- 1Laboratório de Ecologia e Conservação da Megafauna Marinha, Instituto de Oceanografia, Universidade Federal do Rio Grande, Rio Grande, Brazil
- 2Laboratório de Ecologia e Conservação de Tetrápodes Marinhos e Costeiros, Universidade da Região de Joinville, São Francisco do Sul, Brazil
- 3Grupo de Estudos de Mamíferos Aquáticos do Rio Grande do Sul, Torres, Brazil
- 4Departamento de Ciências Biológicas, Universidade Estadual de Santa Cruz, IIhéus, Brazil
- 5Laboratório de Mamíferos Aquáticos e Bioindicadores, Faculdade de Oceanografia, Universidade do Estado do Rio de Janeiro, Rio de Janeiro, Brazil
The franciscana is endemic to subtropical coastal waters of Brazil, Uruguay, and Argentina, and is the only living species of the family Pontoporiidae. It is regarded as the most endangered cetacean in the western South Atlantic. Five management units are recognized (Franciscana Management Areas, FMAs – sensu Secchi et al., 2003a), with abundance estimates ranging from a few hundred to around 15,000 dolphins. Low reproductive potential and short life span make this species highly susceptible to current non-natural removal rates. Bycatch in gillnet fisheries occurs in high levels since the 1960s in Uruguay and 1980s in Brazil and Argentina. Although other threats exist, such as habitat degradation that includes physical (noise) and chemical pollution, depletion of fish stocks and climate change, incidental mortality in gillnets is currently the greatest threat to franciscanas. Fishing-related mortality ranges from approximately 100, in FMA I, to more than 1,000 in FMA III, and exceed from near two (in FMA IV) to more than five times (in FMA III) the maximum allowed sustainable mortality rate, based on potential biological removal (PBR) approach. These numbers indicate that the species is unlikely to cope with the current levels of bycatch and that urgent and extreme reduction on fishing practice and effort are required to avoid collapse of the franciscana and to lower its risk of extinction. Current mortality levels and projected declines resulted in the listing of the franciscana as “Vulnerable” in the IUCN Red List. Recent fisheries regulations were implemented in areas with extensive bycatch in Brazil and were expected to improve the species’ conservation status. There is evidence, however, that this regulation is insufficient to reduce fishing-related mortality to sustainable levels due to either or both lack of compliance and inadequate regulation strategies. Here we provide a comprehensive review on the franciscana ecology and threats and discuss perspectives for its conservation.
Introduction
The franciscana or La Plata River dolphin, Pontoporia blainvillei (Figure 1), is perhaps the most endangered cetacean in the Western South Atlantic (Secchi, 2010). Its life history traits, including low reproductive and survival rates, and short life span, makes it vulnerable to high levels of non-natural mortality (e.g., Danilewicz et al., 2002; Secchi et al., 2003b; Secchi, 2010). The genus Pontoporia belongs to the so-called river dolphins, lineage that diverged well before the radiation of delphinids, with adaptation to fluvial habitats might have insured their survival against environmental changes in the marine ecosystem or the emergence and dominance of delphinids (Cassens et al., 2000). It has been hypothesized that the ancestral of Pontoporia occupied the Paraná basin and reinvaded the marine coastal waters to north and south of the La Plata River mouth (Cozzuol, 1985; Hamilton et al., 2001). This recent ecological reversal was followed by ecologically specialized adaptations that allowed the species to benefit from a productive environment for reproduction, growth and survival demands, and to cope with competitive pressures of the dominant delphinids and predation by sharks and killer whales inhabiting the inner continental shelf of the eastern South America. This adaptation, that allowed franciscana to reach demographic balance of reproduction and mortality, however, is insufficient to respond to non-natural mortality due to the ever increasing impacts from human activities (Secchi, 2006; Cáceres et al., 2020). Franciscana’s restricted distribution to coastal waters makes it vulnerable to a variety of impacts from coastal development (Danilewicz et al., 2009, 2010; Secchi, 2010; Amaral et al., 2018). Habitat degradation such as chemical and physical pollution (noise) and overfishing have affected the species. Nevertheless, the large spatial overlap between the distribution the species and extensive fishing activities has led to high and unsustainable levels of incidental mortality, especially in gillnets, and is currently the greatest danger to franciscanas (e.g., Secchi, 2010).

Figure 1. Group of franciscanas including two adults (longer beaks), two likely juveniles (shorter beaks) and a newborn calf.
Here we present a comprehensive review on the ecology of franciscana and the main impacts that have led it to be the most endangered species in the Western South Atlantic. We also showed that the current levels of bycatch in fisheries are unsustainable and discuss the conservation perspectives to increase the species long-term chances of survival.
Franciscana Ecology
Distribution and Movements
The franciscana is endemic to the western South Atlantic Ocean, ranging from Itaúnas (18°25′S – 30°42′W), southeastern Brazil (Siciliano, 1994), to Golfo Nuevo (42°35′S - 64°48′W), in the Argentinian Patagonia (Crespo et al., 1998; Figure 2). Franciscanas are predominantly coastal, occupying waters beyond the surf zone and out to the 30 or 50 m isobaths (Secchi et al., 1997; Crespo et al., 2010; Danilewicz et al., 2010; Botta et al., 2015). No size, age or sex-related differences in habitat use patterns in relation to depth have been observed in southern Brazil (Danilewicz et al., 2009). Occasional occurrences were recorded in estuaries of Brazil (e.g., Santos et al., 2009), and the species is rather common in the Uruguayan portion of the La Plata River estuary (Praderi, 1986), in the Negro River estuary, Argentina (Failla et al., 2012) and resident in the estuary of Babitonga Bay, Brazil (Cremer and Simões-Lopes, 2005; Sartori et al., 2017). The species is not distributed continuously throughout its range. Siciliano et al. (2002) first reported the existence of two areas in its northern range where franciscanas are extremely rare or absent. These areas were reassessed by Amaral et al. (2018) and reshaped as Gap I, from Santa Cruz (19°57′S), in the state of Espírito Santo, to Barra de Itabapoana (21°18′S), in the state of Rio de Janeiro, Brazil; and Gap II, from Armação dos Búzios (22°44′S) to Piraquara de Dentro (22°59′S), in the state of Rio de Janeiro, Brazil (Figure 2). The extension of the gaps was reduced and the narrowing of the continental shelf, water transparency and lower water temperature, due to local upwelling, seem to be among the reasons for these hiatuses (Siciliano et al., 2002; Amaral et al., 2018). It is suspected that variation in anthropogenic pressures, population demography, and prey availability, among other ecological factors, might influence the extension of the hiatus over time, creating small expansions and retractions on franciscana distribution (Danilewicz et al., 2012). Nevertheless, current genetic information suggests that reproductive isolation is very likely (Cunha et al., 2014).
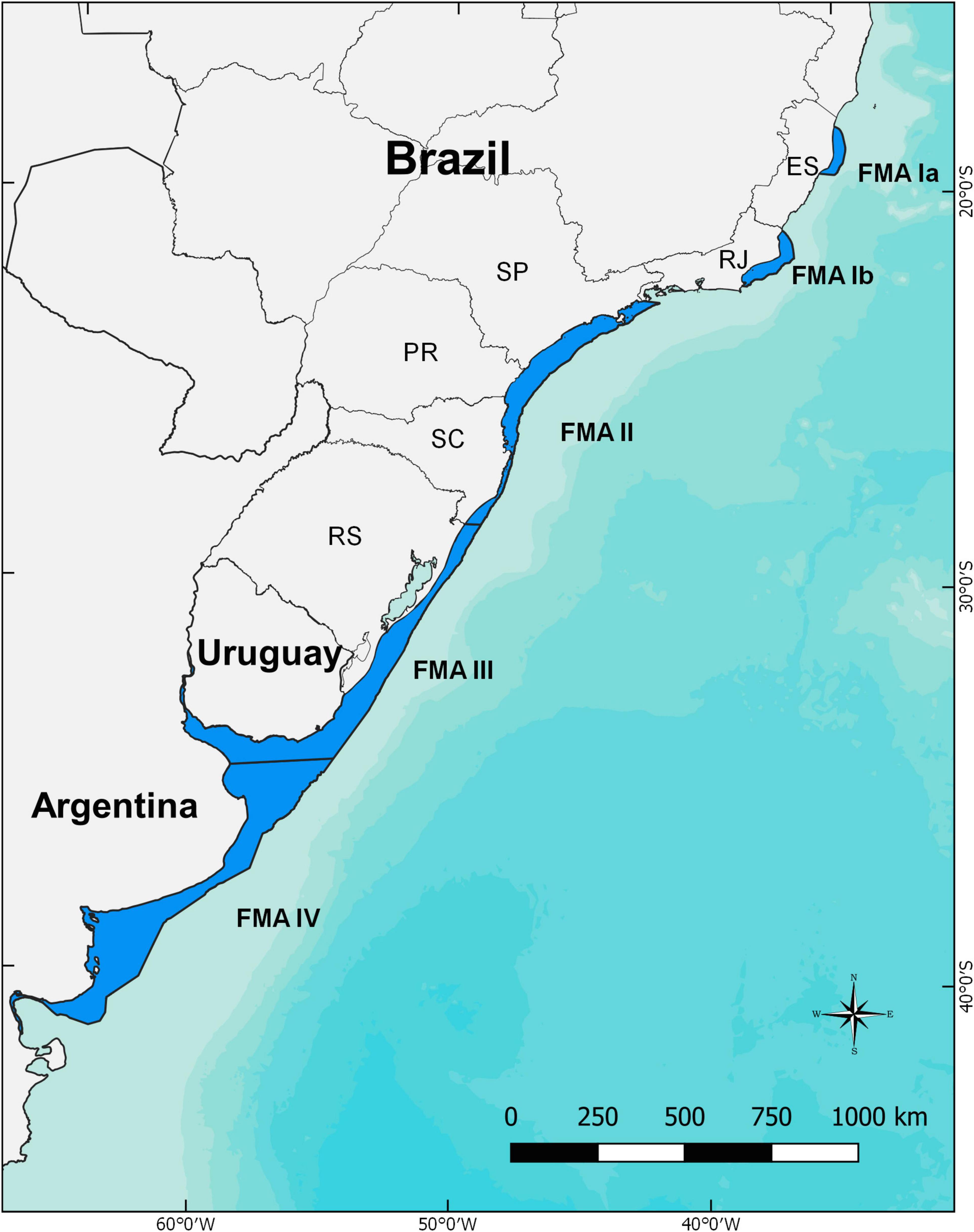
Figure 2. Franciscana distribution in coastal waters of the western South Atlantic and the proposed limits of each Franciscana Management Areas (FMAs – sensu Secchi et al., 2003a). ES, Espírito Santo State; RJ, Rio de Janeiro State; SP, São Paulo State; PR, Paraná State; SC, Santa Catarina State; RS, Rio Grande do Sul State.
Franciscanas do not migrate and probably occupy small spatial ranges (Bassoi et al., 2020). Travels of up to 70–100 km alongside the coast have been documented for a few satellite-tagged individuals off Argentina, although most animals performed much more restricted movements (Wells et al., 2013). In Babitonga Bay, Brazil, the movements of satellite-tagged individuals were very limited, remaining in a very small portion of the bay (Cremer et al., 2013), and home range was smaller in the summer than in the winter season (Cremer et al., 2018b). The species inshore-offshore movements were related to tidal state, and the individuals follow the flow of the current in Bahia Anegada, Argentina (Bordino et al., 1999; Bordino, 2002) and in Babitonga Bay, Brazil (Paitach et al., 2017).
Population Structure/Management Units
Genetics, morphology, distribution, and populational parameters were used by Secchi et al. (2003a) to propose four provisional management units (Franciscana Management Areas, or FMAs), with the following areas of occurrence: FMA I – coastal waters of Espírito Santo and Rio de Janeiro States, Brazil; FMA II – São Paulo, Paraná and Santa Catarina States, Brazil; FMA III – coastal waters of Rio Grande do Sul State, southern Brazil, and Uruguay; and FMA IV – coastal waters of Buenos Aires and Rio Negro Provinces (with occasional records as far south as Chubut), in Argentina. Since then, new genetic and morphological information have indicated the probable subpopulation structure throughout the species range (Lázaro et al., 2004; Mendez et al., 2008, 2010; Véras, 2011; Barbato et al., 2012; Costa-Urrutia et al., 2012; Negri et al., 2012; Cunha et al., 2014; Gariboldi et al., 2015, 2016; Ott et al., 2015), leading to the proposition of new sub-divisions within each FMA (Cunha et al., 2014). Nevertheless, since (i) the degree of differentiation within the FMAs was highly variable, (ii) evidence were founded only on mitochondrial DNA data, and (iii) there is presently no agreement on threshold levels of gene flow, the proposed management units should be accepted, latest refinements in franciscana subpopulation structure were restricted only to locations where the greatest levels of genetic difference were observed (Anonymous, 2015). Accordingly, FMA I was considered an Evolutionarily Significant Unit and was split into two distinct management units (FMA Ia and FMA Ib, Cunha et al., 2014). The other important change was the displacement of the boundary between FMA II and FMA III, moved about 250 km northward, at the central coast of Santa Catarina State (Cunha et al., 2014; Ott et al., 2015). It is not meant to apply new management dogmas to these units, rather, it is strongly recommended that limits and sub-structuring of these FMAs be constantly reassessed as new data become available.
Morphological and molecular data support the existence of distinctive franciscana populations. Evidence of population structuring was first demonstrated through multivariate analysis of morphometric data, which revealed the existence of two geographical forms: a smaller one in the northern part of the species’ range (north of 27°S) and a larger form in the coastal waters of southern Brazil, Uruguay, and Argentina (south of 32°S) (Pinedo, 1991, 1995). Body length of individuals from São Paulo (23°30′S-25°30′S) and Rio de Janeiro (21°35′S-22°25′S) states, Brazil, were significantly different, with animals from Rio de Janeiro being larger (Ramos et al., 2002). Similar growth patterns were observed in cranial dimensions (Ramos et al., 2002) as well as for other body metrics (Barbato et al., 2012). These results indicated that metric differences in body and skull variables were not clinal. Analyses of the highly variable d-loop region of mitochondrial DNA (mtDNA) provided molecular-based support to the existence of these two geographic forms (Secchi et al., 1998). Subsequent studies compared the mtDNA of franciscanas from Uruguay and Argentina with those published by Secchi et al. (1998) and found support for the existence of a large southern population (including animals from Rio Grande do Sul State in Brazil, Uruguay and Argentina), that is markedly distinct from animals inhabiting the waters of Rio de Janeiro State, in Brazil (Ott, 2002; Lázaro et al., 2004). Moreover, fixed genetic differences between the populations were observed, implying essentially no effective genetic exchange (Secchi et al., 1998; Ott, 2002; Lázaro et al., 2004). A pairwise analysis of haplotype distances showed increasing differentiation in the haplotype frequencies with increasing geographic gap, following an isolation-by-distance configuration (Lázaro et al., 2004). These authors and Ott (2002) also suggested that haplotypic frequencies of samples from Claromecó (in Argentina) were significantly different from the remaining southern population. The data presented by Mendez et al. (2008), however, do not fit this model. Rather, they propose that ecological forces can be more important than geographic distance in defining population structuring by regulating gene flow. Franciscanas from Claromecó are more similar to those from coastal marine areas, including those from Uruguay, than to those from the estuarine area of Samborombón Bay, northern Argentina. A similar pattern was observed in the Uruguayan coast (Costa-Urrutia et al., 2012). The authors noticed that, among several sampled areas including marine and estuarine coasts, the most genetically distinct individuals were those collected in the La Plata estuary, regardless of the geographic distance. This suggests that a fine-scale structuring occurs in areas with higher influence of the La Plata River estuary in both Argentina and Uruguay. These areas are shallow, relatively enclosed, and have high abundances of estuary-dependent prey, which make them a suitable habitat for calving. In fact, telemetry studies have demonstrated that individuals from Samborombón Bay have a very restricted movement range of about 20 km (Bordino and Wells, 2005). Furthermore, Rodríguez et al. (2002) found that individuals from this area prey upon different species when compared to those from the coastal oceanic environments. If part of the population has evolved and is mostly adapted to occupy an estuarial niche, intra-specific competition for resources is minimized, representing an advantage for the species as a whole.
Abundance
Abundance estimation for the franciscana has long been both a research priority and a major scientific challenge (Secchi et al., 2001; Danilewicz et al., 2010). Because the species’ small size, elusive behavior, avoidance of engine boats, and cryptic color pattern, it is very rarely sighted in the wild, especially during onboard surveys. Although aerial surveys have been agreed to be the best platform to assess franciscana′s abundance, boat surveys may be useful in small inner water areas (Bordino et al., 1999; Crespo et al., 2002; Cremer and Simões-Lopes, 2008). Nevertheless, as discussed by Danilewicz et al. (2010), the lack of appropriate correction factors for visibility biases (mainly availability bias, i.e., the proportion of time a group is available for detection), have been a major caveat and precluded reliable abundance estimates. Recent experiments applying alternative methodologies (use of helicopters and simultaneous boat/aerial surveys) have been carried out (e.g., Sucunza et al., 2018) and produced significant advances on computing correction factors for the species’ abundance estimations. Currently, no range-wide abundance estimation for franciscana is available. Estimates have been obtained either for a portion or the entire individual FMAs, through aerial surveys carried out by experienced researchers.
In FMA I, Danilewicz et al. (2012) conducted aerial surveys in Espírito Santo State (FMA Ia) and Rio de Janeiro, Brazil (FMA Ib) in 2011. In that study, no sighting was recorded during on-effort time in FMA Ia, despite relatively large survey work, suggesting that abundance of franciscana in FMA Ia is very small, a hypothesis further supported by Cunha et al. (2014), who identified a very low genetic diversity of that population. Further aerial surveys (2018–2019) estimated an abundance of 893 individuals for this area (CV = 0.30) and nearly 2,000 individuals in FMA Ib (CV = 0.46) (Sucunza, 2020), indicating that these are the two smallest populations among all FMAs.
Franciscana abundance in FMA II was estimated at 6,800 animals (CV = 0.26) (Sucunza et al., 2020) in 2008–2009. This value might be slightly underestimated as a few flights occurred when weather conditions were not ideal. Estimates are also available for Babitonga Bay, in Santa Catarina State, Brazil, where an isolated population of franciscanas occurs (Cremer et al., 2012). Boat-based and aerial line-transect surveys estimated, respectively, 50 individuals in the early 2000s (CV = 0.29, Cremer and Simões-Lopes, 2008) and 55 animals in 2011 (Zerbini et al., 2011), which suggests that this population is stable over a 10-year period.
Surveys in FMA III have been conducted only within the Brazilian portion (RS State). The first estimate was computed from aerial surveys conducted over a small region in 1996 (435 km2; 1.5% of its presumed distribution up to the 50 m isobath) (Secchi et al., 2001). Density corrected for availability bias was estimated at 0.66 individuals/km2 (CV = 0.34), and an extrapolated abundance of nearly 42,000 individuals (CV = 0.34) was assumed to the range of the entire population (Secchi et al., 2001). Later, Danilewicz et al. (2010) conducted an aerial survey covering an area from the shoreline to as far as 13 nm (43% of its presumed distribution) and estimated 6,800 individuals (CV = 0.32) in 2004. Surveys to estimate franciscana abundance in Uruguay are a research priority for this FMA.
In FMA IV, a population of nearly 15,000 individuals (CV = 0.42) was estimated from aerial surveys carried out in Argentina, during 2003–2004 (Crespo et al., 2010). This estimate was not corrected for perception bias and density estimated for offshore areas (depths greater than 30 m) was not extrapolated to non-surveyed areas, and it is possible that this estimative is downward biased.
Trophic Ecology
Franciscana is an intermediate trophic-level predator and is likely to play an important role in the food web of coastal subtropical western South Atlantic Ocean.
Franciscanas seem to be opportunistic feeders, eating the most abundant prey in the area. Seasonal fluctuations in diet match patterns of temporal variation in the abundance of the main prey species, and decadal changes in the dolphin’s diet seem to coincide with declines in fish stock abundance (Bassoi et al., 2020). Franciscanas feed predominantly upon several species of fish (e.g., Sciaenidae, Engraulidae, Gadidae, Batrachoididae, Trichiuridae, and Carangidae families), but also mollusks and crustaceans with important variation in prey relative contribution among FMAs (Brownell, 1989; Di Beneditto and Ramos, 2001; Rodríguez et al., 2002; Danilewicz et al., 2002; Cremer et al., 2012; Lopes, 2012; Troina et al., 2016; Henning et al., 2017; Rupil et al., 2019; Campos et al., 2020), totaling around 80 prey species. Prey items are typically smaller than 80 mm in length (Danilewicz et al., 2002). In spite of regional variation, teleosts of the Sciaenidae family (bottom-dweller croakers) are the predominant fish prey and small squids are the main mollusk prey (Di Beneditto and Ramos, 2001; Rodríguez et al., 2002; Danilewicz et al., 2002; Cremer et al., 2012; Henning et al., 2017; Campos et al., 2020). Lactation period seems to range from 6 to 9 months depending on the region and the duration of exclusive milk intake may last a few months until the transition to a mixed diet of milk (Kasuya and Brownell, 1979; Di Beneditto and Ramos, 2001; Rosas and Monteiro-Filho, 2002; Rodríguez et al., 2002; Denuncio et al., 2013; Panebianco et al., 2016). The first solid food items are slow-moving prey such as very small fish and shrimps (e.g., Danilewicz et al., 2002; Rodríguez et al., 2002; Denuncio et al., 2013). Information regarding daily food requirements for wild franciscanas is lacking.
The known predators of franciscanas include killer whales (Orcinus orca) and some other top marine predators, including broadnose sevengill (Notorynchus cepedianus), hammerhead (Sphyrna spp.), sand tiger (Eugomphodus taurus), tiger (Galeocerdo cuvieri), and requiem (Carcharhinus sp.) sharks (Praderi, 1985; Ott and Danilewicz, 1996; Santos and Netto, 2005). There is little overlap in diet with other coastal cetaceans such as bottlenose dolphins (Tursiops spp.), and Guiana dolphins (Sotalia guianensis) (Hardt et al., 2013; Secchi et al., 2016; Cremer et al., 2018b). On the other hand, feeding competition is likely with some predatory fish such as the cutlass fish (Trichiurus lepturus) (Bittar and Di Beneditto, 2009). In some areas, the franciscana’s main prey items are also bycatch of trawling fisheries (Machado et al., 2020).
Reproduction
The franciscana has among the shortest reproductive life cycles of all cetaceans (Danilewicz, 2003). The species can be classified as birth-pulse and birth-flow breeder, depending on the region. Reproduction is markedly seasonal in the southern range, with births occurring in a “pulse” in the austral spring and summer, from October to February (Brownell, 1984; Rosas and Monteiro-Filho, 2002; Danilewicz, 2003; Cremer et al., 2013; Panebianco et al., 2016). In the northernmost part of the species range (FMA I), reproductive seasonality does not seem to occur and births “flow” year-round (Di Beneditto and Ramos, 2001). Although lack of reproductive seasonality was also proposed for franciscanas collected in São Paulo State (Silva et al., 2020) evidence is not strong as the size of the fetuses reported indicate that births are likely to occur during austral spring and summer.
Age and length at attainment of sexual maturity also varies among FMAs (Table 1). Sexual maturity for both males and females is reached at between 2 and 5 years of age. Spatial variation in the mean length at sexual maturity is due to the marked inter-population differences in body size. In the northern range of the species distribution (FMA I and II) franciscana males and females attain sexual maturity between and 3 years of age and total lengths spanning from 112 to 132 cm (Ramos et al., 2000; Di Beneditto and Ramos, 2001; Silva et al., 2020). Annual pregnancy rate was estimated to be 0.36, with an interbirth interval of 2.8 years (Silva et al., 2020). In the southern range (FMA III and IV), franciscanas reach sexual maturity at the age of 3–4 years and length varying from about 126-131 cm in males and from 133 to 140 cm in females (Kasuya and Brownell, 1979; Danilewicz et al., 2000; Danilewicz, 2003; Panebianco et al., 2012, 2016). The annual pregnancy rate was estimated to be 0.65, which is correspondent to an average birth interval of 1.5 years. This implies that within the population, half of the females reproduce yearly and the other half biannually (Danilewicz et al., 2000; Danilewicz, 2003).
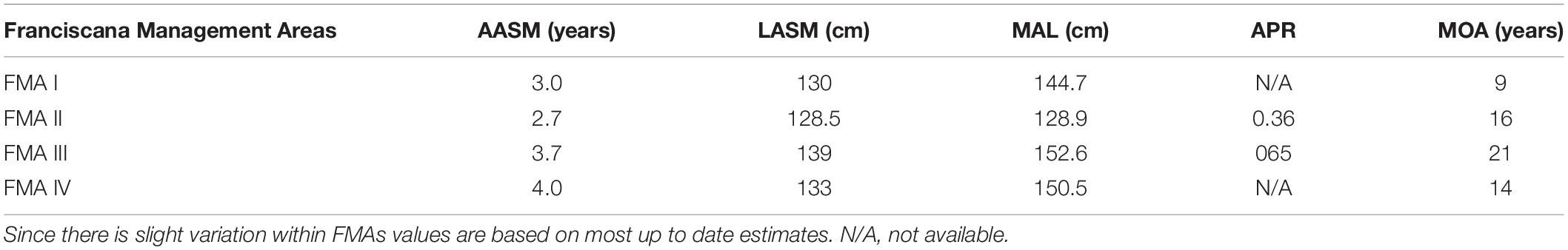
Table 1. Spatial differences in age at attainment of sexual maturity (AASM), body length at attainment of sexual maturity (LASM), modeled asymptotic length (MAL), annual pregnancy rate (APR), and maximum observed age (MOA) of female franciscanas among FMAs (sensu Secchi et al., 2003a).
The gestation period does not vary much geographically, with estimates of between 10.2 and 11.2 months (Kasuya and Brownell, 1979; Harrison et al., 1981; Di Beneditto and Ramos, 2001; Rosas and Monteiro-Filho, 2002; Danilewicz, 2003; Panebianco et al., 2016). Neonatal length and weight were estimated at slightly over 70 cm and 6 kg in the southern range. In parts of the northern range, the newborn calves are about 10 cm smaller and 1–2 kg lighter. Weaned calves are near 1 m long and weigh about 15 kg (Rosas and Monteiro-Filho, 2002; Danilewicz, 2003). Females do not show any evidence of reproductive senescence (Kasuya and Brownell, 1979; Rosas and Monteiro-Filho, 2002; Danilewicz, 2003; Panebianco et al., 2016; Silva et al., 2020). Considering the age of first reproduction, life span, and calving intervals, a female franciscana might produce from four to eight offspring in her lifetime (Danilewicz, 2003), although investment in reproduction seems to vary geographically.
Age and Growth
The franciscana’s life span seems to be around 20 years. The oldest aged franciscana was a 21-year old female and the maximum observed age for males was 17 years (Botta et al., 2010). The age frequency distribution of incidentally caught and washed ashore animals suggests that only a fraction of the population lives more than 12 years, irrespective of their geographic location (Kasuya and Brownell, 1979; Pinedo, 1994; Pinedo and Hohn, 2000; Di Beneditto and Ramos, 2001; Secchi et al., 2003b). This indicates that franciscana has one of the shortest life spans among all cetaceans. Mean asymptotic length for franciscanas from Rio de Janeiro State, Brazil (FMA I), is 117.1 cm for males and 144.7 cm for females (Ramos et al., 2000). For specimens from São Paulo and Paraná states, Brazil (FMA II), mean asymptotic length ranges from 113.3 to 117.3 cm for males and 128.9 to 136.2 cm for females (Barreto and Rosas, 2006; Conversani et al., 2020). In FMA III, individuals from Rio Grande do Sul State, Brazil, present mean asymptotic length ranging from 129.8 to 130.6 cm for males and 146.3 to 152.6 cm for females (Barreto and Rosas, 2006; Botta et al., 2010). For specimens from Uruguay, the asymptotic length is 133.3 cm for males and 153.0 cm for females (Kasuya and Brownell, 1979). In Argentina (FMA IV) the mean asymptotic length is 135.8 cm for males and 150.5 cm for females (Botta et al., 2010). It is worthwhile to note that franciscana variation in size is not clinal as one would expect (Table 1). Franciscanas are larger in their southern range (Rio Grande do Sul State, Brazil; Uruguay and Argentina – FMAs III and IV) followed by animals from the northern range (Rio de Janeiro and Espírito Santo States, Brazil – FMA I) and are smaller in the middle of its range (São Paulo, Paraná and Santa Catarina States, Brazil – FMA II). Body weight also follows the same geographic pattern (e.g., Rosas, 2000; Botta et al., 2006).
Reversed sexual size dimorphism (RSSD) occurs in franciscana (Kasuya and Brownell, 1979; Pinedo, 1995; Higa et al., 2002; Ramos et al., 2002). Females are larger than males in both the total body length as well as weight (Brownell, 1989; Rosas, 2000; Botta et al., 2006). Larger females might represent an evolutionary advantage for small species by allowing gestation and birth of larger, even slightly, calves. Larger calves potentially have a higher likelihood of survival due to several factors including a relatively lower metabolic rate and a lower surface/volume ratio, which reduces heat loss. Predation of larger calves might also be less likely. In fact, most of the odontocetes presenting RSSD are the smaller species in the group (Danilewicz et al., 2004). In general, these species produce relatively larger calves at birth than the other species within the taxonomic group (e.g., Ralls, 1976).
Behavior
The franciscana exhibits a reduced repertoire of surfacing behavior and a small portion of the body is exposed during immersion (Bordino et al., 1999; Cremer and Simões-Lopes, 2005; Cremer et al., 2018b). Group size is small (Figure 1), ranging from one to 13 individuals (Bordino et al., 1999; Cremer and Simões-Lopes, 2005; Cremer and Simões-Lopes, 2008), but solitary individuals are uncommon. Nevertheless, in some occasions many groups may form large aggregations of up to 50 dolphins sharing a relatively small area. Association patterns were investigated through the deployment of satellite-linked tags on franciscanas in Bahia San Blas, Argentina, showing that female–male pairing occurs for extended periods, suggesting that they form reproductive pairs (Wells et al., 2013), considering that these animals were not genetically related (Mendez et al., 2010). This authors also suggest that the groups form functional social units. Considering further the reversed sexed length dimorphism and the absence of secondary sexual characteristics, it is hypothesized that franciscanas have a single-male breeding system (serial monogamy) with guarding strategy to increase mating chances (Danilewicz et al., 2004; Costa-Urrutia et al., 2012; Panebianco et al., 2012; Wells et al., 2013), which is unusual in cetaceans. This is coincident with some evidence that males do not disperse from their social units (Costa-Urrutia et al., 2012). An alternative but not exclusionary hypothesis is that adult males might help provide parental care. This behavior could increase the offspring’s chances of survival. Epimeletic behaviors were recorded in Uruguay and Brazil for franciscana dolphins and were associated to parental care after their neonates being incidentally killed in gillnets (Pilleri, 1971; Cremer et al., 2006).
Bioacoustics
Franciscanas produce pulsed and tonal sounds. Pulsed sounds, as the clicks, are produced in a narrow band high frequency (NBHF) (Melcón et al., 2012), similar to dolphins of the genus Cephalorhynchus, which could be related to either predation avoidance behavior (Morisaka and Connor, 2007) or a consequence of the small size of the animals, hence their sound-producing structures. Tellechea et al. (2017) recorded high and low-frequency click trains in the La Plata River estuary, in Uruguay. Click trains with mean frequency of 130 kHz and bandwith of 20 kHz were recorded for an adult franciscana held in captivity (Von Fersen et al., 1997). Wild neonate produces clicks in frequencies ranging between 37 and 160 kHz (Melcón et al., 2016). Whistles were recently recorded for the species. Their frequencies varied between 0.7 and 23.4 kHz for free ranging individuals and between 8.6 and 94.6 kHz for individuals handled during capture/tagging/release procedures. These sounds were more complex, indicating that they were related to a stressful situation and/or to parental care (Cremer et al., 2017). Tellechea et al. (2017) suggest that franciscana echolocates only sparingly because it takes advantage of passive listening to find the sound-producing preys. The echolocation system has an early but continuing postnatal maturation (Frainer et al., 2015). Neonates are acoustically active at 1-week-old and can produce broad band clicks, including burst pulses, that could be important in the mother-calf communication (Tellechea and Norbis, 2014).
Franciscana Threats
Incidental Mortality in Fishing Nets
Mortality due to bycatch in gillnets is the greatest threat to the franciscana. There is no indication of direct exploitation of the species. Reports of incidental mortality in the shark gillnet fisheries of Punta del Diablo, Uruguay, date back to the early 1940s (Van Erp, 1969). Although gillnetting in southern Brazil began around this time (Haimovici, 1997), gillnet sets for bottom-dwelling fish were only recognized as a major threat to the franciscana in the 1980s (Pinedo, 1994). Bycatch has since been reported from the main fishing communities along most of the species’ range (e.g., Corcuera, 1994; Moreno et al., 1997; Praderi, 1997; Secchi et al., 1997, 2003b; Di Beneditto et al., 1998; Bertozzi and Zerbini, 2002; Rosas et al., 2002; Franco-Trecu et al., 2009; Frizzera et al., 2012; Marcondes et al., 2018). Estimates of incidental capture totaled at least 2,900 animals per year in all management units combined in the early 2000s (e.g., Ott et al., 2002; Secchi et al., 2003b), but these figures are probably underestimated to an unknown extent, primarily due to: (1) the occurrence of catches in other non-monitored categories of fisheries (e.g., active gillnetting, Secchi et al., 1997; trawling, Szephegyi et al., 2015); (2) lack of data on the number of fishers and fishing effort carried out with gillnets (3) limited number of communities where landings are monitored; (4) under-reporting of incidental catches by fishers; and (5) dolphins captured may drop off the net during retrieval (Secchi et al., 2003b). For example, in southern Brazil alone, annual fishing-related mortality can exceed this value (e.g., Prado et al., 2013). Bycatch for each of the FMAs has been estimated from different approaches. A beach monitoring project in FMA Ia recorded an average of 5.9 carcasses/year in the period of 2011/2017, ranging from one to 14 franciscana’ carcasses recorded each year (Marcondes et al., 2018). In just 1 month, in 2006, four franciscanas were incidentally caught in the artisanal fishery from Doce River community, Espírito Santo State, Brazil (Frizzera et al., 2012). For the FMA Ia and Ib together bycatch estimated can be >110/year, based on interviews with fishers (Di Beneditto, 2003; Freitas-Netto and Barbosa, 2003). In FMA II, most complete estimates of mortality also came from near-daily beach carried out from 2016 to 2018. These surveys recorded 1,628 carcasses that represent a mean of 543 individuals/year. Despite the fact that many animals were in advanced state of decomposition, data indicated that most of them died because of incidental capture in fishing nets (Cremer et al., 2018a). Since most carcasses do not wash ashore (see Prado et al., 2013), these mortalities estimated from beach surveys are probably highly underestimated. Bycatch estimates in FMA III and IV have been obtained through monitoring of the fishing fleet. Annual bycatch is higher in FMA III, with estimates of 1,300 animals/year (Ott et al., 2002; Secchi et al., 2003b, 2004) and much higher in some years (Prado et al., 2013, 2016). In FMA IV the annual bycatch was estimated in 350–800 individuals/year (Bordino and Albareda, 2004; Cappozzo et al., 2007; Negri et al., 2012). These annual mortality estimates suggest that bycatch levels are unsustainable (Secchi, 2006; Danilewicz et al., 2009, 2012; Crespo et al., 2010; Zerbini et al., 2011; Prado et al., 2013, 2016) and are fragmenting functional social units, as suggested by some authors (Mendez et al., 2010; Costa-Urrutia et al., 2012). Although franciscana is legally protected in Brazil, Uruguay and Argentina (e.g., Arias et al., 2002; Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio), 2011; Soutullo et al., 2013), regulations addressing the management of gillnet fisheries that propose restrictions with potential to minimize bycatch of franciscana are limited to Brazil (Brasil, 2012). Their effectiveness, however, seems limited either because of lack of enforcement and compliance or due to their limited spatial scope (see section “Conservation Perspectives” below).
Habitat Degradation
Ingestion of Debris
Ingestion of plastic debris by cetaceans for long time has been a global concern (e.g., Laist, 1997). Analyses of stomach contents have shown that franciscana is also susceptible to the ingestion of many sorts of debris including discarded fishing gear (Bassoi, 1997; Bastida et al., 2000; Danilewicz et al., 2002; Denuncio et al., 2011). For instance, in Rio Grande do Sul State, southern Brazil, stomach contents of franciscanas (n = 36) contained pieces of nylon net (17% of the stomachs), cellophane, and plastic fragments (6%) (Bassoi, 1997). The rate of debris ingestion by franciscanas varies spatially with higher values observed in northern Argentina, where cellophane, fishing debris, and plastic were found in 45, 32, and 16% of the stomachs, respectively (Bastida et al., 2000). According to these authors, fishing-related debris were more often found in stomachs of specimens collected along the shore of the La Plata River estuary while, in contrast, cellophane debris were more abundant (100% greater) in animals stranded on the marine coast. In the northern range (FMA I), plastic debris were found in nearly 15% of the stomach contents of franciscana (Di Beneditto and Awabdi, 2013). Regardless of the fact the ingestion may occur directly or indirectly through the prey, the effects of such debris ingestion on the health status of individual franciscanas have not been determined, and the population-level implications are uncertain, but should not be ignored.
Chemical Pollution
There is no evidence that coastal oil spills have affected franciscanas. On the other hand, trace elements, organobrominated compounds and andorganochlorines compounds have been documented in the tissues of this species along its distribution range. O’Shea et al. (1980) were the first to analyze the concentration of organochlorine in franciscanas incidentally killed in fisheries off Uruguay. Much later, the presence of these pollutants in tissues of franciscanas were investigated in Argentina (e.g., Borrell et al., 1995, 1997) and Brazil (Castello et al., 2000). Comparatively, the concentrations of DDTs in Brazil and Argentina were lower than in Uruguay. A wide range of organochlorine residues has been discovered in the blubber of franciscanas from the Brazilian coastal waters (Kajiwara et al., 2004; Lailson-Brito et al., 2011). In contrast to the lower residue levels of CHLs, HCB, HCHs, and heptachlor epoxide, the concentrations of DDT’s and PCB’s are surprisingly, the highest compared to those of north Atlantic dolphins, possibly reflecting high levels of industrialization or poor ecological enforcement in Brazil (Kajiwara et al., 2004).
With regard to trace elements, Seixas et al. (2008) found that the concentrations of selenium (Se), total mercury (Hg), and organic mercury (OrgHg) were higher in the livers and kidneys of franciscanas from Rio Grande do Sul State than Rio de Janeiro State, Brazil. For both areas, the values were of the same order of magnitude as those reported in earlier studies with the same species from Brazil (Lailson-Brito et al., 2002; Kunito et al., 2004; Seixas et al., 2007) and Argentina (Marcovecchio et al., 1994; Gerpe et al., 2002; Denuncio et al., 2017; Romero et al., 2017). Franciscana livers showed higher concentrations of mercury, zinc, and copper relative to concentrations in other organs, whereas their highest cadmium concentrations were mostly found in kidneys (Marcovecchio et al., 1990; Gerpe et al., 2002; Lailson-Brito et al., 2002; Kajiwara et al., 2004; Seixas et al., 2008). The concentrations of these trace elements seem to be positively correlated with age (e.g., Seixas et al., 2008). For example, mercury is an exogenous and harmful metal (no benefit at any concentration), which accumulates in the tissues of higher food web organisms (such as marine mammals) as they grow (Caurant et al., 1994; Feroci et al., 2005; Polizzi et al., 2014; Baptista et al., 2016; Kehrig et al., 2016; Romero et al., 2016). Conversely, selenium is acknowledged as an essential element for metabolic activity of aquatic mammals, performing as a protective agent against the toxicity of exogenous metals such as mercury and silver (Feroci et al., 2005; Baptista et al., 2016). Recently, Ti concentrations and metalloprotein detoxification in livers of franciscanas dolphins from southeastern Brazil were assessed (Monteiro et al., 2020).
In general, the observed trace elements concentrations are considered low by the authors when compared to those found in delphinids. However, it is important to highlight that the franciscana is smaller than the majority of delphinids, weighting nearly half the weight of an adult Guiana dolphin (Sotalia guianensis), another coastal species. Therefore, the contamination does not become less relevant because of the observed concentrations, since the doses may be very similar to those of delphinids. It is possible that the adverse effects also occur in the franciscana. Likewise, the synergic effects of the pollutants reported for the species must be taken in consideration. Other emergent contaminants, such as pyrethroid insecticides and sunscreen agents, also represent a potential threat that should be evaluated (e.g., Alonso et al., 2015).
A wide set of contaminants was already reported in franciscana’s tissues’ along its distribution. In particular, the concentrations found in populations under the influence of La Plata river, Argentina and in southeastern Brazil. Due to its coastal habits, franciscana will always be highly exposed to elevated concentrations of contaminants. In fact, recent data shows that even those contaminants banned for decades can still be found in its tissues. This could be related to the remobilization of contaminated sediments (i.e., dragging), inappropriate storage of old stocks of these pollutants, or even their cycle in the biotic compartment. In this context, it is important to highlight the PCBs concentrations in franciscanas from the southeastern region in Brazil, which is an important immunosuppressor. Additionally, the input and incorporation of emerging contaminants of unknown effects to mammals is also of concern. Hence, franciscanas are exposed to a cocktail of substances that are, mostly, immunosuppressors and/or present deleterious effects over the reproductive process. Then, it is possible for those contaminants to be exerting pressure over this species, altering reproductive cycles and increasing its susceptibility to pathogens.
The most concerning impact by chemical pollution on franciscanas is much likely being experienced by the small population (<700) the inhabits the FMA Ia. This isolated population of franciscanas, was impacted and is under direct and indirect effects of the iron ore tailing due to Fundão dam collapse, in Minas Gerais state, Brazil, that reached the outfall of Doce river. This has been considered the most catastrophic environmental disaster in Brazil (Garcia et al., 2017) and it produced an unprecedented pollution load and habitat modification in coastal waters off FMA Ia. In this sense, long-term monitoring is required to assess the status of this population.
Coastal Development
Harbors represent a big threat for coastal habitats as they bring together many impacts related to dredging, noise pollution and chemical pollution that can affect directly and indirectly the franciscanas (Pinheiro et al., 2019; Marcondes et al., 2020). Franciscanas do not approach to harbors, and in Babitonga Bay, Brazil, they represent a loss of habitat (Cremer et al., 2018b). The development of harbor activities is growing fast on the south and southeast coast of Brazil, at least from Santa Catarina State to Espírito Santo State, Brazil (considering the area of franciscana distribution). In Espírito Santo state, where is located the smallest franciscana population (FMA Ia), 14 new harbors were proposed, which represent one harbor complex for every 20 km of coastline (Pinheiro et al., 2019). Along with that, this same area was recently contaminated with around 62 million tons of Fe-enriched tailings through the Doce River, in an episode that became known as the “Mariana disaster,” which is likely to have significant impact on franciscana and its ecosystem (e.g., Frainer et al., 2016; Hatje et al., 2017).
Depletion of Fish Stocks
Historical catch records of commercial fishes have demonstrated a decline in yearly landings of the sciaenids Micropogonias furnieri and Macrodon ancylodon in southern Brazil (Haimovici, 1997; Haimovici, 1998). This is consistent with a reduction in the occurrence of these two species in franciscana’s diet (Bassoi and Secchi, 2000; Secchi et al., 2003b). Micropogonias furnieri has been heavily exploited by gillnet and trawl fisheries for more than three decades (Reis, 1992; Haimovici, 1998) and a drastic decrease in the density of juveniles in coastal waters has been observed (Ruffino and Castello, 1992). During that same period, M. ancylodon and M. furnieri decreased drastically from 41 to 7%, and from 27.5 to 4% in the frequency of occurrence, respectively, in the diet of franciscana (Secchi et al., 2003b). Haimovici (1998) showed that stocks of these sciaenid species have been greatly exploited and are currently at very low levels in the region. On the other hand, frequency of occurrence of cutlassfish Trichiurus lepturus and the sciaenid Umbrina canosai in the diet of the franciscana increased from about 5 and 3% in the late 1970s, to about 39 and 20% in the mid 1990s, respectively. Trichiurus lepturus, together with Cynoscion guatucupa, represents 47% of the total estimated bony fish biomass in this region (Haimovici et al., 1996). Both species have only experienced moderate fishing pressure (Haimovici, 1997, 1998). While C. guatucupa has always been the most important prey for franciscana, T. lepturus has had only a little importance in the franciscana’s diet in the past. However, now it is the second most important prey for the species in this region. These values suggest that changes in the franciscana diet are related to the reduced availability of certain prey species due to their over-exploitation. Although the effects of this major dietary change on the franciscana are unknown, the sharing prey with extensive fisheries may affect the trophic dynamics and have negative energetic implications for the species in some areas (e.g., Machado et al., 2020).
Assessment and Conservation Perspectives
Potential Biological Removal (PBR) for Franciscana
The PBR is defined as the maximum number of animals that may be removed from the population by non-natural causes, while allowing it to reach or maintain its optimum sustainable level (Wade, 1998). The sustainability of the estimated annual bycatch of franciscana was assessed through the PBR approach for each FMA, as follow:
Where Nmin is the 20th percentile of a log-normal distribution based on the estimated abundance of franciscanas (N); Rmax is the maximum net productivity level; and Rf is a recovery factor. Nmin is used instead of N to account for imprecision in the abundance estimates. The Rmax values used in the PBR analysis of up to 4% is considered reasonable for small cetaceans impacted by fisheries (Wade, 1998). The recommended Rf values varied from 0.1 for populations considered endangered by the IUCN (Hilton-Taylor, 2000), or poorly studied, up to 1.0 for populations considered of low risk and well- studied. Since franciscana is currently classified as “Vulnerable” by the IUCN (Zerbini et al., 2017), is declining, with some populations with much higher risk of collapse than others (Secchi and Wang, 2002; Secchi and Fletcher, 2004; Secchi, 2006), and there are uncertainties in mortality estimates, a Rf of 0.1 was used.
Fishing-related mortality ranges from approximately 100, in FMA I, to more than 1,000 in FMA III, and exceed from near two in FMA IV to more than fivefold in FMA III the maximum allowed sustainable mortality rate base on potential biological removal (PBR) approach (Table 2). These numbers reveal that species cannot sustain the current levels of bycatch and that urgent and extreme restrictions on fishing practice and effort are necessary to avoid collapse of franciscana and to reduce its risk of extinction.
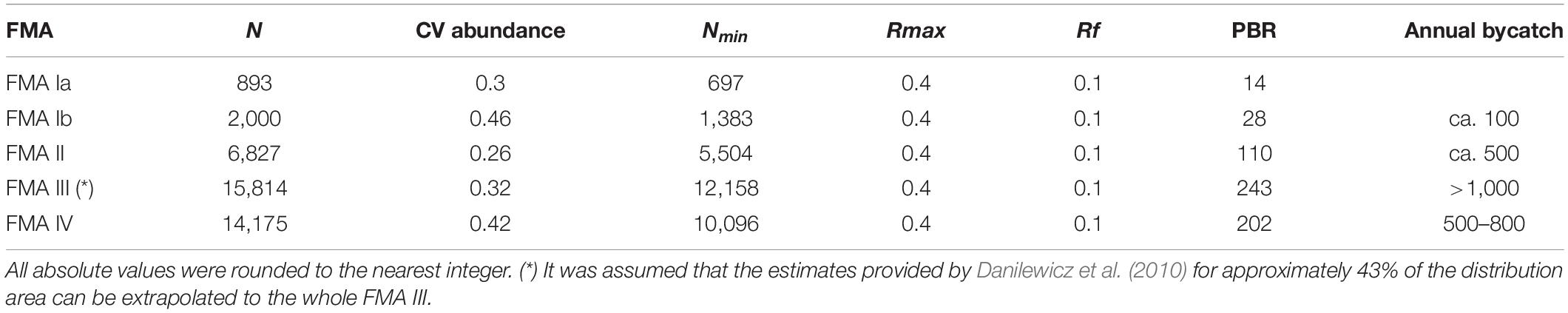
Table 2. Franciscana abundance estimate (N) and coefficient of variation (CV), minimum abundance (Nmin), maximum net reproductive rate (Rmax), recovery factor (Rf), approximate annual bycatch and maximum sustainable bycatch valued based on the potential biological removal approach (PBR, sensu Wade, 1998), for each Franciscana Management Area (FMA – sensu Secchi et al., 2003a).
Although the PBR approach has a clear advantage that management actions linked to direct human-related kills no longer rely on detecting negative population trend, but on simply detecting a mortality level that would cause depletion (Wade, 1998), it does not capture population-specific differences in life history traits, described here, that shape population dynamics and its potential to respond to impact (e.g., Secchi and Fletcher, 2004; Secchi, 2006; Cáceres et al., 2020). Viability analyses using population-specific estimates of abundance, fisheries-related incidental mortality and vital rates suggested that levels of bycatch were not sustainable and that projected probability of decline was high for all FMAs in a time frame of 25 years (Secchi and Fletcher, 2004; Secchi, 2006), which is coherent with the PBR values of this present study. Projected declines resulted in the listing of franciscana as “Vulnerable” in the IUCN Red List (Zerbini et al., 2017).
Conservation Perspectives
Even though franciscana is legally protected in Brazil, Uruguay and Argentina (Arias et al., 2002; Weiskel et al., 2002; Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio), 2011; Soutullo et al., 2013), threats to the species are far from being mitigated. Although other potential sources of non-natural mortality, as described in this present study, also affect the franciscanas in a difficult way to scale, the greatest threat to the species is incidental capture in gillnets (Secchi, 2010). Some efforts have been made to evaluate the effectiveness of methods for reducing bycatch. Pingers were tested in Argentina in a small-scale fishery and a highly significant reduction in bycatch was demonstrated (Bordino et al., 2002), but a dinner bell effect was observed with the attraction of southern sea lions (Otaria flavescens), which depredated the catch hence, discouraging the continuity of that study. Later, with the advancement of new pingers, experiments carried out in Argentina with Dolphin Pinger 70 kHz of Future Oceans (Bordino, 2018), and in Brazil with Banana Pinger of Fishtek Marine (Paitach et al., 2018), demonstrated the potential of “seal safe” devices for franciscanas deterrence. Despite the experiments showed a significant displacement of the franciscanas from the vicinity of the ensonified area (Bordino, 2018; Paitach et al., 2018), both studies were focused on the behavior response to a single pinger, and questions remain regarding the long-term effectiveness of the pingers in a real fishing situation, arranged in an array of many pingers along a gillnet. Understanding the behavior of franciscanas in the vicinity of the nets, with and without pingers, is crucial for designing technological alternatives for bycatch mitigation. Acoustically reflective gillnets, in turn, were not efficient in reducing bycatch (Bordino et al., 2013). Berninsone et al. (2020) investigated the potential of switching gillnets to bottom longlines to reduce franciscana bycatch rates in a small-scale artisanal fishery in Argentina. They concluded that the use of bottom longlines result in significant mitigation of current bycatch of franciscana dolphins, but it requires the acceptance and compliance of the fishers before implementation (e.g., Brownell et al., 2019).
In Brazil, where most of the franciscana bycatch occurs, gillnet fishery had been carried out unregulated for several decades. The number of boats, the dimension of the nets and the duration of the fishing trips increased with no rules. Despite the frequent and significant mortality of several endangered species, such as cetaceans, sea turtles, birds and sharks, as well as the decline of some target species, no enforcement could take place. Lack of regulation markedly increased fishing effort and the catch of the already depleted stocks. One of the most affected species is the franciscana. In 2012, a joint Interministerial Regulation of the Ministry of Environment and Ministry of Fisheries and Aquiculture (hereafter referred to as INI12/2012) was published aiming at regulating gillnet fisheries in southern and southeastern Brazil, between the States of Rio Grande do Sul to Espírito Santo, distribution limits of franciscana in the country (Brasil, 2012).
Although less conservative in several aspects than the recommendations in the Brazilian Action Plan for the Conservation of the Franciscana (Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio), 2011), several rules of this INI 12/2012 establish limits on the gillnet fishing effort (Brasil, 2012). Maximum allowed gillnet length was reduced and varied according to boat size. If the boat length is modified, the net length will follow the smaller previous category. Gillnet fishing permits were halted. New boats are not allowed to have gillnet fishing permits and boats operating with a different gear cannot change to gillnetting. No gillnet fishing is allowed between 15 May and 15 June, except for the artisanal fishery. A mosaic no-fishing areas for gillnets with different levels of restriction were established along the entire region regulated by INI12/2012 (Figure 3): (i) no powered boat can fish within the first nautical mile (ii) only rowing boats can operate within the first nautical mile but with nets no longer than 1000 m; (iii) no powered boat can operate within the 5 nm between the southern border of Brazil (with Uruguay) to Albardão lighthouse (located approximately 120 km further to the north); (iv) to the north of Albardão lighthouse, no gillnetting is allowed for commercial gillnetting within 4 nm up to the northern limit of Rio Grande do Sul state and within 3 nm from this point to the remaining area concerned in this Regulation; (v) no gillnetting is allowed within the 15 nm off the Jurubatiba National Park, in Rio de Janeiro state. All boats longer than 15 m are obligated to carry and turn on Virtual Monitoring System (VMS) when at sea so their fishing location can be monitored. Fishing license will be lost in case of infraction to the Regulation. These rules were set to be implemented in areas with extensive bycatch in Brazil and were expected to improve the species’ conservation status. However, they were never effectively implemented, either due to the lack of awareness in small fishing communities, or the lack of enforcement. Furthermore, some rules proposed in the INI 12/2012, such as the functioning of steering committees and the implementation of on-board observer programs, under the responsibility of the Federal Government, have yet to be established. While lack of enforcement persists, no assessment regarding the effectiveness of the regulation can be made. Evidence, however, indicates that the level of franciscana’s fishing-related mortality in Brazil remains high, in the order of several hundreds to a few thousands, which is not different from the numbers observed in years that preceded the regulations (e.g., Prado et al., 2013, 2016; Cremer et al., 2018a). Moreover, a recent case study showed that even with full compliance, the current fishing regulations cannot reduce franciscana bycatch to sustainable levels in southern Brazil (Prado et al., 2021). Therefore, these regulations are proved insufficient to reduce fishing-related mortality to sustainable levels due to either or both lack of enforcement and, hence, compliance, and insufficient reduction in fishing effort.
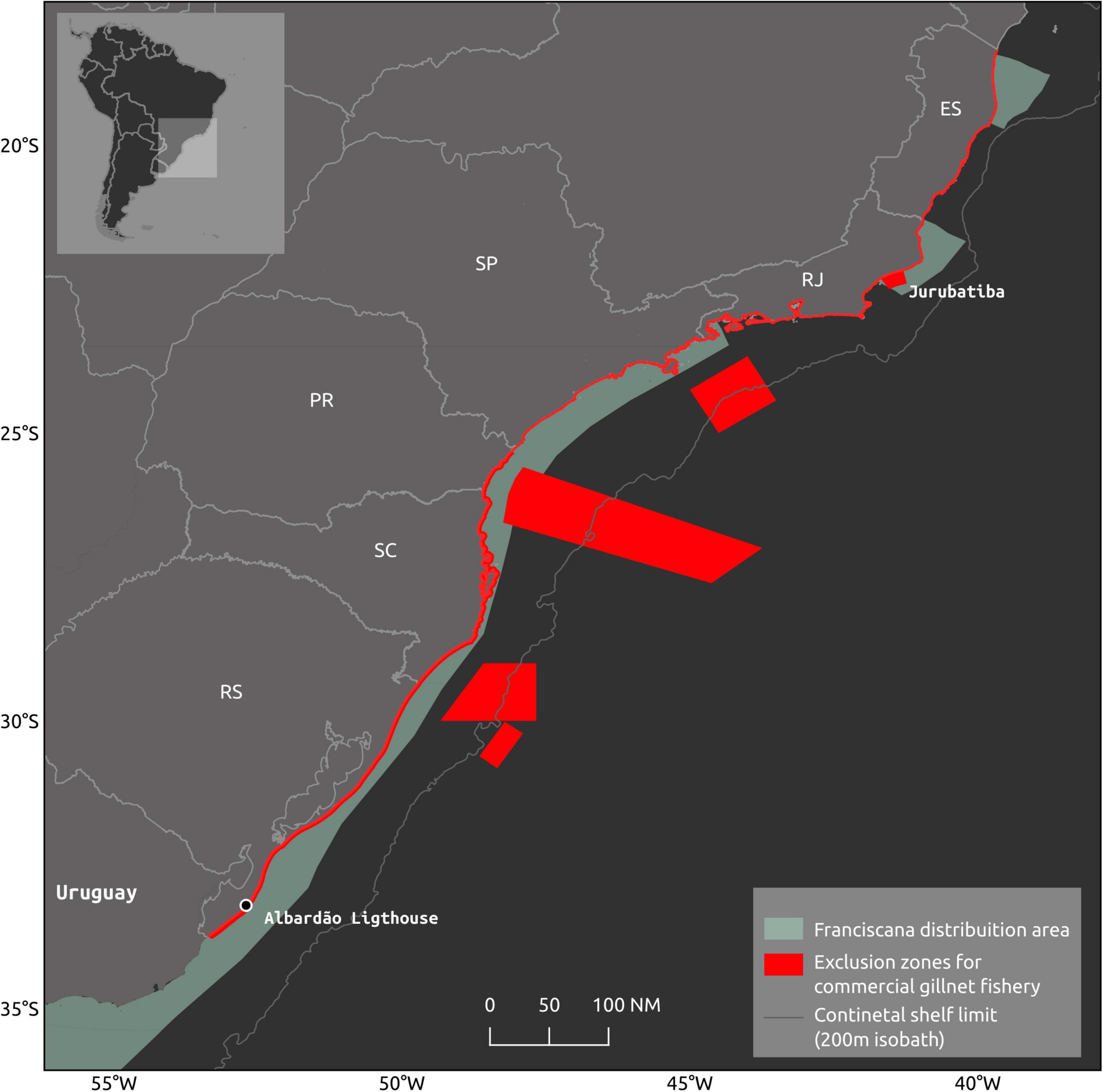
Figure 3. Exclusion areas for commercial gillnet fishery in southern and southeastern States of Brazil: Rio Grande do Sul – RS, Santa Catarina – RS, Paraná – PR, São Paulo – SP, Rio de Janeiro – RJ, and Espírito Santo – ES.
Recently, franciscana and other six threatened small cetaceans, according to the IUCN Red List of Threatened Species, were assessed by worldwide experts to determine the extent to which ex situ management could, together with strengthen in situ conservation efforts, increase likelihood of success (Taylor et al., 2020). It was stressed that the species is relatively abundant in much of its range and there is still the potential for successful in situ conservation and long-term viability of franciscanas in the wild, hence that it is currently not a high-priority candidate for ex situ management (Secchi et al., 2020). If ex situ management is considered in the future, improved knowledge is necessary about the requirements for successful handling and maintenance of animals under human care and preparation for their subsequent release (Secchi et al., 2020). It was further recommended that a capture/tag/release program for franciscanas in Brazil and Argentina should be expanded and include biological data gathering in support of rehabilitation/release of live by-caught and stranded franciscanas and to inform the possible future development of an action plan using the One Plan approach (sensu Byers et al., 2013; Taylor et al., 2020).
Meanwhile, an immediate and significant bycatch reduction, as a result of a drastic decrease of the total fishing effort, and habitat restoration is, perhaps, the only short-term options for reducing the risk of franciscana’s collapse (e.g., Prado et al., 2021). These authors identified two bycatch hotspots areas in Rio Grande do Sul state coast (part of FMA III), south of Brazil, and recommended that they should be designated as no-fishing zones for gillnets. One of the recommended gillnet no-take area is off the Albardão region, a strong candidate to become a marine protected area as it represents an important feeding and breeding ground for endangered loggerhead sea turtle and elasmobranchs, respectively (Vooren and Klippel, 2005; Monteiro et al., 2016). Prado et al. (2021) also recommended that total fishing effort outside these areas should drop considerably. These sort of management options are extremely unpopular among fishers (e.g., Murray et al., 2000; Brownell et al., 2019), especially if the closure areas encompass significant proportions of their fishing grounds, which is the case in the study of Prado et al. (2021). In fact, any action with higher likelihood of effectiveness will, necessarily, result in lowering absolute fishing effort or changing location of main fishing grounds, which, in turn, may have negative socio-economic impact. Although the implementation of the exclusion zones for gillnetting and limiting fishing effort as recommended by Prado et al. (2021) will strongly distress the status quo, they followed international guidelines (e.g., International Whaling Commission (IWC), 2016; Food and Agriculture Organization (FAO), 2021) and were founded on robust scientific evidence and on a clearly defined management goal to reduce risk of collapse of franciscana’s population in southern Brazil. It is worthwhile noting, however, that reducing fishing effort will be beneficial for franciscana, and also for the economic viability of the fishery, especially if fisheries agency is focused on long-term outcomes by aligning economic and conservation objectives. Therefore, key stakeholders need to work together and to build participative multi-fisheries management and other ecosystem-based conservation strategies in order to increase the chances of franciscana long-term survival while maintaining sustainable fishing activities that are relevant to some communities that are socioeconomically vulnerable.
In that sense, extensive overfishing (e.g., Haimovici and Cardoso, 2017; Cardoso et al., 2018; Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio), 2018) and prolonged ineptitude of federal fisheries agency toward a proper management impelled local fishers in Rio Grande do Sul State to lead a movement that ended in the sanction of the Sustainable Fisheries State Policy Act in 2018 (RS State Law 15,223/2018). This Law banned all bottom trawling up to 12 miles from the State’s ca. 570 km of marine coast. Scientific evidence indicated that the ban would have ecosystem benefits, by reducing impact on endangered species and facilitating the recovery of fish stocks, which in turn may promote continued revenues for small-scale and industrial fisheries (Cardoso et al., 2018, 2021). According to these authors, the establishment of the trawl exclusion zones would promote habitat restoration and a rapid increase in the biomass of important sciaenid species for gillnet fisheries including white croaker, striped weakfish, king fish (Macrodon atricauda) and Argentine croaker (Umbrina canosai). Although trawl fisheries do not represent a direct threat to franciscana, they are responsible for a high bycatch of juvenile sciaenids that are both important prey of franciscana and potential recruits for the gillnet fishery. If biomass of sciaenid fishes increases, it is expected that gillnet fishery can have higher yield with lower fishing effort.
The trawl fishery ban and the regulation of gillnetting represent important advances regarding fisheries management in Brazil. If fully complied and improved as recommended by Prado et al. (2021), in the case of gillnet fishery, these regulations can enhance the conservation status of franciscana and several other endangered coastal species as well as promote the recovery of some fish stocks and their habitats. Franciscana mortality data in recent decades allow us to infer that the species probably was once the most abundant small cetacean in coastal waters off the western South Atlantic, and certainly played, and might still play, an important role in the trophic web of this ecosystem. The evidence presented the present study shows that this situation is certainly the result of increasing human pressure on the coastal zone, with the depletion of its natural resources and very high and unsustainable fishing effort, that leads to an unsustainable fishing-related mortality. Franciscana is the most endangered small cetacean in the entire western South Atlantic and the only living member of the family Pontoporiidae. Losing the franciscana represents the loss of a unique evolutionary lineage that survived to several changes on the planet, but that could become extinct in a few decades.
Author Contributions
ES conceived this study. All authors wrote the first draft, revised, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling Editor declared a past co-authorship with one of the authors ES.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Funding
We are indebted to the Organization for the Conservation of South American Aquatic Mammals – YAQU PACHA e.V, Nuremberg Zoo for continuously funding the franciscana research. This research was partially supported by an environmental offset measure established through a Consent Decree/Conduct Adjustment Agreement between Petrorio and the Brazilian Ministry for the Environment, with the Brazilian Biodiversity Fund – FUNBIO as implementer. Financial support was also provided by the National Council for Research and Development (CNPq) and Coordination for the Improvement of Higher Education Personnel (CAPES). CNPq provided a research fellowship to ES (PQ 310597/2018-8), MC (PQ 10477/2017-4), and JL-B (PQ 311481/2017-5). Funding was also provided by CNPq to ES through Edital Universal (Grant # 426503/2018-0). UERJ/FAPERJ provides a research fellowship (Prociência) to JL-B. MC receives research support from Petrobras and the Fundo de Apoio à Pesquisa – FAP/UNIVILLE. CAPES provided free access to many relevant journals through the portal “Periódicos CAPES.”
Acknowledgments
We thank our research teams ECOMEGA at FURG, Projeto Toninhas at Univille, GEMARS and MAQUA at UERJ. R. Claudino prepared the map of the gillnet fishing exclusion zones. The first draft of the manuscript was presented and used to guide management recommendation made in the scope of the Ex Situ Option for Cetacean Conservation workshop focused on Franciscana which was held in San Clemente, Argentina. This is a research contribution of contribution of the Research Group Ecologia e Conservação da Megafauna Marinha – ECOMEGA-FURG/CNPq.
References
Alonso, M. B., Feo, M. L., Corcellas, C., Gago-Ferrero, P., Bertozzi, C. P., Marigo, J., et al. (2015). Toxic heritage: maternal transfer of pyrethroid insecticides and sunscreen agents in dolphins from Brazil. Env. Poll. 207, 391–402. doi: 10.1016/j.envpol.2015.09.039
Amaral, K. B., Danilewicz, D., Zerbini, A., Di Beneditto, A. P., Andriolo, A., Alvares, D. J., et al. (2018). Reassessment of the franciscana Pontoporia blainvillei (Gervais & d’Orbigny, 1844) distribution and niche characteristics in Brazil. J. Exp. Mar. Biol. Ecol. 508, 1–12. doi: 10.1016/j.jembe.2018.07.010
Anonymous. (2015). Report of the Vii Workshop for the Conservation of the Franciscana (Pontoporia Blainvillei). São Francisco do Sul Santa Catarina, Brazil 6–8 October 2015. Anonymous.
Arias, A., da Rocha, J., Weiskel, H. W., Fidelix, L., De Haro, C., Cremer, M., et al. (2002). Report of the working group on legislation and education. Lat. Am. J. Aq. Mamm. 1, 67–70.
Baptista, G., Kehrig, H. A., Di Beneditto, A. P. M., Hauser-Davis, R. A., Almeida, M. G., Rezende, C. E., et al. (2016). Mercury, selenium and stable isotopes in four small cetaceans from the Southeastern Brazilian coast: influence of feeding strategy. Env. Poll. 218, 1298–1307. doi: 10.1016/j.envpol.2016.08.088
Barbato, B. H. A., Secchi, E. R., Di Beneditto, A. P. M., Ramos, R. M. A., Bertozzi, C., Marigo, J., et al. (2012). Geographical variation in franciscana (Pontoporia blainvillei) external morphology. J. Mar. Biol. Assoc. U.K. 92, 1645–1656. doi: 10.1017/S0025315411000725
Barreto, A. S., and Rosas, F. C. W. (2006). Comparative growth analysis of two populations of Pontoporia blainvillei on the Brazilian coast. Mar. Mamm. Sci. 22, 644–653. doi: 10.1111/j.1748-7692.2006.00040.x
Bassoi, M. (1997). Avaliação da Dieta Alimentar de Toninha, Pontoporia blainvillei (Gervais and D’Orbigny, 1844), Capturadas Acidentalmente na Pesca Costeira de Emalhe no Sul do Rio Grande do Sul. Bachelor Dissertation. Rio Grande: Fundação Universidades do Rio Grande, 68.
Bassoi, M., and Secchi, E. R. (2000). Temporal variation in the diet of franciscana Pontoporia blainvillei (Cetacea, Pontoporiidae) as a consequence of fish stocks depletion off southern Brazil. IV Encontro para a Coordenação da Pesquisa e Conservação da Franciscana, Pontoporia blainvillei, no Atlântico Sul Ocidental. Working paper No. 9. Porto Alegre.
Bassoi, M., Sheperd, J. G., Secchi, E. R., Moreno, I. B., and Danilewicz, D. (2020). Oceanographic processes driving the feeding ecology of franciscana dolphin off Southern Brazilian coast. Cont. Shelf Res. 201:104124. doi: 10.1016/j.csr.2020.104124
Bastida, R., Rivero, L., and Rodríguez, D. (2000). Presencia inusual de elementos de origen antrópico en los contenidos estomacales de la franciscana (Pontoporia blainvillei). IV Encontro para a Coordenação da Pesquisa e Conservação da Franciscana, Pontoporia blainvillei, no Atlântico Sul Ocidental. Working paper No. 26. Sorrento.
Berninsone, L. G., Bordino, P., Gnecco, M., Foutel, M., Machay, A. I., and Werner, T. B. (2020). Alternative to mitigate the bycatch of franciscana dolphin (Pontoporia blainvillei) in Argentina. Front. Mar. Sci. 7:699.
Bertozzi, C. P., and Zerbini, A. N. (2002). Incidental mortality of franciscana (Pontoporia blainvillei) in the artisanal fishery of Praia Grande, São Paulo State, BrazilLat. Am. J. Aq. Mamm. 1, 153–160.
Bittar, V. T., and Di Beneditto, A. P. M. (2009). Diet and potential feeding overlap between Trichiurus lepturus (Osteichthyes: perciformes) and Pontoporia blainvillei (Mammalia: cetacea) in northern Rio de Janeiro, Brazil. Zoologia 26, 374–378.
Bordino, P. (2002). Movement patterns of franciscana dolphins (Pontoporia blainvillei) in Bahia Anegada, Buenos Aires, Argentina. Lat. Am. J. Aq. Mamm. 1, 71–76.
Bordino, P. (2018). Understanding the Sonar Behavior of Franciscana Dolphins in Response to Active Pingers. Final Report. Available online at: https://www.bycatch.org/sites/default/files/r_Franciscana%20CPODS_FINAL_140918.pdf (accessed December 15, 2019).
Bordino, P., and Albareda, D. (2004). Incidental mortality of Franciscana dolphin Pontoporia blainvillei in coastal gillnet fisheries in northern Buenos Aires, Argentina. Technical Paper IWC-SC/56/SM11.
Bordino, P., Kraus, S., Albareda, D., Fazio, A., Palmerio, A., Mendez, M., et al. (2002). Reducing incidental mortality of franciscana dolphin Pontoporia blainvillei with acoustic warning devices attached to fishing nets. Mar. Mamm. Sci. 18, 833–842. doi: 10.1111/j.1748-7692.2002.tb01076.x
Bordino, P., Mackay, A. I., Werner, T. B., Northridge, S. P., and Read, A. J. (2013). Franciscana bycatch is not reduced by acoustically reflective or physically stiffened gillnets. End. Sp. Res. 21, 1–12. doi: 10.3354/esr00503
Bordino, P., Thompson, G., and Iniguez, M. (1999). Ecology and behaviour of the Franciscana dolphin Pontoporia blainvilleiin Bahía Anegada, Argentina. J. Cet. Res. Manage. 1, 213–222.
Bordino, P., and Wells, R. S. (2005). “Radiotracking of franciscana dolphins (Pontoporia blainvillei) in bahia samborombon, buenos aires, Argentina,” in Proceeding of the 16th Biennial Conference on the Biology of Marine Mammals, (San Diego, CA).
Borrell, A., Pastor, T., Aguilar, A., Corcuera, J., and Monzón, F. (1995). DDTs and PCBs in La Plata dolphins (Pontoporia blainvillei) from Argentina: age and sex trends. Europ. Res. Cet. 8, 273–276.
Borrell, A., Pastor, T., Aguilar, A., Corcuera, J., and Monzón, F. (1997). Contaminación por DDT y PCBs en Pontoporia blainvillei de águas argentinas: variación com la edad y el sexo. Anais do 2o. Encontro sobre a Coordenação de Pesquisa e Manejamento da Franciscana. Editora FURG. Rio Grande: 62–69.
Botta, S., Muelbert, M. M. C., and Secchi, E. R. (2006). Morphometric relationships of franciscana dolphin, Pontoporia blainvillei (Cetacea), off Rio Grande do Sul coast, southern Brazil. Lat. Am. J. Aq. Mamm. 5, 117–123.
Botta, S., Secchi, E. R., Albuquerque, C., Hohn, A., Da Silva, V. M. F., Santos, M. C. O., et al. (2015). Ba/Ca ratios in teeth reveal habitat use patterns of dolphins. Mar. Ecol. Prog. Series 521, 249–263. doi: 10.3354/meps11158
Botta, S., Secchi, E. R., Muelbert, M. M., Danilewicz, D., Negri, M. F., Cappozzo, H. L., et al. (2010). Age and growth of franciscana dolphins, Pontoporia blainvillei (Cetacea: pontoporiidae) incidentally caught off southern Brazil and northern Argentina. J. Mar. Biol. Ass. U. K. 90, 1493–1500. doi: 10.1017/s0025315410001141
Brasil (2012). Instrução Normativa Interministerial INI 12/2021. 22 de Agosto de 2012. MPA/MMA. Diario Oficial da Uniao, 22 de Agosto de 2014 1. 39–40.
Brownell, R. L. (1984). Review of reproduction in platanistid dolphins. Rep. Int. Whal. Commn. 6, 149–158.
Brownell, R. L. (1989). “Franciscana, Pontoporia blainvillei (Gervais and d’Orbigny 1844),” in Handbook of Marine Mammals, Vol. 4, eds S. H. Ridgway and R. J. Harrison (London: Academic Press), 45–67.
Brownell, R. L., Reeves, R. R., and Read, A. J. (2019). Bycatch in gillnet fisheries threatens critically endangered small cetaceans and many others. End. Sp. Res. 40, 285–296. doi: 10.3354/esr00994
Byers, O., Lees, C., Wilcken, J., and Schwitzer, C. (2013). ‘The One Plan approach: the philosophy and implementation of CBSG’s approach to integrated species conservation planning’. WAZA Magazine 14, 2–5.
Cáceres, M. O., Cáceres-Saez, I., Secchi, E. R., Negri, M. F., Panebianco, M. V., and Cappozzo, H. L. (2020). Assessing the growth rate of endangered Franciscana dolphin in Argentina, South America. Reg. Stud. Mar. Sci. 40:101479. doi: 10.1016/j.rsma.2020.101479
Campos, L. B., Lopes, X. M., Silva, E., and Santos, M. C. O. (2020). Feeding habits of the franciscana dolphin (Pontoporia blainvillei) in southeastern Brazil. J. Mar. Biol. Ass. U.K. 100, 301–313. doi: 10.1017/s0025315420000120
Cappozzo, H. L., Negri, M. F., Pérez, F. H., Albareda, D., Monzón, F., and Corcuera, J. F. (2007). Incidental mortality of franciscana dolphin (Pontoporia blainvillei) in Argentina. Lat. Am. J. Aq. Mamm. 6, 127–137.
Cardoso, L. G., Abdallah, P. R., Haimovici, M., and Dumont, L. F. C. (2018). Protect Vulnerable Habitats and Species from Bottom Trawling. Relatório Final – Produtos 1 e 2 – Termo de Referência N. BRA-0142-2017accessed. Available online at: https://demersais.furg.br/images/producao/Cardoso_et_al_2018_Relatorio_deslocamento_arrasto_de_fundo_12_mn_final.pdf (accessed January 10, 2020).
Cardoso, L. G., Haimovici, M., Abdallah, P. R., Secchi, E. R., and Kinas, P. G. (2021). Prevent bottom trawling in southern Brazil. Science 372:138. doi: 10.1126/science.abh0279
Cassens, I., Vicario, S., Waddell, V. G., Balchowsky, H., Van Belle, D., Ding, W., et al. (2000). Independent adaptation to riverine habitats allowed survival of ancient cetacean lineages. Proc. Natl. Acad. Sci. U.S.A. 97, 11343–11347. doi: 10.1073/pnas.97.21.11343
Castello, H. P., Junin, M., Rotman, F., and Sartí, G. C. (2000). Análisis de Contaminantes Organoclorados y Metales Pesados en Franciscana, Pontoporia Blainvillei, de Argentina y Brasil. Report of the Third Workshop for Coordinated Research and Conservation of the Franciscana Dolphin (Pontoporia blainvillei) in the Southwestern Atlantic. Bonn: UNEP/CMS, 46–50.
Caurant, F., Amiard, J. C., Amiard-Triquet, C., and Sauriau, P. G. (1994). Ecological and biological factors controlling the concentrations of trace elements (As, Cd, Cu, Hg, Se, Zn) in delphinids Globicephala melas from the North Atlantic Ocean. Mar. Ecol. Prog. Series 103, 207–219. doi: 10.3354/meps104207
Conversani, V., Silva, D., Barbosa, R. A., Hohn, A. A., and Santos, M. C. O. (2020). Age and growth of franciscana, Pontoporia blainvillei, and Guiana, Sotalia guianensis, dolphins from southeastern Brazil. Mar. Mam Sci. 2020, 1–15.
Corcuera, J. (1994). Incidental mortality of franciscanas in Argentine waters: the threat of small fishing camps. Rep. Int. Whal. Commn 15, 291–294.
Costa-Urrutia, P., Abud, C., Secchi, E. R., and Lessa, E. P. (2012). Population genetic structure and social kin associations of franciscana dolphin, pontoporia blainvillei. J. Hered. 103, 92–102. doi: 10.1093/jhered/esr103
Cozzuol, M. A. (1985). The Odontoceti of the “Mesopotamiense” of the Parana River ravines. Systematic review. Invest. Cet. 17, 39–51.
Cremer, M. J., Barreto, A. S., Castilho, P. V., Dick, J., Domit, C., Barbosa, C. B., et al. (2018a). Franciscana Dolphin Mortality in Franciscana Management Area II (FMA II), Southern Brazil. SM WP03.
Cremer, M. J., Hardt, F. A. S., and Júnior, A. J. T. (2006). Evidence of epimeletic behavior involving a Pontoporia blainvillei calf (Cetacea, Pontoporiidae). Biotemas 19, 83–86.
Cremer, M. J., Holz, A. C., Bordino, P., Wells, R. S., and Simões-Lopes, P. C. (2017). Social sounds produced by franciscana dolphins, Pontoporia blainvillei (Cetartiodactyla, Pontoporiidae). J. Acoust. Soc. Am. 141, 2047–2054. doi: 10.1121/1.4978437
Cremer, M. J., Holz, A. C., Sartori, C. M., Schulze, B., Paitach, R. L., and Simões-Lopes, P. C. (2018b). “Behavior and ecology of endangered species living together: long-term monitoring of resident sympatric dolphin populations,” in Advances in Marine Vertebrate Research in Latin America, eds M. R. Rossi-Santos and C. W. Finkl (Berlin: Springer). doi: 10.1007/978-3-319-56985-7_17
Cremer, M. J., Pinheiro, P. C., and Simões-Lopes, P. C. (2012). Prey consumed by Guiana dolphin Sotalia guianensis (Cetacea, Delphinidae) and franciscana dolphin Pontoporia blainvillei (Cetacea, Pontoporiidae) in an estuarine environment in southern Brazil. Iheringia. Série Zool. 102, 131–137. doi: 10.1590/s0073-47212012000200003
Cremer, M. J., Sartori, C. M., Holz, A. C., Schulze, B., Santos, N. Z., Alvez, A. K. M., et al. (2013). Franciscana strandings on the north coast of Santa Catarina State and insights into birth period. Biotemas 26, 133–139.
Cremer, M. J., and Simões-Lopes, P. C. (2005). The occurrence of Pontoporia blainvillei (Gervais & d’Orbigny) (Cetacea, Pontoporiidae) in an estuarine area in southern Brazil. Rev. Bras. Zool. 22, 717–723. doi: 10.1590/s0101-81752005000300032
Cremer, M. J., and Simões-Lopes, P. C. (2008). Distribution, abundance and density estimates of franciscanas, Pontoporia blainvillei (Cetacea: Pontoporiidae), in Babitonga bay, southern Brazil. Rev. Bras. Zool. 25, 397–402. doi: 10.1590/s0101-81752008000300003
Crespo, E. A., Harris, G., and González, R. (1998). Group size and distributional range of the franciscana, Pontoporia blainvillei. Mar. Mamm. Sci. 14, 845–849. doi: 10.1111/j.1748-7692.1998.tb00768.x
Crespo, E. A., Pedraza, S. N., Grandi, M. F., Dans, S. L., and Graffo, G. V. (2010). Abundance and distribution of endangered Franciscana dolphins (Pontoporia blainvillei) in Argentine waters and conservation implications. Mar. Mammal Sci. 26, 17–35. doi: 10.1111/j.1748-7692.2009.00313.x
Crespo, E. A., Secchi, E. R., Dalla Rosa, L., Kinas, P. G., Danilewicz, D., and Bordino, P. (2002). Report of the working group of abundance estimates Special Issue on the Biology and Conservation of Franciscana. Lat. Am. J. Aq. Mamm. 1, 65–66.
Cunha, H. A., Medeiros, B. V., Barbosa, L. A., Cremer, M. J., Marigo, J., Lailson-Brito, J., et al. (2014). Population structure of the endangered franciscana dolphin (Pontoporia blainvillei): reassessing management units. PLoS One 9:e85633. doi: 10.1371/journal.pone.0085633
Danilewicz, D., Claver, J. A., Carrera, A. L. P., Secchi, E. R., and Fontoura, N. F. (2004). Reproductive biology of male franciscanas (Pontoporia blainvillei) (Mammalia: cetacea) from Rio Grande do Sul, southern Brazil. Fish. Bull. 102, 581–592.
Danilewicz, D., Moreno, I. B., Ott, P. H., Tavares, M., Azevedo, A. F., Secchi, E. R., et al. (2010). Abundance estimate for a threatened population of franciscana dolphins in southern coastal Brazil: uncertainties and management implications. J. Mar. Biol. Assoc. U.K. 90, 1649–1657. doi: 10.1017/s0025315409991482
Danilewicz, D., Rosas, F., Bastida, R., Marigo, J., Muelbert, M., Rodriguez, D., et al. (2002). Special issue on the biology and conservation of franciscana. Lat. Am. J. Aq. Mamm. 1, 25–42. doi: 10.31931/fmbc.v22i2.2019.25-25
Danilewicz, D., Zerbini, A. N., Andriolo, A., Secchi, E. R., Sucunza, F., Ferreira, E., et al. (2012). Abundance and Distribution of an Isolated Population of Franciscana Dolphins (Pontoporia blainvillei) in Southeastern Brazil: red alert for FMA I? Paper SC/64/SM17 presented to the IWC Scientific Committee. Panama City.
Danilewicz, D. S. (2003). Reproduction of female franciscana (Pontoporia blainvillei) in Rio Grande do Sul, southern Brazil. Lat. Am. J. Aq. Mamm. 2, 67–78.
Danilewicz, D. S., Secchi, E. R., Ott, P. H., Moreno, I. B., Bassoi, M., and Martins, M. B. (2009). Habitat use patterns of franciscana dolphin (Pontoporia blainvillei) off southern Brazil in relation to water depth. J. Mar. Biol. Assoc. U.K. 89, 943–949. doi: 10.1017/s002531540900054x
Danilewicz, D. S., Secchi, E. R., Ott, P. H., and Moreno, I. M. (2000). Analysis of the age at sexual maturity and reproductive rates of franciscana (Pontoporia blainvillei) from Rio Grande do Sul, southern Brazil. Com. Mus. Ciên. Tecnol. PUCRS 13, 89–98.
Denuncio, P., Viola, M., Machovsky-Capuska, G. E., Raubenheimer, D., Blasina, G., Machado, R., et al. (2017). Population variance in prey, diets and their macronutrient composition in an endangered marine predator, the Franciscana dolphin. J. Sea Res. 129, 70–79. doi: 10.1016/j.seares.2017.05.008
Denuncio, P. E., Bastida, R., Dassis, M., Giardino, G., Gerpe, M., and Rodríguez, D. H. (2011). Plastic ingestion in Franciscana dolphins, Pontoporia blainvillei(Gervais andd’Orbigny, 1844), from Argentina. Mar. Poll. Bull. 62, 1836–1841. doi: 10.1016/j.marpolbul.2011.05.003
Denuncio, P. E., Bastida, R. O., Danilewicz, D., Morón, S., Rodríguez-Heredia, S., and Rodríguez, D. H. (2013). Calf chronology of the franciscana dolphin (Pontoporia blainvillei): birth, onset of feeding, and duration of lactation in coastal waters of Argentina. Aq. Mamm. 39, 73–80. doi: 10.1578/am.39.1.2013.73
Di Beneditto, A. P. M. (2003). Interactions between gillnet fisheries and small cetaceans in northern Rio de Janeiro, Brazil: 2001-2002. Lat. Am. J. Aq. Mamm. 2, 79–86.
Di Beneditto, A. P. M., and Awabdi, D. R. (2013). How marine debris ingestion differs among megafauna species in a tropical coastal area. Mar. Poll. Bull. 88, 86–90. doi: 10.1016/j.marpolbul.2014.09.020
Di Beneditto, A. P. M., and Ramos, R. M. A. (2001). Biology and conservation of the franciscana (Pontoporia blainvillei) in the north of Rio de Janeiro State, Brazil. J. Cetacean Res. Manage. 3, 185–192.
Di Beneditto, A. P. M., Ramos, R. M. A., and Lima, N. R. W. (1998). Fishing activity in northern Rio de Janeiro State (Brazil) and its relation with small cetaceans. Braz. Arch. Biol. Tech. 41, 296–302.
Failla, M., Seijas, V. A., Esposito, R., and Iñíguez, M. A. (2012). Franciscana dolphins, Pontoporia blainvillei, of the Río Negro Estuary, Patagonia, Argentina. Mar. Biodivers Rec. 5:e102.
Feroci, G., Badiello, R., and Fini, A. (2005). Interactions between different selenium compounds and zinc, cadmium and mercury. J. Trace Elem. Med. Biol. 18, 227–234. doi: 10.1016/j.jtemb.2004.09.005
Food and Agriculture Organization (FAO) (2021). Fishing Operations. Guidelines to Prevent and Reduce Bycatch of Marine Mammals in Capture Fisheries. FAO Technical Guidelines for Responsible Fisheries No.1, Suppl. 4. Rome: FAO.
Frainer, G., Huggenberger, S., and Moreno, I. B. (2015). Postnatal development of franciscana’s (Pontoporia blainvillei) biosonar relevant structures with potential implications for function, life history, and bycatch. Mar. Mamm. Sci. 31, 1193–1212. doi: 10.1111/mms.12211
Frainer, G., Siciliano, S., and Tavares, D. C. (2016). Franciscana Calls for Help: the Short and Long-term Effects of Mariana’s Disaster on Small Cetaceans of South-eastern Brazil. Tech. Doc. SC/66b/SM/04. Cambridge: International Whaling Commission, Bled, Slovenia.
Franco-Trecu, V., Costa, P., Abud, C., Dimitriadis, C., Laporta, P., Passadore, C., et al. (2009). Bycatch of franciscana Pontoporia blainvilleiin uruguayan artisanal gillnet fisheries: an evalution after a twelve-year gap in data collection. Lat. Am. J. Aq. Mamm. 7, 11–22.
Freitas-Netto, R. F., and Barbosa, L. A. (2003). Cetaceans and fishery interactions along the Espírito Santo State, Southeastern Brazil during 1994-2001. Lat. Am. J. Aq. Mamm. 2, 57–60.
Frizzera, F. C., Tosi, C., Pinheiro, H., and Marcondes, M. (2012). Captura acidental de toninha (Pontoporia blainvillei) na costa norte do Espírito Santo, Brasil. Bol Do Mus Biol Mello Leitão 29, 81–86.
Garcia, L. C., Ribeiro, D. B., Roque, F. O., Ochoa-Quintero, J. M., and Lawrence, W. F. (2017). Brazil’s worst mining disaster: corporations must be compelled to pay the actual environmental costs. Ecol. Appl. 27, 5–9. doi: 10.1002/eap.1461
Gariboldi, M. C., Túnez, J. I., Dejean, C. B., Failla, M., Vitullo, A. D., Negri, M. F., et al. (2015). Population genetics of Franciscana dolphins (Pontoporia blainvillei): introducing a new population from the southern edge of their distribution. PLoS One 10:e0132854. doi: 10.1371/journal.pone.0132854
Gariboldi, M. C., Túnez, J. I., Failla, M., Hevia, M., Panebianco, M. V., Paso Viola, M. N., et al. (2016). Patterns of population structure at microsatellite and mitochondrial DNA markers in the franciscana dolphin (Pontoporia blainvillei). Ecol. Evol. 6, 8764–8776. doi: 10.1002/ece3.2596
Gerpe, M., Rodríguez, D., Moreno, V. J., Bastida, R. O., and Moreno, J. D. (2002). Accumulation of heavy metals in the franciscana (Pontoporia blainvillei) from Buenos Aires Province, Argentina. Lat. Am. J. Aq. Mamm. 1, 95–106.
Haimovici, M. (1998). Present state and perspectives for the southern Brazil shelf demersal fisheries. Fish. Manag. Ecol. 5, 277–289. doi: 10.1046/j.1365-2400.1998.540277.x
Haimovici, M., and Cardoso, L. G. (2017). Long-term changes in the fisheries in the Patos Lagoon estuary and adjacent coastal waters in Southern Brazil. Mar. Biol. Res. 13, 135–150. doi: 10.1080/17451000.2016.1228978
Haimovici, M., Martins, A. S., and Vieira, P. C. (1996). Distribuição e abundância de peixes teleósteos sobre a plataforma continental do Sul do Brasil. Rev. Brasil. Biol. 56, 27–50.
Hamilton, H., Caballero, S., Collins, A. G., and Brownell, R. L. Jr. (2001). Evolution of river dolphins. Proc. Royal Soc. London Series B 268, 549–556.
Hardt, F. A. S., Cremer, M. J., Tonello, A. J., Bellante, A., and Buffa, G. (2013). Use of carbon and nitrogen stable isotopes to study the feeding ecology of small coastal cetacean populations in southern Brazil. Biota Neotrop. 13, 90–98. doi: 10.1590/s1676-06032013000400009
Harrison, R. J., Bryden, M. M., McBrearty, D., and Jr, R. B. (1981). The ovaries and reproduction in Pontoporia blainvillei (Cetacea: platanistidae). J. Zool. 193, 563–580. doi: 10.1111/j.1469-7998.1981.tb01504.x
Hatje, V., Pedreira, R. M. A., De Rezende, C. E., Schettini, C. A. F., De Souza, G. C., Marin, D. C., et al. (2017). The environmental impacts of one of the largest tailing dam failures worldwide. Sci. Rep. 7, 1–13.
Henning, B., Carvalho, B. S., Pires, M. M., Bassoi, M., Marigo, J., Bertozzi, C., et al. (2017). Geographical and intrapopulation variation in the diet of a threatened marine predator, Pontoporia blainvillei (Cetacea). Biotropica 50, 157–168. doi: 10.1111/btp.12503
Higa, A., Hingst-Zaher, E., and Vivo, M. D. (2002). Size and shape variability in the skull of Pontoporia blainvillei (Cetacea: pontoporiidae) from the Brazilian coast. Lat. Am. J. Aq. Mamm. 1, 145–152.
Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) (2011). Plano de Ação Nacional Para a Conservação do Pequeno Cetáceo – Toninha (Pontoporia blainvillei) [National Action Plan for the Conservation of the Small Cetacean - Franciscana (Pontoporia blainvillei)]. Brasília: Diretoria de Conservação da Biodiversidade [in Portuguese].
Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) (2018). Livro Vermelho da Fauna Brasileira Ameaçada de Extinção, VI – Peixes, 1st Edn. Brasília: Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio).
International Whaling Commission (IWC) (2016). A Conservation Management Plan for Franciscana (Pontoporia blainvillei). IWC/66/CC11. Cambridge: IWC, 22.
Kajiwara, N., Matsuoka, S., Iwata, H., Tanabe, S., Rosas, F. C. W., Fillmann, G., et al. (2004). Contamination by persistent organochlorines in cetaceans incidentally caught along Brazilian coastal waters. Arch. Env. Contam. Toxic. 46, 124–134. doi: 10.1007/s00244-003-2239-y
Kasuya, T., and Brownell, R. L. (1979). Age determination, reproduction, and growth of the franciscana dolphin, Pontoporia blainvillei. Sci. Rep. Whal. Res. Inst. 31, 45–67.
Kehrig, H. A., Hauser-Davis, R. A., Seixas, T. G., Pinheiro, A. B., and Di Beneditto, A. P. M. (2016). Mercury species, selenium, metallothioneins and glutathione in two dolphins from the southeastern Brazilian coast: mercury detoxification and physiological differences in diving capacity. Env. Poll. 213, 785–792. doi: 10.1016/j.envpol.2016.03.041
Kunito, T., Nakamura, S., Ikemoto, T., Anan, Y., Kubota, R., Tanabe, S., et al. (2004). Concentration and subcellular distribution of trace elements in liver of small cetaceans incidentally caught along the Brazilian coast. Mar. Poll. Bull. 49, 574–587. doi: 10.1016/j.marpolbul.2004.03.009
Lailson-Brito, J., Azeredo, M. A. A., Malm, O., Ramos, R. A., Di Beneditto, A. P. M., and Saldanha, M. F. (2002). Trace metals in liver and kidney of the franciscana (Pontoporia blainvillei) from the northern coast of Rio de Janeiro State, Brazil. Lat. Am. J. Aq. Mamm. 1, 107–114.
Lailson-Brito, J., Dorneles, P. R., Azevedo-Silva, C. E., Azevedo, A. F., and Vidal, L. G. (2011). Organochlorine concentrations in franciscana dolphins, Pontoporia blainvillei, from Brazilian waters. Chemosphere. 84, 882–887. doi: 10.1016/j.chemosphere.2011.06.018
Laist, D. W. (1997). “Impacts of marine debris: entanglement of marine life in marine debris including a comprehensive list of species with entanglement and ingestion records,” in Marine Debris–Sources, Impacts and Solutions, eds J. M. Coe, and D. B. Rogers (New York, NY: Springer-Verlag), 99–139. doi: 10.1007/978-1-4613-8486-1_10
Lázaro, M., Lessa, E. P., and Hamilton, H. (2004). Geographic genetic structure in the franciscana dolphin (Pontoporia blainvillei). Mar. Mamm. Sci. 20, 201–214. doi: 10.1111/j.1748-7692.2004.tb01151.x
Lopes, X. M. (2012). Hábitos alimentares de Toninha, Pontoporia blainvillei (Gervais e D’Orbigny, 1844) (Mammalia, Cetacea), no sul do Estado de São Paulo e norte do Paraná, Brasil. Master’s Dissertation, Universidade Estadual Paulista. Rio claro, 92. Available online at: http://hdl.handle.net/11449/99539
Machado, R., Oliveira, L. R., Ott, P. H., Haimovici, M., Cardoso, L. G., Milmann, L., et al. (2020). Trophic overlap between marine mammals and fisheries in subtropical waters in the western South Atlantic. Mar. Ecol. Prog. Ser. 639, 215–232. doi: 10.3354/meps13284
Marcondes, D. S., Nunes, T. Y., Fonseca, G. F., Moura, S. P. G., Belleghem, T. V., and Domit, C. (2020). Atividades de derrocagem subaquática e potenciais impactos em golfinhos costeiros: avaliação, monitoramento e medidas de mitigação. Bol. Soc. Bras. Mastoz. 89, 135–145.
Marcondes, M. C. C., Angeli, M., Fontes, F., Palazzo Júnior, J. T., Campos, R., Dapper, C., et al. (2018). Report on Franciscana Fisheries Interaction in FMA Ia, Brazil. Paper submitted to the 68 IWC Scientific Meeting, SC/67B/SM/03. Cambridge: IWC, 17.
Marcovecchio, J. E., Gerpe, M. S., Bastida, R. O., Rodríguez, D. H., and Morón, S. G. (1994). Environmental contamination and marine mammals in coastal waters from Argentina: an overview. Sci. Tot. Env. 154, 141–151. doi: 10.1016/0048-9697(94)90084-1
Marcovecchio, J. E., Moreno, V. J., Bastida, R. O., Gerpe, M. S., and Rodríguez, D. H. (1990). Tissue distribution of heavy metals in small cetaceans from the Southwestern Atlantic Ocean.Mar. Poll. Bull. 21, 299–304. doi: 10.1016/0025-326x(90)90595-y
Melcón, M. L., Failla, M., and Iñíguez, M. A. (2012). Echolocation behavior of franciscana dolphins (Pontoporia blainvillei) in the wild. J. Acoust. Soc. Am. 131, EL448–EL453.
Melcón, M. L., Failla, M., and Iñíguez, M. A. (2016). Towards understanding the ontogeny of echolocation in franciscana dolphins (Pontoporia blainvillei). Mar. Mamm. Sci. 32, 1516–1521. doi: 10.1111/mms.12336
Mendez, M., Rosembaum, H. C., and Bordino, P. (2008). Conservation genetics of the franciscana dolphin in Northern Argentina: population structure, by-catch impacts and management implications. Conserv. Genet. 9, 419–435. doi: 10.1007/s10592-007-9354-7
Mendez, M., Rosembaum, H. C., Subramaniam, A., Yackulic, C., and Bordino, P. (2010). Isolation by environmental distance in mobile marine species: molecular ecology of franciscana dolphins at their southern range. Mol. Ecol. 19, 2212–2228. doi: 10.1111/j.1365-294x.2010.04647.x
Monteiro, D. S., Estima, S. C., Gandra, T. B. R., Silva, A. P., Bugoni, L., Swimmer, Y., et al. (2016). Long-term spatial and temporal patterns of sea turtle strandings in southern Brazil. Mar. Biol. 163:247.
Monteiro, F., Lemos, L. S., De Moura, J. F., Rocha, R. C. C., Moreira, I., Di Beneditto, A. P. M., et al. (2020). Total and subcellular Ti distribution and detoxification processes in Pontoporia blainvillei and Steno bredanensis dolphins from Southeastern Brazil. Mar. Poll. Bull. 153:110975. doi: 10.1016/j.marpolbul.2020.110975
Moreno, I. B., Ott, P. H., and Danilewicz, D. S. (1997). Análise Preliminar do Impacto da Pesca Artesanal Costeira Sobre Pontoporia blainvillei no Litoral Norte do Rio Grande do Sul, Sul do Brasil. Anais do2° Encontro Sobre Coordenação de Pesquisa e Manejo da Franciscana. Rio Grande: Editora da Furg, 31–41.
Morisaka, T., and Connor, R. C. (2007). Predation by killer whales (Orcinus orca) and the evolution of whistle loss and narrow-band high frequency clicks in odontocetes. J. Evol. Biol. 20, 1439–1458. doi: 10.1111/j.1420-9101.2007.01336.x
Murray, K. T., Read, A. J., and Solow, A. R. (2000). The use of time/area closure to reduce bycatch of harbour porpoise: lessons from the Gulf of Maine sink gillnet fishery. J. Cetacean Res. Manage. 2, 135–141.
Negri, M. F., Denuncio, P., Panebianco, M. V., and Cappozzo, H. L. (2012). Bycatch of franciscana dolphins Pontoporia blainvillei and the dynamic of artisanal fisheries in the species’ southernmost area of distribution. Braz. J. Ocean 60, 149–158. doi: 10.1590/s1679-87592012000200005
O’Shea, T. J., Brownell, J. R., Clark, J. D., Walker, W. A., Gay, M. A., and Lamont, T. G. (1980). Organochlorine pollutants in small cetaceans from the Pacific and south Atlantic Oceans, November 1968-June 1976. Pestic Mon. J. 14, 35–46.
Ott, P. H. (2002). Diversidade Genética e Estrutura Populacional de Duas Espécies de Cetáceos do Atlântico Sul Ocidental: Pontoporia Blainvillei e Eubalaena Australis. Rio Grande: Universidade Federal do Rio Grande do Sul.
Ott, P. H., and Danilewicz, D. S. (1996). Presence of franciscana dolphins (Pontoporia blainvillei) in the stomach of a killer whale (Orcinus orca) stranded in southern Brazil. Mammalia 62, 605–609.
Ott, P. H., Domit, C., Siciliano, S., and Flores, P. A. C. (2015). Memórias do VII Workshop Para Acoordenação de Pesquisa e Conservação de Pontoporia Blainvillei (Gervais & d’Orbigny, 1844). Available online at: www.pontoporia.org (accessed January 10, 2020).
Ott, P. H., Secchi, E. R., Moreno, I. B., Danilewicz, D., Crespo, E. A., Bordino, P., et al. (2002). Report of the working group on fishery interactions. Lat. Am. J. Aq. Mamm. 1, 55–64.
Paitach, R. L., Amundin, M., and Cremer, M. J. (2018). “A “Seal safe” acoustic deterrent may mitigate the most serious threat to franciscana dolphin,” in XII Congreso de la SOLAMAC, Lima, Peru. Livro de Resumos do XII Congreso de la SOLAMAC, 258.
Paitach, R. L., Simões-Lopes, P. C. A., and Cremer, M. J. (2017). Tidal and seasonal in-fluences in dolphin habitat use in a southern Brazilian estuary. Sci. Mar. 81, 49–56. doi: 10.3989/scimar.04495.25a
Panebianco, M. V., Del Castillo, D. L., Denuncio, P. E., Negri, M. F., Bastida, R., Failla, M., et al. (2016). Reproductive biology of female franciscana dolphins (Pontoporia blainvillei) from Argentina. Mar. Biol. Assoc. U.K. 96, 831–840. doi: 10.1017/s002531541500137x
Panebianco, M. V., Negri, M. F., and Cappozzo, H. L. (2012). Reproductive aspects of male franciscana dolphins (Pontoporia blainvillei) off Argentina. Anim. Rep. Sci. 131, 41–48. doi: 10.1016/j.anireprosci.2012.02.005
Pilleri, G. (1971). On the La Plata dolphin Pontoporia blainvillei off the Uruguayan coast. Invest. Cetacea 3, 69–73.
Pinedo, M. C. (1991). Development and Variation of the Franciscana Pontoporia blainvillei. Ph.D. thesis. Santa Cruz, CA: University of California.
Pinedo, M. C. (1994). Review of small cetacean fishery interactions in southern Brazil with special reference to the franciscana, Pontoporia blainvillei. Rep. Int. Whal. Commn. 15, 251–259.
Pinedo, M. C. (1995). Development and variation in external morphology of the franciscana, Pontoporia blainvillei. Rev. Bras. Biol. 55, 85–96.
Pinedo, M. C., and Hohn, A. A. (2000). Growth layer patterns in teeth from the franciscana, Pontoporia blainvillei: developing a model for precision in age estimation. Mar. Mamm. Sci. 16, 1–27. doi: 10.1111/j.1748-7692.2000.tb00901.x
Pinheiro, F. C. F., Pinheiro, H. T., Teixeira, J. B., Martins, A. S., and Cremer, M. J. (2019). Opportunistic development and environmental disaster threat franciscana dolphins in the Southeast of Brazil. Trop. Cons. Sci. 12:1940082919847886.
Polizzi, P., Romero, M., Boudet, L., Das, K., Denuncio, P., Rodríguez, D., et al. (2014). Metallothioneins pattern during ontogeny of coastal dolphin, Pontoporia blainvillei, from Argentina. Mar. Pollut. Bull. 80, 275–281. doi: 10.1016/j.marpolbul.2013.10.037
Praderi, R. (1985). Relaciones entre Pontoporia blainvillei (Mammalia: Cetacea) y tiburones (Selachii) de aguas Uruguayas. Com. Zoo. Mus. Hist. Nat. Montevideo 11, 1–19.
Praderi, R. (1986). Comentarios sobre la distribución de Pontoporia blainvillei en aguas del Rio de La Plata. Actas I Reunión de Trabajo de Especialistas en Mamíferos Acuáticos de América del Sur, Buenos Aires. 206–214.
Praderi, R. (1997). “Análisis comparativo de estadísticas de captura y mortalidad incidental de Pontoporia blainvillei en Uruguay durante 20 años,” in Anais do 2° Encontro sobre Coordenação de Pesquisa e Manejo da Franciscana, eds M. C. Pinedo and A. S. Barreto (Rio Grande: Fundação Universidades do Rio Grande), 42–53.
Prado, J. H. F., Kinas, P. G., Pennino, M. G., Seyboth, E., Silveira, F. R. G., Ferreira, E. C., et al. (2021). Definition of no-fishing zones and fishing effort limits to reduce franciscana bycatch to sustainable levels in southern Brazil. Animal Cons. doi: 10.1111/acv.12679
Prado, J. H. F., Mattos, P. H., Silva, K. G., and Secchi, E. R. (2016). Long-term seasonal and interannual patterns of marine mammal strandings in subtropical western South Atlantic. PLoS One 11:e0146339. doi: 10.1371/journal.pone.0146339
Prado, J. H. F., Secchi, E. R., and Kinas, P. G. (2013). Mark-recapture of the endangered franciscana dolphin (Pontoporia blainvillei) killed in gillnet fisheries to estimate past bycatch from time series of stranded carcasses in southern Brazil. Ecol. Indicators. 32, 35–41. doi: 10.1016/j.ecolind.2013.03.005
Ralls, K. (1976). Mammals in which females are larger than males. Quart. Rev. Biol. 51, 245–276. doi: 10.1086/409310
Ramos, R. M. A., Di Beneditto, A. P. M., and Lima, N. R. W. (2000). Growth parameters of Pontoporia blainvillei in northern Rio de Janeiro, Brazil. Aq. Mamm. 26, 65–75.
Ramos, R. M. A., Di Beneditto, A. P. M., Siciliano, S., Santos, M. C. O., Zerbini, A. N., and Bertozzi, C. (2002). Morphology of the franciscana (Pontoporia blainvillei) off southeastern Brazil: sexual dimorphism, growth and geographic variation. Lat. Am. J. Aq. Mamm. 1, 129–144.
Reis, E. G. (1992). An Assessment of the Exploitation of the White Croaker Micropogonias furnieri (Pisces, Sciaenidae) by the Artisanal and Industrial Fisheries in Coastal Waters of Southern Brazil. Doctoral dissertation. Norwich: University of East Anglia.
Rodríguez, D. H., Rivero, L., and Bastida, R. O. (2002). Feeding ecology of the franciscana (Pontoporia blainvillei) in marine and estuarine waters of Argentina. Lat. Am. J. Aq. Mamm. 1, 77–94.
Romero, M. B., Polizzi, P., Chiodi, L., Das, K., and Gerpe, M. (2016). The role of metallothioneins, selenium and transfer to offspring in mercury detoxification in franciscana dolphins (Pontoporia blainvillei). Mar. Poll. Bull. 109, 650–654. doi: 10.1016/j.marpolbul.2016.05.012
Romero, M. B., Polizzi, P., Chiodi, L., Robles, A., Das, K., and Gerpe, M. (2017). Metals as chemical tracers to discriminate ecological populations of threatened franciscana dolphins (Pontoporia blainvillei) from Argentina. Environm. Sc. Poll. Res. 24, 3940–3950. doi: 10.1007/s11356-016-7970-9
Rosas, F. C. W. (2000). Interações Com a Pesca, Mortalidade, Idade, Reprodução e Crescimento de Sotalia Guianensis e Pontoporia blainvillei (Cetacea, Delphinidae e Pontoporiidae) no Litoral Sul do Estado de São Paulo e litoral do Estado do Paraná, Brasil. Tese de Doutoramento. Curitiba: Universidade Federal do Paraná.
Rosas, F. C. W., and Monteiro-Filho, E. L. A. (2002). Reproductive parameters of Pontoporia blainvillei (Cetacea, Pontoporiidae), on the coast of São Paulo and Paraná States, Brazil. Mammalia 66, 231–245.
Rosas, F. C. W., Monteiro-Filho, E. L. A., and Oliveira, M. R. (2002). Incidental catches of franciscana (Pontoporia blainvillei) on the southern coast of São Paulo State and the coast of Paraná State. Brazil. Lat. Am. J. Aq. Mamm. 1, 161–168.
Ruffino, M. L., and Castello, J. P. (1992). Alterações na fauna acompanhante da pesca do camarão barba-ruça (Artemesia longinaris) nas imediações da barra de Rio Grande, RS. Nerítica 7, 43–55.
Rupil, G. M., Barbosa, L., Marcondes, M. C. C., Carvalho, B. M., and Farro, A. P. C. (2019). Franciscana dolphin (Pontoporia blainvillei) diet from Northern Espírito Santo State coast, Brazil. Rev. Biotemas 32, 87–96.
Santos, M. C. D. O., Oshima, J. E. D. F., and Silva, E. D. (2009). Sightings of franciscana dolphins (Pontoporia blainvillei): the discovery of a population in the Paranaguá estuarine complex, Southern Brazil. Braz. J. Ocean. 57, 57–63. doi: 10.1590/s1679-87592009000100006
Santos, M. C. O., and Netto, D. F. (2005). Killer whale (Orcinus orca) predation on a franciscana dolphin (Pontoporia blainvillei) in Brazilian waters.Lat. Am. J. Aq. Mamm. 4, 69–72.
Sartori, C. M., Paitach, R. L., and Cremer, M. J. (2017). Photo-identification of franciscanas (Pontoporia blainvillei) in Babitonga Bay, Santa Catarina State, Brazil. J. Cet. Res. Manage. 16, 49–55.
Secchi, E. R. (2006). Modeling the Population Dynamics and Viability Analysis of Franciscana (Pontoporia blainvillei) and Hector’s Dolphins (Cephalorhynchus hectori) Under the Effects of Bycatch in Fisheries, Parameter Uncertainty and Stochasticity. Ph.D. thesis. Dunedin: University of Otago.
Secchi, E. R. (2010). “Review on the threats and conservation Status of Franciscana, Pontoporia blainvillei (Cetacea, Pontoporiidae),” in Biology, Evolution and Conservation of River Dolphins within South America and Asia, Vol. 1, eds J. M. Shostell and M. Ruiz-Garcia (Hauppauge, NY: Nova Science Publishers Inc.), 323–339.
Secchi, E. R., Botta, S., Weigand, M. M., Lopez, L. A., Fruet, P. F., Genoves, R. C., et al. (2016). Long-term and gender-related variation in the feeding ecology of common bottlenose dolphins inhabiting a subtropical estuary and the adjacent marine coast in the western South Atlantic. Mar. Biol. Res. 13, 121–134. doi: 10.1080/17451000.2016.1213398
Secchi, E. R., Danilewicz, D., and Ott, P. H. (2003a). Applying the phylogeographic concept to identify franciscana dolphin stocks: implications to meet management objectives. J. Cetacean Res. Manage. 5, 61–68.
Secchi, E. R., and Fletcher, D. (2004). Modelling Population Growth and Viability Analysis for four Franciscana Stocks: Effects of Stock-Specific Differences in Life Traits, Fishing Bycatch, Parameter Uncertainty and Stochasticity. Technical Paper IWC - SC/56/SM20. Porto Alegre.
Secchi, E. R., Kinas, P. G., and Muelbert, M. (2004). Incidental catches of franciscana in coastal gillnet fisheries in the Franciscana Management Area III: period 1999-2000. Lat. Am. J. Aq. Mamm. 3, 61–68.
Secchi, E. R., Ott, P. H., Crespo, E. A., Kinas, P. G., Pedraza, S. N., and Bordino, P. (2001). A first estimate of franciscana (Pontoporia blainvillei) abundance off southern Brazil. J. Cet. Res. Manag. 3, 95–100.
Secchi, E. R., Ott, P. H., and Danilewicz, D. S. (2003b). “Effects of fishing by-catch and conservation status of the franciscana dolphin, Pontoporia blainvillei,” in Marine Mammals: Fisheries, Tourism and Management Issues, eds N. Gales, M. Hindell, and R. Kirkwood (Collingwood: CSIRO Publishing), 174–191.
Secchi, E. R., and Wang, J. Y. (2002). Assessment of the conservation status of a franciscana (Pontoporia blainvillei) stock in the Franciscana Management Area III following the IUCN Red List process. Lat. Am. J. Aq. Mamm. 1, 183–190.
Secchi, E. R., Wang, J. Y., Murray, B., Rocha-Campos, C. C., and White, B. N. (1998). Populational differences between franciscanas, Pontoporia blainvillei, from two geographical locations as indicated by sequences of mtDNA control region. Can. J. Zool. 76, 1622–1627. doi: 10.1139/cjz-76-9-1622
Secchi, E. R., Zerbini, A. N., Bassoi, M., Dalla Rosa, L., Möller, L. M., and Rocha-Campos, C. C. (1997). Mortality of franciscanas, Pontoporia blainvillei, in coastal gillnetting in southern Brazil: 1994-1995. Rep. Int. Whal. Commn. 47, 653–658.
Secchi, E. R., Zerbini, A. N., Wells, R. W., and Bastida, R. (2020). “Is the franciscana (Pontoporia blainvillei) a candidate for ex situ conservation?,” in Ex Situ Options for Cetacean Conservation. Report of the 2018 Workshop, Nuremberg, Germany, pp.xx-yy. Occasional Paper of the IUCN Species Survival Commission No.66, eds B. L. Taylor, G. Abel, P. Miller, F. Gomez, L. von Fersen, D. DeMaster, et al. (Gland: IUCN).
Seixas, T. G., Kehrig, H. A., Costa, M., Fillmann, G., Di Beneditto, A. P. M., Secchi, E. R., et al. (2008). Total mercury, organic mercury and selenium in liver and kidney of a South American coastal dolphin. Environ. Poll. 154, 98–106. doi: 10.1016/j.envpol.2008.01.030
Seixas, T. G., Kehrig, H. A., Fillmann, G., Di Beneditto, A. P. M., Souza, C. M. M., Secchi, E. R., et al. (2007). Ecological and biological determinants of trace elements accumulation in liver and kidney of Pontoporia blainvillei. Sci. Total Environ. 385, 208–220. doi: 10.1016/j.scitotenv.2007.06.045
Siciliano, S. (1994). Review of small cetaceans and fisheryinteractions in coastal waters of Brazil. Rep. Int. Whal. Commn. 15, 241–250.
Siciliano, S., Di Beneditto, A. P., and Ramos, R. M. A. (2002). A toninha, Pontoporia blainvillei (Gervais & d’Orbigny, 1844) (Mammalia, Cetacea, Pontoporiidae), nos estados do Rio de Janeiro e Espírito Santo, costa sudeste do Brasil: caracterizações dos habitats e fatores de isolamento das populações. Bol. Mus. Nac. 476, 1–15.
Silva, D. F., Barbosa, R. A., Conversani, V. R. M., Botta, S., Hohn, A. A., and Santos, M. C. O. (2020). Reproductive parameters of franciscana dolphins (Pontoporia blainvillei) of Southeastern Brazil. Mar. Mamm. Sci. 36, 1291–1308. doi: 10.1111/mms.12720
Soutullo, A., Clavijo, C., and Martiìnez-Lanfranco, J. A. (eds) (2013). Especies Prioritarias Para la Conservacioìn en Uruguay. Vertebrados, Moluscos Continentales y Plantas Vasculares. SNAP/DINAMA/MVOTMA y DICYT/MEC. Montevideo: 222.
Sucunza, F. (2020). Estimativas de Densidade e Dbundância da Toninha (Pontoporia blainvillei) a Partir de Levantamentos Aéreos: Produção e Aplicação de Fatores de Correção. Tese de Doutorado. Juiz de Fora: Universidade Federal de Juiz de Fora.
Sucunza, F., Danilewicz, D., Andriolo, A., Azevedo, A. F., Secchi, E. R., and Zerbini, A. N. (2020). Distribution, habitat use, and abundance of the endangered franciscana in southeastern and southern Brazil. Mar. Mamm. Sci. 36, 421–435. doi: 10.1111/mms.12650
Sucunza, F., Danilewicz, D., Cremer, M., Andriolo, A., and Zerbini, A. N. (2018). Refining estimates of availability bias toimprove assessment of the conservation status of an endangered dolphin. PLoS One 13:e0194213. doi: 10.1371/journal.pone.0194213
Szephegyi, M., Franco-Trecu, V., Doño, F., Reyes, F., Forselledo, R., and Crespo, E. A. (2015). “Primer relevamiento sistemático de captura incidental de franciscanas en la flota uruguaya de arrastre de fondo costero,” in Memórias do VII Workshop Para a Coordenação de Pesquisa e Conservação de Pontoporia blainvillei (Gervais & d’Orbigny, 1844), 22-24 de outubro de 2010, (Florianópolis).
Taylor, B. L., Abel, G., Miller, P., Gomez, F., von Fersen, L., and DeMaster, D., et al. (eds) (2020). Ex situ options for cetacean conservation. Report of the 2018 workshop, Nuremberg, Germany. Occasional Paper of the IUCN Species Survival Commission No. 66. Gland: IUCN.
Tellechea, J. S., and Norbis, W. (2014). Sound characteris-tics of two neonatal Franciscana dolphins (Pontoporia blainvillei). Mar. Mamm. Sci. 30, 1573–1580. doi: 10.1111/mms.12122
Tellechea, J. S., Perez, W., Olsson, D., Lima, M., and Norbis, W. (2017). Feeding habits of franciscana dolphins (Pontoporia blainvillei): echolocation or passive listening? Aq. Mamm. 43, 440–448.
Troina, G., Botta, S., Secchi, E. R., and Dehairs, F. (2016). Ontogenetic and sexual characterization of the feeding habits of franciscanas, Pontoporia blainvillei, based on tooth dentin carbon and nitrogen stable isotopes. Mar. Mamm. Sci. 32, 1115–1137. doi: 10.1111/mms.12316
Véras, E. (2011). Estrutura Populacional e História Filogeográfica da Toninha (Pontoporia blainvillei). Master thesis. Porto Alegre: Pontifícia Universidade Católica do Rio Grande do Sul.
Von Fersen, L., Kamminga, C., and Seidl, A. (1997). Estudios preliminares sobre el comportamiento de un ejemplar de franciscana (Pontoporia blainvillei) en Mundo Marino, Argentina. [Preliminary studies on the behavior of one individual of franciscana dolphin (Pontoporia blainvillei) in Mundo Marino, Argentina (DT10)]. Report of the Third Workshop for Coordinated Research and Conservation of the Franciscana Dolphin, Buenos Aires. Buenos Aires, 30–33.
Vooren, C. M., and Klippel, S. (2005). Ações Para a Conservação de Tubarões e Raias no Sul do Brasil. Porto Alegre: Igaré, 262.
Wade, P. R. (1998). Calculating limits to the allowable human-caused mortality of cetaceans and pinnipeds.Mar. Mam. Sci. 14, 1–37. doi: 10.1111/j.1748-7692.1998.tb00688.x
Weiskel, H. W., Bordino, P., and Arias, M. A. (2002). Gillnets and conservation of the franciscana dolphin (Pontoporia blainvillei) in Argentina: a policy perspective. Lat. Am. J. Aq. Mamm. 1, 183–190.
Wells, R. S., Bordino, P., and Douglas, D. C. (2013). Patterns of social association in the franciscana, Pontoporia blainvillei. Mar. Mamm. Sci. 29, E520–E528.
Zerbini, A. N., Secchi, E., Crespo, E., Danilewicz, D., and Reeves, R. (2017). Pontoporia blainvillei (errata version published in 2018). The IUCN Red List of Threatened Species 2017: e.T17978A123792204. Available online at: https://dx.doi.org/10.2305/IUCN.UK.20173.RLTS.T17978A50371075.en. (accessed April 17, 2020).
Zerbini, A. N., Secchi, E. R., Danilewicz, D., Andriolo, A., Laake, J. L., and Azevedo, A. (2011). Abundance and Distribution of the Franciscana (Pontoporia blainvillei) in the Franciscana Management Area II (southeastern and southern Brazil). Paper SC/62/SM7 presented to the IWC Scientific Committee, Agadir. Cambridge: IWC.
Keywords: Pontoporia blainvillei, cetaceans, western South Atlantic, fisheries, bycatch, habitat degradation, pollution
Citation: Secchi ER, Cremer MJ, Danilewicz D and Lailson-Brito J (2021) A Synthesis of the Ecology, Human-Related Threats and Conservation Perspectives for the Endangered Franciscana Dolphin. Front. Mar. Sci. 8:617956. doi: 10.3389/fmars.2021.617956
Received: 15 October 2020; Accepted: 18 June 2021;
Published: 30 July 2021.
Edited by:
Diego Horacio Rodriguez, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), ArgentinaReviewed by:
Humberto Luis Cappozzo, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), ArgentinaElisabeth Slooten, University of Otago, New Zealand
Copyright © 2021 Secchi, Cremer, Danilewicz and Lailson-Brito. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eduardo R. Secchi, ZWR1LnNlY2NoaUBmdXJnLmJy
 Eduardo R. Secchi
Eduardo R. Secchi M. J. Cremer
M. J. Cremer D. Danilewicz3,4
D. Danilewicz3,4 J. Lailson-Brito
J. Lailson-Brito