- 1Laboratório de Avaliação e Promoção da Saúde Ambiental, Instituto Oswaldo Cruz (Fiocruz), Rio de Janeiro, Brazil
- 2GEMM-Lagos, Araruama, Brazil
- 3Universidade Federal do Estado do Rio de Janeiro, Rio de Janeiro, Brazil
- 4Marine Mammal Institute, Hatfield Marine Science Center, Oregon State University, Corvallis, OR, United States
- 5Leibniz Center for Tropical Marine Ecology (ZMT), Systems Ecology Group, Bremen, Germany
- 6Departamento de Química, Pontifícia Universidade Católica do Rio de Janeiro, Rio de Janeiro, Brazil
- 7Laboratório de Biodiversidade, Instituto Oswaldo Cruz (Fiocruz), Rio de Janeiro, Brazil
Marine mammals are considered excellent ocean health sentinels and are ubiquitously exposed to chemical contaminants worldwide. The Guiana dolphin (Sotalia guianensis) is a near-threatened dolphin species from Brazil with unknown population size data. This indicates the need for assessments regarding deleterious effects that may arise from exposure to chemical contamination, especially metals. After entry in the organism, these compounds are subject to internal subcellular compartmentalization, which in turn alters their bioavailability. However, almost no assessments regarding subcellular metal contents in marine mammals are available. In this context, metal compartmentalization was determined in three subcellular fractions for three toxic elements, Cd, Hg and Pb, by inductively coupled plasma mass spectrometry (ICP-MS) in Guiana dolphin kidney and liver samples from Southeastern Brazil. Differential metal-detoxification mechanisms were observed for the three elements, where metallothionein (MT) detoxification was postulated for only for Pb, while Cd and Hg were poorly associated to MT, and mostly present in the insoluble fraction, indicating low bioavailability. This is the first report on subcellular metal compartmentalization in Guiana dolphins and indicates that critical biochemical detoxification data is obtained through subcellular fraction analyses in marine mammals. This indicates an emerging study field for this type of assessment, which may, in turn, aid in conservation efforts.
Introduction
Metal and metalloid contamination in aquatic ecosystems is a growing concern worldwide, since these elements may lead to potential toxic effects and display the ability to bioaccumulate and/or biomagnify throughout the trophic web (Censi et al., 2006). Biomonitoring efforts concerning metal and metalloid contamination scenarios, however, mostly determine total concentrations. Conversely, after entry in the organism, metals and metalloids undergo internal subcellular compartmentalization, which alters their bioavailability, and, consequently, potential deleterious effects. For example, intracellular metal storage tends to increase metal and metalloid concentrations in certain organs, but, at the same time, reduces the toxicological risk inherent to the presence of these contaminants in the organism (Decataldo et al., 2004). Therefore, total metal concentrations are, in fact, unreliable biological tools to reveal the actual adverse effects of metal and metalloid contamination in exposure risk assessments (Decataldo et al., 2004; Marijić et al., 2016), and reporting only total metal concentrations and inferring deleterious toxicological effects is not justified.
Because of this, the evaluation of metal and metalloid toxicokinetics must consider both their distribution within the cell and solubility characteristics. For example, metals and metalloids present in soluble cellular fractions are bioavailable and may lead to toxic cellular effects while insoluble metals are inert and, thus, biologically unavailable (Wallace et al., 2003; Dragun et al., 2015). Organisms utilize several detoxifying pathways in order to remove these contaminants from the body, where detoxification proteins [such as metallothionein (MT), MT-like proteins and glutathione] and enzymes are present mainly in the soluble cellular fraction, while insoluble fractions contain metal-rich granules, organelles, and cellular debris (Wallace et al., 2003; Marijić et al., 2016).
Cetaceans are considered excellent sentinel species concerning environmental pollution (Kehrig et al., 2016), and several studies have been carried out regarding total metal and metalloid concentrations in different species worldwide. For example, trace metals have been determined in the liver and kidney of the Franciscana dolphin Pontoporia blainvillei from the northern coast of Rio de Janeiro State, Brazil (Lailson-Brito et al., 2002), in liver, kidney, brain, lung, muscle of striped dolphins (Stenella coeruleoalba) found stranded along the Murcia coastline, in the Mediterranean Sea (Martínez-López et al., 2019), in the kidney, liver, and muscle of common dolphins (Delphinus delphis) from Portugal (Monteiro et al., 2016), among many others However, almost no assessments regarding subcellular metal contents in these animals are available worldwide, and only one report is available for the southern hemisphere (Monteiro et al., 2019). It is important to note that this type of assessment is an important step to further understand the potentially toxic effects of these pollutants in marine mammals, with direct relation to humans and public health. For example, many of the food items consumed by these animals are also consumed by humans, so these assessments may further knowledge on the contamination of ingested fish species.
Distributed from Nicaragua to Santa Catarina, Brazil, the Guiana dolphin (Sotalia guianensis) inhabits exclusively coastal waters, estuaries, and bays, reaching a maximum depth of 50 m and are especially vulnerable to coastal pollution (Flores and Da Silva, 2009). Categorized as a small cetacean, its food habits mainly include teleosts, such as the largehead hairtail (Trichiurus lepturus) and squid (Loligo spp.) (Di Beneditto and Siciliano, 2007). As a long-lived (>33 years) top predator, it is considered an important ocean health sentinel species for several pollutants (de Moura et al., 2014). This species is highly vulnerable due to inhabiting exclusively coastal areas, as these regions are more likely to receive a high number of contaminants released by human activities. It is currently listed in the Brazilian Red Book of Threatened Species of Fauna as “vulnerable,” considered one of the ten most endangered species in the state of Rio de Janeiro (ICMBIO, 2016), and classified as “near threatened” by the IUCN (Secchi et al., 2019). Several studies conducted using Guiana dolphin samples concerning metal concentrations are available (de Carvalho et al., 2008; Dorneles et al., 2008; de Moura et al., 2012; Lailson-Brito et al., 2012; Kehrig et al., 2016; Salgado et al., 2018), although none exist regarding subcellular metal distributions, which are more adequate in assessing both environmental metal bioavailability and potential biochemical deleterious effects (Decataldo et al., 2004).
In this context, the present study aimed to assess the intracellular distribution of three toxic elements, cadmium, lead and mercury, for the first time in Guiana dolphins found stranded along the Southeastern coast of Brazil. These data will become valuable baselines in order to detect negative impacts of the increasing human activities observed along the Brazilian coast which may lead to reproductive and developmental effects in dolphins. In addition, they may aid in verifying potential effects of environmental risk mitigation actions.
Methodology
Study Area, Sampling, and Sample Processing
Guiana dolphins were sampled from a study area comprised approximately 300 km of coastline, from Saquarema (south, 22°55′12″S; 42°30′37″W) to São João da Barra (north, 21°38′25″S; 41°03′04″W), located in the state of Rio de Janeiro, Southeastern Brazil (Figure 1). This is relatively near the oil and gas exploration area of Campos Basin, one of the highest productivity areas in Brazil. One individual found stranded in the city of Rio de Janeiro (distant about 100 km from Saquarema), was included in the study as it was found in 2002 and is a juvenile, which we thought would be interesting to assess.

Figure 1. Sampling locations of Guiana dolphin (Sotalia guianensis) specimens found stranded or caught as bycatch in the state of Rio de Janeiro, southeastern Brazil. P. S. River-Paraíba do Sul River.
The detected metals in the study area mostly originate from the Paraíba do Sul River, which flows through the states of Minas Gerais, São Paulo, and Rio de Janeiro, three of the most developed states in Brazil. The Paraíba do Sul river basin is occupied by many industrial parks and cities, and receives effluents from several anthropogenic activities, such as agricultural runoff, industrial waste, and domestic sewage, thus categorized as highly polluted, especially by metals (Miguens et al., 2016). Its waters supply over 20 million people (De Carvalho and Torres, 2002). Another water body, the São João River, also flows through the state of Rio de Janeiro, running into the Atlantic Ocean in the study region. The São João River and its tributaries have suffered destructive action of its natural resources over the years, with wood exploitation and disorganized and illegal population occupation on its banks, through the expansion of agriculture, livestock and subdivisions without sanitation infrastructure, and its waters and sediment have also been reported as containing high metal content (Paixão et al., 2013), although studies are still scarce in the region.
Guiana dolphin specimens were found stranded or incidentally captured as bycatch in artisanal fisheries between 2002 and 2012. Only fresh to moderately fresh (stages 2–3) carcasses were evaluated, according to the decomposition scale of Geraci and Lounsbury (1993). Liver (n = 21) and kidney (n = 9) samples were removed from each specimen, frozen at −80°C, subsequently freeze-dried for at least 48 h (Liotop 101, Liobrás, São Paulo, Brazil) and then crushed with mortar and pestle for tissue homogenization. Total body length, maturity stage and sex were determined for each specimen. According to the literature, all specimens were adults, as maturity is reached at 170 cm and 164 cm for males and females, respectively, except for individual #34, considered a juvenile (Rosas and Monteiro Filho, 2002). Table 1 displays the morphometric data for each specimen.
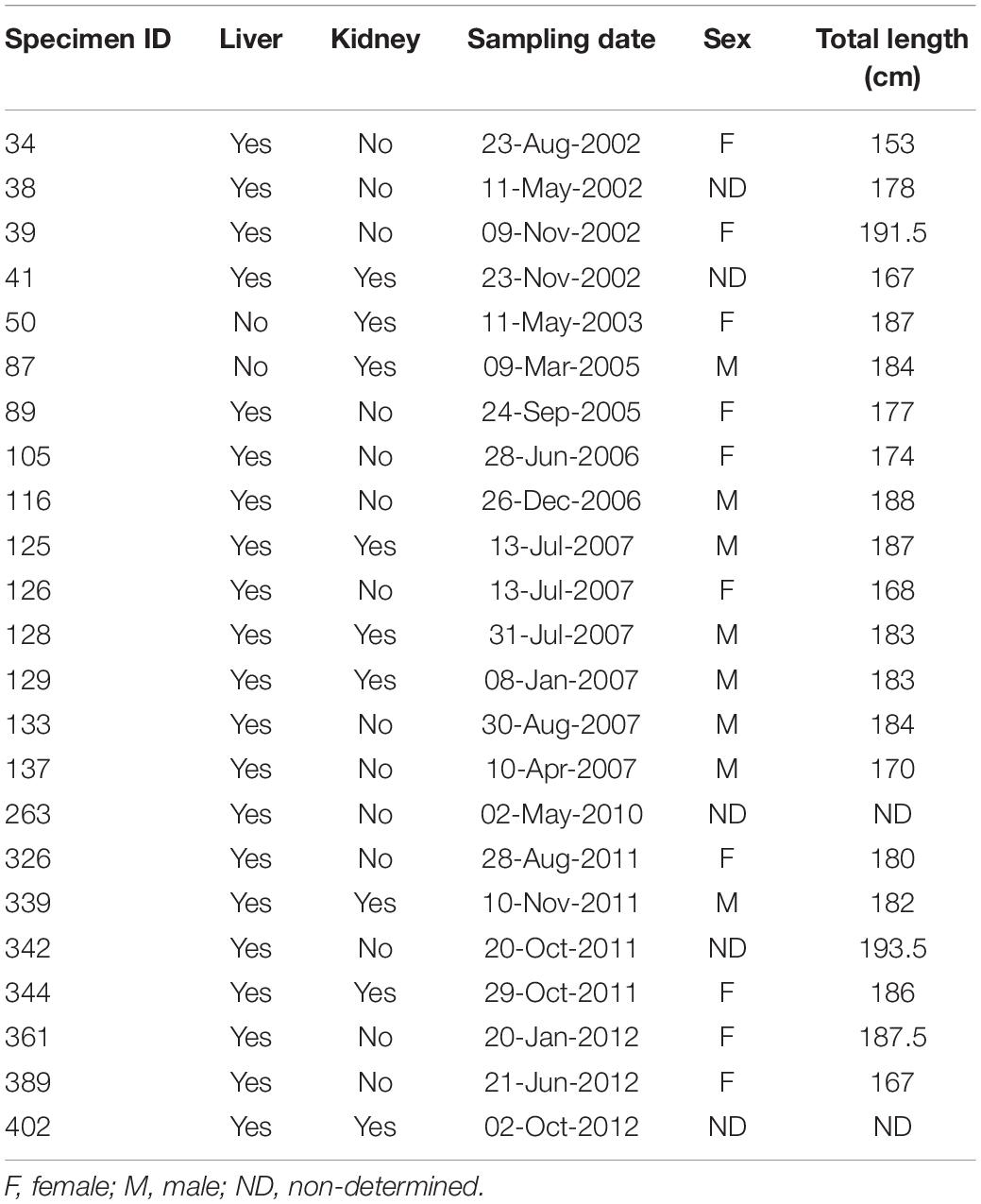
Table 1. Biomorphometric data, sampling date, for each Guiana dolphin (Sotalia guianensis) specimen evaluated in the present study.
Preparation of the Insoluble and Soluble Subcellular Fractions
Insoluble and soluble subcellular fractions were prepared from a modified MT purification procedure according to Erk et al. (2002). This procedure has been also applied to human blood and serum samples (Kizek et al., 2001; Petrlovà et al., 2006) and is comparable to the procedure carried out by Decataldo et al. (2004) used to assess subcellular metal fractionation in different dolphin samples. Briefly, about 150 mg of each freeze-dried sample was manually homogenized in sterile polypropylene microtubes in a Tris–Hcl buffer solution (pH 8.6) containing phenyl-methyl-sulphonyl-fluoride as a protease inhibitor and β-mercaptoethanol as the reducing agent. The homogenized samples were first centrifuged at 20.000 × g for 60 min at 4°C in a microcentrifuge (Eppendorf, 540R, São Paulo, Brazil). After the centrifugation, the precipitates were separated and labeled the insoluble fraction (ISF), which contains mostly insoluble and thus, non-bioavailable, elements which are poorly associated to MT detoxification (Decataldo et al., 2004). Aliquots of these partially purified supernatants, which comprise non-heat-resistant biomolecules (Decataldo et al., 2004), were then transferred to new microtubes, labeled the heat-denaturable fraction (HDF) and frozen at −80°C until analysis. The rest of the partially purified supernatants were heated at 70°C on a heating block for 10 min and centrifuged again in the same conditions, this time for 30 min. After centrifugation, the supernatants were separated, labeled the purified heat-stable thermostable fraction (PHSF), comprising heat-stable protein biomolecules, such as MT and MT-like proteins among others, which may bind to metals in detoxification attempts (Decataldo et al., 2004), and frozen at −80°C until analysis.
Determination of Metal and Metalloid Contents
Metal content was determined in the three sub-cellular fractions, ISF, HDF, and PHSF. Aliquots of each fraction acid-digested with bidistilled nitric acid overnight, followed by heating at 100°C on a heating block for 4 h, cooled and made up to 10 mL with ultra-pure water (resistivity > 18 MΩ cm). The metals and metalloids were determined on an Elan DRC II (Perkin Elmer Sciex, Norwalk, CT, United States) spectrometer. Quality control was performed by a strict blank control and the analyses of technical replicates and certified reference materials (DORM-4, NRC, Canada). Certified reference material recoveries ranged from 92 to 107% (Cd–92%; Hg–107%; Pb–95%) and were considered appropriate for the present study (Eurachem, 2014; Ishak et al., 2015). External calibration was performed for all analytes, using calibration solutions prepared by appropriate dilutions of a mixed standard solution (Merck IV). All analytical curves presented correlation coefficients > 0.995 for the assessed analytes. 102Rh was used as the internal standard. Five readings for each sample were obtained. The limits of quantification (LQ) were calculated as 10 Sb/slope, where Sb is the standard deviation of 10 blank measures and the slope is the angular coefficient of the analytical curve (Eurachem, 2014). The LQ for Cd, Hg and Pb were of 0.002, 0.024, and 0.003 mg kg–1, respectively.
Statistical Analyses
The Kruskal-Wallis test was applied at a significance level of p < 0.05 to verify potential differences among Cd, Hg, and Pb contents in liver and kidneys between sampling years. The test was conducted separately by each type of tissue, per year of sampling for each sample, although the minimum sample of n = 5 for this test (Choi et al., 2003; Minitab, 2020) was reached only for one-year (2007). Thus, the results are only indicative of potential trends, and should only be further evaluated if the sample sizes per year increase in future assessments.
Results
Metal Content and Subcellular Profiles
Cd in kidney and liver was mostly concentrated in the insoluble fraction, with the heat-denaturable and heat-stable fractions <LQ. This indicates that non-bioavailable concentrations of this element, and that the MT detoxification pathway does not seem to be induced by Cd levels in the evaluated Guiana dolphin specimens. Hg in kidney and liver was also mostly concentrated in the insoluble fraction. Pb in kidney was mostly concentrated in the insoluble fraction, although some specimens presented heat-denaturable fraction in kidney, while liver presented both heat-denaturable fraction and purified heat-stable fraction concentrations. No trend for higher or lower Cd, Hg, or Pb values in a given year were observed for either kidney or liver as per the Kruskal Wallis test (p > 0.05). However, as indicated in the statistical analyses section, the sample size analyzed herein was small per year (n = 4 for 2002, n = 1 for 2003, n = 2 for 2005, n = 2 for 2006, n = 6 for 2007, n = 1 for 2010, n = 4 for 2011, and n = 3 for 2012) and studies with higher sample sizes may indicate different trends. The Cd, Pb, and Hg metal compartmentalization results in Guiana dolphin liver and kidney are displayed in Figure 2.
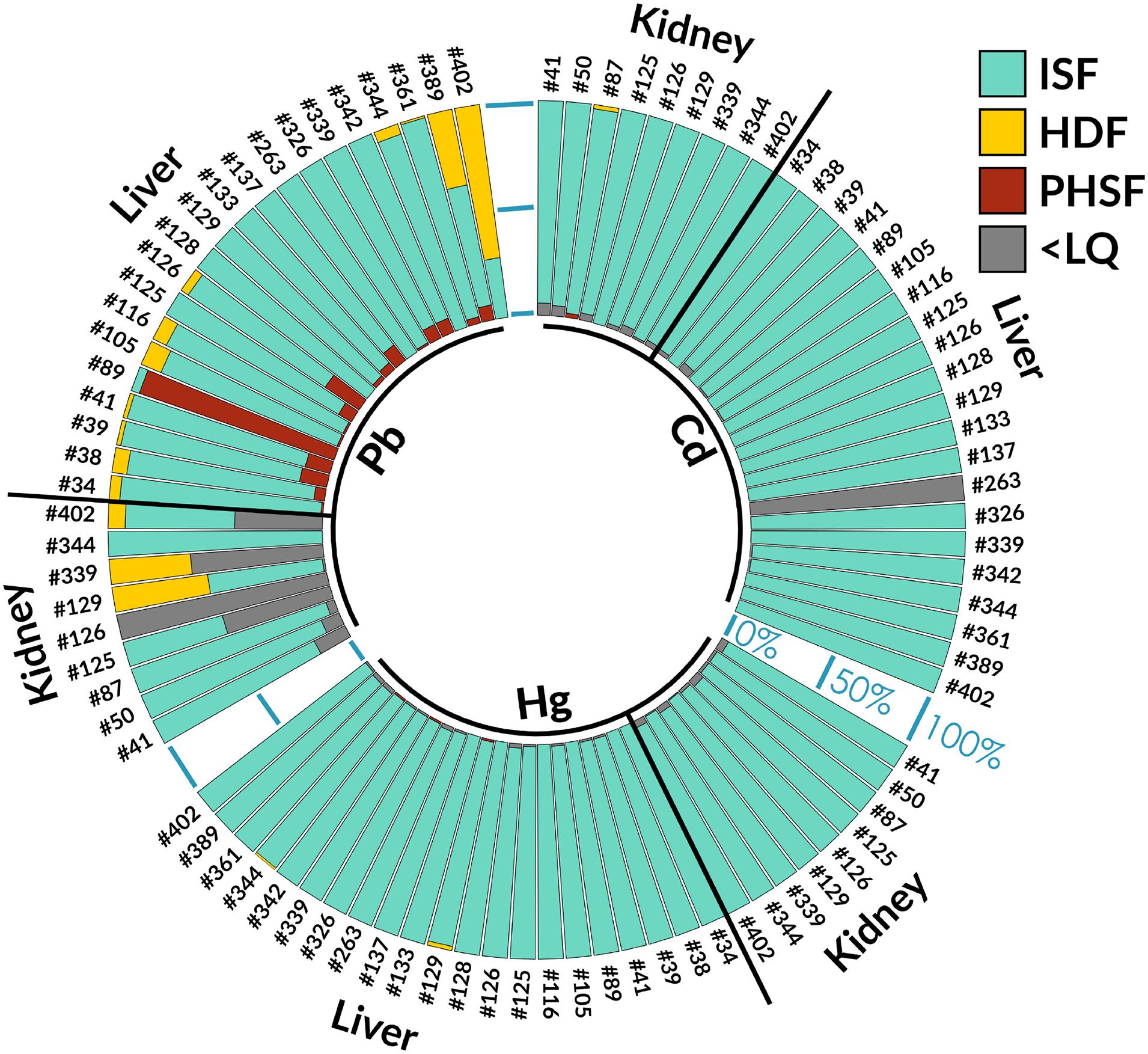
Figure 2. Cd, Pb, and Hg metal compartmentalization results in Guiana dolphin (Sotalia guianensis) liver and kidney. ISF, insoluble fraction; HDF, heat-denaturable fraction; PHSF, purified heat-stable fraction; <LQ, lower than the limit of quantification.
Discussion
Subcellular compartmentalization occurs after metal entry in the organism, altering metal bioavailability. Therefore, the total metal burden of an organism in not indicative of toxicologically active content, and total metal accumulation has been indicated as a poor metal toxicity predictor by several authors for different organisms (Geffard et al., 2010; Marijić et al., 2016). In this regard, soluble tissue metal levels have been reported as a promising biological tool in estimating metal exposure in various organisms, like mussels and fish (Filipovi and Raspor, 2003; Dragun et al., 2015; Marijić et al., 2016), while reports in marine mammals are still scarce.
The MT detoxification pathway, occurring mainly in the cytosolic subcellular fraction, is one of the main metal and metalloid detoxification mechanisms (Decataldo et al., 2004), and has been reported in many marine mammals, such as seals, sea lions, whales, and dolphins (Olafson and Thompson, 1974; Lee et al., 1977; Ridlington et al., 1981; Das et al., 2000; Cáceres-Saez et al., 2016; Kehrig et al., 2016), although studies are extremely scarce on the subject due to the notorious difficulties in studying these animals and the aforementioned differences in metal concentrations, bioavailability, and metabolism.
Cd in both kidney and liver, mostly concentrated in the insoluble fraction in the present study, contrasts with other reports evaluating Cd subcellular metal profiles in dolphins from other species. For example, the study carried out by Decataldo et al. (2004) analyzed 13 Stenella coeruleoalba and Tursiops truncatus specimens stranded along the southern coasts of Italy from 1991 to 1999 and reported the presence of Cd in all three subcellular fractions. In another study, Cd was found in both the insoluble fraction and the heat-denaturable fraction in Steno bredanensis specimens sampled off the coast of Rio de Janeiro, in five livers, three kidneys and six muscle samples obtained in 2005, 2007, and 2011, with no stable pattern observed, which seems to indicate a certain amount of detoxification attempts by MT (Monteiro et al., 2019). For example, both Mediterranean Stenella coeruleoalba and Tursiops truncatus have been reported as feeding preferentially on squid, considered the main source of Cd transference to marine mammals (Bustamante et al., 1998), and mid-level carnivorous fish species that feed on crustaceans and small fish (i.e., cod and hake, respectively), (Würtz and Marrale, 1993; Blanco et al., 2001) and Steno bredanensis feed on cephalopods and both oceanic and coastal fish (Dorneles et al., 2007; Melo et al., 2010), although the species feeding ecology is still relatively poorly known (Lodi and Hetzel, 1999). Although a feeding overlap of Guiana dolphins with Steno bredanensis in Brazil concerning certain prey items prey is noted, i.e., squid (Loligo plei and L. sanpaulensis; Di Beneditto and Siciliano, 2007; Di Beneditto et al., 2017), Guiana dolphins also feed on the top-level carnivorous largehead hairtail fish, which usually contain high metal levels, especially mercury. However, differences in metal metabolism and metal bioavailability, leading to differential intracellular metal compartmentalization and deleterious effects (Decataldo et al., 2004; Marijić and Raspor, 2006; Monteiro et al., 2019) must also be taken into account, although no reliable systematic assessments on how differential metal levels affect the induction process of MT detoxification pathways are available. In addition, protective effects of essential metals against toxic elements are also important, such as the protective effect of Fe, Se, and Zn against Hg toxicity. In fact, one very interesting study has reported a mercury detoxification mechanism via mercury selenide formation in Sotalia guianensis from the coast of Rio de Janeiro, including histological assessment evidence (Lailson-Brito et al., 2012). Furthermore, evidence of protective effect of Cu, Cr and Se against Cd and Co against Pb have been observed in several organisms such as fish and several mammals (mice, rats, and dolphins) (Land et al., 2018).
The Hg findings reported herein, also mostly concentrated in the insoluble fraction, also corroborates previous studies carried out with other dolphins, where the Hg cytosol fraction potentially associated to MT is low and that Hg does not seem to be mediated by the MT detoxification pathway in marine mammals (Decataldo et al., 2004; Pedrero et al., 2012). This indicates that other Hg detoxification mechanisms are in place, and some authors have postulated that Hg bind to other proteins after the formation of Se-trisulfide groups, or that Se:Hg correlations may lead to the formation of insoluble Se:Hg granules in some organs, such as the liver (Chen et al., 1974; Nigro and Leonzio, 1996), although confirmation can only be obtained through histological assessments.
The presence of hepatic Pb in both the heat-denaturable fraction and purified heat-stable fraction is expected, as the liver, as the highest metabolically active organ effectively carries out detoxification processes, followed by kidney excretion (Taub, 2004). Therefore, this metal compartmentalization indicates a certain degree of MT-mediated detoxification for this element in Guiana dolphin liver. Recent studies on the subcellular distribution of metals in another dolphin assessed off the coast of Rio de Janeiro, rough-toothed dolphins (Steno bredanensis), corroborate the findings reported herein regarding Pb detoxification by MT. However, a greater allocation of this element in insoluble fractions of hepatic and renal tissues is observed in Guiana dolphins, whereas for rough-toothed dolphins the thermostable fraction was the most significant in this regard (Monteiro et al., 2019), indicating MT-mediated detoxification.
In a recent study carried out on subcellular metal distributions and MT associations in rough-toothed dolphin specimens sampled off the coast of Rio de Janeiro, MT detoxification was mostly noted for Cd, and Pb, while Hg displayed lower MT-associations (Monteiro et al., 2019). In the present study, only Pb displayed any MT-mediated detoxification, due to contents in the purified heat-stable fraction. This may be due to differential detoxification mechanisms in Guiana dolphins compared to rough-toothed dolphins. Although rough-toothed dolphins exhibit usually offshore habits, and would thus, be probably less exposed to anthropogenic activity effects than Guiana dolphins, which exhibit coastal habits (Gannier and West, 2005), in Brazil, rough-toothed dolphins exhibit coastal habits (Jefferson, 2009; Bisi et al., 2013). Thus, the previous explanation applied for comparisons between metal detoxification mechanisms in other dolphin species proposed by Monteiro et al. (2019) is not valid. These authors postulate that coastal species, due to higher metal exposure, may exhibit rapid overwhelming of cellular defense systems which, consequently, become unable to adequately excrete these contaminants through MT detoxification, usually a preferential detoxification route for metals, tending to store metal in non-soluble fractions, while offshore species, due to oceanic contaminant diffusion with increasing distance from the coast (James, 2002), may not be so quickly overwhelmed, and, thus, able mitigate metals in the soluble fraction through MT detoxification. However, it has been reported that metals in the insoluble form metals are usually less toxic than in the soluble form (Magalhães et al., 2015). Therefore, the presence of hepatic Pb in both the heat-denaturable fraction and purified heat-stable fraction, indicates some MT-mediated detoxification for this element in Guiana dolphin, although to what extent this detoxification is adequate to reduce intracellular body-burdens, still requires further investigation, and the hepatic and renal Cd and Hg mostly in the insoluble fraction, as well as a portion of hepatic and most renal Pb, in the assessed Guiana dolphins seem to indicate storage in non-bioavailable form, potentially resulting in non-deleterious effects. Further assessments combining multidisciplinary approaches, however, are required to verify this hypothesis in Guiana dolphins, such as general health, immunological, reproductive, and ecological assessments.
Conclusion
This is the first report assessing the subcellular compartmentalization and consequent MT-detoxification pathway of Pb, Cd, and Hg in Guiana dolphins. The findings indicate preferential storage in non-bioavailable form of hepatic and renal Cd and Hg, while Pb seems to be mostly stored in non-bioavailable form in kidneys, but displaying a certain amount of MT-mediated detoxification in liver, both of which may reduce the toxic effects of these metals in Guiana dolphins, although further assessments combining multidisciplinary approaches, i.e., general health, immunological, reproductive, and ecological assessments, are required to verify this hypothesis in this species. The exclusive coastal habitat of this species, shared with densely human inhabited environments, poses numerous threats to its populations, including bycatch in artisanal gillnets and high levels of contaminant loads and emerging diseases. The combined effects of these factors are rapidly driving Guiana dolphins to many uncertainties regarding its future, with an urgent need for action. In addition, as it presents unknown population sizes, there is a need for further assessments regarding ecological trends for the species that may arise from the type of chemical contamination assessed herein, representing, therefore, an emerging study field for this type of assessment, which may, in turn, aid in evaluating environmental contamination mitigation and conservation efforts. Further analyses include MT determinations and statistical evaluations concerning correlations between these metalloproteins and the detected metals, as well as size-exclusion chromatography coupled to ICP-MS assessments, to categorize proteins by size and metal-binding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This animal study was reviewed and approved by the Brazilian Ministry of the Environment, through SISBIO licenses no. 19665-1, nos. 32550-1 and 32550-2.
Author Contributions
RH-D: data curation, visualization, writing original draft, writing review and editing, and supervision. LF: data investigation and data curation. LL and JM: data investigation, data curation, writing original draft, and writing review and editing. RR: data investigation and data curation. TS: funding acquisition, writing original draft, writing review and editing, and supervision. RZ: data investigation and supervision. SS: project administration, data curation, visualization, writing original draft, writing review and editing, and supervision. All authors contributed to the article and approved the submitted version.
Funding
TS acknowledges Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq for financial support in the form of a productivity grant. RH-D acknowledges support from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro – FAPERJ). SS acknowledges support from Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (Produtividade em Pesquisa: 306076/2019-5) and INOVA Fiocruz.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Bisi, T. L., Dorneles, P. R., Lailson-Brito, J., Lepoint, G., Azevedo, A. F., Flach, L., et al. (2013). Trophic Relationships and Habitat Preferences of Delphinids from the Southeastern Brazilian Coast Determined by Carbon and Nitrogen Stable Isotope Composition. Plos One 8:e82205. doi: 10.1371/journal.pone.0082205
Blanco, C., Salomón, O., and Raga, J. A. (2001). Diet of the bottlenose dolphin (Tursiops truncatus) in the western Mediterranean Sea. J. Mar. Biol. Asso. U K. 81, 1053–1058. doi: 10.1017/s0025315401005057
Bustamante, P., Caurant, F., Fowler, S., and Miramand, P. (1998). Cephalopods as a key of the transfer of cadmium to top marine predators. Sci. Total Environ. 220, 71–80. doi: 10.1016/s0048-9697(98)00250-2
Cáceres-Saez, I., Polizzi, P., Romero, B., Dellabianca, N. A., Guevara, S. R., Goodall, R. N. P., et al. (2016). Hepatic and renal metallothionein concentrations in Commerson’s dolphins (Cephalorhynchus commersonii) from Tierra del Fuego, South Atlantic Ocean. Mar. Pollut. Bull. 108, 263–267. doi: 10.1016/j.marpolbul.2016.03.061
Censi, P., Spoto, S. E., Saiano, F., Sprovieri, M., Mazzola, S., Nardone, G., et al. (2006). Heavy metals in coastal water systems. Case Study Northwest. Gulf Thailand Chemosphere. 64, 1167–1176.
Chen, R. W., Whanger, P. D., and Fang, S. C. (1974). Diversion of mercury binding in rat tissues by selenium: A possible mechanism of protection. Pharmacol. Res. Commun. 6, 571–579. doi: 10.1016/s0031-6989(74)80006-8
Choi, W., Lee, J., Huh, M., and Kang, S.-H. (2003). An Algorithm for Computing the Exact Distribution of the Kruskal–Wallis Test. Commun. Statist. Simulat. Comput. 32, 1029–1040. doi: 10.1081/sac-120023876
Das, K., Debacker, V., and Bouquegneau, J. M. (2000). Metallothioneins in marine mammals. Cell. Mol. Biol. 46, 283–294.
de Carvalho, C. E. V., Di Beneditto, A. P. M., Souza, C. M. M., Ramos, R. M. A., and Rezende, C. E. (2008). Heavy metal distribution in two cetacean species from Rio de Janeiro State, south-eastern Brazil. J. Mar. Biol. Assoc. UK 88, 1117–1120. doi: 10.1017/S002531540800032
De Carvalho, C. E. V., and Torres, J. P. (2002). “The ecohydrology of the Paraíba Do Sul River, Southeast Brazil,” in The Ecohydrology of South American Rivers and Wetlands, ed. Michael E. McClain (Venice: IAHS Special Publication), 179–191.
Di Beneditto, A. P. M., Badia, C. C. V., and Siciliano, S. (2017). On the feeding habit of the Guiana Dolphin Sotalia guianensis (van Bénedén, 1864) (Mammalia: Cetartiodactyla: Delphnidae) in southeastern Brazil (∼22°S): has there been any change in more than two decades?. J. Threat. Taxa 9, 9840–9843. doi: 10.11609/jott.2745.9.2.9840-9843
de Moura, J. F., Hacon, S. S., Vega, C. M., Hauser-Davis, R. A., de Campos, R. C., and Siciliano, S. (2012). Guiana dolphins (Sotalia guianensis, Van Benédén 1864) as indicators of the bioaccumulation of total mercury along the coast of Rio de Janeiro state, Southeastern Brazil. Bull Environ. Contam. Toxicol. 88, 54–59. doi: 10.1007/s00128-011-0448-z
de Moura, J. F., Hauser-Davis, R. A., Lemos, L., Emin-Lima, R., and Siciliano, S. (2014). Guiana dolphins (Sotalia guianensis) as marine ecosystem sentinels: ecotoxicology and emerging diseases. Rev. Environ. Contaminat. Toxicol. 228, 1–29. doi: 10.1007/978-3-319-01619-1_1
Decataldo, A., Di Leo, A., GIandomenico, S., and Cadelliccio, N. (2004). Association of metals (mercury, cadmium and zinc) with metallothionein-like proteins in storage organs of stranded dolphins from the Mediterranean Sea (Southern Italy). J. Environ. Monitor. 6, 361–367. doi: 10.1039/b315685k
Di Beneditto, A., and Siciliano, S. (2007). Stomach contents of the marine tucuxi dolphin (Sotalia guianensis) from Rio de Janeiro, south-eastern Brazil. J. Mar. Biol. Assoc. U K. 87, 253–254. doi: 10.1017/s0025315407053647
Dorneles, P. R., Lailson-Brito, J., Dos Santos, R., Costa, P., Malm, O., Azevedo, A. F., et al. (2007). Cephalopods and cetaceans as indicators of offshore bioavailability of cadmium off Central South Brazil Bight. Environ. Pollut. 148, 352–359. doi: 10.1016/j.envpol.2006.09.022
Dorneles, P. R., Lailson-Brito, J., Fernandez, M. A., Vidal, L. G., Barbosa, L. A., Azevedo, A. F., et al. (2008). Evaluation of cetacean exposure to organotin compounds in Brazilian waters through hepatic total tin concentrations. Environ. Pollut. 156, 1268–1276. doi: 10.1016/j.envpol.2008.03.007
Dragun, Z., Marijić, V., Vuković, M., and Raspor, B. (2015). “Metal bioavailability in the Sava River water,” in The Sava River, eds R. Milacić, J. Ščančar, and M. Paunović (Berlin: Springer-Verlag), 123–155.
Erk, M., Ivanković, D., Raspor, B., and Pavicić, J. (2002). Evaluation of different purification procedures for the electrochemical quantification of mussel metallothioneins. Talanta 57, 1211–1218. doi: 10.1016/s0039-9140(02)00239-4
Eurachem (2014). Eurachem Guide: The Fitness for Purpose of Analytical Methods - A Laboratory Guide to Method Validation and Related Topics. Nat. Sci. 2, 1–70.
Filipovi, V., and Raspor, B. (2003). Metallothionein and metal levels in cytosol of liver, kidney and brain in relation to growth parameters of Mullus surmuletus and Liza aurata from the Eastern Adriatic Sea. Water Res. 37, 3253–3262. doi: 10.1016/s0043-1354(03)00162-3
Flores, P. A. C., and Da Silva, V. M. F. (2009). “Tucuxi and Guiana Dolphin (Sotalia fluviatilis and S. guianensis),” in Encyclopedia of Marine Mammals, 2nd Edn, eds W. F. Perrin, B. Würsig, and J. G. M. Thewissen (Amsterdam: Elsevier), 1188–1192.
Gannier, A., and West, K. L. (2005). Distribution of the rough-toothed dolphin (Steno bredanensis) around the Windward Islands (French Polynesia). Pacific Sci. 59, 17–24. doi: 10.1353/psc.2005.0007
Geffard, A., Satelet, H., Garric, J., Biaganti-Risbourg, S., Delahaut, L., and Geffard, O. (2010). Subcellular compartmentalization of cadmium, nickel, and lead in Gammarus fossarum: Comparison of methods. Chemosphere 78, 822–829. doi: 10.1016/j.chemosphere.2009.11.051
Geraci, J. R., and Lounsbury, V. (1993). Marine Mammals Ashore: A Field Guide for Strandings. Texas: Texas A&M Sea Grant Publications.
Ishak, I., Rosli, F. D., Mohamed, J., and Ismail, M. F. M. (2015). Comparison of digestion methods for the determination of trace elements and heavy metals in human hair and nails. Malaysian J. Med. Sci. 6, 11–20. doi: 10.1007/bf02784086
James, I. D. (2002). Modelling pollution dispersion, the ecosystem and water quality in coastal waters: a review. Environ. Model. Soft. 17, 363–385. doi: 10.1016/s1364-8152(01)00080-9
Jefferson, T. A. (2009). “Rough-toothed dolphin: Steno bredanensis,” in Encyclopedia of Marine Mammals, 2nd Edn, eds W. F. Perrin, B. Wursig, and J. G. M. Thewissen (San Diego, CA: Academic Press.), 990–992.
Kehrig, H. A., Hauser-Davis, R. A., Seixas, R. G., Pinheiro, A. B., and Di Beneditto, A. P. (2016). Mercury species, selenium, metallothioneins and glutathione in two dolphins from the southeastern Brazilian coast: Mercury detoxification and physiological differences in diving capacity. Environ. Pollut. 213, 785–792. doi: 10.1016/j.envpol.2016.03.041
Kizek, R., Trnková, T. L., and Palcek, E. (2001). Determination of metallothionein at the femtomole level by constant current stripping chronopotentiometry. Analytic. Chem. 73, 4801–4807. doi: 10.1021/ac010126u
Lailson-Brito, J., Azeredo, M., Malm, O., Ramos, R. M., Beneditto, A. P., and Saldanha, M. C. (2002). Trace metals in liver and kidney of the franciscana (Pontoporia blainvillei) from the northern coast of Rio de Janeiro State, Brazil. Latin Am. J. Aquat. Mammals 1, 107–114.
Lailson-Brito, J., Cruz, R., Dorneles, P. R., Andrade, L., Azevedo, A. F., Fragoso, A. B., et al. (2012). Mercury-Selenium Relationships in Liver of Guiana Dolphin: The Possible Role of Kupffer Cells in the Detoxification Process by Tiemannite Formation. Plos One 7:e42162. doi: 10.1371/journal.pone.0042162
Land, S. N., Rocha, R. C. C., Bordon, I. C., Saint’Pierre, T. D., Ziolli, R. L., and Hauser-Davis, R. A. (2018). Biliary and hepatic metallothionein, metals and trace elements in environmentally exposed neotropical cichlids Geophagus brasiliensis. J. Trace Elem. Med. Biol. 50, 347–355. doi: 10.1016/j.jtemb.2018.07.023
Lee, S. S., Mate, B. R., von, der Trenck, K. T., Rimerman, R. A., and Buhler, D. R. (1977). Metallothionein and the subcellular localization of mercury and cadmium in the Californian sea lion. Compar. Biochem. Physiol. 57, 45–53. doi: 10.1016/0306-4492(77)90076-4
Lodi, L., and Hetzel, B. (1999). Rough-toothed dolphin, Steno bredanensis, feeding behaviors in Ilha Grande Bay, Brazil. Biociências 7, 29–42.
Magalhães, D. P., Marques, M. R. C., Baptista, D. F., and Buss, D. B. (2015). Metal bioavailability and toxicity in freshwaters. Environ. Chem. Lett. 13, 69–87. doi: 10.1007/s10311-015-0491-9
Marijić, V. F., and Raspor, B. (2006). Age- and tissue-dependent metallothionein and cytosolic metal distribution in a native Mediterranean fish, Mullus barbatus, from the Eastern Adriatic Sea. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 143, 382–387. doi: 10.1016/j.cbpc.2005.05.019
Marijić, V. F., Dragun, Z., Perić, M. S., Kepčija, R. M., Gulin, V., Velki, M., et al. (2016). Investigation of the soluble metals in tissue as biological response pattern to environmental pollutants (Gammarus fossarum example). Chemosphere 154, 300–309. doi: 10.1016/j.chemosphere.2016.03.058
Martínez-López, E., Peñalver, J., Escriña, A., Lara, L., Gens, M. J., María Dolores, E., et al. (2019). Trace metals in striped dolphins (Stenella coeruleoalba) stranded along the Murcia coastline, Mediterranean Sea, during the period 2009-2015. Chemosphere 229, 580–588. doi: 10.1016/j.chemosphere.2019.04.214
Melo, C. L. C., dos Santos, R. A., Bassoi, M., Araújo, A. C., Brito, J. L., Dorneles, P., et al. (2010). Feeding habits of delphinids (Mammalia: Cetacea) from Rio de Janeiro State, Brazil. J. Mar. Biol. Asso. U K. 90, 1509–1515. doi: 10.1017/s0025315409991639
Miguens, F. C., de Oliveira, M. L., Ferreira, A. O., Barbosa, L. R., de Melo, E. J. T., and de Carvalho, C. E. V. (2016). Structural and elemental analysis of bottom sediments from the Paraíba do Sul River (SE, Brazil) by analytical microscopy. J. South Am. Earth Sci. 66, 82–96. doi: 10.1016/j.jsames.2015.12.009
Minitab (2020). Data considerations for Kruskal-Wallis Test. Available online at: https://support.minitab.com/en-us/minitab-express/1/help-and-how-to/modeling-statistics/anova/how-to/kruskal-wallis-test/before-you-start/data-considerations/ (accessed on November 9, 2020).
Monteiro, F., Lemos, L. S., de Moura, J. F., Rocha, R. C. C., Moreira, I., Di Beneditto, A. P., et al. (2019). Subcellular metal distributions and metallothionein associations in rough-toothed dolphins (Steno bredanensis) from Southeastern Brazil. Mar. Pollut. Bull. 146, 263–273. doi: 10.1016/j.marpolbul.2019.06.038
Monteiro, S. S., Pereira, A. T., Costa, E., Torres, J., Oliveira, I., Bastos-Santos, J., et al. (2016). Bioaccumulation of trace element concentrations in common dolphins (Delphinus delphis) from Portugal. Mar. Pollut. Bull. 113, 400–407. doi: 10.1016/j.marpolbul.2016.10.033
Nigro, M., and Leonzio, C. (1996). Intracellular storage of mercury and selenium in different marine vertebrates. Mar. Ecol. Prog. Ser. 135, 17–143.
Olafson, R. W., and Thompson, J. A. J. (1974). Isolation of heavy metal binding proteins from marine vertebrates. Mar. Biol. 28, 83–86. doi: 10.1007/bf00396298
Paixão, P. V., Takase, I., and Stapelfeldt, D. M. A. (2013). Dosagem de Metais em Sedimentos da Bacia do Rio São João no Estado do Rio de Janeiro. Brazil: Congresso Brasileiro de Quimica.
Pedrero, Z., Ouerdan, L., Mounicou, S., Lobinski, R., Monperrus, M., and Amouroux, D. (2012). Identification of mercury and other metals complexes with metallothioneins in dolphin liver by hydrophilic interaction liquid chromatography with the parallel detection by ICP MS and electrospray hybrid linear/orbital trap MS/MS. Metallomics 4, 473–479. doi: 10.1039/c2mt00006g
Petrlovà, J., Potesil, D., Mikelova, R., Blastik, O., Adam, V., Trnkova, L., et al. (2006). Attomole voltammetric determination of metallothionein. Electrochim. Acta 51, 5112–5119. doi: 10.1016/j.electacta.2006.03.078
Ridlington, J. W., Chapman, D. C., Goeger, D. E., and Whanger, P. D. (1981). Metallothionein and Cu-chelatin: characterization of metal-binding proteins from tissues of four marine animals. Compar. Biochem. Physiol. B 70, 91–104.
Rosas, F. C. W., and Monteiro Filho, E. L. A. (2002). Reproduction of the estuarine dolphin (Sotalia guianensis) on the coast of Paranà, southern Brazil. J. Mammal. 83, 507–515. doi: 10.1644/1545-1542(2002)083<0507:roteds>2.0.co;2
Salgado, L. D., Rosa, S. M., and de Azevedo, J. C. R. (2018). Concentrations of metals in liver of Guiana dolphins (Sotalia guianensis) from an estuary in Southeast of Brazil. Ecotoxicol. Environ. Contaminat. 13, 51–61.
Secchi, E., Santos, M. C., de, O., and Reeves, R. (2019). Sotalia Guianensis. The IUCN Red List of Threatened Species 2018. Available online at: http://dx.doi.org/10.2305/IUCN.UK.2018-2. RLTS.T181359A144232542.en (accessed on October 4, 2019).
Wallace, W. G., Lee, B.-G., and Luoma, S. N. (2003). Subcellular compartmentalization of Cd and Zn in two bivalves. I. Significance of metal-sensitive fractions (MSF) and biologically detoxified metal (BDM). Mar. Ecol. Prog. Ser. 249, 183–197.
Keywords: odontocetes, subcellular metal fractionation, metal detoxification, bioavailability, contamination
Citation: Hauser-Davis RA, Figueiredo L, Lemos L, de Moura JF, Rocha RCC, Saint’Pierre T, Ziolli RL and Siciliano S (2020) Subcellular Cadmium, Lead and Mercury Compartmentalization in Guiana Dolphins (Sotalia guianensis) From Southeastern Brazil. Front. Mar. Sci. 7:584195. doi: 10.3389/fmars.2020.584195
Received: 16 July 2020; Accepted: 16 November 2020;
Published: 14 December 2020.
Edited by:
Lyne Morissette, M – Expertise Marine, CanadaReviewed by:
Danielle Kreb, Conservation Foundation for Rare Aquatic Species of Indonesia, IndonesiaTatiana Bisi, Rio de Janeiro State University, Brazil
Copyright © 2020 Hauser-Davis, Figueiredo, Lemos, de Moura, Rocha, Saint’Pierre, Ziolli and Siciliano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachel Ann Hauser-Davis, cmFjaGVsLmhhdXNlci5kYXZpc0BnbWFpbC5jb20=; cmFjaGVsLmRhdmlzQGlvYy5maW9jcnV6LmJy
 Rachel Ann Hauser-Davis1,2*
Rachel Ann Hauser-Davis1,2* Jailson Fulgêncio de Moura
Jailson Fulgêncio de Moura Rafael C. C. Rocha
Rafael C. C. Rocha Tatiana Saint’Pierre
Tatiana Saint’Pierre