- 1Instituto Español de Oceanografía, Center Oceanogràfic de les Balears, Palma, Spain
- 2Staatliches Museum für Naturkunde in Stuttgart, Stuttgart, Germany
- 3Department of Animal Physiology, Faculty of Biological Sciences, Complutense University of Madrid, Madrid, Spain
The genus Coris contains 28 valid species, most of them with an Indo-Pacific distribution and only two species recognized from the eastern Atlantic: Coris atlantica and C. julis. This last species has a large variability in its coloration, which caused that during the first half of XIX century the sexes were considered as different species. Two liveries have been described for C. julis: (i) primary or “giofredi,” which corresponds to females and primary-phase males and is considered common for Atlantic and Mediterranean populations; and (ii) the secondary or “julis” which corresponds to secondary-phase males, which is different for Atlantic and Mediterranean populations. In this study we demonstrate the existence of two sibling species within the C. julis species complex. Morphological and molecular analyses showed that this species complex is composed of two species: (i) C. julis, distributed mainly in the Mediterranean, from which the species was originally described; and (ii) C. melanura, a species described by Lowe in 1839 which is resurrected here, mainly distributed in the eastern Atlantic and western Mediterranean. We also discuss the possible speciation event to understand the contemporary distribution patterns of Coris species in the eastern Atlantic and Mediterranean.
Introduction
The accurate delineation of species is of paramount importance to quantify and preserve biodiversity. However, this process is difficult when trying to tell apart closely related taxa (Mayr, 1947; Coyne and Orr, 2004). In this sense, genetic divergence of independent lineages is not always reflected by a clear phenotypic differentiation, because constraints of adaptation to similar environments may lead to morphological conservatism (Lavoué et al., 2011). Delineation of species is particularly acute in reef fish, whose conspicuous colors are often the only available character to distinguish taxa (Randall, 1998; DiBattista et al., 2012).
The genus Coris Lacepède, 1801 belongs to the family Labridae and contains 28 valid species, most of them with an Indo-Pacific distribution and a preference to inhabit rocky shores (Randall, 1999, 2013; Parenti and Randall, 2018; Fricke et al., 2021). Coris julis (Linnaeus, 1758), reported from the northeastern Atlantic and the Mediterranean, and Coris atlantica Günther, 1862, reported from the African Atlantic coast, are currently the only two species representing this genus outside the Indo-Pacific (Randall, 1999; Parenti and Randall, 2018). Most species of Coris are beautifully colored, to the point that Günther (1880) said they “belong to the most gorgeously colored kinds of the whole class of fishes.” The color pattern is of great diagnostic importance in the genus, but the radical changes of color that occur with sex change frequently led to incorrectly describing different species for each color phase (Randall, 1999).
Coris julis was originally described from various Mediterranean Sea localities including Genoa, Venice, Rome, and Naples (Italy), Marseille (France) and Crete (see Fricke et al., 2021). Due to the large variability in the coloration of this species, during the first half of XIX century the two liveries were described as different species. Thus, Julis speciosa Risso, 1827 from Nice (France), Julis mediterranea Risso, 1827 from Nice (France), Julis festiva Cuvier and Valenciennes, 1839 from Brest (France), Julis vulgaris Cuvier and Valenciennes, 1839 and Julis melanura Lowe, 1839 from Madeira Island (Portugal) were considered by Day (1880) as synonyms of males of C. julis, while Labrus giofredi Risso, 1810 from Nice (France) was considered a synonym of females. Additional synonyms include Julis azorensis Fowler, 1919 from the Azores, Julis cettii Rafinesque, 1810 from Palermo (Sicily, Italy), Anarchichas fusellus Naro, 1847 from the Adriatic Sea, Labrus iulis Brünnich, 1768 from Marseille (France), Labrus keslik Lacepède, 1801 from Istanbul (Turkey), Sparus niloticus Hasselquist, 1762 from Egypt, Labrus paroticus Linnaeus, 1758 from the Mediterranean Sea, Labrus perdica Forsskål, 1775 in Niebuhr (1775: 34, xi) from Istanbul (Turkey), Coris taeniatus Steindachner, 1863 possibly from the Canary Islands, and Julis vulgaris Fleming, 1828 from Cornwall (United Kingdom).
Subsequently, in the eastern Atlantic, Julis azorensis Fowler, 1919, was described from the Azores and again considered as a synonym of C. julis by Bauchot and Quignard (1973). Later, Fowler (1936) considered C. atlantica, described from Sierra Leone, as a junior synonymy of C. julis. This synonymy was revised by Roux (1961), who listed the morphological differences between the two species and clearly stated the validity of both, but some authors kept considering C. atlantica as a synonym (Blache et al., 1970; Eschmeyer et al., 1998), probably due to the phenotypic variation within C. julis. However, Guillemaud et al. (2000) found a high genetic divergence between C. atlantica and C. julis and considered both species as valid representatives of the Coris genus in the eastern Atlantic.
While C. atlantica seems to be restricted to the western-central African coast from Senegal to Angola, including the Cape Verde Islands (Guillemaud et al., 2000; Fricke et al., 2021), the C. julis species complex is widely distributed from Norway to Senegal, including the Azores, Madeira, the Canary Islands and the Mediterranean (Quignard and Pras, 1986; Guillemaud et al., 2000; Fricke et al., 2021). The rainbow wrasse C. julis is a protogynous hermaphroditic species with diandry (Michel et al., 1987; Kuwamura et al., 2020). Two liveries have been described for this species: (i) a primary one also called “giofredi” corresponding to females and primary-phase males which is considered common for both the Atlantic and the Mediterranean populations, and (ii) a secondary one also called “julis” corresponding to secondary-phase males, which is different for Atlantic and Mediterranean specimens (Roede, 1966; Sanchez-Delgado, 1981). Despite the geographic segregation of the secondary livery, since the late 1980’s the Atlantic secondary-phase male livery has been reported from several scattered locations in the western Mediterranean, such as Alboran Island, Cap Tres Forques (Morocco), Gulf of Lions (Laurent and Lejeune, 1988), Catalan coast (Corbera et al., 1996; Zabala et al., 2005), and Balearic Islands (Martino and Grau, 2010).
Although secondary-phase males with Atlantic livery are not predominant in any of these Mediterranean locations, they can be quite frequent and abundant. In this sense, Laurent and Lejeune (1988) reported mixed populations of males showing the Atlantic and Mediterranean liveries in Alboran Island, Cap Tres Forques and Gulf of Lions. In the latter area, the Atlantic livery was particularly abundant, representing between 10 and 20% of all secondary-phase males (Laurent and Lejeune, 1988). Moreover, up to eight individuals showing this livery were collected widespread in the Balearic Islands between 2006 and 2010, four of them obtained during a very short time span (spring of 2010), after contacting recreational fishermen (Martino and Grau, 2010).
To explain the cohabitation in the same locations of the Atlantic and Mediterranean types of C. julis, Laurent and Lejeune (1988) already suggested an absence of inter-fecundity between them. In fact, deep genetic divergence between Mediterranean and Atlantic individuals has been observed using different molecular markers (Guillemaud et al., 2000; Aurelle et al., 2003; Fruciano et al., 2011; Landi et al., 2014). These authors considered that such genetic differences represent the variability between different populations (Atlantic vs Mediterranean) within C. julis, but Landi et al. (2014) suggested that the high interspecific distances found between specimens from Portugal and the central Mediterranean could be attributed to failure to recognize synonyms as valid species. The aim of the present study is to provide evidence for the existence of a C. julis species complex, and the resurrection of Coris melanura (Lowe, 1839). In order to stabilize the nomenclature within this complex, a redescription of C. julis (Linnaeus, 1758) is also provided.
Materials and Methods
Morphological Descriptions
Material of Coris melanura and C. julis are deposited in the ichthyological collections of the Museu de História Natural do Funchal (MMF), the Museu de la Naturalesa de les Illes Balears (MNIB) of the Societat de Història Natural de les Illes Balears, the Marine Fauna Collection of the Instituto Español de Oceanografía in the Centro Oceanográfico de Málaga (CFM-IEOMA), and the Staatliches Museum für Naturkunde in Stuttgart (SMNS).
Morphometric measurements and external observations for all specimens follows Randall (1999). The description and nomenclature of the head lateral line system follows Kasumyan (2003). The fin-ray formulae follow Fricke (1983).
Molecular Analyses
A piece of the right pectoral fin was removed from fresh specimens and preserved in 96% ethanol. A total of 19 samples were used for molecular analyses, of which 15 belonged to C. melanura and four to C. julis.
DNA was extracted from this tissue using the DNeasy Blood and Tissue Extraction kit (Qiagen, West Sussex, United Kingdom). Polymerase chain reaction (PCR) was used to amplify three partial mitochondrial genes (mtDNA): 12s rRNA with primers 12SL1091/12SH1478 (Kocher et al., 1989), the cytochrome c Oxidase subunit I (COI; DNA barcode) with primers FF2d / FR1d (Ivanova et al., 2007) and the cytochrome b (CYTB) with primers L14724 / H15175 (Palumbi, 1996). PCR was performed in 25 μl volume: 17.7 μl ddH2, 2.5 μl Mangobuffer (Bioline), 1 μl DNTPs, 1.75 μl MgCl2, 0.5 μl each primer (each 10 pmol), 0.05 μl TAQ (Bioline), and 1 μl DNA. The PCR thermal profile used for both mitochondrial genes was: initial stage of 96°C for 5 min; then 35 cycles at 94°C for 60 s, 50/54/54°C for 60 s and 72°C for 60 s, followed by a final extension at 72°C for 10 min. PCR products were purified using the QIAquickR PCR Purification Kit (QIAGEN). Both heavy and light strands were sequenced on an ABI 3130 sequencer (Applied Biosystems).
Sequences were imported into BioEdit 7.0.5.2. (Hall, 1999) and checked for quality and accuracy with nucleotide base assignment. Multiple sequence alignments (MSA) were obtained with ClustalW (Thompson et al., 1994). The DNA sequences obtained for three mitochondrial fragments were deposited in the GenBank database1 under the following numbers: MW970114-NW970131; MW979469-MW979486; MZ044564-MZ044581; MZ230640; MZ230729; and MZ275234.
Additionally, a search of sequences for the three mitochondrial fragments was performed of all species belonging to the genus Coris from the GenBank and the Barcode of Life Data System (BOLD SYSTEMS:2; Ratnasingham and Hebert, 2007). The sequences were previously published in the following references: Guillemaud et al. (2000), Almada et al. (2002), Barber and Bellwood (2005), Westneat and Alfaro (2005), Arnal et al. (2006), Read et al. (2006), Ward and Holmes (2007), Kazancioglu et al. (2009), Steinke et al. (2009, 2016, 2017), Matschiner et al. (2011), Hubert et al. (2012), Victor et al. (2013), Landi et al. (2014), Aiello et al. (2017) and Delrieu-Trottin et al. (2019).
Genetic distance (p-distance) and number of base differences between pair of sequences of each mitochondrial fragment were calculated with MEGA v.7.1 (Tamura et al., 2013). The average values of both genetic indices between our study samples and GenBank and BOLD SYSTEMS sequences were compared.
The sequences were also used to investigate the phylogenetic relationships among the Coris species included here. To do so, phylogenetic trees based on Bayesian Inference (BI) were reconstructed. The optimal substitution model of molecular evolution for 12s rRNA fragment was Kimura 2-parameter (Kimura, 1980) plus gamma, while for COI and CYTB the optimal model was the Hasegawa-Kishino-Yano model with invariable sites and gamma distribution (HKY+G+I; Hasegawa et al., 1985). These models were selected following the Bayesian Criteria (BIC) using MEGA. BI was performed with MrBayes v.3.2.1 (Ronquist et al., 2012) conducting four independent MCMC runs (with four chains each) for 15 million generations, sampling every 2,000 generations and discarding the first 25% of samples as burn-in. This scheme was applied for all the fragments. Convergence was assessed by effective sample size (ESS) calculation and was visualized using TRACERv.1.5. Halichoeres marginatus (Hodge et al., 2012) was included as an outgroup for the phylogenetic analyses of 12s rRNA and CYTB, while Thalassoma purpureum (Delrieu-Trottin et al., 2019) was added as an outgroup for COI.
In addition, to elucidate species boundaries within Coris species, a delimitation analysis was performed using the Poisson Tree Process (bPTP; Zhang et al., 2013) method for each mitochondrial fragment. For this purpose, we used the Bayesian trees which were previously performed in MrBayes for each fragment as input data. The calculation was conducted on the bPTP webservice3 excluding outgroups, with 250,000 MCMC generations, thinning set to 100 and burning at 25% and performing a Bayesian search. The probability of each node to represent a species node was calculated with the Bayesian solution considered the frequency of the nodes across the sampling.
Lastly, for the Coris species distributed in eastern Atlantic and Mediterranean, median-joining haplotype networks based on 12s rRNA, COI and CYTB fragments were generated by using Popart 1.7 (Leigh and Bryant, 2015).
The sampling localities of the morphologically and genetically analyzed specimens in the present study are shown in Figure 1.
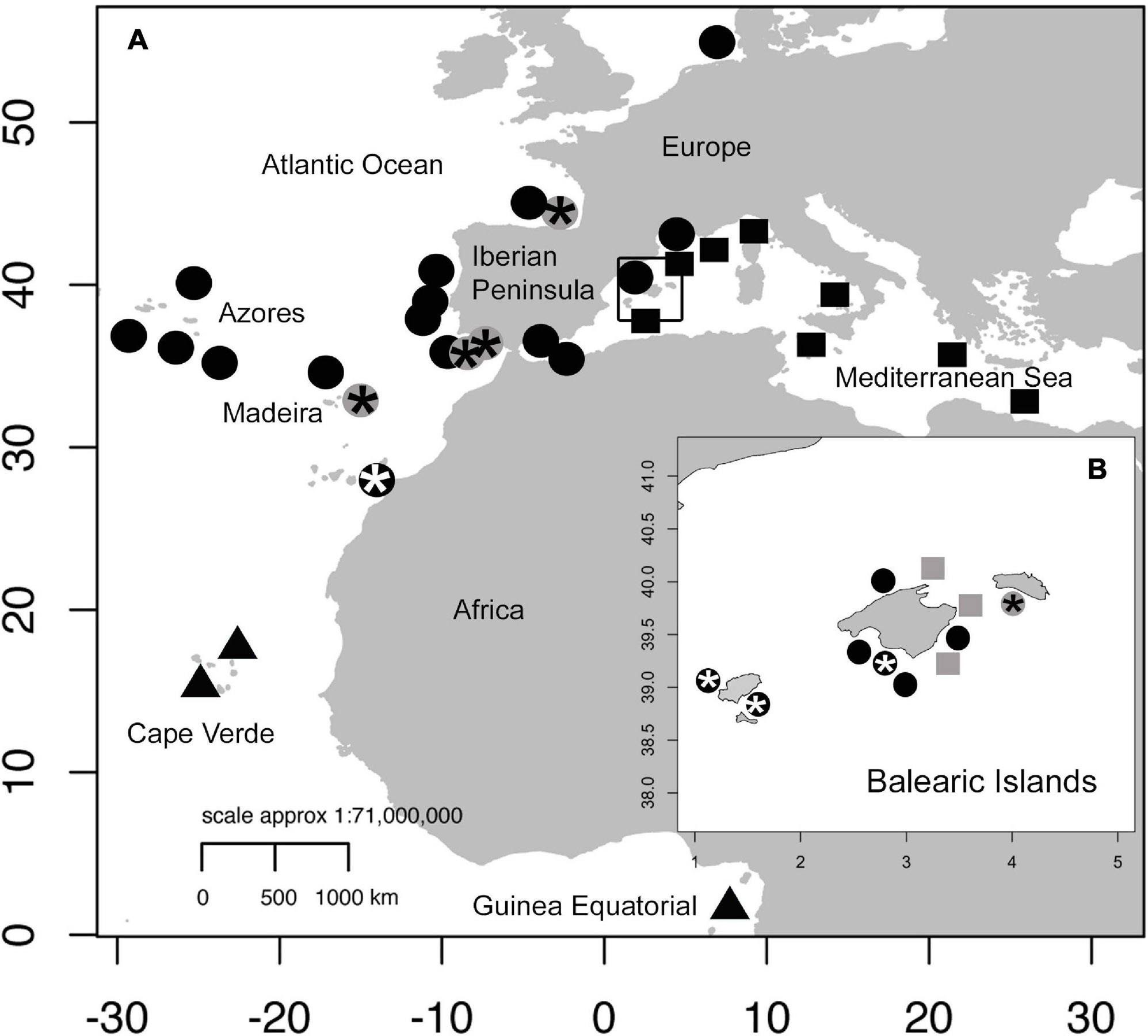
Figure 1. Map of Atlantic and Mediterranean sampling locations for Coris species. (A) Coris samples at each location for morphological (Laurent and Lejeune, 1988; Zabala et al., 2005; Martino and Grau, 2010) and molecular studies (Guillemaud et al., 2000; Aurelle et al., 2003; Arnal et al., 2006; Kazancioglu et al., 2009; Matschiner et al., 2011; Landi et al., 2014; BOLD Systems sequences) are indicated as follows: Coris atlantica in triangles; C. melanura in circles and C. julis in squares. (B) Coris specimens reported from Balearic Islands are shown. The Coris specimens collected in the present study for molecular and morphological analyses from Madeira, Portugal, Cantabria Sea, Gulf of Cadiz and Balearic Islands are indicated in gray, and those specimens used only for morphological analysis are indicated in asterisk.
Results
Coris melanura (Lowe, 1839) (Blacktail rainbow wrasse) (Figures 2, 3).

Figure 2. Photographs of fresh and preserved specimens of Coris melanura showing the primary livery. (A) CFM-IEOMA 7441 from La Caleta, Cádiz, Gulf of Cádiz, Atlantic Ocean (the dark mark between DII and DIII is evident in this specimen); (B) CFM-IEOMA 7416 from Balearic Islands, Mediterranean Sea; (C) CFM-IEOMA 7417 from Santander, Bay of Biscay, Atlantic Ocean; (D,E) CFM-IEOMA 7422 and CFM-IEOMA7424 from Faro, Gulf of Cadiz, Atlantic Ocean, respectively. The fresh and preservation in formalin color are disposed in right and left site, respectively.
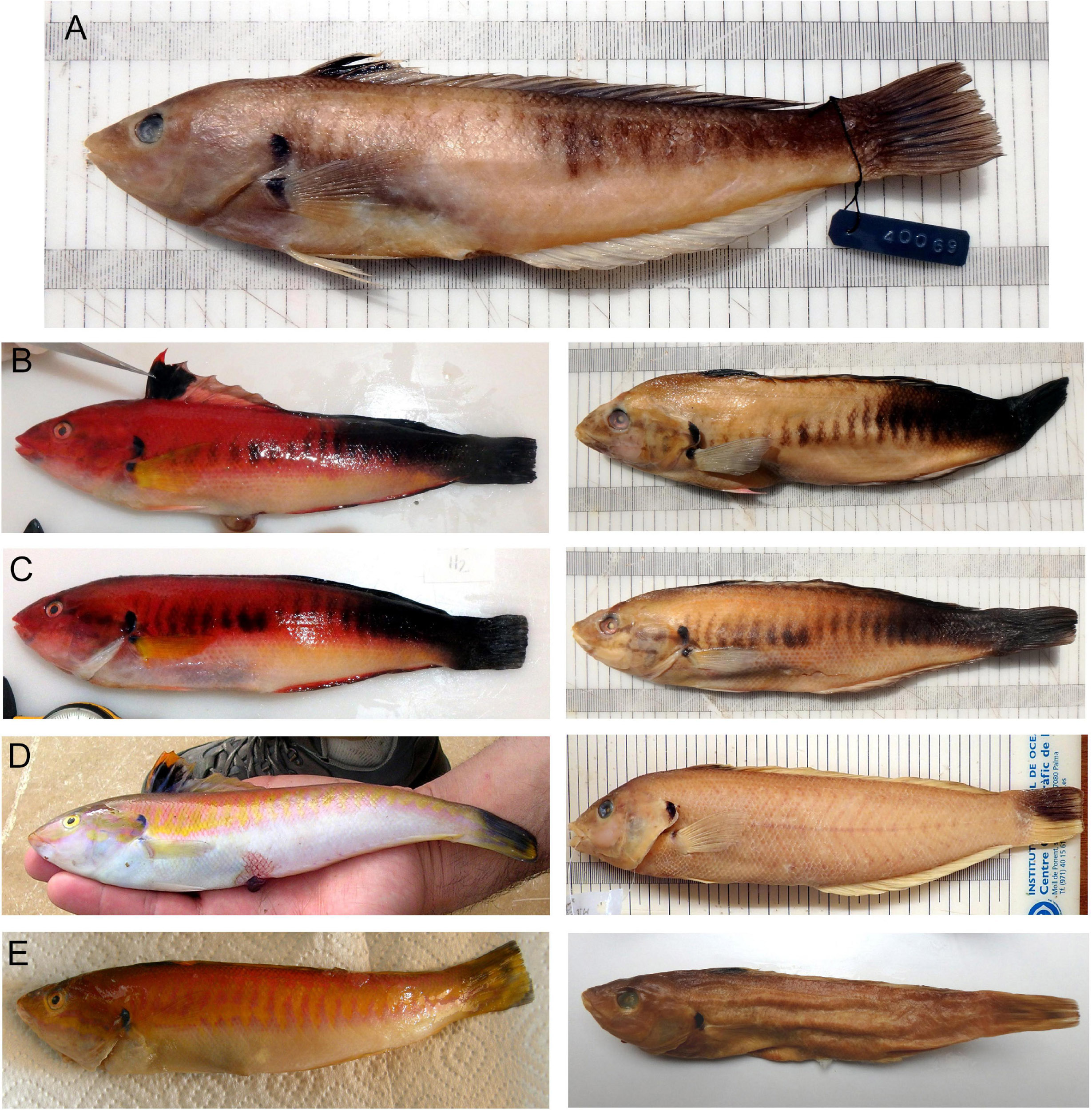
Figure 3. Photographs of fresh and preserved neotype and other material of Coris melanura showing the secondary livery (males). (A) Neotype MFF 40069 from Madeira, Atlantic Ocean; (B,C) other material CFM-IEOMA7428 and CFM-7427 from Santander, Bay of Biscay, Atlantic Ocean, respectively; (D,E) other material MNIB 177 and MNIB 182 from Mallorca and Ibiza, Balearic Islands, Mediterranean Sea, respectively. The fresh and preservation in formalin color are disposed in right and left site, respectively.
Taxonomy
Julis melanura Lowe, 1839: 85 (off Madeira; holotype: lost; neotype as designated below).
Coris (Hologymnosus) taeniatus Steindachner, 1863: 1189, pl. 2 (fig. 1) [Java, Indonesia (in error, probably Canary Islands); syntypes: NMW 25675-76 (2)].
Julis azorensis Fowler, 1919: 204, fig. 2 (Horta, Fayal Island, Azores, northeastern Atlantic; holotype: USNM 42127).
Neotype
MMF 40069, 184 mm SL, Madeira; Portugal, uncertain locality, J. Silva, June 2009.
Other Material
MMF036809 (one specimen), Madeira, Portugal, Cais do Carvao (32°38′11″N, 16°56′15″W), F. França, March 2005; MMF 3468, MMF 3474 (one specimen), Mid-Atlantic uncertain locality, Hydrothermal Vent Field, Mid-Atlantic Ridge, J. Collins, June 1952; MMF 3616 (one specimen), Madeira, Portugal, uncertain locality, February 1953; MMF 3752 (one specimen), Madeira, Portugal, uncertain locality, 5 June 1953, MMF 46659 (one specimen), Madeira, Portugal, uncertain locality, J. Silva, 12 May 2017; CFM-IEOMA 7441, 161 mm SL, Cádiz, Spain, La Caleta (36°52′43″N, 6°30′44″W) 3 m depth, trap, F. Ordines, 22 April 2021; CFM-IEOMA 7417 to 7420 and CFM-IEOMA-7425 to 7428 and (eight specimens), Cantabrian Sea, Spain, Santander (43°28′51″N, 3°51′14″W) 5 m depth, angling, I. Bolado, 20 July 2020; CFM-IEOMA-7421 to 7424 (four specimens), Algarve, Portugal, Faro (36°58′56″N, 7°57′48″W) 3 m depth, angling, I. Ruiz-Jarabo, 19 October 2020; CFM-IEOMA 7416, Balearic Islands, Spain, Menorca Channel (39°53′15″N, 3°52′24″E) 77 m depth, bottom trawl, R/V Miguel Oliver, Cruise MEDITS_0519, St. 34, E. Massutí, 9 June 2019; MNIB 177, Balearic Islands, Spain, southern Mallorca (39°15′14″N, 2°57′1″E) >80 m depth, angling, J. M. Lucas, 4 May 2010; MNIB 182, Balearic Islands, Spain, Es Vedrà, western Ibiza (38°51′33N″, 1°9′58″E) 50 m depth, angling, A. Box, 26 April 2010; MNIB 181, Balearic Islands, Spain, eastern Ibiza (38°58′4″N, 1°33′1″E) >80 m depth, angling, A. Box, 4 July 2010; MNIB 183, Balearic Islands, Spain, Es Vedrà, western Ibiza (38°51′33N″, 1°9′58″E) 50 m depth, angling, A. Box, 26 April 2010; SMNS 16792, Canary Islands, Fuerteventura; SMNS 16968, Madeira; SMNS 22618, Canary Islands, La Palma.
Diagnosis
Coris melanura is characterized within the genus Coris by the following characters: soft dorsal-fin rays 12; lateral-line scales 72–74 + 1; pectoral rays 13; body depth 4.0–5.1 in SL; pelvic-fin tip not extending to the vertical through pectoral-fin tip; primary livery (females and initial-phase males) with a thin black line extending on the upper half of body from behind the orbit to almost half of caudal fin rays, a small triangular dark mark present at the membrane between the second and third dorsal spines (sometimes barely visible), and 6–8 longitudinal series of small red dots surrounding the white belly from lower edge of pectoral-fin base to anus; and secondary livery (secondary phase males) with black caudal fin and a longitudinal series of black, yellow or red (sometimes two of this colors present) vertically elongated spots (bars) along the body side.
Type Material
We hereby designate the specimen MMF 40069 as the neotype of Julis melanura Lowe, 1839. The individual is characterized by the following traits: soft dorsal-fin rays 12; lateral-line scales 74 + 1; pectoral rays 13; body depth 4.5 in SL; pelvic-fin tip not extending to the vertical through pectoral-fin tip; it presents a secondary livery (secondary phase male); preserved coloration is mainly pale, with whole posterior upper third of the body including the caudal fin black, and a longitudinal series of gray (darker to the caudal fin) vertical bars present along the side of the body, extending from near the pectoral-fin origin to the caudal peduncle where they merge with the black color of the peduncle; a black spot on upper side of pectoral axil and opercle; dorsal fin with an anterior black spot extending to the membrane between third and fourth spines, but not to the edge which is pale (Figure 3).
Description
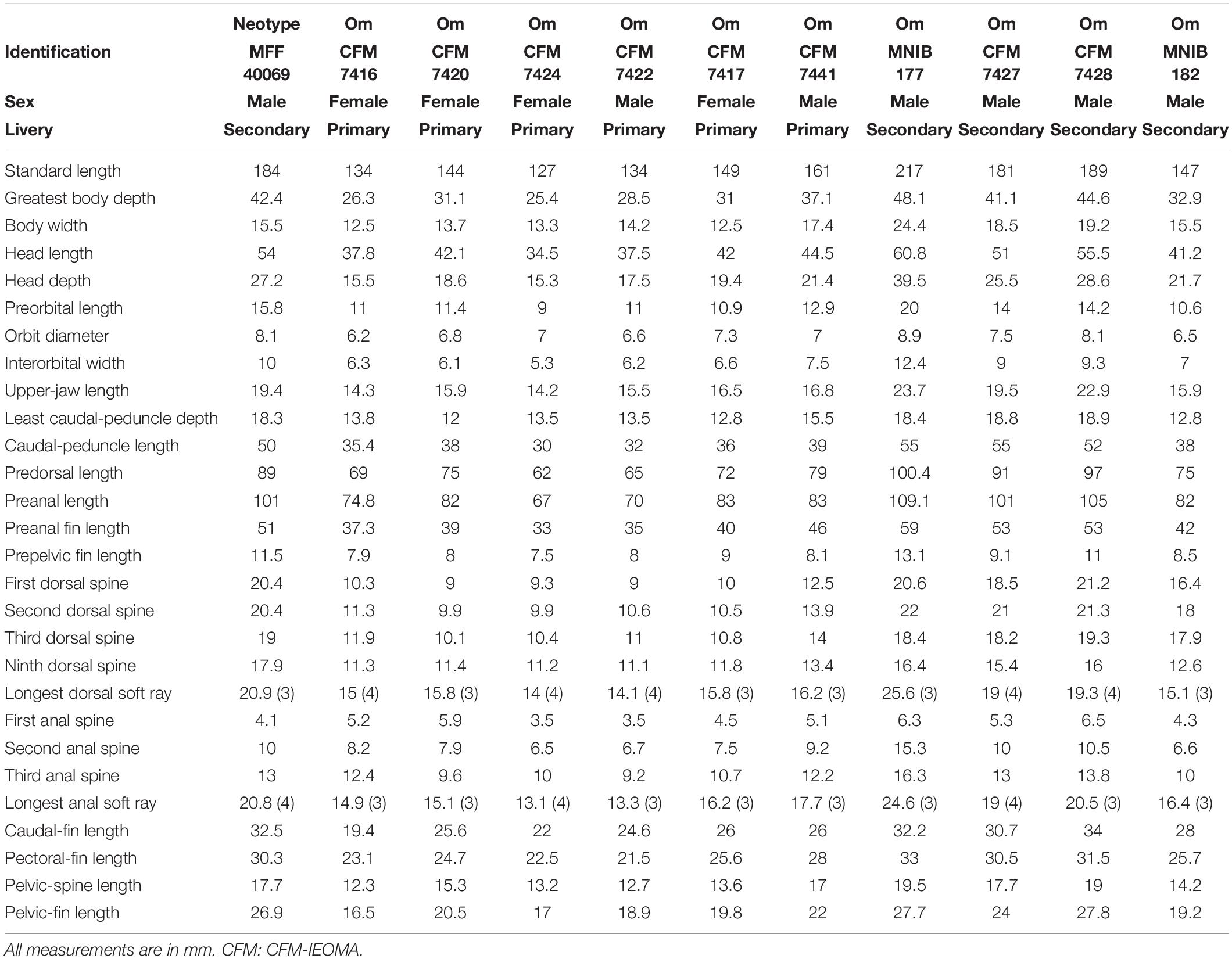
Dorsal fin IX, 12; anal fin III, 12; all soft dorsal and anal rays branched; pectoral fin 13, all branched except the uppermost; pelvic fin I, 5; caudal fin (iii), ii, 12, ii, (iii). Gill rakers (first arch, upper + lower limbs): 4–6 [4 (2), 5 (18), 6 (2)] + 9–10 [9 (5), 10 (15), 11 (2)], total 14–16 [14 (7), 15 (11), 16 (4)]; branchiostegal rays: 6; pored lateral-line scales: 73–74 [72 (6), 73 (12), 74 (7)] + 1 (larger scale located on caudal fin base); scales above lateral line to origin of dorsal fin: 4–5 [4 (3), 5 (22)]; scales below lateral line to origin of anal fin: 21–23 [21 (6), 22 (12), 23 (6)]; vertebrae: 25 including the urostyle (10 precaudal and 15 caudal vertebrae). Absolute and relative values of morphometric measurements are given in Tables 1, 2.
Body slender, moderately compressed, its width 1.7–2.7 in body depth at origin of dorsal fin (maximum depth); body depth 4.0–5.1 in standard length (SL); head small, its length 3.3–3.8 in SL; snout pointed, its length 3.0–3.9 in head length (HL); eye small, the orbit diameter 4.8–7.0 in HL, clearly situated in the anterior part of the head, with post-orbital length (1.7–2.0 in HL) much larger than snout; interorbital space convex, the least bony width 4.4–7.0 in HL; least caudal peduncle depth and caudal peduncle length 2.3–2.8 and 2.4–3.8 in HL, respectively.
Mouth terminal, small and slightly oblique, the maxilla extending to a vertical through anterior nostril, upper-jaw length 4.2–5.9 in HL; front of upper and lower jaws with a pair of large recurved canine teeth, those in the upper jaw more spaced allowing the lower jaw pair of canines fitting between the uppers when mouth closed; second tooth on upper jaw, spaced from first one, and almost two thirds the length of the first and recurved; second tooth followed by a row of ten teeth, first tooth in this row already much smaller than the second, and getting progressively smaller and less pointed, last three blunt; a canine tooth on the upper jaw on the corner of the mouth, slightly smaller than second frontal teeth and recurved anteriorly; second tooth on lower jaw about two thirds of the first, recurved, followed by a row of 9–10 teeth, first tooth in this row already smaller than the second, getting progressively smaller and less pointed; both jaws present an shorter inner row of much smaller conical to blunt posteriorly teeth. Upper pharyngeal plates each with 15–20 teeth (variable even between both plates in the same individual), pointed in the anterior area to conical and blunt posteriorly, with three molars on the posterior side of the medial edge, the largest about twice the size of the second largest; lower T-shaped pharyngeal plate with a total of 33–45 teeth in total (medial and transverse limbs); medial limb with 11–16 teeth distributed as one single most anterior tooth a little larger than the rest and pointed, and 5–6 transverse rows of 1–3 teeth each; all teeth in the medial limb conical and pointed to blunt posteriorly; transverse limb of lower pharyngeal plate with 23–30 teeth distributed in three rows of teeth, the most posterior one with a very large triangular molar (about three and a half times larger than the second larger molar), flanked by three teeth, the first one much larger than the second and second larger than third which is conical; middle row with 10–11 molariform teeth that describe a curve in order to surround the large posterior molar, three teeth in the middle the largest of this row and similar; anterior row of teeth in the transverse limb interrupted in the middle by the medial limb, with 3–4 very similar in size small molar teeth in each side of medial limb (one individual did not present this row on one side); tongue narrow, with a rounded anterior end.
Ventral edge of preopercle free to a vertical at the space between the posterior nostril and anterior edge of orbit; posterior free edge of preopercle about two thirds of ventral free edge, ending slightly below the lower edge of orbit. Anterior nostril small and tubular placed in front of the eye barely above the center of pupil; posterior nostril a small slit vertically oriented, situated half pupil diameter behind anterior nostril. Seven to eight [6 (14), 7 (7), and 8 (4)] suborbital pores from behind center of eye to below middle front of orbit; three pores that radiate from the suborbital canal, one located in front of middle of orbit and slightly anterior and below anterior nostril, the other two located near the upper maxilla, one placed slightly anterior to a vertical through the anterior nostril, the other one above posterior third of upper lip; four pores on the lower free edge of preopercle [4 (23), 3 (2)], continuing anteriorly as three pores in mandibular series; one pore on preopercle angle, and three on the upper free edge of preopercle, continuing with two pores [2 (23), 3 (2)], the uppermost almost meeting the level of the temporal canal; supraorbital canal with four pores starting anteriorly above posterior nostril (three individuals also presented an extra pore above anterior nostril) and ending posteriorly above the orbit at a vertical through the posterior edge of eye; supraorbital canal extends anteriorly to a pore located on the snout at a vertical through the most anterior pore above the lip radiating from the suborbital canal; temporal canal with seven pores, starting anteriorly behind and slightly below the upper edge of the orbit (3, anterior to the vertical through the upper free edge of preopercle); upper temporal canal starting with a single central pore located at the edge of the ending angle of scales, which is located slightly anteriorly to a vertical through posterior edge of orbit, then four pores distributed flanking the scales, the most posterior one located at a vertical through fourth pore in the temporal canal.
Scales cycloid; smaller scales on nape extending forward slightly anterior to a vertical through posterior edge of orbit; rest of the head naked; scales in the chest smaller than scales on sides of the body; scales on chest progressively smaller ventrally and anteriorly; lateral line starting in the free upper edge of opercle, approximately paralleling dorsal profile to space between ninth and tenth soft rays, then descending sharply to body midline and straight again until the base of the caudal fin; last lateral line pored scale on base of caudal fin larger than the rest; no scales on base of dorsal, anal, and paired fins; progressively smaller scales on base of caudal fin, covering caudal rays to about one fifth of caudal fin length.
Origin of dorsal fin above fifth lateral line scale, the predorsal fin length 3.3–4.3 in SL; distance between first and second dorsal spines about half the distance between second and third; dorsal spines very flexible, similarly to rays; individuals showing the primary livery (females and initial-phase males): first three dorsal spines not elongated, the first 3.6–4.7 in HL, second 3.2–4.3 in HL, third usually the largest 3.1–4.3 in HL, then spines very even or gently decreasing until the eighth, which is equal or slightly shorter than ninth (2.8–3.7 in HL); individuals showing the secondary livery (secondary-phase males): first three dorsal spines longer than the rest, the first 2.5–3.3 in HL, second usually the longest 2.3–3.4 in HL, third 2.3–4.2 in HL, then sharply decreasing from third to fourth, from fourth to eight spines very even or gently decreasing until the eighth, which is equal or slightly shorter than ninth (2.8–3.5 in HL); the longest dorsal soft ray are the third or fourth (2.3–2.9 in HL); origin of anal fin below soft dorsal origin, the preanal fin length 1.7–2.0 in SL; all anal spines very flexible and abruptly increasing in size, the first 5.1–13.2 in HL, second 4–6.2 in HL and third 3.1–4.6 in HL; the longest anal soft ray are the third or fourth (2.4–2.8 in HL); caudal fin convex, its length 1.4–1.9 in HL; pectoral fin pointed, its length 1.5–1.9 in HL, being the longest its second ray; origin of pelvic fins at the vertical of lower base of pectoral fins, the prepelvic length 3.4–3.9 in SL; pelvic fin length 1.9–2.6 in HL, being the longest its second soft ray, not elongated, when extended the distance between its tips and anus is 2.2–4.9 in HL.
Coloration
Primary livery (females and initial-phase males): Body brown (of variable intensity) dorsally from snout to caudal fin; eye yellow or orange; a black line (dark brown anteriorly) as thick as about the width of 1–3 scales along sides of body, extending from behind the orbit to caudal fin base, sometimes even reaching the posterior half of this fin; this line not vanishing in individuals preserved in formalin or ethanol, but increasing its contrast (Figure 2); lower half of the body white; a yellow band extending from below lower lip (orange-pink on the head) passes through the pectoral fin base and reaches the caudal-fin base; this yellow band disappears completely upon preservation but in some of the small individuals a dark line, hidden when fresh, replaces it; 6–8 longitudinal series of small red dots surrounding the white belly from the lower edge of pectoral fin base; pectoral and pelvic fins translucent; a bluish-black spot on upper side of pectoral axil and opercle; dorsal fin yellow or-orange, in some individuals brownish near the base, except for a small triangular dark mark of varying intensity (sometimes barely visible) and size, situated at the membrane between the second and third dorsal spines, which originates near the upper part of the second dorsal spine, not reaching the middle of the membrane; anal fin almost completely covered by a yellow band, but tips and base translucent at least anteriorly; caudal fin yellowish-orange, except for the black line crossing the side of the body that extends into caudal fin and the upper and lower corners which are translucent or whitish. One of the two males with primary livery showing barely visible transverse gray bars along the side, projecting from the black line toward the yellow band (Figure 2).
Secondary livery (males): Back and sides down to approximately the level of the upper origin of the pectoral fin with a variable background color (usually vivid red, pallid pink or greenish) (Figure 3); lower part of the body white or yellowish; caudal fin completely black in most individual analyzed (but see section “Remarks” below), with the black color extending to the caudal peduncle and last rays of the dorsal fin, in some individuals including the whole posterior upper third of the body; a longitudinal series of black, yellow or red vertical bars present along the side of the body, extending from near the pectoral-fin origin to the to the caudal peduncle where they merge completely with the black color of the peduncle in the darkest individuals; pectoral translucent or yellowish; pelvic fins pinkish; a bluish-black spot on upper side of pectoral axil and opercle; dorsal fin with an anterior black spot extending to the third spine, sometimes also on part of the membrane between third and fourth spines, but not to the edge which is vividly red; the remainder of this fin with two longitudinal bands: one near the base similar to the dorsal background color, the other near the margin, colored dark gray to black posteriorly; anal fin with two longitudinal bands similar to those on dorsal fin.
Remarks
The primary livery well agrees with the description of Julis azorensis Fowler, 1919. Although the description does not mention the thin black line extending from behind the orbit to caudal fin base, it is still barely visible in the holotype deposited at the National Museum of Natural History, Washington D.C., United States, USNM 42127 (Fricke et al., 2021). Moreover, the holotype also shows a “well defined dark line from pectoral axil to caudal base little before middle” which has also appeared in some of our smaller individuals when have been preserved, replacing the yellow band which disappears fast in contact with both ethanol or formalin. This livery is also coincident with some of the characteristics cited in the description of Julis festiva in Cuvier and Valenciennes (1839), such as the presence of the triangular dark mark (“bleue” in that description) between the second and third dorsal spines and the presence of small red dots surrounding the belly (“son ventre d’un beau rose vif”), although it is remarked the presence of “première bandelette, composée de petites lunules bleu noirâtre, étendue depuis l’angle de l’opercule jusque sur la queue, et par une seconde bande jaune semée de points rouges, qui prend naissance dans l’aisselle de la pectorale, et qui va jusque sur la queue” which may remind the vertical bars (lunules) of the secondary livery. However, we have never observed the vertical bars in individuals without elongated first dorsal spines which is the case of that used in the description by Valenciennes, neither the presence of the vertical bars concurrent with the presence of the yellow band from pectoral axil to the caudal fin. Therefore, the identity of J. festiva cannot be determined with certainty.
However, the secondary livery of our individuals is clearly coincident with the description of Julis melanura Lowe, 1839, in which the author points out that the species is clearly distinguished by the “deep blackness of the caudal and hinder part of the tail or body,” a trait not mentioned in the description of J. festiva (Lowe, 1839).
The secondary livery had been described as the color pattern of Atlantic secondary-phase males. In the Gulf of Lions, Laurent and Lejeune (1988) found an individual with the same livery as those described by Sanchez-Delgado (1981) from the Cantabrian Sea. Both works described as one of the most prominent differences with Mediterranean secondary livery, the black caudal fin, with black color extending forward to the caudal peduncle and last dorsal-fin rays, even occupying the “medial region of the posterior third of the body” (Laurent and Lejeune, 1988). The largest individuals analyzed in the present work were from the Balearic Islands (220 and 222 mm SL; MNIB 177 and MNIB 181, respectively), and showed a general clearer coloration and the black colored area on the caudal peduncle was reduced to the upper half of caudal fin and the last dorsal rays. The smaller individuals analyzed that presented elongated first three dorsal spines and vertical bars along the body sides (secondary-phase males) showed a caudal fin coloration predominantly yellow but mottled with gray, suggesting the vertical bars and the elongation of the dorsal spines may occur earlier than the black coloration in the caudal fin during the transition from primary to secondary livery. This is the intermediate step between primary and secondary liveries, differing from the typical secondary Atlantic livery described by Sanchez-Delgado (1981) and Laurent and Lejeune (1988).
Coris julis (Linnaeus, 1758) (Mediterranean rainbow wrasse) (Figures 4, 5).

Figure 4. Panels (A–D) show photographs of specimen CFM-IEOMA 7429 from Cala Sant Vicenç, Mallorca, Balearic Islands, Mediterranean Sea, showing the primary livery of Coris julis. (A) Swimming before entering in the trap; (B) just after being caught, out of water; (C) back in water again before anesthesia; (D) just after death; (E) after preservation in formalin. Panel (F) shows the preserved coloration of neotype of Labrus perdica Niebuhr (1775) SMNS 11609 from Greece.

Figure 5. Photographs of fresh and preserved specimens of Coris julis. (A) neotype of Labrus julis Linnaeus, 1758, Labrus cettii Rafinesque, 1810, Labrus giofredi Risso, 1810 and Julis speciosa Risso, 1827 CFM-IEOMA 7433 from Eastern Mallorca, Balearic Islands, Mediterranean Sea, showing the typical coloration of the secondary livery (male); (B) paratype CFM-IEOMA 7432 from Punta Tomàs, Mallorca, Balearic Islands, Mediterranean Sea, showing an intermediate coloration between primary and secondary liveries without elongation of first dorsal spines. The fresh and preservation in formalin color are disposed in right and left site, respectively.
Taxonomy
Labrus julis Linnaeus, 1758: 284 (Genoa, Italy, Mediterranean Sea; neotype as designated below).
Labrus paroticus Linnaeus, 1758: 284 [probably Mediterranean Sea; syntypes: NRM 3 (5)].
Sparus niloticus Hasselquist, 1762: 387 [Nile River, near Cairo, Egypt (locality is erroneous, is Mediterranean Sea); no types known; not available, published in a rejected work (ICZN, Opinion 57); original spelling “Sparvs niloticvs” due to historical typeset, equals “Sparus niloticus”; based on Hasselquist, 1757: 341, also a rejected work (ICZN Opinion 57)].
Labrus iulis Brünnich, 1768: 54 (Marseille, France, Mediterranean Sea; incorrect subsequent spelling of Labrus julis Linnaeus, 1758).
Labrus perdica Fabricius [ex Forsskål] in Niebuhr, 1775: 34, xi [Istanbul, Turkey, Sea of Marmara; neotype as designated below; authorship according to Fricke (2008): 48].
Labrus keslik Lacepède, 1801: 453, 523 (Istanbul, Turkey, Sea of Marmara; unneeded replacement name for Labrus perdica Niebuhr, 1775).
Labrus cettii Rafinesque (ex Cetti), 1810: 23, 54 (Sardinia Island, Italy, Mediterranean Sea; neotype as designated below).
Labrus giofredi Risso, 1810: 228, pl. 9 (fig. 23) [Nice, France, northwestern Mediterranean Sea; syntypes: MNHN (lost); neotype as designated below).
Julis giofredi var. I: Risso, 1827: 310 (Nice, France, northwestern Mediterranean Sea).
Julis giofredi var. II: Risso, 1827: 310 (Nice, France, northwestern Mediterranean Sea).
Julis mediterranea Risso, 1827: 309 (Nice, France, northwestern Mediterranean Sea; Risso specimen, possible holotype: MNHN B-0867].
Julis mediterranea var. I: Risso, 1827: 309 (Nice, France, northwestern Mediterranean Sea).
Julis speciosa Risso, 1827: 311, pl. 9 (fig. 20) [Nice, France, northwestern Mediterranean Sea; syntypes: MNHN (lost); neotype as designated below].
Julis vulgaris Fleming, 1828: 210 (Cornwall, England; unneeded replacement name for Labrus julis Linnaeus, 1758); Valenciennes in Cuvier and Valenciennes, 1839: 361, pl. 384).
Julis festiva Valenciennes in Cuvier and Valenciennes (1839): 374 (Brest, France, northeastern Atlantic; holotype: MNHN A-9244).
Anarchichas fusellus (Naro, 1847) (ex Chiereghini) 1847: col 115 [Adriatic Sea; not available, Chiereghini name mentioned in synonymy of Julis giofredi Risso (=Labrus giofredi Risso, 1810)].
Neotype
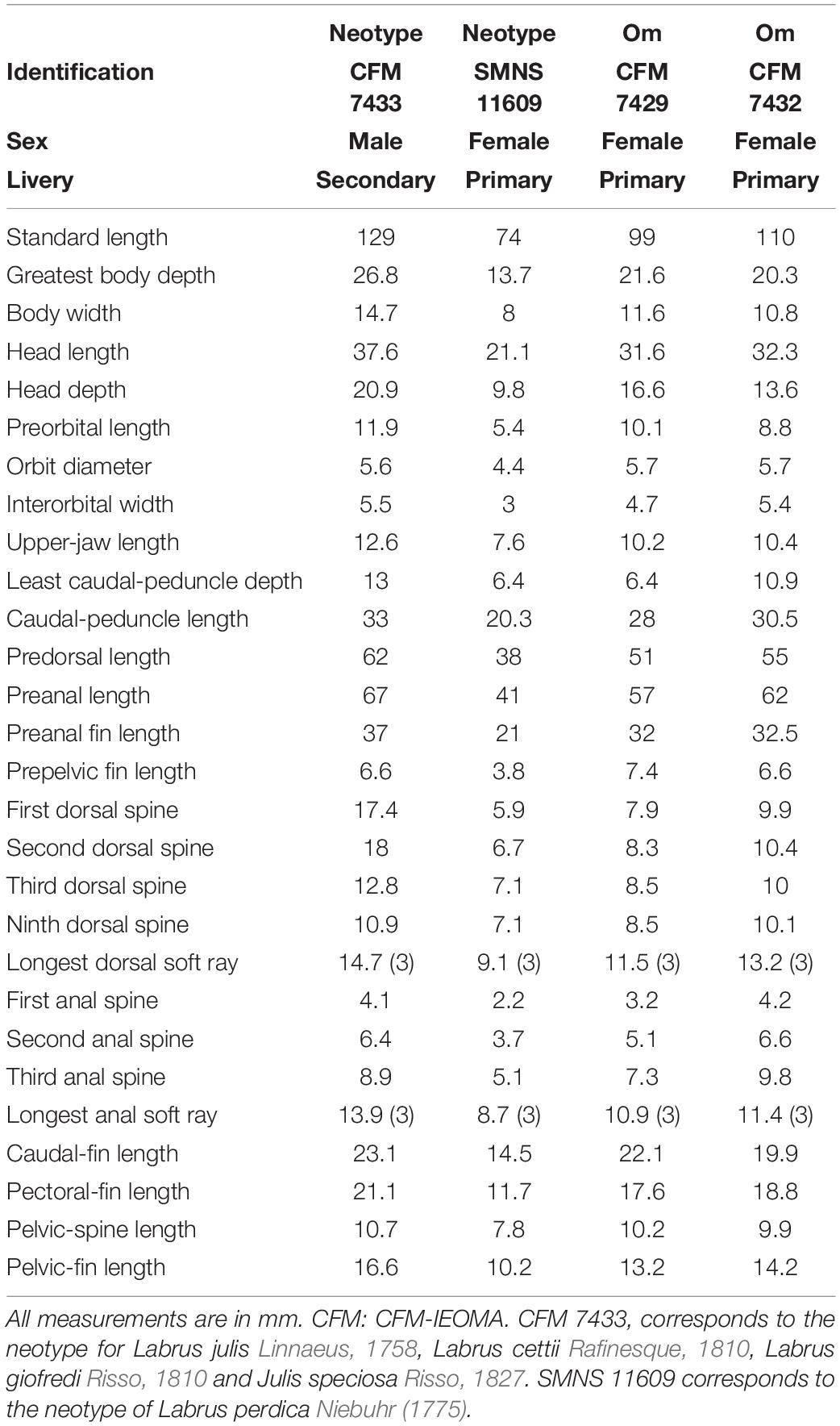
CFM-IEOMA 7433, 129 mm SL, Balearic Islands, Spain, eastern Mallorca (39°27′1″N, 3°20′15″E) 52 m depth, bottom trawling, R/V Miguel Oliver, Cruise MEDITS_0520, St. 86, F. Ordines, 19 June 2020, is hereby selected as the neotype of Labrus julis Linnaeus, 1758, Labrus cettii Rafinesque, 1810, Labrus giofredi Risso, 1810 and Julis speciosa Risso, 1827 (fig. 5); SMNS 11609, 74 mm SL, Varkisa, Greece, V. Tsiomos, 21 December 1990, is hereby selected as the neotype of Labrus perdica Fabricius 1775 in Niebuhr, 1775.
Other Material
MNIB 110 (one specimen), Balearic Islands, Spain, west Mallorca, Port d’Andratx, <30 m depth, angling, A. M. Grau, 9 September 1996; MNIB 179 and 180 (four specimens), Balearic Islands, Spain, east Mallorca, Punta Amer, angling, A. M. Grau, 23 August 2010; MNIB 86 (one specimen), Balearic Islands, Spain, west Mallorca, Port d’Andratx, A. M. Grau, 25 August 2006; CFM-IEOMA 7429 to 7431 (three specimens), Balearic Islands, Spain, northern Mallorca, Cala Sant Vicenç (39°55′28″N, 3°4′0.5″E) 3 m depth, trap, F. Ordines, 18 October 2020; CFM-IEOMA 7432-7434-7435 (three specimens), Balearic Islands, Spain, northern Mallorca, Punta Tomàs (39°57′32″N, 3°11′18″E) 25 m depth, angling, J. Orejuela, 9 June 2019; CFM-IEOMA 7436 to 7440 (five specimens), Balearic Islands, Spain, northeastern Mallorca, Cala Rajada, (39°43′4″N, 3°28′56″E) 48 m depth, angling, L. Kunig, 9 June 2020; SMNS 51 (one specimen), France, ex Museum Tübingen, July 1841; SMNS 401, Hvar Island, Croatia, ex Museum Milano, June 1854; SMNS 419 (two specimens), Hvar Island, Croatia, ex Museum Milano, June 1854; SMNS 721, Nice, France, von Elsässer, November 1859; SMNS 963 (two specimens), Trieste, Italy, Adriatic Sea, December 1862; SMNS 8597 (two specimens), Cala Millor, Mallorca, Balearic Islands (39°36′29″N, 3°23′25″E—39 °26′27″N, 3°4″E), Spain, 0–1.5 m depth, R. Fricke, 17 July 1981; SMNS 8640 (two specimens), Cala Millor, Mallorca, Balearic Islands (39°36′29″N, 3°23′25″E—39°26′27″N, 3°4″E), Spain, R. Fricke, 17 July 1981; SMNS 8647, south of Pula (44°50′30″N, 13°50′30″E), Croatia, R. Fricke, 9 June 1978; SMNS 8649 (three specimens), south of Pula (44°50′30″N, 13°50′30″E), Croatia, R. Fricke, 14 June 1978; SMNS 8656, south of Pula (44°50′30″N, 13°50′30″E), Croatia, R. Fricke, 11 June 1978; SMNS 9370, Fornells, Menorca, Balearic Islands (40°03′45″N, 4°08′08″E), Spain, 0.2–1.0 m depth, R. Fricke, 4 September 1989; SMNS 9386 (four specimens), Fornells, Menorca, Balearic Islands (40°03′45″N, 4°08′08″E), Spain, 0.2–1.2 m depth, R. Fricke, 6 September 1989; SMNS 9379, 10 km southeastern Mahon, Menorca, Balearic Islands, Spain, ca. 39°50′N 4°19′E, R. Fricke, 12 September 1989; SMNS 9587, Varkisa (37°43′12″N, 23°55′32″E), Greece, A. Kodakos, 3 November 1989; SMNS 9611 (two specimens), Varkisa (37°43′12″N, 23°55′32″E), Greece, A. Kodakos, 30 October 1989; SMNS 9863 (24 specimens), Varkisa (37°43′12″N, 23°55′32″E), Greece, V. Tsiomos, 21 December 1989; SMNS 11609 (12 specimens), Varkisa (37°43′12″N, 23°55′32″E), Greece, V. Tsiomos, 21 December 1990; SMNS 12000, Giglio Island (42°22′06″N, 10°52′33″E), Italy, 22 m depth, I. Koch, 1 April 1991; SMNS 12448 (two specimens), Cala Saona, Formentera, Balearic Islands (38°41′42.6″N, 1°23′20.7″E), Spain, 1–2.5 m depth, R. Fricke, 28 September 1991; SMNS 12449 (two specimens), Cala Saona, Formentera, Balearic Islands (38°41′42.6″N, 1°23′20.7″E), Spain, 1–2.5 m depth, R. Fricke, 30 September 1991; SMNS 12454, Cala Saona, Formentera, Balearic Islands (38°41′42.6″N, 1°23′20.7″E), Spain, 1–2.5 m depth, R. Fricke, 1 October 1991; SMNS 14720, Osor, Cres Island, Croatia, 2 m depth, M. Grabert, September 1993; SMNS 16688 (two specimens), Karataş, Adana, Turkey, 10–13 m depth, 9 April 1995; SMNS 21365, Ischia Island (40°42′20″N, 13°51′36″E—40°42′09″N, 13°51′51″E), Italy, 0–2 m depth, R. Fricke, 31 May 1999; SMNS 25986, Genoa, Italy, Eisenhardt, 1819; SMNS 26008, Monaco, Herzog von Urach, 1888.
Diagnosis
Coris julis is characterized within the genus Coris by the following characters: soft dorsal-fin rays 12; lateral-line scales 72–74 + 1; pectoral rays 13; body depth 4.2–5.4 in SL; pelvic-fin tip not extending to the vertical through pectoral-fin tip; primary livery (juveniles, females and initial-phase males) with a striped longitudinal pattern usually brown on back and below a darker purple-brown stripe that extends from the snout to the caudal-fin base; secondary livery (males) with an orange stripe extending from snout to caudal-fin base which is indented throughout the body, and a black cuneiform band extending below the orange indented band, from pectoral axil to about the vertical through anal-fin origin.
Description
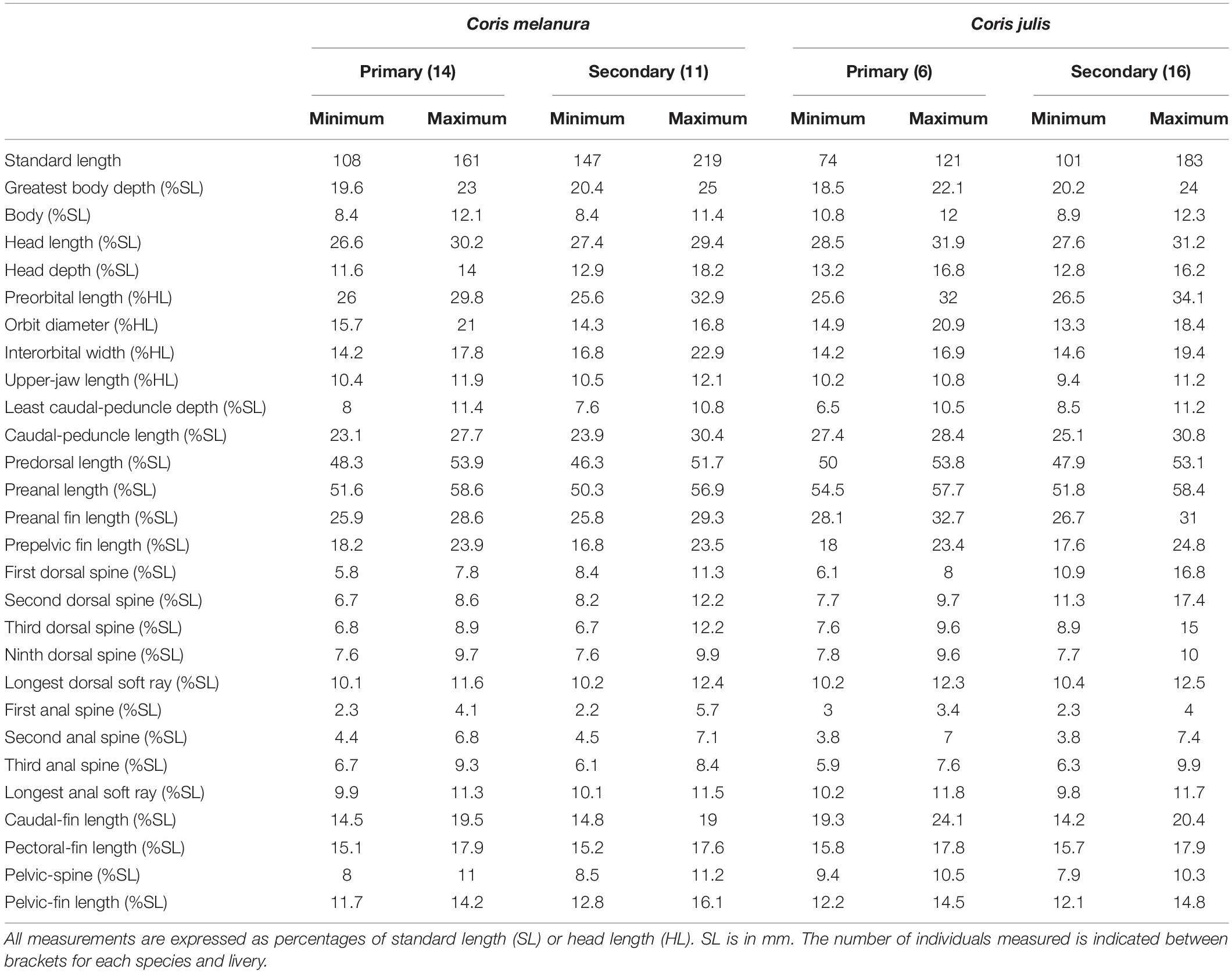
Dorsal fin IX, 12; anal fin III, 12; all soft dorsal and anal rays branched; pectoral fin 13, all branched except the uppermost; pelvic fin I, 5; caudal fin (iii), ii, 12, ii, (iii). Gill rakers (first arch): 5 [4 (3), 5 (18), 6 (2)] + 10 [9 (9), 10 (14)], total 13–16 [13 (2), 14 (8), 15 (11), 16 (2)]; branchiostegal rays 6; pored lateral-line scales 73 [72 (7), 73 (11), 74 (5)] + 1 (larger, located on caudal-fin base); scales above lateral line to origin of dorsal fin 5 [4 (4), 5 (19)]; scales below lateral line to origin of anal fin 21–23 [21 (7), 22 (9), 23 (7)]; vertebrae 25 including the urostyle (10 precaudal and 15 caudal vertebrae). Absolute and relative values of morphometric measurements are given in Tables 2, 3.
Body slender, moderately compressed, its width 1.7–2.4 in body depth; body depth at origin of dorsal fin (maximum depth) 4.2–5.4 in SL; least caudal-peduncle depth 2.5–3.2 in head length, 9.2–10.9 in SL; head small, its length 3.1–3.6 in SL; snout pointed, its length 2.9–3.9 in HL; eye small, orbit diameter 4.8–7.5 in HL, clearly situated on the anterior part of the head, with post-orbital length (1.7–2.1 in HL) much larger than snout; interorbital space convex, the least bony width 5.2–7.0 in HL; caudal-peduncle length 2.5–4.9 in HL.
Mouth terminal, small and slightly oblique; maxilla extending to a vertical through anterior nostril; upper-jaw length 4.0–5.7 in HL; front of upper and lower jaws with a pair of large recurved canine teeth, those in the upper jaw more spaced allowing the lower jaw pair fitting in between when mouth closed; second tooth on upper jaw recurved, with a space toward the first one, and almost two-thirds of the length of the first; second tooth followed by a row of 9–11 teeth, first tooth in this row already much smaller than the second, and getting progressively smaller and less pointed, last three blunt; a canine tooth on the upper jaw in the corner of the mouth, slightly smaller than second frontal teeth, recurved anteriorly; second tooth on lower jaw about two-thirds of the length of the first, recurved, followed by a row of 9–10 teeth, first tooth in this row already smaller than the second, getting progressively smaller and less pointed; both jaws with a shorter inner row of much smaller, conical to blunt posterior teeth. Upper pharyngeal plates each bearing 15–25 teeth (variable even between the two plates of the same individual), pointed in the anterior area, conical and blunt posteriorly, with three molars on the posterior side of the median edge, the largest one about twice the size of the second largest; lower pharyngeal plate T-shaped, with a total of 35–43 teeth (on median and transverse limbs); median limb bearing 10–15 teeth, with a single, anteriormost tooth pointed and a little larger than the others, and 5–6 transverse rows of 1–3 teeth each; all teeth in the median limb conical, anteriorly pointed, posteriorly blunt; transverse limb of lower pharyngeal plate with three rows of teeth, the posteriormost with a very large triangular molar (about 3.5 times larger than the second largest molar), flanked by three teeth, the first much larger than the second, and the second larger than the third, which is conical; middle row curved, surrounding the large posterior molar, with 10–12 molariform teeth, with the three median teeth largest, similar in length; anterior tooth row in the transverse limb interrupted in the middle by the medial limb, with 3–6 small molar teeth on each side of the median limb which are very similar in size. Tongue narrow, with a rounded anterior end.
Ventral edge of preopercle free to a vertical through the space between posterior nostril and anterior edge of orbit; length of posterior free edge of preopercle about two thirds of ventral free edge, ending slightly below the lower edge of orbit. Anterior nostril small and tubular, situated in front of eye barely above the center of pupil; posterior nostril a small slit, oriented vertically, placed half a pupil diameter behind anterior nostril. Suborbital pores 6–9 [6 (12), 7 (5), 8 (3), 9 (3)], arranged between behind center of eye and below middle front of orbit; three pores radiate from suborbital canal, one located in front of middle of orbit and slightly anterior and below of anterior nostril, the other two located near the upper maxilla, one situated slightly anteriorly to a vertical through anterior nostril, the other above posterior third of upper lip; 4 [4 (25), 5 (1)] pores on the free lower edge of preopercle, continuing anteriorly with three pores in mandibular series; one pore on preopercular angle, and 3–4 [3 (18), 4 (5)] on the free upper edge of preopercle, continuing with two pores, the uppermost almost meeting level of temporal canal; supraorbital canal with four pores starting anteriorly above posterior nostril (one individual presented an extra pore above anterior nostril) and ending posteriorly above orbit at a vertical through posterior margin of eye; supraorbital canal extending anteriorly to a pore located on the snout at a vertical through anteriormost pore above the lip radiating from the suborbital canal; temporal canal with 7–9 pores [7 (17), 8 (5), 9 (1)], starting anteriorly behind and slightly below upper edge of orbit (three anterior to the vertical through the upper free edge of preopercle); upper temporal canal starting with a single central pore located at the edge of the ending angle of scales, which is located slightly anteriorly to a vertical through posterior edge of orbit, then 4 [4 (21), 5 (2)] pores distributed flanking the scales, the most posterior one located at a vertical through fourth pore of temporal canal.
Scales cycloid; smaller scales on nape extending forward to a vertical through posterior edge of orbit; remainder of head naked; scales on chest smaller than scales on sides of body; scales on chest progressively smaller ventrally and anteriorly; lateral line starting behind the free upper edge of opercle, approximately parallel to dorsal profile to space between ninth and tenth dorsal-fin soft rays, then sharply descending to body midline, and then again straight toward caudal-fin base; last pored lateral-line scale on base of caudal fin larger than the rest; no scales on base of dorsal, anal, or paired fins; progressively smaller scales on base of caudal fin, covering caudal-fin rays to about one-fifth of caudal-fin length.
Origin of dorsal fin situated above fifth lateral-line scale, predorsal-fin length 3.2–4.0 in SL; distance between first and second dorsal spines about half the distance between second and third spines; dorsal-fin spines flexible, but rather rigid in the largest individual, although not pungent; in individuals showing primary (females, initial-phase males) and intermediate liveries the anterior three dorsal-fin spines not elongated, length of the first spine 3.6–5.0 in HL, second 3.1–4.0 in HL, third 3.0–4.0 in HL, then spines very even or gently decreasing until the eighth which is equal or slightly shorter than the ninth (3.0–3.9 in HL); individuals showing the secondary livery (males): first three dorsal spines longer than the remainder, first spine 1.8–2.6 in HL, second usually the longest, 1.8–2.5 in HL, third 2.0–3.5 in HL, then sharply decreasing from third to fourth, from fourth to eight spines very even or gently decreasing until the eighth which is equal or slightly shorter than the ninth (3.0–4.0 in HL); the longest dorsal soft ray are the third or fourth (2.3–3.1 in HL); origin of anal fin situated below soft dorsal-fin origin, preanal-fin length 1.7–1.9 in SL; all anal-fin spines flexible, increasing in size, the first 7.1–13.1 in HL, second 4.1–8.2 in HL and third 3.1–5.3 in HL; anal-fin soft rays similar in size, third and fourth usually the longest, 2.4–3.1 in HL; caudal fin convex, its length 1.3–2.0 in HL; pectoral fin pointed, its length 1.7–2.0 in HL, second ray longest; origin of pelvic fins at a vertical of lower pectoral-fin base, prepelvic-fin length 3.1–3.7 in SL; pelvic-fin length 1.9–2.6 in HL, second pelvic-fin soft ray longest, not elongated, distance between extended pelvic-fin tip and anus 2.8–6.6 in HL.
Coloration
We found the two main coloration patterns already described in detail in numerous works: the primary and secondary liveries, plus an intermediate color pattern found in only one of the collected individuals, but commonly seen in others while diving during the sampling (Figures 4, 5).
Primary livery (females and primary-phase males): Color changing very rapidly depending on the environment. As an example, these changes were followed for one of the sampled individuals which was photographed swimming before getting caught, out of water just after getting caught, put back in water again and finally after death (Figure 4). In general, body coloration is defined, in the most heterogeneous or “striped” coloration type within this livery, by a brown back that covers the sides to the level of the lateral line, below there may be a thin line of white dots that separates the back coloration from a usually darker purple-brown stripe that extends from the snout to the caudal-fin base, its lower margin level with lower margin of orbit; eye orange; lower half of the body white, with the exception of a yellow stripe extending from below mouth to caudal-fin base; a bluish-black spot on upper side of pectoral axil (barely visible in the smaller individuals) and opercle; pectoral and pelvic fins translucent; dorsal and anal fins variable but most individuals with yellow or red translucent longitudinal stripes extending from base to tips; caudal fin yellow and red, with translucent distal corners. We observed live individuals without the dotted white line, a darker brown back and a lighter purple-brown stripe, which instead of the striped appearance showed a uniform purple-brown color in the upper half of the body and a barely visible yellow stripe in the lower half. All the “striped” individuals we collected showed this uniform coloration after death. After preservation in formalin, the upper half of the body remained brown without any distinguishable stripe or line, the yellow stripe was completely lost in the lower half, which was completely white on the belly and whitish-gray from the anal fin origin to the caudal fin (Figure 4).
Intermediate livery (intermediate-phase males): Back purple-brown; eye orange; an orange-brown stripe below from snout through the eye and the opercle upper edge and to caudal fin base; this band is indented throughout its length; lower half of body white; a bluish-black spot on upper side of pectoral axil and opercle; pectoral and pelvic fins translucent; anal fin yellowish; dorsal fin yellowish but with some black in the first two spines and membranes; caudal fin gray near its base but yellow posteriorly except for the gray distal corners (Figure 5).
Secondary livery (males): Back typically greenish or turquoise but may also be purple-brown (this variation not seen in large individuals); eye orange or yellow; an orange stripe extending from snout through the eye and upper edge of opercle to caudal-fin base; this band is indented throughout the body; lower half of body white to anus, turning rather grayish posteriorly; a black cuneiform band extending below the orange indented band from pectoral axil to about the vertical through anal-fin origin, getting wider and overlapping the indented stripe posteriorly; the black cuneiform band is rimmed on its lower margin by a thin, blue line which extends anteriorly to the upper lip; a bluish-black spot on upper side of pectoral axil and opercle; pectoral and pelvic fins translucent; dorsal fin with an anterior black spot covering the membranes before the third spine, and sometimes even extending to membrane between third and fourth spines, but not to the margin which is vividly red; remainder of dorsal fin light orange and lilac, usually with an orange-lilac-orange striped pattern which extends from the base to the tips; anal fin white, light orange and lilac, usually striped white-orange-lilac-orange from the base to the tips; caudal fin coloration variable, with predominating yellowish orange, sometimes mixed with green or turquoise, except for the grayish upper and lower distal corners (Figure 5).
Remarks
Roede (1966) described the coloration of seven individuals showing an intermediate coloration. In most of them the indented band was vague but noticeable although “it was gray-brown instead of bright orange,” but two of the individuals showed an indented band in orange although the rest was more similar to the primary livery and the individuals did not present elongated first dorsal spines. This seems to be a similar pattern to that we found in CFM-7432.
Synonymy and Neotype Designations
Coris julis has a complicated synonymy; as we now find that two closely related species co-occur in the western Mediterranean, this synonymy needs to be clarified before the two species can be properly named. Therefore, we here discuss the names in this species complex in detail.
Labrus julis was originally described by Linnaeus (1758: 284), based on multiple sources including Gronow (1756: 28, “Labrus oblongus, nigricans, lateribus linea alba utrinquesinuata longitudinali variis, cauda indivisa”) from the Mediterranean Sea, and Artedi (1738a,b: 34; 1738b: 53; “Labrus palmaris varius, dentibus duobus majoribus maxillae superioris”), which was again based on multiple historical sources, starting with Aristotle, Athenaeus, Aelianus, Oppianus and Galenus, and also including the following: Bellon (1553: 254, “Iulis” from Venice, Italy and Marseille, France, fig. based on Labrus bergylta; 256, “Phycis,” fig. based on Coris julis, from Marseille, France), Rondelet (1554: 180, “Iulis”), Gesner (1558: 549, “Iulis”) from Venice (Italy) and Greece, Salviani (1558: 217, fig., “Julia”; 219, “Julis”) from Rome (Italy), Gesner (1598: 14, “Iulis”), Aldrovandi (1638: 39, “Iulis authoris”), Jonston (1649: 45, pl. 14, fig. 3, “Iulis”), Charleton (1668: 133, “Julis”), Willughby (1686: 324, “Julis”), Ray (1713: 138, “Julis”). Linnaeus (1758) from Genoa (Italy), but his description was based on specimens from various Mediterranean localities, including Marseille (France), Genoa, Venice, Rome, Naples (Italy) and Crete (Greece). No type specimens of Labrus julis Linnaeus, 1758 are extant (Fricke et al., 2021); they may have never been preserved. Although most of the illustrations in the historical literature show the color pattern of the secondary livery (males) of Coris julis, we cannot be sure that only this species is included in the historical sources. In order to clarify and stabilize the taxonomic identity of this taxon, CFM-IEOMA 7433 is hereby selected as the neotype of Labrus julis Linnaeus, 1758 (Figure 5), whereas CFM-IEOMA 7429 and 7432 are selected as paratypes (Figures 4, 5). The type locality is thus restricted to eastern Mallorca (39°27′1″N, 3°20′15″E). The neotype is diagnosed as follows: soft dorsal-fin rays 12; lateral-line scales 72 + 1; pectoral rays 13; body depth 4.8 in SL; pelvic-fin tip not extending to the vertical through pectoral-fin tip; it presents a secondary livery (males); back turquoise; an orange stripe extending from snout through the eye and upper edge of opercle to caudal-fin base; this band is indented throughout the body; lower half of body white to grayish posteriorly; a black cuneiform band extending below the orange indented band from the vertical through pelvic fin tip to the vertical through anal-fin origin, getting wider and overlapping the indented stripe posteriorly; the black cuneiform band is rimmed on its lower margin by a thin, blue line which extends anteriorly to the upper lip; a bluish-black spot on upper side of pectoral axil and opercle; dorsal fin with an anterior black spot covering the membranes before the third spine, but not to the margin which is vividly red with an orange stripe (Figure 5). We chose a fresh specimen that was available for genetic examination; therefore, the neotype locality is situated as close as practical to the original western Mediterranean syntype localities.
Labrus paroticus was described by Linnaeus (1758: 284), allegedly from India, with reference to the manuscript to the then unpublished work Linnaeus (1764: 76, with locality “America”). Five syntypes are known (NRM 3), which probably originated from the Mediterranean Sea, and refer to the primary livery of Coris julis. Subsequent authors, including Bauchot and Quignard (1973: 431), treated the name as a junior synonym of Coris julis (Linnaeus, 1758).
Sparus niloticus was described by Hasselquist (1762: 387), allegedly from the Nile River, near Cairo (Egypt), based on Hasselquist (1757: 341). Both Hasselquist works were rejected by ICZN (Opinion 57, 1914); therefore, the taxon is not available. It was placed in the synonymy of Coris julis (Linnaeus, 1758) by Fricke (2008: 47); the type locality was obviously erroneous, and the material probably originated from the Mediterranean coast of Egypt.
Labrus iulis was described by Brünnich (1768: 54) from Marseille (France), obviously based on the primary livery of Coris julis, together with a variety b which may have been based on the primary livery of Coris melanura; this has been considered as an incorrect subsequent spelling of Labrus julis Linnaeus, 1758 (Fricke et al., 2021). None of the material is extant; it may have never been preserved.
Labrus perdica was originally described by Fabricius [ex Forsskål] in Niebuhr (1775: 34, xi), based on material collected by P. S. Forsskål in the Sea of Marmara near Istanbul (Turkey); the color description apparently identifies this taxon as the primary livery of Coris julis (see Fricke, 2008: 48), although it might be confused with C. melanura. No type material of this taxon is extant (Fricke, 2008: 48; Fricke et al., 2021). In order to clarify and stabilize the taxonomic identity of this taxon, SMNS 11609 is selected as the neotype of Labrus perdica Fabricius, 1775 (fig. 4), fixing it as a junior synonym of Coris julis. The neotype is diagnosed as follows: soft dorsal-fin rays 12; lateral-line scales 73 + 1; pectoral rays 13; body depth 5.4 in SL; pelvic-fin tip not extending to the vertical through pectoral-fin tip; it presents a primary livery; the preserved coloration is brown in the upper half of the body without any distinguishable stripe or line, and the lower half pale; a black spot on opercle and another barely visible on the upper side of pectoral axil; all fins transparent-pale (Figure 4).
Labrus infuscus was described by Walbaum (1792: 249) from the Mediterranean Sea, based on Gronow (1763: 70, No. 238, “Labrus ex livido-brunneus, capite subacuto”), which again was based on Klein (1749: 45, pl. 8, fig. 5, “Maenas in ventre flavicans”).
No type material of this taxon is extant (Fricke et al., 2021). Although Parenti and Randall (2000: 15) placed this taxon in the synonymy of Coris julis (Linnaeus, 1758), an inspection of the sources reveals that the color pattern and body shape does not match this species; it is rather based on a species of Symphodus; probably S. cinereus (Bonnaterre, 1788). We may therefore exclude Labrus infuscus from our considerations.
Labrus subfuscus was described by Walbaum (1792: 254); although no locality was stated, it should have been New York, United States, as the description was based on the “Labrus, Blackfish; zu Neuyork” of Schöpf (1788: 156bis). No type material of this taxon is extant (Fricke et al., 2021). Although Parenti and Randall (2000: 15, 43) treated this taxon as a junior synonym of Coris julis (Linnaeus, 1758), it is evident that it is in fact a junior synonym of Tautoga onitis (Linnaeus, 1758). Therefore, Labrus subfuscus may be excluded from our considerations as well.
Labrus keslik was described by Lacepède (1801: 453, 523) from Istanbul, Turkey, Sea of Marmara; the name is an unneeded replacement name for Labrus perdica Niebuhr (1775), and therefore does not need to be considered here.
Labrus cettii was described by Rafinesque (1810: 23, 54) from Sardinia Island, Italy, Mediterranean Sea, based on Cetti (1777: 122, “Iulis”). The species was characterized by having a red iris; a blue blotch on the opercle; body dark above, white below; a yellow stripe along the sides of the body. This coloration might refer to the primary livery of either Coris melanura or Coris julis. No type material of this taxon is extant (Fricke et al., 2021). In order to clarify and stabilize the taxonomic identity of this taxon, CFM-IEOMA 7433 from eastern Mallorca is selected as the neotype of Labrus cettii Rafinesque, 1810 (Figure 5), fixing it as a junior synonym of Coris julis. Diagnosis of neotype see above.
Labrus giofredi was described by Risso (1810: 228, pl. 9, fig. 23) from Nice, France, northwestern Mediterranean Sea. Judging from the description and illustration, it was based on the primary livery Coris julis. No type material of this taxon is extant, and former MNHN material has been lost (Fricke et al., 2021). In order to clarify and stabilize the taxonomic identity of this taxon, CFM-IEOMA 7433 from eastern Mallorca is selected as the neotype of Labrus giofredi Risso, 1810 (Figure 5), fixing it as a junior synonym of Coris julis. Diagnosis of neotype see above.
Risso (1827: 310) distinguished two varieties of Julis giofredi (Risso, 1810), which were cited by Fricke et al. (2021) as Julis giofredi var. argentata and Julis giofredi var. fuscoviolacea, both from Nice, France, northwestern Mediterranean Sea. However, these were not named by Risso (1827), who just used “Var. I” and “Var. II.” We are not aware of any available use of these variety names; therefore, they can be neglected for the purpose of this work.
Julis mediterranea was described by Risso (1827: 309) from Nice, France, northwestern Mediterranean Sea. A Risso specimen and possible holotype could be detected, MNHN B-0867 (Fricke et al., 2021). The color and morphological description including the presence of “une raie longitudinal, dentée ou en zigzag, d’un bel orange” and “la nageoire dorsal est très relevée à son origine et ornée d’une grande tache rouge bleue” clearly refers to the secondary livery (male) of Coris julis.
Risso (1827: 310) distinguished two varieties of Julis mediterranea Risso, 1827, which were cited by Fricke et al. (2021) as Julis mediterranea var. pallidula and Julis mediterranea var. veridula, both from Nice, France, northwestern Mediterranean Sea. However, these were not named by Risso (1827), who just used “Var. I” and “Var.” We are not aware of any available use of these variety names; therefore, they can be neglected for the purpose of this work.
Julis speciosa was described by Risso (1827: 311, pl. 9, fig. 20) from Nice, France, northwestern Mediterranean Sea. According to the color description, it is based on the primary livery of either Coris melanura or C. julis. No type material of this taxon is extant (Fricke et al., 2021). In order to clarify and stabilize the taxonomic identity of this taxon, CFM-IEOMA 7433 from eastern Mallorca is selected as the neotype of Julis speciosa Risso, 1827 (Figure 5), fixing it as a junior synonym of Coris julis. Diagnosis of neotype see above.
Julis vulgaris was described by Fleming (1828: 210) from Cornwall, England; it represents an unneeded replacement name for Labrus julis Linnaeus, 1758, which was also used by Valenciennes in Cuvier and Valenciennes (1839: 361, pl. 384).
Julis festiva was described by Valenciennes in Cuvier and Valenciennes (1839: 374) based on a specimen from Brest, France, northeastern Atlantic. According to the color description, it is based on the primary livery of C. melanura, but see section “Remarks” within C. melanura description. The holotype is deposited at Muséum National d’Histoire Naturelle, Paris, MNHN A-9244 (Fricke et al., 2021).
Anarchichas fusellus was described by Naro (1847: col 115) from the Adriatic Sea. The name is not available, as it is a manuscript name of Chiereghini that was mentioned in synonymy of “Julis giofredi Risso” (=Labrus giofredi Risso, 1810), which defines it as a junior synonym of Coris julis.
Coris (Hologymnosus) taeniatus was described by Steindachner (1863: 1189, pl. 2, fig. 1) from Java, Indonesia; the type locality was in error, and the specimens probably originated from the Canary Islands. According to the color description and the figure, it is based on the primary livery of C. melanura. The syntypes are deposited at Naturhistorisches Museum Wien, NMW 25675-25676, two specimens (Fricke et al., 2021).
Julia azorensis was described by Fowler (1919: 204, fig. 2) from Horta, Fayal Island, Azores, northeastern Atlantic. According to the color description, the figure and the photographs of the holotype deposited at the National Museum of Natural History, Washington D.C., United States, USNM 42127 (Fricke et al., 2021), it is based on an individual of C. melanura presenting the primary livery (see section “Remarks” within C. melanura description).
Key to the Northeastern Atlantic and Mediterranean Species of Coris
1a. Pelvic fins clearly extending across a vertical through pectoral fin tip ………………………………………………. C. atlantica.
1b. Pelvic fins not extending across a vertical through pectoral fin tip …………………………………………………………………………….. 2
2a. Primary livery (juveniles, females and initial-phase males) with a thin black line on the sides of the body, about 1–3 scales wide, extending along the flanks from behind the orbit to caudal fin rays, even reaching the posterior half of this fin in some individuals; this line not vanishing, but increasing in contrast when preserved in formalin or ethanol; 6–8 longitudinal series of small red dots surrounding the white belly from the lower edge of pectoral fin base; a small triangular dark mark present at the membrane between the second and third dorsal spines (barely visible in small individuals).
Secondary livery (males) presenting a longitudinal series of vertical bars (usually black, red or yellow) along the body flanks; except for the smaller individuals, the caudal fin (at least the upper half) and frequently the posterior upper third of the body is black; first and second dorsal spines 2.5–3.3 and 2.3–3.4 in head length, respectively………………………………………………….. C. melanura.
2b. Primary livery (juveniles, females and initial-phase males) lacking the black thin line along the flanks as described above, the dark mark in the membrane between the second and third dorsal spines and the small red dots surrounding the belly.
Secondary livery (males) presenting an orange indented band along the flanks from snout to caudal fin base; caudal fin predominantly orange yellowish or this color mixed with the green or turquoise present in the back; first and second dorsal spines 1.8–2.6 and 1.8–2.5 in head length, respectively. ………………………………………………………… C. julis.
Genetics and Phylogeny
A total of 368 (79 variable sites, 73 informative sites), 600 (196 variable sites and 186 informative sites) and 384 (184 variable sites and 87 informative sites) base pairs (bp) for 12s rRNA, COI and CYTB mitochondrial fragments were sequenced, respectively, for Coris melanura and C. julis.
The average interspecific divergence for the 12s rRNA fragment between any two species of Coris was 7.3% and 23.8 bp differences. No genetic differences were detected between C. melanura and those GenBank sequences designated as C. julis and collected from the Northeast Atlantic (Azores and Portugal coast; Table 4). However, wider values of genetic distance and pb differences were observed with the C. julis specimens from the Mediterranean (2.1%; 7.2 bp differences), followed by C. atlantica (7.4%; 24 bp differences).
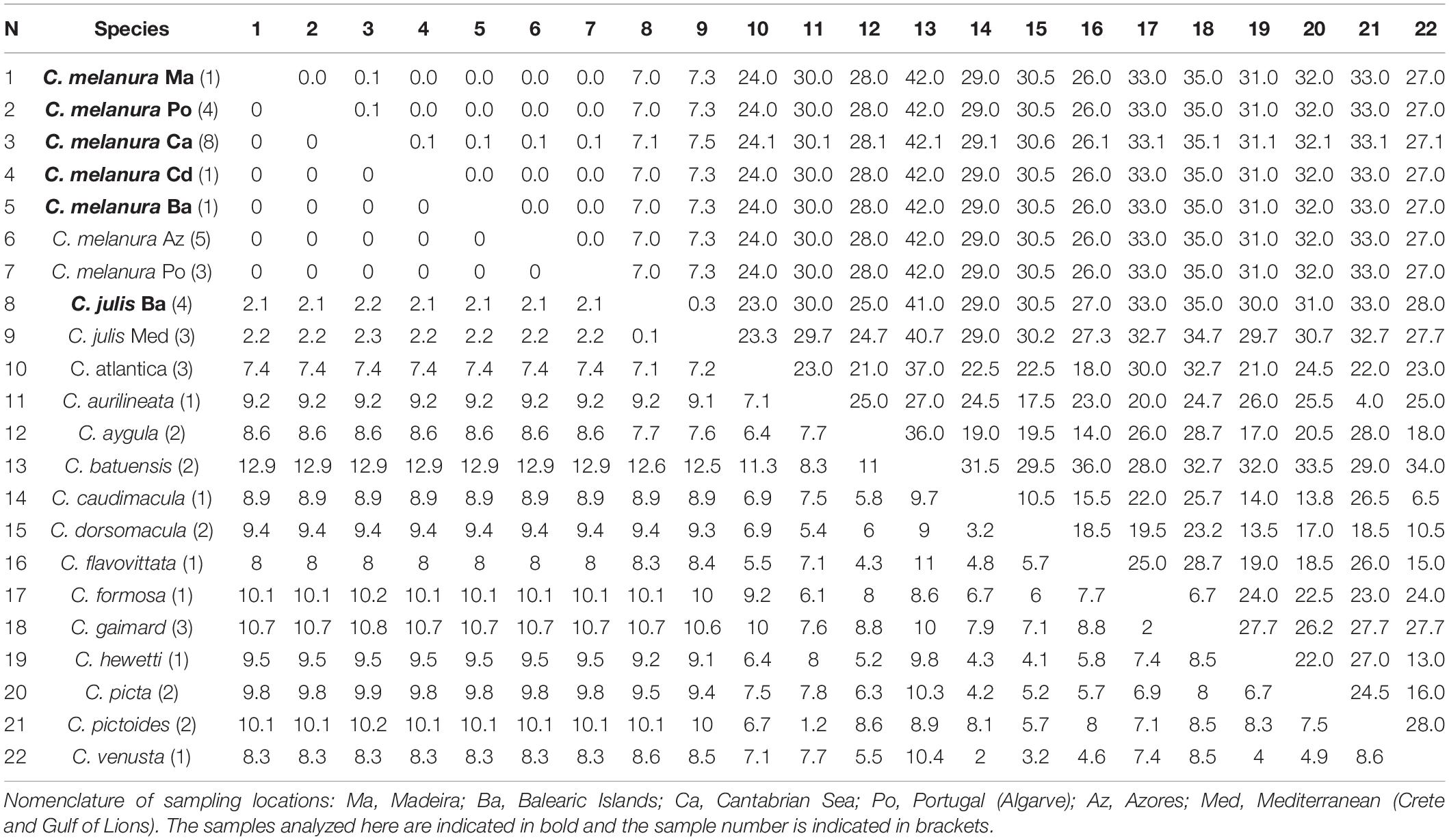
Table 4. Mean genetic distances (%) and numbers of base differences for 12s rRNA from Coris species below and above the diagonal, respectively.
In the case of the COI gene, the average interspecific divergence between any two species of Coris was 14.3% and 80.5 pb differences. No genetic differences were observed between C. melanura and GenBank sequences assigned as C. atlantica and two sequences as C. julis (both sequences are listed as unverified in GENBANK: KJ768228-29) all these samples are from the northeast Atlantic (Portugal coast; Table 5). Coris melanura showed high values of genetic distance with C. julis from Mediterranean (Balearic Islands, Italy and Turkey; 6.0%; 33.6 bp differences). Greater genetic differences were also observed when we compared to C. atlantica (14.3%; 80.5 bp differences), which includes two sequences identified as C. atlantica from Equatorial Guinea (Southeast Atlantic).
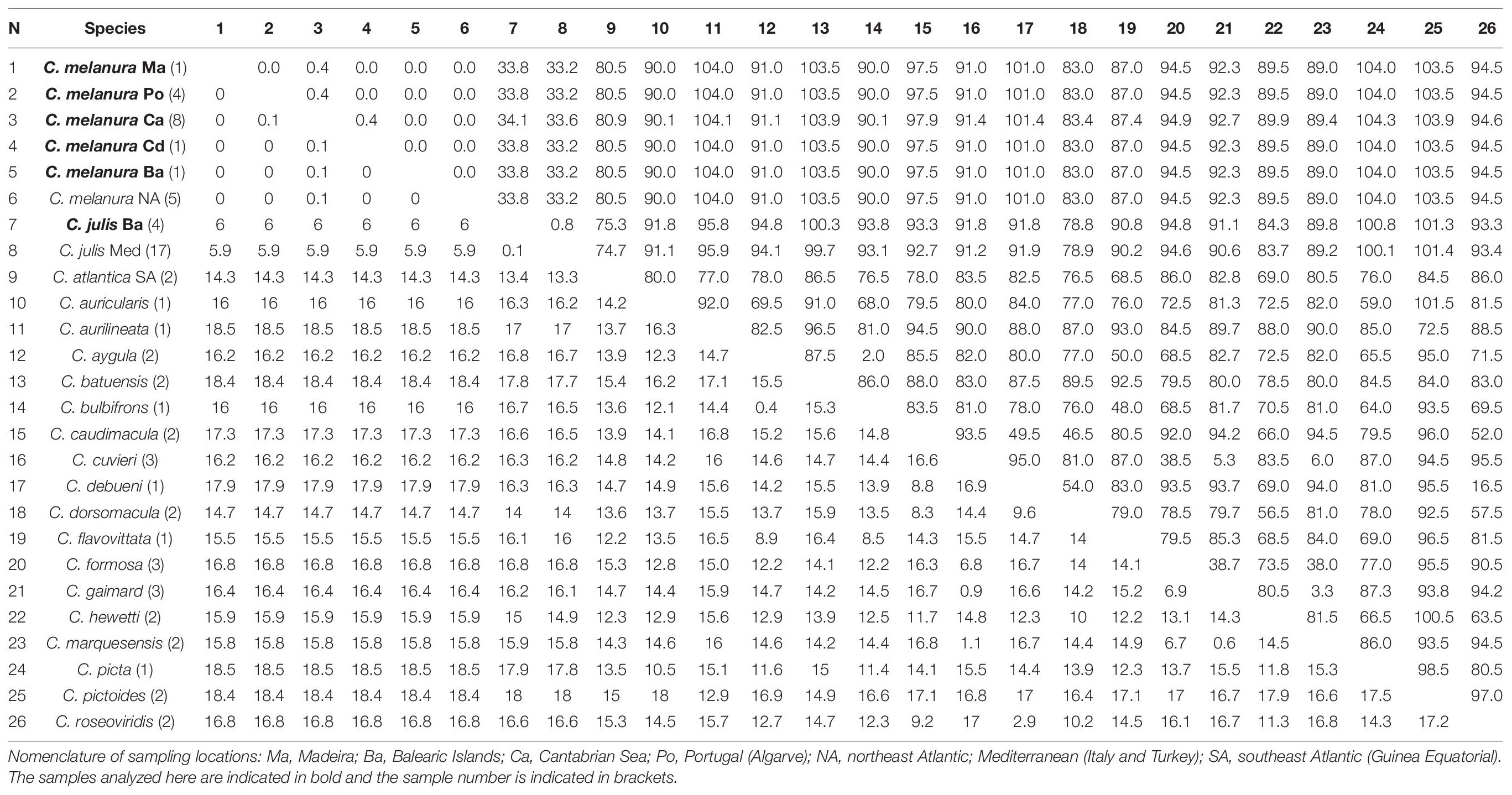
Table 5. Mean genetic distances (%) and numbers of base differences for COI from Coris species below and above the diagonal, respectively.
Based on the CYTB gene, the average interspecific divergence between the species of Coris included in the present study was 17.3% and 52 pb differences. Coris melanura showed the lowest genetic distance with GenBank sequences assigned as C. julis from Northeast Atlantic (0.3%; 1 bp differences; Table 6), but with greater differences with C. julis specimens here collected (8.1%; 24 bp differences).
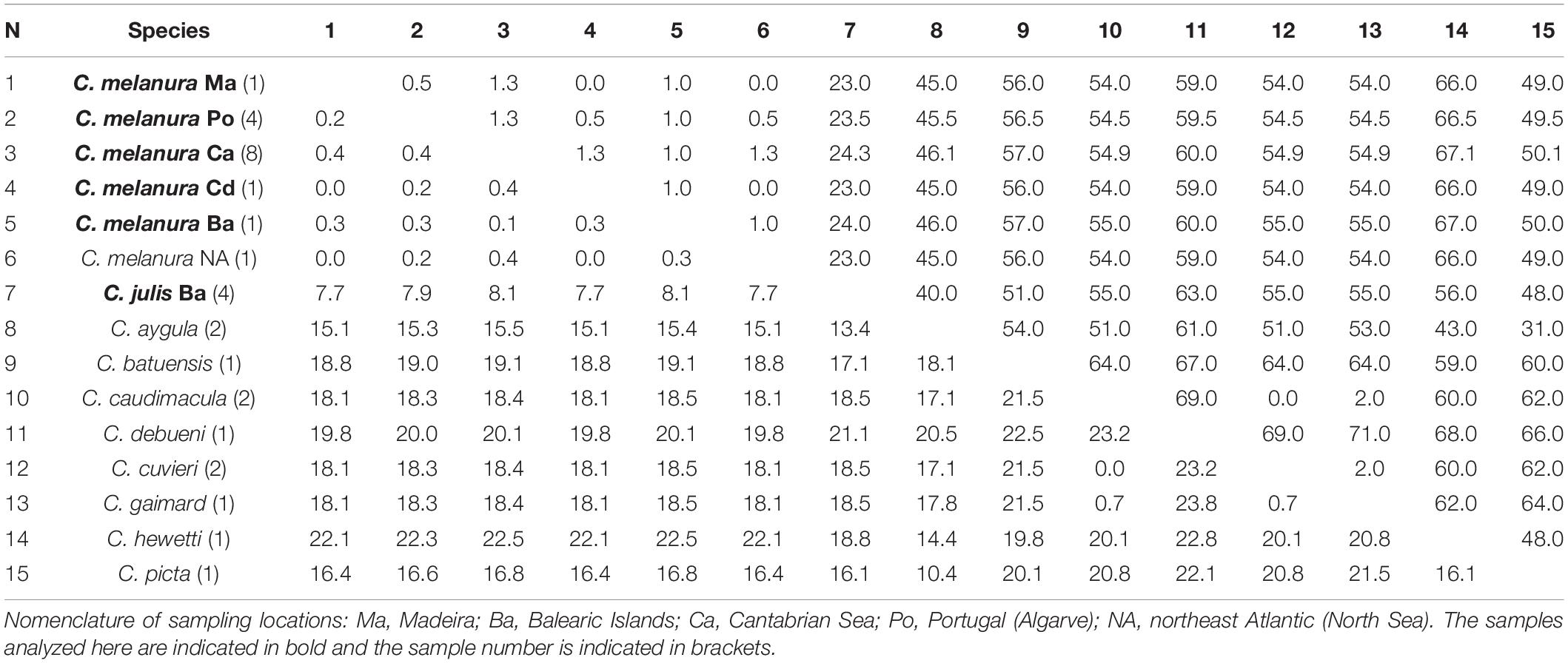
Table 6. Mean genetic distances (%) and numbers of base differences for CYTB from Coris species below and above the diagonal, respectively.
In all phylogenetic trees, C. melanura and C. julis form a monophyletic group (PP = 1) which is sister clade to C. atlantica (PP > 0.95) (Figures 6–8).
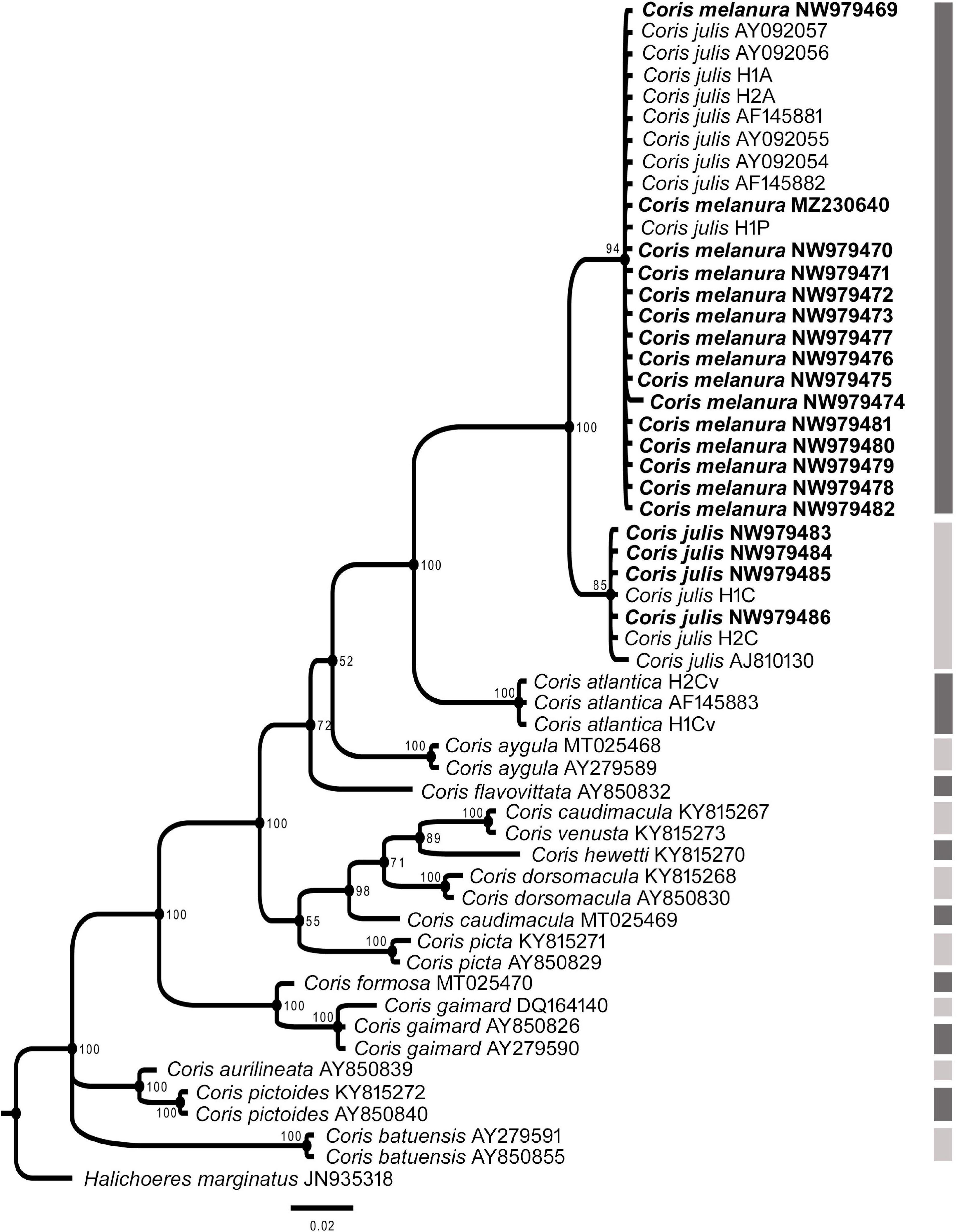
Figure 6. Phylogenetic relationship based on Bayesian Inference (BI) for 12s rRNA fragment for Coris species. Posterior probabilities (%) and GenBank accession numbers are indicated near the nodes and the species name, respectively. Species delimitation analysis using Poisson Tree Process (bPTP) is indicated by lateral dark and light gray side bars. The samples analyzed here are indicated in bold.
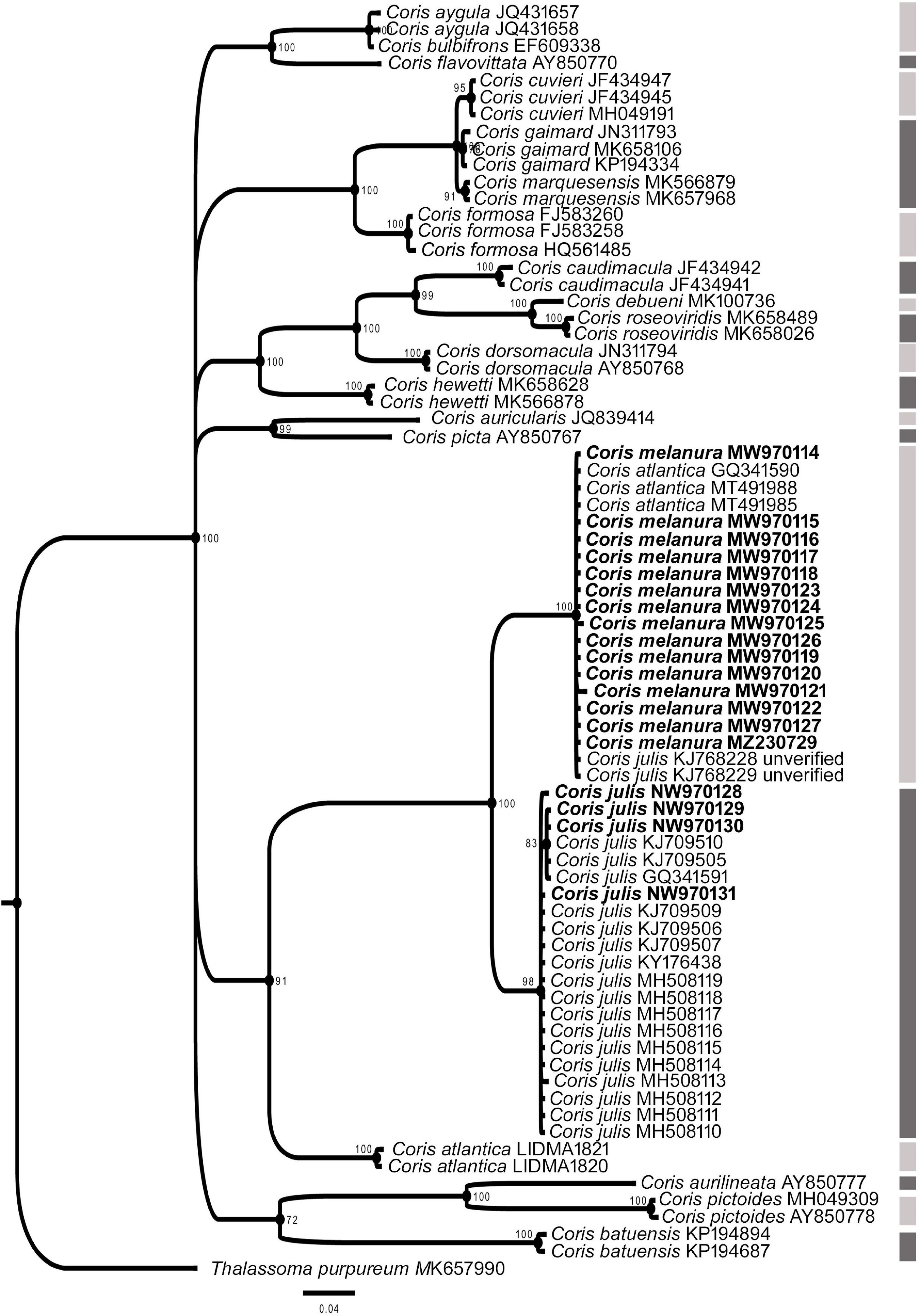
Figure 7. Phylogenetic relationship based on Bayesian Inference (BI) for COI fragment for Coris species. Posterior probabilities (%) and GenBank and BOLD Systems accession numbers are indicated near the nodes and the species name, respectively. Species delimitation analysis using Poisson Tree Process (bPTP) is indicated by lateral dark and light gray side bars. The samples analyzed here are indicated in bold.
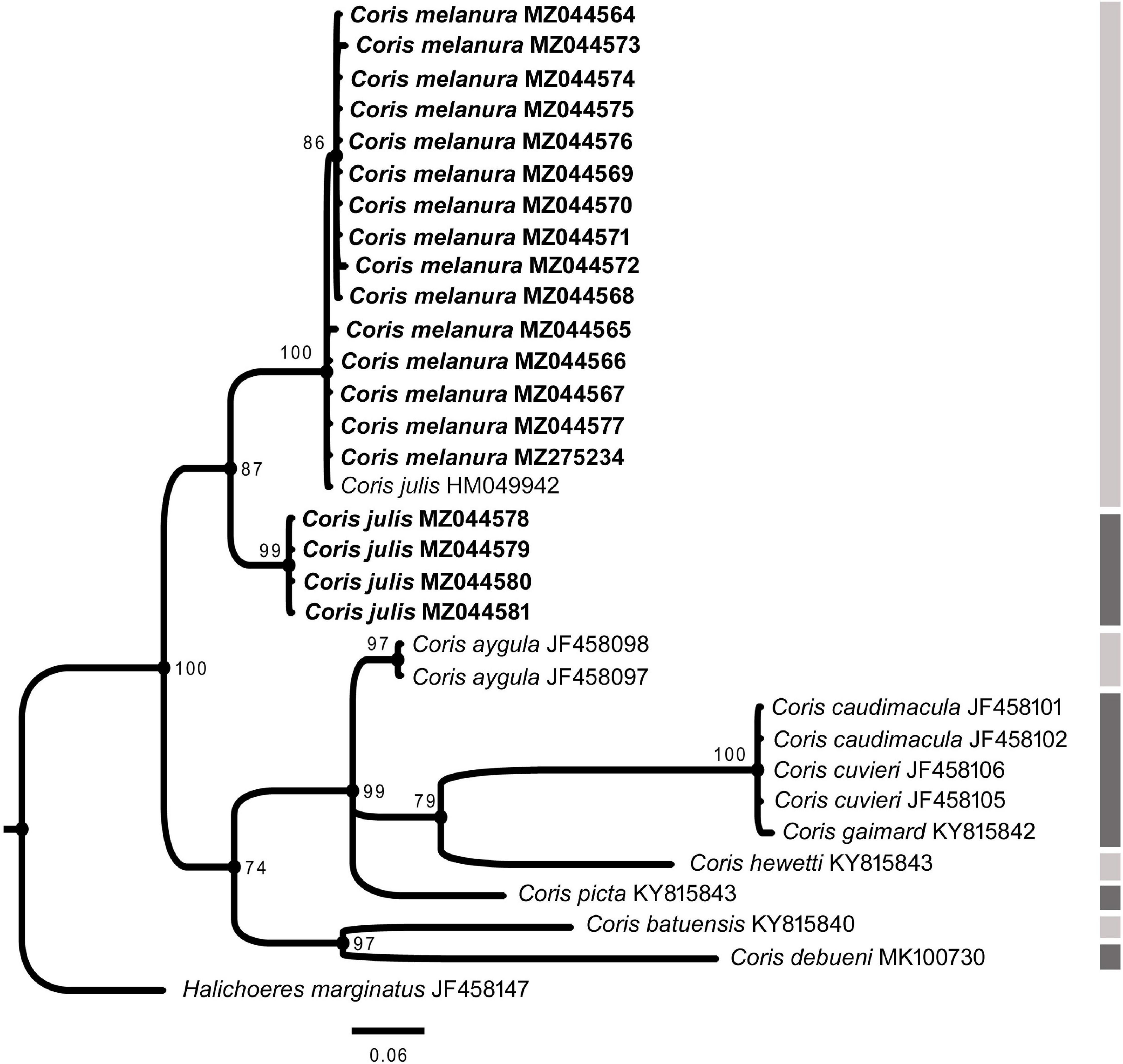
Figure 8. Phylogenetic relationship based on Bayesian Inference (BI) for CYTB fragment for Coris species. Posterior probabilities (%) and GenBank accession numbers are indicated near the nodes and the species name, respectively. Species delimitation analysis using Poisson Tree Process (bPTP) is indicated by lateral dark and light gray side bars. The samples analyzed here are indicated in bold.
The results of the Poisson Tree Process recovered at least 17, 18 and eight putative species cluster for 12S, COI and CYTB fragments, respectively. Among them are C. julis and C. melanura for the three genes (Figures 6–8), thus supporting the results obtained by morphological, genetic distances and phylogenetic analyses.
In agreement with phylogenetic analyses, the haplotype networks showed no shared haplotype for the Coris species distributed in the Atlantic and Mediterranean (Figure 9). In the case of 12s rRNA, C. melanura comprised two haplotypes, one of them included almost all the specimens (20 of 21 samples) of all sampling areas, and for C. julis a more frequent haplotype (six of seven samples) was also observed (Figure 9A). A similar pattern was obtained for COI fragment, C. melanura displayed three haplotypes, one of them being the most frequent (17 of 19 samples) and present in all sampling areas, and C. julis showed four haplotypes, one of them being the most frequent (14 of 21 samples) and also present in all sampling areas (Figure 9B). Lastly, the CYTB fragment showed five haplotypes for C. melanura two of them being the most frequent (8 / 4 of 15 samples), while C. julis displayed only one haplotype (Figure 9C).
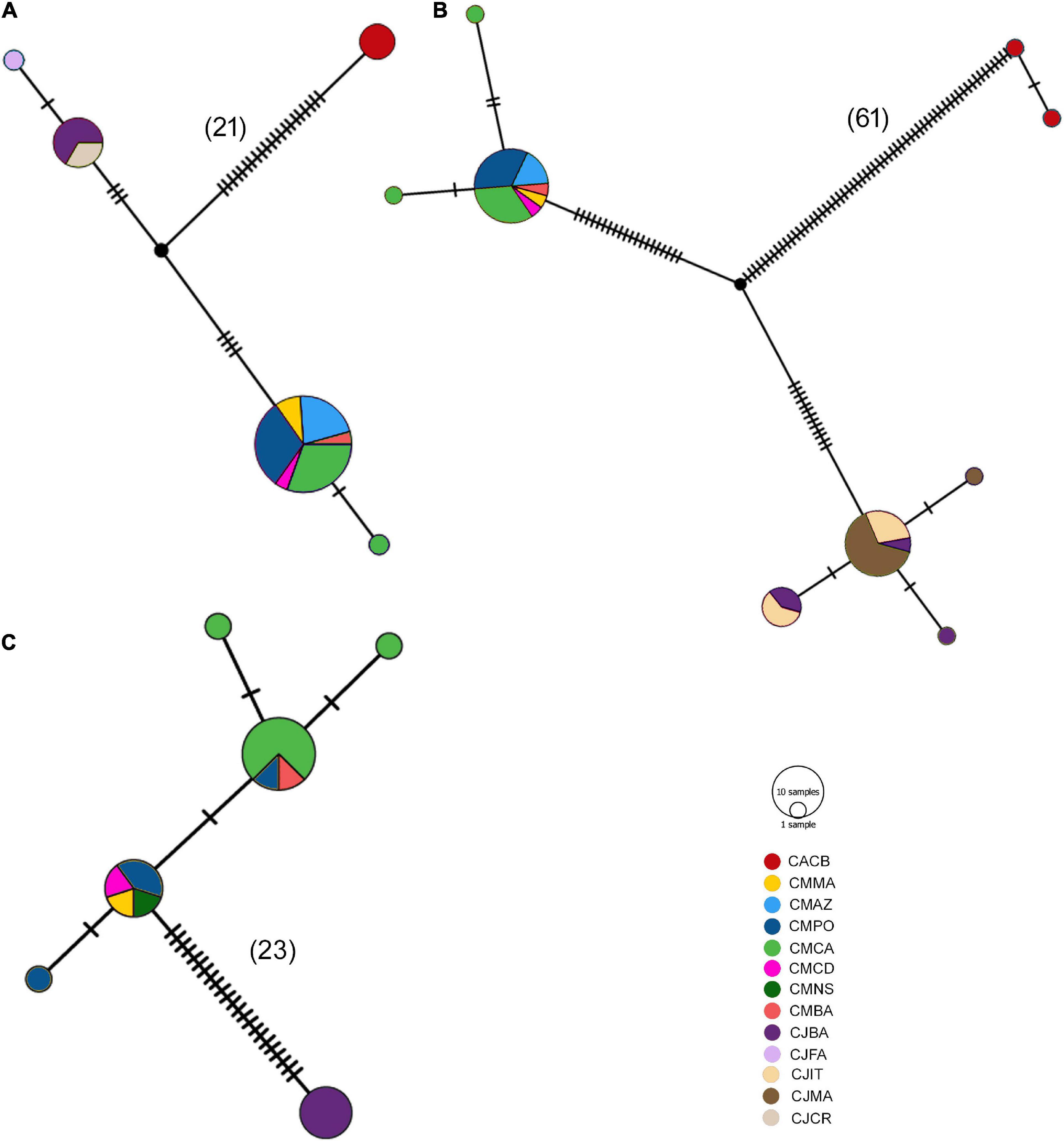
Figure 9. Haplotype network for each fragment: (A) 12S, (B) COI, and (C) CYTB. Nomenclature: CACB, Coris atlantica Cape Verde; CMMA, Coris melanura Madeira; CMAZ, C. melanura Azores; CMPO, C. melanura Portugal; CMCA, C. melanura Cantabria Sea; CMNS, C. melanura North Sea; CMBA, C. melanura Balearic Islands; CJBA; Coris julis Balearic Islands; CJFA; C. julis France; CJIT, C. julis Italy; CJMA, C. julis Malta; CJCR, C. julis Crete. Mutation steps are indicated as diagonal bars, and steps larger than 20 are also indicated in parenthesis.
Discussion
Our morphological and molecular analyses confirmed the hypothesis that Coris julis is a species complex composed of two sibling species: C. julis, distributed mainly in the Mediterranean, from which the species was originally described, and C. melanura (Lowe, 1839), that we propose as a valid species with the oldest available name, distributed mainly in the northeastern Atlantic and western Mediterranean.
The primary and secondary liveries of C. melanura and those of C. julis showed a different color pattern. Although the differences in the secondary livery between Atlantic and Mediterranean morphotypes had already been documented (e.g., Sanchez-Delgado, 1981; Michel et al., 1987; Laurent and Lejeune, 1988; Martino and Grau, 2010), this is the first time that differences in the primary livery are reported. Another difference between the two species is the size at which the change of primary to secondary livery occurs. The only sampled individual showing primary livery of C. melanura from the Balearic Islands was 13.4 cm SL, whereas the rest of individuals showing this livery ranged from 10.8 to 16.1 cm SL and were sampled in the Atlantic. By contrast, all the sampled individuals of C. julis above 12.1 cm SL showed the secondary or intermediate (only one individual) liveries. This is similar to the results reported by Roede (1966) from the Gulf of Lions, who analyzed 265 individuals and found that the primary livery only appeared in individuals smaller than 12 cm SL, and those reported by Bacci and Razzauti (1957) who analyzed 746 individuals from the west coast of Sardinia and the coast off Livorno (northern Tyrrhenian Sea) and only found the primary livery at lengths smaller than 15 cm total length (i.e., smaller than 13 cm SL).
Interspecific divergences between C. melanura and C. julis were high, averaging 2.1, 6, and 8.1% for 12s rRNA, COI and CYTB, respectively. Divergence values for COI fragment exceeding the standard threshold for marine fish species delimitation which is 2% for this fragment (Hubert et al., 2008; Ward et al., 2009). These large interspecific divergence values for COI fragment have already been reported by Landi et al. (2014) within C. julis between Atlantic and Mediterranean, suggesting the presence of two valid species. In this sense, it is proposed that those species with more than 2% of genetic divergence for the COI fragment are generally reproductively isolated (April et al., 2012). For the other two fragments used here, there is no a standard species delimitation value. However, Grant and Bowen (1998) proposed a range of divergence in marine sister species based on three different fragments (CYTB, ND4/5 and COI) ranging from of 3.4–12.4%. Considering this, the divergence values for the three fragments fall within the range of divergences supporting the validity of both species.
These findings are also supported by the phylogenetic analyses of the three fragments, which showed two clearly divergent lineages between those specimens morphologically identified as C. melanura and C. julis. The close phylogenetic relationship of these both species could indicate a recent divergence event, as recorded in other fish species in the same study area, such as Symphodus tinca vs remaining Symphodus species (Hanel et al., 2002), Pagellus acarne vs P. bogaraveo (Meynard et al., 2012) and Raja montagui vs R. polystigma (Ramírez-Amaro et al., 2018). Aurelle et al. (2003), based on mtDNA sequences, estimated 1–2 Mya of divergence time between Atlantic and Mediterranean Coris julis populations. Considering the close phylogenetic relationship between C. melanura and C. julis, the divergence time suggested by Aurelle et al. (2003) could be proposed as the time in which both species diverged. That places this speciation process after the Messinian Salinity Crisis and the re-opening of Gibraltar Strait. However, age estimation studies, employing a molecular clock, and nuclear markers are needed to confirm this hypothesis.
Environmental conditions during interglacial and glacial episodes in eastern Atlantic could have played a critical role as driver of diversification of Coris genus in this region, as proposed in several marine species (Freitas et al., 2019; Ávila et al., 2019). Coris specimens from Atlantic waters could have adapted to colder temperatures and also colonized, via the Strait of Gibraltar, the warm Mediterranean basin, as it has been described for other marine taxa (e.g., Muths et al., 2010; Taboada and Pérez-Portela, 2016). In this sense, C. melanura and C. julis show differences in their thermal ranges, being cold water and warm water species, respectively. While C. melanura is a species more related to the Northeast Atlantic, but present in the western Mediterranean, C. julis is more related to the Mediterranean, being also present in the adjacent Atlantic waters, where does not show a clear distribution. In fact, C. julis does not seem to reach the northern Iberian Peninsula (Sanchez-Delgado, 1981), being only present in the Gulf of Cadiz in the closer area to the Strait of Gibraltar (probably not reaching the latitude of Cadiz according to our in situ observations). Moreover, Mediterranean individuals showing the Atlantic livery (C. melanura) seem to be more common in the Gulf of Lions, one of the coldest areas in the Mediterranean, where males with this livery can represent an important part of the male population of Coris specimens (Laurent and Lejeune, 1988). Otherwise, in warmer areas of the Mediterranean, differences in depth range between both species can be seen. In the Balearic Islands, while those specimens with typical morphology of C. julis are commonly distributed between the surface and 25 m depth (Fischer et al., 2007), although they can reach depths of more than 80 m (Martino and Grau, 2010), C. melanura has only been recorded from 55 to 160 m depth (Martino and Grau, 2010 and present study).
The presence of C. melanura specimens in the Mediterranean (Laurent and Lejeune, 1988; Corbera et al., 1996; Zabala et al., 2005; Martino and Grau, 2010), suggest the existence of a sympatric between C. melanura and C. julis, that would cover those neighboring areas to Gibraltar Strait, as the Gulf of Cadiz and the Alboran Sea, as well as to northward the western Mediterranean, at least to the Gulf of Lions. A similar pattern of secondary contact zone has been suggested for other sister species of Coris, C. gaimard and C. cuvieri, both species have been recorded at Christmas Island, an adjacent zone located on the border of its geographical distribution, the Indian and Pacific oceans, respectively (Ahti et al., 2016). According to Montanari et al. (2014), closely related fishes that have arisen by allopatric speciation are likely to hybridize upon secondary contact, but our results showed that there is no overlap of haplotypes between C. melanura and C. julis, suggesting that hybridization between these two species may not occur in the wild, reforcing the conclusion that the speciation process between these two species is completed.
Data Availability Statement
The DNA sequences obtained for three mitochondrial fragments were deposited in the GenBank database (http://www.ncbi.nlm.nih.gov/genbank/) under the following numbers: MW970114-NW970131; MW979469-MW979486; MZ044564-MZ044581; MZ230640; MZ230729; and MZ275234.
Author Contributions
SR-A and FO conceived and coordinated the present study. RF revised the morphological description and leaded the historical review of species synonyms. SR-A, FO, RF, IR-J, IB, and EM collected some samples and revised the manuscript. All authors approved the final manuscript.
Funding
This study was funded by the project LIFE IP INTEMARES, coordinated by the Biodiversity Foundation of the Ministry for the Ecological Transition and the Demographic Challenge. It receives financial support from the European Union’s LIFE programme (LIFE15 IPE ES 012). SR-A was supported by a postdoctoral contract co-funded by the Regional Government of the Balearic Islands and the European Social Fund. The MEDITS surveys are co-funded by the European Union through the European Maritime and Fisheries Fund (EMFF), within the National Program of collection, management and use of data in the fisheries sector and support for scientific advice regarding the Common Fisheries Policy.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank M. Cojan, J. Orejuela, E. Cifre, and L. Kunig to help in the collection of samples and Francisco Sanchez and M. C. Iglesias for providing us with specialized bibliography. We would also like to thank Societat de Història Natural de les Illes Balears (MNIB) and A. M. Grau and G. X. Pons for lending us the Coris samples as well as the Marine Fauna Collection of the Instituto Español de Oceanografía in the Centro Oceanográfico de Málaga, and Lourdes Fernández and Francisca Salmeron for their help with samples storage. We would also further like to thank all the participants in the MEDITS surveys, as well as the crew of R/V Miguel Oliver.
Footnotes
- ^ http://www.ncbi.nlm.nih.gov/genbank/
- ^ https://www.boldsystems.org
- ^ https://species.h-its.org/ptp/
References
Ahti, P. A., Coleman, R. R., DiBattista, J. D., Berumen, M. L., Rocha, L. A., and Bowen, B. W. (2016). Phylogeography of Indo-Pacific reef fishes: sister wrasses Coris gaimard and C. cuvieri in the Red Sea, Indian Ocean and Pacific Ocean. J. Biogeogr. 43, 1103–1115. doi: 10.1111/jbi.12712
Aiello, B. R., Westneat, M. W., and Hale, M. E. (2017). Mechanosensation is evolutionary turned to locomotor mechanics. PNAS 114, 4459–4464. doi: 10.1073/pnas.1616839114
Almada, V. C., Almada, F., Henriques, M., Santos, R. S., and Brito, A. (2002). On the phylogenetic affinities of Centrolabrus trutta and Centrolabrus caeruleus (Perciformes: Labridae) to the genus Symphodus: molecular, meristic and behavioural evidences. Arquipelago Life Mar. Sci. 19A, 85–92.
April, J., Hanner, R. H., Dion-Cote, A. M., and Bernatchez, L. (2012). Glacial cycles as an allopatric speciation pump in north-eastern American freshwater fishes. Mol. Ecol. 22, 409–422. doi: 10.1111/mec.12116
Arnal, C., Vernean, O., and Desdevises, Y. (2006). Phylogenetic relationship sand evolution of cleaning behavior in the family Labridae: importance of body colour pattern. Evol. Biol. 19, 755–763. doi: 10.1111/j.1420-9101.2005.01059.x
Artedi, P. (1738a). Genera Piscium. In Quibus Systema Totum Ichthyologiæ Proponitus Cum Classibus, Ordinibus, Generum Characteribus, Specierum Differentiis, Observationibus Plurimis. Redactis Speciebus 242 Ad Genera 52. Ichthyologiæ Pars 3. Lugduni Batavorum: Conradus Wishoff, 1–84.
Artedi, P. (1738b). Synonymia Nominum Piscium Fere Omnium; in Qua Recensio Fit Nominum Piscium, Omnium Facile Authorum, Qui Undam De Piscibus Scripsere: Uti Graecorum, Romanorum, Barbarorum, Nec Non-Omnium Insequentium Ichthyologorum, Una Cum Nominibus Inquilinis Variarum Nationum. Ichthyologiæ Pars 4. Lugduni Batavorum: Conradus Wishoff.
Aurelle, D., Guillemaud, T., Afonso, P., Morato, T., Wirtz, P., Santos, R. S., et al. (2003). Genetic study of Coris julis (Osteichtyes, Perciformes, Labridae) evolutionary history and dispersal abilities. C. R. Biol. 326, 771–785. doi: 10.1016/j.crvi.2003.08.001
Ávila, S. P., Melo, C., Berning, B., Sá, N., Quartau, R., Rijsdijk, K. F., et al. (2019). Towards a ‘Sea-Level Sensitive’ dynamic model: impact of island ontogeny and glacio-eustasy on global patterns of marine island biogeography. Biol. Rev. 94, 1116–1142. doi: 10.1111/brv.12492
Bacci, G., and Razzauti, A. (1957). Falso gonocorismo in “Coris julis” (L.) 181-189. R.C. Accad. dei Lincei 23, 181–189.
Barber, P. H., and Bellwood, D. R. (2005). Biodiversity hotspots: evolutionary origins of biodiversity in wrasses (Halichoeres: Labridae) in the Indo-Pacific and new world tropics. Mol. Phylogenet.Evol. 35, 235–253. doi: 10.1016/j.ympev.2004.10.004
Bauchot, M. L., and Quignard, J. P. (1973). Labridae (in CLOFNAM I). Check-list of the fishes of the north-eastern Atlantic and the Mediterranean. Paris: UNESCO.
Blache, Cadenat, J., and Stauch, A. (1970). “Clés de détermination des poissons de mer signalés dans l’Athantique oriental (entre le 20° parallele nord et le 15° parallele sud.),” in Proceedings of the Faune tropical Vol. XVIII. Office de la Recherche Scientifique et Technique Outre Mer, Paris.
Bonnaterre, J. P. (1788). Ichthyologie. Tableau Encyclopédique Et Méthodique Des Trois Règnes De La Nature. Paris: Elsevier. doi: 10.5962/bhl.title.11660
Brünnich, M. T. (1768). Ichthyologia Massiliensis, Sistens Piscium Descriptiones Eorumque Apud Incolas Nomina. Accedunt Spolia Maris Adriatici. Paris: Hafniae et Lipsiae. doi: 10.5962/bhl.title.5782
Cetti, F. (1777). “Anfibi e pesci di Sardegna,” in Storia Naturale di Sardegna, eds G. Piatoli and I. Sassari (Zurich: Apvd Christ). doi: 10.5962/bhl.title.5783
Charleton, W. (1668). Onomasticon zoicon, plerorumque animalium differentias and nomina propria pluribus linguis exponens. J. Allestry 50, 1–309.
Corbera, J., Sabateìs, A., and Garcia-Rubies, A. (1996). Peces De Mar De la Peniìnsula Ibeìrica. Barcelona: Ed. Planeta.
Cuvier, G. L. C., and Valenciennes, A. (1839). Histoire Naturelle Des Poissons. Tome treizième. Livre seizième. Des Labroïdes. Tome 13. Paris: Pitois-Levrault, 369–388.
Day, F. (1880). “Labridae,” in The Fishes of Great Britain and Ireland, ed. F. Day (London: Williams and Norgate), 251–270. doi: 10.5962/bhl.title.55922
Delrieu-Trottin, E., Williams, J. T., Pitassy, D., Driskell, A., Hubert, N., Viviani, J., et al. (2019). A DNA barcode reference library of French Polynesian shore fishes. Sci. Data 6:114. doi: 10.1038/s41597-019-0123-5
DiBattista, J. D., Waldrop, E., Bowen, B. W., Schultz, J. K., Gaither, M. R., Pyle, R. L., et al. (2012). Twisted sister species of Pygmy Angelfishes: discordance between taxonomy, coloration, and phylogenetics. Coral Reefs 31, 839–851. doi: 10.1007/s00338-012-0907-y
Eschmeyer, W. N., Ferraris, C. J., Hoang, D., and Long, D. J. (1998). Species of Fishes- in Catalog of Fishes. San Francisco, CA: California Academy of Sciences.
Fischer, S. T., Patzner, R. A., Müller, C. H. G., and Winler, H. M. (2007). Studies on the ichthyofauna of the coastal waters of Ibiza (Balearic Islands, Spain). Rostocker Meeresbiol. Beiträge 18, 30–62.
Fleming, J. (1828). A History Of British Animals, Exhibiting The Descriptive Characters And Systematical Arrangement Of The Genera And Species Of Quadrupeds, Birds, Reptiles, Fishes, Mollusca, And Radiata Of The United Kingdom; Including The Indigenous, Extirpated, And Extinct Kinds, Together With Periodical And Occasional Visitants. Edinburgh: Ball and Bradfute, James Duncan. doi: 10.5962/bhl.title.12859
Fowler, H. W. (1919). The fishes of the United States Eclipse Expedition to West Africa. Proc. U. S. Natl. Museum 56, 195–292. doi: 10.5479/si.00963801.56-2294.195
Fowler, H. W. (1936). The marine fishes of west Africa, based on the collection of the American Museum Congo Expedition 1909 - 1915. Bull. Am. Museum Nat. Hist. 70, 1–1943.
Freitas, R., Romeiras, M., Silva, L., Cordeiro, R., Madeira, P., González, J. A., et al. (2019). Restructuring of the ‘Macaronesia’ biogeographic unit: a marine multi-taxon biogeographical approach. Sci. Rep. 9:15792. doi: 10.1038/s41598-019-51786-6
Fricke, R. (1983). A method of counting caudal fin rays of actinopterygian fishes. Braunschweiger Naturkundliche Schriften 1, 729–733.
Fricke, R. (2008). Authorship, availability and validity of fish names described by Peter (Pehr) Simon Forsskål and Johann Christian Fabricius in the ’Descriptiones animalium’ by Carsten Niebuhr in 1775 (Pisces). Stuttgarter Beiträge zur Naturkunde A Neue Serie 1, 1–76.
Fricke, R., Eschmeyer, W. N., and van der Laan, R. (2021). Eschmeyer’s Catalog Of Fishes: Genera, Species, References. Available online at: http://research.calacademy.org/research/Ichthyology/Catalog/fishcatmain.asp (accessed May 4, 2021).
Fruciano, C., Hanel, R., Debes, P. V., Tigano, C., and Ferrito, V. (2011). Atlantic-Mediterranean and within-Mediterranean molecular variation in Coris julis (L. 1758) (Teleostei, Labridae). Mar. Biol. 158, 1271–1286. doi: 10.1007/s00227-011-1647-1
Gesner, C. (1558). Historiæ Animalium Liber IIII, Qui Est De Piscium Et Aquatilium Animantium Natura. Cvm Iconibvs Singvlorvm Ad Vivvm Expressis Fere Omnib. DCCVI. C. Zurich: Apvd Christ.
Gesner, C. (1598). Fischbuch, das ist außführliche Beschreibung und lebendige Conterfactur aller unnd jeden Fischen. J. Saur, Frankfurt am Meyn 58, 1–202.
Grant, W. S., and Bowen, B. W. (1998). Shallow population histories in deep evolutionary lineages of marine fishes: insights from sardines and anchovies and lessons for conservation. J. Hered. 89, 415–426. doi: 10.1093/jhered/89.5.415
Gronow, L. T. (1756). Museum Ichthyologicum, Sistens Piscium Indigenorum Et Quorundam Exoticorum, Qui In Museo Laur. Theod. Gronovii adservantur, Descriptiones, Ordine Systematico; Accedunt Nonullorum Exoticorum Piscium Icones, Aeri Incisae. Tomus 2. Lugduni Batavorum: Theodor Haak.
Gronow, L. T. (1763). Zoophylacii Gronoviani Fasciculus Primus Exhibens Animalia Quadrupeda, Amphibia Atque Pisces, Quae In Museo Suo Adservat, Rite Examinavit, Systematice Disposuit, Descripsit Atque Iconibus Illustravit Laur. Lugduni Batavorum: Theod. Gronovius, 1–136.
Guillemaud, T., Cancela, M. L., Afonso, P., Morato, T., Santos, R. S., and Wirtz, P. (2000). Molecular insights into the taxonomic status of Coris atlantica (Pisces: Labridae). JMBA 80, 929–933. doi: 10.1017/S0025315400002915
Günther, A. (1862). Catalogue of the Fishes in the British Museum. London: Catalogue of the Acanthopterygii Pharyngognathi and Anacanthini in the collection of the British Museum.
Günther, A. (1880). Report on the shore fishes. Rep. sci. res. voy. H.M.S. Challenger, 1873-76. J. Zool. 1, 1–82. doi: 10.5962/bhl.title.51598
Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 41, 95–98.
Hanel, R., Westneat, M. W., and Sturmbauer, C. (2002). Phylogenetic relationships, evolution of broodcare behavior, and geographic speciation in the Wrasse tribe Labrini. J. Mol. Evol. 55, 776–789. doi: 10.1007/s00239-002-2373-6
Hasegawa, M., Kishino, H., and Yano, T. (1985). Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J. Mol. Evol. 22, 160–174. doi: 10.1007/BF02101694
Hasselquist, F. (1757). Fredric Hasselquists M.D., Societ. Reg. Scient. Upsal. and Stockholm Soc., Iter Palæstinum Eller Resa Til Heliga Landet, Förråttad Ifrån År 1749 til 1752, Med Besfrikningar, Rön, Anmärkningar Öfverde Märkvårdigaste Naturalier, På Hennes Kongl. Maj:ts befallning, utgiven af Carolus Linnæus. Stockholm: Lars Salvius. doi: 10.5962/bhl.title.112563
Hasselquist, F. (1762). D. Friedrich Hasselquists, der Akademien der Wissenschaften zu Stockholm und Upsala Mitglieds, Reise nach Palästina in dem Jahren von 1749 bis 1752. Auf Befehl Ihro Majestät der Königinn von Schweden herausgegeben von Carl Linnaeus. Aus dem Schwedischen [übersetzt von T. H. Gadebusch]. Rostock: Johann Christian Koppem.
Hodge, J. R., Read, C. I., van Herwerden, L., and Bellwood, D. R. (2012). The role of peripheral endemism in species diversification: evidence from the coral reef fish genus Anampses (Family: Labridae). Mol. Phylogenet. Evol. 62, 653–663. doi: 10.1016/j.ympev.2011.11.007
Hubert, N., Hanner, R., Holm, E., Mandrak, N., Taylor, E., Burridge, M., et al. (2008). Identifying Canadian freshwater fishes through DNA barcodes. PLoS One 3:e2490. doi: 10.1371/journal.pone.0002490
Hubert, N., Meyer, C. P., Bruggemann, H. J., Gueìrin, F., Komeno, R. J. L., Espiau, B., et al. (2012). Cryptic diversity in Indo-Pacific coral-reef fishes revealed by DNA- barcoding provides new support to the Centre-of-Overlap hypothesis. PLoS One 7:e28987. doi: 10.1371/journal.pone.0028987
Ivanova, N. V., Zemlak, T. S., Hanner, R., and Hebert, P. D. N. (2007). Universal primer cocktails for fish DNA barcoding. Mol. Ecol. Notes 7, 544–548. doi: 10.1111/j.1471-8286.2007.01748.x
Kasumyan, A. O. (2003). The lateral line in fish: structure, function, and behavior. J. Ichthyol. 43, 175–213.
Kazancioglu, E., Near, T. J., Hanel, R., and Wainwright, P. C. (2009). Influence of sexual selection and feeding functional morphology on diversification rate of parrot fishes (Scaridae). Proc. R. Soc. B 276, 3439–3446. doi: 10.1098/rspb.2009.0876
Kimura, M. (1980). A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16, 111–120. doi: 10.1007/BF01731581
Klein, J. T. (1749). Iacobi Theodori Klein Historiæ Piscium [Promovendæ Missus Primus De Lapillis Eorumque Numero in Craniis Piscium, Cum Præfatione: De Piscium Auditu. Accesserunt I. Anatome tursionum; II. Observata in capite raiæ]. Tomus 5. Gedanii: Schreiber, 1–120.
Kocher, T. D., Thomas, W. K., Meyer, A., Edwards, S. V., Pääbo, S., Villablanca, F. X., et al. (1989). Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. PNAS 86, 6196–6200. doi: 10.1073/pnas.86.16.6196
Kuwamura, K., Sunobe, T., Sakai, Y., Kadota, T., and Sawada, K. (2020). Hermaphroditism in fishes: an annotated list of species, phylogeny, and mating system. Ichthyol. Res. 67, 341–360. doi: 10.1007/s10228-020-00754-6
Landi, M., Dimech, M., Arculeo, M., Biondo, G., Martins, R., Carneiro, M., et al. (2014). DNA barcoding for species assignment: the case of Mediterranean marine fishes. PLoS One 9:e106135. doi: 10.1371/journal.pone.0106135
Laurent, L., and Lejeune, P. (1988). Coexistence en Méditerranée de deux livrées terminales différentes chez la girelle Coris julis (Pisces, Labridae). Cybium 12, 91–95.
Lavoué, S., Miya, M., Arnegard, M. E., McIntyre, P. B., Mamonekene, V., and Nishida, M. (2011). Remarkable morphological stasis in an extant vertebrate despite tens of millions of years of divergence. Proc. R. Soc. B 278, 1003–1008. doi: 10.1098/rspb.2010.1639
Leigh, J. W., and Bryant, D. (2015). PopART: full-feature software for haplotype network construction. Methods Ecol. Evol. 6, 1110–1116. doi: 10.1111/2041-210X.12410
Linnaeus, C. (1758). Systema Naturae. Ed. X. (Systema Naturae Per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, Cum Characteribus, Differentiis, Synonymis, Locis. Tomus I. Editio Decima, Reformata). Zurich: Apvd Christ. doi: 10.5962/bhl.title.542
Linnaeus, C. (1764). Museum S. R. M. Adolphi Friderici Regis Suecorum, Gothorum, Vandalorumque, in Quo Animalia Rariora Imprimis Et Exotica. Tomus Secundi Prodromus. Aves, Amphibia, Pisces. Zurich: Apvd Christ.
Lowe, R. T. (1839). A supplement to a synopsis of the fishes of Madeira. Proc. Zool. Soc. Lond. 1839, 76–92.
Martino, S., and Grau, A. M. (2010). PreseÌncia de la donzella, Coris julis (Linnaeus, 1758), amb lliurea atlaÌntica (Osteichthyes: Labridae) a les Illes Balears (MediterraÌnia occidental). Boll. Soc. d’Història Nat. Les Balears 53, 153–160.
Matschiner, M., Hanel, R., and Salzburger, W. (2011). On the origin and trigger of the notothenioid adaptive radiation. PLoS One 6:e18911. doi: 10.1371/journal.pone.0018911
Mayr, E. (1947). Ecological factors in speciation. Evolution 1, 263–288. doi: 10.1111/j.1558-5646.1947.tb02723.x
Meynard, C. N., Mouillot, D., Mouquet, N., and Douzery, E. J. P. (2012). A Phylogenetic perspective on the evolution of Mediterranean teleost fishes. PLoS One 7:e36443. doi: 10.1371/journal.pone.0036443
Michel, C., Lejeune, P., and Voss, J. (1987). Biologie et comportement des labridés européens. Rev. Franç. d’Aquariol.: Herpétolie 14, 1–80.
Montanari, S. R., Hobbs, J. P. A., Pratchett, M. S., Bay, L. K., and van Herwerden, L. (2014). Does genetic distance between parental species influence outcomes of hybridization among coral reef butterflyfishes? Mol. Ecol. 11, 2757–2770. doi: 10.1111/mec.12762
Muths, D., Davoult, D., Jolly, M. T., Gentil, F., and Jollivet, D. (2010). Pre-zygotic factors best explain reproductive isolation between the hybridizing species of brittle-stars Acrocnida brachiata and A. spatulispina (Echinodermata: Ophiuroidea). Genetica 138, 667–679. doi: 10.1007/s10709-010-9441-4
Naro, G. D. (1847). Sinonimia Moderna Delle Specie Registrate Nell’ Opera Intitolata: “Descrizione De’ Crostacei, De’ Testacei E De’ Pesci Che Abitanno Le Lagune E Golfo Veneto Rappresentati in Figure À Chiaro-Scuro Ed A Colori”. Venice: Antonelli, 1–128. doi: 10.5962/bhl.title.120206
Niebuhr, C. (1775). Descriptiones Animalium Avium, Amphibiorum, Piscium, Insectorum, Vermium; Quae in Itinere Orientali Observavit Petrus Forskål. Hauniae: Post mortem auctoris edidit Carsten Niebuhr, 1–20.
Opinion 57. (1914). Names Dating from Hasselquist’s “Iter Palaestinum,” 1757, and the Translation, 1762, are Untenable. New York, NY: Smithsonian Institution Publication.
Palumbi, S. R. (1996). “Nucleic acids II: the polymerase chain reaction,” in Molecular Systematics. Sunderland, eds D. M. Hillis, C. Moritz, and B. K. Mable (Massachusetts: Sinauer Associates Inc), 205–248.
Parenti, P., and Randall, J. E. (2000). An annotated checklist of the species of the Labroid fish families Labridae and Scaridae. Ichthyol. Bull. J. L. B. Smith Instit. Ichthyol. 68, 1–97.
Parenti, P., and Randall, J. E. (2018). A checklist of wrasses (Labridae) and parrotfishes (Scaridae) of the world: 2017 update. J. Ocean Sci. Found. 30, 11–27. doi: 10.5281/zenodo.1247552
Quignard, J. P., and Pras, A. (1986). “Labridae,” in Fishes of the North-eastern Atlantic and the Mediterranean, eds P. J. P. Whitehead, M. L. Bauchot, J. C. Hureau, J. Nielsen, and E. Tortonese (Paris: UNESCO), 919–942. doi: 10.2307/1444931
Rafinesque, C. S. (1810). Indice D’ittiologia Siciliana; Ossia, Catalogo Metodico Dei Nomi Latini, Italiani, E Siciliani Dei Pesci, Che Si Rinvengono In Sicilia Disposti Secondo Un Metodo Naturale E Seguito Da Un Appendice Che Contiene La Descrizione De Alcuni Nuovi Pesci Siciliani. Messina: G. del Nobolo, 1–70. doi: 10.5962/bhl.title.58965
Ramírez-Amaro, S., Ordines, F., Picornell, A., Castro, J. A., Ramon, M. M., Massutí, E., et al. (2018). The evolutionary history of Mediterranean Batoidea (Chondricththyes: Neoselachii): a molecular phylogenetic approach. Zool. Scr. 47, 686–698. doi: 10.1111/zsc.12315
Randall, J. E. (1998). Zoogeography of shore fishes of the Indo-Pacific region. Zool. Stud. 37, 227–268.
Randall, J. E. (1999). Revision of the Indo-Pacific fishes of the genus Coris, with descriptions of five new species. Indo Pac. Fish. 29, 1–74.
Randall, J. E. (2013). Severn new species of labrid fishes (Coris, Iniistius, Macropharyngodon, Novaculops and Pterogogus) from the Western Indian Ocean. J. Ocean Sci. Found. 7, 1–43. doi: 10.5281/zenodo.1041964
Ratnasingham, S., and Hebert, P. D. N. (2007). BOLD: the barcode of life data system (www.barcodinglife.org). Mol. Ecol. Notes 7, 355–364. doi: 10.1111/j.1471-8286.2007.01678.x
Read, C. I., Bellwood, D. R., and van Herwerden, L. (2006). Ancient origins of the Indo-Pacific coral reef fish Biodiversity: a case study of the leopard wrasse (Labridae: Macropharyngodon). Mol. Phylogenet. Evol. 38, 808–819. doi: 10.1016/j.ympev.2005.08.001
Risso, A. (1810). Ichthyologie de Nice, ou histoire naturelle des poissons du Département des Alpes Maritimes. Paris: F. Schoell, 1–11. doi: 10.5962/bhl.title.7052
Risso, A. (1827). Histoire Naturelle Des Principales Productions De L’europe Méridionale, Et Particulièrement De Celles Des Environs De Nice Et Des Alpes Maritimes. Tome 3. Paris: F. G. Levrault, 1–16. doi: 10.5962/bhl.title.58984
Roede, M. J. (1966). Notes on the labrid fish Coris julis (Linnaeus, 1758) with emphasis on dichromatism and sex. Vie Milieu 17, 1317–1333.
Rondelet, G. (1554). Libri De Piscibus Marinis, In Quibus Veræ Piscium Effigies Expressæ Sunt. Lugduni: M. Bonhomme. doi: 10.5962/bhl.title.64229
Ronquist, F., Teslenko, M., Van Der Mark, P., Ayres, D. L., Darling, A., Hohna, S., et al. (2012). Mrbayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Roux, C. H. (1961). A propos d’un Poisson de la famille des Labridae, de la côte occidentale d’Afrique: Coris atlantica Günther 1862. Bull. Museìum Natl. d’Hist. Nat. 33, 387–390.
Sanchez-Delgado, F. (1981). Contribución al conocimiento de los labridos (familia Labridae) de las costas ibéricas. Parte I. Descripción de las especies. Bol. Instit. Español Oceanogr. 6, 19–57.
Schöpf, J. D. (1788). “Beschreibungen einiger nord-amerikanischer Fische, vorzüglich aus den neu-yorkischen Gewässern,” in Schriften der Berlinischen Gesellschaft Naturforschender Freunde 8, ed. F. Zu (Charleston: Nabu Press).
Steindachner, F. (1863). Ichthyologische Mittheilungen. (VI.) [With subtitles I-IV]. Verhandlungen Kaiserlich Königlichen Zool. Bot. Gesellschaft Wien 13, 1189–1192.
Steinke, D., Connell, A., and Hebert, P. N. (2016). Linking adults and immatures of South African marine fishes1. Genome 59, 959–967. doi: 10.1139/gen-2015-0212
Steinke, D., de Waard, J., Gomon, M., Johnson, J., Larson, H., Lucanus, O., et al. (2017). DNA barcoding the fishes of Lizard Island (Great Barrier Reef). Biodivers. Data J. 5:e12409. doi: 10.3897/BDJ.5.e12409
Steinke, D., Zemlak, T. S., and Hebert, P. D. N. (2009). Barcoding Nemo: DNA-based identifications for the ornamental fish trade. PLoS One 4:e6300. doi: 10.1371/journal.pone.0006300
Taboada, S., and Pérez-Portela, R. (2016). Contrasted phylogeographic patterns on mitochondrial DNA of shallow and deep brittle star across the Atlantic-Mediterranean area. Sci. Rep. 6:32425. doi: 10.1038/srep32425
Tamura, K., Stecher, G., Peterson, D., Filipski, A., and Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. doi: 10.1093/molbev/mst197
Thompson, J. D., Higgins, D. G., and Gibson, T. J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680. doi: 10.1093/nar/22.22.4673
Victor, B. C., Alfaro, M. E., and Sorenson, L. (2013). Rediscovery of Sagittalarva inornata n. gen., n. comb. (Gilbert, 1890) (Perciformes: Labridae), a long-lost deepwater fish from the eastern Pacific Ocean: a case study of a forensic approach to taxonomy using DNA barcoding. Zootaxa 3669, 551–570. doi: 10.11646/zootaxa.3669.4.8
Walbaum, J. J. (1792). Petri Artedi sueci genera piscium. In quibus systema totum ichthyologiae proponitur cum classibus, ordinibus, generum characteribus, specierum differentiis, observationibus plurimis. Redactis speciebus 242 ad genera 52. Ichthyologiae pars III. Ant. Ferdin. 1, 1–3.
Ward, R. D., Hanner, R., and Hebert, P. D. N. (2009). The campaign to DNA barcode all fishes, FISH-BOL. J. Fish Biol. 74, 329–356. doi: 10.1111/j.1095-8649.2008.02080.x
Ward, R. D., and Holmes, B. H. (2007). An analysis of nucleotide and amino acid variability in the barcode region of cytochrome c oxidase I (cox1) in fishes. Mol. Ecol. Notes 7, 899–907. doi: 10.1111/j.1471-8286.2007.01886.x
Westneat, M. W., and Alfaro, M. E. (2005). Phylogenetic relationships and evolutionary history of the reef fish family Labridae. Mol. Phylogenet. Evol. 36, 370–390. doi: 10.1016/j.ympev.2005.02.001
Willughby, F. (1686). De Historia Piscium Libri Quatuor, Jussu and Sumptibus Societatis Regiæ Londinensis editi. Totum Opus Recognovit, Coaptavit, Supplevit, Librum Etiam Primum And Secundum Integros Adjecit Johannes Raius e Societate Regia. Tomus I. Oxonii: Theatro Sheldoniano.
Zabala, M., Garcia-Rubies, A., and Corbera, J. (2005). “Els peixos de les illes Medes i del litoral catalaÌ, guia per observar-los al seu ambient,” in Col⋅leccioì Norai, 8. Edn, ed. D. M. Escola (Badalona: Centre d’Estudis Marins de Badalona).
Keywords: wrasses, divergence, livery, Mediterranean, eastern Atlantic, species delimitation, species complex
Citation: Ramírez-Amaro S, Ordines F, Fricke R, Ruiz-Jarabo I, Bolado I and Massutí E (2021) Genetic and Morphological Evidence to Split the Coris julis Species Complex (Teleostei: Labridae) Into Two Sibling Species: Resurrection of Coris melanura (Lowe, 1839) Redescription of Coris julis (Linnaeus, 1758). Front. Mar. Sci. 8:744639. doi: 10.3389/fmars.2021.744639
Received: 20 July 2021; Accepted: 07 September 2021;
Published: 01 October 2021.
Edited by:
Sophie von der Heyden, Stellenbosch University, South AfricaReviewed by:
Sergio R. Floeter, Federal University of Santa Catarina, BrazilSébastien Lavoué, Universiti Sains Malaysia (USM), Malaysia
Copyright © 2021 Ramírez-Amaro, Ordines, Fricke, Ruiz-Jarabo, Bolado and Massutí. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sergio Ramírez-Amaro, c3JncmFtaXJlejA4QGdtYWlsLmNvbQ==; c2VyZ2lvLnJhbWlyZXpAaWVvLmVz
†These authors have contributed equally to this work and share first authorship
 Sergio Ramírez-Amaro
Sergio Ramírez-Amaro Francesc Ordines1†
Francesc Ordines1† Ronald Fricke
Ronald Fricke