- 1Tropical Aquaculture Research and Development Center, South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Sanya, China
- 2Key Laboratory of South China Sea Fishery Resources Exploitation and Utilization, Ministry of Agriculture, Guangzhou, China
- 3Sanya Tropical Fisheries Research Institute, Sanya, China
- 4Dalian Tianzheng Industry Co., Ltd., Dalian, China
This study evaluated the effects of rotifers enriched with three enhancement products (Nannochloropsis, S.presso, and Algamac 3080) on the body fatty acid composition, growth, survival, jaw deformity, and bone development-related gene expression of the golden pompano larvae. The rotifers enriched with Nannochloropsis were rich in EPA, and the rotifers enriched with S.presso and Algamac 3080 were rich in docosahexaenoic acid (DHA). The level of DHA in Algamac 3080 is higher than that in S.presso. The first feeding started at 3 DPH, and data were collected at 8 DPH. The results showed that the body fatty acid composition of the larvae was basically the same as that of the feeding rotifers. The specific growth rate of S.presso and Algamac 3080 treatment was significantly higher than the un-enriched treatment (P < 0.05). The survival rate of Algamac 3080 treatment was significantly lower than the other treatments (P < 0.05), and the jaw deformity rate of S.presso treatment was significantly lower than the Nannochloropsis and un-enriched treatment (P < 0.05). The expression level of BMP2 and BMP4 in golden pompano larvae were not significantly affected by the enhancement products (P > 0.05), and the expression level of RXRα decreased significantly in the S.presso and Algamac 3080 treatment (P < 0.05). This study indicates that S.presso was an enhancement product more suitable for rotifers for golden pompano larvae. This study provided reliable reference and guidance for the first feeding of golden pompano larvae and also provided more reference data for the study of the mechanism of diet on larval fish bone deformity.
Introduction
In the artificial breeding of marine fish, the choice of feed during first feeding period is particularly important, because they are very vulnerable during this period (Hamre et al., 2013). The critical period for the transformation of endogenous nutrition into exogenous nutrition is also critical for the development of bones and systems of larval fish (Koedijk et al., 2010). Unsuitable feed can lead to nutrient deficiency and starvation, resulting in growth retardation, deformity, and even death (Rice et al., 1987; Gisbert et al., 2004; Waqalevu et al., 2019). Rotifers and Artemia nauplii are widely used in aquaculture due to the advantages of suitable size and easy cultivation (Kotani et al., 2009; Ma et al., 2018). In particular, Brachionus is usually selected as the weaning food for marine finfish (Kobayashi et al., 2008; Kotani et al., 2009).
Furthermore, some studies have revealed that rotifers lack essential nutrients, so they must be enriched before feeding them to fish larvae (Hamre, 2016; Kotani, 2017). Common enriched methods include the use of fresh microalgae or commercial enhancement products, but because microalgae are difficult to operate and cultivate, commercial enhancement products are more widely used in enrichment process (Eryalcin, 2018). In the enriching of live feeds, polyunsaturated fatty acids (PUFA) such as arachidonic acid (ARA, 20:4 n-6), eicosapentaenoic acid (EPA, 20:3 n-3), and docosahexaenoic acid (DHA, 22:6 n-3) are usually the nutrient components that people pay more attention to. They play an important role in neurological and visual development, and stress resistance (Hernández-Cruz et al., 2015). Recent studies have also confirmed that they, especially DHA, are associated with bone and cartilage development in fish and provide a contribution to the prevention of bone deformities (Roo et al., 2009; Izquierdo et al., 2013).
In addition to focusing on growth and survival of larval fish in evaluating the effects of different enriched rotifers, bone deformities are also particularly important. Especially in the early stage, the development of the jaw will affect eating, growth, etc.; and bone deformities will become a potential that affects the later growth and survival rate (Cobcroft et al., 2001; Fraser and de Nys, 2005; Ferraresso et al., 2010). In golden pompano, some osteogenic marker genes have been studied in depth. Among them, bone morphogenetic protein (BMP) and retinoid X receptor (RXR) have been found to be significantly related to bone deformities (Yang et al., 2015; Ma et al., 2018). As a growth factor, BMPs play an important role in morphogenesis during embryonic development, and it is also an osteoinductive factor with osteogenic properties (Nishimura et al., 2012; Marques et al., 2016). RXRs can work with other genes such as IGF, BMP, Hox, or shh in morphogenesis and are related to bone deformation and survival (Cahu et al., 2009). In addition, RXRs can also be used as a transcription factor to regulate gene expression in various biological processes (Zhao et al., 2000; Shulman et al., 2004).
Golden pompano is one of the important economic fishes in the southern coastal areas of China and is popular in consumers for its tender and delicious meat (Ma et al., 2016, 2018). The artificial breeding technology of golden pompano is relatively mature, but the low survival rate and high deformity rate during the first feeding of larval fish are still technical bottlenecks. It is more effective and realistic to optimize the nutrition enriched method of rotifers to solve this problem so we selected an instant algae and two commercial enrichment products and unenhanced rotifers for comparison to explore a reasonable and convenient way of enriching rotifers and provide reference for the optimization of the details of the artificial breeding process of golden pompano.
Materials and Methods
Eggs and Larval Fish Rearing
Fertilized eggs of golden pompano were obtained from Guanghui Aquaculture Hatchery, Hainan Province, China, and were transported to Tropical Aquaculture Research and Development Center, South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Xincun Town and hatched in 500-L fiberglass incubators at 26°C. On 3 days post-hatching (DPH), larvae were stocked into 12 larval rearing tanks (1000-L) at a density of 40 fish L–1. Rearing tanks were supplied with filtered seawater and conducted in indoor flow-through culture system with a daily exchange rate of 200% tank volume. Two air stones were used in each tank to keep the dissolved oxygen level close to saturation. During the experimental period, the water quality parameters were measured daily and maintained at ammonia nitrogen < 0.1 mg L–1, nitrite nitrogen < 0.02 mg L–1, pH 7.9–8.1, salinity 33‰, and dissolved oxygen > 7.0 mg L–1.
Cultivation of Rotifers
This experiment included four dietary treatments with three replicates each, including (1) instant microalgal paste at 8 × 106 cell mL–1 (Nannochloropsis sp., Qingdao Hong Bang Biological Technology Co., Ltd, Qingdao, China), (2) S.presso (Selco S.presso®, INVE Aquaculture) at 350 mg L–1, (3) Algamac 3080® (Aquafauna, United States) at 200 mg L–1, and (4) Rotifers without enrichment served as control. The pure cultures of a strain of Brachionus plicatilis (L-type) with a typical lorica length of about 160 μm were supplied by Haiyou Jiayin Biotechnology Co., Ltd, Qionghai, China. Rotifers were fed with S.parkle® (INVE Aquaculture) at a density of 500 rotifers ml–1. The condition for rotifer culture was set at 24.3 ± 0.7°C, > 5.8 mg dissolved oxygen L–1, pH 7.95–8.11, and 37.5 g L–1 salinity. Prior to enrichment, the rotifers collected from the culture tank were rinsed with filtered seawater on a 100-μm mesh screen. During enrichment, the density of rotifers increased to 1,000 ml–1. After enrichment for 12 h, the rotifers were harvested on a 100-μm mesh screen and fed to the fish larvae. Starting from 3 DPH, the density of rotifers was kept at 15 ml–1 in the larval fish rearing tank. The instant microalgae (Nanno 3600 paste, Reed Mariculture, United States) were also added into all the larval fish tanks at a density of about 1 × 106 cell ml–1 to create a green color background.
Jaw Deformity
A total of 50 fish larvae were randomly collected from each rearing tank to examine the incidence of deformity. The fish were anesthetized by overdosing of Aqui-S (AQUI-S, New Zealand) and fixed in 4% paraformaldehyde. Jaw deformity was assessed by observing under a stereo microscope (Olympus SZ40, Japan) using the criteria described by Cobcroft and Battaglene (2009). The appearance of the jaws of each larvae was rated on a scale of 0–3 according to the jaw malformation index (Cobcroft et al., 2004) modified for golden pompano larvae. A score of 0 indicated a normal jaw (Figure 1A). A score of 0.5 indicated very minor malformation e.g., slightly short lower jaw (Figure 1B), that would not be considered malformation from a commercial perspective. The larvae were defined as malformed when the jaw score reached 1, 2, or 3: Score 1, minor variation from normal structure, some resistance to closing mouth, e.g., snub nose (Figure 1C); Score 2, intermediate where some elements are abnormal in shape or position although limited movement occurs to open and close the mouth, e.g., ventral transposition of the glossohyal (Figure 1D); Score 3, severe malformation where jaw elements have abnormal shape or are in abnormal positions, and do not move to close the mouth, e.g., severe bending Meckel’s cartilage (Figure 1E).
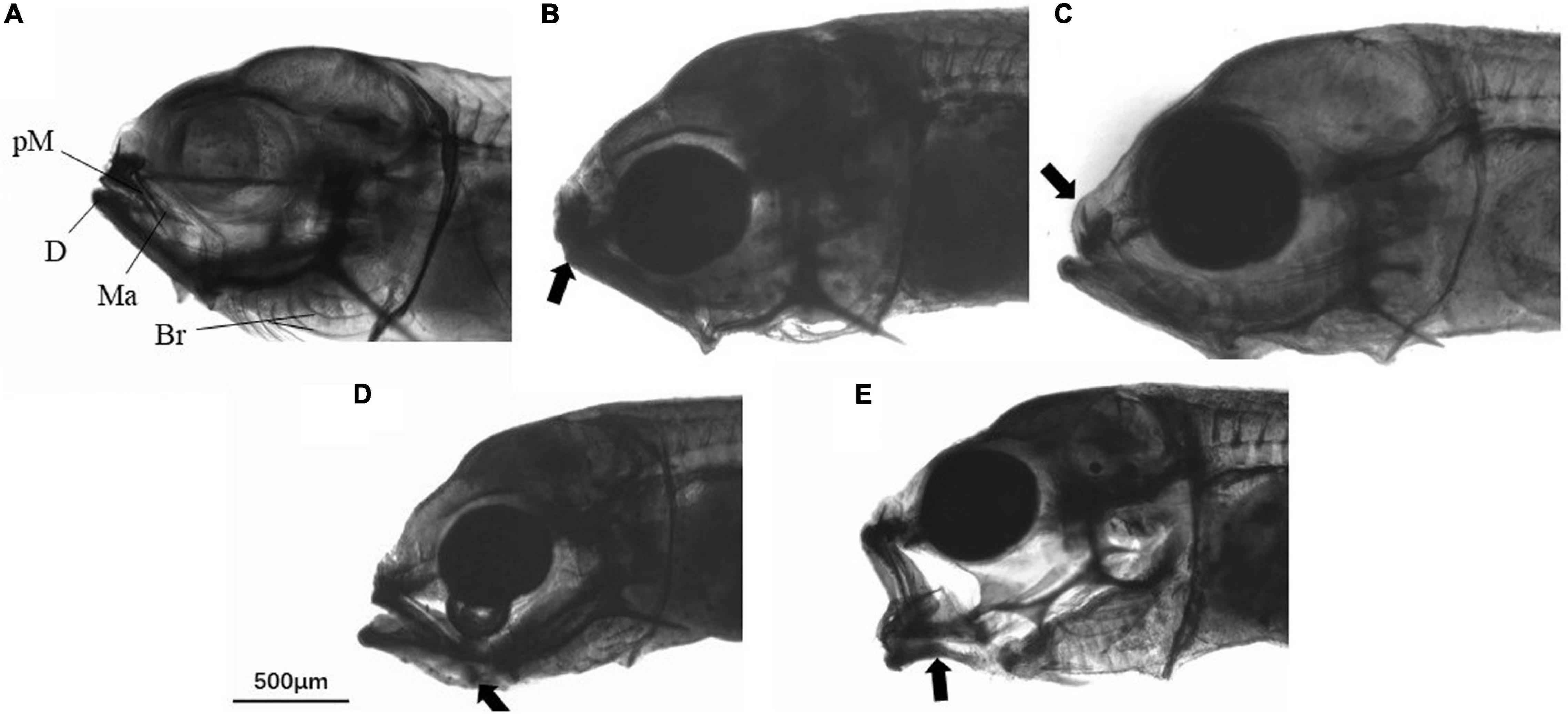
Figure 1. Golden pompano larvae with different jaw malformation index scores. (A) Score 0, (B) score 0.5, (C) score 1, (D) score 2, and (E) score 3. Abbreviations: pM, Premaxilla; Ma, maxilla; D, dentary; Br, branchiostegal rays. The arrow points to the location of the deformity.
Fatty Acids Analysis
After enrichment, four million rotifers from each treatment in three replicates were collected and preserved in liquid nitrogen until analysis. On 8 DPH, 0.5 g fish larvae (wet weight) in five replicates were sampled for fatty acid analyses. All rotifer and fish samples were pre-washed using an ammonium formate solution (0.5 M) to remove salt, and paper tower was used to remove extra water before preservation in liquid nitrogen. The fatty acids were analyzed at South China Sea Fisheries Research Institute, China, following the method described by Ma and Qin (2014).
Gene Expression Analysis
The fish larvae were sampled on 8 DPH. Total RNA was extracted using TRIzol (Invitrogen, United States). RNA integrity was verified by agarose gel electrophoresis. RNA concentration was measured by spectrophotometry (Bioteke Corporation Co., Ltd., China) at 260 nm, and the purity was determined at the OD 260/280 ratio and agarose gel electrophoresis. The RNA was immediately used for cDNA synthesis. Subsequently, reverse transcription was performed on 1 μg of total RNA using TransScript-Uni One-Step gDNA Removal and cDNA Synthesis SuperMix (Transgen Biotech Co., Ltd., China). The synthesized cDNA samples were stored at −20°C until further use.
The primers of BMP4, BMP2, RXRα, and EF1α (Table 1) were previously designed and validated by Yang et al. (2015) and Ma et al. (2018). In quantitative real-time PCR, EF1α was used as the internal reference and amplified. The reaction conditions were as follows: initial denaturation at 95°C for 15 min, 40 cycles of denaturing at 95°C for 10 s, annealing at 58°C for 20 s, and extension at 72°C for 30 s. For each test, three replicates were performed in this study. No template control was included with each assay to verify that PCR master mixes were free of contamination. Dissociation curves were employed to ensure that only one single PCR product was amplified in each gene reaction. After verification of PCR efficiency to be 95–105%, the relative gene expression was calculated using the ΔCT (comparative threshold cycles) (ΔCT = CT of target gene–CT of EF1α, ΔΔCT = sample CT – ΔCT of calibrator sample).

Table 1. Primers of bone morphogenetic protein 2 (BMP2), bone morphogenetic protein 4 (BMP4), retinoid X receptor α (RXRα), and extension factor 1α (EF1α) genes in golden pompano used in qPCR.
Calculations and Statistical Analysis
Specific growth rate (SGR) was calculated as: SGR = 100 × [ln (Wf) – ln (Wi)]/ΔT, where Wf was the final body weight, Wi was the initial body weight, and ΔT was the experimental duration.
The data were expressed as the mean ± standard deviation (SD). Statistical analyses were carried out by PASW Statistics (version 18). Comparisons between different groups were conducted by Tukey’s test, and significant difference was set at P < 0.05. All percentage data were transformed using square root to satisfy the assumptions of ANOVA.
Results
Fatty Acid Composition in Rotifers and Fish Larvae
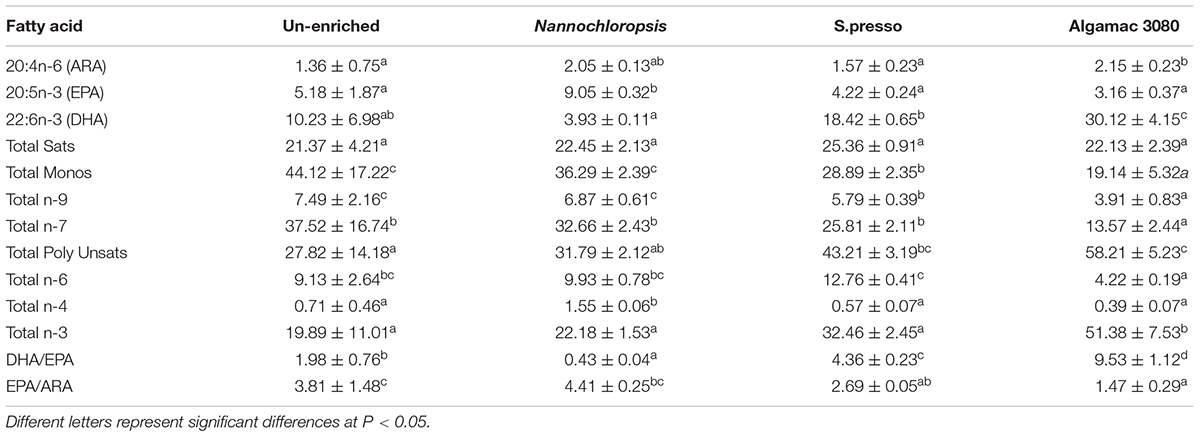
The specific fatty acid composition in rotifers significantly varied between treatments (Table 2). The amount of EPA (20:5n-3) in the rotifers enriched with Nannochloropsis (9.05%) was significantly higher than other treatments (P < 0.05). The EPA was not significantly different between the treatments of S.pressa, Algamac 3080, and un-enriched (P > 0.05). The amount of DHA (22:6n-3) in the rotifers enriched with Algamac 3080 (30.12%) was significantly higher than other treatments (P < 0.05). The DHA in S.pressa treatment was significantly higher than that in Nannochloropsis treatment, while un-enriched treatment was not significantly different from them (P > 0.05). The DHA/EPA of all treatments showed significantly different (P < 0.05), and the order from large to small was: Algamac 3080 (9.53), S.presso (4.36), Un-enriched (1.98), and Nannochloropsis (0.43). The EPA/ARA in S.presso treatment (2.69) and Algamac 3080 treatment (1.47) were significantly lower than un-enriched treatment (3.81, P < 0.05).
The specific fatty acid composition in fish larvae significantly varied between treatments (Table 3). The trend of EPA (20:5n-3) amount in fish larvae was consistent with rotifers, the amount of EPA in the fish larvae of Nannochloropsis treatment (8.09%) was significantly higher than other treatments (P < 0.05). The trend of DHA (22:6n-3) amount in fish larvae was not completely consistent with rotifers. The amount of EPA in Algamac 3080 treatment fish larvae was no significant difference with S.presso treatment (P > 0.05), but it was still significantly higher than the other two treatments (P < 0.05). The DHA/EPA of all treatments showed significantly different (P < 0.05) and had the same trend as rotifers. The EPA/ARA of all treatments showed significantly different (P < 0.05), and the order from large to small was: Un-enriched (3.33), Nannochloropsis (2.33), S.presso (1.28), and Algamac 3080 (0.69).
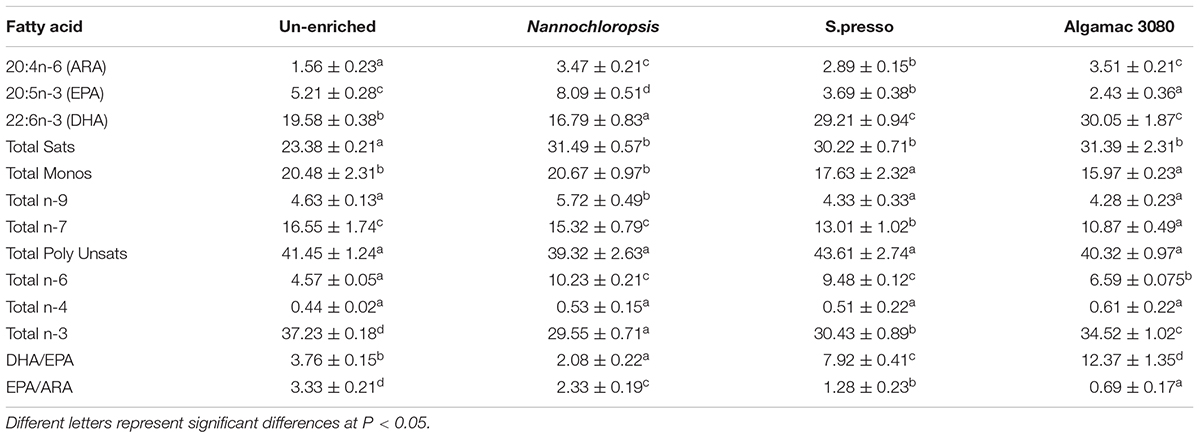
Table 3. Fatty acid composition (% of total fatty acids) in 8 days post-hatching (8-DPH) fish fed enriched and un-enriched rotifers.
Larval Fish Growth and Survival
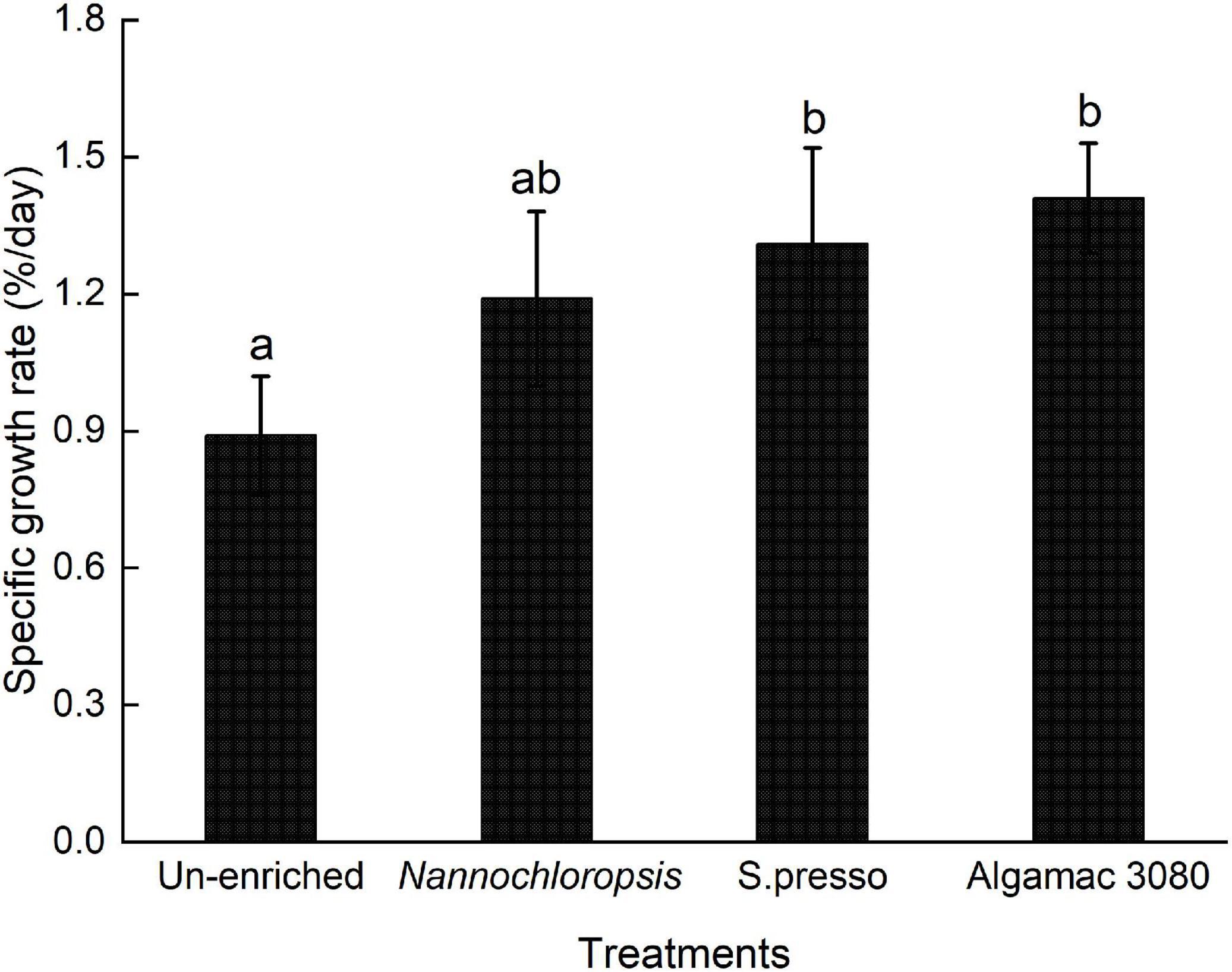
SGR showed significant difference between un-enriched and commercial enrichment products treatments (P < 0.05, Figure 2). The highest SGR were observed in the two commercial enrichment products (S.presso and Algemac 3080), and there was no significant difference between them (P > 0.05).
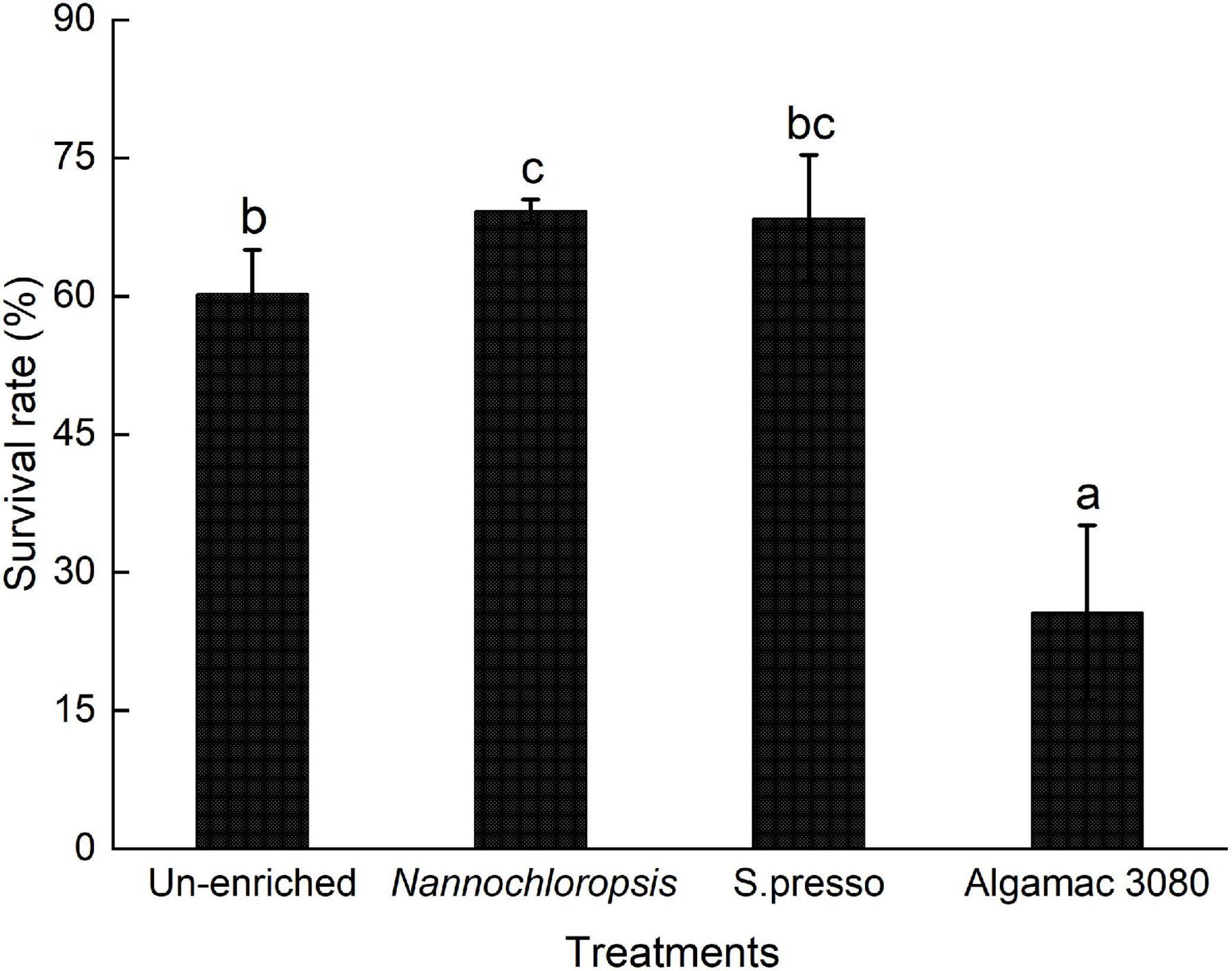
The survival rate of Nannochloropsis treatment was significantly higher than the un-enriched treatment (P < 0.05, Figure 3). Among the two commercial enrichment products treatments, there was no significant difference in survival rate between S.presso and Algamac 3080 treatment (P > 0.05), and the survival rate of Algamac 3080 treatment was significantly lower than all treatments (P < 0.05).
Jaw Deformity
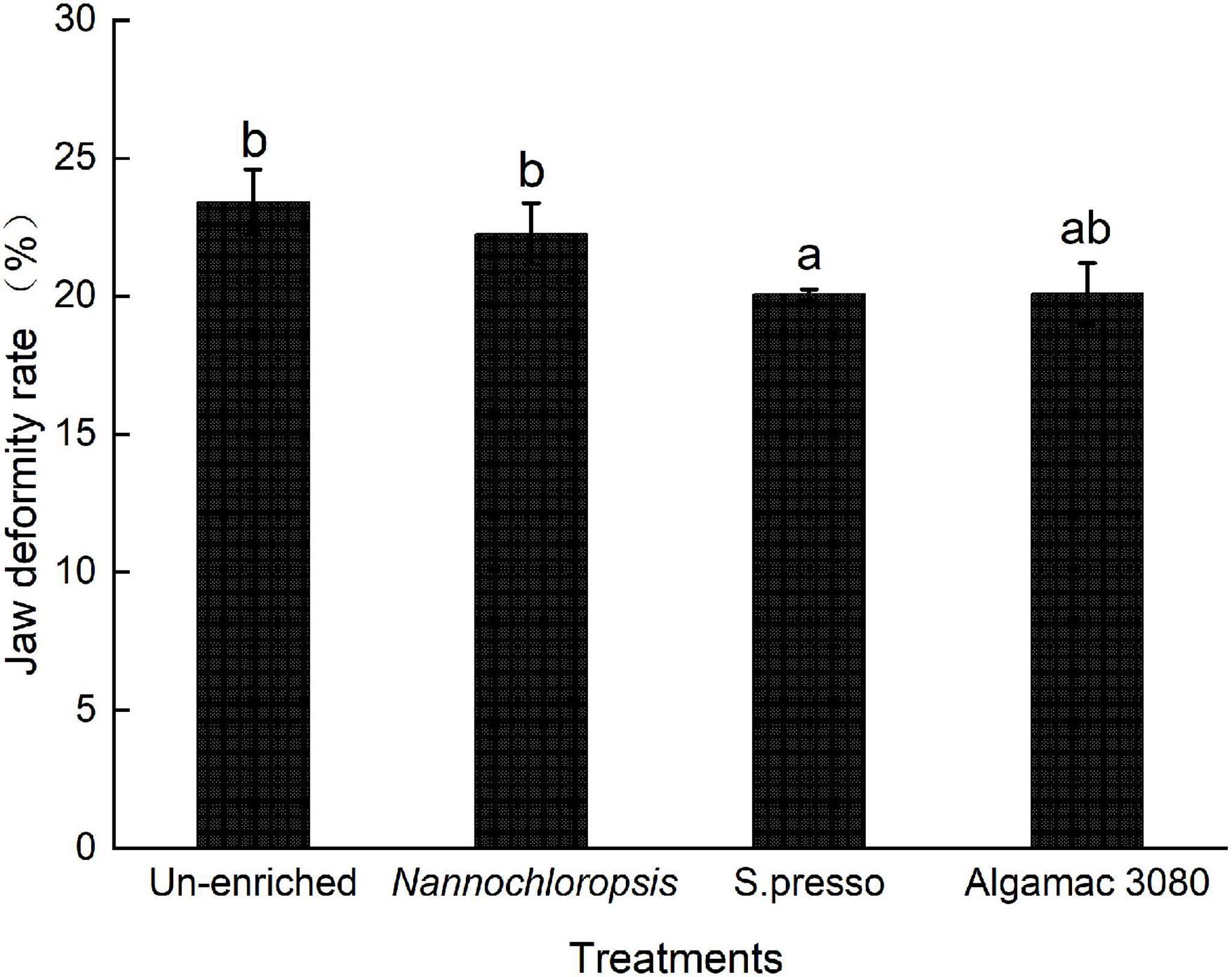
Jaw deformity rate showed no significant difference between Nannochloropsis and un-enriched treatment (P > 0.05, Figure 4), and the rate of S.presso treatment was significantly lower than Nannochloropsis and un-enriched treatment (P < 0.05). There was no significant difference in jaw deformity rate between Algamac 3080 treatment and all treatments (P > 0.05).
Relative Expression Level of Bone Development Related Genes
The expression level of BMP2 showed no significant difference between treatments (P > 0.05, Figure 5). The expression level of BMP4 showed no significant difference between the three enriched treatments and un-enriched treatment (P > 0.05), and S.presso treatment was significantly higher than Nannochloropsis treatment (P < 0.05). The RXRα expression level of the three enriched treatments was significantly lower than the un-enriched treatment (P < 0.05), and there was no significant difference between the two commercial enrichment products treatments (P > 0.05); meanwhile, they were significantly lower than the Nannochloropsis treatment (P < 0.05).
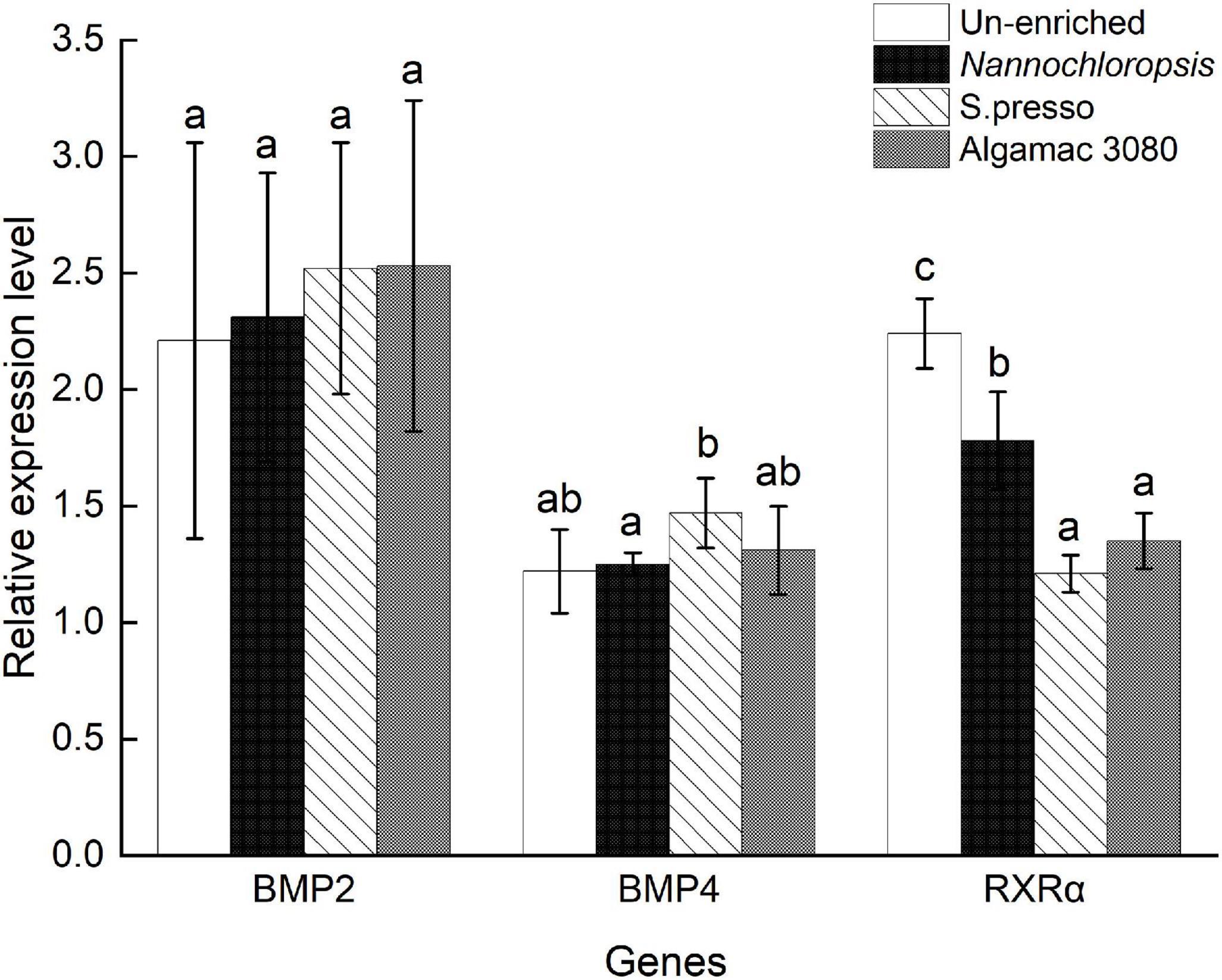
Figure 5. Relative expression level of bone morphogenetic protein 2 (BMP2), bone morphogenetic protein 4 (BMP4), and retinoid X receptor α (RXRα) genes of golden pompano larvae in four enrichment treatments.
Discussion
Effects of Feeding Rotifers Enriched With Different Enhancement Products on Larval Performance
The initial feeding food must reach a level close to the nutritional requirements of the larval fish to be fully utilized (Conceição et al., 2010; Lall and Dumas, 2015). Generally, the utilization of food can be roughly estimated by observing and comparing the body composition of fish (Belal, 2005; Faulk et al., 2005). Rotifers enriched with Nannochloropsis were characterized by high EPA, while rotifers enriched with S.presso had a high DHA state. Judging from the fatty acid composition of golden pompano larvae, such levels of EPA and DHA could be absorbed and utilized. The Algamac 3080-enhanced rotifers had a higher DHA level than S.presso, but its utilization effect in golden pompano larvae did not seem to match the DHA content of rotifers. The optimal diet of young fish should be similar to the content of yolk sac (Barroso et al., 2013; Hauville et al., 2016), but unfortunately there have been no reports on the nutritional composition of yolk sac of golden pompanos. In the artificial breeding eggs of fat snook (Centropomus parallelus) (Barroso et al., 2013) observed a DHA:EPA:ARA ratio of 11.4:2.4:1.0, which is similar to the rotifers’ body composition in the S.presso treatment.
In this study, increasing the DHA in the food had a significant positive effect on the growth of golden pompano larvae. Similar results were found in California halibut Paralichthys californicus larvae (Vizcaíno-Ochoa et al., 2010). Not only DHA, HUFA n-3 (including DHA, EPA, α-linolenic acid) promotes the normal growth and development of larvae (Rodrìguez et al., 1998; Hamre and Harboe, 2008). HUFA n-3 is also an important component of cell membrane and plays a very important physiological function in organisms (Roo et al., 2019). However, in Algamac 3080 treatment which had the highest DHA content of rotifers, the SGR of golden pompano larvae was limited. This phenomenon showed that the growth promotion effect of DHA is only established within a certain limit, and the similar phenomenon was found in the Gilthead Seabream Sparus aurata (Rodriguez et al., 1994).
Excessive DHA supplementation reduced the survival of golden pompano larvae, the survival rate of Algamac 3080 treatment was only about half of un-enriched treatment. However, it is generally believed that DHA is helpful to improve the survival of larvae fish. In seawater carnivorous fish such as Atlantic Cod Gadus Morhua and black sea bass Centropristis striata, DHA improvement in an appropriate range is helpful to improve the survival rate of larvae (Park et al., 2006; Rezek et al., 2010). In other fish such as Senegal sole Solea senegalensis and California halibut Paralichthys californicus, DHA has no significant effect on larval survival (Villalta et al., 2005; Vizcaíno-Ochoa et al., 2010). Some scholars believe that excessive DHA will aggravate lipid peroxidation and cause pathological tissue damage in larval fish, which may lead to death (Betancor et al., 2011). This view may be one of the hypotheses that led to the results of this study, but further research is needed to prove it. In addition, studies have shown that the larvae demand for HUFA N-3 is not only reflected in the number of individual components, but also related to the DHA/EPA ratio (Rodríguez et al., 1997). Seeking a balanced ratio is also the key point to explore the appropriate first feeding to improve survival for larval fish.
Effects of Feeding Rotifers Enriched With Different Enhancement Products on Jaw Deformity
Increased levels of DHA, or DHA/EPA, seemed to contribute to a decrease in the jaw deformity rate of larvae, similar patterns have been observed in larval longfin yellowtail Seriola rivoliana, but the specific role of PUFA n-3 in the bone formation mechanism is still unclear (Cobcroft et al., 2012). However, the effect of reducing jaw deformity rate may be limited to S.presso-level DHA or DNA/EPA, because the survival rate of golden pompano larvae in Algamac 3080 treatment was too low, and the jaw deformity rate does not differ from that of the un-enriched treatment, which may be due to the death from deformity. It is a pity that we did not investigate the cause of death of larval fish in this study, which can provide reference for more rigorous experimental design in similar studies in the future.
First feeding of different enhancement products had a significant effect on the expression of RXRα. From the results, it is not difficult to find that the jaw deformity rate and the expression level of RXRα had same trend. RXRα and α isoform of RAR can form active dimers that cause apoptosis, which is speculated to be a potential cause of bone deformities especially in the head of larval fish (Egea et al., 2001; Villeneuve et al., 2005; Zambonino Infante and Cahu, 2007; Ferraresso et al., 2010; Yang et al., 2015). Studies have shown that, in addition to the metamorphosis period, the expression of RXRα in fish will be upregulated under stress conditions (Cobcroft et al., 2012; Roo et al., 2019). The stress events in this study may be caused by the lack of nutrients in the un-enriched and Nannochloropsis treatment, which is similar to Yang’s result (Yang et al., 2015). However, some studies have found that DHA can stimulate the expression of RXRα (Cahu et al., 2009), contrary to the results of this study, it may be that the lack of other nutrients such as vitamins in the un-enriched and Nannochloropsis treatment of rotifers led to stronger transcription stimulation (Zambonino Infante and Cahu, 2007; Mazurais et al., 2008; Darias et al., 2012). The BMP4 and retinoic acid pathways could produce a synergistic response and aggravate cell apoptosis (Villeneuve et al., 2005). In this study, no consistent changes in BMP4 with the jaw deformity rate were observed. It may not be the main cause of the jaw deformity.
In summary, the use of different enrichment products had caused significant differences in the fatty acid composition of rotifers; had a synergistic effect on the fatty acid composition of golden pompano larvae; and also caused different degrees of growth, survival, and jaw deformity. S.presso and Algamac 3080 treatment had more advantages in growth of golden pompano larvae, Algamac 3080 treatment exposed considerable disadvantages in survival. S.presso treatment had the lowest jaw deformity rate. Therefore, S.presso was a more suitable enrichment product for enriching rotifers for the first feeding of larval golden pompano. In addition, this study also found that BMP2 and BMP4 were not sensitive to changes in different enrichment product. On the contrary, RXRα had an opposite trend to the jaw deformity rate.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The animal study was reviewed and approved by The Animal Care and Use Committee of South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences.
Author Contributions
ZM and TZ conceptualized the study. SZ was responsible for the experimental operation. ZF, RY, and SZ were in charge of the field sampling. ZF and RY conducted the sample determination. ZF prepared and wrote the original draft. ZM and TZ reviewed, edited, and wrote the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by the Hainan Provincial Natural Science Foundation of China (2019CXTD418, 319QN339, and 319MS102), Guangxi Innovation-Driven Development Projects (Guike AA18242031), and Central Public Interest Scientific Institution Basal Research Fund South China Sea Fisheries Research Institute, CAFS (No. 2020TD55).
Conflict of Interest
TZ was employed by the Dalian Tianzheng Industry Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Barroso, M. V., de Carvalho, C. V. A., Antoniassi, R., and Cerqueira, V. R. (2013). Use of the copepod Acartia tonsa as the first live food for larvae of the fat snook Centropomus parallelus. Aquaculture 38, 153–158. doi: 10.1016/j.aquaculture.2013.01.022
Belal, I. E. H. (2005). A review of some fish nutrition methodologies. Bioresour. Technol. 96, 395–402. doi: 10.1016/j.biortech.2003.11.030
Betancor, M. B., Atalah, E., Caballero, M., Benitez-Santana, T., Roo, J., Montero, D., et al. (2011). α-tocopherol in weaning diets for European sea bass (Dicentrarchus labrax) improves survival and reduces tissue damage caused by excess dietary DHA contents. Aquac. Nutr. 17, e112–e122. doi: 10.1111/j.1365-2095.2009.00741.x
Cahu, C. L., Gisbert, E., Villeneuve, L. A. N., Morais, S., Hamza, N., Wold, P., et al. (2009). Influence of dietary phospholipids on early ontogenesis of fish. Aquac. Res. 40, 989–999. doi: 10.1111/j.1365-2109.2009.02190.x
Cobcroft, J. M., and Battaglene, S. C. (2009). Jaw malformation in striped trumpeter Latrislineata larvae linked to walling behaviour and tank colour. Aquaculture 289, 274–282. doi: 10.1016/j.aquaculture.2008.12.018
Cobcroft, J. M., Pankhurst, P. M., Poortenaar, C., Hickman, B., and Tait, M. (2004). Jaw malformation in cultured yellowtail kingfish (Seriola lalandi) larvae. New Zeal. J. Mar. Fresh. 38, 67–71. doi: 10.1080/00288330.2004.9517218
Cobcroft, J. M., Pankhurst, P. M., Sadler, J., and Hart, P. R. (2001). Jaw development and malformation in cultured striped trumpeter Latris lineata. Aquaculture. 199, 267–282. doi: 10.1016/S0044-8486(01)00592-0
Cobcroft, J. M., Shu-Chien, A. C., Kuah, M., Jaya-Ram, A., and Battaglene, S. C. (2012). The effects of tank colour, live food enrichment and greenwater on the early onset of jaw malformation in striped trumpeter larvae. Aquaculture. 35, 61–72. doi: 10.1016/j.aquaculture.2012.05.035
Conceição, L. E. C., Yúfera, M., Makridis, P., Morais, S., and Dinis, M. T. (2010). Live feeds for early stages of fish rearing. Aquac. Res. 41, 613–640. doi: 10.1111/j.1365-2109.2009.02242.x
Darias, M. J., Boglino, A., Manchado, M., Ortiz-Delgado, J. B., Estévez, A., Andree, K. B., et al. (2012). Molecular regulation of both dietary vitamin A and fatty acid absorption and metabolism associated with larval morphogenesis of Senegalese sole (Solea senegalensis). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 161, 130–139. doi: 10.1016/j.cbpa.2011.10.001
Egea, P. F., Rochel, N., Birck, C., Vachette, P., Timmins, P. A., and Moras, D. (2001). Effects of ligand binding on the association properties and conformation in solution of retinoic acid receptors RXR and RAR. J. Mol. Biol. 307, 557–576. doi: 10.1006/jmbi.2000.4409
Eryalcin, K. M. (2018). Effects of different commercial feeds and enrichments on biochemical composition and fatty acid profile of rotifer (Brachionus plicatilis. Müller 1786) and Artemia franciscana. Turk. J. Fish. Aquat. Sci. 18, 81–90. doi: 10.4194/1303-2712-v18_1_09
Faulk, C. K., Holt, G. J., and Davis, D. A. (2005). Evaluation of fatty acid enrichment of live food for yellowtail snapper Ocyurus chrysurus larvae. J. World Aquac. Soc. 36, 271–281. doi: 10.1111/j.1749-7345.2005.tb00331.x
Ferraresso, S., Milan, M., Pellizzari, C., Vitulo, N., Reinhardt, R., Canario, A. V., et al. (2010). Development of an oligo DNA microarray for the European sea bass and its application to expression profiling of jaw deformity. Bmc Genomics. 11:354. doi: 10.1186/1471-2164-11-354
Fraser, M. R., and de Nys, R. (2005). The morphology and occurrence of jaw and operculum deformities in cultured barramundi (Lates calcarifer) larvae. Aquaculture 250, 496–503.. https://doi.org/10.1016/j.aquaculture.2005.04.067,Google Scholar
Gisbert, E., Conklin, D. B., and Piedrahita, R. H. (2004). Effects of delayed first feeding on the nutritional condition and mortality of California halibut larvae. J. Fish Biol. 64, 116–132. doi: 10.1111/j.1095-8649.2004.00289.x
Hamre, K. (2016). Nutrient profiles of rotifers (Brachionus sp.) and rotifer diets from four different marine fish hatcheries. Aquaculture 450, 136–142. doi: 10.1016/j.aquaculture.2015.07.016
Hamre, K., and Harboe, T. (2008). Artemia enriched with high n-3 HUFA may give a large improvement in performance of Atlantic halibut (Hippoglossus hippoglossus L.) larvae. Aquaculture. 277, 239–243. doi: 10.1016/j.aquaculture.2008.02.028
Hamre, K., Yúfera, M., Rønnestad, I., Boglione, C., Conceição, L. E. C., and Izquierdo, M. (2013). Fish larval nutrition and feed formulation: knowledge gaps and bottlenecks for advances in larval rearing. Rev. Aquac. 5, S26–S58. doi: 10.1111/j.1753-5131.2012.01086.x
Hauville, M. R., Main, K. L., Migaud, H., and Gordon Bell, J. (2016). Fatty acid utilization during the early larval stages of Florida pompano (Trachinotus carolinus) and common snook (Centropomus undecimalis). Aquac. Res. 47, 1443–1458. doi: 10.1111/are.12602
Hernández-Cruz, C. M., Mesa-Rodríguez, A., Betancor, M., Haroun-Izquierdo, A., Izquierdo, M., Benítez-Santana, T., et al. (2015). Growth performance and gene expression in gilthead sea bream (Sparus aurata) fed microdiets with high docosahexaenoic acid and antioxidant levels. Aquac. Nutr. 21, 881–891. doi: 10.1111/anu.12213
Izquierdo, M. S., Scolamacchia, M., Betancor, M., Roo, J., Caballero, M. J., Terova, G., et al. (2013). Effects of dietary DHA and α-tocopherol on bone development, early mineralisation and oxidative stress in Sparus aurata (Linnaeus, 1758) larvae. Br. J. Nutr. 109, 1796–1805. doi: 10.1017/S0007114512003935
Kobayashi, T., Nagase, T., Hino, A., and Takeuchi, T. (2008). Effect of combination feeding of Nannochloropsis and freshwater Chlorella on the fatty acid composition of rotifer Brachionus plicatilis in a continuous culture. Fish. Sci. 74, 649–656. doi: 10.1111/j.1444-2906.2008.01570.x
Koedijk, R. M., Folkvord, A., Foss, A., Pittman, K., Stefansson, S. O., Handeland, S., et al. (2010). The influence of first-feeding diet on the Atlantic cod Gadus morhua phenotype: survival, development and long-term consequences for growth. J. Fish Biol. 77, 1–19. doi: 10.1111/j.1095-8649.2010.02652.x
Kotani, T. (2017). “Enrichment of Rotifers and Its Effect on the Growth and Survival of Fish Larvae,” in Rotifers: Aquaculture, Ecology, Gerontology, and Ecotoxicology, eds A. Hagiwara and T. Yoshinaga (Singapore: Springer Singapore), 47–62.
Kotani, T., Genka, T., Fushimi, H., Hayashi, M., Dierckens, K., and Sorgeloos, P. (2009). Effect of cultivation methods on nutritional enrichment of euryhaline rotifer Brachionus plicatilis. Fish. Sci. 75, 975–984. doi: 10.1007/s12562-009-0105-1
Lall, S. P., and Dumas, A. (2015). “3-Nutritional requirements of cultured fish: formulating nutritionally adequate feeds,” in Feed and Feeding Practices in Aquaculture, ed. D. A. Davis (Oxford: Woodhead Publishing), 53–109.
Ma, Z., Hu, J., Yu, G., and Qin, J. G. (2018). Gene expression of bone morphogenetic proteins and jaw malformation in golden pompano Trachinotus ovatus larvae in different feeding regimes. J. Appl. Anim. Res. 46, 164–177. doi: 10.1080/09712119.2017.1282371
Ma, Z., and Qin, J. G. (2014). Replacement of fresh algae with commercial formulas to enrich rotifers in larval rearing of yellowtail kingfish Seriola lalandi (Valenciennes, 1833). Aquac. Res. 45, 949–960. doi: 10.1111/are.12037
Ma, Z., Zheng, P., He, D., Jiang, S., and Qin, J. G. (2016). Effect of feeding Artemia nauplii enriched with different enhancement products on larval performance of golden pompano Trachinotus ovatus (Linnaeus, 1758). Indian J. Fish. 63, 62–69. doi: 10.21077/ijf.2016.63.2.50560-09
Marques, C. L., Fernández, I., Viegas, M. N., Cox, C. J., Martel, P., Rosa, J., et al. (2016). Comparative analysis of zebrafish bone morphogenetic proteins 2, 4 and 16: molecular and evolutionary perspectives. Cell. Mol. Life Sci. 73, 841–857. doi: 10.1007/s00018-015-2024-x
Mazurais, D., Darias, M. J., Gouillou-Coustans, M. F., Le Gall, M. M., Huelvan, C., Desbruyères, E., et al. (2008). Dietary vitamin mix levels influence the ossification process in European sea bass (Dicentrarchus labrax) larvae. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R520–R527. doi: 10.1152/ajpregu.00659.2007
Nishimura, R., Hata, K., Matsubara, T., Wakabayashi, M., and Yoneda, T. (2012). Regulation of bone and cartilage development by network between BMP signalling and transcription factors. J. Biochem. 151, 247–254. doi: 10.1093/jb/mvs004
Park, H. G., Puvanendran, V., Kellett, A., Parrish, C. C., and Brown, J. A. (2006). Effect of enriched rotifers on growth, survival, and composition of larval Atlantic cod (Gadus morhua). ICES J. Mar. Sci. 63, 285–295. doi: 10.1016/j.icesjms.2005.10.011
Rezek, T. C., Watanabe, W. O., Harel, M., and Seaton, P. J. (2010). Effects of dietary docosahexaenoic acid (22:6n-3) and arachidonic acid (20:4n-6) on the growth, survival, stress resistance and fatty acid composition in black sea bass Centropristis striata (Linnaeus 1758) larvae. Aquac. Res. 41, 1302–1314. doi: 10.1111/j.1365-2109.2009.02418.x
Rice, J. A., Crowder, L. B., and Binkowski, F. P. (1987). Evaluating potential sources of mortality for larval bloater (Coregonus hoyi): starvation and vulnerability to predation. Can. J. Fish. Aquat. Sci. 44, 467–472. doi: 10.1139/f87-055
Rodríguez, C., Pérez, J. A., Díaz, M., Izquierdo, M. S., Fernández-Palacios, H., and Lorenzo, A. (1997). Influence of the EPADHA ratio in rotifers on gilthead seabream (Sparus aurata) larval development. Aquaculture 150, 77–89. doi: 10.1016/S0044-8486(96)01472-X
Rodriguez, C., Perez, J. A., Lorenzo, A., Izquierdo, M. S., and Cejas, J. R. (1994). n-3 HUFA requirement of larval gilthead seabream Sparus aurata when using high levels of eicosapentaenoic acid. Comp. Biochem. Physiol. A Physiol. 107, 693–698. doi: 10.1016/0300-9629(94)90371-9
Rodrìguez, C., Pérez, J. A., Badìa, P., Izquierdo, M. S., Fernández-Palacios, H., and Hernández, A. L. (1998). The n–3 highly unsaturated fatty acids requirements of gilthead seabream (Sparus aurata L.) larvae when using an appropriate DHA/EPA ratio in the diet. Aquaculture 169, 9–23. doi: 10.1016/S0044-8486(98)00328-7
Roo, F. J., Hernández-Cruz, C. M., Socorro, J. A., Fernández-Palacios, H., Montero, D., and Izquierdo, M. S. (2009). Effect of DHA content in rotifers on the occurrence of skeletal deformities in red porgy Pagrus pagrus (Linnaeus, 1758). Aquaculture 287, 84–93. doi: 10.1016/j.aquaculture.2008.10.010
Roo, J., Hernández-Cruz, C. M., Mesa-Rodriguez, A., Fernández-Palacios, H., and Izquierdo, M. S. (2019). Effect of increasing n-3 HUFA content in enriched Artemia on growth, survival and skeleton anomalies occurrence of greater amberjack Seriola dumerili larvae. Aquaculture 500, 651–659. doi: 10.1016/j.aquaculture.2018.09.065
Shulman, A. I., Larson, C., Mangelsdorf, D. J., and Ranganathan, R. (2004). Structural determinants of allosteric ligand activation in RXR heterodimers. Cell 116, 417–429. doi: 10.1016/S0092-8674(04)00119-9
Villalta, M., Estévez, A., Bransden, M. P., and Bell, J. G. (2005). The effect of graded concentrations of dietary DHA on growth, survival and tissue fatty acid profile of Senegal sole (Solea senegalensis) larvae during the Artemia feeding period. Aquaculture 249, 353–365. doi: 10.1016/j.aquaculture.2005.03.037
Villeneuve, L., Gisbert, E., Zambonino-Infante, J. L., Quazuguel, P., and Cahu, C. L. (2005). Effect of nature of dietary lipids on European sea bass morphogenesis: implication of retinoid receptors. Br. J. Nutr. 94, 877–884. doi: 10.1079/BJN20051560
Vizcaíno-Ochoa, V., Lazo, J. P., Barón-Sevilla, B., and Drawbridge, M. A. (2010). The effect of dietary docosahexaenoic acid (DHA) on growth, survival and pigmentation of California halibut Paralichthys californicus larvae (Ayres, 1810). Aquaculture 302, 228–234. doi: 10.1016/j.aquaculture.2010.02.022
Waqalevu, V., Honda, A., Dossou, S., Khoa, T. N. D., Matsui, H., Mzengereza, K., et al. (2019). Effect of oil enrichment on Brachionus plicatilis rotifer and first feeding red sea bream (Pagrus major) and Japanese flounder (Paralichthys olivaceus). Aquaculture 510, 73–83. doi: 10.1016/j.aquaculture.2019.05.039
Yang, Q., Zheng, P., Ma, Z., Li, T., Jiang, S., and Qin, J. G. (2015). Molecular cloning and expression analysis of the retinoid X receptor (RXR) gene in golden pompano Trachinotus ovatus fed Artemia nauplii with different enrichments. Fish Physiol. Biochem. 41, 1449–1461. doi: 10.1007/s10695-015-0098-x
Zambonino Infante, J. L., and Cahu, C. L. (2007). Dietary modulation of some digestive enzymes and metabolic processes in developing marine fish: applications to diet formulation. Aquaculture 268, 98–105. doi: 10.1016/j.aquaculture.2007.04.032
Keywords: enrichment, larvae rearing, growth, survival, deformity, fatty acids, gene expression
Citation: Fu Z, Yang R, Zhou S, Ma Z and Zhang T (2021) Effects of Rotifers Enriched With Different Enhancement Products on Larval Performance and Jaw Deformity of Golden Pompano Larvae Trachinotus ovatus (Linnaeus, 1758). Front. Mar. Sci. 7:626071. doi: 10.3389/fmars.2020.626071
Received: 04 November 2020; Accepted: 22 December 2020;
Published: 10 February 2021.
Edited by:
Yen-Ju Pan, National Taiwan Ocean University, TaiwanReviewed by:
Te-Hua Hsu, National Taiwan Ocean University, TaiwanTerry Snell, Georgia Institute of Technology, United States
Copyright © 2021 Fu, Yang, Zhou, Ma and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenhua Ma, emhlbmh1YS5tYUBob3RtYWlsLmNvbQ==; Tao Zhang, emh0XzMwMDBAMTYzLmNvbQ==
 Zhengyi Fu
Zhengyi Fu Rui Yang1,2,3
Rui Yang1,2,3 Zhenhua Ma
Zhenhua Ma