- 1State Key Laboratory of Marine Pollution, City University of Hong Kong, Kowloon, Hong Kong
- 2Department of Veterinary Clinical Sciences, Jockey Club College of Veterinary Medicine and Life Sciences, City University of Hong Kong, Kowloon, Hong Kong
- 3School of Medical and Health Sciences, Tung Wah College, Kowloon, Hong Kong
On top of conventional necropsy, virtopsy (postmortem computed tomography and postmortem magnetic resonance imaging) has been integrated into the Cetacean Stranding Response Programme in Hong Kong since March 2014. To date, 177 out of 240 local stranded cetaceans have been examined by virtopsy. This integration has modernised the characterisation and documentation of cetacean biological health and profiles, and causes of death. During this 6-year period, critical pitfalls regarding logistics, carcass recovery, handling, and preservation have been identified. A strategic management scheme is crucial for the successful incorporation of virtopsy into this pioneer programme. This study explains the workflow of the Cetacean Virtopsy Stranding Response Programme in Hong Kong waters. Difficulties encountered are highlighted and practical solutions to address management issues are proposed to consolidate the stranding response network.
Introduction
Postmortem (PM) investigation generally involves an invasive “body-opening” autopsy in humans (or necropsy, same procedure in animals). Virtopsy, also known as PM imaging, is the examination of a carcass with modern imaging modalities, including the use of postmortem computed tomography (PMCT) and postmortem magnetic resonance imaging (PMMRI) (Kot et al., 2016) prior to conventional autopsy. Indeed, virtopsy can supplement or even partially replace an autopsy (Dirnhofer et al., 2006). Whole-body PMCT and PMMRI are non-invasive techniques that create volumetric image datasets, while two- and three-dimensional (3D) volumetric reconstruction and rendering are performed with advanced visualisation technology (e.g., multiplanar reconstruction and direct volume rendering). These results allow objective visualisation and recapitulation of PM findings prior to conventional necropsy (Kot et al., 2020a,b).
In human forensic medicine, the correlation between findings of whole-body virtopsy and conventional autopsy was first established in 2003 (Thali et al., 2003). PMCT allows excellent identification of osseous lesions, foreign bodies, pathological gas formation, and organ trauma with higher precision over conventional autopsy (Dirnhofer et al., 2006; Aghayev et al., 2007; Thali et al., 2007). PMMRI has proved to be superior in demonstrating soft tissue injury, neurological and non-neurological organ trauma as well as non-traumatic pathology (Thali et al., 2007). Examination with both PMCT and PMMRI has been recommended since these modalities can effectively complement each other (Roberts et al., 2012; Bagó and Kneissly, 2017).
Virtopsy has been applied extensively in veterinary forensic medicine and proved to be conducive to the ease of operation, reduces the risk of contracting zoonotic diseases, aids in result reproducibility, is convenient for data storage, and gives an opportunity to seek a third opinion (Watson and Heng, 2017). It provides initial or complementary evidence on the cause of death of stranded animals (Chan and Kot, 2017; Kot and Martelli, 2017; Kot et al., 2020a,b) and serves as a guide for veterinary pathologists, improving the accuracy in subsequent diagnosis and facilitating the study on disease surveillance.
A pioneer virtopsy project was implemented in March 2014 to advance the Cetacean Stranding Response Programme in Hong Kong (HK) waters (Kot et al., 2015). The role of virtopsy has become pivotal as veterinarians and personnel of the stranding response team became more aware of its strengths (Tsui and Kot, 2015). Discussions regarding the modification of stranding response protocol to accommodate virtopsy, including concerns over manpower, logistics, equipment, and safety, were followed by the standardisation of virtopsy protocols. Standardised virtopsy protocols and techniques for PM pathological diagnosis were adhered to (Chan et al., 2017; Yuen et al., 2017; Kot et al., 2018a, 2019, 2020a,b,c). Pitfalls encountered were addressed with corrective measures to ensure the structural and practical management of the first Cetacean Virtopsy Stranding Response Programme (CVSRP) in the world. The novel scheme of image data reconstruction methodologies has substantially modernised the characterisation and documentation of injuries and deaths of stranded cetaceans in HK waters.
Government agencies, research scientists, and non-governmental organisations are gradually recognising the benefits of implementing virtopsy into the stranding response workflow. In recent years, delegates from Costa Rica, Japan, Mainland China, New Zealand, Taiwan, Thailand and United States have started their virtopsy trials on stranded cetaceans (Chan et al., 2017). In spite of the emerging popularity, literature on standardised stranding protocols and guidelines were limited (Geraci and Lounsbury, 2005) and a comprehensive review on the routine incorporation of virtopsy into the cetacean stranding response programme was absent. The present study aimed to (1) explain the pioneer practice of CVSRP in HK waters; (2) identify the difficulties and pitfalls encountered; and (3) propose practical solutions for addressing management issues and optimising the entire programme.
Materials and Methods
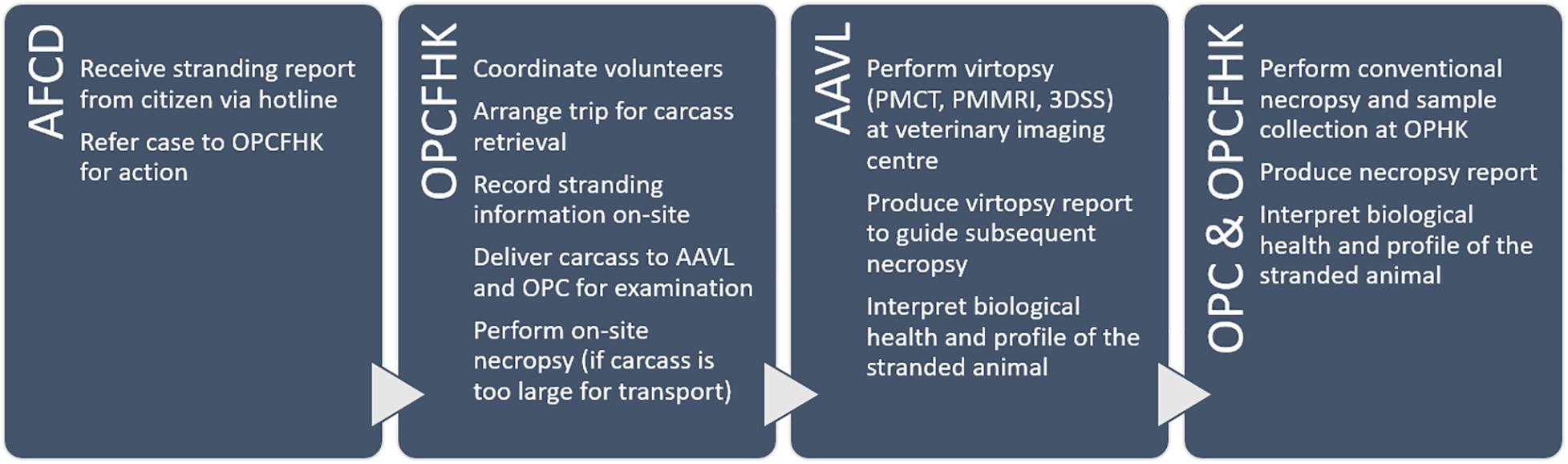
Stranding Response
Cetaceans stranded in HK waters were noted by citizens and reported via telephone hotline (1823). Depending on the location, logistics for carcass recovery were arranged by the Agriculture, Fisheries, and Conservation Department (AFCD). Carcasses were retrieved by the Marine Life Stranding Response Team (MLSRT) from the Ocean Park Conservation Foundation Hong Kong (OPCFHK). On-site information was recorded, including stranding time and location, carcass size, decomposition condition upon recovery, presence of wounds, and entanglements. Decomposition condition was classified using the Smithsonian condition codes: code 1 for live strandings, code 2 for fresh carcasses, code 3 for moderately decomposed carcasses, code 4 for advanced decomposed carcasses, and code 5 for mummified or skeletal remains (Geraci and Lounsbury, 2005). Carcasses were packed in plastic bags, sealed, and transported to the examination facilities for virtopsy by the Aquatic Animal Virtopsy Lab (AAVL) and then for necropsy by Ocean Park Corporation (OPC) and OPCFHK. For carcasses that were incompatible with the scanning modalities (in this study: weight limit of the CT couch was 250 kg; maximum diameter of the gantry aperture was 90 cm) and deemed non-retrievable, on-site necropsy and sample collection were carried out. Head, flippers, and fluke were collected for partial PMCT scans whenever possible. When PM examinations could not be scheduled immediately, carcasses were stored at −20°C until ready.
Virtopsy
AAVL utilised virtopsy techniques to provide insights for the assessment of aquatic animal profiles and biological health locally and globally. The team was in charge of carcass PM imaging, data post-processing (e.g., image reconstruction and rendering), result interpretation, and virtopsy reporting. PMCT was performed on all cases while PMMRI was limited to code 2 and code 3 carcasses. Carcasses were positioned on the examination couches without tilting or bending. Flippers (and dorsal fins, if any) were tightly packed to the body to avoid falling out of the scan’s field of view (Figure 1). PMCT was performed with a Siemens 64-row multi-slice spiral CT scanner Somatom go.Up (Siemens Healthineers, Germary), or a GE Healthcare 16-row multi-slice spiral CT scanner Brivo CT385 (General Electric Company, Wisconsin, United States), or a Toshiba 16-row multi-slice spiral CT scanner Alexion (Toshiba Medical Systems, Japan). Operation of PMCT, an ionising radiological unit, must be performed by a certificated radiological technician or clinician in compliance with the Radiation Ordinance (Cap 303), Laws of HKSAR. PMMRI was performed with a GE SIGNA Explorer 1.5 Tesla MR scanner (General Electric Company, Wisconsin, United States) or a 0.25 Tesla Esaote Vet MR Grande scanner (Esaote Biomedica, Genoa, Italy). Details of scanning parameters may refer to previous publications (Kot et al., 2016, 2018a, 2019, 2020a,b; Yuen et al., 2017). Datasets were saved in DICOM 3.0 format. Data post-processing and evaluation were performed on Aquarius iNtuition workstation (TeraRecon, San Mateo, CA, United States). Diagnosis was made by veterinary radiologists or radiological clinicians who are certificated and experienced to report virtopsy findings.
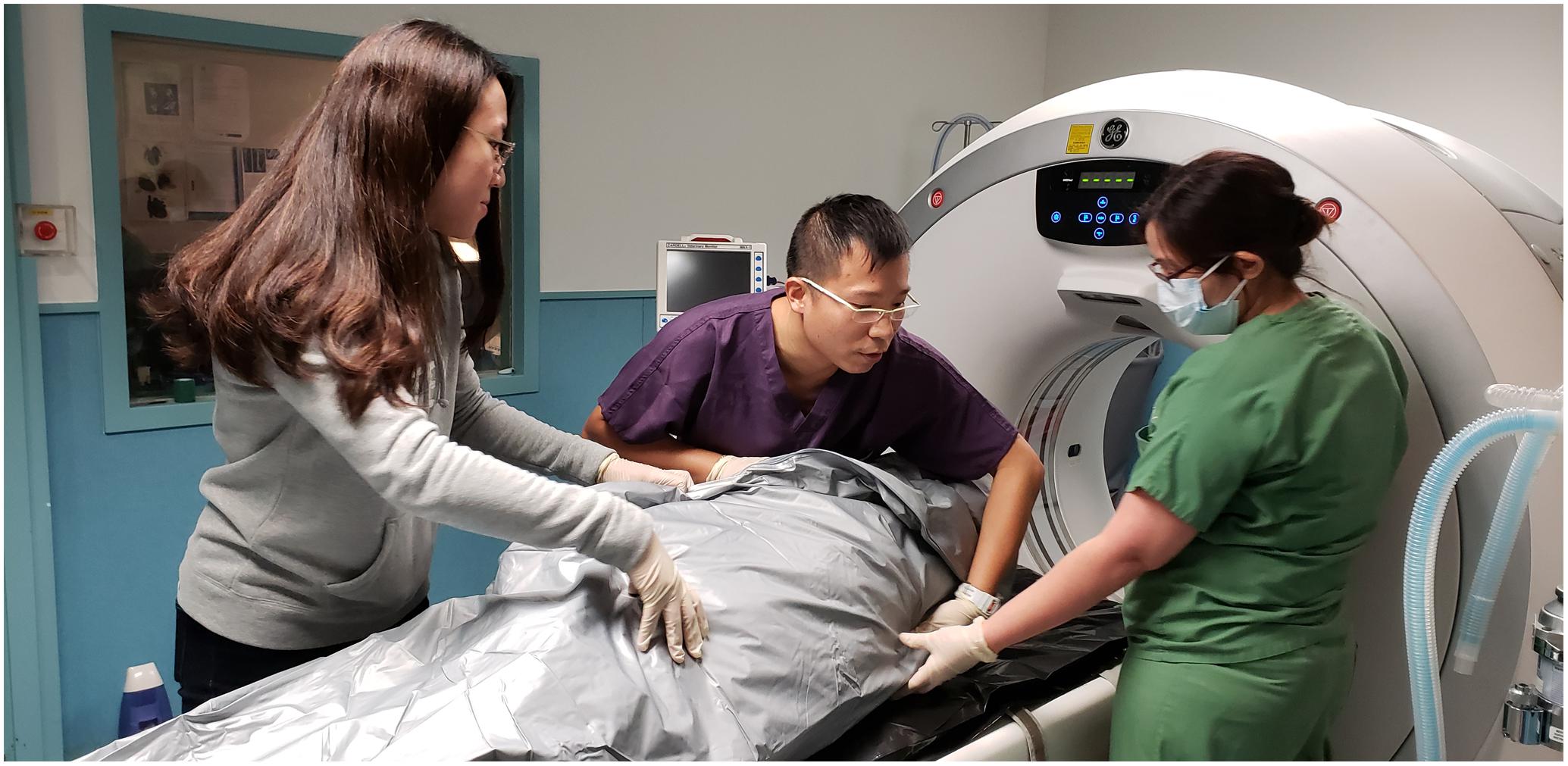
Figure 1. Proper positioning of the carcass in the cadaver bag prior to the PMCT scan is essential, by palpating the flippers and other body landmarks of the carcass.
Conventional Necropsy
After virtopsy, carcasses were transported to OPC for conventional necropsy and sample analysis by in-house veterinarians. In former conventional necropsies, organs were sampled and examined at several cuts to evaluate overall condition. In the pioneer CVSRP, based on the preliminary virtopsy reports, necropsies were guided to any region that showed abnormalities and pathologies in the whole-body PMCT scans. Tiny lesions that could have been missed by random sampling were pinpointed for thorough examination.
All the on-site records, virtopsy results and findings, and necropsy reports were uploaded to a centralised database shared among different parties of the CVSRP. The CVSRP workflow is summarised in Figure 2. All the procedures in this study were reviewed and approved by the AFCD HKSAR (AF GR CON 09/68PT.15).
Results
During a period of 6 years, from 2014 to 2020, 262 cetacean stranding cases were reported in HK waters. A total number of 240 stranded animals were recovered, including 165 Indo-Pacific finless porpoises (Neophocaena phocaenoides) (NP), 53 Indo-Pacific humpback dolphins (Sousa chinensis) (SC), and 22 other species (OT).
Out of the 240 stranded cetaceans recovered, 4 animals (2%) were alive and not retrieved for examination. Virtopsy was performed on 177 carcasses (74%), including 119 NP, 41 SC, and 17 OT. The 59 unscanned carcasses (25%) included 46 NP, 10 SC, and 3 OT that were non-retrievable due to logistic difficulties.
The 177 PMCT scans included 120 whole-body scans (68%) and 57 partial scans (32%) on skulls and flippers in which the carcasses were too large to be entirely retrieved. Eleven out of 177 PMCT scanned carcasses (6%) were also examined by PMMRI. All the virtopsy results were stored and managed under the Cetacean Postmortem Multimedia Analysis Platform, a tailor-made web-based database integrated with a medical viewing system (Chan and Kot, 2017).
Virtopsy provided numerous unique findings that could supplement and might even partially replace conventional necropsy. In this study, atlanto-occipital dissociation was commonly observed in PMCT of the stranded cetaceans (Chan et al., 2017; Kot et al., 2018a). Skeletal anomalies, including osteolytic lesions in vertebra suggestive of spondylolysis or spondylolisthesis, physeal plate fractures or misalignment, and intramedullary bone islands, were also noted in some cases (Kot et al., 2016). These are biological health conditions that could compromise the locomotion of the animals but are easily omitted in routine necropsies. Virtopsy also allowed chronological age estimation by the degree of epiphyseal ossification and closure (Wong et al., 2016), as well as bone mineral density evaluation by quantitative CT, both of which may contribute to the assessment of biological health and profiles.
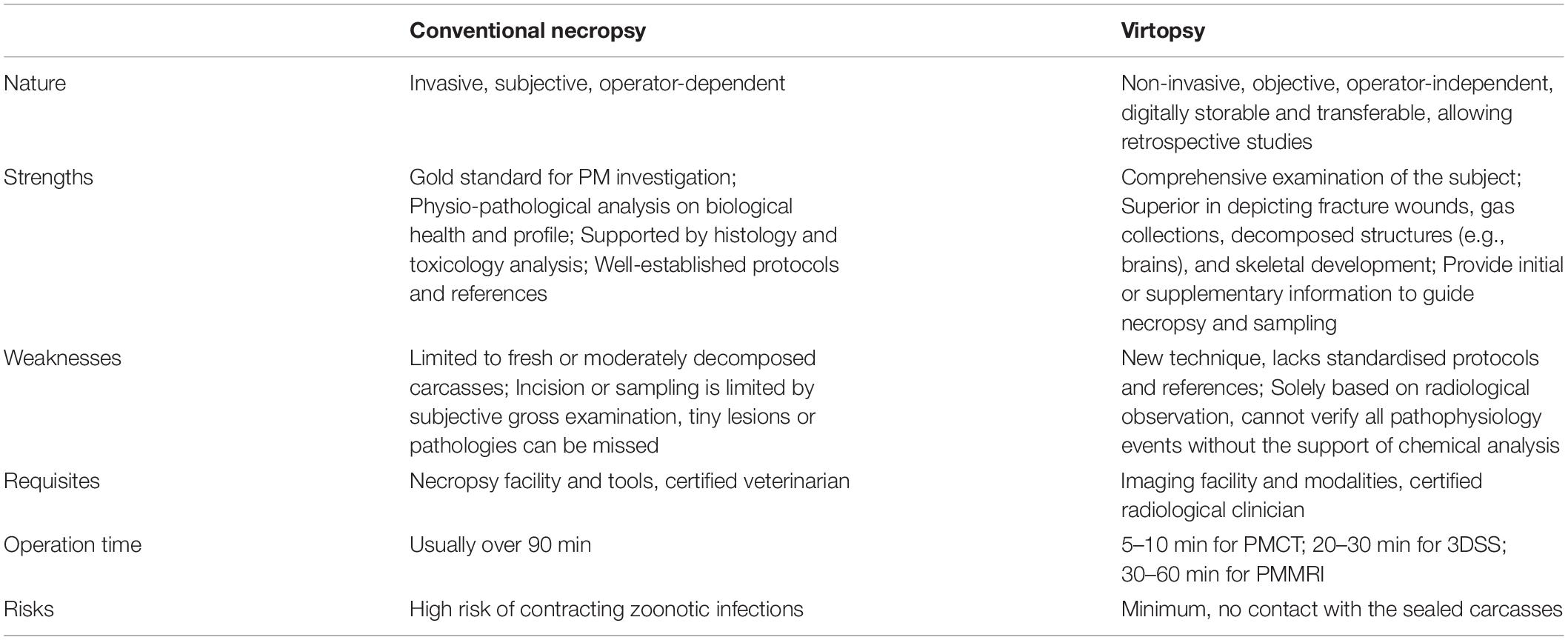
The advantages and disadvantages of virtopsy compared to conventional necropsy is listed in Table 1. Throughout the 6-year programme experience, 4 interrelated pitfalls for the implementation and management of the CVSRP were identified:
Difficulties Locating or Accessing Carcasses
Stranded cetaceans may either be washed ashore, remain afloat, or sink into the sea. Beached carcasses were reported by citizens with only an approximate location. Floating carcasses under a dynamic water current may not stay at their reported stranding sites by the time MLSRT arrives. Adverse weather and poor visibility further hampered the recovery. In the study, despite additional searching efforts from the Marine Region of the HK Police Force, 22 of the 262 reported cases (8%) were not recovered.
Degree of Autolysis
In regards to the decomposition condition among the 240 recovered cetaceans, there were 5 code 1 animals (2%), 6 code 2 animals (3%), 29 code 3 animals (12%), 183 code 4 animals (76%), and 17 code 5 animals (7%). Most of the retrieved carcasses (83%) were putrefied (code 4–5) due to delayed discovery, which severely diminished the necropsy diagnostic value. The decomposition condition was assigned at the time of retrieval. Insufficient cooling of the carcasses might lead to further deterioration before PM examination. Timely stranding discovery and retrieval were crucial for meaningful virtopsy and tissue sampling to investigate their potential cause of death.
Challenges in Carcass Transportation
Carcass transportation was considerably affected by logistic capability. In response to stranding reports, a goods delivery minivan was hired and carcass manoeuvre was done solely by manual effort. The lack of proper lifting equipment prompted occupational hazards to the personnel and failure in retrieving the carcasses. Furthermore, only a few veterinary clinics with CT and MRI units accepted stranded cetaceans for virtopsy. These clinics were usually compacted with narrow hallways that obstructed carcass transportation, not allowing to perform virtopsy on large-sized carcasses. Due to logistic incompatibility, 59 recovered carcasses (25%) were not retrieved for PM examinations.
Improper Carcass Handling
The use of inappropriate packaging materials (e.g., a single-layered plastic bag of unsuitable size) resulted in the release of pungent odour and leakage of bodily fluids from decomposing carcasses, which evoked a series of sanitation issues. Some carcasses were bent inside the bags to facilitate transportation, which undesirably distorted the carcasses (imposing PM changes) and hindered standardised virtopsy scanning (in which a natural body position of the subject is recommended). A human cadaver pouch was used for larger carcasses, but the metallic components (e.g., zippers) casted artifacts in PMCT, which affected image quality and lead to virtopsy result misinterpretation (Figure 3).
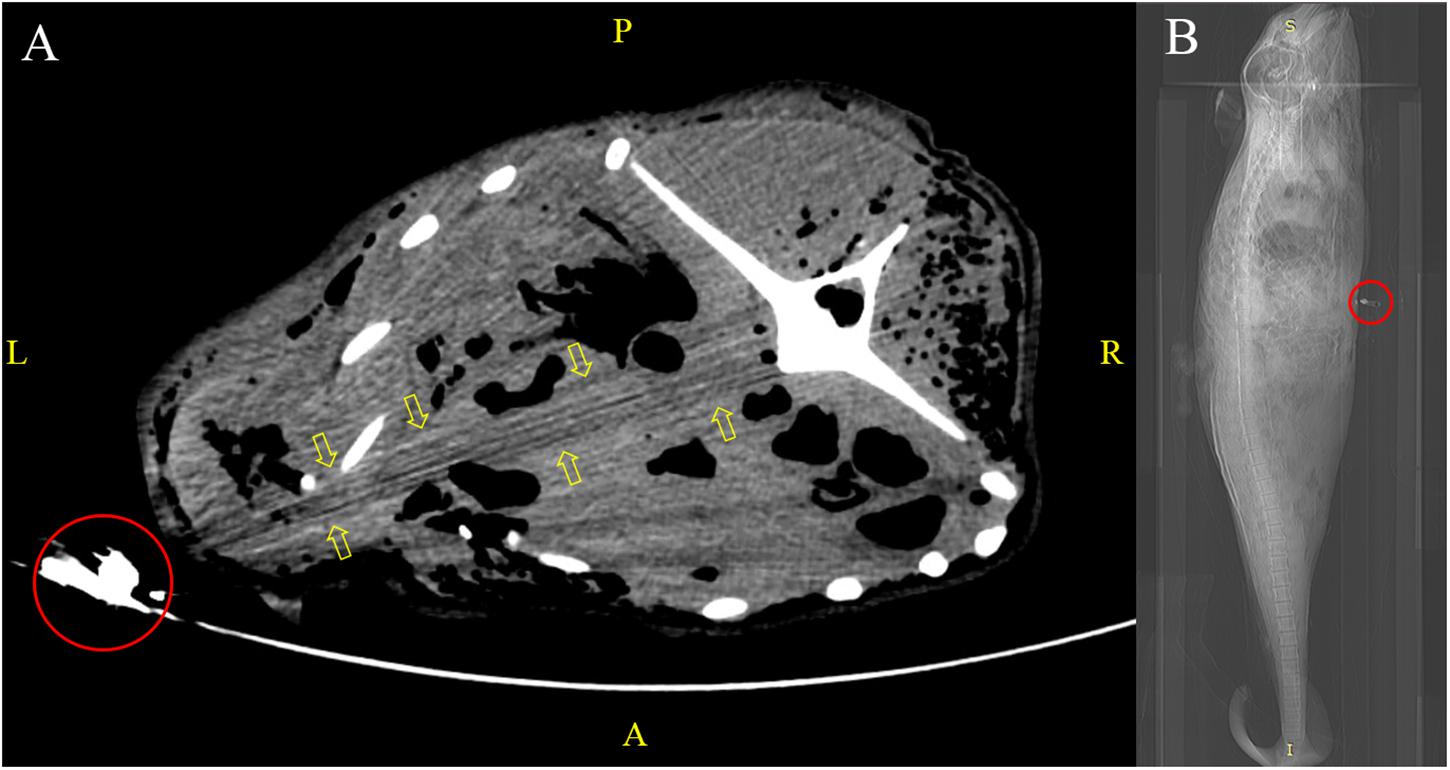
Figure 3. Virtopsy of an adult finless porpoise kept in a human cadaver pouch. (A) Metallic zipper (red circle) of the cadaver bag in the PMCT scan field led to streaking artifacts (yellow arrows) that mimicked an abnormality in adjacent structures. (B) CT scout view of the same case with metallic zipper (red circle) positioned at the thorax region.
When PM examination was not immediately feasible, carcasses were frozen at −20°C. Improper storage could lead to undesirable PM alterations of anatomical structures, which detrimentally affected the evaluation of biological health profiles and causes of death. For instance, a PM cranial deformity was identified in a PMCT scan on a carcass stored in a packed freezer (Figure 4), evidencing that a heavy object was misplaced over the head region during storage. No sign of recent bone remodeling and absence of haematoma in the adjacent soft tissue were observed, which proved the indentation was PM. The slanted level of liquefied brain also indicated that the carcass was not kept in a prone position. The unnatural positioning led to false asymmetry of the organs and distorted the interpretation of virtopsy findings. In addition, while PMCT could be performed on frozen carcasses, data acquisition of PMMRI depends upon fluid molecular mobility, which required up to 24 h for the carcasses to completely thaw prior to scanning (Kot et al., 2020b).
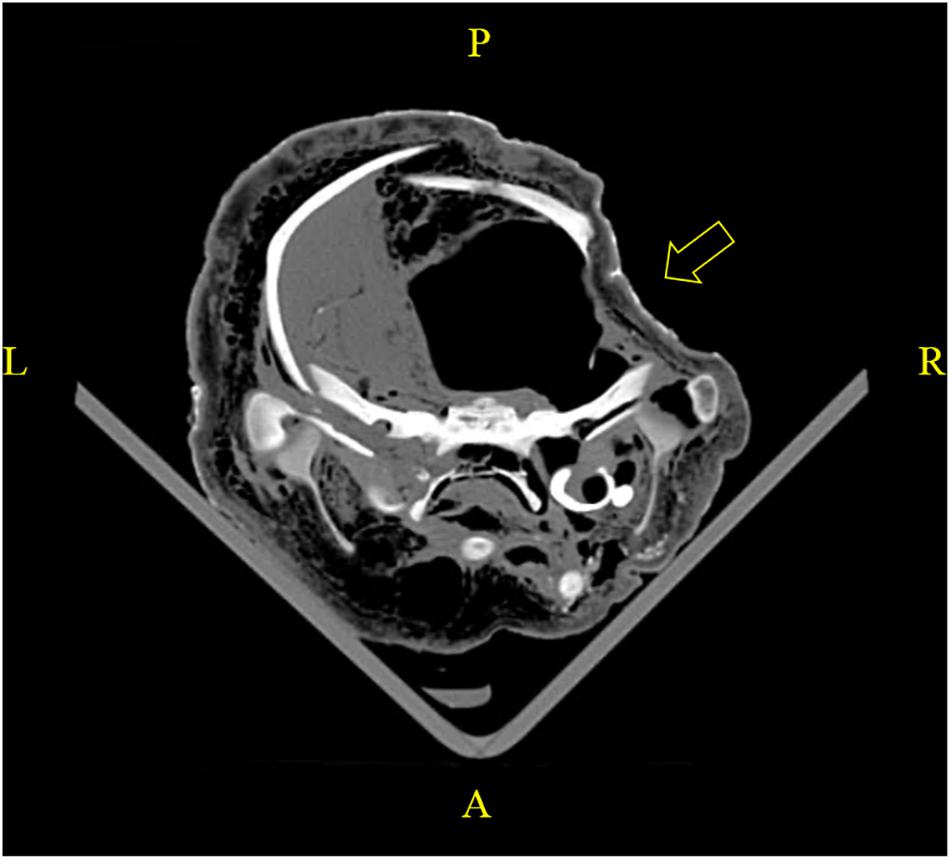
Figure 4. A PMCT revealed improper storage of a carcass in a packed freezer posing undesirable PM alterations. PM cranial deformity was caused by misplacing a heavy object over the fronto-parietal region. No sign of recent bone remodeling and absence of haematoma in the adjacent soft tissue were observed, which proved the indentation was PM. Slanted level of liquefied brain indicated the carcass was frozen with a left lateral approach.
Discussion
Peer-reviewed literature has proven that virtopsy has an excellent applicability both in human and veterinary practice (Dirnhofer et al., 2006; Thali et al., 2007; Ibrahim et al., 2012; Watson and Heng, 2017) with the sole objective of documenting external and internal structures of carcasses using high throughput imaging techniques to facilitate the investigation of cause and manner of death. Although CVSRP shared a similar objective aiming to provide an alternative virtual technique to supplement necroscopic findings of stranded cetaceans for PM investigation, the implementation and management of virtopsy were initially quite laborious. Given the highly variable conditions of cetacean strandings (i.e., random location, number, size, weight, and decomposition condition), management of stranding response and arrangement of routine virtopsy required the personnel involved to adopt these immediate changes.
This study was the first to provide an overview of the pioneer CVSRP management scheme in HK waters. Four pitfalls regarding this CVSRP were recognised, crucial for sample and virtopsy image quality, workflow smoothness, and management quality. We proposed 4 solutions, adopted and adjusted human and veterinary virtopsy project publications to address these interrelated pitfalls, and to facilitate the entire workflow in a more effective and efficient manner.
Development of a Mobile Application for Cetacean Stranding and Sighting Reports
Successful retrieval of stranded carcasses depends on accurate reporting of their location and timely recovery. Previous studies have proven that a simple technological solution can foster a transboundary collaboration to collect biodiversity data, using opportunistic platforms such as ecotourism operations, thus eliminating the cost of specialised research vessels for more cost-effective means of ecosystem monitoring (Gössling, 1999; Harris et al., 2001; Tallis et al., 2008). A mobile application is an effective means to enhance public awareness and improve efficacy in stranding reports. The use of web-based technologies could also facilitate the collection of cetacean antemortem and PM data (i.e., the sighting of live, injured, distressed and stranded animals) (Chan et al., 2017). Different research groups and government agencies worldwide have developed mobile apps for such purposes, for instance, “Dolphin & Whale 9111” from the National Oceanic and Atmospheric Administration of the United States, and “ 2” from the Taiwan Cetacean Society.
2” from the Taiwan Cetacean Society.
Through mobile applications, the concept of “citizen science” could be practiced, in which the general public could participate in large-scale, on-going scientific research activities regarding cetacean sightings and stranding response. The application would aim to (1) allow reporting of cetacean strandings in a simple and effective manner; (2) facilitate reporting of any live, injured or distressed cetacean sighting; (3) provide automatic GPS position tracking; (4) utilise camera function to capture bodily conditions of stranded animals; (5) display stranding reports on a coastal map. With the ease of reporting, accurate positioning, and visual details, stranded cetaceans could be timely recovered to preserve carcass freshness and enhance the diagnostic value of PM investigations, thereby increasing the likelihood to identify the causes of stranding and death. Promotion of this “citizen science” collaboration would be undertaken via the mobile app to recruit general public actively contributing to CVSRP, and foster their engagement in marine conservation.
Tailor-Made Logistics and Equipment for Stranding Response
Given the ad hoc nature of an unpredictable carcass size in a cetacean stranding, it is desirable to designate an adequate logistic support and lifting equipment to transport the carcass from the site of discovery to the PM examination facilities. A vehicle with appropriate lifting aid is essential for safe and efficient carcass manoeuvre, especially when many of the stranded SC or large-sized cetaceans go beyond the capability of the response personnel. A portable radiolucent stretcher with retractable handles for carrying the carcasses (Figure 5) and a trolley for easy driving are recommended to transfer them from the vehicle to the CT or MRI examination couches. Reserving a larger logistic capacity and better equipment support could allow a more efficient response and minimise the risk of occupational hazards.

Figure 5. Tailor-made equipment, a portable radiolucent stretcher with retractable handles, was designed by OPCFHK for carrying the carcasses.
Tailor-Made Packaging and Standardised Preservation Protocol
Seamless carcass packaging is essential to protect personnel from zoonotic diseases and equipment from damages, as well as to preserve carcasses. Carcass bags with handles should be made of durable material and remain robust upon wetting and freezing. The entire bag including the zippers should be radiolucent, allowing complete enclosure of odour and bodily fluids without interfering with virtopsy scanning. The bag should be large enough to contain anticipated carcasses in their natural position without bending. An identification tag with the carcass identity should be attached, so carcasses would not be mixed up upon transportation, examination, and storage among different parties.
Thermal preservation should be executed to minimise PM carcass deterioration upon recovery. Except for mummified remains (code 5), if several waiting hours are anticipated, ice packs should be applied to the external surface of the flank of the body, especially during hot weather (e.g., > 30oC). Whenever possible, dry ice should be added into the oral cavity and blowhole to chill the brain, and into the anus to chill the organs in the lower abdominal region. A proper manner of refrigeration is required for overnight storage. Carcasses should be kept at −20°C in a natural prone position, without being twisted or compressed (National Pathology Accreditation Advisory Council, 2013). For storage of large-sized cetaceans, a walk-in cold room used as a morgue is preferred over a freezer.
A Centralised Facility for Stranded Cetacean PM Examinations
The CVSRP is an on-going project which keeps expanding. In addition to PMCT and PMMRI, 3D surface scanning (3DSS) is the next element to be integrated. 3DSS can improve the surface documentation of carcasses and lead to more graphical insights on the wounds and inflicting tools. It also facilitates the investigation of the potential causes of death (both natural and anthropogenic injuries), thereby posing more advisory measures for cetacean conservation. Trials of 3DSS have been conducted to develop a standardised protocol for biological profiling and PM investigation of stranded cetaceans (Kot et al., 2018b, 2020c).
In the current CVSRP, virtopsy and necropsy were performed at separate facilities which required time- and resource-demanding carcass transportation. A one-stop examination centre for stranded cetaceans should be established, integrating advanced technologies and standard examination methods, including PMCT, PMMRI, 3DSS, and conventional necropsy. With tailor-made transportation gears and accommodations, efficient workflow of PM examination is warranted (Ebert et al., 2015). The planning should also make a reference to human and veterinary forensic settings. Guidelines for facilities and operation of hospital and forensic mortuaries (National Pathology Accreditation Advisory Council, 2013) should be considered and implemented.
A tentative workflow is proposed as follows: Upon receiving a stranding report, response personnel will reach the site by designated transportation. Basic measurements and photos of the carcass and any associated wounds or entanglements will be taken. The carcass will be cleaned and packed in a natural prone position without bending it, into a radiolucent cadaver bag without any metal parts. Cooling measures will be applied when deemed necessary. The carcass will be transferred on a stretcher to the virtopsy facility. Radiological clinician who is certificated and experienced to report virtopsy findings will be notified to setup the scanning modules in advance so that the PMCT scan can be performed instantly upon arrival. After PMCT data acquisition, the carcass bag will be opened to allow 3DSS of the carcass directly on the CT couch. Without altering the carcass position between PMCT and 3DSS, external and internal findings can be directly correlated for proper interpretation of the deceased animal’s biological health and profiles. A typical 3DSS of a stranded cetacean takes about 30 min, preliminary PMCT interpretation will be conducted in the meantime, followed by PMMRI scanning whenever applicable. After the virtopsy session, the carcass will be transferred to the necropsy room for conventional necropsy and tissue sampling with reference to the virtopsy findings. Instead of performing random sectioning of organs, any abnormalities and pathologies observed in the virtopsy will be followed. If refrigeration is necessary, the carcass will be kept in the morgue with a natural prone position and sufficient spacing. Data of the field report, virtopsy, and necropsy reports will be analysed as a whole, and consensus on the assignation of cause of death and biological health concerns will be reached by all of the involved parties, with an addressed degree of confidence (i.e., confirmed, probable or suspected).
A one-stop examination facility could streamline the logistics and minimise carcass decomposition, producing more meaningful biological health data of stranded cetaceans with less resources. Administration complexity could be reduced, quality control and sharing of the PM examination data among different collaborators could be enhanced, and the overall management of the virtopsy integrated stranding response programme could be advanced. This study is a valuable reference for other stranding response programmes worldwide that are interested in integrating virtopsy or other modern diagnostic modalities into their routine workflow.
Data Availability Statement
The datasets generated for this study will not be made publicly available due to restrictions from the Agriculture, Fisheries and Conservation Department of Hong Kong Special Administrative Region. The raw data supporting the conclusions of this article can be made available by contacting Dr. Ng Wai-Chuen (waichuen_ng@afcd.gov.hk), upon reasonable request.
Ethics Statement
Ethical review and approval was not required for this animal study as research activities coincided with the Local Cetacean Stranding Response Programme in Hong Kong, sanctioned by the Agriculture, Fisheries and Conservation Department of Hong Kong Special Administrative Region.
Author Contributions
BK, HT, and DC wrote the first draft of the manuscript. All authors contributed to the conception and design of the study, analysed the data, wrote sections of the manuscript, contributed to field work, data collection, and manuscript revision, read and approved the submitted version.
Funding
This project was funded by the Hong Kong Research Grants Council (Grant No. UGC/FDS17/M07/14), the Marine Ecology Enhancement Fund (Grant Nos: MEEF2017014, MEEF2017014A, MEEF2019010, and MEEF2019010A) and the Marine Ecology & Fisheries Enhancement Funds Trustee Limited. All the funding was awarded to BK (project leader and principal investigator of the funded projects).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the Agriculture, Fisheries and Conservation Department of the Hong Kong Special Administrative Region Government for the continuous support in this project. Sincere appreciation was also extended to veterinarians, staff and volunteers from the Aquatic Animal Virtopsy Lab, City University of Hong Kong, Ocean Park Conservation Foundation Hong Kong and Ocean Park Hong Kong. Special gratitude was owed to technicians in CityU Veterinary Medical Centre and Hong Kong Veterinary Imaging Centre for operating the CT and MR units in the present study. Any opinions, findings, conclusions, or recommendations expressed herein do not necessarily reflect the views of the Marine Ecology Enhancement Fund or Trustee. Special thanks to Dr. María José Robles Malagamba for English editing of this manuscript.
Footnotes
- ^ https://apps.apple.com/us/app/dolphin-whale-911/id698859376
- ^ https://play.google.com/store/apps/details?id=com.larvata.savingwhale.client
References
Aghayev, E., Thali, M. J., Sonnenschein, M., Jackowski, C., Dirnhofer, R., and Vock, P. (2007). Post-mortem tissue sampling using computed tomography guidance. Forensic Sci. Int. 166, 199–203. doi: 10.1016/j.forsciint.2006.05.035
Bagó, Z., and Kneissly, S. (2017). Virtual Autopsy (‘Virtopsy’): a rapid tool to supplement traditional necropsy examination. J. Comp. Pathol. 156:108. doi: 10.1016/j.jcpa.2016.11.173
Chan, D. K. P., and Kot, B. C. W. (2017). “Cetaceans postmortem multimedia analysis platform (CPMAP): Pilot web-accessed database of a virtopsy-driven stranding response program in the Hong Kong waters,” in Proceedings of the 48th International Association for Aquatic Animal Medicine Conference, Cancun.
Chan, D. K. P., Tsui, H. C. L., and Kot, B. C. W. (2017). Database documentation of marine mammal stranding and mortality: current status review and future prospects. Dis. Aquat. Org. 126, 247–256. doi: 10.3354/dao03179
Dirnhofer, R., Jackowski, C., Vock, P., Potter, K., and Thali, M. J. (2006). Virtopsy: Minimally invasive, imaging-guided virtual autopsy. Radiographics 26, 1305–1333. doi: 10.1148/rg.265065001
Ebert, L. C., Flach, P., Schweitzer, W., Leipner, A., Kottner, S., Gascho, D., et al. (2015). Forensic 3D surface documentation at the institute of forensic medicine in zurich – workflow and communication pipeline. J. Forensic Radiol. Imaging 5, 1–7. doi: 10.1016/j.jofri.2015.11.007
Geraci, J. R., and Lounsbury, V. J. (2005). Marine Mammals Ashore: A field Guide for Strandings. Baltimore: National Aquarium in Baltimore.
Gössling, S. (1999). Ecotourism: a means to safeguard biodiversity and ecosystem functions? Ecol. Econ. 29, 303–320. doi: 10.1016/s0921-8009(99)00012-9
Harris, E., Huntley, C., Mangle, W., and Rana, N. (2001). Transboundary Collaboration in Ecosystem Management: Integrating Lessons from Experience. Master’s thesis, Ann Arbor, MI: University of Michigan.
Ibrahim, A. O., Abu Bakar, M. Z., and Noordin, M. M. (2012). Applicability of virtopsy in veterinary practice: A short review. Pertanika J. Trop. Agric. Sci. 35, 1–8.
Kot, B. C. W., Chan, D. K. P., Yuen, H. L. A., and Tsui, H. C. L. (2018a). Diagnosis of atlanto-occipital dissociation: Standardised measurements of normal craniocervical relationship in finless porpoises (genus Neophocaena) using postmortem computed tomography. Sci. Rep. 8:8474. doi: 10.1038/s41598-018-26866-8
Kot, B. C. W., Chan, D. K. P., Yu, M. C. Y., Chau, W. K. L., Lau, A. P. Y., and Tsui, H. C. L. (2018b). “Three-dimensional surface scanning in postmortem investigation of stranded cetaceans: a step-by-step guide for carcass surface documentation,” in Proceedings of the 49th International Association for Aquatic Animal Medicine Conference, Long Beach, CA.
Kot, B. C. W., Chan, D. K. P., Yuen, A. H. L., and Tsui, H. C. L. (2019). Morphological analysis of the foramen magnum in finless porpoise (genus Neophocaena) using postmortem computed tomography 3D volume rendered images. Mar. Mam. Sci. 35, 261–270. doi: 10.1111/mms.12512
Kot, B. C. W., Chu, J., Fernando, N., Gendron, S., Heng, H. G., and Martelli, P. (2015). “Can necropsy go bloodless: Applicability of virtopsy as a routine procedure in stranded cetaceans in the Hong Kong waters,” in Proceedings of the 21st Biennial Conference on the Biology of Marine Mammals, San Francisco, CA.
Kot, B. C. W., Fernando, N., Gendron, S., Heng, H. G., and Martelli, P. (2016). “The virtopsy approach: Bridging necroscopic and radiological data for death investigation of stranded cetaceans in the Hong Kong waters,” in Proceedings of the 47th International Association for Aquatic Animal Medicine Conference (Virginia Beach, VA).
Kot, B. C. W., and Martelli, P. (2017). “To see or not to see: The need for attention on computed tomography features of postmortem change and decomposition in stranded cetaceans,” in Proceedings of the 48th International Association for Aquatic Animal Medicine Conference, Cancun.
Kot, B. C. W., Chung, T. Y. T., Chan, D. K. P., and Tsui, H. C. L. (2020a). Image rendering techniques in postmortem computed tomography: Evaluation of biological health and profile in stranded cetaceans. JoVE. 163:e61701. doi: 10.3791/61701
Kot, B. C. W., Tsui, H. C. L., Chung, T. Y. T., and Lau, A. P. Y. (2020b). Postmortem neuroimaging of cetacean brains using computed tomography and magnetic resonance imaging. Front. Mar. Sci. 7:544037. doi: 10.3389/fmars.2020.544037.
Kot, B. C. W., Tsui, H. C. L., Chung, T. Y. T., Cheng, W. W., Mui, T., Lo, M. Y. L., et al. (2020c). Photogrammetric three-dimensional modeling and printing of cetacean skeleton using an omura’s whale stranded in Hong Kong waters as an example. JoVE 163:e61700. doi: 10.3791/61700.
National Pathology Accreditation Advisory Council (2013). Requirements for the Facilities and Operation of Mortuaries. Available online at: https://www1.health.gov.au/internet/main/publishing.nsf/Content/9E16A59073C155C4CA257BF0001B080B/$File/V0.29%20Mortuaries.pdf [Accessed May 25, 2020].
Roberts, I. S. D., Benamore, R. E., Benbow, E. W., Lee, S. H., Harris, J. N., Jackson, A., et al. (2012). Post-mortem imaging as an alternative to autopsy in the diagnosis of adult deaths: a validation study. Lancet. 379, 136–142. doi: 10.1016/s0140-6736(11)61483-9
Tallis, H., Kareiva, P., Marvier, M., and Chang, A. (2008). An ecosystem services framework to support both practical conservation and economic development. Proc. Natl. Acad. Sci. U.S.A. 105, 9457–9464. doi: 10.1073/pnas.0705797105
Thali, M. J., Jackowski, C., Oesterhelweg, L., Ross, S. G., and Dirnhofer, R. (2007). Virtopsy – the Swiss virtual autopsy approach. Leg. Med. 9, 100–104. doi: 10.1016/j.legalmed.2006.11.011
Thali, M. J., Yen, K., Schweitzer, W., Vock, P., Boesch, C., Ozdoba, C., et al. (2003). Virtopsy, a new imaging horizon in forensic pathology: virtual autopsy by postmortem multislice computed tomography (MSCT) and magnetic resonance imaging (MRI) – a feasibility study. J. Forensic Sci. 48, 386–403.
Tsui, H. C. L., and Kot, B. C. W. (2015). “Cetacean stranding response program with virtopsy as an integral part in Hong Kong: Pitfalls in management,” in Proceedings of the 21st Biennial Conference on the Biology of Marine Mammals, San Francisco, CA.
Watson, E., and Heng, H. G. (2017). Forensic radiology and imaging for veterinary radiologists. Vet. Radiol. Ultrasound. 58, 245–258. doi: 10.1111/vru.12484
Wong, K. H. C., Lam, C. S. C., Ling, A. P. H., Tsui, H. C. L., Wong, B. L. Y., and Kot, B. C. W. (2016). “Utilization of computed tomography in assessing fusion pattern of vertebral non-epiphyseal suture in Indo-Pacific finless porpoise (Neophocaena phocaenoides) in the Hong Kong waters,” in Proceeding of 47th International Association for Aquatic Animal Medicine Conference (Virginia Beach, VA).
Keywords: pitfalls, management, cetacean, stranding, virtopsy
Citation: Tsui HCL, Kot BCW, Chung TYT and Chan DKP (2020) Virtopsy as a Revolutionary Tool for Cetacean Stranding Programs: Implementation and Management. Front. Mar. Sci. 7:542015. doi: 10.3389/fmars.2020.542015
Received: 11 March 2020; Accepted: 10 September 2020;
Published: 06 November 2020.
Edited by:
Karen A. Stockin, Massey University, New ZealandReviewed by:
Gail Schofield, Queen Mary University of London, United KingdomGabriela Hernandez, Servicio Nacional de Salud Animal (SENASA), Costa Rica
Copyright © 2020 Tsui, Kot, Chung and Chan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brian C. W. Kot, YnJpYW5rb3RAeWFob28uY28udWs=
†These authors have contributed equally to this work
 Henry C. L. Tsui
Henry C. L. Tsui Brian C. W. Kot
Brian C. W. Kot Tabris Y. T. Chung
Tabris Y. T. Chung Derek K. P. Chan
Derek K. P. Chan