- 1Zoology Department, University of Otago, Dunedin, New Zealand
- 2Marine Science Department, University of Otago, Dunedin, New Zealand
Hector’s dolphin is a small, endangered dolphin species found exclusively in the inshore coastal waters of New Zealand. We draw on 36 years of involvement in research on Hector’s dolphin, and its subspecies Māui dolphin, to provide an overview of the species’ conservation biology, and summarize the incremental progress towards sustainable management. We offer lessons learned at the interface between science and management. These lessons emphasize the importance of acting early, having clear management goals and ensuring that the area over which protection measures are applied is sufficiently large to be biologically relevant. High-quality information is vital, but gaining appropriate conservation outcomes depends also on social and political processes. We warn that compromise can have high biological costs and that representation on stakeholder groups is usually biased toward extractive users and short-term economic perspectives. In New Zealand, outcomes have depended closely on politics; the greatest gains have been made when relevant government ministers took a special interest. Scientists have crucial roles in every phase of this process. Each country and each species will present their own challenges and opportunities. We trust, however, that lessons learned from Hector’s dolphin conservation will be useful to researchers and managers elsewhere.
Introduction
Key environmental problems are escalating, in many cases toward extirpation of populations or extinction of species (e.g., Hoekstra et al., 2005; Ceballos et al., 2015). The science and management of conservation impacts often follows a predictable sequence. The conservation issue is identified by a researcher, often a graduate student. Discovery of the problem is usually met with denial by the industry or stakeholder group causing the impact, and sometimes also by the management agency. After further investigation, in which the burden of proof usually falls on the researcher, a management initiative may follow—perhaps a small protected area or (often voluntary) changes to practices associated with the impact. For social and political reasons, management agencies attempt to find compromise, but achieving such social approval (Wilhere et al., 2012) often results in actions insufficient to ensure a sustainable outcome, let alone population recovery. A feedback loop usually follows: of research showing the management intervention is insufficient, followed by a small increase in protection, and so on (see also Devillers et al., 2015; Pressey et al., 2017). Where the conservation problem is urgent, the changes may not happen fast enough. This is not a negative or cynical view, especially in relation to mammals (e.g., Hoffmann et al., 2011; Estrada et al., 2017). Even in well-resourced nations, with active conservation agencies and good legal structures, progress can be very slow (Salafsky et al., 2002). Elsewhere, progress toward effective solutions has been too slow to save small cetaceans. The most striking examples of this are baiji (e.g., Turvey et al., 2007) and vaquita (Jaramillo-Legorreta et al., 2019). It seems to us that there has never been a more urgent time for ecologists to focus their research on the conservation problems faced by their study species.
Our case study is of Hector’s dolphin Cephalorhynchus hectori, an endangered dolphin found only in New Zealand waters. More than 40 years ago Dr. Alan Baker, then of the National Museum in Wellington, published a four-page paper that summarized the state of knowledge on Hector’s dolphin at that time (Baker, 1978), including known deaths in gillnet and trawl fisheries. There has been a large amount of research on Hector’s dolphin since the 1980s, much of it focused explicitly on understanding those aspects of the species’ biology that are most important for management of the bycatch problem. This contribution provides a brief summary of that work, and draws from it some pertinent observations about the science and management of bycatch and similar conservation problems. We focus specifically on direct causes of loss, such as dolphins being killed in fishing nets, rather than societal drivers such as globalization or economic growth (cf. Hicks et al., 2016). The societal drivers of conservation impacts are beyond our expertise as biologists, and poorly studied in New Zealand.
Hector’s dolphin is one of four species in the Genus Cephalorhynchus. Each of the four species is small (Hector’s dolphin is among the smallest of all cetaceans) and restricted to the shallow coastal waters of Africa (C. heavisidii), Sth America (C. commersonii and C. eutropia), and New Zealand (C. hectori) (Dawson, 2019). Based on mt-DNA and morphometric differences, Hector’s dolphin is divided into two subspecies; C. hectori hectori in South Island waters, and C. hectori maui in North Island waters (Baker et al., 2002). The North Island subspecies is known as Māui dolphin.
Compared to other mammals, Cephalorhynchus dolphins are relatively long-lived and slow reproducing. Their inshore habitat puts them in direct contact with intensive human activities, including fishing, transport, pollution, marine mining and, potentially, tidal energy generation.
Over time, our work has become increasingly applied. We began our research on Hector’s dolphins in 1984 with the initial aims of studying their behavior, acoustic behavior, and ecology (e.g., Slooten and Dawson, 1988). However, as it became clear that they were routinely caught in fishing nets, our research focus shifted toward quantifying bycatch (e.g., Dawson, 1991) and its impacts (e.g., Slooten and Lad, 1991; Slooten et al., 2000), estimating survival and reproductive rates (Slooten, 1991; Slooten et al., 1992), distribution and habitat use (e.g., Rayment et al., 2010, 2011a; Dawson S. M. et al., 2013) and effectiveness of conservation efforts (Slooten and Dawson, 2010; Slooten and Davies, 2011; Gormley et al., 2012).
Distribution, Abundance and Ecology
The first nationwide survey of Hector’s dolphin was carried out in 1984–85 using a 4 m inflatable boat with two observers (Dawson and Slooten, 1988). This strip-transect survey provided the first reliable data on alongshore distribution of Hector’s dolphins, revealing a relatively small total population that was highly fragmented. Fifteen years later, vessel-based and aerial line-transect surveys resulted in estimates of Hector’s dolphin numbers to 4 nautical miles offshore: 7,270 (CV 0.16: Dawson et al., 2004; Slooten et al., 2004) for the South Island and 111 (CV 0.44: Slooten et al., 2006a) for Māui dolphin off the North Island west coast. The most recent population estimate suggest that Māui dolphin abundance has approximately halved to 57 individuals (1 year and older, in 2016; 95% Confidence Interval 44–75; Cooke et al., 2019).
The most recent South Island surveys, carried out under contract for the Ministry for Primary Industries extended to 20 nautical miles offshore (MacKenzie and Clement, 2014, 2016). These resulted in very similar population estimates for all populations except the east coast of the South Island, where the most recent estimate (MacKenzie and Clement, 2014) is five times higher than the previous estimate (Dawson et al., 2004). Some of the difference clearly arises from the much greater offshore extent of the more recent surveys. However, methodological differences also appear to contribute. The Cloudy Bay—Clifford Bay area, on the northern east coast of the South Island, has been surveyed several times, using different survey methods. Recent mark-recapture estimates of the number of Hector’s dolphins in Cloudy–Clifford Bay (Hamner et al., 2017), from both photo-ID (230; cv = 0.30) and genotype data (269; cv = 0.12; individuals 1 year and older) are roughly a quarter of the most recent line-transect estimate for that area (953, 95% CI 482–1,885; MacKenzie and Clement, 2014). The previous line-transect estimate, for 0–4 nmi offshore, was 162 (95% CI 56–474; Dawson et al., 2004). Again, the greater offshore extent of the most recent line-transect surveys may account for some of this difference, but a fuller investigation of potential biases in the most recent line-transect estimates seems warranted.
If recent aerial surveys are taken at face value, the total population of South Island Hector’s dolphins is estimated at 14,849 (cv = 14%; MacKenzie and Clement, 2016). Even if this number is biased high, it indicates a small total population—in the several thousands, rather than several tens of thousands. All surveys have shown that the population is strongly fragmented, with relatively high-density areas off the west coast and central part of the South Island east coast. Elsewhere, there are small, localized concentrations which are often separated by tens or hundreds of kilometers.
Seasonal surveys of distribution show Hector’s dolphins are strongly clustered in shallow, inshore waters in summer and more evenly distributed with respect to water depth and distance offshore in winter (Slooten et al., 2006b; Rayment et al., 2010). This is true also within Akaroa Harbor, where year-round passive acoustic monitoring has shown a strong seasonal pattern of dolphins using the middle and inner harbor areas far more often in summer than at other times of year (Dawson S. et al., 2013). Analysis of 29 years of sighting data gathered at Banks Peninsula shows the formation of summer hotspots which are stable over time (Brough et al., 2019). Acoustic monitoring of hotspots, and nearby areas used less often, shows disproportionate use of feeding buzzes in hotspots, indicating that foraging opportunities drive their formation (Brough et al., 2020).
Population structure has been assessed via photo-ID surveys in different locations around the South Island. Repeated sightings of the same individuals within each study site, and a lack of re-sightings among sites, indicate that there is little if any movement between study sites more than 90km apart (Bräger, 1998; Fletcher et al., 2002). Data from the areas surveyed most intensively show repeated resightings of photographically identified individuals over periods of more than 20 years and throughout the seasons. Banks Peninsula is the most intensively studied area, with substantial survey effort since 1984. Kernel density analyses reveal that individuals show long-term residence in relatively small home ranges [average home range (k95) = 49.7 km of coastline, Rayment et al., 2009a]. Genetic data confirm this pattern, with at least four genetically different regional populations (North Island, South Island east, west and south coast) and further evidence of genetic structure within those populations (Pichler et al., 1998; Pichler, 2002; Hamner et al., 2012). These genetic differences could not occur if movements were extensive and involved breeding.
The diet of Hector’s dolphins has been studied by examining stomach contents from individuals found dead on beaches or caught in fishing gear (Slooten and Dawson, 1988, 1994; Miller et al., 2013). Diet differs between the east and west coasts of the South Island, though it is not known whether this reflects preference or prey availability. In general Hector’s dolphins eat a wide range of fish species including benthic fish (e.g., sand flounder Rhombosolea plebia, stargazer Crapatalus novaezelandiae, ahuru Auchenoceros punctatus), demersal fish (e.g., red cod Pseudophycis bacchus) as well as mid-water species (e.g., arrow squid Nototadarus sp., Hector’s Lanternfish Lampanyctodes hectoris) and fish found near the water surface (e.g., yellow-eyed mullet Aldrichetta forsteri, Sprat Spattus sp., kahawai Arripis trutta). Hector’s dolphins do not appear to scavenge from gillnets. Fish taken by Hector’s dolphins are substantially smaller (2–35 cm) than those taken in commercial gillnets.
Hector’s dolphins frequently follow inshore trawlers, especially those working in shallow (<30 m) waters trawling for flatfish (Hawke, 1994; Rayment and Webster, 2009). The largest observed group sizes occur in these situations, with 50 or more dolphins following a single trawler (pers. obs). Observations made via echosounder suggest that the dolphins are feeding on fish stirred up by the net (and trawl doors). They may also enter the net as has been observed for other species (e.g., Jaiteh et al., 2012). In any case, association with trawling is a high-risk feeding strategy. The catch rate of Hector’s dolphins trawl fisheries is very poorly known, due to very low levels of observer coverage (Slooten and Dawson, 2017). There have, however, been many documented mortalities in trawl gear (e.g., Dawson, 1991; Baird and Bradford, 2000; Dragonfly Consulting, 2020). Multiple captures are known to occur, with up to three Hector’s dolphins caught in the same trawl and up to five in the same gillnet (Dawson, 1991; Department of Conservation [DOC], 2020).
Population Growth and Sustainability
Quantifying cetacean abundance poses special difficulties (e.g., Dawson et al., 2008) which are usually shown by relatively high imprecision (CVs of exemplary surveys are typically 20–30% or greater). This is why repeated abundance surveys often have very low power to detect trends (Taylor et al., 2007). In this context estimates of survival, reproductive rates and population growth rates are essential in order to determine whether human impacts are sustainable1. Adult survival rates have been estimated using photographic-identification data and mark-recapture analysis (Slooten et al., 1992; Cameron et al., 1999; du Fresne, 2005; Gormley et al., 2012), and are a key parameter in modeling of population trajectory. In addition, at Banks Peninsula survival rates allow evaluation of the effectiveness of conservation efforts. Survival rates have increased by more than 5% since the 1988 establishment of the Banks Peninsula Marine Mammal Sanctuary (Gormley et al., 2012).
Reproductive rates have been estimated using a combination of information from photo-ID studies and examination of dead dolphins found beachcast or caught in fishing gear. We estimated age at first reproduction (7–9 years old) from ovary, uterus and tooth sections (Slooten, 1991; Slooten and Lad, 1991; Gormley, 2009). Calving interval can be estimated only from repeated sightings of live dolphins and was estimated at 2–3 years (Slooten and Dawson, 1994; Gormley, 2009). Maximum population growth was estimated at 1.8% per year (Slooten and Lad, 1991), which is at the lower end of the range for dolphins (2–4%, Perrin and Reilly, 1984).
One of the simplest approaches to evaluating sustainability is the US Potential Biological Removal (PBR) system (Wade, 1998). This approach allows sustainable levels of impact to be calculated, even if all that is available is an estimate of population size. Default values can be used for maximum population growth rate, if an estimate is not available for the species in question, and for the recovery factor, based on whether the species is endangered, above half of the original population size, or of unknown status. The PBR approach has been extensively tested via simulation (Wade, 1998). A key requirement is that areas used in the PBR calculations must match the regional population structure. If not, small local populations may be exposed to bycatch that is unsustainable at the local scale. Also, if management areas are too large, and bycatch occurs only in part of that area, the abundance of the impacted population will be over-estimated, resulting in population depletion (Barlow et al., 1995; Taylor, 1997, 2005; Wade and Angliss, 1997).
PBRs at an appropriate scale are shown in Figure 1, using 0.1 as the recovery factor suggested for endangered species and 4% as the default maximum population growth rate for cetaceans, and using the most recent population estimates (MacKenzie and Clement, 2014, 2016). The estimated maximum population growth rate for Hector’s dolphin of 1.8% would result in PBRs about half of those shown in Figure 1 (e.g., Slooten and Dawson, 2008). This is a level of total human impact that, if not exceeded, would allow depleted populations to recover to at least half of their original population size (Wade, 1998). Actual bycatch levels have been estimated at 110–150 individuals per year, for all populations combined during the period 2000–2006 (Davies et al., 2008). This is more than an order of magnitude greater than the PBR. The most recent estimate is 58 Hector’s dolphins per year—44 (95% CI 21–80) in gillnets and 14 (95% CI 1–43) in trawl fisheries (Ministry for Primary Industries [MPI] and Department of Conservation [DOC], 2019). More sophisticated risk analyses have confirmed that bycatch levels are unsustainable (see section “Conservation Problems,” below).
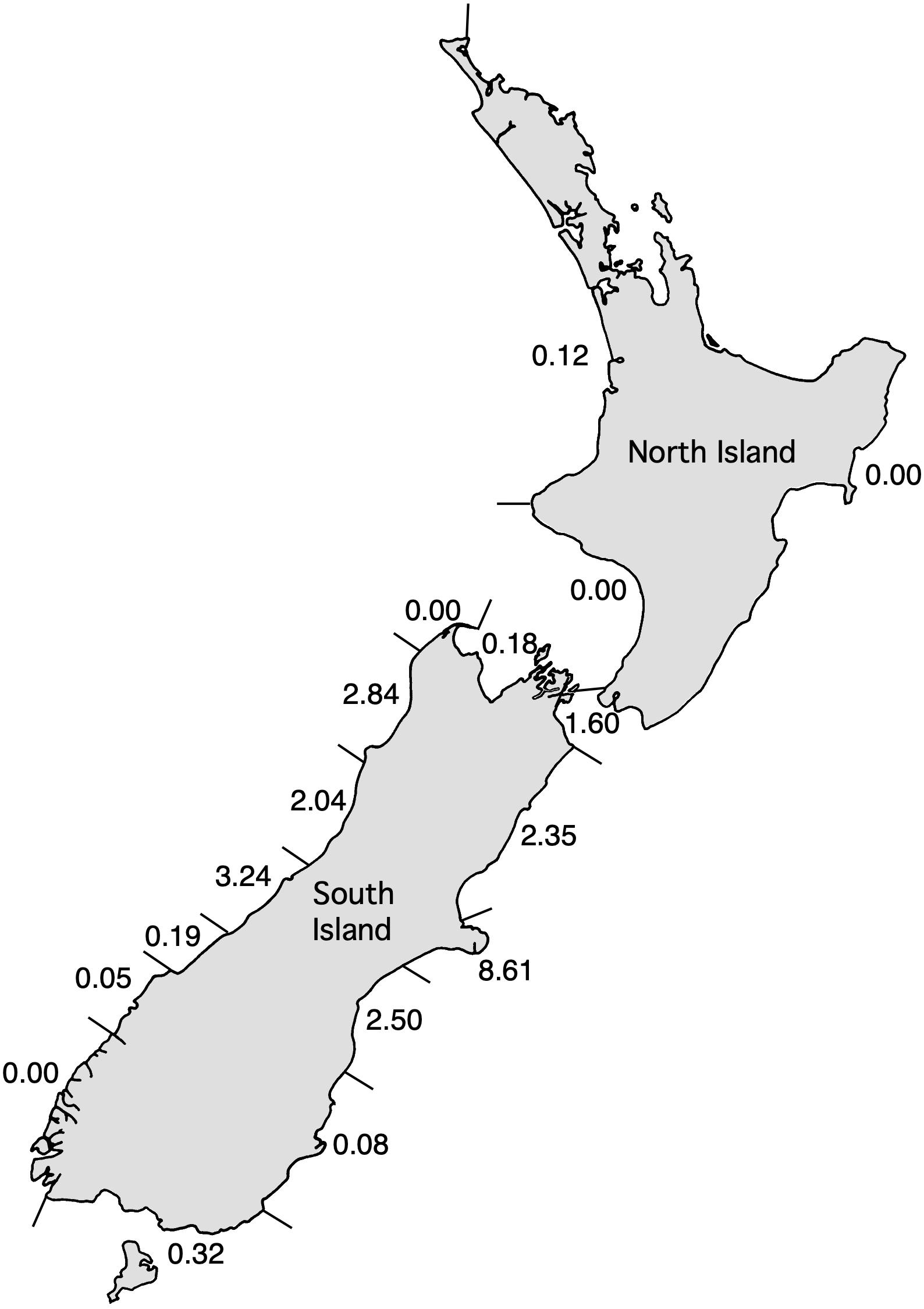
Figure 1. Map showing PBR estimates for regional populations of Māui dolphins (North Island) and Hector’s dolphins (South Island), using the most recent population estimates, and default values of 0.04 for Rmax and 0.1 for recovery factor.
Conservation Problems
Hector’s dolphin is found only in New Zealand waters and is listed as Endangered (Reeves et al., 2013). The North Island subspecies Māui dolphin is listed separately as Critically Endangered (Reeves et al., 2013).
Bycatch in gillnet fisheries is considered the most serious threat to the species, and bycatch in trawl fisheries the second most serious (Department of Conservation [DOC] and MFish, 2007; Currey et al., 2012). The number of dolphins killed each year has been estimated via an observer programme in the commercial gillnet fishery off Canterbury on the east coast South Island (Baird and Bradford, 2000). During 2000–2006, an estimated average of 110–150 dolphins were caught per year nationwide (Davies et al., 2008).
In general, dolphin bycatch is a relatively simple and predictable problem. Over the last three decades it has become clear that dolphin bycatch occurs wherever gillnet and trawl fisheries overlap with dolphin populations (e.g., Perrin et al., 1994; Brownell et al., 2019). An estimated 300,000 small cetaceans are killed each year in gillnet fisheries worldwide (Read et al., 2006). At particular risk are dolphin populations that are endemic, small and/or fragmented (see Table 1 in Dawson et al., 2008).
Risk analyses for Hector’s dolphin have consistently indicated population declines over the last forty years. These analyses have used a wide range of methods including deterministic and stochastic Leslie Matrix models (e.g., Slooten and Lad, 1991; Slooten et al., 2000), deterministic and stochastic Logistic models (e.g., Martien et al., 1999; Burkhart and Slooten, 2003; Slooten, 2007; Slooten and Dawson, 2010) and Bayesian models (e.g., Davies et al., 2008; Gormley, 2009). The results have been very consistent (Slooten and Davies, 2011). For example, Slooten (2007) predicted population declines if fisheries bycatch continues, and recovery to about half of the original population size by 2050 if fisheries mortalities are reduced to zero. Davies et al. (2008) made very similar predictions. Estimates of current population size, as a proportion of 1970 population size, range from 27% (Slooten, 2007) to 34% (Davies et al., 2008).
These analyses did not include dolphins caught in trawl fisheries, or by recreational fishers, and are therefore optimistic. Dolphins are caught in the trawl fishery and in recreational gillnets, but insufficient information is available to estimate the total number caught. The catch rate, per day fishing, appears to be lower in trawl fisheries than in gillnets. However, fishing effort is much higher in trawl fisheries and observer coverage in inshore fisheries has been too low to provide scientifically robust estimates of trawl bycatch.
A recent risk analysis carried out under contract for New Zealand’s fisheries agency (MPI; Roberts et al., 2019) focuses on a much shorter timeframe than the previous risk analyses and provides a more optimistic assessment. The MPI risk analysis depends critically on an unvalidated habitat model, and the overlap between fishing effort and dolphins suggested by that model. The analysis was been critically reviewed an international Expert Panel (Taylor et al., 2018), invited by Ministry of Primary Industries (MPI) and Department of Conservation (DOC) to spend a week in New Zealand in 2018. There has been no formal response to the 37 recommendations of the Expert Panel (Taylor, pers comm.).
Research and management has so far focused on fisheries bycatch, because this is clearly a serious threat, and one that can be managed. Possible additional impacts include pollution, aquaculture, marine mining, port development, disease and, potentially, future tidal energy generation (Department of Conservation [DOC] and MFish, 2007; Stockin et al., 2010; Currey et al., 2012; Roe et al., 2013; Leunissen et al., 2019). There is no evidence for large scale changes in prey abundance and/or availability. Any such changes may be mitigated by the dolphin’s broad diet (Miller et al., 2013). Due to the presence of additional, as yet unquantified impacts on the species, fisheries bycatch needs to be managed in a precautionary manner.
Solutions
The solution to dolphin bycatch is simple: avoid overlap between dolphins and gillnet and trawl fisheries. Achieving that solution, however, is politically difficult. Technical solutions such as pingers (acoustic alarms) have proven effective in reducing harbor porpoise bycatch in controlled experiments (Kraus et al., 1997) but results in fisheries applications have been variable (e.g., Palka et al., 2008), and the approach has not proven effective for several dolphin species (e.g., Dawson S. et al., 2013). Pingers are used voluntarily by gillnetters in Canterbury as part of a mitigation strategy, but there is no evidence that they are effective for Hector’s dolphin (Dawson and Slooten, 2005; Dawson S. et al., 2013).
The only management method known to be effective for Hector’s dolphin is to remove gillnet and trawl fisheries from their habitat. This can be achieved by changing to more selective fishing methods that do not catch dolphins.
Progressively larger protected areas have been created to reduce bycatch of Hector’s dolphins. The process started in 1988 with the creation of the Banks Peninsula Marine Mammal Sanctuary (Figure 2). In 2003, a second protected area was created off the North Island west coast (Figure 2). In both areas, gillnets were prohibited from the shoreline to 4 nautical miles (n mi; 7.4 km) offshore. Inside the harbors, gillnetting is still allowed during the winter months at Banks Peninsula and year-round in other New Zealand harbors. Protection was extended in 2008, 2012, and 2020 (Department of Conservation [DOC], 2008; MFish, 2008; Ministry for Primary Industries [MPI] and Department of Conservation [DOC], 2012, 2019), because bycatch was clearly still unsustainable (e.g., Slooten, 2007; Davies et al., 2008; Currey et al., 2012; Ministry for Primary Industries [MPI] and Department of Conservation [DOC], 2019). Current protection (Figure 2) includes a ban on gillnets that varies in offshore extent from 2 to about 20 n mi from shore. Bans on trawling are much less extensive (Figure 2).
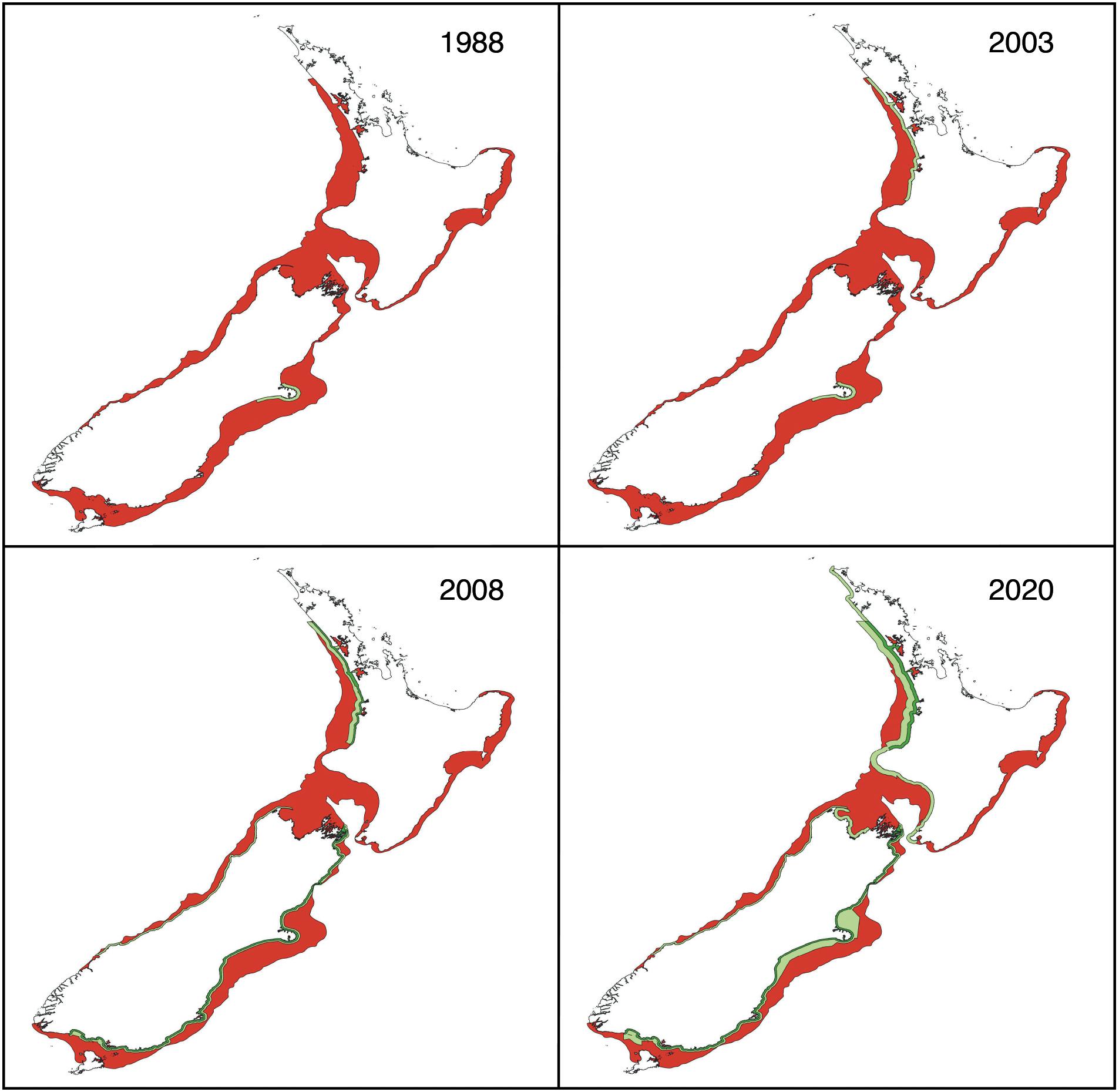
Figure 2. Historical sequence of protection. Hector’s and Māui dolphin habitat is indicated in red, protection from gillnet and trawl fisheries in dark green, and protection from gillnet fisheries only in light green.
Each extension has been a step forward in reducing the overlap between gillnets and Hector’s dolphins (including the North Island subspecies). The 2008 protection measures were predicted to lead to population recovery in areas with the best protection measures, but continued population declines in areas with little or no protection (e.g., west coast of South Island). The total population was predicted to decline by a further 600 or so individuals by 2050 (Slooten and Dawson, 2010). At this time, there is no evidence for population recovery (MacKenzie and Clement, 2016). For example, the Banks Peninsula population has declined rapidly (at a rate of about 6% per year) and in 2012 was assessed as almost stable (rate of decline <1% per year; Gormley et al., 2012). Results for Māui dolphin are similar, with rapid declines in the past and apparently slower declines after partial protection (Cooke et al., 2019).
Therefore, although protection has improved over time, there is no evidence to suggest that it is sufficient for population recovery. In addition, current protection does not yet meet national or international guidelines for marine mammal protection. For example, New Zealand legislation requires population recovery to “non-threatened” as soon as practicable and in any case within 20 years (Marine Mammals Protection Act [MMPA], 1978). United States legislation requires that population recovery, to at least half of the original population size, should not be delayed by more than 10% as a result of fisheries bycatch. To meet this goal would require a much larger reduction in the overlap between dolphins and fishing methods that cause dolphin mortality (gillnet and trawl fisheries).
All abundance surveys of Māui dolphin, irrespective of method, are consistent with decline (Wade et al., 2012). A gillnet fisher voluntarily reported catching a Māui dolphin in 2012, well outside the protected area created in 2003, prompting a small extension of the protected area (Currey et al., 2012). Further extensions in 2020 are shown in Figure 2.
Specific ways in which current protection for Hector’s and Māui dolphin could be improved include:
• Māui dolphin protection has been extended north and south, but does not extend sufficiently far offshore. High gillnet effort just outside the protected area, indicates a high risk of entanglement for any dolphins that stray across its boundary (Figure 2).
• Better protection of harbors. Although the current measures provide protection in North Island harbor entrances, gillnetting continues in most of the harbor habitat.
• Removing concessions allowing amateur gillnetting in the upper reaches of four harbors on Banks Peninsula and in a similar area in Queen Charlotte Sound between 1 April and 30 September and in several other harbors (e.g., Otago Harbor, Okarito Lagoon). Acoustic data from Akaroa Harbor show that, throughout this period, the upper harbor is routinely used by Hector’s dolphins (Dawson S. M. et al., 2013). Therefore gillnetting in this habitat constitutes an avoidable risk.
• Extending protection offshore to the 100 m depth contour. All recent population surveys show that Hector’s dolphins range much further offshore than the current protection measures. Māui dolphin sightings, including public sightings, are also consistent with a range throughout waters less than 100 m deep.
• Increased protection from trawl fisheries.
Every year since 2012, the Scientific Committee of the International Whaling Commission has expressed its concern about the small population of Māui dolphin, and recommended protection from gillnet and trawl fisheries from the shoreline to the 100 m depth contour or 20 nmi offshore—including harbors (e.g., International Whaling Commission [IWC], 2012, 2019). Likewise, the IUCN (after discussion by the Cetacean Specialist Group) recommended protection to the 100 m depth contour throughout Hector’s and Māui dolphin habitat (International Union for Conservation of Nature [IUCN], 2012).
A more general move away from the use of gillnets and trawling, in favor of selective fishing methods would have benefits beyond dolphins. It would promote recovery of depleted fish and bycatch species (including penguins and other seabirds). Enhancing selectivity should benefit sustainability, which would be in fishers’ long-term interest. This could be a win-win; in both ecological and economic terms.
Lessons Learned
Many of the “lessons” learned from deep involvement in the conservation science of Hector’s dolphin are general, and have been previously described by others. Here we seek to emphasize those most relevant to the conservation of small cetaceans, and to Hector’s dolphin in particular.
There is an interesting trade-off between the completeness of the protection measures and the cost of monitoring and evaluating their effectiveness. More restrictive protection is less expensive to monitor. For example, excluding gillnets and trawling from an area is less costly to monitor than having sufficient observers onboard to estimate bycatch. Use of “technical fix” management methods (e.g., pingers, changes to net rigging, etc.) requires comprehensive testing to demonstrate efficacy. In contrast, reducing the overlap between dolphins and fishing methods that kill dolphins only requires demonstration that the area concerned is large enough to meet the required conservation target.
Act Early
Toshio Kasuya received the Kenneth S. Norris Award at the Society for Marine Mammalogy Biennial Conference in Cape Town in 2007. In his acceptance speech, Dr. Kasuya pointed out that, in general, when dealing with marine mammal conservation issues it is essential to act early. Soulé (1980) effectively made the same point in his famous statement that there are no hopeless cases, only expensive ones. The longer protection measures are delayed, the more expensive and extreme they must become to meet the same objective, and the less likely they are to be effective. The corollary of Kasuya’s reminder is that inaction is a management decision.
Clearly Define the Management Goals
It is essential that the process of developing conservation measures starts with clearly outlining the management goals (e.g., Wade, 1998; Salafsky et al., 2002; Foley et al., 2010; Noss et al., 2012). This requires discussion about population recovery and an acceptable rate of recovery (Wade, 1998). For example, US regulations require that fisheries mortality should not delay population recovery by more than 10%. Clear, quantitative goals or principles simplify choices among management options. Options that do not meet these can be eliminated early on in the process (Fernandes et al., 2005). Also, any compromises made, for economic, social or political reasons, would be more explicit. In the Hector’s dolphin case study, successive Ministers have chosen management options that compromise between the interests of the public and the fishing industry. Media statements announcing the 2008 decision on dolphin protection (Figure 2) mentioned that the new protection measures would lead to “fewer dolphins caught”. There was no mention of sustainability or population recovery. Likewise, the New Zealand Minister of Fisheries said about the 2020 decision (Figure 2) that “Fisheries New Zealand … tell me the approach they have recommended best provides for effective protection for the dolphins where this is required, but also minimizes impact on utilization of fisheries resources to the extent possible.” (Nash, 2020).
Quantitative analyses of the effectiveness of management decisions (e.g., Slooten and Dawson, 2010; Slooten, 2013) suggest that the government’s implicit management goal has been to avoid further declines, rather than to allow population recovery. This means holding populations at their current depleted levels. For Māui dolphin, and for other small, vulnerable Hector’s dolphin populations, this dramatically increases their chance of extinction.
Recognize That Protected Areas Need to Be Large, and Biologically Relevant
Calls for bold goals in conservation, such as protection of large areas, have been made many times (e.g., Noss et al., 2012; Wilson, 2016; Dinerstein et al., 2017; Dudley et al., 2018). With respect to individual species, it is obvious that the area in which an impact is removed needs to be large enough to be effective (Edgar et al., 2014). Small protected areas may act shift the problem rather than solve it and can worsen population fragmentation. For example, following the creation of the Banks Peninsula Marine Mammal Sanctuary, fishers shifted their fishing effort to areas immediately north, south and offshore of the sanctuary and continued to catch dolphins. The estimated number of dolphins killed each year in Canterbury (Figure 2) was reduced from an average of 90 (1970–1988) to an average of 28 dolphins per year (1989–2006). While this was clearly an improvement, estimated catches were still well above the PBR of 0.2–0.45 per year for this area (Slooten and Dawson, 2008; Slooten and Davies, 2011). In the long term, it is easier to make a too-big reserve smaller, than a too-small reserve bigger.
Protected area boundaries need to be generous for another reason. Large MPAs are more ecologically sound because they include more of the ecological support system; including prey, shelter, and refuge from natural and anthropogenic risks. This holistic focus is a key feature of the advocacy for and implementation of ecosystem-based frameworks (e.g., Crowder and Norse, 2008; Halpern et al., 2010). Additionally, if the management goal is recovery, the population will need habitat to recover to. In this context, areas from which the target species has been extirpated should be seriously considered for protection.
It seems obvious that protected area boundaries must be established on biological grounds, relevant to the target species. For Hector’s and Māui dolphins that means basing offshore boundaries on water depth, not distance offshore, which is favored by managers. Since they are air breathers, and their diet includes several benthic species (Miller et al., 2013), depth is directly important.
The current approach consists of a complex set of protection measures, extending to different offshore distances for the two fishing methods (gillnetting and trawling) and for different regions. These do not match relevant water depths, or patterns of dolphin distribution. The 2008 Threat Management Plan (TMP) included an option that would have extended to 6 n mi (11.1 km) for the west coast South Island, 18 n mi (33.3 km) for the Banks Peninsula area and 12 n mi (22.2 km) elsewhere. Banning gillnetting and trawling in water less than 100m deep would achieve the same protection much more simply.
In determining boundaries of protected areas, it is worth bearing in mind that estimates of population range usually increase as more data become available. In addition, it is clear that sighting surveys are better at providing data on where animals are, than where they are not. In low-density areas, passive acoustic monitoring, such as echolocation loggers (e.g., T-PODs) can be much more effective than visual surveys for monitoring habitat use and documenting presence (e.g., Koschinski et al., 2003; Rayment et al., 2009b, 2011b; Jaramillo-Legorreta et al., 2019). For example, Hector’s dolphins are rarely seen in the upper reaches of Akaroa Harbor in the middle of winter, but echolocation loggers show they use the area on 41% of the days in winter (Dawson S. M. et al., 2013).
“Stakeholder” Working Groups Are Often Biased Toward Industry
This bias occurs in several ways. Firstly, extractive stakeholders are better represented numerically. This is partly because extractive stakeholders are easier to identify than non-extractive users. For example, New Zealand stakeholder groups addressing marine conservation issues always include fishers, but usually do not include tourist operators, surfers, kayakers etc. Secondly, extractive users are usually much better organized. They typically have associations and lobby groups which represent their interests. Thirdly, extractive users are usually much better funded. This is especially true of commercial users, who can afford to hire scientific and legal experts to represent their interests. Among the ways in which this difference shows is in industry’s ability to hire scientific experts who are able to follow and influence the often highly technical discussions at stakeholder meetings. In contrast, few government officials, and even fewer members of the public, have the necessary scientific training or time to contribute at this level.
Differences in resourcing can also bias the treatment of issues in subtle ways. For example, stakeholder meetings addressing bycatch issues in New Zealand are almost invariably held in Wellington, where the government departments and the main bodies representing commercial and recreational fishing interests are based. Stakeholders not based in Wellington must bear the costs of travel. For small groups representing non-extractive users this can be a substantial hurdle. The post-COVID increase in video conferencing has helped to address this problem.
Do Not Expect Government Officials to Be Neutral or Well-Informed
Scientists tend to assume that public servants are neutral and well-informed on the issues in question and that the process as a whole is fair, neutral and science-based. These are unrealistic expectations. We are not implying dishonesty or incompetence. However, they often have biases forced on them by legislation. For example, the New Zealand Ministry for Primary Industries must allow for extractive use if that is at all possible. Hence there is an inherent bias in favor of fishing, which tends to counteract calls to limit or ban environmentally damaging activities. This has been very clear in discussions over the past 30 + years about protection of Hector’s dolphins, and also in consultation on other Marine Protected Areas. More subtly, government departments often have their own vested interests, for example in down-playing a particular conservation threat that they should have been managing. Outcomes are often influenced by controlling who is involved in relevant discussions (e.g., Brower, 2007), which may include limiting participation of independent experts. Workload issues may prevent government officials from reaching high levels of competence over the range of issues within their responsibility. Pressey et al. (2017) argued that many day-to-day activities of conservation professionals are effectively “displacement activities” (sensu Tinbergen, 1952) because they do not focus explicitly on conservation impact.
Offer the Full Range of Management Options
When discussing different management approaches, it is essential to include the full range of options from “do nothing” to “total protection.” The perceived need to compromise inherently favors a middle option. Also, the likely sustainability of each option should be clearly indicated, so that the consequences of choices are obvious. For example, in the 2008 Threat Management Plan for Hector’s dolphin, the management option most effective at reducing bycatch (option 3) would have allowed only very slow population recovery. The predicted rate of recovery under option 3 is not as high as required under either New Zealand or US legislation (Slooten, 2007) but much higher than recovery potential under the management option that was chosen. Had a broader range of management options been discussed in public consultations, the final decision might have come closer to meeting national and international goals for population recovery. In our case study, public consultation has consistently considered only a highly restricted range of options. For example, the most recent Threat Management Plan (Ministry for Primary Industries [MPI] and Department of Conservation [DOC], 2019) did not include the management recommendations of the IWC and IUCN.
Developing conservation measures is rarely a simple process of setting boundaries for protected areas based on scientific evidence. Instead, the process usually involves many stakeholder groups and considerable compromise. In these negotiations, biological criteria (e.g., avoiding population declines) are rarely seen as a bottom line. Compromise may have high biological costs.
Outcomes May Depend on the Balance of Political Power Within Government
The two government agencies involved in New Zealand, the Ministry for Primary Industries (MPI) and Department of Conservation (DOC) represent different interests and differ greatly in political influence. The Minister for Primary Industries (or Fisheries) has traditionally been a much more senior member of Cabinet than the Minister of Conservation.
MPI, despite its somewhat conflicting responsibilities (to ensure fish stocks and the marine environment are sustainable and to encourage the development of the fishing industry), takes the lead role on issues that involve the impact of fishing. In the marine realm, DOC, in practice, deals only with threatened species, marine mammals, and marine reserves. This means that DOC participates on a narrow range of issues, and is always the junior partner. This imbalance is embodied in the relevant legislation. For example, decisions on controlling fishing are made by the Minister for Primary Industries. The Minister of Conservation has to be “consulted” but his/her agreement is not required. In contrast, relevant decisions taken by the Minister of Conservation (e.g., on Marine reserves or Marine Mammal Sanctuaries) require the “concurrence” (i.e., agreement) of the Minister for Primary Industries.
Robust Studies of the Effectiveness of Management Measures Are Vital
Without studies of effectiveness, management is based on acts of faith (Ahmadia et al., 2015). The research on Hector’s dolphin at Banks Peninsula is, to our knowledge, the longest running intensive study of a management tool intended to reduce marine mammal bycatch. These studies show, for example, a biologically important improvement in survival rates after the sanctuary’s establishment (Gormley et al., 2012). They also show, however, that more needs to be done for this population to recover: the central problem is that the existing protected areas are not big enough (Slooten et al., 2006a; Rayment et al., 2010; Slooten, 2013).
Commitment to Long-Term Research Is Needed
Long-term studies are essential for gathering data on vital rates of long-lived animals, and for other data critical for effective conservation management. Long-term studies have the potential to provide data on year-to-year variability of population parameters such as survival and reproductive rates, and on ecological features such as habitat use. Ultimately, long-term studies provide a reality check on estimates of maximum age from autopsies, and contribute data relevant to questions around environmental change, such as climate change. Despite this, few funding agencies are prepared to commit to long term funding.
Concluding Remarks
One might expect that making conservation gains in a country such as New Zealand is easier than it actually is. After all, New Zealand has good conservation legislation, a comprehensive social welfare system (providing support to anyone put out of work by conservation action), a small, well-educated and relatively affluent population that is generally supportive of conservation, and an enviable history of progressive action in marine conservation (e.g., Ballantine and Gordon, 1979).
Globally, two of the most important problems in marine conservation are lack of the right kind of information, and finding agreement when species of concern cross the waters of several nations. Neither of these apply to management of Hector’s dolphin, which is blessed with robust information on all crucial aspects of the species’ biology, and which, being endemic to NZ, is not directly affected by the actions of other nations.
Even with so many factors in its favor, effective conservation action can be difficult to achieve, and often comes down to political will (Devillers et al., 2015). For Hector’s and Māui dolphin, the most important protection decisions happened when particularly motivated Ministers of Conservation and Fisheries (Helen Clark, Pete Hodgson, and Jim Anderton, respectively) took ownership of the issue and made solutions possible.
The path toward effective protection for Hector’s dolphin has been tortuous. The journey down that path, however, has been rewarding. A sustainable solution has not yet been reached, but our current location on the path is far better than where we started. While it is crucial to focus on the overall goal (in this case ensuring recovery), it is important to recognize and celebrate progress toward it.
There has never been a time when conservation science is more important than it is now.
Author Contributions
Both authors listed have made a substantial, direct and equal intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The long-term research on Hector’s dolphins is the work of a large group of people. Key graduate students include Stefan Bräger, Sam DuFresne, Eduardo Secchi, Deanna Clement, Will Rayment, Trudi Webster, Elanor Miller, Tom Brough, Jesu Valdez, Lindsay Wickman, and Will Carome. For their participation in wide-ranging discussions on science and management we are especially grateful to Simon Childerhouse, Will Rayment, Sam DuFresne, Trudi Webster, Lesley Douglas, and Ian West. We are very grateful for financial and other support for the research from Care for the Wild International, Department of Conservation, Ministry of Fisheries, NZ Nature Safaris, NZ Royal Society, NZ Whale and Dolphin Trust, Royal Forest and Bird Protection Society, University of Otago, Whale and Dolphin Conservation Society and WWF. We are very grateful to Leszek Karsmarski, Bernd Würsig, Jane Watson, and two reviewers for their comments on the manuscript.
Footnotes
- ^ Throughout, “sustainable” means resulting in population growth rates >1.
References
Ahmadia, G. N., Glew, L., Provost, M., Gill, D., Hidayat, N. I., Mangubhai, S., et al. (2015). Integrating impact evaluation in the design and implementation of monitoring marine protected areas. Philos. Trans. R. Soc. B Biol. Sci. 370:20140275. doi: 10.1098/rstb.2014.0275
Baird, S. J., and Bradford, E. (2000). Estimation of Hector’s Dolphin Bycatch from Inshore Fisheries, 1997-98 Fishing Year. Published Client Report on Contract 3024, Funded by Conservation Services Levy. Wellington: Department of Conservation.
Ballantine, W. J., and Gordon, D. P. (1979). New Zealand’s first marine reserve, cape rodney to okakari point, leigh. Biol. Conserv. 15, 273–280. doi: 10.1016/0006-3207(79)90048-x
Baker, A. N. (1978). The status of Hector’s dolphin, Cephalorhynchus hectori (van Beneden), in New Zealand waters. Rep. Int. Whal. Commn. 28, 331–334.
Baker, A. N., Smith, A. N. H., and Pichler, F. B. (2002). Geographical variation in Hector’s dolphin: recognition of a new subspecies of Cephalorhynchus hectori. J. Roy. Soc. NZ. 32, 713–717. doi: 10.1080/03014223.2002.9517717
Barlow, J., Swartz, S. L., Eagle, T. C., and Wade, P. R. (1995). US Marine Mammal Stock Assessments: Guidelines for Preparation, Background, and a Summary of the 1995 Assessments. NOAA Technical Memorandum, NMFS-OPR-95-6 NOAA. Washington, DC: National Oceanic and Atmospheric Administration.
Bräger, S. (1998). Behavioural Ecology and Population Structure of Hector’s Dolphin (Cephalorhynchus hectori). Ph.D Thesis. Dunedin: University of Otago.
Brough, T. E., Rayment, W. J., Slooten, E., and Dawson, S. (2019). Fine scale distribution for a population of New Zealand’s only endemic dolphin (Cephalorhynchus hectori) shows long-term stability of coastal hotspots. Mar. Mam. Sci. 35, 140–163. doi: 10.1111/mms.12528
Brough, T. E., Rayment, W. J., Slooten, E., and Dawson, S. M. (2020). Spatiotemporal distribution of foraging in a marine predator: behavioural drivers of hotspot formation. Mar. Ecol. Prog. Ser. 635, 187–202. doi: 10.3354/meps13198
Brower, A. (2007). Grazing land reform in New Zealand: background, mechanics and result. Rangel. Ecol. Manag. 60, 435–440. doi: 10.2111/1551-5028(2007)60[435:glrinz]2.0.co;2
Brownell, R. L., Reeves, R. R., Read, A. J., Smith, B. D., Thomas, P. O., Ralls, K., et al. (2019). Bycatch in gillnet fisheries threatens critically endangered small cetaceans and many others. Endanger. Species Res. 40, 285–296. doi: 10.3354/esr00994
Burkhart, S. M., and Slooten, E. (2003). Population viability analysis for Hector’s dolphin (Cephalorhynchus hectori): a stochastic population model for local populations. NZ. J. Mar. Freshw. Res. 37, 553–566. doi: 10.1080/00288330.2003.9517189
Cameron, C., Barker, R., Fletcher, D., Slooten, E., and Dawson, S. (1999). Modelling survival of Hector’s dolphins around banks Peninsula, New Zealand. JABES 4, 126–135. doi: 10.2307/1400593
Ceballos, G., Ehrlich, P. R., Barnosky, A. D., García, A., Pringle, R. M., and Palmer, T. M. (2015). Accelerated modern human–induced species losses: entering the sixth mass extinction. Sci. Adv. 5:e1400253. doi: 10.1126/sciadv.1400253
Cooke, J. G., Constantine, R., Hamner, R. M., Steele, D., and Baker, C. S. (2019). Population Dynamic Modeling of the Māui Dolphin based on Genotype Capture- Recapture with Projections Involving Bycatch and Disease Risk. New Zealand Aquatic Environment and Biodiversity Report No. 216. Wellington: Ministry for Primary Industries.
Crowder, L. B., and Norse, E. (2008). Essential ecological insights for marine ecosystem-based management and marine spatial planning. Mar. Policy 32, 772–778. doi: 10.1016/j.marpol.2008.03.012
Currey, R. J. C., Boren, L. J., Sharp, B. R., and Peterson, D. (2012). A Risk Assessment of Threats to Maui’s Dolphins. Wellington: Ministry for Primary Industries, 51.
Davies, N. M., Bian, R., Starr, P., Lallemand, P., Gilbert, D., and McKenzie, J. (2008). Risk Analysis for Hector’s Dolphin and Maui’s Dolphin Subpopulations to Commercial set Net Fishing using a Temporal-Spatial Age-Structured Model. Wellington: Ministry of Fisheries.
Dawson, S. M. (1991). Incidental catch of Hector’s dolphins in inshore gillnets. Mar. Mam. Sci. 7, 283–295. doi: 10.1111/j.1748-7692.1991.tb00103.x
Dawson, S. M. (2019). “Cephalorhynchus dolphins: C. heavisidii, C. eutropia, C. hectori, and C. commersonii,” in Encylopedia of Marine Mammals, eds B. Wūrsig, J. G. M. Thewissen, and K. M. Kovas (London: Elsevier), 166–172.
Dawson, S. M., Fletcher, D., and Slooten, E. (2013). Habitat use and conservation of an endangered dolphin. Endang. Species Res. 21, 45–54. doi: 10.3354/esr00508
Dawson, S., Northridge, S., Waples, D., and Read, A. J. (2013). To ping or not to ping: the use of active acoustic devices in mitigating interactions between small cetaceans and gillnet fisheries. Endang. Species Res. 19, 201–221. doi: 10.3354/esr00464
Dawson, S. M., and Slooten, E. (1988). Hector’s Dolphin Cephalorhynchus hectori: distribution and abundance. Rep. Int. Whal. Commn. 9, 315–324.
Dawson, S. M., and Slooten, E. (2005). Management of gillnet bycatch of cetaceans in New Zealand. J. Cet. Res. Manage. 7, 59–64.
Dawson, S. M., Slooten, E., DuFresne, S., Wade, P., and Clement, D. (2004). Small-boat surveys for coastal dolphins: line-transect surveys for Hector’s dolphins (Cephalorhynchus hectori). Fish. Bull. 201, 441–451.
Dawson, S. M., Wade, P., Slooten, E., and Barlow, J. (2008). Design and field methods for sighting surveys of cetaceans in coastal and riverine habitats. Mammal Rev. 38, 19–49. doi: 10.1111/j.1365-2907.2008.00119.x
Devillers, R., Pressey, R. L., Gretch, A., Kittinger, J. N., Edgare, G. J., Ward, T., et al. (2015). Reinventing residual reserves in the sea: are we favouring ease of establishment over need for protection? Aquat. Conserv. 25, 480–504. doi: 10.1002/aqc.2445
Dinerstein, E., Olson, D., Joshi, A., Vynne, C., Burgess, N. D., Wikramanayake, E., et al. (2017). An ecoregion-based approach to protecting half the terrestrial realm. Bioscience 67, 534–545. doi: 10.1093/biosci/bix014
Department of Conservation [DOC] (2008). Dolphins to Benefit from Further Protection. Wellington: Department of Conservation.
Department of Conservation [DOC] (2020). Hector’s and Māui Dolphin Incident Database. Wellington: Department of Conservation.
Department of Conservation [DOC] and MFish (2007). Hector’s and Maui’s Dolphin Threat Management Plan. Draft for Public Consultation. Wellington: Department of Conservation.
Dragonfly Consulting (2020). Protected Species Bycatch in New Zealand Fisheries. Available online at: https://psc.dragonfly.co.nz/2019v1/released/ (accessed September 12, 2020).
Dudley, N., Jonas, H., Nelson, F., Parrish, J., Pyhälä, A., Stolton, S., et al. (2018). The essential role of other effective area-based conservation measures in achieving big bold conservation targets. Glob. Ecol. Conserv. 15:e00424. doi: 10.1016/j.gecco.2018.e00424
du Fresne, S. (2005). Conservation Biology of Hector’s dolphin. Ph.D Thesis. Dunedin: University of Otago.
Edgar, G. J., Stuart-Smith, R. D., Willis, T. J., Kininmonth, S., Baker, S. C., Banks, S., et al. (2014). Global conservation outcomes depend on marine protected areas with five key features. Nature 506, 216–220. doi: 10.1038/nature13022
Estrada, A., Garber, P. A., Rylands, A. B., Roos, C., Fernandez-Duque, E., Di Fiore, A., et al. (2017). Impending extinction crisis of the world’s primates: why primates matter. Sci. Adv. 3:e1600946. doi: 10.1126/sciadv.1600946
Fernandes, L., Day, J., Lewis, A., Slegers, S., Kerrigan, B., Breen, D., et al. (2005). Establishing representative no-take areas in the great barrier reef: large-scale implementation of theory on marine protected areas. Conserv. Biol. 19, 1733–1744. doi: 10.1111/j.1523-1739.2005.00302.x
Fletcher, D. J., Dawson, S. M., and Slooten, E. (2002). Designing a mark-recapture study to allow for local emigration. JABES 7, 586–593. doi: 10.1198/108571102799
Foley, M. M., Halpern, B. S., Micheli, F., Armsby, M. H., Caldwell, M. R., and Crain, C. M. (2010). Guiding ecological principles for marine spatial planning. Mar. Policy 34, 955–966. doi: 10.1016/j.marpol.2010.02.001
Gormley, A. M. (2009). Population Modelling of Hector’s Dolphin. Ph.D thesis. Dunedin: University of Otago.
Gormley, A. M., Slooten, E., Dawson, S. M., Barker, R., Rayment, W. J., DuFresne, S., et al. (2012). First evidence that marine protected areas can work for marine mammals. J. Appl. Ecol. 49, 474–480. doi: 10.1111/j.1365-2664.2012.02121.x
Halpern, B. S., Lester, S. E., and McLeod, K. L. (2010). Placing marine protected areas onto the ecosystem-based management seascape. Proc. Natl. Acad. Sci. 107, 18312–18317. doi: 10.1073/pnas.0908503107
Hamner, R. M., Pichler, F., Heimeier, D., Constantine, R., and Baker, C. S. (2012). Genetic differentiation and limited gene flow among fragmented populations of New Zealand endemic Hector’s and Maui’s dolphins. Conserv. Genet. 13, 987–1002. doi: 10.1007/s10592-012-0347-9
Hamner, R. M., Constantine, R., Mattlin, R., Waples, R., and Baker, C. S. (2017). Genotype-based estimates of local abundance and effective population size for Hector’s dolphins. Biol. Conserv. 211, 150–160. doi: 10.1016/j.biocon.2017.02.044
Hawke, D. J. (1994). Seabird association with Hector’s dolphins and trawlers at lyttelton harbour mouth. Notornis 41, 206–209.
Hicks, C. C., Crowder, L. B., Graham, N. A., Kittinger, J. N., and Cornu, E. L. (2016). Social drivers forewarn of marine regime shifts. Front. Ecol. Environ. 14:252–260. doi: 10.1002/fee.1284
Hoekstra, J. M., Boucher, T. M., Ricketts, T. H., and Roberts, C. (2005). Confronting a biome crisis: global disparities of habitat loss and protection. Ecol. Lett. 8, 23–29. doi: 10.1111/j.1461-0248.2004.00686.x
Hoffmann, M., Belant, J. L., Chanson, J. S., Cox, N. A., Lamoreux, J., Rodrigues, A. S. L., et al. (2011). The changing fates of the world’s mammals. Philos. Trans. R. Soc. B Biol. Sci. 366, 2598–2610. doi: 10.1098/rstb.2011.0116
International Union for Conservation of Nature [IUCN] (2012). Actions to Avert the Extinctions of Rare Dolphins: Maui Dolphins, Hector’s Dolphins, Vaquita Porpoises and South Asian River and Freshwater Dependent Dolphins and Porpoises. Recommendation from the 2012 World Conservation Congress in Jeju, South Korea. Glund: International Union for Conservation of Nature.
International Whaling Commission [IWC] (2012). Report of the Scientific Committee of the International Whaling Commission. IWC/64/Rep1, Reporting on the Meeting of the Scientific Committee. Panama: International Whaling Commission, 11–23.
International Whaling Commission [IWC] (2019). Report of the Scientific Committee of the International Whaling Commission. IWC/69/Rep1, reporting on the meeting of the Scientific Committee. Nairobi: International Whaling Commission, 10–23.
Jaiteh, V. F., Allen, S. J., Meeuwig, J. J., and Loneragan, N. R. (2012). Subsurface behavior of bottlenose dolphins (Tursiops truncatus) interacting with fish trawl nets in northwestern Australia: implications for bycatch mitigation. Mar. Mam. Sci. 29, E266–E281.
Jaramillo-Legorreta, A. M., Cardenas-Hinojosa, G., Nieto-Garcia, E., Rojas-Bracho, L., Thomas, L., Ver Hoef, J. M., et al. (2019). Decline towards extinction of Mexico’s vaquita porpoise (Phocoena sinus). R. Soc. Open Sci. 6:190598. doi: 10.1098/rsos.19059
Koschinski, S., Culik, B. M., Henriksen, O. D., Tregenza, N., Ellis, G., Jansen, C., et al. (2003). Behavioural reactions of free-ranging porpoises and seals to the noise of a simulated 2 MW windpower generator. Mar. Ecol. Prog. Ser. 265, 263–273. doi: 10.3354/meps265263
Kraus, S., Read, A., Anderson, E., Baldwin, K., Solow, A., Spradlin, T., et al. (1997). Acoustic alarms reduce incidental mortality of porpoises in gill nets. Nature 388:525. doi: 10.1038/41451
Leunissen, E. M., Rayment, W. J., and Dawson, S. (2019). Impact of pile-driving on Hector’s dolphin in lyttelton harbour. N. Z. Mar. Poll. Bull. 142, 31–42. doi: 10.1016/j.marpolbul.2019.03.017
MacKenzie, D., and Clement, D. M. (2014). Abundance and Distribution of ECSI Hector’s Dolphin. NZ Aquatic Environment and Biodiversity Report 123. Wellington: Ministry of Fisheries, 1–83.
MacKenzie, D., and Clement, D. M. (2016). Abundance and Distribution of ECSI Hector’s Dolphin. NZ Aquatic Environment and Biodiversity Report 168. Wellington: Ministry of Fisheries, 1–71.
Martien, K. K., Taylor, B. L., Slooten, E., and Dawson, S. (1999). A sensitivity analysis to guide research and management for Hector’s dolphin. Biol. Conserv. 90, 183–191. doi: 10.1016/s0006-3207(99)00020-8
MFish (2008). Minister Announces New Measures to Protect Dolphins. New Zealand: Ministry of Fisheries.
Miller, E. J., Lalas, C., Dawson, S., Ratz, H., and Slooten, E. (2013). Hector’s dolphin diet: the species, sizes and relative importance of prey eaten by Cephalorhynchus hectori, investigated using stomach content analysis. Mar. Mam. Sci. 29, 606–628.
Marine Mammals Protection Act [MMPA] (1978). New Zealand Marine Mammals Protection Act. New Zealand: Parliamentary Counsel Office.
Ministry for Primary Industries [MPI] and Department of Conservation [DOC] (2012). Review of the Maui’s Dolphin Threat Management Plan. Joint Discussion Paper No: 2012/18. Wellington: Ministry for Primary Industries.
Ministry for Primary Industries [MPI] and Department of Conservation [DOC] (2019). Protecting Hector’s and Māui Dolphin. Consultation on Proposals for an Updated Threat Management Plan. Wellington: Ministry for Primary Industries.
Nash, S. (2020). New Zealand Minister of Fisheries, Stuart Nash, in a letter to the Minister of Conservation, Eugenie Sage. Available online at: https://www.fisheries.govt.nz/dmsdocument/41103-hectors-and-maui-dolphin-threat-management-plan-advice-on-options-and-next-steps-18-october-2019-appendices-briefing-b19-0533 (accessed January 3, 2021).
Noss, R. F., Dobson, A. P., Baldwin, R., Beier, P., Davis, C. R., and Dellasala, D. A. (2012). Bolder thinking for conservation. Conserv. Biol. 26, 1–4. doi: 10.1111/j.1523-1739.2011.01738.x
Palka, D., Rossman, M., van Atten, A., and Orphanides, C. (2008). “Effect of pingers on harbor porpoise and seal bycatch in the US Northeast gillnet fishery,” in Paper Presented SC/60/SM2 at the Scientific Committee of the International Whaling Commission (Santiago).
Perrin, W. F., Donovan, G. P., and Barlow, J. (eds) (1994). Gillnets and Cetaceans: Incorporating the Proceedings of the Symposium and Workshop on the Mortality of Cetaceans in Passive Fishing Nets and Traps. Cambridge: International Whaling Commission.
Perrin, W. F., and Reilly, S. B. (1984). Reproductive parameters of dolphins and small whales of the family Delphinidae. Rep. Int. Whal. Commn. 6, 97–133.
Pichler, F. B. (2002). Genetic assessment of population boundaries and gene exchange in Hector’s dolphin. DOC Science Internal Series 44, Wellington: Department of Conservation, 37.
Pichler, F. B., Baker, C. S., Dawson, S. M., and Slooten, E. (1998). Geographic isolation of Hector’s dolphin populations described by mitochondrial DNA sequences. Conserv. Biol. 12, 676–682. doi: 10.1111/j.1523-1739.1998.96390.x
Pressey, R. L., Weeks, R., and Gurney, G. G. (2017). From displacement activities to evidence-informed decisions in conservation. Biol. Conserv. 212, 337–348. doi: 10.1016/j.biocon.2017.06.009
Rayment, W. J., Clement, D., Dawson, S., Slooten, E., and Secchi, E. (2011a). Distribution of Hector’s dolphin (Cephalorhynchus hectori) off the west coast, South Island, New Zealand, with implications for the management of bycatch. Mar. Mam. Sci. 27, 398–420. doi: 10.1111/j.1748-7692.2010.00407.x
Rayment, W. J., Dawson, S. M., and Slooten, E. (2009b). Trialling an automated passive acoustic detector (T-POD) with Hector’s dolphins (Cephalorhynchus hectori). J. Mar. Biol. Assn. 89, 1015–1022. doi: 10.1017/s0025315409003129
Rayment, W. J., Dawson, S. M., Slooten, E., Bräger, S., DuFresne, S., and Webster, T. (2009a). Kernel density estimates of alongshore home range of Hector’s dolphins at Banks Peninsula. New Zealand. Mar. Mam. Sci. 25, 537–556. doi: 10.1111/j.1748-7692.2008.00271.x
Rayment, W. J., Dawson, S. M., Scali, S., and Slooten, E. (2011b). Listening for a needle in a haystack: passive acoustic detection of dolphins at very low densities. Endang. Species Res. 14, 149–156. doi: 10.3354/esr00356
Rayment, W. J., Dawson, S. M., and Slooten, E. (2010). Seasonal changes in distribution of Hector’s dolphin at Banks Peninsula, New Zealand: implications for protected area design. Aquat. Cons. 20, 106–116.
Rayment, W. J., and Webster, T. R. (2009). Observations of Hector’s dolphins (Cephalorhynchus hectori) associating with inshore fishing trawlers at Banks Peninsula, New Zealand. NZ J. Mar. Freshw. Res. 43, 911–916. doi: 10.1080/00288330909510049
Read, A. J., Drinker, P., and Northridge, S. (2006). By-catches of marine mammals in US fisheries and a first attempt to estimate the magnitude of global marine mammal by-catch. Conserv. Biol. 20, 163–169.
Reeves, R. R., Dawson, S. M., Jefferson, T. A., Karczmarski, L., Laidre, K., O’Corry-Crowe, G., et al. (2013). Cephalorhynchus hectori. The IUCN Red List of Threatened Species 2013: e.T4162A44199757. Available online at: https://10.2305/IUCN.UK.2013-1.RLTS.T4162A44199757.en (accessed September 12, 2020).
Roberts, J. O., Webber, D. N., Roe, W. D., Edwards, C. T. T., and Doonan, I. J. (2019). Spatial Risk Assessment of Threats to Hector’s and Māui Dolphins (Cephalorhynchus hectori). NZ Aquatic Environment and Biodiversity Report No. 214. Wellington: Ministry for Primary Industries.
Roe, W., Howe, L., Baker, E. J., Burrows, L., and Hunter, S. A. (2013). An atypical genotype of Toxoplasma gondii as a cause of mortality in Hector’s dolphins (Cephalorhynchus hectori). Vet. Parasitol. 192, 67–74. doi: 10.1016/j.vetpar.2012.11.001
Salafsky, N., Margoluis, R., Redford, K. H., and Robinson, J. G. (2002). Improving the practice of conservation: a conceptual framework and research agenda for conservation science. Conserv. Biol. 16, 1469–1479. doi: 10.1046/j.1523-1739.2002.01232.x
Slooten, E. (1991). Age, growth and reproduction in Hector’s dolphins. Can. J. Zool. 69, 1689–1700. doi: 10.1139/z91-234
Slooten, E. (2007). Conservation management in the face of uncertainty: effectiveness of four options for managing Hector’s dolphin bycatch. Endang. Species Res. 3, 169–179. doi: 10.3354/esr003169
Slooten, E. (2013). Effectiveness of area-based management in reducing bycatch of the New Zealand (Hector’s) dolphin. Endang. Species Res. 20, 121–130.
Slooten, E., and Davies, N. (2011). Hector’s dolphin risk assessments: old and new analyses show consistent results. J. Roy. Soc. NZ. 42, 49–60.
Slooten, E., and Dawson, S. M. (1988). Studies on Hector’s Dolphin Cephalorhynchus hectori: a progress report. Rep. Int. Whal. Commn. 9, 325–338.
Slooten, E., and Dawson, S. M. (1994). “Hector’s Dolphin Cephalorhynchus hectori (van Beneden, 1881),” in Handbook of Marine Mammals Vol 5, The First Book of Dolphins, eds S. H. Ridgway and R. Harrison (New York, NY: Academic Press), 311–333.
Slooten, E., and Dawson, S. M. (2008). Sustainable levels of human impact for Hector’s dolphin. Open Cons. Biol. J. 2, 37–43. doi: 10.2174/1874839200802010037
Slooten, E., and Dawson, S. M. (2010). Assessing the effectiveness of conservation management decisions: likely effects of new protection measures for Hector’s dolphin (Cephalorhynchus hectori). Aquat. Cons. 20, 334–347. doi: 10.1002/aqc.1084
Slooten, E., and Dawson, S. M. (2017). “Bycatch and PBRs for Maui and Hector’s dolphin,” in Paper Presented SC/667a/HIM07, to the Scientific Committee of the International Whaling Commission (Bled), 9–21.
Slooten, E., Dawson, S. M., and Lad, F. (1992). Survival rates of photographically identified Hector’s dolphins from 1984 to 1988. Mar. Mam. Sci. 8, 327–343. doi: 10.1111/j.1748-7692.1992.tb00049.x
Slooten, E., Dawson, S. M., and Rayment, W. J. (2004). Aerial surveys for coastal dolphins: abundance of Hector’s dolphins off the South Island west coast New Zealend. Mar. Mam. Sci. 20, 477–490. doi: 10.1111/j.1748-7692.2004.tb01173.x
Slooten, E., Dawson, S. M., Rayment, W. J., and Childerhouse, S. J. (2006a). A new abundance estimate for Maui’s dolphin: what does it mean for managing this critically endangered species? Biol. Conserv. 128, 576–581. doi: 10.1016/j.biocon.2005.10.013
Slooten, E., Fletcher, D., and Taylor, B. L. (2000). Accounting for uncertainty in risk assessment: case study of Hector’s dolphin mortality due to gillnet entanglement. Cons. Biol. 14, 1264–1270. doi: 10.1046/j.1523-1739.2000.00099-411.x
Slooten, E., and Lad, F. (1991). Population biology and conservation of Hector’s dolphin. Can. J. Zool. 69, 1701–1707. doi: 10.1139/z91-235
Slooten, E., Rayment, W. J., and Dawson, S. M. (2006b). Offshore distribution of Hector’s dolphins at banks Peninsula: is the banks Peninsula marine mammal sanctuary large enough? NZ J. Mar. Freshw. Res. 40, 333–343. doi: 10.1080/00288330.2006.9517425
Soulé, M. E. (1980). “Thresholds for survival: Maintaining fitness and evolutionary potential,” in Proceedings of the First Conference on Scientific Research in the National Parks, ed. R. M. Linn (Washington, DC: US Dept. Interior), 681.
Stockin, K. A., Law, R. J., Roe, W., Meynier, L., Martinez, E., Duignan, P. J., et al. (2010). PCBs and organochlorine pesticides in Hector’s (Cephalorhynchus hectori hectori) and Maui’s (Cephalorhynchus hectori maui) dolphins. Mar. Poll. Bull. 60, 834–842. doi: 10.1016/j.marpolbul.2010.01.009
Taylor, B. L. (1997). “Defining “population” to meet management objectives for marine mammals,” in Molecular Genetics of Marine Mammals, eds A. E. Dizon, S. J. Chivers, and W. F. Perrin (Lawrence: Allen Press, Inc), 347–364.
Taylor, B. L. (2005). “Identifying units to conserve,” in Marine Mammal Research: Conservation Beyond Crisis, eds J. E. Reynolds, III W. F. Perrin, R. R. Reeves, S. Montgomery, and T. J. Ragen (Baltimore: John Hopkins University Press), 149–164.
Taylor, B. L., Lonergan, M., and Reeves, R. R. (2018). Panel Comments and Recommendations. Unpublished report for Ministry of Primary Industries. Wellington: Ministry for Primary Industries.
Taylor, B. L., Martinez, M., Gerrodette, T., and Barlow, J. (2007). Lessons from monitoring trends in abundance of marine mammals. Mar. Mam. Sci. 23, 157–175. doi: 10.1111/j.1748-7692.2006.00092.x
Tinbergen, N. (1952). “Derived” activities: their causation, biological significance, origin, and emancipation during evolution. Q. Rev. Biol. 27, 1–32. doi: 10.1086/398642
Turvey, S. T., Pitman, R. L., Taylor, B. L., Barlow, J., Akamatsu, T., Barrett, L. A., et al. (2007). First human-caused extinction of a cetacean species? Biol. Lett. 3, 537–540. doi: 10.1098/rsbl.2007.0292
Wade, P. R. (1998). Calculating thresholds to the human-caused mortality of cetaceans and pinnipeds. Mar. Mam. Sci. 14, 1–37. doi: 10.1111/j.1748-7692.1998.tb00688.x
Wade, P. R., and Angliss, R. P. (1997). Guidelines for Assessing Marine Mammal Stocks: Report of GAMMS Workshop, 1996, Seattle, Washington. NOAA Technical Memorandum, NMFS-OPR-12. Wellington: Ministry for Primary Industries.
Wade, P. R., Hamner, R. M., Constantine, R., and Baker, C. S. (2012). “The Potential Biological Removal (PBR) and probability of decline for Maui’s dolphin,” in A Risk Assessment of Threats to Maui’s Dolphins, eds R. J. C. Currey, L. J. Boren, B. R. Sharp, and D. Peterson (Wellington: Ministry for Primary Industries and Department of Conservation), 28–36.
Wilhere, G. F., Maguire, L. A., Scott, M. J., Rachlow, J. L., Goble, D. D., and Svancara, L. K. (2012). Conflation of values and science: response to Noss et al. Conserv. Biol. 26, 943–944. doi: 10.1111/j.1523-1739.2012.01900.x
Keywords: science, conservation, management, hector’s dolphin, Māui dolphin
Citation: Slooten E and Dawson SM (2021) Delays in Protecting a Small Endangered Cetacean: Lessons Learned for Science and Management. Front. Mar. Sci. 8:606547. doi: 10.3389/fmars.2021.606547
Received: 15 September 2020; Accepted: 02 March 2021;
Published: 24 March 2021.
Edited by:
Randall William Davis, Texas A&M University at Galveston, United StatesReviewed by:
Alana Grech, ARC Centre of Excellence for Coral Reef Studies, AustraliaJerome Spitz, Université de la Rochelle, France
Copyright © 2021 Slooten and Dawson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephen M. Dawson, c3RldmUuZGF3c29uQG90YWdvLmFjLm56
 Elisabeth Slooten
Elisabeth Slooten Stephen M. Dawson
Stephen M. Dawson