- 1Monterey Bay Aquarium Research Institute, Moss Landing, CA, United States
- 2Marine Laboratory, University of Guam, Mangilao, GU, United States
Several species of small, red, deep-sea Trachymedusae have been described and then re-described over the past 20 years, leading to some confusion in the scientific literature. This paper provides an overview of three genera (Benthocodon, Crossota, and Pectis) in the family Rhopalonematidae (Cnidaria: Hydrozoa) that have been observed and examined both in the field and in the laboratory. Twenty years of in situ observations in Monterey Bay indicate that two of the genera, Benthocodon and Pectis, are often associated with the benthic boundary layer and can occur in dense patches. They have been observed resting on soft sediments with their subumbrellar surface down but are also found swimming up to several 100 m above the bottom. Individuals in the genus Crossota tend to be solitary and more pelagic in nature. Although Crossota may be found near the bottom as well, down to depths of 4,000 m, they have not been observed resting on the bottom. The three genera are morphologically similar and difficult to distinguish from each other. As a group, they are small (<5 cm) and sometimes darkly pigmented, making in situ identifications challenging. We show that these three genera can be differentiated morphologically and we provide a key to the genera and species common in Monterey Bay. Further, the genera differ in their depth distribution and behavior. Molecular genetics suggest that the genera and species are distinct from each other but that their taxonomy needs revision. This paper reviews the generic characteristics along with species identifications and provides images and video (Supplementary Material) that may be helpful in identification.
Introduction
The Trachymedusae are a diverse group of jellyfish, most of which live below the euphotic zone (Mayer, 1910). In Monterey Bay, California, they can be found in the water column all the way to the ocean floor at 4,000 m. This marks both the deepest extent of the Monterey Submarine Canyon as well as the depth limitation for the remotely operated vehicles (ROVs) used in this study. Three genera (Pectis Haeckel, 1879; Benthocodon Larson and Harbison, 1990; and Crossota Vanhöffen, 1902) in the family Rhopalonematidae are commonly reddish in hue, similar in size, and have been difficult to distinguish from one another. The most common species: Benthocodon pedunculata (Bigelow, 1913) and Pectis profundicola (Naumov, 1971) are benthopelagic and, although patchy, have been recorded in numbers up to 80/m2 (Larson et al., 1992). They often swim in short bursts, usually followed by bouts of sinking (Larson and Harbison, 1990; Larson et al., 1992; Matsumoto et al., 1997). Crossota species are typically found singly in the water column (Thuesen, 2003). Previous observations from human occupied vehicles and remotely operated vehicles consisted mostly of comments about “little red jellies” and the occasional specimen collection. Based on their abundance and mobility, these genera likely represent an ecologically important group (Alldredge, 1984). A similar conclusion was reached by Thuesen and Childress (1994) based on their physiological research which indicated that the metabolism of some deep-sea medusae is close to that of deep-sea fishes and crustaceans.
Representatives of Benthocodon, Crossota, and Pectis are abundant in the deep waters off Monterey and we present a morphological key and a molecular phylogenetic analysis that facilitates identification of each genus and species common to Monterey Bay. Lastly, we summarize field and laboratory observations that provide a better understanding of the natural history of these deep-sea jellies.
Materials and Methods
Field Observations and Collection
Observations and collections were made using remotely operated vehicles from the Monterey Bay Aquarium Research Institute (MBARI) (Robison et al., 2017). The ROV Ventana is depth rated to 1,850 m. ROV Tiburon was, and ROV Doc Ricketts is, depth rated to 4,000 m (http://www.mbari.org/at-sea/vehicles/). High definition video cameras are mounted on these vehicles and the video signal is conveyed to the surface support vessel (ROV Ventana—R/V Point Lobos and R/V Rachel Carson; ROV Tiburon and ROV Doc Ricketts—R/V Western Flier) through optical fibers at the core of the ROV's tether. At the surface, the video signal is viewed on a high-resolution monitor and was recorded initially on digital BetaCam tape, then MBARI switched to a High Definition camera system and recorded on High Definition tape, and we are now recording the video digitally. The observer's comments and descriptions are recorded on the audio track of the recording. Environmental data (depth, location, temperature, dissolved oxygen, and salinity) for each dive are collected and, after each dive, this information is integrated into a database. These data and all frame grabs from video images collected by our ROVs, are annotated into a comprehensive relational database (http://www.mbari.org/vars) that is available to the public.
Specimens were collected in 6.5 l “detritus” samplers, designed for the gentle capture of delicate material in midwater (Youngbluth, 1984), or in a “suction” sampler consisting of a transparent funnel and 2 m of flexible tubing leading back to 12 separate 6-l collection cylinders within the ROV toolsled. For the detritus samplers, the ROV pilot positions the vehicle so that the open cylinder of the sampler encloses the medusa, then the doors at either end are gently closed by hydraulic rams. For the suction sampler, the funnel, attached to a clear plexiglass tube and flexible tubing, can be extended in front of the ROV (https://www.mbari.org/high-frequency-suction-samplers/). When a medusa is near the wide opening of the funnel, the suction pump is turned on and the animal is gently collected and deposited into a sample container.
Molecular Genetic Data
Tissue samples for molecular analysis were collected with the ROVs and either frozen at −80°C or pressed onto Whatman FTA cards (Sigma-Aldrich, St. Louis, MO). Genomic DNA was extracted from frozen tissue samples using a modified CTAB-DTAB protocol (Gustinich et al., 1991). Approximately 300 μl of tissue was placed into a 2 ml Eppendorf tube and 600 μl of “blood lysis buffer” (8% DTAB, 1.5 M NaCl, 100 mM Tris-Cl, pH 8.6, 50 mM EDTA) was added. The tissue was incubated at 68°C for 5 min and then 900 μl of chloroform were added to deproteinize the tissue. The tube was inverted 2–3 times and then spun at 10,000 rpm for 2 min. The supernatant was removed (discarding the lower portion and being careful to avoid the tissue detritus at the interface of phases) and DNA was precipitated from the supernatant by adding 900 μl of ultrapure water and 100 μl of a 5% CTAB solution that contained 0.4 M NaCl. This was followed by gentle inversion of the tube and centrifugation at 10,000 rpm for 2 min. The resulting pellet was resuspended in 300 μl 1.2 M NaCl and 750 μl EtOH were added, the sample was gently mixed and spun at 10,000 rpm for 10 min to precipitate the DNA again, followed by a rinse with 300 μl 70% EtOH. Additional extractions were performed using the Qiagen DNeasy Blood and Tissue Kit following the manufacturer's protocol for animal tissue extractions with some modifications: after the addition of Buffer AL, 200 μl of cold isopropanol was added to each sample. In addition, two elutions were done on each sample for a total of 70 μl genomic DNA. Genomic DNA was also obtained from FTA paper following the manufacturer's protocol. The resulting pellets were resuspended in 50 μl of ultrapure water; 1–2 μl of genomic DNA were used for either 30 or 10 μl PCR reactions.
The nuclear large subunit ribosomal RNA (LSU or 28S) gene was amplified (initial denaturation at 94°C for 2 min; 30 cycles at 94°C for 30 s, 55°C for 30 s, and 72°C for 70 s; final elongation at 72°C for 7 min) using slightly modified (LSUD1F ACCCGCTGAATTTAAGCATA; D3Ca ACGAACGATTTGCACGTCAG or LSUD4Ra AACCAGCTACTAGRYGGTTCGAT) universal primers (Scholin and Anderson, 1994). The PCR product was either sequenced directly from a gel cut or cloned into TOPO TA vector. In both cases, the results from multiple PCR reactions or plasmid DNAs from 2 to 5 individual clones were sequenced individually and as a pool with the SequiTherm EXCEL II Long-Read DNA sequencing kit (Epicenter Technologies) and analyzed on a LI-COR 4200 IR2 instrument. Some of the clones were also sequenced with the BigDye Terminator v.2 sequencing kit and analyzed on an ABI 3100 instrument.
The nuclear small subunit ribosomal RNA (SSU or 18S) gene was amplified using the modified universal primers mitchA and mitchB from (4 cycles of 94°C for 60 s, 58°C for 60 s, 72°C for 2 min, followed by 29 cycles of 94°C for 60 s, 64°C for 60 s, 72°C for 90 s; Medlin et al., 1988). All products were bi-directionally sequenced using BigDye® Terminator v3.1 Cycle Sequencing Kit on an ABI 3100 or ABI 3500 sequencer (Applied Biosystems, Foster City, CA, USA).
Two mitochondrial genes were amplified and sequenced, the mitochondrial cytochrome oxidase one (COI) gene and the gene encoding the large RNA subunit of the mitochondrial ribosome (16S). COI were amplified using the forward primer of Leray et al. (2013) and the Folmer et al. (1994) reverse primer (16 cycles at 94°C for 10 s, starting annealing temperature of 62°C for 30 s that was lowered by 1°C during each subsequent cycle, and 68°C for 60 s, followed by 25 cycles of 94°C for 10 s, 46°C for 30 s, and 68°C for 60 s with a final elongation of 72°C for 10 min). 16S sequences were generated following the protocols described in Bentlage et al. (2018).
Phylogenetic Analyses
All sequences were assembled in Geneious (various versions; Biomatters Limited, NZ, RRID:SCR_010519) and a dataset was compiled that comprised newly generated and previously published sequences of Trachymedusae and Narcomedusae (Table 1 and Figure 1). Individual loci were aligned using MAFTT (v7.205; Katoh and Standley, 2013). Unreliably aligned positions were identified and excluded using Gblocks (Talavera and Castresana, 2007, RRID:SCR_015945), as implemented in the alignment viewer Seaview (version 4; Gouy et al., 2010, RRID:SCR_015059), allowing for smaller blocks, gap positions, and less strict flanking positions. The four resulting alignments were concatenated using the catfasta2phyml script available from https://github.com/nylander/catfasta2phyml.
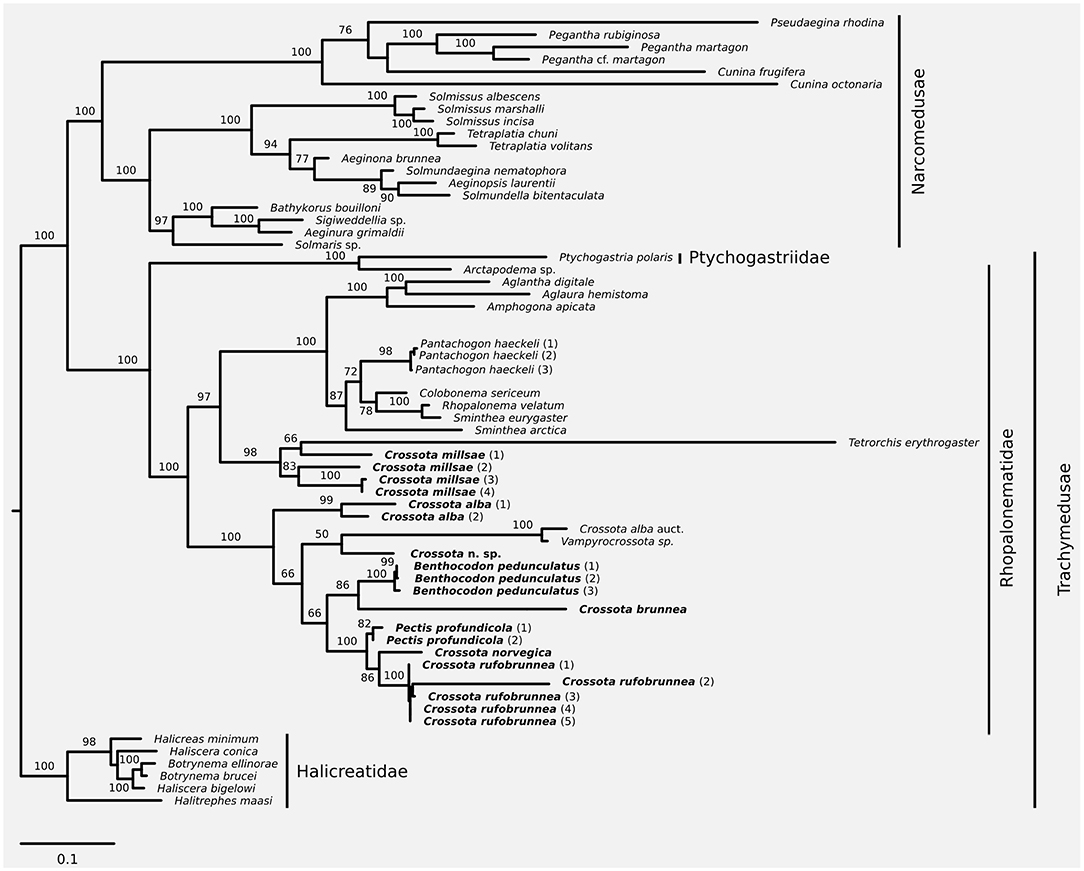
Figure 1. Maximum likelihood phylogeny based on a combined dataset of nuclear 18S and 28S plus mitochondrial 16S and COI. Taxa discussed in this contribution in bold font. Node support was inferred using 1,000 non-parametric bootstrap replicates. Substitution model for phylogenetic reconstruction and bootstrapping was GTR+I+G; scale bar represents substitutions per site.
Partitionfinder (version 1.1.0; Lanfear et al., 2012) was used to identify the appropriate substitution models and best partitioning scheme for inferring phylogenetic relationships from the concatenated alignment; partitioning schemes were evaluated and ranked using the Bayesian information criterion (Bic). Partitioning schemes tested were the following: (1) all loci evolve under the same model (no partitioning), (2) all loci evolve under a different model of evolution (separate 16S, COI, 18S, and 28S partitions), (3) mitochondrial (16S and COI) and nuclear (18S and 28S) loci evolve under a different model, (4) nuclear loci (18S and 28S) evolve under one model while the mitochondrial loci (16S and COI) can be best modeled by separate models, and (5) mitochondrial loci (16S and COI) evolve under one model while the nuclear loci (18S and 28S) can be best modeled separately. The best partitioning scheme was then encoded in RAxML (version 8.2.7; Stamatakis, 2014, RRID:SCR_006086) to infer the maximum likelihood phylogeny. Robustness of the phylogeny was evaluated using 1,000 non-parametric bootstrap replicates using RAxML's rapid bootstrapping algorithm (-fa-m GTRGAMMAI-p $RANDOM-x $RANDOM-# 1000). Following the phylogenetic framework presented in Bentlage et al. (2018), the resulting phylogeny was rooted on the trachymedusan family Halicreatidae.
Results
Phylogeny and Taxonomy
We have observed, collected, and sequenced several specimens of Pectis profundicola previously identified incorrectly as Benthocodon pedunculata (Larson et al., 1992; Matsumoto et al., 1997) and as Voragonema pedunculata (Bouillon et al., 2001; Lindsay and Pagès, 2010), several species of Crossota, and Benthocodon pedunculatus (see discussion for the change in the species name from B. pedunculata to B. pedunculatus).
We increased taxon sampling compared to the recent phylogeny of Bentlage et al. (2018) with respect to Crossota, Benthocodon, and Pectis, and provide information to help discriminate between the three genera while expanding on the taxa and sampling in recent work (Bentlage et al., 2018). We support the earlier finding (Bentlage et al., 2018) that Crossota is not a monophyletic clade (Figure 1) with Crossota millsae (Thuesen, 2003) being most closely related to Tetrorchis erythrogaster (Bigelow, 1909). Note that C. millsae is paraphyletic with respect to Tetrorchis, albeit at low bootstrap support. Overall, the remainder of the genus Crossota, other than C. millsae, were paraphyletic with respect to Benthocodon, Pectis, and Vampyrocrossota. Crossota is separated according to nominal species, supporting their distinction based on morphology (Figures 2, 3). A putatively undescribed species (Crossota n. sp.—Figure 3) photographed and collected in the Gulf of California appears to be the closest relative of Vampyrocrossota and a specimen from Bentlage et al. (2018) that appears to have been misidentified (Crossota alba auct). Benthocodon and Pectis, the other two genera of interest in this contribution are nested firmly within a clade of Crossota species but some nodes remain poorly supported. Monophyly of Benthocodon and Pectis cannot be confirmed or rejected until additional species in these genera have been sampled.
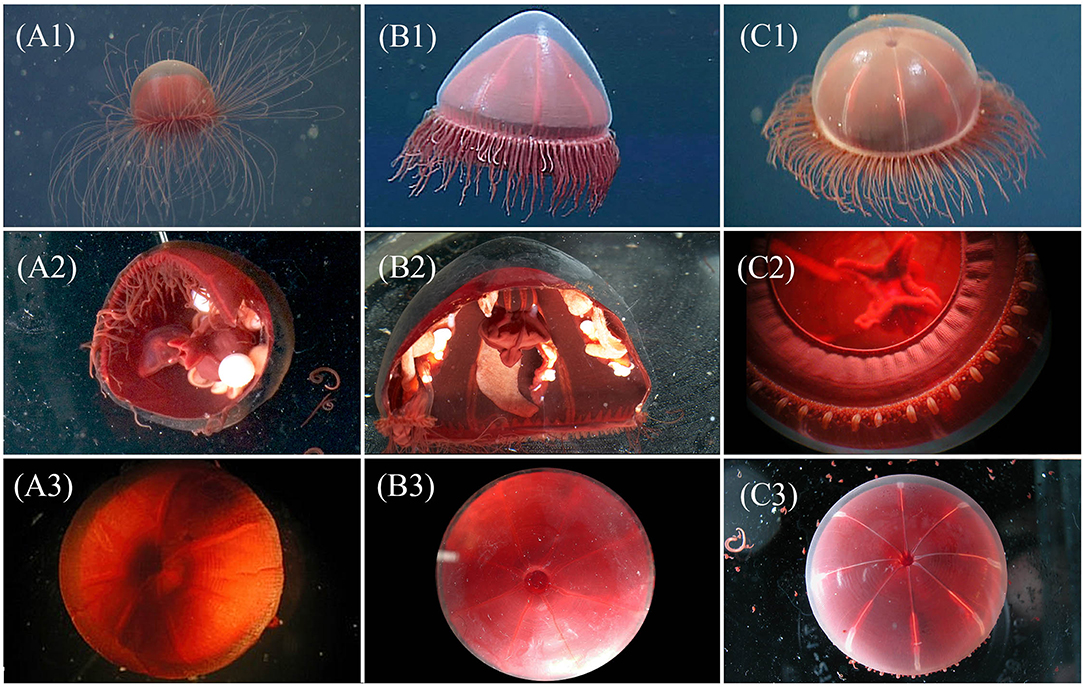
Figure 2. Visual comparison for three genera of “little red jellies” provided for assistance with identification of genera, each image is a unique specimen: Crossota (A1-3), Pectis (B1-3), and Benthocodon (C1-3). Top row, left to right: in situ images for C. rufobrunnea (A1—DR457 @ 968 m in Monterey Bay), P. profundicola (B1—T622 @ 777 m in Monterey Bay), and B. pedunculatus (C1—T1113 @ 3,483 m in Monterey Bay, California) taken by MBARI ROVs. Of the three genera, only Pectis (B1) has centripetal canals and this individual has 8 in each quadrant, irregular in size and just above the canal ring. Row 2 (A2,B2,C2) provides closeup and partial dissections taken in lab for different individuals of C. rufobrunnea (A2—T1023SS1 @ 955 m in Astoria Canyon, Oregon), P. profundicola (B2—T1023SS2 @ 1,117 m in Astoria Canyon, Oregon), and B. pedunculatus (C2—T623b @ 3,607 m in Monterey Bay, California). Note the pendant gonads and absent peduncle of Crossota (A2), and the semi-pendant gonads and short peduncle for Pectis (B2). Benthocodon (C2) shows the folded, per-radial lips, tentacular arrangement, and well-developed velum. See Figure 4 for a better view of the peduncle for which Benthocodon pedunculatus is named. Row 3 (A3,B3,C3) provides a dorsal view of the subumbrella region. The subumbrella for C. rufobrunnea (A3—T766 @ 1,375 m in Monterey Bay, California), unlike both P. profundicola (B3—T834SS9 @ 1,384 m in Monterey Bay, California), and B. pedunculatus (C3—T835SS5 @ 3,285 m in Monterey Bay, California), appears closed and does not allow a view through clear mesoglea into the peduncle.
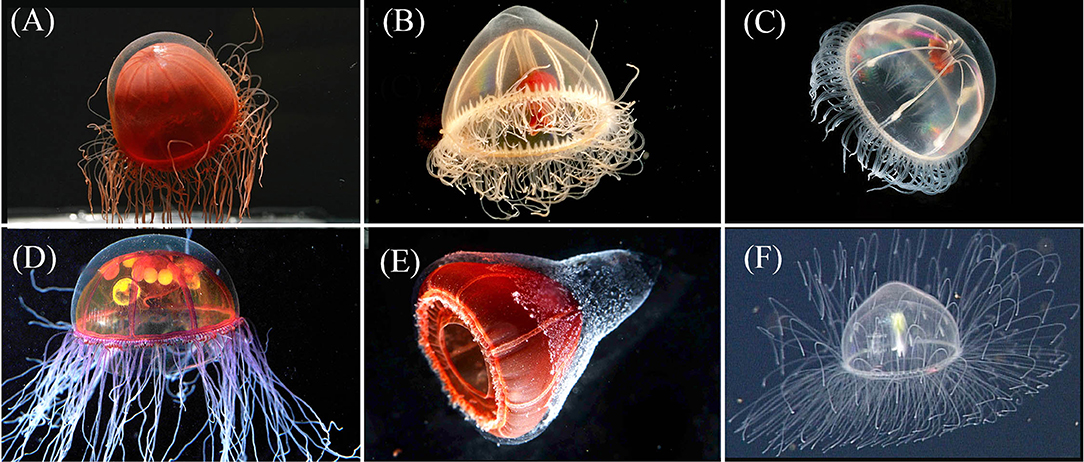
Figure 3. Row 1: The exumbrella of Crossota norvegica [(A)—photo by K. Raskoff from 1,595 m in the Chukchi Basin, Arctic] appears more transparent than its congeners. Pectis tatsunoko [(B)—T1024SS11 from 1,028 m in Astoria Canyon, Oregon] has eight centripetal canals, often irregular in height along each quadrant and are more hemispherical in shape than Benthocodon hyalinus [(C)—photo by K. Raskoff, from 635 m along the Northwind Ridge, Arctic]. Note the presence of a mostly clear peduncle and red lips in both P. tatsunoko and B. hyalinus. Row 2 provides images of two additional species of Crossota that we have observed and were included in the molecular analysis: Crossota millsae [(D)—DR88 @ 2,115 m in Monterey Bay], Crossota n. sp. [(E)—DR726 @ 2,292 m in Mazatlan Basin, Gulf of California], and one that was not included in the molecular analysis Crossota alba [(F)—DR723 @ 2,600 m in Farallon Basin, Gulf of California]. Specimens (A) and (C) were collected in the Arctic *(Raskoff et al., 2010).
Vertical Distribution and Behavior
Over 20 years of in situ observations in Monterey Bay have revealed that Crossota, Benthocodon, and Pectis exhibit distinct depth distributions.
All species of Crossota that we observed found in the water column at depths from 500 to 4,000 m and were never observed on the bottom, although they have been seen swimming in the benthic boundary layer. Crossota swims with a series of strong pulsing contractions, usually followed by a period of quiescence.
Benthocodon species are generally found in deeper waters (>2,500 m) although the type specimen (Crossota pedunculata) was collected in <100 m of water. While Benthocodon hyalinus is found in the water column or near the bottom, Benthocodon pedunculatus is typically found drifting above the sediment (up to 100 m from the bottom) or either on or just above the bottom (see Supplemental Video). Those Benthocodon pedunculatus specimens that are not on the sediment, generally pulse consistently but weakly every few seconds (see Supplemental Video). They are negatively buoyant and sink between pulses, thus maintaining a somewhat constant position on or just above the bottom. They are capable of stronger bouts of swimming, but these tend to be followed by longer sinking/rest periods. Abundance of these medusae was as high as 11 per square meter (based on video transects—Figure 4).
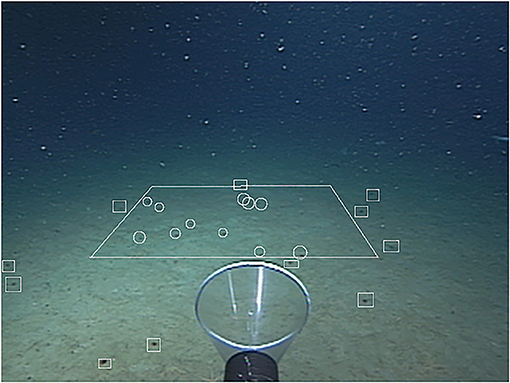
Figure 4. A one square meter quadrat superimposed on video from the ROV Tiburon (04/15/2000, dive #132) to illustrate the abundance of Benthocodon pedunculatus. Medusae within the quadrat (n = 11) are encircled and medusa outside the quadrat have squares drawn around them. Depth is 3,009 m in the Monterey Submarine Canyon.
In Monterey Bay, Pectis specimens can be found throughout the water column with P. tatsunoko in the water column and P. profundicola associated with the benthic boundary layer (Matsumoto et al., 1997) similar to Benthocodon pedunculatus but are usually observed shallower than the latter near the bottom in depths <3,000 m. Pectis profundicola specimens have longer escape bouts of pulsing and shorter rest periods between pulsing bouts than Benthocodon pedunculatus (see Supplemental Video).
Discussion
As more research vehicles spend time in the deeper waters of the ocean collecting specimens and video footage, it is likely that more species within the three genera of Crossota, Benthocodon, and Pectis will be observed and described. This paper is not intended to be a full taxonomic species review of the three genera but is an attempt to provide guidelines for placing initial observations into the appropriate genus as well as provide some preliminary information for those who want to delve deeper into the species within each genus. Full species descriptions will require additional specimen collections and close examination of taxonomic and molecular characters. Nonetheless, phylogeny and imagery provided here demonstrate that the diversity of these taxa is larger than currently described, even in such a well-studied location as the Monterey Submarine Canyon. An issue that remains to be resolved is that the classification of rhopalonematids does not align with its phylogenetic history, as shown by the analyses presented here (Figure 1) and previously (Bentlage et al., 2018).
Naumov (1971) described a new genus and the type species (Voragonema profundicola), based upon a single specimen. He used the presence of prominent centripetal canals as a defining feature rather than the presence of a peduncle. Bouillon et al. (2001) noted that (Naumov, 1971) original description of Voragonema was incomplete, with some of the characters based solely on the holotype illustration that lacked detail. To address this issue, Bouillon et al. (2001) redescribed the genus Voragonema and described a new species, V. laciniata. Galea et al. (2016) proposed that Pectis antarctica (Haeckel, 1879) possesses all of the published characteristics of Voragonema and appears identical to Voragonema laciniata (Bouillon et al., 2001). The genus name Pectis has precedence over Voragonema and, following Galea et al. (2016), Pectis is used in this paper when referring to specimens previously identified as Voragonema. While Pectis pedunculata is in the literature, the type specimen is the same for both this species and Benthocodon pedunculatus and upon examination (by the primary author GIM), the type specimen is lacking centripetal canals and belongs in the genus Benthocodon and not Pectis, therefore Pectis pedunculata cannot be considered as valid.
The genus Crossota was erected in 1902 by Vanhöffen as a trachymedusa with a distinctive dark red coloration, several rows of tentacles, and 8 pendant gonads hanging adjacent to the stomach. Vanhöffen (1902) described two species (C. norvegica and C. brunnea) differentiating C. brunnea (the type species) from C. norvegica by the latter's brighter red coloration. Both Vanhöffen (1902) and Maas (1906) originally grouped Crossota with Ptychogastria (Allman, 1878) due to the arrangement of the tentacles (despite the fact that Crossota lacks the clusters of tentacles and adhesive pads that are characteristic of Ptychogastria). Bigelow (1913) described two new species (C. alba and C. pedunculata) and noted that the presence of a peduncle in these two new species might be sufficient basis to erect a new genus. Ptychogastria has since been placed into the Ptychogastriidae, separate from Crossota (Rhopalonematidae) and the two genera were shown not to be monophyletic (Grange et al., 2017; Bentlage et al., 2018). Ptychogastria can be found in Monterey Bay on both hard and soft sediment with a transparent exumbrella and different colored tentacles making them relatively easy to distinguish from Benthocodon, Pectis, or Crossota.
The third genus of “little red jellies” encountered in the Monterey Submarine Canyon is Benthocodon, a genus described by Larson and Harbison (1990) based upon: (1) the presence of a peduncle, and (2) gonads that are continuous along the upper part of the radial canals with the proximal part of the gonad somewhat detached. Larson and Harbison (1990) erected Benthocodon to accommodate a new species, B. hyalinus described from a single specimen (the type species, Holotype USNM 87603) and a photograph published by Curtsinger (1986) and incorrectly identified as Arctapodema (taken near McMurdo Station Antarctica). Larson and Harbison (1990) also moved Crossota pedunculata (Bigelow, 1913) into the genus Benthocodon along with their newly described species B. hyalinus. Unfortunately, Larson and Harbison (1990) did not match the gender of Benthocodon with the species name upon moving Crossota pedunculata. To match gender (male), the correct spelling is Benthocodon pedunculatus which is how it is referred to in this paper.
At this time, we recognize two species of Pectis (P. profundicola and P. tatsunoko), five species of Crossota (C. alba, C. norvegica, C. millsae, C. brunnea, and C. rufobrunnea), and two Benthocodon species (B. pedunculatus and B. hyalinus) from the west coast of North America and in the Monterey Submarine Canyon in particular. We did not collect enough specimens of either P. tatsunoko or B. hyalinus to include them in the molecular analysis. Two specimens not found in Monterey—Crossota norvegica and a potential new Crossota species collected in the Gulf of California—are included in the phylogeny but Pectis antarctica is not included as we have not had access to specimens. All of the species in this paper can be identified using morphological characters taken from personal observations and existing descriptions. It is possible that further taxonomic work may result in either splitting up the different species of Crossota or combining genera (Pectis, Crossota, Benthocodon, and a fourth genus of a small black jelly-Vampyrocrossota) into a single genus. At this point, we do not have enough information to justify either approach. Consistent identifications will be necessary to interpret the ecological data collected alongside deep-sea jellyfish specimens (cf. Lindsay et al., 2017).
At the generic level, the medusae discussed herein occupy different depth layers of the water column, providing insight into deep-living jelly community structure. Ultimately, accurate taxonomy will give a clearer picture of deep-sea community composition and assembly. For example, gelatinous zooplankton have been shown to be an important component of deep-water food webs as predators and prey (Choy et al., 2017), and clarifying species identities holds potential for further resolving the roles different genera and species play in these food webs (Grange et al., 2017).
The phylogeny presented herein provides further evidence that Crossota is a polyphyletic group. The taxonomic key presented reflects our understanding of the current classification status of “little red jellies” and some of their relatives. It provides an operational framework for identifying these taxa until further taxonomic revisions may provide additional characters and/or a reinterpretation of known characters that align taxonomy with phylogeny in Trachymedusae. Coloration and exumbrellar furrows have previously been used as diagnostic characteristics for species identification of Crossota, Benthocodon, and Pectis but those features need to be validated in the field and post-collection via photographs as both are frequently lost upon collection (Bigelow, 1913; Galea et al., 2016) and coloration is often lost or reduced upon collection (Figure 4) and/or preservation. Crossota specimens sometimes retain the deep red color of their subumbrella upon fixation while both Pectis and Benthocodon specimens often become more faintly colored, the latter to the point of transparency. Consequently, we omitted these coloration and exumbrellar furrows from our key to facilitate the identification of fixed specimens. Both Pectis and Benthocodon have abcission points at the base of the tentacles, Crossota does not.
Key* to the Three Genera (Figure 2)
1a. With distinct centripetal canals (Figures 2B1,B2)…. Pectis
1b. Without distinct centripetal canals (Figures 2A1,C1)…… 2
2a. With subumbrella appearing to have a distinct ’hole’ that leads down to peduncle (Figure 2C3)……….Benthocodon
2b. With subumbrella lacking a ’hole-like’ invagination at the apex (Figures 2A3,B3)…………………………Crossota
Generic Diagnosis
Genus Pectis (Haeckel, 1879)
Pectis (Haeckel, 1879): 266.
Voragonema (Naumov, 1971): 13
Rhopalonematidae with a thick, hemispherical bell. Eight pendant gonads, medusa buds may be present, centripetal canals, and a gastric peduncle. Tentacles in multiple rows and while capable of autotomization, many tentacles remain after collection.
Valid Species
• Pectis antarctica (Haeckel, 1879) [Galea et al. (2016) considers Voragonema laciniata (Bouillon et al., 2001) as a junior synonym]—type species, 11–13 triangular centripetal canals but usually 12; 1,000–1,200 tentacles arranged in 5–6 rows; gonads wide, folded, thick-walled and slightly pendant distally (Haeckel, 1881, p. 5; Figure 2); does have a peduncle—Not included in this study as we have no specimens and have not observed this species in Monterey Bay.
• Pectis profundicola (Naumov, 1971). Original description based on trawled specimen from 6,800 to 8,700 m); Eight conical centripetal canals; ~500 tentacles; gonads unknown; does have a short peduncle; brown manubrium; and clear umbrella. Our specimens differ from the original described species by the latter having a clear subumbrella. We believe that the transparent exumbrella from the original description is likely a collection artifact as some of the preserved specimens that we have collected have lost their pigmentation and in situ images reveal that they did have pigmentation prior to collection (Figures 3, 5). Otherwise, our specimens match the incomplete description provided by Naumov (1971) although we have found specimens with more tentacles (1000–2000) and with irregular centripetal canals as well as specimens with seven, eight, and nine centripetal canals.
• Pectis tatsunoko (Lindsay and Pagès, 2010). Nine triangular centripetal canals originating from the ring canal; ~1,050 tentacles; 6–7 rows of tentacles, rose-orange-colored subumbrella. The coloration of the umbrella and tentacles is not maintained upon preservation. This species was not included in our molecular analysis as we did not have access to enough specimens.
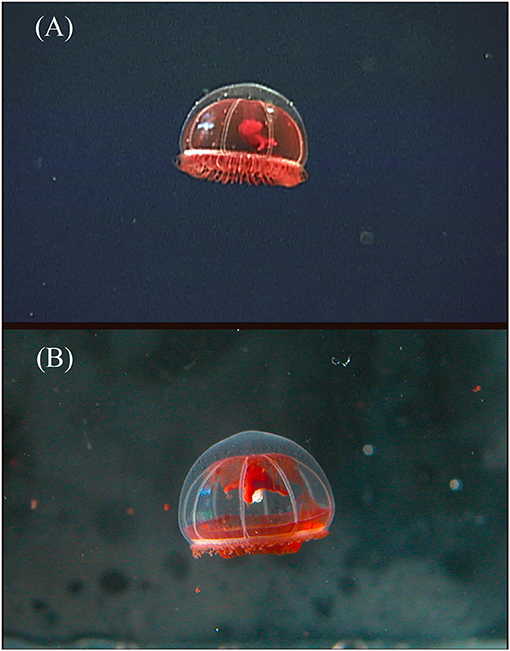
Figure 5. Benthocodon pedunculatus collected on ROV Tiburon dive #836 at a depth of 3,419 m in Monterey Bay, California in 2005. The two photos are of the same specimen, (A) is in situ and (B) is in the laboratory taken just after return to the surface. The red subumbrella had completely disappeared by the time the animal was preserved. This animal survived collection but has autotomized its outer ring of tentacles. The normally long manubrium has also retracted by at least 50% in length between the top and bottom image and the peduncle is harder to observe as well.
Species Key for Pectis
1a. Eight centripetal canals present ……………P. profundicola
1b. More than eight centripetal canals present………………2
2a. More than ten rounded centripetal canals………………………………………………………P. antarctica
2b. Less than 10 pointed centripetal canals…………………… ………………………………………………P. tatsunoko
Genus Crossota (Vanhöffen, 1902)
Crossota (Vanhöffen, 1902): 72.
Rhopalonematidae with a hemispherical bell. Eight tubular or pendant gonads, medusa buds may be present. No centripetal canals. Tentacles in single or multiple rows and while capable of autonomization, many tentacles remain after collection.
Valid Species
• Crossota brunnea (Vanhöffen, 1902) Type species, multiple rows of tentacles (up to 84 tentacles/octant observed); gonads hanging from radial canals next to manubrium, still immature in specimens 17 mm in diameter.
• Crossota norvegica (Vanhöffen, 1902) synonymized with C. brunnea by Bigelow (1913)—C. brunnea var. norvegica. Fewer than 600 tentacles (up to 43/octant observed); eight pendant gonads from near the manubrium and as long as the manubrium in specimens 15–20 mm in diameter; umbrella deep reddish-brown.
• Crossota rufobrunnea (Kramp, 1913)—medusa buds (Theusen, personal communication); 250+ tentacles in one row; eight pendant gonads hanging from radial canals next to short peduncle; umbrella reddish brown.
• Crossota alba (Bigelow, 1913)—~179 tentacles in three rows; eight sausage shaped hanging gonads midway between apex and ring canal; medusa buds not yet observed; long unpigmented peduncle; manubrium darkly pigmented (chocolate-brown to black); subumbrella unpigmented.
• Crossota millsae (Thuesen, 1993)—up to 220 tentacles in one row with clear abscission zones; eight sausage shaped pendant gonads hang from ~1/3 of the way down radial canal from apex; medusa buds sometimes present; no peduncle; bright pink to lavender color to manubrium and gonads; mesoglea clear; umbrella may have burnt-tangerine pigmentation.
Species Key for Crossota
1a. Gonads midway along radial canal; umbrella colorless………………………………………….C. alba
1b. Gonads located nearer manubrium than ring canal, umbrella pigmented………………………………………………2
2a. Gonads located approximately halfway between base of manubrium and mid-point of radial canal, umbrella with burnt-tangerine pigment……………………….C. millsae
2b. Gonads near base of manubrium, coloration not burnt-tangerine………………………………………………3
3a. With one row of 200–250 tentacles …………………………C. rufobrunnea (umbrella deep reddish brown)
3b. With multiple rows of tentacles…………………………4
4a. With 600 or more tentacles in several rows; umbrella pale brown, immature at 17 mm diameter………………………………………………………………C. brunnea
4b. Less than 600 tentacles, umbrella bright red/brown, mature at 18 mm in diameter…………………………………….C. norvegica
Genus Benthocodon (Larson and Harbison, 1990)
Benthocodon (Larson and Harbison, 1990): 22.
Rhopalonematidae with a thick, hemispherical bell. Eight linear to sinuous, flattened, partially pendant gonads occur on the eight radial canals; not known to have medusa buds; centripetal canals absent; manubrium with a well-developed gastric peduncle; tentacles numerous (about 800). Tentacles in multiple rows that tend to autotomize upon collection.
Valid Species
• Benthocodon pedunculatus Type species was first described as Crossota pedunculata (Bigelow, 1913) (Holotype USNM 31057). It was then reclassified as B. pedunculata (Larson and Harbison, 1990). Benthocodon pedunculatus lacks centripetal canals; short peduncle; 8 sausage shaped gonads only attached to radial canals for a short distance and mostly hanging distally; 3–4 rows of tentacles ~350 tentacles; reddish brown pigmentation on subumbrella. Has a deeper depth distribution than Pectis profundicola (down to at least 4,000 m in Monterey Bay). Dense aggregations often observed.
• Benthocodon hyalinus (Larson and Harbison, 1990). Peduncle ¼ or more the length of the manubrium; eight linear to sinuous gonads attached along most of the radial canals becoming pendant distally; lacks pigmentation on the subumbrella, and the gonads extend along most of the length of the radial canals, only at their distal ends are the 8 linear gonads pendant; more than 800 tentacles of different sizes but one type. Reaches up to 4 cm in diameter and has been observed in moderately dense aggregations just above the bottom. This species was not included in the molecular analysis due to lack of sufficient specimens for DNA extraction.
Data Availability Statement
Accession numbers (in bold) in Table 1 represent sequences generated as part of this study. Specific sequence data can be found in GenBank using the provided accession number.
Ethics Statement
All specimens were collected under valid US Scientific Collecting Permit guidelines and restrictions.
Author Contributions
GM, RS, and BR contributed to the conception and design of the study as well as the collection and identification of specimens. GM, KW, and BB performed the DNA extractions. BB performed the phylogenetic analysis. All authors contributed to the manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful for the work of many others who have contributed to this paper (Francesc Pages, Kim Reisenbichler, Chuck Baxter, Esther Chen, Joanna Tenant, Lou Zeidberg, Steve Haddock, and Lynne Christiansen) as well as the crews and pilots of MBARI's research vessels (R/V Point Lobos, R/V Rachel Carson, R/V Western Flier, and the remotely operated vehicles Ventana, Tiburon, and Doc Ricketts). Additionally, BB was supported by the Smithsonian Institution and Guam EPSCoR (National Science Foundation grant number OIA-1457769) and the MBARI staff were supported by the generous support of the David and Lucile Packard Foundation to the Monterey Bay Aquarium Research Institute. Comments and suggestions by three reviewers have greatly enhanced the clarity and content of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2019.00798/full#supplementary-material
Supplemental Video. Four supplemental videos are provided. Benthocodon pendunculatus filmed in the water column on ROV Tiburon dive #837; Benthocodon pedunculatus filmed just above the seafloor during ROV Tiburon dive #894; Pectis profundicola filmed in the water column from ROV Ventana dive #4176; and Crossota millsae filmed in the water column during ROV Doc Ricketts dive #1049. Depths and identifications are on each video clip. All clips were filmed in Monterey Bay, California.
References
Alldredge, A. L. (1984). “The quantitative significance of gelatinous zooplankton as pelagic consumers,” in Flows of Energy and Materials in Marine Ecosystems, ed M. J. R. Fasham (Boston, MA: Plenum Publishing Corporation), 407–433.
Allman, G. J. (1878). “Hydrozoa,” in G. S. Nares, Narrative of a Voyage to the Polar Sea During 1875–76, in H.M.S. Ships Alert and Discovery. With Notes on the Natural History, Vol. 2, ed H. W. Feilden, (London: Sampson Low, Marston, Searle, and Rivington), 290–292.
Bentlage, B., Osborn, K. J., Lindsay, D. J., Hopcroft, R. R., and Collins, A. G. (2018). Loss of metagenesis and evolution of a parasitic life style in a group of open-ocean jellyfish. Mol. Phylogenet. Evol. 124, 50–59. doi: 10.1016/j.ympev.2018.02.030
Bigelow, H. B. (1909). “The Medusae,” in Reports on the Scientific Results of the Expedition to the Eastern Tropical pacific, in charge of Alexander Agassiz, by the U. S. Fish Commission steamer “Albatross” from October, 1904, to March, 1905. XVI. The Medusae. Mem. Mus. Comp. Zoology Harv. Coll. 37, 1–243, plates 1–48. Available online at: http://www.biodiversitylibrary.org/item/30084.
Bigelow, H. B. (1913). “Medusae and Siphonophorae collected by the U.S. Fisheries steamer “Albatross” in the Northwestern Pacific, 1906,” in Proceedings of the United States National Museum, Vol. 44, 1–119. Available online at: https://repository.si.edu/handle/10088/14430
Bouillon, J., Pages, F., and Gili, J. M. (2001). New species of benthopelagic hydromedusae from the Weddell Sea. Polar Biol. 24, 839–845. doi: 10.1007/s003000100289
Choy, C. A., Haddock, S. H. D., and Robison, B. H. (2017). Deep pelagic food web structure as revealed by in situ feeding observations. Proc. Biol. Sci. 284, 20172116. doi: 10.1098/rspb.2017.2116
Folmer, O., Black, M., Hoeh, W., Lutz, R., and Vrijenhoek, R. (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrate. Mol. Mar. Biol. Biotechnol. 3, 294–299.
Galea, H. R., Roder, C., Walcher, C., Wamuth, M., Kohlberg, E., and Fischer, P. F. (2016). Glaciambulata neumayeri gen. et sp. nov., a new Antarctic trachymedusa (Cnidaria: Hydrozoa), with a revision of the family Ptychogastridae. Eur. J. Taxon. 252, 1–30. doi: 10.5852/ejt.2016.252
Gouy, M., Guindon, S., and Gascuel, O. (2010). SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 27, 221–224. doi: 10.1093/molbev/msp259
Grange, L. J., Smith, C. R., Lindsay, D. J., Bentlage, B., and Youngbluth, M. J. (2017). High abundance of the epibenthic Trachymedusa Ptychogastria polaris Allman, 1878 (Hydrozoa, Trachylina) in Subpolar Fjords along the West Antarctic Peninsula. PLoS ONE. 12:e0168648. doi: 10.1371/journal.pone.0168648
Gustinich, S., Manfioletti, G., Del Sal, G., Schneider, C., and Carninci, P. (1991). A fast method for high-quality genomic DNA extraction from whole human blood. BioTech. 11, 298–301.
Haeckel, E. (1879). Das System der Medusen. Erster Teil einer Monographie der Medusen. Denkschriften der Medicinisch-Naturwissenschaftlichen Gesellschaft zu Jena. 1, XX+1–360, 320 plates. Available online at: http://www.biodiversitylibrary.org/item/101461.
Haeckel, E. (1881). Monographie der Medusen. Zweiter Theil. Erste Hälfte: Die Tiefsee-Medusen der Challenger-Reise. Zweite Hälfte: Der Organismus der Medusen. Jena: Gustav Fischer. Available online at: https://www.biodiversitylibrary.org/item/101236#page/36/mode/1up
Katoh, K., and Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. doi: 10.1093/molbev/mst010
Kramp, P. L. (1913). Medusae collected by the “Tjalfe” Expedition (in Greenland waters). Videnskabelige Meddelelser fra Dansk naturhistorisk Forening i Kjøbenhavn. 65, 257–286. Available online at: https://biodiversitylibrary.org/page/36397336
Lanfear, R., Calcott, B., Ho, S. Y. W., and Guindon, S. (2012). PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 29, 1695–1701. doi: 10.1093/molbev/mss020
Larson, R. J., and Harbison, G. R. (1990). Medusae from McMurdo Sound, Ross Sea including the descriptions of two new species, Leuckartiara brownie and Benthocodon hyalinus. Polar Biol. 11, 19–25. doi: 10.1007/BF00236517
Larson, R. J., Matsumoto, G. I., Madin, L. P., and Lewis, L. M. (1992). Deep-sea benthic and benthopelagic medusae, recent observations from submersibles and a remotely operated vehicle. Bull. Mar. Sci. 51, 277–286.
Leray, M., Yang, J. Y., Meyer, C. P., Mills, S. C., Agudelo, N., Ranwez, V., et al. (2013). A new versatile primer set targeting a short fragment of the mitochondrial COI region for metabarcoding metazoan diversity: application for characterizing coral reef fish gut contents. Front. Zool. 10, 34. doi: 10.1186/1742-9994-10-34
Lindsay, D., and Pagès, F. (2010). Voragonema tatsunoko (Trachymedusae: Rhopalonematidae), a new species of benthopelagic medusa, host to the hyperiid amphipod Mimonectes spandli (Physosomata: Mimonectidae). Zootaxa. 2671, 31–39. doi: 10.5281/zenodo.199149
Lindsay, D. J., Grossman, M. M., Bentlage, B., Collins, A. G., Minemizu, R., Hopcroft, R. R., et al. (2017). The perils of online biogeographic databases: a case study with the ‘monospecific’ genus Aegina (Cnidaria, Hydrozoa, Narcomedusae). Mar. Biol. Res. 13, 494–512. doi: 10.1080/17451000.2016.1268261
Maas, O. (1906). Médusen. Rapports Scientifiques. Résultats du voyage du S.Y. Belgica en 1897–1898–1899. Zoologie. R51, 3–32, pls 1–3. Available online at: http://www.biodiversitylibrary.org/item/18741#page/7/mode/1up.
Matsumoto, G. I., Baxter, C., and Chen, E. H. (1997). Observations of the deep-sea trachymedusa Benthocodon pedunculata. Invert. Biol. 116, 17–25. doi: 10.2307/3226920
Mayer, A. G. (1910). Medusae of the world. Hydromedusae, Vols. I & II. Scyphomedusae, Vol. III. Carnegie Institution, Washington. 735, plates 1–76. Available online at: http://www.biodiversitylibrary.org/item/16794#page/7/mode/1up
Medlin, L., Elwood, H. J., Stickel, S., and Sogin, M. L. (1988). The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 71, 491–499. doi: 10.1016/0378-1119(88)90066-2
Naumov, D. V. (1971). Gydroidnye i stsifoidnye medusy iz Kurilo-Kamchatskogo zhelova (Hydromedusae and Scyphomedusae from the Kurile-Kamchatka trench). Trudy Inst. Okeanol. 92, 9–17.
Raskoff, K. A., Hopcroft, R. R., Kosobokova, K. N., Purcell, J. E., and Youngbluth, M. (2010). Jellies under ice: ROV observations from the Arctic 2005 hidden ocean expedition. Deep Sea Res. II. 57, 111–126. doi: 10.1016/j.dsr2.2009.08.010
Robison, B. H., Reisenbichler, K. R., and Sherlock, R. E. (2017). The coevolution of midwater research and ROV technology at MBARI. Oceanogr. 30, 26–37. doi: 10.5670/oceanog.2017.421
Scholin, C. A., and Anderson, D. M. (1994). Identification of species and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). 1. RFLP analysis of SSU rRNA genes. J. Phycol. 30, 744–754. doi: 10.1111/j.0022-3646.1994.00744.x
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Talavera, G., and Castresana, J. (2007). Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 56, 564–577. doi: 10.1080/10635150701472164
Thuesen, E. V. (1993). Vampyrocrossota childressi, a new genus and species of black medusa from the bathypelagic zone off California (Cnidaria: Trachymedusae: Rhopalonematidae). Proc. Biol. Soc. Wash. 106, 190–194.
Thuesen, E. V. (2003). Crossota millsae (Cnidaria: Trachymedusae: Rhopalonematidae), a new species of viviparous hydromedusa from the deep sea off California and Hawaii. Zootaxa. 309, 1–12. doi: 10.11646/zootaxa.309.1.1
Thuesen, E. V., and Childress, J. J. (1994). Oxygen consumption rates and metabolic enzyme activities of oceanic California medusae in relation to body size and habitat depth. Biol. Bull. 187, 84–98. doi: 10.2307/1542168
Vanhöffen, E. (1902). Die acraspeden Medusen der Deutschen Tiefsee-Expedition 1898–1899. I. Trachymedusen. Wissenschaftliche Ergebnisse der Deutschen Tiefsee Expedition “Valdivia”. 3, 55–86. Available online at: https://www.biodiversitylibrary.org/item/16279#page/7/mode/1up
Keywords: Trachymedusae, Crossota, Pectis, Benthocodon, Monterey Submarine Canyon, ROV, phylogenetics
Citation: Matsumoto GI, Bentlage B, Sherlock R, Walz K and Robison BH (2020) “Little Red Jellies” in Monterey Bay, California (Cnidaria: Hydrozoa: Trachymedusae: Rhopalonematidae). Front. Mar. Sci. 6:798. doi: 10.3389/fmars.2019.00798
Received: 26 March 2019; Accepted: 11 December 2019;
Published: 14 January 2020.
Edited by:
Diva Amon, Natural History Museum, United KingdomReviewed by:
Tina Molodtsova, P.P. Shirshov Institute of Oceanology (RAS), RussiaDale R. Calder, Royal Ontario Museum, Canada
Copyright © 2020 Matsumoto, Bentlage, Sherlock, Walz and Robison. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: George I. Matsumoto, bWFnZUBtYmFyaS5vcmc=
 George I. Matsumoto
George I. Matsumoto Bastian Bentlage
Bastian Bentlage Rob Sherlock
Rob Sherlock Kristine Walz
Kristine Walz Bruce H. Robison
Bruce H. Robison