- 1Institute of Marine Environment and Ecology, National Taiwan Ocean University, Keelung, Taiwan
- 2Center of Excellence for the Oceans, National Taiwan Ocean University, Keelung, Taiwan
Noctiluca scintillans is a larger, bioluminescent red-tide dinoflagellate (400–1,000 μm in diameter) that reproduces by sexual or asexual reproduction (binary fission). The process of sexual reproduction in N. scintillans has been thoroughly studied, but the ecological role and the mechanism of shifting from asexual to sexual reproduction have not been fully elucidated. It is believed, however, that sexual reproduction occurs when N. scintillans faces environmental stress. In this study, we tried to determine which factors drive N. scintillans to undergo sexual reproduction and we considered sexual reproduction’s ecological role. We cultured N. scintillans under different conditions of temperature, N. scintillans cell concentration, prey concentration, cultivation time, cultivation volume, light exposure time and physical vibration (simulated wave motion), and counted gametocyte mother cells every 24 h to calculate how the sexual reproduction rate changed over the experimental period. Rises in the sexual reproduction rate or the concentration of gametocyte mother cells only occurred in response to large variations in prey concentration, typically after the exponential phase of N. scintillans population growth. A noticeable upsurge in gametocyte mother cells, from 1% or less to nearly 10% of the total N. scintillans population, occurred when the prey concentration fell below ∼400 cells/mL. This implies that a sudden decrease in prey concentration induces more N. scintillans to shift from trophonts to gametocyte mother cells. We suggest that sexual reproduction may occur in N. scintillans as a response to the post-bloom situation when the dinoflagellate’s food supply has been dramatically depleted, producing large numbers of gametes for an alternative mode of survival after the end of each bloom.
Introduction
Noctiluca scintillans is a heterotrophic dinoflagellate that can be found in temperate and tropical waters around the world and occasionally causes red tides in eutrophic estuaries (Harrison et al., 2011). Although it has been categorized as a harmful algae species (Elbrächter and Qi, 1998), N. scintillans does not itself produce phytotoxic or other toxins (Escalera et al., 2007; Frangópulos et al., 2011). However, it may cause a temporary hypoxic zone or release high levels of ammonia into the surrounding waters when a bloom ends, causing harm to natural shellfish beds and/or aquaculture sites (Miyaguchi et al., 2008; Baek et al., 2009; Bravo and Figueroa, 2014; Zhang et al., 2017). It has a rather large cell size (∼400–1,000 μm in diameter) for a dinoflagellate and floats with the current without swimming actively. It can move vertically by regulating the concentration of ammonia inside the cell, thus controlling its buoyancy (Baek et al., 2009). N. scintillans has bioluminescent properties: when subjected to physical shock, it gives off a blue bioluminescent glow for less than 0.1 s (Valiadi and Iglesias-Rodriguez, 2013; Bravo and Figueroa, 2014). In coastal cities or islands where N. scintillans blooms seasonally, it became a tourist attraction and was given the name “sea sparkle” or “blue tears” (Tsai et al., 2018).
Due to the tendency of N. scintillans to cause damage to fishing grounds (Huang and Qi, 1997; Chalaris, 2010), research on this species started in the early 1800s. Many studies have been published, providing much insight into the global distribution, prey preference and bloom pattern of N. scintillans. However, studies of its morphological characteristic have been highly controversial and much fewer in number. Haeckel (1911) proposed that N. scintillans should be included in the Cystoflagellata within the dinoflagellates, and before that it was classified as a jellyfish. Later it was placed in the newly created order Noctilucales, after observations of the trophont cells by Kofoid (1920). Much later, Zingmark (1970) did an extensive study on the life cycle of N. scintillans, mainly focusing on its sexual reproduction cycle, and came to question whether N. scintillans is in fact a dinoflagellate? Due to the general morphology of the vegetative state and its asexual reproductive process. For instance, N. scintillans has a diploid vegetative cell while other dinoflagellates are haploid (Zingmark, 1970; Pfiester, 1984; Fukuda and Endoh, 2006). This trait and several others make it appear unlike other dinoflagellates (Harrison et al., 2011). Saldarriaga et al. (2004) re-analyzed the genetic sequence of N. scintillans and found that it grouped with other dinoflagellates, but its precise phylogenetic position was still uncertain, depending on the method of analysis. Recently, Fukuda and Endoh (2006) reexamined its life cycle, including sexual reproduction, and recorded more detailed morphological characteristics, giving a new perspective toward placing of N. scintillans among other dinoflagellates.
Studies on Noctiluca scintillans are plentiful, but the mechanism of, including sexual reproduction is still unknown. It is unclear whether this process influences the bloom cycle or plays an important ecological role in its population dynamics. Reproduction in N. scintillans involves both sexual and asexual processes. The vegetative cell, or trophont cell, mostly undergoes binary fission but randomly undergoes gametogenesis, which produces gametes that afterward fuse in pairs form zygotes (Zingmark, 1970; Fukuda and Endoh, 2006; Sathish et al., 2021). Some researchers have suggested that gametogenesis isn’t random, but is tied to environmental stress or other factors, but most have lacked solid evidence for this (Lirdwitayaprasit, 2002; Miyaguchi et al., 2006). Both Zingmark (1970) and Fukuda and Endoh (2006) described gametogenesis in considerable depth, including the fusion of gametes and the development of new vegetative cells from each zygote (Figure 1), but both studies failed to pinpoint the parameters that trigger gametogenesis.
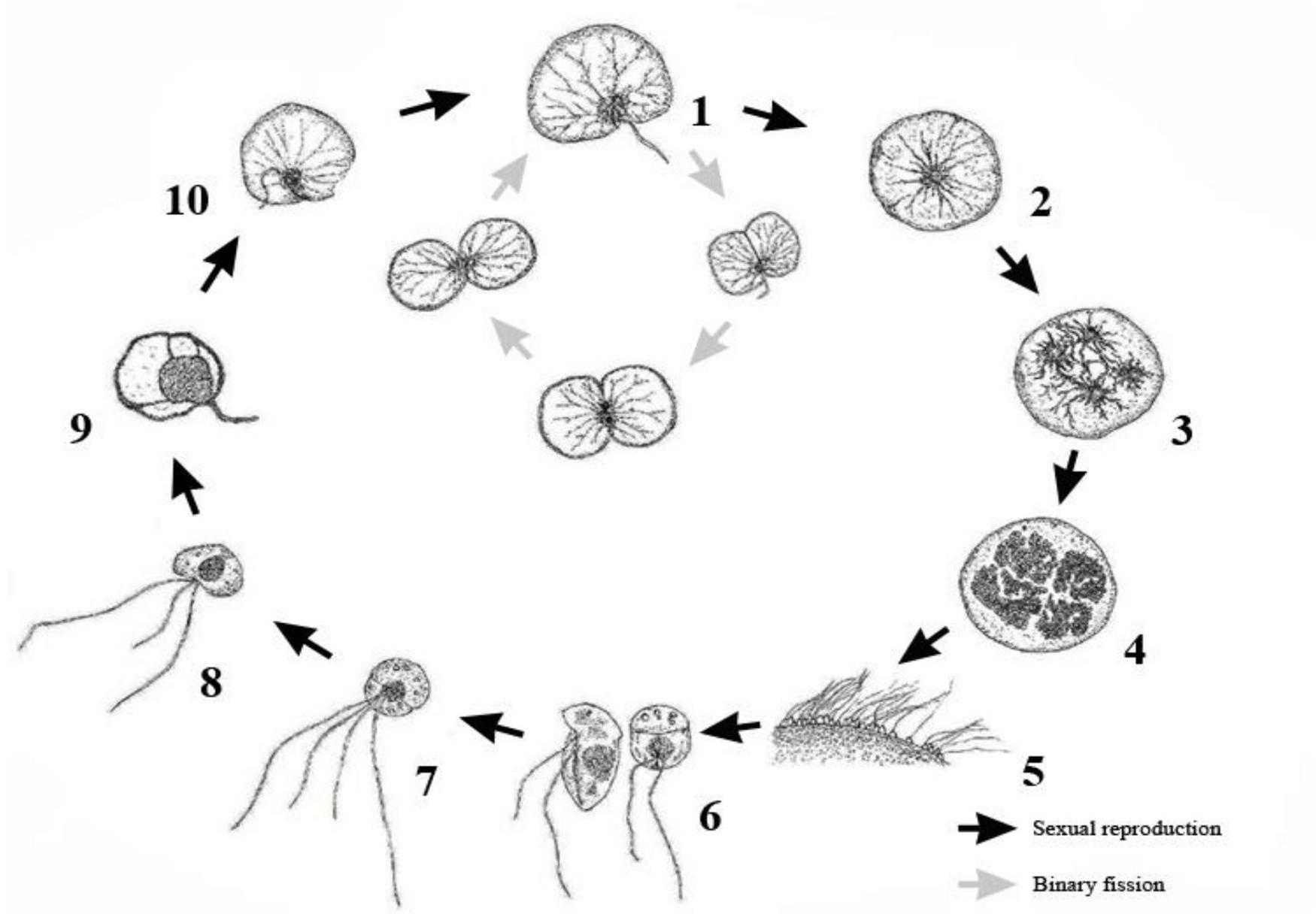
Figure 1. Schematic representation of the complete life cycle of Noctiluca scintillans. Schematic drawing includes both asexual binary fission and sexual reproduction. Redraw from Fukuda and Endoh (2006). 1. Trophont cell. 2. The initiation of gametogenesis, trophont cell turns into a gametocyte mother cell. 3. Two successive nuclear divisions. 4. Nuclei divides until 256–1024 progametes. 5. Progametes mature into gametes (zoospores). 6. Each gametes with two flagella, after leaving the gametocyte mother cell. 7. Zygote with four flagella. 8. Zygote develops into trophont cell. 9. The number of flagella starts decrease and formation of tentacle begins. 10. Tentacle formation is complete, gain phagotrophic capability and growth into mature trophont cell.
Sexual reproduction in Noctiluca scintillans starts with the trophont cell (Figure 2A) shedding its tentacle. The shape of the cell changes from reniform to spherical, followed successively by the concentration of its chromosomes in the nucleus just below the cell surface (Figure 2B), supposed to be one round of meiosis and about 8 rounds of nuclear division (or mitosis), each division taking about 1–2 h (Zingmark, 1970; Fukuda and Endoh, 2006). After the initial successful meiosis, four haploid nuclei are formed that become progametes (Figure 2C), which continue to divide 6–8 times (by mitosis) to 256–1,024 progametes. At the beginning of this stage, the trophont cell has completely transformed into a gametocyte mother cell (Figure 2D), which is visually distinctive from ordinary trophont cells undergoing cell division (Figure 2E). As the gametocyte mother cells reach 1,024 progametes, the progametes cease dividing and each progamete starts to develop into a mature gamete (zoospore) with two flagella. The fully mature gametes are then released into the surrounding water (Figure 2F), leaving the gametocyte mother cell as a hollow husk that decays (Zingmark, 1970; Schnepf and Drebes, 1993; Fukuda and Endoh, 2006; Zhang et al., 2016; Sathish et al., 2021). The gamete of N. scintillans is small compared to a trophont cell, about 20 μm in diameter at most, making it hard to locate and observe. It has morphological affinities with dinoflagellates (Jollos, 1910), with two flagella that differ in length and motion posited in its longitudinal and transverse grooves (Fukuda and Endoh, 2006). These newly formed gametes swim using their flagella, and fuse in pairs into zygotes as homothallism by observing a considerable number of zygotes (conjugants) in the same culture dish (Fukuda and Endoh, 2006). The zygote does not initially have the ability to catch prey, due to its undeveloped tentacle. After the degeneration of three of the four flagella, leaving just one, and the development of a tentacle (Métivier and Soyer-Gobillard, 1988), the zygote has completely transformed into a trophont cell or vegetative cell, marking the end of the sexual reproduction cycle (Fukuda and Endoh, 2006).

Figure 2. Process of gametogenesis in Noctiluca scintillans. (A). Tropont cells of N. scintillans. (B) Stage 1 of gametogenesis. The cell has lost prominent structures, such as its tentacle, and the cell has become spherical. The nucleus (red arrowhead) is just below the cell surface. (C) Stage 3 of gametogenesis, with 4 progametes. (D) Stage 8 of gametogenesis. The nucleus has divided into 128 progametes. (E) The gametocyte mother cell has reached its final stage (stage 12), but the progametes are not yet mature. (F) Stage 14 of gametogenesis. The gametes are being released into surrounding water and can move freely. Scale bar = 200 urn in (A,B), 100 urn in (C,E), 50 urn in (D), and 20 urn in (F).
Sato et al. (1998) suggested that gametogenesis may not occur randomly, but is rather tied to the number of divisions that the trophont cells have undergone. They suggested that after a trophont cell has undergone cell division (binary fission) about 20–24 times, the cell has a high probability to shift to sexual reproduction. The cell division counter for a trophont cell will be reset when a gamete comes into contact with it. However, the most common triggers for sexual reproduction in dinoflagellates are environmental stresses (Zingmark, 1970; Sato et al., 1998; Lirdwitayaprasit, 2002; Miyaguchi et al., 2006; Spector, 2012), including temperature, light exposure time, prey concentration, N. scintillans concentration, cultivation time, cultivation volume and simulated wave motion (Pfiester, 1984; Sato et al., 1998; Spector, 2012; Kremp, 2013; Valiadi and Iglesias-Rodriguez, 2013; Bravo and Figueroa, 2014). In order to confirm this, we cultured N. scintillans under these different sorts environmental stress.
In this study, we try to fill in gaps in knowledge about the sexual reproduction of Noctiluca scintillans, mainly focusing on (1) what triggers gametogenesis, (2) how gametogenesis affects the population of N. scintillans during blooms and (3) whether this process is inducible under laboratory conditions. Later we found that the shift of prey concentrations over time could trigger gametogenesis and developed a method to induce this process in laboratory conditions. The occurrence of sexual reproduction implies a strong connection with the end of bloom in natural environments.
Materials and Methods
Sampling and Culture
A pure cell culture of Noctiluca scintillans was established by isolating a mature cell (trophont) near the mainland Chinese coast in the harbor of Jie-Shou Au, Nangan Island (26°09′06″ N, 119°57′06″ E), Matsu Archipelago, Taiwan. A sterile pipette was used to wash the collected cell several times with 0.2-μm-filtered autoclaved seawater at a salinity of 30 to remove debris in the water column or other organisms attached to the cell (Lee and Hirayama, 1992). This single-cell culture of N. scintillans was fed the green algae Tetraselmis chui (prey) in a Falcon Cell culture 6-well plate (each well holds 10 mL) and maintained at 20°C in a 12:12 light:dark cycle at 100 μmol photons m–2 s–1 in 0.2-μm-filtered seawater at a salinity of 30. The green alga (T. chui) was maintained in the conditions described as above in f/2-filtered seawater (Guillard and Ryther, 1962) at a salinity of 30 in a 250 mL culture flask (BD Falcon). The culture of N. scintillans and T. chui were repeated until the desired concentration and volume were achieved, this culture condition and method were later referred as stock culture.
Tests of Different Environmental Factors
Parameters for the testing of each factor are shown in Table 1. Each factor was tested individually, as the independent variable while the other environmental conditions were those used for culturing N. scintillans as described above. Initial tests were done with a high, low and medium level of each variable factor, which the medium level served as the control—the same condition as in stock culture. For each treatment (factor), N. scintillans were cultured in Falcon Cell culture 6-well plate in triplicate, each well having a total liquid volume of 8 mL. Only the conditions with variation in culture volume was done in a 1,000 mL beker. The initial concentrations of N. scintillans and T. chui in all treatments were measured after added stock solution to each treatment. The concentration of N. scintillans and proportion of gametocyte mother cells were recorded every 24 h under a dissecting microscope (Nikon, SM21000). The sexual reproduction rate was calculated as the proportion of easily identifiable gametocyte mother cells in the whole population.
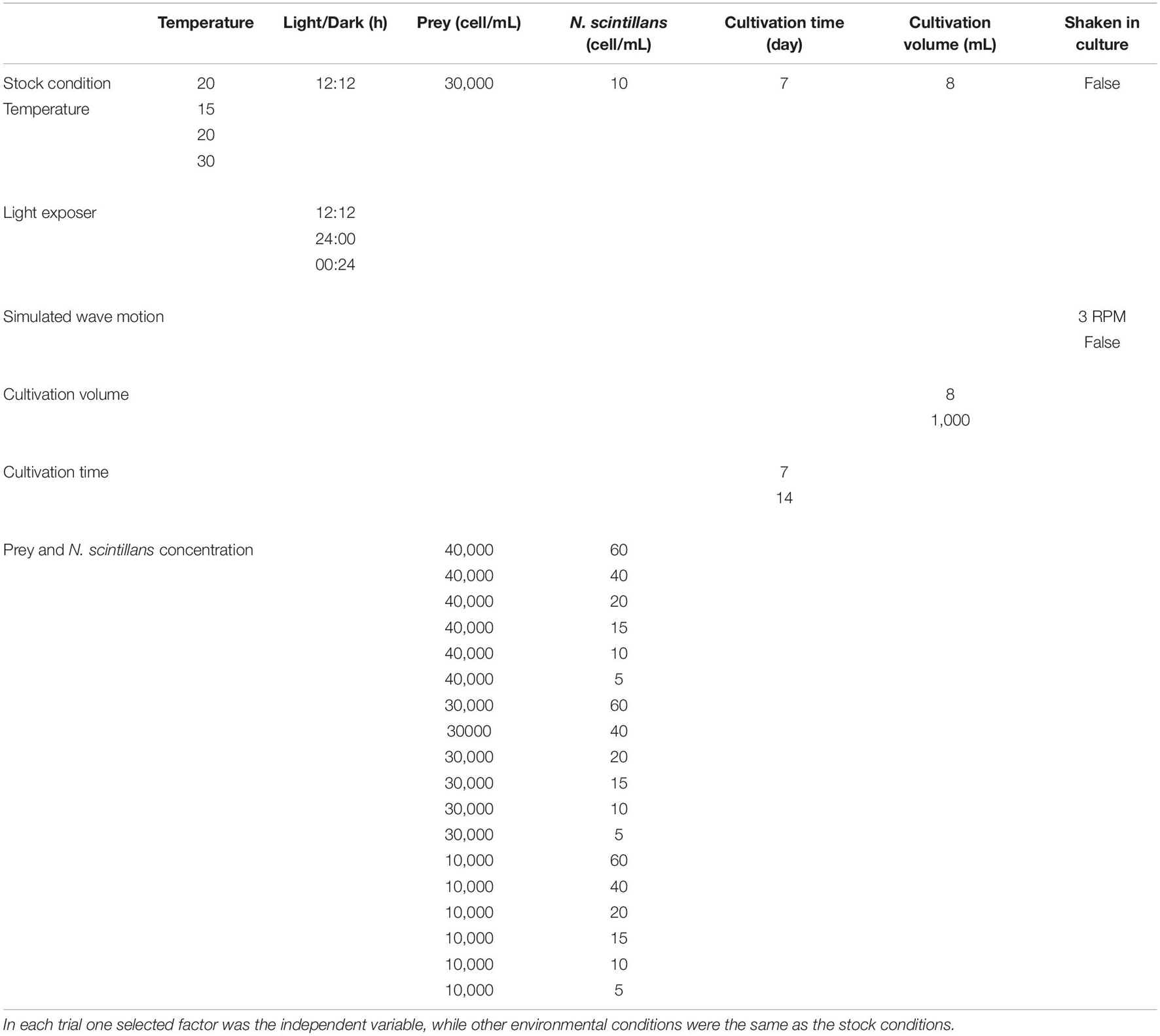
Table 1. All-parameter set for the testing of factors suspected of inducing sexual reproduction in Noctiluca scintillans.
After seeing that culture time has a noticeable influence on the rate of sexual reproduction, a second round of tests was done, repeating all the previous tests but with a longer culture time to confirm whether this was the main influencing factor. After adjusting the culture time, some treatments involving differences in the initial concentration of N. scintillans or prey showed a noticeable increase in gametocyte mother cells compared to other treatment. Therefore, a third round of testing was undertaken, focusing on these two factors, i.e., the initial concentrations of N. scintillans and prey, respectively (Figures 3, 4) with wider range of each variable factor. A noticeable relationship between the initial concentration N. scintillans and prey were observed, causing higher proportion of gametocyte mother cells in N. scintillans populations, but was not definitive enough to come to a conclusion. Hence, a fourth round of testing was commenced, for plotting out the relationship between these factors, focuses on the concentrations of N. scintillans, prey and sexual reproduction rate. The fourth round of testing uses a combination condition picked from previous tests with high sexual reproduction rate, i.e., 10 cells/mL initial N. scintillans concentration with variation of initial prey concentrations (40,000, 50,000, and 60,000 cells/mL), maintained at 20°C in a 12:12 light:dark cycle, a total cultivation volume of 8 mL and a cultivation time of 14 days. N. scintillans, prey concentration and sexual reproduction rate were recorded daily, where sexual reproduction rate was calculated as the proportion of gametocyte mother cells in the whole population.
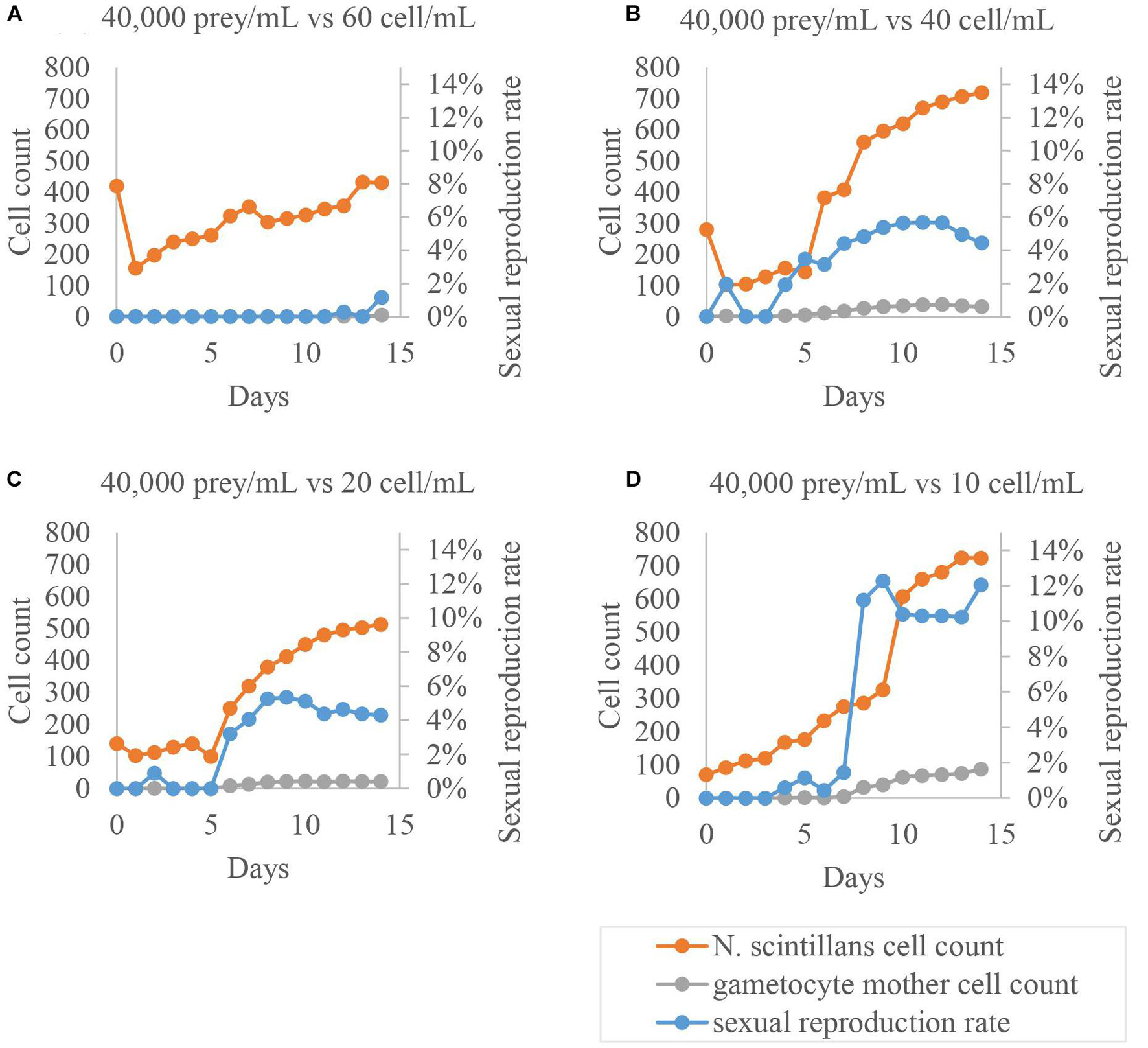
Figure 3. Sexual reproduction rates of cultured Noctiluca scintillans with its various initial cell concentration. Orange dots represent the amount of trophont cells, blue dots show the proportion of gametocyte mother cells to the total population, and gray dots exhibit the amount of gametocyte mother cells. The highest sexual reproduction rate occurred in each condition was lowest in (A), intermediate and mutually similar, about 6% in (B,C), and highest, up to –12%, in (D).
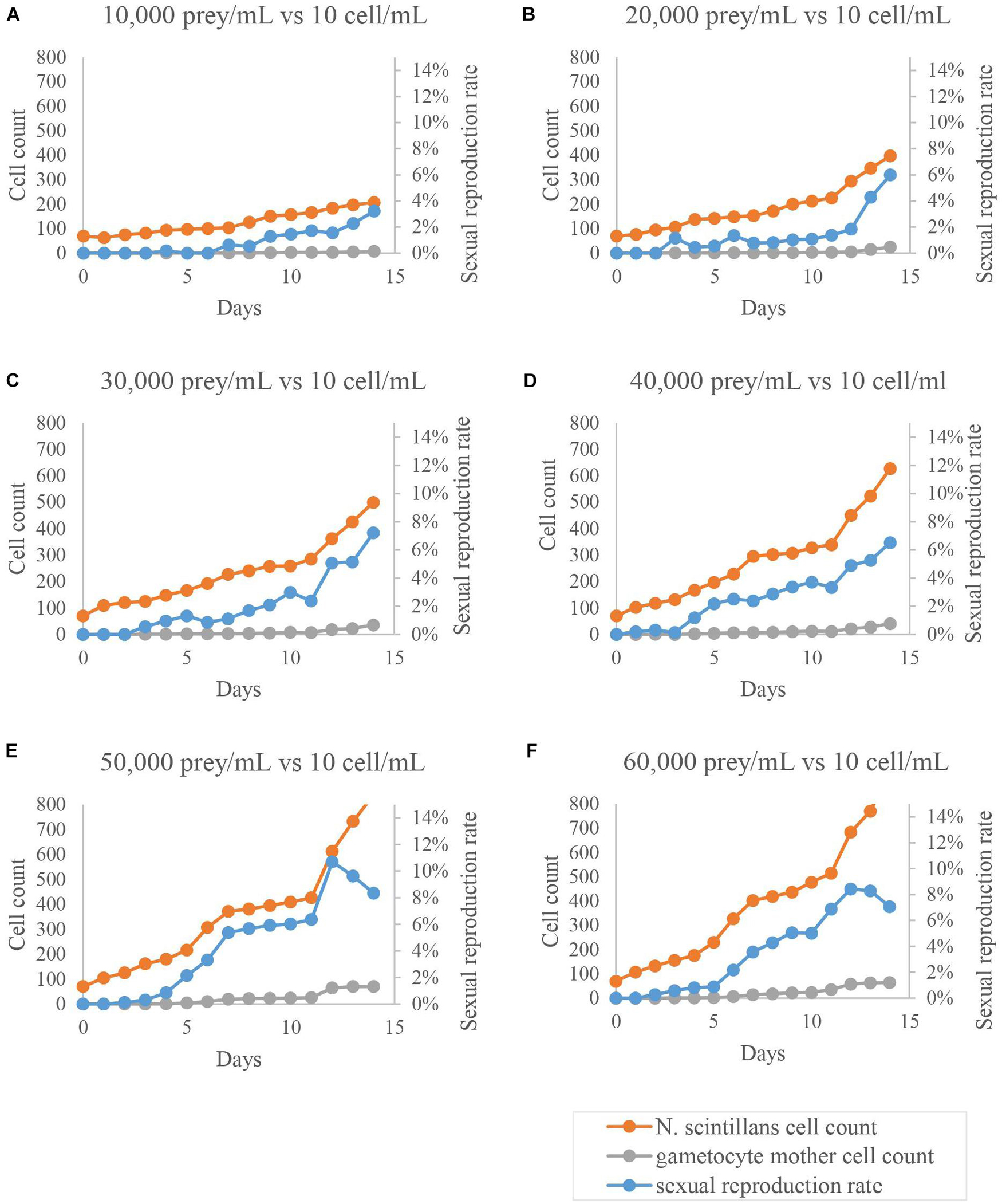
Figure 4. Sexual reproduction rates of cultured Noctiluca scintillans with various initial prey concentration. Orange dots represent the amount of trophont cells, blue dots show the proportion of gametocyte mother cells to the total population, and gray dots exhibit the amount of gametocyte mother cells. All treatments (A–F) showed noticeable increases in sexual reproduction rates as time progressed, where (E,F) reach the two highest values on day 12, ∼11% and 9%, respectively, followed by a decrease.
Enumeration of Trophonts, Gametocyte Mother Cells and Tetraselmis chui Cells
Every 24 h, samples (8 mL) of each triplicate treatment culture were fixed in neutralized formaldehyde (3% final concentration) for quantitative enumeration of N. scintillans (trophont cells), gametocyte mother cells and T. chui. Using the same equipment as above, trophonts and gametocyte mother cells were counted under a dissecting microscope (Nikon, SM21000) at a magnification of 10 ×. For T. chui, a 100 μL subsample was taken from the original fixed sample (8 mL), then placed into a 2 mL centrifuge tube. After thorough shaking, 1 μL of this solution was transferred (or dropped) onto a glass slide and counted under a stereomicroscope (Nikon, Eclipse TS2R) at a magnification of 200 × or higher. All counts were repeated three times, to avoid miscounts.
Shortening the Culture Time
Previous preliminary results of the present study showed how different culture conditions could increase the proportion of gametocyte mother cells in N. scintillans populations and revealed a promising candidate for the main triggering factor of sexual reproduction in N. scintillans. i.e., the relation of relative concentration between N. scintillans and T. chui—the amount of prey that a single N. scintillans trophont cell is distributed or can encounter, referred as encounter rate in the following text. With this knowledge, an alternative culture method was designed to test this theory and to maintain steady production of gametocyte mother cells for materials in future experiments. An initial N. scintillans concentration of 30 cells/mL and T. chui concentration of 5 × 104 cells/mL in a Falcon Cell culture 6-well plate with 0.2-μm-filtered seawater at a salinity of 30 was proceeded. Each well contained a total volume of 8 mL f/2 medium and the plates were maintained at 20°C in a 12:12 light:dark cycle at 100 μmol photons m–2 s–1. Each prey concentration was kept constant at around 5 × 104 cell/mL by adding additional T. chui stock solution in the first 3 days. Each sample of 50 μL water was fixed with Lugol’s solution (3% final concentration) daily to calculate the concentration of prey under a stereomicroscope (Nikon, Eclipse TS2R) by the same process as the initial tests. In addition, direct observation of the trophont cells and gametocyte mother cells in each well took place daily under a dissecting microscope (Nikon, SM21000). The experimental period lasted 14 days initially, but was later shortened to 10 days, due to the highest sexual reproduction mostly occurs on day 8–10. This alternative culture method was later used as reference for designing other test related to N. scintillans sexual reproduction cycle.
Timetable Determination
The number of gametocyte mother cells at each stage of their development was counted under a dissecting microscope in order to verify the duration of each phase of the sexual reproductive process. Staging (Table 2) was based on cytological features recorded in earlier studies (Zingmark, 1970; Sato et al., 1998; Lirdwitayaprasit, 2002; Fukuda and Endoh, 2006; Sathish et al., 2021). Stage 0 to stage 13 is regarded as the transformation from trophont cell to gametocyte mother cell, the production of progametes and the maturing from progametes to gametes. Stage D defines as the discarding of the husk left behind after all or most gametes left the gametocyte mother cell. Stage 14 to stage 17 is the combining of gametes to form zygotes and develop into a trophont cell. To promote growth and to later induce the formation of gametocyte mother cells, three 6-well cell culture plates were then prepared, each with a different initial concentration of N. scintillans, 10, 20, or 30 cell/mL. After transfer, each well was filled with sterilized and filtered seawater to a total volume of 8 mL with prey concentration of 5 × 104 cell/mL, and the well plates were maintained at 20°C under a 12:12 h light:dark cycle. For 72 h, each well was observed hourly under a dissecting microscope (Nikon, SM21000). Upon discovering cells that were about to undergo or had already undergone sexual reproduction, these cells were transferred to a Falcon Cell culture 96-well plate, one cell per well, each well of which contained 1 mL of sterilized 0.2-μm-filtered seawater. For another 72 h, the state of each cell was recorded hourly.
Statistical Analysis
In the third-round of testing, we divided the 14 days experiment into 3 intervals for each treatment: 0–4, 5–9, and 10–14 day. The ANOVA analysis followed by a Tukey test was performed to determine which interval and treatment of the sexual reproduction had a significant difference.
Results
Possible Triggering Factors for Sexual Reproduction
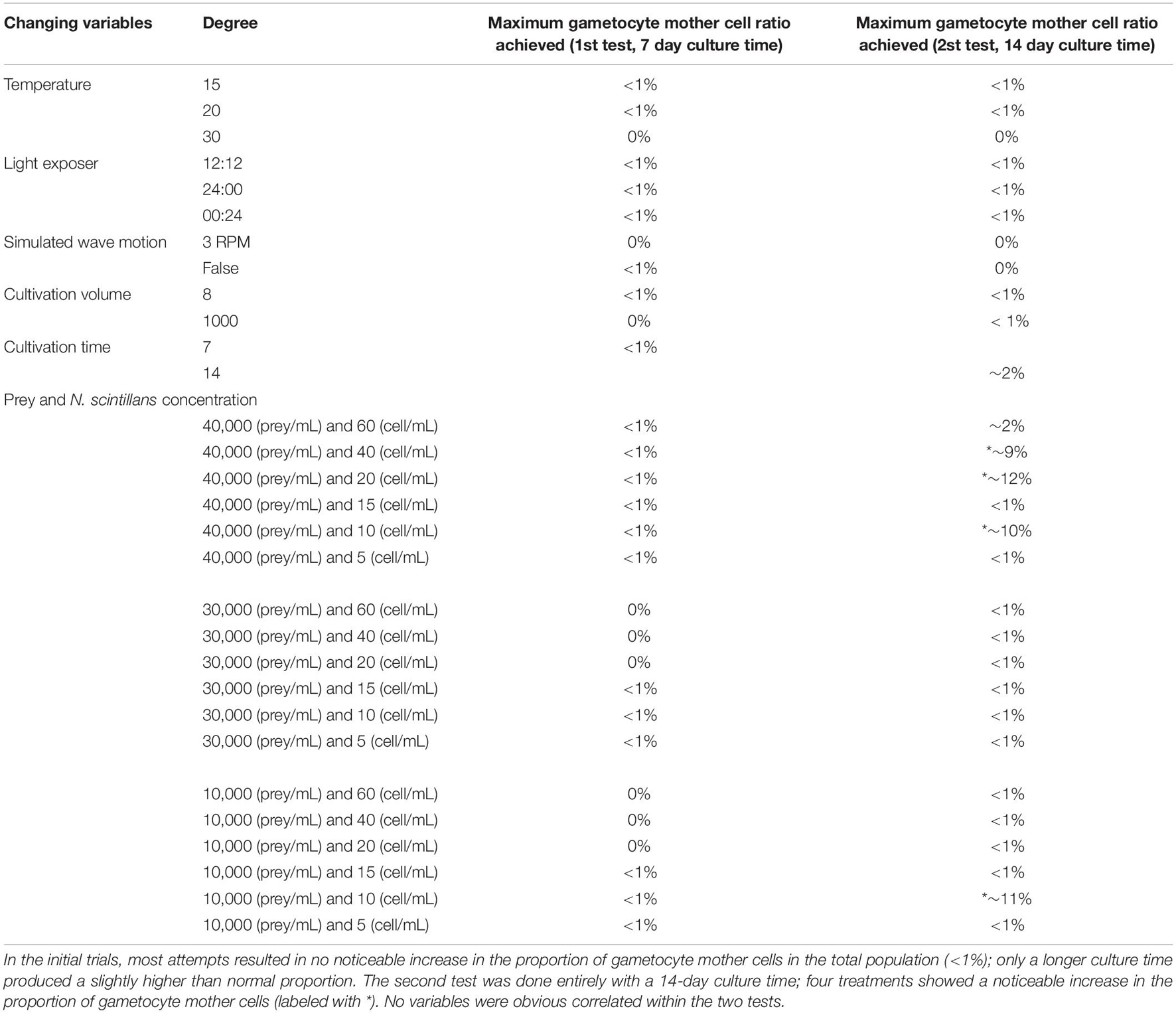
In the preliminary trials, there was no indication that any of the variables tested had a noticeable and consistent influence on the rate of sexual reproduction in Noctiluca scintillans (Table 3). However, a longer culture time was associated with a slightly higher proportion of gametocyte mother cells, with a rise from < 1% initially to nearly 2% after 14 days in culture (Table 3). This may indicate that earlier reports of no increase in the proportion of gametocyte mother cells were due to not waiting long enough. After repeating all tests with a 14 days culture time, most results did not change compared to the 7days culture time (Table 3). However, when the concentration of either prey or N. scintillans varied, a noticeable higher sexual reproduction rate, up to nearly 10% of the total population, was observed. This only occurred in the conditions with the initial concentration of T. chui and N. scintillans (trophont cell) set as: 40,000 prey/mL and 40 trophont cell/mL, 40,000 prey/mL and 20 trophont cell/mL, 40,000 prey/mL and 10 trophont cell/mL and 10,000 prey/mL and 10 trophont cell/mL.
The result for the third-round of testing is shown in Figures 3–6. As shown in Figure 3, each sexual reproduction rate in different initial concentrations of N. scintillans increases significantly on day 10–14 (Figure 6, p < 0.001 in B, C, and D), except for A, which sexual reproduction rate only increased to around 1% on day 14 (Figure 3A). In Figures 4, 5, all experiment conditions with different initial concentration of T. chui, except for A, showed significantly increasing sexual reproduction rates on day 10–14 (Figure 5, p < 0.05 in B, C, and D). By comparing the results of day 10–14 in each experiment conditions, there is a noticeable increase in sexual reproduction rate when the initial concentration of N. scintillans decrease (Figure 6E, p < 0.001) or the initial concentration of T. chui increase (Figure 5G, p < 0.005). However, combining these findings with results from the first and second round of test (Table 3), no obvious connection between these factors and sexual reproduction was evident.
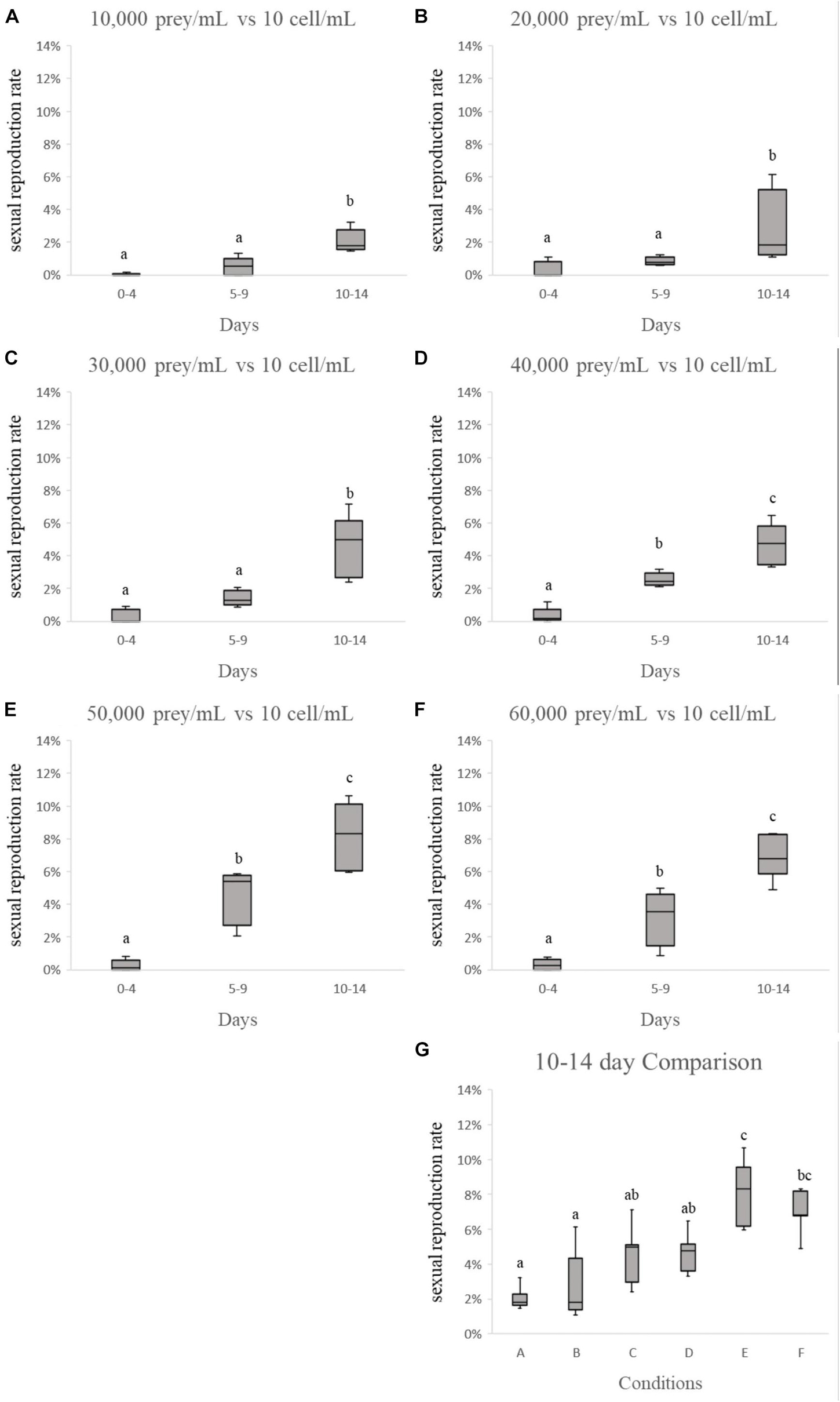
Figure 5. Comparison of “sexual reproduction rates vs. initial prey concentration.” Each sexual reproduction rate in (A–F) increases after 4 days’ culture, and reaches its maximum rate on day 10–14. (G) Shows by increasing the initial prey concentration, the more sexual reproduction occurs.
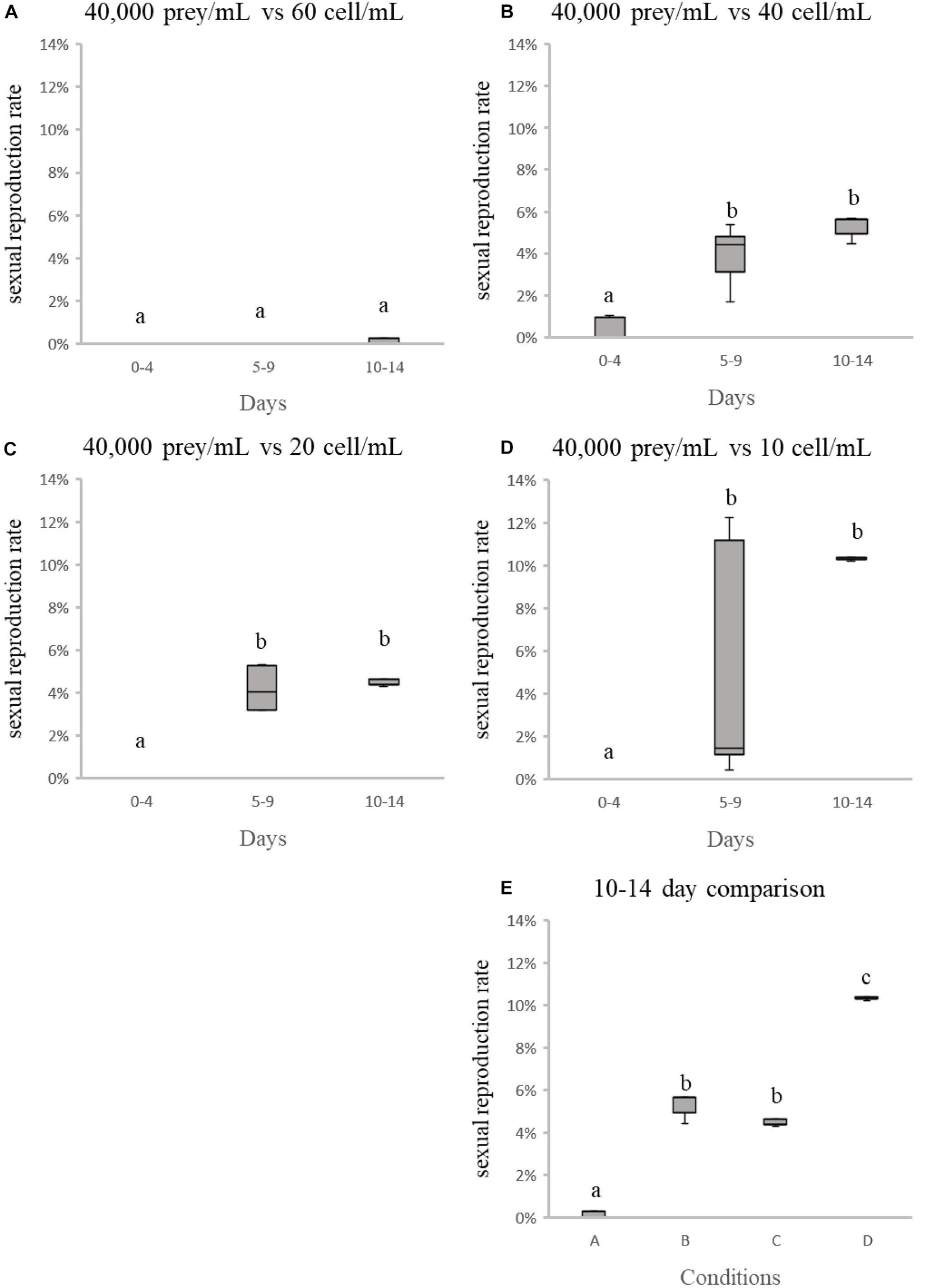
Figure 6. Comparison of “sexual reproduction rates vs. initial Noctiluca scintillans concentration.” Each sexual reproduction rate in (A–D) increases after 4 days’ culture, and reaches its maximum rate on day 10–14. (E) Shows by decreasing the initial N. scintillans concentration, the more sexual reproduction occurs.
Plots of the relation between proportion of gametocyte mother cells, prey concentration and N. scintillans concentration according to the fourth round of testing were shown in Figures 7–10. No noticeable connections between the concentration of prey and the proportion of gametocyte mother cells were observed (Figure 7A). However, there were noticeable connections between the concentration of N. scintillans and the proportion of gametocyte mother cells. In addition, further analysis showed positive correlations of higher proportion of gametocyte mother cells with increasing concentration of N. scintillans (Figure 7B, p < 0.001). However, there was no occurrence of sexual reproduction in most cases when the N. scintillans cell count was lower than 160 cells (20 cell/mL), indicating that the sexual reproduction will only happen after the population size reaches a certain concentration, for instance 20 cell/mL (Figure 7B). There was a noticeable relationship between the amount of prey that each N. scintillans could encounter (T. chui cell count/N. scintillans cell count) and the proportion of gametocyte mother cells: when the encounter rate of prey decreased, the sexual reproduction rate of N. scintillans increased (Figure 8A). The critical value for N. scintillans to undergo sexual reproduction was calculated, excluding the samples of population size under 20 cell/mL showing no sexual reproduction, found to be 35,400 prey cells/N. scintillans. Indicating the lower the prey cells offered to N. scintillans after certain population growth, the more likely it becomes that N. scintillans undergoes sexual reproduction (Figure 8B).
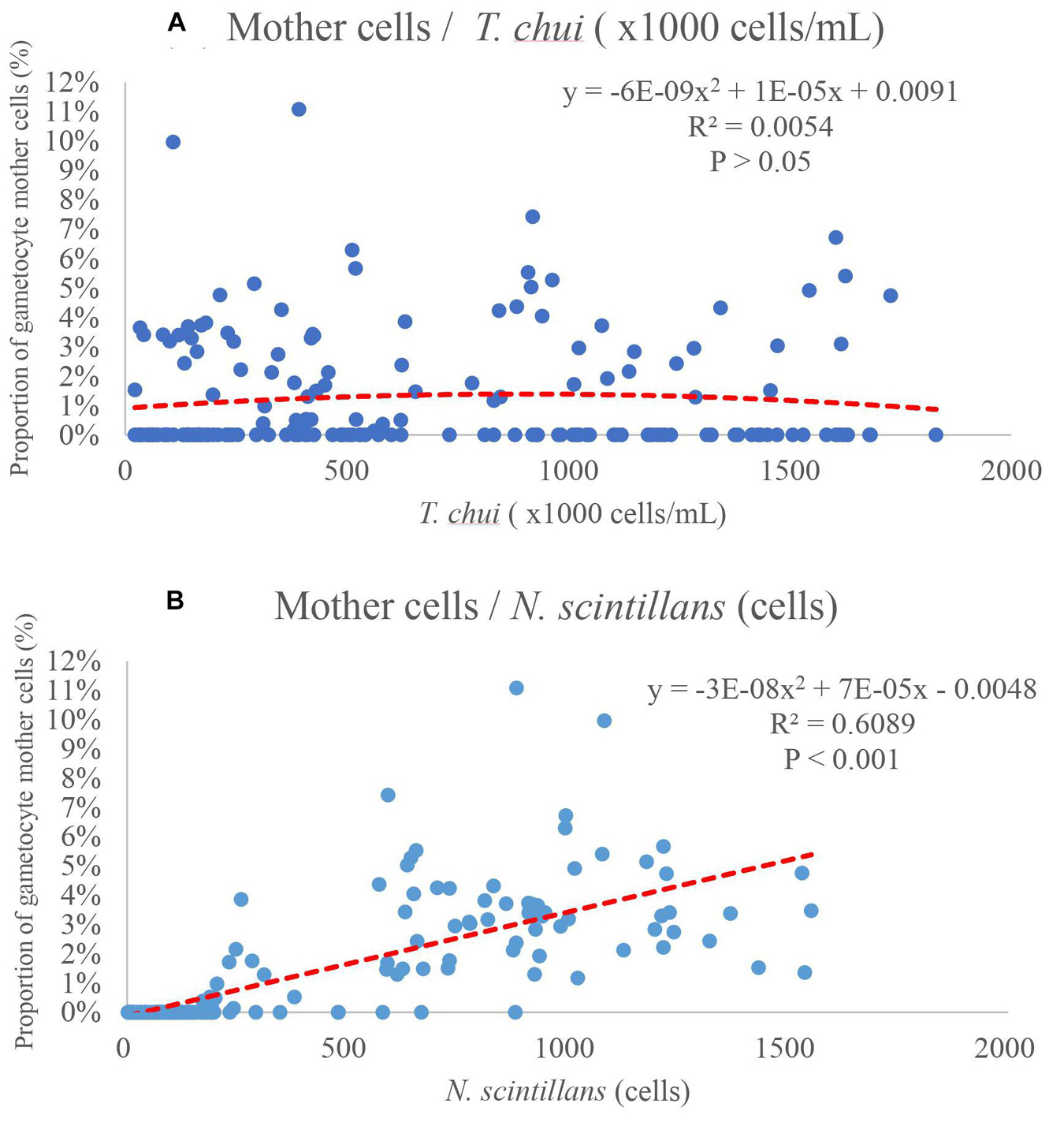
Figure 7. Proportion of gametocyte mother cells, as a function of concentration of Tetraselmis chui or Noctiluca scintillans. (A) The plot shows no obvious correlation between proportion of gametocyte mother cells and prey concentration. (B) The concentration of Noctiluca scintillans has a positive correlation with the proportion of gametocyte mother cells, indicating that with a higher concentration of Noctiluca scintillans, sexual reproduction is more likely to occur. However, most sample data with no sexual reproduction occurrence, fall under 160 cell (20 cell/mL).
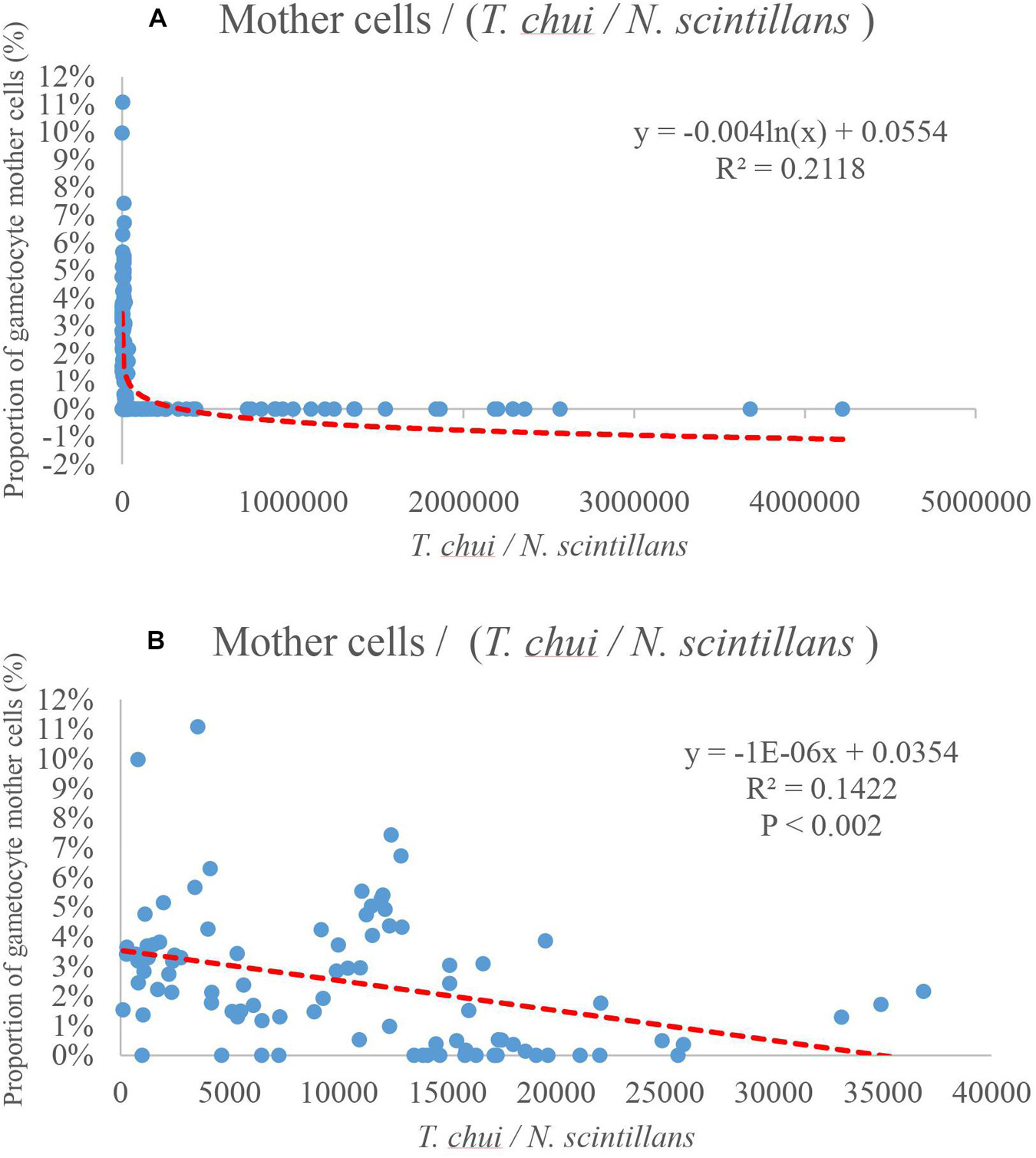
Figure 8. Proportion of gametocyte mother cells as a function of T. chui I N. scintillans. The relationship between the abundance of prey that each N. scintillans could encounter and the proportion of gametocyte mother cells. (A) The proportion of gametocyte mother cells increase as the amount of preys per N. scintillans encounter decreases. (B) The plot only shows the relation between the encounter rate and data with sexual reproduction likely to happen, by eliminating data of N. scintillans population under 160 cells (20 cell/mL). The trend line indicates that when the amount of preys per N. scintillans encountered drops lower than 35,400 prey cells / N. scintillans cells, the proportion of gametocyte mother cells starts increases dramatically.
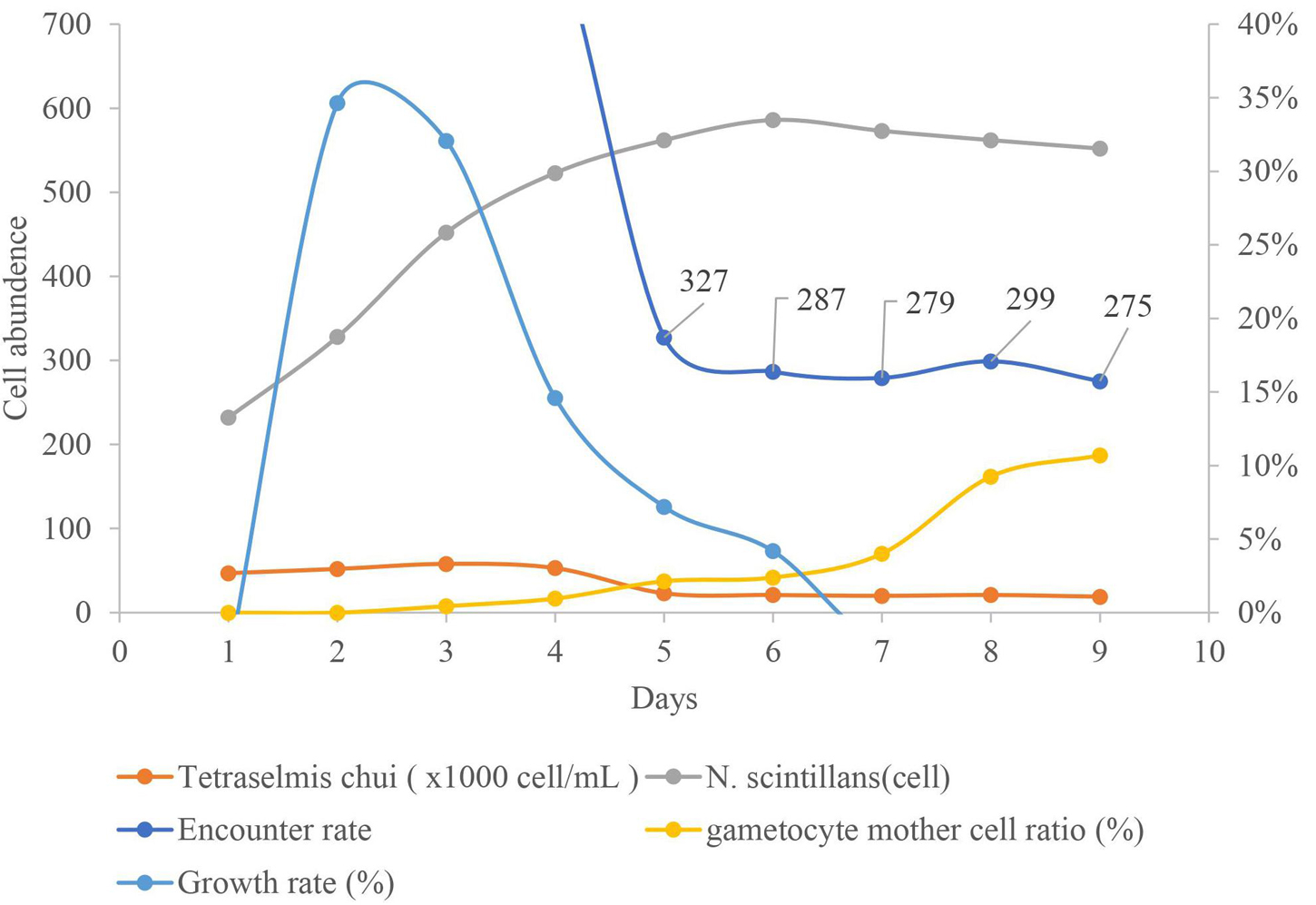
Figure 9. Relations between the densities of Tetraselmis chui and Noctiluca scintillans, proportion of gametocyte mother cells, encounter rate of T. chui to N. scintillans and growth rate of N. scintillans during 10 days in culture time. Prey were added every 24 h for the first 3 days to keep the prey concentration a constant around 5 × 104 cell/mL. At the end of the 4th day or beginning of the 5th day, the proportion of gametocyte mother cells increased noticeably, when the concentration of prey and the prey encounter rate, decreased accordingly.
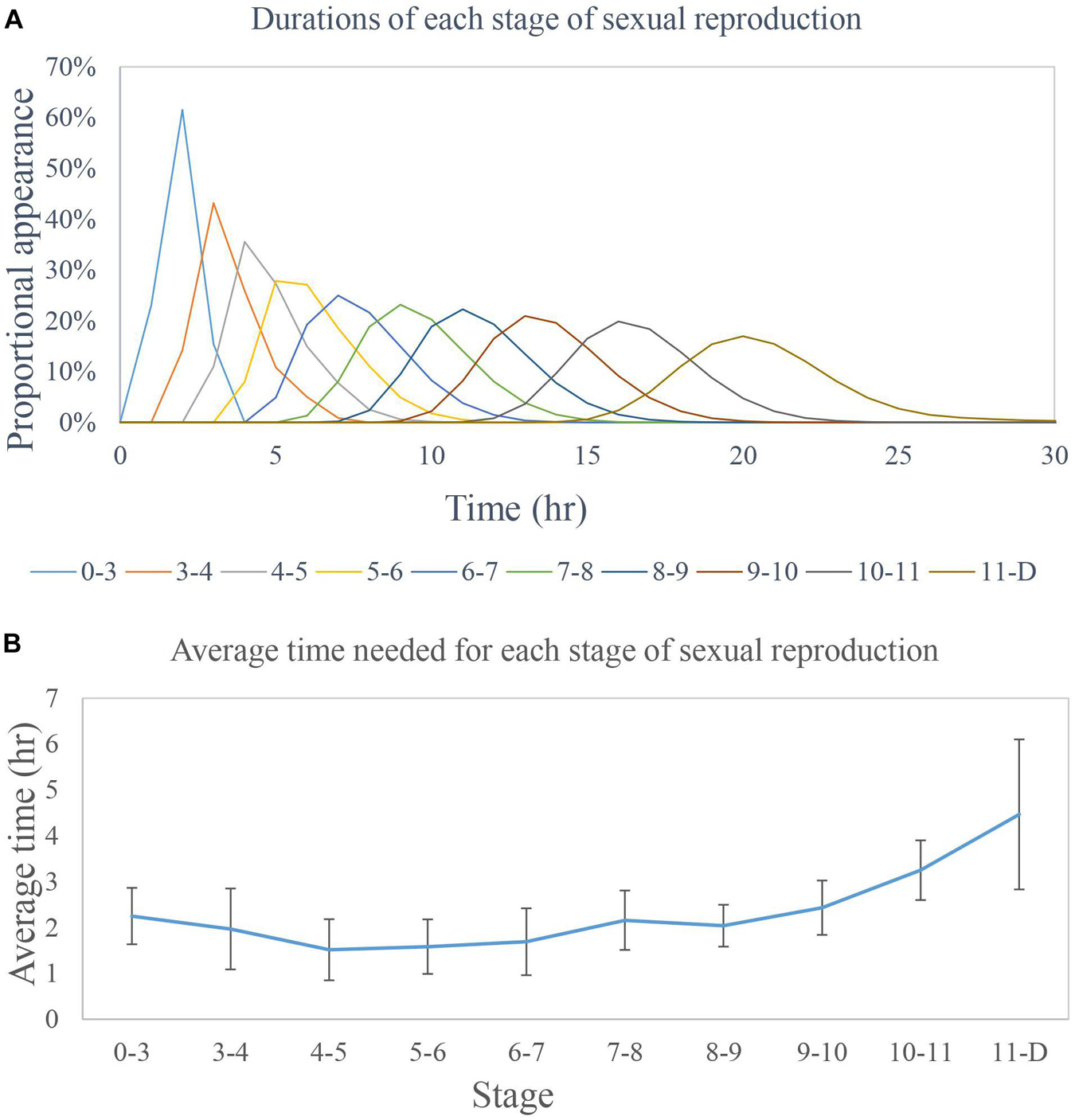
Figure 10. Time requirement for the sexual reproduction cycle of Noctiluca scintillans. (A). Each peak in the graph indicates the modal average time of appearance for the different stages of N. scintillans. The longest recorded time to finish sexual reproduction was 30 h, while the shortest was 13 h. (B) The average time for each stage was 2 h. The time required to reach stage D from stage 11 (i.e., for progametes to mature and be released) varied the most.
Method for Increasing Sexual Reproduction
Although decreasing the encounter rate of N. scintillans and its prey induces the onset of sexual reproduction, this only takes place after the population of N. scintillans has undergone mass reproduction, i.e., following the exponential phase (Figure 9). We found that this process can be speeded up by accelerating the exponential phase. This was accomplished by adding new prey every 24 h for the first 3 days to keep the prey concentration at a constant ca. 5 × 104 cell/mL and then stopping the feeding for the remainder of the culture period. By day 8 or 9 of the experiment, the sexual reproduction rate had increased significantly, to around 10% (Figure 9).
Timetable Determination
Gametocyte mother cells were collected and each cell was cultured in a single well of a 96-well plate. The time necessary for each stage of sexual reproduction from stage 0 to stage D was recorded for each cell (Figure 10A). At stage D the gametocyte mother cells were expelled. Due to the large size difference between the zoospores and the gametocyte mother cells (Zingmark, 1970; Schnepf and Drebes, 1993; Fukuda and Endoh, 2006), we could clearly determine which stage it was in after stage D. The average time needed for transformation between stages before stage D was 2 h (Figure 10B), and the average total time from stage 0 to stage D was 20 h. This may indicate that, when N. scintillans undergoes sexual reproduction the cell will be expelled after 24 h.
Discussion
Factors Inducing Gametocyte Mother Cells
Due to the lack of comparative information about the formation of gametocyte mother cells in N. scintillans, we investigated several possible triggers of sexual reproduction in other dinoflagellates (Baek et al., 2009). Including temperature, N. scintillans concentration, prey concentration, cultivation time, cultivation volume, light exposure time and physical shaking (simulated wave motion) (Schnepf and Drebes, 1993; Sato et al., 1998; Fukuda and Endoh, 2006; Harrison et al., 2011; Bravo and Figueroa, 2014). We found that the trigger might not be as simple as just one factor, like temperature or light exposure time. In one experiment the sexual reproduction rate increased from an average < 1% to > 10% (Table 3), but we were unable to confirm statistically whether this increase was random or not. Two iterations of further testing were needed to narrow down the possible influencing factors (Figures 3, 4). The testing experiments in the present study showed significant increasing in sexual reproduction rate with decreasing of initial N. scintillans cell concentration or increasing in initial prey concentration (Figures 5, 6). However, the results still could not explain which factor was the main contributor toward sexual reproduction. Another noticeable phenomenon occurred in testing experiments with different initial N. scintillans concentrations showed the rapidly dropping trophont cell concentrations after 24 h-cultivation (Figure 3). This may be caused by the sudden exposure to increase of N. scintillans cell concentration or low prey distribution, causing a deleterious effect on the population. In addition, the sexual reproduction rates increased significantly mainly on day 10–14, some on day 5–9, but none on day 0–4 in both test experiments, indicating that the populations needed to grow to a certain concentration then came the sexual reproduction. The testing experiments of the fourth round focusing on N. scintillans concentration and prey concentration showed that either or both of these factors may play an important role in triggering sexual reproduction in N. scintillans.
In Figure 7B, there was a noticeable positive correlation between the concentration of N. scintillans and the proportion of gametocyte mother cells, i.e., the higher the proportion of gametocyte mother cells, the higher the concentration of N. scintillans (p < 0.001). However, this positive correlation will not be able to explain the lack of sexual reproduction in several conditions of experiments with high initial concentration of N. scintillans, shown in previous experiments (Figures 3, 4). In addition, most samples without the occurrence of sexual reproduction landed between 0 and 200 N. scintillans cells. Similar results were shown in Figures 3, 6, where most increasing in sexual reproduction rate only occurred after the 4th day and achieved maximum sexual reproduction rate on the day 10–14, indicating that for sexual reproduction to even happen, the N. scintillans population would have to first undergo growth or asexual reproduction 1–2 times. The data where sexual reproduction could not occur in Figure 8A were ignored in the analysis of Figure 8B. In average, the sexual reproduction occurred after 3–4 days in the present study. It only occurred at/after the exponential phase. Therefore, N. scintillans cell count lower than 160 (20 cell/mL), where the population have not proceeded to twice of the initial size (10 cell/mL), were ignored, for not staying in the exponential phase, to provide a more persuasive relationships between encounter rates and sexual reproduction rates (p < 0.002).
A small fraction of trophonts spontaneously transform into gametogenic cells (gametocyte mother cell) (Calkins, 1899; Ishikawa, 1899; Soyer, 1969, Soyer, 1970a, b; Sato et al., 1998). Sato et al. (1998) suggested that gametogenesis may not occur randomly but is instead tied to the number of times the trophont cells have divided, i.e., Noctiluca scintillans gametogenesis can be artificially induced when each trophont was isolated from a culture of a homogeneous population. After a trophont cell has undergone cell division about 20–24 times, it supposedly has a high probability to shift from binary fission to sexual reproduction. Furthermore, when a gamete encounters another trophont cell, the countdown is supposedly reset to initial state. Fukuda and Endoh (2006) failed in reproducing the results of Sato et al. (1998) by the same method to trigger the differentiation of trophonts into gametocyte mother cell and speculated that a seasonal effect may be involved in triggering gametogenesis. However, we saw no evidence to support either of such claims. If gametogenesis halts after contact with a gamete, then less gametogenesis should have occurred in high concentrations of N. scintillans and more gametogenesis in low concentrations. No such pattern is evident in Figure 7B.
Rates of Encounter Between N. scintillans and Its Prey
In the present study, laboratory cultures showed that below a ratio of 35,400 prey cells to one N. scintillans, a higher sexual reproduction rate was obtained (∼10%; Figure 8B). The alternative culture method resulted in an increase in sexual reproduction on the 8 day of culture (Figure 9). Even though, the encounter rate did not drop below the critical value suggested in Figure 8B. The rate of sexual reproduction started to increase after the prey concentration was not maintained and dropped. With a similar timing as the growth rate decreased, signaling the end of the exponential growth phase. A reduced encounter rate is only effective after the N. scintillans population has undergone mass reproduction, that is, after the exponential growth phase.
Timetable of Sexual Reproduction
Zingmark (1970) estimated the total time of gametogenesis between the formation of the gametocyte mother cell and the mature gametocyte to be approximately 10 h, with approximately 45 min to 1 h intervals for each divisions of the tetrad nuclei. Fukuda and Endoh (2006) observed that the intervals of each division were about 2 h, except for the first two divisions approximately 1 h, and postulated that the first two divisions may correspond to meiosis. However, the average time needed for each stage in the sexual reproduction of N. scintillans in the present study is about 2 h with more than 40 cells observed in each stage and the total time from stage 0 to stage D is about 20 h (Figure 10), showing no difference in intervals between stages. We still have no detail observations on the process from zygote to trophont cell even though under the culture condition with more than 30 gametocyte mother cells mixed in each well and no direct evidence for meiosis postulated by Zingmark (1970) and Fukuda and Endoh (2006) as far.
Cysts or Not?
Zingmark (1970) suggested that the swarmers of Noctiluca scintillans are isogametes. This is later supported by the detailed observation from Fukuda and Endoh (2006), that the zygote develops directly into a large trophonts. However, Schnepf and Drebes (1993) speculated that Noctiluca scintillans appears to be anisogamous, and the zygote does not need to grow to become a large trophont. Hence, the Noctiluca scintillans zygote is a planozygote, ready to take up food in the “normal” way (Schnepf and Drebes, 1993). Resting stage or encystment was not observed in the life cycle of Noctiluca scintillans (Pfiester, 1984), and nuclear events after syngamy were not observed, indicating that in Noctiluca subsequent development occurs in a diploid state, omitting encystment (Fukuda and Endoh, 2006). The fate of the released gametes remains unknown since the connection between gametes and vegetative cells has not been experimentally established (Sato et al., 1998). However, Fukuda and Endoh (2006) did maintain clonal cultures of Noctiluca scintillans throughout the whole life cycle and revealed new details of various stages, especially the transformation from the zygote into a miniscule trophont.
Applications to Ocean Ecology
Natural bloom of N. scintillans most commonly occur as follows: an increasing concentration of phytoplankton leads to an algal bloom. Second, due to this increase in prey or by physical aggregation by seasonal winds, the concentration of N. scintillans increases. Third, the population of N. scintillans grows exponentially by asexual or sexual reproduction and may cause harm to nearby fisheries resources. Fourth, after some time, the population of N. scintillans disappears due to either lack of prey, consumption by larger predators or being carried away by the wind (Miyaguchi et al., 2006; Baek et al., 2009; Harrison et al., 2011; Do Rosário Gomes et al., 2014). After the release of gametes, the cultured gametocyte mother cells are expelled and sink to the bottom of Petri dish with in 24 h in the present study, indicating an alternative pathway for N. scintillans cells to disappear besides the traditional food webs (Figure 9).
The timing of the increase in sexual reproduction must also be considered. Whether a bloom is caused by physical aggregation (e.g., by currents or wind) or by biological factors (e.g., by an increased prey concentration), the population size of N. scintillans starts off relatively small but grow rapidly long with the standing crop of food or prey. After the initial phase of rapid growth, the exponential phase of the N. scintillans population ends along with exhaustion of the food source (Harrison et al., 2011; Spector, 2012). The encounter rate with prey decreases, leading to increased sexual reproduction in N. scintillans, and this in turn becomes a factor contributing to the dissipation of N. scintillans bloom. If the sexual reproduction rate reaches about 10%, this proportion of the population will sink and disappear every 24 h when sexual reproduction is at its peak.
Fukuda and Endoh (2006) failed to reconstruct the program of gametogenesis in N. scintillans purposed from Sato et al. (1998) by isolating a single trophont cell from a culture of a homogeneous population, and in return they suggested that seasonal effect may be involved in triggering gametogenesis.
Hypotheses proposed by former researchers are that sexual reproduction may promote survival by increasing genetic diversity, or that it serves as preparation for the next season’s bloom (Peperzak, 2006; Kremp, 2013). The latter idea assumes that the newly formed zygotes sink to the sea floor and bloom when environmental conditions become favorable. Either hypothesis requires confirmation by further research. Furthermore, the process by which gametes fuse to form zygotes and the development of the subsequent trophont cell are still not well studied (Zingmark, 1970; Fukuda and Endoh, 2006).
Schnepf and Drebes (1993) found that only very few gametocytes developed within a culture vessel and occasionally, the flasks contained a relatively high number (up to about 15%) of microgametocytes. Even though the liberated microgametes swam preferentially on the bottom of the Petri dish under culture condition without any fusion of microgametes observed, and died after about 1 day (Schnepf and Drebes, 1993), Zingmark (1970) observed the dropping of the conjugates (early zygotes) on the bottom of the depression after stopping swimming. The zygotes can be seen to lie on the bottom of the depression for at least 2 days without further development. In most cases the zygotes ceased to develop and eventually died. However, in one case a zygote increased in size and became vacuolate. A nuclear mass and what appeared to be a coiled tentacle also were differentiated. This cell enlarged to about 200 um and then died. The development of zygotes into trophont cells needs further studies to give additional evidence for the development. The zygote-like stages or any intermediate growth stages which would eventually lead to the normal, large trophonts were not observed (Schnepf and Drebes, 1993).
Conclusion
In this study we used traditional culture methods and repeating testing of different factors to discover which factors particularly influence sexual reproduction in Noctiluca scintillans, and how to stimulate this process in the laboratory. The results show that the onset of sexual reproduction is mainly controlled by the encounter rate with prey. After a population of N. scintillans undergoes mass reproduction (e.g., after an exponential growth phase) the encounter rate with prey decreases and an increase in the sexual reproduction rate from the usual 1% to a maximum of at least 10% of the total population will occur. With this knowledge in hand, it was possible to develop a method of culturing to N. scintillans and increasing its sexual reproduction rate to around 10% in under 10 days, which makes it much easier than before to acquire samples for related research, including into the timing of the N. scintillans life cycle. We were able to show that in average every discrete stage in the process of sexual reproduction requires 2 h to finish, but that stage D (when the spent gametocyte mother cell is discarded) varies the most. The total time for release of gametes is around 20 h. These newly acquired facts about N. scintillans provide a more fundamental understanding of these species and how it interacts with the environment.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
S-FT and K-PC designed the research. JL carried out the experiment. S-FT and JL wrote the manuscript. All authors analyzed the data.
Funding
This study was supported by the grants from the Ministry of Science and Technology, Taiwan, ROC (108-2611-M-019-013 and 109-2611-M-019-017).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank to Mark J. Grygier (Center of Excellence for the Oceans, National Taiwan Ocean University, Taiwan) for his constructive criticism and linguistic improvements.
References
Baek, S. H., Shimode, S., Kim, H.-C., Han, M.-S., and Kikuchi, T. (2009). Strong bottom–up effects on phytoplankton community caused by a rainfall during spring and summer in Sagami Bay. Japan. J. Mar. Syst. 75, 253–264. doi: 10.1016/j.jmarsys.2008.10.005
Bravo, I., and Figueroa, R. I. (2014). Towards an ecological understanding of dinoflagellate cyst functions. Microorganisms 2, 11–32. doi: 10.3390/microorganisms2010011
Calkins, G. N. (1899). Mitosis in Noctiluca miliaris and its bearing on the nuclear relations of the protozoa and metazoa. J. Morphol. 15, 711–770. doi: 10.1002/jmor.1050150306
Chalaris, A. (2010). Harmful algal blooms and aquaculture: Are they a problem for farmers in Greece? Fish Farm. 32, 22–25.
Do Rosário Gomes, H., Goes, J. I., Matondkar, S. G. P., Buskey, E. J., Basu, S., Parab, S., et al. (2014). Massive outbreaks of Noctiluca scintillans blooms in the Arabian Sea due to spread of hypoxia. Nat. Commun. 5:4862. doi: 10.1038/ncomms5862
Elbrächter, M., and Qi, Z. (1998). Aspects of Noctiluca (Dinophyceae) population dynamics. Berlin: Springer.
Escalera, L., Pazos, Y., Morono, N., and Reguera, B. (2007). Noctiluca scintillans may act as a vector of toxigenic microalgae. Harmf. Algae 6, 317–320. doi: 10.1016/j.hal.2006.04.006
Frangópulos, M., Spyrakos, E., and Guisande, C. (2011). Ingestion and clearance rates of the red Noctiluca scintillans fed on the toxic dinoflagellate Alexandrium minutum (Halim). Harmf. Algae 10, 304–309. doi: 10.1016/j.hal.2010.11.002
Fukuda, Y., and Endoh, H. (2006). New details from the complete life cycle of the red-tide dinoflagellate Noctiluca scintillans (Ehrenberg) McCartney. Eur. J. Protistol. 42, 209–219. doi: 10.1016/j.ejop.2006.05.003
Guillard, R. R., and Ryther, J. H. (1962). Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 8, 229–239. doi: 10.1139/m62-029
Harrison, P., Furuya, K., Glibert, P., Xu, J., Liu, H., Yin, K. D., et al. (2011). Geographical distribution of red and green Noctiluca scintillans. Chin. J. Oceanol. Limnol. 29, 807–831. doi: 10.1007/s00343-011-0510-z
Huang, C., and Qi, Y. (1997). The abundance cycle and influence factors on red tide phenomena of Noctiluca scintillans (Dinophyceae) in Dapeng Bay, the South China Sea. J. Plankton Res. 19, 303–318. doi: 10.1093/plankt/19.3.303
Ishikawa, C. (1899). Further observations on the nuclear division of Noctiluca. Cambridge: Harvard University
Kofoid, C. A. (1920). A New Morphological Interpretation of the Structure of Noctiluca and its Bearing on the Status of the Cystoflagellata (Haeckel): One Plate, Two Text-Figures, Vol. 19, Berkeley, CA: University of California Publications in Zoology, 317–334.
Kremp, A. (2013). Diversity of dinoflagellate life cycles: facets and implications of complex strategies. London: Geological Society.
Lee, J. K., and Hirayama, K. (1992). The food removal rate by Noctiluca scintillans feeding on Tetraselmis tetrathelle and Gymnodinium nagasakiense. Bull. Facul. Fish. Nagasaki Univ. 71, 169–175.
Lirdwitayaprasit, T. (2002). “Culture of green Noctiluca under laboratory conditions: I. Feeding behavior and sexual reproduction,” in Proceedings of the Fifth IOC/WESTPAC International Science Symposium. Bangkok: IOC/WESTPAC.
Métivier, C., and Soyer-Gobillard, M.-O. (1988). Organization of cytoskeleton during the tentacle contraction and cytostome movement in the dinoflagellate Noctiluca scintillans McCartney. Cell Tissue Res. 251, 359–370. doi: 10.1007/BF00215845
Miyaguchi, H., Fujiki, T., Kikuchi, T., Kuwahara, V. S., and Toda, T. (2006). Relationship between the bloom of Noctiluca scintillans and environmental factors in the coastal waters of Sagami Bay. Japan. J. Plankton Res. 28, 313–324. doi: 10.1093/plankt/fbi127
Miyaguchi, H., Kurosawa, N., and Toda, T. (2008). Real-Time polymerase chain reaction assays for rapid detection and quantification of noctiluca scintillans zoospore. Mar. Biotechnol. 10, 133–140. doi: 10.1007/s10126-007-9031-3
Peperzak, L. (2006). Modelling vegetative growth, gamete production and encystment of dinoflagellates in batch culture. Mar. Ecol. Prog. Ser. 306, 143–152. doi: 10.3354/meps306143
Pfiester, L. A. (1984). 6 - Sexual Reproduction. Dinoflagellates. D. L. Spector. San Diego: Academic Press, 181–199. doi: 10.1016/B978-0-12-656520-1.50010-9
Saldarriaga, J. F., “Max” Taylor, F. J. R., Cavalier-Smith, T., Menden-Deuer, S., and Keeling, P. J. (2004). Molecular data and the evolutionary history of dinoflagellates. Eur. J. Protistol. 40, 85–111. doi: 10.1016/j.ejop.2003.11.003
Sathish, T., Thomas, L. C., and Padmakumar, K. B. (2021). Vegetative and sexual reproduction of bloom-forming dinoflagellate noctiluca scintillans (ehrenberg) mccartney from tropical cochin estuary (southwest coast of india): in-situ and laboratory studies. Thalassas: Int. J. Mar. Sci. 37, 31–37. doi: 10.1007/s41208-020-00247-3
Sato, M. S., Suzuki, M., and Hayashi, H. (1998). The density of a homogeneous population of cells controls resetting of the program for swarmer formation in the unicellular marine microorganism Noctiluca scintillans. Experim. Cell Res. 245, 290–293. doi: 10.1006/excr.1998.4247
Schnepf, E., and Drebes, G. (1993). Anisogamy in the dinoflagellate Noctiluca? Helgoländer Meeresuntersuchungen 47, 265–273. doi: 10.1007/BF02367168
Soyer, M.-O. (1969). L’enveloppe nucléaire chez Noctiluca miliaris Suriray (Dinoflagellata). II. Rôle des ampoules nucléaires et de certains constituants cytoplasmiques dans la mécanique mitotique. J. de Microscopie 8, 709–720.
Soyer, M.-O. (1970a). Etude ultrastructurale de l’endoplasme et des vacuoles chez deux types de Dinoflagellés appartenant aux genres Noctiluca (Suriray) et Blastodinium (Chatton). Zeitschrift für Zellforschung und Mikroskopische Anatomie 105, 350–388. doi: 10.1007/BF00335462
Soyer, M.-O. (1970b). Les ultrastructures liées aux fonctions de relation chez Noctiluca miliaris S.(Dinoflagellata). Zeitschrift für Zellforschung und mikroskopische Anatomie 104, 29–55. doi: 10.1007/BF00340048
Tsai, S.-F., Wu, L.-Y., Chou, W.-C., and Chiang, K.-P. (2018). The dynamics of a dominant dinoflagellate, Noctiluca scintillans, in the subtropical coastal waters of the Matsu archipelago. Mar. Pollut. Bull. 127, 553–558. doi: 10.1016/j.marpolbul.2017.12.041
Valiadi, M., and Iglesias-Rodriguez, D. (2013). Understanding bioluminescence in dinoflagellates-how far have we come? Microorganisms 1, 3–25. doi: 10.3390/microorganisms1010003
Zhang, S., Chan, K., Shen, Z., Cheung, S., Landry, M., and Liu, H. (2016). A Cryptic marine ciliate feeds on progametes of noctiluca scintillans. Protist 2016:168. doi: 10.1016/j.protis.2016.08.005
Zhang, S., Harrison, P. J., Song, S., Chen, M., Kung, H. S., Lau, W. K., et al. (2017). Population dynamics of Noctiluca scintillans during a bloom in a semi-enclosed bay in Hong Kong. Mar. Pollut. Bull. 121, 238–248. doi: 10.1016/j.marpolbul.2017.06.025
Keywords: dinoflagellate, gametes, gametogenesis, gametocyte mother cell, prey concentration, encounter rate
Citation: Lee JL-N, Chiang K-P and Tsai S-F (2021) Sexual Reproduction in Dinoflagellates—The Case of Noctiluca scintillans and Its Ecological Implications. Front. Mar. Sci. 8:704398. doi: 10.3389/fmars.2021.704398
Received: 02 May 2021; Accepted: 12 October 2021;
Published: 17 November 2021.
Edited by:
Alberto Basset, University of Salento, ItalyReviewed by:
Sara Cristina Antunes, University of Porto, PortugalHelga Do Rosario Gomes, Lamont Doherty Earth Observatory (LDEO), United States
Copyright © 2021 Lee, Chiang and Tsai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kuo-Ping Chiang, a3BjaGlhbmdAbWFpbC5udG91LmVkdS50dw==; Sheng-Fang Tsai, c3RzYWlAbWFpbC5udG91LmVkdS50dw==
 Jeffery Liang-Neng Lee
Jeffery Liang-Neng Lee Kuo-Ping Chiang
Kuo-Ping Chiang Sheng-Fang Tsai
Sheng-Fang Tsai