- Hawaii Institute of Marine Biology, University of Hawai'i at Mānoa, Kaneohe, HI, United States
Coral reef restoration is an attractive tool for the management of degraded reefs; however, conventional restoration approaches will not be effective under climate change. More proactive restoration approaches must integrate future environmental conditions into project design to ensure long-term viability of restored corals during worsening bleaching events. Corals exist along a continuum of stress-tolerant phenotypes that can be leveraged to enhance the thermal resilience of reefs through selective propagation of heat-tolerant colonies. Several strategies for selecting thermally tolerant stock are currently available and range broadly in scalability, cost, reproducibility, and specificity. Different components of the coral holobiont have different utility to practitioners as diagnostics and drivers of long-term phenotypes, so selection strategies can be tailored to the resources and goals of individual projects. There are numerous unknowns and potential trade-offs to consider, but we argue that a focus on thermal tolerance is critical because corals that do not survive bleaching cannot contribute to future reef communities at all. Selective propagation uses extant corals and can be practically incorporated into existing restoration frameworks, putting researchers in a position to perform empirical tests and field trials now while there is still a window to act.
Introduction
Like many natural resources, coral reefs are facing the consequences of climate change. Regardless of contemporary reductions in emissions, Earth is committed to several degrees of warming (Sherwood et al., 2020) that will impact a wide range of ecosystems. Ocean warming is causing increasingly frequent and severe thermal stress events on coral reefs, triggering bleaching that results in physiologically and metabolically impaired corals. When corals become so severely compromised that they are unable to recover from temperature stress, reef ecosystems degrade with immediate and latent consequences (Hughes et al., 2018). Climate change has already caused dramatic losses in coral cover worldwide (Wilkinson, 2004). Without intervention, the rate of change in environmental conditions will likely soon outpace natural flexibility and adaptive capacity (Bay et al., 2017; Matz et al., 2018) to maintain functional coral reefs and the ecosystem services they provide. The serious implications for food security, coastal protection, and biodiversity have compelled the search for active management solutions and expanded interest in reef restoration projects (van Oppen et al., 2017).
Resource managers are increasingly aware that win-win outcomes that conserve biodiversity and maintain human interests may be impossible (McShane et al., 2011). Many research groups and management agencies are calling for immediate exploration of non-conventional management strategies and dramatic human interventions for coral reefs while they can still be effective (Hardisty et al., 2019; National Academies of Sciences Engineering and Medicine, 2019; Anthony et al., 2020). Strategies involving reef restoration are attractive because they offer a direct intervention using physical and logistical techniques that are already established. However, under climate change, conventional restoration approaches must be modified to be more proactive, accounting for future conditions, because restoration using coral stock that is intolerant of climate change will likely be inefficient and ineffective.
These proactive restoration efforts must be conceptually reasonable, have manageable or scalable risk, and be logistically feasible. Here we argue that coupling coral propagation and reef restoration practices with methods for identifying heat-tolerant corals meets these criteria and should be assiduously explored. Selective propagation of local thermally tolerant coral stocks uses existing corals and techniques, can be readily integrated with ongoing restoration programs, and theoretically enhances the temperature resilience of the outplant site. To establish the efficacy of the approach while it still has relevance, field-testing should be performed now.
Aims and Practices of Coral Reef Restoration
Ecological restoration is defined as “the process of assisting the recovery of an ecosystem that has been degraded, damaged, or destroyed” and a successfully restored ecosystem “contains sufficient biotic and abiotic resources to continue its development without further assistance or subsidy” (Society for Ecological Restoration International Science and Policy Working Group, 2004). Since scleractinian corals are the foundation of the coral reef ecosystem, interventions to increase the amount of living hard coral cover are a primary focus in reef restoration projects (Precht and Robbart, 2009), which typically target degradation traceable to anthropogenic activities for which mitigation measures exist (e.g., ship grounding, dredging, localized runoff). Coral reef restoration is still an emerging field undergoing technological and conceptual innovation (Omori, 2019). Improving restoration techniques and furthering ecological knowledge have been the main motivations for reef restoration projects over the past several decades (Bayraktarov et al., 2019) and best practices are emerging with the lessons learned.
The majority of coral reef restoration projects currently involve direct outplanting of whole or fragmented corals chosen opportunistically and transplanted. Fragmentation of hard corals was pioneered and developed by the commercial aquarium trade (Delbeek, 2001) and is utilized extensively by reef restoration practitioners to asexually propagate coral stock for transplantation (Rinkevich, 2014; Boström-Einarsson et al., 2020). The “coral gardening” approach has adapted this technique to include an intermediate nursery phase (either in situ or ex situ). Coral gardening allows time for fragments to recover and grow to an adequate size before outplanting (Rinkevich, 1995, 2005) and represents a “sustainable” source of material for restoration that minimizes continuous harvest from the broader population.
Sexual propagation of corals is also being explored as a potential restoration tool either through directly seeding reef areas with larvae (Doropoulos et al., 2019; Cameron and Harrison, 2020) or using settled spat (from controlled crosses or large-scale wild spawning events) to obtain stock for outplanting (Nakamura et al., 2011; Villanueva et al., 2012). Sexual reproduction allows selective breeding of particular traits and increased genotypic diversity in wildtype crosses, which may improve adaptive and evolutionary potential (Bay et al., 2017; Quigley et al., 2020a). The vast numbers of coral larvae resulting from spawning events and their small individual size means sexual reproductive approaches have the potential to be scaled up in ways that fragmentation cannot. However, sexual reproduction is less tractable than asexual propagation because approaches require more extensive effort and expertise, can vary widely in methodology by species, and are often dependent on seasonal events.
Sexual or asexual approaches to obtaining source material for outplanting could be combined with other proposed restoration strategies (e.g., artificial or augmented substrates meant to convey a settlement or growth advantage to desirable species, thermal preconditioning, heterotrophic feeding, probiotics) to gain synergistic benefits. Regardless of the particular strategy, theoretical consideration of the techniques, consequences, and limitations is crucial to the effectiveness of any restoration project. Practical concerns of resource managers and stakeholders, such as preserving ecosystem services, maintaining biodiversity, retaining or increasing coral cover, and preventing phase shifts, necessitate the ongoing development of restoration techniques.
Coral Reef Restoration Under Climate Change
Climate change presents a major challenge to traditional resource management because increasing atmospheric CO2 is a pan-global driver whose mitigation is outside the purview of any single resource management agency. The inevitability and enormity of the problem has led resource managers and stakeholders to reconsider traditional conservation goals and to start planning for climate change adaptation—managing change rather than maintaining conditions (Palmer et al., 2004; Stein et al., 2013). Climate change is increasingly considered in forestry management planning and the control of terrestrial invasive species (Nagel et al., 2017; Beaury et al., 2020) and interest in resilience-based management of coral reefs is growing (Mcleod et al., 2019).
While it has been assumed that local management actions help mitigate coral bleaching effects by reducing additive and synergistic stressors (Anthony, 2016), recent evidence suggests that recurring incidences of more extreme heat stress may limit the benefits (Hughes et al., 2017). In the past 50 years, approximately 50% of the Great Barrier Reef (Dietzel et al., 2020) and >80% of the Caribbean (Gardner et al., 2003) has been degraded. It is estimated that only 10% of the world’s reefs will persist past the year 2050 (Burke, 2011), as bleaching becomes a nearly annual occurrence (van Hooidonk et al., 2013). Even under best-case emissions trajectories, coral reefs will continue to be negatively transformed by climate change (Hughes et al., 2018; Anthony et al., 2020).
Conventional coral reef restoration is unsuitable under climate change because increasing temperature stress must now be accepted as an established parameter of the environment which will continue to impact newly outplanted corals during restoration (Drury et al., 2017a; Drury and Lirman, 2021). Without the introduction of meaningful adaptive variation there is a mismatch in the speed of adaptation relative to climate change (Matz et al., 2020), leading to local extirpation and limiting the long term persistence of reefs (Bay et al., 2017). Returning a degraded coral reef to its pristine state was once a realistic goal, but in many locations it now appears that priorities must shift to supporting ecosystems that are more resilient to climate change even if they represent modified versions of the ideal state. Fortunately, the existing coral restoration toolbox is diverse and potentially adaptable to proactive restoration objectives (Rinkevich, 2019).
Proactive Coral Reef Restoration
The terms “proactive restoration” and “preemptive restoration” appear occasionally in terrestrial resource management literature (Schweitzer et al., 2014; Muzika, 2017; Schoukens, 2017; Schweiger et al., 2018) especially in fields where there are strong anthropocentric concerns, such as endangered species compliance or timber management. In Foundations of Restoration Ecology (Falk et al., 2006), the authors state, “By proactive, we mean restoration projects that are designed to accomplish more than returning a system to some prior state.” Perhaps the term is not used more frequently, despite the concept being evident in many studies, because there is already a foregone conclusion in terrestrial systems that we will be factoring climate change into the design of management plans for the foreseeable future. We suggest the term “proactive restoration” is applicable to coral reef restoration undertaken in anticipation of environmental change and accounting for expected future conditions.
We advocate combining methods for identifying heat-tolerant coral stock with existing best practices in propagation and outplanting as a viable proactive reef restoration strategy that should be explored in earnest. Using coral stock selected to persist under anticipated future climate conditions should enhance the long-term survivorship of the individual outplanted colonies and consequently reduces wasted effort by practitioners. Transplantation of thermally tolerant individuals can also support adaptation (Bay et al., 2017), with models that include migration and selection for optimal genotypes predicting coral reefs in specific geographic ranges could persist for 100–200 more years (Matz et al., 2020).
Our focus is on the propagation of heat-tolerant colonies selected from a local population for use in restoration projects in the same locale, but less conservative formulations are also possible. Practitioners faced with insufficient thermal-tolerance in a local coral population could consider applying a “climate adjusted provenancing” approach (Prober et al., 2015; Baums et al., 2019) that includes selecting heat-tolerant corals from more distant reefs, representing a hybrid strategy of assisted gene flow (Aitken and Whitlock, 2013) and selective propagation. In contrast to the conventional objective of repairing damage, selective propagation and outplanting could hypothetically be implemented prior to any evidence of degradation in order to preemptively support resilience of coral populations in anticipation of an imminent decline. While integrating thermal resilience in coral populations using this proactive restoration approach is largely untested and subject to issues of scale, reef restoration without climate change planning is already untenable.
Persistence of Heat-Tolerance
Many strategies proposed for human intervention in coral conservation involve moving individual corals along with their algal, bacterial, and viral symbionts, taking advantage of intrinsic adaptive variation within and among populations (Figure 1). Proactive restoration using selected heat-tolerant coral stock requires that the extant thermal tolerance available in local coral populations is sufficient to persist under more stressful future conditions and that propagated individuals retain a significant portion of the heat-tolerance identified in source colonies. Multiple components of the coral holobiont drive phenotypes of interest, but their utility for practitioners may vary.

Figure 1. Variation in the response of corals to heat stress. (A) Colonies of several species on a reef during a bleaching event. (B) Coral fragments of a single species undergoing an artificial aquarium-based heat stress test.
Genetic drivers of thermal tolerance in the coral host are well-supported by experimental evidence on heritability (Dixon et al., 2015; Kirk et al., 2018), the long-term persistence of thermal tolerance after acclimatization (Schoepf et al., 2019), transplantation (Palumbi et al., 2014; Kenkel and Matz, 2016), environmental correlates (Jin et al., 2016), and reproducible bleaching effects (Ritson-Williams and Gates, 2020; Voolstra et al., 2020). This evidence suggests that host genetic effects have the highest translatability of any component of the holobiont and are most useful for practitioners, despite our limited understanding of genotype by environment interactions (Howells et al., 2013; Drury and Lirman, 2021). The utility of host genetic effects does not require any actual data on genomic variants, but can be established through broad-sense (clonal) heritability by measuring phenotype(s) of known individuals.
Corals can also harbor multiple genera of Symbiodiniaceae simultaneously (Silverstein et al., 2012), which can shift in response to natural and experimental heat stress (Baker et al., 2004; Berkelmans and van Oppen, 2006; Cunning et al., 2015b). However, different coral genera have varying levels of tolerance for diverse symbiont assemblages and flexibility in symbiont associations may be genera specific or temporally unstable (Goulet, 2006; Thornhill et al., 2006). Conversely, some corals bleach and recover without shuffling symbionts (Cunning et al., 2016) and recapitulate stress tolerance phenotypes through multiple bleaching events (Fisch et al., 2019; Ritson-Williams and Gates, 2020). There are also fine-scale differences within symbiont genera (Sampayo et al., 2008) and potential genotype level physiological implications (Baums et al., 2014). We suggest that in certain instances symbiont community dynamics may be a translatable factor that influences phenotype in a useful manner for practitioners, but additional data on historical, environmental, and species-specific factors is needed.
The evidence for bacterial translatability is equivocal. Temperature stress is associated with shifts in the microbiome (Bourne et al., 2008; Littman et al., 2011) and corals with more stable microbiomes tend to be more thermally tolerant (Hadaidi et al., 2017; Grottoli et al., 2018). Specific bacteria (Ben-Haim et al., 2003; Thurber et al., 2009; Mouchka et al., 2010) are correlated to bleaching response and specific bacterial functions (Santos et al., 2014) have also been linked to thermal tolerance. Bacterial probiotics used to supplement microbial communities of corals can improve thermal tolerance in laboratory experiments (Rosado et al., 2019). Conversely, corals from different thermal environments have unique microbes that may shift when moved to more stressful environments (Ziegler et al., 2017) and bacterial communities are flexible during development, aging, and bleaching (Littman et al., 2011; Williams et al., 2015; van Oppen and Blackall, 2019). Although the microbiome does play a role in thermal tolerance, the complexity of this component makes it difficult to establish translatability for restoration practitioners.
Science of Coral Stock Selection
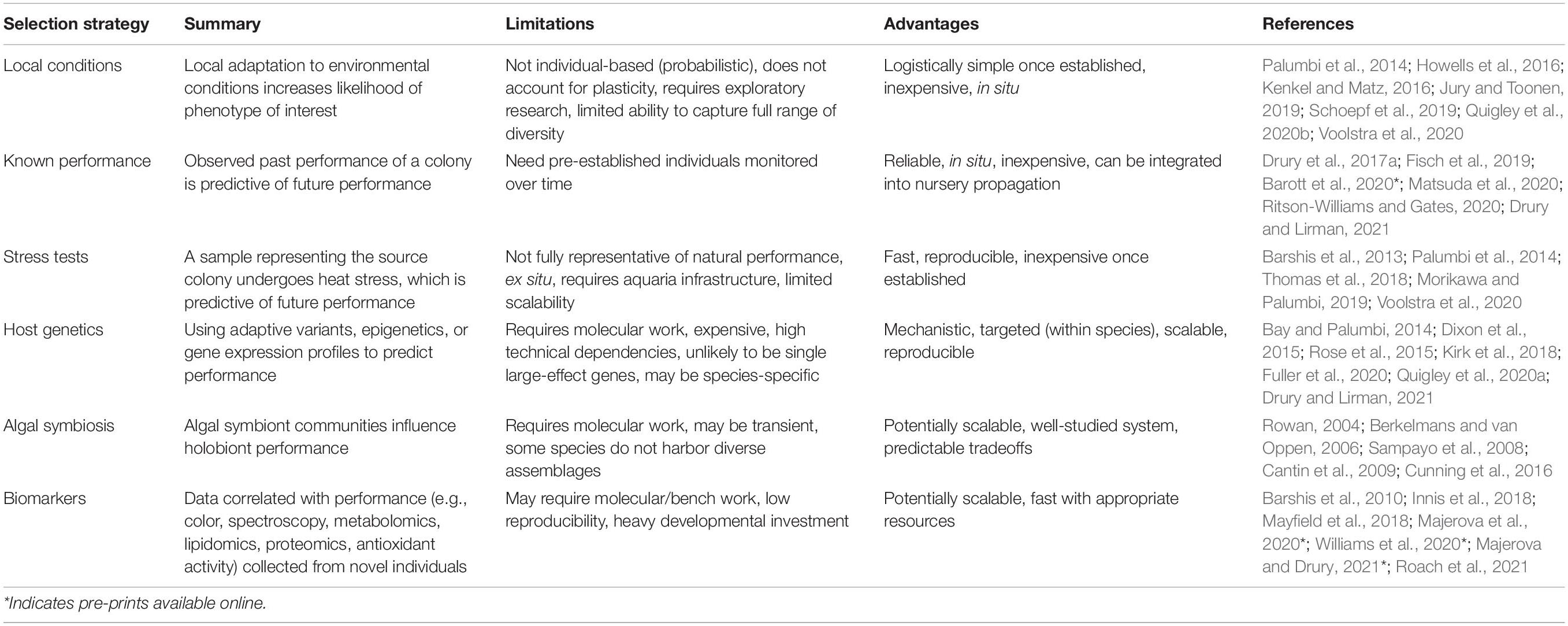
Requisite in any program of selective propagation is the identification of individuals or populations with the desired phenotype. Heat-tolerance phenotypes may be derived from host, algal symbiont, microbial, or synergistic holobiont effects (see above) or inferred from the environment, and should be durable across space and time to be useful to practitioners. Strategies for identifying candidate coral colonies (Table 1) range dramatically in scalability, cost, lag time between conceptualization and usability, and technical dependencies.
There is evidence that each of these strategies has potential to identify a more heat-tolerant stock of corals for propagation than random or opportunistic sampling; however, there are pros, cons, and major unknowns for each. We assume that in situ methods will give more ecologically relevant information, but may not be as scalable or tractable as ex situ methods. While tank-based heat stress tests and molecular assays may be beneficial to research groups or small-scale projects where the investment in screening and tracking individual colonies is acceptable, more scalable solutions are critical for human interventions to have positive impacts on the long-term persistence of reefs. For example, opportunistic selection of coral stock from an area with documented elevated thermal history and/or an above average proportion of non-bleaching corals could be carried out with minimal additions to standard propagation workflows, but may suffer from lower precision. While more technically involved, remote sensing of individual corals across an entire reef potentially combines rapid identification with targeted selection. The most effective selection strategy for a given restoration project will depend on many factors including feasibility (resources, expertise), what prior data is available about the coral populations and the source and outplant sites, which particular coral species are included in the project, and the project scale and timeline.
Trade-Offs in Proactive Coral Reef Restoration
The targeted selection of corals using any of these techniques should be expected to come with trade-offs because there is an ultimate energetic budget allocated across the various metabolic, reproductive, and stress response processes in any organism (Lesser, 2013). Corals are exposed to multiple stressors including high temperatures, acidifying oceans, disease, salinity fluctuations, sediment, nutrients, algal overgrowth, and recurrent storms. It is still unclear whether building coral resilience to one stressor will in turn lead to resistance to multiple stressors (“cross-tolerance”; van Oppen et al., 2017). Previous work shows that growth in benign conditions was lower for stress-tolerant corals (Bay et al., 2017) and faster growth was inversely related to tissue loss after thermal stress (Ladd et al., 2017). High temperature tolerance was also inversely related to low temperature tolerance in transplanted corals (Howells et al., 2013) and migrants from warm climates suffered health consequences during winter (Schoepf et al., 2019). There is also strong experimental support for slower growth in heat-tolerant symbiont communities (Little et al., 2004; Jones and Berkelmans, 2010), likely the result of lower carbon translocation (Cantin et al., 2009), but this effect is diminished under warmer conditions like those corals will face in the future (Cunning et al., 2015a).
Muller et al. (2018) found no relationship between temperature tolerance and disease susceptibility in Caribbean Acroporids at ambient temperatures, but showed disease tolerance was lost under thermal stress, suggesting that a small proportion of the population is tolerant to both stressors. Conversely, Wright et al. (2019) found support for positive responses to multiple stressors. This study showed high correlations between temperature tolerance, calcification under ocean acidification conditions, and disease resistance, suggesting a possible common genetic architecture that could respond positively to selection, providing a mechanism for persistence under multiple stressful conditions (Wright et al., 2019).
At the population level, a potential cost of artificially accelerating local adaptation is reduced genetic or genotypic diversity. Selective propagation does not remove genotypes or standing genetic diversity from a reef (unlike agricultural monoculture), where relatively small numbers of coral genotypes (on the order of dozens) used as focal stock can capture nearly all the genetic diversity of a population (Drury et al., 2017b; Baums et al., 2019). However, selective propagation would shift the allele frequency spectrum, positively affecting patterns of directional selection during heat stress (Bay et al., 2017). Because heat-tolerance is a complex trait (i.e., there is more than one way for a coral to be heat-tolerant), rather than focusing on a single or small number of selected stock genotypes, restoration practitioners can choose to select as wide an assortment of corals as possible that meet the heat-tolerance selection criteria established by an individual project (Baums et al., 2019). This step will also likely result in individuals with multiple interacting pathways and genetic architectures that contribute to heat tolerance. To promote additional genetic diversity, restoration projects may leave substrate available for natural recruitment and emphasize coral survival and reproductive competence to maintain gene flow with other populations.
The evaluation of trade-offs in coral resilience is challenging because of the difficulty in extensive measurement of the many realistic phenotypes of interest, such as partial mortality, wound healing, growth rate, and fecundity (Baums et al., 2019). Changes at ecosystem scale may also be decoupled from experimental trade-offs, such that outcomes defined in one or several genotypes or species obscure broader functional dynamics on actual reefs. Regardless, delaying new interventions because of uncertainty around trade-offs could mean losing key species and functions (Anthony et al., 2020), representing an opportunity cost of non-intervention. A robust coral reef ecosystem is dependent on many coral traits, but we contend that temperature tolerance is of paramount importance in the face of ever-increasing coral bleaching events. While greater fecundity, growth, and structural complexity enhance ecosystem services and long-term capacity for resilience, corals that do not survive cannot contribute at all.
Conclusion
We argue that selection and propagation of heat-tolerant coral stock is a rational option for proactive reef restoration under climate change. We acknowledge risks and unknowns that warrant attention and further exploration, but contend that given the urgency of the situation, this strategy is feasible, relatively conservative, and logistically practical within existing restoration frameworks. Important areas for continued research include developing high-throughput selection methods, investigating the trade-offs in selecting heat-tolerant corals, and assessing the long-term viability of those corals. We also advocate empirical field tests to develop methodology, reveal unknown limitations and drawbacks, and assess real-world performance in preparation for implementing full-scale proactive restoration projects to meet resource management objectives and prepare coral reefs to face the future.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
CC, KH, and CD conceived of and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The National Fish and Wildlife Foundation and the Paul G. Allen Family Foundation were funding partners in this work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with one of the authors CD.
Acknowledgments
We are grateful to Josh Hancock for discussions on proactive restoration and Eva Majerova, Ty Roach, and the Coral Resilience Lab for constructive comments on the manuscript. We dedicate this manuscript to Ruth Gates, who encouraged us to think about interventions to support the long-term persistence of coral reefs. This is SOEST contribution 11343 and HIMB contribution 1856.
References
Aitken, S. N., and Whitlock, M. C. (2013). Assisted gene flow to facilitate local adaptation to climate change. Annu. Rev. Ecol. Evol. Syst. 44, 367–388. doi: 10.1146/annurev-ecolsys-110512-135747
Anthony, K. R. N. (2016). Coral reefs under climate change and ocean acidification: challenges and opportunities for management and policy. Annu. Rev. Environ. Resour. 41, 59–81. doi: 10.1146/annurev-environ-110615-085610
Anthony, K. R. N., Helmstedt, K. J., Bay, L. K., Fidelman, P., Hussey, K. E., Lundgren, P., et al. (2020). Interventions to help coral reefs under global change—a complex decision challenge. PLoS One 15:e0236399. doi: 10.1371/journal.pone.0236399
Baker, A. C., Starger, C. J., McClanahan, T. R., and Glynn, P. W. (2004). Corals’ adaptive response to climate change. Nature 430, 741–741. doi: 10.1038/430741a
Barott, K. L., Huffmyer, A. S., Davidson, J. M., Lenz, E. A., Matsuda, S. B., Hancock, J. R., et al. (2020). Bleaching resistant corals retain heat tolerance following acclimatization to environmentally distinct reefs. bioRxiv [Preprint]. doi: 10.1101/2020.09.25.314203
Barshis, D. J., Ladner, J. T., Oliver, T. A., Seneca, F. O., Traylor-Knowles, N., and Palumbi, S. R. (2013). Genomic basis for coral resilience to climate change. Proc. Natl. Acad. Sci. U. S. A. 110, 1387–1392. doi: 10.1073/pnas.1210224110
Barshis, D. J., Stillman, J. H., Gates, R. D., Toonen, R. J., Smith, L. W., and Birkeland, C. (2010). Protein expression and genetic structure of the coral Porites lobata in an environmentally extreme Samoan back reef: does host genotype limit phenotypic plasticity? Mol. Ecol. 19, 1705–1720. doi: 10.1111/j.1365-294X.2010.04574.x
Baums, I. B., Baker, A. C., Davies, S. W., Grottoli, A. G., Kenkel, C. D., Kitchen, S. A., et al. (2019). Considerations for maximizing the adaptive potential of restored coral populations in the western Atlantic. Ecol. Appl. 29:e01978. doi: 10.1002/eap.1978
Baums, I. B., Devlin-Durante, M. K., and LaJeunesse, T. C. (2014). New insights into the dynamics between reef corals and their associated dinoflagellate endosymbionts from population genetic studies. Mol. Ecol. 23, 4203–4215. doi: 10.1111/mec.12788
Bay, R. A., and Palumbi, S. R. (2014). Multilocus adaptation associated with heat resistance in reef-building corals. Curr. Biol. 24, 2952–2956. doi: 10.1016/j.cub.2014.10.044
Bay, R. A., Rose, N. H., Logan, C. A., and Palumbi, S. R. (2017). Genomic models predict successful coral adaptation if future ocean warming rates are reduced. Sci. Adv. 3:e1701413. doi: 10.1126/sciadv.1701413
Bayraktarov, E., Stewart-Sinclair, P. J., Brisbane, S., Boström-Einarsson, L., Saunders, M. I., Lovelock, C. E., et al. (2019). Motivations, success, and cost of coral reef restoration. Restor. Ecol. 27, 981–991. doi: 10.1111/rec.12977
Beaury, E. M., Fusco, E. J., Jackson, M. R., Laginhas, B. B., Morelli, T. L., Allen, J. M., et al. (2020). Incorporating climate change into invasive species management: insights from managers. Biol. Invasions 22, 233–252. doi: 10.1007/s10530-019-02087-6
Ben-Haim, Y., Zicherman-Keren, M., and Rosenberg, E. (2003). Temperature-regulated bleaching and lysis of the coral Pocillopora damicornis by the novel pathogen Vibrio coralliilyticus. Appl. Environ. Microbiol. 69, 4236–4242. doi: 10.1128/AEM.69.7.4236-4242.2003
Berkelmans, R., and van Oppen, M. J. H. (2006). The role of zooxanthellae in the thermal tolerance of corals: a “nugget of hope” for coral reefs in an era of climate change. Proc. Biol. Sci. 273, 2305–2312. doi: 10.1098/rspb.2006.3567
Boström-Einarsson, L., Babcock, R. C., Bayraktarov, E., Ceccarelli, D., Cook, N., Ferse, S. C. A., et al. (2020). Coral restoration – a systematic review of current methods, successes, failures and future directions. PLoS One 15:e0226631. doi: 10.1371/journal.pone.0226631
Bourne, D., Iida, Y., Uthicke, S., and Smith-Keune, C. (2008). Changes in coral-associated microbial communities during a bleaching event. ISME J. 2, 350–363. doi: 10.1038/ismej.2007.112
Cameron, K. A., and Harrison, P. L. (2020). Density of coral larvae can influence settlement, post-settlement colony abundance and coral cover in larval restoration. Sci. Rep. 10:5488. doi: 10.1038/s41598-020-62366-4
Cantin, N. E., van Oppen, M. J. H., Willis, B. L., Mieog, J. C., and Negri, A. P. (2009). Juvenile corals can acquire more carbon from high-performance algal symbionts. Coral Reefs 28:405. doi: 10.1007/s00338-009-0478-8
Cunning, R., Gillette, P., Capo, T., Galvez, K., and Baker, A. C. (2015a). Growth tradeoffs associated with thermotolerant symbionts in the coral Pocillopora damicornis are lost in warmer oceans. Coral Reefs 34, 155–160. doi: 10.1007/s00338-014-1216-4
Cunning, R., Ritson-Williams, R., and Gates, R. D. (2016). Patterns of bleaching and recovery of Montipora capitata in Kāne ‘ohe Bay, Hawai ‘i, USA. Mar. Ecol. Prog. Ser. 551, 131–139. doi: 10.3354/meps11733
Cunning, R., Silverstein, R. N., and Baker, A. C. (2015b). Investigating the causes and consequences of symbiont shuffling in a multi-partner reef coral symbiosis under environmental change. Proc. Biol. Sci. 282:20141725. doi: 10.1098/rspb.2014.1725
Delbeek, C. J. (2001). Coral farming: past, present, and future trends. Aquarium Sci. Conserv. 3, 171–181. doi: 10.1023/A:1011306125934
Dietzel, A., Bode, M., Connolly, S. R., and Hughes, T. P. (2020). Long-term shifts in the colony size structure of coral populations along the great barrier reef. Proc. Biol. Sci. 287:20201432. doi: 10.1098/rspb.2020.1432
Dixon, G. B., Davies, S. W., Aglyamova, G. A., Meyer, E., Bay, L. K., and Matz, M. V. (2015). Genomic determinants of coral heat tolerance across latitudes. Science 348, 1460–1462. doi: 10.1126/science.1261224
Doropoulos, C., Vons, F., Elzinga, J., ter Hofstede, R., Salee, K., van Koningsveld, M., et al. (2019). Testing industrial-scale coral restoration techniques: harvesting and culturing wild coral-spawn slicks. Front. Mar. Sci. 6:658. doi: 10.3389/fmars.2019.00658
Drury, C., and Lirman, D. (2021). Genotype by environment interactions in coral bleaching. Proc. Biol. Sci. 288:20210177. doi: 10.1098/rspb.2021.0177
Drury, C., Manzello, D., and Lirman, D. (2017a). Genotype and local environment dynamically influence growth, disturbance response and survivorship in the threatened coral, Acropora cervicornis. PLoS One 12:e0174000. doi: 10.1371/journal.pone.0174000
Drury, C., Schopmeyer, S., Goergen, E., Bartels, E., Nedimyer, K., Johnson, M., et al. (2017b). Genomic patterns in Acropora cervicornis show extensive population structure and variable genetic diversity. Ecol. Evol. 7, 6188–6200. doi: 10.1002/ece3.3184
Falk, D., Palmer, M., and Zedler, J. (eds) (2006). Foundations of Restoration Ecology. Washington, DC: Island Press.
Fisch, J., Drury, C., Towle, E. K., Winter, R. N., and Miller, M. W. (2019). Physiological and reproductive repercussions of consecutive summer bleaching events of the threatened Caribbean coral Orbicella faveolata. Coral Reefs 38, 863–876. doi: 10.1007/s00338-019-01817-5
Fuller, Z. L., Mocellin, V. J. L., Morris, L. A., Cantin, N., Shepherd, J., Sarre, L., et al. (2020). Population genetics of the coral Acropora millepora: toward genomic prediction of bleaching. Science 369:eaba4674. doi: 10.1126/science.aba4674
Gardner, T. A., Côté, I. M., Gill, J. A., Grant, A., and Watkinson, A. R. (2003). Long-term region-wide declines in Caribbean corals. Science 301, 958–960. doi: 10.1126/science.1086050
Goulet, T. L. (2006). Most corals may not change their symbionts. Mar. Ecol. Prog. Ser. 321, 1–7. doi: 10.3354/meps321001
Grottoli, A. G., Dalcin Martins, P., Wilkins, M. J., Johnston, M. D., Warner, M. E., Cai, W.-J., et al. (2018). Coral physiology and microbiome dynamics under combined warming and ocean acidification. PLoS One 13:e0191156. doi: 10.1371/journal.pone.0191156
Hadaidi, G., Röthig, T., Yum, L. K., Ziegler, M., Arif, C., Roder, C., et al. (2017). Stable mucus-associated bacterial communities in bleached and healthy corals of Porites lobata from the Arabian Seas. Sci. Rep. 7:45362. doi: 10.1038/srep45362
Hardisty, P., Roth, C. H., Silvey, P. J., Mead, D., and Anthony, K. R. N. (2019). Reef Restoration and Adaptation Program – Investment Case. A Report Provided to the Australian Government from the Reef Restoration and Adaptation Program. Townsville, Qld: Australian Institute of Marine Science.
Howells, E. J., Abrego, D., Meyer, E., Kirk, N. L., and Burt, J. A. (2016). Host adaptation and unexpected symbiont partners enable reef-building corals to tolerate extreme temperatures. Glob. Chang. Biol. 22, 2702–2714. doi: 10.1111/gcb.13250
Howells, E. J., Berkelmans, R., van Oppen, M. J. H., Willis, B. L., and Bay, L. K. (2013). Historical thermal regimes define limits to coral acclimatization. Ecology 94, 1078–1088. doi: 10.1890/12-1257.1
Hughes, T. P., Anderson, K. D., Connolly, S. R., Heron, S. F., Kerry, J. T., Lough, J. M., et al. (2018). Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359, 80–83. doi: 10.1126/science.aan8048
Hughes, T. P., Kerry, J. T., Álvarez-Noriega, M., Álvarez-Romero, J. G., Anderson, K. D., Baird, A. H., et al. (2017). Global warming and recurrent mass bleaching of corals. Nature 543, 373–377. doi: 10.1038/nature21707
Innis, T., Cunning, R., Ritson-Williams, R., Wall, C. B., and Gates, R. D. (2018). Coral color and depth drive symbiosis ecology of Montipora capitata in Kāne‘ohe Bay, O‘ahu, Hawai‘i. Coral Reefs 37, 423–430. doi: 10.1007/s00338-018-1667-0
Jin, Y. K., Lundgren, P., Lutz, A., Raina, J.-B., Howells, E. J., Paley, A. S., et al. (2016). Genetic markers for antioxidant capacity in a reef-building coral. Sci. Adv. 2:e1500842. doi: 10.1126/sciadv.1500842
Jones, A., and Berkelmans, R. (2010). Potential costs of acclimatization to a warmer climate: growth of a reef coral with heat tolerant vs. sensitive symbiont types. PLoS One 5:e10437. doi: 10.1371/journal.pone.0010437
Jury, C. P., and Toonen, R. J. (2019). Adaptive responses and local stressor mitigation drive coral resilience in warmer, more acidic oceans. Proc. Biol. Sci. 286:20190614. doi: 10.1098/rspb.2019.0614
Kenkel, C. D., and Matz, M. V. (2016). Gene expression plasticity as a mechanism of coral adaptation to a variable environment. Nat. Ecol. Evol. 1:14. doi: 10.1038/s41559-016-0014
Kirk, N. L., Howells, E. J., Abrego, D., Burt, J. A., and Meyer, E. (2018). Genomic and transcriptomic signals of thermal tolerance in heat-tolerant corals (Platygyra daedalea) of the Arabian/Persian Gulf. Mol. Ecol. 27, 5180–5194. doi: 10.1111/mec.14934
Ladd, M. C., Shantz, A. A., Bartels, E., and Burkepile, D. E. (2017). Thermal stress reveals a genotype-specific tradeoff between growth and tissue loss in restored Acropora cervicornis. Mar. Ecol. Prog. Ser. 572, 129–139. doi: 10.3354/meps12169
Lesser, M. P. (2013). Using energetic budgets to assess the effects of environmental stress on corals: are we measuring the right things? Coral Reefs 32, 25–33. doi: 10.1007/s00338-012-0993-x
Little, A. F., van Oppen, M. J. H., and Willis, B. L. (2004). Flexibility in algal endosymbioses shapes growth in reef corals. Science 304, 1492–1494. doi: 10.1126/science.1095733
Littman, R., Willis, B. L., and Bourne, D. G. (2011). Metagenomic analysis of the coral holobiont during a natural bleaching event on the great barrier reef. Environ. Microbiol. Rep. 3, 651–660. doi: 10.1111/j.1758-2229.2010.00234.x
Majerova, E., Carey, F., Drury, C., and Gates, R. (2020). Preconditioning improves bleaching susceptibility in the reef-building coral Pocillopora acuta through modulations in autophagy pathway. Authorea doi: 10.22541/au.158274769.98869554
Majerova, E., and Drury, C. (2021). A BI-1 mediated cascade improves redox homeostasis during thermal stress and prevents oxidative damage in a preconditioned reef-building coral. bioRxiv [Preprint]. doi: 10.1101/2021.03.15.435543
Matsuda, S. B., Huffmyer, A. S., Lenz, E. A., Davidson, J. M., Hancock, J. R., Przybylowski, A., et al. (2020). Coral bleaching susceptibility is predictive of subsequent mortality within but not between coral species. Front. Ecol. Evol. 8:178. doi: 10.3389/fevo.2020.00178
Matz, M. V., Treml, E. A., Aglyamova, G. V., and Bay, L. K. (2018). Potential and limits for rapid genetic adaptation to warming in a great barrier reef coral. PLoS Genet. 14, e1007220–e1007219. doi: 10.1371/journal.pgen.1007220
Matz, M. V., Treml, E. A., and Haller, B. C. (2020). Estimating the potential for coral adaptation to global warming across the Indo-West Pacific. Glob. Chang. Biol. 26, 3473–3481. doi: 10.1111/gcb.15060
Mayfield, A. B., Chen, Y.-J., Lu, C.-Y., and Chen, C.-S. (2018). The proteomic response of the reef coral Pocillopora acuta to experimentally elevated temperatures. PLoS One 13:e0192001. doi: 10.1371/journal.pone.0192001
Mcleod, E., Anthony, K. R. N., Mumby, P. J., Maynard, J., Beeden, R., Graham, N. A. J., et al. (2019). The future of resilience-based management in coral reef ecosystems. J. Environ. Manage. 233, 291–301. doi: 10.1016/j.jenvman.2018.11.034
McShane, T. O., Hirsch, P. D., Trung, T. C., Songorwa, A. N., Kinzig, A., Monteferri, B., et al. (2011). Hard choices: making trade-offs between biodiversity conservation and human well-being. Biol. Conserv. 144, 966–972. doi: 10.1016/j.biocon.2010.04.038
Morikawa, M. K., and Palumbi, S. R. (2019). Using naturally occurring climate resilient corals to construct bleaching-resistant nurseries. Proc. Natl. Acad. Sci. U. S. A. 116, 10586–10591. doi: 10.1073/pnas.1721415116
Mouchka, M. E., Hewson, I., and Harvell, C. D. (2010). Coral-associated bacterial assemblages: current knowledge and the potential for climate-driven impacts. Integr. Comp. Biol. 50, 662–674. doi: 10.1093/icb/icq061
Muller, E. M., Bartels, E., and Baums, I. B. (2018). Bleaching causes loss of disease resistance within the threatened coral species Acropora cervicornis. Elife 7:e35066. doi: 10.7554/eLife.35066
Muzika, R. M. (2017). Opportunities for silviculture in management and restoration of forests affected by invasive species. Biol. Invasions 19, 3419–3435. doi: 10.1007/s10530-017-1549-3
Nagel, L. M., Palik, B. J., Battaglia, M. A., and D’Amato, A. W. (2017). Adaptive silviculture for climate change: a national experiment in manager-scientist partnerships to apply an adaptation framework. J. For. 115, 167–178. doi: 10.5849/jof.16-039
Nakamura, R., Ando, W., Yamamoto, H., Kitano, M., Sato, A., Nakamura, M., et al. (2011). Corals mass-cultured from eggs and transplanted as juveniles to their native, remote coral reef. Mar. Ecol. Prog. Ser. 436, 161–168. doi: 10.3354/meps09257
National Academies of Sciences Engineering and Medicine (2019). A Research Review of Interventions to Increase the Persistence and Resilience of Coral Reefs. Washington, DC: National Academies Press.
Omori, M. (2019). Coral restoration research and technical developments: what we have learned so far. Mar. Biol. Res. 15, 377–409. doi: 10.1080/17451000.2019.1662050
Palmer, M., Bernhardt, E., Chornesky, E., Collins, S., Dobson, A., Duke, C., et al. (2004). ECOLOGY: ecology for a crowded planet. Science 304, 1251–1252. doi: 10.1126/science.1095780
Palumbi, S. R., Barshis, D. J., Traylor-Knowles, N., and Bay, R. A. (2014). Mechanisms of reef coral resistance to future climate change. Science 344, 895–898. doi: 10.1126/science.1251336
Precht, W., and Robbart, M. (2009). “Coral reef restoration,” in Coral Reef Restoration Handbook, ed. W. F. Precht (Boca Raton, FL: CRC Press), 1–24. doi: 10.1201/9781420003796.ch1
Prober, S., Byrne, M., McLean, E., Steane, D., Potts, B., Vaillancourt, R., et al. (2015). Climate-adjusted provenancing: a strategy for climate-resilient ecological restoration. Front. Ecol. Evol. 3:65. doi: 10.3389/fevo.2015.00065
Quigley, K. M., Bay, L. K., and Oppen, M. J. H. (2020a). Genome-wide SNP analysis reveals an increase in adaptive genetic variation through selective breeding of coral. Mol. Ecol. 29, 2176–2188. doi: 10.1111/mec.15482
Quigley, K. M., Randall, C. J., van Oppen, M. J. H., and Bay, L. K. (2020b). Assessing the role of historical temperature regime and algal symbionts on the heat tolerance of coral juveniles. Biol. Open 9:bio047316. doi: 10.1242/bio.047316
Rinkevich, B. (1995). Restoration strategies for coral reefs damaged by recreational activities: the use of sexual and asexual recruits. Restor. Ecol. 3, 241–251. doi: 10.1111/j.1526-100X.1995.tb00091.x
Rinkevich, B. (2005). Conservation of coral reefs through active restoration measures: recent approaches and last decade progress. Environ. Sci. Technol. 39, 4333–4342. doi: 10.1021/es0482583
Rinkevich, B. (2014). Rebuilding coral reefs: does active reef restoration lead to sustainable reefs? Curr. Opin. Environ. Sustain. 7, 28–36. doi: 10.1016/j.cosust.2013.11.018
Rinkevich, B. (2019). The active reef restoration toolbox is a vehicle for coral resilience and adaptation in a changing world. J. Mar. Sci. Eng. 7:201. doi: 10.3390/jmse7070201
Ritson-Williams, R., and Gates, R. D. (2020). Coral community resilience to successive years of bleaching in Kane ‘ohe Bay, Hawai ‘i. Coral Reefs 39, 757–769. doi: 10.1007/s00338-020-01944-4
Roach, T. N. F., Dilworth, J., Christian Martin, H., Daniel Jones, A., Quinn, R., and Drury, C. (2021). Metabolomic signatures of coral bleaching history. Nat. Ecol. Evol. 5, 495–503. doi: 10.1038/s41559-020-01388-7
Rosado, P. M., Leite, D. C. A., Duarte, G. A. S., Chaloub, R. M., Jospin, G., da Rocha, U. N., et al. (2019). Marine probiotics: increasing coral resistance to bleaching through microbiome manipulation. ISME J. 13, 921–936. doi: 10.1038/s41396-018-0323-6
Rose, N. H., Seneca, F. O., and Palumbi, S. R. (2015). Gene networks in the wild: identifying transcriptional modules that mediate coral resistance to experimental heat stress. Genome Biol. Evol. 8, 243–252. doi: 10.1093/gbe/evv258
Rowan, R. (2004). Coral bleaching: thermal adaptation in reef coral symbionts. Nature 430:742. doi: 10.1038/430742a
Sampayo, E. M., Ridgway, T., Bongaerts, P., and Hoegh-Guldberg, O. (2008). Bleaching susceptibility and mortality of corals are determined by fine-scale differences in symbiont type. Proc. Natl. Acad. Sci. U. S. A. 105, 10444–10449. doi: 10.1073/pnas.0708049105
Santos, H. F., Carmo, F. L., Duarte, G., Dini-Andreote, F., Castro, C. B., Rosado, A. S., et al. (2014). Climate change affects key nitrogen-fixing bacterial populations on coral reefs. ISME J. 8, 2272–2279. doi: 10.1038/ismej.2014.70
Schoepf, V., Carrion, S. A., Pfeifer, S. M., Naugle, M., Dugal, L., Bruyn, J., et al. (2019). Stress-resistant corals may not acclimatize to ocean warming but maintain heat tolerance under cooler temperatures. Nat. Commun. 10:4031. doi: 10.1038/s41467-019-12065-0
Schoukens, H. (2017). Proactive habitat restoration and the avoidance of adverse effects on protected areas: development project review in Europe after Orleans. J. Int. Wildl. Law Policy 20, 125–154. doi: 10.1080/13880292.2017.1346349
Schweiger, A. H., Boulangeat, I., Conradi, T., Davis, M., and Svenning, J.-C. (2018). The importance of ecological memory for trophic rewilding as an ecosystem restoration approach. Biol. Rev. Camb. Philos. Soc. 94, 1–15. doi: 10.1111/brv.12432
Schweitzer, C., Clark, S. L., Gottschalk, K. W., Stringer, J., and Sitzlar, R. (2014). Proactive restoration: planning, implementation, and early results of silvicultural strategies for increasing resilience against gypsy moth infestation in upland oak forests on the Daniel Boone National Forest, Kentucky. J. For. 112, 401–411. doi: 10.5849/jof.13-085
Sherwood, S. C., Webb, M. J., Annan, J. D., Armour, K. C., Forster, P. M., Hargreaves, J. C., et al. (2020). An assessment of earth’s climate sensitivity using multiple lines of evidence. Rev. Geophys. 58:e2019RG000678. doi: 10.1029/2019RG000678
Silverstein, R. N., Correa, A. M. S., and Baker, A. C. (2012). Specificity is rarely absolute in coral–algal symbiosis: implications for coral response to climate change. Proc. Biol. Sci. 279, 2609–2618. doi: 10.1098/rspb.2012.0055
Society for Ecological Restoration International Science and Policy Working Group (2004). SER International Primer on Ecological Restoration. Quincy, FL: Society for Ecological Restoration International.
Stein, B. A., Staudt, A., Cross, M. S., Dubois, N. S., Enquist, C., Griffis, R., et al. (2013). Preparing for and managing change: climate adaptation for biodiversity and ecosystems. Front. Ecol. Environ. 11:502–510. doi: 10.1890/120277
Thomas, L., Rose, N. H., Bay, R. A., López, E. H., Morikawa, M. K., Ruiz-Jones, L., et al. (2018). Mechanisms of thermal tolerance in reef-building corals across a fine-grained environmental mosaic: lessons from Ofu, American Samoa. Front. Mar. Sci. 4:434. doi: 10.3389/fmars.2017.00434
Thornhill, D. J., LaJeunesse, T. C., Kemp, D. W., Fitt, W. K., and Schmidt, G. W. (2006). Multi-year, seasonal genotypic surveys of coral-algal symbioses reveal prevalent stability or post-bleaching reversion. Mar. Biol. 148, 711–722. doi: 10.1007/s00227-005-0114-2
Thurber, R. V., Willner Hall, D., Rodriguez-Mueller, B., Desnues, C., Edwards, R. A., Angly, F., et al. (2009). Metagenomic analysis of stressed coral holobionts. Environ. Microbiol. 11, 2148–2163. doi: 10.1111/j.1462-2920.2009.01935.x
van Hooidonk, R., Maynard, J. A., and Planes, S. (2013). Temporary refugia for coral reefs in a warming world. Nat. Clim. Change 3, 508–511. doi: 10.1038/nclimate1829
van Oppen, M. J. H., and Blackall, L. L. (2019). Coral microbiome dynamics, functions and design in a changing world. Nat. Rev. Microbiol. 17, 557–567. doi: 10.1038/s41579-019-0223-4
van Oppen, M. J. H., Gates, R. D., Blackall, L. L., Cantin, N., Chakravarti, L. J., Chan, W. Y., et al. (2017). Shifting paradigms in restoration of the world’s coral reefs. Glob. Chang. Biol. 23, 3437–3448. doi: 10.1111/gcb.13647
Villanueva, R. D., Baria, M. V. B., and dela Cruz, D. W. (2012). Growth and survivorship of juvenile corals outplanted to degraded reef areas in Bolinao-Anda Reef Complex, Philippines. Mar. Biol. Res. 8, 877–884. doi: 10.1080/17451000.2012.682582
Voolstra, C. R., Buitrago-López, C., Perna, G., Cárdenas, A., Hume, B. C. C., Rädecker, N., et al. (2020). Standardized short-term acute heat stress assays resolve historical differences in coral thermotolerance across microhabitat reef sites. Glob. Chang. Biol. 26, 4328–4343. doi: 10.1111/gcb.15148
Wilkinson, C. R. (2004). Global Coral Reef Monitoring Network: Status of Coral Reefs of the World 2004. Townsville, Qld: Australian Institute of Marine Science.
Williams, A., Chiles, E. N., Conetta, D., Pathmanathan, J. S., Cleves, P. A., Putnam, H. M., et al. (2020). Metabolome shift associated with thermal stress in coral holobionts. bioRxiv [Preprint]. doi: 10.1101/2020.06.04.134619
Williams, A. D., Brown, B. E., Putchim, L., and Sweet, M. J. (2015). Age-related shifts in bacterial diversity in a reef coral. PLoS One 10:e0144902. doi: 10.1371/journal.pone.0144902
Wright, R. M., Mera, H., Kenkel, C. D., Nayfa, M., Bay, L. K., and Matz, M. V. (2019). Positive genetic associations among fitness traits support evolvability of a reef-building coral under multiple stressors. Glob. Chang. Biol. 25, 3294–3304. doi: 10.1111/gcb.14764
Keywords: coral bleaching, thermal tolerance, selective propagation, climate change, restoration
Citation: Caruso C, Hughes K and Drury C (2021) Selecting Heat-Tolerant Corals for Proactive Reef Restoration. Front. Mar. Sci. 8:632027. doi: 10.3389/fmars.2021.632027
Received: 21 November 2020; Accepted: 27 April 2021;
Published: 26 May 2021.
Edited by:
Iliana B. Baums, Pennsylvania State University (PSU), United StatesReviewed by:
Kátia Cristina Cruz Capel, University of São Paulo, BrazilAnastazia T. Banaszak, National Autonomous University of Mexico, Mexico
Copyright © 2021 Caruso, Hughes and Drury. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlo Caruso, Q2FybG9SZWVmQGdtYWlsLmNvbQ==
 Carlo Caruso
Carlo Caruso Kira Hughes
Kira Hughes Crawford Drury
Crawford Drury