- 1Stanford University, Hopkins Marine Station, Pacific Grove, CA, United States
- 2Cawthron Institute, Nelson, New Zealand
- 3Institute of Marine Sciences, University of California, Santa Cruz, Santa Cruz, CA, United States
- 4Southwest Fisheries Science Center, NOAA National Marine Fisheries Service, La Jolla, CA, United States
- 5Golden Honu Services of Oceania, Newport, OR, United States
- 6Golden Honu Services of Oceania, Honolulu, HI, United States
- 7Marine Biology Program, The University of Hawai’i at Mānoa, Honolulu, HI, United States
- 8Port of Nagoya Public Aquarium, Nagoya, Japan
- 9Usa Marine Biological Institute, Kochi University, Kôchi, Japan
- 10Hawaii Preparatory Academy, Kamuela, HI, United States
The North Pacific Loggerhead sea turtle (Caretta caretta) undergoes one of the greatest of all animal migrations, nesting exclusively in Japan and re-emerging several years later along important foraging grounds in the eastern North Pacific. Yet the mechanisms that connect these disparate habitats during what is known as the “lost years” have remained poorly understood. Here, we develop a new hypothesis regarding a possible physical mechanism for habitat connectivity – an intermittent “thermal corridor” – using remotely sensed oceanography and 6 juvenile loggerhead sea turtles that formed part of a 15 year tracking dataset of 231 individuals (1997–2013). While 97% of individuals remained in the Central North Pacific, these 6 turtles (about 3%), continued an eastward trajectory during periods associated with anomalously warm ocean conditions. These few individuals provided a unique opportunity to examine previously unknown recruitment pathways. To support this hypothesis, we employed an independently derived data set using novel stable isotope analyses of bone growth layers and assessed annual recruitment over the same time period (n = 33, 1997–2012). We suggest evidence of a thermal corridor that may allow for pulsed recruitment of loggerheads to the North American coast as a function of ocean conditions. Our findings offer, for the first time, the opportunity to explore the development of a dynamic ocean corridor for this protected species, illuminating a longstanding mystery in sea turtle ecology.
Introduction
Conservation of long-lived pelagic organisms, like sea turtles, that navigate whole ocean basins remains one of the most daunting challenges in marine science. Six of the seven species of sea turtles are listed as critically endangered, endangered, or vulnerable by the IUCN and are subject to numerous threats on land, in the Exclusive Economic Zones (EEZs) of nations, and in the open ocean (IUCN, 2018). For sea turtles, we have long known where they nest but Archie Carr’s “lost year” puzzle, for which he called out as the “most substantial of all obstacles to understanding the ecology of sea turtles” (Carr, 1980), is now understood to be the “lost decades” for some species.
Even the at-sea movements of well-known sea turtle species such as loggerheads (Caretta caretta) have until recently been a mystery. Only 25 years ago, many assumed that loggerheads found in the eastern North Pacific Ocean originated from undiscovered nesting sites in the region, as it was hard to imagine a juvenile sea turtle undertaking a 12,000 km transpacific migration from its natal beaches in Japan to the North American coast (Nichols et al., 2000; Seminoff et al., 2012). The daunting presence of one of Darwin’s “impassable” barriers (Darwin, 1872), the Eastern Pacific Barrier (EPB) (Ekman, 1953), usually prevents the dispersal of marine fauna from the central to eastern Pacific Ocean (see Briggs, 1961; Lessios and Robertson, 2006; Baums et al., 2012). But phylogeographic studies have provided insight into the pathways of colonization across this biogeographic barrier for several small (Baums et al., 2012) and large marine species into the eastern Pacific, including sea turtles (Dutton et al., 2014). For North Pacific loggerhead sea turtles, the combination of genetic analyses (Bowen et al., 1995), flipper tag recoveries, and satellite tracked individuals (Uchida and Teruya, 1988; Resendiz et al., 1998; Nichols et al., 2000) eventually confirmed the North Pacific population as a single, widely dispersed, genetically distinct stock. Nesting exclusively in Japan, hatchlings from this population undertake developmental migrations that we now know span the entire North Pacific ocean basin (Bowen et al., 1995; Nichols et al., 2000). Foraging hotspots for juveniles have been identified in the Central North Pacific (CNP) (Polovina et al., 2004, 2006; Kobayashi et al., 2008; Abecassis et al., 2013) and in the eastern North Pacific off the Baja California Peninsula (BCP), Mexico (Peckham et al., 2011; Wingfield et al., 2011; Seminoff et al., 2014). Upon maturity, these turtles return to their natal beaches in Japan to reproduce, residing in the western Pacific for the remainder of their lives (Nichols et al., 2000; Watanabe et al., 2011).
Throughout their range, North Pacific loggerheads are subject to high rates of fisheries-related mortality (Gilman et al., 2007; Peckham et al., 2008). While we now have knowledge of their movements within their core oceanic and coastal habitats, our understanding of the connectivity between these disparate locations is fragmented, leaving critical gaps in our ability to effectively assess and protect this cryptic species. Briscoe et al. (2016) examined the basin-scale movement patterns of 231 satellite tracked North Pacific juveniles between 1997–2013, revealing an extensive pelagic phase within the oceanic waters of the CNP. Of these individuals, tracked for up to 4.9 years at a time, many displayed east-west movement patterns within their oceanic habitat, but only 30% moved into the eastern Pacific. Even fewer (n = 6 turtles, ∼3%) transitioned into the California Current Large Marine Ecosystem (CCLME) (Figure 1A). And yet over 43,000 individuals from this population are known to congregate along the coastal waters of the BCP at any given time (Seminoff et al., 2014). Aging analysis has shown that these juveniles are long-term residents (20+ years) (Turner Tomaszewicz et al., 2015) and the differential fitness and reproductive outcomes achieved by coastal recruits suggests this area represents important developmental grounds for some portion of this population (Peckham et al., 2011; Seminoff et al., 2014; Turner Tomaszewicz et al., 2015, 2017).
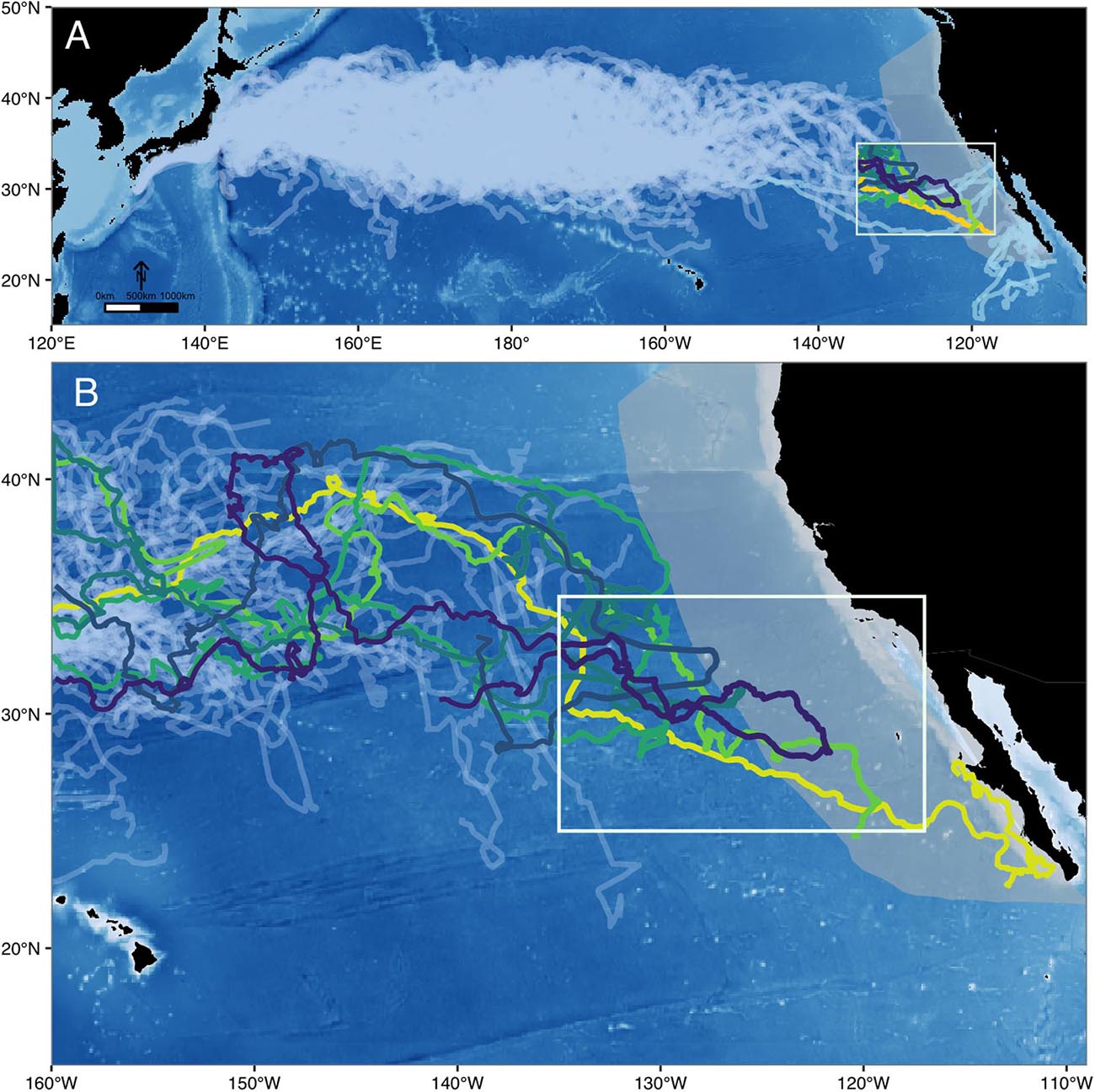
Figure 1. State-space modeled distribution of (A) 231 electronically tagged juvenile loggerhead sea turtles across the North Pacific Ocean (1997 – 2013), deployed off Japan and in the Central North Pacific (light gray). CCLME region shown in gray. While individuals released from the North American coast (light blue) were not included in the study because they represent a larger age-size class, they are shown here to characterize the full habitat use of individuals from this population. (B) The eastern North Pacific study region, utilized by the 6 individuals that migrated from the Central North Pacific towards the North American coast study area (white box).
How and why some loggerheads recruit to the eastern Pacific while others remain in the CNP is unresolved. Despite one of the largest tracking sets of any migratory species, evidence of only 6 out of 231 individuals moving across the EPB and into the eastern Pacific is perplexing, but corresponds with recent findings by Turner Tomaszewicz et al. (2017) that revealed this ontogenetic habitat shift may be infrequent or variable. Historically, the lack of data on recruitment and the prolonged, slow-growing nature of loggerheads has limited our visibility into this important ontogenetic shift for the North Pacific population. But for the first time, the routes of these 6 “sentinel” individuals illuminate this lost years puzzle and have provided us with the first empirical insight into a recruitment pathway this cryptic species.
Here, we extend the meta-analysis of these 231 tracks by Briscoe et al. (2016) and follow the unique movements of the 6 individuals that progressed beyond the CNP towards the North American coast (Figure 1B). First, we review several well-established connections between loggerhead movement and their environment to develop a theory for recruitment. We then explore the evolution of favorable oceanographic conditions along the migratory corridor identified by these 6 individuals. Finally, we add an additional line of evidence using an independently derived data set documenting loggerhead recruitment to the BCP region over the same time period (1997–2012), to compare estimated annual recruitment in years of oceanographic variability. Our findings offer a promising hypothesis underlying habitat connectivity and a new interpretation of this longstanding mystery in sea turtle ecology.
Thermal Hypothesis
Temperature is a fundamental driver of sea turtle distribution (Epperly et al., 1995). As ectotherms, core body temperature is inherently tied to the surrounding environment and thus, sea turtles rely on thermal cues to both select (or avoid) habitats and initiate long-distance movements (Bentivegna, 2002; McMahon and Hays, 2006; Hawkes et al., 2007; Báez et al., 2011). In the North Pacific, loggerheads exhibit strong fidelity to the 18°C isotherm, a widely used proxy for the highly productive Transition Zone Chlorophyll Front (TZCF) (Polovina et al., 2000). Individuals have been shown to track the position of the TZCF over annual and interannual cycles, which serves as both a transport and foraging mechanism for many highly mobile species across the North Pacific Ocean (Polovina et al., 2001).
For loggerheads, defining associations with sea surface temperature (SST) now extend beyond distribution, as understanding and anticipating SST changes have become pivotal to dynamic fisheries management strategies in the North Pacific. Identification of loggerhead-SST affinities in the open ocean motivated the development of one of the first the dynamic bycatch avoidance tools, TurtleWatch (Howell et al., 2008), used by the Hawaii-based longline fishery to mitigate interactions by avoiding waters within thermally optimal habitat. In the CCLME, increased loggerhead bycatch within the California Drift Gillnet fishery has historically correlated with anomalously warm water periods associated during El Niño Southern Oscillation (ENSO). This association led to the establishment of temperature-based operational guidelines for the fishery and since 2003, a time-area closure is enacted when an El Niño event has been forecast or declared (NMFS, 2000).
For North Pacific juveniles, SST conditions may even play a role in the timing of recruitment and ontogenetic shift patterns. In the western Pacific, Ascani et al. (2016) identified the effects of Pacific Decadal Oscillation (PDO) induced changes in SST and ocean circulation on the recruitment of neonate loggerheads into the open ocean. During certain PDO phases, the strength of the Kuroshio Extension Current serves as a physical barrier preventing (or facilitating) turtles from reaching productive TZCF waters. In the CNP, Briscoe et al. (2016) showed that an extended residency was in part due to eastward moving turtles turning around upon reaching cool oceanographic conditions in the eastern North Pacific. Included as part of several intrinsic and extrinsically-motivated hypotheses, they theorized that interannual variability may influence east-west movements and habitat connectivity. Indeed, between 1997 and 2012, the eastern North Pacific experienced high variability in oceanographic conditions (Fleming et al., 2016). Warm and cool episodes were characterized by fluctuating ENSO indices at intervals of approximately 2–4 years, as well as local-forcing events that resulted in anomalous conditions (Sydeman et al., 2006; Fleming et al., 2016; Fiedler and Mantua, 2017). With these physical variations came associated shifts in biological productivity, trophic structure, and species distribution (Hughes, 2000; Mackas et al., 2006; Ainley and Hyrenbach, 2010; Hazen et al., 2014). For turtles queued up in the eastern CNP, we posited that exceptionally warm conditions may provide the environmental cues and thermal requirements necessary to create an ecological bridge - a temporary pathway known to connect two suitable but disparate and distinct habitat regions for highly migratory species (Fromentin et al., 2014; Briscoe et al., 2017) – thus facilitating movement into the CCLME. Based on our previous findings of the behavior of the 231 juveniles, the well-established relationships between movement and temperature, and an exploration of ocean conditions within the region identified by our 6 sentinel loggerheads, we explored the hypothesis that years with anomalously warm conditions could facilitate the recruitment of juvenile loggerheads into the eastern North Pacific and to the Baja California foraging habitat.
Below, we detail our observations and assessments used to support this proposed hypothesis and discuss the importance of future experiments to validate this work. Additional information on materials and methods can be found in the Supplementary Materials section.
Observations
The 6 satellite tracked sea turtles evaluated in this study were deployed at the same date-time and location (176°E, 32.7°N, 4-May-2005, Supplementary Table 1), providing a unique opportunity to concurrently monitor the timing and long-term movements of juveniles into the eastern North Pacific. Impressively, these 6 individuals transmitted an average of 1,116 days (more than 3 years) ± 137 days (range: 898–1,247 days) and over an average distance of 17,115 km ± 2,884 km (range: 12,533 – 20,395 km). All loggerheads tracked the 18°C isotherm across the CNP and moved into the eastern North Pacific during the winter months (October–March), when the TZCF migrates to its southern-most position (Bograd et al., 2004) (Supplementary Figure 1). Of these individuals, only two successfully migrated beyond 130°W (Figures 2B,C); the remaining 4 eventually reversed direction and returned to the CNP (Figures 2A,D–F).
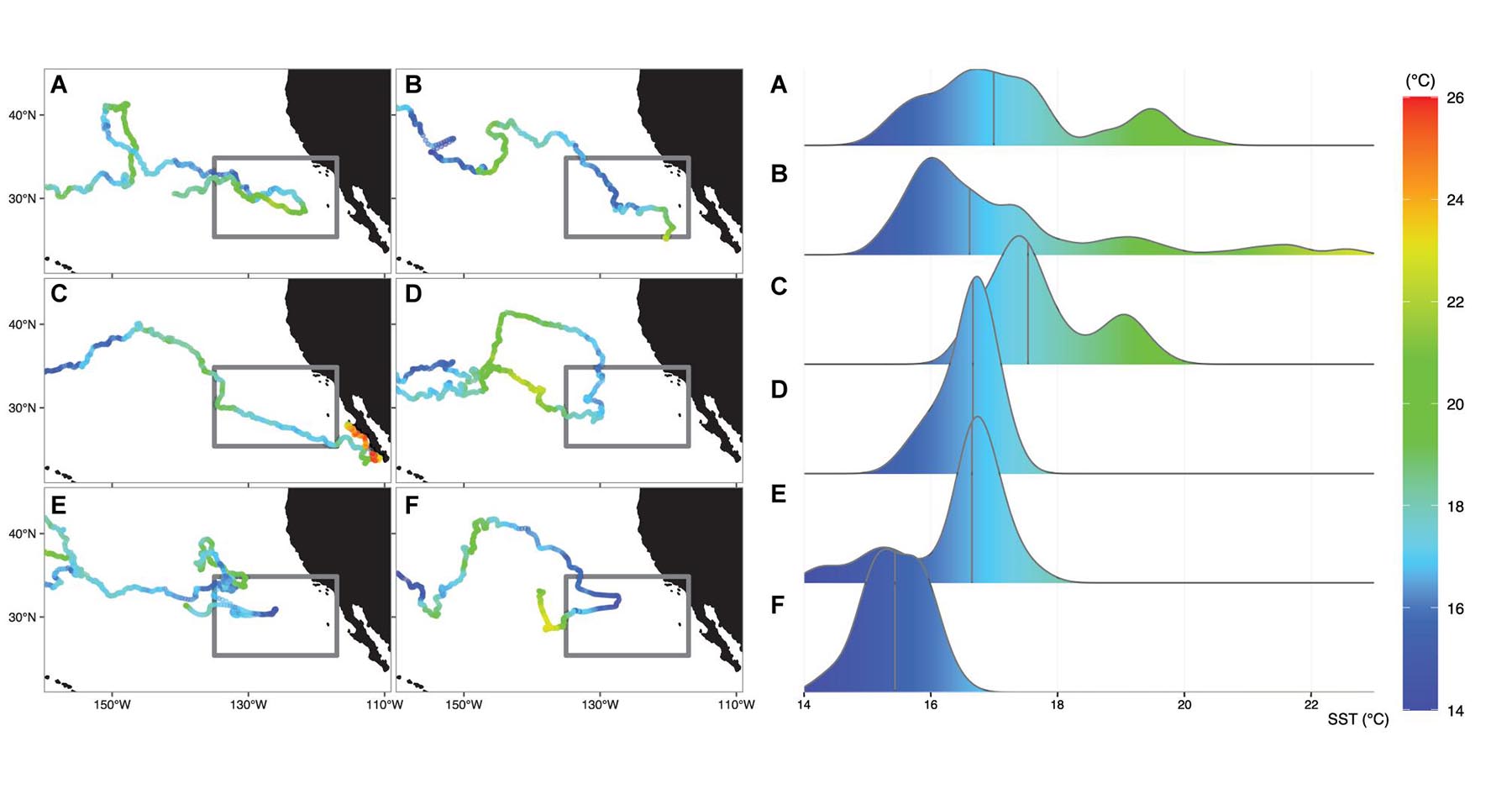
Figure 2. Left panel: Daily sea surface temperatures (SST) extracted under the 6 satellite-tracked individuals that moved into the eastern North Pacific study region (gray box). Turtles (A,D–F) did not complete migration to the North American coast, but instead reversed direction and moved towards the Central North Pacific. Turtles (B,C) successfully moved through the study region in an eastward direction. Right panel: Density distribution of the corresponding daily SSTs experienced by each turtle upon entering the study region and until reaching the location of maximum eastward longitude. Vertical line represents the median SST value.
A cursory examination of the time-matched remotely sensed thermal conditions within the eastern North Pacific study area (Figure 2) showed grouped SSTs to be slightly cooler for the individuals that reversed direction (turtles A, D–F); median: 16.6°C, range: 14.2°C – 20.4°C), compared to the two successful migrators (turtles B and C; median 17.1°C, range 15.1°C – 22.6°C, Supplementary Table 2). Most notably, the two successful migrators tracked more eastward moving days in water warmer than 18°C (46 and 43 days for turtles B and C, respectively, compared to those that reversed direction (31 days for turtle A and 0 days for turtles D-F, Supplementary Table 2). The two juveniles that transitioned towards the coast did so between April-July, suggesting an arrival that would align with peak coastal productivity (Jacox et al., 2015a). Of those that turned around, turtles D, E, and F spent less than 2.5 months in the region before returning to the CNP by April-May. Turtle A arrived within the study area in early March and continued an eastward trajectory until early August. A close inspection of the bimodal distribution in SSTs for this individual (Figure 2A, right panel) indicated that this turtle encountered the warmest SSTs during an area-restricted search just prior to turning around (Figure 2A, left panel). Further investigation of the mesoscale bio-physical processes may shed light on the behavioral movements for this individual.
While these 6 juveniles provided a descriptive understanding of SSTs experienced within the eastern North Pacific, we caveat that the small sample size of satellite tracked individuals limited our ability to draw robust conclusions between real-time environmental conditions and behavioral responses, especially given unknown biophysical lags. Despite this, these individuals provided us with the first-ever glimpse into the seasonal timing of recruitment and the specified location of a migratory pathway, greatly enhancing our previous understanding of these unknown routes.
With this information, we explored the thermal conditioning of this eastern North Pacific pathway with respect to interannual variability. ENSO is a well-documented phenomena (Emery and Hamilton, 1985; Liu and Alexander, 2007) and considerable attention has been paid to changing ocean conditions in the eastern North Pacific driven by this broad scale climatic forcing mechanism. However, it should be noted that oceanographically warm or cool conditions may evolve in the absence of an ENSO event. Recent studies using regional ocean circulation models have begun to provide insight into the importance of regional forcing as a driver of interannual SST variability (Frischknecht et al., 2015; Jacox et al., 2015b), as evidenced by recent marine heatwaves within the California Current Large Marine Ecosystem (CCLME, e.g., the Blob), (Bond et al., 2015; Di Lorenzo and Mantua, 2016; Fiedler and Mantua, 2017). To investigate correlative relationships between remote, broad-scale climate forcing and localized conditions, we compared the monthly SST anomalies in the study region with those in the equatorial Pacific (Niño 3.4, a widely used index of ENSO events) (Trenberth, 1997) over the 15 year study period (Supplementary Figure 2). Our analyses showed only a moderate correlation in phase and amplitude (r = 0.42) These results were similar to those found in a study by Fiedler and Mantua (2017), highlighting the occurrence of more short-term trends in anomalous conditions in the CCLME in the absence of an El Niño or La Niña event (e.g., 2005 and 2012).
Using the timing and location of our sentinel tracks, we characterized the oceanographic conditions that turtles would experience during the two previously identified time periods: (1) the winter months (Oct-Mar) preceding each year of recruitment when the turtles were approaching the eastern CNP (the typical turn-around location); and (2) the following summer months (Apr-Sep) when the turtles were crossing the EPB for coastal recruitment. To account for both remotely forced and localized trends in variability in the absence of ENSO, we classified “warm” and “cool” conditions based on the auto-correlative properties of SST within the study area, as current conditions are influenced by previous ones, and as previously noted, time lags often exist between an environmental state and an animal’s response (Ainley and Hyrenbach, 2010). Within the study area, we found serial correlation of regional SST conditions to persist for up to 3 months (r = 0.51 at 3 months), with statistically significant correlative properties diminishing thereafter (Supplementary Figure 3). In the North Pacific, anomalous SSTs tend to peak in the winter but can persist into the subsequent summer months (Fiedler and Mantua, 2017). For this reason, a warm (or cool) period represented a recruitment year summer (hereafter referred to as “summer, Year 0”) and its preceding winter (“winter, Year −1”). Each period was classified as warm (or cool) if the regional or remotely forced 3-month running mean of SST anomalies exceeded a 0.5°C (−0.5°C) threshold for 3 or more consecutive months. Over the entire study period, we classified 6 warm and 5 cool periods. Warm periods encompassed five ENSO events (1997–1998, 2002–03, 2004–05, 2006–07, 2009–10) and 2005, a year of delayed upwelling and anomalously warm conditions in the California Current (Mackas et al., 2006; Peterson et al., 2006), though not officially an El Niño. Cool periods included the 1998–99, 2000–01, 2007–08, and 2010–11 La Niña events, and 2012, which was considered a cool year without the presence of a La Niña (Fiedler and Mantua, 2017).
To explore our hypothesis through an independent line of evidence, we examined the annual recruitment of loggerheads in relation to these thermal conditions using the humeri of loggerheads stranded along the BCP between 2003 and 2012 (Turner Tomaszewicz et al., 2017). The recruitment year, and then an annual recruitment count, was determined by extending the work of Turner Tomaszewicz et al. (2017). In this study, the humeri were examined using skeletochronology and stable isotope analyses to identify the timing of ontogenetic habitat shifts. Specifically, Turner Tomaszewicz et al. (2017) reported a distinct differentiation in isotope values for 33 loggerheads that revealed the size and age when each turtle transitioned from oceanic waters to the North American coast. Here we continued this analysis to determine the year this habitat shift occurred for each of these individuals. This consisted of assigning the year of stranding to the outermost bone layer and back-assigning the corresponding year to each sequential bone growth layer until the recruitment was dated for each of these 33 individuals, thereby providing an annual turtle recruitment count (Supplementary Materials). Twenty of these individuals had recruitment years associated with warm conditions while a total of 13 individuals recruited during years associated with cool conditions. Annual recruitment during warm ocean conditions was significantly greater than annual recruitment during cool conditions (one-tailed Mann-Whitney, P = 0.0201 Figure 3).
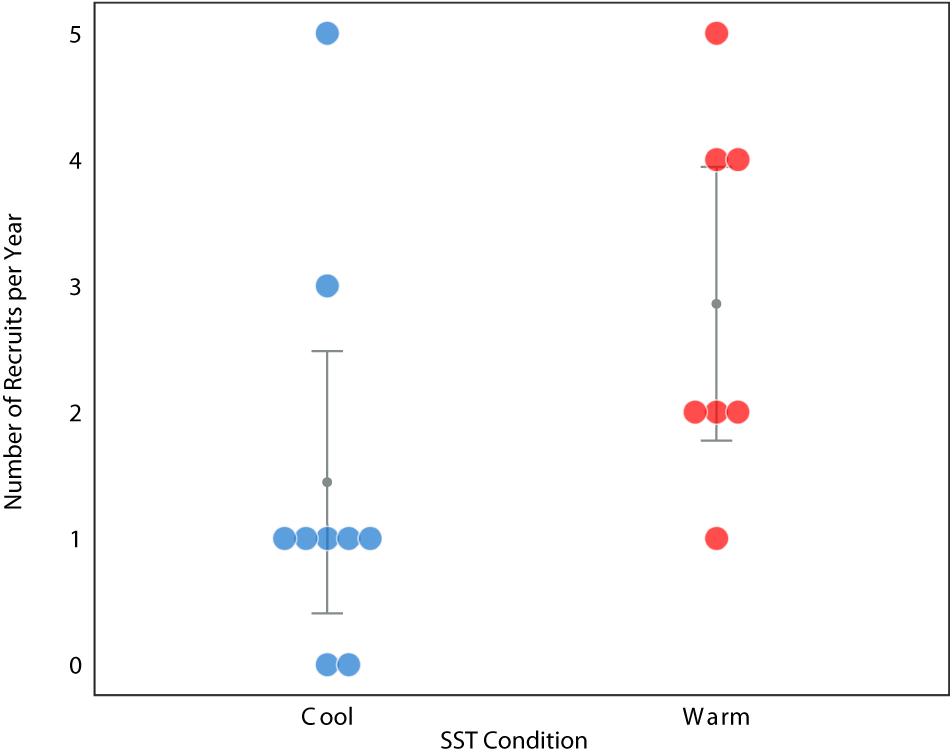
Figure 3. Relative number of annual juvenile loggerhead sea turtle recruits during years of cool (blue) and warm (red) regional ocean conditions in the eastern North Pacific Ocean study area (Figure 1B) between 1997–2012, n = 33. Raw data values are shown with color dots. Error bars and dark circle represent the grouped median and standard error.
Discussion
Out of our previously published 15-year study tracking the multi-year movements of 231 satellite tagged turtles, the movements of just 6 sentinel individuals examined here offer the first empirical insight into a previously unknown migratory pathway and the capability to offer a new interpretation to explain a long-standing mystery of one of the ocean’s greatest migrants. The tracking of these individuals, coupled with remotely sensed oceanographic conditions and novel methods in sequential sampling of annual bone growth layers, provided us with the ability to elucidate juvenile recruitment in relation to favorable ocean conditions. From these data, we propose a new hypothesis regarding a possible physical mechanism for habitat connectivity and provide the first evidence of a “thermal corridor” that would allow for pulsed recruitment of loggerheads from the Central North Pacific to the North American coast.
The spatial composites of SST anomalies across the study region illustrate the year-to-year variability in oceanographic conditions that were associated with annual recruitment to the eastern Pacific (Figure 4). During warm periods, it appears a transport pathway opened intermittently as a function of the warm sea surface temperatures, thereby creating a thermal corridor that may facilitate successful recruitment across the Eastern Pacific Barrier to coastal foraging grounds (Figure 4D). The corridor was not only present during the summer but showed a preconditioning of warmer temperatures in the preceding winter months (Figure 4B). Such anomalous conditions, especially if sustained for several months at a time, may provide key environmental cues to animals concentrated in the eastern edge of the CNP that the thermal corridor is opening. By contrast, during cool years the thermal corridor appears to close (Figures 4A,C), leading to relatively weak or no coastal recruitment. These conditions possibly prompt eastward-moving turtles to turn around and remain in the CNP. The one exception was 2007, which met the criteria for cool conditions but experienced relatively high recruitment (n = 5, Figure 3). In this instance, individuals may have exploited the warm but receding summer SST anomalies (0.1°C–0.3°C) that persisted as the California Current transitioned from a moderate El Niño to a strong La Niña event.
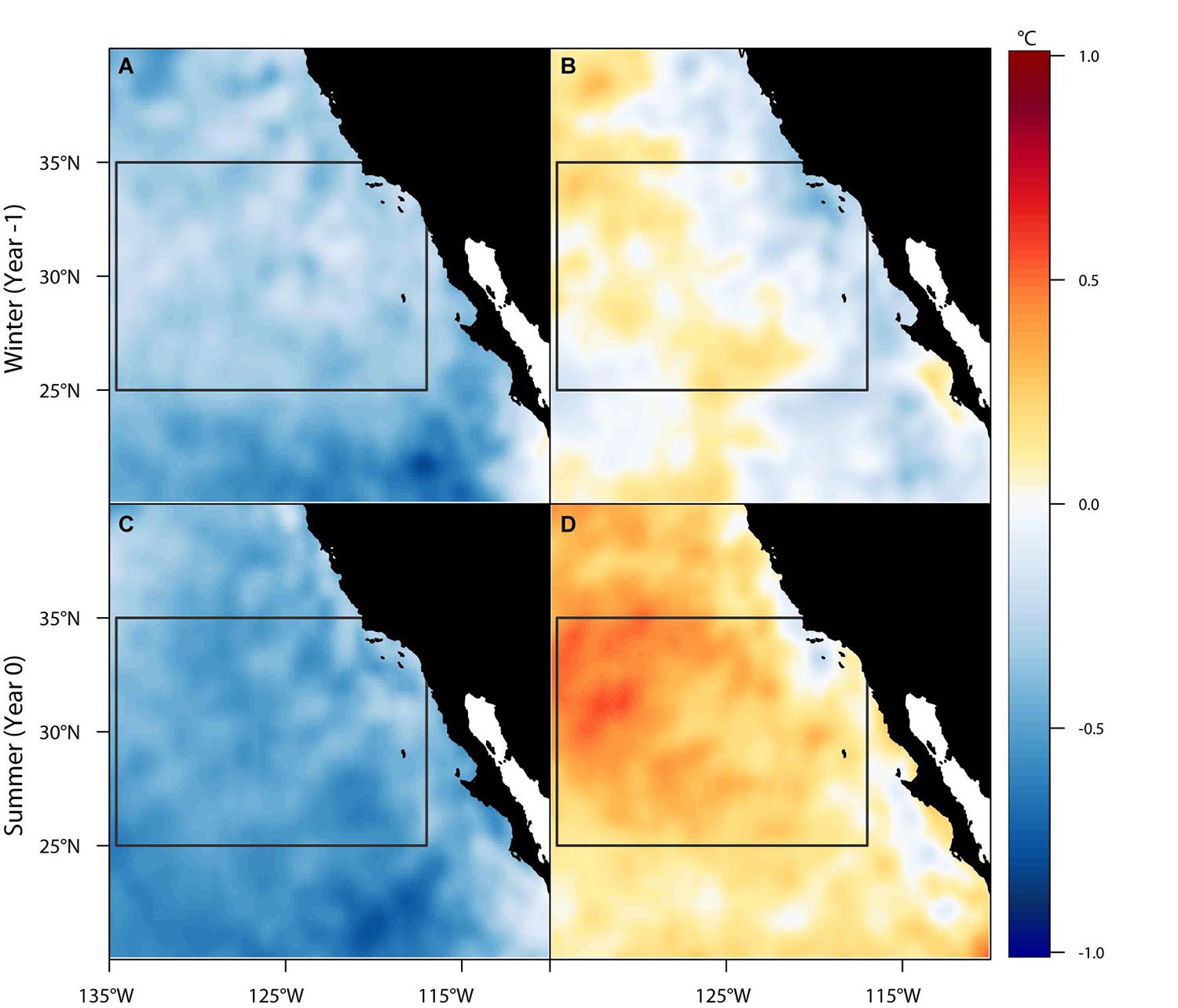
Figure 4. Six-month mean spatial composites of monthly SST anomalies for cool (A,C) and warm (B,D) ocean conditions during recruitment year summers (Apr-Sep, Year 0) and their preceding winters (Oct-Mar, Year -1). The region utilized by the six easternmost transiting juvenile loggerhead sea turtles is outlined in gray and represents the location of a thermal corridor that may facilitate the successful recruitment to coastal foraging grounds.
The idea that fluctuations in ocean-atmosphere conditions can modulate recruitment dynamics is not new but is less frequently demonstrated. For North Pacific loggerheads, the theory that eastern boundary recruitment may be tied to changing ocean conditions is in agreement with several independently established observations. First, the aforementioned role of SST as a significant predictor in sea turtle movement and distribution (Spotila and Standora, 1985), and thus their well-documented coupling and sensitivity to thermal conditions (e.g., McMahon and Hays, 2006; Hawkes et al., 2007). Second, interannual variability and anomalous SSTs have been shown to influence migration strategies of several other highly mobile species in the North Pacific, including albacore (Thunnus alalonga) (Kimura et al., 1997), eels (Anguila japonica) (Lin et al., 2017), and northern elephant seals (Mirounga angustirostris) (Abrahms et al., 2018). Finally, we drew upon the highly relevant studies that have linked interannual and decadal oscillations to indices of loggerhead sea turtle recruitment. As previously mentioned, Ascani et al. (2016) provided evidence of recruitment and survival of neonate North Pacific loggerheads into the TZCF as modulated by synchronous basin-wide changes in SST associated with the PDO. In a negative PDO phase, the Kuroshio Extension Current is strong, acting as a barrier that prevents northward transport into the productive TZCF that is otherwise favored during a positive phase. In the North Atlantic, Báez et al. (2011) have linked environmental variability to east-west migrations for juvenile loggerheads. The western Mediterranean serves as a staging area for late stage juveniles preparing to return to the western Atlantic (Eckert et al., 2008). During positive phases of the North Atlantic Oscillation, strong winds and cool SSTs impede migration back to the western Atlantic, forcing an accumulation of loggerheads around the Strait of Gibraltar and a significant increase in strandings (Báez et al., 2011).
These studies, combined with advancements in biologging and satellite telemetry have greatly enhanced our ability to understand how migratory animals move through their environment. However, tracking individuals through multiple life history stages remains inherently difficult (Hazen et al., 2012a), especially for a long-lived, slow growing species that remains in the juvenile stage for up to several decades (Turner Tomaszewicz et al., 2015). Despite the unique opportunity to observe recruitment during an otherwise cryptic life history stage, our multi-year dataset represents only a snapshot of this important developmental period. At present, the small number of individuals that moved into the eastern North Pacific limits the power associated with our ability to fully test our hypothesis. However, exploring the behavior of a unique subset of the population should not be underestimated. McMahon and Hays (2006) and Caughley and Gunn (1996) aptly noted that such limitations in sample size should not preclude crucial life-history information from being gathered or studied; rather it is imperative that endangered animals be studied while they can, so that the most informed conservation and management decisions can be made (McMahon and Hays, 2006). This is especially true given that another dataset of this magnitude (i.e., several hundred individuals from a single population over a 15-year period) may be quite difficult to reassemble. Our preliminary evaluation provides novel insights into the existence of a dynamic ocean corridor, but future satellite tagging and bone stable isotope studies focused within the eastern North Pacific are necessary to experimentally test our hypothesis under varying ocean conditions. In the interim, other lines of evidence by Allen et al. (2013), Eguchi et al. (2018), and Welch et al. (2019) provide strong support to the proposed thermal corridor. Recently, the ephemeral presence of large numbers of juveniles off the United States West Coast coincided with the 2014–2015 El Niño and 2015–2016 marine heatwave (Eguchi et al., 2018; Welch et al., 2019) and triggered the operational closure of the California Drift Gillnet fishery. A study by Allen et al. (2013) confirmed that the influx of these small juveniles into the Southern California Bight during anomalously warm water events were in fact open ocean recruits from the CNP and not displaced coastal residents. Distinct stable isotope values provided evidence to dismiss the theory that these individuals were already BCP coastal recruits following warmer water northwards. These studies, combining data from loggerhead aerial surveys, at-sea-sightings, stranding records, and tissue samples support our hypothesis of a thermal corridor facilitating coastal recruitment during anomalously warm ocean conditions.
Oceanographic conditions are expected to become more extreme with climate change, leading to shifts in the distributions and migratory pathways of sea turtles, sea birds, marine mammals, and pelagic fishes (Hazen et al., 2012b). If a thermal corridor across the Eastern Pacific Barrier were to open more frequently in a warming ocean, it may result in an increase in the relative abundance of loggerheads and other highly migratory marine megafauna shifting from the Central North Pacific to foraging grounds along the eastern Pacific coast (e.g., Pacific bluefin tuna (Kitagawa et al., 2007), white sharks (Tanaka et al., 2021), and northern elephant seals (Abrahms et al., 2018). Finally, a change in the distribution of protected species can pose increased conservation challenges. For the North Pacific loggerhead, it could mean higher exposure to significant regional bycatch not only for the Baja California coast (Peckham et al., 2008), but for other potentially important North American foraging grounds, including the Southern California Bight (Welch et al., 2019).
As the planet undergoes unprecedented changes in the anthropogenic era, barriers to species dispersal that were once considered “impassable,” like the Eastern Pacific Barrier, are being redefined and so are the associated ecological implications. Understanding mechanisms facilitating migration between discrete habitats and behavioral responses of species, like the North Pacific loggerhead, is crucial to interpreting the life history, ontogenetic habitats, and threats these species face at sea. For sea turtles, Archie Carr’s “lost years” puzzle is still one of most substantial of all obstacles to understanding the ecology and conservation challenges of sea turtles, but multiple emerging technologies and analyses can now be integrated to elucidate this challenging problem. The puzzle is far from complete, but the picture is coming into view.
Data Availability Statement
Publicly available datasets were analyzed in this study.
Author Contributions
DB and LC conceived the hypothesis and its evaluation. TS, MK, and HO provided animal care. GB, MR, TS, and MK conducted fieldwork including tag deployment. DB, CT, and LC conceived and designed methodology. DP, GB, JP, DB, CT, and JS acquired and analyzed data. DB and CT performed formal analyses. DB led and CT, LC helped with the drafting of the manuscript. All authors contributed to the draft revisions and gave final approval for publication.
Funding
Funding for DB was provided by the Crowder Lab at Hopkins Marine Station, Stanford University. Funding for CT was provided by NMFS Southwest Fisheries Science Center and the National Research Council Research Associateship. Funding for LC was provided by the Department of Biology and by the Woods Institute for the Environment, Stanford University.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to gratefully acknowledge the personnel at Port of Nagoya Public Aquarium, S. Hoyt Peckham and the community and members of Grupo Tortuguero de las Californias for collection of bone samples from BCP, Mexico. The authors wish to thank Steven Bograd and Elliott Hazen for their constructive feedback in the early stages of this work, and the reviewers for their helpful contributions that improved this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.630590/full#supplementary-material
References
Abecassis, M., Senina, I., Lehodey, P., Gaspar, P., Parker, D., Balazs, G., et al. (2013). A model of loggerhead sea turtle (Caretta caretta) habitat and movement in the oceanic North Pacific. PLoS One 8:e73274. doi: 10.1371/journal.pone.0073274
Abrahms, B., Hazen, E. L., Bograd, S. J., Brashares, J. S., Robinson, P. W., Scales, K. L., et al. (2018). Climate mediates the success of migration strategies in a marine predator. Ecol. Lett. 21, 63–71. doi: 10.1111/ele.12871
Ainley, D. G., and Hyrenbach, K. D. (2010). Top-down and bottom-up factors affecting seabird population trends in the California current system (1985–2006). Prog. Oceanogr. 84, 242–254. doi: 10.1016/j.pocean.2009.10.001
Allen, C. D., Lemons, G. E., Eguchi, T., Leroux, R. A., Fahy, C. C., Dutton, P. H., et al. (2013). Stable isotope analysis reveals migratory origin of loggerhead turtles in the Southern California Bight. Mar. Ecol. Prog. Ser. 472, 275–285. doi: 10.3354/meps10023
Ascani, F., Van Houtan, K. S., Di Lorenzo, E., Polovina, J. J., and Jones, T. T. (2016). Juvenile recruitment in loggerhead sea turtles linked to decadal changes in ocean circulation. Glob. Change Biol. 22, 3529–3538. doi: 10.1111/gcb.13331
Báez, J. C., Bellido, J. J., Ferri-Yáñez, F., Castillo, J. J., Martín, J. J., Mons, J. L., et al. (2011). The North Atlantic Oscillation and sea surface temperature affect loggerhead abundance around the Strait of Gibraltar. Sci. Marina 75, 571–575. doi: 10.3989/scimar.2011.75n3571
Baums, I. B., Boulay, J. N., Polato, N. R., and Hellberg, M. E. (2012). No gene flow across the Eastern Pacific Barrier in the reef−building coral Porites lobata. Mol. Ecol. 21, 5418–5433. doi: 10.1111/j.1365-294x.2012.05733.x
Bentivegna, F. (2002). Intra-Mediterranean migrations of loggerhead sea turtles (Caretta caretta) monitored by satellite telemetry. Mar. Biol. 141, 795–800. doi: 10.1007/s00227-002-0856-z
Bograd, S. J., Foley, D. G., Schwing, F., Wilson, C., Laurs, R., Polovina, J., et al. (2004). On the seasonal and interannual migrations of the transition zone chlorophyll front. Geophys. Res. Lett. 31, 1–5.
Bond, N. A., Cronin, M. F., Freeland, H., and Mantua, N. (2015). Causes and impacts of the 2014 warm anomaly in the NE Pacific. Geophys. Res. Lett. 42, 3414–3420. doi: 10.1002/2015gl063306
Bowen, B., Abreu-Grobois, F., Balazs, G., Kamezaki, N., Limpus, C., and Ferl, R. (1995). Trans-Pacific migrations of the loggerhead turtle (Caretta caretta) demonstrated with mitochondrial DNA markers. Proc. Natl. Acad. Sci. U.S.A. 92, 3731–3734. doi: 10.1073/pnas.92.9.3731
Briggs, J. C. (1961). The east Pacific barrier and the distribution of marine shore fishes. Evolution 15, 545–554. doi: 10.1111/j.1558-5646.1961.tb03184.x
Briscoe, D., Parker, D., Bograd, S., Hazen, E., Scales, K., Balazs, G., et al. (2016). Multi-year tracking reveals extensive pelagic phase of juvenile loggerhead sea turtles in the North Pacific. Move. Ecol. 4, 1–12.
Briscoe, D. K., Hobday, A. J., Carlisle, A., Scales, K., Eveson, J. P., Arrizabalaga, H., et al. (2017). Ecological bridges and barriers in pelagic ecosystems. Deep Sea Res. II 140, 182–192. doi: 10.1016/j.dsr2.2016.11.004
Carr, A. (1980). Some problems of sea turtle ecology. Am. Zool. 20, 489–498. doi: 10.1093/icb/20.3.489
Caughley, G., and Gunn, A. (1996). Conservation Biology in Theory and Practice. Cambridge, MA: Blackwell Science.
Darwin, C. (1872). The Origin of Species by Means of Natural Selection. Garden City, NY: Doubleday & Co.
Di Lorenzo, E., and Mantua, N. (2016). Multi-year persistence of the 2014/15 North Pacific marine heatwave. Nat. Clim. Change 6, 1042–1047. doi: 10.1038/nclimate3082
Dutton, P. H., Jensen, M. P., Frey, A., Lacasella, E., Balazs, G. H., Zárate, P., et al. (2014). Population structure and phylogeography reveal pathways of colonization by a migratory marine reptile (Chelonia mydas) in the central and eastern Pacific. Ecol. Evol. 4, 4317–4331. doi: 10.1002/ece3.1269
Eckert, S. A., Moore, J. E., Dunn, D. C., Van Buiten, R. S., Eckert, K. L., and Halpin, P. N. (2008). Modeling loggerhead turtle movement in the Mediterranean: importance of body size and oceanography. Ecological Applications 18, 290–308. doi: 10.1890/06-2107.1
Eguchi, T., Mcclatchie, S., Wilson, C., Benson, S. R., Leroux, R. A., and Seminoff, J. A. (2018). Loggerhead turtles (Caretta caretta) along the US west coast: abundance, distribution, and anomalous warming of the North Pacific. Front. Mar. Sci. 5:1–15. doi: 10.3389/fmars.2018.00452
Emery, W. J., and Hamilton, K. (1985). Atmospheric forcing of interannual variability in the northeast Pacific Ocean: connections with El Niño. J. Geophys. Res. Oceans 90, 857–868.
Epperly, S. P., Braun, J., Chester, A. J., Cross, F. A., Merriner, J. V., and Tester, P. A. (1995). Winter distribution of sea turtles in the vicinity of Cape Hatteras and their interactions with the summer flounder trawl fishery. Bull. Mar. Sci. 56, 547–568.
Fiedler, P. C., and Mantua, N. J. (2017). How are warm and cool years in the California Current related to ENSO? J. Geophys. Res. Oceans 122, 5936–5951. doi: 10.1002/2017jc013094
Fleming, A. H., Clark, C. T., Calambokidis, J., and Barlow, J. (2016). Humpback whale diets respond to variance in ocean climate and ecosystem conditions in the California Current. Glob. Change Biol. 22, 1214–1224. doi: 10.1111/gcb.13171
Frischknecht, M., Münnich, M., and Gruber, N. (2015). Remote versus local influence of ENSO on the California current system. J. Geophys. Res. Oceans 120, 1353–1374. doi: 10.1002/2014jc010531
Fromentin, J. M., Reygondeau, G., Bonhommeau, S., and Beaugrand, G. (2014). Oceanographic changes and exploitation drive the spatio−temporal dynamics of Atlantic bluefin tuna (Thunnus thynnus). Fish Oceanogr. 23, 147–156. doi: 10.1111/fog.12050
Gilman, E., Kobayashi, D., Swenarton, T., Brothers, N., Dalzell, P., and Kinan-Kelly, I. (2007). Reducing sea turtle interactions in the Hawaii-based longline swordfish fishery. Biol. Conserv. 139, 19–28. doi: 10.1016/j.biocon.2007.06.002
Hawkes, L. A., Broderick, A. C., Coyne, M. S., Godfrey, M. H., and Godley, B. J. (2007). Only some like it hot - quantifying the environmental niche of the loggerhead sea turtle. Divers. Distrib. 13, 447–457. doi: 10.1111/j.1472-4642.2007.00354.x
Hazen, E. L., Jorgensen, S., Rykaczewski, R. R., Bograd, S. J., Foley, D. G., Jonsen, I. D., et al. (2012b). Predicted habitat shifts of Pacific top predators in a changing climate. Nat. Clim. Change 3, 234–238. doi: 10.1038/nclimate1686
Hazen, E. L., Maxwell, S. M., Bailey, H., Bograd, S. J., Hamann, M., Gaspar, P., et al. (2012a). Ontogeny in marine tagging and tracking science: technologies and data gaps. Mar. Ecol. Prog. Ser. 457, 221–240. doi: 10.3354/meps09857
Hazen, E. L., Schroeder, I. D., Peterson, J., Peterson, B., Sydeman, W. J., Thompson, S. A., et al. (2014). ““Oceanographic and Climatic Drivers and Pressures,”,” in The California Current Integrated Ecosystem Assessment: Phase III Report, eds C. J. Harvey, N. Garfield, E. L. Hazen, and G. D. Williams (Washington, DC: NOAA).
Howell, E. A., Kobayashi, D. R., Parker, D. M., Balazs, G. H., and Polovina, A. (2008). TurtleWatch: a tool to aid in the bycatch reduction of loggerhead turtles Caretta caretta in the Hawaii-based pelagic longline fishery. Endang Species Res 5, 267–278. doi: 10.3354/esr00096
Hughes, L. (2000). Biological consequences of global warming: is the signal already apparent? Trends Ecol. Evol. 15, 56–61. doi: 10.1016/s0169-5347(99)01764-4
IUCN (2018). IUCN. Available online at: https://www.iucnredlist.org/
Jacox, M. G., Bograd, S. J., Hazen, E. L., and Fiechter, J. (2015b). Sensitivity of the California Current nutrient supply to wind, heat, and remote ocean forcing. Geophys. Res. Lett. 42, 5950–5957. doi: 10.1002/2015gl065147
Jacox, M. G., Fiechter, J., Moore, A. M., and Edwards, C. A. (2015a). ENSO and the California Current coastal upwelling response. J. Geophys. Res. Oceans 120, 1691–1702. doi: 10.1002/2014jc010650
Kimura, S., Sugimoto, M. N., and Takashige. (1997). Migration of albacore, Thunnus alalunga, in the North Pacific Ocean in relation to large oceanic phenomena. Fish. Oceanogr. 6, 51–57. doi: 10.1046/j.1365-2419.1997.00029.x
Kitagawa, T., Boustany, A. M., Farwell, C. J., Williams, T. D., Castleton, M. R., and Block, B. A. (2007). Horizontal and vertical movements of juvenile bluefin tuna (Thunnus orientalis) in relation to seasons and oceanographic conditions in the eastern Pacific Ocean. Fish. Oceanogr. 16, 409–421. doi: 10.1111/j.1365-2419.2007.00441.x
Kobayashi, D. R., Polovina, J. J., Parker, D. M., Kamezaki, N., Cheng, I. J., Uchida, I., et al. (2008). Pelagic habitat characterization of loggerhead sea turtles, Caretta caretta, in the North Pacific Ocean (1997–2006): Insights from satellite tag tracking and remotely sensed data. J. Exp. Mar. Biol. Ecol. 356, 96–114. doi: 10.1016/j.jembe.2007.12.019
Lessios, H. A., and Robertson, D. R. (2006). Crossing the impassable: genetic connections in 20 reef fishes across the eastern Pacific barrier. Proc. Biol. Sci. 273, 2201–2208. doi: 10.1098/rspb.2006.3543
Lin, Y.-F., Wu, C.-R., and Han, Y.-S. (2017). A combination mode of climate variability responsible for extremely poor recruitment of the Japanese eel (Anguilla japonica). Sci. Rep. 7:44469.
Liu, Z., and Alexander, M. (2007). Atmospheric bridge, oceanic tunnel, and global climatic teleconnections. Rev. Geophys. 45, 1–34.
Mackas, D., Peterson, W., Ohman, M., and Lavaniegos, B. (2006). Zooplankton anomalies in the California Current system before and during the warm ocean conditions of 2005. Geophys. Res. Lett. 33, 1–7.
McMahon, C. R., and Hays, G. C. (2006). Thermal niche, large−scale movements and implications of climate change for a critically endangered marine vertebrate. Glob. Change Biol. 12, 1330–1338. doi: 10.1111/j.1365-2486.2006.01174.x
Nichols, W., Resendiz, A., Seminoff, J., and Resendiz, B. (2000). Transpacific migration of a loggerhead turtle monitored by satellite telemetry. Bull. Mar. Sci. 67, 937–947.
NMFS (2000). Biological Opinion. Endangered Species Act Section 7 Consultation on Authorization to Take Listed Marine Mammals Incidental to Commercial Fishing Operations Under Section 101(a)(5)(E) of the Marine Mammal Protection Act for the California/Oregon Drift Gillnet Fishery. Silver Spring, MD: NMFS.
Peckham, S. H., Maldonado-Diaz, D., Koch, V., Mancini, A., Gaos, A., Tinker, M. T., et al. (2008). High mortality of loggerhead turtles due to bycatch, human consumption and strandings at Baja California Sur. Mexico, 2003 to 2007. Endang. Species Res. 5, 171–183. doi: 10.3354/esr00123
Peckham, S. H., Maldonado-Diaz, D., Tremblay, Y., Ochoa, R., Polovina, J., Balazs, G., et al. (2011). Demographic implications of alternative foraging strategies in juvenile loggerhead turtles Caretta caretta of the North Pacific Ocean. Mar. Ecol. Prog. Ser. 425, 269–280. doi: 10.3354/meps08995
Peterson, W. T., Emmett, R., Goericke, R., Venrick, E., Mantyla, A., Bograd, S. J., et al. (2006). The state of the California Current, 2005-2006: warm in the north, cool in the south. California Cooper. Ocean. Fish. Invest. Rep. 47, 1–46.
Polovina, J., Uchida, I., Balazs, G., Howell, E. A., Parker, D., and Dutton, P. (2006). The Kuroshio extension bifurcation region: a pelagic hotspot for juvenile loggerhead sea turtles. Deep Sea Res. II 53, 326–339. doi: 10.1016/j.dsr2.2006.01.006
Polovina, J. J., Balazs, G. H., Howell, E. A., Parker, D. M., Seki, M. P., and Dutton, P. H. (2004). Forage and migration habitat of loggerhead (Caretta caretta) and olive ridley (Lepidochelys olivacea) sea turtles in the central North Pacific Ocean. Fish Oceanogr. 13, 36–51. doi: 10.1046/j.1365-2419.2003.00270.x
Polovina, J. J., Howell, E., Kobayashi, D. R., and Seki, M. P. (2001). The transition zone chlorophyll front, a dynamic global feature defining migration and forage habitat for marine resources. Prog. Oceanogr. 49, 469–483. doi: 10.1016/s0079-6611(01)00036-2
Polovina, J. J., Kobayashi, D. R., Parker, D. M., Seki, M. P., and Balazs, G. H. (2000). Turtles on the edge: movement of loggerhead turtles (Caretta caretta) along oceanic fronts, spanning longline fishing grounds in the central North Pacific, 1997–1998. Fish. Oceanogr. 9, 71–82. doi: 10.1046/j.1365-2419.2000.00123.x
Resendiz, A., Resendiz, B., Nichols, W. J., Seminoff, J. A., and Kamezaki, N. (1998). First confirmed east-west transpacific movement of a loggerhead sea turtle,Caretta caretta, released in Baja California. Mexico. Pac. Sci. 52, 151–153.
Seminoff, J., Alfaro Shigueto, J., Amorocho, D., Arauz, R., Baquero, A., Chacón, D., et al. (2012). “”Biology and conservation of sea turtles in the eastern Pacific Ocean: a general overview”,” in Sea Turtles of the Eastern Pacific: Advances in Research and Conservation, eds J. A. Seminoff and B. P. Wallace (Tucson: University of Arizona Press), 301.
Seminoff, J. A., Eguchi, T., Carretta, J., Allen, C. D., Prosperi, D., Rangel, R., et al. (2014). Loggerhead sea turtle abundance at a foraging hotspot in the eastern Pacific Ocean: implications for at-sea conservation. Endang. Species Res. 24, 207–220. doi: 10.3354/esr00601
Spotila, J. R., and Standora, E. A. (1985). Environmental constraints on the thermal energetics of sea turtles. Copeia 694–702. doi: 10.2307/1444763
Sydeman, W. J., Bradley, R. W., Warzybok, P., Abraham, C. L., Jahncke, J., Hyrenbach, K. D., et al. (2006). Planktivorous auklet (Ptychoramphus aleuticus) responses to ocean climate, 2005: unusual atmospheric blocking? Geophys. Res. Lett. 33, 1–5.
Tanaka, K. R., Van Houtan, K. S., Mailander, E., Dias, B. S., Galginaitis, C., O’Sullivan, J., et al. (2021). North Pacific warming shifts the juvenile range of a marine apex predator. Sci. Rep. 11:3373. doi: 10.1038/s41598-021-82424-9
Turner Tomaszewicz, C. N., Seminoff, J. A., Avens, L., Goshe, L. R., Peckham, S. H., Rguez-Baron, J. M., et al. (2015). Age and residency duration of loggerhead turtles at a North Pacific bycatch hotspot using skeletochronology. Biol. Conserv. 186, 134–142. doi: 10.1016/j.biocon.2015.03.015
Turner Tomaszewicz, C. N., Seminoff, J. A., Peckham, S. H., Avens, L., and Kurle, C. M. (2017). Intrapopulation variability in the timing of ontogenetic habitat shifts in sea turtles revealed using δ15N values from bone growth rings. J. Anim. Ecol. 86, 694–704. doi: 10.1111/1365-2656.12618
Uchida, S., and Teruya, H. (1988). “Transpacific migration of a tagged loggerhead Caretta caretta,” in Proceedings of the International Symposium on Sea Turtles, Himeji.
Watanabe, K. K., Hatase, H., Kinoshita, M., Omuta, K., Bando, T., Kamezaki, N., et al. (2011). Population structure of the loggerhead turtle Caretta caretta, a large marine carnivore that exhibits alternative foraging behaviors. Mar. Ecol. Prog. Ser. 424, 273–283. doi: 10.3354/meps08989
Welch, H., Hazen, E. L., Briscoe, D. K., Bograd, S. J., Jacox, M. G., Eguchi, T., et al. (2019). Environmental indicators to reduce loggerhead turtle bycatch offshore of Southern California. Ecol. Indic. 98, 657–664. doi: 10.1016/j.ecolind.2018.11.001
Keywords: loggerhead sea turtle, distribution, satellite remote sensing, sea surface temperature, habitat connectivity, migration, corridors
Citation: Briscoe DK, Turner Tomaszewicz CN, Seminoff JA, Parker DM, Balazs GH, Polovina JJ, Kurita M, Okamoto H, Saito T, Rice MR and Crowder LB (2021) Dynamic Thermal Corridor May Connect Endangered Loggerhead Sea Turtles Across the Pacific Ocean. Front. Mar. Sci. 8:630590. doi: 10.3389/fmars.2021.630590
Received: 17 November 2020; Accepted: 12 February 2021;
Published: 08 April 2021.
Edited by:
Alastair Martin Mitri Baylis, South Atlantic Environmental Research Institute, Falkland IslandsReviewed by:
Gail Schofield, Queen Mary University of London, United KingdomPaolo Luschi, University of Pisa, Italy
Copyright © 2021 Briscoe, Turner Tomaszewicz, Seminoff, Parker, Balazs, Polovina, Kurita, Okamoto, Saito, Rice and Crowder. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dana K. Briscoe, ZGFuYWticmlzY29lQGdtYWlsLmNvbQ==
 Dana K. Briscoe
Dana K. Briscoe Calandra N. Turner Tomaszewicz4
Calandra N. Turner Tomaszewicz4 Jeffrey A. Seminoff
Jeffrey A. Seminoff Hitoshi Okamoto
Hitoshi Okamoto