- 1Laboratorio de Ecología General, Departamento de Biología, Facultad Experimental de Ciencias, University of Zulia, Maracaibo, Venezuela
- 2Centre for Tropical Water and Aquatic Ecosystem Research, James Cook University, Townsville, QLD, Australia
- 3Fundación Fauna Caribe Colombiana (FFCC), Barranquilla, Colombia
The Guiana dolphin (Sotalia guianensis) home range is located across Central and South American countries, in coastal habitats in the Caribbean and Atlantic Ocean. Its distribution is scattered, with multiple population centers which are under threats that vary based on local realities. We compiled and assessed biological data from multiple sources (published and unpublished data) to improve our understanding regarding the Maracaibo Lake Management Unit, which is an isolated and unique population core of this species. We identified at least two distinguishable population centers throughout the Maracaibo Lake System, one in the northern portion—in the Gulf of Venezuela, and another in the southern portion of the Maracaibo Lake itself. Both centers have differences in some biological aspects (e.g., group size and habitat use), but similarities in the human-induced pressures (e.g., intentional take, habitat degradation, and traditional use). We detailed the uses of Guiana dolphin (consumptive and non-consumptive) by community members, including the use as talismans for indigenous fishers and consumption of its meat as a religious belief (Easter period), and dolphin watching tours carried out by local companies. In one artisanal port, at least 15 animals are intentionally taken annually to be used for local consumption, shark-bait, or trade; however, we acknowledge that this annual take is likely an underestimate. Further research is needed to clarify how and at what magnitude mentioned and other key-threats are impacting over Guiana dolphin MU in the Maracaibo Lake System.
Background
The Guiana dolphin (Sotalia guianensis) inhabits in shallow waters in coastal marine, brackish, and freshwater environments (Flores et al., 2010). This small cetacean Management Units (MU) are isolated and under multiple human-induced pressures. In Venezuela, the Guiana dolphin is categorized as Vulnerable, due to habitat degradation, intentional take, by-catch, and highly polluted home range (Romero et al., 2001; Barrios-Garrido et al., 2015).
However, within the Maracaibo Lake MU, there are still important knowledge gaps regarding the habitat use, threats, distribution, and ecology of the Guiana dolphin (Barrios-Garrido et al., 2016; Espinoza-Rodríguez et al., 2019). The Guiana dolphin MU in the Maracaibo Lake is an isolated population center that needs to be investigated due to its genetic and anatomic uniqueness (Caballero et al., 2007, 2010, 2018; De Turris-Morales et al., 2010a).
Data Compiled
Here we summarized data available from multiple sources including literature review, market-based assessments, in situ observations (21 artisanal ports or landing sites), unpublished data, newspaper, in-depth (key-informants) and semi-structured interviews of indigenous and local fishers regarding information about the presence, sale, and use of Guiana dolphin in the Maracaibo Lake System (Figure 1). Aged between 21 and 78 years old, our respondents (n = 82) were 23 women and 59 men. Indigenous respondents were considered and approved by the community clan leaders. Lastly, we also assessed stranding network records from the environmental entities databases (unpublished data).
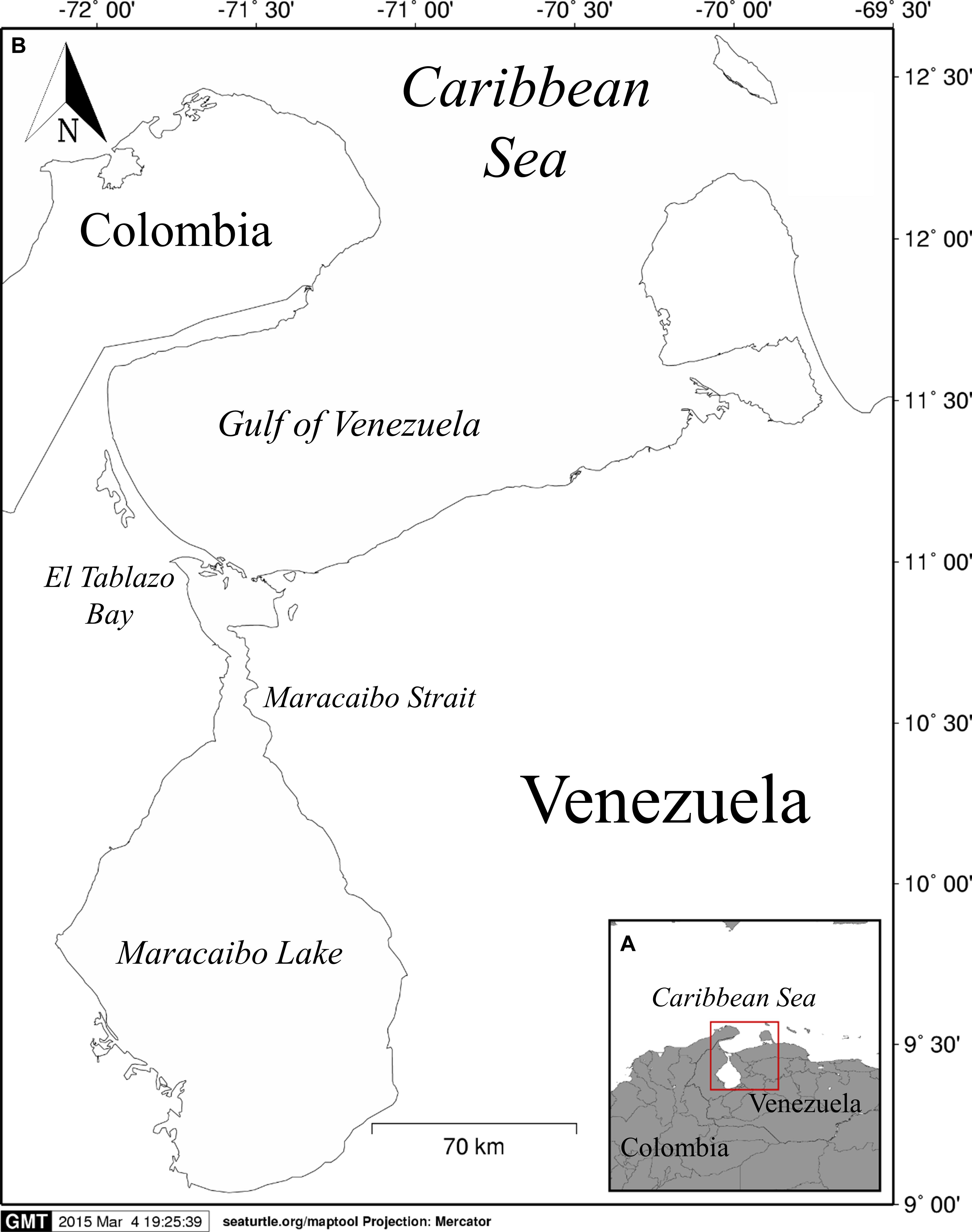
Figure 1. Study area. (A) Geographical location of the Maracaibo Lake System within Venezuela, showing its relative position within the Caribbean Sea. (B) Detail of the four aquatic habitat that form the Maracaibo Lake System: Gulf of Venezuela, El Tablazo Bay, Maracaibo Strait, and the Maracaibo Lake.
Main Findings—Regional Problems, and Local Pressures
The Guiana dolphin is the most common and frequently sighted cetacean in the Maracaibo Lake System; and is also responsible for the most stranding records (Casinos et al., 1981; Romero et al., 2001; Bolaños-Jiménez et al., 2015). Guiana dolphins present two main population cores in the Maracaibo Lake System (Caballero et al., 2010; Barrios-Garrido et al., 2015). The first one in the Gulf of Venezuela; where there is an estimated abundance of 1.34 individuals per km2, with groups composed of 2–5 individuals, and aggregations as large as one-hundred (Espinoza-Rodríguez et al., 2019). The second population core inside the Maracaibo Lake System has an abundance estimation of 1.66 individuals per km2, within an area of 249.2 km2, based on research carried out in the western-central area of this aquatic habitat (Delgado-Ortega, 2012).
In the Gulf of Venezuela, Guiana dolphin sightings have been recorded year-round with significant variations between seasons (wet/dry season). The wet season generally has the highest number of sightings, group size, and frequency of encounter (Barrios-Garrido et al., 2016). Their distribution within the area is heterogeneous. Dolphins were observed mainly in the southern portion of the Gulf of Venezuela, north El Tablazo Bay. We found significantly larger groups inside the navigation channel; this area is considerably deeper and it is where dolphins have exhibited most of their activities.
The behavioral data available on Guiana dolphins in the Gulf of Venezuela indicated that dolphins were rarely seen resting, spending approximately 38% of the time either traveling or feeding. Dolphins appeared to be active during both day and night; nonetheless, we recorded larger number of groups in the afternoons. Also, diurnal observations suggest that feeding peaks occur during the incoming tide, possibly following prey movements. The latter is also correlated with seabird and dolphin associations that often occurred during surveys, with most frequently associated seabird being Fregata magnificens (Espinoza-Rodríguez et al., 2015). These associations were generally seen when fish and dolphins congregations were resulted in high energy activity at the surface.
Group sizes in the Maracaibo Lake were similar to the groups observed in the Gulf of Venezuela, ranging from 1 to 8 individuals (Barrios-Garrido et al., 2015). Their distribution in this area was significantly correlated with low salinities, and water transparency, resulting in heterogeneous habitat usage (Delgado-Ortega, 2012). There were fewer sightings during the dry season, similar to the groups observed in other areas of the Maracaibo Lake System. It is suggested that these fluctuations throughout its geographical range are likely related to environmental, physicochemical changes, and prey availability.
In Maracaibo Lake, this species exhibits a hunting strategy where larger individuals encircle prey, move aggressively, attack by hitting strongly with their fluke or beak, and capture prey once it reaches the water surface. Adult dolphins then proceed to feed the calves (De Turris-Morales et al., 2010b). Further research is needed to understand if this strategy is similar in the northern population center.
Between 1999 and 2015 evidence suggests that, if captured (intentionally or otherwise), most individuals were slaughtered for uses such as human consumption, shark-bait (artisanal longline fishery), or trade (Barrios-Garrido et al., 2017; Sanchez and Briceño, 2017; Altherr and Hodgins, 2018) (Figure 2). We estimate that at least 15 animals per year are intentionally taken in one port, to be used as mentioned. We also identified the existence of commercial use of Guiana dolphins in local markets (either by-caught or intentionally taken). The Guiana dolphin meat is often consumed during Easter, similar to other aquatic meats (in line with local religious traditions and magical beliefs and rites) (Montiel-Villalobos and Barrios-Garrido, 2005; Barros et al., 2010; Rojas-Cañizales et al., 2015, 2020). Key-informants affirmed that this consumption, related to religious ritual, is linked to Christian practice to avoid “red meat” during Easter. Some interviewees confirmed that Guiana dolphins are used as talismans for indigenous fishers, who claimed that their presence in the waters is linked with productive periods (Barrios-Garrido and Montiel-Villalobos, 2016) (Figure 2).
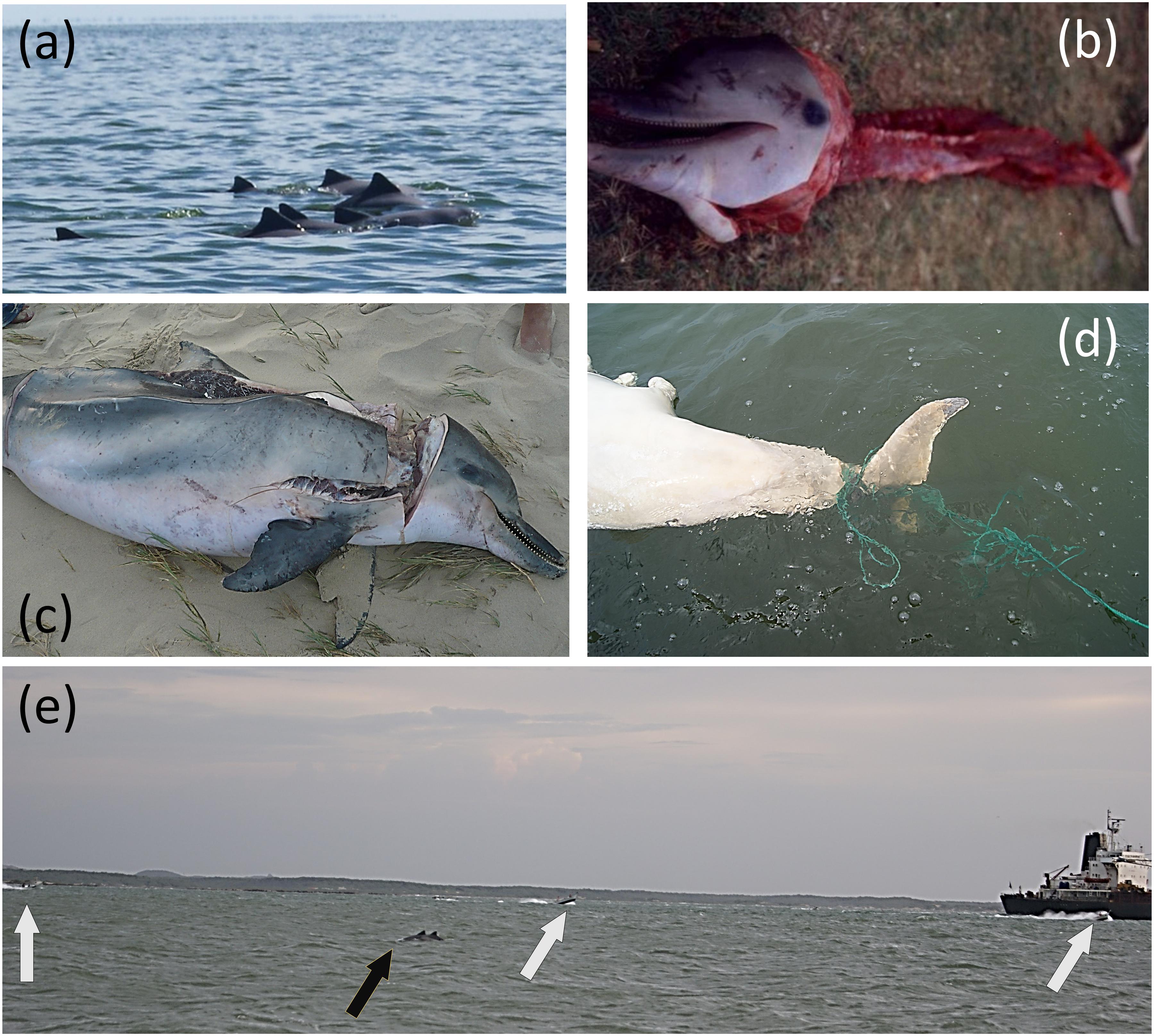
Figure 2. Evidences of the impacts of the detailed treaths over the Guiana dolphin in the Maracaibo Lake System. (a) Group of Guiana dolphin swinging next to oil marks in the water. (b) Harvested juvenile Guiana dolphin, used for human consumption. (c) Guiana dolphin individual with marks of knife cuts and its dorsal muscle removed, individual used as shark bait in the artisanal longline fishery. (d) Guiana dolphin fluk entangled with artisanal longline to capture blue crap (Callinectes spp.) in the Maracaibo Lake. (e) Guiana dolphin group (black arrow) traveling in the navigation channel, surrounded by oil tanker and artisanal boats (white arrows).
There are some tourism initiatives reliant on Guiana dolphins as target species, especially in the southern portion of the Maracaibo Lake (Hoyt and Iñíguez, 2008). However, some fishers argued that they have to compete with dolphins for fish and, as a result, they have used boat engines or knocked on the boats to generate noise to move Guiana dolphin groups away from their nets. They also affirmed that during those periods entangled calves are commonly found freshly dead and discarded.
Our assessment of the local use of Guiana dolphins is likely an underestimate. Some respondents noted that they have witnessed the intentional capture of tens of dolphins in only 1 day using nylon gillnets as an artisanal seine fishery. This intentional capture practice may reach unsustainable levels in some areas where decimated populations are rarely observed nowadays (e.g., eastern portion of the Maracaibo Lake). No further details were provided about these intentional capture events due to the illegality of this practice.
Recommendations and Future Management Plans
High levels of Guiana dolphin mortality is related to fishing activities (Figure 2). However, there is a lack of standardized population assessments to compare abundance, population trends, distribution, and fisheries-related mortality of Guiana dolphin in the Maracaibo Lake System. Genetic identification to solve the links between northern (Gulf of Venezuela) and southern (Maracaibo Lake) populations are needed (Caballero et al., 2018). Further research is needed in the area to improve current knowledge of the species, including in other data-poor zones; e.g., Capatarida (Falcon State), and Ceuta (Zulia state) (Espinoza-Rodríguez et al., 2019). Conservation strategies focused on Guiana dolphins in the Maracaibo Lake System are strongly recommended (e.g., stranding network, specialized research centers, among others); moreover, a risk assessment of Guiana dolphin mortality is required to inform management decisions.
Further research is needed to have a better perspective about key threats of the Guiana dolphin MU in the Maracaibo Lake System, especially in the following human-related pressures: heavy metal bioaccumulation, boat noise effects, direct capture, habitat degradation, artisanal fishery impacts, oil extraction, and transportation activities, algal and duckweed blooms, and marine debris interaction (entanglement and ingestion). All of these mentioned potential threats have been previously described in the study area (Delgado-Ortega, 2012; Barrios-Garrido et al., 2015, 2016; Sanchez and Briceño, 2017; Espinoza-Rodríguez et al., 2019) (Figure 2).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Division de Estudios Basicos Sectoriales (DEBS), Experimental Faculty of Sciences at University of Zulia. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. All of our interviewees provided oral consent (mentioned in the protocol) to participate on this research as stated in our authorizations. Our human research data gathered between 2008 and 2011 was authorized by the “Division de Estudios Basicos Sectoriales (DEBS)” from the Experimental Faculty of Science at University of Zulia, and our human research data gathered between 2014 and 2015 was authorized by the Human Ethics Committee at James Cook University, under the ethics permit number: H5704. The animal study was reviewed and approved by the Venezuelan Environmental Ministry (Ministerio para el Poder Popular del Ambiente) now the Ministry of Eco-Socialism and Water, under the permit for animal research and biological data collection memorandum number 0038.
Author Contributions
HB-G had the initial idea, designed the study, and lead the research team. KD-M led the evaluation of the northern population center of Sotalia guianensis. NE-R led the evaluation of the southern population center of Sotalia guianensis. All authors contributed substantially in the data compilation, analysis, and writing process and approved this manuscript for publication if this is the case.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the local fishers and Indigenous clan leaders who allow us to conduct this research in their communities. We recognize the important role of tens of volunteers of the Laboratory of General Ecology at the University of Zulia, who participated for years gathering the research data. We are appreciative of Gabriela Delgado-Ortega and Joanmyra Carrasquero for logistical support in the field. We thank Rachel Miller, Ryan Pearson, and Brad Nahill, for their help in improving the English of this manuscript. We want to recognize all the researchers that for decades have helped to improve our understanding regarding cetaceans in Venezuela. Finally, we thank to Frontiers in Marine Science for the waiver received to complete this publication.
References
Altherr, S., and Hodgins, N. (2018). SMALL CETACEANS, BIG PROBLEMS: A Global Review of The Impacts of Hunting on Small Whales, Dolphins and Porpoises: PRO Wild. Chippenham: Animal Welfare Institute, Whale and Dolphin Conservation.
Barrios-Garrido, H., Boher-Bentti, S., De Turris-Morales, K., Espinoza-Rodriguez, N., Ferrer-Pérez, A., Herrera-Trujillo, O. L., et al. (2015). “Tonina costera, Sotalia guianensis,” in Libro Rojo de la Fauna Venezolana, eds J. P. Rodriguez, A. Garcia-Rawlins, and F. Rojas-Suarez (Caracas: Provita, Fundacion Empresas Polar).
Barrios-Garrido, H., Bolivar, J., Benavides, L., Viloria, J., Dugarte-Contreras, F. N., and Wildermann, N. (2017). Evaluación de la pesquería de palangre artesanal y su efecto en la Raya Hocicona (Dasyatis guttata) en Isla Zapara-Golfo de Venezuela. Latin Am. J. Aquat. Res. 45, 302–310. doi: 10.3856/vol45-issue2-fulltext-6
Barrios-Garrido, H. A., De Turris-Morales, K., Delgado-Ortega, G., Nash, C. M., and Espinoza-Rodriguez, N. (2016). Acoustic Parameters of Guiana Dolphin (Sotalia guianensis) whistles in the Southern Gulf of Venezuela. Aquat. Mamm. 42, 127–136. doi: 10.1578/AM.42.2.2016.127
Barrios-Garrido, H. A., and Montiel-Villalobos, M. G. (2016). Strandings of Leatherback turtles (Dermochelys coriacea) along the western and southern coast of the Gulf of Venezuela. Herpetol. Conserv. Biol. 11, 244–252.
Barros, T., Jiménez-Oraá, M., Heredia, H. J., and Seijas, A. E. (2010). Artificial incubation of wild-collected eggs of American and Orinoco crocodiles (Crocodylus acutus and C. intermedius), Guárico and Zulia, Venezuela. Conserv. Evidence 7, 111–115.
Bolaños-Jiménez, J., Balladares, C., Barrios-Garrido, H., Bermúdez-Villapol, L., De Turris, K., Espinoza, N., et al. (2015). Varamientos de cetáceos en Venezuela: 1988-2014. Mamm. Notes 2:238.
Caballero, S., Hollatz, C., Rodríguez, S., Trujillo, F., and Baker, C. S. (2018). Population structure of riverine and coastal dolphins Sotalia fluviatilis and Sotalia guianensis: patterns of nuclear and mitochondrial diversity AND implications for conservation. J. Heredity 109, 757–770. doi: 10.1093/jhered/esy049
Caballero, S., Trujillo, F., Vianna, J. A., Barrios-Garrido, H., Montiel, M., Beltrán-Pedreros, S., et al. (2010). Mitochondrial DNA diversity, differentiation and phylogeography of the South American riverine and coastal dolphins Sotalia fluviatilis and Sotalia guianensis. Latin Am. J. Aquat. Mamm. 8, 69–79. doi: 10.5597/lajam00155
Caballero, S., Trujillo, F., Vianna, J. A., Barrios-Garrido, H., Montiel, M. G., Beltrán-Pedreros, S., et al. (2007). Taxonomic status of the genus Sotalia: species level ranking for “tucuxi” (Sotalia fluviatilis) and “costero” (Sotalia guianensis) dolphins. Mar. Mamm. Sci. 23, 358–386. doi: 10.1111/j.1748-7692.2007.00110.x
Casinos, A., Bisbal, F., and Boher, S. (1981). Sobre tres ejemplares de Sotalia fluviatilis del lago de Maracaibo (Venezuela)(Cetacea, Delphinidae). Publ. Dept. Zool. Barcelona 7, 93–96.
De Turris-Morales, K., Barrios-Garrido, H., Espinoza-Rodríguez, N., and Delgado-Ortega, G. (2010a). “Caracteres no métricos de dos poblaciones del delfín estuarino (Sotalia guianensis) en el Sistema del Lago de Maracaibo,” in Proceedings of the Memorias de las XII Jornadas Nacionales de Investigación y Postgrado, XIIJNIP-092-2010, (Maracaibo: Universidad del Zulia, Facultad Experimental de Ciencias).
De Turris-Morales, K., Delgado-Ortega, G., Espinoza-Rodríguez, N., Montiel-Villalobos, M., and Barrios-Garrido, H. (2010b). Nota sobre el comportamiento alimenticio del Delfín Estuarino (Sotalia guianensis) en la costa occidental del Lago de Maracaibo. Ecotrópicos 23, 114–116.
Delgado-Ortega, G. (2012). Distribución espacial y temporal de la Tonina del Lago (Sotalia guianensis) en la costa occidental del Sistema de Maracaibo. (Biologist Research Thesis Manuscript). Maracaibo: University of Zulia.
Espinoza-Rodríguez, N., Carrasquero, J., De Turris-Morales, K., Delgado-Ortega, G., and Barrios-Garrido, H. (2015). Asociaciones entre aves marinas y Sotalia guianensis en el sur del Golfo de Venezuela. Caldasia 37, 309–318. doi: 10.15446/caldasia.v37n2.54381
Espinoza-Rodríguez, N., De Turris-Morales, K., Shimada, T., and Barrios-Garrido, H. (2019). Guiana Dolphin (Sotalia guianensis) in the southern Gulf of Venezuela: seasonal distribution, group size, and habitat use. Regional Stud. Mar. Sci. 32:100874. doi: 10.1016/j.rsma.2019.100874
Flores, P. A. C., Bazzalo, M., Caballero, S., Santos, M. C. O., Rossi-Santos, M. R., Trujillo, F., et al. (2010). Proposed English common name for the neotropical delphinid Sotalia guianensis (P.-J. Van Beneden, 1864). Latin Am. J. Aquat. Mamm. 8, 179–181. doi: 10.5597/lajam00167
Hoyt, E., and Iñíguez, M. (2008). Estado del Avistamiento de Cetáceos en América Latina. Chippenham: WDCS.
Montiel-Villalobos, M., and Barrios-Garrido, H. (2005). Observaciones sobre la distribución y situación actual del Manatí Trichechus manatus (Sirenia: Trichachidae) en el Sistema del Lago de Maracaibo. Anartia 18, 1–12.
Rojas-Cañizales, D., Espinoza-Rodríguez, N., Petit-Rodríguez, M. J., Palmar, J., Mejías-Balsalobre, C., Wildermann, N., et al. (2020). Marine turtle mortality in a southern Caribbean artisanal fishery: a threat for immature green turtles. Region. Stud. Mar. Sci. 38:101380. doi: 10.1016/j.rsma.2020.101380
Rojas-Cañizales, D., Rodriguez, M. A., Suarez-Villasmil, L., and Barrios-Garrido, H. (2015). Evaluación preliminar de la tasa de crecimiento de neonatos de la hicotea, Trachemys callirostris callirostris (Testudines: Emydidae) en cautiverio. Mem. Fundación La Salle de Ciencias Naturales 72, 91–99.
Romero, A., Agudo, I., Green, S., and Di Sciara, G. N. (2001). Cetaceans of Venezuela: Their distribution and conservation status. NOAA Technical Report NMFS 151. A Technical Report of the Fishery Bulletin. Washington, DC: US Department of Commerce, 1–60.
Keywords: intentional take, by-catch capture, ambient noise, heavy metals, management plan, aquatic bushmeat, small cetaceans, southern caribbean
Citation: Barrios-Garrido H, De Turris-Morales K and Espinoza-Rodriguez NE (2021) Guiana Dolphin (Sotalia guianensis) in the Maracaibo Lake System, Venezuela: Conservation, Threats, and Population Overview. Front. Mar. Sci. 7:594021. doi: 10.3389/fmars.2020.594021
Received: 12 August 2020; Accepted: 29 December 2020;
Published: 27 January 2021.
Edited by:
Diego Horacio Rodriguez, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), ArgentinaReviewed by:
David Ainley, H.T. Harvey and Associates, United StatesSalvatore Siciliano, Oswaldo Cruz Foundation (Fiocruz), Brazil
Copyright © 2021 Barrios-Garrido, De Turris-Morales and Espinoza-Rodriguez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hector Barrios-Garrido, aGVjdG9yLmJhcnJpb3NnYXJyaWRvQG15LmpjdS5lZHUuYXU=
 Hector Barrios-Garrido
Hector Barrios-Garrido Kareen De Turris-Morales
Kareen De Turris-Morales Ninive Edilia Espinoza-Rodriguez
Ninive Edilia Espinoza-Rodriguez