- 1School of Biological Sciences, Georgia Institute of Technology, Atlanta, GA, United States
- 2Foreign Commonwealth Office, St Helena Government, Jamestown, Saint Helena
- 3Research and Conservation Department, Georgia Aquarium, Atlanta, GA, United States
- 4Ch’ooj Ajauil AC, Cancun, Mexico
- 5Marine Megafauna Foundation, Truckee, CA, United States
- 6Environment and Natural Resources Directorate, St Helena Government, Jamestown, Saint Helena
- 7Saint Helena National Trust, Jamestown, Saint Helena
- 8Large Marine Vertebrates Research Institute Philippines, Bohol, Philippines
A reliable aggregation of whale sharks (Rhincodon typus) takes place in waters surrounding the remote South Atlantic island of St. Helena from December to May each year, peaking in January. Using photographic identification (photo-ID), a total of 277 individual sharks were identified over the course of the study, consisting of a 1.1:1 sex ratio of male and female sharks, ranging from 5 to 12 m in total length, with 86% of males and 51% of females likely to be mature. Modified maximum likelihood methods of the photo-ID data estimated ∼102 individual whale sharks at any one time, each residing within the study site for a mean of 19 days with a decline to complete absence at ∼75 days following initial identification. Interannual periodicity was observed for some (n = 34) sharks at the site. Eyewitness accounts of mating behavior have been reported by reliable local observers on two separate occasions, which comprise the first observations of copulation in this species and are consistent with the size and sex demographics of the population. Horizontal movements away from the island proved difficult to track, due to deep-diving behavior that either damaged or caused premature detachment of the archival satellite tags, however, some individuals showed large scale movement away from the island towards both Africa and South America. Acoustic telemetry showed that animals use the habitats around the entire island, but are focused on the leeward side, particularly from James Bay to Barn Cap. Due to its likely role in the reproductive ecology of the whale shark, St. Helena represents a critical habitat for this endangered species and deserves concerted research and conservation efforts.
Introduction
The whale shark (Rhincodon typus) can be found circumglobally in tropical and warm temperate seas, and often forms reliable coastal aggregations, typically in response to high prey abundance (Rowat and Brooks, 2012). The species has suffered a > 50% decline in global population over the last 75 years, leading to a conservation status of “Endangered” on the IUCN Red List of Threatened Species (Pierce and Norman, 2016). Understanding the ability of whale shark populations to recover from these declines is vital for the conservation of the species, but little is known about their reproduction and some life-history characteristics. Genetic evidence suggests that whale shark populations are distinct between the Atlantic and Indo-Pacific, with clear differentiation between sharks sampled in the Gulf of Mexico and Indo-Pacific locations (i.e., Vignaud et al., 2014). Coupled with movement information from broad-scale whale shark photo-identification studies, such as Norman et al. (2017), this indicates that whale sharks in the Atlantic represent a functionally separate population from those in the Indo-Pacific (Pierce and Norman, 2016). The lack of movement or genetic data form outside the Gulf of Mexico and Caribbean region within the Atlantic makes it difficult to identify finer-scale structure within this ocean basin. In addition, there is a lack of information about the biology and ecology of whale shark populations in the South Atlantic relative to other ocean basins (Norman et al., 2017). Data are lacking on the formation of coastal feeding aggregations of juvenile sharks in this region, which tend to occur in areas of seasonal upwelling (de la Parra Venegas et al., 2011) or other high-productivity habitats. Similarly, the ecology of adult whale sharks, which are often sighted at volcanic islands far removed from continental shelf habitats (Ramírez-Macías et al., 2017), is poorly understood in general and in the Atlantic in particular.
The tropical South Atlantic Ocean has only a few oceanic islands in the habitable range of whale sharks, namely St. Peter and St. Paul Rocks (Brazil), Fernando de Noronha (Brazil), Trindade and Martim Vaz (Brazil), Ascension (United Kingdom), and St. Helena (United Kingdom), whereas the Pacific, North Atlantic, and Indian oceans are replete with oceanic islands. Whale sharks have been sighted at all of these Atlantic locations; however, the significance and role of these areas in whale shark biology and ecology is understudied (Hazin et al., 2008). Previous work at the archipelago of St. Peter and St. Paul in the Equatorial Atlantic region documented putative reproductive behaviors and indicators of sexual activity in whale sharks. Examples of this included a mature male shark that exhibited mating behaviors in proximity to a vessel, and large females that were observed with distended abdomen and potential mating scars suggesting pregnancy and/or possible mating attempts, respectively (Macena and Hazin, 2016). These results suggest the importance of the tropical Atlantic in terms of whale shark population demographics and reproductive ecology, although further research is needed to confirm these observations.
Whale shark aggregations can be explored through a variety of different research methods and techniques. Photographic identification is a non-invasive tool that uses stable markings to identify individuals within a population and has been employed on a variety of marine species, including turtles (Schofield et al., 2008), cetaceans (Hammond et al., 1990), and manta rays (Marshall et al., 2011). Whale sharks also have natural body markings that make them suitable study targets for photographic identification (Arzoumanian et al., 2005) and this technique has been commonly used to estimate population demographics and structure, such as sex ratios and size ranges, throughout all sites where whale sharks are encountered (Norman et al., 2017). Furthermore, photographic identification can be used to explore aggregation structure such as seasonality and individual residency by documentation of repeat encounters with individuals over time (McCoy et al., 2018). These repeat encounters can be used in mark-recapture models to estimate population sizes (e.g., McCoy et al., 2018) and residency through modified maximum likelihood methods (e.g., Fox et al., 2013), such as lagged identification rates, providing a valuable tool to explore whale shark sites globally.
Satellite and acoustic telemetry are two other techniques that have been commonly used on whale sharks worldwide to explore a variety of research questions relating to movement ecology. These methods involve attaching satellite and/or acoustic transmitters that passively record or actively report a suite of data depending on the tag. Satellite telemetry is a vital tool to explore horizontal movements as well as vertical diving behavior and profiles. Insights from these tags have shown that whale sharks are capable of very large horizontal movements (e.g., Hueter et al., 2013) and able to conduct deep dives into the bathypelagic zone (e.g., Tyminski et al., 2015). Acoustic telemetry can help explore residency patterns (such as site use and fidelity) (Cagua et al., 2015), fine-scale horizontal movements, and validate photographic identification data (Cochran et al., 2019). Used in combination, these techniques can elucidate both the large and fine-scale movements of whale sharks in a particular area.
To redress the lack of information on whale sharks in the South Atlantic, and to help understand their reproductive biology, here we report the first data on the biology of whale sharks around St. Helena Island. We have compiled historical data curated by the St. Helena Government (SHG) and collected new information during research expeditions to the island in 2015, 2016, 2018, and 2019. We used photographic identification from these dedicated expeditions to explore the population demographics of St. Helena whale sharks. Additionally, satellite and acoustic telemetry were used in combination to explore the large and fine-scale movement and residency patterns. Finally, we used modified maximum likelihood methods to further understand the population demographics of whale sharks at St. Helena.
Materials and Methods
Study Site
Saint Helena is a rocky volcanic island in the South Atlantic Ocean, approximately 2,000 km west of Angola, and 3,500 km east of Brazil (Figure 1). It is one of the most remote inhabited islands in the world, with a population of ca. 4,300 people, and is part of the United Kingdom Overseas Territory (UKOT) of St. Helena, Ascension, and Tristan da Cunha. The island is surrounded by steep cliffs on all sides and the water depth increases sharply as you move away from the island in all directions, down to the South Atlantic abyssal plain at approximately 4,200-4,500 m depth. The island has a mild and stable subtropical climate, heavily influenced by southeast trade winds, which also create a stark difference in navigability between the calmer leeward side facing the northwest, and the rougher windward side facing southeast. The island is home to a substantial portion of UK marine biodiversity (Brown, 2013; Bormpoudakis et al., 2019) and has high rates of endemism for both terrestrial (Gray et al., 2019) and marine life (Roberts et al., 2002).
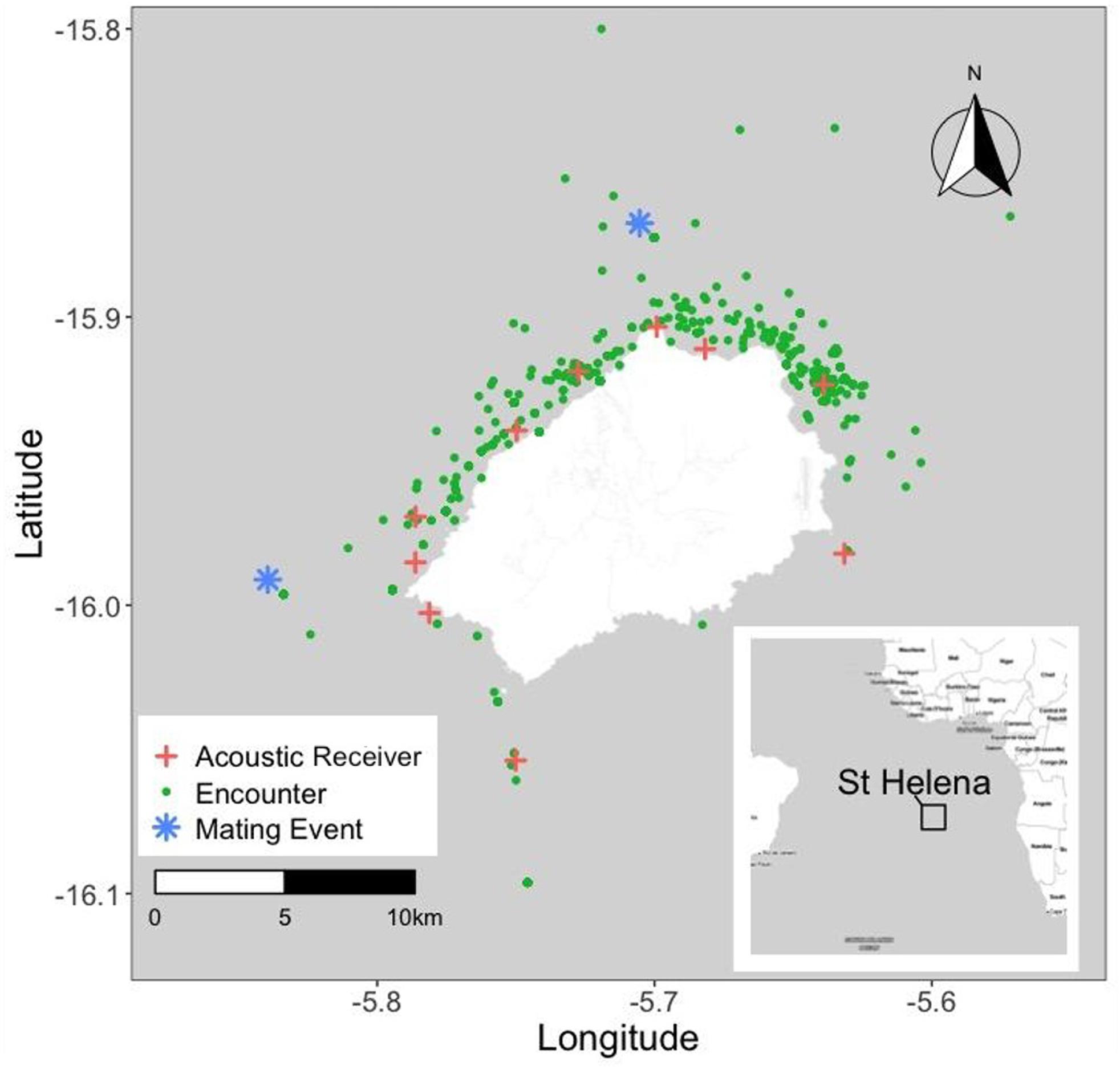
Figure 1. St. Helena study site, showing whale shark sightings from 1999 to 2019 inclusive as green dots. Asterisks show sites of eyewitness accounts of mating behavior. Crosses show the sites of receivers in the acoustic array deployed since 2016. Inset: location of St. Helena in the South Atlantic.
Visual Observations
The Environment Natural Resources and Planning Directorate (ENRD) of St. Helena Government has maintained an incidental sightings database of whale sharks, which are locally also called bone sharks, since 1999. Any boater, fisher or member of the general public who saw a whale shark was encouraged to report the sighting to ENRD resource managers for inclusion in the database. This database represents opportunistic sightings of whale sharks by marine users and is mainly driven by fishermen, some recreational users, and three tourism operators, all of whom are on the water year-round. Sightings form the first four Georgia Aquarium expeditions dedicated to the study of whale sharks in St. Helena are also included in this database. These took place in January 2015, January-February 2016, February 2018, and March 2019 and their timing was determined by seasonal occurrence trends noted in the earlier sightings database.
During these four expeditions, visual observations took place from cabin-cruiser style boats. On the expeditions in 2015 and 2016, an equal effort search pattern was deployed that incorporated the entire circumference of the island. In subsequent years, search effort was focused on areas of known whale shark abundance to improve efficiency. Upon seeing a whale shark near the surface, snorkelers were deployed to observe and photograph animals for submission to the Wildbook for Whale Sharks photographic identification database1 (Arzoumanian et al., 2005). Whale sharks were sexed visually by the presence/absence of claspers, measured with laser photogrammetry following Rohner et al. (2011), and photographed to document distinguishing features and/or injuries. Maturity in males was determined by the external morphology of the claspers. Sharks with claspers extending beyond the pelvic fins, with a thick and calcified appearance, were considered mature; claspers extending past the pelvic fins but not being calcified in appearance were considered sub-adult (i.e., immature), and claspers not extending past the pelvic fins and not calcified were also classes as immature (Norman and Stevens, 2007). Maturity in females cannot be accurately assessed externally, however, maturity is thought to occur ∼9.0 m total length (Acuña-Marrero et al., 2014), although size at maturation may differ between ocean regions (Hueter et al., 2013). Conservatively, female whale sharks over 9 m were considered mature while those under 8 m were considered immature. Those in the ambiguous 8-9 m range were excluded from maturity assessments due to uncertainty in female size at maturity (Macena and Hazin, 2016). In 2016, multiple laser photogrammetry photos were taken during each encounter to generate mean values and improve accuracy of size estimates (Rohner et al., 2011). The number of sightings was compared across years and months to explore seasonal and interannual trends in sighting reports from the ENRD dataset. Sex ratios, number of individuals, size estimates, and resighting rates were explored from the Wildbook dataset. Maps were created using the ggmap package in R studio (Kahle and Wickham, 2019). Figures were created using the ggplot2 package in R studio (Wickham, 2016).
Telemetry
Satellite Telemetry
Multiple types of satellite tags were used to address questions of whale shark movements on a regional spatial scale. These tags included pop-up satellite archival tags (Wildlife Computer Mk10/MiniPAT and Desert Star SeaTagMOD/SeaTagGeo) and smart position only (Wildlife Computer SPOT253-C/SPOT6/SPLASH10) tags. All tags were attached to the dorsal surface of the whale shark, along the lateral aspect at the base of the first dorsal fin. Wildlife Computers SPOT253-C tags in towed configuration were used to track whale shark movements in near-real-time using Doppler locations generated by the CLS-ARGOS satellite system. These tags were affixed to a whale shark on a long braided stainless wire leader attached to a Wildlife Computers titanium intradermal dart applied by pole spear. The section of leader in the skin of the animal was covered in heat-shrink wrap to minimize abrasion. Wildlife Computers MiniPAT archival tags were used to generate horizontal movement tracks using light-based location estimates, as well as recording vertical movements and water temperature. Sensors were set to sample at 3-min intervals. The MiniPAT tag has a self-preservation circuit that severs the tag from the leader if the tag depth exceeds a pre-set value of either 1,650 or 1,850 m. MiniPAT tags were affixed to the animals with short leaders of Dyneema braided synthetic material that was heat shrink wrapped to minimize skin abrasion. Wildlife Computers SPLASH10 towed archival tags were also used on a leader similar to that of the SPOT253-C tags.
Transmissions that had quality scores2 under 1 were removed from further analyses as these transmissions are accompanied by greater uncertainty and error. Tracks were then filtered to remove floating tags from the dataset using methods similar to Hearn et al. (2013). Briefly, changes in latitude and longitude were assessed on each tag and compared to observations from known floating tags to apply a filtering method for all tags. The tracks that fit these criteria were determined to be floating and removed from further analysis. MiniPAT most probable tracks (MPT) were generated using the GPE3 processing tool in the Wildlife Computers web portal3, which estimates animal movements from tag data and user input variables such as maximum animal swim speed. Datasets were explored to look for any unrealistic movements such as >200 km/day. No tags were physically recovered during this study and therefore complete time-series datasets were not available for further analyses.
Acoustic Telemetry
To investigate fine-scale habitat usage and observer-independent seasonality, an acoustic array was established around the island in 2016 (six receivers) and 2017 (an additional four receivers). This consisted of Vemco VR2 passive acoustic receivers deployed at prominent features on the bottom, within no-decompression diving depth of the surface, i.e., <30 m depth. The array aimed to allow coverage around the entire island; however, accessibility on the southeast windward side of the island was limited due to weather and safe diving conditions. Depending on the site, receivers were affixed with plastic cable-ties to mooring ropes on subsurface buoys or to rebar embedded in plastic 20 L buckets filled with concrete. The acoustic receivers were not range tested due to field constraints; however, we speculate the range would be on the upper end of previous range tests (>530 m) due to the open ocean, steeply sloped, and deep nature of the water surrounding St. Helena (Cagua et al., 2013). Vemco V16 acoustic transmitters were affixed to whale sharks using leaders similar to those of the MiniPAT tags described above in both the 2016 and 2018 field seasons. Receivers were downloaded and maintained on an approximately annual basis.
Site use and fidelity were explored by analyzing the number of detections, individuals, and days that were documented at each receiver location. Receivers that had high total numbers of detections likely represent areas where sharks were remaining for periods of time and therefore may be indicative of site fidelity. Total number of individuals detected by each receiver was explored to investigate sites that may indicate habitats frequented by multiple individuals. Lastly, the number of days that each receiver detected an individual shark was explored to investigate site fidelity, as receivers that were visited multiple times throughout the course of the study may be important fidelity sites or nearby migratory routes. Spatial residence (Rspatial) was calculated by dividing the number of days a shark was detected by an individual receiver by the total number of days it was documented in the array. When comparing between years Rspatial values were only calculated for receivers that were active for both years because the addition of receivers in later years could influence results as they could increase the number of days an individual was detected in the array through increased search effort.
Individual residency was explored by analyzing the number of detections by individuals throughout the year and calculating both minimum (Rmin) and maximum (Rmax) residence indices. Sharks that were not detected on the array were removed from analysis as these would skew results and may be indicative of tag loss or failure rather than residence patterns. Minimum residence index (Rmin) was calculated by dividing the number of days an individual was detected in the array by the period between initial tagging and the end of the study. Maximum residence index (Rmax) was calculated by dividing the number of days an individual was detected in the array by the total number of days between initial tagging and last detection. Both indices are directly affected by study duration, which can bias animals that were tagged later in the course of the study or individuals that were detected over short periods of time (Cochran et al., 2019), therefore, both results are reported in an attempt to provide upper and lower values for true residency.
Lagged Identification Rate
The photographic identification data were downloaded from Wildbook for Whale Sharks and used to calculate the lagged identification rate (LIR), which is the probability that an individual will be re-sighted at the site after a certain time lag (Whitehead, 2001). The LIR was estimated using the “Movement” module in program SOCPROG 2.7 (Whitehead, 2009). Eight models were fitted against various parameters for population closure, emigration, reimmigration and mortality (Table 1). The quasi-Akaike information criterion (QAIC) was used to account for data overdispersion (Whitehead, 2007). We used the ΔQAIC to select the best-fit model based on a ΔQAIC of ≤2 providing considerable support (Burnham and Anderson, 2003), whilst subjectively weighing the number of biologically relevant parameters estimated, and the comparability to other studies using these methods. The best-fit model was then bootstrapped for 100 repetitions to generate confidence intervals (95%) and standard errors (Buckland and Garthwaite, 1991).
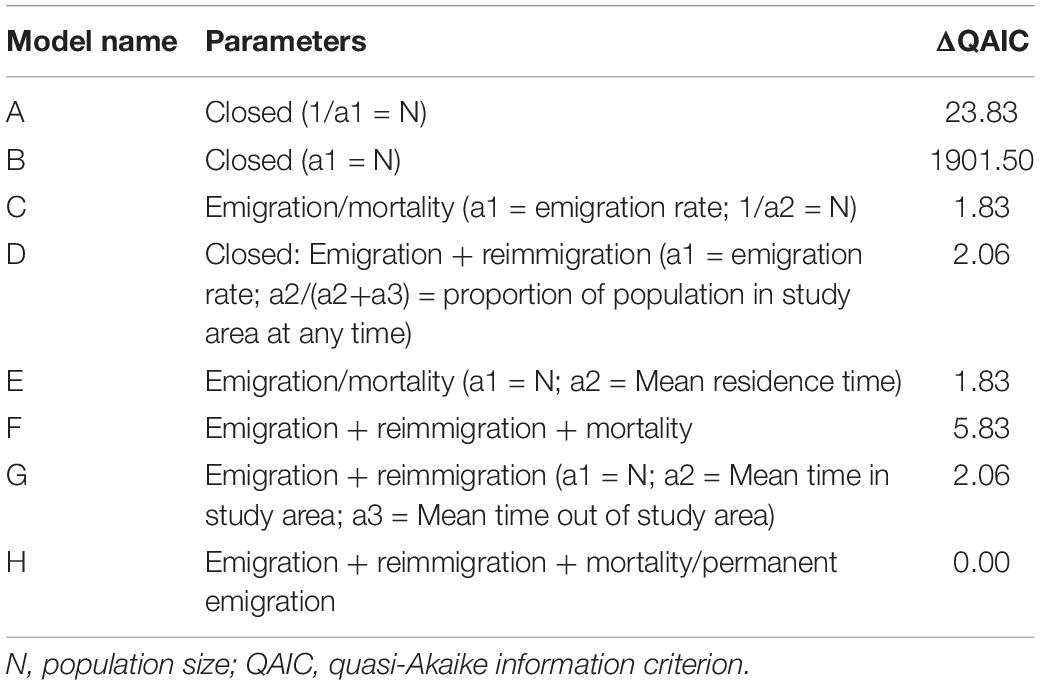
Table 1. Model results for maximum likelihood methods using parameters to test for population closure, mortality or permanent emigration, reimmigration, and residency as preset in program SOCPROG 2.7 (Whitehead, 2009).
Results
Visual Observations
Sighting Database
The ENRD sighting database contained 985 whale shark sightings between February 1999 and May 2019 (Figure 2). These data do not represent individual identification of whale sharks, but rather the total number of encounters over the time period. Most of these sightings were unaccompanied individual sharks, with only 150 encounters documenting more than one individual. There was distinct seasonality in whale shark encounters with 85.5% of all encounters being documented in the months of January, February, and March. This season appears to begin with whale sharks arriving around the island in November and December, however, sightings increased sharply starting in January. January had the highest number of sightings, with 38.5% of the total (Figure 3), whereas February and March had the next highest number of sightings with 28.9% and 18.1%, respectively. Encounters from visual observations showed a drastic decline in sightings from April to December with sharks seen infrequently in the surrounding waters over these months. Annually, whale shark sightings have increased towards the end of the study period with peaks corresponding with dedicated research expeditions and associated increased search effort.
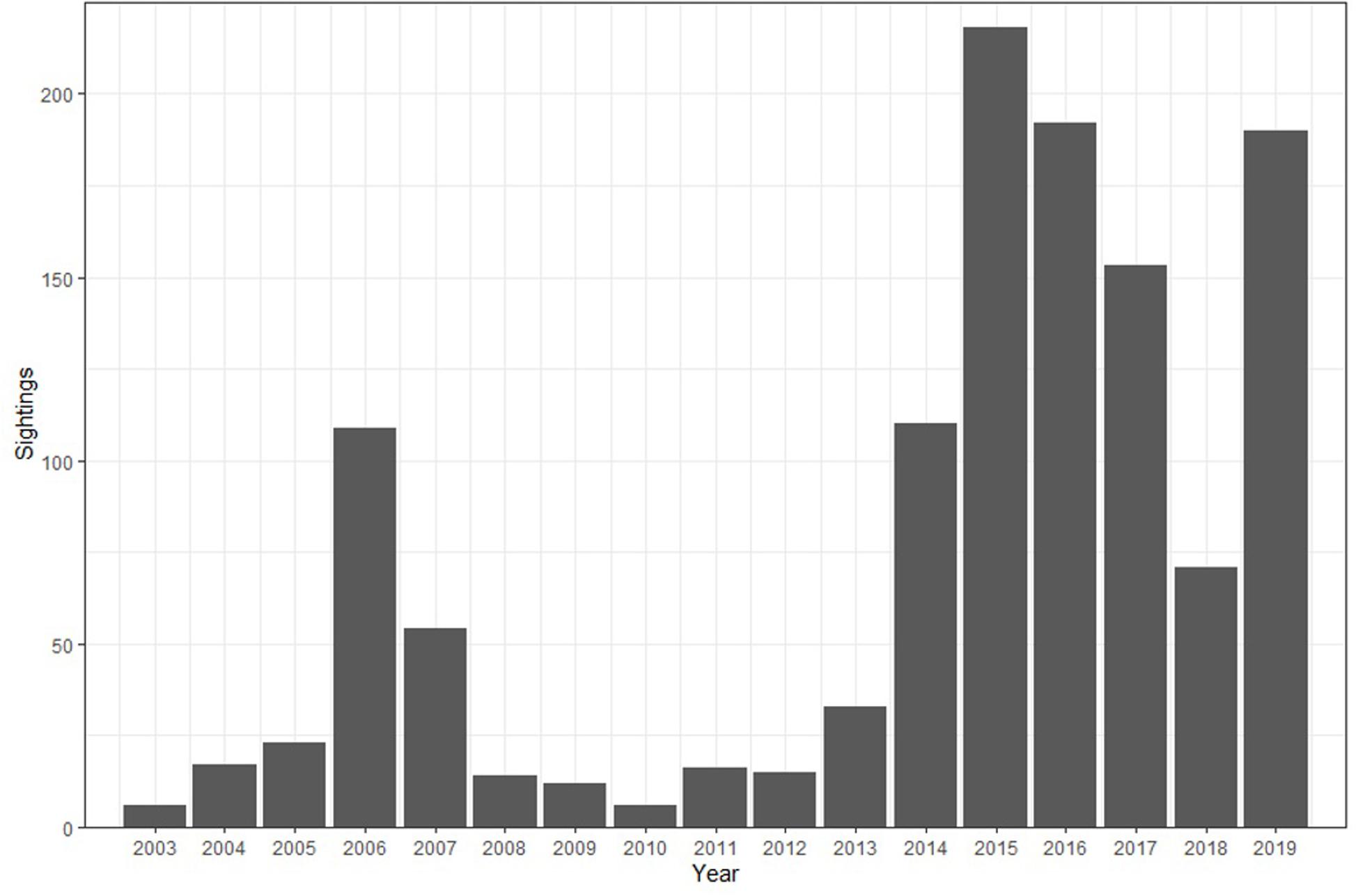
Figure 2. Sightings per year of whale sharks since 2003. Dedicated expeditions were conducted in 2015, 2016, 2018, and 2019. Note year 1999 is not displayed on the figure to eliminate gaps in coverage.
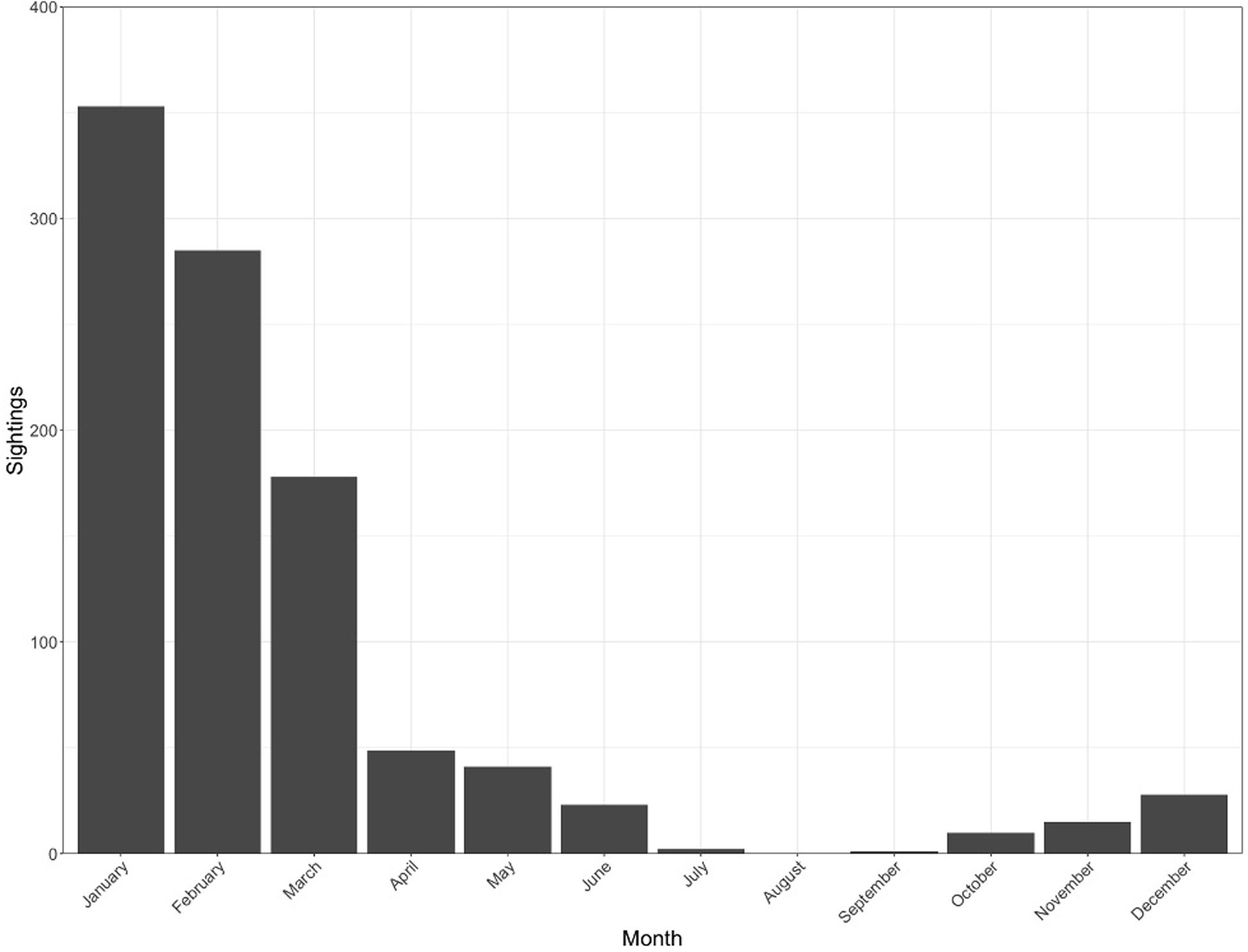
Figure 3. Sightings per month aggregated across all years since 1999, showing seasonality peaking between January and March.
On any given day 1-40 whale sharks were seen around St. Helena from both the ENRD database and dedicated research expeditions. A few extraordinary aggregations of over 30 individuals were noted, including an event off Egg Island on the 17th of January 2014, at Barn Cap on the 21st of January 2015, off Sugarloaf on 22nd of January 2015, and another in Flagstaff Bay on 13th February 2019. These aggregations were remarkable for being feeding events whereas most other sightings around St. Helena did not involve obvious feeding behavior. Whale sharks at St. Helena were seen feeding at the surface using the active surface suction-feeding mode described by Motta et al. (2010), but more often, they were observed swimming calmly 1-5 m below the surface. A range of different sizes was seen at these feeding events; however, the sharks involved were not visibly different in size (at ∼7-9 m) from those encountered at other times. Qualitative plankton tows taken at the time of the 2015 aggregation event indicated the presence of substantial numbers of fish eggs, associated with nearby schools of skipjack tuna (Katsuwonus pelamis) that were suspected to be spawning at the surface.
Mating Events and Reproductive Behavior
Putative mating behaviors of whale sharks were reported to ENRD staff on two occasions during the reporting period. These events took place in 2005 and 2007 at opposite ends of the island, both on popular fishing grounds, both approximately 16 km from shore. The observations were made by two different people and each was unaware of the observations of the other. The 2005 event was reported by the island’s chief fisheries officer at the time, whereas the 2007 event was reported by a career professional fisherman. On January 28, 2005 on the New Shoval fishing ground (5°50′21.97″W, 15°59′28.12″S; see Figure 1) the chief fisheries officer reported:
“Two whale sharks mating! Came together - - one on [its] back swimming below the other one, then came belly to belly, very near to the surface for a few minutes. Came alongside the boat, lifted its head out of the water. Quite a few remoras on them - - were pale white.”
On February 15, 2007 on the Dawson’s fishing ground (15°52′2.83″S, 5°42′19.25″W and see Figure 1) a professional fisherman reported:
“Saw two smaller ones (male) and one larger female. The two males were trying to mate with the female. Saw the male going belly to belly with female and other male also trying to get in there!”
While photo and video documentation were not made of these events, both observers are competent naturalists and would be expected to recognize mating behavior among sharks. In addition, during the 2019 expedition photo documentation was made of potential pre-copulatory behaviors and post-copulatory wounds. A male whale shark followed a female whale shark and positioned his rostrum towards the posterior of the female which resulted in contact of the female’s caudal fin against the male’s snout (Figure 4). Furthermore, a female whale shark was encountered with abrasions on her left pectoral fin that may be indicative of recent mating attempts (Figure 5).

Figure 4. Solicited contact between a male and female whale shark with the male positioning his snout on the caudal fin of the female.

Figure 5. Abrasions on the pectoral fin of a female whale shark that may be indicative of recent mating behavior.
Photographic Identification
The Wildbook database contained 462 encounters from April 2013 to May 2019 with 277 individual sharks identified. The majority of all individual sharks (n = 180, 65.4%) were encountered only once across the study period (Supplementary Figure 1). Of the individuals that were encountered more than once (n = 97), a total of 35% (n = 34) were seen between seasons with an average of 1.4 seasons per individual (range:1-4 seasons). Sex was determined for 257 sharks. Of these 53% (n = 137) were males, and 47% (n = 120) were females, an approximately even sex ratio. Sex ratios did not differ between month, with January exhibiting a 1.07:1 sex ratio and February and March exhibiting a 1.42:1 sex ratio of males to females. Of the male sharks, 108 were assessed for clasper morphology with 86.1% (n = 93) being identified as mature and 13.8 % (n = 15) identified as subadult (n = 9) and immature (n = 6). Size estimates were documented for 89 female and 107 male whale sharks and yielded an average size of 8.1 m (Figure 6). The average estimated size was 7.9 and 8.4 m for male and female whale sharks, respectively. Assuming a size at maturity of 9 m for female whale sharks and removing eight sharks in the ambiguous 8-9 m range, 50.6 % (n = 41) of female sharks with length estimates were conservatively determined to be mature. Laser photogrammetry from the 2016 expedition yielded an average total length of 9.51 m ± 0.18 (mean ± s.e) (8.55–10.79 m, n = 15). During the 2018 and 2019 expeditions, a total of 154 unique individuals were sighted and detailed observations of injuries were documented. Injuries were documented on 56.4% (n = 87) of all individuals and were mainly minor and well-healed in nature. A few instances of well-healed major injuries were documented including a shark with its entire dorsal fin missing, a shark with some very large apparent bite marks, and a shark with entanglement wounds around its head and mouth, however, major wounds and fresh wounds were not a common occurrence.
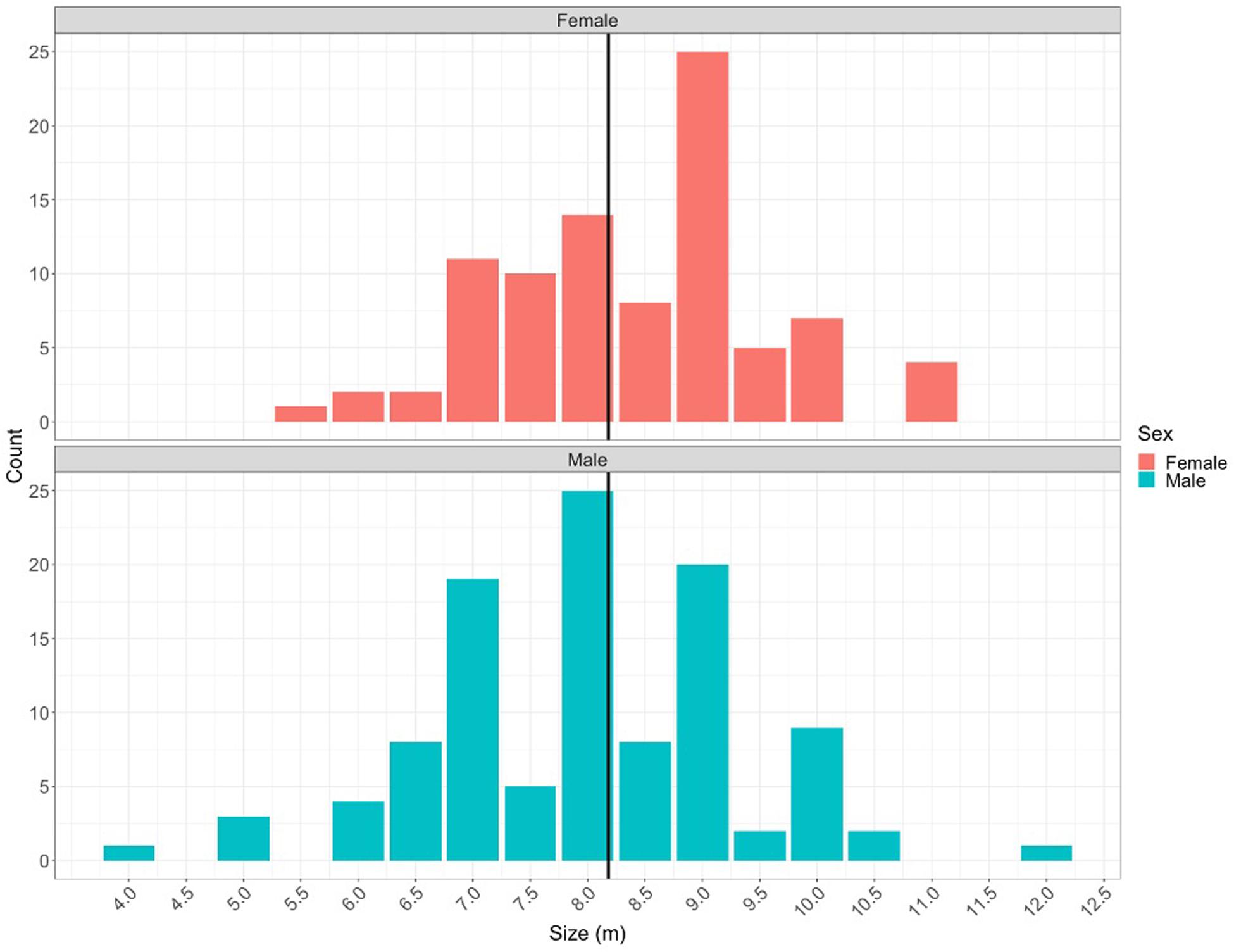
Figure 6. Size frequency of male (teal) and female (red) whale sharks encountered in St. Helena. The black vertical line represents the average of all sharks encountered at 8.1 m.
Telemetry
Satellite Telemetry
Horizontal and vertical movements
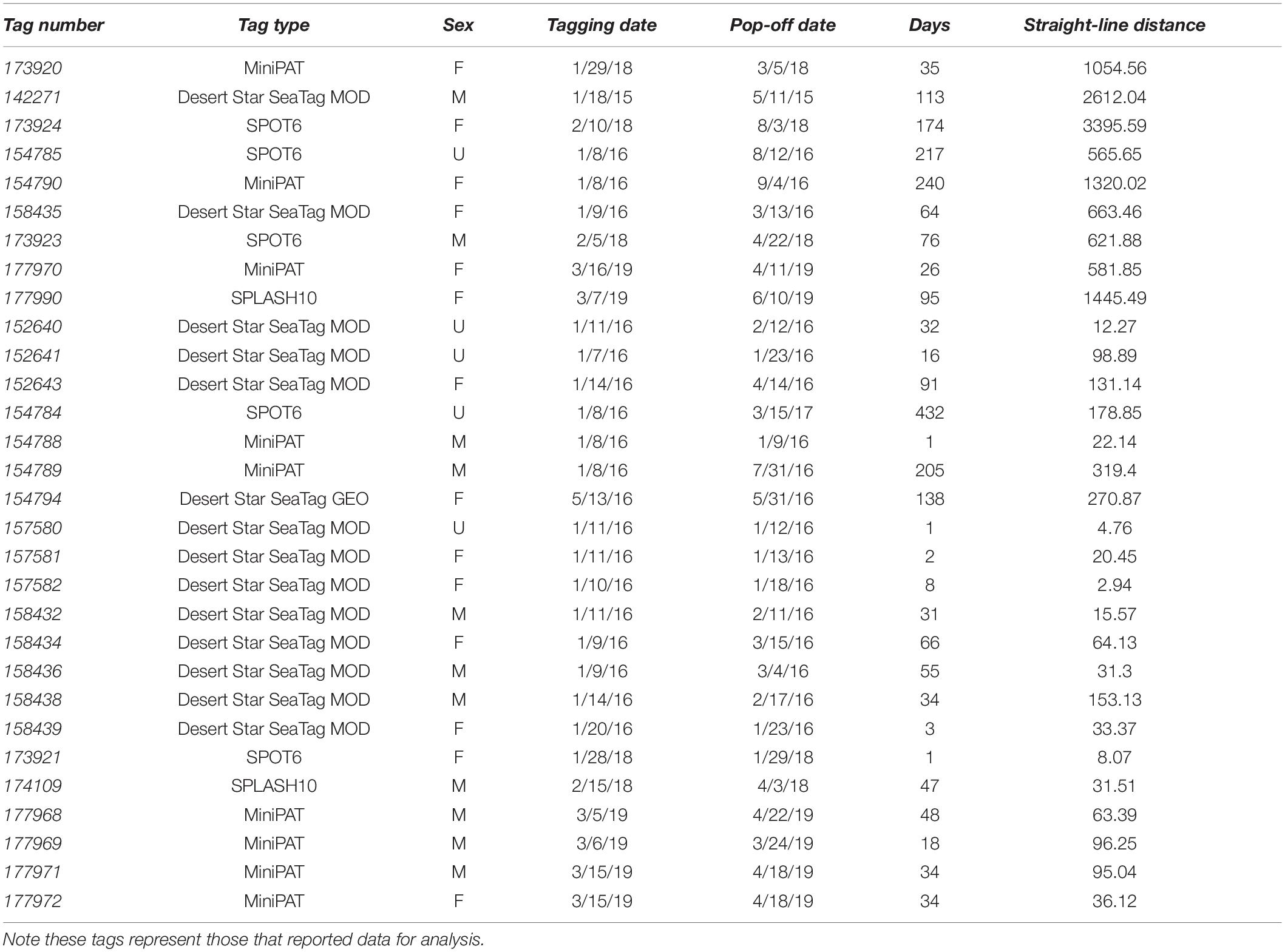
While a total of fifty satellite tags were placed on whale sharks, only thirty reported reliable data from which horizontal movements could be evaluated. There were no unrealistic movements (>200 km/day) observed in any of the tag datasets. Among tags that reported data, an average deployment duration of 74.84 ± 19.71 days was obtained (Table 2). The average straight-line distance between tagging and pop-off was 465 km with a median distance of 97 km. The majority of tags (n = 21) remained near St. Helena (Figure 7) and sixteen sharks reported straight-line distances of less than 100 km between tagging and pop-off locations, over an average of 24.8 days. No obvious habitat use was observed in the tracks as there appeared to be substantial individual variation in how animals moved around the island. Additionally, many tags (n = 14) released prematurely due to the crush depth being reached shortly after the animals left the proximity of St. Helena.
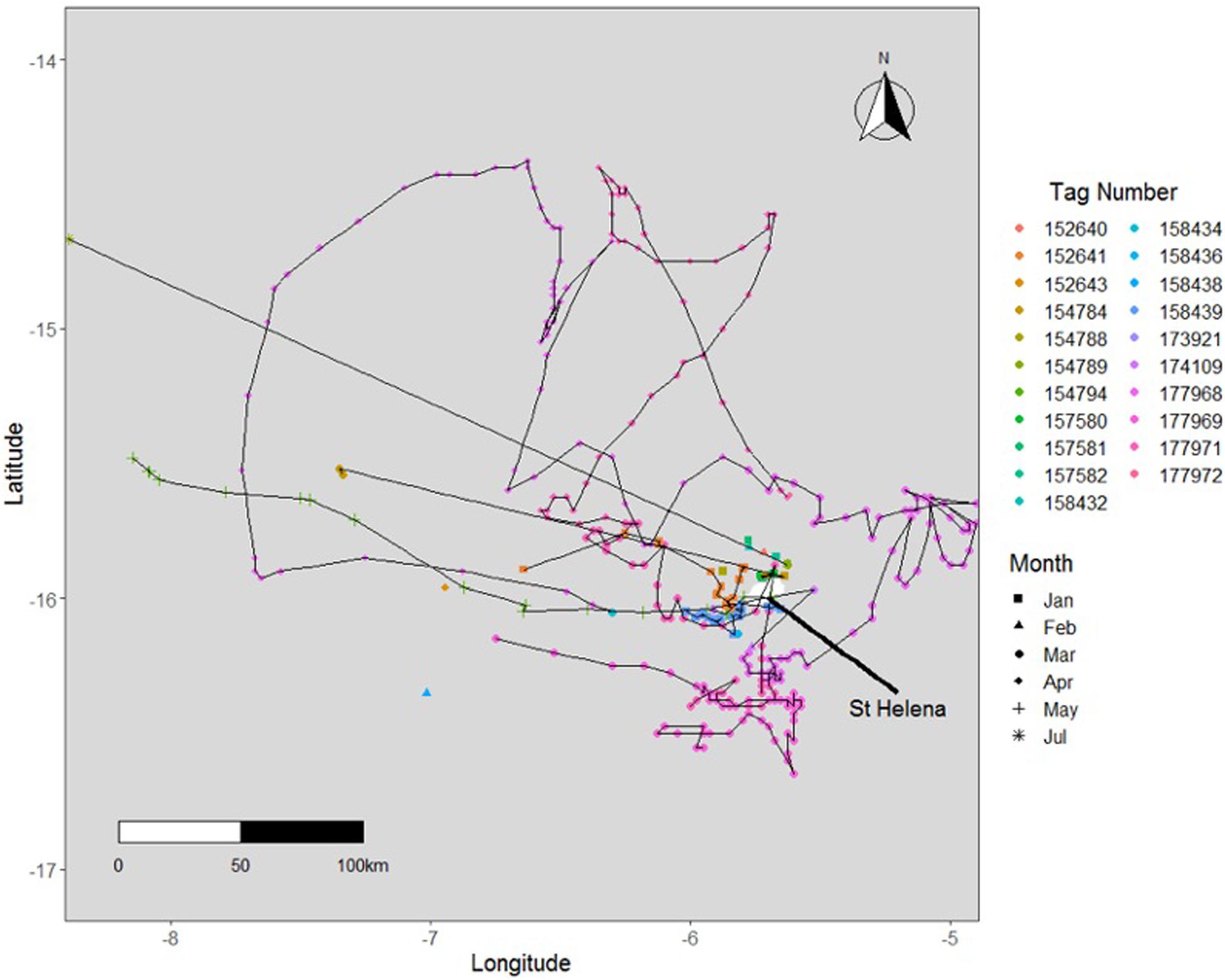
Figure 7. Tracks of satellite-tagged whale sharks from St Helena 2015-2019 representing individuals that have travelled short distances (<300 km) from St. Helena.
Five tagged individuals were seen to venture away from St. Helena, recording straight-line distances of over 1,000 km over an average of 131 days (Table 2). One tagged individual (tag #173924) swam to the northeastern coast of Brazil, near the archipelago of Fernando de Noronha, a distance of 3,395 km from the tagging location (Figure 9). These were class 1 location reports representing reliable transmissions with improved accuracy, but no other data packets were received, and thus a complete track could not be generated. Another individual (tag #173920) traveled 1,054 km east of the tagging location, towards the western coast of Namibia and Angola (Figure 9). One individual (tag #142271) moved to the Gulf of Guinea near the coast of Nigeria, over 2,500 km distance (Figure 9). This tag washed ashore shortly after detachment, before it was able to relay its data payload, and therefore a track could not be generated for this individual. Three sharks (#177990, #154790, and #154785) traveled towards Ascension Island, with one individual (tag #154785), returning towards St. Helena, after which its tag released in August of 2016 (Figure 8). Two sharks were in close proximity to Ascension Island during the months of April, May, and June.
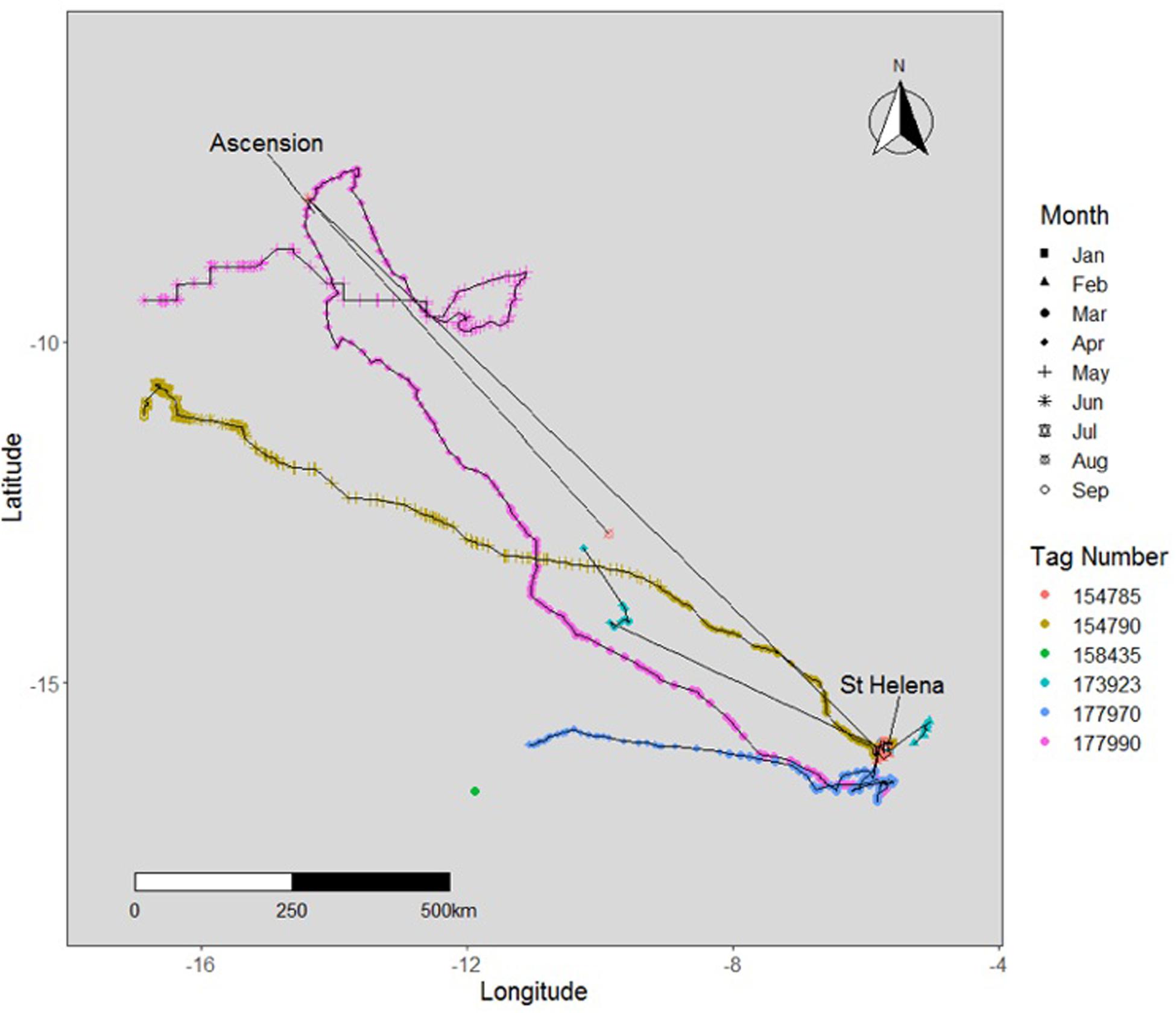
Figure 8. Tracks of satellite-tagged whale sharks from St Helena 2015-2019 representing individuals that have traveled intermediate distances (500-1500 km) toward Ascension Island from St. Helena.
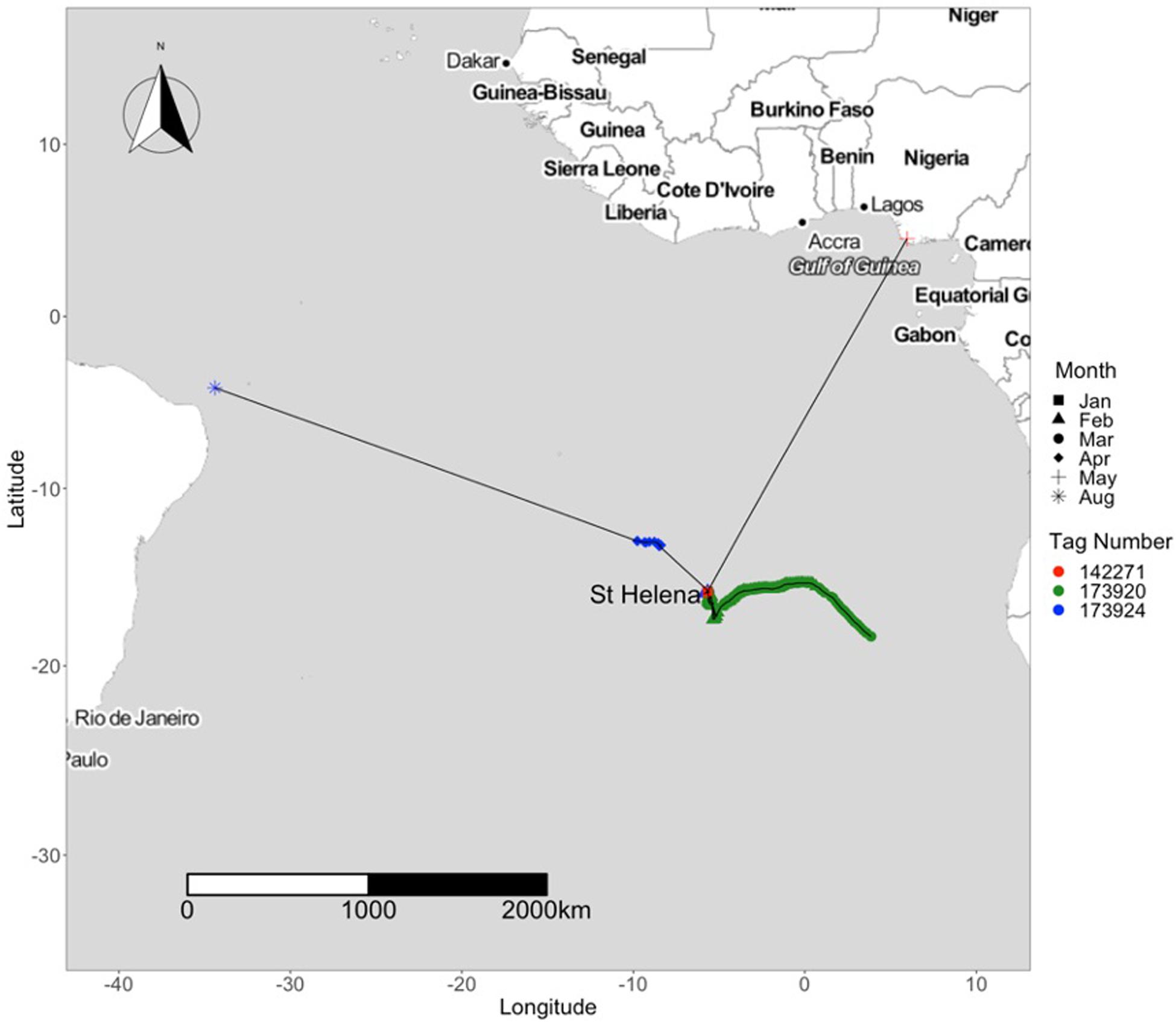
Figure 9. Tracks of satellite-tagged whale sharks from St Helena 2015-2019 representing individuals that have traveled greater distances (>1000 km) from St. Helena.
In general, there was north westerly movement away from the island, with only a handful (n = 4) of individuals venturing east. Variations in individual movement patterns showed that some individuals left the surrounding water relatively quickly after being tagged, whereas others appeared to spend more time navigating in closer proximity to St. Helena. Eight out of nine MiniPAT tagged sharks (5 males and 3 females) reported diving data for analysis. There were 509 daily dives reported, with a median daily maximum depth of 619 m and a maximum depth overall of 1,879 m (Figure 10). The 500-700 m depth range accounted for 65.6% (n = 334) of all maximum daily dives. It is also worth noting that 6.6% of all daily dives were to depths greater than 1,000 m (n = 34); evidently these dives took place once the individuals left the proximity of St. Helena.
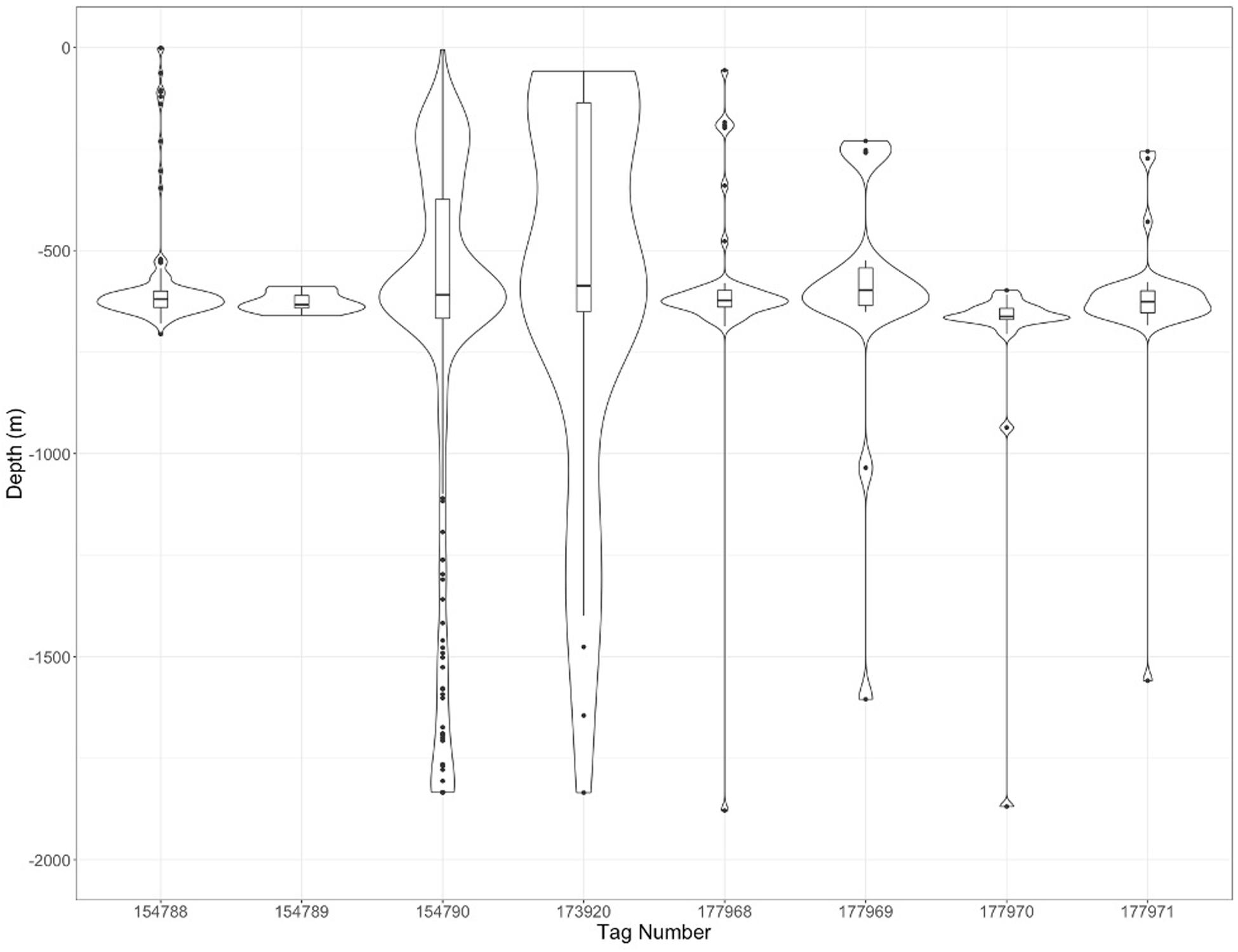
Figure 10. Diving behavior of individual whale sharks from St. Helena. Black line in boxplot represents the mean diving depth while the extent of the boxes represent the interquartile range.
Acoustic Telemetry
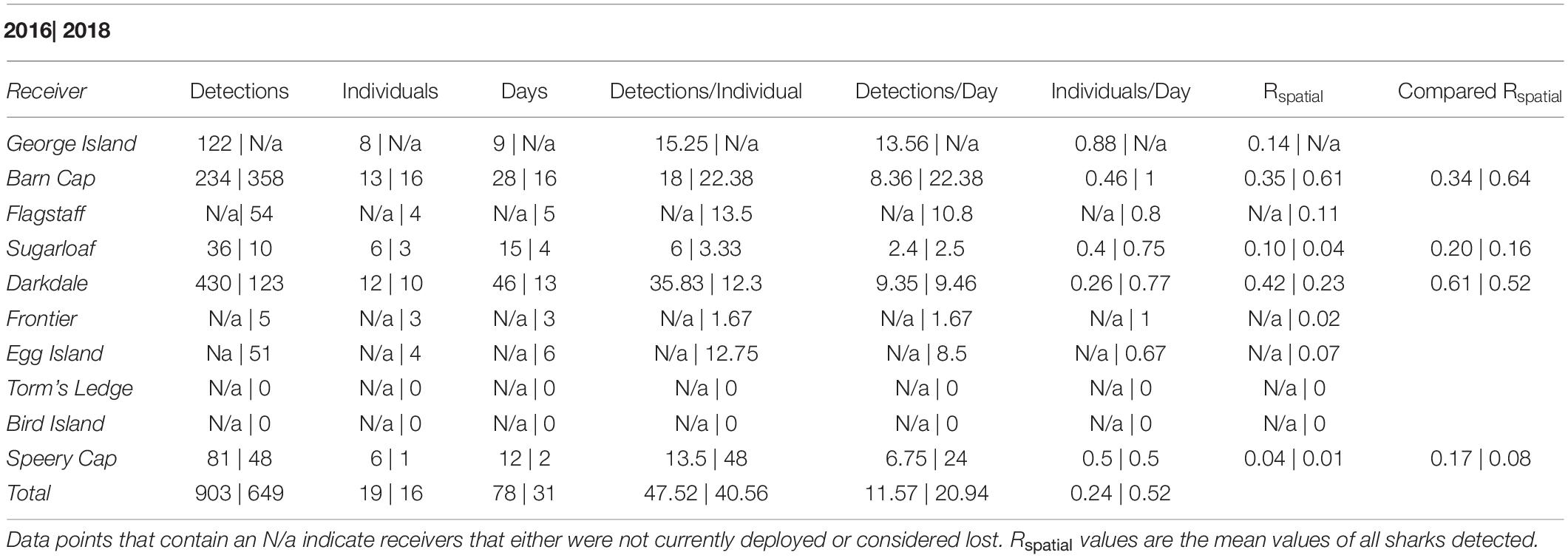
Data were downloaded in both the 2018 and 2019 field seasons, representing information from the 2016 and 2018 field seasons. There was an initial total of six receivers deployed in 2016 with coverage around the island (Barn Cap, Darkdale, Sugarloaf, Speery Cap, George Island, and Torm’s Ledge). An additional five receivers (Frontier, Bird Island, Flagstaff, Torm’s Ledge replacement, and Egg Island) were deployed in 2017 to reduce gaps in coverage (Figure 11). A total of 43 individual sharks were tagged with acoustic transmitters. Eight tagged individuals (18.6%) were not detected on any of the receivers and two receivers, Torm’s Ledge and Bird Island, did not have any detections recorded in the dataset. Tagged individuals were detected 49.6 ± 9.37 times at 2.5 ± 0.24 receivers over both years (Figure 12). There were 903 detections of 19 individuals over 78 distinct days in 2016. In 2018, there were 649 detections of 16 individuals over 31 distinct days (Table 3). Individual sharks were detected in the array over an average of 5.7 (range 1-27) days.
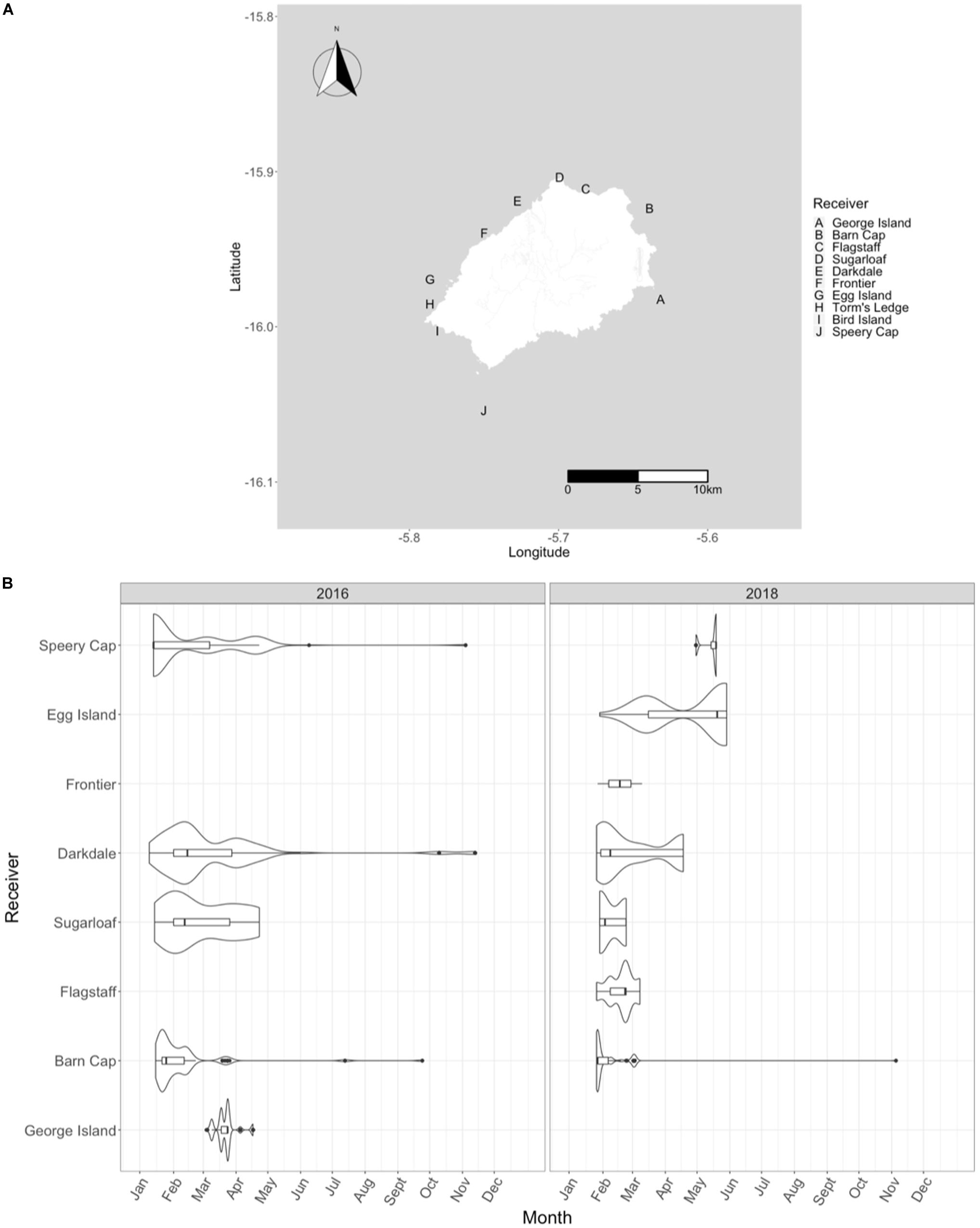
Figure 11. Acoustic telemetry data from tagged whale sharks in St. Helena. Panel (A) shows the names and locations of the acoustic receivers. Panel (B) is a violin plot of the number of detections per year. The line in each boxplot represents the mean while the extent of the boxes represent the interquartile range. Further information regarding each receiver is provided in Table 3.
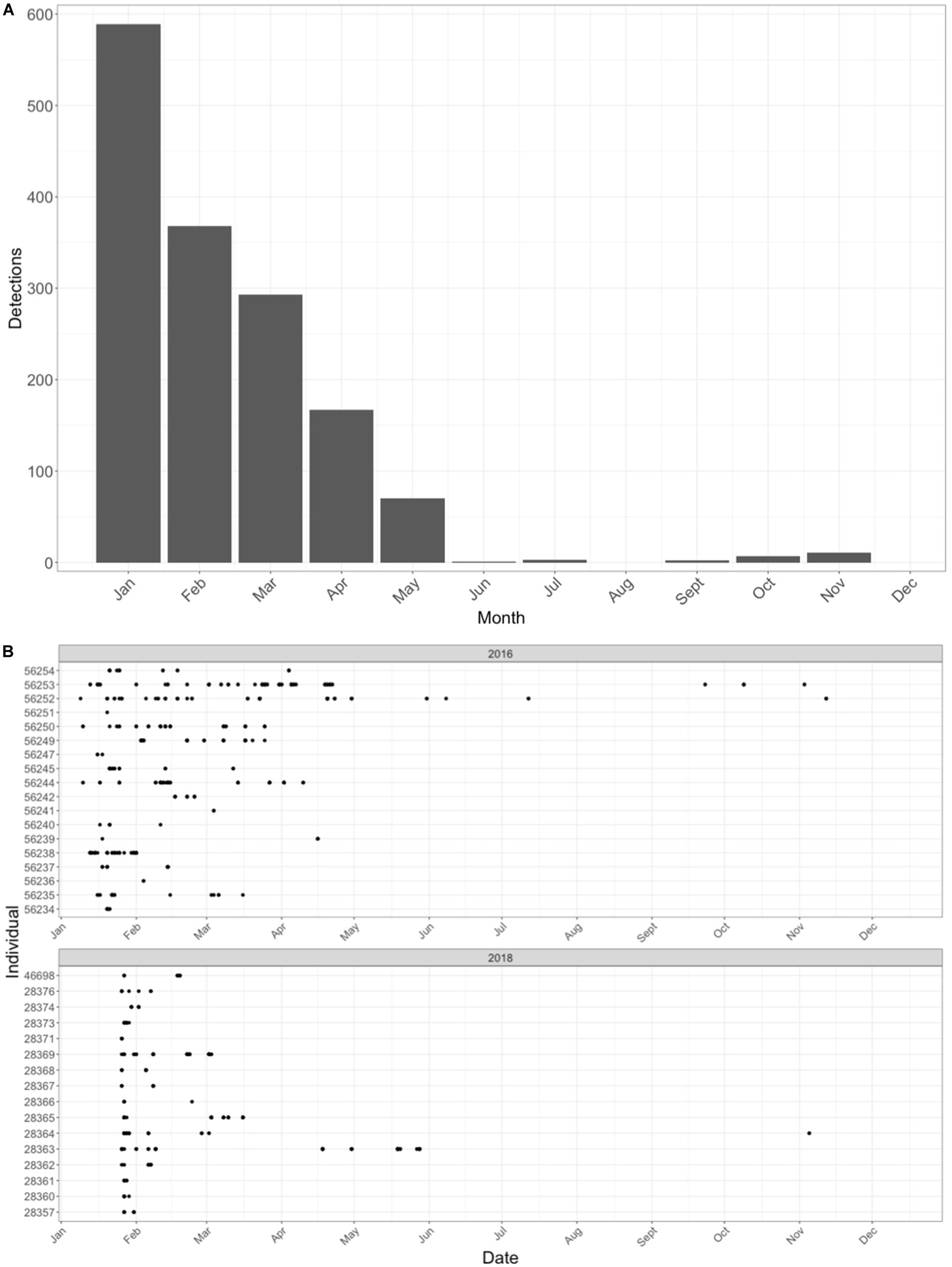
Figure 12. Acoustic telemetry data from tagged whale sharks in St. Helena. Panel (A) shows the number of detections per month. Panel (B) shows the detections of individual sharks in 2016 and 2018 over the course of the year.
Barn Cap and the wreck of the Darkdale recorded 73.5 and 73.9% of all detections in 2016 and 2018, respectively. Rspatial values were calculated for each receiver from each individual and showed similar site use and fidelity results. In 2016, Darkdale and Barn Cap led receivers with values of 0.42 and 0.35, respectively. In 2018, Barn Cap and the Darkdale reported values of 0.61 and 0.25, respectively. When comparing Rspatial values from receivers that were active for both years similar patterns were seen, however, annual variations in receiver use were also documented with Barn Cap increasing from 0.34 to 0.64 from 2016 to 2018 (Table 3).
The majority of individual detections were documented in January and declined steadily until June, in which detections were effectively zero (Figure 12). When removing detections that were recorded at the start of a second season, there were on average 35.8 days (range 0-185) between first and last detection. On average whale sharks exhibited an Rmax of 0.24 (range: 0-1) and an Rmin value of 0.008 (Supplementary Table 1). Three individual sharks were recorded returning to St. Helena waters at the beginning of a new season after their initial tagging seasons. These individuals returned during the months of September and November and were documented at Darkdale, Barn Cap, and Speery Cap.
There was one confirmed instance of tag loss, wherein an individual that was acoustically tagged in 2018 was re-sighted in 2019 and no acoustic tag was present. However, the overall frequency of tag shedding at this location is unknown. Of further note, the George Island receiver was not recovered for maintenance during the 2018 field season and therefore battery life was uncertain and tagged individuals in 2018 were likely not detected due to low battery. Furthermore, the receiver at Speery Cap in 2016 recorded 938 (81.9% of all detections) detections of one tagged individual (#56232) over the course of 31 h, with detections being recorded every few minutes during that period. The likely cause for this observation is the shedding of a transmitter in close proximity to the receiver, leading to continuous detections over that time period. This tagged individual was only recorded at Speery Cap and was removed from further analysis. It should also be noted that the maximum depth rating for the acoustic tags was significantly less than the average daily maximum dive depth for whale sharks at St. Helena (determined from archival satellite tags), so it is likely that a significant number of tags were lost due to crushing at depth.
Lagged Identification Rate
Models C, E, and H had substantial support (ΔQAIC < 2; Table 1). However, models C and E produced fewer parameter estimates than did model H, whilst biasing towards single-sighting data. Model H best-fit the empirical data (ΔQAIC = 0), described through a combination of residency, population size, reimmigration and mortality or permanent emigration. The LIR declined steadily to zero, from 1 to 64 – 88 (mean 75.4) days, followed by an increase between 268 and 470 (mean 376.9) days and again at mean 742.4, 1306.4, and at 2218.4 days, suggesting an interannual periodicity of some sharks at the site (Supplementary Figure 2). The model estimated a mean 102.1 ± 35.7 (95% CI 21.1 – 146.4) whale sharks at the study site at any one time, each residing a mean 18.9 ± 22.5 (95% CI 0.5 – 70.8) days at the study site whilst spending 32.8 ± 50.7 (95% CI 2.5 – 164.4) days outside the site. Mortality or permanent emigration was estimated at 0.00056 ± 0.00039 (95% CI 0.0001 – 0.0014), an apparent survival of 0.796 ± 0.857 year–1.
Discussion
Population Characterization
The whale shark population that visits St. Helena is a seasonal aggregation of mostly adult male and female animals in approximately equal ratio. This is unusual in two ways; first, because most known aggregation sites are dominated by juvenile animals, and second, because most aggregations are dominated by males, in ratios varying from 3:1 to 10:1 or more (Norman et al., 2017). Macena and Hazin (2016) found a similar trend in seasonality and size in the Equatorial Atlantic at St. Peter and St. Paul Rocks, with peak whale shark sightings occurring between January and June, and sharks averaging 8.27 m (2.5-14 m) in total length. However, the sex ratio of 14 sharks observed at that location differs from St. Helena, with a 3.6:1 ratio of females to males recorded. These authors also reported reproductive behaviors and suggested that these oceanic islands in the Atlantic play a significant role in whale shark reproduction. The unique presence of approximately equal numbers of mature adults of both sexes at St. Helena supports this hypothesis. St. Helena is unique in hosting a significant population of adult whale sharks, with similar numbers of both sexes, and appears to play an important role in their reproductive ecology.
The whale sharks documented at St. Helena were estimated 4-12 m in length with average lengths around 8 m, which is the upper end of those seen at most coastal aggregations (4.5-8.5 m), but smaller than some of large females regularly seen at Darwin’s Arch in the Galapagos (9-13.5 m) (Norman et al., 2017). However, there are known biases of visual estimates with larger sharks often being underestimated in size (Perry et al., 2018). Laser photogrammetry of 15 sharks yielded an average length of 9.15 m, and the average sizes of whale sharks encountered in St Helena may be closer to the laser photogrammetry data, although that sample size is small. During some expeditions there were individuals that were visually estimated to be larger than 12 m total length, however, visual estimates should all be assessed with caution (Perry et al., 2018). Uncertainties and errors in visual length estimates can easily influence the maturity assessment for female whale sharks reported here and should be interpreted cautiously until more accurate methods to ascertain female maturity status are developed. Nonetheless, the size ranges and clasper morphology of whale sharks in St. Helena further confirms the mature status of the population (Rowat and Brooks, 2012), although immature and smaller (∼5 m) sharks were also occasionally encountered. The large number of individuals that have been encountered only once or a handful of times shows the transient and open nature of this population. The presence of feeding whale sharks may indicate that sharks are seasonally arriving in St. Helena to feed allowing them opportunistic chances to mate due to the presence of mature conspecifics of the opposite sex, or that the feeding is opportunistic, and that mating is the main driver of their presence at the island.
Saint Helena appears to be a globally important site for whale sharks, not only due to the occurrence of adults and possible mating, but due to the number of animals. The maximum likelihood methods estimated a mean ∼102 whale sharks at any one time, considerably higher than that estimated at coastal sites in the Indo-Pacific using the same methods (e.g., 16, Southern Leyte, Philippines, Araujo et al., 2017; 35, Mafia Island, Tanzania, Prebble et al., 2018), yet similar to that observed in offshore Qatar (116, Prebble et al., 2018) or in the Gulf of Mexico (136, de la Parra, unpub. data). This is a considerable number of whale sharks, and highlights the importance of St. Helena for the species. The LIR revealed that some whale sharks have an interannual periodicity at the site, following a complete absence of individuals after ∼75 days – an interesting feat given the remoteness of the site and the large proportion of single-sighted whale sharks. However, this result follows the seasonal nature of the aggregation with peaks occurring between January and March, ∼90 days. This philopatry to the site has also been observed in adult whale sharks at Ningaloo Reef in Australia (Norman and Morgan, 2016) and at Donsol, Philippines (McCoy et al., 2018), and is common amongst juvenile-dominated coastal aggregations (Norman et al., 2017).
Although a high level of transience was overall observed, they do appear to spend some time in St. Helena as estimated through the LIR and acoustic telemetry. Whale sharks spent a mean 19 days in St. Helena before departing the site, similar to that observed in Qatar (18 days, Prebble et al., 2018), the Red Sea (12 days, Cochran et al., 2016) or Honduras (12 days, Fox et al., 2013), yet lower than that estimated at coastal sites such as Donsol (50 days, McCoy et al., 2018) or Tanzania (31 days, Prebble et al., 2018). These estimates of residency using photo-ID data are supported by the acoustic telemetry findings, where detection peaks at various stations were 2–3 weeks apart (Figure 12). The estimated apparent survival was high given the inferred transience within this population. However, it is similar to that observed in Donsol (0.78 year–1, McCoy et al., 2018), Southern Leyte (0.74 year–1, Araujo et al., 2017), or among non-scarred individuals at Ningaloo Reef (Australia) as estimated through different methods (0.82 year–1, Lester et al., 2020), and contrasting with that observed in Tanzania (0.97 year–1, Prebble et al., 2018) or scarred individuals at Ningaloo Reef (0.88 year–1, Lester et al., 2020). However, this could be attributed to the lack of the model’s ability to distinguish mortality from permanent emigration (Holmberg et al., 2008). Some permanent emigration is likely as evidenced by the LIR falling to zero after around 75 days (Whitehead, 2001).
Mating Events and Reproductive Behavior
Reproductive behaviors in elasmobranchs have proven difficult to study due to a lack of direct observations. However, existing knowledge of elasmobranch reproductive behaviors as summarized by Pratt and Carrier (2001); Carrier et al. (2004) can help elucidate the suspected mating and reproductive behavior that was documented for whale sharks in St. Helena. Generally, elasmobranch courtship begins when one individual signals to another that they are reproductively receptive, with either olfaction and/or motor displays being primary sensory cues. During this time males may cease feeding, become aggressive, and display dominance behaviors to others. A female may be approached by either a solitary male or group of males that follow her and focus on or “nose” her cloaca. The male’s mouth is then used to make and maintain contact with the female, typically by grasping the pectoral fin. After attaining a hold of the female, copulatory behaviors likely vary by species/female cooperation and can occur anywhere in the water column from the seafloor to the sea surface. In successful copulation, clasper insertion occurs and the female becomes relatively motionless with copulation taking anywhere from a few seconds to hours.
Pre-copulatory, copulatory, and post-copulatory behaviors were all observed at St. Helena. The two eyewitness accounts of mating in the ENRD/SHG sightings database support the notion that the Central Atlantic plays a role in whale shark reproductive ecology and, to our knowledge, are the only documented observations of mating in this species. One of these accounts was provided by the island’s chief fisheries officer at the time, and the other by a career professional fisher, and we found both to be credible witnesses. On the 2019 expedition, members of our team witnessed following behavior and solicited contact between the snout of a male and the caudal fin of a female suggesting that this may be pre-copulatory behavior. However, copulatory behaviors such as rotation or insertion of claspers were not observed. Dominance behavior was observed in St. Helena between two male whale sharks with the larger male (est. 9 m) positioning himself above and behind the smaller male (est. 8 m) shark and appearing to actively direct him towards the bottom of the water column, similar in description to two male sand tiger sharks (Carcharias taurus) in captivity that circled and tailed one another until the alpha male forced the beta male out of the area prior to copulation (Gordon, 1993). Whether dominance hierarchies and behaviors are common in whale sharks has yet to be proven as they were rarely documented throughout the rest of the expeditions. Furthermore, one female whale shark was encountered in St. Helena that appeared to bear tell-tale pectoral mating scars that were similar to those reported by Macena and Hazin (2016). However, the relatively low frequency of pectoral mating scars on females may not be unusual as whale sharks are filter feeders and their teeth are not as prominent as other species potentially making mating scars less noticeable. Direct mating observations of great white sharks reported by a fur seal observer mentioned that they had positioned belly-to-belly and became motionless (Francis, 1996), a shared observation between both reports of mating in St. Helena. Taken together, these pieces of evidence support the idea that St. Helena is a mating area for whale sharks.
Although observations of mating have remained elusive for whale sharks, there have been some reports of whale shark reproductive behaviors that have been observed elsewhere. Whale shark tour guides operating in Donsol, Philippines have reported reproductive-like behaviors (McCoy et al., 2018) and researchers have observed an 8 m male whale shark unfurling its clasper and banking in this same area (Miranda et al., in revision). Additionally, four male whale sharks were observed at a nearby shoal displaying belly to belly behaviors, however, no female was observed in close proximity, although visibility was poor (Miranda et al., in revision). These behaviors are similar to aerial footage of a male whale shark rolling over on its back in proximity to another smaller, presumably female, individual that was recorded at Ningaloo Reef, Australia (T. Klein, pers. comm.). A 9.5 m male whale shark was observed rolling over and rotating his claspers in proximity to a boat in the Seychelles (D. Rowat, pers. comm in Macena and Hazin, 2016.). Additionally, male whale sharks at the Georgia Aquarium and Okinawa Churaumi Aquarium have also been recorded doing this behavior as well (A. Dove unpub. data; Matsumoto et al., 2019). A male shark near St. Peter and St. Paul’s rocks exhibited similar behavior, rolling over in proximity to a fishing vessel and repeating this behavior three times over the course of 10 minutes (Macena and Hazin, 2016). A mature male whale shark Okinawa Churaumi Aquarium has been observed biting the pectoral fins of the females in the exhibit, a display of pre-copulatory behaviors (R. Matsumoto pers. comm.). Large females with distended bellies were also observed at St. Peter and St. Paul’s Rocks with markings on their pectoral fins that the authors suggested were caused by mating attempts.
In addition to observed mating and reproductive behavior, there have been a few sightings of neonates in the Central Atlantic. Three male neonate whale sharks (∼56 cm total length) were captured in the Central Atlantic in the Gulf of Guinea near the Equator (Wolfson, 1983). All of these individuals were captured by fishing fleets in waters that ranged in depth from 2,600 to 4,700 m. Additionally, two more neonates were observed in close proximity to St. Peter and St. Paul’s Rocks (Kukuyev, 1996). One individual, a 59 cm female, was captured in a landing net beyond the continental slope off Sierra Leone while the other was found in the stomach of a blue shark caught in the Central Tropical Atlantic (Kukuyev, 1996). Furthermore, two sharks were observed at 1.8–2 m in size from St. Peter and St. Paul’s rocks (Hazin et al., 2008), a size range that is rarely encountered anywhere. These observations further support the idea that the Central Atlantic is a prime area for whale shark reproductive ecology.
Horizontal and Vertical Movements
Whale shark habitat use at St. Helena appeared to incorporate all sides of the island to greater or lesser degree, but the anecdotal perceptions of local residents that whale sharks are concentrated on the leeward side do seem to be somewhat supported by the acoustic array data. Two receiver stations in the north of the island, Barn Cap and the wreck of the Darkdale, recorded 68.3% (1145/1677) of all reception events. Additionally, these receivers led in Rspatial calculations confirming their value as important sites for whale sharks in St. Helena. The Speery Cap receiver recorded 15.1% (254/1677) of all detections and may represent another important location for whale sharks around St. Helena, although this location is on the windward side of the island and limited by surface search effort and accessibility. Furthermore, when exploring Rspatial values between years there were changes in these values suggesting that habitat and site use may vary between seasons. Seasonality of detections in the array matched those of surface-based assessments, viz animals begin to arrive at the island in November and December, with the aggregation peaking in January and petering out by May. This confirms the value of validating visual observations with unsupervised passive acoustic monitoring as suggested by Cagua et al. (2015); Cochran et al. (2019). Residence indices were similar to those from other seasonal sites (Shib Habil: Rmax = 0.36, Rmin = 0.05) and in contrast to sites that had cryptic residency of whale sharks year-round (Mafia: Rmin = 0.24) further supporting the seasonal nature observed from the sighting data (Cagua et al., 2015; Cochran et al., 2019). The maximum residence indexes calculated for tagged sharks shows that they spend roughly 23% of their time, or ∼84 days, in St. Helena waters. This value is similar to the permanent emigration of ∼75 days predicted by the LIR model and both are representative of the seasonality observed in the sighting data.
Our attempts to learn about connectivity between whale shark populations in St. Helena and other geographic locations were hampered by the remoteness of the island and the extreme deep-diving behavior of the tagged animals in adjacent oceanic waters. Remoteness is a problem because any given tag must remain functional on the animal for at least 1,120 km in order to reach the nearest land (Ascension Island) and more than 2,000 km to reach a continental coast. The extreme deep diving behavior of the tagged animals, however, tended to mean that the tags detached prematurely due to the triggering of the crush depth safety release mechanism, long before animals reached another site. In other words, extreme vertical movements prevented us from properly documenting horizontal movements. The deep-diving capabilities of the whale shark appears to exceed current technological capacity to document their biology; new telemetry tools will need to be devised in order to properly document their movement patterns.
The general horizontal movement patterns of whale sharks tagged in this study revealed a northwest movement away from the island. This may indicate that whale sharks are following the prevailing currents which primarily head west towards Brazil and this could potentially be an energy efficient or thermoregulatory-mandated way to travel away from the island, however, they may also be traveling towards other key features as well. The horizontal movements that were documented in this study included several tracks towards Ascension and sometimes back again, which is perhaps not surprising since it is the closest land and one of the only other oceanic islands in the tropical South Atlantic. There was no documentation of any archival or real-time tracks that would suggest use of the habitats of Sysoev, Cardno, and Bonaparte seamounts near St. Helena. Nonetheless, not all sharks exhibited this northwest horizontal movement pattern away from the island, and there appeared to be variation in each individual’s travel pattern. Among one of the most distant tag detachments detected by ARGOS took place in an offshore oilfield near the Niger Delta of Nigeria, in the Gulf of Guinea, some 2,612 km to the NE of St. Helena, on May 11th of 2015. Unfortunately, this tag washed up on a beach near Lagos shortly afterwards, before it had appreciable time floating at the surface to transmit its data payload. Another tag detached from an individual halfway between St. Helena and the coast of Angola and Namibia on March 3, 2018. Sequeira et al. (2014) analyzed a comprehensive data set from logbooks of tuna purse seine fisheries, covering 31 years from 21°N to 15°S and 34°E to 14°E, which showed significant reports of whale sharks along the western coast of Africa south of the Gulf of Guinea. The highest number of documented sightings was from July-September (1153) with April-June having similar numbers (1070); in contrast January–March only had seven encounters over the same time period. A similar hotspot off the coast of Gabon was found from April-September and is thought to be linked to increases in primary productivity related to nutrient runoff from the Congo river, which peaks in April–June (Capietto et al., 2014; Escalle et al., 2016). Whether this means that whale sharks are leaving St. Helena around April to venture to the feed on the western coast of Africa warrants further investigation.
A different tag transmitted a handful of data packets from a location near Fernando de Noronha Island, Brazil, on August 3rd, 2018, more than 3,300 km to the west-northwest of St. Helena, but not enough data were received to generate a track for that tag. In a different study, one female whale shark that was tagged in the Gulf of Mexico traveled 7,213 km over 150 days through the Caribbean Sea, across the equator, and into the South Atlantic Ocean before her tag came off in January (Hueter et al., 2013). This location was in close proximity to where our tag transmitted data, potentially connecting the Gulf of Mexico and the South Atlantic. These results were both tantalizing and frustrating, but they hint that whale sharks may be capable of basin-wide movements across the South Atlantic. This would be consistent with large scale movements by several tagged individuals (Eckert et al., 2002; Hueter et al., 2013).
Two distinct patterns of vertical movement were documented among the animals tagged in this study. Immediately following tagging, and for the duration of their time spent in the waters surrounding the island, all tagged animals dove almost every day to a maximum depth of around 600 m between the hours of 06:00 and 19:00. After leaving the island, the diving pattern abruptly changed from daily 600 m dives, to a much more sporadic diving pattern, but many of these dives attained far greater maximum depths in the bathypelagic zone that were deep enough to trigger tag release/destruction. Extreme diving behavior of the sort has been previously documented in whale sharks and these dives have caused premature release of tags just as reported here (Tyminski et al., 2015). However, many coastal aggregation sites are not adjacent to depths that could result in tag damage. Tyminski et al. (2015) documented a dive to 1,928 m in the Gulf of Mexico, which was determined to be on the bottom at that location, and this observation made the whale shark the deepest diving fish known to science. The purpose of whale sharks’ dives into the bathypelagic zone is not known, however, the dive profiles tend to be V-shaped rather than U-shaped (Tyminski et al., 2015), which suggests that they are not primarily feeding dives. Gleiss et al. (2011) proposed, based on the geometry of the dive profile, that it may provide a mechanism for whale sharks to travel long horizontal distances efficiently (see also Lawson et al., 2019). In considering this possibility, it helps to imagine the behavior not as a dive per se, but rather a glide into the depths at a very shallow angle of descent. Whatever the reason for these dives is, it must be compelling because it subjects the animal to an environmental temperature change from 25°C to around 3°C, and a pressure change of nearly 200 atmospheres, both of which have profound effects on enzyme kinetics, toxicity of certain biochemical species, and cell membrane permeability (Yancey et al., 2002). The net effect of this behavior was that most realized tag missions were less than 1 month in duration, regardless of the programmed mission length. Deep diving behavior of whale sharks clearly warrants dedicated investigation.
Conclusion
Waters adjacent to St. Helena are home to a reliable seasonal population of adult male and female whale sharks and there have been multiple documented instances of sexual behaviors, which leads us to conclude that it is most likely a mating ground for this species, and the only one yet documented. Since the whale shark is an endangered species on the IUCN Red List (Pierce and Norman, 2016), this makes St. Helena waters a particularly important and possibly critical habitat for this species. Future studies around St. Helena should further characterize the extremely regular diving behavior immediately surrounding the island and the extraordinarily deep-diving behavior that takes place over abyssal depths farther from shore, as well as confirming reproduction through either direct observation of reproductive behaviors, or indirect measurements such as blood hormone analysis or underwater ultrasound. Regardless of what future research reveals, it is clear that St. Helena is important habitat for whale sharks and deserving of concerted research efforts.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by the Georgia Institute of Technology and was carried out in accordance with the St. Helena Government environmental ordinance under permit number 2019-SRE-1.
Author Contributions
AD and EC conceived the study. AD designed the survey. AD, EC, CP, DW, RP, SP, AB, LH, BT, KA, and RH conducted the field work. CP, AD, DW, GA, and RP analyzed the data. CP and AD prepared the manuscript with critical input from SP and GA. All authors contributed to the article and approved the submitted version.
Funding
This research was made possible by funding from the Georgia Aquarium, the St. Helena Government, the Saint Helena National Trust, and grant DPLUS039 from the Darwin Foundation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We appreciate the citizens of St. Helena for diligently reporting bone shark (whale shark) sightings to ENRD/SHG and thank the past and present staff of ENRD for curating those data. Thanks to Her Excellency Lisa Honan, Governor of St. Helena for her support and hospitality during three of our expeditions. We also appreciate the tour operators who provided boat support: Jonny Herne, Keith Yon, Craig Yon, Donnie O’Bey, Karl Thrower, Alex Benjamin, and Anthony Thomas. Special thanks to Graham Sim for answering so many questions about his eyewitness account of whale shark mating behavior, and Dr. Judith Brown for helping in the early stages of conceptualizing the expeditions. AD appreciates the support and participation of the Saint Helena National Trust Marine Team, Katie Hindle, Morgan Riley, Lourens Malan, Ross Leo, Alexandra Watts, and Georgia Aquarium’s zoological operations, dive operations and research staff in conducting four difficult expeditions to such a remote location, and the patience of his wife and two children in tolerating his prolonged absences as a result.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.576343/full#supplementary-material
Footnotes
- ^ www.whaleshark.org
- ^ https://www.argos-system.org/support-and-help/faq-localisation-argos
- ^ https://my.wildlifecomputers.com/
References
Acuña-Marrero, D., Jiménez, J., Smith, F., Doherty, P. F. Jr., Hearn, A., Green, J. R., et al. (2014). Whale shark (Rhincodon typus) seasonal presence, residence time and habitat use at Darwin Island, galapagos marine reserve. PLoS One 9:e115946. doi: 10.1371/journal.pone.0115946
Araujo, G., Snow, S., So, C. L., Labaja, J., Murray, R., Colucci, A., et al. (2017). Population structure, residency patterns and movements of whale sharks in Southern Leyte, Philippines: results from dedicated photo-ID and citizen science. Aquat. Conserv. Mar. Freshw. Ecosyst. 27, 237–252. doi: 10.1002/aqc.2636
Arzoumanian, Z., Holmberg, J., and Norman, B. (2005). An astronomical pattern-matching algorithm for computer-aided identification of whale sharks Rhincodon typus. J. Appl. Ecol. 42, 999–1011. doi: 10.1111/j.1365-2664.2005.01117.x
Bormpoudakis, D., Fish, R., Leo, D., and Smith, N. (2019). South Atlantic Natural Capital Project; Cultural ecosystem services in St Helena. Peterborough: JNCC.
Brown, J. (2013). Mapping St Helena’s Marine Biodiversity to Create A Marine Management Plan. 1st Annual Report. Available at https://urlzs.com/fFXV (accessed November 14, 2019).
Buckland, S. T., and Garthwaite, P. H. (1991). Quantifying precision of mark-recapture estimates using the bootstrap and related methods. Biometrics 47, 255–268. doi: 10.2307/2532510
Burnham, K. P., and Anderson, D. (2003). Model Selection and MultiModel Inference. New York, NY: Springer.
Cagua, E. F., Berumen, M. L., and Tyler, E. (2013). Topography and biological noise determine acoustic detectability on coral reefs. Coral Reefs 32, 1123–1134. doi: 10.1007/s00338-013-1069-2
Cagua, E. F., Cochran, J. E., Rohner, C. A., Prebble, C. E., Sinclair-Taylor, T. H., Pierce, S. J., et al. (2015). Acoustic telemetry reveals cryptic residency of whale sharks. Biol. Lett. 11:20150092. doi: 10.1098/rsbl.2015.0092
Capietto, A., Escalle, L., Chavance, P., Dubroca, L., de Molina, A. D., Murua, H., et al. (2014). Mortality of marine megafauna induced by fisheries: insights from the whale shark, the world’s largest fish. Biol. Conserv. 174, 147–151. doi: 10.1016/j.biocon.2014.03.024
Carrier, J. C., Pratt, H., and Castro, J. I. (2004). “Reproductive biology of elasmobranchs,” in Biology of Sharks and Their Relatives, eds J. C. Carrier, J. A. Musick, and M. R. Heithaus (Milton Park: Taylor & Francis), 269–286. doi: 10.1201/9780203491317.ch10
Cochran, J., Braun, C., Cagua, E., Campbell, M. Jr., Hardenstine, R., Kattan, A., et al. (2019). Multi-method assessment of whale shark (Rhincodon typus) residency, distribution, and dispersal behavior at an aggregation site in the Red Sea. PLoS One 14:e0222285. doi: 10.1371/journal.pone.0222285
Cochran, J., Hardenstine, R., Braun, C., Skomal, G., Thorrold, S., Xu, K., et al. (2016). Population structure of a whale shark Rhincodon typus aggregation in the Red Sea. J. Fish Biol. 89, 1570–1582.
de la Parra Venegas, R., Hueter, R., Cano, J. G., Tyminski, J., Remolina, J. G., Maslanka, M., et al. (2011). An unprecedented aggregation of whale sharks, Rhincodon typus, in Mexican coastal waters of the Caribbean Sea. PLoS One 6:e18994. doi: 10.1371/journal.pone.0018994
Eckert, S. A., Dolar, L. L., Kooyman, G. L., Perrin, W., and Rahman, R. A. (2002). Movements of whale sharks (Rhincodon typus) in South-east Asian waters as determined by satellite telemetry. J. Zool. 257, 111–115. doi: 10.1017/s0952836902000705
Escalle, L., Gaertner, D., Chavance, P., De Molina, A. D., Ariz, J., and Merigot, B. (2016). Consequences of fishing moratoria on catch and bycatch: the case of tropical tuna purse-seiners and whale and whale shark associated sets. Biodivers. Conserv. 25, 1637–1659. doi: 10.1007/s10531-016-1146-2
Fox, S., Foisy, I., De La Parra Venegas, R., Galván Pastoriza, B., Graham, R., Hoffmayer, E., et al. (2013). Population structure and residency of whale sharks Rhincodon typus at Utila, Bay Islands, Honduras. J. Fish Biol. 83, 574–587. doi: 10.1111/jfb.12195
Francis, M. P. (1996). Observations on a pregnant white shark with a review of reproductive biology. Great White Sharks 157:172.
Gleiss, A. C., Norman, B., and Wilson, R. P. (2011). Moved by that sinking feeling: variable diving geometry underlies movement strategies in whale sharks. Funct. Ecol. 25, 595–607. doi: 10.1111/j.1365-2435.2010.01801.x
Gordon, I. (1993). Pre-copulatory behaviour of captive sandtiger sharks, Carcharias taurus. Environ. Biol. Fishes 38, 159–164. doi: 10.1007/978-94-017-3450-9_14
Gray, A., Wilkins, V., Pryce, D., Fowler, L., Key, R. S., Mendel, H., et al. (2019). The status of the invertebrate fauna on the South Atlantic island of St Helena: problems, analysis, and recommendations. Biodivers. Conserv. 28, 275–296. doi: 10.1007/s10531-018-1653-4
Hammond, P. S., Mizroch, S. A., and Donovan, G. P. (1990). Individual Recognition of Cetaceans: Use of Photo-Identification and Other Techniques to Estimate Population Parameters: Incorporating the Proceedings of The Symposium and Workshop on Individual Recognition and the Estimation of Cetacean Population Parameters. Cambridge, MA: International Whaling Commission.
Hazin, F., Vaske Júnior, T., Oliveira, P., Macena, B., and Carvalho, F. (2008). Occurrences of whale shark (Rhincodon typus Smith, 1828) in the Saint Peter and Saint Paul archipelago, Brazil. Braz. J. Biol. 68, 385–389. doi: 10.1590/s1519-69842008000200021
Hearn, A. R., Green, J. R., Espinoza, E., Peñaherrera, C., Acuña, D., and Klimley, A. P. (2013). Simple criteria to determine detachment point of towed satellite tags provide first evidence of return migrations of whale sharks (Rhincodon typus) at the Galapagos Islands, Ecuador. Anim. Biotelem. 1:11. doi: 10.1186/2050-3385-1-11
Holmberg, J., Norman, B., and Arzoumanian, Z. (2008). Robust, comparable population metrics through collaborative photo-monitoring of whale sharks Rhincodon typus. Ecol. Appl. 18, 222–233. doi: 10.1890/07-0315.1
Hueter, R. E., Tyminski, J. P., and de la Parra, R. (2013). Horizontal movements, migration patterns, and population structure of whale sharks in the Gulf of Mexico and northwestern Caribbean Sea. PLoS One 8:e71883. doi: 10.1371/journal.pone.0071883
Kahle, D., and Wickham, H. (2019). ggmap: spatial visualization with ggplot2. R J. 5, 144–161. doi: 10.32614/rj-2013-014
Kukuyev, E. (1996). The new finds in recently born individuals of the whale shark Rhiniodon typus (Rhiniodontidae) in the Atlantic Ocean. J. Ichthyol. 36:203.
Lawson, C. L., Halsey, L. G., Hays, G. C., Dudgeon, C. L., Payne, N. L., Bennett, M. B., et al. (2019). Powering Ocean giants: the energetics of shark and ray Megafauna. Trends Ecol. Evol. 34, 1009–1021. doi: 10.1016/j.tree.2019.07.001
Lester, E., Meekan, M. G., Barnes, P., Raudino, H., Rob, D., Waples, K., et al. (2020). Multi-year patterns in scarring, survival and residency of whale sharks in Ningaloo Marine Park, Western Australia. Mar. Ecol. Prog. Ser. 634, 115–125. doi: 10.3354/meps13173
Macena, B. C., and Hazin, F. H. (2016). Whale shark (Rhincodon typus) seasonal occurrence, abundance and demographic structure in the mid-equatorial Atlantic Ocean. PLoS One 11:e0164440. doi: 10.1371/journal.pone.0164440
Marshall, A. D., Dudgeon, C., and Bennett, M. (2011). Size and structure of a photographically identified population of manta rays Manta alfredi in southern Mozambique. Mar. Biol. 158, 1111–1124. doi: 10.1007/s00227-011-1634-6
Matsumoto, R., Matsumoto, Y., Ueda, K., Suzuki, M., Asahina, K., and Sato, K. (2019). Sexual maturation in a male whale shark (Rhincodon typus) based on observations made over 20 years of captivity. Fish. Bull. 117, 78–86. doi: 10.7755/fb.117.1-2.9
McCoy, E., Burce, R., David, D., Aca, E. Q., Hardy, J., Labaja, J., et al. (2018). Long-term photo-identification reveals the population dynamics and strong site fidelity of adult whale sharks to the coastal waters of Donsol, Philippines. Front. Mar. Sci. 5:271. doi: 10.3389/fmars.2018.00271
Motta, P. J., Maslanka, M., Hueter, R. E., Davis, R. L., De la Parra, R., Mulvany, S. L., et al. (2010). Feeding anatomy, filter-feeding rate, and diet of whale sharks Rhincodon typus during surface ram filter feeding off the Yucatan Peninsula, Mexico. Zoology 113, 199–212.
Norman, B. M., Holmberg, J. A., Arzoumanian, Z., Reynolds, S. D., Wilson, R. P., Rob, D., et al. (2017). Undersea constellations: the global biology of an endangered marine megavertebrate further informed through citizen science. BioScience 67, 1029–1043. doi: 10.1093/biosci/bix127
Norman, B. M., and Morgan, D. L. (2016). The return of “Stumpy” the whale shark: two decades and counting. Front. Ecol. Environ. 14, 449–450. doi: 10.1002/fee.1418
Norman, B. M., and Stevens, J. D. (2007). Size and maturity status of the whale shark (Rhincodon typus) at Ningaloo Reef in Western Australia. Fish. Res. 84, 81–86. doi: 10.1016/j.fishres.2006.11.015
Perry, C. T., Figueiredo, J., Vaudo, J. J., Hancock, J., Rees, R., and Shivji, M. (2018). Comparing length-measurement methods and estimating growth parameters of free-swimming whale sharks (Rhincodon typus) near the South Ari Atoll, Maldives. Mar. Freshw. Res. 69, 1487–1495. doi: 10.1071/mf17393
Pierce, S. J., and Norman, B. (2016). Rhincodon typus. The IUCN Red List of Threatened Species 2016: e.T19488A2365291. Available online at: http://dx.doi.org/10.2305/IUCN.UK.2016-1.RLTS.T19488A2365291.en (accessed August 08, 2019).
Pratt, H. L., and Carrier, J. C. (2001). A review of elasmobranch reproductive behavior with a case study on the nurse shark, Ginglymostoma cirratum. Environ. Biol. Fishes 60, 157–188. doi: 10.1007/978-94-017-3245-1_11
Prebble, C. E., Rohner, C. A., Pierce, S. J., Robinson, D. P., Jaidah, M. Y., Bach, S. S., et al. (2018). Limited latitudinal ranging of juvenile whale sharks in the Western Indian Ocean suggests the existence of regional management units. Mar. Ecol. Prog. Ser. 601, 167–183. doi: 10.3354/meps12667
Ramírez-Macías, D., Queiroz, N., Pierce, S. J., Humphries, N. E., Sims, D. W., and Brunnschweiler, J. M. (2017). Oceanic adults, coastal juveniles: tracking the habitat use of whale sharks off the Pacific coast of Mexico. PeerJ 5:e3271. doi: 10.7717/peerj.3271
Roberts, C. M., McClean, C. J., Veron, J. E., Hawkins, J. P., Allen, G. R., McAllister, D. E., et al. (2002). Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 295, 1280–1284. doi: 10.1126/science.1067728
Rohner, C., Richardson, A., Marshall, A., Weeks, S., and Pierce, S. (2011). How large is the world’s largest fish? Measuring whale sharks Rhincodon typus with laser photogrammetry. J. Fish Biol. 78, 378–385. doi: 10.1111/j.1095-8649.2010.02861.x
Rowat, D., and Brooks, K. (2012). A review of the biology, fisheries and conservation of the whale shark Rhincodon typus. J. Fish Biol. 80, 1019–1056. doi: 10.1111/j.1095-8649.2012.03252.x
Schofield, G., Katselidis, K. A., Dimopoulos, P., and Pantis, J. D. (2008). Investigating the viability of photo-identification as an objective tool to study endangered sea turtle populations. J. Exp. Mar. Biol. Ecol. 360, 103–108. doi: 10.1016/j.jembe.2008.04.005
Sequeira, A. M., Mellin, C., Fordham, D. A., Meekan, M. G., and Bradshaw, C. J. (2014). Predicting current and future global distributions of whale sharks. Glob. Change Biol. 20, 778–789. doi: 10.1111/gcb.12343
Tyminski, J. P., de la Parra-Venegas, R., Cano, J. G., and Hueter, R. E. (2015). Vertical movements and patterns in diving behavior of whale sharks as revealed by pop-up satellite tags in the eastern Gulf of Mexico. PLoS One 10:e0142156. doi: 10.1371/journal.pone.0142156
Vignaud, T. M., Maynard, J. A., Leblois, R., Meekan, M. G., Vázquez-Juárez, R., Ramírez-Macías, D., et al. (2014). Genetic structure of populations of whale sharks among ocean basins and evidence for their historic rise and recent decline. Mol. Ecol. 23, 2590–2601. doi: 10.1111/mec.12754
Whitehead, H. (2001). Analysis of animal movement using opportunistic individual identifications: application to sperm whales. Ecology 82, 1417–1432. doi: 10.1890/0012-9658(2001)082[1417:aoamuo]2.0.co;2
Whitehead, H. (2007). Selection of models of lagged identification rates and lagged association rates using AIC and QAIC. Commun. Stat. Simul. Comput. 36, 1233–1246. doi: 10.1080/03610910701569531
Whitehead, H. (2009). SOCPROG programs: analysing animal social structures. Behav. Ecol. Sociobiol. 63, 765–778. doi: 10.1007/s00265-008-0697-y
Wolfson, F. (1983). Records of seven juveniles of the whale shark, Rhiniodon typus. J. Fish Biol. 22, 647–655. doi: 10.1111/j.1095-8649.1983.tb04224.x
Keywords: mating, movement ecology, diving, aggregation, demographics, remote, elasmobranch
Citation: Perry CT, Clingham E, Webb DH, de la Parra R, Pierce SJ, Beard A, Henry L, Taylor B, Andrews K, Hobbs R, Araujo G and Dove ADM (2020) St. Helena: An Important Reproductive Habitat for Whale Sharks (Rhincodon typus) in the Central South Atlantic. Front. Mar. Sci. 7:576343. doi: 10.3389/fmars.2020.576343
Received: 25 June 2020; Accepted: 05 October 2020;
Published: 10 November 2020.
Edited by:
David Wells, Texas A&M University at Galveston, United StatesReviewed by:
Mourier Johann, Institut de Recherche Pour le Développement, FranceEric Hoffmayer, Southeast Fisheries Science Center (NOAA), United States
Copyright © 2020 Perry, Clingham, Webb, de la Parra, Pierce, Beard, Henry, Taylor, Andrews, Hobbs, Araujo and Dove. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cameron T. Perry, Y3BlcnJ5NjBAZ2F0ZWNoLmVkdQ==; cGVycnljbmJjQGdtYWlsLmNvbQ==
 Cameron T. Perry
Cameron T. Perry Elizabeth Clingham
Elizabeth Clingham D. Harry Webb3
D. Harry Webb3 Rafael de la Parra
Rafael de la Parra Simon J. Pierce
Simon J. Pierce LeeAnn Henry
LeeAnn Henry Kenickie Andrews
Kenickie Andrews Gonzalo Araujo
Gonzalo Araujo Alistair D. M. Dove
Alistair D. M. Dove