E., Zollner, P. A., and Garity, S. C. (2005). Inter-specific variation in avian responses to human disturbance. J. Appl. Ecol. 42, 943–953. doi: 10.1111/j.1365-2664.2005.01071.x
Bonenfant, M., and Kramer, D. L. (1996). The influence of distance to burrow on flight initiation distance in the woodchuck, Marmota monax. Behav. Ecol. 7, 299–303. doi: 10.1093/beheco/7.3.299
Borkenhagen, K., Corman, A.-M., and Garthe, S. (2017). Estimating flight heights of seabirds using optical rangefinders and GPS data loggers: a methodological comparison. Mar. Biol. 165:17. doi: 10.1007/s00227-017-3273-z
Bradbury, G., Trinder, M., Furness, B., Banks, A. N., Caldow, R. W. G., and Hume, D. (2014). Mapping seabird sensitivity to offshore wind farms. PLoS One 9:e106366. doi: 10.1371/journal.pone.0106366
Burger, J., and Gochfeld, M. (1991). Human distance and birds: tolerance and response distances of resident and migrant species in India. Environ. Conserv. 18, 158–165. doi: 10.1017/S0376892900021743
Camphuysen, C. J. (1998). Beached bird surveys indicate decline in chronic oil pollution in the North Sea. Mar. Pollut. Bull. 36, 519–526. doi: 10.1016/S0025-326X(98)80018-0
Camphuysen, C. J., and Garthe, S. (2004). Recording foraging seabirds at sea: standardised recording and coding of foraging behaviour and multi-species foraging associations. Atl. Seab. 6, 1–32.
Camphuysen, C. J., Lavaleye, M. S. S., and Leopold, M. F. (1999). Vogels, Zeezoogdieren en Macrobenthos bij het Zoekgebied voor Gaswinning in Mijnbouwvak Q4 (Noordzee). Texel: Netherlands Institute for Sea Research.
Carney, K. M., and Sydeman, W. J. (1999). A review of human disturbance effects on nesting colonial waterbirds. Waterbirds Int. J. Waterbird Biol. 22, 68–79. doi: 10.2307/1521995
Cockrem, J. F. (2007). Stress, corticosterone responses and avian personalities. J. Ornithol. 148, 169–178. doi: 10.1007/s10336-007-0175-8
Ecorys, Deltares, and OceaniC Development (2012). Blue Growth: Scenarios and Drivers for Sustainable Growth from the Oceans, Seas and Coasts: Maritime Sub-Function Profile Report Short Sea Shipping (1.2). Available at: https://webgate.ec.europa.eu/maritimeforum/en/node/2946 (accessed November 18, 2018).
Elliott, K. H., Ricklefs, R. E., Gaston, A. J., Hatch, S. A., Speakman, J. R., and Davoren, G. K. (2013). High flight costs, but low dive costs, in auks support the biomechanical hypothesis for flightlessness in penguins. Proc. Natl. Acad. Sci. U.S.A. 110, 9380–9384. doi: 10.1073/pnas.1304838110
Fernández-Juricic, E., Blumstein, D. T., Abrica, G., Manriquez, L., Adams, L. B., Adams, R., et al. (2006). Relationships of anti-predator escape and post-escape responses with body mass and morphology: a comparative avian study. Evol. Ecol. Res. 8, 731–752.
Fowler, G. S. (1999). Behavioral and hormonal responses of Magellanic penguins (Spheniscus magellanicus) to tourism and nest site visitation. Biol. Conserv. 90, 143–149. doi: 10.1016/S0006-3207(99)00026-9
Frid, A., and Dill, L. (2002). Human-caused disturbance stimuli as a form of predation risk. Conserv. Ecol. 6:11. doi: 10.5751/ES-00404-060111
Fridell, E., Boteler, B., Tröltzsch, J., Winnes, H., Parsmo, R., Kowalczyk, U., et al. (2015). Future Scenarios. Deliverable 1.4. BONUS Research Project Sustainable Shipping and Environment of the Baltic Sea Region (SHEBA). Available at: https://www.sheba-project.eu/imperia/md/content/sheba/deliverables/sheba_d1.4_final.pdf (accessed November 18, 2018).
Garthe, S., and Hüppop, O. (1994). Distribution of ship-following seabirds and their utilization of discards in the North Sea in summer. Mar. Ecol. Prog. Ser. 106, 1–9. doi: 10.3354/meps106001
Garthe, S., and Hüppop, O. (2004). Scaling possible adverse effects of marine wind farms on seabirds: developing and applying a vulnerability index. J. Appl. Ecol. 41, 724–734. doi: 10.1111/j.0021-8901.2004.00918.x
Garthe, S., Hüppop, O., and Weichler, T. (2002). Anleitung zur erfassung von seevögeln auf see von schiffen. Seevögel 23, 47–55.
Garthe, S., Markones, N., Hüppop, O., and Adler, S. (2009). Effects of hydrographic and meteorological factors on seasonal seabird abundance in the southern North Sea. Mar. Ecol. Prog. Ser. 391, 243–255. doi: 10.3354/meps08170
Garthe, S., Schwemmer, P., Paiva, V. H., Corman, A.-M., Fock, H. O., Voigt, C. C., et al. (2016). Terrestrial and marine foraging strategies of an opportunistic seabird species breeding in the Wadden Sea. PLoS One 11:e0159630. doi: 10.1371/journal.pone.0159630
Garthe, S., Sonntag, N., Schwemmer, P., and Dierschke, V. (2007). Estimation of seabird numbers in the German North Sea throughout the annual cycle and their biogeographic importance. Vogelwelt 128, 163–178.
Gill, J. A. (2007). Approaches to measuring the effects of human disturbance on birds: measuring the effects of human disturbance on birds. Ibis 149, 9–14. doi: 10.1111/j.1474-919X.2007.00642.x
Gill, J. A., Norris, K., and Sutherland, W. J. (2001). Why behavioural responses may not reflect the population consequences of human disturbance. Biol. Conserv. 97, 265–268. doi: 10.1016/S0006-3207(00)00002-1
Gill, J. A., Sutherland, W. J., and Watkinson, A. R. (1996). A Method to quantify the effects of human disturbance on animal populations. J. Appl. Ecol. 33, 786–792. doi: 10.2307/2404948
Glover, H. K., Guay, P.-J., and Weston, M. A. (2015). Up the creek with a paddle; avian flight distances from canoes versus walkers. Wetl. Ecol. Manag. 23, 775–778. doi: 10.1007/s11273-015-9411-9
Goss-Custard, J. D., Burton, N. H. K., Clark, N. A., Ferns, P. N., McGrorty, S., Reading Christopher, J., et al. (2006a). Test of a behavior-based individual-based model: response of shorebird mortality to habitat loss. Ecol. Appl. 16, 2215–2222.
Goss-Custard, J. D., Triplet, P., Sueur, F., and West, A. D. (2006b). Critical thresholds of disturbance by people and raptors in foraging wading birds. Biol. Conserv. 127, 88–97. doi: 10.1016/j.biocon.2005.07.015
Goss-Custard, J. D., Caldow, R. W. T., Clarke, R. T., Durell, S., Urfi, J., and West, Y. D. (1995a). Consequences of habitat loss and change to populations of wintering migratory birds: predicting the local and global effects from studies of individuals. Ibis 137, S56–S66. doi: 10.1111/j.1474-919X.1995.tb08458.x
Goss-Custard, J. D., Clarke, R. T., Briggs, K. B., Ens, B. J., Exo, K.-M., Smit, C., et al. (1995b). Population consequences of winter habitat loss in a migratory shorebird. I. Estimating model parameters. J. Appl. Ecol. 32, 320–336. doi: 10.2307/2405099
Green, J. A. (2011). The heart rate method for estimating metabolic rate: review and recommendations. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 158, 287–304. doi: 10.1016/j.cbpa.2010.09.011
Greenewalt, C. H. (1962). Dimensional relationships for flying animals. Smithson. Misc. Collect. 144, 1–46.
Guay, P.-J., McLeod, E. M., Cross, R., Formby, A. J., Maldonado, S. P., Stafford-Bell, R. E., et al. (2013). Observer effects occur when estimating alert but not flight-initiation distances. Wildl. Res. 40, 289–293. doi: 10.1071/WR13013
Guillemette, M. (1994). Digestive-Rate constraint in wintering common eiders (Somateria mollissima): implications for flying capabilities. Auk 111, 900–909. doi: 10.2307/4088822
Heinemann, D. (1981). A range finder for pelagic bird censusing. J. Wildl. Manag. 45, 489–493. doi: 10.2307/3807930
HELCOM (2018). State of the Baltic Sea – Second HELCOM Holistic Assessment 2011-2016. Baltic Sea Environment Proceedings 155. Available at: www.helcom.fi/baltic-sea-trends/holistic-assessments/state-of-the-baltic-sea-2018/reports-and-materials/ (accessed September 25, 2018).
Hirschfeld, A., and Attard, G. (2017). Vogeljagd in Europa -analyse von abschusszahlen und auswirkungen der jagd auf den erhalt bedrohter vogelarten. Ber. Vogelschutz 53, 15–42.
Hirschfeld, A., and Heyd, A. (2005). Mortality of migratory birds caused by hunting in Europe: bag statistics and proposals for the conservation of birds and animal welfare. Ber. Vogelschutz 42, 47–74.
Horswill, C., and Robinson, R. A. (2015). Review of Seabird Demographic Rates and Density Dependence. JNCC Report No. 552. Peterborough: Joint Nature Conservation Committee.
Kaiser, M. J., Galanidi, M., Showler, D. A., Elliott, A. J., Caldow, R. W. G., Rees, E. I. S., et al. (2006). Distribution and behaviour of Common Scoter Melanitta nigra relative to prey resources and environmental parameters. Ibis 148, 110–128. doi: 10.1111/j.1474-919X.2006.00517.x
Kelsey, E. C., Felis, J. J., Czapanskiy, M., Pereksta, D. M., and Adams, J. (2018). Collision and displacement vulnerability to offshore wind energy infrastructure among marine birds of the Pacific Outer Continental Shelf. J. Environ. Manage. 227, 229–247. doi: 10.1016/j.jenvman.2018.08.051
Knapton, R. W., Petrie, S. A., and Herring, G. (2000). Human disturbance of diving ducks on Long Point Bay, Lake Erie. Wildl. Soc. Bull. 28, 923–930.
Krüger, T. (2016). On the effects of kitesurfing on waterbirds – a review. Inf. Naturschutz Niedersachs. 1, 3–66.
Laursen, K., and Frikke, J. (2008). Hunting from motorboats displaces Wadden Sea eiders Somateria mollissima from their favoured feeding distribution. Wildl. Biol. 14, 423–433. doi: 10.2981/0909-6396-14.4.423
Lima, S. L., and Dill, L. M. (1990). Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640. doi: 10.1139/z90-092
Madsen, J. (1985). Impact of disturbance on field utilization of pink-footed geese in West Jutland, Denmark. Biol. Conserv. 33, 53–63. doi: 10.1016/0006-3207(85)90004-7
Madsen, J. (1995). Impacts of disturbance on migratory waterfowl. Ibis 137, S67–S74. doi: 10.1111/j.1474-919X.1995.tb08459.x
Madsen, J. (1998). Experimental refuges for migratory waterfowl in Danish wetlands. II. Tests of hunting disturbance effects. J. Appl. Ecol. 35, 398–417. doi: 10.1046/j.1365-2664.1998.00315.x
Madsen, J., and Fox, A. D. (1995). Impacts of hunting disturbance on waterbirds - a review. Wildl. Biol. 1, 193–207. doi: 10.2981/wlb.1995.0025
Markones, N., Guse, N., Borkenhagen, K., Schwemmer, H., and Garthe, S. (2015). Seevogel - Monitoring 2014 in der Deutschen. Vilm: AWZ von Nord - und Ostsee.
Markones, N., Schwemmer, H., and Garthe, S. (2013). Seevogel-Monitoring 2011 / 2012 in der Deutschen. Vilm: AWZ von Nord- und Ostsee.
Matczak, M. (2018). QUO VADIS Exploring the Future of Shipping in the Baltic Sea. Turku: Baltic LINes.
May, R., Masden, E. A., Bennet, F., and Perron, M. (2019). Considerations for upscaling individual effects of wind energy development towards population-level impacts on wildlife. J. Environ. Manage. 230, 84–93. doi: 10.1016/j.jenvman.2018.09.062
Mayhew, P. W. (1988). The daily energy intake of European wigeon in winter. Ornis Scand. 19, 217–223. doi: 10.2307/3676562
McFadden, T. N., Herrera, A. G., and Navedo, J. G. (2017). Waterbird responses to regular passage of a birdwatching tour boat: implications for wetland management. J. Nat. Conserv. 40, 42–48. doi: 10.1016/j.jnc.2017.09.004
McHuron, E. A., Schwarz, L. K., Costa, D. P., and Mangel, M. (2018). A state-dependent model for assessing the population consequences of disturbance on income-breeding mammals. Ecol. Model. 385, 133–144. doi: 10.1016/j.ecolmodel.2018.07.016
Mendel, B., Schwemmer, P., Peschko, V., Müller, S., Schwemmer, H., Mercker, M., et al. (2019). Operational offshore wind farms and associated ship traffic cause profound changes in distribution patterns of Loons (Gavia spp.). J. Environ. Manage. 231, 429–438. doi: 10.1016/j.jenvman.2018.10.053
Mendel, B., Sonntag, N., Schwemmer, P., Dries, H., Guse, N., Müller, S., et al. (2008). Profiles of Seabirds and Waterbirds of the German North and Baltic Seas. Distribution, Ecology and Sensitivities to Human Activities within the Marine Enviroment. Bonn: Bundesamt für Naturschutz.
Merkel, F. R., Mosbech, A., and Riget, F. (2009). Common Eider (Somateria mollissima) feeding activity and the influence of human disturbances. Ardea 97, 99–107. doi: 10.5253/078.097.0112
Møller, A. P. (2008). Flight distance and population trends in European breeding birds. Behav. Ecol. 19, 1095–1102. doi: 10.1093/beheco/arn103
Møller, A. P., Samia, D. S. M., Weston, M. A., Guay, P.-J., and Blumstein, D. T. (2014). American exceptionalism: population trends and flight initiation distances in birds from three continents. PLoS One 9:e107883. doi: 10.1371/journal.pone.0107883
Mori, Y., Sodhi, N. S., Kawanishi, S., and Yamagishi, S. (2001). The effect of human disturbance and flock composition on the flight distances of waterfowl species. J. Ethol. 19, 115–119. doi: 10.1007/s101640170007
Nimon, A. J., Schroter, R. C., and Stonehouse, B. (1995). Heart rate of disturbed penguins. Nature 374:415. doi: 10.1038/374415a0
Norberg, U. M. (1996). “Energetics of flight,” in Avian Energetics and Nutritional Ecology, ed. C. Carey (Boston, MA: Springer), 199–249. doi: 10.1007/978-1-4613-0425-8_7
Nudds, R. L., and Bryant, D. M. (2000). The energetic cost of short flights in birds. J. Exp. Biol. 203, 1561–1572.
R Core Team (2016). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rodgers, J. A., and Schwikert, S. T. (2002). Buffer-zone distances to protect foraging and loafing waterbirds from disturbance by personal watercraft and outboard-powered boats. Conserv. Biol. 16, 216–224. doi: 10.1046/j.1523-1739.2002.00316.x
Rodgers, J. A., and Smith, H. T. (1995). Set-back distances to protect nesting bird colonies from human disturbance in Florida. Conserv. Biol. 9, 89–99. doi: 10.1046/j.1523-1739.1995.09010089.x
Ronconi, R. A., and Clair, C. C. S. (2002). Management options to reduce boat disturbance on foraging black guillemots (Cepphus grylle) in the Bay of Fundy. Biol. Conserv. 108, 265–271. doi: 10.1016/S0006-3207(02)00126-X
Samia, D. S. M., Nakagawa, S., Nomura, F., Rangel, T. F., and Blumstein, D. T. (2015). Increased tolerance to humans among disturbed wildlife. Nat. Commun. 6:8877. doi: 10.1038/ncomms9877
Schwemmer, P., Mendel, B., Sonntag, N., Dierschke, V., and Garthe, S. (2011). Effects of ship traffic on seabirds in offshore waters: implications for marine conservation and spatial planning. Ecol. Appl. 21, 1851–1860. doi: 10.1890/10-0615.1
Smit, C. J., and Visser, G. J. M. (1993). Effects of disturbance on shorebirds: a summary of existing knowledge from the Dutch Wadden Sea and Delta area. Wader Study Group Bull. 68, 6–19.
Sonntag, N., Schwemmer, H., Fock, H. O., Bellebaum, J., and Garthe, S. (2012). Seabirds, set-nets, and conservation management: assessment of conflict potential and vulnerability of birds to bycatch in gillnets. ICES J. Mar. Sci. 69, 578–589. doi: 10.1093/icesjms/fss030
Steven, R., Pickering, C., and Guy Castley, J. (2011). A review of the impacts of nature based recreation on birds. J. Environ. Manage. 92, 2287–2294. doi: 10.1016/j.jenvman.2011.05.005
Sutherland, W. J. (1998). The effect of local change in habitat quality on populations of migratory species. J. Appl. Ecol. 35, 418–421. doi: 10.1046/j.1365-2664.1998.00320.x
Swennen, C., Leopold, M. F., and De Bruijn, L. L. M. (1989). Time-stressed oystercatchers, Haematopus ostralegus, can increase their intake rate. Anim. Behav. 38, 8–22. doi: 10.1016/S0003-3472(89)80061-2
Sæther, B.-E., and Bakke, Ø. (2000). Avian life history variation and contribution of demographic traits to the population growth rate. Ecology 81, 642–653. doi: 10.2307/177366
Tasker, M. L., Jones, P. H., Dixon, T. I. M., and Blake, B. F. (1984). Counting seabirds at sea from ships: a review of methods employed and a suggestion for a standardized approach. Auk 101, 567–577.
Thaxter, C., and Burton, N. (2009). High Definition Imagery for Surveying Seabirds and Marine Mammals: A Review of Recent Trials and Development of Protocols. Thetford: British Trust for Ornithology.
Thaxter, C. B., Wanless, S., Daunt, F., Harris, M. P., Benvenuti, S., Watanuki, Y., et al. (2010). Influence of wing loading on the trade-off between pursuit-diving and flight in common guillemots and razorbills. J. Exp. Biol. 213, 1018–1025. doi: 10.1242/jeb.037390
Thiel, M., Nehls, G., Bräger, S., and Meissner, J. (1992). The impact of boating on the distribution of seals and moulting ducks in the Wadden Sea of Schleswig-Holstein. Neth. Inst. Sea Res Publ. Ser. 20, 221–233.
Urfi, A. J., Goss-Custard, J. D., Lev, S. E. A., and Durell, D. (1996). The ability of oystercatchers Haematopus ostralegus to compensate for lost feeding time: field studies on individually marked birds. J. Appl. Ecol. 33, 873–883. doi: 10.2307/2404958
von Nordheim, H., Boedeker, D., and Krause, J. C. (2006). Progress in Marine Conservation in Europe: Natura 2000 Sites in German Offshore Waters. Berlin: Springer-Verlag. doi: 10.1007/3-540-33291-X
Walker, B. G., Boersma, P. D., and Wingfield, J. C. (2006). Habituation of adult Magellanic Penguins to human visitation as expressed through behavior and corticosterone secretion. Conserv. Biol. 20, 146–154. doi: 10.1111/j.1523-1739.2005.00271.x
Webb, A., and Durinck, J. (1992). “Counting birds from ship,” in Manual for Aeroplane and Ship Surveys of Waterfowl and Seabirds, eds J. Komdeur, J. Bertelsen, and G. Cracknell (Slimbridge: IWRB Special Publication),24–37.
Weimerskirch, H., Prudor, A., and Schull, Q. (2017). Flights of drones over sub-Antarctic seabirds show species- and status-specific behavioural and physiological responses. Polar Biol. 41, 259–266. doi: 10.1007/s00300-017-2187-z
Weston, M. A., McLeod, E. M., Blumstein, D. T., and Guay, P.-J. (2012). A review of flight-initiation distances and their application to managing disturbance to Australian birds. Emu Austral Ornithol. 112, 269–286. doi: 10.1071/MU12026
Wetlands International (2018). Waterbird Population Estimates. Available at: wpe.wetlands.org (accessed November 1, 2018).
Wickham, H. (2016). ggplot2: Elegant Graphics for Data Analysis. New York, NY: Springer-Verlag. doi: 10.1007/978-3-319-24277-4
Wildfowl and Wetlands Trust (2015). International Single Species Action Plan for the Conservation of the Long-tailed Duck Clangula hyemalis. Available at: https://www.unep-aewa.org/sites/default/files/publication/aewa_ts57_issap_ltd.pdf (accessed March 5, 2019).
Williams, J. M., Tasker, M. L., Carter, I. C., and Webb, A. (1995). A method of assessing seabird vulnerability to surface pollutants. Ibis 137, S147–S152. doi: 10.1111/j.1474-919X.1995.tb08435.x
Wisniewska, D. M., Johnson, M., Teilmann, J., Rojano-Doñate, L., Shearer, J., Sveegaard, S., et al. (2016). Ultra-high foraging rates of Harbor porpoises make them vulnerable to anthropogenic disturbance. Curr. Biol. 26, 1441–1446. doi: 10.1016/j.cub.2016.03.069
Keywords: seabirds, ship traffic, disturbance, behavior, escape distance, vulnerability index, risk-disturbance hypothesis, marine spatial planning
Citation: Fliessbach KL, Borkenhagen K, Guse N, Markones N, Schwemmer P and Garthe S (2019) A Ship Traffic Disturbance Vulnerability Index for Northwest European Seabirds as a Tool for Marine Spatial Planning. Front. Mar. Sci. 6:192. doi: 10.3389/fmars.2019.00192
Received: 29 January 2019; Accepted: 26 March 2019;
Published: 11 April 2019.
Edited by:
Jessica Redfern, Southwest Fisheries Science Center (NOAA), United StatesReviewed by:
Zachary Adam Schakner, United States Army Corps of Engineers, United StatesBob Furness, University of Glasgow, United Kingdom
Copyright © 2019 Fliessbach, Borkenhagen, Guse, Markones, Schwemmer and Garthe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katharina Leonia Fliessbach, ZmxpZXNzYmFjaEBmdHotd2VzdC51bmkta2llbC5kZQ==
 Katharina Leonia Fliessbach
Katharina Leonia Fliessbach Kai Borkenhagen
Kai Borkenhagen Philipp Schwemmer
Philipp Schwemmer